Abstract
Background
Consensus has not been reached on what constitutes an optimal diet in individuals with prediabetes and type 2 diabetes mellitus (T2DM), especially between low-carbohydrate options.
Objectives
We compared 2 low-carbohydrate diets with 3 key similarities (incorporating nonstarchy vegetables and avoiding added sugars and refined grains) and 3 key differences (incorporating compared with avoiding legumes, fruits, and whole, intact grains) for their effects on glucose control and cardiometabolic risk factors in individuals with prediabetes and T2DM.
Methods
Keto-Med was a randomized, crossover, interventional trial. Forty participants aged ≥18 years with prediabetes or T2DM followed the well-formulated ketogenic diet (WFKD) and the Mediterranean-plus diet (Med-Plus) for 12 weeks each, in random order. The diets shared the 3 key similarities noted above. The Med-Plus incorporated legumes, fruits, and whole, intact grains, while the WFKD avoided them. The primary outcome was the percentage change in glycated hemoglobin (HbA1c) after 12 weeks on each diet. Secondary and exploratory outcomes included percentage changes in body weight, fasting insulin, glucose, and blood lipids; average glucose from continuous glucose monitor (CGM), and nutrient intake.
Results
The primary analysis was of 33 participants with complete data. The HbA1c values did not differ between diets at 12 weeks. Triglycerides decreased more for the WFKD [percentage changes, −16% (SEM, 4%) compared with −5% (SEM, 6%) for the Med-Plus; P = 0.02] and LDL cholesterol was higher for the WFKD [percentage changes, +10% (SEM, 4%) compared with −5% (SEM, 5%) for the Med-Plus; P = 0.01]. Weight decreased 8% (SEM, 1%) compared with 7% (SEM, 1%) and HDL cholesterol increased 11% (SEM, 2%) compared with 7% (SEM, 3%) for the WFKD compared with the Med-Plus, respectively; however, there was a significant interaction of diet × order for both. Participants had lower intakes of fiber and 3 nutrients on the WFKD compared with the Med-Plus. Twelve-week follow-up data suggest the Med-Plus is more sustainable.
Conclusions
HbA1c values were not different between diet phases after 12 weeks, but improved from baseline on both diets, likely due to several shared dietary aspects. The WFKD led to a greater decrease in triglycerides, but also had potential untoward risks from elevated LDL cholesterol and lower nutrient intakes from avoiding legumes, fruits, and whole, intact grains, as well as being less sustainable. This trial was registered at clinicaltrials.gov as NCT03810378.
Keywords: Mediterranean, ketogenic, diet, diabetes, prediabetes, HbA1c, metabolomic, intervention, human
Introduction
Consensus has not been reached on what constitutes an optimal diet in individuals with prediabetes and type 2 diabetes mellitus (T2DM). In 2018–2019, both Diabetes UK and the American Diabetes Association (ADA) released nutrition guidelines for preventing and managing diabetes (1, 2), endorsing an individualized approach and agreeing on several key nutrition recommendations, including: 1) incorporating nonstarchy vegetables; and 2) minimizing added sugars and refined grains. Diabetes UK also emphasized considering food patterns over macronutrient composition and gave the highest rating for the Mediterranean diet for patients with and at risk for T2DM. In contrast, the ADA did not recommend a specific, single dietary pattern, but recommended several, including the Mediterranean diet and low-carbohydrate (low-carb) and very low-carb diets. In addition, for the first time, the ADA suggested reducing overall carbohydrate intake to decrease blood glucose levels and noted that in select adults with T2DM, low- or very low-carb eating patterns were viable approaches.
Despite this guidance, little research exists to make informed decisions on the benefits and risks of low-carb diet patterns that differ in the level of carbohydrate restriction, particularly in individuals with or at risk for T2DM. Instead, previous studies have focused on comparing low-carb to low-fat eating patterns (3–10). In addition, most studies in this area have not prioritized matching the intervention diets on key nutrition recommendations that are agreed upon by proponents across a wide variety of diet types that emphasize consumption of nonstarchy vegetables and discourage consumption of added sugars and refined grains (11).
Our study is unique in comparing 2 low-carb diets—the well-formulated ketogenic diet (WFKD) and the Mediterranean-plus diet (Med-Plus)—that both incorporate 3 key nutrition messages endorsed by diabetes organizations (1, 2): including nonstarchy vegetables, restricting added sugars, and limiting refined grains. The main differences between the 2 diets involve legumes, fruits, and whole, intact grains, which are avoided for the WFKD and included for the Med-Plus. These 3 food groups are consistently recommended by national and international public health organizations based on extensive evidence of cardiovascular benefits of fiber, antioxidants, and the vitamins and minerals characteristic of those food groups (2, 12–15). We hypothesized that after 12 weeks on each diet, glycated hemoglobin (HbA1c) values would not be different, but would be similarly improved from baseline due to the 3 shared dietary characteristics of including nonstarchy vegetables, restricting added sugars, and limiting refined grains, but the WFKD would have more adverse health and cardiometabolic risks due to the avoidance of legumes, fruits, and whole, intact grains.
Methods
Study design
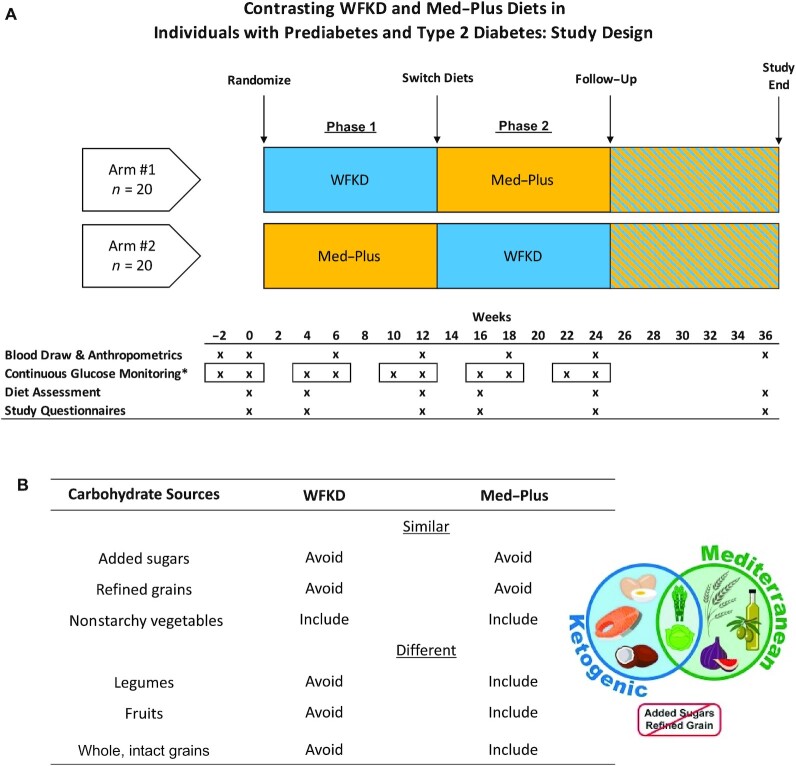
The Keto-Med study was a single-site, randomized, crossover clinical trial comparing 2 metabolically distinct diets—the WFKD compared with the Med-Plus—among individuals with prediabetes and T2DM. The primary outcome was the percentage change in HbA1c from baseline after 12 weeks on each diet; secondary outcomes included fasting insulin and blood lipid values. Exploratory outcomes included 3 continuous glucose monitor (CGM) metrics (average glucose, time-in-range, and CV), as well as assessing diet quality and adherence throughout the study and for 12 weeks following the diet intervention. The study design is illustrated in Figure 1A. Microbiome data generated in this trial are not included here due to complexity of the findings and space limits; it is anticipated that these findings will be presented in a separate publication.
FIGURE 1.
(A) Keto-Med randomized trial study design. *CGM worn for a 2-week interval at each time, as indicated by the square borders. (B) Key similarities and differences for the 2 Keto-Med diet patterns. Abbreviations: CGM, continuous glucose monitoring; Med-Plus, Mediterranean-plus diet; WFKD, well-formulated ketogenic diet.
Procedures for this study were followed in accordance with the ethical standards from the Helsinki Declaration and were approved by the Stanford University Human Subjects Committee (institutional review board protocol 49218). All study participants provided written informed consent.
Modifications to the study design due to coronavirus disease 2019
A portion of this study was impacted by the coronavirus disease 2019 (COVID-19) shelter-in-place orders (initiated 16 March 2020). A full description of the study modifications is provided in the Supplemental Methods. Briefly, participants whose end-of-phase blood draws were scheduled during the initial shelter-in-place order were asked to extend the duration of their assigned diets until research staff were able to restart in-person blood draw visits in early June 2020. All data collection that was designed from the onset to be collected remotely continued without disruption during the shelter-in-place, with data collected according to the original timeline; this included online surveys, dietary recalls (via phone call), CGMs, and stool kits (mailed directly to participants). Of all study variables, blood pressure data were the most severely impacted by COVID-19; no blood pressure results are presented due to a high proportion of missing data.
Participants
The inclusion criteria were adults ≥18 years of age with a diagnosis of prediabetes (HbA1c 5.7–6.4% or fasting glucose 100–125 mg/dL) (16) or T2DM (HbA1c ≥6.5% or fasting glucose ≥126 mg/dL) (16). Participants were recruited from the San Francisco Bay area. Recruitment strategies included mass mailings to e-mail lists (e.g., prior study participants, individuals who expressed an interest in past studies and asked to be added to a mailing list) and patient referrals from the clinic of coinvestigator Sun H Kim. Participants were required to have a stable dietary history, defined as neither adding nor eliminating a major food group in their diet for at least the previous month. Race and ethnicity were self-reported.
The exclusion criteria were weight <110 lbs (50 kg); BMI ≥40 kg/m2; LDL cholesterol >190 mg/dL; systolic blood pressure >160 mmHg; or diastolic blood pressure >90 mmHg. Participants were also excluded if they were taking certain antihyperglycemic medications (e.g., insulin, glucagon-like peptide 1 receptor agonists, or sodium-glucose cotransporter 2 inhibitors) or weight loss medications. A full description of the exclusion criteria is available at clinicaltrials.gov; this trial was registered at clinicaltrials.gov as NCT03810378.
Participant and public involvement
The public was not involved in the original design of the study. Once enrolled, participants were encouraged to act as citizen scientists and provide suggestions and/or feedback as to any modifications that would improve the conduct of the study. Once the study was completed, the participants were all invited to a group results presentation and were encouraged to help share and disseminate the research results once published.
Sample size
The sample size was determined by resource availability.
Randomization
Eligible participants were randomly assigned to 1 of 2 diet sequences: the WFKD for 12 weeks (phase 1) and then the Med-Plus for 12 weeks (phase 2), or the opposite order. Randomization was stratified by prediabetes compared with T2DM status. Diet randomization was performed using an allocation sequence determined by computerized, random-number generation in block sizes of 4 by a statistician not involved in the intervention delivery or data collection. Participants did not learn of their diet sequence until they had completed all baseline laboratory measures and surveys.
Dietary intervention
The study intervention involved having all participants maintain 2 dietary patterns, the WFKD and Med-Plus, for 12 weeks each. Ad libitum intake was advised, and participants were guided to follow 2 sets of dietary guidelines that shared 3 important similarities and 3 important differences.
During the WFKD phase, participants were counseled to sustain nutritional ketosis by limiting carbohydrates to 20–50 g/day and keeping proteins to ∼1.5 g/kg ideal body weight/day, with the remaining kcals coming from fats. This meant they were to exclude legumes, most fruits (limited amounts of some berries were allowed), all grains, and all sugars. These criteria are based on the recommendations of Volek and Phinney (17). Participants were also instructed to consume >3 servings/day of nonstarchy vegetables and maintain adequate mineral and fluid intake for the ketogenic state (sodium, 3–5 g/day; potassium, 3–4 g/day) (18).
During the Med-Plus phase, participants were encouraged to sustain a Mediterranean diet based on recommendations from the Mediterranean Diet Pyramid (19), with the additional restriction of avoiding added sugars and refined grains (hence, the referral to this as the Med-Plus). Instructions were to follow a mostly plant-based diet that included vegetables (including starchy vegetables); legumes; fruits; whole, intact grains; nuts; and seeds, with fish as the primary animal protein and olive oil as the primary fat.
In both dietary phases, whole foods were promoted and all processed foods and added sugars were strongly discouraged (Figure 1B). There was no prescribed washout period between intervention phases; therefore, participants immediately began either the WFKD (if on the Med-Plus first) or the Med-Plus (if on the WFKD first) for the second 12-week phase.
Participants received weekly, individual nutrition counseling and education sessions by a registered dietitian and certified diabetes educator via email, phone, or videotelephony (Zoom Video Communications), with phase-transition meetings occurring face-to-face prior to the COVID-19 pandemic. Participants needing extra support or expressing greater interest in learning more about their assigned diet were provided with additional sessions as requested, up to a maximum of 3 times per week.
Food-delivery period
Participants were provided, at no cost, with all meals and snacks for the first 4 weeks of each phase of the study (i.e., weeks 1–4 and weeks 13–16) by a San Francisco Bay area food-delivery service (Methodology; https://www.gomethodology.com/) (20). Research staff worked closely with the food-delivery company to develop a set of menu offerings to match a high-quality ketogenic diet and a high-quality Mediterranean diet. During those 4 weeks, meals were delivered once per week, with 7 days’ worth of menu items per delivery. Notably, Methodology is a dairy-free and gluten-free operation.
Although weight loss was not discouraged, the study diet design did not include a prescribed energy restriction and was not intended to be a weight loss study. Participants were told to eat until they were satiated throughout the study. In collaboration with the Methodology culinary team, it was determined that it would not be possible to tailor the delivery of different energy intake levels for different participants in different weeks. The decision was made to estimate a single, total energy level that was likely to satiate the study participants with the greatest energy needs, so that no one would need to seek any foods outside of those provided. That level was determined to be 2800 kcal. This meant that all participants received a weekly set of menu items designed to provide ∼2800 kcal/day to allow for ad libitum intake. It was expected that most participants would not need or choose to eat 2800 kcal/day to be satiated. They were instead instructed to consume an equal proportion of each menu item over the course of the week to maintain a similar balance of nutrients, regardless of the total energy intake (20).
Self-provided period
For each of the 2 diet types and intervention phases, the 4 weeks of food delivery were followed by 8 weeks of the participants purchasing their own foods (i.e., self-provided). At this point, given that they were no longer restricted to the food-delivery service being dairy and gluten free, both groups were able to add dairy to their diets (e.g., dairy cream in coffee for the WFKD and whole-fat yogurt for the Med-Plus), and the Med-Plus group was able to add whole, intact wheat back into their diet (e.g., farro, wheat berries). Otherwise, all other guidelines used during the food-delivery weeks were used to guide the participants in this period. A recipe booklet was provided to all participants for both dietary phases, and suggestions for study-compliant menu items at local restaurants for both diets were also provided. Additional details of the diets are provided elsewhere (20).
Follow-up period
For the 12-week follow-up (following completion of the two 12-week diet phases), participants could choose to follow whatever dietary pattern they preferred (e.g., fully or partially following either the WFKD or Med-Plus, a combination, or neither).
Collection of dietary intake
Two types of dietary data were collected. For the primary reporting data, 3 unannounced 24-hour dietary recalls—a structured interview intended to capture detailed information about food and drink intakes—were administered within a 1-week window (on 2 weekdays and 1 weekend day) of each major time point via telephone by a trained nutritionist using Nutrition Data System for Research (NDS-R; Nutrition Coordinating Center) (21). Second, throughout the study, participants were encouraged to log their food intake using the Cronometer app (Cronometer Pro, Nutrition Tracking Software for Professionals; https://cronometer.com/pro).
Antihyperglycemic medications
To minimize the risk of hypoglycemia, individuals with T2DM were instructed to stop sulfonylurea medications before starting the WFKD and to reduce the dose by 50% before starting the Med-Plus. Sulfonylurea medications were restarted or increased for persistent hyperglycemia (>180 mg/dL).
Measures of adherence and satisfaction to the WFKD and Med-Plus
An average of available dietary recalls and records at each time point were used to calculate adherence scores at 6 time points from NDS-R diet data to measure degrees of adherence to the dietary recommendations. A description of score components is provided in Supplemental Tables 1 and 2. A standardized range of scores was created for both diets (1–10, with 10 being the highest level of adherence). Adherence scores are reported as means ± SDs. While participants were completing the WFKD phase of the study, they were provided with blood ketone monitors and strips (Abbott Precision Xtra Blood Glucose & Ketone Monitoring System) to measure ketones 3 times per week before breakfast (fasting state). Additionally, participants completed a fasting venous blood draw at 7 time points throughout the study, and levels of β-hydroxybutyrate were analyzed from plasma samples. These 2 measures provided feedback on whether participants lowered their carbohydrate intake enough to be in ketosis and provided an objective, biological measurement of adherence to the WFKD. Data on ketone blood levels during the WFKD phase are described in detail elsewhere (20). There was no parallel biochemical parameter for Med-Plus adherence. Data on food and diet satisfaction and alterations to diet and physical activity due to COVID-19 are described in detail elsewhere (20).
Anthropometric and metabolic data
After an overnight fast of 10–12 hours, all participants visited the Clinical and Translational Research Unit (CTRU) or Menlo Medical Laboratory (during COVID-19) at 7 time points: prebaseline, baseline, weeks 4 and 12 (phase 1), weeks 16 and 24 (phase 2), and week 36 (follow-up). Height, weight, and blood pressure were measured at the CTRU. Height was measured at the first visit to the nearest 0.1 cm using a Seca wall-mounted stadiometer. Body weight was recorded without shoes to the nearest 0.1 kg using a calibrated Scale-tronix clinical scale. After 5 minutes of sitting and resting, CTRU nurses obtained 3 blood pressure readings on the right arm 1 minute apart. These were collected automatically using a Welch Allyn, Spot Vital Signs LXi. If a participant's blood pressure was over 160/90 mmHg, they rested another 5 minutes before taking another measurement. During COVID-19, participants had blood drawn at the Menlo Medical Laboratory and were asked to self-report their weight; an alternate approach to collecting blood pressure data was not identified.
Fasting blood draws were completed at either the CTRU or Menlo Medical Laboratory via venipuncture by trained nurses or phlebotomists. HbA1c and glucose levels were measured within 2 hours of collection of fresh blood at the CTRU using the Siemens or DCA 2000+ instrument for HbA1c and the Nova glucose analyzer for glucose. Alanine aminotransaminase (ALT), red blood cell count, hemoglobin, and hematocrit data were collected and measured using standard protocols. All measurements were performed on fresh blood within 2 hours of collection.
Insulin and lipid concentrations were analyzed at the Core Laboratory for Clinical Studies (Washington University) (22–26). Insulin was analyzed by radioimmunoassay. Total cholesterol and triglycerides were measured by enzymatic method and HDL cholesterol was measured by direct method on the Roche cobas c501 using Roche cobas reagents. LDL cholesterol was calculated using the Friedewald equation (25). If triglycerides were >400 mg/dL, LDL cholesterol was measured using a direct method on the Roche cobas c501 using Sekisui reagents.
Participants completed validated questionnaires about their quality of life, perceived cognitive function, wellness, and gastrointestinal symptoms, including stool types, at baseline, during weeks 4 and 12 of each diet phase, and at follow-up (27–30).
Continuous glucose monitoring
Dexcom G6 CGM devices (Dexcom, Inc.) that provide interstitial glucose concentrations every 5 minutes were provided for participants at 5 time points to use for 10 days at a time. Average glucose and time-in-range (TIR) values are considered the most important CGM metrics for clinical decision-making, and CV is the standard metric for glucose variability (31); these 3 factors are presented as exploratory outcomes. A glucose management indicator was not calculated, as 14 days of data are generally recommended for this metric and only 10 days were available. TIR was defined as the percentage of time between 70 and 140 mg/dL for individuals with prediabetes and between 70 and 180 mg/dL for individuals with T2DM. Participants were not blinded to these blood glucose readings. Data were securely retrieved using Dexcom's Clarity software for clinics.
Data management
Study data were collected and managed using REDCap (Research Electronic Data Capture) (32, 33). The Stanford REDCap platform (http://redcap.stanford.edu) is developed and operated by the Stanford Medicine Research Informational Technology team.
Statistical analysis
Participant demographics and baseline clinical characteristics are summarized by arm as n (%) and median (IQR) for categorical and continuous variables, respectively. To assess any differences in each variable between the 2 arms, the absolute standardized differences (ASDs) are presented, where values of ≤0.2, 0.5, and ≥0.8 correspond to small, medium, and large differences, respectively.
The primary outcome was the percentage change in HbA1c from baseline after 12 weeks from each diet phase. To visualize its distribution, we present the median and IQR. We used a 2-sided likelihood ratio test and a linear mixed model to evaluate the differences between diets (the WFKD compared with the Med-Plus) while adjusting for the fixed effects of order (e.g., study arm) and prediabetes compared with T2DM status and for a random effect to account for the correlated observations of each participant. Additionally, we investigated an interaction term for a differential effect of diet × order from the crossover design to assess interpretability of the main effect for diet. For each outcome (in absolute values), we present the means and 95% CIs for each phase and changes from baseline by diet order.
The primary analysis was a complete case analysis and uses participants’ week 12 values in each phase (or last available lab value). As prespecified in the statistical analysis plan, participants who did not complete both phases (i.e., crossover) were excluded. We used similar analyses for our secondary outcomes of fasting glucose, fasting insulin, LDL cholesterol, HDL cholesterol, triglycerides, ALT, and weight and our exploratory outcome of average glucose from CGM. Quantile-quantile plots were used to assess the normality of model residuals. In a secondary analysis, we investigated a potential difference in diet effects by diabetes status using an interaction term. Additionally, we evaluated the means and 95% CIs of the primary outcome for each diet, to assess improvements from baseline.
Total energy, macronutrient intake, and dietary adherence are presented by arm and diet phase as means (SDs). Nutrient intakes are presented by arm and diet phase as medians (IQRs). To assess any differences in nutrient intakes between the 2 arms, ASDs are presented.
For the exploratory CGM analysis, only data from participants who provided data for the 5 primary time points were considered (weeks 0, 4, 12, 16, and 24), as this allowed for comparisons of glucose responses at baseline and across the different diet phases. For these participants, we analyzed blood glucose control through the CGM metrics of average glucose, TIR, and CV. To visualize the distribution of the different metrics, we present medians and IQRs in violin plots. For our exploratory analysis, we present the means and 95% CIs for each metric by diet, diabetes status, and delivery method. Individuals with prediabetes were analyzed separately from those with T2DM, because these groups have different glucose target ranges (31). In addition, the food-delivery and self-provided periods were also separated in the analysis because of higher adherence to the diets during the food-delivery period (20). We present bar charts with means and SEs by diet and study phase for the following exploratory analyses: gastrointestinal symptoms, quality of life, perceived cognitive function, and wellness.
Weight change is known to impact HbA1c values (34). Therefore, in a prespecified sensitivity analysis of the primary outcome, we adjusted for the baseline weight and percentage weight change from baseline. In a sensitivity analysis for the potential impact of the COVID-19 pandemic, we compared the 2 diets in the first phase only. For this analysis, we used a linear regression model and adjusted for the baseline value and diabetes status; in a separate evaluation, we also adjusted for the percentage weight change from baseline. Because sulfonylurea medications are known to alter HbA1c values, we carried out a sensitivity analysis to exclude participants who took different dosages of this class of medication between diets.
All analyses were completed using R version 3.6.2. A significance level of 0.05 was set for all analyses. No correction was applied for multiple comparisons, and secondary and exploratory analyses should be interpreted accordingly.
Results
Randomization and demographic characteristics
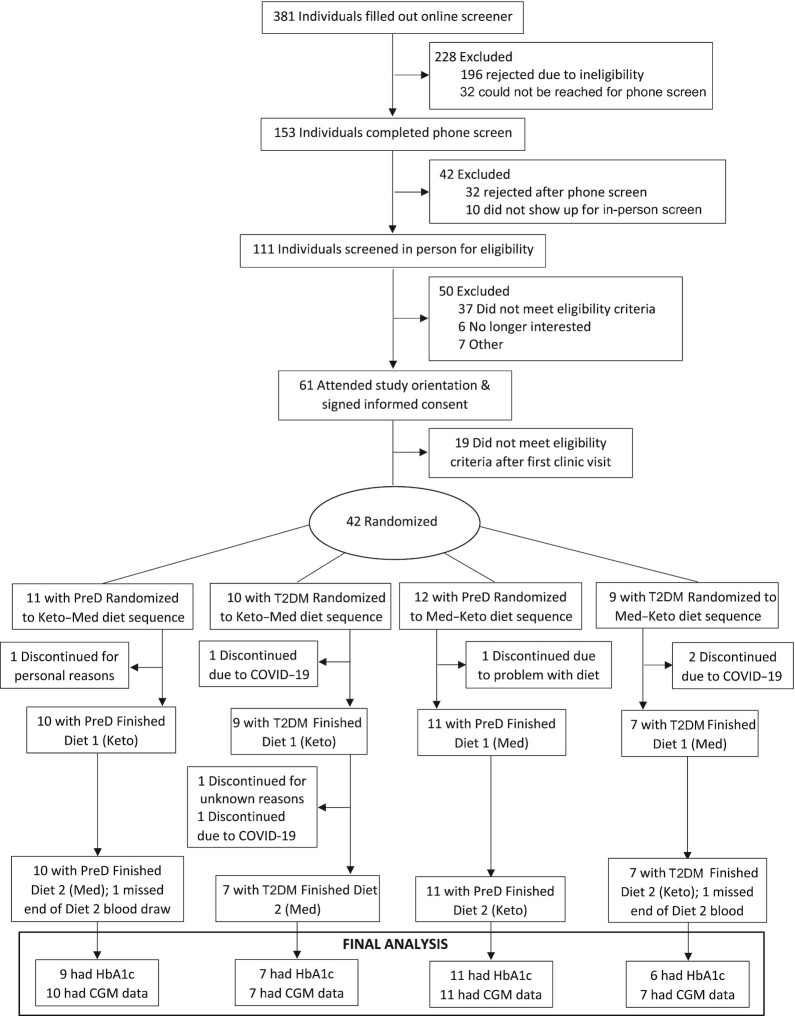
Participant enrollment began on 5 June 2019 and continued through 21 February 2020. The date of final follow-up data collection was 4 December 2020. Of the 381 potential participants who completed an initial online screener, 42 were randomly assigned to the intervention arms (Figure 2). Two of these 42 randomized participants dropped out before beginning their first dietary phase. Of the remaining 40 participants who initiated their participation in the study, 4 discontinued due to disruptions related to COVID-19 and 1 participant discontinued for unknown reasons. Two participants missed the blood draw at the end of the second diet phase due to COVID-19, but had CGM data available. Baseline sociodemographic, anthropometric, and metabolic characteristics for the primary analysis population (n = 33) with complete data are shown in Table 1. Slightly more than half of participants were male (61%), roughly half were non-Hispanic white (45%), and most were college educated (85%); 61% had prediabetes and 39% had T2DM. Ages ranged from 41 to 77 years (median, 60.5 years) and BMIs ranged from 22.7 to 39.7 kg/m2. Based on ASD values, we did not observe any large differences between arms.
FIGURE 2.
Consolidated Standards of Reporting Trials participant flow for the Keto-Med randomized trial. Abbreviations: CGM, continuous glucose monitoring; COVID-19, coronavirus disease 2019; HbA1c, glycated hemoglobin; PreD, prediabetes; T2DM, type 2 diabetes mellitus.
TABLE 1.
Keto-Med randomized trial participants’ baseline sociodemographic, anthropometric, and metabolic characteristics1
| Characteristics | WFKD → Med-Plus | Med-Plus → WFKD | Total | ASD |
|---|---|---|---|---|
| (n = 16) | (n = 17) | (n = 33) | ||
| Gender, n (%) | — | — | — | 0.17 |
| Male | 9 (56.2) | 11 (64.7) | 20 (60.6) | — |
| Female | 7 (43.8) | 6 (35.3) | 13 (39.4) | — |
| Diagnosis, n (%) | — | — | — | 0.17 |
| Prediabetes | 9 (56.2) | 11 (64.7) | 20 (60.6) | — |
| T2DM | 7 (43.8) | 6 (35.3) | 13 (39.4) | — |
| Age, years | 55.7 [52.6–67.4] | 61.0 [55.1–67.3] | 60.5 [52.7–67.3] | 0.09 |
| Highest level of education achieved, n (%) | — | — | — | 0.61 |
| High school graduate | 0 (0.0) | 2 (11.8) | 2 (6.1) | — |
| Some college | 2 (12.5) | 1 (5.9) | 3 (9.1) | — |
| College degree | 6 (37.5) | 6 (35.3) | 12 (36.4) | — |
| Some postgraduate school | 1 (6.2) | 2 (11.8) | 3 (9.1) | — |
| Postgraduate degree | 7 (43.8) | 6 (35.3) | 13 (39.4) | — |
| Race or ethnicity, n (%) | — | — | — | 0.62 |
| Non-Hispanic white | 9 (56.2) | 6 (35.3) | 15 (45.5) | — |
| Hispanic or Latinx | 2 (12.5) | 5 (29.4) | 7 (21.2) | — |
| Asian | 4 (25.0) | 4 (23.5) | 8 (24.2) | — |
| Black or African American | 0 (0.0) | 1 (5.9) | 1 (3.0) | — |
| Native Hawaiian or Pacific Islander | 1 (6.2) | 1 (5.9) | 2 (6.1) | — |
| Weight, kg | ||||
| Both sexes | 90.0 [79.8–101.3] | 84.6 [75.9–93.5] | 87.5 [75.9–95.8] | 0.29 |
| Male | 95.0 [ 81.0–104.0] | 81.3 [ 76.0–93.5] | 86.2 [74.3–95.8] | — |
| Female | 85.3 [ 77.4–93.5] | 90.2 [ 75.5–93.5] | 89.1 [76.5–97.3] | — |
| BMI, kg/m2 | ||||
| Both sexes | 30.4 [27.6–35.6] | 31.0 [27.1–35.2] | 30.6 [27.1–34.6] | 0.11 |
| Male | 35.5 [30.2–36.0] | 31.3 [28.4–37.2] | 28.1 [24.9–30.6] | — |
| Female | 27.4 [24.5–29.4] | 29.5 [ 26.4–33.3] | 32.7 [29.6–36.2] | — |
| Blood pressure, mmHg | ||||
| Systolic | 126.5 [113.5–132.0] | 123.5 [113.5–129.8] | 122.0 [113.5–128.5] | 0.27 |
| Diastolic | 75.0 [68.3–80.5] | 72.8 [68.1–77.9] | 74.5 [69.0–79.5] | 0.09 |
| HbA1c levels, % | ||||
| All participants | 6.1 [5.9–6.8] | 6.0 [5.6–6.6] | 6.0 [5.7–6.9] | 0.23 |
| Prediabetes | 6.0 [5.7–6.0] | 5.70 [5.5–6.0] | 5.8 [5.7–6.0] | — |
| T2DM | 6.9 [6.5–7.2] | 6.85 [6.7–7.0] | 7.0 [6.0–7.5] | — |
| Fasting glucose, mg/dL | ||||
| All participants | 120.0 [111.8–134.3] | 118.0 [106.0–134.0] | 115 [105–133] | 0.01 |
| Prediabetes | 114.0 [108.0–117.0] | 106.0 [101.0–117.5] | 110 [100.5–115.3] | — |
| T2DM | 138.0 [123.5–142.0] | 147.0 [137.0–161.5] | 137 [122–149] | — |
| Fasting insulin, µIU/mL | ||||
| All participants | 14.7 [12.5–22.1] | 16.5 [11.5–19.2] | 15.4 [12.1–24.3] | 0.06 |
| Prediabetes | 14.4 [12.0–22.1] | 18.0 [13.3–19.4] | 16.2 [12.0–23.9] | — |
| T2DM | 14.7 [13.7–19.6] | 12.4 [7.8–17.9] | 14.9 [12.4–25.3] | — |
| Blood lipids, mg/dL | ||||
| LDL cholesterol | 101.0 [78.0–115.3] | 116.0 [93.0–129.0] | 107 [75–132] | 0.49 |
| HDL cholesterol | 47.0 [41.0–56.0] | 46.0 [42.0–56.0] | 48 [41–54] | 0.09 |
| Triglycerides | 99.5 [88.8–131.0] | 108.0 [82.0–163.0] | 105 [88–157] | 0.19 |
| Alanine aminotransferase, IU/L | 26.5 [20.8–36.5] | 25.0 [20.0–34.0] | 24 [17–40] | 0.06 |
| Medication, n | ||||
| Metformin only | 4 | 6 | 10 | — |
| Metformin + sulfonylurea | 5 | 3 | 8 | — |
| Other | 1 | 0 | 1 | — |
Values are n (%) and median [IQR]. Abbreviations: ASD, absolute standardized difference; HbA1c, glycated hemoglobin; Med-Plus, Mediterranean-plus diet; T2DM, type 2 diabetes mellitus; WFKD, well-formulated ketogenic diet.
Dietary adherence
While receiving food deliveries, diet adherence scores (range 1–10, where 10 indicated the highest level of adherence) were similar for both diets [WFKD, mean ± SD, 7.6 ± 2.1 (range, 1.2–9.7); Med-Plus, mean ± SD, 7.3 ± 1.5 (range, 3.5–9.6)]. Adherence for both diets was higher in the 4-week food-delivery period than during the self-provided food period, but the scores were again similar between diets for the self-provided period [WFKD, mean ± SD, 5.8 ± 2.8 (range, 0.3–9.7); Med-Plus, mean ± SD, 5.5 ± 1.5 (range, 1.7–8.3)]. Within diets, variability was highest when participants were on the WFKD and self-providing foods. Comparisons of individual adherence during different periods of the study (baseline, food-delivery period, self-provided period, and 12-week follow-up) for each diet (WFKD and Med-Plus), stratified by participants with prediabetes and T2DM, are shown in Supplemental Figures 1and2. Also available in graphical and table displays in Supplemental Figure 3 and Supplemental Tables 3–7 are intake data by diet type and food-delivery compared with self-provided periods for kcals and macronutrient distribution, types of carbohydrate, whole compared with refined grains, types of fat, and animal compared with plant protein. A comprehensive description of dietary adherence is presented elsewhere (20).
Nutrient intake
Reported energy intake during each of the 2 intervention periods, food-delivery and self-provided, was approximately 250–300 kcal/day lower compared to baseline, which should be taken into consideration when interpreting nutrient intake data (Supplemental Table 3).
Food-delivery period
When contrasting changes in nutrient intakes from baseline between the 2 diets at the end of the 4-week food-delivery periods, when adherence was highest, intake was higher (ASD >0.5) for the WKFD for 3 nutrients (vitamins B12 and D and selenium) and higher for the Med-Plus for fiber and 7 nutrients (thiamin, iron, folate, magnesium, and vitamins B6, C, and E; see Supplemental Table 8). On average, participants on the WFKD increased their nutrient intakes of vitamins B12, C, and E and omega-3 fats and decreased their nutrient intakes of thiamin, folate, and calcium compared to baseline (see Supplemental Table 9). On average, participants on the Med-Plus increased their nutrient intakes of folate, omega-3 fats, and vitamins C and E and decreased their nutrient intakes of vitamin B12 and calcium compared to baseline. The food-delivery company is a dairy-free (and gluten-free) operation, and this likely explained the decreases in calcium for both diet phases.
Self-provided period
When contrasting changes in nutrient intakes from baseline between the 2 diets at the end of the 8-week self-provided periods, when adherence was lower than in the food-delivery phase, intake was not higher for the WFKD for any nutrients, but was higher (ASD >0.5) for the Med-Plus for fiber and 3 nutrients (vitamin C, folate, and magnesium; see Supplemental Table 10). On average, participants on the WFKD increased their nutrient intake of vitamin E and decreased their nutrient intakes of thiamin, folate, and iron compared to baseline (see Supplemental Table 11). On average, participants on the Med-Plus did not increase or decrease intake of any nutrient during the self-provided period compared to baseline.
Primary and secondary analyses
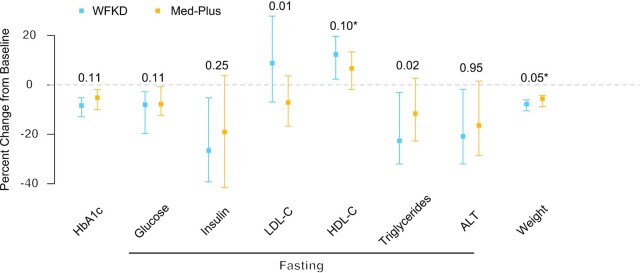
As shown in Figure 3, HbA1c levels decreased for both diets, but we did not observe a significant difference between diets [mean percentage changes: WFKD, −9% (95% CI: −11% to −7%) and Med-Plus, −7% (95% CI: −9% to −5%); P = 0.11]. We present main effect estimates and SEs for all variables in the primary analysis model in Supplemental Table 12. For secondary outcomes, the WFKD led to a significantly greater decrease in triglycerides (P = 0.02) and significantly increased LDL cholesterol concentrations relative to a decrease for the Med-Plus (P = 0.01). Absolute values of outcome variables are presented for baseline and by diet phase and order in Supplemental Table 13; changes in outcome variables relative to baseline are presented by diet phase and order in Supplemental Table 14. We note that better outcomes were observed for those starting with the WFKD in the first phase. Significant interaction effects of diet × order were detected for weight and HDL cholesterol percentage change (P = 0.01 and 0.001, respectively; Supplemental Table 14). Given the interaction effect for weight, we present weight change over time by diet order (i.e., arm) in Figure 4. Here, we observe a difference between arms at week 12 after phase 1 but not phase 2.
FIGURE 3.
Percent change from baseline by diet (n = 33). Presented by diet (the WFKD compared with the Med-Plus), median (square), and IQR (bars) of percentage changes from baseline in HbA1c, fasting glucose, fasting insulin, fasting LDL cholesterol, fasting HDL cholesterol, fasting triglycerides, fasting ALT, and weight (including some self-reported weight changes during COVID-19). Also, P values (above) from a likelihood ratio test for diet show differences in a linear, mixed-effect model after adjusting for order, diabetes status, and correlated observations. *Significant interaction effect of diet × order (P < 0.05). Abbreviations: ALT, alanine aminotransferase; COVID-19, coronavirus disease 2019; HbA1c, glycosylated hemoglobin; Med-Plus, Mediterranean-plus diet; WFKD, well-formulated ketogenic diet.
FIGURE 4.
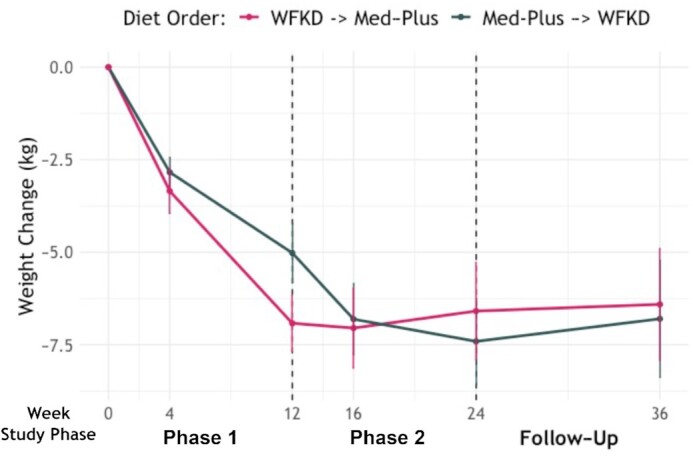
Weight change from baseline by phase and diet order (n = 33). The only time point at which the weight loss difference was significant was at week 12, at the end of the first diet phase, with a mean difference of 6.9 kg (SEM, ±0.8 kg) compared with 5.0 kg (SEM, ±0.80 kg) for the WFKD and Med-Plus, respectively (P = 0.04; paired t-test). Abbreviations: Med-Plus, Mediterranean-plus diet; WFKD, well-formulated ketogenic diet.
The findings from a secondary analysis to evaluate a significant interaction effect in outcomes’ percentage changes from baseline for diet × diabetes status are presented in Supplemental Table 15. The only notable finding was a modestly greater decrease in ALT for the participants with prediabetes on the Med-Plus [mean, −0.17 (95% CI: −0.29 to −0.05) compared with −0.04 (95% CI: −0.25 to 0.16) for prediabetes compared with T2DM, respectively] and, alternatively, a modestly greater decrease in ALT for participants with T2DM on the WFKD [mean, −0.1 (95% CI: −0.21 to 0) compared with −0.14 (95% CI: −0.35 to 0.06) for prediabetes compared with T2DM, respectively; interaction diet × diabetes status P = 0.04].
Exploratory CGM analysis
Descriptive data from CGM are presented to complement the primary HbA1c outcome data. The exploratory analysis population had data available for 35 participants (i.e., 2 participants completed the protocol and provided CGM data, but were unable to attend a clinic visit and provide a blood sample due to COVID-19). To investigate each metric, we present its mean and 95% CI by diet phase, diabetes status (prediabetes compared with T2DM), and food-provision period. The decrease in the average glucose value from CGM data was significantly lower during the WFKD compared with the Med-Plus, based on the linear mixed model (−8% compared with −2%; P = 0.03). For participants with prediabetes, the effects of the diet on blood glucose were moderate, as expected, and were greater for the WFKD. Both diets increased the TIR (70–140 mg/dL for prediabetes), with a greater increase for the WFKD. For the participants with T2DM, both diets decreased average glucose levels and increased TIR (70–180 mg/dL for T2DM), with the WFKD having a greater absolute effect (Supplemental Figure 4; Supplemental Table 16). Both in participants with prediabetes and participants with T2DM, both diets decreased the CV. Again, the WFKD had a greater effect in both populations.
Sensitivity analyses
Sensitivity analyses were conducted to examine whether the primary HbA1c outcome might have been affected by other variables that displayed significant changes during the course of the study. However, these analyses had low power due to the small sample size, and their results should be interpreted with caution. We did not observe a significant difference in HbA1c values between diets when adjusting for baseline weight and percentage weight change (P = 0.45), nor when adjusting for percentage weight change alone (P = 0.45).
In an effort to address possible COVID-19-related changes in the study protocol, data were analyzed for phase 1 only. Our analysis population includes 36 participants that completed phase 1 before the COVID-19 shelter-in-place orders disrupted the study (we note 3 of these participants were not able to contribute blood samples in phase 2). Participants on the WFKD observed a significantly better percentage change from baseline for HbA1c values compared to those on the Med-Plus when adjusting for diabetes status (P = 0.03; 5%; 95% CI: 1%–10%). In a separate evaluation adjusting for the percentage weight change from baseline in the model, we did not observe a significant difference between diets (P = 0.16; Supplemental Table 17). After phase 1, participants on the WFKD had a slightly greater decrease in weight compared to those on the Med-Plus [−6.9 kg (95% CI: −8.4 to −5.5) compared with −4.9 kg (95% CI: −6.4 to −3.4), respectively].
We performed a sensitivity analysis for differential usage of sulfonylurea medication. Out of 7 participants taking sulfonylureas at baseline, 3 used the same dosage for both diets and 4 used different dosages between diets. When excluding these 4 participants, we observed significant differences for both HbA1c and HDL cholesterol values between diets, favoring the WFKD (Supplemental Table 18).
Follow-up data
Diet data
The follow-up average scores were intermediate between the baseline and self-provided scores for both the WKFD and Med-Plus (Supplemental Figure 2). Also, at follow-up, the diet adherence scores were higher for the Med-Plus than for the WKFD (i.e., the follow-up diet of the primary analysis population was more similar to the Med-Plus than to the WKFD). On average, the participants reported consuming fewer calories, an increased percentage of total energy from fat, and a lower intake from carbohydrates relative to baseline. Participants were consuming similar distributions of types of fat (saturated compared with unsaturated) and protein (animal compared with plant) compared to baseline amounts. Although added sugar intake decreased and fiber intake (insoluble and soluble) increased during the intervention phases, participants were consuming similar levels at follow-up compared to baseline. Intake of grains dropped during the intervention and remained lower than baseline levels at follow-up. Participants were consuming fewer refined grains but also fewer whole grains at follow-up relative to baseline (Supplemental Figure 3; Supplemental Tables 3–7).
Clinical data
Twelve weeks after completing both diet phases, relative to baseline, on average, the participants maintained decreased HbA1c values, fasting glucose levels, and weight (CGM data were not collected at follow-up). HDL cholesterol levels were higher at follow-up than at baseline. Fasting insulin, triglyceride, and ALT data were similar at follow-up compared to baseline. The only factor that was observed to be more adverse at follow-up was an elevated LDL cholesterol concentration (Supplemental Table 19).
Quality of life
Participants were surveyed at 6 time points to capture any changes in their quality of life, perceived cognitive function, wellness, and gastrointestinal symptoms, including stool types. There were no observable differences in the distributions for any of the measures, either across time points or between the diets (Supplemental Table 20, gastrointestinal habits; Supplemental Figure 5, gastrointestinal symptoms; Supplemental Figure 6, Bristol stool scale; Supplemental Figure 7, quality of overall health, life, and cognitive abilities; and Supplemental Figure 8, pain).
Adverse events
During the trial, there were 4 adverse events reviewed by the study physician (SHK). One was an elevated ALT level likely related to the study (the participant was on the WFKD during the time of the event). Two of the 4 adverse events that were possibly but probably not related to the study were a kidney infection and exacerbation of eczema (the participants were on the WFKD during the time of their event). The fourth was a transient ischemic attack that occurred during the study follow-up, and was probably not related to the study (the participant was on the Med-Plus during the time of the event).
Discussion
In this randomized, crossover intervention trial among adults with prediabetes or T2DM, the 12-week percentage change in HbA1c, the primary study outcome, improved significantly for participants on both diets, but was not significantly different between the WFKD and Med-Plus phases. Significant differences in 2 of the clinical values were mixed: triglyceride changes favored the WFKD, while LDL cholesterol changes favored the Med-Plus. There was a general trend towards improvements for both diet phases for most of the secondary outcomes, but no significant differences between diets in the primary analysis for fasting glucose, insulin, HDL cholesterol, and ALT data. Average glucose as determined by CGM, an exploratory outcome, decreased more for participants on the WFKD compared with those on the Med-Plus. In sensitivity analyses addressing changes in medications or looking at only the first phase of the crossover, differences between diets for HbA1c and HDL cholesterol values shifted to statistical significance, favoring the WFKD. The dietary adherence assessment indicated similar levels of average adherence between the 2 diets during both the food-delivery period and self-provided period. Differences in nutrient intakes during the intervention favored the Med-Plus. At the 12-weeks follow-up, the participants’ dietary patterns were more similar to the Med-Plus, suggesting a potential for greater sustainability of the Med-Plus in the long term.
Our findings corroborate previous evidence indicating that HbA1c levels can be significantly reduced on both a ketogenic diet (35–38) and a Mediterranean diet (39–44), especially if the diet is restricted in added sugars and refined carbohydrates (2). The primary question of our study sets it apart from previous studies: do people with an impaired glucose metabolism experience greater metabolic benefits or harms when restricting legumes, fruits, and whole, intact grains in addition to avoiding added sugars and refined grains? For this reason, we designed the WFKD and Med-Plus to be matched on 3 specific dietary factors (including nonstarchy vegetables and avoiding added sugars and refined grains) and mismatched on 3 specific dietary factors (the WFKD avoids and the Med-Plus includes legumes, fruits, and whole grains).
While both dietary patterns produced several health benefits, including a greater decrease in triglyceride concentrations for the WFKD, the WFKD also induced a few changes of potential concern. First, the WFKD significantly increased LDL cholesterol. Increases in LDL cholesterol and decreases in triglycerides are common effects of low-carb diets, and have been linked with a shift in the LDL cholesterol particle size that is associated with a decreased cardiovascular risk (45–48). However, since we did not fractionate LDL by size, we cannot dismiss the potential harms of the observed LDL cholesterol increase on the WFKD. Second, the WFKD also induced a decrease in fiber intake, which increased on the Med-Plus. However, through the production of beta-hydroxybutyrate, the WFKD has been suggested to reduce some of the requirement for butyrate, which is likely responsible for some of the of the benefits of dietary fiber (49). Finally, in addition to fiber, participants had greater decreases in essential nutrients on the WFKD (folate, vitamin C, and magnesium) than on the Med-Plus. Collectively, these potential harms likely relate to avoiding legumes, fruits, and whole, intact grains on the WFKD, and temper enthusiasm for avoiding these food groups, which is consistently recommended by national and international public health organizations (2, 12–15).
Several aspects of the design and implementation were strengths of our study. First, the crossover design allowed participants to serve as their own controls. Second, the initial 4-week period of food delivery facilitated high adherence, while the latter 8 weeks of self-provided foods in the 12-week diet phases increased generalizability. Third, the 12-week follow-up after completion of the 2 intervention phases provided insights regarding the poststudy stability of diet behaviors. Fourth, we chose a well-established clinical value as the primary outcome: HbA1c. Fifth, we assessed an extensive set of well-studied secondary clinical outcomes to evaluate overall cardiometabolic health. Sixth, it proved valuable to include and collect CGM data, particularly because the COVID-19 shelter-in-place disruption did not impact home collection of CGM data as much as it did HbA1c data obtained from blood samples drawn in research clinics. Seventh, extensive diet data collection using state-of-the-art NDS-R allowed us to assess adherence—an important metric in free-living trials (50)—and to compare nutrient intakes. Finally, we believe an important design strength was emphasizing high-quality dietary choices for both diet phases (50), as exemplified by using the WFKD for the ketogenic diet phase and by an emphasis on avoiding refined grains and added sugars for the Mediterranean diet phase.
Our study had several limitations. First, the conduct of the study was disrupted in several ways by COVID-19. Access to our primary clinical research facility was disrupted, resulting in some participants extending their time on the diet phase they were on when the shelter-in-place orders began. Fortunately, most participants that faced this disruption agreed to simply continue with their assigned diet phase until we were able to collect an end-phase blood sample; for some, this meant staying on that particular diet phase for up to 24 weeks, rather than 12. To address this, we collected whatever data we could at the original 12-week time point (e.g., survey data, stool samples, CGM), and then collected additional data when blood-drawing facilities became available again. In addition, the study health educator maintained frequent contact with and support of participants during these delays. Unfortunately, concerns about the pandemic and the extended delays led to 4 participants dropping out who might have otherwise completed the study. Also, the pandemic was a problem for the collaborating partner that provided food to study participants, and 12 of the participants did not receive delivered food for the first 4 weeks of their second phase of the crossover. Fortunately, adherence among those participants was determined to be similarly high compared to adherence among those who did receive food delivery during that study phase. In addition, during the pandemic, while we were able to manage blood collections through an alternative site, weight or blood pressure data were not able to be measured. To continue collecting participants’ weight data, we accepted self-reported home weighing from those participants. We were not able to find an alternate approach to measure blood pressure; thus, blood pressure data are not presented. A second limitation was the relatively small sample size, although the crossover design was helpful in maximizing the control of many potentially confounding variables. A third limitation was the increased risk of making a type 1 error that resulted from testing several secondary outcomes and using post hoc comparisons for outcomes in which a significant interaction was observed. Finally, the lack of a washout period may be considered a limitation; yet, we believe the 12-week diet phase durations were likely sufficient to eliminate or minimize any carryover effects.
Although reducing carbohydrate intake is often recommended as a strategy to control blood glucose in patients with prediabetes and T2DM, the metabolic benefits and risks of extreme versus moderate carbohydrate restriction are unclear. We found that both the WFKD and Med-Plus were associated with improved blood glucose and reduced body weight, both of which occurred without guidance on caloric restriction and may relate to the similarities in the 2 diet patterns, which limited added sugars and refined sugars. Although the WFKD was associated with improved glucose control on several CGM metrics, the differences in HbA1c values were modest despite 50% lower carbohydrate intake on the WFKD compared with the Med-Plus (<20% compared with <40% of total calories from carbohydrates, respectively). In addition, the rise in LDL cholesterol, decrease in fiber intake, and greater potential for nutrient deficiencies on the WFKD may be concerning; longer-term studies will be needed to understand these associations with clinical outcomes. Collectively, these comparative outcomes do not support a benefit sufficient to justify avoiding legumes, whole fruits, and whole, intact grains to achieve the metabolic state of ketosis. In a clinical setting, patients should be supported in choosing a dietary pattern that fits their needs and preferences. There should be less focus on promoting 1 particular diet approach as best; rather, clinicians should allow patients to make an informed choice to help them establish which approach is most suitable for them. Finally, regardless of benefits, diets need to be sustainable, and our study suggests it was difficult for participants to maintain the WFKD, as well as to achieve and maintain the similarities in the 2 diets in terms of restricting added sugars and refined grains.
Supplementary Material
ACKNOWLEDGEMENTS
This research would not have been possible without the contributions of the following individuals and groups. Reyna Berania, Cynthia To, and Amanda Calzada were study research assistants. The nutritionists and dietitians who helped with the study included Mandy Murphy Carroll, Antonella Dewell, Susan Kirkpatrick, and Alex Schiavuzzi. Julie Nguyen and Leslie Peng, from the food delivery company Methodology, were crucial to the study's success, as were the nurses and staff from the Stanford Clinical Translational Research Unit. And finally, the study would not have been possible without the participation of our incredible study volunteers.
All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The lead author, CDG (the manuscript's guarantor), affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
The authors’ responsibilities were as follows—CDG: designed the research project; CDG, MJL, DP, LA, and SHK: wrote the initial and final drafts of the manuscript; CP, LRD, AC, KMC, AC, CCD, and JLR: provided feedback and critical revisions of the manuscript; KMC: conducted the statistical analyses; and all authors: read and approved the final manuscript.
Notes
All authors report no conflicts of interest.
Supported by generous research gifts (to CDG and Dr. Justin Sonnenburg) from John and Meredith Pasquesi, Sue and Bob O'Donnell, and the Teton Fund, as well as the National Heart, Lung, and Blood Institute (NIH T32HL007034 to MJL, AC), Stanford Clinical Translational Science Award (NIH UL1TR001085 and TL1R001085), and Stanford Diabetes Research Center (NIH P30DK116074).
The study funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Supplemental Methods, Supplemental Tables 1–20, and Supplemental Figures 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ADA, American Diabetes Association; ALT, alanine aminotransferase; ASD, absolute standardized difference; CGM, continuous glucose monitoring; COVID-19, coronavirus disease 2019; CTRU, Clinical and Translational Research Unit; HbA1c, glycated hemoglobin; low-carb, low carbohydrate; Med-Plus, Mediterranean-plus diet; NDS-R, Nutrition Data System for Research; T2DM, type 2 diabetes mellitus; TIR, time-in-range; WFKD, well-formulated ketogenic diet.
Contributor Information
Christopher D Gardner, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Matthew J Landry, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Dalia Perelman, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Christina Petlura, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Lindsay R Durand, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Lucia Aronica, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Anthony Crimarco, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Kristen M Cunanan, Quantitative Sciences Unit, Department of Medicine, Stanford University, Stanford, CA, USA.
Annie Chang, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Christopher C Dant, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Jennifer L Robinson, Stanford Prevention Research Center, Department of Medicine, School of Medicine, Stanford University, Stanford, CA, USA.
Sun H Kim, Division of Endocrinology, Gerontology and Metabolism, Department of Medicine, Stanford University Medical Center, Stanford, CA, USA.
Data Availability
The data that support the findings of this study are available from the corresponding author, CDG, upon reasonable request.
References
- 1. Dyson P, Twenefour D, Breen C, Duncan A, Elvin E, Goff Let al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabetes Med. 2018;35(5):541–7. [DOI] [PubMed] [Google Scholar]
- 2. Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod Jet al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BSet al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–90. [DOI] [PubMed] [Google Scholar]
- 4. Yancy Jr WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140(10):769–77. [DOI] [PubMed] [Google Scholar]
- 5. Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory Jet al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140(10):778–85. [DOI] [PubMed] [Google Scholar]
- 6. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. [DOI] [PubMed] [Google Scholar]
- 7. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RRet al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A to Z Weight Loss Study: A randomized trial. JAMA. 2007;297(9):969–77. [DOI] [PubMed] [Google Scholar]
- 8. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg Iet al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41. [DOI] [PubMed] [Google Scholar]
- 9. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SDet al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPet al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landry MJ, Crimarco A, Gardner CD. Benefits of low carbohydrate diets: a settled question or still controversial?. Curr Obes Rep. 2021;10(3):409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th edition. U.S. Department of Agriculture and U.S. Department of Health and Human Services; 2020. [Google Scholar]
- 13. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJet al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AVet al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245–71. [DOI] [PubMed] [Google Scholar]
- 15. Nishida C, Uauy R, Kumanyika S, Shetty P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 2004;7(1a):245–50. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association. 2 . Classification and diagnosis of diabetes: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(Suppl 1):S15–33. [DOI] [PubMed] [Google Scholar]
- 17. Volek JS, Phinney SD. The art and science of low carbohydrate living: an expert guide to making the life-saving benefits of carbohydrate restriction sustainable and enjoyable. First ed. Dublin (OH): Beyond Obesity LLC; 2011. [Google Scholar]
- 18. Phinney S, Volek J. The importance of managing potassium and sodium as part of a well-formulated ketogenic diet. [Internet]. San Francisco: Virta Health Corp; 2019. [Accessed 2021 Sep 9]. Available from: https://www.virtahealth.com/blog/potassium-sodium-ketogenic-diet. [Google Scholar]
- 19. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing Eet al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6):1402S–6S. [DOI] [PubMed] [Google Scholar]
- 20. Landry MJ, Crimarco A, Perelman D, Durand LR, Petlura C, Aronica Let al. Adherence to ketogenic and Mediterranean study diets in a crossover trial: the Keto-Med randomized trial. Nutrients. 2021;13(3):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. [DOI] [PubMed] [Google Scholar]
- 22. Bystrom C, Sheng S, Zhang K, Caulfield M, Clarke NJ, Reitz R. Clinical utility of insulin-like growth factor 1 and 2; determination by high resolution mass spectrometry. PLoS One. 2012;7(9):e43457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterson JI, Young DS. Evaluation of the hexokinase/glucose-6-phosphate dehydrogenase method of determination of glucose in urine. Anal Biochem. 1968;23(2):301–16. [DOI] [PubMed] [Google Scholar]
- 24. Rambaldi DC, Reschiglian P, Zattoni A, Johann C. Enzymatic determination of cholesterol and triglycerides in serum lipoprotein profiles by asymmetrical flow field-flow fractionation with on-line, dual detection. Anal Chim Acta. 2009;654(1):64–70. [DOI] [PubMed] [Google Scholar]
- 25. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26. Miller WG, Myers GL, Sakurabayashi I, Bachmann LM, Caudill SP, Dziekonski Aet al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010;56(6):977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svedlund J, Sjödin I, Dotevall G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33(2):129–34. [DOI] [PubMed] [Google Scholar]
- 28. Winham DM, Hutchins AM. Perceptions of flatulence from bean consumption among adults in 3 feeding studies. Nutr J. 2011;10(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–4. [DOI] [PubMed] [Google Scholar]
- 30. Hanmer J, Jensen RE, Rothrock N. A reporting checklist for HealthMeasures’ patient-reported outcomes: ASCQ-Me, Neuro-QoL, NIH Toolbox, and PROMIS. J Patient Rep Outcomes. 2020;4(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester Tet al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal Let al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gummesson A, Nyman E, Knutsson M, Karpefors M. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(9):1295–305. [DOI] [PubMed] [Google Scholar]
- 35. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DEet al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13(5):689–711.e1. [DOI] [PubMed] [Google Scholar]
- 36. Hall KD, Guo J, Courville AB, Boring J, Brychta R, Chen KYet al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat Med. 2021;27(2):344–53. [DOI] [PubMed] [Google Scholar]
- 37. Yuan X, Wang J, Yang S, Gao M, Cao L, Li Xet al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WWet al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. 2019;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sleiman D, Al-Badri MR, Azar ST. Effect of Mediterranean diet in diabetes control and cardiovascular risk modification: a systematic review. Front Public Health. 2015;3:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Esposito K, Maiorino MI, Ciotola M, Di Palo C, Scognamiglio P, Gicchino Met al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151(5):306–14. [DOI] [PubMed] [Google Scholar]
- 42. Maiorino MI, Bellastella G, Petrizzo M, Gicchino M, Caputo M, Giugliano Det al. Effect of a Mediterranean diet on endothelial progenitor cells and carotid intima-media thickness in type 2 diabetes: follow-up of a randomized trial. Eur J Prev Cardiol. 2017;24(4):399–408. [DOI] [PubMed] [Google Scholar]
- 43. Itsiopoulos C, Brazionis L, Kaimakamis M, Cameron M, Best JD, O'Dea Ket al. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis. 2011;21(9):740–7. [DOI] [PubMed] [Google Scholar]
- 44. Toobert DJ, Glasgow RE, Strycker LA, Barrera M, Radcliffe JL, Wander RCet al. Biologic and quality-of-life outcomes from the Mediterranean lifestyle program: a randomized clinical trial. Diabetes Care. 2003;26(8):2288–93. [DOI] [PubMed] [Google Scholar]
- 45. Sharman MJ, Kraemer WJ, Love DM, Avery NG, Gómez AL, Scheett TPet al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. 2002;132(7):1879–85. [DOI] [PubMed] [Google Scholar]
- 46. Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83(5):1025–31. [DOI] [PubMed] [Google Scholar]
- 47. Sharman MJ, Gómez AL, Kraemer WJ, Volek JS. Very low-carbohydrate and low-fat diets affect fasting lipids and postprandial lipemia differently in overweight men. J Nutr. 2004;134(4):880–5. [DOI] [PubMed] [Google Scholar]
- 48. Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WWet al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol. 2018;17(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wood RJ, Fernandez ML, Sharman MJ, Silvestre R, Greene CM, Zern TLet al. Effects of a carbohydrate-restricted diet with and without supplemental soluble fiber on plasma low-density lipoprotein cholesterol and other clinical markers of cardiovascular risk. Metabolism. 2007;56(1):58–67. [DOI] [PubMed] [Google Scholar]
- 50. Gardner CD, Crimarco A, Landry MJ, Fielding-Singh P. Nutrition study design issues—important issues for interpretation. Am J Health Promot. 2020;34(8):951–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, CDG, upon reasonable request.