Abstract
Objectives
To examine the association between maternal depressive symptoms in the immediate postnatal period and offspring’s behavioural outcomes in a large cohort of term-born and preterm-born toddlers.
Design and participants
Data were drawn from the Developing Human Connectome Project. Maternal postnatal depressive symptoms were assessed at term-equivalent age, and children’s outcomes were evaluated at a median corrected age of 18.4 months (range 17.3–24.3).
Exposure and outcomes
Preterm birth was defined as <37 weeks completed gestation. Maternal depressive symptoms were assessed with the Edinburgh Postnatal Depression Scale (EPDS). Toddlers’ outcome measures were parent-rated Child Behaviour Checklist 11/2–5 Total (CBCL) and Quantitative Checklist for Autism in Toddlers (Q-CHAT) scores. Toddlers’ cognition was assessed with the Bayley Scales of Infant and Toddler Development—Third Edition (Bayley-III).
Results
Higher maternal EPDS scores were associated with toddlers’ higher CBCL (B=0.93, 95% CI 0.43 to 1.44, p<0.001, f2=0.05) and Q-CHAT scores (B=0.27, 95% CI 0.03 to 0.52, p=0.031, f2=0.01). Maternal EPDS, toddlers’ CBCL and Q-CHAT scores did not differ between preterm (n=97; 19.1% of the total sample) and term participants. Maternal EPDS score did not disproportionately affect preterm children with respect to CBCL or Q-CHAT scores.
Conclusions
Our findings indicate that children whose mothers reported increased depressive symptoms in the early postnatal period, including subclinical symptoms, exhibit more parent-reported behavioural problems in toddlerhood. These associations were independent of gestational age. Further research is needed to confirm the clinical significance of these findings.
Keywords: MENTAL HEALTH, Child & adolescent psychiatry, Developmental neurology & neurodisability, Depression & mood disorders, NEONATOLOGY
Strengths and limitations of this study.
Prospective study with a large sample, using multiple imputation to reduce non-response bias.
Maternal depressive symptoms assessed as a continuous variable, providing more nuanced information about the significance of subclinical symptoms.
Maternal depressive symptoms assessed earlier than in previous studies, enabling recognition of early screening opportunities for families.
Potential common method variance bias through parent-completed child behavioural assessments.
Unknown paternal and parental factors, such as comorbid psychiatric conditions, that may confound our findings.
Introduction
Postnatal depression affects approximately 12% of mothers worldwide.1 In contrast to ‘baby blues’, which is a state of emotional lability that affects between 13.7% and 76.0% of women in the first few days after birth and typically resolves spontaneously within 2 weeks,2 postnatal depression is more severe and starts in the first few months postpartum.1 Stressful life events have been linked to a heightened risk of developing postnatal depression3; for example, mothers of preterm infants have a significantly higher risk of postpartum depression compared with mothers of term infants,4 likely due to heightened stress associated with perinatal complications.5
Women with postnatal depression tend to be less responsive to their baby’s needs and to display less affection.6 Therefore, in the short-term, postpartum depression may affect mother–infant interactions7 and in the long term, it may lead to alterations in brain development,8 emotional difficulties,9 less secure attachment, cognitive and behavioural problems in childhood and a possible increased risk of autism spectrum disorder (ASD).10 11 Large cohort studies, such as the Avon Longitudinal Study of Parents and Children, have shown that these associations are even evident when maternal depression is measured on a continuum of symptoms rather than a dichotomous diagnosis,12–14 supporting the notion that elevated subdiagnostic psychiatric symptoms can also negatively impact on children’s development.15
Studies investigating the underlying causes that may link maternal postnatal depression to child outcomes have implicated several biological and environmental variables. For instance, genetic and epigenetic factors have been shown to both mediate and mitigate the intergenerational transmission of psychiatric disorders,16 while lower quality parenting, interparental conflict and socioeconomic deprivation have been shown to exacerbate children’s developmental risk of emotional and behavioural problems.11 In addition, being born preterm (ie, <37 weeks’ gestation, as per the WHO definition)17 has been associated with alterations in early brain development18 as well as neurological, behavioural and cognitive problems in childhood and beyond.19 20 Therefore, it is complex to disentangle the possible effects of postnatal maternal mental health and those of perinatal clinical factors on specific outcomes in preterm children, as these may involve both maternal psychosocial and biological variables, as well as child preterm-related neurodevelopmental morbidity.
Furthermore, a question that remains unanswered is whether preterm birth (PTB) accentuates the association between maternal postnatal depression and child outcome. Two theoretical frameworks exist that hypothesise certain infants may be influenced differently by external stimuli: the diathesis stress model proposes that certain vulnerability factors make affected infants more prone to suboptimal environmental influences with subsequent poorer outcomes,21 22 whereas the differential susceptibility model frames such factors as plasticity mediating, thus leading to poorer outcomes in negative environments, as well as better outcomes in supportive environments.22 23 Previous studies investigating differential susceptibility have shown mixed findings studying a range of environmental and clinical exposures,24 25 with child outcomes including attachment, internalising and externalising behaviour and academic competence.25 Both low birth weight in term infants (small for gestational age, SGA)26 and PTB23 24 27 have been explored as distinct potential susceptibility factors. This distinction is based on the different pathophysiological processes underlying the respective conditions of SGA and PTB, both, or a combination, of which can cause low birth weight.28 For example, SGA is a marker of intrauterine growth restriction related to placental dysfunction,29 whereas PTB can be caused by a multitude of factors, including infection and inflammation.30
Given that mothers of preterm children experience elevated levels of distress,31 are at high risk of developing postnatal depression,32 and that preterm children themselves are vulnerable to psychiatric sequelae,33 in addition to investigating the association between very early maternal postnatal depressive symptoms and child behavioural and emotional outcomes, we further aimed to investigate the interaction between PTB and maternal depressive symptoms on child outcomes. Previous work focusing on the differential susceptibility of preterm born children to various environmental stimuli, as described above, had not yet studied maternal depressive symptoms as a proposed exposure. We specifically aimed to investigate the continuum of maternal depressive symptoms rather than solely focussing on clinically significant maternal depression, so as to provide more nuanced information about the importance of subclinical depressive symptoms on child outcomes. We hypothesised that early postnatal maternal depressive symptoms would be more elevated in mothers of preterm compared with term infants and that these would impact preterm children’s behavioural and emotional outcomes to a greater degree than their term counterparts.
Methods
Sample
Participants were enrolled in the Developing Human Connectome Project (DHCP, http://www.developingconnectome.org/), a neuroimaging-focused project, with eligibility criteria including pregnant women (aged ≥16 years) with a gestational age of 20–42 weeks, and newborn infants aged 24–44 weeks; infants enrolled in the DHCP had MRI at term-equivalent age. Exclusion criteria for the DHCP included: contraindications to MRI, babies being too unwell to tolerate a scan and language difficulties preventing informed consent.34 Toddlers were invited to the Centre for the Developing Brain, St Thomas’ Hospital, London, for neurodevelopmental assessment at 18 months post expected delivery date; appointments were made according to family availability as close as possible to this time point. Inclusion criteria for our follow-up study were: mother and baby attendance for MRI at term-equivalent age; completed toddler neurodevelopmental assessment. These inclusion criteria were met by 509 toddlers by the date of closure for this analysis (26 February 2020). Of the 509 toddlers, 51 were one of a twin pregnancy, and three were one of a triplet pregnancy; the sample contained 22 sibling pairs and one set of triplets.
Maternal variables
Maternal age, parity, body mass index (BMI), ethnicity and postcode were collected at enrolment into the DHCP study. Our sample was ethnically representative of the surrounding geographical area. Parity was coded as 0, 1, 2 or ≥3 previous children. Index of Multiple Deprivation (IMD) rank was computed from the current maternal postcode using the 2019 IMD classification; it combines locality specific information about income, employment, education, health, crime, housing and living environment, thus providing a proxy for family socioeconomic status.35 Lower IMD rank corresponds to greater social deprivation. Our sample was generally less deprived than the surrounding geographical areas, as well as the UK as a whole, reflecting trends observed in other UK longitudinal studies.36
Maternal depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EPDS)37 on the day of infant’s MRI at term-equivalent age. Mothers of infants born at term were tested in the first few weeks postnatally, whereas mothers of preterm-born infants were tested once they reached term-corrected age. The EPDS is a 10-item screening questionnaire completed by mothers, with higher scores reflecting a higher likelihood of depressive disorders. A score of 13 can be used as a cut-off, indicating high-level symptoms, although a cut-off of 11 maximises the sensitivity and specificity of the screening tool for depression.38 Mothers completed the EPDS independently in a private room in our centre, with no interaction with the researcher. Participants were informed that the results would be discussed with them and consented to information being shared with their general practitioner in the case of high scores. The EPDS questionnaire was scored by a member of the DHCP team.34
Child variables
Infant clinical characteristics were gathered from clinical notes where available, or from maternal report, and included: sex, gestational age at birth, birth weight and pregnancy size (singleton/twin/triplet).
Behavioural outcomes were assessed using the Child Behaviour Checklist/11/2–5 (CBCL), a parent-completed 100-item questionnaire, in which the parent rates the child’s behaviour over the preceding 2 months using a 3-point Likert scale (‘not true’, ‘somewhat or sometimes true’ and ‘very true or often true’). Responses are categorised into syndrome profiles, and these are subsequently grouped into internalising (emotional reactivity, anxiety/depression, somatic complaints and withdrawal), externalising (attention problems, aggressive behaviour) and total (internalising, externalising, sleep and other) problem scales. Higher scores indicate increased emotional and behavioural problems. Total scores are classified into a normal range (<83rd centile, T<60), borderline range (83rd-90th centile, T 60–63) and clinical range (>90th centile, T≥64).39 The CBCL is known to have high reliability, validity and cross-informant agreement for measuring children’s emotional and behavioural problems.39
We used the Quantitative Checklist for Autism in Toddlers (Q-CHAT) as an additional behavioural screening tool to broaden the exploration of mental health outcomes in toddlers. The Q-CHAT is a parent-completed 25-item questionnaire, in which the child’s behaviour is scored on a 5-point (0–4) frequency scale. Higher total scores correspond to a higher frequency of behaviours also observed in autism spectrum conditions. The Q-CHAT shows good test–retest reliability, face validity and specificity, yet poor positive predictive value for autism,40 41 highlighting that higher Q-CHAT scores may reflect developmental immaturity rather than autism.41
Cognitive assessment was performed using the Bayley Scales of Infant and Toddler Development–Third Edition (Bayley-III). The Bayley-III provides scores for a child’s overall cognitive, language and motor development. The cognitive standardised composite score was used in this study; scores between 70 and 84 indicate mild cognitive impairment, scores between 55 and 69 indicate moderate impairment and scores lower than 55 indicate severe impairment.42 Reliability and validity of the Bayley-III have been shown to be robust,43 although some studies report its underestimation of developmental problems.44
Assessments were carried out by staff experienced in the neurocognitive assessments of toddlers.
Analysis
Descriptive statistics and one-way Analysis of Variance tests were performed in IBM SPSS Statistics for Windows V.25. All other analyses were carried out in Stata V.16.
Multiple imputation (MI) was carried out to account for missing data in CBCL (11/509, 2.24%), Q-CHAT (9/509, 1.8%), maternal EPDS (73/509, 14.3%), maternal BMI (27/509, 5.3%) and IMD rank (3/509, 0.6%). Variables were imputed simultaneously using the ‘mi impute chained’ procedure that performs imputation by chained equations. The imputation models had the same structural form as the analysis models and included all variables that appear in the corresponding analysis models (maternal EPDS, maternal BMI, multiple pregnancy, parity, IMD rank, gestational age at birth, birth weight, sex, corrected age at assessment and Bayley III Cognitive Composite score). In the imputation models, we also included variables that were associated with the incomplete variables at the 20% level. As such, maternal age was included in the imputation model because it was found to be a significant predictor of the total CBCL raw score (p=0.001), the Q-CHAT score (p=0.021) and EPDS score (p=0.122) when it was included as an independent variable in regression models.
Maternal EPDS and CBCL were imputed using Poisson regression; Q-CHAT, maternal BMI and the IMD rank were imputed using linear regression. Fourty MI data sets were created. To assess the stability of our MI parameters, we extracted the Monte Carlo error of each parameter estimate and examined whether the error for the coefficient was less than 10% of the parameter’s SE estimate. MI estimates were used for the primary analyses and compared with the estimates from complete-case (CC, individuals who had no missing data preimputation) analyses. Conditional normality was inspected in the CC analyses using QQ plots of the residuals of the models. Sensitivity analyses with and without extreme values were conducted. Initially, we fit the model using all available data, constructed the residuals and examined the QQ plot. Extreme values were then removed, models refitted without these values, and new QQ plots of residuals constructed again to check for any new extreme values. This process was repeated as many times as needed to remove all extreme values. During this process, the resulting estimates from the models were being examined as to whether they had substantially changed. We found that the removal of extreme values did not make any difference to the estimated parameters, and hence present the results from the full sample.
The analysis models were multiple linear regressions fitted using the ‘mi estimate’ procedure, which estimates effects after application of Rubin’s rules.45 To account for the small amount of clustering in our data (twin/triplet siblings), the models’ SEs were obtained using Stata’s robust cluster estimator ‘vce(cluster idvar)’. For continuous variables, Cohen’s f2 effect sizes were calculated using , where is the R2 value from a regression model that includes the variable of interest as well as all the covariates used in the model, and is the R2 value from the regression model that includes only the covariates.46 47 For binary variables, Cohen’s f2 effect sizes were produced after estimating first the Cohen’s d using the formula: , where k is the number of groups. As a measure of dispersion, Cohen’s d used the average root mean-square error over the MI data sets. Adjusted R2 values after MI were extracted after estimating the model with the user-written ‘mibeta’ command with the ‘fisherz’ option,48 which calculates R2 measures for linear regression with MI data. The significance of the joint effect of the categorical variable parity was assessed using ‘mi test’, which performs Wald tests of composite linear hypotheses.
Primary outcome measures were children’s total CBCL raw score and Q-CHAT score. Secondary outcome measures were CBCL internalising and externalising scores. The effect of maternal EPDS score was adjusted for IMD rank, maternal age, maternal BMI, maternal parity, pregnancy size and the following child variables: continuous gestational age, birth weight, Bayley-III cognitive composite score and corrected age at assessment. The interaction between PTB and maternal depressive symptoms was explored using a CC analysis in both CBCL and Q-CHAT models, using a dichotomised measure of gestational age. EPDS, CBCL and Q-CHAT scores were compared between term (≥37 weeks gestation) and preterm infants (<37 weeks gestation) using the CC data set. Our regressions were, thus, run two times: with and without the interaction term.
As all mothers had their EPDS score measured near term (or term-corrected in the case of mothers of preterm infants), we further investigated the association between time elapsing between baby’s birth and mother’s EPDS assessment and EPDS score, in order to avoid erroneously identifying ‘baby blues’ in mothers of term-born infants versus postnatal depression in mothers of preterm infants. This post hoc analysis was performed using Poisson regression.
Patient and public involvement
The current study was developed in consultation with the Weston Programme for Family Centered Research, which involves parents to define what research is valuable to them and to allow them to lead it with support from the scientists in the Centre for the Developing Brain.
Results
Descriptive statistics
Our sample of 509 toddlers was followed up at a median-corrected age of 18.4 months (range 17.3–24.3 months). 51 (10.0%) of these were twins, and 3 (0.59%) were triplets. Of the 509, 21 (4.13%) mothers scored above a clinical cut-off (≥13) on the EPDS37 38; the distribution of maternal EPDS scores is shown graphically in online supplemental figure 1. Demographic data are shown in table 1. Complete data were available for 400 (78.6%) participants. Missing data were imputed and thus all 509 subjects were included in the primary and secondary analyses. One participant was excluded from the cognition analysis after examining the quintiles of the residuals against the theoretical quintiles of a normal distribution. The mean CBCL T score was 46.9 (SD 9.5) (table 1); using CBCL-specified cut-offs,39 449 (90.2%) of participants had a CBCL score in the normal range, 30 (6.0%) were borderline and 19 (3.8%) scored in the clinical range. The mean Q-CHAT score was 30.5 (SD 9.3) (table 1). The mean Bayley III Cognitive Composite score in our sample was 100 (SD 11.4) (table 1), which corresponds to the standardised test mean42; 480 (94.3%) of participants had a normal cognitive score, 24 (4.7%) had mild impairment, 5 (1%) had moderate impairment and nil had severe impairment. This distribution is not dissimilar from that of the normative sample.42
Table 1.
Sociodemographic, maternal and clinical characteristics (n=509).
| Variable | Number (%)* |
| Sex: male | 274 (53.8) |
| Index of multiple deprivation (IMD) quintiles | |
| 1 (least deprived) † | 65 (12.8) |
| 2 | 87 (17.2) |
| 3 | 108 (21.3) |
| 4 | 173 (34.2) |
| 5 (most deprived) | 73 (14.4) |
| Gestational age at birth (weeks), median (range) | 39.7(20–43) |
| Gestational category | |
| Extremely preterm (<28 weeks) | 18 (3.5) |
| Very preterm (28–32 weeks) | 28 (5.5) |
| Late preterm (32–37 weeks) | 51 (10.0) |
| Term (≥37 weeks) | 412 (80.9) |
| Birth weight (g), median (range) | 3290 (450–4750) |
| Multiple pregnancy | 54 (10.6) |
| Maternal parity | |
| 0 | 332 (65.2) |
| 1 | 124 (24.4) |
| 2 | 32 (6.3) |
| 3+ | 21 (4.2) |
| Maternal BMI (kg/m2), median (range) | 23.2 (15.3–43.6) |
| Maternal age at infant’s birth (years), mean (SD) (range) | 34.2 (4.8) (17–52) |
| Maternal ethnicity | |
| White | 272 (53.4) |
| Black/Black British | 56 (11.0) |
| Asian/Asian British | 28 (5.5) |
| Chinese | 18 (3.5) |
| MixedWhite and Asian | 4 (0.8) |
| MixedWhite and Black | 4 (0.8) |
| Any other | 30 (5.9) |
| Do not wish to answer | 9 (1.8) |
| No data | 88 (17.3) |
| Bayley III cognitive composite score, mean (SD) (range) | 100 (11.4) (55–125) |
| CBCL total T score, mean (SD) (range) | 46.9 (9.5) (28–69) |
| Q-CHAT total score, mean (SD) (range) | 30.5 (9.3) (8–70) |
| EPDS score, median (range) | 4 (0–28) |
| EPDS score, n (%) | |
| <13 | 415 (8.2) |
| ≥13 | 21 (4.1) |
| No data | 73 (14.3) |
*Unless otherwise specified.
†Quintile 1 corresponds to the highest, least deprived, IMD rankings.
bmjopen-2021-058540supp001.pdf (93.2KB, pdf)
Association between maternal EPDS score and toddler CBCL and Q-CHAT scores
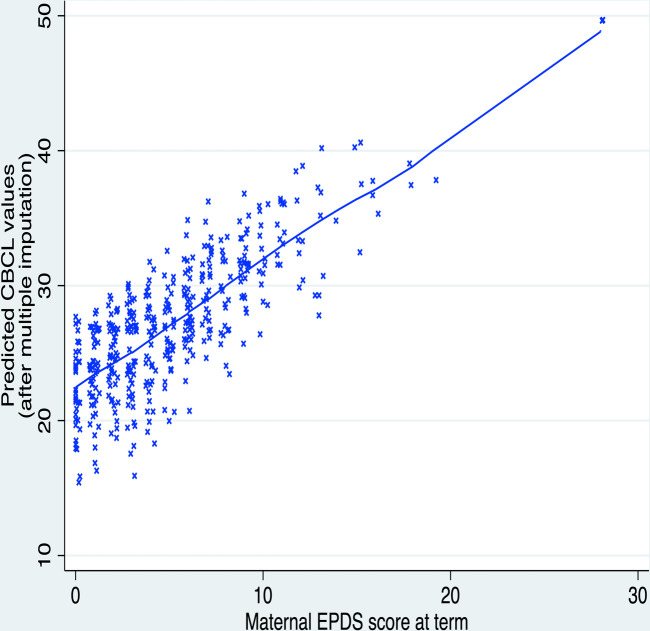
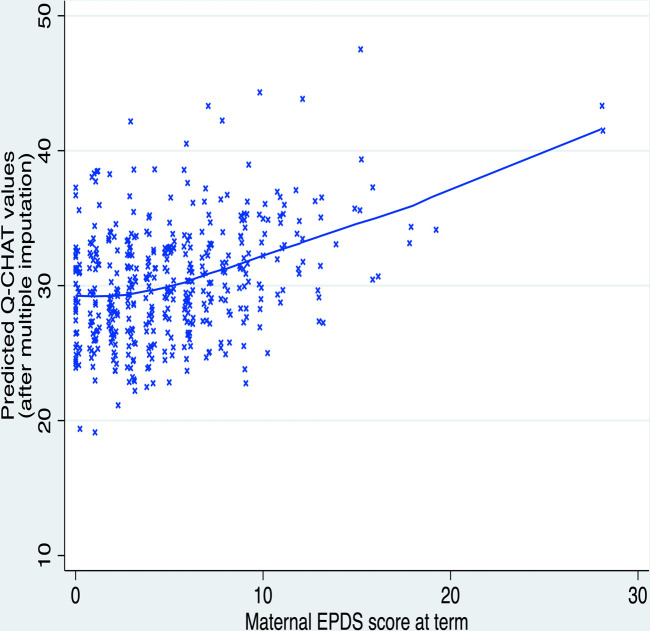
Predictors of children’s CBCL and Q-CHAT scores after MI are shown in table 2. Higher maternal EPDS score was associated with children’s higher CBCL total score (B=0.93, 95% CI 0.43 to 1.44, p<0.001, f2=0.05) and Q-CHAT score (B=0.27, 95% CI 0.03 to 0.52, p=0.031, f2=0.01) (table 2). These associations are presented graphically in figure 1 and figure 2, respectively. Boys had higher CBCL and Q-CHAT scores than girls. Higher Q-CHAT scores were associated with lower IMD rank (ie, greater socioeconomic deprivation) and lower Bayley-III cognitive composite scores. Parity was not a significant predictor of outcome in any of the models (table 2).
Table 2.
CBCL and Q-CHAT model predictors using multiple imputation without interaction (Cf. (online supplemental table 3) for complete-case analysis)
| CBCL | QCHAT | |||||
| B (95% CI) | p | f2 | B (95%CI) | p | f2 | |
| Maternal EPDS | 0.93 (0.43 to 1.44) | <0.001 *** | 0.05 | 0.27 (0.03 to 0.52) | 0.031* | 0.01 |
| Maternal BMI | −0.09 (−0.44 to 0.26) | 0.621 | – | 0.06 (-0.13 to 0.24) | 0.538 | – |
| Multiple pregnancy | 3.15 (-3.07 to 9.37) | 0.320 | – | 1.33 (-2.62 to 5.28) | 0.509 | – |
| Parity | ||||||
| 1 | −2.52 (−5.96 to 0.93) | 0.151 | – | −2.14 (−4.02 to -0.27) | 0.025 † | – |
| 2 | −3.23 (−9.16 to 2.70) | 0.285 | – | 0.88 (-1.99 to 3.75) | 0.548 | – |
| 3+ | −1.37 (−8.36 to 5.61) | 0.699 | – | −0.49 (−4.57 to 3.60) | 0.815 | – |
| IMD rank | −1.48 (−3.33 to 0.37) | 0.117 | – | −1.50 (−2.60 to -0.40) | 0.008 ** | 0.02 |
| Gestational age at birth (weeks) | 0.10 (-0.65 to 0.85) | 0.786 | – | 0.26 (-0.17 to 0.70) | 0.233 | – |
| Birth weight (kg) | 0.56 (-2.65 to 3.78) | 0.731 | – | −1.24 (−2.93 to 0.46) | 0.151 | – |
| Sex:female | −4.14 (−6.96 to -1.31) | 0.004 ** | 0.06 | −1.95 (−3.42 to -0.48) | 0.009 ** | 0.05 |
| Corrected age at assessment (months) | −0.90 (−2.17 to 0.37) | 0.166 | – | −0.16 (−0.91 to 0.59) | 0.677 | – |
| Cognition | −0.05 (−0.20 to 0.09) | 0.467 | – | −0.27 (−0.35 to -0.20) | <0.001 *** | 0.12 |
CBCL model adjusted R2=0.0676. Q-CHAT model adjusted R2=0.193.
Effect size (Cohen’s f2, calculated from squared part correlations for predictors significant to 0.05): 0.02=small, 0.15=medium and 0.35=large0.46
– Indicates data not given, as predictor not significant to 0.05.
**p<0.05; **p<0.01; ***p<0.001.
†Wald test of whole parity variable in Q-CHAT model: F(3, 476.9) = 1.88, p=0.133.
B, unstandardised coefficient; CBCL, Child Behaviour Checklist score at 18 months; Cognition, infant Bayley III score at 18 months; Maternal EPDS, maternal Edinburgh Postnatal Depression Scale score at term-equivalent age; Corrected age at assessment (months), age at behavioural assessment, corrected for gestational age; Parity, dummy variable, one/two/three+ previous child(ren); Multiple pregnancy, dummy variable of twin/triplet pregnancy; Q-CHAT, Quantitative Checklist for Autism in Toddlers score at 18 months.
Figure 1.
Children’s predicted CBCL scores at 18 months are positively correlated to the maternal EPDS score at term-equivalent age. CBCL, Child Behaviour Checklist score; EPDS, Edinburgh Postnatal Depression Scale.
Figure 2.
Children’s predicted Q-CHAT scores at 18 months are positively correlated to the maternal EPDS score at term-equivalent age. EPDS, Edinburgh Postnatal Depression Scale; Q-CHAT, Quantitative Checklist for Autism in Toddlers.
Maternal EPDS score did not disproportionately affect preterm children with respect to CBCL or Q-CHAT scores (table 3).
Table 3.
CBCL and Q-CHAT model predictors using complete-case analysis with interaction of ‘EPDS x Term’
| CBCL | Q-CHAT | |||
| B (95% CI) | p | B (95% CI) | p | |
| Maternal EPDS | 0.89 (−0.24 to 2.02) | 0.121 | 0.24 (−0.28 to 0.75) | 0.365 |
| Maternal BMI | −0.01 (−0.39 to 0.37) | 0.955 | 0.00 (−0.16 to 0.17) | 0.982 |
| Multiple pregnancy | 1.76 (−6.65 to 10.17) | 0.681 | 0.97 (−2.07 to 4.01) | 0.532 |
| Parity | ||||
| 1 | −2.75 (−6.49 to 0.99) | 0.149 | −1.42 (−3.30 to 0.46) | 0.139 |
| 2 | −3.49 (−10.36 to 3.37) | 0.317 | 0.16 (−2.84 to 3.16) | 0.917 |
| 3+ | −1.17 (−9.69 to 7.35) | 0.788 | −1.13 (−4.24 to 1.98) | 0.476 |
| IMD rank | −1.41 (−3.54 to 0.73) | 0.195 | −1.68 (−2.64 to -0.72) | 0.001 ** |
| Gestation: term | 1.25 (−8.34 to 10.85) | 0.797 | 2.64 (−1.74 to 7.02) | 0.236 |
| Birth weight (kg) | −1.01 (−4.08 to 2.05) | 0.516 | −2.25 (−3.73 to -0.78) | 0.003 ** |
| Sex: female | −4.64 (−7.83 to -1.44) | 0.005 ** | −2.22 (−3.72 to -0.71) | 0.004 ** |
| Corrected age at assessment (months) | −0.83 (−2.27 to 0.62) | 0.261 | −0.39 (−1.18 to 0.04) | 0.335 |
| Cognition | −0.03 (−0.20 to 0.14) | 0.720 | −0.22 (−0.29 to −0.15) | <0.001 *** |
| EPDS x Gestation:Term | −0.01 (−1.30 to 1.28) | 0.991 | −0.02 (−0.60 to 0.56) | 0.950 |
*p<0.05; **p<0.01; ***p<0.001.
CBCL model adjusted R2=0.0865. Q-CHAT model adjusted R2=0.215.
B, unstandardised coefficient; CBCL, Child Behaviour Checklist score at 18 months; Cognition, infant Bayley III score at 18 months; Maternal EPDS, maternal Edinburgh Postnatal Depression Scale score at term-equivalent age; EPDS x Gestation:Term, interaction term between maternal EPDS score and term gestation at birth; Corrected age at assessment (months), age at behavioural assessment, corrected for gestational age; Parity, dummy variable, one/two/three+ previous child(ren); Multiple pregnancy, dummy variable of twin/triplet pregnancy; Q-CHAT, Quantitative Checklist for Autism in Toddlers score at 18 months; Gestation: term, dummy variable, term (≥37 weeks) versus preterm (<37 weeks) gestation at birth.
Association between maternal EPDS score and toddler CBCL internalising and externalising scores
Higher maternal EPDS score was associated with both internalising (B=0.22, 95% CI 0.08 to 0.36, p<0.01, f2=0.03) and externalising (B=0.40, 95% CI 0.20 to 0.61, p<0.001, f2=0.05) symptoms in children (online supplemental table 1 and 2), respectively). Comparison of the imputed model analyses to the CC analyses showed that results were consistent for the CBCL model (online supplemental table 3). Comparison for the Q-CHAT model showed that maternal EPDS was a significant predictor in the imputed model, but not in the CC analysis (online supplemental table 3).
Effect of time-lag between baby’s birth and mother’s EPDS assessment and EPDS score
Mothers who gave birth prematurely (<37 weeks gestation) had their EPDS score assessed on average 7.7 weeks later post delivery than mothers who gave birth at term (preterm participants M=8.9 (SD 4.8), term participants M=1.2 (SD 1.3); t(99.4)=15.5, p<0.001). The time-lag between birth and EPDS assessment did not predict maternal EPDS score, and there was no evidence of a significant interaction between gestation and birth-to-assessment time-lag (online supplemental tables 4 and 5, respectively).
Discussion
Principal findings
Our results showed that more maternal self-reported depressive symptoms shortly after birth were associated with greater parent-reported toddlers’ behavioural problems. Given that fewer than 5% of the mothers in our cohort had EPDS scores above a clinical threshold,37 our findings indicate that even subclinical depressive symptoms—that is, not only diagnostic postnatal depression—adversely impact children’s behavioural outcomes. In addition, our cohort was typically developing with few CBCL scores reaching a concerning threshold; our results could be interpreted within the conceptual framework of mental illness lying on a continuum with typical behavioural traits.49 Our findings further showed that PTB did not influence the association between self-reported maternal depressive symptoms and parent-reported infants’ behavioural outcomes in toddlerhood. This indicates that in this context, PTB may not be regarded as a vulnerability or plasticity factor. Interestingly, mothers of preterm infants did not report more depressive symptoms compared with mothers of term infants in this study.
Comparison to prior literature
Our results with respect to internalising and externalising symptoms are in line with previous studies, including large population cohort studies, which showed an association between postnatal maternal depression and young children’s emotional and behavioural problems.11 Another previous study in 18-month old toddlers found that maternal depression was associated with internalising and dysregulated behaviour, but not externalising symptoms.50 This difference between our and Conroy et al’s findings may have arisen from their exclusion of infants born <36 weeks and their use of a clinical diagnosis of depression for mothers, rather than the continuous self-reported approach we employed. Interestingly, our finding that even subclinical depressive symptoms may adversely impact parent-reported child behavioural outcomes is in line with recent data showing that low-level as well as high-level depressive symptoms are associated with internalising and externalising symptoms in children aged 3 years.51
The results showing an association between maternal postnatal depressive symptoms and the Q-CHAT are less robust and need to be interpreted with caution. First, these results must be viewed in the context of the Q-CHAT having a low positive predictive value for autism, with the measure perhaps being more reflective of developmental immaturity.41 Although some prior studies have shown an association between antenatal maternal depression and offspring’s ASD,10 52 and postnatal depression has been suggested as a potential focus of cross-domain studies of ASD,53 there is no clear aetiological role of maternal postnatal depression in the development of ASD per se. Also, given that mothers with ASD are more likely to suffer from perinatal depression than mothers without ASD,54 and ASD is highly heritable,55 maternal depression may actually be a confounding rather than causative factor in our observed results. Overall, therefore, our findings with respect to the Q-CHAT do not provide support for a role of maternal depression in the aetiology of autism traits, but rather suggest that maternal depression can influence toddler behaviour.
The finding that preterm infants were not disproportionately affected by maternal depressive symptom supports. Hadfield et al’s findings that maternal distress at 9 months did not differentially impact very preterm (<34 weeks) or late preterm (34–36+6 weeks) infants with respect to socioemotional outcomes, although paternal distress did have an impact on very preterm infants’ outcomes.24 However, our results differ from Gueron-Sela et al’s finding that very preterm (28–33 weeks) 12-month old infants’ social outcomes were more influenced by maternal emotional distress at 6 months than term infants’ outcomes.23 The inconsistent findings may be due to methodological differences: for instance, our infant assessment being conducted at 18 months corrected age when social competency is more developed, our assessment of maternal depressive symptoms being in the very early postnatal period, or our use of the CBCL and Q-CHAT tools as markers of toddler behaviour. Importantly, the lack of support for a diathesis–stress or differential susceptibility model of maternal mental state on preterm infants in our study must be viewed in the context of our results also showing no difference in CBCL and Q-CHAT scores between term and preterm infants. This is in contrast to the existing literature that preterm infants are more likely than term infants to develop behavioural problems, such as ADHD, in childhood and adolescence.20 33 It is possible that the phenotypes of neurodevelopmental and neuropsychiatric disorders assessed with the chosen behavioural measures may not be sufficiently expressed at 18 months corrected age.56 In addition, as briefly discussed above, much of the existing literature emphasises the risk of extreme (<28 weeks) or very preterm (28–33 weeks) birth on later behavioural outcomes,20 33 whereas only 3.5% and 5.5% of our participants fell within the extreme and very preterm group, respectively, and we, thus, may not have the power to show any subtle effects.
Strengths and limitations of the study
The strengths of this study lie primarily in its large sample and prospective data collection. Moreover, the use of MI methodology has facilitated retention of a complete dataset, thus minimising non-response bias and increasing parameter precision. A strength in comparison to prior population cohort studies is that we assessed very early maternal depressive symptoms, and our sample is perhaps more representative of today’s society—with increasing maternal age—than large cohort studies conducted in the 1990 s–2000s. Given the complex interplay of biological and environmental factors in the aetiology of behavioural disorders, the inclusion of a substantive proportion of preterm infants in our cohort also offers an important insight into the role of PTB in behavioural outcomes; moreover, our results represent the full gestational spectrum, rather than discrete gestational categories. In addition, using maternal depressive symptoms as a continuous, rather than dichotomous, variable allows a more nuanced understanding of the role maternal postnatal depressive symptoms may play in influencing children’s outcomes.
There are several limitations to this study that necessitate our findings to be considered with caution. First, differences in birth-to-EPDS-assessment time lags are a potential confounder, given the time-sensitive nature of early-onset temporary baby blues and late-onset pathological postnatal depression. Mothers of infants born at term were assessed early postdelivery, within the period one would anticipate baby blues to present, whereas mothers of preterm participants were on average assessed later, when postnatal depression predominates.1 57 Although our post hoc analyses showed that the time elapsed from birth to EPDS assessment was not associated with maternal EPDS score, providing reassurance that our assessments of mothers of term-born infants were not inflated by the common temporary symptoms of baby blues, it is possible that we did not capture the full extent of late-onset depressive symptoms in mothers of term-born infants. This may explain why maternal EPDS scores did not differ between preterm and term groups in our complete data set analysis, contrary to the current literature,31 as well as why our rate of postpartum depression, using an EPDS cut-off of 13, was low (4.1%) compared with the previously documented UK community prevalence rate of 8.9% at 8 weeks postpartum.58 Our results must, therefore, be interpreted with some caution.
Second, although statistical techniques were used to impute missing data and mitigate this problem, 14.3% of maternal EPDS scores were missing. This rate of missingness may relate to some mothers being reluctant to complete a questionnaire at the time their child is having an MRI or due to simultaneous childcare duties. Third, a number of important confounders that are likely to affect children’s behavioural outcomes were not assessed in this study, including genetic risk for psychiatric disorders,59 parental psychiatric comorbidities,50 chronicity of postnatal depressive symptoms,51 antenatal maternal depression, paternal depression, subsequent parent–infant attachment, and interparental conflict.11 Thus, we are unable to conclude whether our observed associations between early postnatal maternal depressive symptoms and children’s behavioural outcomes are moderated or mediated by other parental and/or psychiatric factors.
Fourth, while our study included a reasonable proportion of preterm infants (97/509, 19%), our sample was not random, as preterm children were selectively recruited for the DHCP; indeed, preterm infants are over-represented in our sample when compared with the UK population incidence (7.3%),60 which may limit the study’s generalisability to the general population. This over-representation of preterm infants may explain why our mean maternal age is higher than the national mean age of 30.7,61 given that increasing maternal age is associated with increased risk of adverse pregnancy outcomes.62 Our observed large maternal age range in itself also poses a limitation on the generalisability of our findings to the general population, and further research would be necessary to identify a possible moderation effect of high maternal age on both EPDS scores and child behavioural outcomes. Furthermore, although a 19% prevalence of PTB is high for a community sample, the proportion of very and extreme preterm infants in our sample is small, and this may not have provided sufficient power to detect any differential susceptibility effect of PTB on outcomes.
Sixth, the effect sizes of the association between maternal EPDS score and behavioural problems were small; this raises questions regarding the clinical significance of our findings and potentially explains some of the inconsistency between this and previous studies. Even within our analyses, the association between maternal depressive symptoms and Q-CHAT scores was not observed in our CC analysis, thus calling into question the validity of this result. It is also important to highlight again the poor positive predictive value of the Q-CHAT for autism41; higher Q-CHAT scores do not imply a diagnosis of ASD, and this distinction may also explain the contrast to previous studies.
Finally, it is well documented that maternal depression influences reporting of Q-CHAT63 and CBCL scores.64 Our study used maternal report of maternal depressive symptoms, and our outcome measures were parent-completed questionnaires; despite the CBCL showing good cross-informant agreement,39 it is, thus, possible that reporting bias with common method variance could have skewed our results.
Implications of our findings
Of greatest importance to clinicians and policymakers is our finding that even subclinical self-reported maternal depressive symptoms are associated with parent-reported behavioural outcomes of offspring. This has significant implications for the risk stratification of women and their babies in the postnatal period, during which contact with medical professionals is already established. Identifying high-risk families and providing appropriate supportive measures at the early postnatal stage may help to prevent future psychiatric morbidity.
Future research
Further follow-up of large cohorts of preterm and term infants, to an age when behavioural phenotypes may become more pronounced, is needed to investigate whether the long-term developmental trajectories of term and ex-preterm infants are differentially susceptible to changes of postnatal maternal mental health. Future research should consider both maternal and paternal mental health as well as socioeconomic and environmental factors on child outcomes. Such follow-up should use independent, objective assessments of child behavioural outcomes in order to avoid the common method variance inherent to parent-reported measures. Finally, it is crucial for future research to elucidate the interplay of biochemical and neurodevelopmental changes that may mediate and confound the translation of environmental exposures into distal behavioural phenotypes.
Conclusion
This prospective longitudinal cohort study found no evidence to support the concept of PTB as a vulnerability or plasticity factor with respect to the effect of maternal depressive symptoms on behavioural development. However, we showed that early subclinical maternal postnatal depressive symptoms were associated with behavioural problems in children on parent-reported measures. This adds to the increasing body of literature indicating the role of subclinical and early postnatal depressive symptoms in the aetiology of childhood behavioural disorders. These findings are of great relevance to child and public health, and further research may strengthen its implications for developing strategies to facilitate effective screening and support for women and children, enabling all to reach their full potential.
Supplementary Material
Footnotes
Twitter: @drirakleine, @PretermResearch
Contributors: We thank all DHCP investigators for their contribution to the study. We thank Dr Oliver Gale-Grant MRes (Centre for the Developing Brain, King’s College London; Department of Forensic & Neurodevelopmental Sciences, King’s College London) for providing the IMD rank data. We are very grateful to the families who generously took part in this research.
Conceptualisation: SC, DE, CN. Methodology: IK, GV, SC, AP, DE, CN. Investigation: SF, AC. Data curation: IK, AL. Formal analysis: IK, GV, AP, CN. Writing—original draft preparation: IK. Writing—review and editing: IK, GV, AL, SF, AC, SC, AP, DE, CN. Visualisation: IK, GV. Funding acquisition: SC, DE. Supervision: AP, DE, CN. Guarantor: CN.
Funding: The DHCP project was funded by the European Research Council under the European Union Seventh Framework Programme (FR/2007-2013)/ERC Grant Agreement number 319456. The authors acknowledge infrastructure support from the National Institute for Health Research Mental Health Biomedical Research Centre at South London, Maudsley NHS Foundation Trust, King’s College London, the National Institute for Health Research Mental Health Biomedical Research Centre at Guys, and St Thomas’ Hospitals NHS Foundation Trust. The study was also supported in part by the Engineering and Physical Sciences Research Council/Wellcome Trust Centre for Medical Engineering at King’s College London (grant WT 203148/Z/16/Z) and the Medical Research Council (UK) (grants MR/K006355/1 and MR/L011530/1) and the MRC Centre for Neurodevelopmental Disorders at King’s College London. AP receives a NIHR SI award (NF-SI-0617-10120). AL is supported by the UK Medical Research Council (MR/N013700) and King’s College London member of the MRC Doctoral Training Partnership in Biomedical Sciences. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Competing interests: ADE received financial support from the EU-AIMS-Trials (European Research Council under the European Union Seventh Framework Programme) as co-principal investigator. ADE received consulting fees from Chiesi Farmaceutici (advice on neuroprotection in newborn infants) and Medtronix (unpaid participation in Scientific Advice Committee). ADE has a patent on Xenon as an organ protectant (Number P023708WO). ADE was Chair of the Data Monitoring and Ethics Committee for the Baby-Oscar Trial, and served on the Data Monitoring and Ethics Committee for the PAEN Trial. There are no other relationships or activities that could appear to have influenced the submitted work.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by UK National Research Ethics Authority (14/LO/1169) and conducted in accordance with the World Medical Association’s Code of Ethics (Declaration of Helsinki). Written informed consent was given by children’s carer(s) at recruitment into the study.
References
- 1.Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord 2017;219:86–92. 10.1016/j.jad.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 2.Rezaie-Keikhaie K, Arbabshastan ME, Rafiemanesh H, et al. Systematic review and meta-analysis of the prevalence of the maternity blues in the postpartum period. J Obstet Gynecol Neonatal Nurs 2020;49:127–36. 10.1016/j.jogn.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 3.Beck CT. Predictors of postpartum depression: an update. Nurs Res 2001;50:275–85. 10.1097/00006199-200109000-00004 [DOI] [PubMed] [Google Scholar]
- 4.de Paula Eduardo JAF, de Rezende MG, Menezes PR, et al. Preterm birth as a risk factor for postpartum depression: a systematic review and meta-analysis. J Affect Disord 2019;259:392–403. 10.1016/j.jad.2019.08.069 [DOI] [PubMed] [Google Scholar]
- 5.Blom EA, Jansen PW, Verhulst FC, et al. Perinatal complications increase the risk of postpartum depression. the generation R study. BJOG 2010;117:1390–8. 10.1111/j.1471-0528.2010.02660.x [DOI] [PubMed] [Google Scholar]
- 6.Beck CT. Postpartum depression: a metasynthesis. Qual Health Res 2002;12:453–72. 10.1177/104973202129120016 [DOI] [PubMed] [Google Scholar]
- 7.Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health 2003;6:263–74. 10.1007/s00737-003-0024-6 [DOI] [PubMed] [Google Scholar]
- 8.Lebel C, Walton M, Letourneau N, et al. Prepartum and postpartum maternal depressive symptoms are related to children's brain structure in preschool. Biol Psychiatry 2016;80:859–68. 10.1016/j.biopsych.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 9.Goodman SH, Rouse MH, Connell AM, et al. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011;14:1–27. 10.1007/s10567-010-0080-1 [DOI] [PubMed] [Google Scholar]
- 10.Hagberg KW, Robijn AL, Jick S. Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin Epidemiol 2018;10:1599–612. 10.2147/CLEP.S180618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014;384:1800–19. 10.1016/S0140-6736(14)61277-0 [DOI] [PubMed] [Google Scholar]
- 12.Van Batenburg-Eddes T, Brion MJ, Henrichs J, et al. Parental depressive and anxiety symptoms during pregnancy and attention problems in children: a cross-cohort consistency study. J Child Psychol Psychiatry 2013;54:591–600. 10.1111/jcpp.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker ED, Jaffee SR, Uher R, et al. The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depress Anxiety 2011;28:696–702. 10.1002/da.20856 [DOI] [PubMed] [Google Scholar]
- 14.Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry 2013;70:1312–9. 10.1001/jamapsychiatry.2013.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleine I, Falconer S, Roth S, et al. Early postnatal maternal trait anxiety is associated with the behavioural outcomes of children born preterm <33 weeks. J Psychiatr Res 2020;131:160–8. 10.1016/j.jpsychires.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci 2009;10:446–57. 10.1038/nrn2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Preterm birth, 2018. Available: https://www.who.int/news-room/fact-sheets/detail/preterm-birth [Accessed May 18, 2022].
- 18.Dimitrova R, Pietsch M, Christiaens D, et al. Heterogeneity in brain microstructural development following preterm birth. Cereb Cortex 2020;30:4800–10. 10.1093/cercor/bhaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzallen GC, Sagar YK, Taylor HG, et al. Anxiety and depressive disorders in children born preterm: a meta-analysis. J Dev Behav Pediatr 2021;42:154–62. 10.1097/DBP.0000000000000898 [DOI] [PubMed] [Google Scholar]
- 20.Allotey J, Zamora J, Cheong-See F, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG 2018;125:16–25. 10.1111/1471-0528.14832 [DOI] [PubMed] [Google Scholar]
- 21.Jaekel J, Pluess M, Belsky J, et al. Effects of maternal sensitivity on low birth weight children's academic achievement: a test of differential susceptibility versus diathesis stress. J Child Psychol Psychiatry 2015;56:693–701. 10.1111/jcpp.12331 [DOI] [PubMed] [Google Scholar]
- 22.Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: differential susceptibility to environmental influences. Curr Dir Psychol Sci 2007;16:300–4. [Google Scholar]
- 23.Gueron-Sela N, Atzaba-Poria N, Meiri G, et al. The caregiving environment and developmental outcomes of preterm infants: diathesis stress or differential susceptibility effects? Child Dev 2015;86:1014–30. 10.1111/cdev.12359 [DOI] [PubMed] [Google Scholar]
- 24.Hadfield K, O'Brien F, Gerow A. Is level of prematurity a risk/plasticity factor at three years of age? Infant Behav Dev 2017;47:27–39. 10.1016/j.infbeh.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 2009;135:885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- 26.Pluess M, Belsky J. Prenatal programming of postnatal plasticity? Dev Psychopathol 2011;23:29–38. 10.1017/S0954579410000623 [DOI] [PubMed] [Google Scholar]
- 27.DeMaster D, Bick J, Johnson U, et al. Nurturing the preterm infant brain: Leveraging neuroplasticity to improve neurobehavioral outcomes. Pediatr Res 2019;85:166–75. 10.1038/s41390-018-0203-9 [DOI] [PubMed] [Google Scholar]
- 28.World Health organization (who) . Global nutrition targets 2025: low birth weight policy brief 2014.
- 29.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 30.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. The Lancet 2008;371:75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson C, Redshaw M, Gray R, et al. Risk of psychological distress in parents of preterm children in the first year: evidence from the UK millennium cohort study. BMJ Open 2015;5:e007942. 10.1136/bmjopen-2015-007942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigod SN, Villegas L, Dennis C-L, et al. Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: a systematic review. BJOG 2010;117:540–50. 10.1111/j.1471-0528.2009.02493.x [DOI] [PubMed] [Google Scholar]
- 33.Johnson S, Wolke D. Behavioural outcomes and psychopathology during adolescence. Early Hum Dev 2013;89:199–207. 10.1016/j.earlhumdev.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 34.Lautarescu A, Victor S, Lau-Zhu A, et al. The factor structure of the Edinburgh postnatal depression scale among perinatal high-risk and community samples in London. Arch Womens Ment Health 2022;25:157–69. 10.1007/s00737-021-01153-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministry of Housing, Communities & Local Government . The English indices of deprivation 2019, 2019. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
- 36.Boyd A, Golding J, Macleod J, et al. Cohort Profile: The ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111–27. 10.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987;150:782–6. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 38.Levis B, Negeri Z, Sun Y, et al. Accuracy of the Edinburgh postnatal depression scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ 2020;371:m4022. 10.1136/bmj.m4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles, 2000. [Google Scholar]
- 40.Allison C, Baron-Cohen S, Wheelwright S, et al. The Q-CHAT (quantitative checklist for autism in toddlers): a normally distributed quantitative measure of autistic traits at 18-24 months of age: preliminary report. J Autism Dev Disord 2008;38:1414–25. 10.1007/s10803-007-0509-7 [DOI] [PubMed] [Google Scholar]
- 41.Allison C, Matthews FE, Ruta L, et al. Quantitative checklist for autism in toddlers (Q-CHAT). A population screening study with follow-up: the case for multiple time-point screening for autism. BMJ Paediatr Open 2021;5:e000700. 10.1136/bmjpo-2020-000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ed. Bayley N. Bayley Scales of Infant and Toddler Development. In: Harcourt assessment. 3rd Edition, 2006. [Google Scholar]
- 43.Albers CA, Grieve AJ. Review of Bayley scales of infant and toddler development-third edition. J Psychoeduc Assess 2007;25:180–90. [Google Scholar]
- 44.Anderson PJ, De Luca CR, Hutchinson E, et al. Underestimation of developmental delay by the new Bayley-III scale. Arch Pediatr Adolesc Med 2010;164:352–6. 10.1001/archpediatrics.2010.20 [DOI] [PubMed] [Google Scholar]
- 45.Rubin DB. Inference and missing data. Biometrika 1976;63:581–92. 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 46.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Erlbaum Associates L, 1988. [Google Scholar]
- 47.Selya AS, Rose JS, Dierker LC, et al. A Practical Guide to Calculating Cohen's f(2), a Measure of Local Effect Size, from PROC MIXED. Front Psychol 2012;3:111. 10.3389/fpsyg.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harel O. The estimation of R 2 and adjusted R 2 in incomplete data sets using multiple imputation. J Appl Stat 2009;36:1109–18. [Google Scholar]
- 49.Plomin R, Haworth CMA, Davis OSP. Common disorders are quantitative traits. Nat Rev Genet 2009;10:872–8. 10.1038/nrg2670 [DOI] [PubMed] [Google Scholar]
- 50.Conroy S, Pariante CM, Marks MN, et al. Maternal psychopathology and infant development at 18 months: the impact of maternal personality disorder and depression. J Am Acad Child Adolesc Psychiatry 2012;51:51–61. 10.1016/j.jaac.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 51.Kingston D, Kehler H, Austin M-P, et al. Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS One 2018;13:e0195365. 10.1371/journal.pone.0195365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiggins LD, Rubenstein E, Daniels J, et al. A phenotype of childhood autism is associated with preexisting maternal anxiety and depression. J Abnorm Child Psychol 2019;47:731–40. 10.1007/s10802-018-0469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babb JA, Deligiannidis KM, Murgatroyd CA, et al. Peripartum depression and anxiety as an integrative cross domain target for psychiatric preventative measures. Behav Brain Res 2015;276:32–44. 10.1016/j.bbr.2014.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pohl AL, Crockford SK, Blakemore M, et al. A comparative study of autistic and non-autistic women's experience of motherhood. Mol Autism 2020;11:3. 10.1186/s13229-019-0304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colvert E, Tick B, McEwen F, et al. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry 2015;72:415–23. 10.1001/jamapsychiatry.2014.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egger HL, Emde RN. Developmentally sensitive diagnostic criteria for mental health disorders in early childhood: the diagnostic and statistical manual of mental disorders-IV, the research diagnostic criteria-preschool age, and the diagnostic classification of mental health and developmental disorders of infancy and early childhood-revised. Am Psychol 2011;66:95–106. 10.1037/a0021026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henshaw C. Mood disturbance in the early puerperium: a review. Arch Womens Ment Health 2003;6 Suppl 2:s33–42. 10.1007/s00737-003-0004-x [DOI] [PubMed] [Google Scholar]
- 58.Heron J, O'Connor TG, Evans J, et al. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord 2004;80:65–73. 10.1016/j.jad.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 59.Biel MG. Parental psychiatric symptoms and children's outcomes: toward understanding and responding to intergenerational risk in child psychiatry. J Am Acad Child Adolesc Psychiatry 2018;57:632–3. 10.1016/j.jaac.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 60.National Institute for Health and Care Excellence . Preterm labour and birth (NICE guideline 25), 2015. Available: https://www.nice.org.uk/guidance/ng25 [Accessed January 27, 2019]. [PubMed]
- 61.Office for National Statistics . Birth characteristics in England and Wales: 2020, 2022. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2020#birth-characteristics [Accessed February 5, 2022].
- 62.Londero AP, Rossetti E, Pittini C, et al. Maternal age and the risk of adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth 2019;19:261. 10.1186/s12884-019-2400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goh DA, Gan D, Kung J, et al. Child, maternal and demographic factors influencing caregiver-reported autistic trait symptomatology in toddlers. J Autism Dev Disord 2018;48:1325–37. 10.1007/s10803-018-3471-7 [DOI] [PubMed] [Google Scholar]
- 64.Friedlander S, Weiss DS, Traylor J. Assessing the influence of maternal depression on the validity of the child behavior checklist. J Abnorm Child Psychol 1986;14:123–33. 10.1007/BF00917228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058540supp001.pdf (93.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request.