Abstract
Objective
The mechanical thrombectomy (MT) benefit is related to the degree of reperfusion achieved. First pass effect (FPE) is defined as complete/near revascularisation of the large-vessel occlusion (modified Thrombolysis in Cerebral Infarction (mTICI) 2c-3) after a single device pass. This study assessed the health benefit and economic impact of achieving FPE for acute ischaemic stroke (AIS) patients from the Spanish National Health System (NHS) perspective.
Design
A lifetime Markov model was used to estimate incremental costs and health outcomes (measured in quality-adjusted life-years (QALYs)) of patients that achieve FPE. A subanalysis of the Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischaemic Stroke (STRATIS) registry was performed to obtain clinical outcomes. The base case included all patients that achieved at least a final mTICI ≥2 b, while the alternative scenario included all patients regardless of their final mTICI (0–3). Treatment costs were updated to reflect current practice based on expert panel consensus, while other acute and long-term costs were obtained from a previous cost-effectiveness analysis of MT performed in Spain. Sensitivity analyses were performed to assess the model’s robustness.
Setting
Spanish healthcare perspective.
Participants
AIS patients in Spain.
Interventions
FPE following MT.
Outcome measures
The model estimated QALYs, lifetime costs and net monetary benefit for the FPE and non-FPE group, depending on the inclusion of reperfusion groups and formal care costs.
Results
STRATIS subanalysis estimated significantly better clinical outcomes at 90 days for the FPE group in all scenarios. In the base case, the model estimated lifetime cost saving per patient of €16 583 and an incremental QALY gain of 1.2 years of perfect health for the FPE group. Cost savings and QALY gains were greater in the alternative scenario (-€44 289; 1.75). In all scenarios, cost savings were driven by the long-term cost reduction.
Conclusion
Achieving FPE after MT can lead to better health outcomes per AIS patient and important cost savings for the Spanish NHS.
Keywords: HEALTH ECONOMICS, Stroke, Interventional radiology, Neuroradiology
Strengths and limitations of this study.
A Markov model estimated the lifetime health and cost implications of achieving first pass effect (FPE) in acute ischaemic stroke patients treated with mechanical thrombectomy in Spain from the National Health System perspective.
The model allows to quantify the benefits of aiming mechanical thrombectomy techniques that may increase the FPE success rates.
A limitation of this study is that clinical efficacy and patient characteristics were based on the Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischaemic Stroke (STRATIS) registry, which included centres outside Spain.
Another limitation is that some model parameters, such as acute and long-term costs have been derived from literature, which have been validated by clinical experts.
Introduction
The annual number of strokes in the European Union is forecasted to increase by 34% in 2035, mainly due to its ageing population. With improving survival rates after stroke, almost 1 million more people will be living with a stroke as a chronic condition, rising from 3.7 million in 2015 to 4.6 million in 2035.1 It is estimated that the incidence and prevalence of strokes will increase by 35% and 31%, respectively, in Spain by 2035,2 which will inevitably raise the associated economic burden.
Mechanical thrombectomy (MT) is the most effective reperfusion treatment used in acute ischaemic stroke (AIS) management in patients with large vessel occlusion (LVO).3 4 Its cost-effectiveness has already been demonstrated in Spain; improving functional outcomes is associated with a higher quality of life and reduced health costs, leading to €44 378 in savings per patient compared with thrombolysis with intravenous tissue-type plasminogen activator (IV-tPA) alone.5
Clinical evidence suggests that the number of passes during an MT inversely correlates with the functional outcome of the procedure.6 7 Achieving complete/near revascularisation of the LVO (modified Thrombolysis in Cerebral Infarction (mTICI) 2c-3) after a single pass with MT, known as first pass effect (FPE), is associated with significant improvements in clinical outcomes and can be considered an independent predictor of good functional outcomes.8 Recent studies have begun to try to identify factors or predictors of FPE that may impact the choice of thrombectomy device and technique in the future.8–11
The objective of this study is to assess the health outcome benefits and economic impact of achieving FPE for the AIS patients from the National Health System (NHS) perspective in Spain.
Methods
Model structure
A previously published cost-effectiveness model comparing MT+IV-tPA with IV-tPA alone in a Spanish NHS setting was modified to reflect only patients that received MT treatment which afterwards were stratified to reflect those who achieved FPE and those who did not (non-FPE),5 and allowed to estimate lifetime health and costs outcomes of the two patient groups. As in the previous modelling, this analysis is also over the patient’s lifetime and from the Spanish NHS perspective. The model was developed using Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA).
The model had a two-phase structure, consisting of an acute-subacute phase from stroke onset to 90 days, and a rest-of-life phase 91 days after stroke to the end of patient’s life. In the acute–subacute phase, patients enter the model once reperfusion status (FPE vs non-FPE) has been determined, and then are assigned to one of the seven mutually exclusive health states based on modified Ranking Scale (mRS; 0-no symptoms, 6-death) to reflect several degrees of disability at 90 days. Afterwards, patients enter a Markov structure, from 91 days after the stroke to the end of the patients’ life. In this phase, patients could remain in the same health state or transition to different states during each annual cycle until the end of life, depending on the occurrence of a recurrent stroke or death from other causes (age-specific and gender-specific mortality). A half-cycle correction was used to account for transitions occurring in the middle of a cycle.
Patient population
The model simulates a hypothetical cohort of 1000 patients with clinic-demographic characteristics based on the STRATIS registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischaemic Stroke).12 STRATIS registry patients were classified into 2 groups: patients with a final mTICI ≥2 b (used for the base case analysis), and patients with final mTICI (0–3) (used for the alternative scenario). Afterwards, patients in both groups were stratified into FPE and non-FPE groups.
Clinical data
Clinical data were obtained from a subanalysis of the STRATIS registry12 in which FPE and Non-FPE groups were compared. Moreover, it was considered that patients were at risk of experiencing adverse events (symptomatic intracranial haemorrhage and malignant cerebral oedema) during the acute–subacute phase; therefore, adverse events data were also obtained from STRATIS registry subanalysis.
Categorical variables were compared by using χ2 test and Mantel-Haenszel χ2 test when appropriate. Proportion differences were compared by z-test both one-sided and two-sided tests are performed (considering 5% and 2.5% significance level, respectively). All statistical analyses were performed by using SAS V.9.4.
Background age/gender-related mortality was obtained from the latest available Life Table in Spain (data from 2018)13 and relative risks of death by mRS score were used to adjust age/gender-related mortality14 to account for the increased risks observed among stroke survivors (online supplemental table A1 and A2). Recurrence stroke rates were obtained from Mohan et al15 (online supplemental table A3).
bmjopen-2021-054816supp001.pdf (109.2KB, pdf)
Quality of life
Health outcomes were measured using quality-adjusted life-years (QALYs), a measure that weights life-years gained with an intervention by its utility value. Utilities assigned to health states can take values from 0 (death) to 1 (optimal health) and negative values (state worse than death). Utilities by mRS score were obtained from Rivero-Arias et al,16 with values ranging from 0.93 (mRS 0) to −0.54 (mRS 5) (online supplemental table A4).
Costs
The study considered the Spanish NHS perspective, consequently, only direct medical costs were considered, including treatment and adverse events management costs, acute and long-term care costs. Treatment costs were updated to reflect the costs for each patient group (FPE vs non-FPE) and were kept in line with the new treatment approaches according to local practice based on a panel of experts’ consensus. Treatment costs in both groups FPE and non-FPE included the costs of AIS diagnosis, and adjunctive IV-tPA in 30% of the cases according to local practice (online supplemental table A5).
Adverse events, acute and long-term costs by mRS score were kept consistent with the previous model.5 For each scenario, a second analysis was performed to include formal care costs such as nursing/residential costs. All costs are presented in Euros and were inflated to reflect Euros in 2020 (table 1). Costs and health outcomes were discounted at an annual discount rate of 3% consistent with the relevant health technology assessment guidelines for Spain.17
Table 1.
Adjusted acute and long-term costs (Euros 2020)
| mRS | Acute costs | Annual long-term cost | |
| Total acute care cost (€) | Total long-term healthcare cost (without nursing and residential care cost) (€) | Total including nursing and residential care cost (€) | |
| mRS 0 | 4718 | 1340 | 2767 |
| mRS 1 | 5242 | 1489 | 3074 |
| mRS 2 | 5766 | 1638 | 3382 |
| mRS 3 | 6468 | 23 250 | 33 061 |
| mRS 4 | 7187 | 25 833 | 53 339 |
| mRS 5 | 7906 | 28 417 | 67 400 |
| mRS 6 | 4046 | ||
Note: Table adapted from de Andrés-Nogales et al.5
mRS, modified Ranking Scale.
Economic model outcomes and sensitivity analysis
The model estimates the lifetime total costs and QALYs for each patient group. To quantify the net economic value of FPE, the net monetary benefit (NMB) was calculated, considering a willingness-to-pay (WTP) threshold of €30 000/QALY, (NMB=(Incremental QALYs×WTP) − Incremental Costs).18 19
Deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were conducted to evaluate results’ robustness.20 DSA assigns a one-way variation to input parameters including discount rates, mRS at 90 days, age, health states utilities, recurrent stroke rates, relative risk of death, and all costs (treatment, acute and long-term costs). In PSA, 10 000 Monte Carlo simulations were run after assigning a probability distribution to all key parameters simultaneously (mRS scores at 90 days (Dirichlet), mortality relative risks (lognormal), starting age (normal), utilities (beta) and costs (gamma)), to account for the general uncertainty around model inputs.5
Patient and public involvement
This study was conducted without patient and public involvement. Therefore, patients were not involved in the study design, reporting or interpretation of the findings.
Results
Based on STRATIS subanalysis, the mean age of stroke considered in the model was 68 years. Both groups have similar characteristics at baseline. Descriptive statistics on the FPE and non-FPE groups are reported in online supplemental tables A6–A9.
Our results suggest that the FPE group had significantly better clinical outcomes at 90 days after stroke compared with the non-FPE group in the base case scenario (mRS 0–2: 66.2% vs 54.6%, p<0.005). Similar results in the alternative scenario were observed (mRS 0–2: 66.9% vs 50.6%, p<0.0001) (figure 1). Adverse events results across scenario populations are presented in online supplemental table A10.
Figure 1.
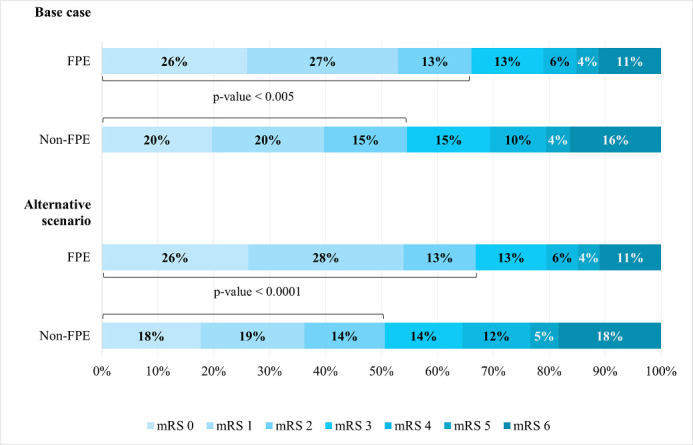
mRS outcomes at 90 days base case and alternative scenario. FPE, first pass effect; mRS, modified Rankin Score.
In the base case scenario, the model estimates an average lifetime cost per patient equal to €97 206 for the FPE group and €113 790 for the non-FPE group. Of these, 83% were associated with long-term costs. Overall, the FPE group generated a cost reduction of €16 583 per patient in a lifetime horizon. Cost reductions are predicted to be greater when nursing/residential care cost are included, leading to a savings of €30 072 per patient.
In terms of health outcomes, the model estimates that achieving FPE lead to a QALY gain of 1.2 years (7.89 vs 6.69), while the number of independent people at 90 days is also projected to increase by 116 (662 vs 546) in this hypothetical cohort. However, there is an estimated increase in the total number of recurrent strokes in the FPE group due to patients living longer (283 vs 257).
The model suggests that achieving FPE lead to a NMB of €52 634 considering a WTP of €30 000/QALY gained. The NMB was expected to increase to €66 122 when nursing/residential care cost are considered. FPE provides greater net economic value demonstrating higher efficacy with lower costs from the payer perspective in a lifetime time horizon (table 2). In the alternative scenario, similar results were observed, which may confirm the greater benefits that achieving FPE (between 32% and 47% higher) may provide when all patients regardless their final mTICI are considered (QALY gain of 1.75 years and €21 910 cost reduction; when considering nursing/residential costs, a cost reduction of €44 289 and an NMB of €96 684) (table 2).
Table 2.
Summary of base case and alternative scenario results
| Costs | Base case | Alternative scenario | ||||
| FPE | Non-FPE | Incremental | FPE | Non-FPE | Incremental | |
| Treatment (€) | 9086 | 10 432 | −1346 | 9086 | 10 432 | −1346 |
| Adverse event costs (€) | 269 | 582 | −313 | 238 | 551 | −314 |
| Acute costs (€) | 5259 | 5353 | −94 | 5250 | 5387 | −137 |
| Long term care costs (€) | 79 296 | 94 263 | −14 968 | 78 039 | 98 469 | −20 430 |
| Long term care costs (with nursing/residential care cost) (€) | 144 072 | 172 527 | −28 456 | 141 678 | 184 487 | −42 809 |
| Recurrent stroke costs (€) | 3297 | 3160 | 137 | 3313 | 2997 | 316 |
| Total costs (€) | 97 206 | 113 790 | −16 583 | 95 925 | 117 836 | −21 910 |
| NMB (€) | 52 634 | 74 306 | ||||
| Total costs (with nursing/residential care cost) (€) | 161 982 | 192 054 | −30 072 | 159 565 | 203 854 | −44 289 |
| NMB (with nursing/residential care cost) (€) | 66 122 | 96 684 | ||||
| Total QALYs | 7.89 | 6.69 | 1.2 | 7.96 | 6.21 | 1.75 |
| Total life years | 10.99 | 10.06 | 0.92 | 11.03 | 9.71 | 1.32 |
FPE, first pass effect; NMB, net monetary benefit; QALYs, quality-adjusted life-years.
Sensitivity analysis
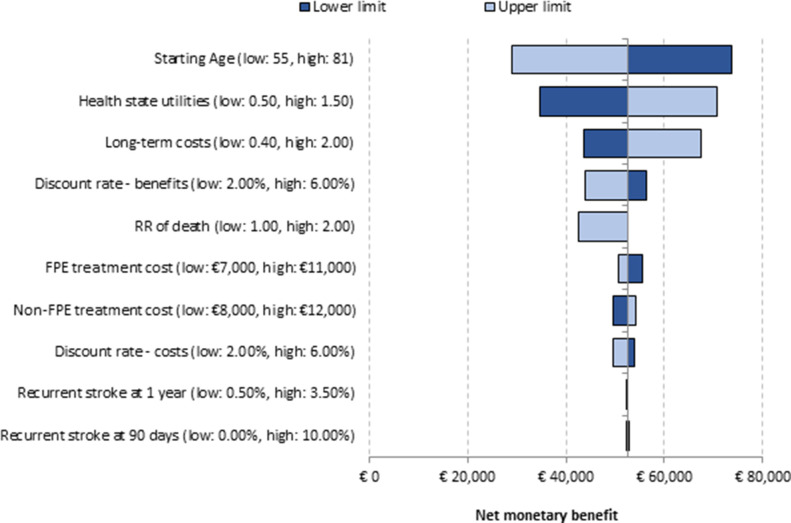
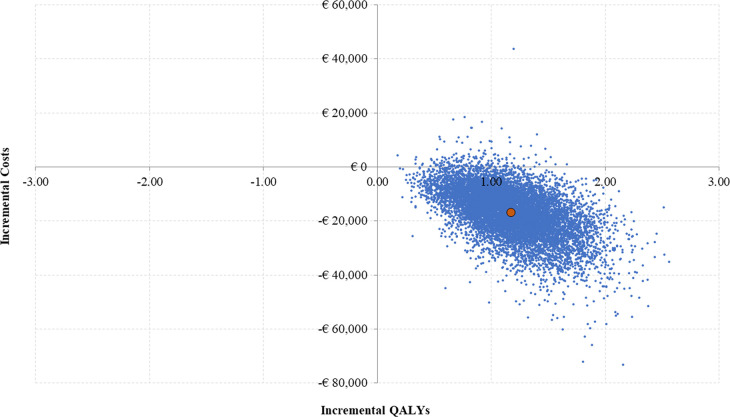
According to the DSA, in both scenarios (base case and alternative), the key drivers of the analysis included long-term care costs, starting age, health state utilities by mRS score and relative risk of death. However, none of these key parameters changed the direction of the results; therefore, in all the simulations, the NMB remained positive (minimum value: €28 884; maximum value: €73 620), showing the results were robust to input parameters variations (figure 2). In the PSA, FPE was estimated to be cost neutral or cost saving in 98.4%of the Monte Carlo simulations (figure 3).
Figure 2.
Tornado diagram of deterministic sensitivity analysis. FPE, first pass effect; RR, relative risk.
Figure 3.
Probabilistic sensitivity analysis. QALYs, quality-adjusted life-years.
Discussion
Clinical evidence suggests that achieving FPE after a single pass is associated with favourable outcomes after an MT procedure.6 7 Our study estimated the health gains from achieving FPE and examined the associated economic impact from the Spanish NHS perspective over a lifetime horizon.
Clinical outcomes, based on a subanalysis from the STRATIS registry, showed that achieving mTICI 2c-3 reperfusion after a single pass leads to significantly better overall mRS distribution and functional independence (mRS 0–2). The difference in the proportion in mRS 0–2 between FPE and non-FPE groups ranged between 11.5% and 16.3% depending on the cohort of patients (figure 1). Similar findings have been described in the literature.8 21 22 An analysis of North American Solitaire Acute Stroke Registry conducted by Zaidat et al suggested that if patients achieved mTICI 3, the FPE lead to better clinical outcomes compared with the rest of the cohort that did not achieve FPE (61.3% vs 35.3%, p<0.0001).8 The meta-analysis by Abbasi et al reported on the association between FPE and clinical outcomes finding higher rates of functional independence for FPE compared with Non-FPE (56% vs 41%, p<0.01) and lower mortality (17% vs 25%, p<0.01).21 Furthermore, a recent meta-analysis that conducted a per-pass analysis of recanalisation and health outcomes in thrombectomy22 suggests that the likelihood of functional independence in patients with final successful recanalisation decreased after each pass. On the first pass, 55% of patients achieved mRS 0–2 (p value: 0.033), while rates progressive declined after each subsequent pass, dropping to 26% for patients who required five or more passes for successful recanalisation. The results of our analysis also confirm improved health outcomes from achieving FPE and are therefore coherent with existing literature.
The base case results suggest that achieving FPE yields better health outcomes than non-FPE group, providing an incremental QALY gain of 1.2, equivalent to 438 days in perfect health. From the cost perspective, the FPE group is associated with lower healthcare costs, leading to a cost saving of €16 583 and €30 072 when considering nursing/residential healthcare costs (table 2). QALYs and cost savings resulted to be greater in the alternative scenario: the FPE group lead to 1.75 additional QALYs per patient (or 657 days in full health) and €21 910 in savings (€44 289 when considering nursing/residential healthcare costs). The greater results observed in the alternative scenario can be explained by a slight increase in good functional outcomes in the FPE group, accompanied by a decrease in the mRS 0–2 in the non-FPE group, which contributed to an even larger incremental difference between FPE and non-FPE outcomes in this scenario.
Cost savings in both scenarios were mainly driven by reductions in long-term costs associated with the management of functionally dependent patients. Furthermore, all results were tested by performing DSA and PSA, which demonstrated that our results are robust. In both scenarios and subscenarios, the non-FPE group was associated with lower health benefits and higher healthcare costs.
Improved health outcomes are generally associated with economic benefits. Even though there is less literature available on cost-implications from FPE, a recently published study23 estimated the short-term cost implications of FPE in several countries, including Spain. The authors estimated the procedural/hospitalisation and annual care costs differences considering a 1-year time horizon. Similar to our work, the study showed lower procedural/hospitalisation and annual care costs for patients that achieved FPE versus non-FPE across countries considered. Furthermore, our findings are compatible with other studies undertaken in the USA that have demonstrated that achieving TICI 3 lead to healthcare and societal cost savings relative to achieving TICI 2b for LVO.24 25
Overall, the results of this study showed that raising the FPE rate will not only increase the quality of life for patients, but also decrease the overall healthcare costs. Achieving FPE can potentially be one of the primary goals in the treatment of patients with ischaemic stroke due to LVO from both a clinical and economic perspective. Because this analysis was performed from the Spanish NHS perspective, only the direct costs are considered. There could be larger savings associated with achieving FPE if indirect costs, such as informal care and productivity losses, were included.
To our knowledge, this is one of the first studies that aim to evaluate the lifetime health and cost implications of achieving FPE in AIS patients in Spain from the NHS perspective. Among the strengths of this study are the Markov structure (allows to better reflect the patient pathway in terms of lifetime costs and benefits) and the inclusion of comprehensive diagnostics and treatments costs, main adverse events management and recurrent strokes, to account for all health outcomes and associated costs after a stroke.
This study has some limitations. First, clinical efficacy and patient characteristics were based on the STRATIS registry, which included centres outside Spain. Moreover, the study’s reliance on observational data may limit the result’s interpretation due to the potential effect that unmeasured confounders (eg, quality of stroke care, procedural technique) could have on the mRS score variation between groups. Furthermore, the STRATIS registry is based on specific stent retrievers and might not be applicable to other types of devices with different safety and efficacy profile. Also, the average age for a stroke onset in Spain might be higher than our assumption for all patients (68 years), which could potentially lead to an overestimation of health outcomes. However, age was included in the DSA, varied to an upper limit of 81 years, and this did not lead to dramatic changes in the results as the NMB remained positive in all scenarios. Third, patients were assumed to remain in a given mRS score until they experienced a recurrent stroke or death. Other factors that may have an effect on mRS scores, such as comorbidities, were not included. However, this aspect could affect both patient cohorts equally considering there are no differences in the baseline characteristics, nonetheless further studies on mRS decline in the long term are encouraged. Acute and long-term costs were obtained from the original cost-effectiveness model and the same limitations for costs would apply. Finally, resource consumption was based on a panel of experts’ consensus and clinical practice and subject to heterogeneity between centres. However, these assumptions were tested in the DSA and PSA and did not alter the overall results.
Conclusion
Achieving FPE after MT can lead to important healthcare cost saving and better functional clinical outcomes per patient compared with not achieving FPE. Costs saving to the Spanish NHS ranged from -€16 583 to -€44 289 depending on the patient cohort and long-term costs. Increasing FPE rates will lead to greater cost savings to the healthcare system.
Supplementary Material
Acknowledgments
The authors acknowledge Medtronic and Valeska Seguel Ravest for its support and editorial assistance.
Footnotes
Contributors: EGD, CR-P, AF-P, RM-M, JOQ, JZ, AT and MM-G: contributed to the design, data collection, analysis, interpretation and drafting, reviewing, and revising the manuscript. NHM-K, OOZ and DL: reviewing and revising the manuscript. EGD contributed as guarantor.
Funding: This study was sponsored by Medtronic.
Competing interests: EGD, CR-P, AF-P, RM-M, JOQ and JZ declare no conflicts of interest. MM-G is a proctor and consultant of Medtronic. AT is a consultant, proctor and advisor of Medtronic (Consultancy Anaconda, Balt, Stryker and Perflow). NHM-K is a scientific consultant regarding trial design and conduct for Medtronic. OOZ is a consultant for Neuravi/Cerenovus, Stryker, Penumbra and Medtronic. DL is an imaging core laboratory consultant for Cerenovus, Genentech, Medtronic and Stryker.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study included a post hoc analysis of an existing study, therefore, institutional review board approval was not obtained for this analysis. Moreover, no research approval was required for model input parameters that were obtained from literature or based on panel of experts consensus.
References
- 1.Stevens E, Emmett E, Wang Y. The burden of stroke in Europe report Stroke Alliance Eur; 2017: 131. ISBN 978-1-5272-0858-2. [Google Scholar]
- 2.Luengo-Fernandez R, Violato M, Candio P, et al. Economic burden of stroke across Europe: a population-based cost analysis. Eur Stroke J 2020;5:17–25. 10.1177/2396987319883160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019;4:6–12. 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 5.de Andrés-Nogales F, Álvarez M, de Miquel María Ángeles, MÁ deM, et al. Cost-effectiveness of mechanical thrombectomy using stent retriever after intravenous tissue plasminogen activator compared with intravenous tissue plasminogen activator alone in the treatment of acute ischaemic stroke due to large vessel occlusion in Spain. Eur Stroke J 2017;2:272–84. 10.1177/2396987317721865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai Y, Pu J, Wang H, et al. Impact of retriever passes on efficacy and safety outcomes of acute ischemic stroke treated with mechanical thrombectomy. Cardiovasc Intervent Radiol 2018;41:1909–16. 10.1007/s00270-018-2022-0 [DOI] [PubMed] [Google Scholar]
- 7.Bai X, Zhang X, Yang W, et al. Influence of first-pass effect on recanalization outcomes in the era of mechanical thrombectomy: a systemic review and meta-analysis. Neuroradiology 2021;63:795-807. 10.1007/s00234-020-02586-7 [DOI] [PubMed] [Google Scholar]
- 8.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018;49:660–6. 10.1161/STROKEAHA.117.020315 [DOI] [PubMed] [Google Scholar]
- 9.Zaidat OO, Haussen DC, Hassan AE, et al. Impact of stent retriever size on clinical and angiographic outcomes in the STRATIS stroke thrombectomy registry. Stroke 2019;50:441–7. 10.1161/STROKEAHA.118.022987 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TN, Malisch T, Castonguay AC, et al. Balloon guide catheter improves revascularization and clinical outcomes with the solitaire device: analysis of the North American solitaire acute stroke Registry. Stroke 2014;45:141–5. 10.1161/STROKEAHA.113.002407 [DOI] [PubMed] [Google Scholar]
- 11.Di Maria F, Kyheng M, Consoli A, et al. Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke 2021;16:20–8. 10.1177/1747493020923051 [DOI] [PubMed] [Google Scholar]
- 12.Mueller-Kronast NH, Zaidat OO, Froehler MT, et al. Systematic evaluation of patients treated with Neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS registry. Stroke 2017;48:2760–8. 10.1161/STROKEAHA.117.016456 [DOI] [PubMed] [Google Scholar]
- 13.Instituto Nacional de Estadıstica . 2018 Mortality tables of Spanish population National results. In: INEbase, 2018. www.ine.es [Google Scholar]
- 14.Slot KB, Berge E, Sandercock P, et al. Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke 2009;40:1585–9. 10.1161/STROKEAHA.108.531533 [DOI] [PubMed] [Google Scholar]
- 15.Mohan KM, Wolfe CDA, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489–94. 10.1161/STROKEAHA.110.602615 [DOI] [PubMed] [Google Scholar]
- 16.Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341–54. 10.1177/0272989X09349961 [DOI] [PubMed] [Google Scholar]
- 17.López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para La evaluación económica aplicada a LAS tecnologías sanitarias. Gac Sanit 2010;24:154–70. 10.1016/j.gaceta.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 18.Sacristán JA, Oliva J, Del Llano J, et al. ¿Qué ES Una tecnología sanitaria eficiente en España? Gac Sanit 2002;16:334–43. 10.1016/S0213-9111(02)71933-X [DOI] [PubMed] [Google Scholar]
- 19.De Cock E, Miravitlles M, González-Juanatey JR, et al. Valor umbral del coste POR año de vida ganado para recomendar La adopción de tecnologías sanitarias en España: evidencias procedentes de Una revisión de la literatura. PharmacoEconomics Spanish Res Artic 2007;4:97–107. 10.1007/BF03320930 [DOI] [Google Scholar]
- 20.Briggs AA. Decision modelling for health economic evaluation, 2006: 2–3. ISBN: 0191592978. [Google Scholar]
- 21.Abbasi M, Liu Y, Fitzgerald S, et al. Systematic review and meta-analysis of current rates of first pass effect by thrombectomy technique and associations with clinical outcomes. J Neurointerv Surg 2021;13:212–6. 10.1136/neurintsurg-2020-016869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larco JA, Abbasi M, Liu Y. Per-pass analysis of recanalization and good neurological outcome in thrombectomy for stroke: systematic review and meta-analysis. Interv Neuroradiol 2021. 10.1177/15910199211028342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaidat OO, Ribo M, Mattle HP. Health economic impact of first-pass success among patients with acute ischemic stroke treated with mechanical thrombectomy: a United States and European perspective. J Neurointerv Surg 2020:1–7. 10.1136/neurintsurg-2020-016930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunz WG, Almekhlafi MA, Menon BK, et al. Public health and cost benefits of successful reperfusion after thrombectomy for stroke. Stroke 2020;51:899–907. 10.1161/STROKEAHA.119.027874 [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Khunte M, Gandhi D, et al. Implications of achieving TICI 2B vs TICI 3 reperfusion in patients with ischemic stroke: a cost-effectiveness analysis. J Neurointerv Surg 2020;12:1161–5. 10.1136/neurintsurg-2020-015873 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-054816supp001.pdf (109.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.