Abstract
Background
Despite the intense global research endeavour to improve the treatment of patients with COVID-19, the current therapy remains insufficient, resulting in persisting high mortality. Severe cases are characterised by a systemic inflammatory reaction driven by the release of pro-inflammatory cytokines such as IL-6 and tumour-necrosis-factor alpha (TNF-α). TNF-α-blocking therapies have proved beneficial in patients with chronic inflammatory diseases and could therefore pose a new treatment option in COVID-19. Hitherto, no results from randomised controlled trials assessing the effectiveness and safety of infliximab—a monoclonal antibody targeting TNF-α—in the treatment of COVID-19 have been published.
Methods
In this phase-2 clinical trial, patients with COVID-19 and clinical and laboratory signs of hyperinflammation will be randomised to receive either one dose of infliximab (5 mg/kg body weight) in addition to the standard of care or the standard of care alone. The primary endpoint is the difference in 28-day mortality. Further assessments concern the safety of infliximab therapy in COVID-19 and the influence of infliximab on morbidity and the course of the disease. For the supplementary scientific programme, blood and urine samples are collected to assess concomitant molecular changes. The Ethics Committee of the Friedrich Schiller University Jena (2021-2236-AMG-ff) and the Paul-Ehrlich-Institute (4513/01) approved the study.
Discussion
The results of this study could influence the therapy of patients with COVID-19 and affect the course of the disease worldwide, as infliximab is globally available and approved by several international drug agencies.
Trial registration
The trial was registered at clinicaltrials.gov (NCT04922827, 11 June 2021) and at EudraCT (2021-002098-25, 19 May 2021).
Keywords: COVID-19, Infectious diseases, Respiratory infections, Molecular diagnostics, Randomised controlled trial
Background
Introduction
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to an ongoing global pandemic. Thus far, despite intense global research, no causal therapy has been established for this severe condition. Although many cases are limited to mild symptoms, there are large inter-individual differences in the course of the disease and 10–20% of hospitalised patients will develop pronounced hypoxia requiring intensive care therapy [1–3]. The cause of these differences remains unknown; however, old age, obesity and pulmonary and cardiovascular conditions are known risk factors for severe COVID-19 [1]. Also, the pulmonary damage of severe cases appears to be inflicted by a hyperinflammatory state. This so-called cytokine storm [4, 5] is characterised by a systemic release of proinflammatory mediators, including, among others, interleukin-6 (IL-6) and tumour-necrosis-factor alpha (TNF-α).
TNF-α is a proinflammatory cytokine produced predominantly by macrophages and regulates a number of inflammatory functions including the release of other cytokines and the induction of fever. In patients with severe COVID-19, the production of TNF-α and IL-6 is sustained by circulating monocytes [6]. It has been shown that the plasma levels of TNF-α correlate with disease severity [7]. Apart from its role in COVID-19, TNF-α plays a major role in several chronic inflammatory diseases and immunosuppressive therapies targeting TNF-α have proved beneficial in these conditions. TNF-α may therefore pose a promising target for the treatment of severe cases of COVID-19 exhibiting hyperinflammation.
Infliximab is a chimeric monoclonal antibody directed against TNF- α. It is able to bind both the soluble and membrane-bound form and induces apoptosis in cells expressing TNF- α, hereby promoting its anti-inflammatory effects (reviewed in [8]). In the European Union, infliximab is approved for rheumatoid arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and psoriasis with its clinical efficacy and safety proven in many randomised controlled trials. It has, however, been reported that anti-TNF- α therapy may increase the risk of severe and opportunistic infections such as tuberculosis [9] especially when combined with corticosteroid therapy [10]. Conversely, a meta-analysis showed a lower 28-day mortality following infliximab therapy in sepsis [11].
For COVID-19, the effect of anti-inflammatory and immunomodulatory therapy has been tested with different pharmaceuticals. Part of the standard therapy at the time of publication is the corticosteroid dexamethasone which was introduced following the positive effects on mortality seen in the RECOVERY trial [12]. Tocilizumab, a monoclonal IL-6 antibody, and Janus kinase inhibitors such as baricitinib can be considered in the treatment of patients with moderate disease severity according to the German national guideline for the therapy of COVID-19 [13, 14]. On the other hand, therapy with anakinra, a recombinant IL-1 antagonist is not recommended as it led to increased mortality [15, 16]. In a retrospective analysis of 24 patients treated at Jena University Hospital, we found that patients receiving infliximab showed reduced mortality (14.2% vs. 35.3%) [17]. In a phase-2 proof-of-concept trial, infliximab did not reduce blood C-reactive protein levels in hospitalised patients with COVID-19 when compared with standard therapy [18]. Thus far, however, no published randomised controlled trial analysed the effect of infliximab in addition to standard therapy on mortality, morbidity and the course of the disease in a patient collective with defined hyperinflammation.
Aim
The overall aim of this randomised, controlled, multicentre, open-label phase II clinical trial is the improvement of the survival of patients with COVID-19 at high risk for severe disease by repurposing a clinically established and globally available anti-inflammatory pharmaceutical for the treatment of a clearly defined subpopulation of patients. Due to the global extent of the pandemic, even small differences in survival or other clinically relevant outcomes could lead to a profound impact on the ability to overcome the pandemic. In addition, the trial integrates a supplementary scientific programme which seeks to collect blood and urine samples to assess concomitant molecular changes in future analyses.
Methods and analysis
Study design
This study is a randomised, controlled, multicentre, open-label phase II clinical study. Patient recruitment commenced in July 2021 at Jena University Hospital and aims to enrol 88 patients. The last follow-up is planned for August 2022. Figure 1 summarises the study design including the study events.
Fig. 1.
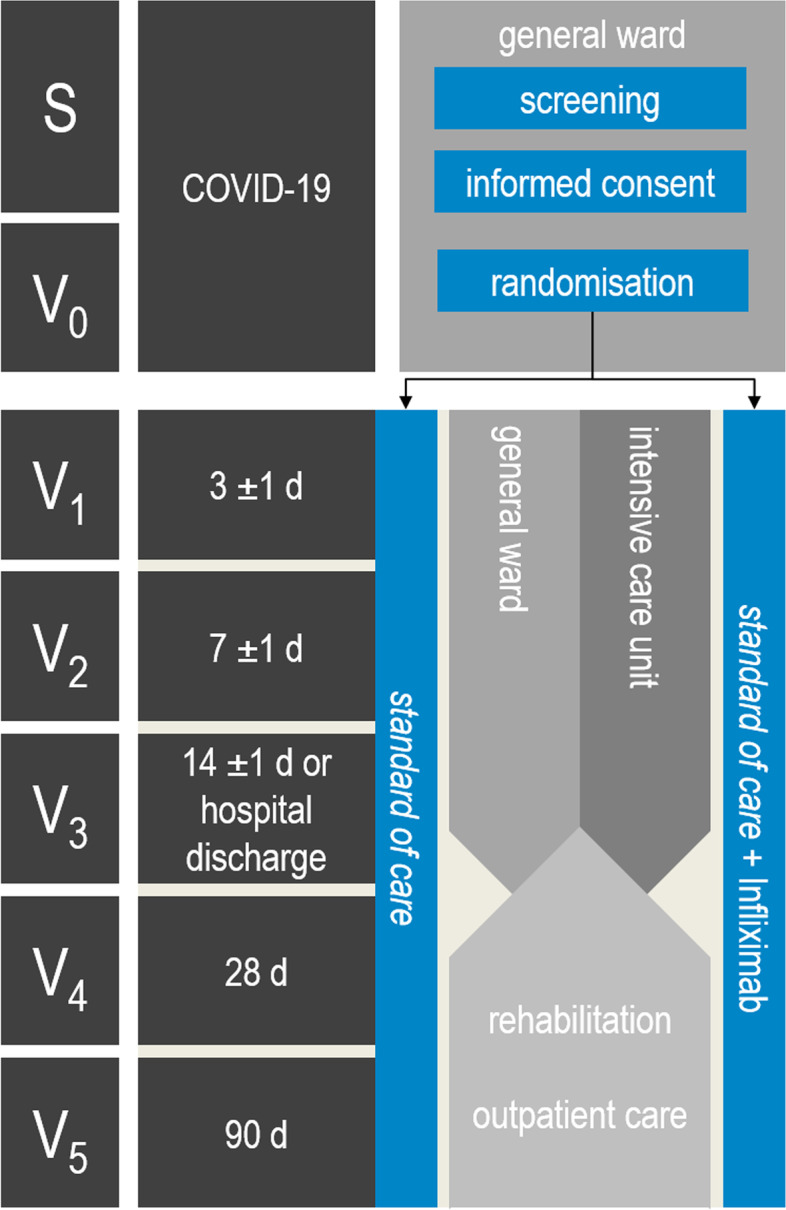
Study design. Hospitalised patients with COVID-19 (positive SARS-CoV-2 PCR testing) with pulmonary symptoms and hyperinflammation are eligible for study inclusion. Patient screening and obtainment of informed consent takes place in the general ward (S). Subsequently, patients are randomly allocated to the control (standard of care, SOC) or interventional group (SOC + infliximab) (V0). Study events take place 3 ± 1 (V1), 7 ± 1 (V2) and 14 ± 1 days after randomisation or at hospital discharge (V3). Follow-ups take place on days 28 (V4) and 90 (V5) after randomisation
Study objectives and outcome measures
The primary endpoint of this study is the difference in 28-day mortality of patients with COVID-19 receiving one dose of infliximab in addition to the standard of care (interventional group) compared with patients receiving standard of care (control group).
Secondary outcome measures include the assessment of the effect of infliximab on
The frequency of adverse and severe adverse events by day 90 after randomisation to assess the safety of infliximab in patients with COVID-19
An excessive inflammatory response
The morbidity and prognosis of patients with COVID-19
The incidence of cardiomyopathy on days 3 and 7 after randomisation
as well as the characterisation of the analytical cohort and course of the disease (see also Table 1).
Table 1.
Secondary endpoints
| Safety of the TNF-α-antibody Infliximab in the treatment of patients with severe COVID-19 | |
| ▶ Frequencies of adverse events (AEs) and serious adverse events (SAEs) | |
| Assessment of the effect of infliximab on an excessive immune response in patients with COVID-19 | |
| ▶ Change in the interleukin-6 (IL-6) concentration in blood from randomisation to day 7 (V2) and day 14 (V3) after randomisation | |
| ▶ Change in the ferritin concentration in blood from randomisation to day 7 (V2) and day 14 (V3) after randomisation | |
| ▶ Change in the lymphocyte count from randomisation to day 7 (V2) and day 14 (V3) after randomisation | |
| Assessment of the effect of infliximab in patients with severe COVID-19 on morbidity and prognosis | |
| ▶ Ventilation-free days until day 28 (V4) after randomisation | |
| ▶ Renal replacement therapy-free days until day 28 (V4) after randomisation | |
| ▶ Vasopressor-free days until day 28 (V4) after randomisation | |
| ▶ Rate of occurrence of severe acute respiratory syndrome (ARDS) until day 28 (V4) after randomisation (Berlin criteria and PaO2/FiO2 ≤ 100 mmHg with PEEP ≥ 5 cmH2O [19]) | |
| ▶ WHO-COVID-19-Progression Scale on day 7 (V2), 14 (V3) and 28 (V4) after randomisation | |
| ▶ Rate of admission to the intensive care unit after randomisation up to day 28 (V4) after randomisation | |
| ▶ Length of hospital stay up to day 28 (V4) after randomisation | |
| ▶ Length of intensive care unit stay up to day 28 (V4) after randomisation | |
| ▶Mortality rates at day 14 (V3) and day 90 (V5) after randomisation | |
| ▶ EQ5D-3L (health-related quality of life): visual analogue scale value and sub-domain ratings at day 90 (V5) after randomisation | |
| ▶ EQ5D-3L: index value at day 90 (V5) after randomisation | |
| ▶ Frequencies of COVID-19 (long-term) sequelae (positive ratings in checklist) | |
|
▶ Incidence of cardiomyopathy at day 3 (V1) and/or day 7 (V2) after randomisation left-ventricular EF < 52 % in men and < 54 % in women, according to the American Society of Echocardiography [20] and the European Association of Cardiovascular Imaging or 10% reduction, if previously reduced [21–24] |
Study setting
Patients treated for COVID-19 are recruited in the general ward of up to 10 hospitals in Germany. The list of recruiting centres can be found under ClinicalTrials.gov (NCT04922827).
Study population
Hospitalised adult patients with COVID-19 (positive SARS-CoV-2 PCR-testing) with typical symptoms of pulmonary involvement and hyperinflammation are eligible for study enrolment, if all inclusion criteria and none of the exclusion criteria are met. Table 2 lists the inclusion and exclusion criteria for study eligibility. The study population consists of two groups, the interventional and the control group.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | |
| ▶ Age ≥ 18 years | |
| ▶ Infection with SARS-CoV-2 (virus detection by means of a PCR test not older than 72 h) | |
| ▶ Bipulmonary infiltrates (detection by means of chest X-ray or computed tomography) | |
| ▶ COVID inflammation score ≥ 10 [25] | |
| ▶ Serum or plasma ferritin concentration ≥ 500 ng/ml | |
| ▶ Arterial oxygen saturation ≤ 93% when breathing ambient air | |
| ▶ Written informed consent from the patient | |
| ▶ Potentially childbearing women: negative pregnancy test | |
| Exclusion criteria | |
| Contraindications for infliximab | |
| ▶ Allergy to infliximab (or any of the other ingredients of the medication) or to other murine proteins | |
| ▶ Active or latent tuberculosis | |
| ▶ Acute or chronic hepatitis B | |
| ▶ Severe infections such as invasive fungal infections, bacterial sepsis, or abscesses | |
| ▶ Opportunistic infections (e.g. pneumocystosis, listeriosis) | |
| ▶ Moderate or severe heart failure (NYHA class III/IV) | |
| ▶ Immunosuppression (e.g. organ transplantation, AIDS, leukopenia) | |
| ▶ Malignancies or lymphoproliferative diseases or chemotherapy within the last 4 weeks | |
| ▶ Multiple sclerosis or peripheral demyelinating diseases, including Guillain-Barré syndrome | |
| ▶ Treatment with other biologics for approved indications of infliximab (e.g. for rheumatoid arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, psoriasis) | |
| Further exclusion criteria | |
| ▶ Autoimmune disease treated with a biological | |
| ▶ Current treatment with TNF-α antibodies, convalescent plasma, bamlanivimab, or other experimental treatments for COVID-19 not considered in national guidelines | |
| ▶ High-flow oxygen therapy, non-invasive/invasive ventilation (WHO-COVID-19 progression scale > 5, [26]) | |
| ▶ Pre-existing long-term ventilation or home oxygen therapy | |
| ▶ Child-Pugh C liver cirrhosis | |
| ▶ Pregnancy or breastfeeding | |
| ▶ Life expectancy < 90 days due to other medical conditions | |
| ▶ Limitation or discontinuation of therapy (e.g. refusal of artificial ventilation) | |
| ▶ Participation in another interventional study | |
| ▶ Previous participation in this study | |
| ▶ Interdependence between the patient and the coordinating investigator or other members of the study team |
Randomisation and intervention
Patients are randomly allocated in a 1:1 ratio to either the control group (standard of care, SOC) or interventional group (SOC + infliximab). Randomisation is performed within 6 hours after study enrolment using an online randomisation tool (PARANDIES) by a member of the study team. Within 3 h after randomisation, infliximab (Remsima®, Celltrion Healthcare, Hungary) is administered intravenously in a dosage of 5 mg/kg body weight over the course of 2 h. The subsequent treatment follows current recommendations for the treatment of COVID-19.
Study outline
Table 3 summarises all measurements and variables of the study for each scheduled study event based on the SPIRIT guidelines [27]. Baseline and demographic data will be recorded at enrolment (V0). Recording of clinical data and blood sampling will be performed in the clinical setting at V0 and 3 ± 1 days (V1), 7 ± 1 days (V2) and 14 ± 1 days after randomisation/at hospital discharge (V3). Transthoracic echocardiography for the assessment of cardiomyopathy will be performed at V1 and V2. For ethical reasons, all patients regardless of group allocation will receive the current standard of care for the treatment of COVID-19 at the discretion of the treating physician in accordance with the current national guideline. Patients receiving infliximab should not receive other TNF-α antibodies or experimental treatments. COVID-19-specific therapies will be documented.
Table 3.
Study outline (SPIRIT)
| Domain | sub-domain/variable | S | V0 | V1 | V2 | V3 | V4 | V5 |
|---|---|---|---|---|---|---|---|---|
| Enrolment | ||||||||
| screening | inclusion and exclusion criteria, informed consent, pregnancy test | X | ||||||
| randomisation | randomisation within 6 hours after study enrolment and | X | ||||||
| Intervention | ||||||||
| study medication | start study medication within 3 hours after randomisation | X | ||||||
| Assessments | ||||||||
| adverse events (AEs) and severe AEs | X | X | X | X | X | X | ||
| demographic variables | age, sex, body height and weight | X | ||||||
| (SARS-CoV-2) medical history | date of: first record of SARS-CoV-2 and first symptoms, symptoms of SARS-CoV-2 infection, SARS-Cov-2 immunisation status, date of hospital admission, type of admission, place before patient transfer/admission, nicotine abuse, family history of heart attack, physical activity, accompanying infections | X | ||||||
| cardiovascular/general comorbidities and previous findings |
Charlson comorbidity index, heart attack, congestive heart failure, coronary heart disease, angina pectoris, valvular heart disease, arrhythmias, arterial hypertension, peripheral arterial disease Previous findings echocardiography: rhythm, imaging quality, ejection fraction, pericardial effusion, aortic/mitral/tricuspid/pulmonary valve stenosis and/or insufficiency, VCI diameter, VCI collapse, RAP, SPAP, MPAP, right ventricular function |
X | ||||||
| clinical status and vital signs |
COVID-19 inflammation score [25], WHO-COVID-19-Progression Scale [26] consciousness, Glasgow Coma Scale, CAM-ICU, blood pressure, mean arterial blood pressure, heart rate, oxygen saturation and FiO2, paO2 / FiO2 ratio, respiratory rate, urine production |
X | X | X | X | X | ||
| organ replacement therapy | administration of oxygen, high-flow oxygen therapy, non-invasive ventilation, invasive ventilation, ECMO, kidney and liver replacement | X | X | X | X | |||
| organ dysfunction | acute encephalopathy, thrombocytopenia, arterial hypoxemia, arterial hypotension, renal dysfunction, metabolic acidosis, septic shock | X | X | X | X | |||
| sepsis-3-criteria | SOFA-score | X | X | X | X | |||
| medication | antiplatelet drugs, anticoagulation, immunosuppressants, angiotensin converting enzyme inhibitors, catecholamines | X | X | X | X | |||
| blood and urine tests: routine | COVID-19-Panel (i.a. troponin, NT-proBNP, PCT, sodium, potassium, chloride, calcium, iron, phosphate, alpha-1antitrypsin, urea, creatinine, bilirubin, albumin, ASAT, ALAT, Gamma-GT, AP, cholinesterase, GLDH, LDH, CK, CK-MB, haptoglobin, haematocrit, haemoglobin, thrombocytes, antithrombin-III, base excess (B.E.) art., bicarbonate (SBC) art., pH art., lactate, Quick, LDL-cholesterol, HDL-cholesterol, HbA1c, IL-6, ferritin, triglycerides, fibrinogen, leucocytes, lymphocytes, D-Dimer, partial thromboplastin time) | X | X | X | X | |||
|
blood and urine sampling: supplementary scientific programme |
date and time | X | X | X | ||||
| virology | SARS-CoV-2 PCR | X | X | X | X | |||
| transthoracic echocardiography (TTE) | rhythm, quality, ejection fraction, pericardial effusion, aortic/mitral/tricuspid/pulmonary valve stenosis and/or insufficiency, VCI diameter, VCI collapse, RAP, SPAP, MPAP, right ventricular function | X | X | |||||
| clinical endpoints and cardiovascular events after randomisation |
thromboembolic events; cardiovascular events: cardiopulmonary resuscitation, arrhythmia, cardiomyopathy/reduced left ventricular function, STEMI/NSTEMI, angina pectoris, valve stenosis, others |
X | X | X | X | X | ||
| cumulative endpoints | ventilation-free days, vasopressor-free days, renal replacement therapy-free days, occurrence of severe acute respiratory distress syndrome (Berliner criteria+ PaO2/FiO2 ≤100 mmHg with PEEP ≥5 cm H2O), medication prohibited by the study protocol and SARS-CoV-2-specific therapies | X | ||||||
| survival status /place of treatment | survival status/date of death, current place of treatment | X | X | X | X | X | ||
| general disease progression/patient history after randomisation | current residence, hospital re-admissions, infections, rehabilitation and outpatient therapies | X | X | |||||
| COVID-19 (long-term) sequelae | checklist (i.a. dyspnoea, taste and smelling disorders, psychological sequela) | X | ||||||
| quality of life: EQ-5D-3L | EQ-5D-3L | X | X | |||||
| end of study data and cumulative treatment data | irregular end of participation, withdrawal of consent to participate, ICU treatment and length of ICU stay since randomisation, length of hospital stay since hospital admission and since randomisation, pregnancy | X | ||||||
V0: randomisation und administration of Infliximab (interventional group) | V1: 3 ± 1 d after randomisation V2: 7 ± 1 d after randomisation
V3: 14 ± 1 d after randomisation or up to 2 days before planned hospital discharge | V4: 28 d (up to 35 d) after randomisation | V5: 90 d (up to 97 d) after randomisation
Patients discharged from hospital are monitored by the study team and will be followed up via telephone interviews and questionnaires at 28 and 90 days after randomisation. Aside from checklists for sequelae of COVID-19, health-related quality of life will be assessed via EQ-5D-3L. AEs and SAEs will be assessed and documented at all study events following randomisation.
Supplementary scientific programme
Within the supplementary scientific programme, the blood and urine samples will be collected and stored for the investigation of translational research questions by analysing biomarkers of organ, metabolic and immunological function and regulation (study visits V1 through V3). In addition, the comparison of the course of disease of patients with severe COVID-19 and previously generated datasets from patients with sepsis and healthy subjects is planned [28].
Sample size and statistical analysis
Sample size planning was based on unpublished retrospective data from Jena University Hospital (April 2020–January 2021, part of the data set in [29]). In 31 patients, the 28-day mortality was 50% in SOC and 12.2% in patients with SOC + infliximab. Under these assumptions, a two-sided Fisher’s exact test at significance level alpha = 0.05 has a power of 95%, if 40 patients are included in each of the two groups. Allowing for a dropout rate of 10%, the total number of patients to be randomised is 88 (2 × 44). We used nQuery 7.0 (Statistical Solutions Ltd, Cork, Ireland) for sample size planning. The full analysis set population (FAS) includes all patients enrolled and randomised to the study with at least one observation after randomisation. Patients will be analysed as randomised (intention-to-treat principle). The primary efficacy analysis will be conducted in the FAS. All variables collected will be analysed in the FAS. The per-protocol collective includes all patients in the intention-to-treat collective who do not have major protocol deviations. In sensitivity analysis, the primary efficacy analysis will be repeated in the per-protocol collective. If there are differences between randomised and actual treatment, an additional ‘as treated’ sensitivity analysis will be performed. An interim-analysis is not planned. Data analysts will be blinded.
Statistical analysis
In general, all data collected will be analysed using descriptive methods in the two groups and in total. This includes at least the number of collected and missing values, mean, standard deviation, minimum, quartiles including median and maximum for metric variables as well as frequency analyses for ordinal and categorical data. The values of the day of randomisation (V0) are used as baseline.
The primary endpoint of the study is the mortality rate at day 28 after randomisation. The null hypothesis H0: πSOC=πSOC+Infliximab is tested two-sided against the alternative hypothesis H1: πSOC≠πSOC+Infliximab using a Fisher’s exact test at significance level alpha = 0.05. Absolute and relative frequencies of patients who died are reported for both groups, respectively. The relative risk, the risk difference, and the number needed to treat (NNT) are given as effect measures, each with 95% confidence intervals.
The secondary endpoints will be analysed by appropriate statistical tests depending on the distributional properties of the outcome measures and type of data. Further exploratory analysis may be added, if the results of the descriptive analysis warrant this.
Dissemination
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines were followed in the reporting of this study protocol [27]. In order to disseminate the results to the scientific community and health care professionals, the study group will publish and present the results in peer-reviewed journals and at appropriate scientific conferences in accordance with the Consolidated Standards of Reporting Trials (CONSORT) criteria [30]. The reporting of the secondary results and results of the supplementary scientific programme will adhere to the strengthening the reporting of observational studies in epidemiology (STROBE) criteria [31] and transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) criteria [32], where applicable. Authorship eligibility will follow the recommendations of the International Committee of Medical Journal Editors (ICMJE). For the public and other relevant groups, the results will be disseminated via mass and social media.
The datasets analysed during the current study and statistical code are available from the corresponding author on reasonable request, as is the full protocol.
Ethics
Informed consent
Designated study physicians will obtain written informed consent from patients willing to participate. For the supplementary scientific programme, written informed consent will be obtained separately. Patients incapable of giving informed consent are not eligible for study participation. Patients may withdraw consent at any given time without providing a reason. In this case, some datasets may be continued to be stored and used if necessary either to demonstrate the effect of the medication used, protect legitimate interests of the patient or to comply with the obligation to submit complete drug approval documents in accordance with the German Medicinal Products Act (AMG).
Ethics and regulatory approval, study registration and data management
The study in its current version (version 1.0, 10 May 2021) was approved by the Ethics Committee of the Friedrich-Schiller-University Jena (2021-2236-AMG-ff, 27 May 2021) and the Paul-Ehrlich-Institute (4513/01, 27 May 2021) and is in accordance with the Declaration of Helsinki and the AMG. It is registered at ClinicalTrials.gov (NCT04922827) and EudraCT (2021-002098-25). The web-based data entry takes place via an encrypted data link (HTTPS) with pseudonymised patient identification numbers. The data management software (OpenClinica, Waltham, Massachusetts, USA) conforms to the Good Clinical Practice guidelines (21 CFR Part 11). The data is stored on servers of the Centre for Clinical Studies Jena and Jena University Hospital. Patient datasets for collaborating study groups will be transferred in anonymized form. The Centre for Clinical Studies Jena will perform trial monitoring in all study centres according to a monitoring plan. In addition, a data safety monitoring board (DSMB) was established consisting of clinicians and a statistician who are independent from the funding source and study team. The board will convene once per year or when necessary and assist the principal investigator (PI) in evaluating the safety of the study medication according to a DSMB charta. The PI may stop or terminate the study if the recruitment rate is insufficient, new scientific evidence prohibits its continuation or for reasons of patient safety. Any changes to the study protocol must be submitted to and approved by the appropriate committee in an amendment.
Discussion
This randomised controlled trial includes a targeted approach for the use of an anti-inflammatory therapy with the TNF-α-antibody infliximab in a selected group of patients with COVID-19 and clearly defined hyperinflammation. The multi-centre design facilitates the transferability of study results to hospitals of similar healthcare level. Should infliximab prove to be superior to standard therapy, this could be reflected in a reduced disease severity and mortality. The results of this study could influence the therapy of patients with COVID-19 and affect the course of the disease worldwide, as infliximab is globally available and approved by several international drug agencies.
Due to the novelty of the disease COVID-19 and the transience of the ever-expanding evidence on the effects of new therapeutic approaches on morbidity and mortality, we decided to choose SOC as the comparator to not deny patients state-of-the-art therapeutic options. During the course of this trial, the standard treatment regimens of patients with COVID-19 may change, if new evidence for certain treatments become available. Currently, other anti-inflammatory treatment regimens are being tested in patients with severe cases. Should these become part of the standard of care, this may improve the mortality in patients with clinical deterioration in the control group and hereby affect the primary endpoint of this trial.
The open-label design may overestimate the therapeutic effect due to lack of placebo. However, for ethical reasons, patients in the control SOC-group should receive anti-inflammatory treatments according to the current clinical evidence and recommendations at the discretion of the treating physician in case of clinical deterioration. Here, the open-label study design enables the treating physician to identify which group the patient belongs to without unblinding.
We are aware that the course of the pandemic has been subject to strong fluctuations caused by changing transmissibility and virulence of SARS-CoV-2. This study was planned and initiated at a time when particularly severe cases of COVID-19 resulted in high morbidity and mortality. Currently, we cannot predict whether with increasing population immunity and lower virulence of the prevalent strains the severity of COVID-19 will again increase to such an extent that the recruitment of this study can be completed. However, the planned molecular analyses of the patients’ biomaterials over the course of the disease will result in a gain in scientific knowledge, even if the sample size is not sufficient to answer the primary end-point.
Due to the high incidence of COVID-19 worldwide and the immense effects of the pandemic on societies, health care and economy systems, any progress in the treatment of this new disease would constitute a great success by not only affecting the individual patient but healthcare systems and economies as a whole.
Trial status
The current version of the study protocol is 1.0 (10 May 2021). Recruitment commenced 18 June 2021. Recruitment is expected to be completed by April 2023.
Acknowledgements
We thank Dr. Julia Josephine Müller, Centre for Clinical Studies Jena, for her support with the project management.
Abbreviations
- AE
Adverse event
- AMG
German Medicinal Products Act
- ARDS
Acute respiratory disease syndrome
- CONSORT
Consolidated Standards of Reporting Trials
- COVID-19
Coronavirus disease 2019
- DSMB
Data safety monitoring board
- FAS
Full analysis set
- IL-6
Interleukin-6
- PI
Principal investigator
- SAE
Severe adverse even
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus type 2
- STROBE
Strengthening the reporting of observational studies in epidemiology
- TNF-α
Tumour necrosis factor alpha
- TRIPOD
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
Authors’ contributions
Principal investigators: AS, SMC; conception of the study: SMC, AS; contribution to study design: SMC, CN, FB, PB, US, MB, PR, AS; on-site implementation: SMC, CN, PB, PR; AS; drafting of the case report forms: CN, PB, SMC, PR. planning of sample size and statistical analysis: US, PB; funding acquisition and BMBF grant holder: SMC. drafting of the manuscript: SMC, CN, PB; revision of the manuscript prior to submission: SMC, CN, FB, PB, US, MB, PR, AS. All authors read and approved the final manuscript. Patients and/or the public were not involved in the design, conduct, reporting and dissemination of this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. The primary sponsor of the study is the Friedrich-Schiller-University Jena (contact via uniklinikum-jena.de). This work is funded by the German Federal Ministry of Education and Research (grant number BMBF 03COV08 to SMC). Celltrion Healthcare (Hungary, contract: I 5479) will provide a part of the study medication. The sponsor, funding source and manufacturer of the study medication had no role in the design of the study and will not have any role in its execution, analyses, interpretation of the data or decision to publish results.
Availability of data and materials
No datasets were generated or analysed during the development of the current study protocol. Relevant data will be available upon reasonable request to the corresponding author after publication, while adhering to ethical and data privacy restrictions.
Declarations
Ethics approval and consent to participate
The study in its current version (version 1.0, 10 May 2021) was approved by the Ethics Committee of the Friedrich-Schiller-University Jena (2021-2236-AMG-ff, 27 May 2021) and the Paul-Ehrlich-Institute (4513/01, 27 May 2021) and is in accordance with the Declaration of Helsinki and the AMG.
Written informed consent will be obtained from all patients participating in this study (s. Methods section for further details).
Patients are insured against potential harm from trial participation as required by AMG.
Consent for publication
No identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. Consent materials are available from the corresponding author on request.
Competing interests
Outside of the study, AS has received consulting fees from AbbVie, Amgen, Celltrion, Janssen, MSD, Mundipharma, and Takeda; lecture fees and support for travel accommodation from AbbVie, Amgen, FalkFoundation, Janssen, MSD, and Takeda; and research funding from Abbvie and Celltrion. All other authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–6. [DOI] [PMC free article] [PubMed]
- 4.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–87. 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed]
- 5.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–7. 10.1016/S2213-2600(20)30216-2. Epub 2020 Apr 27. [DOI] [PMC free article] [PubMed]
- 6.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. 10.1016/j.chom.2020.04.009. Epub 2020 Apr 21. [DOI] [PMC free article] [PubMed]
- 7.Guo J, Wang S, Xia H, Shi D, Chen Y, Zheng S, et al. Cytokine signature associated with disease severity in COVID-19. Front Immunol. 2021;12:681516. doi: 10.3389/fimmu.2021.681516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitoma H, Horiuchi T, Tsukamoto H, Ueda N. Molecular mechanisms of action of anti-TNF-alpha agents - comparison among therapeutic TNF-alpha antagonists. Cytokine. 2018;101:56–63. doi: 10.1016/j.cyto.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108(2):240–248. doi: 10.1038/ajg.2012.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterman MT, Sandborn WJ, Colombel JF, Peyrin-Biroulet L, Robinson AM, Zhou Q, et al. Crohn’s disease activity and concomitant immunosuppressants affect the risk of serious and opportunistic infections in patients treated with adalimumab. Am J Gastroenterol. 2016;111(12):1806–1815. doi: 10.1038/ajg.2016.433. [DOI] [PubMed] [Google Scholar]
- 11.Lv S, Han M, Yi R, Kwon S, Dai C, Wang R. Anti-TNF-alpha therapy for patients with sepsis: a systematic meta-analysis. Int J Clin Pract. 2014;68(4):520–528. doi: 10.1111/ijcp.12382. [DOI] [PubMed] [Google Scholar]
- 12.Recovery Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malin JJ, Spinner CD, Janssens U, Welte T, Weber-Carstens S, Schalte G, et al. Key summary of German national treatment guidance for hospitalized COVID-19 patients: key pharmacologic recommendations from a national German living guideline using an evidence to decision framework (last updated 17.05.2021) Infection. 2022;50(1):93–106. doi: 10.1007/s15010-021-01645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Grana C, Schmucker C, et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;3:CD013881. doi: 10.1002/14651858.CD013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9(3):295–304. [DOI] [PMC free article] [PubMed]
- 17.Stallmach A, Kortgen A, Gonnert F, Coldewey SM, Reuken P, Bauer M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure-a cautionary case series. Crit Care. 2020;24(1):444. doi: 10.1186/s13054-020-03158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher BA, Veenith T, Slade D, Gaskell C, Rowland M, Whitehouse T, et al. Namilumab or infliximab compared with standard of care in hospitalised patients with COVID-19 (CATALYST): a randomised, multicentre, multi-arm, multistage, open-label, adaptive, phase 2, proof-of-concept trial. Lancet Respir Med. 2022;10(3):255–266. doi: 10.1016/S2213-2600(21)00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 21.Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care. 2011;1(1):6. doi: 10.1186/2110-5820-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berrios RAS, O'Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: a systematic review and meta-analysis. J Crit Care. 2014;29(4):495–499. doi: 10.1016/j.jcrc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Landesberg G, Gilon D, Meroz Y, Georgieva M, Levin PD, Goodman S, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33(7):895–903. doi: 10.1093/eurheartj/ehr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller-Werdan U, Ebelt H, Wilhelm J, Wimmer R, Buerke M, Werdan K. Mikrozirkulationsstörung, zytopathische Hypoxie und septische Kardiomyopathie. In: Werdan K, Müller-Werdan U, Schuster H-P, Brunkhorst FM, editors. Sepsis und MODS. Berlin, Heidelberg: Springer Berlin Heidelberg; 2016. pp. 131–151. [Google Scholar]
- 25.La Rosee F, Bremer HC, Gehrke I, Kehr A, Hochhaus A, Birndt S, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34(7):1805–1815. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e7. [DOI] [PMC free article] [PubMed]
- 27.Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coldewey SM, Neu C, Baumbach P, Scherag A, Goebel B, Ludewig K, et al. Identification of cardiovascular and molecular prognostic factors for the medium-term and long-term outcomes of sepsis (ICROS): protocol for a prospective monocentric cohort study. BMJ Open. 2020;10(6):e036527. doi: 10.1136/bmjopen-2019-036527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuken PA, Ruthrich MM, Hochhaus A, Hammersen J, Bauer M, La Rosee P, et al. The impact of specific cytokine directed treatment on severe COVID-19. Leukemia. 2021;35(12):3613–3615. doi: 10.1038/s41375-021-01411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the development of the current study protocol. Relevant data will be available upon reasonable request to the corresponding author after publication, while adhering to ethical and data privacy restrictions.


