Abstract
Background
Coronavirus disease 2019 (COVID-19) has triggered the disruption of health care on a global scale. With Italy tangled up in the pandemic response, oncology care has been largely diverted and cancer screenings suspended. Our multicenter Italian study aimed to evaluate whether COVID-19 has impacted access to diagnosis, staging, and treatment for patients newly diagnosed with colorectal cancer (CRC), compared with pre-pandemic time.
Methods
All consecutive new CRC patients referred to 8 Italian oncology institutions between March and December 2020 were included. Access rate and temporal intervals between date of symptoms onset, radiological and cytohistological diagnosis, treatment start and first radiological evaluation were analyzed and compared with the same months of 2019.
Results
A reduction (29%) in newly diagnosed CRC cases was seen when compared with 2019 (360 vs 506). New CRC patients in 2020 were less likely to be diagnosed with early stage (stages I-II-III) CRC (63% vs 78%, P < .01). Gender and sidedness were similar regardless of the year. The percentage of tumors with any mutation among BRAF, NRAS, and KRAS genes were significantly different between the 2 years (61% in 2020 vs 50% in 2019, P = .04). Timing of access to cancer diagnosis, staging, and treatment for patients with CRC has not been negatively affected by the pandemic. Significantly shorter temporal intervals were observed between symptom onset and first oncological appointment (69 vs 79 days, P = .01) and between histological diagnosis and first oncological appointment (34 vs 42 days, P < .01) during 2020 compared with 2019. Fewer CRC cases were discussed in multidisciplinary meetings during 2020 (38% vs 50%, P = .01).
Conclusions
Our data highlight a significant drop in CRC diagnosis after COVID-19, especially for early stage disease. The study also reveals a remarkable setback in the multidisciplinary management of patients with CRC. Despite this, Italian oncologists were able to ensure diagnostic–therapeutic pathways proper operation after March 2020.
Keywords: colorectal cancer, COVID-19, diagnostic delay, therapeutic delay, multidisciplinary discussions
This article evaluates whether COVID-19 has affected access to diagnosis, staging, and treatment for patients with colorectal cancer.
Implications for Practice.
With Italy becoming the first European country severely hit by the coronavirus disease 2019 (COVID-19) crisis, Italian medical oncologists had to elaborate a prompt response to mitigate the pandemic’s effects on patients with cancer. This work reports on the effectiveness of the efforts made by Italian oncology departments to ensure diagnostic—therapeutic pathways proper operation in response to COVID-19. Timing of access to cancer diagnosis, staging, and treatment for patients with colorectal cancer (CRC) has not been negatively affected by COVID-19, although the analysis highlights an alarming drop in CRC diagnoses throughout 2020 and an increase in the diagnosis of non-early stage cancer.
Introduction
From the first stirrings in Wuhan city to its worldwide rapid spread, Coronavirus Disease 2019 (COVID-19) has marked a turning point toward the post-pandemic era.1,2
In early 2020, the global healthcare systems, suddenly flooded with patients with COVID-19, had to promptly reallocate human and economic resources to effectively tackle the emergency.
Italy was the first European country severely hit by this unparalleled crisis. Mitigation efforts, such as lockdowns’ institutions and reorganization of basic health care services, were taken to flatten the curve on coronavirus.3
Since the first pandemic wave, patients with cancer appeared at major risk of contracting coronavirus and developing severe infection-related complications.4-6 Therefore, oncologists’ daily clinical practice has been derailed according to national and international recommendations7-9 in order to prevent viral transmission and keep the health facilities from collapsing, in some cases at the expense of strenuous efforts and distress among health care professionals.10,11
As a result of managing such a frail population within a pandemic context, some evidence has already attested a decline in new cancer diagnoses after March 2020.12,13 As COVID-19 continues to rage, these estimates are expected to rise further with realistic rebounds in terms of cancer mortality as well as social and health care costs.14
According to national statistics,15 colorectal cancer (CRC) stands as the second leading cause of cancer death considering both sexes. However, the large-scale adoption of screening programs and, more recently, the implementation in the clinical practice of multidisciplinary diagnostic–therapeutic pathways have significantly impacted on CRC prognosis.16
After the COVID-19 outbreak and the failure to maintain cancer screening invitations, a decrease in fecal occult blood tests and routine colonoscopies was registered in Italy according to the Italian National screening Network.17,18 Similar trends have been observed in Europe and the US.19,20
Some of the critical aspects elicited by the 2020 global health crisis are still reverberating on Oncology care. Our multicenter Italian study aimed to evaluate the impact of COVID-19 outbreak on CRC patients’ likelihood of receiving timely diagnosis, staging and treatment after March 2020 compared with pre-pandemic time in Italy.
Materials and Methods
Study Design and Population
Patient data were retrieved from the COVID-DELAY study (“Evaluation of COVID-19 impact on DELAYing diagnostic–therapeutic pathways of cancer patients in Italy”). Aim of this study was to assess whether the COVID-19 outbreak impacted the patients’ with CRC likelihood of receiving timely diagnosis and access to treatment in 2020, by assessing total number of new diagnoses, access rate (number of patients/month) and temporal intervals between date of symptoms onset, diagnosis, first oncological appointment, treatment start and first radiological reassessment, taking the same period of 2019 as comparison. The impact of COVID outbreak on the stage of disease at diagnosis in 2020 compared with 2019 was also evaluated as a secondary objective.
Eight Institutions provided data about all new CRC diagnoses reported during the study period. Clinical records of all consecutive newly diagnosed CRC patients referred to the 8 Oncology Departments (Supplementary Table S1) between March and December 2020 and between March and December 2019 were reviewed. Ethical approval to conduct this study was obtained by the respective local ethical committees on human experimentation of each participating center, after previous approval by the coordinating center (“Comitato Etico Regionale delle Marche—C.E.R.M.”, Reference Number 2021 139).
Patients were included if they were 18 years or older, had histologically proven diagnosis of CRC between March and December 2020 or 2019, received at least one type of oncological treatment (either surgery, radiotherapy, or systemic therapy) after diagnosis, and had available data about radiological diagnosis, cytohistological diagnosis, and treatment start. Patients with recurrent CRC, gastrointestinal (GI) metastases from cancer of a different organ, or GI malignancies other than CRC were excluded. Mean monthly access rate (number of patients/month) and temporal intervals between date of symptoms onset, radiological diagnosis, cytohistological diagnosis, first oncological appointment, treatment start, and first radiological reassessment in 2020 were computed and compared with those of the same period in 2019.
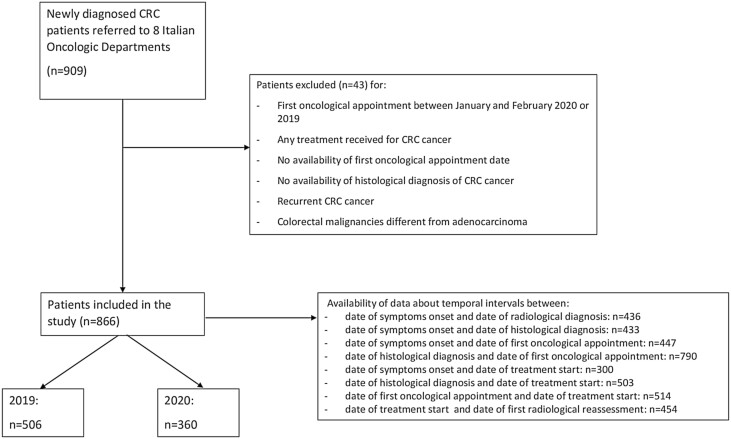
Data of patients who had their CRC diagnosis after first oncological appointment (as per standard practice of referral Hospitals) were not included in the calculation of these specific temporal intervals to avoid negative values (Fig. 1). Baseline (at diagnosis) data about patient, tumor and treatment characteristics were also retrieved from medical records and differences between the 2 years were analyzed.
Figure 1.
Flow diagram of COVID-DELAY study population.
Subgroup analyses were also performed by specifically investigating the study objectives in the lockdown period and based on the infection rate of the provinces where patients with CRC were diagnosed (high- vs medium/low-infected provinces).21 To note, April 1, 2020-June 30, 2020 (instead of March 8, 2020-May 4, 2020) was considered as a reference time period for the lockdown, since a conventional time interval of approximately 1 month between diagnosis and first oncological appointment was expected. Patients were then categorized according to the number of new CRC diagnoses in the hospital where they were treated: high volume (≥150 new diagnoses in the investigated 2-year period) vs low/medium volume (<150 diagnoses).
Statistical Analysis
A 20% reduction of newly diagnosed CRC cases in the pandemic year (2020) compared with 2019 was postulated. Therefore, assuming a 95% CI (95%CI) range of 10% (±5%), a sample size of at least 250 newly diagnosed CRC patients in 2019, corresponding to 200 new diagnoses in 2020 was required to test the null hypothesis.
Descriptive statistics were used to report baseline patient, disease and treatment characteristics. Categorical variables will be reported as either fractions or percentage; continuous variables either as mean, standard deviation (if normally distributed), or as median and interquartile range (if not-normally distributed). Differences between 2020 and 2019 were analyzed using Fisher’s exact test or chi-square test for categorical variables and paired Student’s t test, or the Mann-Whitney U test for continuous variables as appropriate. All statistical analyses were performed using R 3.6.0 (R Foundation for Statistical Computing) and a 2-sided P-value of <.05 was deemed statistically significant.
Results
Population
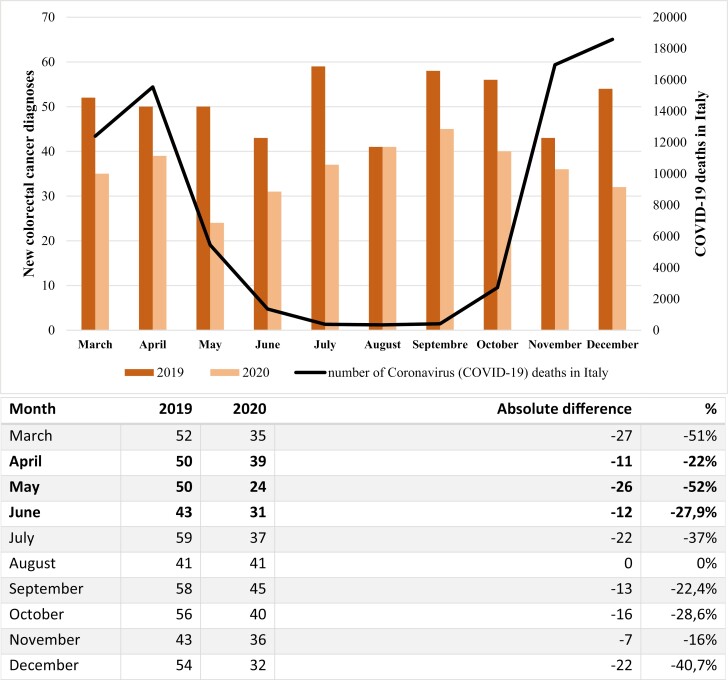
A total of 866 patients was included in our study (Fig. 1). By comparing newly diagnosed CRC cases in 2020 (n = 360) with 2019 (n = 506), a remarkable reduction (−29%) was noted. The mean monthly access rates were 36 in 2020 and 51 in 2019, which revealed a statistically significant decrease (access rate ratio = 0.71, P < .01) (Fig. 2). Median age was similar (70 vs 71 years old, P = .29) between the 2 cohorts. Male patients accounted for 53% and 57%, in the 2 cohorts respectively (P = .29). The sidedness of disease was left in 211 of cases in 2020 and in 265 of cases in 2019 (60% vs 55%, P = .24). Looking at all CRC with available data on mutational status, the percentage of tumors with any mutation among BRAF, NRAS, and KRAS genes was significantly different in the 2 years (61% in 2020 vs 50% in 2019, P = .04).
Figure 2.
Monthly differences of new colorectal cancer diagnoses between 2019 and 2020. April, May, and June 2020 (in bold type) were considered as the lockdown timeframe.
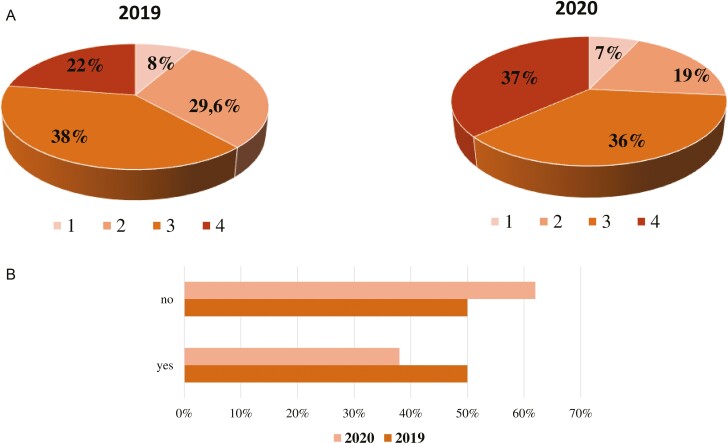
Most notably, significant differences emerged between 2020 and 2019 looking at the clinical stage at diagnosis. Specifically, after COVID-19 outbreak patients with CRC were more likely to be diagnosed with stage IV disease (37% in 2020 vs 22% in 2019, P < .01) (Fig. 3a). Despite the different staging, ECOG PS at treatment start was comparable between the 2 years, with a similar percentage of CRC patients showing ECOG PS 0 (59% in 2020 vs 57% in 2019, P = .61). Looking at the treatment received, a higher percentage of patients with CRC were administered a first-line systemic treatment in 2020 with respect to 2019 (29% vs 16%), with a statistically significant difference in comparison to other treatments, such as adjuvant or neoadjuvant chemotherapy and follow-up after surgery (P < .01). Regarding radiotherapy, no statistically significant difference was noted between pandemic and pre-pandemic period (19% vs 19% respectively, P = 1.00).
Figure 3.
(a) Difference of colorectal cancer stages at diagnosis between 2019 and 2020 (percentages were calculated on the total number of new diagnoses each year taking into account also the number of unknown stages at diagnosis). (b) Difference of colorectal cancer cases discussed during multidisciplinary team meetings between 2019 and 2020.
Remarkably, the number of cases discussed within the framework of multidisciplinary team meetings significantly varied among the 2 years, with a reduction of CRC cases discussed during pandemic (38% vs 50% pre-pandemic, P < .01) (Fig. 3b). However, COVID-19 outbreak did not impact the enrollment of patients in the context of clinical trials (P = .35). Demographic, clinicopathological, and treatment characteristics by year of treatment are summarized in Table 1.
Table 1.
Patients’ characteristics by year of diagnosis.
| Characteristic | 2019 (%) | 2020(%) | P-valuea |
|---|---|---|---|
| Patients | 506 | 360 | |
| Monthly access rate | 51 | 36 | <.01b |
| Median age (IQR) | 71 (range 31-95) | 70 (range 21-94) | .29 |
| Gender | .29 | ||
| Female | 218 (43) | 169 (47) | |
| Male | 288 (57) | 191 (53) | |
| Asymptomatic disease onset*c | 108 (21) | 63 (18) | .11 |
| Sidedness of primitive lesionc | .24 | ||
| Right (until splenic flexure) | 213 (45) | 142 (40) | |
| Left | 265 (55) | 211 (60) | |
| Stage at diagnosisc | <.01b | ||
| Stages I-II-III | 388 (78) | 225 (63) | |
| Stage IV | 112 (22) | 130 (37) | |
| Mutational statusc | .04b | ||
| Wild type | 88 (50) | 58 (39) | |
| Mutant | 87 (50) | 92 (61) | |
| Treatmentc | <.01b | ||
| Adjuvant | 133 (27) | 101 (29) | |
| Neoadjuvant | 71 (14) | 58 (16) | |
| Metastatic | 79 (16) | 102 (29) | |
| Adjuvant post-metastasectomy | 14 (3) | 8 (2) | |
| Follow up | 194 (40) | 83 (24) | |
| MTD discussionc | <.01b | ||
| Yes | 253 (50) | 138 (38) | |
| No | 250 (50) | 221 (62) | |
| Treatment within clinical trialsc | .35 | ||
| Yes | 5 (1) | 6 (2) | |
| No | 417 (99) | 301 (98) | |
| Radiotherapyc | 1.00 | ||
| Yes | 71 (19) | 51 (19) | |
| No | 311 (81) | 224 (81) | |
| ECOG PS at start of treatmentc | .61 | ||
| 0 | 192 (57) | 156 (59) | |
| 1 | 130 (38) | 90 (34) | |
| 2 | 15 (4) | 16 (6) | |
| 3 | 2 (1) | 1 (0) |
Chi-square test comparing proportions between 2019 and 2020. P-values were calculated excluding unknown values.
Statistically significant (P < .05).
% has been calculated excluding NA (not available) values.
Abbreviations: IQR, interquartile range; MDT, multidisciplinary team; ECOG PS, Eastern Cooperative Oncology Group performance status.
Time Intervals
Timing of access to cancer diagnosis, staging, and treatment for patients with CRC has not been negatively affected by the pandemic (Table 2 and Supplementary Fig. S1). In particular, time intervals between symptoms onset and radiological diagnosis (median 27 vs 29 days in 2020 and 2019 respectively, P = .42) and between symptoms onset and histological diagnosis (median 30 vs 33 days, P = .34) were similar between the 2 years. Data analysis showed even significantly shorter temporal intervals during 2020 between symptoms onset and first oncological appointment (median 69 vs 79 days, P = .01) and between histological diagnosis and first oncological appointment (median 34 vs 42 days, P < .01).
Table 2.
Temporal intervals between date of symptoms onset, radiological diagnosis, cytohistological diagnosis, first oncological appointment, treatment start, and first radiological reassessment by year of diagnosis.
| Time interval | 2019 Median, days (IQR) | 2020 Median, days (IQR) | P-valuea |
|---|---|---|---|
| Symptom onset/radiological diagnosis | 29 (51) | 27 (60) | .42 |
| Symptom onset/histological diagnosis | 33 (53) | 30 (59) | .34 |
| Symptom onset/first oncological appointment | 79 (68) | 69 (73) | .01 |
| Histological diagnosis/first oncological appointment | 42 (45) | 34 (41) | <.01b |
| Symptom onset/treatment start | 99 (71) | 83 (86) | .06 |
| Histological diagnosis/treatment start | 55 (44) | 49 (42) | .06 |
| First oncological appointment/treatment start | 14 (21) | 16 (17) | .12 |
| Treatment start/first radiological evaluation | 110 (100) | 96 (48) | <.01b |
Mann-Whitney U test comparing time intervals between 2019 and 2020. P-values were calculated excluding patients with unknown values. Data of patients who had their colorectal cancer diagnosis after first oncological appointment (as per standard practice of referral Hospitals) were also excluded in the calculation of these specific temporal intervals.
Statistically significant (P <.05).
Abbreviation: IQR, interquartile range.
Focusing on systemic treatment initiation, 2020- and 2019-time intervals between symptoms onset and treatment start (median 83 vs 99 days, P = .06), first oncological appointment and treatment start (median 16 vs 14 days, P = .12) resulted similarly. A statistically significant reduction in 2020 in terms of temporal intervals between treatment start and first radiological revaluation (median 96 vs 110 days, P < .01) was noted.
Subgroup Analysis
Subgroup analyses were conducted in order to identify differences in minor groups of patients with CRC by time of the year, provincial infection rate, and hospital volume.
The decline in terms of new CRC diagnoses was greater, albeit not significant, in the lockdown period compared with the other months examined (percentage drop 34% vs 27%, P = .53). May was the most affected month by the decrease in new CRC diagnosis (−52%) (Fig. 2).
Regarding the percentage of patients referring to hospitals in high-infected vs low/medium-infected provinces, no significant difference was noted during the pandemic (72% in 2020 vs 69% in 2019, P = .32). In addition, no difference emerged about patients with CRC referring to low/medium volume vs high-volume hospitals between years (58% vs 60%, P = .59).
Discussion
After triggering a dramatic and rapidly evolving health care system transformation, COVID-19 would have left its permanent mark on cancer care by the end of 2020.
With our country at the forefront of such unrivaled struggle, Italian oncologists have been standing as a barrier to secure their system leak-tight against COVID-19 raging threat.11,22
Unprecedented times called for desperate measures to rationalize the use of essential health care services and to spare hospital facilities capacity. Cancer screening programs and elective oncological procedures were suspended or postponed, routine clinical practices switched to telemedicine and tumor boards redeployed in order to limit avoidable hospital admissions and centralize resource allocation.23-25
In such watershed events, patients with cancer were among the first to feel the void in disaster unpreparedness.26,27
Although COVID-19 has faded the public perception of cancer as the affliction of our time, concerning issues have been shortly raised about the dire consequences of neglecting the long-running cancer epidemic within the ongoing COVID-19 pandemic.28
Since the burden of diagnostic delays will be likely passed on tomorrow’s Oncology, a great turmoil within the cancer research community is currently fueled by the effects of COVID-19 on cancer survival for the years to come.14
As we are navigating our second pandemic year, the present work aims to uncover the outcome of the actions taken to manage the pandemic crisis of oncology standards of care in Italy throughout 2020. By comparing pre- and post-pandemic time intervals within the patients’ with management system, to our knowledge this is the first Italian real-world study to investigate the result of the efforts made to ensure diagnostic—therapeutic pathways proper operation in response to COVID-19.
Mirroring earlier findings from other countries,29-32 our results highlight a sharp decline (−29%) in CRC diagnoses in 2020 (n = 360) compared with the same period of 2019 (n = 506), with the steepest decrease during the lockdown period (-34%). To support these findings, Kaufman et al in their US cross-sectional evaluation reported CRC as the second tumor after breast cancer to suffer from a loss in newly identified cases in the first pandemic peak.29 On this topic also, Morris et al conducted a population-based study in England demonstrating a concerning decrease in the number of detected and treated CRC cases in 2020. According to their report however, by October 2020 the monthly average reduction had returned to 2019 levels.33
Unlike previous investigations, our study’s extended follow-up period demonstrated that a slightly lower, but still alarming, drop (−27%) persisted after the first pandemic wave in the rest of 2020, with December as the most affected month (−40.7%). In line with this, the larger gap compared with pre COVID-19 time has been identified by the Netherland Cancer Registry in the period following lockdown’s institution and the temporary pausing of cancer screenings. In this regard, Dinmohamed et al also suggested that such a backlog on cancer diagnoses might progress more significantly over time, even after the resumption of screening programs.30
Most strikingly, our analysis shows a significant drop of early stage (stages I-II-III) CRC during pandemic compared with 2019 (63% vs 78%, P < .01). Along these lines, London et al described a drastic decline of −84.5% for CRC screening during lockdown.13 Unfortunately, cancer does not wait and missed cases will be eventually diagnosed at a later stage, as confirmed by the higher proportion of stage IV CRC diagnoses in 2020 (37% vs 22%, P < .01). With the best treatment chances the sooner colon cancer is detected, these data draw attention to the prognostic implication of the much-feared “upstaging effect” on cancer outcome and the paradigmatic difference of therapeutic target between curative and palliative setting.
While it is too soon to accurately determine how such fewer cases of early stage CRC in the post-pandemic will impact on cancer survival, preliminary data from a wide UK modeling study have warningly predicted for CRC the highest growth (15-16%) of the mortality rate up to 5 years after diagnosis, compared with other malignancies including breast cancer.34,35
Moreover, with a Healthcare system close to collapse, we must not disregard the additional financial toxicity that our findings might inflict in terms of productivity losses and welfare-related costs. As estimated by Gheorghe et al, the greater economic loss will result from CRC premature deaths and later stage disease presentation, with the load of excess in cancer deaths outdoing that COVID-19 related (due to the difference in the median age of the people affected).36
On the other hand, despite the hard times, our results proved that Italian Medical oncologists met the challenge of preventing cancer patients from being left orphan of care. No leakage in the management system of CRC patients emerged in terms of temporal intervals at any step of the diagnostic—therapeutic pathway. Contrary to what was expected and previous evidence from the French ONCOCARE-COV Study,37 our findings specifically demonstrated during the pandemic a better performance of Italian Oncology Departments. This proved shorter time between diagnosis and treatment could be partly traced to the reduced number of new cancer patients in 2020, easing the pressure on a pandemic distressed system and accelerating patients’ encounters compared to 2019. Additionally, the late-stage presentation shown after COVID-19, generally precluding a surgical approach, might have hastened the referral to medical oncologists. Besides, this observed unexpected and positive trend in 2020 patients’ management might have also been catalyzed by the extensive use of telemedicine and supported by the unwavering resilience of health care providers, as demonstrated by multiple sources.10,11,25
With the multimodal interdisciplinary approach as a cornerstone of modern oncology practice, a growing body of literature has focused on the technical, other than financial and relational issues affecting tumor boards as a result of COVID-19 limitations.38-40 Of note, our study as first reported a significant setback in the multidisciplinary discussion of CRC patients with a significant decrease in the number of the cases reviewed in 2020 compared with the previous year (38% vs 50%, P < .01).
We acknowledge that the present investigation has potential limitations as a retrospective study. Nevertheless, as the cooperative effort of a multicentered national collaboration, our data provide a valuable and thorough insight on cancer care during our country’s first pandemic year.41 On the same ground, it also considers regional dissimilarities in cancer incidence and local administrations’ distinct responses to contain the COVID-19 tidal wave. More importantly, while former publications are mainly based on informatics data analysis from National Cancer Registries, our real-world study, sifting through medical records of 866 patients, is less affected by potential reporting biases during the chaotic times examined.
Conclusions
Gathering together all findings, our study sheds light on the good outcome of the challenges tackled by Italian Oncology Departments to guarantee the tightness of diagnostic-therapeutic pathways and mitigate the effects of COVID-19.
With critical diagnostic delays looming over this persistently evolving scenario, it is of utmost priority to gauge their long-haul impact on cancer survival in order to raise the bar of Oncology care to novel strategies and avoid escalating collateral damages.
As the pandemic continues to strain our health care system, our data lay the ground for far-reaching investigations on COVID-19 repercussions on the decades to come. Clearly, more effort and participation are warranted to advance this matter. On this point, the study is still ongoing to assess outcome data.
Supplementary Material
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study.
Contributor Information
Giulia Mentrasti, Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona, Italy.
Luca Cantini, Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona, Italy.
Clizia Zichi, Department of Oncology, University of Turin, Ordine Mauriziano Hospital, Torino, Italy.
Nicola D’Ostilio, ASL2 Abruzzo, Ospedale Floraspe Renzetti, Lanciano, Italy.
Fabio Gelsomino, Division of Oncology, Department of Oncology and Hematology, University Hospital of Modena, Modena, Italy.
Erika Martinelli, UOC Oncologia ed Ematologia, Dipartimento di Medicina di Precisione, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Rita Chiari, Medical Oncology, Ospedali Riuniti Padova Sud, Monselice, Italy.
Nicla La Verde, Department of Oncology, Ospedale Luigi Sacco, ASST Fatebenefratelli Sacco, Milano, Italy.
Renato Bisonni, Department of Oncology, Ospedale Augusto Murri di Fermo, Fermo, Italy.
Valeria Cognigni, Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona, Italy.
Giada Pinterpe, Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona, Italy.
Federica Pecci, Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona, Italy.
Antonella Migliore, Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona, Italy.
Giacomo Aimar, Department of Oncology, University of Turin, Ordine Mauriziano Hospital, Torino, Italy.
Francesca De Vita, Department of Oncology, University of Turin, Ordine Mauriziano Hospital, Torino, Italy.
Donatella Traisci, Medical Oncology, ASL2 Abruzzo, Ospedale San Pio da Pietralcina, Vasto, Italy.
Andrea Spallanzani, Division of Oncology, Department of Oncology and Hematology, University Hospital of Modena, Modena, Italy.
Giulia Martini, UOC Oncologia ed Ematologia, Dipartimento di Medicina di Precisione, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Linda Nicolardi, Medical Oncology, Ospedali Riuniti Padova Sud, Monselice, Italy.
Maria Silvia Cona, Department of Oncology, Ospedale Luigi Sacco, ASST Fatebenefratelli Sacco, Milano, Italy.
Maria Giuditta Baleani, Department of Oncology, Ospedale Augusto Murri di Fermo, Fermo, Italy.
Marco Luigi Bruno Rocchi, Biomolecular Sciences Department, University of Urbino, Urbino, Italy.
Rossana Berardi, Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona, Italy.
Ethics Statements
The study was conducted in accordance with the precepts of Good Clinical Practice and the ethical principles of the Declaration of Helsinki. Written informed consent was provided by all patients. Results presented in this article contain no personally identifiable information from the study. The study was approved by the respective local ethical committees on human experimentation of each participating center, after previous approval by the coordinating center (“Comitato Etico Regionale delle Marche—C.E.R.M.”, Reference Number 2021 139).
Funding
None declared.
Conflict of Interest
Rita Chiari: BMS, MSD, Roche, Pfizer, AZD, Takeda, Amgen, Boheringer, Novartis (C/A); Fabio Gelsomino: Servier, Eli Lilly, Bristol-Myers Squibb (H), Iqvia, Merck Serono, Amgen (C/A); Nicla La Verde: Novartis, Roche, Pfizer, MSD, Gentili, AstraZeneca (C/A), Eisai (RF); Rossana Berardi: AstraZeneca, Boehringer Ingelheim, Novartis, MSD, Otsuka, Eli-Lilly, Roche (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Author Contributions
Conception/design: G.M., L.C., R.B. Provision of study material or patients: G.M., L.C., C.Z., N.D., F.G., E.M., R.C., N.L.V., R.B., G.A., F.D.V., D.T., A.S., G.M., L.N., M.S.C., M.G.B., V.C., G.P., F.P., A.M. Collection and/or assembly of data: G.M., L.C., C.Z., N.D., F.G., E.M., R.C., N.L.V., R.B., G.A., F.D.V., D.T., A.S., G.M., L.N., M.S.C., M.G.B., V.C., G.P., F.P., A.M. Data analysis and interpretation: G.M., L.C., M.L.B.R., R.B. Manuscript writing: G.M., L.C., V.C., G.P., F.P., A.M., M.L.B.R., R.B. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Keesara S, Jonas A, Schulman K.. COVID-19 and health care’s digital revolution. N Engl J Med. 2020;382(23):e82. 10.1056/nejmp2005835. [DOI] [PubMed] [Google Scholar]
- 2. Sohrabizadeh S, Yousefian S, Bahramzadeh A, et al. A systematic review of health sector responses to the coincidence of disasters and COVID-19. BMC Public Health 2021;21(6):709. 10.1186/S12889-021-10806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Payedimarri AB, Concina D, Portinale L, et al. Prediction models for public health containment measures on COVID-19 using artificial intelligence and machine learning: a systematic review. Int J Environ Res Public Heal 2021;18(9):4499. 10.3390/ijerph18094499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yekedüz E, Utkan G, Ürün Y.. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. 2020;141:92-104. 10.1016/j.ejca.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218-1223. 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee AJX, Purshouse K.. COVID-19 and cancer registries: learning from the first peak of the SARS-CoV-2 pandemic. Br J Cancer. 2021;124(11):1777-1–784.. 10.1038/s41416-021-01324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cancer Patient Management During the COVID-19 Pandemic | ESMO. Accessed April 13, 2022.https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic.
- 8. Ueda M, Martins R, Hendrie PC, et al. Managing Cancer Care during the COVID-19 pandemic: agility and collaboration toward a common goal. JNCCN J Natl Compr Cancer Netw 2020;1-4. 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 9. Dalu D, Rota S, Cona MS, et al. A proposal of a “ready to use” COVID-19 control strategy in an oncology ward: utopia or reality?. Crit Rev Oncol Hematol. 2021;157:103168. 10.1016/j.critrevonc.2020.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berardi R, Torniai M, Cona MS, et al. Social distress among medical oncologists and other healthcare professionals during the first wave of COVID-19 pandemic in Italy. ESMO Open 2021;6(2):100053. 10.1016/j.esmoop.2021.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ballatore Z, Bastianelli L, Merloni F, et al. Scientia potentia est: how the Italian world of oncology changes in the COVID-19 pandemic. JCO Glob Oncol 2020;6:1017-1023. 10.1200/GO.20.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kempf E, Lamé G, Layese R, et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer. 2021;150:260-267. 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. London JW, Fazio-Eynullayeva E, Palchuk MBet al. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657-665. https://doi.org/101200/CCI2000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharpless NE. COVID-19 and cancer. Science. 2020;368(6497):1290. 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 15. I numeri del cancro in Italia 2020 | Associazione Italiana Registri Tumori. Accessed April 5, 2022.https://www.registri-tumori.it/cms/pubblicazioni/i-numeri-del-cancro-italia-2020.
- 16. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386-1422. 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 17. Blanco GDV, Calabrese E, Biancone L, et al. The impact of COVID-19 pandemic in the colorectal cancer prevention. Int J Colorectal Dis. 2020;35(10):1951-1954. 10.1007/s00384-020-03635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rapporto sulla ripartenza degli screening - settembre 2020 | Osservatorio Nazionale Screening. Available at https://www.osservatorionazionalescreening.it/content/rapporto-sulla-ripartenza-degli-screening-settembre-2020. Accessed April 5, 2022
- 19. Patel S, Issaka RB, Chen E, et al. Colorectal Cancer Screening and COVID-19. Am J Gastroenterol. Am J Gastroenterol. 2021;116(2):433-434. 10.14309/ajg.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suárez J, Mata E, Guerra A, et al. Impact of the COVID-19 pandemic during Spain’s state of emergency on the diagnosis of colorectal cancer. J Surg Oncol. 2021;123(1):32-36. 10.1002/jso.26263. [DOI] [PubMed] [Google Scholar]
- 21. Riccardo F, Ajelli M, Andrianou XDet al. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Euro Surveill. 2020;25(49):2000790. 10.2807/1560-7917.ES.2020.25.49.2000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curigliano G. How to guarantee the best of care to patients with cancer during the COVID-19 epidemic: the Italian experience. Oncologist 2020;25(6):463-467. 10.1634/theoncologist.2020-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curigliano G, Banerjee S, Cervantes A, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31(10):1320-1335. 10.1016/j.annonc.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riera R, Bagattini ÂM, Pacheco RLet al. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311-323. https://doi.org/101200/GO2000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perrin PB, Pierce BS, Elliott TR.. COVID-19 and telemedicine: a revolution in healthcare delivery is at hand. Heal Sci Reports 2020;3:e166. 10.1002/HSR2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsamakis K, Gavriatopoulou M, Schizas D, et al. Oncology during the COVID-19 pandemic: challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20(1):441-447. 10.3892/ol.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scalea JR. The distancing of surgeon from patient in the era of COVID-19: bring on the innovation. Ann Surg. 2020;272(1):e18-e19. 10.1097/SLA.0000000000003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horn L, Garassino M.. COVID-19 in patients with cancer: managing a pandemic within a pandemic. Nat Rev Clin Oncol 2021;18(1):1-2. 10.1038/s41571-020-00441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic [published correction appears in JAMA Netw Open. 2020;3(9):e2020927]. JAMA Netw Open. 2020;3(8):e2017267. 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750-751. 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jones D, Neal RD, G Duffy SR, et al. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21 (6):748-750. 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yabroff KR, Wu X-C, Negoita S, et al. Association of the COVID-19 pandemic with patterns of statewide cancer services. J Natl Cancer Inst. 2022;114(6):907-909. 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morris EJA, Goldacre R, Spata E, et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(3):199-208. 10.1016/S2468-1253(21)00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study [published correction appears in Lancet Oncol. 2021;22(1):e5]. Lancet Oncol. 2020;21(8):1023-1034. 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gheorghe A, Maringe C, Spice J, et al. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID-19 pandemic: a national population-based modelling study in England, UK. Eur J Cancer. 2021;152:233-242. 10.1016/j.ejca.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brugel M, Carlier C, Essner C, et al. Dramatic changes in oncology care pathways during the COVID-19 pandemic: the French ONCOCARE-COV study. Oncologist 2021;26(2):e338-e341. 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heraudet L, Domblides C, Daste A, et al. Adaptation of multidisciplinary meeting decisions in a medical oncology department during the COVID epidemic in a less affected region of France: a prospective analysis from Bordeaux University Hospital. Eur J Cancer. 2020;135:98-100. 10.1016/j.ejca.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schroeder BA, Cuevas E, Graber JJ.. Multidisciplinary tumor boards present technical and financial challenges in the COVID-19 era. Ann Oncol. 2021;32(7):933. 10.1016/j.annonc.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gross MW, Läubli H, Cordier D.. Multidisciplinary tumor boards as videoconferences – a new challenge in the COVID-19 era. Ann Oncol. 2021;32(4):572-573. 10.1016/j.annonc.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cantini L, Mentrasti G, Russo GL, et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): fewer cases and higher stages from a real-world scenario. ESMO Open. 2022;7(7):100406. 10.1016/J.ESMOOP.2022.100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.