Abstract
We have performed a physiological analysis of the effects of high-osmolarity media on gas1Δ cells. The reductions in the duplication time, number of pluribudded cells, hypersensitivity to Calcofluor and sodium dodecyl sulfate, and chitin level indicate a partial suppression of the mutant phenotype. GAS1 deletion was found to be lethal in the absence of the Bck1 and Slt2 (Mpk1) proteins of the cell integrity pathway.
The cell wall of yeast cells preserves osmotic integrity and determines the morphology of the cells during budding growth and the processes of mating, sporulation, and pseudohyphal growth. Its constituents, β1,3-/β1,6-glucans, mannoproteins, and chitin, are normally present in proportions of about 50 to 60%, 40%, and 1 to 2% of the cell wall dry weight, respectively (15). Several proteins are involved in cell wall biogenesis. Fks1p and Fks2p are the putative catalytic subunits of the plasma membrane β1,3-glucan synthase; Chs1, Chs2, and Chs3 are responsible for the synthesis of chitin; and several proteins are involved in the synthesis and elaboration of β1,6-glucan and cell wall mannoproteins (CWPs) (15). The assembly of the polymers occurs outside the cell. Several pieces of evidence indicate that the glycosylphosphatidylinositol-CWPs (GPI-CWPs) are convalently linked to β1,3-glucan through a β1,6-glucan chain, whereas proteins with internal repeats (PIR-CWPs) can be directly linked to it (22). The β1,3-glucan molecules can be also linked to some chitin molecules (13). Moreover, a fraction of proteins corresponding to about 2% of total CWPs are bound to chitin through short branches of the β1,6-glucan that are resistant to β1,3-glucanase (11, 14). One putative cell wall polymer cross-linker is Gas1p, a GPI-containing glycoprotein (17). In contrast with GPI-CWPs that at the cell surface undergo a probable transglycosylation reaction that ends with the loss of part of GPI, Gas1p retains the glycolipid and remains anchored to the plasma membrane. The increased alkali solubility of the glucan and the release in the medium of β1,3-glucan and mannoproteins shown by the gas1Δ mutant have suggested for Gas1p a cross-linker function (16, 20). The gas1 cells appear to respond to the weakening of the cell wall by activating a complex set of reactions, among which are a 10-fold increase in the chitin level, a 3-fold increase in the CWP1 mRNA level, a 20-fold increase in the β1,3-glucanase-resistant cross-links between GPI-CWPs and chitin, and the triggering of Fks2p expression (11, 16, 20). Some of these responses are common to other cell wall mutants and could be part of a rescue mechanism activated by cell wall stresses.
The rationale behind the experiments described here is that if gasIΔ cells trigger a compensatory response to protect cell integrity, the osmolarity of the growth medium would affect this response. The genetic interactions between the GAS1 gene and the pathway that governs cell integrity have also been analyzed.
The growth kinetics parameters of the parental Saccharomyces cerevisiae W303-1B (MATα ade2-1 his3-11,15 trp1-1 ura3-1 leu2-3,112 can1-100) strain and the derived WB2d (gas1::LEU2) strain grown in YNB-glucose minimal medium (6.7 g of Difco yeast nitrogen base [without amino acids] per liter and 2% glucose supplemented with 50 mg of the appropriate amino acids and uracil and 100 mg of adenine per liter) at 30°C in the presence of an osmotic stabilizer are shown in Table 1. No significant effect on the duplication time (Td) of the parental strain was observed with 0.5 M KCl and other osmotic supports (data not shown). In contrast, the Td of the gas1-null mutant was about 40 min shorter in the presence of an osmotic support, and the length of the lag phase elapsing from the inoculum to the initiation of the exponential growth was reduced (data not shown). In addition, the percentage of pluribudded cells at the stationary phase decreased from 40% to about 4 to 6%, although a high percentage of budded cells were still present (last two columns in Table 1). Since the effects are present as well in 1 M sorbitol, they are due to the increase in osmolarity and not to the action of specific cations.
TABLE 1.
Growth parameters of gas1 cells in osmotically stabilized media
| Strain (growth condition) | Td (min)a | % in different growth phases
|
||
|---|---|---|---|---|
| Exponential-phase total budded cells | Stationary phase
|
|||
| Total budded cells | Pluribudded cellsb | |||
| W303-1B | 120 | 75 | 20 | <1 |
| W303-1B (0.5 M KCl) | 130 | 66 | 20 | <1 |
| WB2d | 190 | 73 | 78 | 41 |
| WB2d (0.5 M KCl) | 150 | 85 | 70 | 5 |
| WB2d (0.5 M NaCl) | 150 | 80 | 66 | 4 |
| WB2d (1 M sorbitol) | 150 | 80 | 45 | 6 |
Td was measured as an increase in cell number (mean of three different experiments).
Two or more buds.
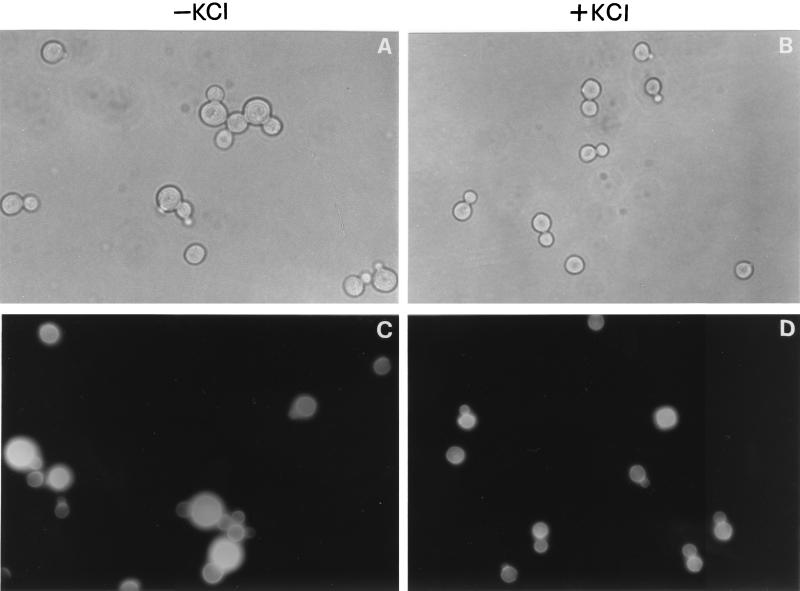
A microscopic examination indicated that gas1Δ cells in 0.5 M KCl lose the typical swollen aspect and are slightly smaller, and cells carrying two or more buds (pluribudded cells), characteristic of the “aggregated” phenotype of gas1, are almost absent (Fig. 1A and B). These results indicate that the increase in osmolarity of the growth medium ameliorates the growth kinetics parameters and the morphological defects of the gas1Δ mutant.
FIG. 1.
Effects of an increase in osmolarity of the growth medium on the morphology of gas1Δ cells. (A and B) WB2d cells (gas1Δ) grown until the stationary phase at 30°C in YNB-glucose or YNB-glucose supplemented with 0.5 M KCl. (C and D) CF staining of exponentially growing WB2d cells under the same conditions.
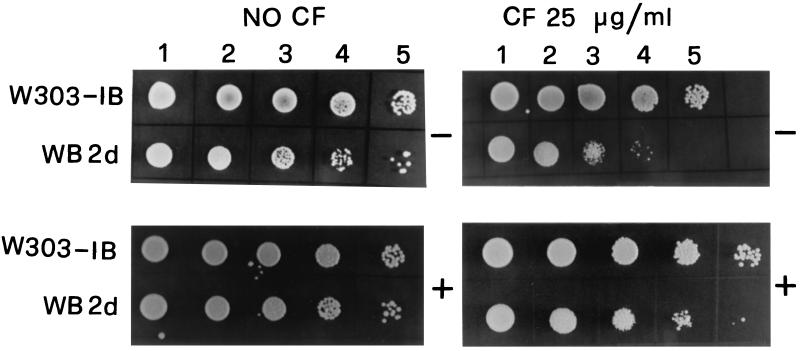
In order to gain further insight into the effects of the increase of the external osmolarity on gas1Δ cells, we tested the cell viability and the sensitivity of growth to Calcofluor white (CF) (Sigma, St. Louis, Mo.), a fluorescent stain that binds to nascent chitin molecules and prevents their assembly in microfibrils (9). gas1Δ mutant is hypersensitive to CF (16). Different dilutions of cells from cultures grown in YNB-glucose medium or in the same medium supplemented with 0.5 M KCl were spotted on YPD (1% peptone, 2% yeast extract, 2% dextrose) plates in the presence or absence of 25 μg of CF per ml. gas1Δ cells, grown in the presence of 0.5 M KCl, showed a slight increase in cell viability and were less sensitive to the perturbing action of CF, whereas no differences were observed in the parental strain (Fig. 2).
FIG. 2.
Cell viability and sensitivity to Calcofluor of gas1Δ cells. Exponentially growing W303-1B and WB2d cells were grown in YNB-glucose in the absence (−) or presence (+) of 0.5 M KCl. At a cell density of 5 × 106/ml, 5 μl of a concentrated suspension (lanes 1) and of 10× (lanes 2), 1,000× (lanes 4), and 10,000× (lanes 5) dilutions was spotted on YPD agar plates with or without CF.
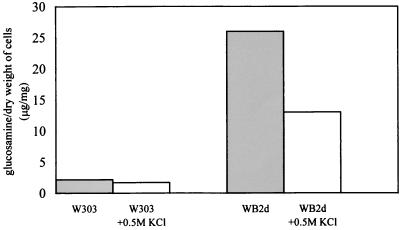
To assess if growth in osmotically supported medium affects chitin accumulation, we measured the chitin level in gas1Δ and its parental strain. The chitin present in the undigestible material obtained after a prolonged treatment with Zymolyase 100T of the alkali-insoluble fraction was measured as described previously (16). The amount of chitin present in gas1Δ cells grown in YNB-glucose medium was approximately 10-fold higher than in the parental strain, in agreement with the previously reported data (16), whereas in cells grown in 0.5 M KCl, the amount of chitin showed a 50% decrease (Fig. 3). This result is also consistent with that obtained by staining of chitin with CF, which showed a less intense fluorescence on the cell surface of WB2d cells grown in the presence of the osmotic support (Fig. 1C and D).
FIG. 3.
Chitin levels in gas1 cells grown in an osmotically supported medium. The assays were performed in duplicate, and the values are the means of two different experiments. The standard deviation was no more than 10%.
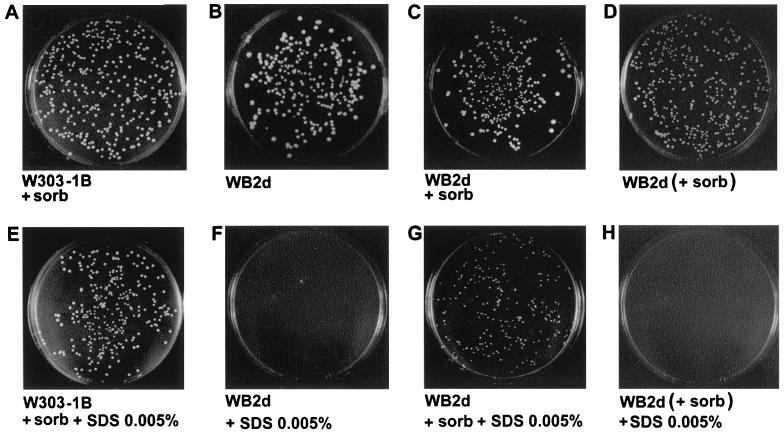
We have shown in a previous work that GAS1-null mutation increases the sensitivity of cell growth to sodium dodecyl sulfate (SDS), a strong destabilizing agent for the plasma membrane that easily penetrates the mutant due to the increased porosity of the cell wall (16, 18, 23). Cells exponentially growing in YNB-glucose to a density of 5 × 106 cells per ml were plated respectively on YNB-glucose plates containing SDS or SDS plus 1 M sorbitol or, as a control, on plates without SDS (Fig. 4). Cells of the parental strain were unaffected by the presence of 0.005% SDS in both the presence of 1 M sorbitol (Fig. 4A and E) and in the absence of sorbitol (data not shown). On the other hand, gas1Δ cells were unable to grow with 0.005% SDS (Fig. 4B and F), but they could form microcolonies if sorbitol was present in the plate (Fig. 4G). Thus, sorbitol partially suppresses the lethal effect of SDS on gas1Δ cells and appears to have a rapid protective effect on the cell wall of the mutant. The opposite shift gave results in agreement with this rapid effect of sorbitol. gas1Δ cells pregrown in YNB-glucose medium containing 1 M sorbitol (+ sorb in Fig. 4) were not able to grow on plates containing only 0.005% SDS (Fig. 4H). As a control, the cells were also plated in the absence of SDS, and the lack of any effect on growth indicates that the hypo-osmotic shock is not responsible for this observed lethal effect (Fig. 4D).
FIG. 4.
Effect of the osmotically stabilized medium on the sensitivity to SDS of a gas1-null mutant. (A, B, C, E, F, and G) W303-1B and WB2d cells exponentially growing in YNB-glucose were diluted, and about 500 cells were plated on solid medium in the presence or absence of SDS and 1 M sorbitol as indicated. (D and H) WB2d cells exponentially growing in YNB-glucose plus 1 M sorbitol (+ sorb) were plated on solid medium in the presence or absence of SDS, but without sorbitol.
Genetic interactions between GAS1 and components of the PKC1-MAP kinase cascade.
It has been proposed that the cell wall weakened by the loss of Gas1p could less efficiently counteract the turgor pressure, thus generating the swollen phenotype of gas1 cells (20). This condition would create stress on the plasma membrane that, similarly to the condition of hypotonic shock, could open stretch-activated ion channels, whose presence has been shown in yeast (7, 10), and activate a cellular response. It is known that a decrease in the osmolarity of the growth medium activates the PKC1–mitogen-activated protein (MAP) kinase pathway in S. cerevisiae (8), inducing an increase in tyrosine phosphorylation and protein kinase activity of Slt2p (Mpk1p) (2, 10). Deletion of PKC1 determines an osmotically remedial cell lysis phenotype at all temperatures, while deletion of elements of the downstream MAP kinase cascade, BCK1-MKK1/MKK2-MPK1, determines a milder phenotype for cell lysis that requires a temperature of at least 37°C. It has been proposed that PKC1 could govern a bifurcated pathway (16). We have previously shown that the combination of deletion of GAS1 and PKC1 determines synthetic lethality (16). Here we analyzed the effects of GAS1 deletion and of single elements of the MAP kinase cascade downstream to PKC1. The double heterozygous diploid strains DL247ΔG (relevant genotype, bck1::URA3/BCK1 gas1::LEU2/GAS1) and DL453ΔG (mpk1::TRP1/MPK1 gas1::LEU2/GAS1), both derived from the parental strain 1788 (a/α leu2-3/leu2-3 trp1-1/trp1-1 ura3- 52/ura3-52 his4/his4 can1r/can1r), were sporulated and dissected on YPD plates (1% peptone, 2% yeast extract, 2% dextrose) supplemented with 0.5 M KCl, and sporulation was carried out at 24°C to prevent cell lysis. Analysis of the spore progeny of 18 tetrads for each diploid indicated that the bck1Δ gas1Δ (Ura+ Leu+) or mpkΔ gas1Δ (Trp+ Leu+) double mutant spores were inviable. Thus, the deletion of GAS1 determines synthetic lethality as well in combination with the inactivation of BCK1 and the last element of the pathway, MPK1. This result suggests that the colethality found between the GAS1 and PKC1 deletions is not simply the sum of severe cell wall defects brought about by the single inactivations. The triggering of the cellular response to cell wall defects of the gas1-null mutant could require the BCK1-MKK1/MKK2-MPK1 module and not other pathways governed by PKC1. Moreover, the lethality of the double-null mutant gas1 pkc1, gas1 bck1, and gas1 mpk1 spores, even in osmotically supported medium, suggests that the increased osmolarity of the growth medium does not completely abolish the dependence from the cell integrity pathway, at least during gas1Δ spore germination.
The effects of the overexpression of the GAS1 gene in a pkc1 disruptant were tested. The pkc1-null mutant strain GPY1115 (MATa pkc1::HIS3 leu2-3,112 ura3-52 his3-Δ200 trpl-Δ90 suc2Δ9 ade2-101) was transformed with a multicopy YEp24 plasmid carrying the GAS1 gene. Transformants were tested for the capability of growing in the absence or presence of 0.5 M KCl. The growth dependence of pkc1Δ cells from osmotically stabilized medium was not suppressed by overproduction of Gas1p (data not shown). Thus, the role of GAS1 in cell wall assembly cannot suppress the previously described defects in cell wall construction of the pkc1-null mutant (21).
The results of our physiological analysis support the hypothesis that the cell wall of gas1 cells is weakened, giving rise to membrane stretching that could activate a rescue mechanism through the cell integrity signaling pathway. In this regard, it has been shown that the extracts from a gas1Δ mutant stimulate the GDP-GTP exchange activity toward Rho1p, which is known to bind and activate Pkc1p (1). Moreover, our results are consistent with the recent findings that the level of the phosphorylated activated form of Slt2p (Mpk1p) is higher in a gas1Δ mutant than in its isogenic wild-type strain and decreases in the presence of sorbitol (M. Molina, personal communication).
Consistent with our hypothesis on the compensatory role of chitin, in gas1Δ cells, there is 50% less chitin in cells grown in the high-osmolarity medium than in those grown in the low-osmolarity medium. The residual accumulation of this polymer suggests that the presence of an osmotic support does not completely abolish the dependence from chitin, probably because of the severe defect in the cell wall assembly of gas1Δ cells. It has been demonstrated that the chitin synthase activity assayed with isolated membranes from S. cerevisiae and Candida albicans cells is higher in cells grown in low- versus high-osmolarity media (4, 5). Posttranslational regulatory mechanisms of the enzyme activity have been proposed (5). Moreover, since a local deposition of chitin has been shown at the hyphal apices of fungal cells (6, 12), a local activation of chitin synthase by membrane stretching subsequent to the softening of the cell wall has also been proposed (5).
Finally, the results obtained indicate a dual role of the osmotic support. The first role is due to the effect of high osmolarity on cell wall porosity. A rapid reduction in the porosity of the cell wall of the mutant is suggested by the decreased sensitivity to SDS acquired by the cells plated in the presence of sorbitol. This is in agreement with the previous observations that the shrinking of the cell envelope in hypertonic solutions significantly reduces cell wall porosity (3). This chemical-physical effect can be easily revealed in the mutant, because its cell wall defects lead to an increase in the cell wall porosity (18). The second role of the increased external osmolarity could be exerted at the level of the cell integrity pathway, as mentioned above. While the increase in the difference in osmolarity, as in a hypo-osmotic shock, is known to activate the pathway, a reduction in the osmolarity gradient switches it off, and this could bring about an attenuation of the cell wall stress response. Thus, the beneficial effect of the osmotic support on gas1Δ cell growth can be abscribed to both a physical action at the level of the cell wall matrix and to a partial suppression of the signal generated by the cell wall damage. It cannot be excluded that the signaling of cell wall defects could involve other sensors as well, such as those belonging to the Wsc or Mid protein families (19, 24), that could converge on the PKC1-MAP kinase pathway.
Acknowledgments
We are grateful to Maria Molina for helpful discussions. We thank Marina Vai and Enzo Martegani for useful comments; David Levin for having provided us with the 1788, DL453, and DL247 strains; Gherard Parravicini for the GPY1115 strain; Lucia Panzeri for help in tetrad dissections; and Antonio Grippo for preparing the figures.
This work was supported by grants to L.P. (MURST-University of Milan Cofin 1997 and MURST 60% 1998). L.F. was a recipient of a fellowship from Prassis-Sigma Tau Italy.
A.T. and L.F. contributed equally to this work.
REFERENCES
- 1.Bickle M, Delley P A, Schmidt A, Hall M. Cell wall integrity modulates RHO1 activity via the exchange factor ROM2. EMBO J. 1998;8:2235–2245. doi: 10.1093/emboj/17.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenport K R, Sohasky M, Kamada Y, Levin D E, Gustin M C. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J Biol Chem. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- 3.De Nobel J G, Klis F M, Munnik T, Priem J, Van Den Ende H. An assay of relative cell wall porosity in Saccharomyces cerevisiae, Kluyveromyces lactis and Schizosaccharomyces pombe. Yeast. 1990;6:483–490. doi: 10.1002/yea.320060605. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande M, O'Donnell R, Gooday G W. Regulation of chitin synthase activity in the dimorphic fungus Benjaminiella poitrasii by external osmotic pressure. FEMS Microbiol Lett. 1997;152:327–332. doi: 10.1111/j.1574-6968.1997.tb10447.x. [DOI] [PubMed] [Google Scholar]
- 5.Gooday G, Shofield D A. Regulation of chitin synthesis during growth of fungal hyphae: the possible participation of membrane stress. Can J Bot. 1995;73(Suppl. 1):S114–S121. [Google Scholar]
- 6.Gooday G. The dynamics of hyphal growth. Mycol Res. 1995;99:385–394. [Google Scholar]
- 7.Gustin M, Zhou X, Mortinc B, Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988;242:762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- 8.Gustin M C, Albertcyn J, Alexander M, Davenport K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–1300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herth W. Calcofluor White and Congo Red inhibit chitin microfibrillar assembly of Poterioochromonas: evidence for a gap between polymerization and microfibril formation. J Cell Biol. 1980;87:442–450. doi: 10.1083/jcb.87.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamada Y, Jumg U S, Piotrowski J, Levin D E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- 11.Kapteyn J C, Ram A F J, Groos E M, Kollar R, Montijn R C, Van Den Ende H, Llobell A, Cabib E, Klis F M. Altered extent of cross-linking of β1,6-glucosylated mannoproteins to chitin in Saccharomyces cerevisiae mutants with reduced cell wall β1,3-glucan content. J Bacteriol. 1997;179:6279–6284. doi: 10.1128/jb.179.20.6279-6284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz D, Rosenberger R F. Hyphal wall synthesis in Aspergillus nidulans: effect of protein synthesis inhibition and osmotic shock on chitin insertion and morphogenesis. J Bacteriol. 1971;108:184–190. doi: 10.1128/jb.108.1.184-190.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollar R, Petrakova E, Ashwell G, Robbins P W, Cabib E. Architecture of the cell wall. J Biol Chem. 1995;270:1170–1178. doi: 10.1074/jbc.270.3.1170. [DOI] [PubMed] [Google Scholar]
- 14.Kollar R, Reinhold B B, Petrakova E, Yeh H J C, Ashwell G, Degonova J, Kapteyn J C, Klis F M, Cabib E. Architecture of the yeast cell wall. J Biol Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- 15.Orlean P. Biogenesis of yeast wall and surface components. Yeast. 1997. III:229–362. [Google Scholar]
- 16.Popolo L, Gilardelli D, Bonfante P, Vai M. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1Δ mutant of Saccharomyces cerevisiae. J Bacteriol. 1997;179:463–469. doi: 10.1128/jb.179.2.463-469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popolo L, Vai M. The Gas1 glycoprotein, a putative wall polymer cross-linker. Biochim Biophys Acta. 1999;1426:385–400. doi: 10.1016/s0304-4165(98)00138-x. [DOI] [PubMed] [Google Scholar]
- 18.Popolo L, Vai M, Gatti E, Porello S, Bonfante P, Balestrini R, Alberghina L. Physiological analysis of mutants indicates involvement of Saccharomyces cerevisiae GPI-anchored protein Gp115 in morphogenesis and cell separation. J Bacteriol. 1993;175:1879–1885. doi: 10.1128/jb.175.7.1879-1885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajavel M, Philip B, Buehrer B M, Errede B, Levin D E. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:3969–3976. doi: 10.1128/mcb.19.6.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ram A F J, Kapteyn J C, Montijn R C, Caro L H P, Douwes J E, Baginsky W, Mazur P, Van Den Ende H, Klis F M. Loss of the plasma membrane-bound protein Gas1p in Saccharomyces cerevisiae results in the release of β1,3-glucan into the medium and induces a compensation mechanism to ensure cell wall integrity. J Bacteriol. 1998;180:1418–1424. doi: 10.1128/jb.180.6.1418-1424.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roemer T, Parravicini G, Payton M A, Bussey H. Characterization of the yeast (1,6)β-glucan biosynthetic components, Kre6p and Skn1p, and genetic interactions between the PKC1 pathway and extracellular matrix. J Cell Biol. 1994;127:567–579. doi: 10.1083/jcb.127.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smits G J, Kapteyn J C, van de Ende H, Klis F M. Cell wall dynamics in yeast. Curr Opin Microbiol. 1999;2:348–352. doi: 10.1016/s1369-5274(99)80061-7. [DOI] [PubMed] [Google Scholar]
- 23.Vai M, Orlandi I, Cavadini P, Alberghina L, Popolo L. Candida albicans homologue of GGP1/GAS1 gene is functional in Saccharomyces cerevisiae and contains the determinants for glycosylphosphatidylinositol attachment. Yeast. 1996;12:361–368. doi: 10.1002/(SICI)1097-0061(19960330)12:4%3C361::AID-YEA920%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 24.Verna J, Lodder A, Lee K, Vagts A, Ballester R. A family of genes required for the maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:13804–13809. doi: 10.1073/pnas.94.25.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]