1 |. INTRODUCTION
The nature of human existence has been the subject of philosophic debate for centuries, and more recently it has also become a topic of biological interest. Advances in our understanding of the human microbiome have led to the concept of humans as holobionts or symbiomes, integrated multitaxon units of biological organization. Indeed, an intimate relationship exists between host and microbiome, resulting in codependence to maintain a homeostatic state considered to represent health. Conversely, a dysbiotic interaction can lead to one of an ever-increasing list of pathologic conditions. While causal relationships remain to be definitively established in a number of instances, it is nonetheless apparent that many aspects of human health and disease have a microbiome component.
Carcinogenesis, by definition, involves a profound disruption of cell and tissue homeostasis. Though the etiology of cancer is complex and multifactorial, the microbiome is important, and a viral component may be the proximate cause of as much as a fifth of human cancers worldwide.1 Carcinogenic viruses usually have latent forms, and include Epstein-Barr virus, hepatitis B and C viruses, human immunodeficiency virus type 1, human herpes virus 8, human papilloma virus, Merkel cell polyomavirus and human T-cell leukemia virus type 1. Human endogenous retroviruses have also long been suspected as oncogenic, although they may require transactivation by other viruses, such as Epstein-Barr virus.2 By comparison, the role of bacteria in the etiology of cancer was historically more narrowly appreciated and less thoroughly investigated. That was to change, however, in the 1980s when pioneering and heroic (self-infection) work by Marshall and Warren3 established Helicobacter pylori as a cause of gastric inflammatory disease. This provided the foundation for studies establishing a link with stomach cancer, and by 1994 H. pylori became the first bacterial species to be recognized by the World Health Organization as a definite cause of cancer in humans. The H. pylori story precipitated a burgeoning of interest in the relationship between bacteria and cancer. At the forefront of this field of bacterial oncopathogenicity was epidemiologic correlations between periodontal disease and cancers of the head and neck, as well as of the colon and pancreas.4–6 Moreover, interactions between oral bacteria and host cells were increasingly recognized as producing proliferative, prosurvival phenotypes recalcitrant to apoptotic cell death.7–9 Although not universally accepted at the time,10 the notion that cancers and periodontitis both represent lesions that fail to heal11 has gained traction, and considerable evidence has accumulated supporting a causal relationship between oral bacteria and cancers. Another interesting parallel to emerge is that the role of oral bacteria in head and neck cancers, as with periodontitis, depends more on community composition and action rather than any one specific organism.
2 |. BACTERIA ASSOCIATED WITH ORAL AND ORODIGESTIVE CANCER
Cancers of the head and neck region are predominantly squamous cell carcinomas,12 and include carcinomas of the oropharynx (including the base of the tongue), generally referred to as oropharyngeal squamous cell carcinomas, along with cancers of the oral squamous cells present most frequently on the anterior of the tongue, lips, floor of the mouth, and gingiva.13 Human papilloma virus is the predominant risk factor for oropharyngeal squamous cell carcinomas, and the traditional risk factors of tobacco use and alcohol consumption are also operational.14,15 While tobacco and alcohol are also risk factors for many oral squamous cell carcinomas, only a small fraction of them are attributable to human papilloma virus, and about 15% have no known risk factors.16 Indeed, smoking and alcohol consumption do not appear to be associated with gingival squamous cell carcinoma,17 and the lesions mimic the appearance of periodontal disease.18 Several studies have shown periodontitis to be a major risk factor for oral squamous cell carcinoma,19–21 and periodontal pathogens have similarly been associated with the lesions. The catalog of organisms associated with oral squamous cell carcinoma has been extensively reviewed in several recent publications.16,22–27 Clarity in this area, however, is hindered by the different sampling methodologies employed. Oral rinses, saliva, and serum only provide an indication of the presence of particular organisms, with no regard to the spatial relationship with the tumor. Tumor scrapings, homogenates, and swabs indicate surface colonization and/or penetration of the tumor site, whereas biopsy sections stained by immunohistochemistry show intracellular localization of the bacteria. These caveats notwithstanding, certain trends have emerged. Traditional periodontal pathogens, such as Porphyromonas gingivalis, Fusobacterium nucleatum, and Treponema denticola, tend to be positively associated with oral squamous cell carcinoma. In contrast, organisms more usually considered oral commensals, such as the mitis group streptococci, are often negatively associated and display anticancer properties in vivo.15,22,28–35 Interestingly, other more pathogenic streptococci, such as Streptococcus anginosus and peptostreptococci, can show a positive correlation with oral squamous cell carcinoma.17,36,37 Accumulating evidence also implicates microbial dysbiosis in the upper digestive tract as a risk factor in the etiology of esophageal squamous cell carcinoma.38–40 Further, there is decreased diversity of the oral microbiota along with enrichment of Porphyromonas and Prevotella in esophageal squamous cell carcinoma, compared with dysplasia and healthy controls. Higher levels of gram-negative anaerobes/microaerophiles are associated with esophagitis and Barrett’s esophagus, whereas a streptococcal-predominant microbiota resides in the normal esophagus.41 Salivary levels of P. gingivalis are associated with the progression of esophageal squamous cell carcinoma,39 and P. gingivalis is overabundant in esophageal cancerous tissue.42 Moreover, detection of P. gingivalis in oral squamous cell carcinoma or in esophageal squamous cell carcinoma lesions is associated with a poor prognosis.42–46 While further studies will increase precision in the field, the current pattern is one of a dysbiotic microbial community enriched in potentially carcinogenic organisms such as P. gingivalis and with an underrepresentation of homeostatic commensals, contributing to the development of oral squamous cell carcinoma and esophageal squamous cell carcinoma.
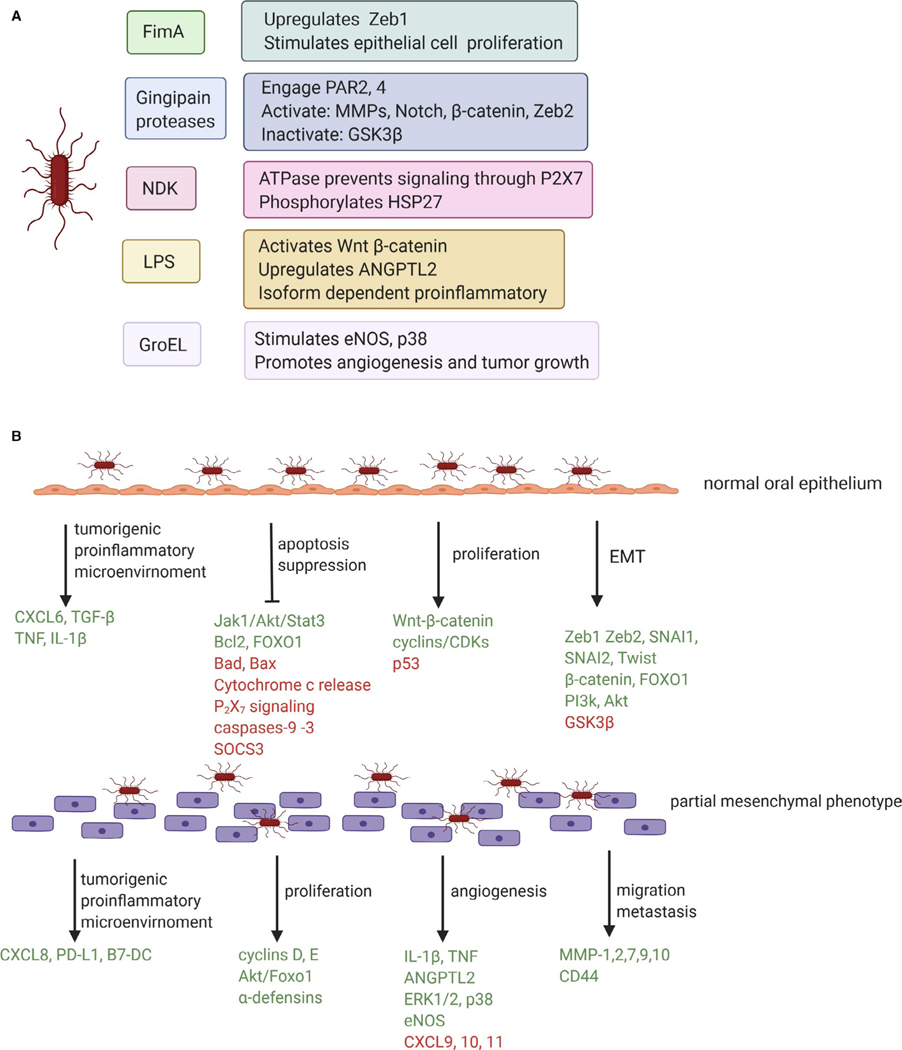
A theoretical mechanistic framework for a bacterial contribution to carcinogenesis includes modulation of the balance of host cell proliferation and death, creation of a proinflammatory microenvironment, and generation of carcinogenic metabolites.47 As the majority of studies of bacterial function are at the single species levels, we shall address the potential tumorigenic activity of P. gingivalis as a monoinfection (Figure 1), before turning our attention to community aspects of the disease.
FIGURE 1.
Potential tumorigenic activity of Porphyromonas gingivalis in oral squamous cell carcinoma. A, Effector molecules of P. gingivalis that have been associated with a tumorigenic function. B, Tumorigenic effects of P. gingivalis on the oral epithelium. Green font denotes components that are activated or increased in messenger RNA or protein amounts. Red font denotes components that are suppressed or decreased in messenger RNA or protein amounts. EMT, epithelial-mesenchymal transition; LPS, lipopolysaccharide; NDK, nucleoside diphosphate kinase
3 |. CELL LIFE AND DEATH
Tumor cells, by definition, have the capacity for unregulated and unlimited proliferation. Enhancement of proliferation by bacteria is therefore a protumorigenic feature, and one prominently displayed by P. gingivalis. Transcriptional profiling of gingival epithelial cells transiently or persistently infected with P. gingivalis shows an upregulation of genes involved in cell proliferation.48–51 Consistent with this, P. gingivalis can induce accelerated progression of primary gingival epithelial cells through the S-phase of the cell cycle by modulation of cyclin/cyclin-dependent kinase activity and by reducing the level of the p53 tumor suppressor protein.52 More rapid proliferation is dependent on the presence of the FimA protein,46 the structural component of the major fimbrial adhesin of P. gingivalis. In addition to fimbriae, the gingipain proteases of P. gingivalis may also contribute to cell proliferation through activation of Notch signaling,53 and through proteolytic degradation and activation of beta-catenin along with disassociation of the beta-catenin destruction complex.54 Nuclear translocation and accumulation of active beta-catenin fragments drives the activity of the beta-catenin-dependent, pro-proliferative T-cell factor/lymphoid enhancer factor promoter.54 P. gingivalis lipopolysaccharide can also activate the Wnt-beta-catenin pathway to stimulate proliferation of gingival progenitor cells.55 P. gingivalis can thus deploy multiple effector molecules to manipulate host cell pathways that control cell division, a theme that recurs in many aspects of the interface between the bacterium and host, as shall be discussed further in subsequent sections.
Studies of noncancer-derived epithelial cells thus reveal the potential for P. gingivalis to initiate uncontrolled proliferation. Investigation of the responses of cells derived from tumors shows that P. gingivalis can reinforce these phenotypes in transformed cells. P. gingivalis was found to increase proliferation of oral squamous cell carcinoma cell lines by regulating cyclin D1 expression through the micro–ribonucleic acid (microRNA) 21/PDCD4/AP-1 negative feedback signaling pathway,56 and by FimA interactions with CXC chemokine receptor 4 and activation of phospho-AKT1-phospho-Forkhead Box O1 signaling.57 Acceleration through the G1 phase of the cell cycle, along with upregulation of cyclins D1 and E, has also been reported in immortalized gingival epithelial cells.58 Additionally, in oral tumor cells derived from the alveolus, P. gingivalis can enhance expression of alpha-defensins, which have been found to elevate proliferation through intersecting with epidermal growth factor receptor signaling.59 Similarly, infection of esophageal squamous cell carcinoma cells with P. gingivalis stimulates cell proliferation and promotes tumor growth in vivo in a mouse xenograft model.42 Mechanisms that have been documented include upregulation of microRNA 194, which in turn targets the GRHL3 transcription factor and modulates GRHL3/PTEN/Akt signaling,60 along with activation of nuclear factor kappa B and subsequent upregulation of cyclin D1, c-Myc, and matrix metalloproteinases.61
A host homeostatic mechanism to protect against relentless cell proliferation is programmed cell death, or apoptosis. Successful tumors, therefore, are able to avoid these apoptotic mechanisms, and antiapoptotic proteins such as Bcl-2 are often overexpressed in cancer cells while proapoptotic proteins, such as Bad, are inactivated.62 P. gingivalis has adopted a multitiered approach to the suppression of apoptotic cell death in primary epithelial cells. A secreted enzyme, nucleoside diphosphate kinase, functions as an adenosine triphosphate synthase and prevents adenosine triphosphate–dependent apoptosis mediated through the purinergic receptor P2X7.63 Additionally, nucleoside diphosphate kinase phosphorylation of heat shock protein 27 curtails cytochrome C release and caspase-9 activation, thus stalling apoptosis.64 Indeed, many of the antiapoptotic activities of P. gingivalis target the intrinsic apoptotic pathway at the mitochondrial membrane, in part through stimulation of signaling through the Janus kinase 1/Alpha serine/threonine kinase (Akt)/Signal Transducer and Activator of Transcription 3 pathway.65,66 Increased expression of Bcl2, along with a decrease in proapoptotic factors such as Bax and Bad, tips the ratio of these interacting proteins toward stabilization of the mitochondrial membrane and resistance to apoptosis, and thus activity of the downstream caspases including caspase-9 and the executioner caspase-3 is suppressed.46,65,67,68 In another tier of antiapoptotic activity, P. gingivalis upregulates the levels of microRNA 203, which leads to inhibition of the proapoptotic signaling molecule Suppressor of Cytokine Signaling 3.69 The multipurpose transcriptional regulator Forkhead Box O1 is also a target of P. gingivalis, which through dephosphorylation of Forkhead Box O1 serine residues induces antiapoptotic programs in epithelial cells.70 In immortalized oral epithelial cells, similar responses involving upregulation of Bcl2, along with activation of phosphatidylinositol-4,5-bisphosphate 3 kinase/Akt and beta-catenin-dependent signaling induces anoikis resistance in P. gingivalis–infected cells.71
In addition to contributing to the development of a tumor, resistance to programmed cell death has clinical implications, as many chemotherapeutic agents function through induction of apoptosis in transformed cells. Both in vitro and in vivo studies support this concept. For example, repeated infection of oral squamous cell carcinoma cells with P. gingivalis diminishes susceptibility to taxol72; and in murine models, tumor xenografts composed of P. gingivalis–infected oral squamous cell carcinoma cells showed resistance to taxol through activation of Notch1 signaling.73
4 |. METASTASIS
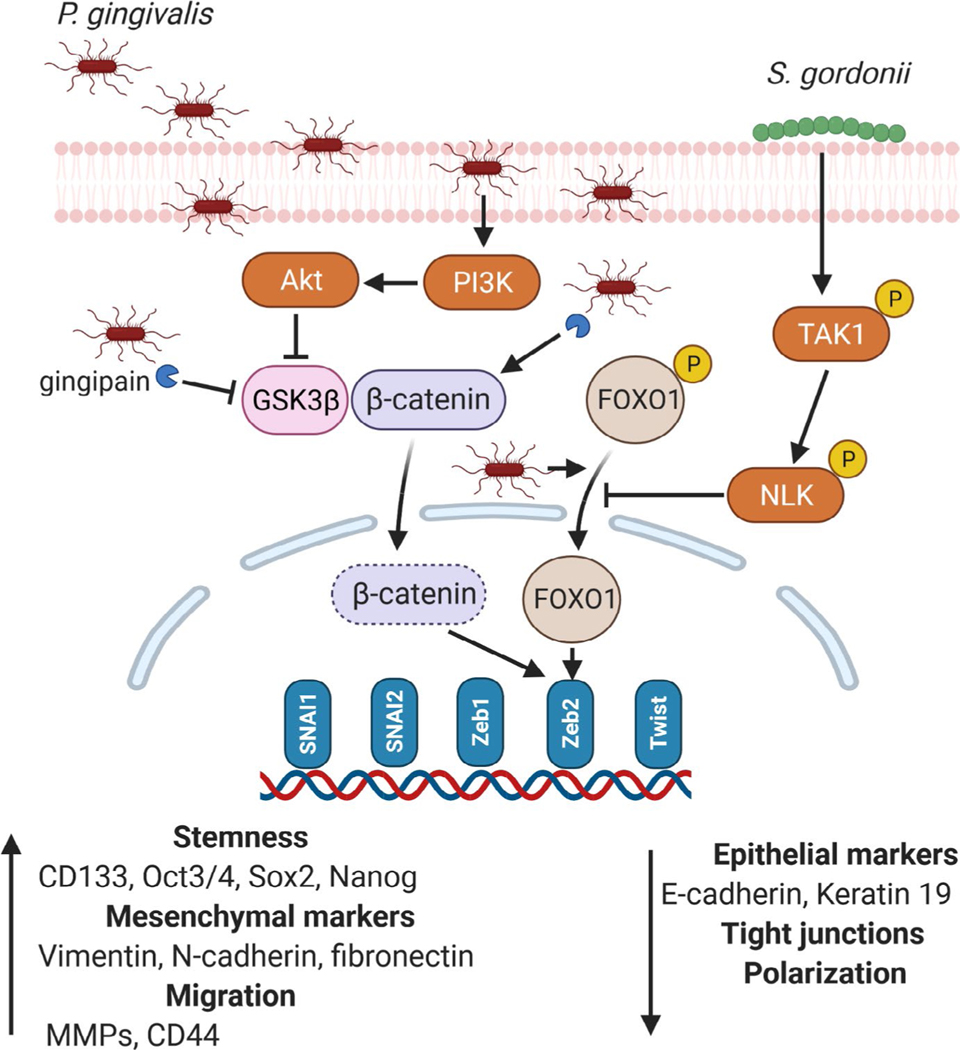
Another hallmark of tumor malignancy is metastasis, a process by which cancer cells spread throughout the body. Early events in metastasis involve the epithelial-mesenchymal transition, whereby epithelial cells lose tight junctions and polarization, and acquire mesenchymal properties including motility and a cancer stem cell–like phenotype that is capable of seeding new tumors.74 Epithelial-mesenchymal transition is a component of normal processes such as embryogenesis and wound healing and is controlled by a complex regulatory network that converges on a series of transcription factors, such as Zinc Finger E-box Binding Homeobox 1 and 2, Snail Family Transcriptional Repressors 1 and 2, and Twist. Originally considered a binary condition, epithelial-mesenchymal transition is now thought to represent a spectrum of states through which cells can transition in either direction.75,76 P. gingivalis shows a remarkable ability to impact the activity of the pathways that control epithelial-mesenchymal transition and induce at least a partial shift toward the mesenchymal state29,72,77–79 (Figure 2). Zinc Finger E-box Binding Homeobox 1 is elevated through FimA-dependent signaling, whereas Zinc Finger E-box Binding Homeobox 2 is regulated by gingipain processing and activation of beta-catenin, along with dephosphorylation and activation of Forkhead Box O1.29,79 P. gingivalis infection of epithelial cells also increases cancer stem cell markers, such as CD44 and CD133, and enhances migration.29,72,77–79 Migration and metastasis of epithelial cells can be facilitated by host matrix metalloproteinase enzymes, which degrade extracellular matrix and basement components. P. gingivalis can increase production of several matrix metalloproteinases, including matrix metalloproteinase-1, 2, 7, 9, and 10, from primary and transformed oral epithelial cells.29,72,78,80,81 Moreover, in invasive oral squamous cell carcinoma lines, P. gingivalis gingipains stimulate proteinase-activated receptors 2 and 4, which increases signaling through Extracellular signal-regulated kinase 1/2-Ets1, p38/Heat Shock Protein 27, and nuclear factor kappa B pathways, consequently elevating matrix metalloproteinase-9 proenzyme expression.80,82 Activation of matrix metalloproteinase-9 is then enhanced by gingipain processing. Infection of oral squamous cell carcinoma cells by P. gingivalis also elevates cell migration, as well as tumorsphere formation, through integrin alpha V/Focal Adhesion Kinase signaling.83 Epithelial-mesenchymal transition in esophageal squamous cell carcinoma cells is promoted by intracellular P. gingivalis, which induces the formation of transforming growth factor beta–dependent Smads–Yes1-associated transcriptional regulator–Tafazzin–TEA domain transcription factor 1 complexes. These protein aggregations orchestrate the activity of several transcription factors that promote epithelial-mesenchymal transition and stem-like traits.44 Furthermore, P. gingivalis can augment the secretion and bioactivity of transforming growth factor beta through upregulation of its surface docking receptor, GARP.44
FIGURE 2.
Impact of Porphyromonas gingivalis on pathways associated with epithelial-mesenchymal transition and the mitigating effect of Streptococcus gordonii. Transcription factors upregulated by P. gingivalis are shown, although the pathways have not been defined in all cases. MMPs, metalloproteinases. P indicates phosphorylation
5 |. ANGIOGENESIS
Developing tumors require a blood supply to provide nutrients. Additionally, to metastasize, tumors need a route to enter the circulation, and this is provided by tumor blood vessels. Thus, angiogenesis is necessary for both tumor development and metastasis. Interleukin-1beta (IL-1β) and tumor necrosis factor, which are secreted by epithelial cells in response to P. gingivalis,70,84–86 are proangiogenic. IL-1β activates endothelial cells to produce vascular endothelial growth factor, and tumor necrosis factor contributes to an angiogenic microenvironment.87 Lipopolysaccharide from P. gingivalis also increases production of Angiopoietin-like Protein 2 from transformed gingival epithelial cells.88 Angiopoietin-like Protein 2 can induce angiogenesis and also increase IL-1β and tumor necrosis factor production through an autocrine loop. P. gingivalis lipopolysaccharide evokes angiogenic responses directly in endothelial cells by activating the mitogen-activated protein kinase Extracellular signal-regulated kinase 1/2.89 Proangiogenic outcomes will be reinforced by lower levels of the angiostatic cytokines CXC motif ligands 9, 10, and 11,90 which P. gingivalis suppresses transcriptionally through downregulation of interferon regulatory factor 1 and reducing the amounts of Stat1.91 Angiogenic properties are also exhibited by the GroEL protein of P. gingivalis, which stimulates endothelial nitric oxide synthase production and p38 mitogen-activated protein kinase signaling. Consequently, GroEL enhances migration of endothelial progenitor cells and promotes angiogenesis and tumor growth in animal models.92
6 |. INFLAMMATION
In the periodontal space, inflammation is an important ecologic determinant that releases nutrients through the destruction of host tissue. P. gingivalis is an inflammophilic organism that utilizes these nutrients for growth.93 Interestingly, to avoid adverse effects of inflammation, P. gingivalis can selectively suppress bactericidal aspects, thus creating a dysbiotic inflammatory microenvironment.8,94 Uncontrolled inflammation is also considered a major driver of tumorigenesis,90 as inflammation can disrupt stromal integrity, and cytokine-promoted proliferation will facilitate tumor growth.95 Hence, an inability to resolve inflammation provides a mechanistic link between periodontitis and carcinogenesis. Various cytokines and CXC family chemokines have been reported to increase oral squamous cell carcinoma growth and increase cancer cell migration.96,97 Serum levels of interleukin-6 (IL-6) are higher in oral squamous cell carcinoma patients and associated with a worse prognosis,98 and CXC motif ligand 8 (interleukin-8) is increased in the saliva of patients with oral squamous cell carcinoma.99 Of note, epithelial cells possess pathways to constrain cytokine production and maintain homeostasis. For example, A20 (TNFAIP3), a ubiquitin-editing enzyme, dampens IL-6 and CXC motif ligand 8 production through modulation of nuclear factor kappa B activity.100 Moreover, the serine phosphatase SerB of P. gingivalis antagonizes production of CXC motif ligand 8 from primary gingival epithelial cells through dephosphorylation of the p65 subunit of nuclear factor kappa B.101 Similar restraint mechanisms are also observed in innate immune cells. P. gingivalis activation of Janus kinase 3 curtails production of IL-6 and tumor necrosis factor through ubiquitination dependent Wnt3 degradation.102 Nonetheless, despite the presence of both host and bacterial mechanisms to dampen cytokine/chemokine production, once cells have become transformed, P. gingivalis stimulates secretion of IL-6 and CXC motif ligand 8.103 In addition to canonical activation of pattern-recognition receptors, cytokines/chemokines can be stimulated by P. gingivalis through modulation of microRNA expression. For example, P. gingivalis can suppress mi-205–5p production, which promotes cytokine synthesis through the Janus kinase/Signal Transducer and Activator of Transcription pathway.104 The implications of increased cytokine levels are many. IL-6 and CXC motif ligand 8 can increase matrix metalloproteinase levels and cell invasiveness, as well as modulate expression of genes involved in regulation of the cell cycle and apoptosis.90,105 CXC motif ligand 8 can stimulate proliferation through transactivation of the epidermal growth factor receptor.106 Epidermal growth factor itself can also be induced by P. gingivalis, which will contribute to the induction of epithelial-mesenchymal transition, as will transforming growth factor beta 1 and tumor necrosis factor, which are also upregulated by P. gingivalis.77,107
The interleukin-23/interleukin-17 axis, which regulates homeostasis in the periodontium,108 is strongly protumorigenic, at least in colorectal cancer.109 P. gingivalis can incite production of these cytokines,110,111 although the carcinogenic potential has yet to be investigated.
Programmed death-ligand 1 (B7-H1, CD274) plays a crucial role in the control of T-cell function and survival. Programmed death-ligand 1 expression is upregulated in invasive oral squamous cell carcinoma cells,112 and tissue samples of oral squamous cell carcinoma express both programmed death-ligand 1 and another ligand of the programmed death receptor programmed death-1, namely B7-DC.113 In both oral squamous cell carcinoma cell lines and primary gingival epithelial cells, P. gingivalis can upregulate programmed death-1 and B7-DC.114 Mechanistically, peptidoglycan from P. gingivalis, packaged in outer membrane vesicles, is internalized by host cells and induces ligand expression by activating serine/threonine kinase RIP2–dependent signaling.115 Enhanced programmed death-ligand 1 and B7-DC expression may lead to anergy and apoptosis of activated T-cells and contribute to the resistance of tumor cells to host immune responses.114
Immunoevasion can also be promoted by P. gingivalis through modulation of macrophage activity. P. gingivalis inhibits phagocytosis of oral squamous cell carcinoma cells by macrophages, and in an animal model increases the relative amount of protumorigenic M2 macrophages in the tumor-associated macrophage population.116 Additionally, secretion of the tumor-enhancing molecules interleukin-1alpha, CC chemokine ligand 3, and chemokine ligand 5 is elevated in macrophages challenged with P. gingivalis. The ability of P. gingivalis to modulate macrophage function may depend on the amount of sphingolipid in the bacterial membrane.117 P. gingivalis also disrupts immune surveillance by generating myeloid-derived dendritic suppressor cells, which functionally resemble myeloid-derived suppressor cells associated with oncogenesis. Through interaction with Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin, the Mfa1-component fimbriae of P. gingivalis mediate invasion into monocytes, which promotes differentiation to apoptosis-resistant indoleamine 2,3-dioxygenase–competent myeloid-derived dendritic suppressor cells. These myeloid-derived dendritic suppressor cells induce immune tolerance through increased FOXP3+ Treg responses.57
7 |. TOXIC METABOLITES
P. gingivalis secretes a variety of metabolic end products as a result of its asaccharolytic metabolism; however, study of the carcinogenic potential of these is scant. Volatile sulfur compounds, such as hydrogen sulfide, methyl mercaptan, dimethyl sulfide, and dimethyl disulfide, are cytotoxic, and hydrogen sulfide in particular may also be genotoxic and stimulate cell proliferation.118 Short-chain fatty acids, such as butyrate and propionate, are produced in abundance by P. gingivalis and influence the physiology of epithelial and immune cells through serving as energy sources.119,120 Hence, an imbalance in the levels of short-chain fatty acids in the tumor microenvironment has the potential to impact cell proliferation and differentiation; however, the matter requires experimental investigation. Butyric acid produced by P. gingivalis can contribute to activation of the Epstein-Barr virus lytic cycle. Butyric acid inhibits histone deacetylases, thus increasing histone acetylation and the transcriptional activity of the Epstein-Barr virus BZLF1 gene, which encodes ZEBRA, a master regulator of the transition from latency to the lytic replication cycle.121
8 |. IN VIVO STUDIES
In vivo studies support the carcinogenic potential of P. gingivalis. In the 4-nitroquinoline-1-oxide tongue squamous cell carcinoma model, P. gingivalis increased both the size and the number of tumors.122 The development of carcinomas was associated with enhanced free fatty acid production, both in the tongue and in the serum of 4-nitroquinoline-1-oxide–treated mice, a shift that can also be observed in oral squamous cell carcinoma.122 Promotion of oral squamous cell carcinoma progression by P. gingivalis in the 4-nitroquinoline-1-oxide model has been corroborated in an independent study, which further showed P. gingivalis invasion increased infiltration of oral lesions with immunosuppressive CD11b+ myeloid cells and myeloid-derived suppressor cells.123 In a subcutaneous transplantation model of oral squamous cell carcinoma in mice, repeated infection with penicillin/streptomycin-treated P. gingivalis resulted in an increase in tumor growth and volume.116 Similarly, in a murine floor-of-mouth model, mice injected with P. gingivalis–challenged oral squamous cell carcinoma cells exhibited a greater tumor burden.83 Oral infection of conventional mice has been found to enhance Zinc Finger E-box Binding Homeobox 1 levels in gingival tissues, indicating the potential for P. gingivalis to initiate epithelial-mesenchymal transition in vivo.29
9 |. COMMUNITY ACTION
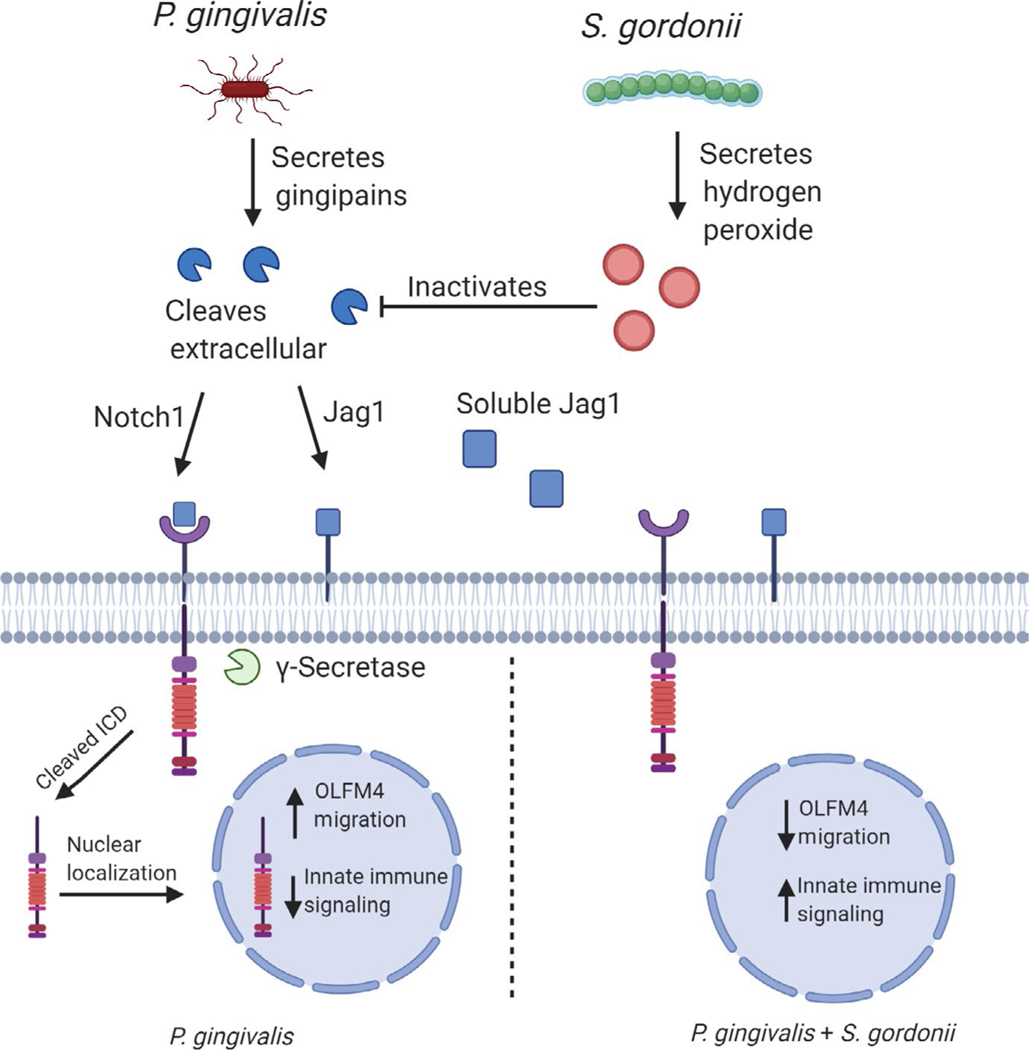
Similar to periodontal diseases, there is a diverse and complex microbial community associated with oral squamous cell carcinoma and esophageal squamous cell carcinoma.16,22,24,27 Bacteria in communities communicate with one another and exchange nutritional substrates to optimize physiology and metabolism. Functional specialization among community participants leads to codependence, and the community, rather than individual species, emerges as the pathogenic unit. Host responses to bacterial communities, including epithelial derived, are clearly distinct from responses to individual constituent organisms.85,124,125 Community pathogenicity, or nososymbiocity, is well studied in periodontal disease, and similar concepts appear applicable to the microbial involvement in tumorigenesis.14,35,126 As documented herein, P. gingivalis clearly has the potential to impinge upon the host in a manner consistent with a carcinogenic outcome. What, then, of the ability of other community participants to modulate the action of P. gingivalis? Synergistic interactions have been demonstrated in the 4-nitroquinoline-1-oxide model, in which coinfection with F. nucleatum and P. gingivalis promotes cancer progression.127 The ability of organisms, such as many oral streptococcal and Neisseria species, to generate the carcinogen acetaldehyde from alcohol could also enhance the tumorigenic potential of heterotypic communities.128,129 On the other hand, interactions with oral streptococci may mitigate the tumorigenic potential of P. gingivalis. S. gordonii can prevent P. gingivalis–induced gingival epithelial cell proliferation,94 and through suppression of P. gingivalis–induced Zinc Finger E-box Binding Homeobox 2 upregulation79 (Figure 2). Mechanistically, S. gordonii activates the TAK1-NLK pathway, which negatively regulates Forkhead Box O1–dependent regulation of Zinc Finger E-box Binding Homeobox 2. Indeed, coinfection of gingival epithelial cells with S. gordonii overrides much of the transcriptional program differentially regulated by P. gingivalis and returns the cells closer to the homeostatic state.130,131 One of the major pathways transcriptionally induced by P. gingivalis, but suppressed in a dual-species context with S. gordonii, is the Notch signaling pathway.131 Activation of Notch signaling proceeds through increased expression of the Notch1 receptor and the Jagged1 agonist. Following Jagged1-Notch1 engagement, the Notch1 extracellular domain is cleaved by P. gingivalis gingipain proteases to activate signaling. The Notch downstream effector olfactomedin 4, a prosurvival glycoprotein,132 participates in epithelial cell migratory, proliferative, and inflammatory responses to P. gingivalis. Olfactomedin 4 has relevance in the oral cavity, as it accumulates in the secretome of head and neck squamous cell carcinomas and is a potential biomarker for the disease.133 Antagonism by S. gordonii involves inhibition of gingipain activity by secreted hydrogen peroxide, which prevents cleavage of the Notch1 extracellular domain (Figure 3). Such abilities position S. gordonii, and potentially other peroxide-producing streptococci, as a homeostatic commensal in tumorigenesis, an organism that functions to maintain eubiosis.94 It is interesting to note that in periodontitis S. gordonii has properties of an accessory pathogen, an organism that enhances the virulence of more overtly pathogenic species,134,135 reinforcing the importance of ecologic context in the pathogenic potential of an organism or community. Antagonism between oral streptococci and P. gingivalis is consistent with many of the in vivo studies showing a trend of reduced streptococci and elevated P. gingivalis associated with tumors. However, as with periodontal disease, it is difficult to discern whether disease results from the relative abundance of the organism or if the relative abundance of the organism reflects its competitiveness in the disease microenvironment.
FIGURE 3.
Interplay between Porphyromonas gingivalis and Streptococcus gordonii in the activation of Notch1 signaling and upregulation of olfactomedin 4 (OLFM4). Activation of Notch signaling is induced through increased expression of the Notch1 receptor and the Jagged1 (Jag1) agonist. In addition, Jagged1 is released in response to P. gingivalis, leading to paracrine activation. Following Jagged1-Notch1 engagement, the Notch1 extracellular domain is cleaved by P. gingivalis gingipain proteases. Antagonism by S. gordonii involves inhibition of gingipain activity by secreted hydrogen peroxide. Olfactomedin 4 is involved in epithelial cell migratory, proliferative and inflammatory responses to P. gingivalis. ICD: intracellular domain
One characteristic of the “community as pathogen” model is that the identities of constituent bacteria are less important than the functions provided by the metagenome. Study of the metatranscriptome of human oral squamous cell carcinoma tumors found microbial metabolic activities such as iron transport, tryptophanase activity, peptidase activities, and superoxide dismutase were better correlated with disease than was community composition.136 In support of this concept, a comparison of microbiotas associated with oral squamous cell carcinoma in different countries revealed functional rather than compositional similarities.137 Moreover, studies of the microbiota in the 4-nitroquinoline-1-oxide model show consistent patterns of metabolic signatures associated with disease.138 Genes associated with bacterial chemotaxis, flagellar assembly, and lipopolysaccharide biosynthesis in particular have been correlated with oral squamous cell carcinoma,33,34,137,139 and lipopolysaccharide biosynthesis genes are also enriched in gingival squamous cell carcinoma patients.
10 |. CULPRITS OR INNOCENT BYSTANDERS
The etiology of cancer is multifactorial, involving multiple genetic and environmental predisposing factors, which may operate in a temporally defined manner. Defining the contribution of any individual factor is thus fraught with difficulty. Deciphering the role of bacteria becomes even more complicated when incorporating the notion that heterotypic communities operate as a functional unit.140 Nonetheless, compelling models that accommodate the existing data are being developed. Al-Hebshi et al27 have proposed the “Passenger-Turning-Driver” microbiome model for oral squamous cell carcinoma. This holds that the oral microbiome is not involved in the initiation of disease. Rather, the nature of the tumor microbiome is a “passenger” event resultant from selection within the tumor microenvironment. As this competitively fit microbiome develops, there is increased expression of proinflammatory components and a transition to a dysbiotic, or “driver,” state that enhances tumorigenesis by sustaining chronic inflammation. A related model, which has also been applied to colorectal cancer,126 derives from the polymicrobial synergy and dysbiosis model of periodontal disease.141 Polymicrobial synergy and dysbiosis posits that driver mutations in host cells begin to establish a tumor microenvironment that selects for a microbial community that can vary among sites. In instances where there is a relative decrease in homeostatic commensals, such as S. gordonii, and a relative overabundance of gram-negative anaerobes, such as P. gingivalis, the community will have tumorigenic potential. Suppression of programmed cell death, stimulation of uncontrolled epithelial cell proliferation, along with a more mesenchymal, migratory phenotype all contribute to tumor development. Dysbiotic inflammation further contributes to oncogenesis while also sustaining colonization by inflammophilic organisms such as P. gingivalis through a reciprocating feed-forward loop. In terms of the passenger/driver metaphor, this may be characterized as the “hitchhiker-turned-car-jacker” model. These models have several features in common, and both may be operational in different contexts.
11 |. CONCLUSIONS
What was once considered at best fanciful and at worst “fake science” is now undeniable: bacteria such as P. gingivalis have a role to play in certain cancers, particularly those of the oral cavity and orodigestive region. The nature of that role requires further research, including large longitudinal and intervention studies. In one sense, however, it is immaterial whether certain bacteria have a causal role or simply have a competitive advantage in the tumor microenvironment. These cancers are often only detected at advanced stages; therefore, any consistent associations occurring between the microbiome/metatranscriptome and disease may provide a foundation for discovering novel targets for early detection and diagnosis.
Funding information
NIH, Grant/Award Number: DE011111, DE012505, DE017921, DE023193, DE028166, DE026727.
REFERENCES
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Cao M, Iduma P, Karachaliou N, Santarpia M, Blanco J, Rosell R. Human endogenous retroviruses and cancer. Cancer Biol Med. 2016;13:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. [DOI] [PubMed] [Google Scholar]
- 4.Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013;34:2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heikkilä P, But A, Sorsa T, Haukka J. Periodontitis and cancer mortality: register-based cohort study of 68,273 adults in 10-year follow-up. Int J Cancer. 2018;142:2244–2253. [DOI] [PubMed] [Google Scholar]
- 6.Michaud DS, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atanasova KR, Yilmaz O. Looking in the Porphyromonas gingivalis cabinet of curiosities: the microbium, the host and cancer association. Mol Oral Microbiol. 2014;29:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinane DF, Galicia JC, Gorr SU, Stathopoulou PG, Benakanakere M P. gingivalis interactions with epithelial cells. Front Biosci. 2008;13:966–984. [DOI] [PubMed] [Google Scholar]
- 11.Cugini C, Klepac-Ceraj V, Rackaityte E, Riggs JE, Davey ME Porphyromonas gingivalis: keeping the pathos out of the biont. J Oral Microbiol. 2013;5:19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA Cancer J Clin. 2015;65:401–421. [DOI] [PubMed] [Google Scholar]
- 13.Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol. 2010;46:414–417. [DOI] [PubMed] [Google Scholar]
- 14.Bornigen D, Ren B, Pickard R, et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci Rep. 2017;7:17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganly I, Yang L, Giese RA, et al. Periodontal pathogens are a risk factor of oral cavity squamous cell carcinoma, independent of tobacco and alcohol and human papillomavirus. Int J Cancer. 2019;145:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perera M, Al-Hebshi NN, Speicher DJ, Perera I, Johnson NW. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J Oral Microbiol. 2016;8:32762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Tan X, Zhao X, et al. Composition and function of oral microbiota between gingival squamous cell carcinoma and periodontitis. Oral Oncol. 2020;107:104710. [DOI] [PubMed] [Google Scholar]
- 18.Seoane J, Varela-Centelles PI, Walsh TF, Lopez-Cedrun JL, Vazquez I. Gingival squamous cell carcinoma: diagnostic delay or rapid invasion? J Periodontol. 2006;77:1229–1233. [DOI] [PubMed] [Google Scholar]
- 19.Shin YJ, Choung HW, Lee JH, Rhyu IC, Kim HD. Association of periodontitis with oral cancer: a case-control study. J Dent Res. 2019;98:526–533. [DOI] [PubMed] [Google Scholar]
- 20.Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao QW, Zhou DS, Peng HJ, Ji P, Liu DS. Association of periodontal disease with oral cancer: a meta-analysis. Tumour Biol. 2014;35:7073–7077. [DOI] [PubMed] [Google Scholar]
- 22.Fitzsimonds ZR, Rodriguez-Hernandez CJ, Bagaitkar J, Lamont RJ. From beyond the pale to the pale riders: the emerging association of bacteria with oral cancer. J Dent Res. 2020;99:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irfan M, Delgado RZR, Frias-Lopez J. The oral microbiome and cancer. Front Immunol. 2020;11:591088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Hu Y, Zhou X, Liu S, Han Q, Cheng L. Role of oral bacteria in the development of oral squamous cell carcinoma. Cancers. 2020;12:2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Rosa GRM, Gattuso G, Pedulla E, Rapisarda E, Nicolosi D, Salmeri M. Association of oral dysbiosis with oral cancer development. Oncol Lett. 2020;19:3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Healy CM, Moran GP. The microbiome and oral cancer: more questions than answers. Oral Oncol. 2019;89:30–33. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hebshi NN, Borgnakke WS, Johnson NW. The microbiome of oral squamous cell carcinomas: a functional perspective. Curr Oral Health Rep. 2019;6:145–160. [Google Scholar]
- 28.Baraniya D, Jain V, Lucarelli R, et al. Screening of health-associated oral bacteria for anticancer properties in vitro. Front Cell Infect Microbiol. 2020;10:575656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sztukowska MN, Ojo A, Ahmed S, et al. Porphyromonas gingivalis initiates a mesenchymal-like transition through ZEB1 in gingival epithelial cells. Cell Microbiol. 2016;18:844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C, Geng F, Shi X, et al. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl Microbiol Biotechnol. 2019;103:1393–1404. [DOI] [PubMed] [Google Scholar]
- 31.Yang CY, Yeh YM, Yu HY, et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front Microbiol. 2018;9:862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt BL, Kuczynski J, Bhattacharya A, et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS One. 2014;9:e98741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Hebshi NN, Nasher AT, Maryoud MY, et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Liu Y, Zheng HJ, Zhang CP. The oral microbiota may have influence on oral cancer. Front Cell Infect Microbiol. 2019;9:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin JM, Luo T, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Microbial communities associated with primary and metastatic head and neck squamous cell carcinoma—a high fusobacterial and low streptococcal signature. Sci Rep. 2017;7:9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooper SJ, Crean SJ, Lewis MA, Spratt DA, Wade WG, Wilson MJ. Viable bacteria present within oral squamous cell carcinoma tissue. J Clin Microbiol. 2006;44:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rai AK, Panda M, Das AK, et al. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch Microbiol. 2021;203:137–152. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Winckler B, Lu M, et al. Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PLoS One. 2015;10:e0143603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017;77:6777–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu G, Gail MH, Shi J, et al. Association between upper digestive tract microbiota and cancer-predisposing states in the esophagus and stomach. Cancer Epidemiol Biomarkers Prev. 2014;23:735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A. 2004;101:4250–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao S, Li S, Ma Z, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer. 2016;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo ZC, Jumatai S, Jing SL, Hu LL, Jia XY, Gong ZC. Bioinformatics and immunohistochemistry analyses of expression levels and clinical significance of CXCL2 and TANs in an oral squamous cell carcinoma tumor microenvironment of Prophyromonas gingivalis infection. Oncol Lett. 2021;21:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi YJ, Jiao YL, Chen P, et al. Porphyromonas gingivalis promotes progression of esophageal squamous cell cancer via TGFβ-dependent Smad/YAP/TAZ signaling. PLoS Biol. 2020;18:e3000825. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Chen MF, Lu MS, Hsieh CC, Chen WC Porphyromonas gingivalis promotes tumor progression in esophageal squamous cell carcinoma. Cell Oncol (Dordr). 2021;44:373–384. [DOI] [PubMed] [Google Scholar]
- 46.Gao S, Liu Y, Duan X, et al. Porphyromonas gingivalis infection exacerbates oesophageal cancer and promotes resistance to neoadjuvant chemotherapy. Br J Cancer. 2021;125(3):433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garrett WS. Cancer and the microbiota. Science. 2015;348:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Handfield M, Mans JJ, Zheng G, et al. Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell Microbiol. 2005;7:811–823. [DOI] [PubMed] [Google Scholar]
- 49.Geng F, Liu J, Guo Y, et al. Persistent exposure to Porphyromonas gingivalis promotes proliferative and invasion capabilities, and tumorigenic properties of human immortalized oral epithelial cells. Front Cell Infect Microbiol. 2017;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D, Hou J, Wu Y, et al. Distinct gene expression characteristics in epithelial cell-Porphyromonas gingivalis interactions by integrating transcriptome analyses. Int J Med Sci. 2019;16:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mans JJ, Lamont RJ, Handfield M. Microarray analysis of human epithelial cell responses to bacterial interaction. Infect Disord Drug Targets. 2006;6:299–309. [DOI] [PubMed] [Google Scholar]
- 52.Kuboniwa M, Hasegawa Y, Mao S, et al. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008;10:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Attar A, Alimova Y, Kirakodu S, et al. Activation of Notch-1 in oral epithelial cells by P. gingivalis triggers the expression of the antimicrobial protein PLA2-IIA. Mucosal Immunol. 2018;11:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Sztukowska M, Wang Q, et al. Noncanonical activation of β-catenin by Porphyromonas gingivalis. Infect Immun. 2015;83:3195–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou L, Dorfer CE, Chen L, Fawzy El-Sayed KM Porphyromonas gingivalis lipopolysaccharides affect gingival stem/progenitor cells attributes through NF-κB, but not Wnt/β-catenin, pathway. J Clin Periodontol. 2017;44:1112–1122. [DOI] [PubMed] [Google Scholar]
- 56.Chang C, Wang H, Liu J, et al. Porphyromonas gingivalis infection promoted the proliferation of oral squamous cell carcinoma cells through the miR-21/PDCD4/AP-1 negative signaling pathway. ACS Infect Dis. 2019;5:1336–1347. [DOI] [PubMed] [Google Scholar]
- 57.Arjunan P, Meghil MM, Pi W, et al. Oral pathobiont activates anti-apoptotic pathway, promoting both immune suppression and oncogenic cell proliferation. Sci Rep. 2018;8:16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan C, Xu X, Tan L, Lin L, Pan Y. The effects of Porphyromonas gingivalis on the cell cycle progression of human gingival epithelial cells. Oral Dis. 2014;20:100–108. [DOI] [PubMed] [Google Scholar]
- 59.Hoppe T, Kraus D, Novak N, et al. Oral pathogens change proliferation properties of oral tumor cells by affecting gene expression of human defensins. Tumour Biol. 2016;37:13789–13798. [DOI] [PubMed] [Google Scholar]
- 60.Liang G, Wang H, Shi H, et al. Porphyromonas gingivalis promotes the proliferation and migration of esophageal squamous cell carcinoma through the miR-194/GRHL3/PTEN/Akt axis. ACS Infect Dis. 2020;6:871–881. [DOI] [PubMed] [Google Scholar]
- 61.Meng F, Li R, Ma L, et al. Porphyromonas gingivalis promotes the motility of esophageal squamous cell carcinoma by activating NF-κB signaling pathway. Microbes Infect. 2019;21:296–304. [DOI] [PubMed] [Google Scholar]
- 62.Farrow SN, Brown R. New members of the Bcl-2 family and their protein partners. Curr Opin Genet Dev. 1996;6:45–49. [DOI] [PubMed] [Google Scholar]
- 63.Yilmaz O, Yao L, Maeda K, et al. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee J, Roberts JS, Atanasova KR, Chowdhury N, Yilmaz O. A novel kinase function of a nucleoside-diphosphate-kinase homologue in Porphyromonas gingivalis is critical in subversion of host cell apoptosis by targeting heat-shock protein 27. Cell Microbiol. 2018;20:e12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao L, Jermanus C, Barbetta B, et al. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol. 2010;25:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakhjiri SF, Park Y, Yilmaz O, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. [DOI] [PubMed] [Google Scholar]
- 69.Moffatt CE, Lamont RJ Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Q, Sztukowska M, Ojo A, Scott DA, Wang H, Lamont RJ. FOXO responses to Porphyromonas gingivalis in epithelial cells. Cell Microbiol. 2015;17:1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoppe T, Kraus D, Probstmeier R, Jepsen S, Winter J. Stimulation with Porphyromonas gingivalis enhances malignancy and initiates anoikis resistance in immortalized oral keratinocytes. J Cell Physiol. 2019;234:21903–21914. [DOI] [PubMed] [Google Scholar]
- 72.Ha NH, Woo BH, Kim DJ, et al. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015;36:9947–9960. [DOI] [PubMed] [Google Scholar]
- 73.Woo BH, Kim DJ, Choi JI, et al. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to taxol and have higher metastatic potential. Oncotarget. 2017;8:46981–46992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gooding AJ, Schiemann WP. Epithelial-mesenchymal transition programs and cancer stem cell phenotypes: mediators of breast cancer therapy resistance. Mol Cancer Res. 2020;18(9):1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jolly MK, Ward C, Eapen MS, et al. Epithelial-mesenchymal transition, a spectrum of states: role in lung development, homeostasis, and disease. Dev Dyn. 2017;247:346–358. [DOI] [PubMed] [Google Scholar]
- 77.Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. J Periodontal Res. 2018;53:565–574. [DOI] [PubMed] [Google Scholar]
- 78.Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz O. Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic pathogen, Porphyromonas gingivalis. Front Cell Infect Microbiol. 2017;7:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohshima J, Wang Q, Fitzsimonds ZR, et al. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc Natl Acad Sci U S A. 2019;116:8544–8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inaba H, Sugita H, Kuboniwa M, et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16:131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ha NH, Park DG, Woo BH, et al. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine. 2016;86:64–72. [DOI] [PubMed] [Google Scholar]
- 82.Inaba H, Amano A, Lamont RJ, Murakami Y. Involvement of protease-activated receptor 4 in over-expression of matrix metalloproteinase 9 induced by Porphyromonas gingivalis. Med Microbiol Immunol. 2015;204:605–612. [DOI] [PubMed] [Google Scholar]
- 83.Kamarajan P, Ateia I, Shin JM, et al. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020;16:e1008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Groeger S, Meyle J. Oral mucosal epithelial cells. Front Immunol. 2019;10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramage G, Lappin DF, Millhouse E, et al. The epithelial cell response to health and disease associated oral biofilm models. J Periodontal Res. 2017;52:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milward MR, Chapple IL, Wright HJ, Millard JL, Matthews JB, Cooper PR. Differential activation of NF-κB and gene expression in oral epithelial cells by periodontal pathogens. Clin Exp Immunol. 2007;148:307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carmi Y, Dotan S, Rider P, et al. The role of IL-1β in the early tumor cell-induced angiogenic response. J Immunol. 2013;190:3500–3509. [DOI] [PubMed] [Google Scholar]
- 88.Ohno T, Yamamoto G, Hayashi JI, et al. Angiopoietin-like protein 2 regulates Porphyromonas gingivalis lipopolysaccharide-induced inflammatory response in human gingival epithelial cells. PLoS One. 2017;12:e0184825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koo TH, Jun HO, Bae SK, et al. Porphyromonas gingivalis, periodontal pathogen, lipopolysaccharide induces angiogenesis via extracellular signal-regulated kinase 1/2 activation in human vascular endothelial cells. Arch Pharm Res. 2007;30:34–42. [DOI] [PubMed] [Google Scholar]
- 90.Sahingur SE, Yeudall WA. Chemokine function in periodontal disease and oral cavity cancer. Front Immunol. 2015;6:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun. 2013;81:2288–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin FY, Huang CY, Lu HY, et al. The GroEL protein of Porphyromonas gingivalis accelerates tumor growth by enhancing endothelial progenitor cell function and neovascularization. Mol Oral Microbiol. 2015;30:198–216. [DOI] [PubMed] [Google Scholar]
- 93.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Metzner B, Hofmann C, Heinemann C, et al. Overexpression of CXC-chemokines and CXC-chemokine receptor type II constitute an autocrine growth mechanism in the epidermoid carcinoma cells KB and A431. Oncol Rep. 1999;6:1405–1410. [DOI] [PubMed] [Google Scholar]
- 97.Christofakis EP, Miyazaki H, Rubink DS, Yeudall WA. Roles of CXCL8 in squamous cell carcinoma proliferation and migration. Oral Oncol. 2008;44:920–926. [DOI] [PubMed] [Google Scholar]
- 98.Park DG, Woo BH, Lee BJ, et al. Serum levels of interleukin-6 and titers of antibodies against Porphyromonas gingivalis could be potential biomarkers for the diagnosis of oral squamous cell carcinoma. Int J Mol Sci. 2019;20:2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.St John MA, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–935. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Mooney EC, Xia XJ, Gupta N, Sahingur SE. A20 restricts inflammatory response and desensitizes gingival keratinocytes to apoptosis. Front Immunol. 2020;11:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takeuchi H, Hirano T, Whitmore SE, Morisaki I, Amano A, Lamont RJ. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-κB RelA/p65. PLoS Pathog. 2013;9:e1003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu L, Yakoumatos L, Ren J, et al. JAK3 restrains inflammatory responses and protects against periodontal disease through Wnt3a signaling. FASEB J. 2020;34:9120–9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yee M, Kim S, Sethi P, Duzgunes N, Konopka K Porphyromonas gingivalis stimulates IL-6 and IL-8 secretion in GMSm-K, HSC-3 and H413 oral epithelial cells. Anaerobe. 2014;28:62–67. [DOI] [PubMed] [Google Scholar]
- 104.Li J, Li L, Wang X, Xiao L Porphyromonas gingivalis inhibition of microRNA-205–5p expression modulates proinflammatory cytokines in gingival epithelial cells. Biochem Genet. 2020;58(4):566–579. [DOI] [PubMed] [Google Scholar]
- 105.Karpinski TM. Role of oral microbiota in cancer development. Microorganisms. 2019;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joh T, Kataoka H, Tanida S, et al. Helicobacter pylori-stimulated interleukin-8 (IL-8) promotes cell proliferation through transactivation of epidermal growth factor receptor (EGFR) by disintegrin and metalloproteinase (ADAM) activation. Dig Dis Sci. 2005;50:2081–2089. [DOI] [PubMed] [Google Scholar]
- 107.Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adh Migr. 2018;12:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kajikawa T, Wang B, Li X, et al. Frontline science: activation of metabolic nuclear receptors restores periodontal tissue homeostasis in mice with leukocyte adhesion deficiency-1. J Leukoc Biol. 2020;108:1501–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glowczyk I, Wong A, Potempa B, et al. Inactive gingipains from P. gingivalis selectively skews T cells toward a TH17 phenotype in an IL-6 dependent manner. Front Cell Infect Microbiol. 2017;7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng WC, van Asten SD, Burns LA, et al. Periodontitis-associated pathogens P. gingivalis and A. actinomycetemcomitans activate human CD14+ monocytes leading to enhanced Th17/IL-17 responses. Eur J Immunol. 2016;46:2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirai M, Kitahara H, Kobayashi Y, et al. Regulation of PD-L1 expression in a high-grade invasive human oral squamous cell carcinoma microenvironment. Int J Oncol. 2017;50:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Groeger S, Howaldt HP, Raifer H, Gattenloehner S, Chakraborty T, Meyle J. Oral squamous carcinoma cells express B7-H1 and B7-DC receptors in vivo. Pathol Oncol Res. 2017;23:99–110. [DOI] [PubMed] [Google Scholar]
- 114.Groeger S, Domann E, Gonzales JR, Chakraborty T, Meyle J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology. 2011;216:1302–1310. [DOI] [PubMed] [Google Scholar]
- 115.Groeger S, Denter F, Lochnit G, Schmitz ML, Meyle J Porphyromonas gingivalis cell wall components induce programmed death ligand 1 (PD-L1) expression on human oral carcinoma cells by a receptor-interacting protein kinase 2 (RIP2)-dependent mechanism. Infect Immun. 2020;88:e00051-e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu S, Zhou X, Peng X, et al. Porphyromonas gingivalis promotes immunoevasion of oral cancer by protecting cancer from macrophage attack. J Immunol. 2020;205:282–289. [DOI] [PubMed] [Google Scholar]
- 117.Rocha FG, Moye ZD, Ottenberg G, et al. Porphyromonas gingivalis sphingolipid synthesis limits the host inflammatory response. J Dent Res. 2020;99:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen LH, Ma W, Wang DD, et al. Association between sulfur-metabolizing bacterial communities in stool and risk of distal colorectal cancer in men. Gastroenterology. 2020;158:1313–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care. 2010;13:715–721. [DOI] [PubMed] [Google Scholar]
- 120.Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-modulated metabolites at the interface of host immunity. J Immunol. 2017;198:572–580. [DOI] [PubMed] [Google Scholar]
- 121.Imai K, Inoue H, Tamura M, et al. The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie. 2012;94:839–846. [DOI] [PubMed] [Google Scholar]
- 122.Wu JS, Zheng M, Zhang M, et al. Porphyromonas gingivalis promotes 4-nitroquinoline-1-oxide-induced oral carcinogenesis with an alteration of fatty acid metabolism. Front Microbiol. 2018;9:2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wen L, Mu W, Lu H, et al. Porphyromonas gingivalis promotes oral squamous cell carcinoma progression in an immune microenvironment. J Dent Res. 2020;99:666–675. [DOI] [PubMed] [Google Scholar]
- 124.Bostanci N, Meier A, Guggenheim B, Belibasakis GN. Regulation of NLRP3 and AIM2 inflammasome gene expression levels in gingival fibroblasts by oral biofilms. Cell Immunol. 2011;270:88–93. [DOI] [PubMed] [Google Scholar]
- 125.Ebersole JL, Peyyala R, Gonzalez OA. Biofilm-induced profiles of immune response gene expression by oral epithelial cells. Mol Oral Microbiol. 2019;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flynn KJ, Baxter NT, Schloss PD. Metabolic and community synergy of oral bacteria in colorectal cancer. mSphere. 2016;1(3):e001 02–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Binder Gallimidi A, Fischman S, Revach B, et al. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6:22613–22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pavlova SI, Jin L, Gasparovich SR, Tao L. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology. 2013;159:1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muto M, Hitomi Y, Ohtsu A, et al. Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: implications for carcinogenesis in upper aerodigestive tract. Int J Cancer. 2000;88:342–350. [PubMed] [Google Scholar]
- 130.Mans JJ, von Lackum K, Dorsey C, et al. The degree of microbiome complexity influences the epithelial response to infection. BMC Genom. 2009;10:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fitzsimonds ZR, Liu C, Stocke KS, et al. Regulation of olfactomedin 4 by Porphyromonas gingivalis in a community context. ISME J. 2021;15(9):2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang X, Huang Q, Yang Z, Li Y, Li CY. GW112, a novel antiapoptotic protein that promotes tumor growth. Cancer Res. 2004;64:2474–2481. [DOI] [PubMed] [Google Scholar]
- 133.Marimuthu A, Chavan S, Sathe G, et al. Identification of head and neck squamous cell carcinoma biomarker candidates through proteomic analysis of cancer cell secretome. Biochim Biophys Acta. 2013;1834:2308–2316. [DOI] [PubMed] [Google Scholar]
- 134.Whitmore SE, Lamont RJ. The pathogenic persona of community-associated oral streptococci. Mol Microbiol. 2011;81:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuboniwa M, Houser JR, Hendrickson EL, et al. Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat Microbiol. 2017;2:1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yost S, Stashenko P, Choi Y, et al. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int J Oral Sci. 2018;10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Perera M, Al-Hebshi NN, Perera I, et al. Inflammatory bacteriome and oral squamous cell carcinoma. J Dent Res. 2018;97:725–732. [DOI] [PubMed] [Google Scholar]
- 138.Stashenko P, Yost S, Choi Y, et al. The oral mouse microbiome promotes tumorigenesis in oral squamous cell carcinoma. mSystems. 2019;4(4):e000329–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang SF, Huang HD, Fan WL, et al. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral Oncol. 2018;77:1–8. [DOI] [PubMed] [Google Scholar]
- 140.Hajishengallis G, Lamont RJ. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016;24:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]