Abstract
Physical activity (PA) is a primary non-pharmacological treatment option for those living with rheumatoid arthritis (RA) and spondyloarthritis (SpA). The aim of this systematic literature review was to summarize and present an updated synthesis of the factors associated with PA in the RA and SpA populations. A tailored search of PubMed (inc. Medline), Web of Science, Embase, APA PsycNET, and Scopus was conducted for research published between 2004 and June 2019. Methodological quality was assessed using The National Institutes of Health (NIH) Quality Assessment Tools for Observational Cohort and Cross-sectional Studies, Case–Control Studies, and Controlled Intervention Studies. Forty RA and eleven SpA articles met the inclusion criteria. Methodological quality was generally fair to good, with two RA studies rated as poor. Correlates are discussed in the sociodemographic, physical, psychological, social, and environmental categories. Environmental factors were not measured in any RA study. In individuals living with RA, consistent positive associations were found between PA and high-density lipoprotein, self-efficacy, and motivation. Consistent negative associations were found for functional disability and fatigue. In individuals with SpA, consistent positive associations were found between PA and quality of life, and consistent negative associations with functional disability. Physical and psychological factors are most consistently related with PA parameters in those living with RA and SpA. Many variables were inconsistently studied and showed indeterminant associations. Studies with prospective designs are needed to further understand the factors associated with PA in these populations, especially in those living with SpA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00296-022-05142-z.
Keywords: Physical activity, Correlate, Factor, Rheumatoid arthritis, Spondyloarthritis
Introduction
Spondyloarthritis (SpA) and rheumatoid arthritis (RA) are chronic inflammatory diseases which share the occurrence of fluctuating flares and stable disease activity [1–4]. Clinically, those with SpA (specifically axial spondyloarthritis) often suffer from inflammatory back pain, sacroiliitis, stiffness, and extreme fatigue [5], whereas those with RA often suffer from joint swelling, cartilage damage and synovial joint destruction [6]. Both inflammatory conditions cause disability and are associated with work challenges [7, 8] and reduced health-related quality of life [9]. The prevalence of SpA ranges from 0.20% in South-East Asia to 1.61% in Northern Arctic communities, whereas the prevalence of ankylosing spondylitis (AS) ranges from 0.02% in Sub-Saharan Africa to 0.35% in Northern Arctic communities [10]. In terms of RA, results of a meta-analyses of 67 studies showed the global prevalence to be 460 per 100,000 between the years 1980 to 2019 [11].
Progressive and beneficial pharmacological treatments for SpA and RA include non-steroidal anti-inflammatories, biologic and targeted synthetic disease-modifying antirheumatic drugs [12, 13]. However, in recent European League Against Rheumatism (EULAR) recommendations, physical activity (PA) has been proposed as an integral option for the non-pharmacological management of inflammatory arthritides [14]. The latest World Health Organizations (WHO) 2020 guidelines on physical activity and sedentary behaviour [15] are the same for healthy adults and those with chronic conditions. Here, the recommendations are that all adults should undertake at least 150 to 300 min of moderately intense aerobic PA or 75 to 150 min of vigorously intense PA, whilst also conducting muscle-strengthening PA at least 2 days per week on all major muscle groups. For adults aged 65 years and older, multicomponent PA focusing on strength training and functional balance is also recommended. Despite the recommendations, those with SpA and RA have been shown to be more sedentary and less physically active than their healthy counterparts [16, 17].
People living with SpA and RA receive similar benefits to their healthy counterparts from engaging in regular PA, including increased muscle strength and cardiovascular fitness [18]. However, supplementary benefits of PA in the SpA and RA population include a reduction in disease activity and inflammation [19, 20]. While the aforementioned studies demonstrate how PA interventions can improve general and disease-related health of individuals with RA and SpA, challenges arise both before and after the PA intervention itself. Adherence to PA after intervention is an example of one challenge in the SpA and RA populations [21, 22], yet this review will focus on the initial challenge of identifying the factors that associate with PA behaviour. Correlates of PA provide clinically relevant information that needs early consideration in intervention design to maximise the potential benefits of a PA intervention.
Despite a growing body of research on the correlates of PA, little research has collated the data in those with SpA. Wilcox et al. [23] initially evaluated the correlates of PA in people living with inflammatory arthritis, however, only two studies were identified that included SpA participants. In narrowing the focus to just people living with RA, a more recent review by Larkin and Kennedy [24] reported positive associations between PA and health perception, self-efficacy, motivation, and levels of previous PA. In addition, coerced regulation style, fatigue, and specific physiological variables were negatively associated with PA. Nevertheless, Larkin and Kennedy [24] concluded there were no definite correlates of PA, prompting the need for more research to aid clinical practitioners in supporting PA behaviour in those with RA. To address the current lack of synthesis of PA correlates in those with SpA and the advancing research utilizing objective measures of PA in inflammatory rheumatic conditions, an updated comprehensive review is needed to allow a better understanding of the factors related to PA in people living with SpA and RA. Therefore, the aim of the present work is to provide an updated synthesis of the factors most strongly associated with PA among people living with SpA and RA.
Methods
The protocol for this review was registered online with a prospective registry of systematic reviews (PROSPERO ID: CRD42019138993). Where possible, the ‘Preferred Reporting Items for Systematic Reviews and Meta-analyses’ (PRISMA) recommendations [25] were followed.
Search strategy
A systematic search was conducted using a tailored search strategy in the following databases: PubMed (inc. Medline), Web of Science, Embase, APA PsycNET, and Scopus. The search strategy was developed based on a preliminary search in PubMed to identify relevant terminology, search terms employed by similar reviews into correlates of PA in inflammatory arthritis, and discussions with a subject librarian and the research team. The databases were searched from 2004 to June 2019, to develop and further a review previously conducted by Larkin and Kennedy [24]. An example of the search strategy used is shown below. The search was restricted to articles with human participants and published in English. Additional eligible studies the author became aware of were included.
Example search strategy for PubMed (inc. Medline)
(Spondyloarthr* OR spondyloarthritis[mh] OR “ankylosing spondylitis*” OR ankylosing spondylitis[mh] OR “rheumatoid arthritis” OR rheumatoid arthritis[mh]) AND (“physical activity” OR physical activity[mh] OR exercis* OR exercise[mh] OR stretch* OR yoga* OR train*) AND (determinant* OR correl* OR factor* OR predict* OR participat* OR “exercise belief”) All others are [all fields].
*indicates the use of all possible suffixes.
Selection criteria
Quantitative observational studies and experimental studies that explored correlates of PA in adults (≥ 18 years) with SpA and/or RA were included. Intervention studies were eligible for inclusion if baseline data were reported. Study participants must have had a diagnosis of RA using the American College of Rheumatology (ACR) /EULAR criteria [26]. Due to the limited number of studies, those with SpA were included if they received a doctor diagnosis, but not if the diagnosis was self-reported. SpA diagnoses that were eligible included: axial spondyloarthritis (axSpA); non-radiographic spondyloarthritis (nr-axSpA); ankylosing spondylitis (AS); psoriatic arthritis (PsA); enteropathic arthritis (EnA); reactive arthritis (ReA); and undifferentiated spondyloarthritis (uSpA). Studies utilising self-reported (questionnaires) and objective measures (accelerometers, pedometers, calorimetry, doubly labelled water, and heart rate monitors) of PA were included. For consistency with other reviews [24], PA was defined in this work as ‘any bodily movement produced by skeletal muscles that results in energy expenditure’ [27]. Exercise is a subcategory of PA, that is distinct in definition by its ‘planned, structured and repetitive’ nature, with an ‘objective to improve or maintain physical fitness component(s)’ [27]. Cross-sectional studies where PA was not the dependent variable were included.
Articles were excluded if they were not published between 2004 and June 2019 and if the study participants were aged younger than 18 years. Qualitative studies and studies that exclusively explored functional or physiological variables of PA in SpA and RA were excluded. Studies that included multiple forms of arthritis but did not analyse the data separately were excluded. Studies that reported on physical inactivity were included, but those reporting exclusively on sedentary behaviour were excluded as sedentary behaviour is independent from physical (in)activity [28]. Longitudinal studies in which PA was not the dependent variable were excluded, unless baseline data were reported. Abstracts, conference proceedings, and grey literature were excluded.
Screening and data extraction
The first author conducted the database searches and imported them to the reference manager software Endnote, whereby duplicates were removed. The abstracts were then imported into the review management software Covidence [29] and further duplicates removed. The first author screened all titles and abstracts independently against the selection criteria, with a second author independently screening 40% to determine eligibility. The same method was used for the review and selection of full text articles. During these two stages a consensus approach was used to resolve any disputes. All disputes were resolved without the need for consultation with a third author. Data extraction was conducted by the first author. Data such as participant characteristics, study design, measure of PA, variables analysed, statistical test, and statistical output were extracted.
Methodological quality
Selected articles fulfilling the inclusion criteria were assessed for methodological quality using The National Institutes of Health (NIH) Quality Assessment Tools for Observational Cohort and Cross-sectional Studies and Case–Control studies [30]. These assessment tools are recommended and deemed acceptable [31]. The assessment tools appraise studies based on the following concepts: objectives; population and sample size; timeframe; outcome measure; exposure variables and measures; attrition; blinding; and analysis. Each of the questions were scored as yes, no, cannot determine, not applicable, or not reported as appropriate. A final quality rating of good, fair, or poor is afforded to each study by the reviewer. The lead author independently assessed the quality of studies, with a following two authors independently assessing 50% each. Any disputes were resolved via a consensus method between the authors, and further consultation from a third author was not warranted.
Data synthesis and coding
Following discussions with the research team, meta-analysis was not conducted due to the nature of included studies (e.g., type of PA and statistics reported). Rather, the coding framework employed by Sallis et al. [32] was used to pool and classify the associations between PA parameters and potential correlates into largely positive/negative ( ±), indeterminant / inconsistent (?) or no association (0). Following this framework, univariate tests were reported, even if multivariate tests were presented. The results will be presented using this approach for consistency across studies. Yet, an associations table utilizing the most adjusted or final statistical model for each variable can be seen in Online Resources 1 and 2. Conceptually similar variables were grouped together, for example, “high-density lipoprotein” (HDL) included, HDL, HDL particle, small particle, and large particle concentration. To be consistent with previous reviews [24], the correlates identified were categorised into the following domains: sociodemographic, physical, psychological, social, and environmental.
For studies utilizing multiple measures of PA (i.e., subjective, objective, or multiple subjective), correlations from all PA measures were included. Both categorical (e.g., active vs. inactive, meeting PA recommendations, health-enhancing PA, exercising ≥ 2/week) and continuous PA parameters (e.g., total PA min, step count, time spent in PA intensities, metabolic equivalent of task, physical activity energy expenditure (PAEE), accelerometer counts) were eligible for inclusion. Continuous parameters of PA are reported above categorical parameters of PA if the same study reports both. However, if a variable was analysed as a potential correlate of a categorical PA parameter, but not of a continuous PA parameter, this was included. Up to two PA parameters were included for each study (indicators of total PA considered first), apart from if multiple measures of PA were used, so that one study population is not overrepresented. If more than two types of PA intensity were reported in a study (i.e., light PA (LPA), moderate-intensity PA (MI-PA), vigorous-intensity PA (VI-PA)), the two most intense categories were included. For accelerometer data, PAEE was considered over total energy expenditure (TEE).
Results
Study selection, characteristics and quality
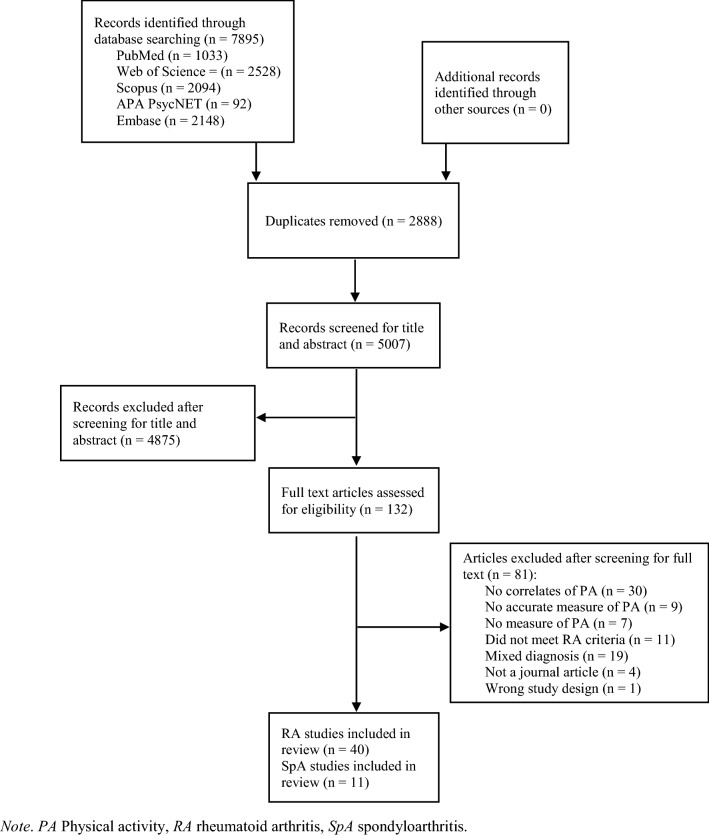
Fifty-one published articles (RA = 40; SpA = 11) were included with the full screening process provided in Fig. 1. The predominant reasons for exclusion were that the studies did not report correlates of PA (n = 30) and that the study included mixed diagnoses, non-specific arthritides or did not analyse RA and SpA data separately (n = 19). A description of each study and the overall methodological quality can be seen in Tables 1 and 2. The number of RA participants ranged from 20 to 3,152, whereas the number of SpA participants ranged from 24 to 2,167. Three RA studies utilized participants from the Swedish Rheumatology Quality Registers [35–37], and a further two used participants from the Swedish RA register [40, 41] . Two RA studies contained the same patients from 3 hospitals in The Netherlands [49, 53]. Two other RA studies used participants and cross-sectional data from the same randomized clinical trial investigating the effectiveness of a PA promotion intervention in the USA [38, 54]. One randomized intervention study [63] investigated the effects of a 12-week low-impact aerobic exercise programme on disease and psychological variables, and aerobic fitness, in those with RA. The randomized intervention study compared a class exercise treatment group, a home exercise treatment group (via videotape), and a control group [63]. Two SpA studies included patients from the Skåne Healthcare Register [78, 79]. Most RA studies had a larger proportion of female participants than male, and four RA studies included only female participants [39, 64, 65, 67]. Twelve RA studies were conducted in the United States and twenty-five were conducted in Europe. Ten of the eleven SpA studies were conducted in Europe. The pooled associations and correlates of PA in those with RA and SpA are displayed in Tables 3 and 4. The results are coded in terms of the specific variable and not necessarily the statistical direction derived from the measure of the variable. Online resources 3 and 4 show the positive and negative statistics for each variable related to PA in all included studies.
Fig. 1.
PRISMA flowchart of study screening and selection process
Table 1.
Description of included RA studies
| Citations | Design | Participants (n) | Age (years) | Sex (F) | PA measure | Location | Methodological Quality |
|---|---|---|---|---|---|---|---|
| Byram et al. [33] | Cross-sectional | RA (165) | 54 | 69% | Customized interview / 2011 Compendium of PA | USA | Fair |
| Conigliaro et al. [34] | Cohort / case–control | RA (30) | 56.07 ± 11.7 | 25 | IPAQ | Italy | Poor |
| Demmelmaier et al. [35] | Longitudinal | RA (2752) | 60 ± 11 | 2,003 | IPAQ-SF (plus ESAI) | Sweden | Good |
| Demmelmaier et al. [36] | Cross-sectional | RA (3152) | 62 (IQR 54–68) | 2,309 | IPAQ-SF (plus ESAI) | Sweden | Good |
| Demmelmaier et al. [37] | Longitudinal | RA (2569) | 60 ± 11 | 1,875 | IPAQ-SF (plus ESAI) | Sweden | Good |
| Ehrlich-Jones et al. [38] | Cross-sectional | RA (185) | 55 ± 14 | 155 | GT1M Actigraph | USA | Good |
| Elkan et al. [39] | Cross-sectional | RA (61) | 60.8 (57.3–64.4) | 61 | IPAQ | Sweden | Fair |
| Eurenius et al. [40] | Cross-sectional | RA (298) | 57 (range 19–90) | 225 | Customized self-report | Sweden | Good |
| Eurenius et al. [41] | Longitudinal | RA (102) | 57 (range 19–84) | 76 | Customized self-report | Sweden | Good |
| Fenton et al. [42] | Cross-sectional | RA (61) | 54.92 ± 12.39 | 67.2.% | GT3X Accelerometer—LPA | UK | Good |
| Greene et al. [43] | Cross-sectional | RA (52) | 61 ± 14.5 | 47 | PADS | USA | Good |
| Hashimoto et al. [44] | Cross-sectional / case control | RA (20) | 69.4 ± 5.1 | 16 | Actigraph mini-motionlogger, omnidirectional accelerometer | Japan | Good |
| Henchoz et al. [45] | Case control / cross-sectional | RA (110) |
53% (40–59) 47% (60–80) |
83 | PAFQ | Switzerland | Good |
| Hernández-Hernández et al. [46] | Case-control / cross-sectional / longitudinal | RA (50) | 54.5 ± 7.4 | 44 | IPAQ and RT3 triaxial accelerometer | Spain | Good |
| Huffman et al. [47] | Cross-sectional / case–control | RA (51) | 55 (46,64) | 36 | RT3 triaxial accelerometer | USA | Good |
| Hugo et al. [48] | Observational | RA (57) | 57.6 ± 10.2 | 73% | Sensewear armband accelerometer | France | Good |
| Hurkmans et al. [49] | Multi-centre Cross -sectional | RA (271) | 62 ± 14 | 178 | SQUASH | The Netherlands | Good |
| Iverson et al. [50] | Longitudinal | RA (573) | 61 ± 12 | 478 | NHSPAQ II | USA | Good |
| Katz et al. [51] | Cross-sectional | RA (158) | 59.2 ± 11.3 | 118 | IPAQ | USA | Good |
| Khoja et al. [52] | Cross-sectional | RA (98) | 58 ± 9 | 83 | Sensewear armband (SWA) biaxial accelerometer | USA | Good |
| Knittle et al. [53] | Multicentre longitudinal | RA (129) | 60.5 ± 13.6 | 88 | SQUASH | The Netherlands | Fair |
| Lee et al. [54] | Cross-sectional | RA (176) | 55 (range 23–86) | 146 | GT1M Actigraph | USA | Fair |
| Løppenthin et al. [55] | Cross-sectional | RA (443) | 60 (range 21–88) | 356 | Leisure time physical activity questionnaire & PAS 2 | Denmark | Good |
| Lundgren et al. [56] | Cross-sectional | RA (95) | 60 ± 11.9 | 71 | PAI | Sweden | Fair |
| Malm et al. [57] | Longitudinal Observational | RA (1387) | 65 ± 15 | 967 | Customized Questionnaire | Sweden | Good |
| Mancuso et al. [58] | Longitudinal / case-control | RA (122) | 49 ± 12 | 84% | PAEI | USA | Good |
| McKenna, et al., [59] | Cross-sectional | RA (75) | 20% ≤ 49, 20% 50–59, 40% 60–69, 20% 70–79 | 48 | The SeanseWear (Pro 3 Armband (SWA) | Ireland | Good |
| Metsios et al. [60] | Cross-sectional | RA (244) | 62.1 (IQR 53.8–69.4) | 174 | IPAQ | UK | Good |
| Mochizuki et al. [61] | Cross-sectional | RA (137) | 64 ± 11.6 | 111 | Liferecorder | Japan | Good |
| Munsterman et al. [62] | Cross-sectional | RA (60) | 51.8 ± 10.4 | 44 | SQUASH | The Netherlands | Good |
| Neuberger et al. [63] | Randomized intervention study | RA (220) | 55.5 (range 40–70) | 82.7% | Self-report: mean mins aerobic exercise / week + mean of other aerobic at T2/3 | USA | Poor |
| Piva et al. [64] | Cross-sectional | RA (47) | 56.5 ± 7 | 47 | SenseWear Professional v 6.1 activity monitor | USA | Good |
| Prioreschi et al. [65] | Case-control / cross-sectional | RA (50) | 48 ± 13 | 50 | Hip-worn Actical accelerometer | South Africa | Good |
| Rongen-van Dartel et al. [66] | Cross-sectional |
RA (167) Low: 42 High: 125 |
Low: 56.78 ± 11.03, High: 54.79 ± 10.59 | Low: 33, High: 67 | Ankle-worn actometer (Actilog version 4.1) | The Netherlands | Good |
| Semanik et al. [67] | Cross-sectional | RA (185) | 70 (range 60–88) | 185 | Yale PA survey | USA | Fair |
| Stavropoulos-Kalinoglou et al. [68] | Cross-sectional | RA (150) |
F: 59 (IQR 55–64) M: 60 (IQR 59–64) |
102 | IPAQ | UK | Good |
| Tierney et al. [69] | Cross-sectional | RA (59) | 60.10 ± 11.27 | 41 | Sensewear armband | Ireland | Good |
| Uutela et al. [70] | Cross-sectional | RA (200) |
AO: 59 ± 11 No AO: 60 ± 13 |
153 | The FIT Index of Kasari | Finland | Good |
| Van den Berg et al. [71] | Observational | RA (252) | 60.5 ± 11.5 | 182 | Customized self-report | The Netherlands | Fair |
| Van der Goes et al. [72] | Cross-sectional | RA (165) |
Inactive: 57 ± 12 Insufficient: 57 ± 12 Recommended: 60 ± 11 |
71% | SQUASH | The Netherlands | Good |
Age presented as mean or median (standard deviation, confidence interval, inter quartile range or range). Sex presented as number or %. RA Rheumatoid Arthritis, OA Osteoarthritis, IPAQ International Physical Activity Questionnaire, IPAQ-SF International Physical Activity Questionnaire – short form, ESAI Exercise Stage Assessment Instrument, PADS Physical Activity and Disability Survey, PAFQ Physical Activity Frequency Questionnaire, SQUASH Short Questionnaire to Assess Health-Enhancing physical activity, NHSPAQ II Nurses Health Study II Physical Activity Questionnaire, PAS 2 Physical Activity Scale, PAI Physical Activity Index, PAEI Paffenbarger Physical Activity and Exercise Index, FIT Index Frequency Intensity Time Index, AO abdominal obesity
Table 2.
Description of included SpA studies
| Citations | Design | Participants (n) | Age | Sex (F) | PA measure | Location | Methodological Quality |
|---|---|---|---|---|---|---|---|
| Arends et al. [73] | Cross-sectional / observational | AS (115) | 44.6 ± 12.1 | 44 | IPAQ, SQUASH, and ActiGraph (GT1M) | The Netherlands | Good |
| Brodin et al. [74] | Observational | AS (50) | 51.5 (22–76) | 16 | Customized scale | Sweden | Fair |
| Brophy et al. [75] | Cohort study | AS (326) | 55 ± 14 | 21% | IPAQ-SF | Wales | Good |
| Fabre et al. [76] | Cross-sectional | axSpA (203) | 46 ± 11.6 | 95 | IPAQ | France | Good |
| Fongen et al. [77] | Cross-sectional / case control | AS (149) | 49.3 ± 11.1 | 39% | IPAQ | Norway | Good |
| Haglund et al. [78] | Cross-sectional | SpA (2167) | 55 ± 14 | 52% | Customized | Sweden | Good |
| Meesters et al. [79] | Cross-sectional | SpA (2167) | 55.4 ± 13.9 | 52% | Customized | Sweden | Good |
| O’Dwyer et al. [80] | Case-control / cross-sectional | AS (39) | 40 ± 9 | 7 | RT3 triaxial accelerometer | Ireland | Good |
| Prince et al. [81] | Multi-centre retrospective observational | AS (52) | 44.8 ± 13.2 | 7 | Customized interview | Australia | Fair |
| Van Genderen et al. [82] | Case control / multi-centre cross-sectional | AS (135) | 51 ± 13 | 54 | Actigraph GT3X triaxial accelerometer | The Netherlands | Good |
| Van Genderen et al. [83] | Case control / cross-sectional | AS (24) | 48 ± 11 | 10 | Tracmor triaxial accelerometer and Baecke | The Netherlands | Good |
Age presented as mean or median (standard deviation, confidence interval, inter quartile range or range). Sex presented as number or %. AS Ankylosing Spondylitis, axSpA Axial Spondyloarthritis, SpA Spondyloarthritis, IPAQ International Physical Activity Questionnaire, IPAQ-SF International Physical Activity Questionnaire – short form, SQUASH Short Questionnaire to Assess Health-Enhancing physical activity
Table 3.
Summary of study results and pooled associations – Rheumatoid arthritis
| Variable | Positive Relationship | Negative Relationship | No Relationship | Assoc | % Studies |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age | 55 | 35, 36, 40, 45, 49, 50, 53, 54bc, 61c, 64ac, 64ac, 65ac, 67a, 69c, 71, 72 | 33, 35, 39a, 41, 43, 46, 46c, 47c, 57, 59c, 62, 63, 66c | ?? | 16/30 53% |
| Gender (female) | 49, 57, 71 | 35, 35, 40, 47c, 55, 59c, 62, 66c, 69c | 33, 36, 41, 43, 54bc, 63, 72 | ?? | 9/19 47% |
| Race /Ethnicity (Caucasian) | 50, 54bc | 33, 43, 47c, 67a | 00 | 2/6 33% | |
| Educational level | 36, 49, 50, 64ac, 64ac, 71 | 35, 43, 54bc, 63, 67a | ?? | 6/11 55% | |
| Employment status | 50, 67a, 69c | 49, 59c, 71 | ? | 3/6 50% | |
| Income | 36 | 35, 35, 67a | 0 | 1/4 25% | |
| Marital status | 50 | 64ac, 64ac, 67a | 0 | 1/4 25% | |
| Living status (children, location) | 36 | 36 | 35, 49, 59c, 71 | 00 | 1/6 17% |
| Smoking | 51b, 69c | 33, 39a, 59c, 71 | 00 | 2/6 33% | |
| Any use of alcohol | 50 | + | 1/1 100% | ||
| Language Comprehension | 36 | 35 | ? | 1/2 50% | |
| Physical | |||||
| RA Duration | 48c, 48c, 49, 64ac, 65ac, 61c, 72 | 33, 35, 35, 36, 39a, 41, 44c, 45, 46, 46c, 47c, 50, 54bc, 57, 63, 64ac, 66c, 67a | 00 | 7/25 28% | |
| Body Mass Index (BMI) / weight / obesity | 61c | 50, 51b, 52c, 52c, 54bc, 65ac, 66c, 68 | 33, 39a, 43, 46, 46c, 47c, 59c, 64ac, 64ac, 69c, 71, 72 | ?? | 8/21 38% |
| Comorbidities | 36, 47c, 64ac | 35, 35, 43, 50, 54bc, 63, 64ac | 00 | 3/10 30% | |
| Disease activity | 33, 34, 45, 46, 47c, 49, 51b, 52c, 55, 59c, 61c, 69c, 72 | 39c, 40, 41, 44c, 46, 46, 46c, 46c, 46c, 48c, 48c, 50, 52c, 59c, 64ac, 64ac, 65ac, 66c | ?? | 13/31 42% | |
| Tender and swollen joint count | 40, 40, 46, 46, 46c, 46c, 57, 57, 63, 66c, 66c | 00 | 0/11 0% | ||
| Radiographic joint damage | 72 | 44c, 57 | 0 | 1/3 33% | |
| Aerobic fitness | 62 | 40, 63 | 0 | 1/3 33% | |
| Strength and muscle function | 51b, 64ac | 40, 41, 63, 64ac | 00 | 2/6 33% | |
| Range of motion | 40, 41 | 0 | 0/2 0% | ||
| Balance | 64ac | 40, 41, 64ac | 0 | 1/4 25% | |
| Function (inability) | 35, 36, 44c, 46, 46c, 47c, 50, 51b, 52c, 52c, 55, 59c, 59c, 61c, 64ac, 64ac, 65ac, 69c | 34, 35, 39a, 40, 41, 42c, 43, 45, 49, 57, 57, 66c | − − | 18/30 60% | |
| Gait Speed | 64ac, 64ac | + | 2/2 100% | ||
| Pain | 36, 53, 55, 61c | 34, 35, 35, 40, 41, 43, 45, 47c, 54bc, 57, 62, 63, 66c | 00 | 4/17 24% | |
| Fatigue | 36, 45, 50, 51b, 55, 55, 55, 55, 58, 63, 66c | 34, 35, 35, 46, 46c, 55, 62 | − − | 11/18 61% | |
| Sleep (good/high) | 51b, 59c | 55, 59c, 66c | ? | 2/5 40% | |
| Stiffness | 34 | − | 1/1 100% | ||
| Other (physical) | |||||
| Waist circumference | 70 | 39a, 47c | 0 | 1/3 33% | |
| Waist to hip ratio | 33 | 33, 46, 46c | 0 | 1/4 25% | |
| Abdominal obesity | 70, 72 | − | 2/2 100% | ||
| Fat Mass Index | 39a | 0 | 0/1 0% | ||
| Lean/fat mass | 51b | + | 1/1 100% | ||
| Body fat | 68 | − | 1/1 100% | ||
| Diabetes | 72 | 33, 55, 66c | 0 | 1/4 25% | |
| Fat Free Mass Index | 39a | 0 | 0/1 0% | ||
| Plasma glucose/insulin | 39a | − | 1/1 100% | ||
| Total cholesterol | 33, 39a | 0 | 0/2 0% | ||
| Cholesterol efflux capacity | 33 | 0 | 0/1 0% | ||
| Low-density lipoprotein | 33, 33, 33, 33, 39a, 52c, 52c | 00 | 0/7 0% | ||
| High-density lipoprotein | 33, 33, 33, 33, 39a, 52c | 33, 33, 52c | + + | 6/9 67% | |
| Tryglycerides | 52c, 72 | 33, 52c | ? | 2/4 50% | |
| Dyslipidaemia | 72 | – | 1/1 100% | ||
| Apolipoprotein A1 | 39a | + | 1/1 100% | ||
| Apolipoprotein B | 39a | 0 | 0/1 0% | ||
| Oxidized LDL | 39a | 0 | 0/1 0% | ||
| Antibodies against phosphorylcholine | 39a | + | 1/1 100% | ||
| Anti-citrullinated protein antibodies | 34, 72 | 0 | 0/2 0% | ||
| Erythrocyte sedimentation rate | 34, 39a, 40, 46, 46c, 47c, 57, 61c, 63, 65ac, 66c | 00 | 0/11 0% | ||
| C-reactive protein | 33, 61c | 34, 40, 46, 46c, 51b, 59c, 59c, 63, 65ac, 66c, 72 | 00 | 2/13 15% | |
| Matrix metalloproteinase (MMP-3) | 61c | 0 | 0/1 0% | ||
| RF positive | 34, 66c | 0 | 0/2 0% | ||
| Cardiovascular risk / disease | 46c, 72 | 46, 46, 46c, 66c | 00 | 2/6 33% | |
| Hypertension | 33, 72 | − | 2/2 100% | ||
| Systolic blood pressure | 52c, 52c | 33 | − | 2/3 67% | |
| Diastolic blood pressure | 52c, 52c | 33 | − | 2/3 67% | |
| Heart rate | 51b | 33 | ? | 1/2 50% | |
| Metabolic syndrome | 46, 72 | 46c | − | 2/3 67% | |
| Nutritional complications | 48c, 48c | − | 2/2 100% | ||
| Augmentation Index | 33 | − | 1/1 100% | ||
| Pulse wave velocity | 33 | 33 | ? | 1/2 50% | |
| Agatson Score | 33 | 0 | 0/1 0% | ||
| Insulin resistance | 33, 52c, 52c | − | 3/3 100% | ||
| RA -related joint surgery | 50 | − | 1/1 100% | ||
| COPD | 66c | 0 | 0/1 0% | ||
| Haemoglobin | 66c | 0 | 0/1 0% | ||
| Psychological | |||||
| Exercise beliefs and (outcome) expectations | 36, 36, 38c, 54bc, 63 | 35, 35, 35, 35, 43 | ?? | 5/10 50% | |
| Motivation | 38c, 47c, 49, 53, 54bc | 47c | + + | 5/6 83% | |
| Subjective Vitality | 42c | + | 1/1 100% | ||
| Self-efficacy | 35, 36, 43, 47c, 47c, 50, 53 | 35, 63, 66c | + + | 7/10 70% | |
| Depression / anxiety | 42c, 51b | 35, 35, 55, 62, 63, 66c | 00 | 2/8 25% | |
| Life worries | 38c | 0 | 0/1 0% | ||
| Health perception (good global health / assessment) | 34, 36, 50, 50, 55, 61c | 34, 35, 35, 40, 41, 57, 59c, 59c | ?? | 6/14 43% | |
| Fear Avoidance Beliefs | 36, 37 | 35, 35, 56 | ? | 2/5 40% | |
| Other (psychological) | |||||
| Life beliefs | 63 | 0 | 0/1 0% | ||
| Health locus of control | 41, 56 | 0 | 0/2 0% | ||
| Pain and impairment relationship | 56 | 0 | 0/1 0% | ||
| Health-related quality of life | 50, 53, 53, 65ac | 46, 46, 46c, 46c, 54bc, 65ac | ?? | 4/10 40% | |
| Individual SF-36 measures (health status) | 34, 34, 50 | 44c, 44c, 44c, 44c, 44c, 44c, 44c, 44c, 65ac, 65ac, 65ac, 65ac, 65ac, 65ac, 65ac, 65ac, 65ac, 66c, 66c, 66c | 00 | 3/23 13% | |
| Arthritis Impact | 34 | 0 | 0/1 0% | ||
| Beliefs about causes of fatigue | 66c, 66c | 0 | 0/2 0% | ||
| Coping strategies (fatigue) | 66c, 66c, 66c, 66c, 66c, 66c | 00 | 0/6 0% | ||
| Fatigue catastrophizing | 66c, 66c, 66c | 0 | 0/3 0% | ||
| Social | |||||
| Social support | 36, 42c | 35 | 35, 35, 35, 43, 49, 63 | 00 | 2/9 22% |
| Other | |||||
| Previous levels of PA | 35, 35, 41 | + | 3/3 100% | ||
| Medications / biologics | 46, 46c, 72 | 39a, 39a, 39a, 47c, 47c, 47c, 47c, 50, 50, 50, 50, 50, 51b, 66c, 66c, 66c, 66c, 72, 72 | 00 | 3/22 14% | |
| Hospital admission /length | 60, 60 | − | 2/2 100% | ||
| Goal Achievement | 53 | 0 | 0/1 0% |
aFemale participants only
bStudies that investigated physical inactivity
cAssociations from objective measures of PA
In the associations column the following applies: + = positive association; −= negative association; ? = indeterminant/inconsistent; 0 = no association. If four or more studies indicate the same association the codes are + + , − −, ?? and 00. The code is based on the percentage of studies supporting an association: 0–33% = 0; 34–59% = ?; and 60–100% = + or −
Table 4.
Summary of study results and pooled associations—Spondyloarthritis
| Variable | Positive relationship | Negative relationship | No Relationship | Assoc | % Studies |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age | 78, 83 | 78 | 74, 76, 82c, 83c | 00 | 2/7 29% |
| Gender (female) | 78 | 78 | 74, 76, 82c, 82c | 00 | 1/6 17% |
| Marital status (married) | 74 | 76 | ? | 1/2 50% | |
| Employment (employed) | 74, 76 | 0 | 0/2 0% | ||
| Educational level | 76, 78, 78 | 0 | 0/3 0% | ||
| Raised children under 12 years | 76 | 0 | 0/1 0% | ||
| Smoking | 78 | 78 | ? | 1/2 50% | |
| Physical | |||||
| Comorbidities | 76 | 0 | 0/1 0% | ||
| Symptom duration | 74, 76 | 0 | 0/2 0% | ||
| Diagnosis duration | 74 | 82c | 82c, 83, 83c | 0 | 1/5 20% |
| Peripheral Joint involvement | 74 | 0 | 0/1 0% | ||
| Disease activity | 74, 76, 82c | 73, 73, 73, 73, 73c, 75, 77, 78 | 73c, 76, 78, 80c, 80c, 82c, 83, 83c | ?? | 8/19 42% |
| Erythrocyte sedimentation rate | 73c | 73, 73 | 0 | 1/3 33% | |
| C-reactive protein | 73c | 73, 73, 76 | 0 | 1/4 25% | |
| Function (inability) | 74 | 73, 73, 73c, 75, 78, 78, 82c, 83c | 76, 80c, 82c, 83 | − − | 8/13 62% |
| Spinal immobility | 74 | 0 | 0/1 0% | ||
| Occiput-to-wall distance | 73 | 73, 73c | 0 | 1/3 33% | |
| Chest expansion | 73, 73, 73c | 0 | 0/3 0% | ||
| Modified Schober test | 73, 73c | 73 | + | 2/3 67% | |
| Lateral spinal flexion | 73c | 73, 73 | 0 | 1/3 33% | |
| Cervical rotation | 73, 73c | 73 | + | 2/3 67% | |
| Radiographic signs | 76 | 0 | 0/1 0% | ||
| Axial pain | 76 | 0 | 0/1 0% | ||
| Fatigue | 83, 83, 83 | 83, 83, 83c, 83c, 83c, 83c, 83c | 00 | 3/10 30% | |
| Psoriatic arthritis subtype | 78 | 78 | ? | 1/2 50% | |
| Undifferentiated spondyloarthritis subtype | 78, 78 | 0 | 0/2 0% | ||
| Inflammatory bowel disease-related arthritis | 78, 78 | 0 | 0/2 0% | ||
| Uveitis | 76 | 0 | 0/1 0% | ||
| Psoriasis | 76 | 0 | 0/1 0% | ||
| Inflammatory bowel disease | 76 | 0 | 0/1 0% | ||
| HLA B27 antigen | 76 | 0 | 0/1 0% | ||
| Inflammatory back pain | 76 | 0 | 0/1 0% | ||
| Arthritis | 76 | 0 | 0/1 0% | ||
| Enthesitis | 76 | 0 | 0/1 0% | ||
| Dactylitis | 76 | 0 | 0/1 0% | ||
| Surgery due to axSpA | 76 | 0 | 0/1 0% | ||
| Body Mass Index (BMI) | 82c | 76, 82c, 83, 83c | 00 | 1/5 20% | |
| Aerobic fitness (VO2max) | 80c | + | 1/1 100% | ||
| Having ankylosing spondylitis | 81 | − | 1/1 100% | ||
| Psychological | |||||
| Health perceptions (overall / disease) | 74 | 74, 76, 83, 83c | 00 | 1/5 20% | |
| Quality of life | 73, 73, 73c, 78, 78 | 80c | + + | 5/6 83% | |
| Motivation | 75, 75, 75 | 75 | 75, 75 | ? | 3/6 50% |
| Perception of exercise | 76, 76 | 0 | 0/2 0% | ||
| Depression | 79 | 76, 78, 78 | 0 | 1/4 25% | |
| Anxiety | 76, 78, 78, 79 | 00 | 0/4 0% | ||
| Environmental | |||||
| Seasonal variation (summer) | 77 | + | 1/1 100% | ||
| Other | |||||
| Previous levels of PA | 74 | + | 1/1 100% | ||
| Medication / biologics | 81 | 74, 76, 76 | 0 | 1/4 25% | |
| Age of anti-TNF therapy | 81 | − | 1/1 100% | ||
| Physiotherapy | 76 | 0 | 0/1 0% |
cAssociations from objective measures of PA
In the associations column the following applies: + = positive association; −= negative association; ? = indeterminant/inconsistent; 0 = no association. If four or more studies indicate the same association the codes are + + , − −, ?? and 00. The code is based on the percentage of studies supporting an association: 0–33% = 0; 34–59% = ?; and 60–100% = + or −
Measurement of PA
Self-reported measures of PA were used in twenty-seven RA studies. Twenty-one RA studies used known self-report questionnaires, whereas six studies applied custom questionnaires or interviews. Objective measures of PA were used in fourteen RA studies. Thirteen RA studies used an accelerometer, and one used a Liferecorder. Nine SpA studies used self-reported measures of PA, whereby, five SpA studies used validated questionnaires, and a further four used customized scales. Objective measures of PA were used in four SpA studies (i.e., accelerometers).
Correlates of PA in rheumatoid arthritis
Sociodemographic
The most frequently studied sociodemographic variables were age and gender. Across studies, age and gender showed inconsistent associations with PA, yet the direction of associations were largely split between inverse (age, 53%; female gender, 47%) or no relationship. Higher educational level and employment status were indeterminately associated with PA parameters; however, over half of the associations were reported as positive (educational level, 55%; and employment status, 50%). Non-significant associations were found for race/ethnicity, income, marital status, living status, and smoking.
Physical
Consistent negative associations with PA were reported for functional disability (60%) and fatigue (61%). However, Løppenthin et al. [55] utilized the five domains of the Multidimensional Fatigue Inventory (MFI-20 [84]), contributing four negative and one non-significant association to the pooling. Removing this study would categorise fatigue as inconsistently related to PA parameters, with only 54% of associations negative. Although disease activity and body mass index (BMI) received inconsistent summary codes, 42% and 38% of associations were negative. Disease activity was measured using the Disease Activity Score 28 (DAS28) in 19 studies, the Simplified Disease Activity Index (SDAI) in 2 studies, and the Rheumatoid Arthritis Disease Activity Index (RADAI) in 2 studies (based on included associations). The pooled associations between sleep and PA were indeterminant, with two associations supporting a positive relationship (40%). Non-significant associations with PA were consistently reported for RA duration, comorbidities, tender and swollen joint count, strength and muscle function, and pain.
Of the biochemical variables, HDL was consistently positively related to PA parameters (67%), whereas low-density lipoprotein (LDL) was unrelated. Additional variables demonstrating consistent and non-significant associations with PA parameters include erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and cardiovascular risk.
Psychological
In adults with RA, motivation was consistently and positively related to PA in 5 of the 6 comparisons (83%), in addition to, self-efficacy in 7 of 10 comparisons (70%). Motivation was assessed in 5 studies, using a variety of scales (Customized scale based on the Perceived Competence Scale, n = 1; an adapted scale (for endurance and strength training motivation) previously used in those with arthritis, n = 1; the Treatment Self-Regulation Questionnaire (TSRQ), n = 2; and a 6-item scale for assessing motivation for increasing PA (confidence to maintain an active lifestyle) based on the Interaction Model of Client Health Behavior (IMCHB), n = 1). Self-efficacy was assessed in 8 studies, also using a range of assessment tools (Exercise Self-Efficacy Scale (ESES), n = 2; Arthritis Self-Efficacy Scale (ASES), n = 1; an adapted scale (for endurance and strength training self-efficacy) previously used in those with arthritis, n = 1; Lorig’s Self Efficacy Scale (for managing arthritis), n = 1; goal self-efficacy subscale of the Self-Regulation Skills Battery, n = 1; the mean of two confidence measures, n = 1; and the Self-Efficacy Scale 28 (for fatigue), n = 1). One study investigated subjective vitality, whereby a positive correlation with light PA was found (r = 0.27, p < 0.05) [42]. Positive associations were seen for exercise beliefs and outcome expectations (50%), health perception (43%) and health-related quality of life (40%), yet these resulted in inconsistent summary codes. The psychological variables most consistently reporting non-significant associations with PA were depression/anxiety (75%), coping strategies for fatigue (100%) and the individual components of the 36-Item Short Form Survey (SF-36; 87%). Studies utilizing the individual components of the SF-36 were grouped due to the disparity of studies reporting total quality of life versus the individual components.
Social
The relationship between social support and PA parameters were examined in six studies. Within these six studies, 6 of 9 associations between social support and PA were non-significant (67%).
Environmental
Environmental variables were not examined in any of the included RA studies.
Other
Three associations from two studies (100%) found that already established PA at baseline was a significant positive predictor of PA. Eurenius et al. [41] found that high baseline PA predicted high levels of PA after one year (OR 3.85, 95% CI 1.67–9.09). Similarly, Demmelmaier et al. [35] identified three trajectories of PA over two years, and previously established PA was a significant positive predictor of being in the stable high vs the decreasing or stable low trajectory. Further, negative associations were reported in one study between PA and the number and length of hospital admissions [60]. Medications and biological treatment were not significantly related to PA parameters. Negative associations were found for corticosteroid use [46] and glucocorticoid therapy [72].
Pooled associations based on most adjusted or final model statistics
Online Resource 1 shows the pooled associations when considering the final model and adjusted associations for each variable. When pooled in this fashion, the indeterminant relationships of employment status shifted to unrelated. Further, the positive relationships of alcohol use and gait speed changed to unrelated. The negative relationships of functional disability, abdominal obesity, nutritional complications, and hypertension shifted to inconsistent. Fatigue was negatively related to PA parameters, yet when pooling for the most adjusted or final models this relationship changed to unrelated. This association change remained the same when removing the results reported by Løppenthin et al. [55] which contributed five independent domains of fatigue. The inconsistent pooled results for disease activity, triglycerides, health perception, fear avoidance beliefs, and health-related quality of life all shifted to unrelated.
Pooled associations with only objective measures of PA
The main differences between the pooled associations from all studies and the pooled associations from studies using objective measures of PA are as follows (all studies vs objective PA studies): age (16/30, 53%, ?? vs. 6/10, 60%, − −); gender (9/19, 47%, ?? vs. 4/5, 80%, − −); race/ethnicity (2/6, 33%, 00 vs. 1/2, 50%, ?); educational level (6/11, 55%, ?? vs. 2/3, 67%, +); smoking (2/6, 33%, 00 vs. 1/2, 50%, ?); RA duration (7/25, 28%, 00 vs. 5/11, 45%, ??); comorbidities (3/10, 30%, 00 vs. 2/4, 50%, ?); disease activity (13/31, 42%, ?? vs. 5/18, 28%, 00); strength and muscle function (2/6, 33%, 00 vs. 1/2, 50%, ?); balance (1/4, 25%, 0 vs. 1/2, 50%, ?); fatigue (11/18, 61%, − − vs. 1/2, 50%, ?); sleep (2/5, 40%, ? vs. 1/3, 33%, 0); metabolic syndrome (2/3, 67%, − vs. 0/1, 0%, 0); HDL (6/9, 67%, ++ vs. 1/2, 50%, ?); exercise beliefs and outcome expectations (5/10, 50%, ?? vs. 2/2, 100%, +); depression / anxiety (2/8, 25%, 00 vs. 1/2, 50%, ?); health perception (6/14, 43%, ?? vs. 1/3, 33%, 0); social support (2/9, 22%, 00 vs. 1/1, 100%, +). Some variables were only assessed in studies using self-reported measures of PA and therefore comparisons cannot be made (e.g., income, fear avoidance beliefs).
Correlates of PA in Spondyloarthritis
Sociodemographic
In adults with SpA, none of the sociodemographic variables showed consistent positive or negative associations with PA. Marital status and smoking were negatively related to PA in 1 of 2 associations (50%), resulting in inconsistent summary scores. Age and gender were consistently not associated with PA; however, in two studies (29%) and one study (17%) respectively, positive associations were found. Some evidence suggested that variables such as employment status, education and raising children under 12 years old, were also unrelated to PA.
Physical
The two most frequently studied physical variables were functional disability and disease activity. Functional disability was the only physical variable that was consistently and negatively associated with PA parameters (62% of 13 associations). The summary score between disease activity and PA was deemed indeterminant, however, 42% of 19 associations were negative. Disease activity was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in 8 studies and the Ankylosing Spondylitis Disease Activity Score (ASDAS-CRP) in 4 studies (based on included associations). One study utilized the total Bath Ankylosing Spondylitis Metrology Index (BASMI) score as an indicator of spinal immobility and found it unrelated with PA [74]. However, Arends et al. [73] investigated the separate components of spinal mobility in relation to PA using three different measures of PA (International Physical Activity Questionnaire (IPAQ), Short QUestionnaire to ASsess Health-enhancing physical activity (SQUASH) and Actigraph, GT1M). The Modified Schober test and cervical rotation were positively related to PA in 2 of 3 (67%) comparisons. One positive association was found between lateral spinal flexion and accelerometer measured PA (33%), whereas one negative association was reported between occiput-to-wall distance and PA measured using the SQUASH (33%). Chest expansion was unrelated to PA parameters. The direction of these associations indicates that PA is positively related to spinal mobility. In one study [83], negative associations were seen for reduced activity, mental and physical fatigue (30%), however other sub-categories of the MFI-20-G were non-significant (70%).
In one study, SpA subtypes such as inflammatory bowel disease (IBD)-related arthritis and uSpA were not significantly associated with meeting WHO MI-PA or VI-PA recommendations, however, one negative relationship (OR 0.72, 95% CI 0.56–0.92, p = 0.009) between the diagnosis of PsA and meeting the VI-PA WHO recommendations was found [78]. Extra-musculoskeletal manifestations, BMI, ESR and CRP were also unrelated to PA.
Psychological
Of the psychological variables, only higher quality of life was consistently and positively related to increased PA parameters (83%) in adults with SpA. Although motivation was only investigated in one study, the indeterminant summary code is not overly representative of the findings from this study. Brophy et al. [75] investigated motivation using the Behavioural Regulation in Exercise Questionnaire (BREQ-2) and found relationships between motivation, individual motivation regulations and PA. Positive associations between intrinsic (β slope 1320 (960–1680), p < 0.05) and identified motivation (β slope 994 (651.5–1336), p < 0.05) and PA were found. Further, introjected motivation and external motivation were unrelated to PA, while amotivation (β slope − 667 (− 1242 to − 92), p < 0.05) was negatively related. The findings by Brophy et al. [75] are consistent with the tenets of self-determination theory showing that autonomous motivations towards exercise had the greatest influence on PA levels [85]. Thus, moving forward, motivation will be discussed as being positively related to PA in those with SpA. Health perceptions, depression, and anxiety were unrelated to PA, yet the associations were derived from only three studies.
Social
Social variables were not examined in any of the included SpA studies.
Environmental
Seasonal variation was the only environmental factor explored. One cross-sectional comparative study [77] showed a positive relationship (p ≤ 0.02) between total weekly PA energy expenditure and summer months (i.e., as compared to winter).
Other
In one study [74], results of multiple logistic regression analyses showed that previous levels of PA positively predicted PA after two years (OR 37.03, 95% CI 3.85–370.00, p = 0.002). Physiotherapy and the use of biologics or medication were unrelated to PA. Yet, one multicentre observational study limited to retrospective data, reported that age at onset of anti-Tumor Necrosis Factor alpha (anti-TNFα) therapy was negatively related to PA, whereas anti-TNFα use was associated with increased PA [81].
Pooled associations based on most adjusted or final model statistics
Considering the results of the pooled associations of PA based on the most adjusted or final models for each variable (see Online Resource 2), the association of employment status and symptom duration shifted (non-significant to indeterminant). Further, the indeterminant association of disease activity changed to unrelated.
Pooled associations with only objective measures of PA
The main differences between the pooled associations from all studies and the pooled associations from studies using objective measures of PA are as follows (all studies vs objective PA studies): disease activity (8/19, 42%, ?? vs. 1/7, 14%, 00); ESR (1/3, 33%, 0 vs. 1/1, 100%, −); CRP (1/4, 25%, 0 vs. 1/1, 100%, −); lateral spinal flexion (1/3, 33%, 0 vs. 1/1, 100%, +); and quality of life (5/6, 83%, + + vs. 1/2, 50%, ?). Several variables were only measured in studies using self-reported measures of PA and therefore comparisons cannot be made (e.g., employment status, comorbidities, motivation, and previous levels of PA).
Discussion
This systematic review provides a needed update of the multifaceted correlates of PA in RA, in a field advancing in pharmacological treatments and more frequently utilizing objective measures of PA. To the best of our knowledge, this is also the first systematic review to synthesise the correlates of PA among adults living with SpA. A total of 40 RA and 11 SpA studies were reviewed with correlates examined in the following domains: sociodemographic; physical; psychological; social; and environmental.
Main correlates of PA—Rheumatoid Arthritis
The current systematic review extends and updates Larkin and Kennedy’s [24] review that was limited to ten studies with mainly cross-sectional designs (8/10) and only one study utilizing objective measures of PA. The pooled results of the RA studies in this review support those presented by Larkin and Kennedy [24]. Motivation, self-efficacy, and previous levels of PA were positively associated with PA parameters. Fatigue and plasma glucose were negatively related with PA. HDL was consistently positively related with PA parameters. However, the positive association of health perception with PA was indeterminant in this review. Additional positive associations identified via this latest review include, any use of alcohol, gait speed, lean fat/mass, apolipoprotein A1 and subjective vitality. Additional negative associations include functional inability, stiffness, abdominal obesity, body fat, hypertension, blood pressure, metabolic syndrome, nutritional complications, insulin resistance, RA-related joint surgery, and hospital admissions/length. The most frequently studied variables showing a lack of consistent findings include age, gender, educational level, BMI, disease activity, exercise beliefs, health perception, and health-related quality of life.
This review did not find any of the sociodemographic variables to be consistently related to PA in those with RA. Some evidence that any use of alcohol (100%, n = 1/1) was positively related to PA was reported, however, this was investigated in one study, and was unrelated to PA when the results where pooled based on the most adjusted or final models for each variable. Age and female gender were inconsistently related to PA; however, the associations were predominantly negative (age, 53%, n = 16/30; female gender, 47%, n = 9/19) and non-significant. The trend towards a negative relationship is somewhat consistent with findings in the general population of older adults, whereby higher age and female sex are associated with a decreased likelihood of persistent PA over ten years [86]. The reason for inconsistent or indeterminant pooled associations between sociodemographic variables and PA is likely to be multifaceted. For example, the age ranges between samples varies considerably, with studies utilizing both narrow (i.e., 40–70 years) [63] and wide (i.e., 19–90 years) [40] age ranges. Further, a gender imbalance is observed in many studies, with a female dominance of participants. Assessment of PA may also be a factor, as when pooling only associations of objective measures of PA, female gender was consistently and negatively related to PA (80%, n = 4/5). A possible reason for the negative association between female gender and PA could be that females report greater disease activity and physical function, compared to males with RA [87]. Race/ethnicity was consistently unrelated to PA parameters, yet, caution should be taken in the interpretation of this result, as racial or ethnic minorities were not proportionately represented in some studies. For example, one study reported that 71% of participants were Caucasian, whereas 2% were Asian, and 22% African American [47]. In another study of 573 individuals with RA, 94% of the participants were Caucasian [50].Of the physical variables, functional disability was the most consistent and negative correlate of PA parameters (60%, n = 18/30). Although the findings were more inconsistent when considering the most adjusted or final model statistics, this finding corroborates recent reports linking worse physical functioning to lower levels of PA in those with early RA [88] and PA maintenance over a 7-year period [89]. Further, fatigue was negatively related to PA (61%, n = 11/18), yet the results may be distorted due to one study [55] utilizing the five separate domains of the MFI-20. Univariately, Løppenthin et al. [55] found four of the five domains of fatigue to be inversely associated with regular PA. However, in the multivariate model, only reduced activity due to fatigue and physical fatigue remained significant. Predominantly, our findings support recent longitudinal studies showing a negative relationship between fatigue and PA [89, 90]. Interestingly, disease activity was inconsistently related to PA, however, almost half of the associations were negative (42%, n = 13/31). Disease activity was unrelated to PA when pooling the results based on the most adjusted statistics or when considering studies utilizing objective measures of PA. One possible reason for the inconsistency in results might relate to whether participants were experiencing a flare when PA was measured. Further, the relationship between disease activity and PA might be dependent on the contribution of other variables in explaining PA. For example, recent reports have shown no association between PA and disease activity, when controlling for age and fatigue [91]. BMI and obesity have been shown to negatively associate with PA in the general population [86] and those with hip osteoarthritis [92], yet our findings suggest this relationship is more inconsistent in people living with RA (38%, n = 8/21). This inconsistency in findings is not surprising considering the complex nature of energy expenditure, which is largely determined by body composition and body weight [93]. In support of a previous review [24], RA duration and pain was unrelated to PA parameters. The most consistent physiological variable positively related to PA parameters was HDL (67%, n = 6/9). Other physiological variables, especially those detecting inflammation (i.e., ESR and CRP), were unrelated to PA.
Self-efficacy was one of the most consistent psychological variables positively related with PA (70%, n = 7/10). Greater self-efficacy has been positively associated with PA in healthy populations [94] and women with other long-term conditions such as fibromyalgia [95]. This review adds to the evidence supporting the association between self-efficacy and PA previously reported by Larkin and Kennedy [24], with the addition of six more studies (seven associations) in the RA population. Inconsistent findings were reported for exercise beliefs and expectations, health perception (including global health/assessment), and health-related quality of life. Motivation was the most consistent psychological variable positively related to PA (83%, n = 5/6) in this review. This positive relationship is supported by studies highlighting the importance of autonomous motivation in predicting PA participation levels in 207 RA members of the National Rheumatoid Arthritis Society [96] and in increased engagement in moderate-to-vigorous PA (MVPA) during a randomized controlled trial [97]. Two studies investigating motivation regulation style (on the self-determination theory continuum), showed an autonomous regulatory style to be associated with higher PA, and a more controlling regulatory style to relate to lower PA. The importance of autonomous regulations in fostering PA behaviour is supported by the findings of a systematic review exploring the relationship between self-determination theory constructs (e.g., autonomous vs. controlled motivation) and PA [85].
Social variables in this review were limited to social support and no significant relationship was found. This non-significant relationship was surprising as research conducted with people living with knee and hip osteoarthritis, has reported that perceptions of autonomy support from their spouse regarding PA to be positively related to PA, while perceived pressure from a female spouse (to a male partner), has been shown to be negatively related to PA [92]. In the current review, positive (n = 2), negative (n = 1), and non-significant relationships (n = 6) were found between social support and PA parameters. The variability in results may be due to the differences in assessment methods (Social Support for Exercise Behaviors Scale, 2 studies; Important Other Climate Questionnaire, 1 study; Health Care Climate Questionnaire, 1 study; Medical Outcomes Study Social Support Survey, 2 studies), type of PA parameter (e.g., total PA, trajectory of PA, health-enhancing PA or LPA), or the type of individual assessed for social support (e.g., spouse, friend, rheumatologist). Further research is needed to explore this variability and the factors that affect the relationship between social correlates and PA behaviour.
No environmental correlates were investigated in the RA studies. Of the other variables, and in concordance with two other reviews [23, 24], previous levels of PA were shown to be positively associated with PA parameters (100%, n = 3/3).
Main correlates of PA—Spondyloarthritis
In SpA studies, positive associations with PA were found for components of spinal mobility (Modified Schober test and cervical rotation), maximum volume of oxygen (VO2max), quality of life, motivation, previous PA and seasonal variation. However, in a model predicting PA after two years, spinal immobility (total BASMI) was not significant [74]. Negative associations with PA were found for functional disability, having AS, being amotivated, and age at onset of anti-TNFα therapy. Indeterminant associations were found for marital status, smoking, disease activity, and PsA subtype. All other variables were not significantly associated with PA parameters.
None of the sociodemographic variables were positively or negatively related to PA in those with SpA. Gender and age were unrelated to PA in this review. However, total self-reported PA, in 24 AS patients (aged 48 ± 11 [83]) and meeting the WHO MI-PA recommendations in 2,167 SpA patients (aged 55 ± 14 [78]) were positively associated with age in two studies (29%, n = 2/7). Marital status and smoking were inconsistently related to PA and employment status, education and children raised under 12 years old were unrelated in a limited number of studies.
Of the physical variables, functional disability was frequently investigated, with studies consistently supporting a negative correlation with PA parameters (62%, 8/13). This is supported by recent findings from a prospective cohort study which found that time spent in MVPA was associated with better function [98]. Surprisingly, spinal immobility, measured via total BASMI score was only reported in one study [74]. Here, a non-significant association with PA was reported. However, Arends et al. [73] reported on the relationship between PA and the separate domains of spinal mobility, and together, the direction of associations indicates that higher PA is related to better spinal mobility. This association has also been supported in relation to time spent in MVPA [98], yet the evidence from exercise programmes is more conflicting and lacks clarity [99]. Longitudinal studies are needed to help clarify the relationship between PA and spinal mobility, and the types of PA most related.
Mixed and inconsistent associations were found between PA and disease activity, with studies reporting positive (n = 3), negative (n = 8) and non-significant (n = 8) associations. Such disparity in findings is commensurate with a review investigating the correlates of PA in people with arthritis [23]. The inconsistency in results may partially be due to the intensity of PA measured, for example, one study showed that higher disease activity (measured via the BASDAI) was associated with greater LPA in adults with AS aged ≥ 52 years, but not associated with MVPA in AS patients across all ages [82]. A further explanation for the inconsistency in results may be that some individuals with SpA may participate in PA as a reaction to disease activity, in comparison to those who may start PA engagement earlier as a preventative measure against disease activity. Interestingly, only one study investigated whether SpA subtype was associated with PA [78]. In multivariate analyses, uSpA and IBD-related arthritis were not significantly associated with meeting the MI-PA or VI-PA recommendations, however, PsA was negatively related to meeting VI-PA recommendations. One possible explanation is that individuals with PsA typically experience more inflammation of the peripheral joints than other SpA subtypes [100] and have separate clinical presentations. Due to the limited number of studies, more research is needed to determine potential differences between SpA subtype and PA parameters.
Of the psychological variables, a self-reported better quality of life was associated with higher PA (83%, n = 5/6) in those with SpA. This association is supported in an online survey of 445 Danish individuals with SpA [101] and those with hip osteoarthritis [92]. Motivation to exercise was investigated in one study [75], whereby intrinsic motivation was positively related to PA levels. Using a path analysis, autonomous forms of motivation (i.e., intrinsic motivation and identified regulation) were positively associated to PA, whereas amotivation was negatively associated. Regulation style has also been found to be a significant determinant of PA in those with RA [49]. The importance of motivation is further highlighted in a behaviour change intervention for those with axial spondyloarthritis. Those undertaking the intervention aimed at supporting PA motivation had improved health-enhancing PA and spinal mobility [102]. Despite the apparent importance of motivation on PA behaviour [85], little research has been conducted in those with SpA. Future research should focus on longitudinal study designs or the utilization of ecological momentary assessment methods [103] to yield insight into the causal and fluctuating relationships between PA motivation and PA behaviour.
Previous levels of PA were found to positively correlate to PA [74]. Based on retrospective data, one study reported that individuals who started anti-TNFα therapy earlier had greater gains in PA [81]. Seasonal variation (summer) was positively associated with PA energy expenditure in one study [77].
Recommendations for future research
Many studies adopted a cross-sectional design and therefore causal effects cannot be determined. To establish the causal relationships between variables and PA, prospective and interventional studies are required. In both RA and SpA studies, psychological variables (namely motivation) are underrepresented despite significant associations and the support of intervention studies. In those with SpA, negative and non-significant associations between mental and physical fatigue, and PA were identified, yet fatigue was only evaluated in one study. Research is needed to determine if a stable relationship between fatigue and PA exists in those with SpA. Self-efficacy is positively related to PA in those with RA, yet self-efficacy was not measured in any of the SpA studies. Studies investigating correlates of PA in the axSpA population was generally sparse. Only one eligible study, investigated diagnoses of SpA other than nr-axSpA and AS. The lack of data reported for more peripheral SpA subtypes (e.g., PsA), poses a challenge to clinicians aiming to support PA behaviour. Considering PA is highly recommended in those with SpA, a greater effort is needed to verify the determinants of PA and evaluate potential differences between subtypes. Researchers would do well to employ longitudinal and interventional studies to explore functional ability, in addition to supporting PA self-efficacy and autonomous motivation in these populations. Whilst the reasons for inconsistent results are likely to be multifaceted, the presence of flares have been shown to impact PA behaviour in those with RA and SpA [104]. Although difficult to measure, no eligible studies in this review utilized flares as a variable. Future research should incorporate flares as a variable, with the aim to provide clinically useful information regarding disease activity and its impact on PA.
Strengths and limitations
This systematic review provides an up-to-date and valuable synthesis of the correlates of PA in those with RA and SpA, which can help inform clinical practitioners and intervention design to support the initiation and maintenance of PA. However, several strengths and limitations of this review are apparent. The systematic review included an array of variables, measures of PA (i.e., self-report and objective) and parameters of PA (e.g., total PA, meeting PA recommendations, MVPA). Multiple measures and parameters of PA allow for a more complete account of PA correlates but somewhat hinders the ability to define and conclude the strength of any relationship (i.e., via meta-analysis). A limited number of studies reporting potential correlates of PA in the SpA population met the inclusion criteria. This was somewhat alleviated by permitting studies that included a doctor diagnosis of SpA, instead of the having to meet a classification criterion. While this allows greater opportunity for the exploration of PA correlates in SpA studies, the uniformity between patients is less stringent than if a classification criterion was applied. RA studies were included if the study cohort met the ACR/EULAR criteria [26], which should be considered when comparing the RA and SpA studies and the potential correlates of PA identified. Further, ten SpA studies (n = 10/11) were conducted in Europe, therefore, it is difficult to extrapolate these results to SpA populations outside of Europe who may have differing living conditions, health systems and treatment options. The findings are more generalizable to female persons with RA and male persons with SpA. Female participants were represented more than males in most RA studies, while four RA studies included female participants only. Participation rate of eligible persons and sample size justification were often not reported. The review was limited to fourteen RA (n = 14/40) and four SpA studies (n = 4/11) that utilized objective measures of PA. The remaining studies employed self-report measures of PA, which increases the risk of bias. Self-reported measures of PA may be subject to recall bias, with over or under-reporting of activity possible [105, 106]. The ability to evaluate causal relationships was limited due to the large number of studies adopting a cross-sectional design. Two RA studies were assessed as having a ‘poor’ quality rating, due to unclear reporting [34] and a greater than 15% differential drop-out rate between intervention groups [63].
Conclusions
The aim of this review was to identify potential and consistent correlates of PA in those living with RA and SpA. Few consistent associations with PA were found in the RA and SpA populations. In individuals with RA, consistent positive associations were found between PA and HDL, self-efficacy and motivation, and consistent negative associations with functional disability and fatigue. In individuals with SpA, consistent positive associations were found between PA and quality of life, and consistent negative associations with functional disability. Prospective studies investigating the correlates of PA in these populations are needed, especially in those with SpA. These findings should inform future research and the design of interventions aiming to promote PA in people with RA and SpA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Bath Institute for Rheumatic Diseases (BIRD) and the University of Bath for funding Thomas’s PhD studentship. We would also like to thank Peter Bradley (Subject Librarian) at the University of Bath for his help with search terms and databases.
Author contributions
T.A. Ingram, R. Sengupta, M. Standage, R. Barnett, and P.C. Rouse meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, were responsible for conceptualisation / design of the work or data acquisition, analysis, or interpretation. The authors reviewed and revised the work for intellectual content, approved the final version for publication, and take responsibility for the accuracy and integrity of the work.
Funding
TI received a match-funded PhD studentship from the Bath Institute for Rheumatic Diseases (BIRD) and the University of Bath to conduct the programme of research that this systematic literature review is associated with.
Declarations
Conflict of interest
Dr. Raj Sengupta has previously held unrelated grants from UCB, Pfizer, Abbvie and Novartis and received honoraria for giving talks from Abbvie, Biogen, Lilly, UCB, Novartis and Pfizer. Rosie Barnett has received unrelated funding from UCB. Thomas Ingram, Prof. Martyn Standage, and Dr. Peter Rouse have no disclosures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas Ingram, Email: tai23@bath.ac.uk.
Raj Sengupta, Email: rajsen99@gmail.com.
Martyn Standage, Email: m.standage@bath.ac.uk.
Rosie Barnett, Email: rlb60@bath.ac.uk.
Peter Rouse, Email: p.c.rouse@bath.ac.uk.
References
- 1.Cooksey R, Brophy S, Gravenor MB, Brooks CJ, Burrows CL, Siebert S. Frequency and characteristics of disease flares in ankylosing spondylitis. Rheumatology (Oxford) 2010;49(5):929–932. doi: 10.1093/rheumatology/kep435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wendling D, Prati C. Flare in axial spondyloarthritis. The dark side of the outcome. Ann Rheum Dis. 2016;75:950–951. doi: 10.1136/annrheumdis-2016-209218. [DOI] [PubMed] [Google Scholar]
- 3.Bykerk VP, Bingham CO, Choy EH, Lin D, Alten R, Christensen R, et al. Identifying flares in rheumatoid arthritis: reliability and construct validation of the OMERACT RA Flare Core Domain Set. RMD Open. 2016;26(2):e000225. doi: 10.1136/rmdopen-2015-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechman K, Tweehuysen L, Garrood T, Scott DL, Cope AP, Galloway JB, Ma MHY. Flares in rheumatoid arthritis patients with low disease activity: predictability and association with worse clinical outcome. J Rheumatol. 2018;45(11):1515–1521. doi: 10.3899/jrheum.171375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun J, Sieper J. Ankylosing spondylitis. The Lancet. 2007;369(9570):1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 7.Martindale J, Shukla R, Goodacre J. The impact of ankylosing spondylitis/axial spondyloarthritis on work productivity. Best Pract Res Clin Rheumatol. 2015;29(3):512–523. doi: 10.1016/j.berh.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Bertin P, Fagnani F, Duburcq A, Woronoff AS, Chauvin P, Cukierman G, et al. Impact of rheumatoid arthritis on career progression, productivity, and employability: The PRET Study. Joint Bone Spine. 2016;83(1):47–52. doi: 10.1016/j.jbspin.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global prevalence of spondyloarthritis: a systematic review and meta-regression analysis. Arthritis Car Res. 2016;68(9):1320–1331. doi: 10.1002/acr.22831. [DOI] [PubMed] [Google Scholar]
- 11.Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–877. doi: 10.1007/s00296-020-04731-0. [DOI] [PubMed] [Google Scholar]
- 12.Poddubnyy D, Sieper J. Treatment of axial spondyloarthritis: what does the future hold? Current Rheumatol Rep. 2020;22(9):47. doi: 10.1007/s11926-020-00924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, Ansari RA, Ahmed J. Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract. 2018;27(6):501–507. doi: 10.1159/000493390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rausch Osthoff AK, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(9):1251–1260. doi: 10.1136/annrheumdis-2018-213585. [DOI] [PubMed] [Google Scholar]
- 15.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Dwyer T, O’Shea F, Wilson F. Physical activity in spondyloarthritis: a systematic review. Rheumatol Int. 2015;35(3):393–404. doi: 10.1007/s00296-014-3141-9. [DOI] [PubMed] [Google Scholar]
- 17.Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health. 2012;9(7):1036–1048. doi: 10.1123/jpah.9.7.1036. [DOI] [PubMed] [Google Scholar]
- 18.Rausch Osthoff AK, Juhl CB, Knittle K, Dagfinrud H, Hurkmans E, Braun J, et al. Effects of exercise and physical activity promotion: meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis. RMD Open. 2018;4(2):e000713. doi: 10.1136/rmdopen-2018-000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sveaas SH, Bilberg A, Berg IJ, Provan SA, Rollefstad S, Semb AG, et al. High intensity exercise for 3 months reduces disease activity in axial spondyloarthritis (axSpA): a multicentre randomised trial of 100 patients. Br J Sports Med. 2020;54:292–297. doi: 10.1136/bjsports-2018-099943. [DOI] [PubMed] [Google Scholar]
- 20.Verhoeven F, Tordi N, Prat C, Demougeot C, Mougin F, Wendling D. Physical activity in patients with rheumatoid arthritis. Joint Bone Spine. 2016;83(3):265–270. doi: 10.1016/j.jbspin.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 21.McDonald MT, Siebert S, Coulter EH, McDonald DA, Paul L. Level of adherence to prescribed exercise in spondyloarthritis and factors affecting this adherence: a systematic review. Rheumatol Int. 2019;39(2):187–201. doi: 10.1007/s00296-018-4225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezzat AM, MacPherson K, Leese J, Li LC. The effects of interventions to increase exercise adherence in people with arthritis: a systematic review. Musculoskeletal Care. 2015;13(1):1–18. doi: 10.1002/msc.1084. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox S, Der Ananian C, Sharpe PA, Robbins J. Correlates of physical activity in persons with arthritis: review and recommendations. J Phys Act Health. 2005;2(2):230–252. doi: 10.1123/jpah.2.2.230. [DOI] [Google Scholar]
- 24.Larkin L, Kennedy N. Correlates of physical activity in adults with rheumatoid arthritis: a systematic review. J Phys Act Health. 2014;11(6):1248–1261. doi: 10.1123/jpah.2012-0194. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 27.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 28.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary behaviour research network (SBRN) – terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org
- 30.National Heart, Lung, and Blood Institute. Study quality assessment tools. Bethesda: U.S. Department of Health & Human Services. Available at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 31.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Med Res. 2020 doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of and adolescents. Med Sci Sports Exerc. 2000;32(5):963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Bryam KW, Oeser AM, Linton MF, Fazio S, Stein CM, Ormseth MJ. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018;24(8):417–421. doi: 10.1097/RHU.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conigliaro P, Triggianese P, Ippolito F, Lucchetti R, Chimenti MS, Perricone R. Insights on the role of physical activity in patients with rheumatoid arthritis. Drug Dev Rev. 2014;75(S1):S54–56. doi: 10.1002/ddr.21196. [DOI] [PubMed] [Google Scholar]
- 35.Demmelmaier I, Dufour AB, Nordgren B, Opava CH. Trajectories of physical activity over two years in persons with rheumatoid arthritis. Arthritis Care Res. 2016;68(8):1069–1077. doi: 10.1002/acr.22799. [DOI] [PubMed] [Google Scholar]
- 36.Demmelmaier I, Bergman P, Nordgren B, Jensen I, Opava CH. Current and maintained health-enhancing physical activity in rheumatoid arthritis: a cross-sectional study. Arthritis Care Res. 2013;65(7):1166–1176. doi: 10.1002/acr.21951. [DOI] [PubMed] [Google Scholar]
- 37.Demmelmaier I, Björk A, Dufour AB, Nordgren B, Opava CH. Trajectories of fear-avoidance beliefs on physical activity over two years in people with rheumatoid arthritis. Arthritis Care Res. 2018;70(5):695–702. doi: 10.1002/acr.23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrlich-Jones L, Lee J, Semanik P, Cox C, Dunlop D, Chang RW. Relationship between beliefs, motivation, and worries about physical activity and physical activity participation in persons with rheumatoid arthritis. Arthritis Care Res. 2011;63(12):1700–1705. doi: 10.1002/acr.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkan AC, Håkansson N, Frostegård J, Hafström I. Low level of physical activity in women with rheumatoid arthritis is associated with cardiovascular risk factors but not with body fat mass—a cross sectional study. BMC Musculoskelet Disord. 2011;12:13. doi: 10.1186/1471-2474-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eurenius E, Stenström CH. Physical activity, physical fitness, and general health perception among individuals with rheumatoid arthritis. Arthritis Rheum. 2005;53(1):48–55. doi: 10.1002/art.20924. [DOI] [PubMed] [Google Scholar]
- 41.Eurenius E, Brodin N, Lindbald S, Opava CH, PARA Study Group Predicting physical activity and general health perception among patients with rheumatoid arthritis. J Rheumatol. 2007;34(1):10–15. [PubMed] [Google Scholar]
- 42.Fenton SAM, Veldhuijzen van Zanten JJCS, Metsios GS, Rouse PC, Yu C, Kitas GD, Duda JL. Autonomy support, light physical activity and psychological well-being in rheumatoid arthritis: a cross-sectional study. Ment Health Phys Act. 2018;14:11–18. doi: 10.1016/j.mhpa.2017.12.002. [DOI] [Google Scholar]
- 43.Greene BL, Haldeman GF, Kaminski A, Neal K, Lim SS, Conn DL. Factors affecting physical activity behaviour in urban adults with arthritis who are predominantly African-American and female. Phys Ther. 2006;86(4):510–519. doi: 10.1093/ptj/86.4.510. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto T, Yoshiuchi K, Inada S, Shirakura K, Wada N, Takeuchi K, Matsushita M. Physical activity of elderly patients with rheumatoid arthritis and healthy individuals: an actigraph study. Biopsychosoc Med. 2015;9:19. doi: 10.1186/s13030-015-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henchoz Y, Bastardot F, Guessous I, Theler JM, Dudler J, Vollenweider P, So A. Physical activity and energy expenditure in rheumatoid arthritis patients and healthy controls. Rheumatology (Oxford) 2012;51(8):1500–1507. doi: 10.1093/rheumatology/kes067. [DOI] [PubMed] [Google Scholar]
- 46.Hernández-Hernández V, Ferraz-Amaro I, Díaz-González F. Influence of disease activity on the physical activity of rheumatoid arthritis patients. Rheumatology (Oxford) 2014;53(4):722–731. doi: 10.1093/rheumatology/ket422. [DOI] [PubMed] [Google Scholar]
- 47.Huffman KM, Pieper CF, Hall KS, St Clair EW, Kraus WE. Self-efficacy for exercise, more than disease-related factors, is associated with objectively assessed exercise time and sedentary behaviour in rheumatoid arthritis. Scand J Rheumatol. 2015;44(2):106–110. doi: 10.3109/03009742.2014.931456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hugo M, Mesen-Cetre N, Pierreisnard A, Schaeverbeke T, Gin H, Rigalleau V. Energy expenditure and nutritional complications of metabolic syndrome and rheumatoid cachexia in rheumatoid arthritis: an observational study using calorimetry and actimetry. Rheumatology (Oxford) 2016;55(7):1202–1209. doi: 10.1093/rheumatology/kew038. [DOI] [PubMed] [Google Scholar]
- 49.Hurkmans EJ, Maes S, de Gucht V, Knittle K, Peeters AJ, Ronday HK, Vliet Vlieland TPM. Motivation as a determinant of physical activity in patients with rheumatoid arthritis. Arthritis Care Res. 2010;62(3):371–377. doi: 10.1002/acr.20106. [DOI] [PubMed] [Google Scholar]
- 50.Iversen MD, Frits M, von Heideken J, Cui J, Weinblatt M, Shadick NA. Physical activity and correlates of physical activity participation over three years in adults with rheumatoid arthritis. Arthritis Care Res. 2017;69(10):1535–1545. doi: 10.1002/acr.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz P, Margaretten M, Trupin L, Schmajuk G, Yazdany J, Yelin E. Role of sleep disturbance, depression, obesity, and physical inactivity in fatigue in rheumatoid arthritis. Arthritis Care Res. 2016;68(1):81–90. doi: 10.1002/acr.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoja SS, Almeida GJ, Wasko MC, Terhorst L, Piva SR. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res. 2016;68(4):424–431. doi: 10.1002/acr.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knittle KP, De Gucht V, Hurkmans EJ, Vliet Vlieland TPM, Peeters AJ, Ronday HK, Maes S. Effect of self-efficacy and physical activity goal achievement on arthritis pain and quality of life in patients with rheumatoid arthritis. Arthritis Care Res. 2011;63(11):1613–1619. doi: 10.1002/acr.20587. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Dunlop D, Ehrlich-Jones L, Semanik P, Song J, Manheim L, Chang RW. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res. 2012;64(4):488–493. doi: 10.1002/acr.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Løppenthin K, Esbensen BA, Østergaard M, Jennum P, Tolver A, Aadahl M, Thomsen T, Midtgaard J. Physical activity and the association with fatigue and sleep in Danish patients with rheumatoid arthritis. Rheumatol Int. 2015;35(10):1655–1664. doi: 10.1007/s00296-015-3274-5. [DOI] [PubMed] [Google Scholar]
- 56.Lundgren S, Olausson SA, Bergström G, Stenström CH. Physical activity and pain among patients with rheumatoid arthritis – a cognitive approach. Adv Physiother. 2005;7(2):77–83. doi: 10.1080/14038190510010322. [DOI] [Google Scholar]
- 57.Malm K, Bergman S, Andersson M, Bremander A, the BARFOT study group, Predictors of severe self-reported disability in RA in a long-term follow-up study. Disabil Rehabil. 2015;37(8):686–691. doi: 10.3109/09638288.2014.939773. [DOI] [PubMed] [Google Scholar]
- 58.Mancuso CA, Rincon M, Sayles W, Paget SA. Psychosocial variables and fatigue: a longitudinal study comparing individuals with rheumatoid arthritis and healthy controls. J Rheumatol. 2006;33(8):1496–1502. [PubMed] [Google Scholar]
- 59.McKenna S, Tierney M, O’Neill A, Fraser A, Kennedy N. Sleep and physical activity: a cross-sectional objective profile of people with rheumatoid arthritis. Rheumatol Int. 2018;38(5):845–853. doi: 10.1007/s00296-018-4009-1. [DOI] [PubMed] [Google Scholar]
- 60.Metsios GS, Stavropoulos-Kalinoglou A, Treharne GJ, Nevill AM, Sandoo A, Panoulas VF, et al. Disease activity and low physical activity associate with number of hospital admissions and length of hospitalisation in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R108. doi: 10.1186/ar3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mochizuki T, Yano K, Ikari K, Kawakami K, Hiroshima R, Momohara S. Relationship between achievement of physical activity goal and characteristics of patients with rheumatoid arthritis. Mod Rheumatol. 2018;28(4):606–610. doi: 10.1080/14397595.2017.1371104. [DOI] [PubMed] [Google Scholar]
- 62.Munsterman T, Takken T, Wittink H. Low aerobic capacity in physical activity not associated with fatigue in patients with rheumatoid arthritis: a cross-sectional study. J Rehabil Med. 2013;45(2):164–169. doi: 10.2340/16501977-1073. [DOI] [PubMed] [Google Scholar]
- 63.Neuberger GB, Aaronson LS, Gajewski B, Embretson SE, Cagle PE, Loudon JK, Miller PA. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57(6):943–952. doi: 10.1002/art.22903. [DOI] [PubMed] [Google Scholar]
- 64.Piva SR, Almeida GJM, Wasko MCM. Association of physical function and physical activity in women with rheumatoid arthritis. Arthritis Care Res. 2010;62(8):1144–1151. doi: 10.1002/acr.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prioreschi A, Hodkinson B, Avidon I, Tikly M, McVeigh J. The clinical utility of accelerometry in patients with rheumatoid arthritis. Rheumatology (Oxford) 2013;52(9):1721–1727. doi: 10.1093/rheumatology/ket216. [DOI] [PubMed] [Google Scholar]
- 66.Rongen-van Dartel SA, Repping-Wuts H, van Hoogmoed D, Knoop H, Bleijenberg G, van Riel PLCM, Fransen J. Relationship between objectively assessed physical activity and fatigue in patients with rheumatoid arthritis: inverse correlation of activity and fatigue. Arthritis Care Res. 2014;66(6):852–860. doi: 10.1002/acr.22251. [DOI] [PubMed] [Google Scholar]
- 67.Semanik P, Wilbur J, Sinacore J, Chang RW. Physical activity behaviour in older women with rheumatoid arthritis. Arthritis Rheum. 2004;51(2):246–252. doi: 10.1002/art.20245. [DOI] [PubMed] [Google Scholar]
- 68.Stavropoulos-Kalinoglou A, Metsios GS, Smith JP, Panoulas VF, Douglas KMJ, Jamurtas AZ, Koutedakis Y, Kitas GD. What predicts obesity in patients with rheumatoid arthritis? An investigation of the interaction between lifestyle and inflammation. Int J Obes. 2010;34:295–301. doi: 10.1038/ijo.2009.220. [DOI] [PubMed] [Google Scholar]
- 69.Tierney M, Fraser A, Purtill H, Kennedy N. Profile of energy expenditure in people with rheumatoid arthritis. Disabil Health J. 2015;8(4):514–520. doi: 10.1016/j.dhjo.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Uutela T, Kautiainen H, Järvenpää S, Salomaa S, Hakala M, Häkkinen A. Waist circumference based abdominal obesity may be helpful as a marker for unmet needs in patients with RA. Scand J Rheumatol. 2014;43(4):279–285. doi: 10.3109/03009742.2013.858769. [DOI] [PubMed] [Google Scholar]
- 71.Van den Berg MH, de Boer IG, le Cessie S, Breedveld FC, Vliet Vlieland TPM. Most people with rheumatoid arthritis undertake leisure-time physical activity in the Netherlands: an observational study. Aust J Physiother. 2007;53(2):113–118. doi: 10.1016/S0004-9514(07)70044-2. [DOI] [PubMed] [Google Scholar]
- 72.Van der Goes MC, Hoes JN, Cramer MJ, van der Veen MJ, van der Werf JH, Bijlsma JWJ, Jacobs JWG. Identifying factors hampering physical activity in longstanding rheumatoid arthritis: what is the role of glucocorticoid therapy? Clin Exp Rheumatol. 2014;32(2):155–161. [PubMed] [Google Scholar]
- 73.Arends S, Hofman M, Kamsma YPT, van der Veer E, Houtman PM, Kallenberg CGM, Spoorenberg A, Brouwer E. Daily physical activity in ankylosing spondylitis: validity and reliability of the IPAQ and SQUASH and the relation with clinical assessments. Arthritis Res Ther. 2013;15(4):R99. doi: 10.1186/ar4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brodin N, Opava CH. Predicting general health perception and exercise habits in ankylosing spondylitis. Adv Physiother. 2007;9(1):23–30. doi: 10.1080/14038190601090901. [DOI] [Google Scholar]
- 75.Brophy S, Cooksey R, Davies H, Dennis MS, Zhou SM, Siebert S. The effect of physical activity and motivation on function in ankylosing spondylitis: a cohort study. Semin Arthritis Rheum. 2013;42(6):619–626. doi: 10.1016/j.semarthrit.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fabre S, Molto A, Dadoun S, Rein C, Hudry C, Kreis S, et al. Physical activity in patients with axial spondyloarthritis: a cross-sectional study of 203 patients. Rheumatol Int. 2016;36(12):1711–1718. doi: 10.1007/s00296-016-3565-5. [DOI] [PubMed] [Google Scholar]
- 77.Fongen C, Halvorsen S, Dagfinrud H. High disease activity is related to low levels of physical activity in patients with ankylosing spondylitis. Clin Rheumatol. 2013;32(12):1719–1725. doi: 10.1007/s10067-013-2320-5. [DOI] [PubMed] [Google Scholar]
- 78.Haglund E, Bergman S, Petersson IF, Jacobsson LTH, Strömbeck B, Bremander A. Differences in physical activity patterns in patients with spondyloarthritis. Arthritis Care Res. 2012;64(12):1886–1894. doi: 10.1002/acr.21780. [DOI] [PubMed] [Google Scholar]
- 79.Meesters JJL, Petersson IF, Bergman S, Haglund E, Jacobsson LTH, Bremander A. Sociodemographic and disease-related factors are associated with patient-reported anxiety and depression in spondyloarthritis patients in the Swedish SpAScania cohort. Clin Rheumatol. 2014;33(11):1649–1656. doi: 10.1007/s10067-014-2699-7. [DOI] [PubMed] [Google Scholar]
- 80.O’Dwyer T, O’Shea F, Wilson F. Decreased physical activity and cardiorespiratory fitness in adults with ankylosing spondylitis: a cross-sectional controlled study. Rheumatol Int. 2015;35(11):1863–1872. doi: 10.1007/s00296-015-3339-5. [DOI] [PubMed] [Google Scholar]
- 81.Prince DS, McGuigan LE, McGirr EE. Working life and physical activity in ankylosing spondylitis pre and post anti-tumor necrosis factor-alpha therapy. Int J Rheum Dis. 2014;17(2):165–172. doi: 10.1111/1756-185x.12018. [DOI] [PubMed] [Google Scholar]
- 82.Van Genderen S, Boonen A, van der Heijde S, Heuft L, Luime J, Spoorenberg A, et al. Accelerometer quantification of physical activity and activity patterns in patients with ankylosing spondylitis and population controls. J Rheumatol. 2015;42(12):2369–2375. doi: 10.3899/jrheum.150015. [DOI] [PubMed] [Google Scholar]
- 83.Van Genderen S, van den Borne C, Geusens P, van der Linden S, Boonen A, Plasqui G. Physical functioning in patients with ankylosing spondylitis: comparing approaches of experienced ability with self-reported and objectively measured physical activity. J Clin Rheumatol. 2014;20(3):133–137. doi: 10.1097/RHU.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 84.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- 85.Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act. 2012;9:78. doi: 10.1186/1479-5868-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith L, Gardner B, Fisher A, Hamer M. Patterns and correlates of physical activity behaviour over 10 years in older adults: prospective analyses from the English Longitudinal Study of Ageing. BMJ Open. 2015;5(4):e007423. doi: 10.1136/bmjopen-2014-007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahlmén M, Svensson B, Albertsson K, Forslind K, Hafström I, BARFOT Study Group Influence of gender on assessments of disease activity and function in early rheumatoid arthritis in relation to radiographic joint damage. Ann Rheum Dis. 2010;69(1):230–233. doi: 10.1136/ard.2008.102244. [DOI] [PubMed] [Google Scholar]
- 88.Qvarfordt M, Andersson MLE, Larsson I. Factors influencing physical activity in patients with early rheumatoid arthritis: a mixed-methods study. SAGE Open Med. 2019;7:2050312119874995. doi: 10.1177/2050312119874995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bremander A, Malm K, Andersson ML, BARFOT study group Physical activity in established rheumatoid arthritis and variables associated with maintenance of physical activity over a seven-year period – a longitudinal observation study. BMC Rheumatol. 2020;4:53. doi: 10.1186/s41927-020-00151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lange E, Gjertsson I, Mannerkorpi K. Long-time follow up of physical activity level among older adults with rheumatoid arthritis. Eur Rev Aging Phys Act. 2020;17:10. doi: 10.1186/s11556-020-00242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toyoshima Y, Yajima N, Nemoto T, Namiki O, Inagaki K. Relationship between disease activity level and physical activity in rheumatoid arthritis using a triaxial accelerometer and self-reported questionnaire. BMC Res Notes. 2021;14(1):242. doi: 10.1186/s13104-021-05666-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stubbs B, Hurley M, Smith T. What are the factors that influence physical activity participation in adults with knee and hip osteoarthritis? A systematic review of physical activity correlates. Clin Rehabil. 2015;29(1):80–94. doi: 10.1177/0269215514538069. [DOI] [PubMed] [Google Scholar]
- 93.Westerterp K. Control of energy expenditure in humans. Eur J Clin Nutr. 2017;71:340–344. doi: 10.1038/ejcn.2016.237. [DOI] [PubMed] [Google Scholar]
- 94.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34(12):1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 95.Oliver K, Cronan TA. Correlates of physical activity among women with fibromyalgia syndrome. Ann Behav Med. 2005;29:44–53. doi: 10.1207/s15324796abm2901_7. [DOI] [PubMed] [Google Scholar]
- 96.Yu CA, Rouse PC, Van Zanten JVJCS, Metsios GS, Ntoumanis N, Kitas GD, Duda JL. Motivation-related predictors of physical activity engagement and vitality in rheumatoid arthritis patients. Health Psychol Open. 2015;2(2):2055102915600359. doi: 10.1177/2055102915600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fenton SAM, Van Zanten JVJCS, Metsios GS, Rouse PC, Yu CA, Ntoumanis N, Kitas GD, Duda JL. Testing a self-determination theory-based process model of physical activity behaviour change in rheumatoid arthritis: results of a randomized controlled trial. Transl Behav Med. 2021;11(2):369–380. doi: 10.1093/tbm/ibaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coulter EH, McDonald MT, Cameron S, Siebert S, Paul L. Physical activity and sedentary behaviour and their associations with clinical measures in axial spondyloarthritis. Rheumatol Int. 2020;40(3):375–381. doi: 10.1007/s00296-019-04494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Regnaux JP, Davergne T, Roren PC, A, Rannou F, Boutron I, Lefevre-Colau MM, Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019;10(10):CD011321. doi: 10.1002/14651858.CD011321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rossou E, Sultana S. Early spondyloarthritis in multiracial society: differences between gender, race, and disease subgroups with regard to first symptom at presentation, main problem that the disease is causing to patients, and employment status. Rheumatol Int. 2012;32(6):1597–1604. doi: 10.1007/s00296-010-1680-2. [DOI] [PubMed] [Google Scholar]
- 101.Rasmussen JO, Primdahl J, Fick W, Bremander A. Physical activity in people with axial spondyloarthritis and the impact of overall attitudes, barriers, and facilitators: a cross-sectional study. Musculoskeletal Care. 2020;18(4):510–518. doi: 10.1002/msc.1495. [DOI] [PubMed] [Google Scholar]
- 102.O’Dwyer T, Monaghan A, Moran J, O’Shea F, Wilson F. Behaviour change intervention increases physical activity, spinal mobility and quality of life in adults with ankylosing spondylitis: a randomised trial. J Physiother. 2017;63(1):30–39. doi: 10.1016/j.jphys.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Taylor IM, Ntoumanis N, Standage M, Spray CM. Motivational predictors of physical education students’ effort, exercise intentions, and leisure-time physical activity: a multilevel linear growth analysis. J Sport Exerc Psychol. 2010;32(1):99–120. doi: 10.1123/jsep.32.1.99. [DOI] [PubMed] [Google Scholar]
- 104.Jacquemin C, Molto A, Servy H, et al. Flares assessed weekly in patients with rheumatoid arthritis or axial spondyloarthritis and relationship with physical activity measured using a connected activity tracker: a 3-month study. RMD Open. 2017;3(1):e000434. doi: 10.1136/rmdopen-2017-000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neilson HK, Robson PJ, Friedenreich CM, Csizmadi I. Estimating activity energy expenditure: how valid are physical activity questionnaires. Am J Clin Nutr. 2008;87(2):279–291. doi: 10.1093/ajcn/87.2.279. [DOI] [PubMed] [Google Scholar]
- 106.Ferrari P, Friedenreich C, Matthews CE. The role of measurement error in estimating levels of physical activity. Am J Epidemiol. 2007;166(7):832–840. doi: 10.1093/aje/kwm148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.