Abstract
In patients with head and neck cancer, irradiation (IR)-sensitive salivary gland (SG) tissue is highly prone to damage during radiotherapy (RT). This leads to SG hypofunction and xerostomia. Xerostomia is defined as the subjective complaint of dry mouth, which can cause other symptoms and adversely affect the quality of life. In recent years, diagnostic techniques have constantly improved with the emergence of more reliable and valid questionnaires as well as more accurate equipment for saliva flow rate measurement and imaging methods. Preventive measures such as the antioxidant MitoTEMPO, botulinum toxin (BoNT), and growth factors have been successfully applied in animal experiments, resulting in positive outcomes. Interventions, such as the new delivery methods of pilocarpine, edible saliva substitutes, acupuncture and electrical stimulation, gene transfer, and stem cell transplantation, have shown potential to alleviate or restore xerostomia in patients. The review summarizes the existing and new diagnostic methods for xerostomia, along with current and potential strategies for reducing IR-induced damage to SG function. We also aim to provide guidance on the advantages and disadvantages of the diagnostic methods. Additionally, most prevention and treatment methods remain in the stage of animal experiments, suggesting a need for further clinical research, among which we believe that antioxidants, gene transfer, and stem cell transplantation have broad prospects.
1. Introduction
Head and neck cancer is defined as a localized malignant tumor of the head and neck. A commonly used treatment regime includes surgery combined with RT [1]. Despite the continuous progress in RT techniques, damage to the surrounding healthy cells or tissues is possible. The SGs proliferate slowly and are sensitive to IR; therefore, damage to SGs is common and irreversible [2–4]. The potential mechanism of IR leading to the loss of SG function has been studied in animal models, which may be related to DNA damage, loss of acinar cell number, increase in reactive oxygen species (ROS), decrease in proliferation and differentiation ability of stem/progenitor cells, and abnormal calcium signaling. In later stages, it may be associated with changes in blood vessels, glandular fibrosis, and inflammation [2, 5, 6].
SG dysfunction can lead to reduced salivary secretion, resulting in xerostomia, which significantly affects the quality of life of patients [1]. Saliva, although more than 99% water, contains many important functional components [7]. The proteins in saliva include mucin-, immunoglobulin-, and proline-rich proteins, which play vital roles in lubrication, antibacterial activity, defense, and protection of the teeth [8–10]. Additionally, other components of saliva can help digest food, regulate pH, strengthen the sense of taste, neutralize harmful substances, and promote wound healing [11]. Therefore, if SG function is damaged, leading to xerostomia, it will inevitably have an impact on the whole mouth, causing oral dryness, mucosal atrophy and ulcer, mastication and swallowing difficulties, caries, infection, and other serious consequences [1, 12].
A MEDLINE/PubMed search was conducted using the terms “xerostomia” OR, “salivary gland hypofunction” AND, “diagnosis” OR, “treatment” OR, “prevention” OR, “questionnaire” OR, “saliva flow rate measurement and oral moisture-checking device” OR, “imaging techniques” OR, “advances in RT” OR, “antioxidants” OR, “botulinum toxin” OR, “submandibular gland transfer” OR, “growth factors” OR, “rapamycin and limonene” OR, “saliva substitutes” OR, “pharmacological salivation agents” OR, “acupuncture and electrical stimulation” OR, “hyperbaric oxygen therapy” OR, “gene therapy” OR, “stem cells.” Published articles from 2010 to 2022 were included, and some significant references were also reviewed. After the initial search, literature with incomplete data and low credibility were excluded. Since many of the methods in the prevention and treatment parts mentioned in this article still lack sufficient clinical trials to prove their effectiveness, we have included some results from animal experiments to demonstrate the potential value of the methods.
This article discusses the xerostomia diagnostic methods that are currently being used and those being newly developed, as well as the current and potential strategies for SG recovery and presents their advantages and disadvantages. The primary aim is to provide guidance for further research in this field.
2. Diagnosis
2.1. Questionnaire
Questionnaires, which are not limited to the assessment of dry mouth but also cover other complications, play a significant role in evaluating xerostomia. They are low-cost, easy to complete by patients and can be evaluated by clinicians. This method can also be used for long-term detection. However, the questionnaires are subjective; therefore, they cannot always reflect the SG function [13, 14].
The Xerostomia Questionnaire, Xerostomia Inventory, Summated Xerostomia Inventory, and visual analog scale are commonly used to evaluate xerostomia, and their effectiveness has been confirmed [15–18]. In addition, the Groningen RT-Induced Xerostomia questionnaire, which was developed in 2010, was used to evaluate the degree of xerostomia and salivary viscosity and is the only tool explicitly developed in the RT-induced xerostomia population [14, 19]. The Groningen RT-Induced Xerostomia questionnaire contains 14 items that are measured during the daytime and nighttime, allowing it to distinguish between a patient's xerostomia in different time frames [19]. It has good reliability, responsiveness, and criterion validity, but Assas et al. found that the construct validity of the questionnaire was indeterminate [14, 19]. The Multidisciplinary Salivary Gland Society questionnaire was created in 2021. It mainly quantified the symptoms of xerostomia, including 20 questions and two scoring systems (Q3 for question answering and Q10 for the visual analog scale). The reliability coefficients of both scoring systems were ≥0.9; however, more clinical trials are needed to further validate the questionnaire. The developers of the MGSG questionnaire recommend using the Q10 system because it is easier for patients to understand and more accurate in translating into different languages [20].
2.2. Saliva Flow Rate Measurement and Oral Moisture-Checking Device
Many studies have shown a moderate correlation between saliva flow rate and xerostomia, so it can also be an indicator of xerostomia. In addition, the saliva flow rate measurement can better reflect the SG function, which is more accurate and reliable than the questionnaires in the evaluation of SG function [21–23]. For some patients, measuring unstimulated whole saliva (UWS), stimulated whole saliva (SWS), or the saliva of a single SG or minor SGs is essential for a clinical diagnosis [24]. The current and new methods for measuring saliva flow rates are summarized in Table 1.
Table 1.
Summary of measurement methods of saliva flow rate.
| Type | Methods | Procedure | Pros | Cons |
|---|---|---|---|---|
| Unstimulated whole saliva [UWS] | Draining method | Allow the saliva to flow naturally down the lower lip and collect it in a graduated container [21] | No effect of slow rate [25] More representative of unstimulated secretion [25] Good repeatability [11] Easy use [11] |
Time-consuming [26] Unattractive [26] Remaining saliva in oral [27] Need good collaboration [25] |
| Spitting Method | Allow the saliva to cumulate at the bottom of the mouth for a while and then spit into a graduated container [21] | Less evaporation of saliva [25] Good repeatability [11] Easy use [11] |
Time-consuming [26] Unattractive [26] Incomplete spit [27] Stimulatory effects [25] Need good collaboration [25] |
|
| Swab method | Allow the saliva to be absorbed with pre-weighed cotton rolls and reweighed at the end of the collection period [21] | Low cost [25] Easy use [25] Suitable for less- or non-collaborative patients [25] |
Risk of swallowing [25] Possible stimulatory effect [28] |
|
| Suction method | Allow the saliva to be sucked into a graduated container by a negative pressure suction device in the closed or open suction method [21] | High reliability [29] Time-saving [29] No strict demands for Collaboration [29] |
Require skilled personnel [29] Possible stimulatory effect [28] |
|
| BokaFlo™ | Place BokaFlo™ disposable device under the subject's tongue to collect saliva, then the device was removed and placed on the BokaFlo™ instrument for measurement [30] | High sensitivity [30] High specificity [30] Comfortable [30] Time-saving [30] |
Underestimate saliva flow rate [30] | |
|
| ||||
| Stimulated whole saliva [SWS] | Acid stimulation method | Place a solution of 2% citric acid on each side of the tongue every 30 seconds for five minutes and collect saliva [21] | Get more saliva in a short time [31] | Change the composition of saliva [25] Change salivary pH [25] Need collaboration [25] |
| Chewing method | Collect saliva after chewing an unflavored gum base or paraffin wax [21] | Get more saliva in a short time [31] | Change the composition of saliva [25] Change salivary pH [25] Uncontrollable chewing force and force duration [32] |
|
|
| ||||
| Major salivary gland | Parotid gland | Place the Lashley cup at the mouth of the parotid gland catheter to collect saliva from the parotid gland [32] | Not invasive [25] | Time-consuming [33] Complex procedure [33] Required skilled personnel [33] |
| Submandibular gland and sublingual gland | Use a Wolff saliva collector to collect saliva from the submandibular and sublingual gland [32] | Not invasive [33] | Time-consuming [33] Complex procedure [33] Require skilled personnel [33] |
|
| Minor salivary gland | Iodine-starch filter paper method | Place the iodine-starch filter paper on the lower lip for a period of time and then scan and digitize with an image scanner [34] | Comfortable [34] Easy use [34] Larger area of lower lip [34] |
No large volume collected [34] Contain harmful iodine [34] Not for people allergic to iodine [34] |
| Electronic sialometry device | Measure the electrical resistance of a filter paper that has absorbed saliva, providing an estimate of saliva volume [35] | Easy use [35] Low cost [35] Quick [34] |
No large volume collected [35] | |
| Standard filter paper | Place a standard filter paper on the buccal mucosa to absorb saliva and weigh it [36] | Not invasive [25] Easy use [36] |
No large volume collected [36] | |
An oral moisture-checking device is used to diagnose xerostomia by measuring the oral moisture of the lingual and buccal mucosa [37]. The results of oral moisture measurements have a weak positive correlation with saliva flow rate, possibly because the chief complaint of xerostomia is not always correlated with saliva flow rate [37]. Third-generation oral moisture-checking devices have been widely used in xerostomia diagnosis. Furthermore, Fukushima et al. first confirmed the effectiveness of a fourth-generation device and its reliability in all age groups. However, the force of the instrument sensor placed on the oral mucosa is difficult to control [37, 38]. In conclusion, the oral moisture-checking device appears to have excellent prospects.
2.3. Imaging Techniques
RT-induced gland atrophy and necrosis can lead to pathological findings such as reduced volume, increased heterogeneity, and unclear boundaries of the gland, which can evaluate SG function and then predict xerostomia [3]. Contrast-enhanced computed tomography with high-density structural and spatial resolution can quickly examine the appearance and cysts of the SGs with ease. However, it has certain ionizing radiation; therefore, ultrasound imaging and magnetic resonance imaging (MRI) can be used to evaluate SG function in non-invasive conditions without ionizing radiation [3, 39]. Ultrasound imaging is used to evaluate gland function based on the information obtained from ultrasound scans, such as size, inflammation, and homogeneity [40]. It is cost-effective but does not adequately reveal lesions [39, 41]. MRI evaluates the degree of SG injury based on the decrease in volume and increase in signal intensity of T2-weighted images [42]. The MRI detection of SGs is highly sensitive, expensive, and heavily affected by metals [3, 42]. Salivary gland scintigraphy is an imaging technique used to measure the uptake and excretion of SGs using the radioactive tracer Technetium-99m pertechnetate [43]. Salivary gland scintigraphy has the advantages of being noninvasive, easy to perform, reproducible, and well tolerated by patients. However, the lack of accurate quantitative reference values and the absence of standardized protocols limit its widespread application [43, 44].
Sialography is another important imaging method the allows a better prediction of xerostomia by assessing the status of the salivary ducts [45]. Owing to the drawbacks of X-ray sialography, such as the injection of contrast agents, ionizing radiation, and the considerable risk of intubation failure, an advanced magnetic resonance (MR) sialography has been proposed [3, 46]. MR sialography uses saliva itself as the contrast medium, which enables the technique to observe changes in a small amount of saliva in the salivary ducts to effectively assess IR-induced xerostomia [46]. In general, MR sialography offers high security, high accuracy, and has the potential for development [3, 46].
3. Prevention
3.1. Advances in RT
Compared to traditional RT, intensity-modulated radiation therapy (IMRT) can adjust IR according to the shape of the target region. It can maximize the IR dose to tumors and reduce the dose that endangers normal tissues and organs, thereby improving the therapeutic effect [47]. Several studies have found that IMRT can efficiently preserve or restore SG function better than conventional RT [47, 48]. Analysis by Ge et al. also showed that the health condition and cognitive function were significantly better in patients in the IMRT group than in the conventional RT group [47]. Volumetric modulated arc therapy (VMAT) is a promising treatment technique [49] that significantly increases the number of beams, improves efficiency, and reduces the uncertainty of equipment [50, 51]. Compared with IMRT, VMAT has advantages such as dose sparing, improved uniformity, reduced IR range, and aiding in alleviating acute dysphagia [49, 52].
In recent years, intensity-modulated proton therapy (IMPT), an emerging and promising treatment for head and neck cancer, is showing reduced toxicity to healthy tissues compared to IMRT and VMAT [53, 54]. This is because the Bragg peak phenomenon of proton beam therapy can generate a more favorable dose distribution curve than photon-based RT techniques [54]. However, IMPT is still challenged by uncertainty in the particle range and the difficulty of adapting to complex anatomical structures [53].
With the development of science and technology, new RT techniques have gradually replaced traditional RT techniques, providing excellent locoregional control and toxicity reduction [51]. However, owing to more professional operating techniques and expensive equipment, popularizing new RT techniques is difficult.
3.2. Antioxidants
Maintaining a normal calcium concentration in acinar cells is a key factor in stimulating salivary secretion, but the presence of ROS will disrupt the normal transfer of intracellular calcium, as shown in Figure 1 [55]. Several experiments have shown that the initial level of ROS in SG cells increases after IR, and the following radioactive protective agents are able to scavenge oxygen free radicals and inhibit oxidative stress.
Figure 1.
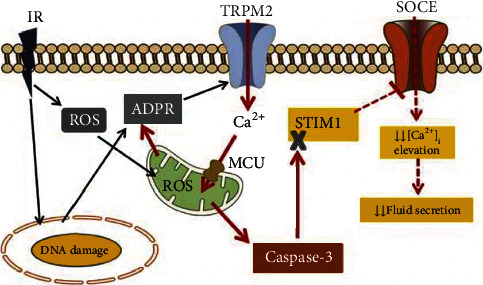
Model showing the early consequences of RT and mechanisms of persistent decrease in salivary secretion. Adenosine diphosphate ribose (ADPR) is the intracellular ligand that binds to and gates. TRPM2: transient receptor potential melastatin 2. MCU: mitochondrial Ca2+ uniporter. SOCE: Store-operated Ca2+ entry. STIM1: stromal interaction molecule 1, IR: irradiation. ROS: reactive oxygen species [55].
TEMPOL is a superoxide dismutase (SOD) analog [56, 57]. In mouse models, TEMPOL has been reported to protect against IR-induced SG injury [57, 58]. MitoTEMPO, as a mitochondria-targeted antioxidant, is similar to TEMPOL, but it contains lipophilic cationic triphenyl, which enables it to easily penetrate the lipid bilayer and accumulate in the mitochondria [59]. Thus, all TEMPOL nitrogen oxides can reduce IR-induced transient receptor potential melastatin-2 activation by scavenging H2O2 or ROS, inhibit caspase-3, prevent the decrease in stromal interaction molecule 1 protein, maintain the store-operated Ca2+ entry pathway of normal Ca2+ entry mechanism, and protect acinar cells and microvascular endothelial cells [56, 60]. It can also selectively protect normal cells from the harmful effects of IR without affecting the radiosensitivity of tumor cells [56, 58, 60]. This is because such substances may be rapidly converted to hydroxylamine in tumors [58]. However, these TEMPOL nitrogen oxides cannot resolve the quality of saliva, such as the decrease in lysozyme level [56].
Alpha-lipoic acid, a natural compound with strong antioxidant effects, can chelate metal ions, inhibit the formation of oxygen free radicals, and regenerate many antioxidants. SG cells can be protected by preserving the signals induced by parasympathetic innervation and releasing regeneration signals that promote cell proliferation. When the dose is sufficient, it can also radiosensitize tumor cells [4, 61]. Epigallocatechin 3-gallate (EGCG), a phenolic antioxidant, inhibits free radical chain reactions by capturing peroxide free radicals. It is superior to other catechins because of the six phenolic hydroxyl groups in its structure [62]. A certain dose of EGCG effectively prevented apoptosis in IR-injured epithelial cells and protects against oxidative stress and inflammatory cell infiltration [63, 64]. However, the absorption and oral bioavailability of EGCG are low, and its role in the physiological conditions of SGs after an injury has not been studied extensively [65].
Erythropoietin, an endogenous glycoprotein hormone, increases when IR damages SG microvessels, ischemia, and hypoxia. Recombinant human erythropoietin has been shown to balance SOD and ROS levels [56, 66]. Notably, recombinant human erythropoietin administration may also activate erythropoietin receptors in cancer cells, making them IR resistant [66]. The specific mechanism of erythropoietin function in the glands and methods to reduce its tumor-protective effect are aspects of future research. Cordycepin, also known as 3-deoxyadenosine, has been reported to clear ROS and inhibit mitochondrial damage [67–69]. It is known to promote the mRNA expression levels of alpha-amylase 1 and aquaporin-5. However, cordycepin can be rapidly deaminated by adenosine deaminase in vivo; currently, the only solution to its short half-life is to increase its dosage [69].
Amifostine is a broad-spectrum cell protector and the only US Food and Drug Administration-approved drug with a radiological protective effect [70]. Alkaline phosphatase levels were higher in normal tissues than in tumor cells. Amifostine is hydrolyzed to reactive sulfhydryl compounds (WR-1065) by alkaline phosphatase in normal tissues, which can also play a role in scavenging oxygen free radicals and protecting cellular substructures to selectively prevent injury caused by IR [70, 71]. Its tumor-protective effect is controversial, but there is currently no evidence that amifostine reduces the efficacy of RT [72, 73]. Systemic administration of amifostine has serious side effects such as acute cutaneous and mucosal toxicity, hypotension, hypocalcemia, and vomiting [70]. Retroductal cannulation and injection can bypass systemic circulation, provide direct glandular access, and be locally administered to the SGs, reducing hypotensive effects compared with intravenous administration [74].
3.3. Botulinum Toxin
BoNT has been shown to inhibit SNAREs involved in acetylcholine release at the neuroglandular junction and receptors involved in acinar cell granule exocytosis to prevent xerostomia. Therefore, it can temporarily atrophy SGs and reduce the number of granules secreted from acinar cells. This may make acinar cells significantly less sensitive to IR, which protects the SGs [75–77].
A study found that mice that received intraglandular injections of BoNT showed increased salivary flow rate, increased glandular weight, and decreased periductal fibrosis after RT compared to that in noninjected animals. This suggests that BoNT has an anti-inflammatory effect that can attenuate RT-induced periductal fibrosis and neutrophil infiltration [78]. In addition, studies have reported that BoNT is safe and effective in humans. It also has a radiosensitizing effect on tumors and can be effectively used in combination with RT [79, 80].
Notably, several studies have shown a positive effect of BoNT on the prevention of xerostomia, although the use of BoNT requires more clinical trials since most of the current studies are limited to animal experiments [81].
3.4. Submandibular Gland Transfer
Recent studies have demonstrated that transferring the submandibular gland to the submental space can effectively reduce IR damage to the SGs and prevent xerostomia. Seikaly et al. reported that submandibular gland transfer was better than oral pilocarpine in preventing xerostomia, leading to an improved quality of life [82, 83]. However, submandibular gland transfer also has complications caused by surgery and many contraindications. Additionally, the potential risk associated with this method of incorrectly interpreting submandibular gland images resulted in a higher IR dose to the submandibular gland [84–86]. In this regard, to reduce the impact of IR on the submandibular gland, transferring a submandibular gland to the patient's forearm during RT and re-transplanting the gland back to its original position after RT was proposed. This study showed that IR-induced SG hypofunction is reduced. However, forearm transfer could only serve as a potential preventive approach until further validation [87, 88].
3.5. Growth Factors
Although the mechanism of xerostomia recovery is still unclear, it is noteworthy that in addition to vascular and nerve recovery, the related molecular regulatory mechanisms have been discussed widely. Pathways such as Wnt/β-catenin, Hedgehog, PDGF-FGF, Chrm1/HB-EGF, and laminin/integrin are thought to play important roles in xerostomia recovery. The PDGF-FGF pathway explains the possible mechanism of interaction between epithelial cells and neural crest-derived mesenchymal stem cells. The Wnt/β-catenin pathway plays a key role in the formation of branching morphology. Transient activation of the Wnt/β-catenin pathway was observed to reduce SG injury caused by RT, which may be related to its role in inhibiting apoptosis and preservation of functional SGs cells. However, a specific explanation of the above mechanism is beyond the scope of this review [89–91].
RT-induced xerostomia is associated with p53-dependent apoptosis; therefore, a variety of growth factors are considered to prevent this injury [92]. In this review, various typical growth factors have been described. Insulin-like growth factor-1 has the potential to treat xerostomia as assessed in animal experiments which may be mediated through increased levels of sirtuin-1. Sirtuin-1 promotes DNA repair in cells and maintains the activation of protein atypical kinase C zeta, ultimately promoting inhibition of SG dysfunction and apoptosis by stimulating activation of the endogenous Akt pathway [93–96]. Similarly, keratinocyte growth factor-1, hepatocyte growth factor, and epidermal growth factor are also believed to inhibit apoptosis through the Akt pathway to protect SGs and can positively affect stem cell proliferation [97–99].
In addition, vascular endothelial growth factor is believed to improve blood flow in SGs and restore their function [100]. However, vascular endothelial growth factor treatment is mostly limited to animal experiments, and further research is needed to determine whether it can be applied in clinics for xerostomia treatment.
3.6. Rapamycin and Limonene
Rapamycin and rapalogue, CCI-799, can induce autophagy by inhibiting target mTOR complex 1 to maintain SG homeostasis [101–104]. CCI-799 can increase SOD expression to suppress excessive ROS, which may be related to autophagy. Furthermore, CCI-799 has been shown to restore salivary flow rate, increase amylase levels, and inhibit the compensatory proliferation of cells [59, 104, 105]. Therefore, rapamycin and CCI-799 have potential use in xerostomia.
The presence of aldehyde dehydrogenase 3A1 (ALDH3A1) prevented excessive aldehyde accumulation from damaging the SGs. RT led to a decrease in ALDH3A1, which further promoted acinar cell apoptosis and reduced spheroid formation [92, 106]. Saiki et al. identified an aldehyde dehydrogenase activator, limonene, to be safer and more acceptable than previous Alda89 (safrole) [106, 107]. It has been experimentally confirmed that limonene can decrease aldehyde levels and improve SG function without protecting the growth of tumors that also contain ALDH3A1 [106]. However, its application is limited owing to its higher doses [108].
4. Treatment
4.1. Saliva Substitutes
Saliva substitutes are one of the most effective measures to relieve xerostomia caused by RT and have antibacterial and preventive effects on tooth demineralization. However, they can only be retained for a short time in the oral cavity and may trigger allergic reactions in patients [109–112]. An edible saliva substitute like oral moisturizing jelly is noteworthy because it contains buffering agents, has a neutral pH, and can improve the swallowing ability of patients, in addition to relieving xerostomia. This resolves the concern regarding commercially available saliva substitutes not being recommended owing to the use of preservatives [109, 113]. Additionally, hyaluronic acid solutions at certain concentrations are similar to saliva in terms of viscosity, elastic modulus, and network structure. They exhibit antibacterial and antioxidant effects, making them a potential candidate for a saliva substitute [12, 114].
4.2. Pharmacological Salivation Agents
Pilocarpine is an imidazole-based alkaloid and as a typical muscarinic M3 receptor agonist, it can act on SGs to increase the saliva flow rate. It can also promote the supplementation of adenocytes, which may be due to the promotion of SOX2+ cell activity [1, 115]. Research is being conducted to develop targeted delivery methods to minimize the side effects of drugs. Malallah et al. believe that fast-disintegrating buccal tablets containing pilocarpine can be rapidly dissolved or decomposed orally. This method can stimulate SGs and attenuate the off-target effect of the drug, but evidence to prove its use in the clinic is lacking [116]. Another idea is the use of oral adhesives, which can improve the retention time of drugs at treatment sites. Chitosan and other substances used have shown adhesion, stability, and controlled slow release to the mucosa [117].
4.3. Acupuncture and Electrical Stimulation
Acupuncture, which uses extremely thin solid metal needles inserted into a suitable subcutaneous area, is a low-risk treatment that has been reported to boost salivary secretion [118]. The mechanism of acupuncture in the treatment of xerostomia remains unclear. However, there are two possible explanations. First, acupuncture stimulates the nervous system to produce neuropeptides that have nutritional and anti-inflammatory effects on SGs. Second, acupuncture has a direct effect on SG blood flow [119]. However, most studies on acupuncture have significant heterogeneity and low comparability [120].
Similar to acupuncture, electrical stimulation has been included in clinical studies as a treatment with fewer side effects. Electrical pulses can stimulate nerves and affect the SGs, such as transcutaneous electrical nerve stimulation which is believed to directly stimulate the auriculotemporal nerve [121]. Though these studies have revealed some positive effects, the evidence is insufficient. The instruments need to be refined in shape and material to provide the appropriate electrical impulse to activate the nerves [122, 123].
4.4. Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy (HBOT) has the ability to affect cytokine responses, induce local angiogenesis, and mobilize stem cells [124, 125] which suggests its potential in the treatment of SG dysfunction. HBOT has been shown to improve xerostomia, the sense of taste, and the swallowing ability of patients [126, 127]. However, most of these studies lack a sufficient sample size and appropriate control groups. The efficacy of treatment is controversial due to factors such as the placebo effect and patient adaptation to xerostomia [126, 128]. The optimal start time of HBOT after RT and the number of treatments still need further research [126, 128]. Moreover, HBOT is not widely accepted by patients because of its prohibitive cost and inability to fully restore SG function [127].
4.5. Gene Therapy
After RT, the absence of a large amount of primary fluid and damage to the acinar cells results in the inhibition of the reabsorption of ions from primary saliva by SG ducts, which may lead to an osmotic gradient between the ductal epithelium and fluid in the ducts [129, 130]. In this case, a convenient water channel can be constructed on duct epithelial cells by transferring human aquaporin-1 (hAQP1) cDNA to assist fluid secretion and relieve xerostomia, as shown in Figure 2 [129, 130].
Figure 2.
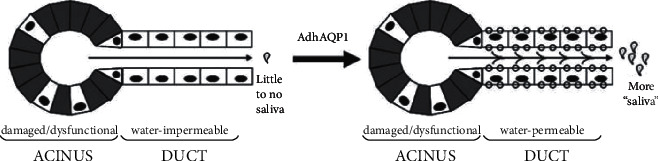
Schematic diagram of the mechanism of improving SG function after hAQP1 expression. Damaged SGs on the left and aquaporin-mediated SGs on the right. The gray acinar cells show they are damaged or dysfunctional by RT. The ductal epithelial cells with minimal damage by RT and surviving acinar cells are white with black nucleus. The small circle on the ductal epithelium cell represents expression of hAQP1. After the successful construction of hAQP1, more fluid could be secreted from the duct epithelial cells, and thus more “saliva” could be secreted into the mouth. However, the “saliva” here is different from the saliva secreted by acinar cells in terms of concrete components [129].
There are two main ways to transfer hAQP1 to SGs in animal models: viral vectors and nonviral vectors [129, 131, 132]. Viral vectors have a higher efficiency than nonviral vectors, but they are more likely to trigger immune rejection in hosts than nonviral vectors [132, 133].
The adenoviral vector is one of the most commonly used vectors in gene therapy, with high transduction efficiency [134]. This vector has been successfully used in clinical trials [129, 135, 136]. Owing to the immune rejection in hosts, a recombinant serotype 5 adenoviral vector-encoding hAQP1, AdhAQP1 can only provide an effective therapeutic outcome for a short time in animal experiments. However, a phase I trial showed that xerostomia was relieved in 5 of the 11 subjects for 2-3 years, and the parotid flow rate remained significantly elevated 3–4.7 years after treatment [135–138]. This may be related to the lack of methylation of the human cytomegalovirus promoter in human SG epithelial cells [139].
Another commonly used viral vector is the serotype 2 adeno-associated viral (AAV2) vector, which triggers milder immune rejection in the host [140]. The results obtained in irradiated miniature pigs suggest that the use of AAV2 as a transduction vector may serve as a way to increase salivary flow over a longer period than AdhAQP1 [140]. The ultrasound-assisted gene transfer method is noteworthy. In animal experiments, ultrasound-assisted gene transfer can generate a “sonoporation” effect to assist gene transfer to SG cells without introducing viral antigens [131, 141]. In experiments to improve SG function in irradiated miniature pigs, this approach achieved a therapeutic efficacy comparable to that of AdhAQP1 and reduced the risk of immune responses in the host triggered by viral vectors [131].
In addition to encoding hAQP1 to improve xerostomia, the prevention of IR-induced SG hypofunction by adenovirus-encoded growth factor and neurotrophic factor deserves further investigation [142–144]. It has been shown that injection of neurturin adenovirus into mouse SGs prior to IR can reduce parasympathetic cell apoptosis, thereby inhibiting IR-induced decline in SG function in mice through acetylcholine signaling, which is essential for SG development and regeneration [144–146].
4.6. Stem Cells
Stem cells are thought to play a vital role in SG formation and recovery from damage, as shown in Figure 3 [147]. Most of the existing treatment methods involve temporary improvement of xerostomia. However, the application of stem cells in its treatment provides the possibility for the long-term recovery of SG tissue and secretory function [148]. SG stem cells can be collected in advance and implanted after RT. The collection of stem cells often depends on their marker expression profiles. According to known literature, c-kit (CD117) is the most studied marker, CD49F, CD29, CD24, and CD133 also have potential as markers and should be used in combination to improve accuracy [149]. The potential of SG cells to aid their recovery after RT was confirmed by isolating the c-Kit cell population, producing a salisphere in vitro, and transplanting it [150, 151]. Salisphere transplantation can not only replace the lost proliferative cells of the SGs post-RT but may also benefit patients' endogenous cells [152]. In addition, SG organoids can be produced using 3D extracellular matrices, which can make cell differentiation similar to that of SGs to obtain a better 3D structure. For clinical applications, SG organoids have been made with biocompatible magnetic nanoparticles [148, 153–155].
Figure 3.
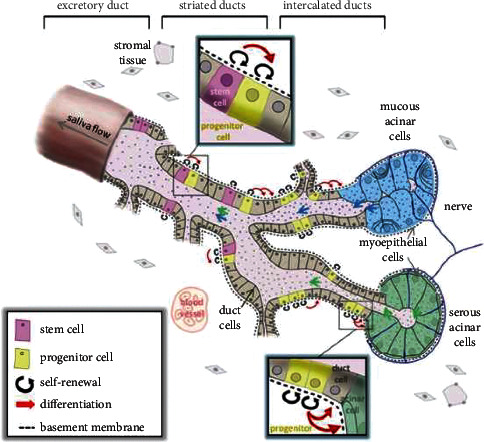
Schematic diagram showing the location, proliferation, and differentiation of stem and progenitor cells in SGs [147].
Furthermore, the isolated stem cell population that can be used for clinical treatment needs to be assessed. The impact of the patient's age, the number of cells to be transplanted during treatment, the accuracy of transplant cells delivered to the desired site, and confirmation of genomic stability during cell culture to prevent potential cancer cells from being transplanted into the patient are challenges for stem cell applications [147, 156]. Though the role of stem cells in restoring SGs is still unclear, there is a view that SG renewal is affected more by the proliferation of acinar cells [157].
In addition to SG cells, stem cells derived from other parts of the body have also been reported, among which adipose-derived stem cells (ADSCs) are the most promising. Meanwhile, mesenchymal stem cells from the bone marrow, labial mucosa, and dental pulp have also proved valuable in treating xerostomia [148]. It has been suggested that ADSCs can be obtained by non-invasive surgery, and their potential for induction of SGs cells has been confirmed, where this transdifferentiation can be facilitated by platelet-rich fibrin [158, 159]. However, there are studies that did not observe significant transdifferentiation of ADSCs into SG. ADSCs also secrete paracrine factors to maintain amylase secretion. Although the mechanism of action remains controversial, the positive effects of ASCs on SGs are certain [160]. Notably, ASCs were included in phase I/II clinical trials and showed good safety and efficacy [161]. Bone marrow mesenchymal stem cells are also shown to increase the expression of SDF1-CXCR4, Bcl-2, and other proteins after transplantation under hypoxic conditions, thus promoting cell proliferation and differentiation, leading to the recovery of SGs [162]. Injection of labial stem cell extracts improved blood vessel, nerve, and cell recovery in mice, and increased saliva flow rates by 50–60% [163]. Dental pulp stem cells can be easily obtained, and their anti-inflammatory effects, multipotent differentiation properties, and migration to damaged tissues illustrate their potential for application in the field of xerostomia treatment [148, 164]. Although they have potential in the treatment of xerostomia along with SGs cells, issues such as efficacy, preservation, transport methods, and safety remain formidable challenges.
5. Conclusion
We found improvements in most diagnostic methods but with shortcomings. Most of the prevention and treatment methods are restricted to animal experiments, requiring further clinical research, where antioxidants, gene transfer, and stem cell transplantation have promising developmental and therapeutic prospects.
Contributor Information
Heng Bo Jiang, Email: hengbojiang@foxmail.com.
Jianmin Han, Email: hanjianmin@bjmu.edu.cn.
Data Availability
All data, figures, and tables in this review paper are labeled with references.
Conflicts of Interest
The authors declare that there are no conflicts of interest..
Authors' Contributions
Yanli Li, Xuehan Li, and Runxuan Pang contributed equally to the paper.
References
- 1.Emmerson E., May A. J., Berthoin L., et al. Salivary glands regenerate after radiation injury through sox2-mediated secretory cell replacement. EMBO Molecular Medicine . 2018;10(3) doi: 10.15252/emmm.201708051.e8051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jasmer K. J., Gilman K. E., Munoz Forti K., Weisman G. A., Limesand K. H. Radiation-induced salivary gland dysfunction: mechanisms, therapeutics and future directions. Journal of Clinical Medicine . 2020;9(12):p. 4095. doi: 10.3390/jcm9124095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu V. W. C., Leung K. Y. A review on the assessment of radiation induced salivary gland damage after radiotherapy. Frontiers Oncology . 2019;9:p. 1090. doi: 10.3389/fonc.2019.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheikholeslami S., Khodaverdian S., Dorri-Giv M., et al. The radioprotective effects of alpha-lipoic acid on radiotherapy-induced toxicities: a systematic review. International Immunopharmacology . 2021;96 doi: 10.1016/j.intimp.2021.107741.107741 [DOI] [PubMed] [Google Scholar]
- 5.Redman R. S. On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotechnic & Histochemistry . 2008;83(3-4):103–130. doi: 10.1080/10520290802374683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Subedi K. P., Zheng C., Ambudkar I. Mitochondria-targeted antioxidant protects against irradiation-induced salivary gland hypofunction. Scientific Reports . 2021;11(1):p. 7690. doi: 10.1038/s41598-021-86927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruyama Y., Nishimoto Y., Umezawa K., et al. Comparison of oral metabolome profiles of stimulated saliva, unstimulated saliva, and mouth-rinsed water. Scientific Reports . 2022;12(1):p. 689. doi: 10.1038/s41598-021-04612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frenkel E. S., Ribbeck K. Salivary mucins in host defense and disease prevention. Journal of Oral Microbiology . 2015;7(1) doi: 10.3402/jom.v7.29759.29759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabian T. K., Hermann P., Beck A., Fejerdy P., Fabian G. Salivary defense proteins: their network and role in innate and acquired oral immunity. International Journal of Molecular Sciences . 2012;13(4):4295–4320. doi: 10.3390/ijms13044295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor G. B., Carpenter G. H. Regulation of salivary gland function by autonomic nerves. Autonomic Neuroscience . 2007;133(1):3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Thakkar J. P., Lane C. J. Hyposalivation and xerostomia and burning mouth syndrome: medical management. Oral and Maxillofacial Surgery Clinics of North America . 2022;34(1):135–146. doi: 10.1016/j.coms.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Winter C., Keimel R., Gugatschka M., Kolb D., Leitinger G., Roblegg E. Investigation of changes in saliva in radiotherapy-induced head neck cancer patients. International Journal of Environmental Research and Public Health . 2021;18(4):p. 1629. doi: 10.3390/ijerph18041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J., Andablo-Reyes E., Mighell A., Pavitt S., Sarkar A. Dry mouth diagnosis and saliva substitutes-a review from a textural perspective. Journal of Texture Studies . 2021;52(2):141–156. doi: 10.1111/jtxs.12575. [DOI] [PubMed] [Google Scholar]
- 14.Assas M., Wiriyakijja P., Fedele S., Porter S., Ni Riordain R. Evaluating the measurement properties of patient-reported outcome measures in radiotherapy-induced xerostomia. Oral Diseases . 2021;27(5):1097–1105. doi: 10.1111/odi.13416. [DOI] [PubMed] [Google Scholar]
- 15.van der Putten G. J., Brand H. S., Schols J. M. G. A., de Baat C. The diagnostic suitability of a xerostomia questionnaire and the association between xerostomia, hyposalivation and medication use in a group of nursing home residents. Clinical Oral Investigations . 2011;15(2):185–192. doi: 10.1007/s00784-010-0382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago P. H. R., Song Y., Hanna K., Nair R. Degrees of xerostomia? A rasch analysis of the xerostomia inventory. Community Dentistry and Oral Epidemiology . 2020;48(1):63–71. doi: 10.1111/cdoe.12504. [DOI] [PubMed] [Google Scholar]
- 17.Thomson W. M., van der Putten G. J., de Baat C., et al. Shortening the xerostomia inventory. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics . 2011;112(3):322–327. doi: 10.1016/j.tripleo.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai S., Ghezzi E. M., Ship J. A. Development of a visual analogue scale questionnaire for subjective assessment of salivary dysfunction. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics . 2001;91(3):311–316. doi: 10.1067/moe.2001.111551. [DOI] [PubMed] [Google Scholar]
- 19.Beetz I., Burlage F. R., Bijl H. P., et al. The groningen radiotherapy-induced xerostomia questionnaire: development and validation of a new questionnaire. Radiotherapy & Oncology . 2010;97(1):127–131. doi: 10.1016/j.radonc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Buchholzer S., Faure F., Tcheremissinoff L., et al. Novel multidisciplinary salivary gland society (msgs) questionnaire: an international consensus. The Laryngoscope . 2022;132(2):322–331. doi: 10.1002/lary.29731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navazesh M., Christensen C. M. A comparison of whole mouth resting and stimulated salivary measurement procedures. Journal of Dental Research . 1982;61(10):1158–1162. doi: 10.1177/00220345820610100901. [DOI] [PubMed] [Google Scholar]
- 22.Eisbruch A., Rhodus N., Rosenthal D., et al. How should we measure and report radiotherapy-induced xerostomia? Seminars in Radiation Oncology . 2003;13(3):226–234. doi: 10.1016/s1053-4296(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 23.Memtsa P. T., Tolia M., Tzitzikas I., et al. Validity and reliability of the Greek version of the xerostomia questionnaire in head and neck cancer patients. Supportive Care in Cancer . 2017;25(3):847–853. doi: 10.1007/s00520-016-3471-0. [DOI] [PubMed] [Google Scholar]
- 24.Saleh J., Figueiredo M. A. Z., Cherubini K., Salum F. G. Salivary hypofunction: an update on aetiology, diagnosis and therapeutics. Archives of Oral Biology . 2015;60(2):242–255. doi: 10.1016/j.archoralbio.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Bellagambi F. G., Lomonaco T., Salvo P., et al. Saliva sampling: methods and devices. An overview. TrAC, Trends in Analytical Chemistry . 2020;124 doi: 10.1016/j.trac.2019.115781.115781 [DOI] [Google Scholar]
- 26.Wolff A., Herscovici D., Rosenberg M. A simple technique for the determination of salivary gland hypofunction. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics . 2002;94(2):175–178. doi: 10.1067/moe.2002.126023. [DOI] [PubMed] [Google Scholar]
- 27.Running C. A., Hayes J. E. Expectation and expectoration: information manipulation alters spitting volume, a common proxy for salivary flow. Physiology & Behavior . 2016;167:180–187. doi: 10.1016/j.physbeh.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Michishige F., Kanno K., Yoshinaga S., Hinode D., Takehisa Y., Yasuoka S. Effect of saliva collection method on the concentration of protein components in saliva. Journal of Medical Investigation . 2006;53(1-2):140–146. doi: 10.2152/jmi.53.140. [DOI] [PubMed] [Google Scholar]
- 29.Jones J. M., Watkins C. A., Hand J. S., Warren J. J., Cowen H. J. Comparison of three salivary flow rate assessment methods in an elderly population. Community Dentistry and Oral Epidemiology . 2000;28(3):177–184. doi: 10.1034/j.1600-0528.2000.280303.x. [DOI] [PubMed] [Google Scholar]
- 30.Fallon B. S., Chase T. J., Cooke E. M., et al. The use of Bokaflo™ instrument to measure salivary flow. BMC Oral Health . 2021;21(1):p. 191. doi: 10.1186/s12903-021-01477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarino C., Bagan L., Murillo-Cortes J., Calvo J., Bagan J. Stimulated whole salivary flow rate: the most appropriate technique for assessing salivary flow in sjogren syndrome. Medicina Oral, Patología Oral y Cirugía Bucal . 2021;26(3):e404–e407. doi: 10.4317/medoral.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navazesh M., Kumar S. K., University of Southern California School of Dentistry Measuring salivary flow: challenges and opportunities. Journal of the American Dental Association . 2008;139(Suppl):35S–40S. doi: 10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- 33.Chen A., Wai Y., Lee L., Lake S., Woo S. B. Using the modified schirmer test to measure mouth dryness: a preliminary study. Journal of the American Dental Association . 2005;136(2):164–170. doi: 10.14219/jada.archive.2005.0137. [DOI] [PubMed] [Google Scholar]
- 34.Satoh-Kuriwada S., Iikubo M., Shoji N., Sakamoto M., Sasano T. Diagnostic performance of labial minor salivary gland flow measurement for assessment of xerostomia. Archives of Oral Biology . 2012;57(8):1121–1126. doi: 10.1016/j.archoralbio.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Gotoh S., Watanabe Y., Fujibayashi T. Development of an electronic device for sialometry of minor salivary glands. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology . 2013;116(3):301–305. doi: 10.1016/j.oooo.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z., Shen M. M., Liu X. J., Si Y., Yu G. Y. Characteristics of the saliva flow rates of minor salivary glands in healthy people. Archives of Oral Biology . 2015;60(3):385–392. doi: 10.1016/j.archoralbio.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima Y., Yoda T., Araki R., et al. Evaluation of oral wetness using an improved moisture-checking device for the diagnosis of dry mouth. Oral Science International . 2017;14(2):33–36. doi: 10.1016/s1348-8643(17)30017-4. [DOI] [Google Scholar]
- 38.Takano T., Kugimiya Y., Morita K., Tazawa S., Ueda T., Sakurai K. Intra- and inter-investigator reliabilities of oral moisture measured using an oral moisture-checking device. Journal of Oral Rehabilitation . 2020;47(4):480–484. doi: 10.1111/joor.12919. [DOI] [PubMed] [Google Scholar]
- 39.Afzelius P., Nielsen M. Y., Ewertsen C., Bloch K. P. Imaging of the major salivary glands. Clinical Physiology and Functional Imaging . 2016;36(1):1–10. doi: 10.1111/cpf.12199. [DOI] [PubMed] [Google Scholar]
- 40.Cheng S. C., Ying M. T., Kwong D. L., Wu V. W. Sonographic appearance of parotid glands in patients treated with intensity-modulated radiotherapy or conventional radiotherapy for nasopharyngeal carcinoma. Ultrasound in Medicine and Biology . 2011;37(2):220–230. doi: 10.1016/j.ultrasmedbio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Cantisani V., David E., Sidhu P. S., et al. Parotid gland lesions: multiparametric ultrasound and mri features. Ultraschall in der Medizin . 2016;37(5):454–471. doi: 10.1055/s-0042-109171. [DOI] [PubMed] [Google Scholar]
- 42.Nomayr A., Lell M., Sweeney R., Bautz W., Lukas P. Mri appearance of radiation-induced changes of normal cervical tissues. European Radiology . 2001;11(9):1807–1817. doi: 10.1007/s003300000728. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y. C., Chen H. Y., Hsu C. H. Recent advances in salivary scintigraphic evaluation of salivary gland function. Diagnostics . 2021;11(7):p. 1173. doi: 10.3390/diagnostics11071173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itonaga T., Tokuuye K., Mikami R., et al. Mathematical evaluation of post-radiotherapy salivary gland function using salivary gland scintigraphy. British Journal of Radiology . 2022;95(1130) doi: 10.1259/bjr.20210718.20210718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ou D., Zhang Y., He X., et al. Magnetic resonance sialography for investigating major salivary gland duct system after intensity-modulated radiotherapy of nasopharyngeal carcinoma. International Journal of Clinical Oncology . 2013;18(5):801–807. doi: 10.1007/s10147-012-0464-y. [DOI] [PubMed] [Google Scholar]
- 46.Astreinidou E., Roesink J. M., Raaijmakers C. P., et al. 3d mr sialography as a tool to investigate radiation-induced xerostomia: feasibility study. International Journal of Radiation Oncology, Biology, Physics . 2007;68(5):1310–1319. doi: 10.1016/j.ijrobp.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 47.Ge X., Liao Z., Yuan J., et al. Radiotherapy-related quality of life in patients with head and neck cancers: a meta-analysis. Supportive Care in Cancer . 2020;28(6):2701–2712. doi: 10.1007/s00520-019-05077-5. [DOI] [PubMed] [Google Scholar]
- 48.Vergeer M. R., Doornaert P. A., Rietveld D. H., Leemans C. R., Slotman B. J., Langendijk J. A. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. International Journal of Radiation Oncology, Biology, Physics . 2009;74(1):1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 49.Cui T., Ward M. C., Joshi N. P., et al. Correlation between plan quality improvements and reduced acute dysphagia and xerostomia in the definitive treatment of oropharyngeal squamous cell carcinoma. Head & Neck . 2019;41(4):1096–1103. doi: 10.1002/hed.25594. [DOI] [PubMed] [Google Scholar]
- 50.Snider J. W., Paine C. C. Sticky stuff: xerostomia in patients undergoing head and neck radiotherapy-prevalence, prevention, and palliative care. Annals of Palliative Medicine . 2020;9(3):1340–1350. doi: 10.21037/apm.2020.02.36. [DOI] [PubMed] [Google Scholar]
- 51.Thind K., Roumeliotis M., Mann T., et al. Increasing demand on human capital and resource utilization in radiation therapy: the past decade. International Journal of Radiation Oncology, Biology, Physics . 2022;112(2):457–462. doi: 10.1016/j.ijrobp.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Vanetti E., Clivio A., Nicolini G., et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field imrt. Radiotherapy & Oncology . 2009;92(1):111–117. doi: 10.1016/j.radonc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Moreno A. C., Frank S. J., Garden A. S., et al. Intensity modulated proton therapy (impt)—the future of imrt for head and neck cancer. Oral Oncology . 2019;88:66–74. doi: 10.1016/j.oraloncology.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon H. G., Ahn Y. C., Oh D., et al. Early clinical outcomes of intensity modulated radiation therapy/intensity modulated proton therapy combination in comparison with intensity modulated radiation therapy alone in oropharynx cancer patients. Cancers . 2021;13(7):p. 1549. doi: 10.3390/cancers13071549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X., Gong B., de Souza L. B., et al. Radiation inhibits salivary gland function by promoting Stim1 cleavage by caspase-3 and loss of soce through a trpm2-dependent pathway. Science Signaling . 2017;10(482) doi: 10.1126/scisignal.aal4064.eaal4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizrachi A., Cotrim A. P., Katabi N., Mitchell J. B., Verheij M., Haimovitz-Friedman A. Radiation-induced microvascular injury as a mechanism of salivary gland hypofunction and potential target for radioprotectors. Radiation Research . 2016;186(2):189–195. doi: 10.1667/RR14431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu L., Wang Y., Cotrim A. P., et al. Effect of tempol on the prevention of irradiation-induced mucositis in miniature pigs. Oral Diseases . 2017;23(6):801–808. doi: 10.1111/odi.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cotrim A. P., Hyodo F., Matsumoto K. I., et al. Differential radiation protection of salivary glands versus tumor by tempol with accompanying tissue assessment of tempol by magnetic resonance imaging. Clinical Cancer Research . 2007;13(16):4928–4933. doi: 10.1158/1078-0432.CCR-07-0662. [DOI] [PubMed] [Google Scholar]
- 59.Farhood B., Ashrafizadeh M., Khodamoradi E., et al. Targeting of cellular redox metabolism for mitigation of radiation injury. Life Sciences . 2020;250 doi: 10.1016/j.lfs.2020.117570.117570 [DOI] [PubMed] [Google Scholar]
- 60.Liu X., Cotrim A., Teos L., et al. Loss of Trpm2 function protects against irradiation-induced salivary gland dysfunction. Nature Communications . 2013;4(1):p. 1515. doi: 10.1038/ncomms2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J. H., Jeong B. K., Jang S. J., et al. Alpha-lipoic acid ameliorates radiation-induced salivary gland injury by preserving parasympathetic innervation in rats. International Journal of Molecular Sciences . 2020;21(7):p. 2260. doi: 10.3390/ijms21072260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng Z., Xu Z. W., Wen W. S., Wang R. S. Tea polyphenols protect against irradiation-induced injury in submandibular glands’ cells: a preliminary study. Archives of Oral Biology . 2011;56(8):738–743. doi: 10.1016/j.archoralbio.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Ohno S., Yu H., Dickinson D., et al. Epigallocatechin-3-Gallate modulates antioxidant and DNA repair-related proteins in exocrine glands of a primary sjogren’s syndrome mouse model prior to disease onset. Autoimmunity . 2012;45(7):540–546. doi: 10.3109/08916934.2012.710860. [DOI] [PubMed] [Google Scholar]
- 64.Xie L. W., Cai S., Zhao T. S., Li M., Tian Y. Green tea derivative (−)-Epigallocatechin-3-Gallate (egcg) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radical Biology and Medicine . 2020;161:175–186. doi: 10.1016/j.freeradbiomed.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Sulistiyani E., Brimson J. M., Chansaenroj A., et al. Epigallocatechin-3-Gallate protects pro-acinar epithelia against salivary gland radiation injury. International Journal of Molecular Sciences . 2021;22(6):p. 3162. doi: 10.3390/ijms22063162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Q., Wang Y., Cui Z., Ma X., Shi H., Zhang W. Erythropoietin plays a protective role in submandibular gland hypofunction induced by irradiation. Journal of Oral and Maxillofacial Surgery . 2021;79(6):1373–1383. doi: 10.1016/j.joms.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Jaiboonma A., Kaokaen P., Chaicharoenaudomrung N., et al. Cordycepin attenuates salivary hypofunction through the prevention of oxidative stress in human submandibular gland cells. International Journal of Medical Sciences . 2020;17(12):1733–1743. doi: 10.7150/ijms.46707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X. T., Li H. C., Li C. B., Dou D. Q., Gao M. B. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of cordyceps militaris. American Journal of Chinese Medicine . 2010;38(6):1093–1106. doi: 10.1142/S0192415X10008494. [DOI] [PubMed] [Google Scholar]
- 69.He M. T., Lee A. Y., Park C. H., Cho E. J. Protective effect of cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro. Nutrition Research and Practice . 2019;13(4):279–285. doi: 10.4162/nrp.2019.13.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Safak G., Celiker M., Tumkaya L., et al. Comparison of effects of dexmedetomidine and amifostine against X-ray radiation-induced parotid damage. Radiation and Environmental Biophysics . 2022;61(2):241–253. doi: 10.1007/s00411-022-00964-8. [DOI] [PubMed] [Google Scholar]
- 71.Bhide S. A., Miah A. B., Harrington K. J., Newbold K. L., Nutting C. M. Radiation-induced xerostomia: pathophysiology, prevention and treatment. Clinical Oncology . 2009;21(10):737–744. doi: 10.1016/j.clon.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Riley P., Glenny A. M., Hua F., Worthington H. V. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database of Systematic Reviews . 2017;7 doi: 10.1002/14651858.CD012744.CD012744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee M. G., Freeman A. R., Roos D. E., Milner A. D., Borg M. F. Randomized double-blind trial of amifostine versus placebo for radiation-induced xerostomia in patients with head and neck cancer. Journal of Medical Imaging and Radiation Oncology . 2019;63(1):142–150. doi: 10.1111/1754-9485.12833. [DOI] [PubMed] [Google Scholar]
- 74.Varghese J. J., Schmale I. L., Mickelsen D., et al. Localized delivery of amifostine enhances salivary gland radioprotection. Journal of Dental Research . 2018;97(11):1252–1259. doi: 10.1177/0022034518767408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teymoortash A., Sommer F., Mandic R., et al. Intraglandular application of botulinum toxin leads to structural and functional changes in rat acinar cells. British Journal of Pharmacology . 2007;152(1):161–167. doi: 10.1038/sj.bjp.0707375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teymoortash A., Muller F., Juricko J., et al. Botulinum toxin prevents radiotherapy-induced salivary gland damage. Oral Oncology . 2009;45(8):737–739. doi: 10.1016/j.oraloncology.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Jahn R., Scheller R. H. Snares--Engines for membrane fusion. Nature Reviews Molecular Cell Biology . 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 78.Zeidan Y. H., Xiao N., Cao H., Kong C., Le Q. T., Sirjani D. Botulinum toxin confers radioprotection in murine salivary glands. International Journal of Radiation Oncology, Biology, Physics . 2016;94(5):1190–1197. doi: 10.1016/j.ijrobp.2015.12.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mueller J., Langbein T., Mishra A., Baum R. P. Safety of high-dose botulinum toxin injections for parotid and submandibular gland radioprotection. Toxins . 2022;14(1):p. 64. doi: 10.3390/toxins14010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ansiaux R., Baudelet C., Cron G. O., et al. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clinical Cancer Research . 2006;12(4):1276–1283. doi: 10.1158/1078-0432.CCR-05-1222. [DOI] [PubMed] [Google Scholar]
- 81.Teymoortash A., Pfestroff A., Wittig A., et al. Safety and efficacy of botulinum toxin to preserve gland function after radiotherapy in patients with head and neck cancer: a prospective, randomized, placebo-controlled, double-blinded phase I clinical trial. PLoS One . 2016;11(3) doi: 10.1371/journal.pone.0151316.e0151316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seikaly H., Jha N., Harris J. R., et al. Long-term outcomes of submandibular gland transfer for prevention of postradiation xerostomia. Archives of Otolaryngology - Head and Neck Surgery . 2004;130(8):956–961. doi: 10.1001/archotol.130.8.956. [DOI] [PubMed] [Google Scholar]
- 83.Rieger J. M., Jha N., Lam Tang J. A., Harris J., Seikaly H. Functional outcomes related to the prevention of radiation-induced xerostomia: oral pilocarpine versus submandibular salivary gland transfer. Head & Neck . 2012;34(2):168–174. doi: 10.1002/hed.21682. [DOI] [PubMed] [Google Scholar]
- 84.Sood A. J., Fox N. F., O’Connell B. P., et al. Salivary gland transfer to prevent radiation-induced xerostomia: a systematic review and meta-analysis. Oral Oncology . 2014;50(2):77–83. doi: 10.1016/j.oraloncology.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Okoturo E. M., Trivedi N. P., Kekatpure V., et al. A retrospective evaluation of submandibular gland involvement in oral cavity cancers: a case for gland preservation. International Journal of Oral and Maxillofacial Surgery . 2012;41(11):1383–1386. doi: 10.1016/j.ijom.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 86.Wu X., Yom S. S., Ha P. K., Heaton C. M., Glastonbury C. M. Submandibular gland transfer: a potential imaging pitfall. American Journal of Neuroradiology . 2018;39(6):1140–1145. doi: 10.3174/ajnr.A5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hagen R., Scheich M., Kleinsasser N., Burghartz M. Two-stage autotransplantation of human submandibular gland: a novel approach to treat postradiogenic xerostomia. European Archives of Oto-Rhino-Laryngology . 2016;273(8):2217–2222. doi: 10.1007/s00405-015-3752-0. [DOI] [PubMed] [Google Scholar]
- 88.Burghartz M., Ginzkey C., Hackenberg S., Hagen R. Two-stage autotransplantation of the human submandibular gland: first long-term results. The Laryngoscope . 2016;126(7):1551–1555. doi: 10.1002/lary.25854. [DOI] [PubMed] [Google Scholar]
- 89.Liu F., Wang S. Molecular cues for development and regeneration of salivary glands. Histology & Histopathology . 2014;29(3):305–312. doi: 10.14670/HH-29.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu F. Functional restoration of salivary glands after radiotherapy: roles of Wnt and Hedgehog pathways. Stem cells and cancer stem cells. Stem Cells and Cancer Stem Cells . 2012;8:287–295. [Google Scholar]
- 91.Serrano Martinez P., Giuranno L., Vooijs M., Coppes R. P. The radiation-induced regenerative response of adult tissue-specific stem cells: models and signaling pathways. Cancers . 2021;13(4):p. 855. doi: 10.3390/cancers13040855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen S. B., Vissink A., Limesand K. H., Reyland M. E. Salivary gland hypofunction and xerostomia in head and neck radiation patients. Journal of the National Cancer Institute Monographs . 2019;2019(53) doi: 10.1093/jncimonographs/lgz016.lgz016 [DOI] [PubMed] [Google Scholar]
- 93.Limesand K. H., Said S., Anderson S. M. Suppression of radiation-induced salivary gland dysfunction by igf-1. PLoS One . 2009;4(3) doi: 10.1371/journal.pone.0004663.e4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meyer S., Chibly A. M., Burd R., Limesand K. H. Insulin-like growth factor-1-mediated DNA repair in irradiated salivary glands is sirtuin-1 dependent. Journal of Dental Research . 2017;96(2):225–232. doi: 10.1177/0022034516677529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grundmann O., Fillinger J. L., Victory K. R., Burd R., Limesand K. H. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy igf-1 administration. BMC Cancer . 2010;10(1):p. 417. doi: 10.1186/1471-2407-10-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chibly A. M., Wong W. Y., Pier M., et al. aPKCζ-dependent repression of yap is necessary for functional restoration of irradiated salivary glands with IGF-1. Scientific Reports . 2018;8(1):p. 6347. doi: 10.1038/s41598-018-24678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi J. S., Shin H. S., An H. Y., Kim Y. M., Lim J. Y. Radioprotective effects of keratinocyte growth factor-1 against irradiation-induced salivary gland hypofunction. Oncotarget . 2017;8(8):13496–13508. doi: 10.18632/oncotarget.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoon Y. J., Shin H. S., Lim J. Y. A hepatocyte growth factor/met-induced antiapoptotic pathway protects against radiation-induced salivary gland dysfunction. Radiotherapy & Oncology . 2019;138:9–16. doi: 10.1016/j.radonc.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Cho J. M., Yoon Y. J., Lee S., et al. Retroductal delivery of epidermal growth factor protects salivary progenitors after irradiation. Journal of Dental Research . 2021;100(8):883–890. doi: 10.1177/0022034521999298. [DOI] [PubMed] [Google Scholar]
- 100.Yamamura Y., Yamada H., Sakurai T., et al. Treatment of salivary gland hypofunction by transplantation with dental pulp cells. Archives of Oral Biology . 2013;58(8):935–942. doi: 10.1016/j.archoralbio.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 101.Diegel C. R., Cho K. R., El-Naggar A. K., Williams B. O., Lindvall C. Mammalian target of rapamycin-dependent acinar cell neoplasia after inactivation of apc and pten in the mouse salivary gland: implications for human acinic cell carcinoma. Cancer Research . 2010;70(22):9143–9152. doi: 10.1158/0008-5472.CAN-10-1758. [DOI] [PubMed] [Google Scholar]
- 102.Laplante M., Sabatini D. M. Regulation of Mtorc1 and its impact on gene expression at a glance. Journal of Cell Science . 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gera J., Lichtenstein A. The mammalian target of rapamycin pathway as a therapeutic target in multiple myeloma. Leukemia and Lymphoma . 2011;52(10):1857–1866. doi: 10.3109/10428194.2011.580478. [DOI] [PubMed] [Google Scholar]
- 104.Morgan-Bathke M., Lin H. H., Ann D. K., Limesand K. H. The role of autophagy in salivary gland homeostasis and stress responses. Journal of Dental Research . 2015;94(8):1035–1040. doi: 10.1177/0022034515590796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morgan-Bathke M., Harris Z. I., Arnett D. G., et al. The rapalogue, cci-779, improves salivary gland function following radiation. PLoS One . 2014;9(12) doi: 10.1371/journal.pone.0113183.e113183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saiki J. P., Cao H., Van Wassenhove L. D., et al. Aldehyde dehydrogenase 3a1 activation prevents radiation-induced xerostomia by protecting salivary stem cells from toxic aldehydes. Proceedings of the National Academy of Sciences of the USA . 2018;115(24):6279–6284. doi: 10.1073/pnas.1802184115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen C. H., Cruz L. A., Mochly-Rosen D. Pharmacological recruitment of aldehyde dehydrogenase 3a1 (Aldh3a1) to assist Aldh2 in acetaldehyde and ethanol metabolism in vivo. Proceedings of the National Academy of Sciences of the USA . 2015;112(10):3074–3079. doi: 10.1073/pnas.1414657112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roblegg E., Coughran A., Sirjani D. Saliva: an all-rounder of our body. European Journal of Pharmaceutics and Biopharmaceutics . 2019;142:133–141. doi: 10.1016/j.ejpb.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 109.Nuchit S., Lam-Ubol A., Paemuang W., et al. Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: a randomized controlled trial. Supportive Care in Cancer . 2020;28(6):2817–2828. doi: 10.1007/s00520-019-05132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hahnel S., Behr M., Handel G., Burgers R. Saliva substitutes for the treatment of radiation-induced xerostomia-a review. Supportive Care in Cancer . 2009;17(11):1331–1343. doi: 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- 111.Spirk C., Hartl S., Pritz E., et al. Comprehensive investigation of saliva replacement liquids for the treatment of xerostomia. International Journal of Pharmaceutics . 2019;571 doi: 10.1016/j.ijpharm.2019.118759.118759 [DOI] [PubMed] [Google Scholar]
- 112.Lopez Jornet P., Hernandez L., Gomez Garcia F., Galera Molero F., Pons-Fuster Lopez E., Tvarijonaviciute A. A clinical study on the efficacy and tolerability of a new topical gel and toothpaste in patients with xerostomia: a randomized controlled trial. Journal of Clinical Medicine . 2021;10(23):p. 5641. doi: 10.3390/jcm10235641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dalodom S., Lam-Ubol A., Jeanmaneechotechai S., et al. Influence of oral moisturizing jelly as a saliva substitute for the relief of xerostomia in elderly patients with hypertension and diabetes mellitus. Geriatric Nursing . 2016;37(2):101–109. doi: 10.1016/j.gerinurse.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 114.Cirillo N., Vicidomini A., McCullough M., et al. A hyaluronic acid-based compound inhibits fibroblast senescence induced by oxidative stress in vitro and prevents oral mucositis in vivo. Journal of Cellular Physiology . 2015;230(7):1421–1429. doi: 10.1002/jcp.24908. [DOI] [PubMed] [Google Scholar]
- 115.Berk L. Systemic pilocarpine for treatment of xerostomia. Expert Opinion on Drug Metabolism and Toxicology . 2008;4(10):1333–1340. doi: 10.1517/17425255.4.10.1333. [DOI] [PubMed] [Google Scholar]
- 116.Malallah O. S., Garcia C. M. A., Proctor G. B., Forbes B., Royall P. G. Buccal drug delivery technologies for patient-centred treatment of radiation-induced xerostomia (dry mouth) International Journal of Pharmaceutics . 2018;541(1-2):157–166. doi: 10.1016/j.ijpharm.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Laffleur F., Rottges S. Buccal adhesive chitosan conjugate comprising pilocarpine for xerostomia. International Journal of Biological Macromolecules . 2019;135:1043–1051. doi: 10.1016/j.ijbiomac.2019.05.219. [DOI] [PubMed] [Google Scholar]
- 118.O’Sullivan E. M., Higginson I. J. Clinical effectiveness and safety of acupuncture in the treatment of irradiation-induced xerostomia in patients with head and neck cancer: a systematic review. Acupuncture in Medicine . 2010;28(4):191–199. doi: 10.1136/aim.2010.002733. [DOI] [PubMed] [Google Scholar]
- 119.Assy Z., Brand H. S. A systematic review of the effects of acupuncture on xerostomia and hyposalivation. BMC Complementary and Alternative Medicine . 2018;18(1):p. 57. doi: 10.1186/s12906-018-2124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonomo P., Stocchi G., Caini S., et al. Acupuncture for radiation-induced toxicity in head and neck squamous cell carcinoma: a systematic review based on pico criteria. European Archives of Oto-Rhino-Laryngology . 2022;279(4):2083–2097. doi: 10.1007/s00405-021-07002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singla V., Vb T., Devi P., Singla N., Aggarwal N. Evaluation of the effects of transcutaneous electrical nerve stimulation (tens) on the whole salivary flow rate in healthy adult subjects—a clinical study. South Asian Research Journal of Oral and Dental Sciences . 2019;1(1):7–14. doi: 10.36346/sarjods.2019.v01i01.002. [DOI] [Google Scholar]
- 122.Tulek A., Mulic A., Hogset M., Utheim T. P., Sehic A. Therapeutic strategies for dry mouth management with emphasis on electrostimulation as a treatment option. International Journal of Dentistry . 2021;2021:21. doi: 10.1155/2021/6043488.6043488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salimi F., Saavedra F., Andrews B., FitzGerald J., Winter S. C. Trans-cutaneous electrical nerve stimulation to treat dry mouth (xerostomia) following radiotherapy for head and neck cancer. A systematic review. Annals of Medicine and Surgery . 2021;63 doi: 10.1016/j.amsu.2021.01.094.102146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thom S. R., Bhopale V. M., Velazquez O. C., Goldstein L. J., Thom L. H., Buerk D. G. Stem cell mobilization by hyperbaric oxygen. American Journal of Physiology - Heart and Circulatory Physiology . 2006;290(4):H1378–H1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 125.Forner L., Hyldegaard O., von Brockdorff A. S., et al. Does hyperbaric oxygen treatment have the potential to increase salivary flow rate and reduce xerostomia in previously irradiated head and neck cancer patients? A pilot study. Oral Oncology . 2011;47(6):546–551. doi: 10.1016/j.oraloncology.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 126.Ravi P., Vaishnavi D., Gnanam A., Krishnakumar Raja V. B. The role of hyperbaric oxygen therapy in the prevention and management of radiation-induced complications of the head and neck—a systematic review of literature. Journal of Stomatology, Oral and Maxillofacial Surgery . 2017;118(6):359–362. doi: 10.1016/j.jormas.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 127.Cankar K., Finderle Z., Jan J. The effect of hyperbaric oxygenation on postradiation xerostomia and saliva in patients with head and neck tumours. Caries Research . 2011;45(2):136–141. doi: 10.1159/000324811. [DOI] [PubMed] [Google Scholar]
- 128.Gerlach N. L., Barkhuysen R., Kaanders J. H., Janssens G. O., Sterk W., Merkx M. A. The effect of hyperbaric oxygen therapy on quality of life in oral and oropharyngeal cancer patients treated with radiotherapy. International Journal of Oral and Maxillofacial Surgery . 2008;37(3):255–259. doi: 10.1016/j.ijom.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 129.Baum B. J., Zheng C., Alevizos I., et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncology . 2010;46(1):4–8. doi: 10.1016/j.oraloncology.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baum B. J., Zheng C., Cotrim A. P., et al. Transfer of the Aqp1 cdna for the correction of radiation-induced salivary hypofunction. Biochimica et Biophysica Acta (BBA)—Biomembranes . 2006;1758(8):1071–1077. doi: 10.1016/j.bbamem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 131.Wang Z., Zourelias L., Wu C., Edwards P. C., Trombetta M., Passineau M. J. Ultrasound-assisted nonviral gene transfer of Aqp1 to the irradiated minipig parotid gland restores fluid secretion. Gene Therapy . 2015;22(9):739–749. doi: 10.1038/gt.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baum B. J., Wellner R. B., Zheng C. Gene transfer to salivary glands. International Review of Cytology . 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- 133.Vitolo J. M., Baum B. J. The use of gene transfer for the protection and repair of salivary glands. Oral Diseases . 2002;8(4):183–191. doi: 10.1034/j.1601-0825.2002.02865.x. [DOI] [PubMed] [Google Scholar]
- 134.Yao X. L., Nakagawa S., Gao J. Q. Current targeting strategies for adenovirus vectors in cancer gene therapy. Current Cancer Drug Targets . 2011;11(7):810–825. doi: 10.2174/156800911796798896. [DOI] [PubMed] [Google Scholar]
- 135.Baum B. J., Alevizos I., Zheng C., et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cdna for radiation-induced salivary hypofunction. Proceedings of the National Academy of Sciences of the USA . 2012;109(47):19403–19407. doi: 10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alevizos I., Zheng C., Cotrim A. P., et al. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Therapy . 2017;24(3):176–186. doi: 10.1038/gt.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alevizos I., Zheng C., Cotrim A. P., et al. Immune reactivity after adenoviral-mediated aquaporin-1 cdna transfer to human parotid glands. Oral Diseases . 2017;23(3):337–346. doi: 10.1111/odi.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Samuni Y., Baum B. J. Gene delivery in salivary glands: from the bench to the clinic. Biochimica et Biophysica Acta—Molecular Basis of Disease . 2011;1812(11):1515–1521. doi: 10.1016/j.bbadis.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheng C., Baum B. J., Liu X., et al. Persistence of Haqp1 expression in human salivary gland cells following Adhaqp1 transduction is associated with a lack of methylation of hcmv promoter. Gene Therapy . 2015;22(9):758–766. doi: 10.1038/gt.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao R., Yan X., Zheng C., et al. Aav2-Mediated transfer of the human aquaporin-1 cdna restores fluid secretion from irradiated miniature pig parotid glands. Gene Therapy . 2011;18(1):38–42. doi: 10.1038/gt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Passineau M. J., Zourelias L., Machen L., Edwards P. C., Benza R. L. Ultrasound-assisted non-viral gene transfer to the salivary glands. Gene Therapy . 2010;17(11):1318–1324. doi: 10.1038/gt.2010.86. [DOI] [PubMed] [Google Scholar]
- 142.Cotrim A. P., Sowers A., Mitchell J. B., Baum B. J. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Molecular Therapy . 2007;15(12):2101–2106. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- 143.Zheng C., Cotrim A. P., Rowzee A., et al. Prevention of radiation-induced salivary hypofunction following hkgf gene delivery to murine submandibular glands. Clinical Cancer Research . 2011;17(9):2842–2851. doi: 10.1158/1078-0432.CCR-10-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferreira J. N. A., Zheng C., Lombaert I. M. A., et al. Neurturin gene therapy protects parasympathetic function to prevent irradiation-induced murine salivary gland hypofunction. Molecular Therapy—Methods & Clinical Development . 2018;9:172–180. doi: 10.1016/j.omtm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Knox S. M., Lombaert I. M. A., Reed X., Vitale-Cross L., Gutkind J. S., Hoffman M. P. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science . 2010;329(5999):1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Knox S. M., Lombaert I. M. A., Haddox C. L., et al. Parasympathetic stimulation improves epithelial organ regeneration. Nature Communications . 2013;4(1):p. 1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pringle S., Van Os R., Coppes R. P. Concise review: adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells . 2013;31(4):613–619. doi: 10.1002/stem.1327. [DOI] [PubMed] [Google Scholar]
- 148.Chansaenroj A., Yodmuang S., Ferreira J. N. Trends in salivary gland tissue engineering: from stem cells to secretome and organoid bioprinting. Tissue Engineering Part B Reviews . 2021;27(2):155–165. doi: 10.1089/ten.TEB.2020.0149. [DOI] [PubMed] [Google Scholar]
- 149.Nevens D., Nuyts S. The role of stem cells in the prevention and treatment of radiation-induced xerostomia in patients with head and neck cancer. Cancer Medicine . 2016;5(6):1147–1153. doi: 10.1002/cam4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lombaert I. M. A., Brunsting J. F., Wierenga P. K., et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One . 2008;3(4) doi: 10.1371/journal.pone.0002063.e2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Feng J., van der Zwaag M., Stokman M. A., van Os R., Coppes R. P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiotherapy & Oncology . 2009;92(3):466–471. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 152.Pringle S., Maimets M., van der Zwaag M., et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells . 2016;34(3):640–652. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- 153.Serrano Martinez P., Cinat D., Luijk P., et al. Mouse parotid salivary gland organoids for the in vitro study of stem cell radiation response. Oral Diseases . 2021;27(1):52–63. doi: 10.1111/odi.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tanaka J., Mishima K. In vitro three‐dimensional culture systems of salivary glands. Pathology International . 2020;70(8):493–501. doi: 10.1111/pin.12947. [DOI] [PubMed] [Google Scholar]
- 155.Lombaert I., Movahednia M. M., Adine C., Ferreira J. N. Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cells . 2017;35(1):97–105. doi: 10.1002/stem.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rocchi C., Emmerson E. Mouth-watering results: clinical need, current approaches, and future directions for salivary gland regeneration. Trends in Molecular Medicine . 2020;26(7):649–669. doi: 10.1016/j.molmed.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 157.Aure M. H., Arany S., Ovitt C. E. Salivary glands: stem cells, self-duplication, or both? Journal of Dental Research . 2015;94(11):1502–1507. doi: 10.1177/0022034515599770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kawakami M., Ishikawa H., Tanaka A., Mataga I. Induction and differentiation of adipose-derived stem cells from human buccal fat pads into salivary gland cells. Human Cell . 2016;29(3):101–110. doi: 10.1007/s13577-016-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dai T. Q., Zhang L. L., An Y., et al. In vitro transdifferentiation of adipose tissue-derived stem cells into salivary gland acinar-like cells. American Journal of Translational Research . 2019;11(5):2908–2924. [PMC free article] [PubMed] [Google Scholar]
- 160.Kim J. W., Kim J. M., Choi M. E., Kim S. K., Kim Y. M., Choi J. S. Adipose-derived mesenchymal stem cells regenerate radioiodine-induced salivary gland damage in a murine model. Scientific Reports . 2019;9(1) doi: 10.1038/s41598-019-51775-9.15752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gronhoj C., Jensen D. H., Vester-Glowinski P., et al. Safety and efficacy of mesenchymal stem cells for radiation-induced xerostomia: a randomized, placebo-controlled phase 1/2 trial (mesrix) International Journal of Radiation Oncology, Biology, Physics . 2018;101(3):581–592. doi: 10.1016/j.ijrobp.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 162.Mulyani S. W. M., Astuti E. R., Wahyuni O. R., Ernawati D. S., Ramadhani N. F. Xerostomia therapy due to ionized radiation using preconditioned bone marrow-derived mesenchymal stem cells. European Journal of Dermatology . 2019;13(2):238–242. doi: 10.1055/s-0039-1694697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Su X., Liu Y., Bakkar M., et al. Labial stem cell extract mitigates injury to irradiated salivary glands. Journal of Dental Research . 2020;99(3):293–301. doi: 10.1177/0022034519898138. [DOI] [PubMed] [Google Scholar]
- 164.Kichenbrand C., Velot E., Menu P., Moby V. Dental pulp stem cell-derived conditioned medium: an attractive alternative for regenerative therapy. Tissue Engineering Part B Reviews . 2019;25(1):78–88. doi: 10.1089/ten.TEB.2018.0168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, figures, and tables in this review paper are labeled with references.


