Abstract
Using representational difference analysis, we isolated novel meningococcal restriction-modification (R-M) systems. NmeBI, which is a homologue of the R-M system HgaI of Pasteurella volantium, was present in meningococci of the ET-5 complex and of lineage III. NmeAI was found in serogroup A, ET-37 complex, and cluster A4 meningococci. NmeDI was harbored by meningococci of the ET-37 complex and of cluster A4, but not by serogroup A meningococci. Two of the R-M systems, NmeBI and NmeDI, were located at homologous positions between the phenylalanyl-tRNA synthetase genes pheS and pheT, which appeared to be a preferential target for the insertion of foreign DNA in meningococci. The distribution of the three R-M systems was tested with 103 meningococcal strains comprising 49 sequence types. The vast majority of the strains had either NmeBI, NmeAI, or both NmeAI and NmeDI. Using cocultivation experiments, we could demonstrate that NmeBI, which was present in ET-5 complex meningococci, was responsible for a partial restriction of DNA transfer from meningococci of the ET-37 complex to meningococci of the ET-5 complex.
Neisseria meningitidis (the meningococcus) is a leading cause of bacterial meningitis in infants and adolescents (40, 42, 52). This gram-negative diplococcus is entirely restricted to the human host, where it colonizes the nasopharynx and is transmitted by aerosols. As described for Bacillus subtilis, Streptococcus pneumoniae, and Haemophilus influenzae, meningococci are naturally competent for transformation with free DNA (reviewed in reference 34). Transformation is enhanced by a 10-bp DNA uptake sequence specific to neisseriae (17, 21, 45). Natural transformation of meningococci contributes to the genetic diversity of meningococci by horizontal gene transfer (2, 25, 36, 60, 61; M. C. Maiden, B. Malorny, and M. Achtman, Letter, Mol. Microbiol., 21:1297–1298, 1996). Hence, analysis of a large variety of meningococcal strains by multilocus enzyme electrophoresis (MLEE) (10) and multilocus sequence typing (MLST) (35) revealed the presence of a large number of different meningococcal clones. On the other hand, clonal expansion results in the maintenance of single lineages, especially during epidemic spread (2). Even in the Northern hemisphere, where meningococcal disease is endemic and outbreaks are rare, several clonal lineages appear to be rather stable in the population. Of the large variety of clonal lineages, most cases of serogroup C disease are caused by ET-37 complex meningococci and derivatives of the cluster A4, whereas serogroup B disease is most frequently caused by ET-5 complex meningococci or meningococci of the lineage III (1).
The transformation process in neisseriae differs from the models developed for natural transformation of gram-positive bacteria and of H. influenzae because it has been demonstrated that significant amounts of DNA enter the cells as double-stranded DNA (5, 11). This difference may be very important because, after uptake, the double-stranded DNA, in contrast to single-stranded DNA, may be a target for restriction-modification (R-M) systems present in N. meningitidis. Such R-M systems are widely distributed among bacteria. The simplest bacterial R-M systems are type II R-M systems, which comprise distinct DNA restriction and modification enzymes and which require no cofactors other than magnesium ions (4, 58). Methylation of adenosyl or cytosyl residues by the modifying enzyme results in the protection of cellular DNA against cleavage by cellular restriction endonucleases (ENases). Type II R-M systems, which recognize nonsymmetrical sites on the target DNA, have been designated as type IIS. Usually, two different DNA methyltransferases (MTases) are present in type IIS R-M systems, each specific to one of the two strands of DNA. MTases share common functional motifs (31) and seem to have evolved from a common ancestor. In contrast, ENases of different R-M systems show only minor evolutionary relationships and seem to have evolved independently (27). Horizontal gene transfer of R-M systems between bacterial species has been described, and it has been suggested that this transfer contributes to the wide distribution of bacterial R-M systems (28). Examples for this phenomenon are sequence similarities between gonococcal and Haemophilus R-M systems (22, 47).
Several R-M systems have been reported in the genus Neisseria. A single strain of N. gonorrhoeae (the gonococcus) can harbor as many as seven different R-M systems (48). MTase activity of several gonococcal R-M systems has also been observed in meningococci (38). According to the Restriction Enzyme Database (REBASE), which is a collection of information about restriction ENases, MTases, and the microorganisms from which they have been isolated, thus far eight R-M systems have been solely identified in meningococci (3, 39a, 44). However, the recognition sites of only three of the meningococcal R-M systems (NmeCI, NmeRI, and NmeSI) are known, only two R-M systems have been cloned (NmeSI and NmeSDI), and NmeI to -IV have been defined by their restriction activities in cellular lysates. Nevertheless, considering that natural transformation of meningococci with chromosomal DNA has been suggested to involve double-stranded DNA, investigations of the interplay of R-M systems, natural transformation, and population structure of meningococci are highly desirable.
We describe here three novel meningococcal R-M systems which are differentially distributed among the most important meningococcal lineages. We characterized these R-M systems and investigate the role of one of them in meningococcal transformation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The meningococcal strains Z2491 (serogroup A, subgroup IV-1, The Gambia, 1983) and 2120 (serogroup C, ET-37 complex, Germany, 1997) have been described previously (17, 60). MC58 (serogroup B, ET-5 complex, United Kingdom, 1983) was a kind gift from E. R. Moxon (Oxford, United Kingdom). Chromosomal DNAs of 103 meningococcal strains comprising 49 sequence types (STs), which have been described recently (35), were kindly provided by M. Achtman (Berlin, Germany). N. lactamica 4691 was a reference strain provided by the German Type Culture Collection (Deutsche Sammlung von Mikroorganismen und Zellkulturen Braunschweig, Germany). Streptomycin-resistant derivatives of the meningococcal strains were selected by plating 5 × 109 CFU on agar containing 500 μg of streptomycin per ml. Meningococci were grown on GC medium base (Difco, Detroit, Mich.) at 37°C in 5% CO2 or in proteose-peptone broth (Difco) at 37°C, both with supplement VX (Difco). When appropriate, 100 μg of kanamycin, 7 μg of chloramphenicol, or 500 μg of streptomycin per ml were added to the medium. Escherichia coli DH5α was used as host for plasmid manipulations. E. coli was grown on Luria-Bertani (LB) agar (Difco) at 37°C in the presence of 100 μg of ampicillin, 30 μg of chloramphenicol, or 30 μg of kanamycin per ml, when appropriate. Antibiotics were purchased from Sigma (Deisenhofen, Germany).
Recombinant DNA techniques.
Restriction enzymes and DNA-modifying enzymes were purchased from New England Biolabs (Beverly, Mass.). Chromosomal DNA from N. meningitidis was purified on a CsCl gradient as described previously (49). Minipreparations of recombinant plasmids were made by the alkaline lysis method (41). RDA was performed essentially as described previously (53) with the following modifications: (i) chromosomal DNA of the tester strain was partially digested with Sau3AI and size-selected fractions were used for subtractive hybridizations and (ii) due to the partial digestion of the tester DNA, only one round of hybridization was performed. Transformation of meningococci was performed as described previously (19). For DNA-DNA dot blot hybridizations, 20 μl of suspensions of 1010 CFU/ml of H2O were dotted onto nylon membranes (Macherey-Nagel, Düren, Germany). Southern and colony blot hybridizations were performed as described previously with digoxigenin-labeled probes (24). RNA was prepared as described previously (23). For RNA-DNA dot blot hybridizations, 20 μg of RNA was dotted onto nylon membranes, immobilized by using a UV cross-linker (Stratagene Europe, Amsterdam, The Netherlands), and hybridized with probes, which were labeled with [α-32P]dCTP (3,000 Ci/mmol; Amersham, Braunschweig, Germany) with the Multiprime DNA labeling system (Amersham). A probe of the siaA gene (16), which is a constitutively expressed gene of the capsular polysaccharide biosynthesis locus in serogroup B, C, W135, and Y meningococci (13, 51), was used as a positive control. Oligonucleotides were purchased from ARK Scientific (Darmstadt, Germany) and are listed in Table 1. PCR was performed on a thermocycler obtained from Biometra (Göttingen, Germany). The thermostable DNA polymerase AmpliTaq was purchased from Perkin-Elmer (Weiterstadt, Germany). Automated DNA sequencing was performed on an Applied Biosystems model 377 (Foster City, Calif.) by using the dye terminator cycle method with AmpliTaq. Nucleotide sequence data were analyzed with Lasergene sequence analysis software (DNAstar, Madison, Wis.). DNA and protein sequences were compared with the GenBank and SWISS-PROT databases on the BLAST server hosted by the National Center for Biotechnology Information (Bethesda, Md.). Further DNA comparisons were made with the preliminary sequence data released by the meningococcus genome sequencing project at the Sanger Centre (Cambridge, United Kingdom; http://www.sanger.ac.uk/Projects/N_meningitidis/).
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′) | Target DNA | Positiond |
|---|---|---|---|
| ATGAAAAACAGTAAGTTAAAGG | nmeAIMa | 398,743–398,764e | |
| HC194 | CTAACATTCGATATTATCAAC | nmeAIM | 399,808–399,788a |
| HC160 | ATGATGATAGGGGCTTCT | nmeBIMa | 183–200f |
| HC161 | CTATTTTCCTATTCTCTCTAC | nmeBIM | 1,241–1,222f |
| HC223 | TGCCGAAAAAGTCAGACAAG | nmeDIMa | 701–720g |
| HC224 | TCATAGTAGTTCTCCAAATAAG | nmeDIM | 1,910–1,889g |
| HC204 | GCCATGAGTAGGAGAATTGC | pheTb | 906,543–906,524e |
| HC205 | CAACGTGAACGACTTGCGC | pheSb | 904,654–904,672e |
| UE43 | ATGAAAAGAATTCTTTGCATTACAGGTACC | siaAc | 174–202h |
| UE31 | TTAAAGATTCAAATCGATAA | siaA | 1,307–1,288h |
| HC202 | GTCGACGCAGGCCTGCGTCGAC | Restriction site linker |
This study.
pheS and pheT encode the alpha and beta chains of the PheRS.
siaA is a constitutively expressed gene of the capsular polysaccharide biosynthesis locus (13, 51).
Nucleotide positions are according to the numbering of the published sequences indicated in footnotes e to g.
Continuous nucleotide sequence of the N. meningitidis Z2491 (http://www.sanger.ac.uk/Projects/N_meningitidis/).
EMBL accession no. AJ132413.
EMBL accession no. AJ238948.
EMBL accession no. M95053.
Cocultivation experiments and plasmids.
Cocultivation of meningococci was performed as described previously (18). Briefly, equal volumes of bacterial suspensions (optical density at 600 nm of 0.2) of the kanamycin-resistant donor and of the streptomycin-resistant recipient strain were combined and incubated at 37°C for 3 h. Serial dilutions were taken from the combined cultures and plated on GC agar supplemented with both kanamycin and streptomycin. The following controls of the cocultivation experiments were carried out. (i) Experiments done in the presence of 40 μg of DNase I (Sigma) per ml abrogated transformation, indicating that DNA transfer was not due to conjugation (data not shown). (ii) Transformants were tested for capsular serogroup by latex agglutination with the Directigen Meningitis Combo Test (Becton Dickinson, Meylan, France). All transformants expressed the capsule of the recipient strain, demonstrating that the selective marker of the donor strain carrying the kanamycin resistance gene, but not the streptomycin resistance gene, was transferred. Donor strains carried the kanamycin resistance gene as a selective marker either in the lst gene or in the hrtA locus. The plasmids used for the construction of the mutants were as follows. pBluescript SK(+) and pCR-Script were purchased from Stratagene Europe. Plasmids pCR-Script-lst and pHC6, as well as pCR-Script-lst/Kan and pHC6.1, have been described recently (12, 55). Briefly, a 1,467-bp PCR fragment comprising the lst gene of N. meningitidis, which encodes the α-2,3-sialyltransferase (20), was cloned into the vector pCR-Script resulting in pCR-Script-lst. Subsequently, the kanamycin resistance gene was ligated into the HincII site of the lst gene resulting in pCR-Script-lst/Kan. pHC6 comprises a 4.4-kb EcoRI DNA fragment of N. meningitidis, which harbors the hrtA locus. The kanamycin resistance gene was cloned into the BstEII site of pHC6, resulting in pHC6.1. To introduce HgaI restriction sites (5′-GACGC-3′) adjacent to the kanamycin resistance gene, an oligonucleotide linker (HC202) was inserted into the HincII site of pCR-Script-lst and into the BstEII site of pHC6, respectively. The StuI site within the linker was the target site to insert the kanamycin resistance gene. A 1,258-bp fragment containing the kanamycin resistance gene was isolated from pUK4K (Pharmacia, Freiburg, Germany; accession no. X06404) by HincII digestion. The chloramphenicol acetyltransferase (CAT) gene was isolated from the plasmid pTnMax5 (accession no. Z50120) by HindIII digestion, resulting in a 1,001-bp fragment harboring the CAT gene and an fd terminator sequence (tfd).
RESULTS
In this study, novel meningococcal R-M systems were identified by representational difference analysis (RDA). RDA has been designed to compare highly related genomes by subtractive hybridization and kinetic enrichment of unique DNA (33). DNA fragments are isolated which are present in only one of two genomes. To isolate meningococcus-specific DNA fragments not present in nonpathogenic N. lactamica, two independent RDAs were performed with meningococcal strains of different clonal lineages as tester strains, i.e., the serogroup B meningococcal strain MC58 (ET-5 complex) and the serogroup A meningococcal strain Z2491 (subgroup IV-1). N. lactamica was used as the driver strain. In the case of the RDA involving the tester strain MC58, 23 distinct DNA fragments were isolated. In the case of the RDA involving the tester strain Z2491, 11 distinct DNA fragments were isolated. Sequence analysis of the meningococcus-specific fragments revealed homologies to R-M systems for 2 of the 34 fragments. These two, and a third novel meningococcal R-M system, which was also differentially distributed in clonal lineages of meningococci, are described below.
HgaI homologue NmeBI.
The meningococcus-specific fragment B1/14 (688 bp) was isolated by RDA and hybridized with the tester strain MC58 but not with the subgroup IV-1 strain Z2491. Using this fragment as a probe, overlapping chromosomal DNA fragments of strain MC58 were cloned, and 3,824 bp were sequenced. The 229 bp at the 5′ end and the 205 bp at the 3′ end of the sequence were 95 and 98% identical, respectively, to parts of two open reading frames (ORF) of the preliminary sequence data of strain Z2491 provided by the Sanger Centre (http://www.sanger.ac.uk/Projects/N_meningitidis/). The deduced amino acid sequences of these ORFs shared homologies with the alpha and beta chains of the phenylalanyl-tRNA synthetase (PheRS) of Escherichia coli (SWISS-PROT accession nos. P08312 and P07395), respectively. The 3,390 bp (EMBL accession no. AJ132413) inserted between the genes encoding the alpha and beta chains of PheRS were specific to MC58 and were not found in Z2491, in which a different insertion of 1,803-bp was present (Fig. 1A). The insertion in MC58 comprised three ORFs (Fig. 1A). The deduced amino acid sequence of ORF 1 (352 amino acids [aa]) showed 56% identity and 70% similarity to the cytosine-5-specific (m5C) MTase M.HgaI-1, and the deduced amino acid sequence of ORF 2 (473 aa) showed 42% identity and 58% similarity to the ENase R.HgaI of the R-M system HgaI of Pasteurella volantium (SWISS-PROT accession nos. P25282 and P43418, respectively). The deduced amino acid sequence of the third ORF (171 aa) shared no noteworthy homologies with known proteins deposited in the SWISS-PROT database. The A+T content of the 3,390-bp insertion specific to MC58 was about 67%, whereas the A+T content of the adjacent regions was about 50%, which is in the range of the A+T content of the meningococcal chromosome (48 to 50%) (reviewed in reference 54). These parameters are an indication for the acquisition of this fragment from distant sources by horizontal gene transfer, perhaps from Haemophilus or Pasteurella spp., whose genomic A+T content ranges between 56 and 63% (reviewed in references 9 and 29).
FIG. 1.
(A) Schematic depiction of the insertions between the pheS and pheT genes (encoding the alpha and beta chains of the PheRS) in different meningococcal strains. The length of the insertion in strain Z2491 is 1,803 bp, that in strain MC58 is 3,390 bp, and that in strain 2120 is 3,013 bp. The A+T content is given for the entire insertion and the genes flanking the insertion, respectively. The orientations of the putative ORFs are indicated by arrows. The restriction sites used for the construction of a NmeBI deletion mutant of strain MC58 are indicated. (B) Schematic depiction of the location of the NmeAI system of strain Z2491 based on the preliminary sequence data released by the meningococcus genome sequencing project (http://www.sanger.ac.uk./Projects/N-meningitidis/). The orientations of the putative ORFs are indicated by arrows. The numbers indicate the A+T content of the ORFs and the intergenic regions, respectively.
The HgaI R-M system was previously isolated from the NCTC strain 3438, which formerly was deposited as Haemophilus gallinarum (7, 50). This R-M system belongs to the family of type IIS R-M systems, which recognize asymmetrical DNA sequences (in the case of HgaI, 5′-GCGTC-3′ on one strand and 5′-GACGC-3′ on the other strand), and cut the DNA at a fixed distance outside the recognition sequence. The modification of the nonsymmetrical recognition sequence in the HgaI system is accomplished by two independent m5C-MTases, one MTase specific to each strand. Therefore, the HgaI system comprises two m5C-MTases (M.HgaI-1 and M.HgaI-2) and one ENase (R.HgaI). In contrast, in the meningococcal homologue (designated NmeBI) isolated from MC58 there was only one complete MTase gene. This M.HgaI-1 homologue exhibited the 10 typical motifs of m5C-MTases (31). The deduced amino acid sequence of the target recognition domain between the conserved motifs VIII and IX of M.NmeBI was 63% identical and 78% similar to the Pasteurella enzyme M.HgaI-1. The high homology (42% identity, 58% similarity) of the potential meningococcal ENase to R.HgaI indicated that the two systems share the same target site specificity because ENases are in general much less related to each other than MTases.
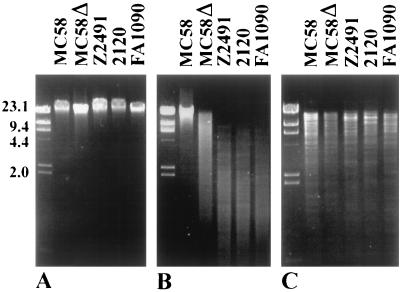
To analyze the function of the predicted MTase, a BspEI/StyI deletion mutant of strain MC58 was constructed (see Fig. 1A), in which parts of both the MTase and the ENase were replaced by the CAT gene. Chromosomal DNA of the wild-type strain and of the NmeBI mutant, respectively, were digested with HgaI. The wild-type DNA was resistant to HgaI digestion, but the DNA of the mutant was restricted (Fig. 2). This finding demonstrated that the MTase encoded by nmeBIM modified the same recognition sequence as the enzyme of Pasteurella volantium. The DNA of the NmeBI mutant was only partially cleaved by HgaI, in contrast to DNA of neisserial strains not belonging to the ET-5 complex. This finding suggested that the chromosomal DNA of the mutant was still partially methylated at the cleavage site, e.g., by the action of an alternative methylase not belonging to the HgaI homologue.
FIG. 2.
Susceptibility of chromosomal DNA to cleavage by HgaI. (A) Uncut chromosomal DNA. (B) Chromosomal DNA digested with HgaI. (C) Chromosomal DNA digested with both HindIII and EcoRI. Lanes: MC58, wild-type strain (ET-5 complex); MC58Δ, NmeBI knockout mutant of MC58; Z2491, N. meningitidis serogroup A (subgroup IV-1); 2120, N. meningitidis serogroup C (ET-37 complex); FA1090, N. gonorrhoeae.
HgiDII homologue NmeAI.
The meningococcus-specific fragment A2/24 (EMBL accession no. AJ243341) was isolated by RDA from strain Z2491. It did not hybridize with the ET-5 complex strain MC58. Nucleotide sequence comparison with the preliminary sequence database released by the meningococcus genome sequencing project of strain Z2491 (http://www.sanger.ac.uk/Projects/N_meningitidis/) revealed that fragment A2/24 was part of two ORFs (Fig. 1B) which shared homologies with a R-M system (designated NmeAI). The deduced amino acid sequence of ORF 1 (351 aa) showed 38% identity and 53% similarity to the MTase M.HgiDII of Herpetosiphon aurantiacus (SWISS-PROT accession no. P25265), a gram-negative rod found in different environmental habitats. The homology was the same for the target recognition domain of the enzyme, which is found between the conserved motifs VIII and IX of m5C-MTases (31). The N-terminal 308 residues of the deduced amino acid sequence of ORF 2 (548 aa) downstream to the meningococcal MTase showed 27% identity and 43% similarity to the protein in the M.HgiDII 3′ region (SWISS-PROT accession no. P25280). The remaining 240 residues shared no homologies with known proteins deposited in the SWISS-PROT database. The A+T content of the first ORF of NmeAI was about 59%, and of the second ORF it was about 62%. This A+T content is approximately 10% higher than that of meningococci (48 to 50%) and that of H. aurantiacus (47 to 55%) (reviewed in reference 26), respectively, suggesting that both meningococci and H. aurantiacus acquired related systems from distant sources.
The HgiDII system is a type II R-M system, which is isoschizomeric to the SalI system (recognition site 5′-GTCGAC-3′). We can rule out the possibility that the meningococcal homologue to HgiDII (NmeAI) is an isoschizomer of SalI, since chromosomal DNA of Z2491 and of the serogroup C strain 2120, which also harbors NmeAI, was susceptible to SalI restriction. The gene encoding the HgiDII MTase has been cloned and characterized, whereas the HgiDII ENase has only been described as enzymatical activity in cellular extracts. Until now, there is no experimental evidence that the ORF downstream to hgiDIIM encodes the cognate ENase (15). Nevertheless, it is conceivable that the MTase encoded by NmeAI is an m5C-MTase, because it exhibits the 10 motifs typical of this type of MTases (31).
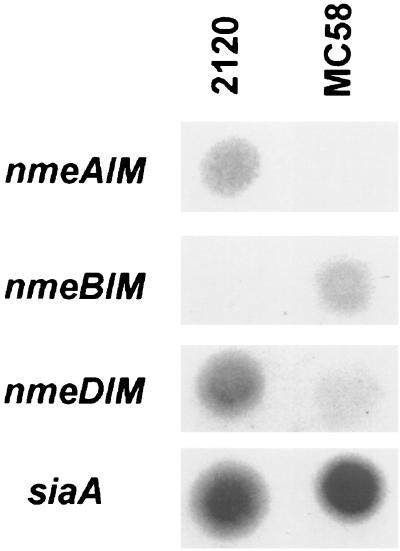
RNA dot blot hybridizations were made to analyze whether the putative MTase was expressed in wild-type meningococci. RNA was isolated from the ET-37 complex strain 2120 and from the ET-5 complex strain MC58. 2120 harbored NmeAI, as did Z2491, and was used for RNA analysis because functional studies described below were performed with this strain. The 3′ region of the MTase gene (PCR product HC193/194) was used as a probe. There was a strong expression of the MTase gene in the ET-37 complex strain but not in the ET-5 complex strain. (Fig. 3). Therefore, although no further functional data were available until now, we could demonstrate active transcription of the methylase gene of NmeAI.
FIG. 3.
RNA dot blot hybridizations. A total of 20 μg of RNA of the ET-37 complex strain 2120 and of the ET-5 complex strain MC58, respectively, were dotted onto nylon membranes and hybridized with probes of the MTase genes of the R-M systems NmeAI, NmeBI, and NmeDI. Hybridization with a probe specific to the siaA gene was performed as positive control because the siaA gene is transcribed in both serogroup B and C meningococci.
pheS-pheT insertion in ET-37 complex meningococci: NmeDI.
In the meningococcal strain MC58, the NmeBI system comprising 3,390 bp was flanked by the genes encoding the alpha and beta chains of the PheRS, respectively. In serogroup A meningococci, an insertion of 1,803-bp with no significant homologies to entries in the GenBank database was present at the same site. We wondered about the structure of the pheS-pheT intergenic region in meningococci of the ET-37 complex. PCR was performed on the ET-37 complex strain 2120 employing the oligonucleotides HC204 and HC205 to amplify the insertion between the pheS and the pheT genes. Sequence analysis revealed that strain 2120 contained an insertion of 3,013 bp (EMBL accession no. AJ238948) with an A+T content of 56% (Fig. 1A). This insertion harbored three ORFs. The deduced amino acid sequence of the first ORF (148 aa) shared a large homology with the HpaII very short patch repair ENase (60% identity and 78% similarity) of Haemophilus parainfluenzae (SWISS-PROT accession no. P36434). The deduced amino acid sequence of the second ORF (420 aa) showed about 25% identity and 40% similarity to various m5C-MTases and contained the 10 conserved motifs of this class of MTases (31). The deduced amino acid sequence of the third ORF (351 aa) showed no significant homologies to known proteins deposited in the SWISS-PROT database. According to the facts that ENases with differing target specificities share less homologies and are typically found close to their cognate MTase, the third ORF may be an ENase. The meningococcal R-M system is designated below as NmeDI. The organization of the three ORFs is the same as that of the HpaII R-M system of H. parainfluenzae (30). Furthermore, the HpaII system is also located next to a tRNA synthetase gene, i.e., the valyl-tRNA synthetase gene (valS). In addition, two other R-M systems from Haemophilus sp. occur close to the valS gene, i.e., the HinP1I system of H. influenzae P1 and the HindIII system of H. influenzae Rd (37). These data demonstrate that in both Haemophilus and meningococci, R-M systems are frequently associated with tRNA synthetase genes. This association either reflects some biological advantage, or the chromosomal integration of horizontally transferred R-M systems frequently encounters tRNA synthetase genes. The methylase gene of NmeDI was actively transcribed in meningococci as shown by RNA dot blot hybridization (Fig. 3).
Distribution of NmeAI, NmeBI, and NmeDI within clonal lineages of meningococci.
The distribution of the R-M systems NmeAI, NmeBI, and NmeDI within clonal lineages of the species N. meningitidis was investigated by DNA-DNA hybridizations. For this purpose, chromosomal DNA of 103 strains belonging to 49 different STs (35) was hybridized with probes derived by PCR (for primers see Table 1) from the methylase genes of NmeAI, NmeBI, and NmeDI, respectively. The strain collection has been used for the establishment of the multilocus sequence typing technique (35) and reflects the genetic diversity of meningococcal lineages. The distribution of the R-M systems among the 49 STs of meningococci is shown in Table 2. Twenty-nine strains, which mostly belong to the ET-5 complex (n = 10) and lineage III (n = 12), were NmeBI positive but negative for NmeAI and NmeDI. Seventy-two strains, including the strains of the ET-37 complex (n = 9), of cluster A4 (n = 7), and of serogroup A (n = 35) were NmeAI positive and NmeBI negative. There were only 2 of 103 strains which were both NmeBI and NmeAI positive. These serogroup X meningococci belonged to the STs 24 and 39. Twenty strains, which included the strains of the ET-37 complex (n = 9) and of cluster A4 (n = 7), were NmeDI positive. This distribution resembled the distribution of NmeAI within the hypervirulent lineages, with the exception of serogroup A meningococci, which did not harbor NmeDI. Four strains of the collection (STs 22, 23, 29, and 35) hybridized with none of the probes. Among the clonal lineages, which are frequently associated with meningococcal disease worldwide, the presence or absence of the R-M systems NmeAI, NmeBI, and NmeDI could be considered as a lineage-specific characteristic. Furthermore, the data suggested that the R-M systems NmeAI and NmeBI exclude each other in one cell and separate the species N. meningitidis into two groups.
TABLE 2.
Distribution of NmeAI, NmeBI, and NmeDI among meningococcal strains of 49 different STsa
| STb | MLEE assignment | No. of analyzed isolates | No. of strains harboring:
|
||
|---|---|---|---|---|---|
| NmeBI | NmeAI | NmeDI | |||
| 1–7 | Subgroups I–VIII | 35 | 35 | ||
| 8–10 | Cluster A4 | 7 | 7 | 7 | |
| 11 | ET-37 complex | 9 | 9 | 9 | |
| 12–14 | Otherc | 3 | 3 | ||
| 15–21 | Other | 8 | 8 | ||
| 22, 23 | Other | 2 | |||
| 24 | Other | 1 | 1 | 1 | |
| 25–28 | Other | 4 | 4 | ||
| 29 | Other | 1 | |||
| 30, 31 | Other | 2 | 2 | ||
| 32–34 | ET-5 complex | 10 | 10 | ||
| 35 | Other | 1 | |||
| 36–38 | Other | 4 | 4 | 4 | |
| 39 | Other | 1 | 1 | 1 | |
| 40–42 | Lineage III | 8 | 8 | ||
| 43, 44 | Related to lineage III (35) | 2 | 2 | ||
| 45, 46 | Lineage III | 2 | 2 | ||
| 47, 48 | Other | 2 | 2 | ||
| 49 | Other | 1 | 1 | ||
Note that STs harboring NmeAI, NmeBI, or NmeDI are indicated. The numbers indicate the number of strains which harbor the R-M system.
The information for the STs was obtained from the MLST website (http://mlst.zoo.ox.ac.uk/).
ETs other than subgroups I to VIII, ET-5 complex, ET-37 complex, cluster A4, and lineage III.
Influence of NmeBI on horizontal gene transfer between meningococcal lineages.
ET-37 complex meningococci harbored NmeAI and NmeDI, whereas strains of the ET-5 complex were positive for NmeBI. Differential distribution has also been observed for opcA (43, 62), and for porin genes (10, 57). Thus, despite recombination inside the neisserial gene pool, meningococcal lineages can be ecologically separated. The reason for this phenomenon is unclear. As a first attempt to elucidate whether differentially distributed R-M systems contribute to sexual isolation, we investigated the impact of NmeBI on transfer of DNA in vitro between ET-37 complex and ET-5 complex strains. Transformation was tested in cocultivation experiments, which appear to resemble transformation in vivo (18). Equal volumes of suspensions of the kanamycin-resistant donor strain and streptomycin-resistant recipient strain were combined and incubated together. The number of transformants resistant to both kanamycin and streptomycin was determined. A streptomycin-resistant derivative of MC58 (ET-5 complex), which harbors NmeBI, was chosen as the recipient in the cocultivation experiment. 2120 (ET-37 complex) was selected as the donor strain. Strain 2120 harbored the kanamycin resistance gene either in the hrtA locus, which we described recently as a highly transforming meningococcal DNA fragment (12), or in the lst gene, which encodes the α-2,3-sialyltransferase (20). As demonstrated above (see Fig. 2), the NmeBI restriction site was isoschizomeric to the HgaI restriction site. Therefore, HgaI restriction sites were cloned adjacent to the kanamycin resistance cassette (see Materials and Methods). The recognition sites were not present in the hrtA locus, the lst gene, and the kanamycin resistance gene, respectively. We assumed that if the HgaI sites were restricted by the ENase of the NmeBI system after uptake of the DNA into the recipient cell, there would be no homologous recombination of the marker genes, including the kanamycin resistance gene, and subsequently no transformants would occur. Six independent experiments were performed in triplicate. Using the hrtA locus as a marker for transformation, the number of transformants was (3.8 ± 0.6)-fold higher, if no restriction sites flanked the kanamycin resistance gene, compared to experiments with donors harboring HgaI recognition sites adjacent to this selective marker gene. This number was (1.5 ± 0.7)-fold, when derivatives of strain 2120 were used, which harbored the kanamycin resistance determinant in the lst gene. Our data demonstrate that NmeBI affected the DNA transfer between ET-37 and ET-5 complex strains.
DISCUSSION
In the present report, we describe three novel meningococcal R-M systems: NmeAI, NmeBI, and NmeDI, which were differentially distributed among the clonal lineages of meningococci. In meningococci of the ET-5 complex and lineage III, we found a homologue to the HgaI system designated NmeBI. In MC58, NmeBI is inserted between the genes encoding the alpha and beta chains of the PheRS. Further investigations of this locus revealed that in ET-37 complex and cluster A4 meningococci a different putative R-M system (NmeDI) was located between the pheS and the pheT. This putative R-M system comprises a very short patch repair ENase homologue, a m5C-MTase, and an ORF with unknown function. In Haemophilus spp., i.e., H. parainfluenzae, H. influenzae P1, and H. influenzae Rd, three R-M systems, i.e., HpaII, HinP1I, and HindIII, occur close to the valS gene. The mechanism of integration of the meningococcal and the Haemophilus R-M systems remained obscure until now. Repeated DNA sequences have not been found at the insertion sites. Therefore, there is no evidence for integration via a mobile genetic element. Nevertheless, the locus next to the tRNA synthetase genes seemed to represent a favored locus for R-M systems both in meningococci and Haemophilus sp. The integration of DNA islands between housekeeping genes has been described recently for the neisserial opcA and ΨopcB regions (62). The analysis of these DNA islands in meningococci and gonococci revealed that they seemed to have been acquired by recombination via conserved flanking housekeeping genes rather than by insertion of mobile genetic elements.
The A+T content of the HgaI homologue NmeBI resembled that of Haemophilus and Pasteurella spp., and it is possible that the system was imported by meningococci from P. volantium, which is a birds' commensal (6). Due to the close contact of domesticated birds with humans, genetic exchange between P. volantium and meningococci is conceivable. The A+T content of the meningococcal HgiDII homologue NmeAI is about 61% and higher than the average meningococcal A+T content. In the case of the type II R-M system HgiDII, which has been described in H. auranticus, the A+T content is also about 60%, which is 10% higher than that of Herpetosiphon spp. (reviewed in reference 26). Therefore, the HgiDII system of H. aurantiacus and the homologue in N. meningitidis, NmeAI, seem to have been imported from sources with a higher A+T content, perhaps from the same source. The A+T content of the NmeDI system is about 56% and higher than the average meningococcal A+T content, which indicates that this system also has been acquired from a distant source with a higher A+T content.
A survey of genetically distinct meningococcal strains, which were characterized by MLST (35), was hybridized with DNA probes derived from the R-M systems described in this study in order to estimate the distribution of the R-M systems among the different clonal lineages of meningococci. With the exception of two STs (STs 24 and 39), NmeBI and NmeAI did not occur simultaneously in one cell. The major clonal groupings causing epidemic and hyperendemic meningococcal disease could be divided into three groups by the meningococcal R-M systems according to their genetic relationships as defined by MLST typing: (i) the lineages causing most cases of serogroup B disease worldwide, i.e., ET-5 complex and lineage III, harbored NmeBI; (ii) serogroup A strains harbored NmeAI; and (iii) the clonal groupings causing most cases of serogroup C disease, i.e., ET-37 complex and cluster A4, harbored NmeAI and NmeDI.
Therefore, NmeDI most likely was acquired by an ancestor of ET-37 and cluster A4 meningococci after serogroup A diverged from this branch or, alternatively, was lost by serogroup A meningococci. A differential distribution has also been shown very recently for another R-M system, the dcmD-dcrD homologue, which was inserted as a DNA island between the housekeeping genes trpE and purK (62). This R-M system was present in meningococcal strains of the STs 25 and 26 and in gonococci.
The clonal distribution of the meningococcal R-M resembled other differentially distributed meningococcal genes. The class 3 porB gene and the opcA gene are found in serogroup A meningococci and in the meningococci of the ET-5 complex and lineage III, whereas meningococci of the ET-37 complex contain a class 2 porB gene and lack the opcA gene (10, 43, 57, 62). These examples are indicative of an ecological separation of ET-37 complex meningococci. Several factors could be responsible for this phenomenon. First, infrequent exchange of DNA due to infrequent cocolonization of the human nasopharynx could isolate a clonal grouping. This scenario is unlikely, because the worldwide geographical distribution of ET-37 complex meningococci does not differ from that of lineages, which appear to exchange DNA. Second, as in the Bacillus system, sequence divergence of genetic loci could be responsible for sexual isolation (39, 59). However, data are not available on the importance of sequence divergence for transformation in meningococci. Finally, in the present study, we raised the question of whether a barrier of transformation was mediated by the presence of the R-M systems. The restriction of DNA transfer by R-M systems has been studied in detail in S. pneumoniae and N. gonorrhoeae. Strains of S. pneumoniae contain either the DpnI or the DpnII restriction system. There was only a minor restriction of the transfer of a plasmid grown on a strain having the DpnI phenotype to a recipient strain with the DpnII phenotype and vice versa in comparison to the transfer of a plasmid grown on the strain having the same phenotype as the recipient strain (32). This effect can be explained by the fact that double-stranded DNA, which is bound to S. pneumoniae cells, is released into the cell as single-stranded DNA (reviewed in reference 34). In neisseriae, however, DNA is recovered in double-stranded form within the cell (5, 11), which renders it susceptible to R-M systems. It has been reported that in the absence of methylation, the transformation of gonococci by a replicative plasmid was restricted by the R-M systems of the recipient strain (46). However, if a nonreplicative plasmid carried a chromosomal DNA fragment, which ought to be integrated into the chromosome by homologous recombination, there was no significant barrier of transformation (45). Concerning conjugative plasmid transfer in gonococci, contradicting results were obtained by two studies. Conjugation between gonococcal strains harbouring different R-M systems seemed to be independent of host-mediated restriction (46), whereas the mobilization of a plasmid from an E. coli donor to a gonococcal recipient was restricted by the recipient and was dependent on the appropriate methylation of the plasmid (8). However, in none of these studies were cocultivation experiments performed, which are an in vitro model for horizontal transfer of chromosomal DNA that resembles the in vivo situation (18). In our study, cocultivation experiments with meningococci revealed the restriction of DNA transformation between ET-37 complex and ET-5 complex meningococci by NmeBI. This effect, however, was rather weak, which might be due to the fact that only two restriction sites were present adjacent to the selective marker gene but not in the marker gene itself or in the chromosomal loci harboring the selective marker. Future experiments should address the cooperative action of several R-M systems resident in one clonal lineage, which should result into a more rigorous cleavage of the DNA fragments transferred. Nevertheless, our report demonstrates for the first time that R-M systems affect genetic transformation of meningococci by chromosomal DNA. We therefore suggest that R-M systems are one factor stabilizing clonal lineages by inhibiting DNA transfer from unrelated clones.
ACKNOWLEDGMENTS
This project was supported by grant V0718/3-1 of the Deutsche Forschungsgemeinschaft to U.V. and M.F.
Mark Achtman is gratefully acknowledged for the kind gift of chromosomal DNAs of meningococcal strains described earlier (35). The N. meningitidis sequencing group at the Sanger Centre is thanked for sharing their data with the scientific community via the Internet.
REFERENCES
- 1.Achtman M. Global epidemiology of meningococcal disease. In: Cartwright K, editor. Meningococcal disease. Chichester, England: John Wiley & Sons, Ltd.; 1995. pp. 159–175. [Google Scholar]
- 2.Achtman M. Microevolution and epidemic spread of serogroup A Neisseria meningitidis—a review. Gene. 1997;192:135–140. doi: 10.1016/s0378-1119(97)00083-8. [DOI] [PubMed] [Google Scholar]
- 3.Bart A, Dankert J, van der Ende A. Operator sequences for the regulatory proteins of restriction modification systems. Mol Microbiol. 1999;31:1277–1278. doi: 10.1046/j.1365-2958.1999.01253.x. [DOI] [PubMed] [Google Scholar]
- 4.Bickle T A, Krüger D H. Biology of DNA restriction. Microbiol Rev. 1993;57:434–450. doi: 10.1128/mr.57.2.434-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas G D, Sparling P F. Entry of double-stranded deoxyribonucleic acid during transformation of Neisseria gonorrhoeae. J Bacteriol. 1981;145:638–640. doi: 10.1128/jb.145.1.638-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackall P J. The avian haemophili. Clin Microbiol Rev. 1989;2:270–277. doi: 10.1128/cmr.2.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown N L, Smith M. Cleavage specificity of the restriction endonuclease isolated from Haemophilus gallinarum (HgaI) Proc Natl Acad Sci USA. 1977;74:3213–3216. doi: 10.1073/pnas.74.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler C A, Gotschlich E C. High-frequency mobilization of broad-host-range plasmids into Neisseria gonorrhoeae requires methylation in the donor. J Bacteriol. 1991;173:5793–5799. doi: 10.1128/jb.173.18.5793-5799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter G R. Pasteurella. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 552–558. [Google Scholar]
- 10.Caugant D A, Mocca L F, Frasch C E, Froholm L O, Zollinger W D, Selander R K. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol. 1987;169:2781–2792. doi: 10.1128/jb.169.6.2781-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaussee M S, Hill S A. Formation of single-stranded DNA during DNA transformation of Neisseria gonorrhoeae. J Bacteriol. 1998;180:5117–5122. doi: 10.1128/jb.180.19.5117-5122.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claus H, Frosch M, Vogel U. Identification of a meningococcal hotspot of transformation by shuttle mutagenesis using signature tagged transposons. Mol Gen Genet. 1998;259:363–371. doi: 10.1007/s004380050823. [DOI] [PubMed] [Google Scholar]
- 13.Claus H, Vogel U, Mühlenhoff M, Gerardy-Schahn R, Frosch M. Molecular divergence of the sia locus in different serogroups of Neisseria meningitidis expressing polysialic acid capsules. Mol Gen Genet. 1997;257:28–34. doi: 10.1007/pl00008618. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey J A, Wallace A B, Cannon J G. The physical map of the chromosome of a serogroup A strain of Neisseria meningitidis shows complex rearrangements relative to the chromosomes of the two mapped strains of the closely related species N. gonorrhoeae. J Bacteriol. 1995;177:6390–6400. doi: 10.1128/jb.177.22.6390-6400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Düsterhöft A, Kröger M. Cloning, sequence and characterization of m5C-methyltransferase-encoding gene, hgiDIIM (GTCGAC), from Herpetosiphon giganteus strain Hpa2. Gene. 1991;106:87–92. doi: 10.1016/0378-1119(91)90569-w. [DOI] [PubMed] [Google Scholar]
- 16.Edwards U, Müller A, Hammerschmidt S, Gerardy-Schahn R, Frosch M. Molecular analysis of the biosynthesis pathway of the alpha-2,8 polysialic acid capsule by Neisseria meningitidis serogroup B. Mol Microbiol. 1994;14:141–149. doi: 10.1111/j.1365-2958.1994.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 17.Elkins C, Thomas C E, Seifert H S, Sparling P F. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol. 1991;173:3911–3913. doi: 10.1128/jb.173.12.3911-3913.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frosch M, Meyer T F. Transformation-mediated exchange of virulence determinants by co-cultivation of pathogenic Neisseriae. FEMS Microbiol Lett. 1992;79:345–349. doi: 10.1111/j.1574-6968.1992.tb14062.x. [DOI] [PubMed] [Google Scholar]
- 19.Frosch M, Schultz E, Glenn Calvo E, Meyer T F. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol Microbiol. 1990;4:1215–1218. doi: 10.1111/j.1365-2958.1990.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert M, Watson D C, Cunningham A M, Jennings M P, Young N M, Wakarchuk W W. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J Biol Chem. 1996;271:28271–28276. doi: 10.1074/jbc.271.45.28271. [DOI] [PubMed] [Google Scholar]
- 21.Goodman S D, Scocca J J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunn J S, Stein D C. The Neisseria gonorrhoeae S.NgoVIII restriction/modification system: a type IIs system homologous to the Haemophilus parahaemolyticus HphI restriction/modification system. Nucleic Acids Res. 1997;25:4147–4152. doi: 10.1093/nar/25.20.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammerschmidt S, Müller A, Sillmann H, Mühlenhoff M, Borrow R, Fox A, van Putten J, Zollinger W D, Gerardy-Schahn R, Frosch M. Capsule phase variation in Neisseria meningitidis serogroup B by slipped strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol. 1996;20:1211–1220. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 24.Hilse R, Hammerschmidt S, Bautsch W, Frosch M. Site-specific insertion of IS1301 and distribution in Neisseria meningitidis strains. J Bacteriol. 1996;178:2527–2532. doi: 10.1128/jb.178.9.2527-2532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobbs M M, Seiler A, Achtman M, Cannon J G. Microevolution within a clonal population of pathogenic bacteria: recombination, gene duplication and horizontal genetic exchange in the opa gene family of Neisseria meningitidis. Mol Microbiol. 1994;12:171–180. doi: 10.1111/j.1365-2958.1994.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 26.Holt J G. Herpetosiphon. In: Staley J T, Holt J G, editors. Bergey's manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 2136–2138. [Google Scholar]
- 27.Jeltsch A, Kröger M, Pingoud A. Evidence for an evolutionary relationship among type-II restriction endonucleases. Gene. 1995;160:7–16. doi: 10.1016/0378-1119(95)00181-5. [DOI] [PubMed] [Google Scholar]
- 28.Jeltsch A, Pingoud A. Horizontal gene transfer contributes to the wide distribution and evolution of type II restriction-modification systems. J Mol Evol. 1996;42:91–96. doi: 10.1007/BF02198833. [DOI] [PubMed] [Google Scholar]
- 29.Kilian M, Biberstein E L. Haemophilus. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 558–569. [Google Scholar]
- 30.Kulakauskas S, Barsomian J M, Lubys A, Roberts R J, Wilson G G. Organization and sequence of the HpaII restriction-modification system and adjacent genes. Gene. 1994;142:9–15. doi: 10.1016/0378-1119(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Cheng X, Klimasauskas S, Mi S, Posfai J, Roberts R J, Wilson G G. The DNA (cytosine-5) methyltransferases. Nucleic Acids Res. 1994;22:1–10. doi: 10.1093/nar/22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacks S A, Springhorn S S. Transfer of recombinant plasmids containing the gene for DpnII DNA methylase into strains of Streptococcus pneumoniae that produce DpnI or DpnII restriction endonucleases. J Bacteriol. 1984;158:905–909. doi: 10.1128/jb.158.3.905-909.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisitsyn N, Wigler M. Cloning the differences between two complex genomes. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz M G, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiden M C, Bygraves J A, Feil E, Morelli G, Russell J E, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant D A, Feavers I M, Achtman M, Spratt B G. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morelli G, Malorny B, Muller K, Seiler A, Wang J, del Valle J, Achtman M. Clonal descent and microevolution of Neisseria meningitidis during 30 years of epidemic spread. Mol Microbiol. 1997;25:1047–1064. doi: 10.1046/j.1365-2958.1997.5211882.x. [DOI] [PubMed] [Google Scholar]
- 37.Nwankwo D O, Moran L S, Slatko B E, Waite Rees P A, Dorner L F, Benner J S, Wilson G G. Cloning, analysis and expression of the HindIII R-M-encoding genes. Gene. 1994;150:75–80. doi: 10.1016/0378-1119(94)90861-3. [DOI] [PubMed] [Google Scholar]
- 38.Ritchot N, Roy P H. DNA methylation in Neisseria gonorrhoeae and other Neisseriae. Gene. 1990;86:103–106. doi: 10.1016/0378-1119(90)90120-g. [DOI] [PubMed] [Google Scholar]
- 39.Roberts M S, Cohan F M. The effect of DNA sequence divergence on sexual isolation in Bacillus. Genetics. 1993;134:401–408. doi: 10.1093/genetics/134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a.Roberts R J, Macelis D. REBASE—restriction enzymes and methylases. Nucleic Acids Res. 1999;27:312–313. doi: 10.1093/nar/27.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salmaso S, Mastrantonio P, Scuderi G, Congiu M E, Stroffolini T, Pompa M G, Squarcione S. Pattern of bacterial meningitis in Italy, 1994. Eur J Epidemiol. 1997;13:317–321. doi: 10.1023/a:1007303502274. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 43.Seiler A, Reinhardt R, Sarkari J, Caugant D A, Achtman M. Allelic polymorphism and site-specific recombination in the opc locus of Neisseria meningitidis. Mol Microbiol. 1996;19:841–856. doi: 10.1046/j.1365-2958.1996.437970.x. [DOI] [PubMed] [Google Scholar]
- 44.Sparling R, Bhatti A R. NmeI, a restriction endonuclease from Neisseria meningitidis. Microbios. 1984;41:73–79. [PubMed] [Google Scholar]
- 45.Stein D C. Transformation of Neisseria gonorrhoeae: physical requirements of the transforming DNA. Can J Microbiol. 1991;37:345–349. doi: 10.1139/m91-056. [DOI] [PubMed] [Google Scholar]
- 46.Stein D C, Gregoire S, Piekarowicz A. Restriction of plasmid DNA during transformation but not conjugation in Neisseria gonorrhoeae. Infect Immun. 1988;56:112–116. doi: 10.1128/iai.56.1.112-116.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein D C, Gunn J S, Piekarowicz A. Sequence similarities between the genes encoding the S.NgoI and HaeII restriction/modification systems. Biol Chem. 1998;379:575–578. [PubMed] [Google Scholar]
- 48.Stein D C, Gunn J S, Radlinska M, Piekarowicz A. Restriction and modification systems of Neisseria gonorrhoeae. Gene. 1995;157:19–22. doi: 10.1016/0378-1119(94)00649-d. [DOI] [PubMed] [Google Scholar]
- 49.Stern A, Nickel P, Meyer T F, So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984;37:447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- 50.Sugisaki H. Recognition sequence of a restriction endonuclease from Haemophilus gallinarum. Gene. 1978;3:17–28. doi: 10.1016/0378-1119(78)90004-5. [DOI] [PubMed] [Google Scholar]
- 51.Swartley J S, Marfin A A, Edupuganti S, Liu L J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syrogiannopoulos G A, Mitselos C J, Beratis N G. Childhood bacterial meningitis in Southwestern Greece: a population-based study. Clin Infect Dis. 1995;21:1471–1473. doi: 10.1093/clinids/21.6.1471. [DOI] [PubMed] [Google Scholar]
- 53.Tinsley C R, Nassif X. Analysis of the genetic differences between Neisseria meningitidis and Neisseria gonorrhoeae: two closely related bacteria expressing two different pathogenicities. Proc Natl Acad Sci USA. 1996;93:11109–11114. doi: 10.1073/pnas.93.20.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vedros N A. Genus I: Neisseriae. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 290–296. [Google Scholar]
- 55.Vogel U, Claus H, Heinze G, Frosch M. Functional characterization of an isogenic meningococcal α-2,3-sialyltransferase mutant: the role of lipooligosaccharide for serum resistance in serogroup B meningococci. Med Microbiol Immunol. 1997;186:159–166. doi: 10.1007/s004300050059. [DOI] [PubMed] [Google Scholar]
- 56.Vogel U, Morelli G, Zurth K, Claus H, Kriener E, Achtman M, Frosch M. Molecular techniques are necessary to distinguish between Neisseria meningitidis isolated from meningococcal disease and healthy contacts. J Clin Microbiol. 1998;36:2465–2470. doi: 10.1128/jcm.36.9.2465-2470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J F, Caugant D A, Morelli G, Koumare B, Achtman M. Antigenic and epidemiologic properties of the ET-37 complex of Neisseria meningitidis. J Infect Dis. 1993;167:1320–1329. doi: 10.1093/infdis/167.6.1320. [DOI] [PubMed] [Google Scholar]
- 58.Wilson G G, Murray N E. Restriction and modification systems. Annu Rev Genet. 1991;25:585–627. doi: 10.1146/annurev.ge.25.120191.003101. [DOI] [PubMed] [Google Scholar]
- 59.Zawadzki P, Roberts M S, Cohan F M. The log-linear relationship between sexual isolation and sequence divergence in Bacillus transformation is robust. Genetics. 1995;140:917–932. doi: 10.1093/genetics/140.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou J, Bowler L D, Spratt B G. Interspecies recombination, and phylogenetic distortions, within the glutamine synthetase and shikimate dehydrogenase genes of Neisseria meningitidis and commensal Neisseria species. Mol Microbiol. 1997;23:799–812. doi: 10.1046/j.1365-2958.1997.2681633.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhou J, Spratt B G. Sequence diversity within the argF, fbp and recA genes of natural isolates of Neisseria meningitidis: interspecies recombination within the argF gene. Mol Microbiol. 1992;6:2135–2146. doi: 10.1111/j.1365-2958.1992.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhu P X, Morelli G, Achtman M. The opcA and ΨopcB regions in Neisseria: genes, pseudogenes, deletions, insertion elements and DNA islands. Mol Microbiol. 1999;33:635–650. doi: 10.1046/j.1365-2958.1999.01514.x. [DOI] [PubMed] [Google Scholar]