Abstract
COVID-19 is a critical pandemic that affected communities around the world, and there is currently no specific drug treatment for it. The virus enters the human cells via spikes and induces cytokine production and finally arrests the cell cycle. Ivermectin shows therapeutic potential for treating COVID-19 infection based on in vitro studies. Docking studies have shown a strong affinity between Ivermectin and some virulence factors of COVID-19. Notably, clinical evidence has demonstrated that Ivermectin with usual doses is effective by both the prophylactic and therapeutic approaches in all phases of the disease. Ivermectin inhibits both the adhesion and replication of the virus. Local therapy of the lung with Ivermectin or combination therapy may get better results and decrease the dose of the drug.
Keywords: Ivermectin, COVID-19, pandemics, SARS-CoV-2, hypoxia-inducible factor-alpha, importin, inflammation, lung
Impact Statement
Ivermectin is naturally derived from the fermentation of Streptomyces avermitilis. Ivermectin is known as a drug with a wide range of pharmacological properties, including antimicrobial, anticancer, and antiviral effects. Ivermectin has a record of safety in human use, with the total distributed doses in one-third of the world population, in the past 30 years. Of note, there is convincing evidence showing the therapeutic potential of Ivermectin for treating COVID-19. Several clinical trials have demonstrated ameliorating effect of Ivermectin on the symptoms of the disease, and a growing number of ongoing clinical trials are evaluating its therapeutic efficacy and safety in patients with various stages of the disease. Moreover, mechanistic studies have indicated that Ivermectin can selectively inhibit molecular targets involved in the replication and infection of the SARS-CoV-2 virus.
COVID-19 and pathogenesis
The lack of specific treatment for the COVID-19 pandemic necessitates the development of effective drugs.1,2 The study on its pathogenesis helps drug or vaccine development against this disease. 3 The virus enters human cells via binding of the spike protein to the angiotensin-converting enzyme-2 receptor (ACE2) in many tissues, especially epithelial cells of human alveoli. 4 After binding, essential changes happen in the spike protein by FURIN, cathepsin, or transmembrane serine protease 2, which are essential events for connecting to the membrane and promoting infection.5–8 FURIN cleavage site probably has an important role in SARS-CoV-2 virulence, because the mutant lacking the FURIN cleavage site shows a reduced replication in Calu3 cells and an attenuated disease burden.9,10 Spike protein has two distinct structures, before and after fusion, with specific features, showing a protective role against immune response. 11 Structural studies on the SARS-CoV-2 spike proteins indicate similarity with the FURIN segment of the sodium channel in human epithelial cells, 12 which makes a competition between them. Molecular studies on SARS-CoV-2 and the host indicate the effect of phosphorylation of virus proteins on the activity of kinases and host cell growth factor receptors. The virus finally induces cytokine production and silences CDK1/2/5 (cyclin-dependent kinase), resulting in cell cycle arrest. 13
COVID-19 infection in the lungs has three main phases: the first or early infection phase is viral replication with somewhat mild symptoms; the second or pulmonary phase is determined by adaptive immunity stimulation and exacerbation of respiratory symptoms; and, in some cases, the third and last phase is a hyperinflammatory state or phase. According to the phase of infection, clinical symptoms range from mild such as fever, fatigue, cough, or myalgia, and sore throat or headache to acute respiratory distress syndromes such as hypoxemia and shortness of breath to shock and failure of organs. 14
Ivermectin
Avermectins are naturally derived from the fermentation of Streptomyces avermitilis. Avermectin-derived drugs include Ivermectin, Selamectin, Duramectin, Eprinomectin, and Abamectin. The discovery of Ivermectin led to the Nobel Prize in Physiology or Medicine in 2015.15–17 Ivermectin is known as a drug with a wide range of pharmacological properties, including antimicrobial, anticancer, and antiviral effects.18–22 Ivermectin is mostly used for onchocerciasis treatment and can be effective against Enterobius vermicularis, Ascaris lumbricoides, Trichuris trichiura, and Strongyloides stercoralis.23–26 Besides, recent studies have shown the antiviral effects of Ivermectin on SARS-CoV-2, HIV-1 (human immunodeficiency virus), Avian Influenza type A, Newcastle disease, yellow fever, and West Nile virus. 27 Ivermectin has a record of safety in human use, with total distributed doses in one-third of the world population, in the past 30 years. 28 In a study, treatment with higher FDA-approved doses of Ivermectin (200 μg/kg) was well-tolerated even with 2000 μg/kg, which is 10 times more than the FDA-approved dose, and only moderate central nervous system toxicity was seen.29–31 Ivermectin should not be used during pregnancy, because safety in pregnancy has not been established. 32
Ivermectin shows therapeutic potential for treating COVID-19 infection
Recent research has shown the strong antiviral properties of Ivermectin by inhibiting the replication of some RNA viruses in vitro.33,34 Ivermectin is selectively concentrated in the pulmonary tissue, about 3 times more than the plasma concentration, and remains in the pulmonary tissue for a long time. 35 There is convincing evidence showing the therapeutic potential of Ivermectin for treating COVID-19. Several clinical trials have demonstrated the ameliorating effects of Ivermectin on the symptoms of the disease, and a growing number of ongoing clinical trials are evaluating its therapeutic efficacy and safety in patients with various stages of the disease. Moreover, mechanistic studies have indicated that Ivermectin can selectively inhibit molecular targets involved in the replication and infection of this virus. Here, we aimed to review published studies, evaluating the effect of Ivermectin on COVID-19 virulence, together with the underlying molecular mechanisms.
In vitro studies
Ivermectin has shown an inhibitory effect on the growth of some RNA viruses, and DNA viruses such as Pseudorabies Virus, SARS-CoV-2, HIV-1, Avian Influenza type A, Newcastle disease, yellow fever, and West Nile virus,27,36–41 An in vitro study showed that the addition of a single dose of Ivermectin (5 μM) to Vero-hSLAM cells 2 h post-infection with Australian SARS-CoV-2 could reduce viral RNA about 5000-fold at 48 h. The IC50 value of the drug at 48 h postinfection, was found 2.2–2.8 μM and no cytotoxicity was observed. 42 This finding warrants further study for the possible benefits of Ivermectin in humans, although it has not been tested in any pulmonary cell lines, which are determinants for SARS-CoV-2 in humans. 28
Clinical evidence
Ivermectin has been reported to be effective by both prophylactic and therapeutic approaches in all phases of the disease from mild to severe.35,43 Primary evidence recommends that treatment with Ivermectin in patients with COVID-19 can reduce mortality, especially in cases that need oxygen or mechanical ventilation. 44
The results from clinical trials that used Ivermectin in a dose ranging from 200 to 1200 μg/kg for three to seven days showed a viral load reduction and promising observations of the disease symptoms. 32 The single standard dose of Ivermectin (9 mg) has shown cases of rapid clinical resolution in severe hospitalized patients of COVID-19.45,46 Other doses, 12 mg/day for —one to two days in patients with body weight < 75 kg, as well as 600 or 300 μg/kg/day for five days, are now under phases II and III of clinical trials for COVID-19. 47 However, these dose plans are more than the approved dose of Ivermectin that is, a single dose (200 μg/kg) for the treatment of strongyloidiasis, and thus ignores consistent exposure. 48 A pilot clinical study conducted on hospitalized adult patients with mild to moderate SARS-CoV-2 infection revealed that add-on therapy of Ivermectin to hydroxychloroquine/azithromycin exerted better effectiveness, shorter hospital stay, and relatively safe compared with controls. Notably, the average time to stay in the hospital was significantly lower in patients received a single oral dose of Ivermectin (200 μg/kg) as add on therapy compared with the controls (7.62 ± 2.75 versus 13.22 ± 5.90 days). Notably, two patients died in the control group, none in the Ivermectin group. 49 The other clinical trial indicated that one or more doses of Ivermectin (200 μg/kg) in addition to usual clinical care could significantly reduce the mortality rate in patients with SARS-CoV-2 infection when compared with the controls (15% versus 25.2%). 50 Moreover, the use of Ivermectin 150 µg/kg in 52 patients after mechanical ventilation showed a potential decrease in hospitalization length and survival compared with 1918 patients who were treated conventionally. 51 In another clinical study, patients (older than 40 years) at emergency rooms were received different medications, including Ivermectin, azithromycin, and oseltamivir; unfortunately, such treatments could not considerably reduce hospitalization risk. 52 Notably, the results of a double-blind, randomized clinical trial in Colombia on 476 adult patients with mild symptoms of COVID-19 showed that treatment with 300 μg/kg per day of Ivermectin for five days did not significantly improve duration of symptoms. 53 However, a meta-analysis of 18 clinical trials evaluating the effect of Ivermectin therapy in hospitalized patients with COVID-19 has shown a 68% reduction in mortality correlated with its usage. 54 Of note, many of the physicians announced their personal experience of using Ivermectin. They believed that the results were satisfying for their patients. They proposed that Ivermectin 12 mg twice daily, alone or with other drugs for five to seven days may be safe for mild–moderate or severe cases of COVID-19 infection in all phases of the disease. They also pointed out that the drug is cost-effective and always available. Moreover, it is being used both prophylactically and for treatment in COVID-19 infection.32,33,35 Notably, Ivermectin is now approved in Peru for at least mild cases of COVID-19. Almost 40 clinical trials are ongoing around the world for evaluating the results of COVID-19 treatment with Ivermectin. 32
Pharmacokinetics of Ivermectin
Ivermectin is found to have low bioavailability. In the blood circulation, 93% of Ivermectin binds to plasma proteins, so a low amount of Ivermectin remains free to be uptaken by the target cells. 55 The plasma concentration of free Ivermectin would be highly lesser than the concentration required to decrease SARS-CoV-2 replication in vitro. 56 After a single oral dose of 150–200 μg/kg of Ivermectin, the plasma concentrations would reach 30–47 ng/mL. 48 Single doses of Ivermectin up to 120 mg are known to be safe and are 10-fold greater than the approved dose by the United States Food and Drug Administration (US FDA), but the Cmax value would be ∼ 250 ng/mL, which is lower than effective in vitro concentrations (IC50: 2.5 μM) against SARS-CoV-2.28,45,46 Notably, after 200 μg/kg injection of Ivermectin in calves, the concentration reached 100 ng/g (about 0.1 μM) in lung tissue, which is not sufficient to get the antiviral effect. 56
Mechanisms behind ameliorating effect of Ivermectin on COVID-19 infection
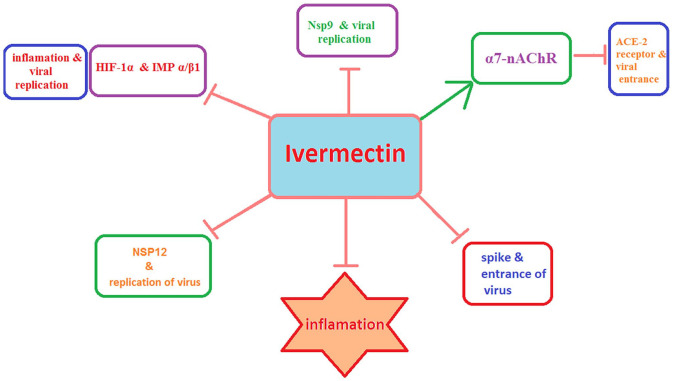
Mechanistic studies have shown that Ivermectin can exert therapeutic effects on both the early stages, including SARS-CoV-2 cell entry and replication, and the late cytokine storm-associated phase of COVID-19. Moreover, Ivermectin has the potential to inhibit viral replication and inflammatory responses through blocking the host cell’s molecular targets (Figure 1). Mechanisms underlying just-mentioned inhibitory actions of Ivermectin on SARS-CoV-2 infection will be discussed in the following sections.
Figure 1.
The main molecular targets of Ivermectin. Ivermectin blocks the SARS-CoV-2 proteins involved in the virus replication including RNA-dependent RNA polymerase enzyme NSP12 and Nsp9. It can also block the virus spike protein and whereby inhibits the cell entry of the virus. In addition, Ivermectin enhances the activity of the host Α7-nAChR, thereby reducing the ACE2-mediated virus entrance. Moreover, Ivermectin can inhibit the cytoplasmic-nuclear shuttling of the host HIF-1α and viral proteins by disrupting IMPα/β1 complex, thereby reducing viral replication and inflammation. (A color version of this figure is available in the online journal.)
Ivermectin directly inhibits the early stages of COVID-19 infection
Molecular ducking studies along with experimental investigations have indicated that Ivermectin can interact with viral proteins that play important roles in the SARS-CoV-2 infection, including those involved in viral replication and/or in the entrance of the virus into the host cells. Ivermectin has been predicted to inhibit the viral entry via hindering the interaction between spike protein and host ACE2, thus directing the virus toward a fruitless infection due to its inability to infiltrate the host cells. 57 While the exact underlying mechanism has yet to be known, docking studies predicted a high binding affinity of Ivermectin for spike protein via hydrogen interaction with Asn487 in the receptor-binding domain (RBD) 58 and with several other residues (LEU492, GLN493, GLY496, TRY505). 59 Moreover, there is an interaction between the alkyl group from Ivermectin and aromatic rings of spike protein residues (TYR449, TYR489, PHE456, LEU455, PHE490). Other docking complex has also predicted five nonpolar interaction residues (Tyr439, Tyr481, Tyr491, Phe483, Leu441) and five polar interaction residues (Arg389, Lys403, Gln479, Gly482, Gln484) had took place between Ivermectin and RBD-spike protein. 58 Among abovementioned residues, the LEU455 and GLN493 possess a high binding affinity with ACE2. In addition, Ivermectin was also found to interact with leucine 91 in the spike protein and histidine 378 of the SARS CoV-2-ACE2 receptor complex. 60 Consequently, Ivermectin may interfere with the attachment of the viral spikes to the human ACE2 receptor, subsequently blocking viral entry, and effectively decreasing the viral infection. 61
After infection, the SARS-CoV-2 binds to the ACE2 receptor by the virus spike protein, subsequently recruiting the host ribosome and reprogramming it to translate the viral RNA to the functional polypeptides. 62 These polypeptides must be auto-cleaved by 3-chymotrypsin like protease (3CLpro) and papain-like proteases (PLpro) to produce NSP12 needed for the viral replication in infected host cells, such as the RNA-dependent RNA polymerase (RdRp) enzyme NSP12. 62 RdRp, called RNA replicase, is a key enzyme in the virulence of RNA viruses since it mainly acts to catalyze the replication of RNA from a primary RNA template in the virus, thereby blocking viral replication. Nsp12, a conserved protein in coronavirus, is an RdRp and responsible for coronavirus transcription or replication. 63 Importantly, RdRp was already predicted as a significant drug target for inhibiting the replication of MERS-CoV and SARS-CoV. 64 More recently, further studies indicated that Ivermectin has a high binding affinity to the Nsp12-RdRp in SARS-CoV-2, interfering with the virus replication. 65
In addition, Ivermectin was also predicted to block the active sites of the virus 3CLpro, which disrupts the viral replication, same as antiviral drugs such as boceprevir, ombitasvir, paritaprevir, tipranavir that have inhibitory effect toward 3CLpro enzyme. 66 An in silico study indicated that Ivermectin (50 μM) inhibited activity of 3CLpro in SARS-CoV-2 by more than 85%. 66 Docking studies also revealed that Ivermectin via its carbonyl group forms stable hydrogen bonds with the active site residues (Cys145 and His41), 66 polar and nonpolar residues of subunit 1, and subunit 2 residues (Thr304 and glu166) of 3CLpro, destabilizing the complex, 58 resulting in the loss of function and consequently decreasing its protease activity.67,68 Recently, blocking the 3CLpro complex has been shown a success in the treatment of SARS-CoV and HIV-1, suggesting 3CLpro a viable target for Ivermectin. 69 Of note, 3CLpro structure is highly conserved among coronaviruses, particularly between SARS-CoV-2 and SARS-CoV. Notably, mutation sequences of 3CLpro protein have shown to be fatal for viruses; thus, the risk of drug resistance from virus evolution is markedly decreased. 70
In addition, the nonstructural protein 9 (Nsp9) is an oligosaccharide/oligonucleotide binding protein that plays an indispensable role in the viral replication. Besides the capacity of Nsp9 to bind viral genomic RNA and mediate viral replication, it can also affect host ribosomal assembly and methylation of mitochondrial rRNA, thereby preventing mitochondrial protein synthesis and thus oxidative phosphorylation, leading to the ATP lowering and the energy loss. Such an effect of Nsp9 has been found to be associated with the symptoms of SARS-CoV-2, including decreased blood pressure following coma, promotion of platelet aggregation, and elevated blood coagulability. 63 These findings together with molecular docking studies showing a strong affinity of Ivermectin to Nsp9 protein suggest this protein as a potential target for the therapeutic armamentarium of the COVID-19 infection. 71
Ivermectin inhibits the inflammation-associated phase of COVID-19
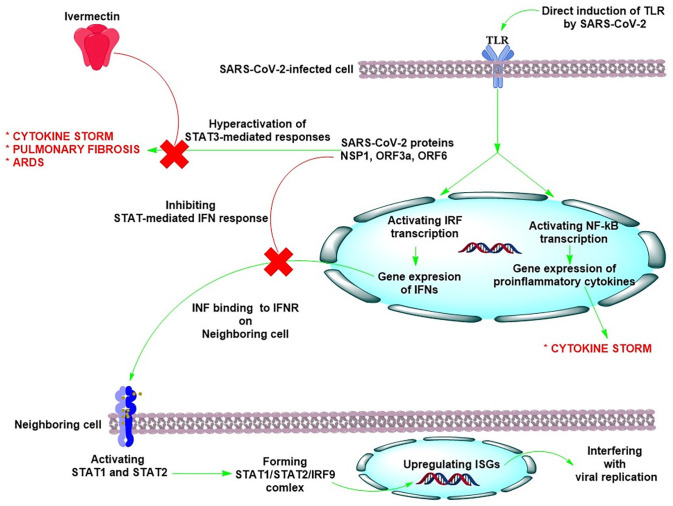
There is growing evidence indicating the anti-inflammatory effects of Ivermectin against the SARS-CoV-2 infection. Toll-like receptors (TLRs) present intracellularly on the host immune cells bind with the virus and detect the viral attack. TLR4 can be activated by SARS-CoV-2 and also by bacterial lipopolysaccharide (LPS) (detected during ICU settings), leading to activation of interferon (INF) regulatory factors (IRFs), nuclear factor kappa B (NF-κB) pathway, and mitogen-activated protein (MAP) kinases causing elevated gene expression of the IFNs as well as pro-inflammatory cytokines and chemokines responsible for cytokine storm.72,73 TLR-mediated activation of IRF transcription is a natural antiviral response of a cell. The produced INFs bind to the IFN-I and IFN-III receptors present on neighboring cells, further activating the downstream STAT signaling pathways, defending against the viral attack. In a normal condition and upon recognition of foreign viruses by the host cell TLRs, STAT1, and STAT2 proteins are predominantly activated and form a transcription factor complex (STAT1/STAT2/IRF9) to translocate into the nucleus and upregulate IFN-stimulated genes (ISGs) to interfere with the viral replication.73,74 Of note, Ivermectin is able to induce the expression of many ISGs, including OASL, IRF9, ISG20, IFIT1, IFIT2, and IF144. 75
For a virus to cause an infection, such antiviral response should be blocked by inhibiting the IFN production. Upon infection, SARS-CoV-2 proteins antagonize the antiviral IFN signaling, 76 consequently, the cells neighboring the infected cell lose out to receive antiviral IFN signals, permitting SARS-CoV-2 virus replication and spread without any barrier (Figure 2). This is one of the main reasons that, at this stage, COVID-19 infection is “hard to detect” clinically. 77 Indeed, the activity of STAT1 and STAT2-mediated IFN response in the host cell is inhibited by SARS-CoV-2 proteins such as the nonstructural protein 1 (NSP1), ORF3a, and ORF6, causing the hyperactivation of STAT3-mediated responses that result in the cytokine storm and a cascade of deleterious events.76,78,79 STAT3 leads to a significant production of inflammatory interleukin (IL)-6 cytokine thorough the elevated macrophage activity in COVID-19 patients. 76 STAT3 is also able to activate integrin αvβ6 and thrombospondin at the lung extracellular matrix, contributing to TGF-β activation and pulmonary fibrosis. 80 The TGF-β activation upregulates the plasminogen activator inhibitor-1 (PAI-1), promoting the continuous expression of IL-6. 80 Eventually, STAT3 and PAI-1 enhance the generation of hyaluronan (HA), causing severe acute respiratory distress syndrome (ARDS) in COVID-19 patients, resulted from diffuse alveolar damage (DAD). 81 Notably, Ivermectin was found to suppress the STAT3 activity, leading to a significant reduction in IL-6 production. In addition, Ivermectin can also induce the ubiquitination-mediated degradation of p21 activated kinase 1 (PAK1), an important mediator that binds to STAT3 for IL-6 gene transcription, interrupting IL-6 expression.76,82 The decrease of STAT3 levels by Ivermectin could also reduce the level of HA, inhibiting ADRS in COVID-19 patients. 81 Of note, an in vivo study showed that Ivermectin can prevent the clinical deterioration through limiting the inflammation of the upper and lower respiratory tracts in SARS-CoV-2-infected hamsters. Transcriptomic analyses of infected lungs revealed that Ivermectin significantly reduced type I and type III IFN responses and modulated other important inflammatory pathways, with a marked reduction of the IL-6/IL-10 ratio and promoting M2 polarization of myeloid cells recruited to the lung. 83
Figure 2.
SARS-CoV-2 activates Toll-like receptors (TLR) on immune cells. The TLR activation results in induction of interferon (INF) regulatory factors (IRFs) and nuclear factor kappa B (NF-κB) transcription which upregulate the expression of IFNs and inflammatory cytokines. The produced INFs bind to the IFN-I and IFN-III receptors present on neighboring cells, further activating the downstream STAT1 and STAT2 proteins that form a transcription factor complex (STAT1/STAT2/IRF9) to translocate into the nucleus and upregulate IFN-stimulated genes (ISGs) to interfere with the viral replication. The activity of STAT1 and STAT2-mediated IFN response is inhibited by SARS-CoV-2 proteins such as the nonstructural protein 1 (NSP1), ORF3a, and ORF6, causing the hyperactivation of STAT3-mediated responses that result in the cytokine storm, pulmonary fibrosis, and acute respiratory distress syndrome (ARDS). Ivermectin can block STAT3-mediated responses. (A color version of this figure is available in the online journal.)
Besides, in ICU settings with increased possibility of bacterial infections, LPS-mediated inflammation can also worsen SARS-CoV-2-mediated inflammation. Notably, oral Ivermectin was found to dose dependently decrease inflammation and improve survival in mice challenged with a lethal dose of LPS. 84 Such an in vivo effect was confirmed by the in vitro studies that indicated Ivermectin could inhibit production of inflammatory cytokines by LPS-challenged macrophages.84–86 In vivo and in vitro studies showed the ability of Ivermectin to prevent LPS-induced production of inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), IL-6, and IL-1 via suppressing NF-κB and MAP kinase pathways.84,85,87 These findings not only support anti-inflammatory effect of Ivermectin in the late stages of SARS-CoV-2 infection, but also indicate the protective effect of this drug against ICU-caused bacterial infection in COVID-19 patients. In mechanism, there is evidence that suggests glycine-gated strychnine-inhibitable chloride channels as a possible target mediating the anti-inflammatory effect of Ivermectin. 54 These glycine receptors are expressed by several kinds of immune cells such as neutrophils and alveolar macrophages as well as vascular endothelium. Activation of glycine receptors on immune and endothelial cells can inhibit inflammatory responses, likely through hyperpolarization of plasma and inhibition of endosomal nicotinamide adenine dinucleotide phosphate oxidase activity.88,89 Of note, there are findings that show Ivermectin as an agonist for glycine receptors can highly increase the activation of such receptors90–93 and whereby prevents LPS-mediated inflammation in macrophages. 86
Ivermectin inhibits molecular targets in lung cells involved in COVID-19 infection
The inhibitory effect of Ivermectin on the host importin (IMP) α/β1 complex has been suggested to be one of the possible mechanisms underlying the antiviral effect of Ivermectin. 41 There are reports that show Ivermectin has the high binding affinity to IMPα and can inhibit activity of IMP α/β1.58,65 IMP α/β1 transports the host proteins in-and-out of the nucleus. The import of a protein into the nucleus requires nuclear localization signal (NLS) to be recognized by the IMPα from the importin heterodimer complex (IMPα/β1), IMPβ1 alone, or the homologues thereof. From there, the cargo protein interacts with the IMPβ1 and is translocated via the nuclear pore complex (NPC) embedded in the nuclear envelope. 94 This nuclear transport complex has been documented to be hijacked by several viruses, such as HIV-1, Dengue Virus, Influenza, as well as SARS-CoV and SARS-CoV-2 to gain access into the nucleus and to facilitate infection. 95
Ivermectin has been found to inhibit the cytoplasmic-nuclear shuttling of viral proteins by disrupting IMPα/β1 complex, thereby reducing viral replication.33,42,44 It has been already found that Ivermectin through this mechanism can affect pseudorabies viruses 40 and a remarkable number of RNA viruses42,96 such as Dengue Virus 1-4, 97 West Nile Virus, 41 Venezuelan Equine Encephalitis Virus, 37 and Influenza. 38 Of note, this mechanism was also recently found to be responsible for reducing the entrance of SARS-CoV-2 proteins into the nucleus and disturbing the reproduction and survival of SARS-CoV-2 virus.32,42 Mechanistically, the Ivermectin binds to the IMPα, blocking the formation of the IMPα/β1 complex, thereby inhibiting the IMPα/β1-dependent nuclear transport activities of viral SARS-CoV-2 proteins. 95
Furthermore, there is also evidence that shows Ivermectin may exert the antiviral activity through inhibiting the formation of the host IMP-α/hypoxia-inducible factor 1-alpha (HIF-1α) complex. 34 HIF-1α is a subunit of a heterodimeric transcription factor that responds to the reduction of oxygen levels in the cellular environment or hypoxia, and adapts the cellular response to oxygen availability.98–100 Through the virus-mediated activation of HIF-1α, the inflammatory response is exacerbated in severe cases of COVID-19 disease. The targets of HIF-1α are all involved in the activation of pro-inflammatory cytokine expression and the subsequent inflammation. 101 HIF-1α activation leads to metabolic reprogramming and virus replication, and inhibition of HIF-1α reduces viral replication significantly. 102 Notably, formation of the IMP-α/HIF-1α complex is required for efficient nuclear translocation of HIF-1α. Ivermectin has been reported to reduce binding activity of the host HIF-1α to the IMP α/β-heterodimer. 103 Upon Ivermectin treatment, nuclear localization and nuclear levels of HIF-1α, as well as HIF-transcriptional activity and HIF-target gene expression were significantly decreased. 103 Based on such evidence, it was suggested that Ivermectin has the potential to inhibit the SARS-CoV-2 replication and the cytokine-mediated pro-inflammatory response through blocking the formation of HIF-1α and IMP α/β1 complex. 34
Another host target involving the antiviral mechanism of Ivermectin against SARS-CoV-2 is α7-nAChR. The activation of nicotinic acetylcholine receptors (nAChR), which respond to the neurotransmitter acetylcholine, the α7 subtype, could increase the viral receptor ACE-2 in airway epithelial cells. Indeed, activation of the α7-nAChR subtype induces overexpression of ACE2 and this is the reason for the vulnerability of patients with chronic obstructive pulmonary disease to severe COVID-19; however, the exact mechanism remains still unclear.104–107 Of note, a low IC50 of Ivermectin (0.156 μM) has been found to inhibit the α7-nAChR,41,108,109 thereby blocking SARS-CoV-2 entrance into the host cells.
Conclusions
Ivermectin could inhibit the proliferation of SARS-CoV-2 in vitro, but the IC50 is 35-fold higher than the concentration of approved clinical doses (9 mg). After single dose of Ivermectin 120 mg, the Cmax would be ∼250 ng/mL, which is lower than the effective concentrations against SARS-CoV-2 in vitro. Although even 2000 μg/kg of Ivermectin (10 times more than FDA-approved dose) was used and rarely CNS toxicity was seen, we should be worried about the probably adverse effects of this drug at higher doses that would be necessary for treating patients with COVID-19.
Although the in vitro studies indicate only the effectiveness of high doses of Ivermectin, several clinical studies have declared cost-effective, easy to availability, and promising results of Ivermectin, even with usual doses. Moreover, the reports show mortality reduction, especially in cases that need oxygen or mechanical ventilation. The effectiveness of Ivermectin, with both preventive and therapeutic approaches, in all phases of the disease has also been demonstrated. To decrease the dose and adverse effects, a combination of Ivermectin with other drugs like hydroxyl chloroquine may be effective. In addition, providing an inhaled formulation to deliver a high dose of Ivermectin locally in the lung, could reduce systemic exposure and may get better results and prevent the adverse effects of the drug.
Besides, Ivermectin shows attractive features that can distinguish its therapeutic potential from other antiviral agents against SARS-Cov-2 infection. Of note, a few FDA-approved drugs are being used on COVID-19 patients at the early stage of infection which may cause less severe infection due to reduced entry of COVID-19 into the cell. Moreover, drugs blocking viral replication would be expected to be of lesser utility in the context of the late cytokine storm-associated phase of COVID-19. Interestingly, Ivermectin is useful in both the early stages and the later stages of the disease. In the early stages, Ivermectin can inhibit SARS-CoV-2 cell entry through blocking both the host molecular targets, such as the α7-nAChR and the ACE2 receptors, and viral molecular targets, such as the spike protein. Ivermectin also can suppress the SARS-CoV-2 replication as evidenced by the reduced viral load in the SARS-CoV-2-infected cells treated by Ivermectin. Molecular and ducking studies indicated that Ivermectin can inhibit the SARS-CoV-2 replication through suppressing the nuclear transfer of SARS-CoV-2 proteins by the host IMP α/β and blocking the activity of the virus proteins including Nsp12-RdRp, Nsp9, and 3CLpro. Ivermectin has also potential to ameliorate the late stage cytokine storm in the COVID-19 patients. Ivermectin has been found to exert anti-inflammatory effect through modulating the signaling pathways and mediators which involve in the inflammatory responses induced by SARS-CoV-2, particularly STAT signaling and chloride channel.
Footnotes
Authors’ Contributions: AAM-B contributed to the conception and design of the work. NB and MM performed the database search and preparing the manuscript. NB and SC revised the manuscript and prepared the figures. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Research Council, Hamadan University of Medical Sciences, Hamadan, Iran [400798125].
ORCID iD: Amir Abbas Momtazi-Borojeni  https://orcid.org/0000-0002-4376-1083
https://orcid.org/0000-0002-4376-1083
References
- 1. Taguchi YH, Turki T. A new advanced in silico drug discovery method for novel coronavirus (SARS-CoV-2) with tensor decomposition-based unsupervised feature extraction. PLoS ONE 2020;15:e0238907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care 2020;57:279–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naqvi AAT, Fatima K, Mohammad T, Fatima U, Singh IK, Singh A, Atif SM, Hariprasad G, Hasan GM, Hassan MI. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta Mol Basis Dis 2020;866:165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Momtazi-Borojeni AA, Banach M, Reiner Ž, Pirro M, Bianconi V, Al-Rasadi K, Sahebkar A. Interaction between coronavirus S-protein and human ACE2: hints for exploring efficient therapeutic targets to treat COVID-19. Angiology 2021;72:122–30 [DOI] [PubMed] [Google Scholar]
- 5. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xia S, Lan Q, Su S, Wang X, Xu W, Liu Z, Zhu Y, Wang Q, Lu L, Jiang S. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduct Target Ther 2020;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson BA, Xie X, Kalveram B, Lokugamage KG, Muruato A, Zou J, Zhang X, Juelich T, Smith JK, Zhang L. Furin cleavage site is key to SARS-CoV-2 pathogenesis. bioRxiv 2020, https://www.biorxiv.org/content/10.1101/2020.08.26.268854v1 [DOI] [PMC free article] [PubMed]
- 11. Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM, Rawson S, Rits-Volloch S, Chen B. Distinct conformational states of SARS-CoV-2 spike protein. bioRxiv 2020, https://www.biorxiv.org/content/10.1101/2020.05.16.099317v1 [DOI] [PMC free article] [PubMed]
- 12. Anand P, Puranik A, Aravamudan M, Venkatakrishnan A, Soundararajan V. SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. eLife 2020;9:e58603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Marrero MC, Polacco BJ, Melnyk JE, Ulferts S, Kaake RM. The global phosphorylation landscape of SARS-CoV-2 infection. Cell 2020;182:685–712.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romagnoli S, Peris A, De Gaudio AR, Geppetti P. SARS-CoV-2 and COVID-19: from the bench to the bedside. Physiol Rev 2020;100:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crump A. Ivermectin: enigmatic multifaceted “wonder” drug continues to surprise and exceed expectations. J Antibiot 2017;70:495–505 [DOI] [PubMed] [Google Scholar]
- 16. Ōmura S, Shiomi K. Discovery, chemistry, and chemical biology of microbial products. Pure Appl Chem 2007;79:581–91 [Google Scholar]
- 17. Guo C, Snowden F. Chairman Mao’s malarial legacy. The Yale Historical Review, p. 56, https://historicalreview.yale.edu/sites/default/files/yhr18.pdf
- 18. Juarez M, Schcolnik-Cabrera A, Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am J Cancer Res 2018;8:317–31 [PMC free article] [PubMed] [Google Scholar]
- 19. Intuyod K, Hahnvajanawong C, Pinlaor P, Pinlaor S. Anti-parasitic drug ivermectin exhibits potent anticancer activity against gemcitabine-resistant cholangiocarcinoma in vitro. Anticancer Res 2019;39:4837–43 [DOI] [PubMed] [Google Scholar]
- 20. Razazan A, Nicastro J, Slavcev R, Barati N, Arab A, Mosaffa F, Jaafari MR, Behravan J. Lambda bacteriophage nanoparticles displaying GP2, a HER2/neu derived peptide, induce prophylactic and therapeutic activities against TUBO tumor model in mice. Sci Rep 2019;9:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barati N, Momtazi-Borojeni AA, Majeed M, Sahebkar A. Potential therapeutic effects of curcumin in gastric cancer. J Cell Physiol 2019;234: 2317–28 [DOI] [PubMed] [Google Scholar]
- 22. Naghibi L, Yazdani M, Momtazi-Borojeni AA, Razazan A, Shariat S, Mansourian M, Arab A, Barati N, Arabsalmani M, Abbasi A, Saberi Z, Badiee A, Jalali SA, Jaafari MR. Preparation of nanoliposomes containing HER2/neu (P5+ 435) peptide and evaluation of their immune responses and anti-tumoral effects as a prophylactic vaccine against breast cancer. PLoS ONE 2020;15:e0243550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lloyd AE, Honey BL, John BM, Condren M. Treatment options and considerations for intestinal helminthic infections. J Pharm Technol 2014;30:130–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campbell S, Soman-Faulkner K. Antiparasitic drugs. Treasure Island, FL: StatPearls Publishing, 2022 [PubMed] [Google Scholar]
- 25. Reza HAM, Rreza G, Nastaran B, Mousa M. Renal hydatid cyst; a rare infectious disease. Oxf Med Case Reports 2019;2019:omz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motevalli Haghi SM, Najm M, Fakhar M, Gholami S, MotevalliHaghi S. Prevalence of Enterobius vermicularis infection among kindergartens of Sari and Babol cities during 2011. J Mazandaran Univ Med Sci 2013;22:240–2 [Google Scholar]
- 27. Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot 2020;73:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaccour C, Hammann F, Ramón-García S, Rabinovich NR. Ivermectin and COVID-19: keeping rigor in times of urgency. Am J Trop Med Hyg 2020;102:1156–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, Hsieh JY, Lasseter KC. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol 2002;42:1122–33 [DOI] [PubMed] [Google Scholar]
- 30. Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci 2020;261:118369. [DOI] [PubMed] [Google Scholar]
- 31. Smit MR, Ochomo EO, Aljayyoussi G, Kwambai TK, Abong’o BO, Chen T, Bousema T, Slater HC, Waterhouse D, Bayoh NM, Gimnig JE, Samuels AM, Desai MR, Phillips-Howard PA, Kariuki SK, Wang D, Ward SA, Ter Kuile FO. Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2018;18:615–26 [DOI] [PubMed] [Google Scholar]
- 32. Vora A, Arora VK, Behera D, Tripathy SK. White paper on Ivermectin as a potential therapy for COVID-19. Indian J Tuberc 2020;67:448–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharun K, Dhama K, Patel SK, Pathak M, Tiwari R, Singh BR, Sah R, Bonilla- Aldana DK, Rodriguez-Morales AJ, Leblebicioglu H. Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19. Ann Clin Microbiol Antimicrob 2020;19:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gonçalves K, Vasconcelos A, Barbirato D, Vasconcelos C, Vasconcelos B. Therapeutic potential of ivermectin for COVID-19. Authorea Preprints 2020, https://www.authorea.com/doi/full/10.22541/au.159050476.60928563
- 35. Banerjee K, Nandy M, Dalai CK, Ahmed SN. The battle against COVID 19 pandemic: what we need to know before we “test fire” Ivermectin. Drug Res 2020;70:337–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 2012;443:851–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lundberg L, Pinkham C, Baer A, Amaya M, Narayanan A, Wagstaff KM, Jans DA, Kehn-Hall K. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res 2013;100:662–72 [DOI] [PubMed] [Google Scholar]
- 38. Götz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Höper D, Kong B-W, Jans DA, Beer M, Haller O. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep 2016;6:23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, de Lamballerie X, Neyts J, Hanson AM, Frick DN, Bolognesi M, Milani M. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother 2012;67:1884–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lv C, Liu W, Wang B, Dang R, Qiu L, Ren J, Yan C, Yang Z, Wang X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res 2018;159:55–62. [DOI] [PubMed] [Google Scholar]
- 41. Yang SNY, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, Jans DA. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res 2020;177:104760. [DOI] [PubMed] [Google Scholar]
- 42. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pareek R, Saxena R, Saxena V. “Ivermectin”—a key in the bunch of keys to unlock the other activities including educational institutions in COVID-19 pandemic. Int J Sci Res 2020;9:939–50 [Google Scholar]
- 44. Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turk J Med Sci 2020;50:611–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DiNicolantonio JJ, Barroso J, McCarty M. Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19. Open Heart 2020;7:e001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmith VD, Zhou J, Lohmer LR. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther 2020;108:762–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Formiga FR, Leblanc R, de Souza Rebouças J, Farias LP, de Oliveira RN, Pena L. Ivermectin: an award-winning drug with expected antiviral activity against COVID-19. J Control Release 2021;329:758–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bray M, Rayner C, Noël F, Jans D, Wagstaff K. Ivermectin and COVID-19: a report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses. Antiviral Res 2020;178:104805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorial FI, Mashhadani S, Sayaly HM, Dakhil BD, AlMashhadani MM, Aljabory AM, Abbas HM, Ghanim M, Rasheed JI. Effectiveness of ivermectin as add-on therapy in COVID-19 management (pilot trial). medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.07.07.20145979v1
- 50. Rajter JC, Sherman M, Fatteh N, Vogel F, Sacks J, Rajter J-J. ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19. medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.06.06.20124461v2 [DOI] [PMC free article] [PubMed]
- 51. Patel A, Desai S. Ivermectin in COVID-19 related critical illness. SSRN J 2020, https://www.isglobal.org/documents/10179/6022921/Patel+et+al.+2020+version+1.pdf
- 52. Szente Fonseca SN, de Queiroz Sousa A, Wolkoff AG, Moreira MS, Pinto BC, Valente Takeda CF, Rebouças E, Vasconcellos Abdon AP, Nascimento ALA, Risch HA. Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: comparative analysis. Travel Med Infect Dis 2020;38:101906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, Díazgranados JA, Oñate JM, Chavarriaga H, Herrera S. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 2021;325:1426–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DiNicolantonio JJ, Barroso-Aranda J, McCarty MF. Anti-inflammatory activity of ivermectin in late-stage COVID-19 may reflect activation of systemic glycine receptors. Open Heart 2021;8:e001655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Audus KL, Knaub SR, Guillot FL, Schaeffer JM. The effect of protein binding on ivermectin uptake by bovine brain microvessel endothelial cells. Vet Res Commun 1992;16:365–77 [DOI] [PubMed] [Google Scholar]
- 56. Lifschitz A, Virkel G, Sallovitz J, Sutra J, Galtier P, Alvinerie M, Lanusse C. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet Parasitol 2000;87:327–38 [DOI] [PubMed] [Google Scholar]
- 57. Choudhury A, Das NC, Patra R, Bhattacharya M, Ghosh P, Patra BC, Mukherjee S. Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach. Future Virol 2021;16:277–91 [Google Scholar]
- 58. Bello M. Elucidation of the inhibitory activity of Ivermectin with host nuclear importin α and several SARS-CoV-2 targets. J Biomol Struct Dyn. Epub ahead of print 10 April 2021. DOI: 10.1080/07391102.2021.1911857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saha JK, Raihan M. The binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2. Struct Chem 2021;32:1985–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo 2020;34:3023–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kalhor H, Sadeghi S, Abolhasani H, Kalhor R, Rahimi H. Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches. J Biomol Struct Dyn 2022;40:1299–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Low ZY, Yip AJW, Lal SK. Repositioning Ivermectin for Covid-19 treatment: molecular mechanisms of action against SARS-CoV-2 replication. Biochim Biophys Acta Mol Basis Dis 2022;1868:166294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romano M, Ruggiero A, Squeglia F, Maga G, Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells 2020;9:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, Wang T, Sun Q, Ming Z, Zhang L. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020;368:779–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sen Gupta PS, Biswal S, Panda SK, Ray AK, Rana MK. Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin. J Biomol Struct Dyn 2020;40:2217–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mody V, Ho J, Wills S, Mawri A, Lawson L, Ebert MC, Fortin GM, Rayalam S, Taval S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun Biol 2021;4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003;300:1763–7 [DOI] [PubMed] [Google Scholar]
- 68. Nukoolkarn V, Lee VS, Malaisree M, Aruksakulwong O, Hannongbua S. Molecular dynamic simulations analysis of ritronavir and lopinavir as SARS-CoV 3CLpro inhibitors. J Theor Biol 2008;254:861–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jamalipour Soufi G, Iravani S. Potential inhibitors of SARS-CoV-2: recent advances. J Drug Target 2021;29:349–64 [DOI] [PubMed] [Google Scholar]
- 70. Silvestrini L, Belhaj N, Comez L, Gerelli Y, Lauria A, Libera V, Mariani P, Marzullo P, Ortore MG, Palumbo Piccionello A. The dimer-monomer equilibrium of SARS-CoV-2 main protease is affected by small molecule inhibitors. Sci Rep 2021;11:9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Littler DR, Gully BS, Colson RN, Rossjohn J. Crystal structure of the SARS-CoV-2 non-structural protein 9, Nsp9. iScience 2020;23:101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zaidi AK, Dehgani-Mobaraki P. The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review. J Antibiot 2022;75:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Park A, Iwasaki A. Type I and type III interferons–induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 2020;27:870–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang C, Sun M, Yuan X, Ji L, Jin Y, Cardona CJ, Xing Z. Enterovirus 71 suppresses interferon responses by blocking Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling through inducing karyopherin-α1 degradation. J Biol Chem 2017;292:10262–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seth C, Mas C, Conod A, Mueller J, Siems K, Kuciak M, Borges I, Ruiz i, Altaba A. Long-lasting WNT-TCF response blocking and epigenetic modifying activities of Withanolide F in human cancer cells. PLoS ONE 2016;11:e0168170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gupta KK, Xu Z, Castellino FJ, Ploplis VA. Plasminogen activator inhibitor-1 stimulates macrophage activation through Toll-like Receptor-4. Biochem Biophys Res Commun 2016;477:503–8 [DOI] [PubMed] [Google Scholar]
- 77. Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. An aberrant STAT pathway is central to COVID-19. Cell Death Differ 2020;27:3209–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yang D, Chu H, Hou Y, Chai Y, Shuai H, Lee AC-Y, Zhang X, Wang Y, Hu B, Huang X. Attenuated interferon and proinflammatory response in SARS-CoV-2–infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J Infect Dis 2020;222:734–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Konno Y, Kimura I, Uriu K, Fukushi M, Irie T, Koyanagi Y, Sauter D, Gifford RJ, Nakagawa S, Sato K. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep 2020;32:108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aluwihare P, Munger JS. What the lung has taught us about latent TGF-β activation. Am J Respir Cell Mol Biol 2008;39:499–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol 2015;194:855–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dou Q, Chen H-N, Wang K, Yuan K, Lei Y, Li K, Lan J, Chen Y, Huang Z, Xie N. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res 2016;76:4457–69 [DOI] [PubMed] [Google Scholar]
- 83. de Melo GD, Lazarini F, Larrous F, Feige L, Kornobis E, Levallois S, Marchio A, Kergoat L, Hardy D, Cokelaer T. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol Med 2021;13:e14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang X, Song Y, Ci X, An N, Ju Y, Li H, Wang X, Han C, Cui J, Deng X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res 2008;57:524–9 [DOI] [PubMed] [Google Scholar]
- 85. Zhang X, Song Y, Xiong H, Ci X, Li H, Yu L, Zhang L, Deng X. Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int Immunopharmacol 2009;9:354–9 [DOI] [PubMed] [Google Scholar]
- 86. Viktorov AV. Ivermectin inhibits activation of Kupffer cells induced by lipopolysaccharide toxin. Antibiot Khimioter 2003;48:3–6 [PubMed] [Google Scholar]
- 87. Ci X, Li H, Yu Q, Zhang X, Yu L, Chen N, Song Y, Deng X. Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam Clin Pharmacol 2009;23:449–55 [DOI] [PubMed] [Google Scholar]
- 88. Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J 2000;14:476–84 [DOI] [PubMed] [Google Scholar]
- 89. McCarty MF, Iloki-Assanga S, Lujan LML, DiNicolantonio JJ. Activated glycine receptors may decrease endosomal NADPH oxidase activity by opposing ClC-3-mediated efflux of chloride from endosomes. Med Hypotheses 2019;123:125–9 [DOI] [PubMed] [Google Scholar]
- 90. Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem 2001;276:12556–64 [DOI] [PubMed] [Google Scholar]
- 91. Lynagh T, Webb TI, Dixon CL, Cromer BA, Lynch JW. Molecular determinants of ivermectin sensitivity at the glycine receptor chloride channel. J Biol Chem 2011;286:43913–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang Q, Lynch JW. A comparison of glycine-and ivermectin-mediated conformational changes in the glycine receptor ligand-binding domain. Int J Biochem Cell Biol 2012;44:335–40 [DOI] [PubMed] [Google Scholar]
- 93. Wheeler M, Ikejema K, Enomoto N, Stacklewitz R, Seabra V, Zhong Z, Yin M, Schemmer P, Rose M, Rusyn I. Glycine: a new anti-inflammatory immunonutrient. Cel Mol Life Sci 1999;56:843–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Caly L, Wagstaff KM, Jans DA. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antiviral Res 2012;95:202–6 [DOI] [PubMed] [Google Scholar]
- 95. Yang SN, Atkinson SC, Fraser JE, Wang C, Maher B, Roman N, Forwood JK, Wagstaff KM, Borg NA, Jans DA. Novel flavivirus antiviral that targets the host nuclear transport importin α/β1 heterodimer. Cells 2019;8:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Azam F, Taban IM, Eid EE, Iqbal M, Alam O, Khan S, Mahmood D, Anwar MJ, Khalilullah H, Khan M. An in-silico analysis of ivermectin interaction with potential SARS-CoV-2 targets and host nuclear importin α. J Biomol Struct Dyn 2022;40:2851–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Azam F, Alabdullah NH, Ehmedat HM, Abulifa AR, Taban I, Upadhyayula S. NSAIDs as potential treatment option for preventing amyloid β toxicity in Alzheimer’s disease: an investigation by docking, molecular dynamics, and DFT studies. J Biomol Struct Dyn 2018;36:2099–117 [DOI] [PubMed] [Google Scholar]
- 98. Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol 2008;141:325–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wilkins SE, Abboud MI, Hancock RL, Schofield CJ. Targeting protein–protein interactions in the HIF system. ChemMedChem 2016;11:773–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gilman NV. Analyses of the 2019 Nobel Prize in physiology or medicine: molecular machinery for cellular oxygen level response. Sci Technol Libr 2020;39:1–27 [Google Scholar]
- 101. Serebrovska ZO, Chong EY, Serebrovska TV, Tumanovska LV, Xi L. Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol Sin 2020;41:1539–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Morris DR, Qu Y, Agrawal A, Garofalo RP, Casola A. HIF-1α modulates core metabolism and virus replication in primary airway epithelial cells infected with respiratory syncytial virus. Viruses 2020;12:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Golan M, Mabjeesh NJ. SEPT9_i1 is required for the association between HIF-1α and importin-α to promote efficient nuclear translocation. Cell Cycle 2013;12:2297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Russo P, Bonassi S, Giacconi R, Malavolta M, Tomino C, Maggi F. COVID-19 and smoking: is nicotine the hidden link? Eur Respir J 2020;55:2001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Leung JM, Yang CX, Sin DD. COVID-19 and nicotine as a mediator of ACE-2. Eur Respir J 2020;55:2001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Olds JL, Kabbani N. Is nicotine exposure linked to cardiopulmonary vulnerability to COVID-19 in the general population? FEBS J 2020;287: 3651–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kashyap VK, Dhasmana A, Massey A, Kotnala S, Zafar N, Jaggi M, Yallapu MM, Chauhan SC. Smoking and COVID-19: adding fuel to the flame. Int J Mol Sci 2020;21:6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Krause RM, Buisson B, Bertrand S, Corringer P-J, Galzi J-L, Changeux J-P, Bertrand D. Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 1998;53:283–94 [DOI] [PubMed] [Google Scholar]
- 109. Chaccour C, Abizanda G, Irigoyen-Barrio Á, Casellas A, Aldaz A, Martínez-Galán F, Hammann F, Gil AG. Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats. Sci Rep 2020;10:17073. [DOI] [PMC free article] [PubMed] [Google Scholar]