Abstract
Coronavirus disease 2019 (COVID-19) is a current pandemic disease caused by a novel severe acute respiratory syndrome coronavirus virus respiratory type 2 (SARS-CoV-2). SARS-CoV-2 infection is linked with various neurological manifestations due to cytokine-induced disruption of the blood brain barrier (BBB), neuroinflammation, and peripheral neuronal injury, or due to direct SARS-CoV-2 neurotropism. Of note, many repurposed agents were included in different therapeutic protocols in the management of COVID-19. These agents did not produce an effective therapeutic eradication of SARS-CoV-2, and continuing searching for novel anti-SARS-CoV-2 agents is a type of challenge nowadays. Therefore, this study aimed to review the potential anti-inflammatory and antioxidant effects of citicoline in the management of COVID-19.
Keywords: COVID-19, Neuroinflammation, Citicoline, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19) is a current pandemic disease caused by a novel severe acute respiratory syndrome coronavirus virus respiratory type 2 (SARS-CoV-2) (Al-Kuraishy et al. 2021a). SARS-CoV-2 is a single-strand RNA virus from the betacoronavireadae family and has a close genetic similarity with other coronaviruses like bat coronavirus, SARS-CoV, and Middle East Respiratory Syndrome coronavirus virus (MERS-CoV) (Al-Gareeb et al. 2021). SARS-CoV-2 initially emerged in Wuhan, China, leading to an unrecognized pneumonia named Wuhan pneumonia. Later, this virus was renamed as a novel coronavirus virus 2019 (nCov2019). After a short period, the world health organization (WHO) notified this disease as a pandemic and renamed this virus as SARS-CoV-2 (Al-Kuraishy et al. 2021b). COVID-19 is regarded as a primary respiratory disease leading to respiratory symptoms identical to those of the flu-like illness characterized by fever, headache, dry cough, dyspnea, myalgia, joint pain, and anosmia (Al-Kuraishy et al. 2021c). Further studies and scrutinized researches revealed that COVID-19 may cause extra-pulmonary manifestations including acute kidney injury, thromboembolic disorders, and gastrointestinal and neurological complications (Al-Kuraishy et al. 2020a). In general, COVID-19 is mostly asymptomatic in about 85% of affected patients. However, 15% of the affected patients presented with severe dyspnea and critical respiratory symptoms due to the propagation of acute lung injury (ALI). In addition, 5% of COVID-19 patients need hospitalization and intensive care unit (ICU) admission due to the development of acute respiratory distress syndrome (ARDS) (Al-Kuraishy et al. 2021d). Critical and severe COVID-19 patients may require invasive oxygen supplementation and mechanical ventilation (Al-Kuraishy et al. 2021d).
Management of COVID-19 patients is mainly supportive and symptomatic relief, since specific anti-SARS-CoV-2 was not developed till now despite development of effective vaccines. Of note, many repurposed agents like ivermectin, remdesivir, and favipiravir were included in different therapeutic protocols in the management of COVID-19 (Carlotti et al. 2020). These agents did not produce effective therapeutic eradication of SARS-CoV-2, and continued search for novel anti-SARS-CoV-2 agents is a of type challenge nowadays (Carlotti et al. 2020).
In the Noble Qur’an, there is something that indicates the greatness of humankind’s creation: we add to the first creation, but they are confused by a new creation (The Noble Qur'an, Surah Q-Verse (15). As well, Imam Ali said: “Do not say what you do not know, but do not say everything you know, for God has imposed on all of your limbs duties that will be used as evidence on the Day of Resurrection.” Therefore, we should search for endogenous or similar agents to be used as a therapeutic tool against inflammatory changes in COVID-19.
Citicoline (CTN) is an endogenous chemical compound known as cystidine-5-diphosphocholine (Jasielski et al. 2020). CTN is commonly available in many dietary sources and is regarded in many countries as a dietary supplement or drugs. CTN has a neuroprotective role through its anti-inflammatory and antioxidant effects (Jasielski et al. 2020; Al-kuraishy et al. 2022). Thus, this critical review aimed to elucidate the potential role of CTN in the management of neurological manifestations in COVID-19.
Pharmacology of citicoline
CTN is a [2R, 3S, 4R, 5R-5(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl) methoxy-hydroxyphosphoryl-2-trimethylazaniumyl-ethyl phosphate] (Fig. 1).
Fig. 1.
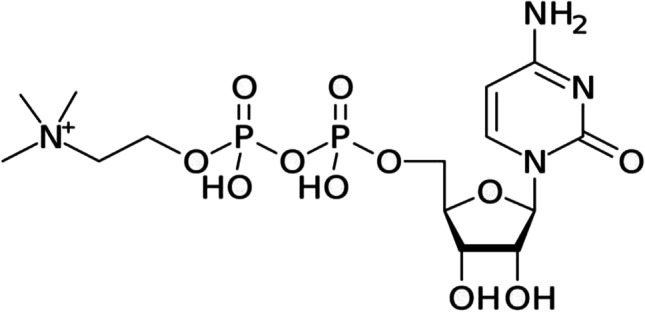
Chemical structure of citicoline
Pharmacokinetic of citicoline
CTN can be used orally and parenterally. In the body, CTN is hydrolyzed to choline and cytidine by dephosphorylation and hydrolysis processes in the intestine (Al-Kuraishy and Al-Gareeb 2020). Choline and cytidine, which cross the blood brain barrier (BBB), are regarded as substrates for neuronal synthesis of phosphatidylcholine (Abbaszadeh et al. 2018). CTN is a water soluble agent with 90% bioavailability after oral administration. CTN reaches its peak plasma level within 1 h following oral administration (Al-Kuraishy and Al-Gareeb 2020). CTN is highly absorbed from intestine, rapidly metabolized by liver enzymes to give inactive metabolites which are eliminated as carbon dioxide, and the remainders are excreted by urine. According to experimental and preclinical studies (Al-Kuraishy and Al-Gareeb 2020; Abbaszadeh et al. 2018), CTN is a safe agent with low toxicity. The effective dose of CTN is 2 g/day. The side effects of CTN are mild and mainly related to gastrointestinal irritation. Though, some studies reported that chronic use of CTN was associated with psychiatric episodes and may have antagonized anti-psychotic drugs. However, a meta-analysis study does not support this claim (Gareri et al. 2015). According to the pharmacokinetic studies, CTN has less interaction with other drugs (Gareri et al. 2015).
Pharmacodynamic of citicoline
CTN preserves the arachidonic acid content of phosphatidylethanolamine and phosphatidylcholine of the neuronal cell membrane. CTN promotes the activity of glutathione reductase and increases synthesis of glutathione with inhibition of phospholipase A (PLA2) activity (Ek et al. 2014). This finding suggests the antioxidant and anti-inflammatory effects of CTN (Ek et al. 2014). Besides, CTN stimulates acetylcholine (Ach) synthesis in the brain by increasing the availability of choline (Abdel-Aziz et al. 2021). As well, CTN maintains the integrity of the inner mitochondrial membrane by inhibiting the catabolism of cardiolipin by inhibition of phospholipase A (PLA2) (Adibhatla and Hatcher 2002). Similarly, CTN stimulates synthesis of cardiolipin, reduces lipid peroxidation, and restores activity of neuronal Na+/K+-ATPase (Piamonte et al. 2020). Furthermore, CTN activates synthesis and release of neurotransmitters like dopamine by stimulating tyrosine hydroxylase (Piamonte et al. 2020; Secades 2019). In addition, CTN improves the release of Ach and noradrenalin, which increases vigilance, learning, and cognitive function (Secades 2019; Al-Kuraishy et al. 2021e).
Moreover, CTN inhibits neuronal excitotoxicity by reducing glutamine concentration in the synaptic cleft by augmenting glutamate uptake through increased expression of glutamate transporters in rat astrocytes (Hurtado et al. 2005; Piotrowska et al. 2022). Therefore, CTN could be effective in the management of ischemic stroke (Piotrowska et al. 2022). However, a meta-analysis revealed that CTN therapy was not associated with beneficial clinical outcomes in patients with ischemic stroke (Shi et al. 2016). Surprisingly, CTN increases the levels of adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicular stimulating hormone (FSH), thyroid stimulating hormone (TSH), and growth hormone (GH) (Abdel-Aziz et al. 2021; Secades 2011). Cavun et al. found that CTN regulates the release of vasopressin by activating presynaptic nicotinic receptors (Çavun et al. 2004).
Neuroprotective effect of citicoline
It has been reported that CTN has neuroprotective activity against different neurodegenerative and traumatic brain disorders through its neuro-restorative effects (Abdolmaleki et al. 2016). As well, CTN has anticonvulsive, sedative, and anxiolytic activities (Abdolmaleki et al. 2016). Of interest, CTN can attenuate neuroinflammation by inhibiting the release of pro-inflammatory cytokines including tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), IL-1β, and monocyte chemoattractant protein 1 (MCP-1) with activation release of anti-inflammatory cytokines IL-10 (Al-Mosawi 2019; Secades 2021). CTN regulates neuronal energy balance by controlling ATP levels and the activity of Na+/K+-ATPase (Piamonte et al. 2020). Similarly, CTN reduced neuronal injury by reversing glutamate transport and associated excitotoxicity (Hurtado et al. 2005; Piotrowska et al. 2022). Furthermore, CTN decreases oxidative stress-induced neuronal cell death and apoptosis by inhibiting lipid peroxidation and activating antioxidant enzyme capacity (Piamonte et al. 2020). Interestingly, CTN improves expression of silent information regulator 1 (SIRT1), which has an anti-apoptotic effect by reducing caspase expression (Krupinski et al. 2002). CTN as well prevents development of endothelial dysfunction by inhibiting disruption of endothelial tight junction in ischemic stroke (Ma et al. 2013). Remarkably, CTN increases neurogenesis, gliogenesis, and synaptogenesis, which attenuates the negative impact of the neurodegenerative process (Martynov and Gusev 2015).
A past review illustrated that clinical trials involving more than 11,000 patients with different neurologic disorders, including acute ischemic stroke (AIS), were significantly ameliorated by CTN treatment compared to the controls (Overgaard 2014). Moreover, a meta-analysis including 1371 patients with AIS from 4 randomized clinical trials revealed that CTN treatment in a dose range of 500–2000 mg/day given within 24 h of AIS led to a significant improvement at 3 months (Saver 2008). CTN improves clinical outcomes in patients with AIS compared to healthy controls [OR = 1.33, 95% CI = 1.10–1.62], (Dávalos et al. 2012). Besides, data from an international CTN trial on AIS, which comprised 2298 AIS patients within 24 h treated by CTN at 2 g/day compared to placebo for 6 weeks, revealed a significant improvement effect of CTN treatment on the primary outcomes (Dávalos et al. 2012).
Furthermore, CTN prevents cerebral vascular impairment-induced cognitive dysfunction (Gareri et al. 2015). Gareri et al. found that CTN therapy at 1 g/day for 1 month in 20 patients with vascular cognitive dysfunction advanced cerebral blood flow and reduced immunogenic reactivity (Gareri et al. 2015). Likewise, a randomized study involving 347 patients with post-stroke cognitive dysfunction treated with CTN illustrated significant neuroprotective effects of CTN against deterioration of cognitive function (Alvarez-Sabín et al. 2013).
In addition, CTN has been shown to be effective in the management of Parkinson disease (PD) by improving the activity of dopaminergic neurons (Que and Jamora 2021). A systematic review showed that CTN was operative in reducing levodopa requirements and associated adverse effects (Que and Jamora 2021).
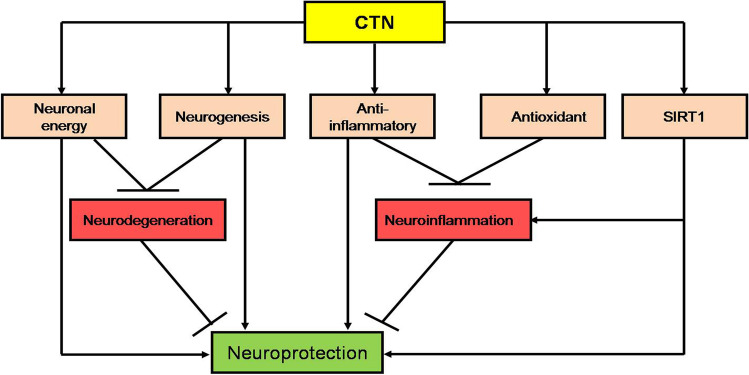
These clinical observations indicated and confirmed the neuroprotective effect of CTN in the management of AIS and degenerative brain diseases through different mechanistic pathways (Fig. 2).
Fig. 2.
Neuroprotective effect of citicoline (CTN): CTN, through its anti-inflammatory and antioxidant effects as well as through induction of neurogenesis and neuronal energy, attenuates neurodegeneration and neuroinflammation, respectively. CTN induces expression of silent information regulator 1 (SIRT1), which inhibits neuroinflammation and produces a direct neuroprotective effect. The final effect of CTN is neuroprotection
COVID-19 and neurological manifestations
Generally, SARS-CoV-2 infection is linked with various neurological manifestations, including dysgeusia and anosmia, due to the neurotropic effect of SARS-CoV-2 (Giacomelli et al. 2020; Al-Kuraishy et al. 2022d). Neurological manifestations have been reported to be found in about 36.4% of COVID-19 patients, counting central and peripheral neurological complications as well as skeletal muscle disorders (Mao et al. 2020; Al-Buhadily et al. 2021). The most common neurological symptoms in COVID-19 are dizziness (16.8%), headache (13.1%), and fatigue (13.0%) (Niazkar et al. 2020). Furthermore, stroke, seizure, ataxia, and confusion were also documented as central neurological complications in COVID-19 patients (Niazkar et al. 2020; Al-Kuraishy et al. 2021f).
In particular, fatigue in COVID-19 patients is developed due to autoantibodies against muscarinic and adrenergic receptors with the development of dysautonomia (Townsend et al. 2021). Correspondingly, neuropsychiatric disorders including depression, psychosis, and anxiety have been reported in COVID-19 patients (Tang et al. 2021). Mazza and colleagues reported that depression and anxiety in COVID-19 survivors were associated with a high inflammatory burden (Mazza et al. 2020). In COVID-19, hyperactive immune responses and neuroinflammation increased the risk of neuropsychiatric complications (Tang et al. 2021). It has been shown in an MRI-based study for taxation of neurological changes in COVID-19 survivors 3 months following discharge, that there were noteworthy structural changes that were consistent with extending neurological symptoms such as cognitive deficits and anosmia (Lu et al. 2020). Of interest, a prospective study that included sixty COVID-19 survivors compared to thirty-nine matched controls found that there were neurological dysfunctions in 55% of COVID-19 survivors as compared with healthy controls (Lu et al. 2020). Thus, the prolonged effect of SARS-CoV-2 infection, even a mild-moderate one, may affect functional and micro-structural brain integrity, resulting in neurological consequences in COVID-19 survivors. Likewise, Paterson et al. demonstrated a high frequency of acute disseminated encephalomyelitis in COVID-19 survivors that was not linked with the initial severity of COVID-19 (Paterson et al. 2020). This finding suggests that SARS-CoV-2 infection, irrespective of its severity, may cause long-term neurological complications, and this may explain neuropsychiatric manifestations in patients with post- COVID-19 survivors.
In addition, delirium was reported in hospitalized patients with severe COVID-19 and may be present in patients with post-COVID-19 (O’Hanlon and Inouye 2020). As well, early presentation of delirium in SARS-CoV-2 infection may predict the development of cognitive dysfunction, mainly in elderly COVID-19 survivors (Rogers et al. 2020). Remarkably, a meta-analysis study publicized that delirium symptoms in COVID-19 patients at the time of admission were connected with poor neurological outcomes (OR = 2.36, 95% CI = 1.80–3.09, P < 0.00001) (Rogers et al. 2020).
Notably, neuropsychiatric symptoms in the acute phase of SARS-CoV-2 infection may lead to fatigue, cognitive impairment, and other neuropsychiatric complications due to cerebral dysfunction (Taquet et al. 2021). Also, a cohort study comprised 236,379 COVID-19 survivors 6 months after acute SARS-CoV-2 infection showed that 56% of COVID-19 survivors developed numerous neuropsychiatric spectrums, mainly with ICU admission (Bulfamante et al. 2020).
Certainly, brainstem injury in acute SARS-CoV-2 infection may lead to cardio-respiratory dysfunction via injury of respiratory and vasomotor centers (Yong 2021). Of note, brainstem dysfunction may continue for long time after acute SARS-CoV-2 infection causing dyspnea and neurological dysfunctions in COVID-19 survivors (Matschke et al. 2020). Advanced expression of ACE2 in the brainstem escalates the susceptibility to SARS-CoV-2 neurotropism and successive inflammatory reaction-induced dysfunction (Matschke et al. 2020). Interestingly, post-mortem studies demonstrated that SARS-CoV-2 proteins and genes were identified in COVID-19 victims (Solomon et al. 2020; Rovere Querini et al. 2020).
Therefore, the fundamental mechanism of neuropsychiatric disorders in COVID-19 might be due to cytokine-induced disruption of the BBB, neuroinflammation, and peripheral neuronal injury, or due to direct SARS-CoV-2 neurotropism (Majolo et al. 2021). Noteworthily, exaggerated inflammatory responses could be the suggested mechanism for the progression of neuropsychiatric and other neurological disorders in COVID-19 (Kumar et al. 2021).
The underlying suggested mechanism for neurological involvement in COVID-19 might be related to the progression of demyelination disorders, as previous coronavirus infections were linked with different neurodegeneration and demyelination (Desforges et al. 2014). In addition, extraordinary expression of ACE2 in some brain regions such as substantia nigra and limbic system may upsurge the interaction between SARS-CoV-2 and neurons with succeeding neurological complications (Chen et al. 2020; Garcia et al. 2021).
These observations suggest that SARS-CoV-2 infections may lead to the occurrence of various neurological manifestations and complications in COVID-19 by complex mechanisms.
Citicoline and COVID-19-induced neurological manifestations
Citicoline and SIRT1
SIRT1 is a mono-ADP ribosyl transferase and NAD-dependent deacylase signaling protein involved in cellular homeostasis and metabolic regulation. SIRT1 has a wide biological effect affecting both longevity and cell survival during acute and chronic oxidative stress-induced injury (Jiao and Gong 2020). In a similar way, SIRT1 regulates inflammatory responses, DNA repair, apoptosis, metabolism, and stress during neuroinflammation (Jiao and Gong 2020). It has been shown that SIRT1 has a protective effect against the development of neuroinflammation in various neurological disorders and could be a therapeutic target in this state (Jiao and Gong 2020). The potential mechanism of SIRT1 against neuroinflammation is related to the inhibition of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α. This SIRT1 effect is mediated by inhibiting expression of disntegrin and metalloproteinase 17 (ADAM17) and tissue metalloproteinase inhibitor 3 (TIMP3) (Fontani 2017). It has been hypothesized that reduction of NAD in aged patients with obesity and diabetes mellitus may increase susceptibility to SARS-CoV-2 infections, since SIRT1 is regarded as a defense mechanism against viral infections (Miller et al. 2020; Al-Kuraishy et al. 2022e). In COVID-19, SIRT1 activity is inhibited, with subsequent loss of anti-inflammatory activity of SIRT1 and the development of an exaggerated inflammatory response due to activation of the ADAM17 inflammatory signaling pathway (Huarachi Olivera and Lazarte 2020; Ferrara and Vitiello 2022). In addition, expression of SIRT1 and AEC2 is increased in the lungs of COVID-19 patients as a compensatory mechanism against SARS-CoV-2 infection-induced hyperinflammation (Pinto et al. 2020; Al-Kuraishy et al. 2022f).
Upregulation of SIRT1 by activators like CTN and resveratrol may attenuate expression of ADAM17 and release of pro-inflammatory cytokines with the development of cytokine storm in COVID-19 (Turana et al. 2021; Giordo et al. 2021). In this state, CTN may modulate expression of ACE2 through activation of SIRT1 and inhibition expression of ADAM17 which increase shedding of ACE2 (Giordo et al. 2021; Al-Kuraishy et al. 2022f).
Of interest, SIRT1 improves neurogenesis and synaptic plasticity as well as enhancement of cognitive functions (Wang et al. 2021a). Experimental study by Wang et al. demonstrated that resveratrol attenuates lead-induced hippocampal injury through activation of neurogenesis by a SIRT-dependent pathway (Wang et al. 2021a). As well, SARS-CoV-2 infection-induced oxidative stress also suppresses SIRT activity (Turana et al. 2021; Al-Kuraishy et al. 2022g). Moreover, the unbalanced p53/SIRT1 axis in SARS-CoV-2 infection may affect lymphocyte homeostasis, causing lymphopenia, and ARDS (Bordoni et al. 2021). Reduction of SIRT1 along with hypercytokinemia in SARS-CoV-2 infection triggers activation of p53, leading to an uncontrolled immunoinflammatory response with the development of neuroinflammation (Bordoni et al. 2021). In this regard, CTN through activation of SIRT1 may attenuate SARS-CoV-2 infection-induced hyperinflammation and neuroinflammation-mediated cognitive dysfunction in COVID-19 patients.
Indeed, the forkhead box O (Foxo), which is a transcription factor involved in the regulation of oxidative stress, apoptosis, inflammatory response, and maturation of lymphocytes, is inhibited by SARS-CoV-2 infection (Cheema et al. 2021). Foxo activators like LOM612 and exportin-1 inhibitors might be effective in reducing SARS-CoV-2 infection-induced oxidative stress and hyperinflammation (Cheema et al. 2021). Sui and colleagues revealed that SIRT1 is regarded as a potent activator of Foxo protein (Sui et al. 2019). Recently, it has been shown that metformin attenuates the progression of diabetic kidney disease through activation of the SIRT1/Foxo axis in rats (Ren et al. 2020). Notably, overexpression of Foxo protein can bind to the SIRT1 promoter to provoke SIRT1 transcription (Chong et al. 2012). Interestingly, SIRT1 increases expression of adenosine monophosphate protein kinase (AMPK) through deacetylation of liver kinase B1 (LKB1). In turn, SIRT1 through stimulation of NAD/NADH increases expression of SIRT1 (Chong et al. 2012). Lin et al. found that activation of AMPK by lycopene can reduce neuroinflammation (Lin et al. 2014). Thus, CTN may play a critical role in reducing dysautonomia in animal model studies through activation of AMPK signaling (Amin et al. 2021).
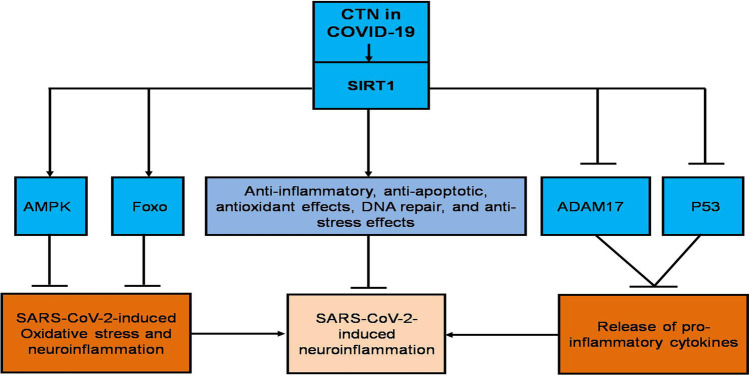
In this notion, CTN through activation of SIRT1/Foxo/AMPK axis (Cacabelos et al. 2019) could be a therapeutic utility in the attenuation of neuroinflammation in COVID-19 (Fig. 3).
Fig. 3.
Citicoline reduces SARS-CoV-2-induced neuroinflammation through a silent information regulator 1 (SIRT1)-dependent pathway: citicoline (CTN) increases expression of forkhead box O (Foxo) and adenosine monophosphate protein kinase (AMPK) with inhibition expression of disintegrin and metalloproteinase 17 (ADAM17) and p53 with subsequent inhibition of SARS-CoV-2-induced oxidative stress, neuroinflammation, and release of pro-inflammatory cytokines leads to inhibition of SARS-CoV-2-induced neuroinflammation
Citicoline, oxidative stress, and hyperinflammation
Notably, oxidative stress is linked with the progression of SARS-CoV-2 infection and COVID-19 severity due to ROS generation, mitochondrial dysfunction, dysregulation of RAS, and reduction of endogenous antioxidant capacity (Cecchini and Cecchini 2020). In turn, oxidative stress triggers release of pro-inflammatory cytokines via activation of inflammatory signaling pathways including nuclear factor kappa B (NF-κB), nod-like receptor pyrin 3 receptor (NLRP3) inflammasome and p38 mitogen activated protein kinase (p38MAPK) (Al-Kuraishy et al. 2022a; Mostafa-Hedeab et al. 2022; Al-Kuraishy et al. 2021g). Pro-inflammatory cytokines and activated inflammatory signaling pathways interacted together in the induction of oxidative stress in SARS-CoV-2 infection (Al-Kuraishy et al. 2022b). Therefore, there is a close relationship between oxidative stress and inflammation in SARS-CoV-2 infection (Al-Kuraishy et al. 2022a; b). A prospective study including 39 patients with mild to moderate COVID-19 compared to 41 patients with severe COVID-19 revealed that higher oxidative stress and inflammatory biomarkers were associated with COVID-19 severity and mortality (Al-Kuraishy et al. 2022b). Remarkably, Mingoti et al. confirmed that higher oxidative stress and inflammatory levels were correlated with a higher risk of neuroinflammation in COVID-19 patients (Mingoti et al. 2022). Indeed, oxidative stress and hyperinflammation induce disruption of the BBB with activation of microglial cells and the development of neuroinflammation (Mingoti et al. 2022).
CTN has been observed to improve human vigilance and working memory by inhibiting oxidative stress levels during neuronal activation (Al-Kuraishy and Al-Gareeb 2020). A prospective study comprised 20 healthy volunteers treated by CTN 500 mg/day for 2 weeks and showed that CTN improved cognitive function with a reduction of oxidative stress biomarker malondialdehyde (MDA) compared to the placebo effect (P < 0.001) (Abdel-Salam et al. 2019). Similarly, CTN attenuates tramadol-induced organ injury by inhibiting the generation of ROS and the development of oxidative stress in rats (Abdel-Salam et al. 2019). CTN significantly reduces expression of MDA with increased expression of antioxidant enzymes and reduced glutathione (GSH) and paraoxonase-1 (PON-1) in rats with experimental cerebral injury (Chen et al. 2021). Systemic oxidative stress with reduction of PON-1 is linked with severity of preeclampsia and primary hypothyroidism (Al-Kuraishy et al. 2018; Al-Naimi et al. 2018).
Therefore, CTN, through its antioxidant effects, may reduce SARS-CoV-2 infection-mediated neuroinflammation and associated cognitive dysfunction in COVID-19 patients. Taken together, the anti-inflammatory and antioxidant effects of CTN could reduce SARS-CoV-2 infection-induced neuroinflammation.
Furthermore, CTN has anti-inflammatory effects by inhibiting the activity of neuronal PLA2, thereby maintaining cardiolipin and sphingomyelin content in the neuron cell membrane and inner mitochondrial membrane (Ek et al. 2014). Increasing activity of secretory PLA2 (sPLA2) is linked with COVID-19 severity (Snider et al. 2021). A prospective study illustrated that sPLA2 levels were increased in children with COVID-19 and correlated with its severity (Bonaz et al. 2020). Thus, PLA2 inhibitors like darapladib and varesplaide could be effective in attenuating inflammatory disorders in COVID-19 (Batsika et al. 2021; Kuypers et al. 2021). Hence, CTN could be effective in reducing COVID-19 severity through inhibition of PLA2 activity.
CTN improves mitochondrial dysfunction by mitoprotective effects including preservation of inner mitochondrial membrane potential, controlling opening of inner mitochondrial membrane and inhibition of mitochondrial PLA2 (Stefano et al. 2021). In SARS-CoV-2 infection, there is noteworthy neuronal mitochondrial dysfunction which linked with development of cognitive dysfunction in COVID-19 patients (Stefano et al. 2021). Particularly, neuronal mitochondrial dysfunction and energy metabolism are severely impaired in severe SARS-CoV-2 infection with neuroinflammation causing cognitive dysfunction (Stefano et al. 2021). Thus, CTN via its anti-inflammatory effects and restoration of neuronal mitochondrial function could be a proposed mechanism for attenuating of COVID-19-induced cognitive impairment.
Citicoline and ubiquitin protease system
The ubiquitin protease system (UPS) is an intracellular proteolytic pathway involved in the regulation of intracellular trafficking, protein synthesis, and folding/degradation of intracellular proteins (Chen et al. 2021). Dysfunction of UPS is linked with the development of different neurological disorders, including Parkinson disease, Alzheimer’s disease, and amyotropic lateral sclerosis (Gong et al. 2016). This intracellular defect in the clearance of many misfolded proteins induces intracellular deposition of misfolded proteins and progression of cytotoxicity (Gong et al. 2016). Modulation of UPS is effective in decreasing inflammatory reactions and responses during different viral infections (Luo 2016). Viral infections may cause dysfunction of the UPS. Of interest, viral infections induce accumulation of ubiquitin conjugates during the replication process, with subsequent inhibition of protein synthesis and development of endoplasmic reticulum stress (Luo 2016). Many viruses avert the function of UPS to inhibit host proteins which interfere with viral replication. As well, UPS is blocked by some viruses to reduce viral clearance (Gong et al. 2016). Herein; inhibition of dysfunctional UPS by selective inhibitors may reduce the severity of SARS-CoV-2 infection (Clemente et al. 2020). It has been reported that CTN modulates the activity of UPS by suppressing proteasome activity, by which CTN can reduce the replication of SARS-CoV-2 and associated inflammatory reactions (Longhitano et al. 2020). CTN, like other proteasome inhibitors like MG132 and lactacistin, can attenuate the early stages of SARS-CoV-2 replication by preventing the release of viral particles from endosomes into the cytosol with inhibition of the p38MAPK pathway (Moutzouris et al. 2010).
These observations suggest that CTN through modulation of UPS can impair SARS-CoV-2 replication and release of pro-inflammatory cytokines. As well, CTN via regulation of dysfunctional UPS can attenuate SARS-CoV-2-induced neuroinflammation and associated degenerative brain diseases.
Citicoline and cholinergic neurotransmission
It has been shown that CTN stimulates synthesis of Ach in the brain by increasing the availability of choline (Abdel-Aziz et al. 2021). CTN improves cognitive function through improvement of cholinergic transmission and associated synaptic plasticity (Abdel-Aziz et al. 2021). Choline from CTN is essential for the synthesis of brain Ach and regulation of the neurochemical process of Ach neurotransmission (Secades 2019). In their study, Piamonte et al. found that CTN can be used as an adjuvant therapy with cholinesterase inhibitors in the management of cognitive dysfunction in patients with Alzheimer’s disease (Piamonte et al. 2020).
It has been proposed that the cholinergic system be regarded as a possible regulator of SARS-CoV-2-induced hypercytokinemia (Courties et al. 2021). A non-interventional study involving 37 COVID-19 patients that examined expression of choline acetyltransferase, acetylcholine esterase, native alpha-7 nicotinic subunit, and its negative duplicate showed that COVID-19 patients without expression of native alpha-7 nicotinic subunit and its negative duplicate increased the risk for release of pro-inflammatory cytokines (Courties et al. 2021). Of note, the alpha-7 nicotinic Ach receptor (α7nAchR) has an anti-inflammatory role by inhibiting the release of pro-inflammatory cytokines from activated macrophages (Koopman et al. 2016). This receptor represents a neuro-immune target in different chronic inflammatory diseases (Koopman et al. 2016). Activation of the anti-inflammatory α7nAchR has been proposed to be a therapeutic target to limit SARS-CoV-2-induced hypercytokinemia (Bonaz et al. 2020). In silico studies observed that SARS-CoV-2 interacts with α7nAchR, thereby reducing the anti-inflammatory effect of this receptor (Alexandris et al. 2021; Al-Kuraishy et al. 2021h). Thus, α7nAchR agonists could inhibit the interaction between SARS-CoV-2 and α7nAchR (Alexandris et al. 2021). In contrast, Hasanagic and Serdarevic proposed that α7nAchR antagonists such as memantine could be effective in the prevention and treatment of SARS-CoV-2 infection by inhibiting ACE2 expression in the respiratory epithelium (Hasanagic and Serdarevic 2020). Insufficiency of cholinergic neurotransmission is linked with the development of delirium and cognitive dysfunction in COVID-19 patients (Hshieh et al. 2008; Al-Kuraishy et al. 2022h). In this state, CTN treatment may improve cholinergic neurotransmission by increasing the anti-inflammatory effect of Ach through α7nAchR-dependent effect. An experimental study demonstrated that activation of α7nAchR by an allosteric modulator attenuates lipopolysaccharide (LPS)-induced neuroinflammation in mice (Abbas and Rahman 2016). As well, CTN treatment can reduce neurotoxicity through activation of cholinergic muscarinic receptors (Galal et al. 2019).
These findings suggest that CTN treatment could be effective in reducing COVID-19-induced cholinergic dysfunction and associated neuroinflammation and cognitive impairment.
Citicoline and dopaminergic neurotransmission
In COVID-19, it has been hypothesized that alteration of dopamine neurotransmission is associated with the pathogenesis of SARS-CoV-2 infection (Nataf 2020). Interestingly, expression of ACE2 is co-expressed with dopa-decarboxylase (DDC), which is the main enzyme for synthesis of dopamine, serotonin, and conversion of histidine to histamine (Nataf 2020). Therefore, downregulation of ACE2 by SARS-CoV-2 infection is linked with a reduction in the levels of dopamine and serotonin. An experimental study demonstrated that administration of the dopamine agonist fenoldopam attenuates pulmonary inflammation and ALI in mice through upregulation of ACE2 (Bone et al. 2017).
Notably, CTN improves dopamine neurotransmission in both the brain and the retina (Rejdak et al. 2002). A comprehensive review illustrated that CTN was effective in the management of degenerative brain diseases (Oddone et al. 2021). Que and Jamora’s systematic review revealed that CTN is effective as an adjuvant therapy in the management of PD through modulation of dopamine neurotransmission and inhibition of apoptosis (Que and Jamora 2021). As well, CTN has an antidepressant effect through improvement of dopamine and serotonin in male mice (Roohi-Azizi et al. 2018).
These results suggest that CTN, through regulating dopamine and serotonin neurotransmission, may attenuate pathological alterations of neurotransmitters in COVID-19 patients.
Citicoline and glutamatergic neurotransmission
Glutamate is an excitatory neurotransmitter in the brain engaged in neurocognitive function. As well, overexpression of glutamate is implicated in the development of different neurological disorders, including epilepsy, stroke, amyotrophic lateral sclerosis, and Alzheimer’s disease (Kotru et al. 2021). Of note, glutamate neurotoxicity and long-term neurological disorders have been linked with different coronavirus infections (Kotru et al. 2021; Al-Gareeb et al. 2022). Previous SARS-CoV epidemics were associated with the development of degenerative brain diseases due to glutamate neurotoxicity (Cataldi et al. 2020). Remarkably, SARS-CoV-2 can exploit metabotropic glutamate receptor 2 (mGluR2) for its entry in the host cells (Wang et al. 2021b). As well, mGluR2 cooperates with ACE2 for internalization of SARS-CoV-2 (Wang et al. 2021b). This interaction induces some neurological manifestations like convulsion, headache, abnormal taste, and anosmia (Wang et al. 2021b; Engin et al. 2021). As well, neuronal injury by direct SARS-CoV-2 neurotropism and associated hyperinflammation and oxidative stress induce excessive release of glutamate (Engin et al. 2021). Glutamate through interaction with N-methyl-D-aspartate (NMDA) receptor triggers progression of neurotoxicity (Al-Kuraishy et al. 2020b).
Therefore, reduction of glutaminergic neurotransmission may attenuate SARS-CoV-2-induced neurotoxicity and neuroinflammation in COVID-19 patients. Of note, CTN decreased neuronal injury through reversal of glutamate transport and associated excitotoxicity (Hurtado et al. 2005; Piotrowska et al. 2022). Likewise, CTN inhibits neuronal excitotoxicity through attenuation of glutamine concentration in the synaptic cleft by augmenting glutamate uptake through increasing expression of glutamate transporters in rat astrocytes (Hurtado et al. 2005; Piotrowska et al. 2022). Thus, CTN has been observed to be effective in treating different neurological disorders by regulating excitotoxicity and glutamate concentrations in COVID-19 patients [18, 125]. A randomized clinical trial revealed that CTN treatment acts as a neuroprotective agent against brain injury induced following cardiac arrest in children (Salamah et al. 2021).
Thus, these findings suggest that CTN treatment could be an effective agent in reducing SARS-CoV-2-induced neurotoxicity.
Citicoline and hypothalamic pituitary axis
In severe SARS-CoV-2 infection, there is a significant reduction in fasting cortisol and ACTH serum levels in COVID-19 patients compared to controls due to impairment of glucocorticoid response and central adrenal insufficiency (Alzahrani et al. 2021). Similarly, severe SARS-CoV-2 infection may induce dysfunction of the hypothalamic-pituitary-thyroid axis, causing central hypothyroidism (Zheng et al. 2021). This dysfunction is correlated with COVID-19 severity in hospitalized patients (Zheng et al. 2021). Likewise, COVID-19 may cause suppression of the hypothalamic-pituitary gonadal axis with the development of infertility (Selvaraj et al. 2021). Indeed, a deficiency of GH in elderly and obese subjects increases their vulnerability to severe SARS-CoV-2 infection due to immunosuppression (Lubrano et al. 2020). Administration of GH in high-risk patients may decrease the risk of COVID-19 severity (Lubrano et al. 2020). The underlying causes of hypothalamic pituitary dysfunction are related to hypoxia, oxidative stress, hyperinflammation, and cytokine storm (Alzahrani et al. 2021; Zheng et al. 2021; Selvaraj et al. 2021).
Interestingly, CTN stimulates the release of ACTH, LH, FSH, TSH, and GH (Abdel-Aziz et al. 2021; Secades 2011). This thrilling effect of CTN may attenuate SARS-CoV-2 infection-induced hypothalamic-pituitary dysfunction, mainly in patients with severe COVID-19.
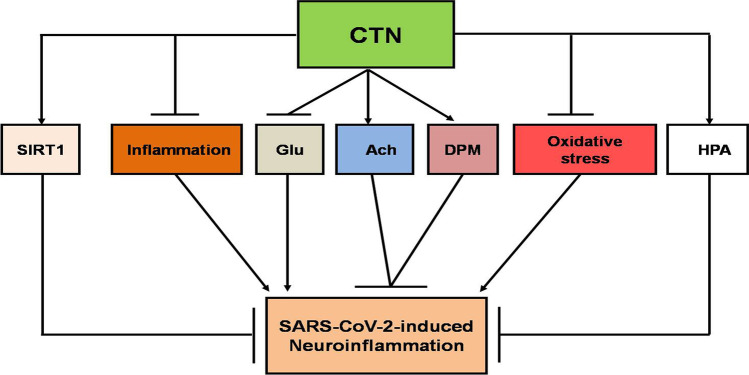
Taken together, in virtue of its anti-inflammatory and antioxidant properties together with modulation of SIRT1, neurotransmission, and hypothalamic pituitary dysfunction, CTN could be effective against neuroinflammation and COVID-19 severity (Fig. 4). Of note, the effective dose of CTN in treating COVID-19 patients is 2 g/day. This effective dose does not interact with most of drugs used in the management of COVID-19 [125].
Fig. 4.
The possible role of citicoline in SARS-CoV-2-induced neuroinflammation: citicoline (CTN) activates the hypothalamic pituitary axis (HPA), acetylcholine (Ach), dopamine (DPM), silent information regulator 1 (SIRT1) and inhibits inflammation, oxidative stress, and glutamine release (Glu), leading to attenuation of SARS-CoV-2-induced neuroinflammation
Conclusion
SARS-CoV-2 infection is linked with various neurological manifestations. The fundamental mechanism of neuropsychiatric disorders in COVID-19 might be due to cytokine-induced disruption of the BBB, neuroinflammation, and peripheral neuronal injury, or due to direct SARS-CoV-2 neurotropism. As well, an exaggerated inflammatory response could be the suggested mechanism for the progression of neuropsychiatric and other neurological disorders in COVID-19. CTN has neuroprotective activity against different neurodegenerative and traumatic brain disorders through its neuro-restorative effects. In virtue of its anti-inflammatory and antioxidant properties, together with modulation of SIRT1, neurotransmission, and hypothalamic-pituitary dysfunction, CTN could be effective against neuroinflammation and COVID-19 severity. Further experimental, preclinical, and clinical studies are warranted to confirm the potential role of CTN in the management of COVID-19.
Author contribution
All the authors contributed to the study conception and design. Data collection and analysis were performed by Hayder M. Al-kuraishy, Ali K. Al-Buhadily, and Ali I. Al-Gareeb. The first draft of the manuscript was written by Hayder M. Al-kuraishy, Ali K. Al-Buhadily, Ali I. Al-Gareeb, Mohammed Alorabi, Nasser A. Hadi Al-Harcan, Maisra M. El-Bouseary, and Gaber E. Batiha, and all the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
All the data generated or analyzed during this study are included in this published article.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hayder M. Al-kuraishy, Email: Hayderm36@yahoo.com
Ali K. Al-Buhadily, Email: alikadhm1977@gmail.com
Ali I. Al-Gareeb, Email: Dr.alialgareeb78@yahoo.com
Mohammed Alorabi, Email: maorabi@tu.edu.sa.
Nasser A. Hadi Al-Harcan, Email: naseer_harchan_55@yahoo.com
Maisra M. El-Bouseary, Email: maysra_mohamed@pharm.tanta.edu.eg
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
References
- Abbas M, Rahman S. Effects of alpha-7 nicotinic acetylcholine receptor positive allosteric modulator on lipopolysaccharide-induced neuroinflammatory pain in mice. Eur J Pharmacol. 2016;783:85–91. doi: 10.1016/j.ejphar.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Abbaszadeh M, Rasooli R, Shadi Mazdaghani MS, Rajaian H, Shamsaei HA (2018) Pharmacokinetics of citicoline after intravenous and intramuscular administration in dogs. Vet Sci Develop. 1 Jan;8(1) 10.4081/vsd.2018.6945.
- Abdel-Aziz N, Moustafa EM, Saada HN. The impact of citicoline on brain injury in rats subjected to head irradiation. Environ Sci Pollut Res Int. 2021;28(8):9742–9752. doi: 10.1007/s11356-020-11101-7. [DOI] [PubMed] [Google Scholar]
- Abdel-Salam O, Youness ER, Mohammed NA, El-Moneim OM, Shaffie N. Citicoline protects against tramadol-induced oxidative stress and organ damage. React Oxygen Species. 2019;7(20):106–120. doi: 10.20455/ros.2019.823. [DOI] [Google Scholar]
- Abdolmaleki A, Moghimi A, Ghayour MB, Rassouli MB. Evaluation of neuroprotective, anticonvulsant, sedative and anxiolytic activity of citicoline in rats. Eur J Pharmacol. 2016;789:275–279. doi: 10.1016/j.ejphar.2016.07.048. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002;70(2):133–139. doi: 10.1002/jnr.10403. [DOI] [PubMed] [Google Scholar]
- Al-Buhadily AK, Hussien NR, Al-Niemi MS, Al-Kuraishy HM, Al-Gareeb AI. Misfortune and spy story in the neurological manifestations of Covid-19. J Pak Med Assoc. 2021;71(Suppl 12):S157–S160. [PubMed] [Google Scholar]
- Alexandris N, Lagoumintzis G, Chasapis CT, Leonidas DD, Papadopoulos GE, Tzartos SJ, et al. Nicotinic cholinergic system and COVID-19: in silico evaluation of nicotinic acetylcholine receptor agonists as potential therapeutic interventions. Toxicol Rep. 2021;8:73–83. doi: 10.1016/j.toxrep.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gareeb AI, Guerreiro SG, Cruz-Martins N, Batiha GE. COVID-19 in relation to hyperglycemia and diabetes mellitus. Al-kuraishy H Front Cardiovasc Med. 2021;8:335. doi: 10.3389/fcvm.2021.644095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GE (2022) Ginkgo biloba in the management of the COVID-19 severity. Arch Pharm e2200188. 10.1002/ardp.202200188 [DOI] [PMC free article] [PubMed]
- Al-kuraishy H, Al-Gareeb A, Al-Omairi N, Cruz-Martins N, Batiha G (2022) Cognitive enhancer effect of citicoline alone or in combination with Panax Ginseng: a prospective human psychometric study. Authorea. 10.22541/au.164865112.22114589/v1
- Al-Kuraishy HM, Al-Gareeb AI. Citicoline improves human vigilance and visual working memory: the role of neuronal activation and oxidative stress. Basic. Clin Neurosci. 2020;11(4):423–432. doi: 10.32598/bcn.11.4.1097.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N, Batiha GE. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type II diabetes mellitus: the anti-inflammatory role of metformin. Front Med (Lausanne) 2021;8:644295. doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Guerreiro SG, Cruz-Martins N, Batiha GE. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med. 2021;8:644095. doi: 10.3389/fcvm.2021.644095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Al-Hussaniy HA, Al-Harcan NAH, Alexiou A, Batiha GE. Neutrophil extracellular traps (NETs) and Covid-19: a new frontiers for therapeutic modality. Int Immunopharmacol. 2022;104:108516. doi: 10.1016/j.intimp.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE (2022a) High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology 1-0 10.1007/s10787-022-00988-y. [DOI] [PMC free article] [PubMed]
- Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. J Lab Physicians. 2018;10(3):276–282. doi: 10.4103/JLP.JLP_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Aljowaie RM, Almutairi SM, Alexiou A, Batiha GE (2022b) The prospective effect of allopurinol on the oxidative stress index and endothelial dysfunction in Covid-19. Inflammation. 24 Feb:1-7 10.1007/s10753-022-01648-7. [DOI] [PMC free article] [PubMed]
- Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Cruz-Martins N, Batiha GE. The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem. 2021;476(11):4161–4166. doi: 10.1007/s11010-021-04232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Fageyinbo MS, Batiha GE. Vinpocetine is the forthcoming adjuvant agent in the management of COVID-19. Future Sci OA. 2022;8(5):FSO797. doi: 10.2144/fsoa-2021-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GE (2021b) The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr 8(649128). 10.3389/fnut.2021.649128 [DOI] [PMC free article] [PubMed]
- Al-Kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GE. Hemolytic anemia in COVID-19. Ann Hematol. 2022;8:1–9. doi: 10.1007/s00277-022-04907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Mostafa-Hedeab G, Kasozi KI, Zirintunda G, Aslam A, et al. Effects of β-blockers on the sympathetic and cytokines storms in Covid-19. Front Immunol. 2021;12:749291. doi: 10.3389/fimmu.2021.749291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Naji MT, Al-Mamorry F. Role of vinpocetine in ischemic stroke and poststroke outcomes: A critical review. Brain Circ. 2020;6(1):1–10. doi: 10.4103/bc.bc_46_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Atanu FO, Batiha GE. Arginine vasopressin and pathophysiology of COVID-19: an innovative perspective. Biomed Pharmacother. 2021;143:112193. doi: 10.1016/j.biopha.2021.112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Gyebi GA, Batiha GE. Covid-19-induced dysautonomia: a menace of sympathetic storm. ASN Neuro. 2021;13:17590914211057635. doi: 10.1177/17590914211057635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Alexiou A, Batiha GE. Impact of sitagliptin on non-diabetic Covid-19 patients. Curr Mol Pharmacol. 2022;15(4):683–692. doi: 10.2174/1874467214666210902115650. [DOI] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Cruz-Martins N, El-Saber Batiha GE. Sequential doxycycline and colchicine combination therapy in Covid-19: the salutary effects. Pulm Pharmacol Ther. 2021;67:102008. doi: 10.1016/j.pupt.2021.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Rauf A, Alhumaydhi FA, Kujawska M, El-Saber Batiha G (2022d) Mechanistic insight and possible mechanism of seizure in Covid-19: the nuances and focal points. CNS Neurol Disord Drug Targets 10.2174/1871527321666220517115227. [DOI] [PubMed]
- Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, Lungnier C. Is ivermectin–azithromycin combination the next step for COVID-19? Biomed Biotechnol Res J (BBRJ) 2020;4(5):101. [Google Scholar]
- Al-Mosawi AJ. The use of citicoline in pediatric neurology and pediatric psychiatry. Austin pediatrics (ISSN: 2381-8999) 2019;6(1):1071–1072. [Google Scholar]
- Al-Naimi MS, Hussien NR, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Levothyroxine improves paraoxonase (PON-1) serum levels in patients with primary hypothyroidism: case–control study. J Adv Pharm Technol Res. 2018;9(3):113–118. doi: 10.4103/japtr.JAPTR_298_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Sabín J, Ortega G, Jacas C, Santamarina E, Maisterra O, Ribo M, et al. Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc Dis. 2013;35(2):146–154. doi: 10.1159/000346602. [DOI] [PubMed] [Google Scholar]
- Alzahrani AS, Mukhtar N, Aljomaiah A, Aljamei H, Bakhsh A, Alsudani N, et al. The impact of COVID-19 viral infection on the hypothalamic-pituitary-adrenal axis. Endocr Pract. 2021;27(2):83–89. doi: 10.1016/j.eprac.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin SN, Khashaba AS, Latif NSA, El Gazzar WB, Hussein UK. Citicoline improved cardiovascular function in animal model of dysautonomia. J Physiol Pharmacol. 2021;72(1):69–80. doi: 10.26402/jpp.2021.1.07. [DOI] [PubMed] [Google Scholar]
- Batsika CS, Gerogiannopoulou AD, Mantzourani C, Vasilakaki S, Kokotos G. The design and discovery of phospholipase A2 inhibitors for the treatment of inflammatory diseases. Expert Opin Drug Discov. 2021;16(11):1287–1305. doi: 10.1080/17460441.2021.1942835. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Sinniger V, Pellissier S. Targeting the cholinergic anti-inflammatory pathway with vagus nerve stimulation in patients with Covid-19? Bioelectron Med. 2020;6(1):15. doi: 10.1186/s42234-020-00051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone NB, Liu Z, Pittet JF, Zmijewski JW. Frontline Science: D1 dopaminergic receptor signaling activates the AMPK-bioenergetic pathway in macrophages and alveolar epithelial cells and reduces endotoxin-induced ALI. J Leukoc Biol. 2017;101(2):357–365. doi: 10.1189/jlb.3HI0216-068RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni V, Tartaglia E, Sacchi A, Fimia GM, Cimini E, Casetti R, et al. The unbalanced p53/SIRT1 axis may impact lymphocyte homeostasis in COVID-19 patients. Int J Infect Dis. 2021;105:49–53. doi: 10.1016/j.ijid.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfamante G, Chiumello D, Canevini MP, Priori A, Mazzanti M, Centanni S, Felisati G. First ultrastructural autoptic findings of SARS-Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86(6):678–679. doi: 10.23736/S0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- Cacabelos R, Carril JC, Cacabelos N, Kazantsev AG, Vostrov AV, Corzo L, et al. Sirtuins in Alzheimer’s disease: SIRT2-related genophenotypes and implications for pharmacoepigenetics. Int J Mol Sci. 2019;20(5):1249. doi: 10.3390/ijms20051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlotti APCP, Carvalho WB, Johnston C, Rodriguez IS, Delgado AF (2020) COVID-19 diagnostic and management protocol for pediatric patients. Clinics (Sao Paulo). 75:e1894. 10.6061/clinics/2020/e1894 [DOI] [PMC free article] [PubMed]
- Cataldi M, Pignataro G, Taglialatela M. Neurobiology of coronaviruses: potential relevance for COVID-19. Neurobiol Dis. 2020;143:105007. doi: 10.1016/j.nbd.2020.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çavun S, Savcı V, Ulus IH. Centrally injected CDP-choline increases plasma vasopressin levels by central cholinergic activation. Fundam Clin Pharmacol. 2004;18(1):71–77. doi: 10.1046/j.0767-3981.2003.00213.x. [DOI] [PubMed] [Google Scholar]
- Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema PS, Nandi D, Nag A. Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19. Open Biol. 2021;11(6):210069. doi: 10.1098/rsob.210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Wang K, Yu J, Howard D, French L, Chen Z, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Dou QP, Liu J, Tang D. Targeting ubiquitin–proteasome system with copper complexes for cancer therapy. Front Mol Biosci. 2021;8:649151. doi: 10.3389/fmolb.2021.649151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16(2):167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente V, D’arcy P, Bazzaro M. Deubiquitinating enzymes in coronaviruses and possible therapeutic opportunities for COVID-19. Int J Mol Sci 2020 n;21(10):3492 10.3390/ijms21103492. [DOI] [PMC free article] [PubMed]
- Courties A, Boussier J, Hadjadj J, Yatim N, Barnabei L, Péré H, et al. Regulation of the acetylcholine/α7nAChR anti-inflammatory pathway in COVID-19 patients. Sci Rep. 2021;11(1):11886. doi: 10.1038/s41598-021-91417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, et al. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380(9839):349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek RO, Serter M, Ergin K, Cecen S, Unsal C, Yildiz Y, Bilgin MD. Protective effects of citicoline on TNBS-induced experimental colitis in rats. Int J Clin Exp Med. 2014;7(4):989–997. [PMC free article] [PubMed] [Google Scholar]
- Engin AB, Engin ED, Engin A. Current opinion in neurological manifestations of SARS-CoV-2 infection. Curr Opin Toxicol. 2021;25:49–56. doi: 10.1016/j.cotox.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Vitiello A. Correction to: the renin-angiotensin system and specifically angiotensin-converting enzyme 2 as a potential therapeutic target in SARS-CoV-2 infections. Naunyn Schmiedeberg's Arch Pharmacol. 2022;395(1):117–118. doi: 10.1007/s00210-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontani F. MMPs, ADAMs and their natural inhibitors in inflammatory bowel disease: involvement of oxidative stress. J Clin Gastroenterol Treat. 2017;3(1):10. doi: 10.23937/2469-584X/1510039. [DOI] [Google Scholar]
- Galal AF, Salem LM, Hassanane MM, Nada SA, Abdel-Salam OM. Citicoline ameliorates neuro- and genotoxicity induced by acute malathion intoxication in rats. J Biosci Appl Res. 2019;5(2):246–261. doi: 10.21608/jbaar.2019.146800. [DOI] [Google Scholar]
- Garcia MA, Barreras PV, Lewis A, Pinilla G, Sokoll LJ, Kickler T, et al. Cerebrospinal fluid in COVID-19 neurological complications: neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm. J Neurol Sci. 2021;427:117517. doi: 10.1016/j.jns.2021.117517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC (2015) The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging 10(1421-9). 10.2147/CIA.S87886 [DOI] [PMC free article] [PubMed]
- Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordo R, Zinellu A, Eid AH, Pintus G. Therapeutic potential of resveratrol in COVID-19-associated hemostatic disorders. Molecules. 2021;26(4):856. doi: 10.3390/molecules26040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Radulovic M, Figueiredo-Pereira ME, Cardozo C. The ubiquitin-proteasome system: potential therapeutic targets for Alzheimer’s disease and spinal cord injury. Front Mol Neurosci. 2016;9:4. doi: 10.3389/fnmol.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanagic S, Serdarevic F (2020) Potential role of memantine in the prevention and treatment of COVID-19: its antagonism of nicotinic acetylcholine receptors and beyond. Eur Respir J 56(2) 10.1183/13993003.01610-2020. [DOI] [PMC free article] [PubMed]
- Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63(7):764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarachi Olivera RE, Lazarte RA. Coronavirus disease (COVID-19) and sirtuins. Rev Fac Cien Med Univ Nac Cordoba. 2020;77(2):117–125. doi: 10.31053/1853.0605.v77.n2.28196. [DOI] [PubMed] [Google Scholar]
- Hurtado O, Moro MA, Cárdenas A, Sánchez V, Fernández-Tomé P, Leza JC, et al. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport. Neurobiol Dis. 2005;18(2):336–345. doi: 10.1016/j.nbd.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Jasielski P, Piędel F, Piwek M, Rocka A, Petit V, Rejdak K. Application of citicoline in neurological disorders: a systematic review. Nutrients. 2020;12(10):3113. doi: 10.3390/nu12103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao F, Gong Z. The beneficial roles of SIRT1 in neuroinflammation-related diseases. Oxid Med Cell Longev. 2020;2020:6782872. doi: 10.1155/2020/6782872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016;113(29):8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotru S, Klimuntowski M, Ridha H, Uddin Z, Askhar AA, Singh G, Howlader MMR. Electrochemical sensing: a prognostic tool in the fight against COVID-19. Trends Analyt Chem. 2021;136:116198. doi: 10.1016/j.trac.2021.116198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Ferrer I, Barrachina M, Secades JJ, Mercadal J, Lozano R. CDP-choline reduces pro-caspase and cleaved caspase-3 expression, nuclear DNA fragmentation, and specific PARP-cleaved products of caspase activation following middle cerebral artery occlusion in the rat. Neuropharmacology. 2002;42(6):846–854. doi: 10.1016/s0028-3908(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Kumar D, Jahan S, Khan A, Siddiqui AJ, Redhu NS, Wahajuddin, et al. Neurological manifestation of SARS-CoV-2 induced inflammation and possible therapeutic strategies against COVID-19. Mol Neurobiol. 2021;58(7):3417–3434. doi: 10.1007/s12035-021-02318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers FA, Rostad CA, Anderson EJ, Chahroudi A, Jaggi P, Wrammert J, et al. Secretory phospholipase A2 in SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-C) Exp Biol Med (Maywood) 2021;246(23):2543–2552. doi: 10.1177/15353702211028560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Huang BR, Yeh WL, Lee CH, Huang SS, Lai CH, et al. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol Aging. 2014;35(1):191–202. doi: 10.1016/j.neurobiolaging.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Longhitano L, Tibullo D, Giallongo C, Lazzarino G, Tartaglia N, Galimberti S, et al. Proteasome inhibitors as a possible therapy for SARS-CoV-2. Int J Mol Sci. 2020;21(10):3622. doi: 10.3390/ijms21103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, et al. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study. EClinicalmedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano C, Masi D, Risi R, Balena A, Watanabe M, Mariani S, Gnessi L. Is growth hormone insufficiency the missing link between obesity, male gender, age, and COVID-19 severity? Obesity (Silver Spring) 2020;28(11):2038–2039. doi: 10.1002/oby.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H. Interplay between the virus and the ubiquitin–proteasome system: molecular mechanism of viral pathogenesis. Curr Opin Virol. 2016;17:1–10. doi: 10.1016/j.coviro.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhang H, Pan Q, Zhao Y, Chen J, Zhao B, Chen Y. Hypoxia/aglycemia-induced endothelial barrier dysfunction and tight junction protein downregulation can be ameliorated by citicoline. Plos One. 2013;8(12):e82604. doi: 10.1371/journal.pone.0082604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majolo F, Silva GLD, Vieira L, Anli C, Timmers LFSM, Laufer S, Goettert MI. Neuropsychiatric disorders and COVID-19: what we know so far. Pharmaceuticals (Basel) 2021;14(9):933. doi: 10.3390/ph14090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynov MY, Gusev EI. Current knowledge on the neuroprotective and neuroregenerative properties of citicoline in acute ischemic stroke. J Exp Pharmacol. 2015;7:17–28. doi: 10.2147/JEP.S63544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Wentzel AR, Richards GA. COVID-19: NAD+ deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Med Hypotheses. 2020;144:110044. doi: 10.1016/j.mehy.2020.110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingoti MED, Bertollo AG, Simões JLB, Francisco GR, Bagatini MD, Ignácio ZM. COVID-19, oxidative stress, and neuroinflammation in the depression route. J Mol Neurosci. 2022;23:1–6. doi: 10.1007/s12031-022-02004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Welson NN, El-Saber Batiha GE, Conte-Junior CA. Selinexor and COVID-19: the neglected warden. Front Pharmacol. 2022;13:884228. doi: 10.3389/fphar.2022.884228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutzouris JP, Che W, Ramsay EE, Manetsch M, Alkhouri H, Bjorkman AM, et al. Proteasomal inhibition upregulates the endogenous MAPK deactivator MKP-1 in human airway smooth muscle: mechanism of action and effect on cytokine secretion. Biochim Biophys Acta. 2010;1803(3):416–423. doi: 10.1016/j.bbamcr.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID-19. J Med Virol. 2020;92(10):1743–1744. doi: 10.1002/jmv.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41(7):1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon S, Inouye SK. Delirium: a missing piece in the COVID-19 pandemic puzzle. Age Ageing. 2020;49(4):497–498. doi: 10.1093/ageing/afaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddone F, Rossetti L, Parravano M, Sbardella D, Coletta M, Ziccardi L, et al. Citicoline in ophthalmological neurodegenerative disease: a comprehensive review. Pharmaceuticals (Basel) 2021;14(3):281. doi: 10.3390/ph14030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard K. The effects of citicoline on acute ischemic stroke: a review. J Stroke Cerebrovasc Dis. 2014;23(7):1764–1769. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piamonte BLC, Espiritu AI, Anlacan VMM. Effects of citicoline as an adjunct treatment for Alzheimer’s disease: a systematic review. J Alzheimers Dis. 2020;76(2):725–732. doi: 10.3233/JAD-200378. [DOI] [PubMed] [Google Scholar]
- Pinto BGG, Oliveira AER, Singh Y, Jimenez L, Gonçalves ANA, Ogava RLT, et al. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J Infect Dis. 2020;222(4):556–563. doi: 10.1093/infdis/jiaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska J, Kryczyk-Poprawa A, Muszyńska B, Pilc A, Opoka W. Application of citicoline in supporting therapy of selected diseases. Acta Poloniae Pharmaceutica - Drug Research. 2022;78(5):591–598. doi: 10.32383/appdr/143279. [DOI] [Google Scholar]
- Que DS, Jamora RDG. Citicoline as adjuvant therapy in Parkinson’s disease: a systematic review. Clin Ther. 2021;43(1):e19–e31. doi: 10.1016/j.clinthera.2020.11.009. [DOI] [PubMed] [Google Scholar]
- Rejdak R, Toczołowski J, Solski J, Duma D, Grieb P. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res. 2002;34(3):146–149. doi: 10.1159/000063658. [DOI] [PubMed] [Google Scholar]
- Ren H, Shao Y, Wu C, Ma X, Lv C, Wang Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol. 2020;500:110628. doi: 10.1016/j.mce.2019.110628. [DOI] [PubMed] [Google Scholar]
- Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohi-Azizi M, Torkaman-Boutorabi A, Akhondzadeh S, Nejatisafa AA, Sadat-Shirazi MS, Zarrindast MR. Influence of citicoline on citalopram-induced antidepressant activity in depressive-like symptoms in male mice. Physiol Behav. 2018;195:151–157. doi: 10.1016/j.physbeh.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Rovere Querini P, De Lorenzo R, Conte C, Brioni E, Lanzani C, Yacoub MR, et al. Post-COVID-19 follow-up clinic: depicting chronicity of a new disease. Acta Biomed. 2020;91(9-S):22–28. doi: 10.23750/abm.v91i9-S.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamah A, Mehrez M, Faheem A, El Amrousy D. Efficacy of citicoline as a neuroprotector in children with post cardiac arrest: a randomized controlled clinical trial. Eur J Pediatr. 2021;180(4):1249–1255. doi: 10.1007/s00431-020-03871-6. [DOI] [PubMed] [Google Scholar]
- Saver JL. Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair. Rev Neurol Dis. 2008;5(4):167–177. [PubMed] [Google Scholar]
- Secades JJ. Citicoline: pharmacological and clinical review, 2010 update. Rev Neurol. 2011;52(Suppl 2):S1–S62. [PubMed] [Google Scholar]
- Secades JJ. Citicoline in the treatment of cognitive impairment. J Neurol Exp Neurosci. 2019;05(1):14–26. doi: 10.17756/jnen.2019-047. [DOI] [Google Scholar]
- Secades JJ. Role of citicoline in the management of traumatic brain injury. Pharmaceuticals (Basel) 2021;14(5):410. doi: 10.3390/ph14050410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj K, Ravichandran S, Krishnan S, Radhakrishnan RK, Manickam N, Kandasamy M. Testicular atrophy and hypothalamic pathology in COVID-19: possibility of the incidence of male infertility and HPG axis abnormalities. Reprod Sci. 2021;28(10):2735–2742. doi: 10.1007/s43032-020-00441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi PY, Zhou XC, Yin XX, Xu LL, Zhang XM, Bai HY. Early application of citicoline in the treatment of acute stroke: a meta-analysis of randomized controlled trials. J Huazhong Univ Sci Technolog Med Sci. 2016;36(2):270–277. doi: 10.1007/s11596-016-1579-6. [DOI] [PubMed] [Google Scholar]
- Snider JM, You JK, Wang X, Snider AJ, Hallmark B, Zec MM et al. (2021) Group IIA secreted phospholipase A 2 is associated with the pathobiology leading to COVID-19 mortality. J Clin Invest. 131(19) 10.1172/JCI149236. [DOI] [PMC free article] [PubMed]
- Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological features of Covid-19. N Engl J Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Ptacek R, Ptackova H, Martin A, Kream RM. Selective neuronal mitochondrial targeting in SARS-CoV-2 infection affects cognitive processes to induce ’brain fog’ and results in behavioral changes that favor viral survival. Med Sci Monit Int Med J Exp Clin Res. 2021;27:e930886–e930881. doi: 10.12659/MSM.930886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui M, Chen G, Mao X, Wei X, Chen Y, Liu C, Fan Y (2019) Gegen qinlian decoction ameliorates hepatic insulin resistance by silent information Regulator1 (SIRT1)-dependent deacetylation of forkhead box O1 (FOXO1). Med Sci Monit 25(8544-53). 10.12659/MSM.919498 [DOI] [PMC free article] [PubMed]
- Tang SW, Helmeste D, Leonard B. Inflammatory neuropsychiatric disorders and COVID-19 neuroinflammation. Acta Neuropsychiatr. 2021;33(4):165–177. doi: 10.1017/neu.2021.13. [DOI] [PubMed] [Google Scholar]
- Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L, Moloney D, Finucane C, McCarthy K, Bergin C, Bannan C, Kenny RA. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One. 2021;16(2):e0247280. doi: 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turana Y, Nathaniel M, Shen R, Ali S, Aparasu RR. Citicoline and COVID-19-related cognitive and other neurologic complications. Brain Sci. 2021;12(1):59. doi: 10.3390/brainsci12010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yang G, Wang X, Wen Z, Shuai L, Luo J, et al. SARS-CoV-2 uses metabotropic glutamate receptor subtype 2 as an internalization factor to infect cells. Cell Discov. 2021;7(1):119. doi: 10.1038/s41421-021-00357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wu Z, Bai L, Liu R, Ba Y, Zhang H, et al. Resveratrol improved hippocampal neurogenesis following lead exposure in rats through activation of SIRT1 signaling. Environ Toxicol. 2021;36(8):1664–1673. doi: 10.1002/tox.23162. [DOI] [PubMed] [Google Scholar]
- Yong SJ. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. 2021;12(4):573–580. doi: 10.1021/acschemneuro.0c00793. [DOI] [PubMed] [Google Scholar]
- Zheng J, Cui Z, Shi N, Tian S, Chen T, Zhong X, et al. Suppression of the hypothalamic-pituitary-thyroid axis is associated with the severity of prognosis in hospitalized patients with COVID-19. BMC Endocr Disord. 2021;21(1):228. doi: 10.1186/s12902-021-00896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.