Abstract
Black Americans of low SES have higher colorectal cancer (CRC) incidence than other groups in the US. However, much of the research that identifies CRC risk factors is conducted in cohorts of high SES and non-Hispanic white participants. Adults participants of the Southern Community Cohort Study (N=75,182) were followed for a median of 12.25 years where 742 incident CRCs were identified. The majority of the cohort are non-Hispanic white or Black and have low household income. Cox models were used to estimate hazard ratios (HRs) for CRC incidence associated with sociocultural factors, access to and use of healthcare, and healthy lifestyle scores to represent healthy eating, alcohol intake, smoking and physical activity. The association between Black race and CRC was consistent and not diminished by accounting for SES, access to healthcare or healthy lifestyle (HR=1.34; 95%CI:1.10,1.63). CRC screening was a strong, risk reduction factor for CRC (HR=0.65; 95%CI:0.55,0.78), and among CRC-screened, Black race was not associated with risk. Participants with ≥high school education were at lower CRC risk (HR=0.81; 95%CI:0.67,0.98). Income and neighborhood-level SES were not strongly associated with CRC risk. Whereas individual health behaviors were not associated with risk, participants that reported adhering to ≥3 health behaviors had a 19% (95%CI:1%,34%) decreased CRC risk compared to participants that reported ≤1 behaviors. The association was consistent in fully-adjusted models, although HRs were no longer significant. CRC screening, education, and a lifestyle that includes healthy behaviors lowers CRC risk. Racial disparities in CRC risk may be diminished by CRC screening.
Introduction:
Colorectal cancer (CRC) incidence causes a large disease burden where an estimated 151,030 individuals in the United States will be diagnosed in 2022. The CRC burden especially impacts Black Americans who have the highest CRC incidence of any racial group in the US. (1) The causes of the CRC racial disparity are not completely understood; theorized causes include a combination of differences in socioeconomic status (SES), in screening and access to healthcare, and in the prevalence of healthy behaviors.
Epidemiologic studies show that several health behaviors and lifestyle factors are related to decreased colorectal cancer risk, including non-smoking, maintaining a healthy weight, moderate alcohol intake, physical activity, and healthy diets. (1) These health behaviors are less prevalent among Blacks Americans than non-Hispanic white Americans. (1) However, much of the epidemiological research that provides support for the associations between lifestyle factors and CRC risk has been conducted in cohorts where most individuals are of high socioeconomic position and are non-Hispanic white. (2) Additionally, previous epidemiologic studies provide evidence that lifestyle factors may have weaker associations with health outcomes in Blacks and populations of low socioeconomic position. (3,4) The Southern Community Cohort Study (SCCS) provides an opportunity to investigate associations in a cohort comprised of individuals of low-SES and who are Black Americans. An effective strategy to reduce CRC racial disparities includes identifying risk factors most important to CRC risk in high risk populations.
Herein, we characterize the associations between sociocultural factors, access to healthcare, and lifestyle factors with colorectal cancer risk. Additionally, we evaluate whether the associations between race and CRC risk are influenced by lifestyle, SES and access to care.
Materials and Methods:
Study Population.
The study data arise from the prospective SCCS. (5,6) The SCCS enrolled over 85,000 adult participants from 2002–2009 in 12 states in the southeastern USA. Participants were English-speaking, and age 40–79 at enrollment. The majority of participants (86%) were enrolled at Community Health Centers. The remaining 14% of cohort participants were enrolled using an identical mailed questionnaire sent to stratified random samples of residents in the same 12 states. At study enrollment, all participants completed questionnaires to obtain information on demographics, socioeconomics, cancer screening participation, medical history, and lifestyle factors, such as height, weight, smoking history, alcohol intake, diet during the previous year, sitting time, and physical activity. The SCCS was approved by Institutional Review Boards at Vanderbilt University Medical Center and Meharry Medical College. All participants provided written informed consent. The study was done with ethical standards consistent with the Belmont Report.
Outcome Assessment.
In 2021, SCCS staff performed linkages to state cancer registries and the National Death Index to acquire information on incident colon and rectal cancers as defined by International Classification of Diseases-Oncology codes C180–189, C199, and C209 (N=690) through December 31, 2017.
Exposure Assessment.
We were interested in establishing the associations between sociocultural factors, access to healthcare, and lifestyle with colorectal cancer risk in the SCCS. All exposures were assessed at the baseline interview. Sociocultural factors of interest were race (Black, white, or other), sex (male or female), household income, educational attainment, and neighborhood deprivation index. The neighborhood deprivation index variable represents socioeconomic status measured at the census tract-level to summarize: ownership and type of housing, income measures, household makeup, unemployment, high school graduation rates, occupations, and car ownership. (7,8) Access to health care was operationalized by variables for ever undergoing CRC screening and health insurance status. We evaluated lifestyle factors by investigating the relations between CRC incidence and adhering to the American Cancer Society (ACS) Guideline for Diet and Physical Activity for Cancer Prevention. (9,10) Associations with cancer incidence were assessed for sedentary time, BMI, physical activity, an ACS dietary score, alcohol consumption and smoking status. BMI was calculated using the values for weight and height provided at enrollment. Participants were considered as having met current physical activity recommendations via sports and exercise if they reported ≥150 min/week of moderate activity, ≥75 min/week of vigorous activity or ≥150 min/week of moderate and vigorous activity combined. The ACS dietary score consisted of three component parts for meeting guidelines for: consuming ≥ 4 cups of fruits and vegetables, choosing at least 50% of grains as whole grains, and limiting consumption of processed and red meat (Supplementary Table 1). Sedentary time was defined as the number of hours per day the participant reported sitting. Dietary intake was assessed using a Food Frequency Questionnaire, developed and validated specifically for the diet in the Southeastern US. (11,12) We classified non- and moderate alcohol drinkers as having met the cancer prevention guideline, as defined by the USDA Dietary Guidelines for Americans for moderate drinking as alcohol intake reported as >0 but ≤1 drink/day for women or ≤2 drink/day for men, and heavy drinking as >1 drink/day for women and >2 drinks/day for men. (13) Never smokers met the cancer prevention guideline. Former smokers were defined as participants who had ever smoked and did not report cigarette smoking at enrollment interview.
Healthy Lifestyle Scores – Compliance to the ACS Guidelines on Nutrition and Physical Activity for Cancer Prevention and Nonsmoking:
We created two healthy lifestyle scores to indicate the number of guidelines adhered to from ACS Guidelines on Nutrition and Physical Activity for Cancer Prevention and Nonsmoking. The first healthy lifestyle score was created by counting and summing (0–5) the number of ACS Guidelines on Nutrition and Physical Activity for Cancer Prevention (9,10) the participant met upon entry into the cohort by assigning one point for each of: BMI in the “healthy” category, meeting physical activity guidelines, being a never smoker, being a non or moderate alcohol drinker, and meeting ≥ 1 diet quality expectations. We created a second healthy lifestyle score that did not include BMI, because unlike the other components of the score, BMI is not a health behavior. We did not include sedentary time in the healthy lifestyle scores because sitting time was not associated with CRC in this cohort, and the optimal amount of sedentary time per day is currently undefined.
Analytical Dataset: Participant Eligibility Information.
The current study includes 75,182 participants who met the following inclusion criteria: ≥ two years of follow-up and; no diagnosis of cancer (except non-melanoma skin cancer) before baseline interview. Missing covariate data (0.9–2.8% of participants) were set to sex- and race-specific medians or modes.
Statistical analysis.
Frequency distributions of participant characteristics were tabulated by CRC incidence, and variables of interest. Cox models were used to estimate hazard ratios (HRs) for CRC incidence associated with sociocultural factors, access to healthcare, lifestyle factors and two healthy lifestyle scores. Age was used as the time scale. Entry time in the Cox models was defined as age at enrollment and exit time as age at CRC diagnosis, age at death, loss to follow-up, or December 31, 2017, whichever came first. We evaluated the proportional hazards assumption graphically, and considered it met.
Statistical models included the following variables as potential confounders, measured at baseline interview: race (Black, white, other), sex (male, female), enrollment source (CHC, non-CHC), CRC screening (ever, never participated in colonoscopy or sigmoidoscopy) health insurance status (yes, no), household income (<$15,000, $15,000‐24,999, ≥$25,000), education (<high school, high school, >high school), neighborhood deprivation index (quintiles based on the distribution of neighborhood deprivation index value of all the census tracts in the 12 states that encompass the SCCS recruitment area), body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2), physical activity (meets, does not meet guideline), sedentary time (quartiles), ACS diet quality variable (0–3), smoking status (never, former, current), alcohol intake (women: none, 0< drink/day ≤1, >1 drink/day; men: none, 0< drinks/day ≤2, >2 drinks/day), and family history of CRC diagnosis in a first degree relative (yes, no, unknown).
We also calculated HRs for CRC incidence by variables of interest stratified by sex, race, ever participation in CRC screening and anatomic site. Possible interactions between variables of interest were assessed by likelihood ratio tests to compare main effects models with and without the addition of cross-product terms. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC) in 2022.
Data Availability Statement.
Data available for qualified investigators and can be requested via: southerncommunitystudy.org/
Results:
The prevalence of exposures for SES, access to health care and health behaviors varied by race and case status (Supplemental Tables 1 & 2). In general and by case status, Black participants had lower household income, less educational attainment, and more often lived in areas with lower area-level SES. Black participants also were less likely to report participation in CRC screening.
The variable for Black race was strongly related to CRC and the association was not diminished by accounting for family history, BMI, health behaviors, SES, or access to healthcare (Table 1, and Figure 1). Specifically, the association between Black race and CRC risk in minimally-adjusted models was 1.35 (95%CI: 1.13,1.62), whereas the HR in fully-adjusted models was 1.33 (95%CI: 1.10,1.62). Because Black participants were less likely to report ever undergoing CRC screening, we examined whether the association between race and CRC risk was consistent when stratified by screening status. Among participants that had never been screened and age-eligible for CRC screening at enrollment (≥ age 50 at enrollment), we observed a strong and consistent association between Black race and increased CRC risk (Table 1). However, among participants that were age-eligible for CRC screening at enrollment and who reported ever having CRC screening, the association between Black race and CRC risk was attenuated and 95% CIs crossed unity (fully-adjusted HR for Black race = 1.16; 95%CI: 0.78,1.73).
Table 1.
The association between race and colorectal cancer risk after adjustment for sociocultural factors and access to care.
| Model 1 | Model 2 Model 1 + family history |
Model 3 Model 2 + BMI + health behaviors |
Model 4 Model 3 + SES |
Model 5 Model 4 + access to care |
|||
|---|---|---|---|---|---|---|---|
| Race | Cohort (N) | Cases (N) | HR (95%CI) a | HR (95%CI) b | HR (95%CI) c | HR (95%CI) d | HR (95%CI) e |
| White | 20903 | 168 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Black | 50687 | 549 | 1.35 (1.13 to 1.62) | 1.36 (1.14 to 1.62) | 1.37 (1.15 to 1.64) | 1.35 (1.11 to 1.64) | 1.33 (1.10 to 1.62) |
| Race | Never screened for colorectal cancer, among participants ≥ age 50 at enrollment | ||||||
| White | 6505 | 78 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Black | 16053 | 275 | 1.38 (1.07 to 1.78) | 1.39 (1.07 to 1.79) | 1.44 (1.11 to 1.87) | 1.44 (1.09 to 1.90) | 1.45 (1.09 to 1.91) |
| Race | Ever screened for colorectal cancer, among participants ≥ age 50 at enrollment | ||||||
| White | 6110 | 49 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Black | 9676 | 101 | 1.25 (0.88 to 1.78) | 1.25 (0.88 to 1.77) | 1.18 (0.83 to 1.69) | 1.16 (0.78 to 1.73) | 1.16 (0.78 to 1.73) |
Abbreviations: CI, confidence interval; HR, hazard ratio; Ref., reference; SES, socioeconomic status.
Adjusted for sex, and enrollment source.
Additionally adjusted for family history of colorectal cancer.
Additionally adjusted for BMI, smoking status, alcohol use, physical activity, sedentary time, and an ACS diet score.
Additionally adjusted for household income, education, and neighborhood deprivation.
Additionally adjusted for insurance coverage and colorectal cancer screening participation.
Figure 1.
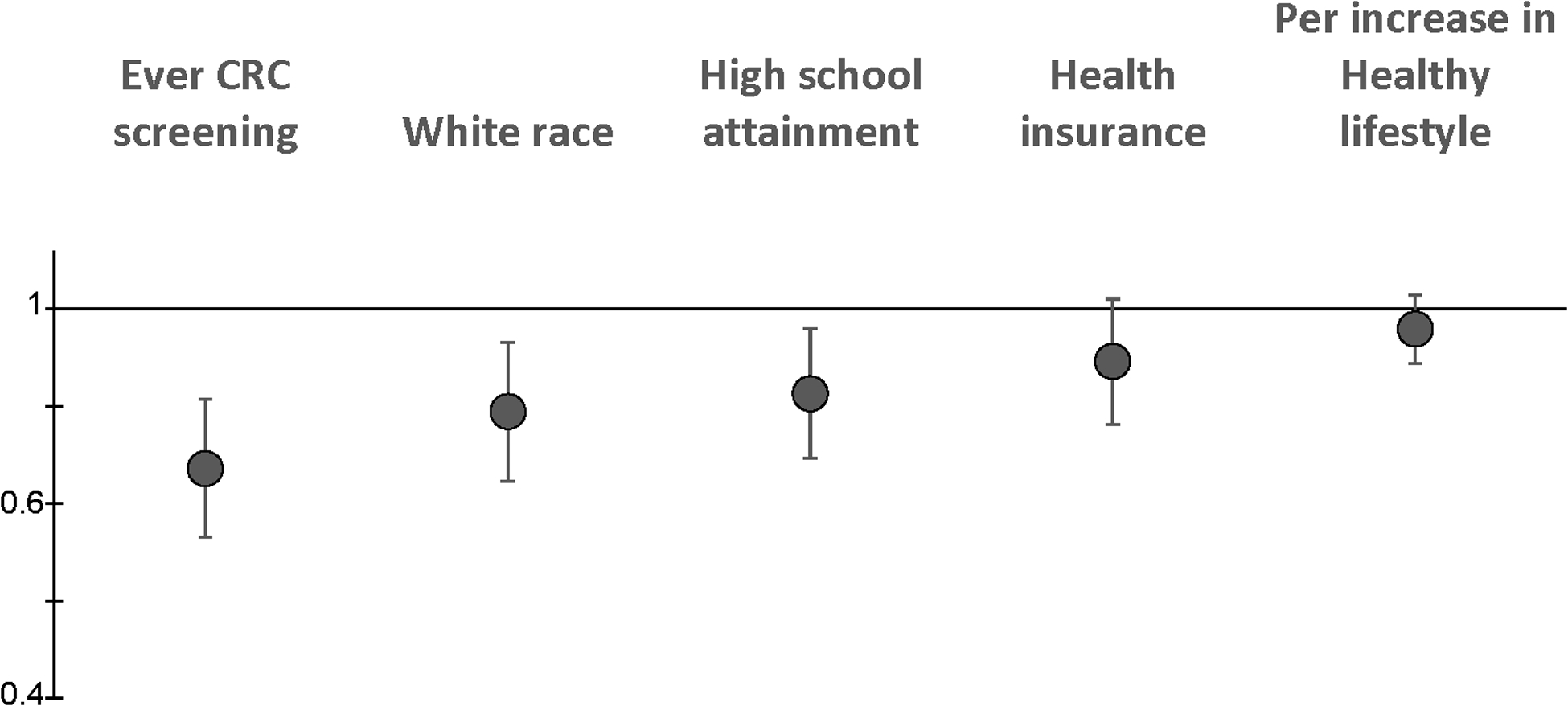
The associations between variables for race, education, access and use of healthcare and adherence to a healthy lifestyle with CRC risk. The Figure displays hazard ratios for the variables most strongly associated with CRC incidence in the cohort: ever participating in CRC screening at enrollment, white race (in comparison to Black race), attaining a high school education, health insurance at enrollment, and a per increase in a healthy lifestyle variable that is a composite variable including smoking status, alcohol use, physical activity, and the ACS diet score. Hazard ratios are adjusted for enrollment source, and the variables presented in the Figure. CRC=colorectal cancer.
In this cohort where most participants are of low SES, household income and neighborhood socioeconomic environment were not strongly associated with CRC risk (Table 2). Participants with greater than a high school education were at lower risk of CRC when compared with participants with less than high school attainment (HR=0.81; 95%CI: 0.67,0.98). Gender was not associated with CRC risk.
Table 2.
Sociocultural factors, access to healthcare, and use of healthcare in association with colorectal cancer incidence.
| Total Analytic Cohort | Black participants | |||||
|---|---|---|---|---|---|---|
| Baseline Characteristic | Cohort N=75,182 | Cases N=742 | HR (95%CI) a | Cohort N=50,687 | Cases N=549 | HR (95%CI) a |
| Sociocultural factors. | ||||||
| Sex | ||||||
| Female | 44337 | 424 | 1 (ref.) | 29476 | 324 | 1 (ref.) |
| Male | 30845 | 318 | 1.18 (1.00 to 1.38) | 21211 | 225 | 1.06 (0.88 to 1.28) |
| Socioeconomic status. | ||||||
| Household income, $ | ||||||
| <15,000 | 41448 | 434 | 1 (ref.) | 30326 | 338 | 1 (ref.) |
| 15,000–24,999 | 16523 | 156 | 0.94 (0.78 to 1.14) | 11263 | 115 | 0.95 (0.76 to 1.28) |
| ≥25,000 | 17211 | 152 | 0.99 (0.79 to 1.24) | 9098 | 96 | 1.08 (0.83 to 1.40) |
| Education | ||||||
| <High school | 21374 | 249 | 0.97 (0.81 to 1.16) | 15824 | 190 | 0.91 (0.74 to 1.11) |
| High school | 24839 | 255 | 1 (ref.) | 17380 | 198 | 1 (ref.) |
| >High school | 28969 | 238 | 0.81 (0.67 to 0.98) | 17483 | 161 | 0.79 (0.63 to 0.98) |
| Neighborhood Deprivation Indexc | ||||||
| Least deprived quintile | 5529 | 46 | 1 (ref.) | 2100 | 24 | 1 (ref.) |
| Quintile 2 | 9066 | 91 | 1.18 (0.83 to 1.69) | 3694 | 46 | 1.08 (0.66 to 1.77) |
| Quintile 3 | 10121 | 82 | 0.90 (0.63 to 1.30) | 4607 | 44 | 0.80 (0.49 to 1.33) |
| Quintile 4 | 15626 | 165 | 1.08 (0.77 to 1.52) | 9711 | 119 | 0.97 (0.62 to 1.51) |
| Most deprived quintile | 34840 | 358 | 1.02 (0.73 to 1.41) | 30575 | 316 | 0.86 (0.57 to 1.32) |
| Access to, and Use of Healthcare. | ||||||
| Insurance status | ||||||
| Uninsured | 30040 | 300 | 1 (ref.) | 21101 | 231 | 1 (ref.) |
| Insured | 45142 | 442 | 0.86 (0.73 to 1.01) | 29586 | 318 | 0.85 (0.70 to 1.02) |
| Colorectal cancer screening | ||||||
| Never | 52804 | 553 | 1 (ref.) | 37344 | 421 | 1 (ref.) |
| Ever | 22378 | 189 | 0.63 (0.53 to 0.76) | 13343 | 128 | 0.65 (0.53 to 0.80) |
Abbreviations: CI, confidence interval; HR, hazard ratio; MET-hrs, metabolic equivalent-hours; Pop, population; Ref., reference.
Adjusted for enrollment source, family history of colorectal cancer, BMI, physical activity, sedentary time, diet quality, alcohol intake, smoking status, and the variables presented in the table.
Other includes all participants who self-identify their race as a race other than non-Hispanic black or non-Hispanic white.
Comparison groups for neighborhood deprivation index were created by dividing participants into quintiles based on the distribution of neighborhood deprivation index value of all the census tracts in the 12 states that encompass the SCCS recruitment area. Q1 includes data from participants in the least deprived quartile of the neighborhood deprivation index.
As previously reported, health care access and use were associated with lower CRC risk in the SCCS (Table 2, Figure 1) (14). Ever undergoing CRC screening via colonoscopy or sigmoidoscopy was the strongest factor associated with reduced CRC incidence in the study. Participants that had health insurance at enrollment were also at decreased risk of CRC.
The majority of cohort members did not meet the ACS Guidelines on Nutrition and Physical Activity for Cancer Prevention and Nonsmoking for BMI and physical activity (Table 3, Supplemental Tables 1 & 2). Participants that were subsequently diagnosed with incident CRC had the following characteristics at enrollment: 21.6% had a BMI between 18.5 and 24.9 kg/m2, 16.6% met the guideline for physical activity set forth in the Physical Activity Guidelines for Americans, 56.6% sat for 8.5 or fewer hours per day (a measure of sedentary time), and 51.5% of cases did not drink alcohol. When stratified by race, Black cases were more likely to be overweight or obese than non-Hispanic white cases (77.6% vs. 72.0%), and more likely to be classified as heavy drinkers (17.1% vs. 11.9%).
Table 3.
The associations between the ACS Guidelines on Nutrition and Physical Activity for Cancer Prevention and Nonsmoking with Colorectal Cancer Incidence.
| Total Analytic Cohort | Black participants | |||||
|---|---|---|---|---|---|---|
| Guideline | Cohort (N) | Cases (N) | HR (95%CI) a | Cohort (N) | Cases (N) | HR (95%CI) a |
| Achieve and maintain a healthy weight throughout life. | ||||||
| Body mass index at baseline (kg/m2) | ||||||
| <18.5 | 867 | 14 | 1.95 (1.13 to 3.38) | 549 | 9 | 1.80 (0.91 to 3.56) |
| 18.5–24.9 | 17897 | 160 | 1 (Ref.) | 11790 | 114 | 1 (Ref.) |
| 25.0–29.9 | 22859 | 239 | 1.10 (0.89 to 1.34) | 14940 | 173 | 1.10 (0.87 to 1.40) |
| ≥30.0 | 33559 | 329 | 1.06 (0.87 to 1.30) | 23408 | 253 | 1.05 (0.82 to 1.33) |
| Be physically active. | ||||||
| Physical activity guideline b | ||||||
| Meets | 14681 | 123 | 1 (Ref.) | 9774 | 90 | 1 (Ref.) |
| Does not meet | 60501 | 619 | 1.07 (0.88 to 1.31) | 40913 | 459 | 1.06 (0.84 to 1.33) |
| Somewhat active | 30245 | 303 | 1.09 (0.88 to 1.35) | 20398 | 225 | 1.08 (0.84 to 1.39) |
| Inactive | 30256 | 316 | 1.05 (0.85 to 1.31) | 20515 | 234 | 1.03 (0.80 to 1.33) |
| Limit sedentary behavior (quartiles of sitting time, hours) | ||||||
| <5.8 | 18707 | 210 | 1 (Ref.) | 12510 | 147 | 1 (Ref.) |
| 5.9–8.5 | 19625 | 210 | 0.98 (0.87 to 1.19) | 12845 | 159 | 1.08 (0.86 to 1.35) |
| 8.6–12.0 | 19430 | 159 | 0.80 (0.65 to 0.98) | 12827 | 123 | 0.88 (0.67 to 1.12) |
| ≥12.1 | 17420 | 163 | 0.97 (0.79 to 1.20) | 12505 | 120 | 0.96 (0.75 to 1.23) |
| Follow a healthy eating pattern. | ||||||
| Diet quality score (number of recommendations met)c | ||||||
| 2–3 | 9008 | 105 | 1 (Ref.) | 6347 | 84 | 1 (Ref.) |
| 1 | 40087 | 396 | 0.90 (0.72 to 1.12) | 27403 | 300 | 0.89 (0.70 to 1.14) |
| 0 | 26087 | 241 | 0.91 (0.71 to 1.15) | 16937 | 165 | 0.86 (0.66 to 1.13) |
| It is best not to drink alcohol. | ||||||
| Alcohol consumption d | ||||||
| None | 34962 | 382 | 1 (Ref.) | 22774 | 278 | 1 (Ref.) |
| Moderate | 26932 | 241 | 0.93 (0.78 to 1.10) | 17722 | 177 | 0.95 (0.78 to 1.16) |
| Heavy | 13288 | 119 | 0.95 (0.76 to 1.20) | 10191 | 94 | 0.97 (0.74 to 1.26) |
| Smoking status | ||||||
| Never | 26997 | 259 | 1 (Ref.) | 18819 | 198 | 1 (Ref.) |
| Ever | 48185 | 483 | 1.14 (0.97 to 1.34) | 31868 | 351 | 1.17 (0.97 to 1.41) |
| Former | 17310 | 211 | 1.21 (1.01 to 1.46) | 10140 | 146 | 1.28 (1.03 to 1.59) |
| Current | 30875 | 272 | 1.07 (0.88 to 1.30) | 21728 | 205 | 1.07 (0.85 to 1.33) |
Abbreviations: ACS, American Cancer Society; HR, hazard ratio; CI, confidence interval; Ref., reference.
Adjusted for sex, race, enrollment source, household income, education, family history of colorectal cancer, insurance coverage, neighborhood deprivation, colorectal cancer screening participation, and the variables presented in the table.
Participants met aerobic physical activity recommendations if they reported ≥ 150 min/week of moderate activity, ≥ 75 min/week of vigorous activity or ≥ 150 min/week of moderate and vigorous activity combined. Participants who did not meet the physical activity guideline were classified into two groups of “somewhat active” and “inactive” based on whether they were above or below the median for total activity (in MET-hrs).
Diet quality variable is created by summing the nutrition-related ACS sub-guidelines met (0–3) related to consumption of grains, red and processed meats, and fruits and vegetables.
Moderate alcohol consumption is defined as 0< drinks/day ≤1 drink/day for women and as 0< drinks/day ≤2 for men.
We created a Diet Quality Score based on the ACS Guidelines on Nutrition for Cancer Prevention, and found no association with CRC (Table 3). Additionally, the component parts of the diet quality score (consuming ≥ 4 cups of fruits and vegetables, choosing at least 50% of grains as whole grains, and limiting consumption of processed and red meat) were not associated with risk (Supplemental Table 3).
Whereas, the associations between individual health behaviors and CRC risk were null, a healthy lifestyle score that included smoking status, alcohol, physical activity, and the ACS diet score was associated with lower CRC risk in models adjusted for sex and race. Specifically, participants that adhered to 3 or 4 guidelines for healthy lifestyle had a 19% decreased CRC risk compared to participants that adhered to ≤1 guideline (HR: 0.81; 95%CI: 0.66,0.99). The association was consistent after adjustment for SES and access to healthcare, although hazard ratios were no longer significant (Table 4). When adherence to BMI weight guidelines were added to the healthy lifestyle score the association with CRC risk was slightly attenuated but the association remained that meeting more guidelines was associated with a non-significant decreased risk of CRC (Table 4). The associations with the healthy lifestyle variable and CRC risk did not vary by sex (P-interaction=0.07), or race (P-interaction=0.37), although associations were less apparent in analyses restricted to Black participants (Supplemental Table 4).
Table 4.
The associations between healthy lifestyle scores and colorectal cancer risk with adjustment for sociocultural factors and access to care.
| Model 1 | Model 2 Model 1 + SES |
Model 3 Model 2 + access to care |
|||
|---|---|---|---|---|---|
| Healthy Lifestyle Scorea | Cohort (N) | Cases (N) | HR (95%CI) c | HR (95%CI) d | HR (95%CI) e |
| 0–1 | 21193 | 202 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 2 | 30137 | 319 | 0.98 (0.82 to 1.17) | 0.99 (0.83 to 1.19) | 1.01 (0.85 to 1.21) |
| 3–4 | 23852 | 221 | 0.81 (0.66 to 0.99) | 0.84 (0.69 to 1.03) | 0.87 (0.71 to 1.06) |
| Healthy Lifestyle Score including BMIb | |||||
| 0–1 | 16020 | 148 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 2 | 28671 | 319 | 1.07 (0.88 to 1.31) | 1.09 (0.89 to 1.32) | 1.10 (0.91 to 1.35) |
| 3–5 | 30491 | 275 | 0.83 (0.68 to 1.02) | 0.86 (0.70 to 1.06) | 0.88 (0.71 to 1.08) |
Abbreviations: CI, confidence interval; HR, hazard ratio; Ref., reference; SES, socioeconomic status.
“Healthy lifestyle score” is a composite variable including smoking status, alcohol, physical activity, and the ACS diet score.
“Healthy lifestyle score including BMI” is a composite variable including BMI, smoking status, alcohol use, physical activity, and the ACS diet score.
Adjusted for sex, race, enrollment source, family history of colorectal cancer.
Additionally adjusted for household income, education, and neighborhood deprivation.
Additionally adjusted for and BMI (in the Healthy Lifestyle Score model), colorectal cancer screening participation, and insurance coverage
Along with CRC screening, race, health insurance coverage, and attaining a high school education or more, adhering to a healthy lifestyle was a consistent risk reduction factor for decreased CRC risk (Figure 1). We evaluated whether the exposures most strongly associated with risk in our study population had differing strengths of the association by anatomic site. We found consistent inverse associations with colon and rectal cancers by CRC screening, race, health insurance coverage, education, and adhering to a healthy lifestyle (Supplemental Table 5).
We also evaluated whether the associations between sociocultural, access to healthcare and lifestyle factors with CRC risk varied by participation in CRC screening. (Supplemental Table 6), and found no differences in our point estimates by health insurance status, neighborhood SES, or individual-level health behaviors and income. Among participants that had ever been screened for CRC, men had a higher CRC risk (HR=1.67; 95%CI: 1.19,2.34). Among participants eligible for CRC screening who has never participated in screening, education was no longer associated with decreased CRC risk (HR for attaining ≥ high school education=1.01; 95%CI: 0.76,1.32).
Discussion:
We examined the relations between healthcare, sociocultural, and lifestyle factors with CRC incidence, and found Black race is strongly related to CRC. Black race is consistently associated with increased CRC risk in the SCCS, and the association does not vary when statistical adjustments are made for variables for health insurance coverage, SES, or lifestyle. Our results are in line with national data that shows Black Americans have higher CRC incidence rates in comparison to other racial groups. Nationally, CRC incidence is 20% higher among Black Americans compared to non-Hispanic whites and 50% higher than incidence in Asian Pacific Islanders. (1)
Higher incidence among Black Americans may partially reflect racial differences in the prevalence of lifestyle factors, such as obesity, although individual health behaviors are not associated with risk in the present study. Black participants in the study, report less participation in CRC screening, and lower CRC screening among Black Americans has been documented by the SCCS and others. (14–17) Other studies have noted that Black Americans less often access healthcare, evidenced by lower follow-up of CRC abnormalities found on screening. (18) Lower CRC screening by Black Americans may be related to having fewer financial resources and less flexibility in daily schedule. (18–20) Lower CRC screening rates and less access to healthcare may mediate the association between Black race and CRC. In support of that assertion, the association between race and CRC risk was attenuated among participants eligible for CRC screening who had ever undergone CRC screening at enrollment (HR for Black race: 1.16; 95% CI: 0.78,1.73). Our results suggest that increasing CRC screening rates and access to preventative services for Black Americans would lessen the racial disparity in CRC risk.
Although the majority of the cohort is of low SES, the Black participants have lower household income, less educational attainment, and more often live in areas with lower area-level SES than the white participants. Low SES may influence health outcomes through less access to medical care, social support, and financial resources, including resources to buy and access nutritious foods. (21) Additionally, unfamiliarity with recommendations for health behaviors may keep individuals from participating in CRC screening and performing healthy behaviors. In support of that theory, previous studies suggest that the association between education and CRC risk is reflective of differences in health behaviors and CRC screening. (22,23) Our study data also supports this theory in that we observed evidence that greater education attainment is associated with a reduction in CRC in analyses including all participants, and analyses restricted to Black participants or participants that had ever been screening for CRC. Other determinants that may cause Black Americans to be at increased CRC risk are increased levels of stress, and the effects of discrimination due to systematic racism, such as lower likelihood of receiving a physician recommendation for CRC screening in comparison to white patients. (24–26)
Whereas individual health behaviors are not strongly associated with CRC incidence in the SCCS, healthy lifestyle taking into account overall adherence to several health behaviors is associated with lower risk of CRC. For instance, adhering to 3 or more health behaviors of non-smoking, moderate alcohol intake, high diet quality and physical activity was associated with a 19% (95%CI:0%,34%) decrease in CRC risk. The association does not vary by sex or race. The lower risk associated with healthy lifestyle is of similar magnitude in association as attaining a high school education which is associated with a 19% (95%CI:2%,33%) decreased CRC risk.
Previous studies report mixed findings on the association between adherence to cancer prevention guidelines for healthy lifestyle and CRC incidence. Two previous studies find weak to null associations between adherence to guidelines set forth by World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and CRC among women (27,28). The WCRF/AICR guideline scores differ from the healthy lifestyle scores in current study in that WCRF/AICR guideline scores also incorporate avoiding adult weight gain as cancer prevention guideline. In two studies that reported analyses specific to Black participants, null associations are also reported (27,29). A noted limitation of the previous studies are the small sample size of Black cases. Additionally, these previous studies, as well as the current study, report low adherence to cancer prevention guidelines by participants. In other cohorts, (28,30–32) whose members are more often of European-ancestry and higher SES, healthy lifestyle has been consistently shown to reduce CRC risk, and the association was consistent across anatomic site. Additionally, data from postmenopausal women enrolled in the Women’s Health Initiative Observational Study find a strong inverse association between an ACS cancer prevention guidelines score and lower CRC risk, where participants that met the most ACS cancer prevention guidelines had a 52% lower risk of colorectal cancer (HR=0.48; 95% CI: 27,68%) compared to participants that met the fewest guidelines (32). The authors did not report race-specific associations. Our data support a role for healthy lifestyle in CRC prevention, however, the magnitude of association is smaller in our cohort that primarily includes Americans of low SES and who are Black.
Our study has limitations including the use of self-reported health behaviors which are susceptible to measurement error. Due to the prospective cohort study design, misclassification is expected to be non-differential and, if present, likely will attenuate study results. Additionally, we use exposure information collected at baseline and do not have pre-enrollment data on risk factors that may contribute to risk across the life course, such as diet and body weight. Importantly, our study also has a number of strengths. The SCCS is a large, prospective, cohort study with comprehensive information on sociocultural and lifestyle factors, and complete follow-up to identify incident CRC cases. The cohort includes underserved at-risk populations seldom included in large numbers in other investigations. The Southern Community Cohort Study is uniquely situated to identify exposures that influence CRC risk in African Americans of very low socioeconomic status, a population with one of the highest colorectal cancer incidence rates in the United States.
Conclusions and Public health significance.
Our study provides evidence that among individuals of low-SES, there are several factors important to reducing CRC risk including race, healthy lifestyle, education, CRC screening and health insurance coverage. The CRC risk-lowering benefits of adhering to a healthy lifestyle through health behaviors did not vary by race, sex or SES. Our findings suggest that CRC incidence will decrease through focused interventions aimed at increasing uptake and access to CRC screening, facilitating Americans’ adherence to maintaining a healthy lifestyle, and lessening the social determinants that uniquely harm Black Americans’ health outcomes.
Supplementary Material
Prevention Relevance Statement:
Colorectal cancer risk may be reduced through screening, higher educational attainment and performing more health behaviors. Importantly, our data show that CRC screening is an important CRC prevention strategy to eliminate the racial disparity in CRC risk.
Acknowledgements:
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R00 CA207848 and R01 CA255318 to S Warren Andersen); the University of Wisconsin-Madison, Office of Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation and the University of Wisconsin Carbone Cancer (grant number P30 CA014520 to HH Bailey which supports S Warren Andersen). The Southern Community Cohort Study (SCCS) is supported by the National Cancer Institute at the National Institutes of Health (grant numbers R01 CA92447, U01 CA202979 to WJ Blot), including special allocations from the American Recovery and Reinvestment Act (grant number 3R01 CA092447‐08S1 to WJ Blot). Data on SCCS cancer cases used in this publication were provided by the Alabama Statewide Cancer Registry; Kentucky Cancer Registry, Lexington, KY; Tennessee Department of Health, Office of Cancer Surveillance; Florida Cancer Data System; North Carolina Central Cancer Registry, North Carolina Division of Public Health; Georgia Comprehensive Cancer Registry; Louisiana Tumor Registry; Mississippi Cancer Registry; South Carolina Central Cancer Registry; Virginia Department of Health, Virginia Cancer Registry; Arkansas Department of Health, Cancer Registry, 4815 W. Markham, Little Rock, AR 72205. The Arkansas Central Cancer Registry is fully funded by a grant from the National Program of Cancer Registries, Centers for Disease Control and Prevention (CDC). Data on SCCS cancer cases from Mississippi were collected by the Mississippi Cancer Registry which participates in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC). Cancer data for SCCS cancer cases from West Virginia have been provided by the West Virginia Cancer Registry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the Mississippi Cancer Registry. The opinions expressed are those of the authors and do not necessarily represent those of the CDC or the West Virginia Cancer Registry.
Footnotes
Author Disclosures: The authors have no conflicts of interest.
References:
- 1.American Cancer Society. Colorectal Cancer Facts & Figures 2020–2022. Atlanta (GA): American Cancer Society; 2020. [Google Scholar]
- 2.Lee DH, Keum N, Giovannucci EL. Colorectal Cancer Epidemiology in the Nurses’ Health Study. Am J Public Health. 2016;106:1599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SS, Signorello LB, Cope EL, McLaughlin JK, Hargreaves MK, Zheng W, et al. Obesity and all-cause mortality among black adults and white adults. Am J Epidemiol. 2012;176:431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren Andersen S, Zheng W, Sonderman J, Shu X-O, Matthews CE, Yu D, et al. Combined Impact of Health Behaviors on Mortality in Low-Income Americans. Am J Prev Med. 2016;51:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved. 2010;21:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren Andersen S, Blot WJ, Shu X-O, Sonderman JS, Steinwandel M, Hargreaves MK, et al. Associations Between Neighborhood Environment, Health Behaviors, and Mortality. Am J Prev Med. 2018;54:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Signorello LB, Cohen SS, Williams DR, Munro HM, Hargreaves MK, Blot WJ. Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104:e98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- 10.Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70:245–71. [DOI] [PubMed] [Google Scholar]
- 11.Signorello LB, Munro HM, Buchowski MS, Schlundt DG, Cohen SS, Hargreaves MK, et al. Estimating nutrient intake from a food frequency questionnaire: incorporating the elements of race and geographic region. Am J Epidemiol. 2009;170:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Signorello LB, Buchowski MS, Cai Q, Munro HM, Hargreaves MK, Blot WJ. Biochemical validation of food frequency questionnaire-estimated carotenoid, alpha-tocopherol, and folate intakes among African Americans and non-Hispanic Whites in the Southern Community Cohort Study. Am J Epidemiol. 2010;171:488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at DietaryGuidelines.gov. [Google Scholar]
- 14.Warren Andersen S, Blot WJ, Lipworth L, Steinwandel M, Murff HJ, Zheng W. Association of Race and Socioeconomic Status With Colorectal Cancer Screening, Colorectal Cancer Risk, and Mortality in Southern US Adults. JAMA Netw Open. 2019;2:e1917995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franks P, Muennig P, Lubetkin E, Jia H. The burden of disease associated with being African-American in the United States and the contribution of socio-economic status. Soc Sci Med 1982. 2006;62:2469–78. [DOI] [PubMed] [Google Scholar]
- 16.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grubbs SS, Polite BN, Carney J, Bowser W, Rogers J, Katurakes N, et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:1928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laiyemo AO, Doubeni C, Pinsky PF, Doria-Rose VP, Bresalier R, Lamerato LE, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta (GA): American Cancer Society; 2017. [Google Scholar]
- 20.Doubeni CA, Corley DA, Zauber AG. Colorectal Cancer Health Disparities and the Role of US Law and Health Policy. Gastroenterology. 2016;150:1052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelan JC, Link BG, Diez-Roux A, Kawachi I, Levin B. “Fundamental causes” of social inequalities in mortality: a test of the theory. J Health Soc Behav. 2004;45:265–85. [DOI] [PubMed] [Google Scholar]
- 22.Mouw T, Koster A, Wright ME, Blank MM, Moore SC, Hollenbeck A, et al. Education and risk of cancer in a large cohort of men and women in the United States. PloS One. 2008;3:e3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104:1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. 1999;896:131–44. [DOI] [PubMed] [Google Scholar]
- 25.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135:531–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman Wallace DA, Baltrus PT, Wallace TC, Blumenthal DS, Rust GS. Black White Disparities in Receiving a Physician Recommendation for Colorectal Cancer Screening and Reasons for not Undergoing Screening. J Health Care Poor Underserved. 2013;24:1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura SJO, Dash C, Rosenberg L, Yu J, Palmer JR, Adams-Campbell LL. Is adherence to diet, physical activity, and body weight cancer prevention recommendations associated with colorectal cancer incidence in African American women? Cancer Causes Control CCC. 2016;27:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petimar J, Smith-Warner SA, Rosner B, Chan AT, Giovannucci EL, Tabung FK. Adherence to the World Cancer Research Fund/American Institute for Cancer Research 2018 Recommendations for Cancer Prevention and Risk of Colorectal Cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2019;28:1469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onyeaghala G, Lintelmann AK, Joshu CE, Lutsey PL, Folsom AR, Robien K, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention guidelines and colorectal cancer incidence among African Americans and whites: The Atherosclerosis Risk in Communities study. Cancer. 2020;126:1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aleksandrova K, Pischon T, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Norat T, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romaguera D, Vergnaud A-C, Peeters PH, van Gils CH, Chan DSM, Ferrari P, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96:150–63. [DOI] [PubMed] [Google Scholar]
- 32.Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women’s health initiative. Cancer Prev Res Phila Pa. 2014;7:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available for qualified investigators and can be requested via: southerncommunitystudy.org/


