Abstract
Psychiatric polygenic risk scores (PRS) have potential utility in psychiatric care and prevention, but there are concerns about their implementation. We surveyed 960 US-based practicing child and adolescent psychiatrists' (CAP) about their experiences, perspectives, and potential uses of psychiatric PRS. While 23% of CAP reported that they had never heard of PRS, 10 % of respondents have had a patient/family bring PRS to them and 4% have generated PRS for patients. Though 25% stated they would request PRS if a patient/caregiver asked, 35% indicated that nothing would prompt them to request PRS. Most respondents (54%) believed psychiatric PRS are currently at least slightly useful and 87% believed they will be so in 5 years. More than 70% indicated they would take action in response to a child with a top fifth percentile psychiatric PRS but no diagnosis: 48% would increase monitoring of symptoms, 42% would evaluate for current symptoms, and 4% would prescribe medications. Yet, most respondents were concerned that high-PRS results could lead to overtreatment and negatively impact patients' emotional well-being. Findings indicate emerging use of psychiatric PRS within child and adolescent psychiatry in the US. It is critical to examine the ethical and clinical challenges that PRS may generate and begin efforts to promote their informed and responsible use.
Keywords: genetics, pediatrics, pharmacotherapy, polygenic risk scores, psychiatric practice
1 ∣. INTRODUCTION
There have been recent advances in identifying genomic loci associated with a number of psychiatric conditions, including autism spectrum disorder, attention deficit hyperactivity disorder, schizophrenia, depression, and bipolar disorder (Demontis et al., 2019; Howard et al., 2019; Lam et al., 2019; Ripke et al., 2014; Satterstrom et al., 2019; Stahl et al., 2019; Sullivan et al., 2018). The identification of these genomic loci has made it increasingly possible to generate polygenic risk scores (PRS), which are an estimate of a person's relative genetic susceptibility to a particular disorder that considers all risk variants present in the person's DNA, weighted by the strength of each variant's ostensible association with the disorder in question. PRS can then be used to stratify individuals by risk compared to the general population (Wray et al., 2021).
Psychiatric PRS, most likely in combination with other predictors, have potential utility in psychiatric care and prevention. The usual age of onset for many psychiatric conditions is during childhood, adolescence, or early adulthood (Kessler et al., 2007), and as many as 20% of children and adolescents in the US have a diagnosable psychiatric disorder (CDC, n.d.). Yet, diagnosis is often delayed, inaccurate, or does not take place, compounding impairment. As prediction tools, psychiatric PRS would be most useful before disorder onset. Initial studies suggest that psychiatric PRS-based estimates are promising. For example, current estimates are that an individual at the 99th PRS percentile for schizophrenia has an approximately 6% lifetime chance of developing this disorder, while someone at the 99th PRS percentile for depression has an approximately 30% chance of developing depression.”(Agerbo et al., 2021; Murray et al., 2021; Schaefer, Mendelsohn, Practice, & Committee, 2013). If utilized appropriately (e.g., ensuring any delivery of genetic test results is provided in the context of evidence-based psychiatric genetic counseling [J. C. Austin, 2020]), identifying those at increased risk could allow for more refined resource allocation toward those with the highest risk and potentially decrease the duration of untreated disorders, which is associated with improved clinical outcomes (Dagani et al., 2017; Ghio et al., 2015).
Despite the potential for PRS to improve outcomes for those at risk, concerns about potential harms of psychiatric genetic testing in children have also been raised (Manzini & Vears, 2018). Positive test results could lead to stigmatization, resulting in disruptions in familial or social relationships (Horstkötter, Berghmans, de Ruiter, Krumeich, & de Wert, 2012). For example, parents could potentially lower the degree to which they support their child's educational, employment, or social aspirations (Corcoran, Malaspina, & Hercher, 2005). Stigmatization may also influence the formation of a child's developing identity (Laegsgaard & Mors, 2008) or minimize a child's expectations for their future (Corcoran et al., 2005). Finally, positive results may trigger anxiety that could lead to the development of other psychiatric issues (Dar-Nimrod, Zuckerman, & Duberstein, 2013; Lebowitz, 2019; Wilhelm et al., 2009).
Thus, critical clinical, ethical, and policy questions remain. For example, which children and adolescents, if any, should be tested and when, if at all, should children and adolescents be tested? In addition, the utility of PRS and how PRS should be used continue to be debated (Curtis, 2019; Lázaro-Muñoz, Torgerson, & Pereira, 2021; Murray et al., 2021; Palk, Dalvie, de Vries, Martin, & Stein, 2019; Zheutlin & Ross, 2018). Given the position of child and adolescent psychiatrists (CAP) as key stakeholders in the implementation of psychiatric PRS, it is critical to understand their perspectives toward use of PRS for psychiatric conditions in this population. Here we report CAP knowledge and experience with PRS, their perceived utility of PRS compared to other psychiatric genetic testing, their concerns about how psychiatric PRS could be used in child and adolescent psychiatry, how they anticipate they would respond to PRS results in their clinical practice, and their perception of the appropriateness of using psychiatric PRS for screening purposes. This study helps inform the clinical, ethical, and policy challenges raised by the use of PRS for psychiatric conditions.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Sampling
Survey participants were recruited from publicly available listservs, professional organizations, national conferences, and professional meetings. Web searches were conducted to identify other publicly available contact information for CAP including social media, journal publications, and academic department websites. The survey was electronically distributed with two reminder emails over a four-week period in June 2020. Participants were compensated with a $10 gift card of their choice. The study was approved by the Baylor College of Medicine Institutional Review Board (protocol number H-46219), and informed consent was obtained from all participants.
2.2 ∣. Survey
A survey was developed to assess CAP current practice, knowledge, and perceptions toward genetic testing and was divided into three sections including general genetic testing, pharmacogenomics, and PRS. The 47-question survey (see supplementary materials) was developed based on current literature with input from an expert panel consisting of CAP, psychologists, genetic counselors, bioethicists, lawyers, and an anthropologist using a modified Delphi method (Linstone & Turoff, 1975). Initial items were generated predominantly based on extant literature on psychiatrists' views of genetic testing by members of the research team with experience in survey design. The draft items were then reviewed by our expert panel, who were asked to comment specifically on content validity, comprehensiveness, overlap with other constructs, and readability and clarity. Responses were analyzed and incorporated into revised versions for rereview. The process was repeated until consensus was met for survey content. Questions specific to PRS were used to learn about CAP knowledge, experience, current and potential utility, concerns, and appropriateness of PRS screening, the results of which we present here (see Soda et al., 2021 for results on knowledge and perceptions of utility of genetic testing in the evaluation of autism spectrum disorder). If participants selected “I have never heard of PRS” as the response to the initial self-reported knowledge of PRS question, display logic was used to skip participants to the next section without asking additional PRS questions. The entire survey took approximately 15–20 min to complete and was administered in English using Qualtrics.
2.2.1 ∣. Self-rated knowledge of PRS
Respondents rated their knowledge of PRS by selecting one of the following response options: I have never heard of PRS, very poor, poor, good, or very good.
2.2.2 ∣. Experience
Respondents reported their experience with PRS in their clinical practice in four yes/no questions.
2.2.3 ∣. PRS result graph interpretation
Respondents were presented with an example PRS result graph from impute.me (Impute.me, n.d.; Folkersen et al., 2020) and responded to five statements about what the graph depicted for the individual's risk of developing the condition (Peck, Borle, Folkersen, & Austin, 2021). Response options included Agree, Disagree, and Unsure.
2.2.4 ∣. Potential use of PRS
Respondents selected all applicable items among a list of 11 options about what would prompt them to request or generate a patient's psychiatric PRS. They then indicated what they would do if a child or adolescent with no current psychiatric diagnosis had a high PRS for a psychiatric disorder by selecting all that applied among 13 items. Throughout the survey, “high PRS” was defined as “top 5%.”
2.2.5 ∣. Concerns
Respondents indicated their level of concern about five potential negative outcomes of having a high (top 5%) psychiatric PRS result on a four-point scale with the descriptors: not at all concerned, slightly concerned, somewhat concerned, and very concerned.
2.2.6 ∣. Appropriateness of screening
Respondents indicated the degree of appropriateness to use PRS to screen in five groups and in two contexts on a four-point scale with the following descriptors: very inappropriate, inappropriate, appropriate, and very appropriate.
2.2.7 ∣. Perceived utility
Respondents rated how useful they thought PRS are in child and adolescent psychiatry now and 5 years from now on a four-point scale with the descriptors: not at all useful, slightly useful, moderately useful, and very useful.
2.3 ∣. Analysis
Categorical variables were analyzed using chi-square and logistic regression. When cell sizes were small, Fisher's Exact Test was used to compare the categorical groups. For comparisons of the current or future utility of PRS, McNemar change tests were computed. Finally, associations between variables were examined using correlations.
3 ∣. RESULTS
3.1 ∣. Participant characteristics
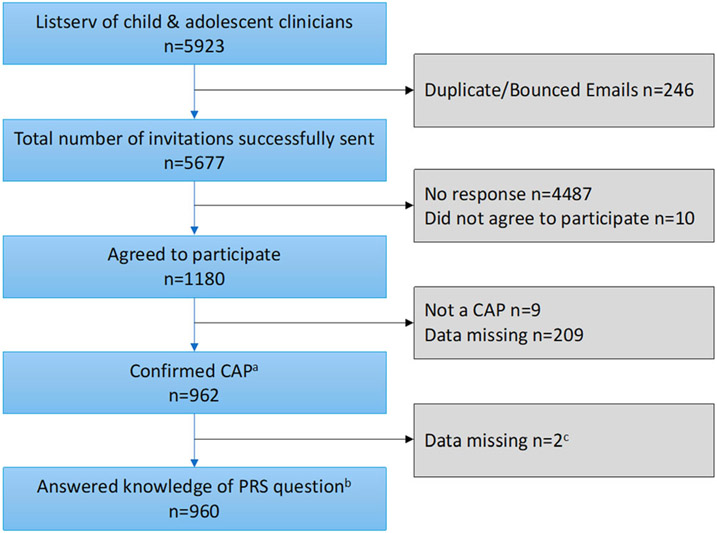
The survey was electronically distributed to 5,677 participants via Qualtrics. Of the 1,180 (20.8%) who agreed to participate, 962 CAP completed the entire survey for an overall 16.9% completion rate, reflecting approximately 11.6% of CAP in current practice in the US (Soda et al., 2021). We then excluded two CAP who did not answer the self-rated knowledge question that determined whether they received the PRS section of the survey, for a final total of 960 respondents reported here (Figure 1).
FIGURE 1.
Study flow. aConfirmed training in child and adolescent psychiatry. bQuestion asked respondents to rate their knowledge of polygenic risk scores (PRS) with five response options: very good, good, poor, very poor, I have never heard of PRS. cWe excluded n = 2 who did not answer the knowledge question because that question determined whether they received questions about PRS.
Participant characteristics for all 960 CAP are shown in Table 1. Among the CAP, 23.4% (n = 225) indicated they had never heard of PRS. There were no differences in awareness of PRS as a function of gender, ethnicity, number of years of clinical practice, percentage of patients with ASD or IDD, or average age of patients. Lack of awareness of PRS was, however, associated with practice setting; 84.5% of CAP who reported that one of their practice settings was at a university medical center (UMC) were aware of PRS (OR = 1.89, 95% CI = 1.25–2.84, p = .003), as compared to persons who were not at a UMC (74.4%). Fewer CAP in private practice settings were aware of PRS (70.1%), as compared to those who did not list an affiliation with a private practice (80.5%; OR = .57, 95% CI = .42–.77, p < .001). CAP who reported awareness of PRS (n = 735) were then asked additional questions regarding knowledge, experience, perceived utility of, concerns about, and potential uses of this information in clinical practice and screening.
TABLE 1.
CAPG participant characteristics
| Participant characteristics | Total sample | Heard of PRS | Not heard of PRS | Statistical comparison |
|---|---|---|---|---|
| Gender (n = 956) | ||||
| Female | 476 (50) | 350 (74) | 125 (56) | χ2 (4) = 6.23, p = .183 |
| Male | 454 (47) | 361 (80) | 92 (41) | |
| Prefer not to say | 26 (3) | 20 (77) | 6 (3) | |
| Other | 1 (<1) | 1 (<1) | 0 (0) | |
| Trans male | 1 (<1) | 1 (<1) | (0) | |
| Ethnicity (n = 955)a | ||||
| White/European American | 665 (70) | 506 (69) | 159 (71) | χ2 (9) = 7.59, p = .576 |
| Asian | 95 (10) | 75 (10) | 20 (9) | |
| Prefer not to say | 54 (5) | 46 (6) | 8 (3) | |
| Mixed ethnicity/race | 33 (4) | 25 (3) | 8 (3) | |
| Black/African American | 37 (4) | 25 (3) | 12 (5) | |
| Hispanic/Latinx | 36 (4) | 25 (3) | 11 (5) | |
| Other | 17 (2) | 14 (2) | 3 (1) | |
| Middle Eastern/Mediterranean | 13 (1) | 11 (1) | 2 (<1) | |
| American Indian/native American | 4 (<1) | 4 (<1) | 0 (0) | |
| Native Hawaiian/Pacific Islander | 1 (<1) | 1 (<1) | 0 (0) | |
| Number years in clinical Practice as CAP (n = 960) | ||||
| 16+ years: | 532 (55) | 420 (57) | 112 (50) | χ2 (5) = 7.27, p = .201 |
| 6–10 years: | 179 (18) | 136 (18) | 43 (19) | |
| 11–15 years: | 167 (17) | 116 (16) | 51 (23) | |
| 1–5 years: | 61 (6) | 47 (6) | 14 (6) | |
| CAP fellow: | 19 (2) | 15 (2) | 4 (2) | |
| Resident: | 2 (<1) | 1 (<1) | 1 (< 1) | |
| % patients with ASD or IDD (n = 835) | ||||
| None or N/A | 33 (4) | 25 (4) | 8 (4) | χ2 (4) = 5.42, p = .248 |
| 1–25% | 655 (79) | 486 (77) | 169 (83) | |
| 26–50% | 101 (12) | 81 (13) | 20 (10) | |
| 51–75% | 10 (<1) | 8 (1) | 2 (1) | |
| 75–100% | 36 (4) | 32 (5) | 4 (2) | |
| Average % of patients (n = 960) | ||||
| Under the age of 12 | 28% | 28% | 28% | F (1, 957) = 0.00, p = .962 |
| Ages 12–17 | 38% | 38% | 39% | F (1, 957) = 1.46, p = .227 |
| Ages 18 and up | 25% | 24% | 27% | F (1, 957) = 2.42, p = .119 |
| Main practice settinga (n = 955) | ||||
| Private practice | 381 | 270 (71) | 111 (29) | χ2 (1) = 11.42, p < .001 |
| Clinic | 267 | 217 (81) | 50 (19) | χ2 (1) = 4.57, p = .033 |
| Hospital | 132 | 106 (80) | 26 (20) | χ2 (1) = 1.19, p = .275 |
| University medical center | 227 | 192 (85) | 35 (15) | χ2 (1) = 10.65, p = .001 |
| Community agency | 114 | 86 (75) | 28 (25) | χ2 (1) = 0.09, p = 763 |
| Psychiatric hospital | 83 | 65 (78) | 18 (22) | χ2 (1) = 0.16, p = .694 |
| Other | 71 | 52 (73) | 19 (27) | χ2 (1) = 0.47, p = .492 |
| Government | 31 | 23 (74) | 8 (26) | χ2 (1) = 0.10, p = .752 |
| Retired | 25 | 22 (88) | 3 (12) | χ2 (1) = 1.87, p = .171 |
| Emergency room | 20 | 17 (85) | 3 (15) | χ2 (1) = 0.81, p = .368 |
| Military (1,361 total practice settings) | 10 | 5 (50) | 5 (50) | χ2 (1) = 3.97, p = .046 |
Note: Sample sizes for individual analyses varied slightly due to missing data.
Abbreviation: PRS, polygenic risk scores.
Respondents selected all that apply.
3.2 ∣. Self-rated knowledge & PRS result interpretation
Among CAP who reported awareness of PRS, over three-quarters (77%) rated their knowledge about PRS as poor (50.1%) or very poor (26.9%), while the remaining 23% rated their knowledge as good (19.6%) or very good (3.4%). Practice site was related to level of self-reported knowledge, with almost one-third (32.6%) of CAP in UMC settings reporting good or very good knowledge of PRS, as compared to only 20% of respondents in non-UMC settings (OR = 1.93, 95% CI = 1.32–2.82, p < .001). There was no significant association (OR = .78, 95% CI = .54–1.13, p = .186) between CAP in private practice settings who reported good to very good knowledge of PRS (27%) as compared to CAP who were not in a private practice setting (24.5%) Finally, ratings of good or very good knowledge were not related to length of time in practice (OR = .88, 95% CI = .60–1.29, p = .522) when comparing persons with <11 years of practice (24.6%) to persons with ≥11 years of experience (22.3%). Only 26.8% (n = 177) of participants responded correctly to all five statements when interpreting the example PRS result graph from impute.me. The average number of items correct among all CAP was 3.45 (SD = 1.57), and the average number of items correct among those who got one or more question wrong was 2.88 (SD = 1.47). There was a weak correlation between self-rated knowledge and PRS result graph interpretation scores, r = 0.16 (p < .001), with better self-rated knowledge associated with higher graph interpretation scores.
3.3 ∣. Experience
Despite their reported poor knowledge about PRS, some respondents are beginning to see PRS in their clinical practice, with 10.1% (n = 74) indicating that they had a patient or a family bring them PRS that they had obtained without the clinician's involvement, and 4.1% reported they recommended testing for psychiatric PRS in the previous 12 months. We observed a weak correlation between recommending testing for psychiatric PRS and better self-rated knowledge, r = .17 (p < .001). There was no correlation between recommending testing for psychiatric PRS and years of experience or practice setting (all p > .05). Additionally, 4.1% of respondents reported that they had requested or generated psychiatric PRS for a patient. When asked how those PRS were generated, 40% reported they were obtained from a research study, 28.8% from a direct-to-consumer provider, 11.4% from an online tool (e.g., impute.me), and 14.3% indicated they did not know how the PRS were generated. Seven percent of respondents also reported that a patient or their parent or guardian had asked them about psychiatric PRS in the previous 12 months.
3.4 ∣. Perceived utility and Concerns
More than half of respondents thought that PRS have some utility in child and adolescent psychiatry, with 54.1% indicating they thought PRS were currently at least slightly useful (49% slightly useful, 4% moderately useful, 1% very useful). They also believed that PRS will become more useful in the future, with significantly more respondents (86.7%) noting that psychiatric PRS will be at least slightly useful in child and adolescent psychiatry in 5 years (X2 [1] = 220.16, p < .001; 54% slightly useful, 29% moderately useful, 4% very useful).
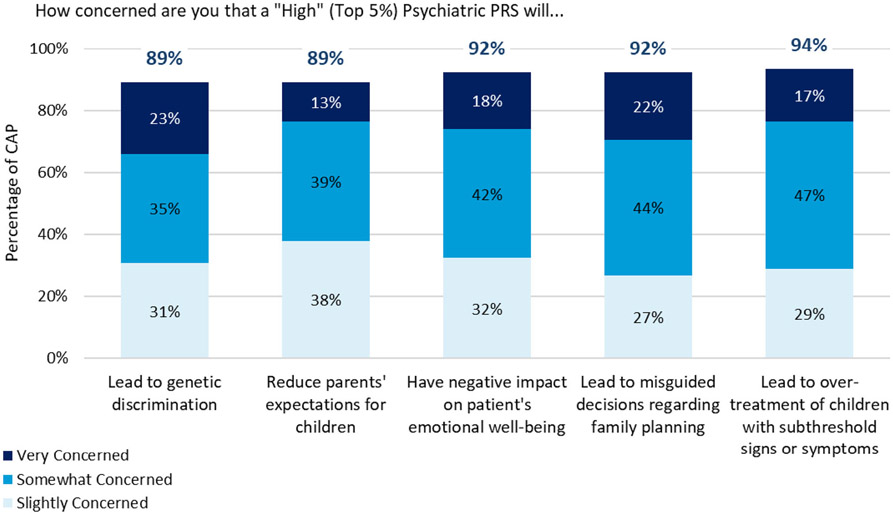
Respondents, however, also endorsed several concerns that PRS could lead to negative outcomes (Figure 2). A strong majority of respondents indicated they were at least slightly concerned about all listed potential negative outcomes (>89% of respondents for each), with the largest number of respondents choosing “somewhat concerned” for each.
FIGURE 2.
Percentage of CAP who endorsed any level of concern that a “high” (top 5%) psychiatric polygenic risk scores (PRS) could cause each outcome. Respondents rated their level of concern on a 4-point scale: not at all concerned. Slightly concerned. Somewhat concerned. Very concerned
3.5 ∣. CAP potential use of PRS
A third of respondents (34.6%) indicated that nothing would prompt them to request or generate a patient's psychiatric PRS, while a number of participants endorsed several motivations: 24.9% reported that they would request or generate a patient's psychiatric PRS if requested by a patient or parent/guardian, 20.5% in response to refractory symptoms, 20.6% for diagnostic clarification, 15.1% to assess risk for a psychiatric condition when a patient has signs or symptoms, 13.6% to assess risk for a psychiatric condition when an asymptomatic patient has a family history, and 12.5% for medication side effects.
CAP were asked what they would do in response to receiving a high (top fifth percentile) psychiatric PRS result for a child or adolescent with no current psychiatric diagnosis. More than 70% (71.2%) reported that they would take some action with the patient and/or family in response to the high PRS: 47.5% would increase monitoring of symptoms, 42.1% would evaluate the patient for symptoms related to psychiatric disorders, 17.6% would recommend modifying aspects of the child or adolescent's life to decrease stress, 17.3% would refer the adolescent or child to a genetic specialist, 14.6% would recommend lifestyle changes, 13.1% would recommend modifying parenting styles or practices, 5% would have first degree relatives tested, 4.5% would recommend psychotherapy, and 3.9% would prescribe medications to help decrease risk. Twenty-nine percent endorsed only items that did not include taking a specific action with the patient or family, including noting they would use online resources to learn more, request a consult from a genetic specialist, and/or do nothing.
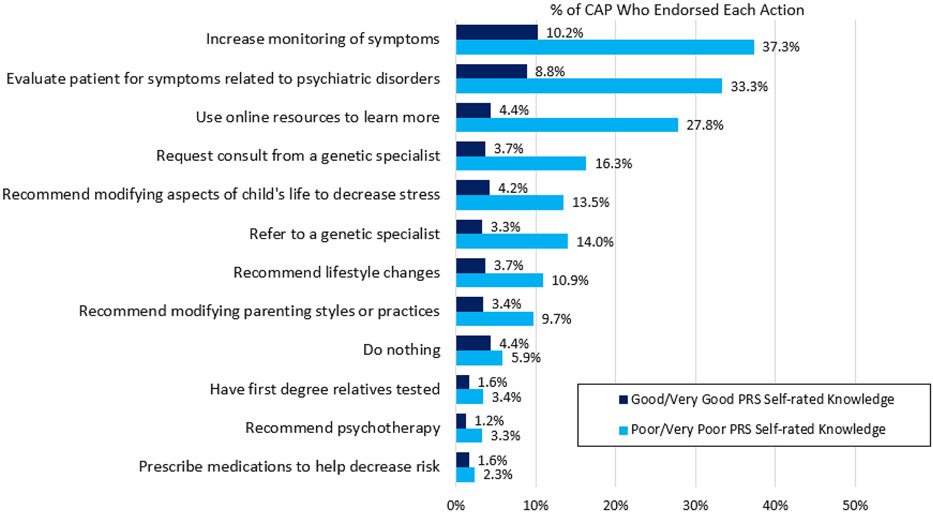
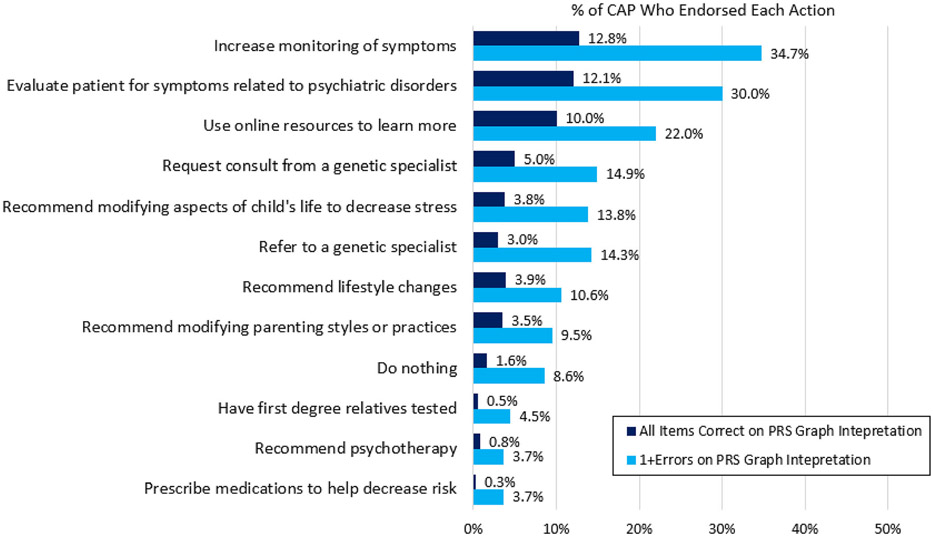
We also examined the differences in these endorsed actions between those who self-rated their knowledge about PRS as good or very good vs. poor or very poor (Figure 3), and between those who answered all items on the PRS graph interpretation measure correctly vs. those who answered one or more questions incorrectly (Figure 4). Respondents with self-reported good to very good knowledge about PRS were more likely to report they would prescribe medication to reduce risk (OR = 2.46, 95% CI = 1.16–5.28, p = .019) or would do nothing (OR = 2.84, 95% CI = 1.73–4.66, p < .001). Respondents with self-reported good to very good knowledge about PRS were less likely to report they would use online resources to learn more (OR = .41, 95% CI = .27–.63, p < .001). Respondents who answered all items correctly on the PRS graph interpretation measure were more likely to report that they would evaluate the patient for symptoms related to psychiatric disorders (OR = 1.55, 95% CI = 1.10–2.18, p = .011) and use online resources to learn more (OR = 1.77, 95% CI = 1.24–2.51, p = .002), and were less likely to report they would prescribe medications to help decrease risk (OR = 0.23, 95% CI = 0.05–0.96, p = .044).
FIGURE 3.
P endorsed hypothetical actions in response to a high (top 5%) psychiatric polygenic risk scores (PRS) in a child or adolescent with no current psychiatric diagnosis by self-rated knowledge. Respondents chose all that applied
FIGURE 4.
CAP endorsed hypothetical actions in response to a high (top 5%) psychiatric polygenic risk scores (PRS) in a child or adolescent with no current psychiatric diagnosis by PRS graph interpretation. respondents chose all that applied
3.6 ∣. CAP attitudes toward PRS testing and psychiatric PRS screening
We assessed CAP's attitudes toward using PRS testing in a range of patients and family members for whom psychiatric PRS could potentially be used in the future. About half of respondents felt that it was appropriate or very appropriate to screen first degree relatives of patients diagnosed with a psychiatric disorder and a high (top fifth percentile) psychiatric PRS (58.6% of respondents), and first episode patients (48.3%). More than a third felt it was appropriate or very appropriate to screen first degree relatives of patients diagnosed with a psychiatric disorder (36.48%) and children or adolescents with subthreshold signs or symptoms of a disorder (34.6%). Finally, 24.8% felt it was appropriate or very appropriate to screen first-degree relatives of an asymptomatic patient with a high PRS. There were no group differences in responses about the appropriateness of PRS testing between those who self-rated their knowledge of PRS as good or very good vs. poor or very poor.
Finally, CAP were largely unsupportive of using PRS testing to screen for risk of psychiatric conditions in other contexts, with 90.3% of respondents indicating it was inappropriate or very inappropriate to screen embryos for psychiatric PRS, and 95.7% indicating it was inappropriate or very inappropriate to screen the general population for psychiatric PRS.
4 ∣. DISCUSSION
In this study of CAP' knowledge, experience, and attitudes toward the use of PRS, we found that some respondents have already encountered PRS in their clinical practice. Further, most respondents thought that PRS already have some utility and anticipated PRS will have greater utility in child and adolescent psychiatry in the future. Many also reported that a number of scenarios would prompt them to generate a patient's psychiatric PRS, and many would take actions in response to an undiagnosed child or adolescent's high-psychiatric PRS. While respondents also endorsed multiple concerns and a general low level of knowledge of PRS, these results suggest that CAP may be open to clinical implementation of psychiatric PRS. On the other hand, nearly a quarter of the sample reported that they had never heard of PRS, and 35% reported that nothing would prompt them to generate or request PRS. These results highlight how knowledge and use of psychiatric PRS in the clinical setting is truly in flux.
Though few respondents have already generated, requested, or recommended testing for psychiatric PRS for a patient, some CAP reported that patients and families are beginning to ask about PRS. With increasing interest around PRS in both academic and popular literature (McKay, n.d.; Robbins, Garde, & Feuerstein, 2018), it is likely CAP will soon see more patients and families asking about PRS. Given that a quarter of our sample reported they would request or generate a patient's psychiatric PRS if asked to do so by a patient or their parent/guardian, some CAP may be implementing psychiatric PRS into their clinical practice in the not-too-distant future. This raises concerns, as there is currently no indication for using psychiatric PRS in the clinical context, nor are PRS part of recommended practice. Further, even if they do not generate PRS or recommend the testing themselves, it is now relatively simple to obtain PRS online using raw data from direct-to-consumer genetic testing (i.e., by uploading raw data to websites like impute.me) (Impute.me, n.d.; Folkersen et al., 2020). Accordingly, some respondents reported that they have had patients or their families bring PRS to them, some of which they reported were generated online.
Respondents also indicated that they would take a number of actions in response to a high PRS for a hypothetical child or adolescent who did not yet have a psychiatric diagnosis, yet the current reliability and predictive power of psychiatric PRS are modest and vary between disorders. Some of the actions endorsed by respondents may be reasonable, requesting a consult from a genetics colleague, evaluating the child for symptoms related to psychiatric disorders, or using online resources to learn more. Other actions, however, may raise concerns, including recommending modifying the child's life to decrease stress or modifying parent styles or practices, or prescribing medications to help decrease risk. Interestingly, the top concern among respondents was the risk of high PRS leading to overtreatment of children with subthreshold signs or symptoms. However, those who reported greater self-rated knowledge about PRS, and thus may feel more confident making clinical decisions based on PRS, were more likely to indicate they would recommend medications to decrease risk for children with no diagnosis but a high-psychiatric PRS. Interestingly, those who performed well on the PRS Graph Interpretation task were less likely to say they would prescribe medications. This suggests that these two groups—those with greater self-rated knowledge and those who interpreted the graph correctly—do not comprise the same people. Indeed, though there was a positive correlation between the two, it was a weak association.
Given respondents' overall self-reported low level of knowledge of PRS, poor performance interpreting an example PRS result graph, and the weak correlation between self-rated knowledge of PRS and performance interpreting the example PRS result graph, CAP endorsements of several actions that may be less appropriate indicate it is imperative that further research explore how these PRS are currently being used and best practices for implementation into child and adolescent psychiatry care. As substantiated by CAP-endorsed concerns around the use of PRS in the clinical setting, there are risks to using this information inappropriately. One could imagine some practices offering psychiatric PRS as the “latest and greatest” clinical test. If clinicians then misinterpret PRS or make clinical decisions based on poorly performing PRS, real harm could be done to patients. Our data highlight the largely untapped opportunity for interdisciplinary collaboration between psychiatrists and clinical genetics professionals in this area (J. Austin, Inglis, & Hadjipavlou, 2014) (genetic counselors in different geographical areas with various areas of expertise can be found using the www.findageneticcounselor.com tool). Importantly, genetic information has the potential for positive or negative reactions from patients and their families. Indeed, the manner in which this information is delivered is crucial in determining outcome (Ashtiani, Makela, Carrion, & Austin, 2014). A collaborative approach between psychiatry and clinical genetics would ensure the implementation of any genetic testing information in the context of evidence based psychiatric genetic counseling, which has been shown to result in positive patient outcomes (J. C. Austin, 2020).
Given that respondents did not rate the current utility of psychiatric PRS very high, it was surprising to see the level of support for PRS testing across a range of groups, including first episode patients and children or adolescents with subthreshold symptoms. Respondents, however, were far less supportive of using psychiatric PRS testing to screen the general population, or to screen embryos generated via in vitro fertilization cycles. As noted elsewhere (Lázaro-Muñoz, Pereira, Carmi, & Lencz, 2021), polygenic embryo screening amplifies a number of ethical and policy issues already present in screening embryos for monogenic conditions, and it also poses new challenges, including the ability to screen for risk for psychiatric conditions, as well as traits (e.g., height, intelligence) (Karavani et al., 2019; Lencz et al., 2021; Turley et al., 2021).
Our results should be interpreted within several limitations. Though we successfully surveyed 11.6% of CAP in the US, our sample may not be representative of the larger group, and it is possible there may be participation bias in our sample. For instance, those who had never heard of PRS may have been less likely to respond, or those with more favorable views toward PRS may have been more likely to participate. We did not collect data from those who did not respond to the survey, thus we are unable to assess whether respondents differed meaningfully from non-responders. A further limitation is the large number of statistical comparisons, without correction for multiple testing. The exploratory nature of understanding the perceptions of CAP regarding PRS will require confirmation in more representative samples. Finally, some questions, such as the self-rated knowledge question, may be prone to social desirability bias. Despite these limitations, this survey provides critical insight into CAP knowledge, experience, and attitudes toward the use of psychiatric PRS in child and adolescent psychiatry, and as such, it can inform needs for future research and best practices.
5 ∣. CONCLUSION
Given the rapidly emerging use of psychiatric PRS in different contexts, including child and adolescent psychiatry, and the growing numbers of samples of psychiatric GWAS, which will give rise to more reliable PRS for more psychiatric disorders, it is critical to begin educational efforts for clinicians and patients to examine the ethical and clinical challenges that PRS may generate, and for relevant professional organizations to develop practice recommendations to help guide clinicians as to how to use these tools. Our findings highlight some of the most pressing issues and underscore the urgency to take action.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Human Genome Research Institute and the National Institute of Mental Health of the National Institutes of Health, grants R00HG008689 (Lázaro-Muñoz), 3R00HG008689-05S1 (Lázaro-Muñoz, Storch), R01MH128676 (Pereira, Lázaro-Muñoz, Storch), and P50HD103555 (Storch) for use of the Clinical and Translational Core facilities. The views expressed are those of the authors alone, and do not necessarily reflect views of NIH, Baylor College of Medicine, or Harvard Medical School.
Footnotes
CONFLICT OF INTEREST
Dr. Pereira reports research grants from the NIH. Ms. Muñoz reports no biomedical financial interests or potential conflicts of interest. Dr. Small reports no biomedical financial interests or potential conflicts of interest. Dr. Soda reports research grants from the NIH and the Foundation of Hope for Research and Treatment of Mental Illness. Ms. Torgerson reports no biomedical financial interests or potential conflicts of interest. Ms. Sanchez reports no biomedical financial interests or potential conflicts of interest. Dr. Austin reports research grants (unrelated to this work) from the Canadian Institutes of Health Research, NIH, Pfizer, and Genome BC, and is supported by the Canada Research Chairs Program and BC mental health and substance use services. Dr. Storch receives research support from NIH, Texas Higher Education Coordinating Board, Houston Community Foundation and Ream Foundation. He receives book royalties from Elsevier, Wiley, Oxford, Springer, Jessica Kingsley, and American Psychological Association. He is a consultant for Biohaven Pharmaceuticals and owns stock in NView. Dr. Lázaro-Muñoz reports research grants from the NIH.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
De-identified survey data are available upon request from the corresponding author. Data will be made available to researchers whose proposed use of the data has been approved by the study team.
REFERENCES
- Agerbo E, Trabjerg BB, Børglum AD, Schork AJ, Vilhjálmsson BJ, Pedersen CB, … Musliner KL (2021). Risk of early-onset depression associated with polygenic liability, parental psychiatric history, and socioeconomic status. JAMA Psychiatry, 78(4), 387–397. 10.1001/jamapsychiatry.2020.4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtiani S, Makela N, Carrion P, & Austin J (2014). Parents' experiences of receiving their child's genetic diagnosis: A qualitative study to inform clinical genetics practice. American Journal of Medical Genetics. Part A, 164A(6), 1496–1502. 10.1002/ajmg.a.36525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J, Inglis A, & Hadjipavlou G (2014). Genetic counseling for common psychiatric disorders: An opportunity for interdisciplinary collaboration. The American Journal of Psychiatry, 171(5), 584–585. 10.1176/appi.ajp.2014.13101421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JC (2020). Evidence-based Genetic counseling for psychiatric disorders: A road map. Cold Spring Harbor Perspectives in Medicine, 10(6), a036608. 10.1101/cshperspect.a036608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (n.d.). Mental Health Surveillance Among Children—United States, 2005–2011. https://www.cdc.gov/mmwr/preview/mmwrhtml/su6202a1.htm [PubMed]
- Corcoran C, Malaspina D, & Hercher L (2005). Prodromal interventions for schizophrenia vulnerability: The risks of being “at risk.”. Schizophrenia Research, 73(2–3), 173–184. 10.1016/j.schres.2004.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D (2019). Clinical relevance of genome-wide polygenic score may be less than claimed. Annals of Human Genetics, 83(4), 274–277. 10.1111/ahg.12302 [DOI] [PubMed] [Google Scholar]
- Dagani J, Signorini G, Nielssen O, Bani M, Pastore A, de Girolamo G, & Large M (2017). Meta-analysis of the interval between the onset and management of bipolar disorder. Canadian Journal of Psychiatry Revue Canadienne De Psychiatrie, 62(4), 247–258. 10.1177/0706743716656607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar-Nimrod I, Zuckerman M, & Duberstein PR (2013). The effects of learning about one's own genetic susceptibility to alcoholism: A randomized experiment. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 15(2), 132–138. 10.1038/gim.2012.111 [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, … Neale BM (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics, 51(1), 63–75. 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkersen L, Pain O, Ingason A, Werge T, Lewis CM, & Austin J (2020). Impute.me: An open-source, non-profit tool for using data from direct-to-consumer Genetic testing to calculate and interpret polygenic risk scores. Frontiers in Genetics, 11, 578. 10.3389/fgene.2020.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio L, Gotelli S, Cervetti A, Respino M, Natta W, Marcenaro M, …Belvederi Murri M (2015). Duration of untreated depression influences clinical outcomes and disability. Journal of Affective Disorders, 175, 224–228. 10.1016/j.jad.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Horstkötter D, Berghmans R, de Ruiter C, Krumeich A, & de Wert G (2012). “We are also normal humans, you know?” views and attitudes of juvenile delinquents on antisocial behavior, neurobiology and prevention. International Journal of Law and Psychiatry, 35(4), 289–297. 10.1016/j.ijlp.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, … McIntosh AM (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience, 22(3), 343–352. 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impute.me. (n.d.). Next generation consumer genetic testing and analysis https://impute.me/
- Karavani E, Zuk O, Zeevi D, Barzilai N, Stefanis NC, Hatzimanolis A, … Carmi S (2019). Screening human embryos for polygenic traits has limited utility. Cell, 179(6), 1424–1435.e8. 10.1016/j.cell.2019.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, & Ustun TB (2007). Age of onset of mental disorders: A review of recent literature. Current Opinion in Psychiatry, 20(4), 359–364. 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laegsgaard MM, & Mors O (2008). Psychiatric genetic testing: Attitudes and intentions among future users and providers. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, 147(3), 375–384. 10.1002/ajmg.b.30609 [DOI] [PubMed] [Google Scholar]
- Lam M, Chen C-Y, Li Z, Martin AR, Bryois J, Ma X, … Huang H (2019). Comparative genetic architectures of schizophrenia in east Asian and European populations. Nature Genetics, 51(12), 1670–1678. 10.1038/s41588-019-0512-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro-Muñoz G, Pereira S, Carmi S, & Lencz T (2021). Screening embryos for polygenic conditions and traits: Ethical considerations for an emerging technology. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 23(3), 432–434. 10.1038/s41436-020-01019-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro-Muñoz G, Torgerson L, & Pereira S (2021). Return of results in a global survey of psychiatric genetics researchers: Practices, attitudes, and knowledge. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 23(2), 298–305. 10.1038/s41436-020-00986-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz MS (2019). The implications of Genetic and other biological explanations for thinking about mental disorders. The Hastings Center Report, 49(Suppl 1), S82–S87. 10.1002/hast.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Backenroth D, Granot-Hershkovitz E, Green A, Gettler K, Cho JH, … Carmi S (2021). Utility of polygenic embryo screening for disease depends on the selection strategy. eLife, 10, e64716. 10.7554/eLife.64716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstone H, & Turoff M (1975). The Delphi method: Techniques and applications. In Technometrics (Vol. 18). 10.2307/3150755 [DOI] [Google Scholar]
- Manzini A, & Vears DF (2018). Predictive psychiatric Genetic testing in minors: An exploration of the non-medical benefits. Journal of Bioethical Inquiry, 15(1), 111–120. 10.1007/s11673-017-9828-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay B (n.d.). Risk Scores Assess Ties Between Genes and Obesity, Disease—WSJ. https://www.wsj.com/articles/new-risk-scores-assess-ties-between-genes-and-obesity-disease-11555599600 [Google Scholar]
- Murray GK, Lin T, Austin J, McGrath JJ, Hickie IB, & Wray NR (2021). Could polygenic risk scores be useful in psychiatry?: A review. JAMA Psychiatry, 78(2), 210–219. 10.1001/jamapsychiatry.2020.3042 [DOI] [PubMed] [Google Scholar]
- Palk AC, Dalvie S, de Vries J, Martin AR, & Stein DJ (2019). Potential use of clinical polygenic risk scores in psychiatry—Ethical implications and communicating high polygenic risk. Philosophy, Ethics, and Humanities in Medicine: PEHM, 14(1), 4. 10.1186/s13010-019-0073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L, Borle K, Folkersen L, & Austin J (2021). Why do people seek out polygenic risk scores for complex disorders, and how do they understand and react to results? European Journal of Human Genetics, 30, 1–7. 10.1038/s41431-021-00929-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA, … Psychosis Endophenotypes International Consortium. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins R, Garde D, & Feuerstein A (2018). What's a polygenic risk score and how good is it at predicting disease? STAT https://www.statnews.com/2018/12/14/polygenic-risk-predict-disease/ [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, Rubeis SD, An J-Y, … Buxbaum JD (2019). Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell, 80(3), 568–584.e23. 10.1016/j.cell.2019.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GB, Mendelsohn NJ, Practice P, & Committee G (2013). Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 15(5), 399–407. 10.1038/gim.2013.32 [DOI] [PubMed] [Google Scholar]
- Soda T, Pereira S, Small BJ, Torgerson LN, Muñoz KA, Austin J, … Lázaro-Muñoz G (2021). Child and adolescent Psychiatrists' perceptions of utility and self-rated knowledge of Genetic testing predict usage for autism Spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 60(6), 657–660. 10.1016/j.jaac.2021.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, … Sklar P (2019). Genome-wide association study identifies 30 loci associated with bipolar disorder. Nature Genetics, 51(5), 793–803. 10.1038/s41588-019-0397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen G, … Psychiatric Genomics Consortium. (2018). Psychiatric genomics: An update and an agenda. The American Journal of Psychiatry, 175(1), 15–27. 10.1176/appi.ajp.2017.17030283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley P, Meyer MN, Wang N, Cesarini D, Hammonds E, Martin AR, … Visscher PM (2021). Problems with using polygenic scores to select embryos. New England Journal of Medicine, 385(1), 78–86. 10.1056/NEJMsr2105065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm K, Meiser B, Mitchell PB, Finch AW, Siegel JE, Parker G, & Schofield PR (2009). Issues concerning feedback about genetic testing and risk of depression. The British Journal of Psychiatry: the Journal of Mental Science, 194(5), 404–410. 10.1192/bjp.bp.107.047514 [DOI] [PubMed] [Google Scholar]
- Wray NR, Lin T, Austin J, McGrath JJ, Hickie IB, Murray GK, & Visscher PM (2021). From basic science to clinical application of polygenic risk scores: A primer. JAMA Psychiatry, 78(1), 101–109. 10.1001/jamapsychiatry.2020.3049 [DOI] [PubMed] [Google Scholar]
- Zheutlin AB, & Ross DA (2018). Polygenic risk scores: What are they good for? Biological Psychiatry, 83(11), e51–e53. 10.1016/j.biopsych.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified survey data are available upon request from the corresponding author. Data will be made available to researchers whose proposed use of the data has been approved by the study team.