Abstract
The aim of the present European Stroke Organisation guideline is to provide clinically useful evidence-based recommendations on the management of patients with intracranial atherosclerotic disease (ICAD). The guidelines were prepared following the Standard Operational Procedure of the European Stroke Organisation guidelines and according to GRADE methodology. ICAD represents a major cause of ischemic stroke worldwide, and patients affected by this condition are exposed to a high risk for future strokes and other major cardiovascular events, despite best medical therapy available. We identified 11 relevant clinical problems affecting ICAD patients and formulated the corresponding Population Intervention Comparator Outcomes (PICO) questions. The first two questions refer to the asymptomatic stage of the disease, which is being increasingly detected thanks to the routine use of noninvasive vascular imaging. We were not able to provide evidence-based recommendations regarding the optimal detection strategy and management of asymptomatic ICAD, and further research in the field is encouraged as subclinical ICAD may represent a big opportunity to improve primary stroke prevention. The second block of PICOs (3–5) is dedicated to the management of acute large vessel occlusion (LVO) ischemic stroke caused by ICAD, a clinical presentation of this disease that is becoming increasingly relevant and problematic, since it is associated with more refractory endovascular reperfusion procedures. An operational definition of probable ICAD-related LVO is proposed in the guideline. Despite the challenging context, no dedicated randomized clinical trials (RCTs) were identified, and therefore the guideline can only provide with suggestions derived from observational studies and our expert consensus, such as the escalated use of glycoprotein IIb-IIIa inhibitors and angioplasty/stenting in cases of refractory thrombectomies due to underlying ICAD. The last block of PICOs is devoted to the secondary prevention of patients with symptomatic ICAD. Moderate-level evidence was found to recommend against the use of oral anticoagulation as preferred antithrombotic drug, in favor of antiplatelets. Low-level evidence based our recommendation in favor of double antiplatelet as the antithrombotic treatment of choice in symptomatic ICAD patients, which we suggest to maintain during 90 days as per our expert consensus. Endovascular therapy with intracranial angioplasty and or stenting is not recommended as a treatment of first choice in high-grade symptomatic ICAD (moderate-level evidence). Regarding neurosurgical interventions, the available evidence does not support their use as front line therapies in patients with high-grade ICAD. There is not enough evidence as to provide any specific recommendation regarding the use of remote ischemic conditioning in ICAD patients, and further RCTs are needed to shed light on the utility of this promising therapy. Finally, we dedicate the last PICO to the importance of aggressive vascular risk factor management in ICAD, although the evidence derived from RCTs specifically addressing this question is still scarce.
Keywords: Intracranial atherosclerotic disease, intracranial artherosclerosis, stroke, guideline
Introduction
Intracranial atherosclerosis or intracranial atherosclerotic disease (ICAD), is a dynamic disease characterized by the development, progression, and complication of atherosclerotic plaques affecting major intracranial arteries. 1 ICAD may represent the most common cause of ischemic stroke in patients of Asian ancestry and is also very prevalent as a cause of stroke among Hispanics and Africans.2–4 Moreover, it may be responsible for up to 10% of ischemic stroke in Caucasians, 4 whereas population-based and necropsy studies suggest that intracranial atherosclerotic plaques could be more common in Caucasian patients.5–7 Therefore, considering the distribution of the world’s population, ICAD may represent a major cause of stroke and vascular cognitive impairment globally. 8 Besides its particular importance as a global health problem, other relevant arguments may justify dedicating a specific guideline on ICAD. First, it is an aggressive entity and patients affected by symptomatic ICAD are exposed to a very high risk of recurrent ischemic events, despite best medical therapy, as observed in the available randomized-controlled clinical trials. 9 Second, although research on ICAD is an evolving field, with relevant studies in the last years, substantial uncertainty remains as which are the best treatments for this disease, especially for high-risk patients. 10 Third, the rapidly increasing use of cerebral vascular imaging to guide therapeutic decisions in the hyperacute phase of stroke, is making ICAD recognition highly accessible for a growing number of patients. In this context, ICAS may emerge as either the potential cause of the ischemic event, or as a coexisting disease whose prognostic significance, both for the acute event and for the assessment of the patients risk of having future strokes, is still poorly understood. Fourth, intracranial arteries have differential anatomic characteristics, which may lead to important peculiarities of the atherosclerotic process affecting them, with potential clinical relevance, such as the hemodynamic impact of intracranial stenosis. 11 And finally, the appearance of vessel-wall imaging techniques has moved our traditional focus from the intracranial stenosis to the intracranial atherosclerotic plaque, 12 thus enabling us assess all the stages of ICAD, including early subclinical non-stenotic phases, which may create a significant opportunity to improve primary stroke prevention. 13
In this guideline, the term ICAD will refer to atherosclerotic plaques affecting major intracranial arteries in any stage of the disease, including non-stenotic ICAD, whereas we will use the term intracranial atherostenosis (ICAS) when the plaque causes a significant luminal narrowing which can be detected by angiographic techniques or transcranial Doppler ultrasound, usually higher than 50%. When the reduction in arterial caliber is severe (>70%) and/or is associated with hemodynamic compromise in its territory, we will talk about high-grade intracranial stenosis.
Hemodynamic compromise caused by a high-grade intracranial stenosis is defined by a significant reduction of anterograde flow in the downstream arterial territory, that prompts activation of collateral circulation aimed to sustain brain tissue perfusion and enhance embolic washout in distal arteries. 14 In case of severe hemodynamic compromise, collateral circulation is insufficient to compensate the decrease in anterograde blood flow caused by the intracranial stenosis, and as a result brain tissue perfusion can no longer be sustained and hypoperfusion can be detected on brain perfusion imaging techniques, which implies that the downstream brain tissue is at risk of infarction. Direct observation of reduced anterograde flow and its effect on brain perfusion depending on collateral compensation can be examined by transcranial Doppler ultrasound and by cerebral perfusion imaging techniques on computed tomography (CT) and magnetic resonance imaging (MRI). The clinical presentation can also suggest hemodynamic compromise, if the patient shows fluctuations in the intensity of neurological deficit after postural changes, postprandial or after blood pressure drops. The infarct pattern in neuroimaging is also an indirect indicator of hemodynamic compromise, when cortical or internal borderzone infarctions appear. We will use intracranial atherostenosis as a short term for intracranial stenosis of presumably atherosclerotic origin, since there are other causes of intracranial stenosis, such as partially recanalized embolic clots, intracranial arterial dissection, infectious and non-infectious vasculitis, reversible cerebral vasoconstriction, arterial vasospasm and others. Nevertheless, the scope of this guideline will be restricted to intracranial stenosis presumably caused by atherosclerosis. Given the diversity of entities capable of producing intracranial arterial lumen reduction, the diagnosis of atherosclerosis as the cause of intracranial stenosis represents a challenge in daily clinical practice, especially in the acute phase of ischemic stroke. First, a complete diagnostic workup to rule out extracranial sources of emboli is needed. Whereas transcranial Doppler ultrasound is useful in the initial detection of intracranial stenosis, confirmation by a noninvasive angiographic technique such as CT or MR angiography is suggested to rule out false positive stenosis. The presence of more than one focal intracranial stenosis affecting several intracranial arteries, in a patient with vascular risk factors for atherosclerosis, speaks in favor of ICAD as the cause of intracranial stenosis. The clinical context is of critical importance to perform a differential diagnosis with other intracranial arteriopathies, such as infectious or noninfectious vasculitis, intracranial dissection, reversible cerebral vasoconstriction, or arterial vasospasm. In this setting, high-resolution arterial-wall MRI is gaining importance in the characterization of intracranial atherosclerotic plaques and their distinction from other causes of intracranial stenosis15,16 Finally, we will categorize ICAD as symptomatic or asymptomatic, depending on whether a cerebral ischemic event can be attributed to the intracranial atherosclerotic plaque or not. Asymptomatic ICAD can be found in stroke-free individuals, but also in stroke patients in whom another known entity is acting as the probable cause of the acute cerebral ischemia, or also when ICAD is affecting several intracranial arteries, with coexistence of one symptomatic intracranial atherostenosis and one or more asymptomatic atherostenoses.
The aim of this guideline is to provide evidence-based recommendations to aid physicians in the treatment of patients with suspected ICAD. The guideline will focus not only on the secondary prevention of patients with symptomatic intracranial atherosclerotic stenosis, but will consider other important clinical and imaging presentations of the disease as well. In this regard, the guideline is divided into three main blocks: (1) management of asymptomatic ICAD, (2) treatment of acute intracranial large vessel occlusion (LVO) caused by ICAD, and (3) management of patients with symptomatic intracranial atherostenosis.
Methods
Composition and approval of the Module Working Group
These guidelines were initiated by the ESO. Two chairpersons (JA and MP) were selected to assemble and coordinate the Guideline Module Working Group (MWG). The final group contained 12 experts (JA, MP, ELC, MZ, AK, GMDM, EM, AK, JC, CK, DB, and PS). The MWG included eight neurologists (of whom one is also a neuroepidemiologist), three neuroradiologists, and one radiologist-epidemiologist; all 12 are experts in cerebrovascular disease with a special interest in intracranial atherosclerosis or stroke. Of the 12 MWG members, all were European. The ESO Guideline Board and Executive Committee reviewed the intellectual and financial disclosures of all MWG members and approved the composition of the group. All participants were asked to disclose any conflict of interest that could influence their participation. The group communicated using e-mail and virtual conferences. The full details of all MWG members and their disclosures is included in Supplemental materials.
Development and approval of clinical questions
This guideline was prepared according to the ESO standard operating procedures (SOP), 17 which are based on the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework. 18 The MWG developed a list of topics and corresponding questions of greatest clinical interest. Questions were formatted using the PICO approach (Population, Intervention, Comparator, and Outcome), and reviewed by two external reviewers as well as members of the ESO Guideline board and Executive Committee. The outcomes were rated by members of the MWG as: critical, important or of limited importance according to GRADE criteria. Final decision on outcomes used a Delphi approach. Results of the outcomes rating for each PICO question are included in the supplement (Supplemental Table 1).
According to GRADE, nine outcomes were considered to be of critical importance (mean score of 7–9): risk of major adverse cardiac events (MACE) including stroke, mortality, major bleeding (including symptomatic intracranial hemorrhage), good functional outcome (defined as a modified Rankin Scale (mRS) 0–2) at 90 days, recurrent ischemic stroke at 30 days, recurrent ischemic stroke at 90 days, long term recurrence of ischemic stroke (annual recurrence), iatrogenic complications (vessel rupture, dissection, etc.), and restenosis/reocclusion within 1 year.
Definition of ICAD
The diagnosis of ICAD is usually made in symptomatic patients after a transient ischemic attack (TIA) or stroke with neurosonological techniques, CT/MRI angiography or digital subtraction angiography (DSA). It can also be made in stroke-free individuals with vascular risk factors. DSA can be considered as the gold standard for the diagnosis of ICAD. High grade ICAD can be diagnosed if one (or both) of two criteria are present: Severity of the stenosis above 70% or hemodynamic compromise in its territory. Additionally, ICAD can be the underlying cause of large vessel occlusion in stroke patients undergoing mechanical thrombectomy. The diagnosis of ICAD in this population can be very challenging. In these patients, the diagnosis of ICAD can be suspected with high probability attending to the characteristics and behavior of the artery at the site of occlusion during thrombectomy maneuvers. Usually, a truncal-type occlusion is seen on the initial DSA series. Then, suboptimal arterial opening with residual stenosis is frequently observed while stent is open or after several stent-retriever passes. Another typical feature is early worsening of arterial caliber after thrombectomy, that can lead to arterial reocclusion. The likelihood that this refractoriness to thrombectomy is caused by ICAD increases if there are other characteristics that lower the probability of an embolic occlusion present, such as absence of a known major cardiac embolic source, preceding transitory symptoms that can be explained by ischemia in the same arterial territory, absence of CT arterial hyperdense sign or MRI susceptibility sign, or watershed-type infarction suggesting hemodynamic compromise caused by a pre-existing stenotic lesion.
Selection of Population, Intervention, Comparator, and Outcome (PICO)
The MWG formulated 11 main PICO questions relevant for ICAS management, with several sub-questions relating to the nine different outcomes defined above (if applicable), different subpopulations, or intervention sub-types, as relevant to each PICO and described below in the PICO header questions (Supplemental Panel 1). These were refined following comments from the ESO Executive Committee and ESO Guidelines Board. Subsequently, ESO Executive Committee and ESO Guidelines Board approved them.
The MWG decided to focus primarily on three patient groups: (1) asymptomatic stroke-free patients with ICAD (primary stroke prevention), (2) hyperacute management of acute stroke patients with an occlusion due to ICAD, and (3) patients with an ischemic stroke or TIA related to high-grade ICAD (secondary stroke prevention).
For primary prevention, we addressed if screening for ICAD (compared to no-screening) is beneficial for the prevention of MACE (PICO 1). We further address if antiplatelet therapy (vs the absence of such treatment) in asymptomatic ICAD patients lowers the risk of MACE (PICO 2). For patients in the hyperacute/acute phase, we examined the effects of additional infusion of glycoprotein IIb/IIIa inhibitors (PICO 3) or adjunct intracranial artery angioplasty/stenting (PICO 4) following mechanical thrombectomy for an acute ischemic stroke due to an ICAD-related LVO. Also concerning the acute phase, intensive blood pressure management (BP target 130–140/80) was compared with permissive hypertension in acute/subacute ischemic stroke or TIA related to high-grade ICAD (PICO 5). Third, we evaluated strategies for secondary prevention in patients with a prior ischemic stroke or TIA related to high-grade ICAD. Namely the following interventions for improving outcome were considered: anticoagulant therapy compared to antiplatelet therapy (PICO 6), dual antiplatelet therapy compared to single antiplatelet therapy (PICO 7), angioplasty and/or stenting plus best medical management (BMT) compared to BMT alone (PICO 8), any neurosurgical interventions plus BMT compared to BMT alone (PICO 9), remote ischemic conditioning plus BMT compared to BMT alone (PICO 10), and aggressive vascular risk factor control, including lipid management compared to BMT alone (PICO 11).
Literature search
For each PICO question, search terms were developed by the MWG and guideline methodologist. Where a validated search strategy was available, this was used or adapted. Where there was a recent relevant systematic review on the question of interest, the corresponding search strategy and results were used and updated as necessary. Search strategies are described in Supplemental materials.
The search was performed by the ESO Guideline methodologist. The following databases were searched: Medline, Embase, and Cochrane from inception to 4 August 2021. Reference lists of review articles, the authors’ personal reference libraries, and previous guidelines were also searched for additional relevant records.
Search results were loaded into the web-based Covidence platform (Health Innovation, Melbourne, Australia) for assessment by the MWG. Two or more MWG members were assigned to independently screen the titles and abstracts of publications registered in Covidence for each PICO question and then assess the full text of studies determined to be potentially relevant. All disagreements were resolved by discussion between the two reviewers or by a third MWG member.
We prioritized randomized-controlled clinical trials (RCTs) but where data were limited, we also considered health registry data analyses, observational studies, and systematic reviews or meta-analyses of observational studies. We considered only studies in humans. We excluded publications with only conference abstracts available.
Data analysis
Data extraction and analysis was performed by the ESO methodologist. In the case that relevant data were not reported in an eligible study, the corresponding author was contacted. In case of no response, the co-authors of the study were also contacted. If no answer was received, data were considered as missing.
Random-effects meta-analyses were conducted using Review Manager (RevMan) software (Cochrane) due to expected heterogeneity in study populations and design. Results were presented as the relevant effect estimates with corresponding 95% confidence intervals (95% CIs). Statistical heterogeneity across studies was assessed using the I2 statistic, and classified as moderate (⩾30%), substantial (⩾50%), or considerable (⩾75%). 19
Evaluation of the quality of evidence and formulation of recommendations
The risk of bias of each included randomized-controlled trial was assessed with the Cochrane Rob2 tool 20 and that of each included observational study with the Cochrane Robins-I tool. 21 As recommended, the evidence synthesis did not use a quality “score” threshold but classified the overall risk of bias at the study level and then in aggregate. 22
The results of data analysis were imported into the GRADEpro Guideline Development Tool (McMaster University, 2015; developed by Evidence Prime, Inc.), For each PICO question, and each outcome, the following were considered: risk of bias based on the type of available evidence (randomized or observational studies); considerations on inconsistency of results; indirectness of evidence, imprecision of results, and other possible bias. GRADE evidence profiles/summary of findings tables were generated and used to prepare recommendations. “Evidence-based Recommendations” were based on the GRADE methodology. The direction, strength and formulation of the recommendations were determined according to the GRADE evidence profiles and the ESO-SOP.17,18
Finally, Expert Consensus Statements were added whenever the PICO group considered that there was insufficient evidence available to provide evidence-based recommendations and where practical guidance is needed for routine clinical practice. The Expert Consensus Statements were based on voting by all expert MWG members. Importantly, these Expert Consensus Statements should not be regarded as evidence-based recommendations, since they only reflect the opinion of the writing group.
Drafting of the document, revision, and approval
Each PICO question was addressed in distinct sections, in line with the updated ESO SOP. 17 First, “analysis of current evidence” summarized current pathophysiological considerations followed by a summary and discussion of the results of the identified RCTs and other studies.
Second, “additional information” was added when more details on the studies referred to in the first section were needed to provide information on key subgroup analyses of the included studies, on ongoing or future RCTs, and on other studies which can provide important clinical guidance on the topic.
Third, an “expert consensus statement” paragraph was added whenever the MWG considered that insufficient evidence was available to provide evidence-based recommendations for situations in which practical guidance is needed for everyday clinical practice.
The guideline document was reviewed several times by all MWG members and modified using a Delphi approach until consensus was reached. The final submitted document was peer-reviewed by two external reviewers, two members of the ESO Guideline Board, and one member of the Executive Committee.
Results: PICO questions
Management of asymptomatic intracranial atherosclerotic disease (ICAD)
PICO 1: In adult stroke-free subjects, is screening compared to no-screening for intracranial atherosclerosis beneficial for the prevention of Major Adverse Cardiovascular Events (MACE) including ischemic stroke?
Analysis of current evidence
With the increasing availability of non-invasive methods able to depict intracranial large arteries, detection of ICAD at its asymptomatic or subclinical stage is becoming more common and feasible at a population-based scale. In our literature search, we have found population-based studies (see Table 1 for GRADEpro ratings of the included studies) revealing the prognostic value of finding asymptomatic intracranial atherosclerosis features, such as intracranial stenosis on transcranial Doppler/color-coded duplex ultrasound or arterial calcification on plain computed tomography.23–26 In this context, the limitations of relying on a single imaging method as a screening tool to diagnose ICAD should be acknowledged. Regarding the diagnosis of ICAD with transcranial ultrasound techniques, as we mentioned in the introduction section, a confirmatory noninvasive angiographic technique may be needed to rule out false positive stenosis. Nevertheless, there is insufficient evidence to support that screening for ICAD in stroke-free persons is beneficial to prevent incident Major Adverse Cardiovascular Events (MACE), including ischemic stroke. To the best of our knowledge, no randomized-controlled clinical trials have been performed targeting specifically this PICO question.
Table 1.
GRADE evidence profile table for PICO 1.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Screening for intracranial atherosclerosis | No screening | Relative (95% CI) | Absolute (95% CI) | ||
| MACE – ICAD patients – adjusted analysis a | ||||||||||||
| 3 | Observational studies | Not serious | Not serious | Not serious | Not serious b | None | 70/303 (23.1%) | 333/2778 (12.0%) | HR 1.85 (1.17–2.95) | 91 more per 1000 (from 19 more to 194 more) | ⨁⨁◯◯ Low | CRITICAL |
| MACE – ICAD patients | ||||||||||||
| 1 | Observational studies | Not serious | Not serious | Not serious | Serious c | None | HR 4.15 (1.01–11.42) | - | ⨁◯◯◯ Very low | CRITICAL | ||
CI: confidence interval; HR: hazard ratio.
Analyses were adjusted for:
Study 1: Age, sex, vascular risk, and presence of carotid plaques.
Study 2: Age, sex, smoking, hypertension, total cholesterol, LDL, HDL, peripheral artery disease, albuminuria, IHD history, diabetes duration, retinopathy, and HbA1C.
Study 3: Factors found significant in univariate analysis were included in a stepwise multivariate Cox proportional hazards regression model with entry criteria of p < 0.20 and exit criteria of p > 0.05 (age, cigarette smoking, hypertension, diabetes mellitus).
Raw numbers are missing for one study, which affects totals and absolute effect calculation.
Result driven by only one study; wide relative confidence interval; since raw data are not available for this study absolute effect cannot be calculated.
Additional information
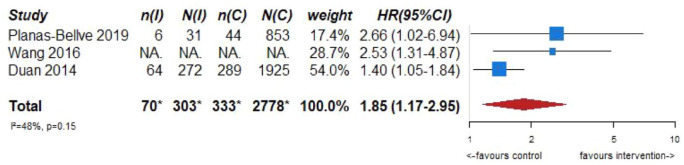
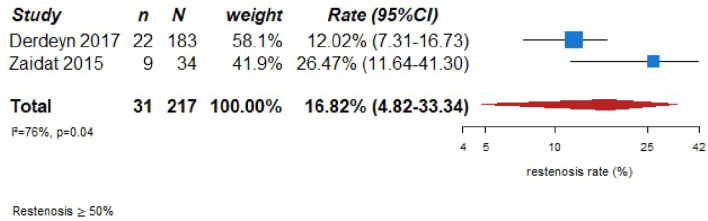
Although there is no evidence to support screening for ICAD in stroke-free individuals as a primary prevention strategy, the prognostic significance of imaging markers of ICAD in asymptomatic persons may deserve an additional comment, giving its increasing importance. In this context, robust observational evidence from population-based studies and studies in stroke-free persons at high vascular risk clearly show an increased risk of MACE and mortality when imaging-based markers of ICAD are present.23–25 Several population-based studies using transcranial Doppler ultrasound as the screening method, reported significant increases in the risk of future strokes,23–25 future ischemic strokes,23–25 future coronary events,23,25 and mortality,23,25 in patients with an asymptomatic intracranial stenosis detected at the beginning of long-term follow-up. Figure 1.1 shows the results of the meta-analysis combining these studies for the association between asymptomatic intracranial stenosis and the risk of future stroke (adjusted hazard ratio (HR) 1.85, 95% CI 1.17–2.95). These data underscore the importance of asymptomatic ICAD in the development of stroke, and thus raise awareness for ICAD amongst clinicians when detected in stroke-free individuals after non-invasive intracranial arterial imaging being performed due to a variety of clinical indications.
Figure 1.1.
PICO 1 – Association between asymptomatic ICAD and risk of future stroke.
*Data from Wang 2016 missing from totals.
In stroke-free individuals from a population-based study, the presence of intracranial carotid artery calcification, as a hallmark of ICAD, was also linked to an increased risk of having future strokes (adjusted HR 4.64, 95% CI 1.44–14.95) and ischemic strokes (adjusted HR 3.52, 95% CI 1.08–11.47; adjusted for age, sex, scanner type, obesity, hypertension, diabetes mellitus, hypercholesterolemia, low high-density lipoprotein cholesterol, smoking, ultrasound carotid plaque score, and calcification volumes in other vessel beds). 26 The amount of intracranial carotid artery calcification has been associated to a higher risk of cognitive decline and dementia (HR per unit increase in calcification volume 1.34, 95% CI 1.01;1.78). 27 Interestingly, from the perspective of medical imaging, numerous non-contrast computed tomography scans of the head are acquired on a daily basis for a variety of indications, where intracranial calcification can be assessed, thus providing the ordering physician with crucial information on cardiovascular risk. In this regard, despite its prognostic value, detection of intracranial arterial calcification is far from being implemented in the clinical routine, unlike the assessment of coronary calcium on thoracic imaging. Regarding quality assessment of these observational studies, although they were not found to have any serious risk of bias, inconsistency, indirectness, or imprecision, due to study design overall quality is low. Randomized-controlled clinical trials in the field are needed to have higher quality data.
PICO 2: In subjects with asymptomatic intracranial atherosclerosis, does antiplatelet treatment compared with no antiplatelet treatment lower the risk of MACE including ischemic stroke?
Analysis of current evidence
Currently, there are no RCTs on subjects with asymptomatic ICAD comparing antiplatelet treatment to no treatment with the risk of MACE and ischemic stroke as an endpoint.
Additional information
Two observational studies, one using transcranial color-coded (TCCS) ultrasound in Caucasians with asymptomatic middle cerebral artery (MCA) stenosis reported outcome in relation to antiplatelet treatment. In this study, after a mean follow-up of 815 days, of the 50 patients included, no patient had an ischemic event in the territory of the asymptomatic MCA stenosis, 42 patients (45 at follow-up) received antiplatelet therapy (either aspirin, or aspirin and dypiridamole, three patients received warfarin), two patients died of non-vascular causes, one had a subdural hematoma. 28 The other study used Magnetic Resonance Angiography (MRA) classification of MCA stenosis. In this review of 1140 MRA examinations, 28 could be classified as having an asymptomatic MCA stenosis. After a mean follow-up of 46.7 months, one patient (out of 28) had suffered a stroke in the territory of the asymptomatic MCA stenosis (five patients had strokes in other territories). Ten patients were on antiplatelet therapy (aspirin or ticlopidine) at baseline and 13 at follow-up (three had warfarin), five patients died due to other non-vascular causes during follow-up, no association of the clinical and imaging markers was associated with subsequent stroke, mortality was associated with higher age (>69 years). 29
Due to the observational character, the low number of overall included patients, potential confounders, and its relative lack of actuality, no clear evidence can be generated from these trials. One explanation for the scarce data might be that potential patients eligible for RCTs including antiplatelet therapy are patients at high vascular risk already receiving this therapy for other reasons. As mentioned in PICO 1, larger population-based observational studies of stroke free – subjects with intracranial atherosclerosis have revealed a significantly higher risk for MACE and stroke.
Although bearing in mind the potential difficulties with such trials, RCTs comparing antiplatelet therapy versus no antiplatelet therapy in patients with asymptomatic ICAD are warranted.
Treatment of an acute intracranial LVO caused by ICAD
Definition of an acute ICAD-related LVO
A high probability of an ICAD-related LVO is assumed if all or most of the following criteria are fulfilled25–29,(1) absence of atrial fibrillation, (2) absence of CT hyperdense sign or MRI susceptibility sign, (3) watershed infarction, (4) truncal-type occlusion, (5) residual stenosis on DSA when stent is open or after three stent-retriever passes or (6) early reocclusion.
General remark about the evidence situation for the hyperacute management of ICAD-related LVOs
Overall, the level of evidence for the hyperacute management of ICAD-related intracranial arterial occlusion is very low. Despite the high incidence of ICAD-related intracranial arterial occlusions (especially in Asian populations in which they are the underlying cause for up to 40% of all large vessel occlusions)30–33 and that ICAD-related intracranial arterial occlusions might pose an independent risk factor for mechanical thrombectomy failure and early reocclusion,34,35 no randomized-controlled clinical trial has evaluated the effects of hyperacute management approaches for these challenging situations.36,37 The authors of these guidelines acknowledge that there is a need for well-designed randomized-controlled clinical trials to answer this clinically important question.
PICO 3: In patients undergoing mechanical thrombectomy for an acute ischemic stroke due to an ICAD-related intracranial arterial occlusion, does infusion of glycoprotein IIb/IIIa inhibitors after initial mechanical thrombectomy, as compared with standard of care, improve functional outcome?
Analysis of current evidence
Our systematic review identified five retrospective studies of very low quality (see Table 2 for the GRADEpro ratings of the included studies) comparing the effects of an additional intraarterial infusion of glycoprotein IIb/IIIa inhibitors after initial mechanical thrombectomy to mechanical thrombectomy alone on functional outcome in patients with an ICAD-related LVO.37–41 Two of these studies were excluded from further analysis due to the following reasons: the first study focused only on vertebrobasilar stroke 40 and the second study did not differentiate clearly enough between large vessel occlusion-stroke due to large arterial atherosclerosis and ICAD-related LVO stroke. 41 The remaining three studies included patients on a consecutive basis, however it remains unclear in these studies on which bases the type of rescue therapy was determined (administration of glycoprotein IIb/IIIa depended on operator’s judgment). All studies were multicenter and primarily done in Asian populations (China and South Korean).37–39
Table 2.
GRADE evidence profile table for PICO 3.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Infusion of glycoprotein IIb/IIIa inhibitors after initial mechanical thrombectomy | Standard of care | Relative (95% CI) | Absolute (95% CI) | ||
| Good functional outcome (mRS 0–2) at 90 days, adjusted a | ||||||||||||
| 3 | Observational studies | Serious b | Not serious | Serious c | Not serious | None | 113/191 (59.2%) | 40/129 (31.0%) | OR 3.20 (1.56–6.55) | 280 more per 1000 (from 102 more to 436 more) | ⨁◯◯◯ Very low | CRITICAL |
| Good functional outcome (mRS 0–2) at 90 days | ||||||||||||
| 3 | Observational studies | Serious b | Not serious | Serious c | Not serious | None | 113/191 (59.2%) | 40/129 (31.0%) | OR 2.97 (1.82–4.84) | 262 more per 1000 (from 140 more to 375 more) | ⨁◯◯◯ Very low |
CRITICAL |
| Symptomatic ICH | ||||||||||||
| 3 | Observational studies | Serious b | Not serious | Serious c | Serious d | None | 17/193 (8.8%) | 25/131 (19.1%) | OR 0.56 (0.21–1.49) | 74 fewer per 1000 (from 144 fewer to 69 more) | ⨁◯◯◯Very low | CRITICAL |
| Mortality at 90 days | ||||||||||||
| 3 | Observational studies | Serious b | Not serious | Serious c | Not serious | None | 10/191 (5.2%) | 28/129 (21.7%) | OR 0.24 (0.11–0.52) | 155 fewer per 1 000 (from 187 fewer to 91 fewer) | ⨁◯◯◯Very low | CRITICAL |
CI: confidence interval; OR: odds ratio.
Adjusting variables:
Study 1: Variables with potential association (p < 0.2 in univariable analyses) (age, sex, smoking, initial NIHSS score, number of MT passes, successful recanalization; patent artery on follow-up; post-procedural antithrombotics).
Study 2: Age, baseline NIHSS score, baseline ASPECTS/pc-ASPECTS, CS 2–4, any ICH, time to revascularization, tirofiban.
Study 3: Age, sex, balloon angioplasty and/or stenting, and variables with p < 0.20 in univariate analyses (immediate reocclusion after first endovascular method, local tirofiban infusion only, final mTICI 2b-3, post procedural reocclusion).
Some or all included studies have a serious risk of bias as assessed with Robins-I.
Intervention or comparator dissimilarity.
Confidence interval unable to exclude substantial benefit or harm.
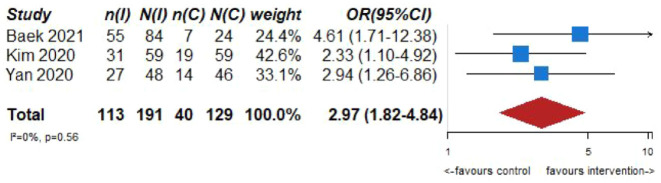
Pooled analysis of the included three studies (191 patients in the intervention and 129 patients in the control group) suggests a positive effect (Odds Ratio (OR) 2.97; 95% CI 1.82–4.84, see Figure 3.1) of the infusion of glycoprotein IIb/IIIa inhibitors on functional outcome (rate of mRS ⩽ 2).37–39 However, it must be noted that we observed substantial heterogeneity between studies with regards to concomitant treatments (i.v. tissue plasminogen activator rates ranged between 30% and 86%) and the target population (one study included only patients with residual stenosis 38 while the other two included patients with refractory occlusions and residual stenosis). Therefore, the pooled estimates must be interpreted very cautious as uncontrollable factors might influence them in both directions. In addition, the lower limit of the 95% CI of the OR crossed 1 in the adjusted analysis (the OR in Figure 3.1 are unadjusted crude OR) of one study for the main outcome (good functional independence), being compatible with high uncertainty of the effects of the intervention. 37 None of the studies showed strong signals of harm such as increased rates of mortality and/or symptomatic intracranial hemorrhage in response to the intervention.37–39
Figure 3.1.
PICO 3 – Association between infusion of glycoprotein IIb/IIIa inhibitors after mechanical thrombectomy, compared to standard of care, and good functional outcome (mRS 0–2) at 90 days, in observational studies.
OR reported for the studies are crude OR calculated by authors based on the raw numbers reported in the articles.
Figure 5.1.
PICO 5 - Risk of bias assessment.
In the largest study with 118 patients, 39 the multivariate analysis suggested a positive effect of glycoprotein IIb/IIIa inhibitors infusion (OR 3.4; 95% CI 1.1–10.1) on the probability of good functional outcome (mRS 0–2). In this study only Tirofiban was used. Two substantial limitations in addition to the retrospective design must be noted: (1) enrollment was done between 2011 and 2016 and since then technological approaches for MT have evolved substantial, which is also reflected by the very low rate of successful reperfusion in the no-Tirofiban group (42.4%) and (2) the intravenous thrombolysis rate was very low in the Tirofiban group with only 33.9% (49.2% in the no-Tirofiban group), which may have had an impact on the safety profile and outcome of the patients. 39 The second largest study (n = 108) 5 was not able to show a positive effect of glycoprotein IIb/IIIa inhibitors infusion on clinical outcome (OR 3.21, 95% CI 0.44–23.5). Intravenous thrombolysis rates were low in this study as well (29.8% in the intervention group and 45.8% in the control group), questioning if these results could be generalized to the European populations, in which i.v. tissue plasminogen activator rates are substantially higher. 42 Also in the last study, the use of glycoprotein IIb/IIIa inhibitors in combination with rescue stenting was associated with better outcomes as compared to rescue stenting alone.37,42 The very large 95% CI in this study further indicates a very high degree of uncertainty and cannot exclude potential negative effects of the intervention. The third study (n = 98) 38 enrolled patients between 2015 and 2019. Their data suggested a positive effect of glycoprotein IIb/IIIa infusion (OR 3.4; 95% CI 1.1–10.2). 38 In this study i.v. tissue plasminogen activator rates were 86% in the intervention and 70.8% in the control arm, comparable to current thrombolysis rates in Europe. However, it must be noted that in this study only patients with residual stenosis (and not refractory occlusion) after thrombectomy were included, limiting the applicability to the general population.
In conclusion, due to serious risk of bias in all these studies, their heterogeneity and their partially inconclusive results an evidence-based suggestion is not possible.
PICO 4: In patients undergoing mechanical thrombectomy for an acute ischemic stroke due to an ICAD-related intracranial arterial occlusion, does angioplasty and/or stenting plus best medical treatment (BMT) after initial mechanical thrombectomy, as compared to BMT alone, improve functional outcome?
Analysis of current evidence
Our systematic review identified four retrospective studies of very low quality comparing the effects of angioplasty and/or stenting plus BMT after initial mechanical thrombectomy to BMT alone on functional outcome in ICAD-related intracranial arterial occlusions (see Table 3 for the GRADEpro ratings of the included studies).37,43–45 One out of the four studies compared LVO patients related to ICAD undergoing angioplasty and/or stenting to non-ICAD LVO patients with similar characteristics. 45 This study suggested that functional outcome did not differ between both groups, which would suggest that rescue therapy in the form of angioplasty and/or stenting appears to be safe. However, for the formulation of the recommendation we did not consider this study as the control group of the study was from a patient population not included in the PICO question. Out of the remaining three studies, one study was monocenter while the other two were multicenter and all of them were done primarily in Asian populations (China and South Korea).37,43,44 As the best medical management in acute stenting procedures is not yet known, it is a potential cause of heterogeneity between the studies.
Table 3.
GRADE evidence profile table for PICO 4.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Angioplasty and/or stenting plus Best Medical Therapy (BMT) after initial mechanical thrombectomy | BMT alone | Relative (95% CI) | Absolute (95% CI) | ||
| Good functional outcome (mRS 0–2) at 90 days | ||||||||||||
| 3 | Observational studies | Serious a | Serious b | Serious c | Serious d | None | 97/159 (61.0%) | 88/200 (44.0%) | OR 2.18 (1.37–3.46) | 191 more per 1000 (from 78 more to 291 more) | ⨁◯◯◯Very low | CRITICAL |
| Good functional outcome (mRS 0–2) at 90 days (sensitivity analysis: Baek et al. intervention group is rescue stenting + mechanical thrombectomy) | ||||||||||||
| 3 | Observational studies | Serious a | Not serious | Serious c | Not serious | None | 54/108 (50.0%) | 33/118 (28.0%) | OR 2.43 (1.38–4.30) | 206 more per 1000 (from 69 more to 346 more) | ⨁◯◯◯Very low | CRITICAL |
| Symptomatic ICH | ||||||||||||
| 3 | Observational studies | Serious a | Not serious | Serious c | Serious e | None | 12/134 (9.0%) | 20/118 (16.9%) | OR 0.56 (0.26–1.22) | 67 fewer per 1000 (from 119 fewer to 30 more) | ⨁◯◯◯Very low | CRITICAL |
| Mortality at 90 days | ||||||||||||
| 3 | Observational studies | Serious a | Not serious | Serious c | Not serious | None | 26/134 (19.4%) | 43/118 (36.4%) | OR 0.52 (0.28–0.95) | 135 fewer per 1000 (from 226 fewer to 12 fewer) | ⨁◯◯◯Very low | CRITICAL |
CI: confidence interval; OR: odds ratio.
Some or all included studies have a serious risk of bias as assessed with Robins-I.
Statistical heterogeneity.
Intervention/comparator dissimilarity.
Wide confidence interval.
Confidence interval unable to exclude substantial benefit or harm.
In two out of three studies43,44 angioplasty and/or stenting was associated with a positive effect on the rate of good functional outcome, while in one study 37 only the combination of angioplasty and/or stenting and infusion of glycoprotein IIb/IIIa inhibitors appeared to be beneficial. An additional problem in evaluating the evidence is, that due to the high variations in rates of additional glycoprotein IIb/IIIa inhibitor infusions (ranging from 10.7% to 77.7%) between groups, the effects of both strategies cannot be clearly distinguished in these studies. No pooled analysis was performed as in two of the studies the groups were mixed (i.e. angioplasty and/or stenting ± glycoprotein IIb/IIIa inhibitor infusion). It is therefore impossible to deduct what the true effect size of angioplasty and/or stenting was in these studies.43,44 Also the 95% CI of the OR (0.97–3.4) in the Baek et al. 37 study crosses one, being compatible with high uncertainty of the effects of the intervention. In none of the studies additional angioplasty and/or stenting was associated with higher rates of symptomatic intracranial hemorrhage or mortality.37,43,44 We further found one RCT enrolling patients with a large vessel occlusion refractory to mechanical thrombectomy in China (ANGEL REBOOT, clintrials.gov identifier NCT05122286). They are randomizing patients either to bailout angioplasty or further thrombectomy passes/stop of procedure. This study might improve the evidence on this topic, although it must be noted that it does not require ICAD as an underlying cause of primary thrombectomy failure.
In the largest study (n = 207), 43 which used propensity-score matching for improving the comparability between the control and intervention groups, angioplasty and/or stenting in LVO patients with a refractory occlusion due to ICAD was associated with significantly higher rates of good functional outcome (36.4% in the intervention group and 19.7% in the control group). Patients were enrolled between 2015 and 2018 in multiple Chinese centers. 43 The second largest study 37 distinguished between patients receiving only additional angioplasty and/or stenting and receiving additional angioplasty and/or stenting plus infusion of glycoprotein IIb/IIIa inhibitors. Patients were enrolled between 2010 and 2018 in three South Korean centers. There was no significant effect of additional angioplasty and/or stenting on outcome but additional angioplasty and/or stenting plus the infusion of glycoprotein IIb/IIIa inhibitors was significantly associated with better outcome. However, the very high rate of good outcome (84.3%) in the angioplasty and/or stenting plus glycoprotein IIb/IIIa inhibitor group raises suspicions of other underlying factors influencing how patients were selected for a treatment strategy. 37 The third study 44 was monocenter and included 45 patients out of which 17 underwent stenting. Glycoprotein IIb/IIIa inhibitors were more often used in the stenting group with 35.3% compared to 10.7% in the control group. Overall, the authors found that stenting was associated with better functional outcome (defined as mRS 0–2) with 35.3% in the intervention and 7.1% in the control group.
Due to the serious risk of bias in all studies, high variations in concomitant treatments and unclear patient selection criteria an evidence-based suggestion is not possible in our opinion.

PICO 5: In patients with an acute ischemic stroke or transient ischemic attack related to a high-grade intracranial atherosclerosis causing hemodynamic compromise, does permissive or induced hypertension, as compared to conventional blood pressure (BP) management (targeting normotension), during the acute phase, improve outcome?
Analysis of current evidence
Blood pressure management in patients during the acute phase of ischemic stroke still remains a matter of debate. Treatment with specific blood pressure lowering agents like glyceryl trinitrate patches and candesartan showed their significant effect on blood pressure lowering, but failed to prove any beneficial effect on clinical outcome.46,47 A recent post-hoc analysis of the Efficacy of Nitric Oxide in Stroke (ENOS) trial indicated a shift toward a worse outcome measured by the modified Rankin Scale by day 90 (OR 1.46, 95% CI 1.01–2.11). 48 In a more recent trial of patients treated with intravenous thrombolysis comparing intensive versus standard blood pressure lowering treatment did not show a significant difference in the effect on death or disability at 90 days, but did show increased mortality caused by intensive blood pressure lowering treatment (OR 1.52, 95% CI 1.09–2.13). 49 All of the trials included patients regardless of the atherosclerosis status of the intracranial arteries. The European Stroke Organisation (ESO) and American Heart Association/American Stroke Association (AHA/ASA) guidelines specify evidence-based upper limits of blood pressure in all ischemic stroke patients and also in patients treated with intravenous thrombolysis and mechanical thrombectomy.50,51 Neither of the guidelines refer specifically to blood pressure management in patients with acute ischemic stroke and ICAD. The AHA/ASA guideline states that induced hypertension in acute ischemic stroke is not well established. 51 Regarding the ESO general guidelines on acute BP management, induced hypertension in cases of clinical deterioration due to hemodynamic compromise is suggested only as a rescue therapy after other conservative measures to improve brain hemodynamics have been tried. 50
It is a matter of deep concern whether blood pressure should be lowered intensely in patients with acute ischemic stroke and intracranial arterial stenosis as it may cause cerebral perfusion compromise. Mechanism preventing hypoperfusion in such patients has been established.52,53
Our systematic review has not identified any RCT answering which BP regimen is the most favorable during the acute phase of stroke in patients with ICAD. We have although identified one RCT (see Table 4 for GRADEpro ratings) comparing intensive (target SBP < 120 mmHg) and modest (target SBP < 140 mmHg) blood pressure lowering in patients with >50% or occlusion of the distal internal carotid artery (ICA) or the M1 segment of the MCA in patents with a recent ipsilateral ischemic stroke. 54 It has included mainly patients between 7 and 42 days following the index ischemic stroke. Therefore, the trial should be rather considered as an early secondary prevention trial. It has a randomized-controlled, single-blinded design. Patients with blood pressure exceeding 140 mmHg were eligible. Patients with intractable hypertension (systolic BP ⩾ 150 mmHg with more than 3 antihypertensives) were excluded. The allocated blood pressure target was maintained for 24 weeks following randomization. The primary endpoint was the volume change of white matter lesions between baseline and 24 weeks on FLAIR imaging. In the intensive blood pressure lowering group the median lesion growth at follow up was 4.9 cm3 versus 2.2 cm3 in the modest blood pressure lowering group and failed to show non-inferiority. The secondary radiological endpoints included ischemic lesion volume change in the ipsilateral hemisphere to the symptomatic intracranial stenosis between baseline and 24 weeks and new ischemic lesions on 24-week FLAIR image. For both endpoints the results were inconclusive. Secondary clinical endpoints were recurrent stroke, myocardial infarction and vascular death during 24 weeks.
Table 4.
GRADE evidence profile Table for PICO 5.
| Question: Permissive or induced hypertension compared to conventional blood pressure (BP) management (target normotension) for outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Setting: patients with an acute ischemic stroke or transient ischemic attack related to a high-grade intracranial atherostenosis causing severe hemodynamic compromise. | ||||||||||||
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Permissive or induced hypertension | conventional blood pressure (BP) management (target normotension) | Relative (95% CI) | Absolute (95% CI) | ||
| Mortality | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Serious a | None | 0/59 (0.0%) | 0/52 (0.0%) | Not estimable | ⨁⨁⨁◯ Moderate |
CRITICAL | |
CI: confidence interval.
RCT not adequately powered compared to required sample size calculation.
For the sake of this PICO we have accepted vascular death as the major clinical outcome. The analysis of the trial (59 patients allocated to intensive and 52 to modest blood pressure control) showed no cases of vascular death during the 24 weeks following randomization. 54
It has to be emphasized that quality assessment revealed a low risk of bias (Figure 5.1)
Additional information
The data on outcome related to cerebral perfusion compromise in patients with acute ischemic stroke due to ICAD is sparse. Furthermore, the correlation between impaired cerebral perfusion, low BP and ICAD for the risk of stroke remains conflicting.55–57 Current evidence does not support the rationale for permissive or induced hypertension routinely in both – acute stroke treatment and prevention in patients with ICAD.
Management of patients with symptomatic intracranial atherosclerosis
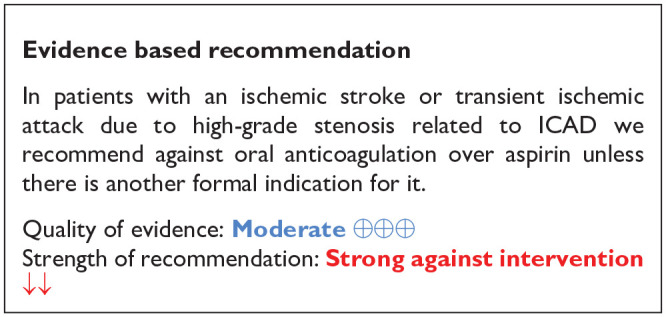
PICO 6: In patients with an ischemic stroke or transient ischemic attack related to a high-grade stenosis related to ICAD and without any formal indication for anticoagulation, does anticoagulant therapy, as compared to antiplatelet therapy, improve outcome?
Analysis of current evidence
Despite intensive medical treatment with antiplatelet agents and aggressive management of vascular risk factors and lifestyle modifications, patients with a recent acute ischemic stroke or transient ischemic attack (TIA) associated with ICAD are at a high risk for early (5.5% at 30 days) and long-term stroke recurrence (14.9% at 1 year and 17.2% at 2 years). 9 In addition to the increased risk of stroke recurrence these patients are also at an increased risk for major adverse cardiovascular events, including a 3.4% probability for myocardial infarction in the first year and a 4.5% risk for all-cause mortality in the first 2 years after the index ischemic stroke or TIA. 9 A third of the patients with ICAD have been reported to have progression of their stenosis during follow-up despite medical treatment, with observational evidence suggesting that this risk can potentially be ameliorated with oral anticoagulants compared to antiplatelet treatment. 58 Given the high risk of cardiovascular events after a recent acute ischemic stroke or TIA associated with ICAD, anticoagulation treatment has been evaluated as a more potent antithrombotic option compared to antiplatelet treatment in the setting of randomized-controlled clinical trials (RCTs).
Our systematic review identified two RCTs (see Table 5 for GRADEpro ratings of the included studies) that have compared anticoagulation treatment with vitamin-k antagonists (VKAs) to single antiplatelet treatment in patients with recent acute ischemic stroke or TIA associated with ICAS. The Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) trial was a double-blinded, multicenter RCT that randomly assigned patients with an ischemic stroke or TIA within 90 days from symptom onset, which was caused by angiographically verified 50%–99% stenosis of a major intracranial artery (carotid, middle cerebral, vertebral, or basilar), to receive either warfarin (with a target international normalized ratio (INR) between 2.0 and 3.0) or aspirin (at a fixed dose of 1300 mg per day). 59 The trial was prematurely terminated after a mean follow-up of 1.8 year due to safety concerns for the patients who had been assigned to receive warfarin. 59 Patients in the warfarin arm had a higher risk for all-cause mortality, major hemorrhage, and myocardial infarction or sudden death. 59 Another open-label, randomized, multicenter trial from Spain evaluated the efficacy of oral anticoagulation with coumadin (with a target INR between 2 and 3) to a fixed dose of 300 mg/day of aspirin in patients with an ischemic stroke between 7 and 90 days from symptom onset associated with a 50%–99% stenosis of the trunk or main branches of the ipsilateral MCA diagnosed by conventional angiography or by at least two noninvasive tests, including transcranial Doppler (TCD), magnetic resonance angiography (MRA) and computed tomography angiography (CTA). 60 After enrolling a total of 28 patients and a mean follow-up of 23.1 ± 10.9 months only two patients in the coumadin group experienced major cardiovascular events. 60
Table 5.
GRADE evidence profile ratings for PICO 6.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Anticoagulation | Antiplatelet | Relative (95% CI) | Absolute (95% CI) | ||
| Recurrent IS | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Very seriousa,b | None | 49/303 (16.2%) | 57/294 (19.4%) | OR 0.80 (0.52–1.22) | 33 fewer per 1000 (from 83 fewer to 33 more) | ⨁⨁◯◯ Low |
CRITICAL |
| MACE | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Very seriousa,b | None | 72/303 (23.8%) | 66/294 (22.4%) | OR 1.07 (0.73–1.57) | 12 more per 1000 (from 50 fewer to 88 more) | ⨁⨁◯◯ Low |
CRITICAL |
| Major bleeding | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Seriousb,c,d | None | 25/303 (8.3%) | 9/294 (3.1%) | OR 2.75 (1.28–5.90) | 49 more per 1000 (from 8 more to 126 more) | ⨁⨁⨁◯ Moderate |
CRITICAL |
| Mortality | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Seriousb,c,d | None | 30/303 (9.9%) | 13/294 (4.4%) | OR 2.38 (1.21–4.66) | 55 more per 1000 (from 9 more to 133 more) | ⨁⨁⨁◯ Moderate |
CRITICAL |
CI: confidence interval; OR: odds ratio; IS: ischemic stroke; MACE: major adverse cardiovascular events.
Confidence intervals unable to exclude substantial benefit or harm.
Evidence provided predominantly by only one adequately powered study.
Wide confidence interval.
Large effect size.
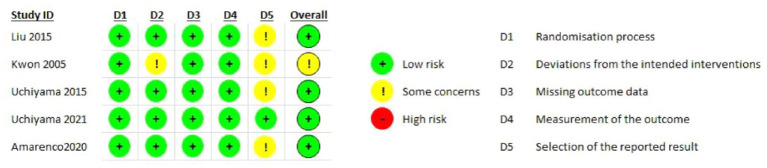
Quality assessment revealed a low risk of bias for both trials (Figure 6.1)
Figure 6.1.
PICO 6 - Risk of bias assessment.
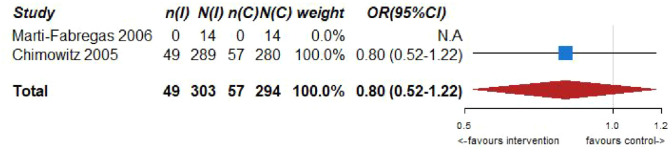
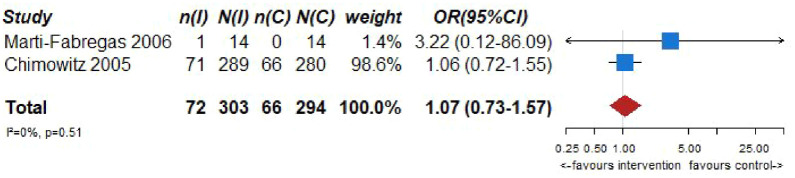
Pooled analyses of these two trials59,60 (303 patients randomized to oral anticoagulation with VKAs and 294 randomized to receive aspirin) suggested no difference in the risk of ischemic stroke recurrence (OR = 0.80, 95% CI: 0.52–1.22; Figure 6.2) or major cardiovascular adverse events (OR = 1.07, 95% CI: 0.73–1.57; Figure 6.3) between patients randomized to INR-guided oral anticoagulation with VKAs or fixed-dose oral aspirin.
Figure 6.2.
PICO 6 – Association between anticoagulation therapy compared to antiplatelet therapy and risk of long term recurrence of IS in RCT.
Figure 6.3.
PICO 6 – Association between anticoagulation therapy compared to antiplatelet therapy and risk of MACE in RCT.
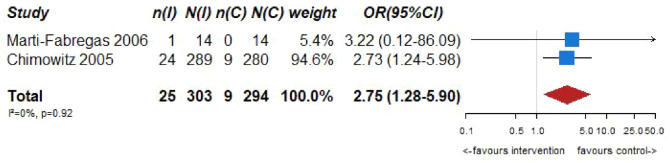
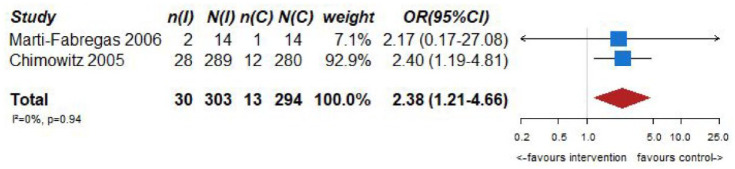
However, VKA treatment increased the risks of major bleeding (OR = 2.75, 95% CI: 1.28–5.90; Figure 6.4) and all-cause mortality (OR = 2.38, 95% CI: 1.21, 4.66; Figure 6.5) compared to aspirin treatment.
Figure 6.4.
PICO 6 – Association between anticoagulation therapy compared to antiplatelet therapy and risk of major bleeding in RCT.
Figure 6.5.
PICO 6 – Association between anticoagulation therapy compared to antiplatelet therapy and mortality in RCT.
No heterogeneity between trials was noticed in any of the outcomes, while pooled estimates were mainly derived from the WASID trial. 59 The results of both trials should be viewed with caution as both studies were underpowered,59,60 and thus there is an increased uncertainty on the true estimates of efficacy outcomes.
Additional information
In the WASID trial only 63% of the time patients randomized to receive VKA had an INR within the pre-specified target range (INR 2.0–3.0). 61 A dose-response effect was uncovered in a post-hoc analysis, suggesting that INRs of less than 2.0 were associated with a higher risk of ischemic stroke and major cardiac events than INRs of 2.0 or greater, whereas INRs greater than 3.0 were associated with a significantly higher risk of major hemorrhages than INRs of 3.0 or less. 61 It needs to be highlighted that the oral anticoagulation regimens evaluated in the aforementioned RCTs were VKAs (warfarin or coumadin, respectively),59,60 with non-vitamin K oral anticoagulants (NOACs) have never been tested in a dedicated RCT of patients with ischemic stroke or TIA associated with ICAD to date. The safety of low-dose rivaroxaban (2.5 mg twice daily) plus aspirin in patients with recent ischemic stroke or TIA (7 –100 days from symptom onset) secondary to intracranial atherosclerotic stenosis of 30%–99% as evidenced by CT or MR angiography is currently being evaluated in a multicenter Canadian RCT (Combination Antithrombotic Treatment for Prevention of Recurrent Ischemic Stroke in Intracranial Atherosclerotic Disease; NCT04142125).
Except for the experimental arms, caution is also warranted in the control arms of the trials reported above as they included only a single antiplatelet agent (aspirin at different doses),59,60 and thus the relative comparison between VKA agents and dual antiplatelet treatment, which is recommended by the current and previous guidelines as the optimal antithrombotic treatment for the first 3 months in patients with a recent ischemic stroke or TIA attributed to ICAS, is unknown.
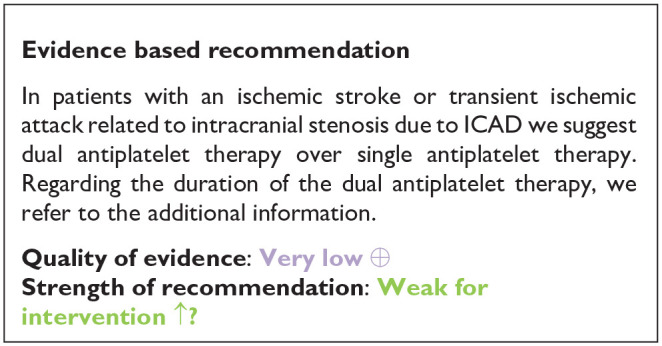
PICO 7: In patients with an ischemic stroke or transient ischemic attack related to intracranial stenosis related to ICAD, does dual antiplatelet therapy, as compared to single antiplatelet therapy, improve outcome?
Analysis of current evidence
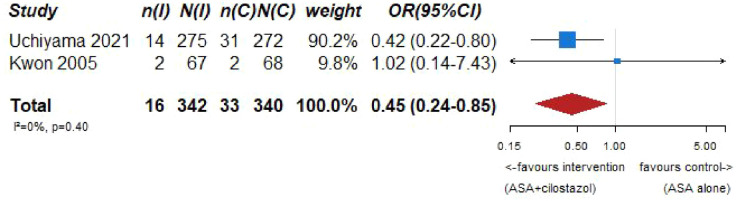
None of the retrieved trials enrolled only patients with ICAD and compared dual antiplatelet therapy with aspirin and P2Y12 inhibitors – clopidogrel or ticagrelor – to single antiplatelet antitherapy (see Table 6 for the GRADEpro ratings of the included trials). Two trials enrolling only patients with symptomatic intracranial stenosis due to ICAD compared dual antiplatelet therapy consisting of aspirin 100 mg/d and cilostazol 200 mg/d (a selective inhibitor of phosphodiesterase type 3), to aspirin 100 mg/d alone: TOSS (Trial of cilOstazol in Symptomatic intracranial arterial Stenosis) and CATHARSIS (Cilostazol-Aspirin Therapy against Recurrent Stroke with Intracranial Artery Stenosis).62,63 TOSS was conducted in South Korea, and CATHARSIS in Japan. Inclusion criteria were ischemic stroke within 2 weeks of randomization in TOSS, and between 2 weeks and 6 months in CATHARSIS. The primary endpoint was the progression of symptomatic intracranial stenosis on MR angiography at 6 months in TOSS and at 2 years in CATHARSIS. TOSS included 135 patients with acute symptomatic stenosis in the middle cerebral artery (M1 segment) or the basilar artery. CATHARSIS included 165 patients with stenosis of the supraclinoid segment of the internal carotid artery, M1 segment of the middle cerebral artery or basilar artery. In TOSS, intracranial stenosis progression at 6 months was less frequent in the cilostazol and aspirin arm compared to aspirin alone (6.7% vs 28.8%, p = 0.008). In CATHARSIS, no significant difference in intracranial stenosis progression at 2 years was detected between cilostazol and aspirin compared to aspirin alone (9.6% vs 5.6%, p = 0.53). In the risk of bias assessment, concerns were raised for the TOSS Trial regarding the high dropout rate, not balanced between arms (29.9% in the cilostazol arm, 20.6% in the placebo arm, see Figure 7.1.), the lack of stroke events during follow-up, and the lack of reporting on intracranial or major hemorrhagic events. The clinical endpoints deemed critical for this guideline were either secondary or not measured at all in TOSS and CATHARSIS, while no significant differences between the two arms were uncovered in the trials.
Table 6.
GRADE evidence profile table for PICO 7.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Dual antiplatelet | Single antiplatet | Relative (95% CI) | Absolute (95% CI) | ||
| Recurrence of ischemic stroke (aspirin + cilostazol vs aspirin alone) | ||||||||||||
| 3 | Randomized trials (overall analysis) | Serious a | Not serious | Serious b | Serious c | None | 15/425 (3.5%) | 31/420 (7.4%) | OR 0.45 (0.24–0.86) | 39 fewer per 1 000(from 55 fewer to 10 fewer) | ⨁◯◯◯ Very low |
CRITICAL |
| Recurrent stroke or death (aspirin + (P2Y12 inhibitors) clopidogrel or ticagrelor vs aspirin alone) | ||||||||||||
| 2 | Randomized trials (subgroup analysis) | Serious d | Not serious | Serious d | Not serious | None | 79/747 (10.6%) | 119/808 (14.7%) | OR 0.69 (0.51–0.93) | 41 fewer per 1000 (from 66 fewer to 9 fewer) | ⨁◯◯◯ Very low |
CRITICAL |
| Risk of MACE including stroke (aspirin + cilostazol vs aspirin alone) | ||||||||||||
| 2 | Randomized trials | Serious b | Not serious | Serious e | Not serious | None | 16/342 (4.7%) | 33/340 (9.7%) | OR 0.45 (0.24–0.85) | 51 fewer per 1000 (from 72 fewer to 13 fewer) | ⨁◯◯◯ Very low |
CRITICAL |
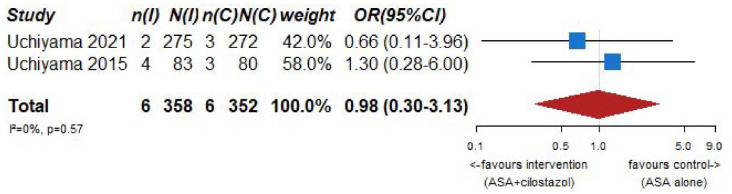
| Risk of major bleeding including ICH (aspirin + cilostazol vs aspirin alone) | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Serious f | Serious c | None | 6/358 (1.7%) | 6/352 (1.7%) | OR 0.98 (0.30–3.13) | 0 fewer per 1000 (from 12 fewer to 34 more) | ⨁◯◯◯ Very low |
CRITICAL |
| Risk of hemorrhagic stroke (aspirin + cilostazol vs aspirin alone) | ||||||||||||
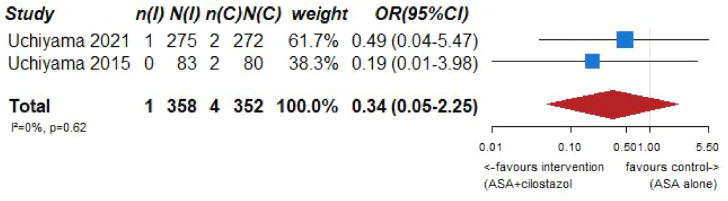
| 2 | Randomized trials | Not serious | Not serious | Not serious | Serious c | None | 1/358 (0.3%) | 4/352 (1.1%) | OR 0.34 (0.05–2.25) | 7 fewer per 1000 (from 11 fewer to 14 more) | ⨁◯◯◯ Very low |
CRITICAL |
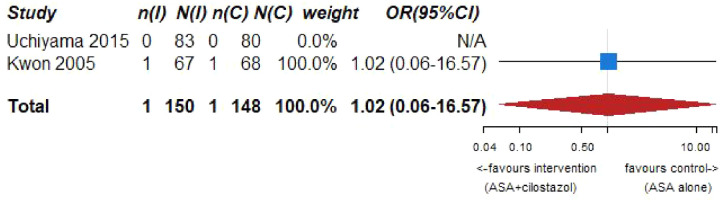
| Mortality (aspirin + cilostazol vs aspirin alone) | ||||||||||||
| 2 | Randomized trials | Serious b | Not serious | Serious c | Serious c | None | 1/150 (0.7%) | 1/148 (0.7%) | OR 1.02 (0.06–16.57) | 0 fewer per 1000 (from 6 fewer to 95 more) | ⨁◯◯◯ Very low |
CRITICAL |
CI: confidence interval; OR: odds ratio.
High dropout rate in TOSS, no Placebo use in CSPS.com.
TOSS included patients with acute ischemic stroke within 2 weeks, CATHARSIS between 2 weeks and 6 months.
In TOSS, during the follow-up period, no strokes or transient ischemic attacks occurred. In CATHARSIS, few endpoint events (large confidence interval). High statistical weight of only 1 study (CSPS.com subanalysis).
Both studies are subanalysis culled from CHANCE and THALES. In the CHANCE subgroup analysis, the endpoint reported on and included here was any stroke, in the THALES subanalysis it was recurrent stroke or death. In CHANCE, Aspirin was combined with Clopidogrel, in THALES with Ticagrelor. Inclusion NIHSS was ⩽3 in CHANCE, ⩽5 in THALES.
Definition of MACE varied across studies.
Definitions of major bleeding varied across trials.
Figure 7.1.
PICO 7 - Risk of bias assessment.
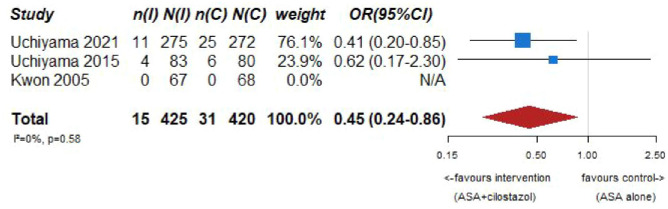
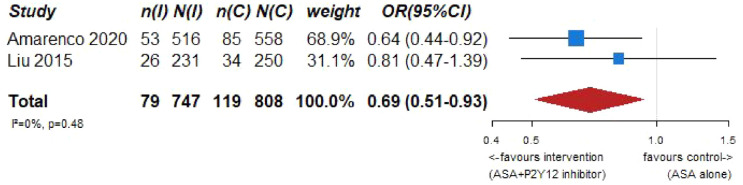
Our systematic literature search identified three subgroup analyses on patients with an acute ischemic stroke and ICAD derived from three large randomized-controlled clinical trials comparing dual antiplatelet therapy with aspirin combined with either clopidogrel, ticagrelor or cilostazol to single antiplatelet therapy for early secondary stroke prevention.64–66 The 3 trials the subgroup analyses were culled from were: (1) Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE), 67 (2) The Acute Stroke or Transient Ischemic Attack Treated With Ticagrelor and ASA for Prevention of Stroke and Death (THALES), 68 (3) Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com). 69 Briefly, CHANCE compared aspirin combined with clopidogrel versus aspirin alone on reducing the 90-day risk of any stroke (ischemic or hemorrhagic) when initiated within 24 h of symptom onset in patients with acute minor stroke (National Institutes of Health Stroke Scale (NIHSS) ⩽ 3) or high-risk TIA. 67 For the CHANCE subanalysis on intracranial stenosis due to ICAD, only patients with baseline MR angiography were included (21% of the overall CHANCE population, i.e. 1089/5170), where intracranial atherostenosis of 50%–99% was observed on MR angiography in 44.2% of patients. The primary endpoint was any stroke (ischemic or hemorrhagic) at 90 days. 64 In THALES, patients with a non-cardioembolic ischemic stroke with NIHSS ⩽ 5 or high-risk transient ischemic attack were randomized to ticagrelor for 30 days or placebo added to aspirin within 24 h of symptom onset. 68 For the THALES subanalysis, we summarized the estimates only of patients with intracranial ipsilateral arterial caliber reduction ⩾30% (9.7% of the overall THALES population, i.e. 1074/11,016), as defined in the post-hoc subanalysis. The primary endpoint was recurrent stroke or death at 30 days. In the aspirin-ticagrelor group, 516 patients had intracranial ipsilateral atherosclerotic stenosis, in the aspirin-only group 558 patients. 66 CSPS.com compared a combination of aspirin and cilostazol to a monotherapy with either aspirin or clopidogrel among patients with MRI confirmed non-cardioembolic ischemic stroke between 8 and 180 days from randomization. Because of a delay in recruitment, the trial was stopped after enrollment of 1884 patients (of an anticipated 4000 patients). 69 For the CSPS.com subanalysis, only patients with intracranial atherostenosis >50% were included in the analysis (n = 547). 65 Of those, 275 were randomized to receive dual antiplatelet therapy with cilostazol and 272 to single antiplatelet therapy. As such, the sample size of the CSPS.com subanalysis, was larger than that of the TOSS or CATHARSIS trials. We pooled three trials combining aspirin with cilostazol in the summary of evidence.
The risk of bias is depicted in Figure 7.1. The summary of evidence for are depicted below (Figure 7.2–7.7).
Figure 7.2.
PICO 7 – Association between aspirin + cilostazol intake, compared to aspirin intake alone, and risk of recurrent IS in RCT.
Figure 7.3.
PICO 7 – Association between aspirin + P2Y12 inhibitor intake, compared to aspirin intake alone, and risk of recurrent IS or death in RCT.
Figure 7.4.
PICO 7 – Association between aspirin + cilostazol intake, compared to aspirin intake alone, and risk of MACE in RCT.
Figure 7.5.
PICO 7 – Association between aspirin + cilostazol intake, compared to aspirin intake alone, and risk of major bleeding in RCT.
Figure 7.6.
PICO 7 – Association between aspirin + cilostazol intake, compared to aspirin intake alone, and risk of hemorrhagic stroke in RCT.
Figure 7.7.
PICD 7 – Association between aspirin + cilostazol intake, compared to aspirin intake alone, and death in RCT.
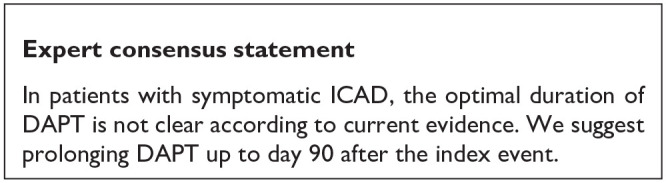
Additional information
The duration of dual antiplatelet therapy (DAPT) varied across trials: 21 days in CHANCE, 30 days in THALES, and at least 6 months in CSPS.com. The SAMMPRIS trial compared best medical therapy (BMT) alone – including the combination of aspirin with clopidogrel over 90 days – versus BMT combined with percutaneous transluminal angioplasty and stenting (PTAS) in patients with a symptomatic 70%–99% intracranial stenosis. BMT alone was superior to PTAS, leading many physicians to opt for a 90-day course of dual antiplatelet therapy among patients with a symptomatic, high-grade intracranial stenosis. 9 The preference toward dual antiplatelet therapy of aspirin and clopidogrel over 90 days was reinforced by a post-hoc study that compared the SAMMPRIS control group to the patients meeting the same qualifying criteria from the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, where no dual antiplatelet therapy was used. After adjusting for baseline characteristics, patients in the SAMMPRIS medical arm had an almost two-fold lower risk the primary endpoint. 70
PICO 8: In patients with an ischemic stroke (IS) or transient ischemic attack (TIA) related to a high-grade stenosis due to ICAD, does angioplasty and/or stenting plus BMT, as compared to BMT alone, improve outcome?
Analysis of current evidence
Two randomized-controlled clinical trials, the SAMMPRIS trial9,71 and the VISSIT trial 72 (see Table 7 for GRADEpro ratings of the included trials), fulfill the criteria to answer the PICO for seven outcomes: recurrent IS within 30 and 90 days, recurrent IS in the long-term follow-up (1 year), risk of MACE (including stroke), major bleeding events (including ICH), restenosis/reocclusion at 1 year and mortality.
Table 7.
GRADE evidence profile ratings for PICO 8.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Does angioplasty and/or stenting plus BMT | BMT alone | Relative (95% CI) | Absolute (95% CI) | ||
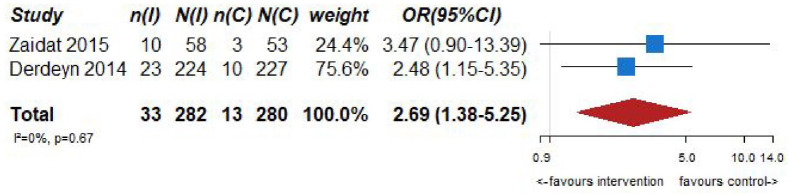
| Recurrent IS at 30 days | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Serious a | Not serious | None | 33/282 (11.7%) | 13/280 (4.6%) | OR 2.69 (1.38–5.25) | 69 more per 1000 (from 17 more to 157 more) | ⨁⨁⨁◯Moderate | CRITICAL |
| Long term recurrence of IS | ||||||||||||
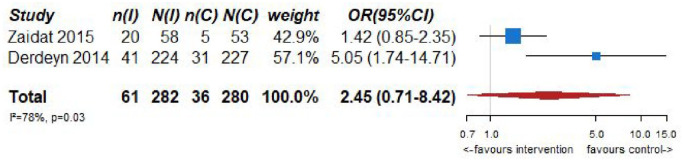
| 2 | Randomized trials | Not serious | Not serious | Serious a | Serious b | None | 61/282 (21.6%) | 36/280 (12.9%) | OR 2.45 (0.71–8.42) | 137 more per 1000 (from 34 fewer to 425 more) | ⨁⨁◯◯Low | CRITICAL |
| MACE | ||||||||||||
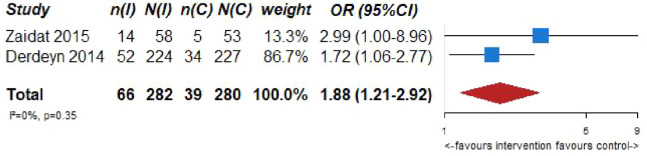
| 2 | Randomized trials | Not serious | Not serious | Serious a | Not serious | None | 66/282 (23.4%) | 39/280 (13.9%) | OR 1.88 (1.21–2.92) | 94 more per 1000 (from 24 more to 182 more) | ⨁⨁⨁◯ Moderate |
CRITICAL |
| Bleeding | ||||||||||||
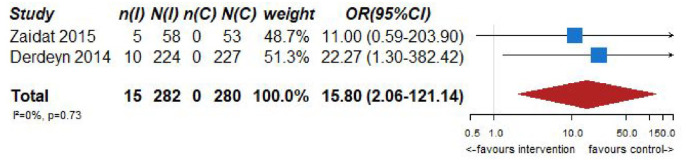
| 2 | Randomized trials | Not serious | Not serious | Serious a | Very serious c | None | 15/282 (5.3%) | 0/280 (0.0%) | OR 15.80 (2.06–121.14) | 0 fewer per 1000 (from 0 fewer to 0 fewer) | ⨁◯◯◯Very low | CRITICAL |
| Mortality | ||||||||||||
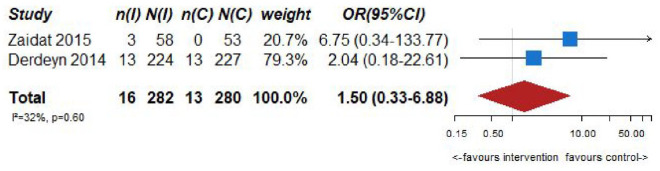
| 2 | Randomized trials | Not serious | Not serious | Serious a | Serious b | Sone | 16/282 (5.7%) | 13/280 (4.6%) | OR 1.50 (0.33–6.88) | 22 more per 1000 (from 31 fewer to 204 more) | ⨁⨁◯◯Low | CRITICAL |
CI: confidence interval; OR: odds ratio.
Older and various generation devices used in the RCTs.
Wide confidence interval.
Very wide confidence interval.
The SAMMPRIS (Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis) trial 9 randomized 451 patients with severe (70%–99%) intracranial atherostenosis recently symptomatic for TIA or IS to aggressive medical management (antiplatelet therapy, intensive management of vascular risk factors and a lifestyle-modification program) or aggressive medical management plus stenting with the Wingspan stent. The primary endpoint was any of the following: stroke or death within 30 days after enrollment, IS in the territory of the qualifying artery beyond 30 days of enrollment, or stroke or death within 30 days after a revascularization procedure of the qualifying lesion during follow-up. Thirty-three (14.7%) of 224 patients in the stenting group and 13 (5.8%) of 227 patients in the medical group had died or had a stroke within 30 days. According to an intention to treat analysis beyond 30 days, 21 (10%) of 210 patients in the medical group and 19 (10%) of 191 patients in the stenting group had a primary endpoint event. A long-term follow-up was performed with a median duration of 32.4 months. At the end of the follow-up 34 (15%) of 227 patients in the medical group and 52 (23%) of 224 patients in the stenting group had a primary endpoint event. 71 Moreover, the stenting group showed more events than the medical group for: any stroke (59 (26%) of 224 patients vs 42 (19%) of 227 patients; p = 0.0468) and major hemorrhage (29 (13%) of 224 patients vs 10 (4%) of 227 patients; p = 0.0009). The study confirmed the early and sustained benefit of BMT on PTAS with the Wingspan system in high-risk patients with atherosclerotic intracranial arterial stenosis.
The VISSIT (Vitesse Intracranial Stent Study for Ischemic Stroke Therapy) trial aimed to evaluate the efficacy and safety of the balloon-expandable stent plus medical therapy versus medical therapy alone in patients with symptomatic intracranial stenosis (>70%). 72 A total of 112 patients were randomized to receive balloon-expandable stent plus medical therapy (stent group; n = 59) or medical therapy alone (medical group; n = 53). Primary outcome measure was a composite of stroke or TIA in the same territory within 12 months of randomization. Primary safety measure was a composite of any stroke, death, or intracranial hemorrhage within 30 days of randomization and any TIA between days 2 and 30 of randomization. The 30-day primary safety end point occurred in more patients in the stent group (14/58; 24.1% (95% CI: 13.9%−37.2%)) versus the medical group (5/53; 9.4% (95% CI: 3.1%−20.7%); p = 0.05). Intracranial hemorrhage within 30 days occurred in five patients in the stent group (5/58; 8.6% (95% CI: 2.9%−19.0%)) versus none in the medical group (95% CI: 0%−5.5%; p = 0.06). The 1-year primary outcome of stroke or TIA occurred in more patients in the stent group (21/58; 36.2% (95% CI: 24.0–49.9)) than in the medical group (8/53; 15.1% (95% CI: 6.7–27.6); p = 0.02) with a similar course of worsening of baseline disability score (14/58; 24.1% (95% CI: 13.9%−37.2%) in the stent group and in the medical group (6/53; 11.3% (95% CI: 4.3%−23.0%; p = 0.09)).
Both the SAMMPRIS and VISSIT trial have several limitations. One of them is that a minimum experience for the participating physicians was defined (e.g. in the VISSIT trial physicians must have placed an intracranial stent in at least 10 patients (for aneurysm or atherosclerosis) in the 12 months prior to site initiation; in the SAMMPRIS trial PTAS was performed by physicians who were selected by a committee of experienced physicians on the basis of their review of procedure notes and outcomes for the 20 most recent consecutive cases of intracranial stenting or angioplasty). The most important is the used Wingspan stent systems. Although the stent has advantages over balloon-expandable stents due to relative ease of delivery, the effectiveness of the self-expanding stent in restoring lumen diameter and preventing restenosis has been debated. The radial force exerted by the Wingspan stent system is comparable lower than that of balloon-expanding stents, which might have impacted results. Furthermore, with newer devices introduced to the market the usage of the Wingspan stent system could be understood as a deviation from the intended intervention, as newer devices might have a better risk/benefit ratio. 73 We therefore downgraded the evidence level from moderate to low.
Quality assessment revealed a low risk of bias for both trials (Figure 8.1) for the seven outcomes.
Figure 8.1.
PICO 8 - Risk of bias assessment.
The pooled analyses of the two trials9,71,72 (282 patients randomized to receive BMT + angioplasty and stenting and 280 patients randomized to receive BMT) suggested a significant difference in the risk of ischemic stroke recurrence at 30 days in favor of the BMT group (OR = 2.69, 95% CI: 1.38–5.25; Figure 8.2) with a concomitant risk profile more beneficial in the BMT group according to the major bleeding risk outcome (OR = 15.80, 95% CI: 2.06–121.14; Figure 8.3) during follow-up. The pooled analysis for overall mortality showed no significant difference between BMT group and BMT + angioplasty and stenting group (OR = 1.50, 95% CI: 0.22–6.88; Figure 8.4). Similarly, the pooled analysis for long term IS recurrence (1 year) did not show a significant difference between the two groups (OR = 2.45, 95% CI: 0.71-8.42; Figure 8.5). Recurrent IS at 90 days was reported only in one trial, in the SAMMPRIS trial.9,71 The pooled analysis for MACE was illustrated in Figure 8.6 and showed a significant difference in favor of BMT group (OR = 1.88, 95% CI: 1.21–2.92). For the outcome measure restenosis/reocclusion it is not possible to completely compare the two trials because only VISSIT trial 72 routinely assessed it in both groups and SAMMPRIS trial9,71,74 assessed only symptomatic restenosis/occlusions. With this limitation, the restenosis rate of 16.82% (95% CI 4.82–33.84) reported in Figure 8.7 is likely underscored.
Figure 8.2.
Pico 8 – Association between angioplasty and/or stenting + BMT compared to BMT and risk of recurrent IS at 30 days in RCT.
Figure 8.3.
PICO 8 – Association between angioplasty and/or stenting + BMT compared to BMT and risk of major bleeding in RCT.
Figure 8.4.
PICO 8 – Association between angioplasty and/or stenting + BMT compared to BMT and overall mortality in RCT.
Figure 8.5.
Pico 8 – Association between angioplasty and/or stenting + BMT compared to BMT and risk of long term recurrence of IS in RCT.
Figure 8.6.
Pico 8 – Association between angioplasty and/or stenting + BMT compared to BMT and risk of MACE in RCT.
Figure 8.7.
PICO 8 – Restenosis rate in the angioplasty and/or stenting + BMT group of RCT.
No heterogeneity between trials was identified in any of the outcomes, but the VISSIT trial 72 was prematurely terminated having enrolled less than one-half of the planned number of patients.
Additional information
There are no RCT data comparing angioplasty and/or stenting to BMT in patients with symptomatic intracranial stenosis 50%–69% and there are no RCT data comparing angioplasty versus angioplasty followed by stenting. In the SAMMPRIS trial 9 183 patients underwent a procedure (four of whom had angioplasty only).
In the SAMMPRIS trial the rate of periprocedural stroke after PTAS was higher than expected and the 30-day rate of stroke or death in the PTAS group (14.7%) is substantially higher than the 4.4%–9.6% rates previously reported with the use of the Wingspan stent in the phase I trial and in two registries.75–77 Conversely, the rate of stroke in the medical-management group was much lower than expected according to WASID trial.59,76–78
In-stent restenosis is not a rare occurrence in intracranial stenosis. A recent metanalysis 79 identified 51 studies with 5043 patients and the pooled incidence rate of in-stent restenosis was 14.8% (95% CI: 11.9%–17.9%). Multiregression analysis revealed that younger patient age was related to higher in-stent restenosis rates (p = 0.019), and vertebrobasilar junction location (p = 0.010) and low residual stenosis (p = 0.018) were two independent risk factors for symptomatic in-stent restenosis rate.
Following SAMMPRIS, 9 several single-center and multicenter trials and registries demonstrated safer periprocedural results with the Wingspan stent.80–87 In a multicenter trial comparing a balloon-expandable stent with the Wingspan self-expanding stent in over 300 patients, Ma et al. 85 demonstrated a 4% periprocedural complication rate, and a total 1-year follow-up stroke, TIA, bleeding and death rate of 7.9% in the Wingspan-treated group.
The WEAVE (Wingspan stEnt system postmArket surVEillance) trial is the largest up to date on-label trial for the self-expandable Wingspan stent system in patients with ICAD. 88 The Wingspan stent system in this setting was safe and the rate of any stroke or death was lower than the target of 4% periprocedural safety set by the FDA. The 1-year follow-up of this cohort was assessed in the WOVEN trial 89 on 12 of the original 24 sites enrolling patients in the WEAVE trial. Including the four patients who had periprocedural events in the WEAVE study, there were 11 strokes or deaths in the 129 patients (8.5%) at the 1-year follow-up.
One of the potential factors explaining these results is the bigger experience of interventionalists as well as by favorable selection of patients meeting the criteria for stenting by strictly following the on-label indications. The premedication regimen with antiplatelet therapy started at least 5 days prior to the stenting and was very strict. Interventionalists were adequately trained. The impact of the experience of the interventionist was demonstrated in the WEAVE trial, as those interventionists who had a case experience of 50 Wingspan stents or greater had no index events in the periprocedural period, and those with less than 50 had a 4.8% periprocedural complication rate.
Data from the recent trials and registries suggest that performing angioplasty and stenting in the early time period, particularly 7 days or less from the qualifying stroke, may result in a higher periprocedural complication rate. A recent metanalysis on intracranial angioplasty and stenting versus medical treatment in intracranial stenosis from 2016, revealed worse long-term outcomes of PTAS compared to medical management [composite outcome of any stroke or death within 1 year (RR = 2.29, 95% CI 1.13–4.66) and 2 years (RR = 1.52, 95% CI 1.04–2.21) 90 in line with the SAMPRIS trial.
The treatment of symptomatic ICAD in Asian population may deserve a separate consideration. Currently the study results of two additional Wingspan trials are pending, the CASSISS trial (China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis) from China 91 and the WICAD study (Wingspan for IntraCranial Atherosclerotic Disease) from Japan. Both trials have demonstrated in early reports similar safety results with the on-label use of the stent.
Two additional aspects that could improve the results of the intracranial angioplasty and stent placement are improvement in device design and point-of-care testing for assessing the magnitude of platelet inhibition with antiplatelet medication. 86 A new generation of balloon-expanding stents with a rapid-exchange platform or new PTA-balloon that can be also used as microcatheters for the stent-implantation may result in superior technical results. 92 Drug-eluting stents93,94 or PTA balloons may also be deployed to decrease the rate of persisting ICAD and restenosis.
There are some unanswered questions in the topic of medical versus neurointerventional treatment of symptomatic severe ICAS, related to well defined clinical scenarios and the improvement of techniques and materials. Pathophysiological mechanisms of stroke may help to select the treatment and this especially affects patients with recurrent ischemic stroke despite well conducted BMT. One of the criticisms about SAMMPRIS trial 9 was that 35.3% of patients included in the PTAS group had not previously failed antithrombotic therapy, according to the Food and Drug Association criteria (deployment of the self-expanding Wingspan stent only in patients having suffered at least two ischemic strokes, attributable to 50% or higher ICAD, while receiving antithrombotic therapy). 76 The combination of artery-to-artery embolism and hemodynamic failure with poor collaterals (typical infarction in a border zone location and pattern) may represent a clue to consider PTCA and stenting in selected patients. The SAMMPRIS trial 9 did not show a benefit in the subgroup of patients with cerebrovascular ischemic events with underlying hypoperfusion with/without poor collaterals,95,96 but the small sample size of this subgroup analysis limits this finding.
Recent advances in neuroimaging technologies allow to determine the underlying pathophysiological mechanism and to add information about plaque features through High Resolution Vessel Wall Imaging MRI.97,98
Another point is the lack of comparative data with new antithrombotic drugs, e.g. ticagrelor, acting as a platelet P2Y12 receptor antagonist, which has been studied among patients with non-cardioembolic minor ischemic stroke or TIA in the SOCRATES (Acute Stroke Or Transient isChemic attack tReated with Aspirin or Ticagrelor and patient outcomES) study.98,99


PICO 9: In patients with an ischemic stroke or transient ischemic attack related to a high-grade stenosis due to ICAD do any neurosurgical intervention plus BMT, compared to BMT alone, improve outcome?
Analysis of current evidence
Our systematic review identified one RCT that compares Extracranial-Intracranial (EC-IC) bypass to medical treatment (see Table 8 for the GRADEpro ratings of the included study). The EC-IC bypass study group trial, published in 1985, was a multicenter RCT that randomly assigned patients with an ischemic stroke or TIA within 90 days from symptom onset, related to stenosis or occlusion of the trunk or major branches of the MCA, stenosis of the ICA at or above C2 or ICA occlusion, to receive either surgery between end-to-side anastomosis of the superficial temporal or occipital artery to a cortical branch of the MCA. 100 All patients were on aspirin (325 mg four times a day) and under blood pressure control. 1377 patients were included, 714 (52%) were randomly assigned to medical treatment and 663 (48%) to surgical therapy (superficial temporal artery to middle cerebral artery bypass). Patients were included between December 1984 and May 1985 and average duration of follow-up was 55.8 months (range 28–90). In the perioperative period of 30 days, 12.2% patients in the surgical arm had a cerebral or retinal ischemic events, compared to 3.4% in the medical arm. Major strokes were also more common in the surgical arm (4.5%) compared to the medical arm (1.3%); excess of major stroke in the perioperative group was 3.2%. Mortality at 30 days was higher in the surgical arm (7/663 due to stroke) compared to medical arm (1/714 due to myocardial infarction). At the end of the study mortality days was higher in the surgical arm (20%) compared to medical arm (17%). Major stroke occurred more frequently in the surgical group (7% vs 5%); and minor stroke occurrence was similar in both groups (19%).
Table 8.
GRADE evidence profile table for PICO 9.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Any neurosurgical interventions plus BMT | BMT alone | Relative (95% CI) | Absolute (95% CI) | ||
| Recurrent stroke at 30 days | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 30/663 (4.5%) | 9/714 (1.3%) | Not estimable | ⨁⨁◯◯ Low |
CRITICAL | |
| Mortality at 30 days | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 7/663 (1.1%) | 1/714 (0.1%) | Not estimable | ⨁⨁◯◯ Low |
CRITICAL | |
CI: confidence interval.
No effect estimate available; only one study available for the outcome.
Quality assessment of the only trial performed is shown in Figure 9.1 and it revealed some concerns about the deviations from the intended intervention with a significant impact on the overall quality of the evidence.
Figure 9.1.
PICO 9 - Risk of bias assessment.
The predefined outcomes of the PICO were not all retrievable. In particular, no data were provided on recurrent IS at 90 days and in the long-term follow-up (12 months), risk of MACE including stroke, major bleeding event inclusive of intracranial hemorrhage and restenosis/reocclusion at 1 year.
The risk of recurrent IS at 30 days was 30/663 (4.5%) in the surgical arm versus 9/714 (1.3%) in the medical arm but these results are affected by the concerns about the risk of bias in the trial (mainly deviations from the intendent protocol). The mortality outcome was 113/663 (17%) in the surgical arm versus 143/714 (20%) in the medical arm. It was affected by an early mortality risk in the surgical arm because of stroke.
Additional information
In 2019 a monocenter study 101 included 63 patients with a recent ischemic stroke related to an occlusion or stenosis >70% of the MCA and/or intracranial segment of the ICA and reduced cerebral perfusion displayed by CT Perfusion (stage II and III: decompensated and ischemic stage). Patients could decide between medical or surgical treatment (superficial temporal artery-middle cerebral artery bypass). The number of ischemic events was 13.3% in the surgical group compared to 48.5% in the medical group (p = 0.003) and the annual stroke risk was 6.7% and 25.6% respectively (p = 0.002). Interestingly, cerebral perfusion improved in the surgical arm. Unfortunately, this study was observational and based on the patient’s choice of treatment without randomization. However, it provides interesting clues in selected patients with reduced cerebral perfusion.
Recently, the ERSIAS a phase II trial has been published. 102 52 patients with symptoms, despite medical treatment, related to occlusion or stenosis ⩾70% of the MCA and/or intracranial segment of the ICA and poor collaterals (ASITN/SIR grades 0–2 on angiography 103 ) were treated by encephaloduroarteriosynangiosis (EDAS). EDAS is an indirect revascularization technique which is mainly used for the treatment of moyamoya disease, to promote the natural tendency of the disease to develop cerebrovascular collaterals. For this purpose, a scalp arteria with a strip of galea is transplanted to a narrow linear dural opening. The distal and proximal vessels could be left open in this procedure as compared to direct bypass techniques. 104 Event rates were compared with propensity-score-match in medically patients treated from SAMMPRIS 9 and COSS (Carotid Occlusion Surgery Study). 105 Ischemic stroke or death occurred in 9.6% of the patients compared to 20% of patients in the SAMMPRIS medical group (p = 0.07), meeting the p < 0.10 threshold for non-futility. Despite the interest of these results, quality of the data is not very high as patients were not randomized and data was compared to historical patients.

PICO 10: In patients with an ischemic stroke or transient ischemic attack related to a high-grade intracranial atherostenosis, does remote ischemic pre-conditioning plus BMT, compared to BMT alone, improve outcome?
Analysis of current evidence
Our systematic review identified two RCTs (see Table 9 for GRADEpro ratings of the included studies) that have compared standard medical care alone to repetitive bilateral arm ischemic preconditioning (BAIPC).106,107 Both were randomized-controlled clinical trials, one including only patients younger than 80 years 107 and one patients 80 years and older. 106 In both studies patients presenting within 7 days of an ischemic stroke or TIA which was caused by a stenosis on MRA or CTA of at least 70% of a major intracranial artery (carotid, middle cerebral, vertebral, or basilar) were randomly assigned to standard medical care or BAIPC plus standard medical care. Patients with concomitant extracranial stenosis were excluded. Standard medical care consisted of either clopidogrel 75 mg/day in combination with atorvastatin 20 mg/day or aspirin 100 mg/day and clopidogrel 75 mg/day in combination with atorvastatin 20 mg/day.
Table 9.
GRADE evidence profile ratings for PICO 10.
| Question: Remote ischemic conditioning plus BMT compared to BMT for outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Setting: In patients with an ischemic stroke or transient ischemic attack related to a high-grade intracranial atherostenosis | ||||||||||||
| Bibliography: | ||||||||||||
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Remote ischemic conditioning plus BMT | BMT | Relative (95% CI) | Absolute (95% CI) | ||
| Recurrent IS at 90 days | ||||||||||||
| 1 | Randomized trials | Serious a | Not serious | Not serious | Serious b | None | 2/38 (5.3%) | 7/30 (23.3%) | OR 0.18 (0.03–0.96) | 181 fewer per 1000 (from 224 fewer to 7 fewer) | ⨁⨁◯◯ Low |
CRITICAL |
| Long term recurrence of IS | ||||||||||||
| 2 | Randomized trials | Serious a | Not serious | Not serious | Serious c | None | 5/68 (7.4%) | 13/58 (22.4%) | OR 0.27 (0.09–0.81) | 152 fewer per 1000 (from 199 fewer to 35 fewer) | ⨁⨁◯◯ Low |
CRITICAL |
| Mortality | ||||||||||||
| 1 | Randomized trials | Serious a | Not serious | Not serious | Very seriousb,d,e | None | 0/30 (0.0%) | 1/28 (3.6%) | OR 0.30 (0.01–7.69) | 25 fewer per 1000 (from 35 fewer to 186 more) | ⨁◯◯◯ Very low |
CRITICAL |
CI: confidence interval; OR: odds ratio.
One item graded serious in ROB2.
Results driven by only one study.
Small sample size.
Wide confidence interval.
Very few events.
The first study included patients younger than 80 years in the BAIPC group (n = 38), who underwent five brief cycles consisting of bilateral upper limb ischemia followed by reperfusion, which was performed twice daily over 300 days. Incidence of recurrent stroke and cerebral perfusion status in BAIPC-treated patients was compared with the control group (n = 30). In the BAIPC group, the incidence of recurrent stroke was 5% and 7.9% at 90 and 300 days, compared to 23.3% and 26.7%, respectively in the control group (p < 0.01). 107
In the second study, patients 80 years and older underwent BAIPC treatment twice daily for 180 consecutive days and consisted of five cycles of simultaneous bilateral upper arm ischemia for 5 min followed by reperfusion for another 5 min. 106 This study included 58 patients consisting of 30 patients in the BAIPC group and 28 controls who underwent sham-BAIPC twice daily and 28 controls. During the 180-day follow-up 2 strokes and 7 TIAs were observed in the BAIPC group compared with 8 strokes and 11 TIAs in the sham BAIPC group (p < 0.05). Moreover, BAIPC had no adverse effects on blood pressure, heart rate, local skin integrity, or plasma myoglobin, and did not induce cerebral hemorrhage but BAIPC reduced plasma high sensitive C-reactive protein, interleukin-6, plasminogen activator inhibitor-1, leukocyte count, and platelet aggregation rate and elevated plasma tissue plasminogen activator (all p < 0.01). In this study 21 cases had to be excluded (15 with incomplete data and 6 were lost to follow-up) 106
Quality assessment revealed a high risk of bias for both trials (Figure 10.1). The high risk of bias is due to an absence of blinding and of appropriate analysis (only per protocol analysis, no intention to treat analysis).
Figure 10.1.
PICO 10 - Risk of bias assessment.
Pooled analyses of these two trials106,107 (68 patients randomized to BAIPC and 58 randomized to receive best medical therapy alone) suggested a risk reduction of long-term ischemic stroke recurrence in the remote ischemic conditioning plus BMT group (OR = 0.27, 95% CI: 0.09–0.81; Figure 10.2). Only the first trial evaluated risk reduction for ischemic stroke recurrence at 90 days and suggested a risk reduction in the remote ischemic conditioning plus BMT group in patients younger than 80 years (OR = 0.18, 95% CI: 0.03–0.96). 107 In contrast, only the second trial evaluated the risk of mortality and suggested a risk reduction in the remote ischemic conditioning plus BMT group in patients 80 years and older (OR = 0.30, 95% CI: 0.01–7.69). 106
Figure 10.2.
PICO 10 – Association between remote ischemic conditioning plus BMT compared to BMT alone and the risk of long term recurrence of IS in RCTs.

PICO 11: In patients with an ischemic stroke or transient ischemic attack related to an intracranial atherostenosis, does aggressive vascular risk factor control, including lipid management, improve outcome?
Analysis of current evidence
Patients with symptomatic atherostenosis have a particularly high risk of recurrent stroke and other major vascular events. It is accepted that general stroke secondary prevention measures should be adopted in patients with symptomatic intracranial atherostenosis, being usually referred to the so-called aggressive control of vascular risk factors. We reviewed current evidence on the effect of intensity of risk factor control in the outcome of symptomatic ICAD patients (stroke recurrence and risk of MACE).
We only found two RCTs regarding risk factor management in symptomatic ICAD. (see Table 10 for the GRADEpro ratings of the included studies) Zhou et al. 108 designed a single-center, randomized, parallel-group clinical trial to assess the effect of different doses of atorvastatin in patients with ischemic stroke or TIA due to MCA or basilar stenosis, including 120 patients. Compared to those that received low-dose atorvastatin (10 mg), stroke recurrence in the same territory was significantly lower in patients that received high-dose atorvastatin (40 mg) after 52 weeks of follow-up (13.5% vs 26.3%, log-rank p = 0.012). Park et al. 54 designed a randomized trial to evaluate intensive control of BP including 132 patients with symptomatic ICAS within 7–42 days after index stroke. After 24 weeks, there were no differences between intensive (target systolic BP < 120 mmHg) or modest (target systolic BP < 140 mmHg) BP control in terms of white matter lesion volume change, infarct growth, stroke recurrence (only one recurrent stroke in each arm) or major vascular events (17 in the intensive and 13 in the modest control groups). Quality assessment revealed a low risk of bias for Park et al trial and some concerns for Zhou et al. trial (see Figure 11.1). Certainty was graded as low for both trials and both outcomes (stroke recurrence and risk of MACE) mainly due to the low number of events and sample size included in the analyses.
Table 10.
GRADE evidence profile ratings for PICO 11.
| Question: Aggressive vascular risk factor control, including lipid management compared to for outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Setting: Patients with an ischemic stroke or transient ischemic attack related to a high-grade ICAS. | ||||||||||||
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Aggressive vascular risk factor control, including lipid management | Relative (95% CI) | Absolute (95% CI) | |||
| Recurrent IS – Target SBP < 120 mmHg versus SBP < 140 mmHg | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very seriousa,b,c,d | None | 1/59 (1.7%) | 1/52 (1.9%) | Not estimable | ⨁⨁◯◯ Low |
||
| Recurrent IS – intensive dose atorvastatin versus low dose atorvastatin | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very seriousb,c | None | 2/37 (5.4%) | 10/38 (26.3%) | Not estimable | ⨁⨁◯◯ Low |
||
| Risk of MACE – target SBP < 120 mmHg versus SBP < 140 mmHg | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very seriousb,c,d | None | 14/59 (23.7%) | 12/52 (23.1%) | Not estimable | ⨁⨁◯◯ Low |
CRITICAL | |
| Mortality – target SBP < 120 mmHg versus SBP < 140 mmHg | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very seriousb,c,d,e | None | 0/59 (0.0%) | 0/52 (0.0%) | Not estimable | ⨁⨁◯◯ Low |
CRITICAL | |
| Mortality – intensive dose atorvastatin versus low dose atorvastatin | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very seriousb,c,e | None | 0/37 (0.0%) | 0/38 (0.0%) | Not estimable | ⨁⨁◯◯ Low |
CRITICAL | |
CI: confidence interval.
Very few events.
Results from only one RCT.
Sample size included in analysis did not match the prespecified sample size for the RCT.
BP target not achieved in the intensive BP lowering group.
No event.
Figure 11.1.
PICO 11 - Risk of bias assessment.
Additional information
Post-hoc analyses from the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, in which patients were treated with standard of care risk factor management at that moment, showed that poorly controlled BP during follow-up was an important risk factor for recurrent stroke and other vascular events. 57 These findings led to the incorporation of intensive risk factor management in the design of the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Compared to WASID, recurrent stroke in the medical arm of the SAMMPRIS trial was reduced by almost 50%, and part of this improvement may be attributed to a better control of vascular risk factors. 70 Similar to WASID, cholesterol and BP control during follow-up were associated with fewer recurrent strokes and vascular events in a post hoc analysis of SAMMPRIS. Moreover, physical inactivity emerged as an important independent predictor of stroke and vascular events in these patients after 3 years of follow-up, 109 highlighting the importance of non-pharmaceutical interventions such as lifestyle altering programs (i.e. in case of the SAMMPRIS trial the INTERVENT® Lifestyle Management Program). 109
Another outcome to be evaluated in patients with stenosis due to ICAD may be stenosis progression, although it is not always related to stroke recurrence. In a secondary analysis of TOSS-2 trial (Trial of cilOstazol in Symptomatic intracranial Stenosis 2), authors conclude that very-high systolic BP level during the short-term period after the index stroke was associated with significantly higher odds of stenosis progression, with no reported effect on clinical prognosis. 110 In the STAMINA observational study using HRVWI, higher reduction of LDLC and longer duration of statin treatment were associated with decreased enhancement volume as a surrogate marker of plaque stability and with decreased stenosis degree, 111 again with no reported clinical effect. We need more evidence that BP control or statins may induce plaque stabilization and/or stenosis regression, and that these radiological outcomes are associated with better clinical outcomes.
The recommended targets for secondary prevention in very-high-risk patients (which includes stroke patients with documented atherosclerosis) in the last ESC/EAS 2019 Dyslipidemia Guidelines are an LDL-C reduction of >50% from baseline and an LDL-C goal of <1.4 mmol/L (<55 mg/dl). 112
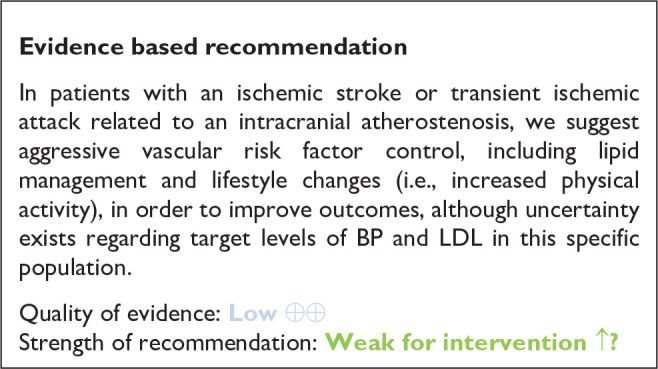
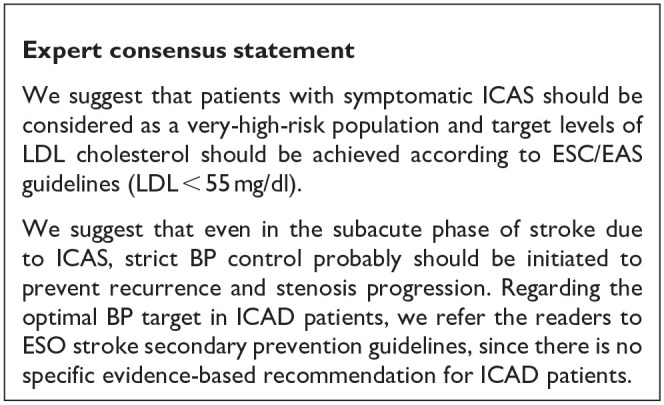
Discussion
Despite the epidemiological importance of ICAD as a major cause of stroke worldwide and the relevance and complexity of the clinical problems affecting ICAD patients, our systematic review disclosed an insufficient degree of evidence for most of the practical questions posed. Indeed, we were able to provide recommendations based on evidence coming from dedicated RCTs in only 5 out of the 11 PICOs. For the remaining PICOs, our suggestions derive from observational studies or from expert consensus, and therefore should be taken with caution. Thus, our results should encourage clinicians and researchers all over the world to conduct well-designed multi-center RCTs on ICAD to find evidence-based answers to solve the scientific questions that are proposed in this guideline. All evidence-based recommendations and expert consensus statements are summarized in Table 11.
Table 11.
Summary of all PICOs.
| Recommendation | Expert consensus statement |
|---|---|
| PICO 1: In adult stroke-free subjects, is screening compared to no-screening for intracranial atherosclerosis beneficial for the prevention of Major Adverse Cardiovascular Events (MACE) including ischemic stroke? | |
| In adult stroke-free subjects, the benefits of screening
programs to detect the presence of asymptomatic intracranial
atherosclerosis are uncertain and therefore we cannot make a
recommendation regarding routine screening for
ICAD. Quality of evidence: Low ⊕⊕ Strength of recommendation: – |
Screening for asymptomatic ICAD in stroke-free individuals to
help assess their vascular risk is not suggested as a prevention
strategy. However, the detection of asymptomatic intracranial atherosclerosis or calcification as an incidental finding on neuroimaging exams implies a significantly higher risk for future major vascular events including stroke. Therefore, patients with asymptomatic intracranial atherosclerosis or calcification, may need to be recognized as harboring a high vascular risk. |
| PICO 2: In subjects with asymptomatic intracranial atherosclerosis, does antiplatelet treatment compared with no antiplatelet treatment lower the risk of MACE including ischemic stroke? | |
| In subjects with asymptomatic intracranial atherosclerosis,
whether antiplatelet treatment lowers the risk of MACE including
ischemic stroke is still uncertain. Therefore, we cannot make a
recommendation regarding antiplatelet therapy. Quality of evidence: – Strength of recommendation: – |
We suggest antiplatelet treatment in subjects with asymptomatic intracranial atherosclerosis after appropriate assessment of the benefit/risk profile on an individual basis. As factors favoring the indication of antiplatelet therapy, we suggest to consider: high or very high vascular risk, presence of severe and/or multiple intracranial stenosis, progression of ICAD, and detection of covert infarctions within the brain territory distal to an intracranial stenosis. As factors against, we suggest to consider those associated with an increased systemic and/or intracranial bleeding risk under antiplatelet therapy. |
| PICO 3: In patients undergoing mechanical thrombectomy for an acute ischemic stroke due to an ICAD-related intracranial arterial occlusion, does infusion of glycoprotein IIb/IIIa inhibitors after initial mechanical thrombectomy, as compared with standard of care, improve functional outcome? | |
| In patients undergoing mechanical thrombectomy for an acute
ischemic stroke due to an ICAD-related intracranial arterial
occlusion, the benefit of the additional infusion of
glycoprotein IIb/IIIa inhibitors after initial mechanical
thrombectomy remains uncertain. Therefore, we cannot make a
recommendation, regarding the routine use of glycoprotein
IIb/IIIa inhibitors in this context based on current evidence.
We suggest enrolling patients in a dedicated
randomized-controlled clinical trial whenever
possible. Quality of evidence: Very Low ⊕ Strength of recommendation: – |
We suggest that if inclusion in a dedicated
randomized-controlled clinical trial is not possible,
glycoprotein IIb/IIIa inhibitors may be used as a rescue
strategy after assessing the bleeding risk for patients with an
acute ischemic stroke suspected to be caused by an underlying
ICAD in case of unsuccessful mechanical
thrombectomy.* *Please refer to the Supplemental material for more detailed instructions |
| PICO 4: In patients undergoing mechanical thrombectomy for an acute ischemic stroke due to an ICAD-related intracranial arterial occlusion, does angioplasty and/or stenting plus best medical management (BMT) after initial mechanical thrombectomy, as compared to BMT alone, improve functional outcome? | |
| In patients undergoing mechanical thrombectomy for an acute
ischemic stroke due to an ICAD-related intracranial arterial
occlusion, whether angioplasty and/or stenting after initial
mechanical thrombectomy improves outcome, remains unknown.
Therefore, we cannot make a recommendation regarding the use of
angioplasty and/or stenting in this context based on current
evidence. We suggest enrolling patients in a dedicated
randomized-controlled clinical trial whenever
possible. Quality of evidence: Very Low ⊕ Strength of recommendation: – |
We suggest that if inclusion in a dedicated
randomized-controlled clinical trial is not possible,
angioplasty and/or stenting may be used as a rescue therapy
after unsuccessful mechanical thrombectomy in patients with an
acute ischemic stroke suspected to be caused by underlying
ICAD.* This suggestion needs to be considered with caution,
since the referred studies with angioplasty and/or stenting in
ICAD-related LVO were focused mainly on Asian patients and their
results might not necessarily be generalizable to other
populations. *Please refer to the Supplemental material for more detailed instructions |
| PICO 5: In patients with an acute ischemic stroke or transient ischemic attack related to a high-grade intracranial atherosclerosis causing hemodynamic compromise, does permissive or induced hypertension, as compared to conventional blood pressure (BP) management (targeting normotension), during the acute phase, improve outcome? | |
| In patients with an acute ischemic stroke or transient ischemic
attack related to high-grade intracranial atherosclerosis
causing severe hemodynamic compromise, we cannot make a
recommendation regarding the use of permissive or induced
hypertension over conventional blood pressure management (target
normotension) during the acute phase, based on current
evidence. Quality of evidence: Moderate ⊕⊕⊕ Strength of recommendation: – |
In patients with high-grade symptomatic intracranial stenosis
and clinical or imaging signs of hemodynamic compromise we
suggest considering induced arterial hypertension as a rescue
treatment option, only after other more conservative measures to
improve cerebral hemodynamics have been tried. In the absence of specific evidence for ICAD patients, we suggest staying aligned with the expert consensus statement of ESO general guidelines on acute BP management. In patients with acute ischemic stroke not treated with reperfusion therapies (intravenous thrombolysis or mechanical thrombectomy) and with clinical deterioration where a hemodynamic mechanism is suspected or shown to be directly responsible for the deterioration, we suggest: • Stopping existing blood pressure lowering therapy, • Administering intravenous fluids and • Introducing non-pharmacological procedures to raise blood pressure Before considering • Careful use of vasopressor agents to increase blood pressure with close monitoring of blood pressure values. |
| PICO 6: In patients with an ischemic stroke or transient ischemic attack related to a high-grade intracranial atherostenosis and without any formal indication for anticoagulation, does anticoagulant therapy, as compared to antiplatelet therapy, improve outcome? | |
| In patients with an ischemic stroke or transient ischemic attack
due to high-grade stenosis related to ICAD we recommend against
oral anticoagulation over aspirin, unless there is another
formal indication for it. Quality of evidence: Moderate ⊕⊕⊕ Strength of recommendation: Strong against intervention ↓↓ |
– |
| PICO 7: In patients with an ischemic stroke or transient ischemic attack related to intracranial atherostenosis, does dual antiplatelet therapy, as compared to single antiplatelet therapy, improve outcome? | |
| In patients with an ischemic stroke or transient ischemic attack
related to intracranial stenosis due to ICAD we suggest dual
antiplatelet therapy over single antiplatelet therapy. Regarding
the duration of the dual antiplatelet therapy, we refer to the
additional information. Quality of evidence: Very low ⊕ Strength of recommendation: Weak for intervention ↑? |
In patients with symptomatic ICAD, the optimal duration of DAPT is not clear according to current evidence. We suggest prolonging DAPT up to day 90 after the index event. |
| PICO 8: In patients with an ischemic stroke (IS) or transient ischemic attack (TIA) related to a high-grade intracranial atherostenosis, does angioplasty and/or stenting plus BMT, as compared to BMT alone, improve outcome? | |
| In patients with an ischemic stroke or transient ischemic attack
related to a high-grade stenosis due to ICAD, we recommend
against angioplasty and/or stenting added to best medical
treatment as first-line treatment. Quality of evidence: Low ⊕⊕ Strength of recommendation: Strong against intervention ↓↓ |
We suggest considering endovascular treatment (angioplasty and/or stenting) as a rescue therapy in selected patients with symptomatic high-grade ICAS after clinical recurrence despite BMT. |
| PICO 9 In patients with an ischemic stroke or transient ischemic attack related to a high-grade intracranial atherostenosis do any neurosurgical intervention plus BMT, compared to BMT alone, improve outcome? | |
| In patients with an ischemic stroke or transient ischemic attack
related to a high-grade stenosis due to ICAD, we recommend
against neurosurgical procedures. Quality of evidence: Low ⊕⊕ Strength of recommendation: Strong against intervention ↓↓ |
– |
| PICO 10: In patients with an ischemic stroke or transient ischemic attack related to a high-grade intracranial atherostenosis, does remote ischemic pre-conditioning plus BMT, compared to BMT alone, improve outcome? | |
| In patients with an ischemic stroke or transient ischemic attack
related to a high-grade stenosis due to ICAD, we suggest
considering ischemic pre-conditioning as an adjuvant to BMT. We
suggest enrolling patients in a dedicated randomized-controlled
clinical trial whenever possible. Quality of evidence: Low ⊕⊕ Strength of recommendation: Weak for intervention ↑? |
– |
| PICO 11: In patients with an ischemic stroke or transient ischemic attack related to an intracranial atherostenosis, does aggressive vascular risk factor control, including lipid management, improve outcome? | |
| In patients with an ischemic stroke or transient ischemic attack
related to an intracranial atherostenosis, we suggest aggressive
vascular risk factor control, including lipid management and
lifestyle changes (i.e., increased physical activity), in order
to improve outcomes, although uncertainty exists regarding
target levels of BP and LDL in this specific
population. Quality of evidence: Low ⊕⊕ Strength of recommendation: Weak for intervention ↑? |
We suggest that patients with symptomatic ICAS should be
considered as a very-high-risk population and target levels of
LDL cholesterol should be achieved according to ESC/EAS
guidelines (LDL < 55 mg/dl). We suggest that even in the subacute phase of stroke due to ICAS, strict BP control probably should be initiated to prevent recurrence and stenosis progression. Regarding the optimal BP target in ICAD patients, we refer the readers to ESO stroke secondary prevention guidelines, since there is no specific evidence-based recommendation for ICAD patients. |
We designed the guideline with the attempt to follow a comprehensive vision of the disease, broadening the research focus, which had been traditionally restricted to the secondary prevention of ICAD. In the endovascular era of stroke, generalization of non-invasive cerebral vascular imaging has led to increased awareness of ICAD and other intracranial vasculopathies in acute ischemic stroke patients. Moreover, the asymptomatic phase of the disease has gained attention recently thanks to population-based studies employing transcranial ultrasound and non-contrast CT to detect intracranial stenosis and intracranial arterial calcification, respectively.23–27 With these concepts in mind, we divided the guideline into three thematic blocks: (1) management of asymptomatic ICAD, (2) treatment of acute intracranial LVO caused by ICAD, and (3) secondary prevention of patients with symptomatic stenosis due to ICAD.
The first block of PICO questions refers to the asymptomatic or subclinical stage of ICAD. We were not able to find dedicated RCTs to provide evidence to answer the two formulated questions. Therefore, we are not able to give any specific evidence-based recommendation regarding whether to screen or not for asymptomatic ICAD, and about how to treat the patients once we discover subclinical ICAD. Our systematic literature review identified four observational studies that consistently found asymptomatic ICAD to be associated with an increased risk for future stroke.23–27 In this context, based on expert consensus, we suggest linking the detection of asymptomatic ICAD with an increased vascular risk and act accordingly in the clinical practice. Regarding the use of antiplatelets, we suggest considering its use in asymptomatic ICAD patients after balancing their benefit and risk on an individual basis, although the result of the expert voting for this point was only marginally positive (7 for vs 5 against).
Our second block is dedicated to the management of acute LVO ischemic stroke related to ICAD. This is an increasingly recognized clinical presentation of the disease in the setting of endovascular treatment for LVO ischemic stroke. First, we propose an operational definition of acute LVO probably caused by ICAD. The following characteristics should prompt the suspicion if most or all of them are present: (1) absence of atrial fibrillation, (2) absence of CT hyperdense sign or MRI susceptibility sign, (3) watershed infarction, (4) truncal-type occlusion, (5) on DSA residual stenosis when stent is open or after three stent-retriever passes or (6) early reocclusion. Overall, we found a low level of evidence to guide clinical practice in case of ICAD-related refractory LVOs after mechanical thrombectomy. No dedicated RCTs were found to answer our PICO questions concerning the use of glycoprotein IIb/IIIa inhibitors or adjuvant angioplasty and/or stenting in these situations. The systematic literature search found retrospective observational studies harboring a considerable risk of bias due to unclear patient selection criteria. Therefore, we are not able to give any specific evidence-based recommendation. After expert consensus in the group, we suggest using glycoprotein IIb/IIIa inhibitors and intracranial angioplasty and/or stenting as a rescue strategy in an escalated manner with the goal to open ICAD-related LVOs, which are refractory to mechanical thrombectomy, although there was a greater discrepancy between the experts in the voting as compared with other PICOs (8 for vs 4 against). The production of new hydrophilic-coated stents for intracranial use could allow the implantation under single-antiplatelet therapy in the near future, thus possibly providing a better safety profile in cases of ICAD-related LVO strokes. 113 Finally, the fifth PICO is dedicated to blood pressure management during the acute phase in ICAD-related acute ischemic stroke, which is especially relevant in high-grade ICAD causing cerebral hemodynamic compromise. Again, we are not able to give any evidence-based recommendation. Besides the general recommendation of preserving hemodynamic stability, the majority of experts in our group voted in favor of considering permissive or induced arterial hypertension as a treatment option in these situations.
All evidence-based recommendations in this guideline appear in the third block of PICOs, dedicated to the secondary prevention after an ICAD-related cerebral ischemic event. Patients with symptomatic ICAD are exposed to a high risk for recurrent ischemic strokes and other major vascular events, despite BMT. 9 For PICO 6, moderate-level evidence coming from two RCTs comparing VKA anticoagulants with antiplatelets was used to base our strong recommendation against anticoagulation as a first-line antithrombotic therapy in symptomatic ICAD.59,60 The systematic literature review for PICO 7 did not find any RCTs specifically comparing DAPT combining aspirin with P2Y12 inhibitors – clopidogrel or ticagrelor – versus aspirin alone in symptomatic ICAD. Our recommendation in favor of DAPT as the preferred antithrombotic regime is therefore supported by a very low level of evidence. This recommendation derives basically from two sources. First, DAPT during the first 90 days after stroke was the treatment used as BMT in the SAMMPRIS trial. 71 And second, post-hoc subgroup analyses of ICAD patients included in RCTs comparing DAPT versus single antiplatelet after high-risk TIA or minor stroke also suggest a benefit from DAPT in these patients.65–68 Probably influenced by the SAMMPRIS treatment regime, the group members agreed to suggest a DAPT duration of 90 days in symptomatic ICAD. Regarding PICO 8, two dedicated RCTs were found to provide low-level evidence to base our strong recommendation against intracranial stenting / angioplasty as a treatment of first choice in symptomatic ICAD.71,72 The group of experts reached the consensus to consider endovascular therapy only as a second-line strategy after BMT failure in highly selected cases. Likewise, our recommendation for PICO 9 is strongly against neurosurgical procedures as a front-line therapy for these patients, which is based on a very low level of evidence coming from one dedicated RCT. 100 In the response to PICO 10, we review the evidence about the use of remote ischemic conditioning for symptomatic ICAD patients. Two RCTs were found, and the quality assessment revealed a high risk of bias, so the level of evidence was deemed to be very low. However, in the absence of signals for harm it could be considered as an adjunct to BMT (weak recommendation for intervention).106,107 Further RCTs on this promising therapy are warranted. The last PICO 11 is dedicated to the concept of aggressive vascular risk factor control, which was introduced for stroke prevention in ICAD patients in the SAMMPRIS trial. 109 Although aggressive risk factor control may at least partially explain the better than expected outcomes of the medical arm group in SAMMPRIS, this regimen was not compared with a more conventional risk factor management. We found one RCT comparing low dose with high dose statins, 111 and another one comparing intensive versus conventional blood pressure control in ICAD patients. 108 Therefore, our recommendation in favor of aggressive risk factor control is considered weak and based on low-grade evidence. Finally, the group reached strong agreement regarding the optimal LDL target suggested for ICAD patients (<55 mg/dl) and the need for strict BP control starting from the subacute phase of stroke.
In conclusion, this ESO guideline attempts to cover the main clinical questions challenging the management of patients with ICAD, from its asymptomatic phase to secondary prevention, considering also the complex situation of acute LVO related to ICAD. The systematic literature review performed allowed us to provide recommendations based on moderate-level evidence in one PICO (6), low level in two (PICOS 7 and 8) and very low in two (PICO 9 and 11). The remaining PICOs were answered with suggestions based on observational studies and expert consenus. Further research in the form of well-designed and conducted RCTs to answer the open questions is highly needed.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221099715 for European Stroke Organisation guidelines on treatment of patients with intracranial atherosclerotic disease by Marios Psychogios, Alex Brehm, Elena López-Cancio, Gian Marco De Marchis, Elena Meseguer, Aristeidis H Katsanos, Christine Kremer, Peter Sporns, Marialuisa Zedde, Adam Kobayashi, Jildaz Caroff, Daniel Bos, Sabrina Lémeret, Avtar Lal and Juan F Arenillas in European Stroke Journal
Footnotes
Author contributions: All listed authors have contributed to the preparation and writing of the manuscript.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have completed a declaration of competing interests and details are available in the Supplemental material.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the development of these guidelines was provided by the European Stroke Organisation, Basel, Switzerland. The author(s) did not receive financial support for the development, writing and/or publication of this guideline.
Ethical approval: Ethical approval was not necessary for the work described in this paper.
Guarantor: Marios-Nikos Psychogios, MD and Juan F. Arenillas, MD, PhD.
ORCID iDs: Marios Psychogios  https://orcid.org/0000-0002-0016-414X
https://orcid.org/0000-0002-0016-414X
Gian Marco De Marchis  https://orcid.org/0000-0002-0342-9780
https://orcid.org/0000-0002-0342-9780
Elena Meseguer  https://orcid.org/0000-0002-7184-2614
https://orcid.org/0000-0002-7184-2614
Christine Kremer  https://orcid.org/0000-0002-5739-6523
https://orcid.org/0000-0002-5739-6523
Marialuisa Zedde  https://orcid.org/0000-0001-7530-818X
https://orcid.org/0000-0001-7530-818X
Sabrina Lémeret  https://orcid.org/0000-0001-8611-1630
https://orcid.org/0000-0001-8611-1630
Supplemental material: Supplemental material for this article is available online.
References
- 1. Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res 2017; 120: 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong KS, Huang YN, Gao S, et al. Intracranial stenosis in Chinese patients with acute stroke. Neurology 1998; 50: 812–813. [DOI] [PubMed] [Google Scholar]
- 3. Kim JT, Yoo SH, Kwon JH, et al. Subtyping of ischemic stroke based on vascular imaging: analysis of 1,167 acute, consecutive patients. J Clin Neurol 2006; 2: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The nNorthern Manhattan Stroke Study. Stroke 1995; 26: 14–20. [DOI] [PubMed] [Google Scholar]
- 5. Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008; 39: 1142–1147. [DOI] [PubMed] [Google Scholar]
- 6. Bos D, van der Rijk MJ, Geeraedts TE, et al. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke 2012; 43: 1878–1884. [DOI] [PubMed] [Google Scholar]
- 7. Tsivgoulis G, Vadikolias K, Heliopoulos I, et al. Prevalence of symptomatic intracranial atherosclerosis in Caucasians: a prospective, multicenter, transcranial Doppler study. J Neuroimaging 2014; 24: 11–17. [DOI] [PubMed] [Google Scholar]
- 8. Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008; 39: 2396–2399. [DOI] [PubMed] [Google Scholar]
- 9. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. New Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013; 12: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ritz K, Denswil NP, Stam OC, et al. Cause and mechanisms of intracranial atherosclerosis. Circulation 2014; 130: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 12. Dearborn JL, Zhang Y, Qiao Y, et al. Intracranial atherosclerosis and dementia: the Atherosclerosis Risk in cCommunities (ARIC) Study. Neurology 2017; 88: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke 2011; 42: S20–S23. [DOI] [PubMed] [Google Scholar]
- 14. Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011; 69: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arenillas JF, Dieleman N, Bos D. Intracranial arterial wall imaging: Techniques, clinical applicability, and future perspectives. Int J Stroke 2019; 14: 564–573. [DOI] [PubMed] [Google Scholar]
- 16. Mandell DM, Matouk CC, Farb RI, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke 2012; 43: 860–862. [DOI] [PubMed] [Google Scholar]
- 17. Steiner T, Dichgans M, Norrving B, et al. European Stroke Organisation (ESO) standard operating procedure for the preparation and publishing of guidelines. Eur Stroke J 2021; 6: Cxxii–cxxxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 19. Higgins J, Green SE. Cochrane handbook for systematic reviews of interventions (updated July 2019). The Cochrane Collaboration, www.training.cochrane.org/handbook (2019, accessed 8 June 2020).
- 20. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 21. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison JK, Reid J, Quinn TJ, et al. Using quality assessment tools to critically appraise ageing research: a guide for clinicians. Age Ageing 2017; 46: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duan JG, Chen XY, Lau A, et al. Long-term risk of cardiovascular disease among type 2 diabetic patients with asymptomatic intracranial atherosclerosis: a prospective cohort study. PLoS One 2014; 9: e106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang HB, Laskowitz DT, Dodds JA, et al. Peak systolic velocity measurements with transcranial Doppler ultrasound is a predictor of incident stroke among the general population in China. PLoS One 2016; 11: e0160967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Planas-Ballvé A, Crespo AM, Aguilar LM, et al. The Barcelona-asymptomatic intracranial atherosclerosis study: subclinical intracranial atherosclerosis as predictor of long-term vascular events. Atherosclerosis 2019; 282: 132–136. [DOI] [PubMed] [Google Scholar]
- 26. Bos D, Portegies ML, van der Lugt A, et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam Study. JAMA Neurol 2014; 71: 405–411. [DOI] [PubMed] [Google Scholar]
- 27. Bos D, Vernooij MW, de Bruijn RF, et al. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimers Dement 2015; 11: 639–647.e1. [DOI] [PubMed] [Google Scholar]
- 28. Kremer C, Schaettin T, Georgiadis D, et al. Prognosis of asymptomatic stenosis of the middle cerebral artery. J Neurol Neurosurg Psychiatry 2004; 75: 1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zaidat OO, Zahuranec DB, Ubogu EE, et al. Asymptomatic middle cerebral artery stenosis diagnosed by magnetic resonance angiography. Neuroradiol 2004; 46: 49–53. [DOI] [PubMed] [Google Scholar]
- 30. Yoon W, Kim SK, Park MS, et al. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurg 2015; 76: 680–686; discussion 686. [DOI] [PubMed] [Google Scholar]
- 31. Wong KS, Li H, Chan YL, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke 2000; 31: 2641–2647. [DOI] [PubMed] [Google Scholar]
- 32. Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke 2014; 16: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bang OY, Saver JL, Liebeskind DS, et al. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J Neurol Sci 2009; 284: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim YW, Hong JM, Park DG, et al. Effect of intracranial atherosclerotic disease on endovascular treatment for patients with acute vertebrobasilar occlusion. Am J Neuroradiol 2016; 37: 2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hwang Y-H, Kim Y-W, Kang D-H, et al. Impact of target arterial residual stenosis on outcome after endovascular revascularization. Stroke 2016; 47: 1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baek J-H, Kim BM, Ihm EH, et al. Clinical outcomes of rescue stenting for failed endovascular thrombectomy: a multicenter prospective registry. J Neurointerv Surg. Epub ahead of print 12 January 2022. DOI: 10.1136/neurintsurg-2021-018308. [DOI] [PubMed] [Google Scholar]
- 37. Baek JH, Jung C, Kim BM, et al. Combination of rescue stenting and antiplatelet infusion improved outcomes for acute intracranial atherosclerosis-related large-vessel occlusion. Front Neurol 2021; 12: 608270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan Z, Shi Z, Wang Y, et al. Efficacy and safety of low-dose tirofiban for acute intracranial atherosclerotic stenosis related occlusion with residual stenosis after endovascular treatment. J Stroke Cerebrovasc Dis 2020; 29: 104619. [DOI] [PubMed] [Google Scholar]
- 39. Kim YW, Sohn SI, Yoo J, et al. Local tirofiban infusion for remnant stenosis in large vessel occlusion: tirofiban ASSIST study. BMC Neurol 2020; 20: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun X, Zhang H, Tong X, et al. Effects of periprocedural tirofiban vs. oral antiplatelet drug therapy on posterior circulation infarction in patients with acute intracranial atherosclerosis-related vertebrobasilar artery occlusion. Front Neurol 2020; 11: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang S, Hao Y, Tian X, et al. Safety of intra-arterial tirofiban administration in ischemic stroke patients after unsuccessful mechanical thrombectomy. J Vasc Interv Radiol 2019; 30: 141–147.e1. [DOI] [PubMed] [Google Scholar]
- 42. Román LS, Menon BK, Blasco J, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 2018; 17: 895–904. [DOI] [PubMed] [Google Scholar]
- 43. Peng F, Wan J, Liu W, et al. Efficacy and safety of rescue stenting following failed mechanical thrombectomy for anterior circulation large vessel occlusion: propensity score analysis. J Neurointerv Surg 2020; 12: 271–273. [DOI] [PubMed] [Google Scholar]
- 44. Baek JH, Kim BM, Kim DJ, et al. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke 2016; 47: 2360–2363. [DOI] [PubMed] [Google Scholar]
- 45. Al Kasab S, Almallouhi E, Alawieh A, et al. Outcomes of rescue endovascular treatment of emergent large vessel occlusion in patients with underlying intracranial atherosclerosis: insights from STAR. J Am Heart Assoc 2021; 10: e020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sandset EC, Bath PM, Boysen G, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011; 377: 741–750. [DOI] [PubMed] [Google Scholar]
- 47. ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet 2015; 385: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woodhouse LJ, Appleton JP, Scutt P, et al. ; ENOS Investigators. Effect of continuing versus stopping pre-stroke antihypertensive agents within 12 h on outcome after stroke: a subgroup analysis of the efficacy of nitric oxide in stroke (ENOS) trial. EClinicalMedicine 2022; 44: 101274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Minhas JS, Wang X, Lindley RI, et al. Comparative effects of intensive-blood pressure versus standard-blood pressure-lowering treatment in patients with severe ischemic stroke in the ENCHANTED trial. J Hypertens 2021; 39: 280–285. [DOI] [PubMed] [Google Scholar]
- 50. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J 2021; 6: II. Ii. 2021/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 52. Rafael H. Cerebral atherosclerosis causes neurogenic hypertension. Stroke 2002; 33: 1180–1181; author reply 1180–1181. [DOI] [PubMed] [Google Scholar]
- 53. Iwasawa E, Ichijo M, Ishibashi S, et al. Acute development of collateral circulation and therapeutic prospects in ischemic stroke. Neural Regen Res 2016; 11: 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park JM, Kim BJ, Kwon SU, et al. Intensive blood pressure control may not be safe in subacute ischemic stroke by intracranial atherosclerosis: a result of randomized trial. J Hypertens 2018; 36: 1936–1941. [DOI] [PubMed] [Google Scholar]
- 55. Yamauchi H, Higashi T, Kagawa S, et al. Impaired perfusion modifies the relationship between blood pressure and stroke risk in major cerebral artery disease. J Neurol Neurosurg Psychiatry 2013; 84: 1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Klijn CJ, Kappelle LJ. Haemodynamic stroke: clinical features, prognosis, and management. Lancet Neurol 2010; 9: 1008–1017. [DOI] [PubMed] [Google Scholar]
- 57. Turan TN, Cotsonis G, Lynn MJ, et al. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007; 115: 2969–2975. [DOI] [PubMed] [Google Scholar]
- 58. Arenillas JF, Molina CA, Montaner J, et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke 2001; 32: 2898–2904. [DOI] [PubMed] [Google Scholar]
- 59. Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. New Engl J Med 2005; 352: 1305–1316. [DOI] [PubMed] [Google Scholar]
- 60. Martí-Fàbregas J, Cocho D, Martí-Vilalta JL, et al. Aspirin or anticoagulants in stenosis of the middle cerebral artery: a randomized trial. Cerebrovasc Dis 2006; 22: 162–169. [DOI] [PubMed] [Google Scholar]
- 61. Ovbiagele B, Cruz-Flores S, Lynn MJ, et al. Early stroke risk after transient ischemic attack among individuals with symptomatic intracranial artery stenosis. Arch Neurol 2008; 65: 733–737. [DOI] [PubMed] [Google Scholar]
- 62. Kwon SU, Cho YJ, Koo JS, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 2005; 36: 782–786. [DOI] [PubMed] [Google Scholar]
- 63. Uchiyama S, Sakai N, Toi S, et al. Final results of cilostazol-aspirin therapy against recurrent stroke with intracranial artery stenosis (CATHARSIS). Cerebrovasc Dis Extra 2015; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu L, Wong KS, Leng X, et al. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology 2015; 85: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Uchiyama S, Toyoda K, Omae K, et al. Dual antiplatelet therapy using cilostazol in patients with stroke and intracranial arterial stenosis. J Am Heart Assoc 2021; 10: e022575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amarenco P, Denison H, Evans SR, et al. Ticagrelor added to aspirin in acute nonsevere ischemic stroke or transient ischemic attack of atherosclerotic origin. Stroke 2020; 51: 3504–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med 2013; 369: 11–19. [DOI] [PubMed] [Google Scholar]
- 68. Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. New Engl J Med 2020; 383: 207–217. [DOI] [PubMed] [Google Scholar]
- 69. Toyoda K, Uchiyama S, Yamaguchi T, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol 2019; 18: 539–548. [DOI] [PubMed] [Google Scholar]
- 70. Chaturvedi S, Turan TN, Lynn MJ, et al. Do patient characteristics explain the differences in outcome between medically treated patients in SAMMPRIS and wasid? Stroke 2015; 46: 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015; 313: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 73. Meyer L, Leischner H, Thomalla G, et al. Stenting with Acclino (flex) for symptomatic intracranial stenosis as secondary stroke prevention. J Neurointerv Surg 2020; 12: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 74. Derdeyn CP, Fiorella D, Lynn MJ, et al. Nonprocedural symptomatic infarction and in-stent restenosis after intracranial angioplasty and stenting in the SAMMPRIS trial (Stenting and Aggressive Medical Management for the Prevention of Recurrent Stroke in Intracranial Stenosis). Stroke 2017; 48: 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zaidat OO, Klucznik R, Alexander MJ, et al. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology 2008; 70: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke 2007; 38: 1531–1537. [DOI] [PubMed] [Google Scholar]
- 77. Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke 2007; 38: 881–887. [DOI] [PubMed] [Google Scholar]
- 78. Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–563. [DOI] [PubMed] [Google Scholar]
- 79. Peng G, Zhang Y, Miao Z. Incidence and risk factors of in-stent restenosis for symptomatic intracranial atherosclerotic stenosis: A systematic review and meta-analysis. AJNR Am J Neuroradiol 2020; 41: 1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miao Z, Zhang Y, Shuai J, et al. Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke 2015; 46: 2822–2829. [DOI] [PubMed] [Google Scholar]
- 81. Wang ZL, Gao BL, Li TX, et al. Outcomes of middle cerebral artery angioplasty and stenting with Wingspan at a high-volume center. Neuroradiol 2016; 58: 161–169. [DOI] [PubMed] [Google Scholar]
- 82. Li TX, Gao BL, Cai DY, et al. Wingspan stenting for severe symptomatic intracranial atherosclerotic stenosis in 433 patients treated at a single medical center. PLoS One 2015; 10: e0139377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jiang WJ, Yu W, Du B, et al. Outcome of patients with ⩾70% symptomatic intracranial stenosis after Wingspan stenting. Stroke 2011; 42: 1971–1975. [DOI] [PubMed] [Google Scholar]
- 84. Zhao T, Zhu WY, Xiong XY, et al. Safety and efficacy of Wingspan stenting for severe symptomatic atherosclerotic stenosis of the middle cerebral artery: analysis of 278 continuous cases. J Stroke Cerebrovasc Dis 2016; 25: 2368–2372. [DOI] [PubMed] [Google Scholar]
- 85. Ma N, Zhang Y, Shuai J, et al. Stenting for symptomatic intracranial arterial stenosis in China: 1-year outcome of a multicentre registry study. Stroke Vasc Neurol 2018; 3: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gao P, Wang D, Zhao Z, et al. Multicenter prospective trial of stent placement in patients with symptomatic high-grade intracranial stenosis. AJNR Am J Neuroradiol 2016; 37: 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu SC, Leung TW, Lee KT, et al. Angioplasty and stenting of atherosclerotic middle cerebral arteries with Wingspan: evaluation of clinical outcome, restenosis, and procedure outcome. AJNR Am J Neuroradiol 2011; 32: 753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alexander MJ, Zauner A, Chaloupka JC, et al. WEAVE trial: final results in 152 on-label patients. Stroke 2019; 50: 889–894. [DOI] [PubMed] [Google Scholar]
- 89. Alexander MJ, Zauner A, Gupta R, et al. The WOVEN trial: Wingspan one-year vascular events and neurologic outcomes. J Neurointerv Surg 2021; 13: 307–310. [DOI] [PubMed] [Google Scholar]
- 90. Tsivgoulis G, Katsanos AH, Magoufis G, et al. Percutaneous transluminal angioplasty and stenting for symptomatic intracranial arterial stenosis: a systematic review and meta-analysis. Ther Adv Neurol Disord 2016; 9: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gao P, Zhao Z, Wang D, et al. China angioplasty and stenting for symptomatic intracranial severe stenosis (CASSISS): A new, prospective, multicenter, randomized controlled trial in China. Interv Neuroradiol 2015; 21: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stracke CP, Meyer L, Fiehler J, et al. Intracranial bailout stenting with the Acclino (Flex) Stent/NeuroSpeed Balloon Catheter after failed thrombectomy in acute ischemic stroke: a multicenter experience. J Neurointerv Surg 2020; 12: 43–47. [DOI] [PubMed] [Google Scholar]
- 93. Wang AY, Chang CH, Chen CC, et al. Leave nothing behind: treatment of intracranial atherosclerotic disease with drug-coated balloon angioplasty. Clin Neuroradiol 2021; 31: 35–44. [DOI] [PubMed] [Google Scholar]
- 94. Remonda L, Diepers M, Berberat J, et al. Drug-coated balloon treatment in symptomatic intracranial high grade stenosis: a retrospective study of 33 patients. Clin Neuroradiol 2021; 31: 45–49. [DOI] [PubMed] [Google Scholar]
- 95. Amin-Hanjani S, Stapleton CJ, Du X, et al. Hypoperfusion symptoms poorly predict hemodynamic compromise and stroke risk in vertebrobasilar disease. Stroke 2019; 50: 495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lutsep HL, Lynn MJ, Cotsonis GA, et al. Does the stenting versus aggressive medical therapy trial support stenting for subgroups with intracranial stenosis? Stroke 2015; 46: 3282–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bai X, Lv P, Liu K, et al. 3D black-blood luminal angiography derived from high-resolution MR vessel wall imaging in detecting MCA stenosis: a preliminary study. AJNR Am J Neuroradiol 2018; 39: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. New Engl J Med 2016; 375: 35–43. [DOI] [PubMed] [Google Scholar]
- 99. Amarenco P, Albers GW, Denison H, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 2017; 16: 301–310. [DOI] [PubMed] [Google Scholar]
- 100. EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. New Engl J Med 1985; 313: 1191–1200–DOI: 10.1056/nejm198511073131904 [DOI] [PubMed] [Google Scholar]
- 101. Ning XJ, Gao Q, Chen C, et al. Effects of superficial temporal artery-middle cerebral artery bypass on hemodynamics and clinical outcomes in the patients with atherosclerotic stenosis in the intracranial segment of internal carotid artery and middle cerebral artery. Clin Neurol Neurosurg 2019; 186: 105510. [DOI] [PubMed] [Google Scholar]
- 102. Gonzalez NR, Jiang H, Lyden P, et al. Encephaloduroarteriosynangiosis (EDAS) revascularization for symptomatic intracranial atherosclerotic steno-occlusive (ERSIAS) Phase-II objective performance criterion trial. Int J Stroke 2021; 16: 701–709. [DOI] [PubMed] [Google Scholar]
- 103. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 104. Matsushima Y, Fukai N, Tanaka K, et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol 1981; 15: 313–320. [DOI] [PubMed] [Google Scholar]
- 105. Powers WJ, Clarke WR, Grubb Rl, Jr, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 2011; 306: 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Meng R, Ding Y, Asmaro K, et al. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurother 2015; 12: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012; 79: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 108. Zhou P, Lu Z, Gao P, et al. Efficacy and safety of intensive statin therapy in Chinese patients with atherosclerotic intracranial arterial stenosis: a single-center, randomized, single-blind, parallel-group study with one-year follow-up. Clin Neurol Neurosurg 2014; 120: 6–13. [DOI] [PubMed] [Google Scholar]
- 109. Turan TN, Nizam A, Lynn MJ, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology 2017; 88: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Park JH, Ovbiagele B, Hong KS, et al. Association of systolic blood pressure with progression of symptomatic intracranial atherosclerotic stenosis. J Stroke 2017; 19: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chung JW, Cha J, Lee MJ, et al. Intensive statin treatment in acute ischaemic stroke patients with intracranial atherosclerosis: a high-resolution magnetic resonance imaging study (STAMINA-MRI study). J Neurol Neurosurg Psychiatry 2020; 91: 204–211. [DOI] [PubMed] [Google Scholar]
- 112. Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG) and ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019; 290: 140–205–DOI: 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 113. Lobsien D, Clajus C, Behme D, et al. Aneurysm treatment in acute SAH with hydrophilic-coated flow diverters under single-antiplatelet therapy: a 3-center experience. AJNR Am J Neuroradiol 2021; 42: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221099715 for European Stroke Organisation guidelines on treatment of patients with intracranial atherosclerotic disease by Marios Psychogios, Alex Brehm, Elena López-Cancio, Gian Marco De Marchis, Elena Meseguer, Aristeidis H Katsanos, Christine Kremer, Peter Sporns, Marialuisa Zedde, Adam Kobayashi, Jildaz Caroff, Daniel Bos, Sabrina Lémeret, Avtar Lal and Juan F Arenillas in European Stroke Journal