Abstract
Recent studies have highlighted the deleterious contributions of B cells to post-stroke recovery and cognitive decline. Different B cell subsets have been proposed based on expression levels of transcription factors (e.g. T-bet) as well as specific surface proteins. CD11b (α-chain of integrin) is expressed by several immune cell types and is involved in regulation of cell motility, phagocytosis, and other essential functions of host immunity. Although B cells express CD11b, the CD11bhigh subset of B cells has not been well characterized, especially in immune dysregulation seen with aging and after stroke. Here, we investigate the role of CD11bhigh B cells in immune responses after stroke in young and aged mice. We evaluated the ability of CD11bhigh B cells to influence pro- and anti-inflammatory phenotypes of young and aged microglia (MG). We hypothesized that CD11bhigh B cells accumulate in the brain and contribute to neuroinflammation in aging and after stroke. We found that CD11bhigh B cells are a heterogeneous subpopulation of B cells, predominantly present in naïve aged mice. Their frequency increases in the brain after stroke in young and aged mice. Importantly, CD11bhigh B cells regulate MG phenotype and increase MG phagocytosis in both ex vivo and in vivo settings, likely by production of regulatory cytokines (e.g., TNF-α). As both APCs and adaptive immune cells with long-term memory function, B cells are uniquely positioned to regulate acute and chronic phases of the post-stroke immune response, and their influence is subset-specific.
Introduction
Age-related changes in the immune system are a determining factor in clinical outcomes of neurological diseases including stroke (1, 2). Work from our lab and others has shown that immune cell populations from aged animals can be significantly different in relative frequency and tissue distribution compared to those from young animals, even in the absence of tissue injury (2–4). After ischemic stroke, aged mice have a smaller infarction size than young mice, however aged mice demonstrate worse neurological outcomes (5–8). Peripheral immune cells are critical to stroke recovery, and aged peripheral immune cells contribute to greater neurological deficits and dystrophic changes in microglia (9, 10). Microglia mount a pro-inflammatory response to infarction, perform phagocytosis, and present antigens for the gradual recruitment of adaptive immune cells (11). After injury, the dural meninges and eventually the brain parenchyma are infiltrated by peripheral leukocytes, further amplifying the immune response (12). Damage to the blood brain barrier (BBB) results in loss of integrity, allowing for a greater influx of peripheral leukocytes into the brain parenchyma (13, 14). Importantly, both brain-resident microglia (MG) and long-lived peripheral B cells undergo significant age-related changes (15–18).
Aged MG contribute to detrimental immune responses, chronic inflammation, and poorer outcomes after brain injury (2, 19). Aged MG have a transcriptomic profile indicative of a chronic pro-inflammatory state, characterized by increased pro-inflammatory cytokine production (e.g., TNF-α, IL-1β, and IL-6), genes associated with host defense, and cell adhesion (20–23). MG are key producers of and are regulated by TNF-α after injury (24). TNF-α participates in injury-mediated MG and astrocyte activation and MG phagocytosis (24–27). TNF-α can be secreted from variety of cell types including long-lived adaptive immune cells such as B cells (28).
B cells have multiple subtypes and diverse roles after stroke (29). For example, IL-10 secretion by regulatory B cells reduces infarct size and inflammation in mice (29, 30), and B cell production of IgG facilitates opsonization of myelin debris for clearance by microvascular endothelial cells and macrophage recruitment (31). Conversely, detrimental responses also arise from B cells. After stroke, brain antigens are exposed to the periphery, and antigen presenting cells from the brain migrate to the cervical lymph nodes, leading to the amplification of harmful auto-reactive lymphocytes and chronic inflammation (32).
Most myeloid cells, as well as B cells, express CD11b for regulation of cell motility and phagocytosis (33, 34). A distinct subset of B cells have been found to accumulate in aged mice and have been studied in the context of autoimmune diseases (35–37). However, less is known regarding the role of aged B cell subsets after stroke (38, 39). Different aged B cells subsets have been proposed based on expression levels of transcription factors (e.g. T-bet) as well as surface proteins including CD11b (40–43). CD11bhigh B cells are often excluded in common approaches in flow cytometric brain immunophenotyping (44–48). Our data shows that relative frequency of CD11bhigh B cells significantly increases with aging and after stroke in both young and aged mice. Sorting peripheral B cells into two subsets of CD11blow and CD11bhigh revealed significant differences in their surface phenotype, inflammatory cytokine production, and phagocytic activity (Fig. 1). We hypothesized that CD11bhigh B cells increase in the brain and contribute to neuroinflammation in aging and after stroke by regulating MG activation and phagocytosis. We demonstrated that CD11bhigh B cells can produce higher levels of TNF-α compared to their CD11blow counterparts, which can activate MG phagocytosis. Taken together, our results emphasize the nuances of immunophenotyping of brain CD11bhigh and CD11blow B cell subsets in age-related neuroinflammation.
FIGURE 1. CD11bhigh B cells increase with aging and have a distinct surface phenotype, increased TNF-α production, and increased phagocytosis.
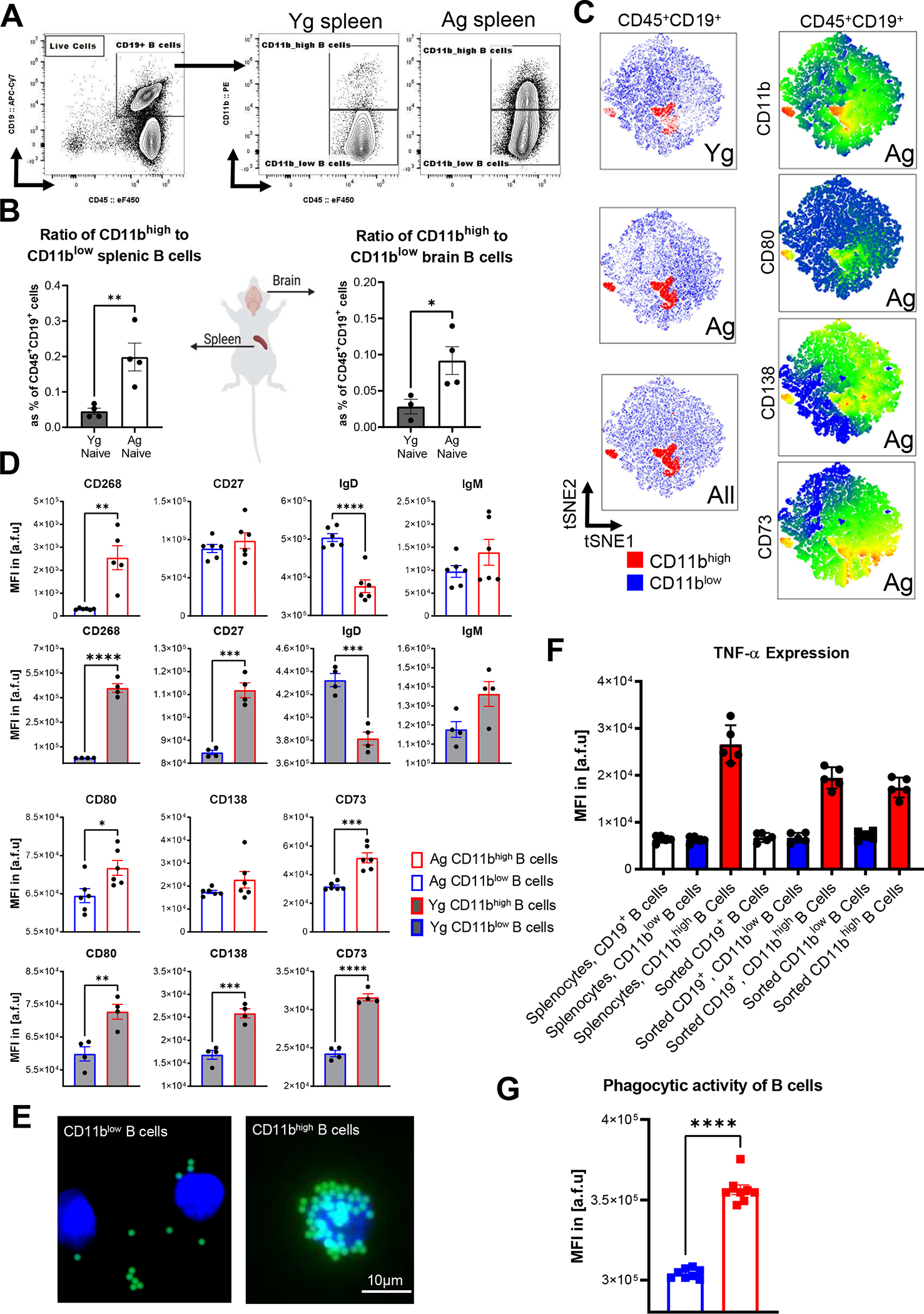
(A) Representative gating strategy from Cells/Singlets/Live/CD45+/CD19+ B cells in naïve Yg and Ag mice. (B) The ratio of CD11bhigh to CD11blow B cells isolated from spleen and brain in Yg (n=4) and Ag (n=5) naïve animals (spleen p=0.0089, brain p=0.0466, unpaired T-test). Absolute counts listed in Table 1. Results reproduced in two independent experiments. (C) The first column represents tSNE plot of CD45+/CD19+ cells demonstrating the CD11bhigh (red) and CD11blow (blue) subpopulations of B cells in Yg, Ag, and combined populations as well as the increase in CD11bhigh B cells with aging. The second column demonstrates a heatmap of the co-expression of B-cell associated surface markers (CD11b, CD80, CD138, and CD73) in Ag B cells. (D) Surface phenotype in MFI of CD45+/CD19+/CD11bhigh compared to CD45+/CD19+/CD11blow B cells isolated from splenocytes of naïve Ag (n=6) and Yg (n=4) mice. Data reproduced in 2 independent experiments. The top two rows show a significant increase in CD268 in Ag (p=0.0011) and Yg (p<0.0001) cells, no significant change in CD27 in Ag (p=0.3944) with an increase in Yg (p=0.0002) cells, a decrease in IgD (Ag p <0.0001 and Yg p= 0.0008) cells, and no significant changes in IgM in Ag (p=0.2075) and Yg (p=0.00529) cells. The bottom two rows show a significant increase in CD80 in Ag (p=0.0208) and Yg (p=0.0064) cells, and an increase in CD138 in Yg (p= 0.0006) with no change in Ag (p = 0.1786) cells, and an increase in CD73 in Ag (p=0.0002) and Yg (p<0.0001) cells. (E) Phagocytosis of 1μm FITC fluorescent beads by sorted Cells/Singlets/Live/CD45+/CD19+ CD11bhigh and CD11blow B-cells from splenocytes isolated from Ag naïve animals (n=8). Representative images of 1μm FITC fluorescent beads with CD11blow (left) CD11bhigh (right) B cells stained with DAPI. (F) FACs detection of TNFα production in CD11bhigh and CD11blow B-cells from sorted CD45+/CD19+/CD11bhigh, sorted CD45+/CD19+/CD11blow, sorted CD45+/CD19+, and splenocytes isolated from Ag naïve animals (n=5). (G) FACs analysis of phagocytosis of fluorescent beads by splenocytes, sorted Cells/Singlets/Live/CD45+/CD19+ B cells, and sorted Cells/Singlets/Live/CD45+/CD19+ CD11bhigh and sorted Cells/Singlets/Live/CD45+/CD19+ CD11blow B-cells from splenocytes isolated from Ag naïve animals (n=5) B, C, D, F, and G results reproduced in two independent experiments. Unpaired T-test. Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, N.S. not significant.
Materials and Methods
Mice:
Young (2–4 months) and aged (18–22 months) C57BL/6J male wild-type (WT) mice from the National Institute on Aging (NIA) were used in this study. PepBoy mice (from Jackson Laboratory) are a congenic strain which carry the differential Ptprc pan leukocyte marker commonly known as CD45.1. C57BL/6 inbred mice express the Ptprc (CD45.2) allele. CD45 is a common b antigen expressed in all leukocytes. It has two different alleles, CD45.1 and CD45. 2, which are functionally identical. This allows for the differentiation of transferred B cells from (CD45.2) mice (donor) to recipient PepBoy (CD45.1) mice, due to the different CD45 alleles (CD45.1 vs CD45.2). All animals were group housed in Tecniplast individually ventilated cage racks, fed a commercially available irradiated, balanced mouse diet (no. 5058, LabDiet, St Louis, MO) and provided corncob bedding. Rooms were maintained at 21–24°C and under a 12:12 h light:dark cycle. All animals were maintained specific pathogen free (see Supplemental Table 1 for list of monitored pathogens). Animal procedures were performed at an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility and were approved by the Animal Welfare Committee at the University of Texas Health Science Center at Houston, TX, USA.
Middle cerebral artery occlusion (MCAO):
Transient focal ischemia was induced under isoflurane anesthesia in young or aged mice for 60 minutes by occlusion of the right middle cerebral artery (49). Body temperature was maintained at 37.0 ± 1.0°C throughout the surgery by an automated temperature control feedback system (TC1000, mouse, CWE Inc., USA). A midline ventral neck incision was made, and unilateral MCAO was performed by inserting a Doccol monofilament (Doccol Corp, Redlands, CA, USA) into the right internal carotid. One hour after ischemia, animals were anesthetized again, and reperfusion was established by withdrawal of the monofilament. Animals were then placed in a recovery cage and were euthanized 72 hours after reperfusion. Sham controls were subjected to same procedure except the suture was not introduced into the middle cerebral artery. Animals were randomly assigned into the stroke and sham surgery groups and single housed in their recovery cages for the first two hours after surgery. Sham and stroke mice were then housed together in their home cages to minimize detrimental effects of social isolation (50). All mice were selected for sham or stroke surgery in a randomized manner and all analyses were performed blinded to surgical conditions. Animals that died due to hemorrhagic transformation or NDS > 3 included 13 aged (out of 35) and 6 young (out of 23). Representative Kaplan-Meier curve included in Fig. 2D.
FIGURE 2. CD11bhigh B cells are increased to both young and aged brains as early as post-MCAO day 7.
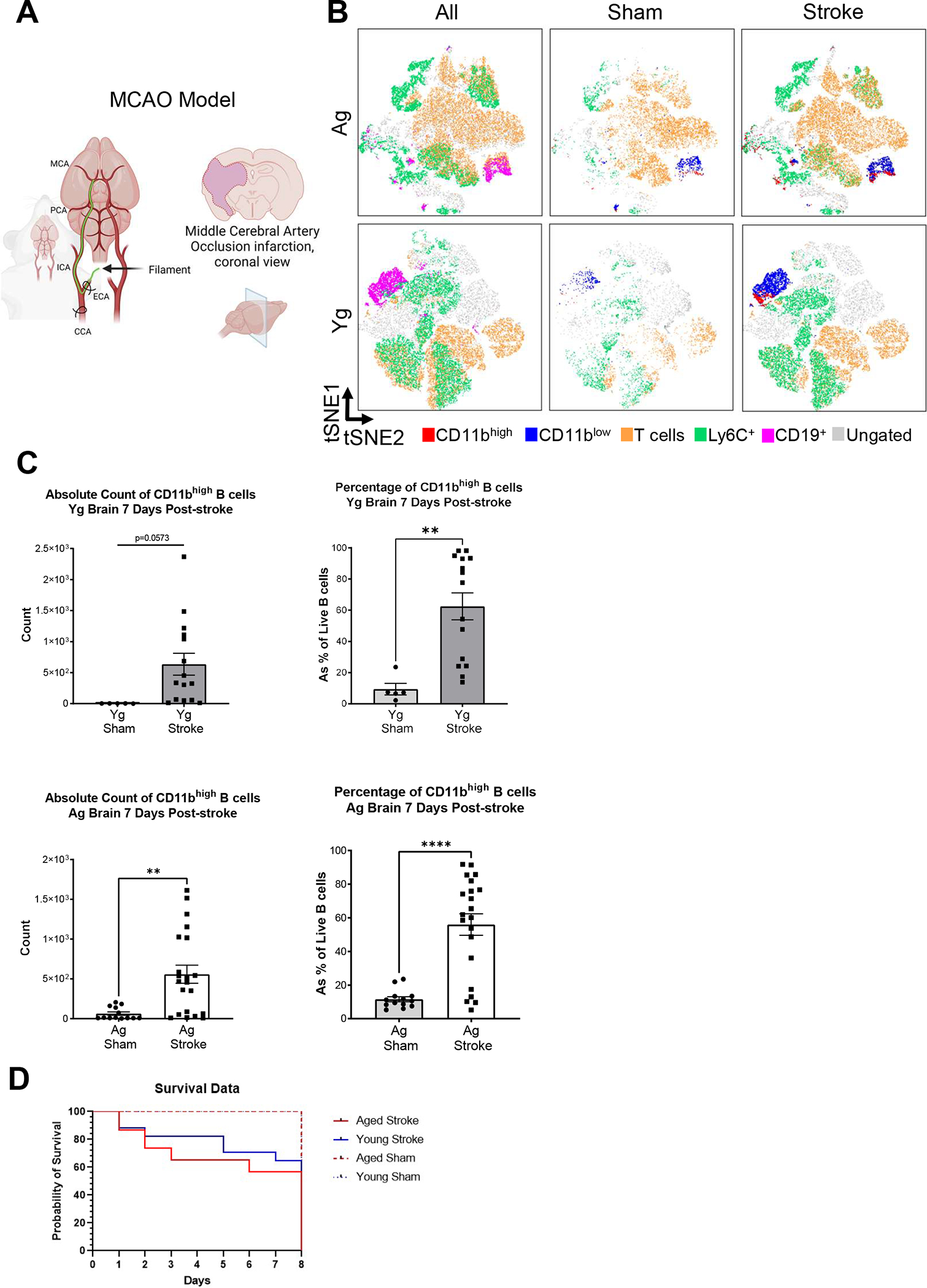
(A) Schematic of the MCAO model used for inducing neurological injury in mice. (B) tSNE plots of live CD45high cells (non-MG immune cells) from sham and stroked homogenized brain from Yg and Ag mice, demonstrating CD19+ B cells (pink), CD11bhigh B cells (red), CD11blow B cells (blue), CD3+ T cells (orange), Ly6C+ monocytes (green), and ungated populations (gray), indicating the increase in CD11bhigh B cells after stroke when compared to sham in both Yg and Ag MCAO mice. (C) Quantification of CD11bhigh and CD11blow B cells isolated from brain, comparing the absolute counts and percentage of CD19+ cells from sham controls to the ipsilateral hemisphere in stroked animals. Conducted in both Yg (n=5–15) and Ag mice (n=13–21). Left the ipsilateral stroked hemisphere in Ag animals demonstrate a significant increase in CD11bhigh B cells (p=0.0019) with Yg cells trending upwards in number (p=0.0573). Right the ipsilateral stroked hemisphere in Ag and Yg animals demonstrate a significant increase in the percentage of CD11bhigh B cells within the total CD45+/CD19+ B cell population (p<0.0001, p=0.0027 respectively). (D) Representative Kaplan-Meier Curve for MCAO mortality in aged and young mice. Results compiled from 6 independent experiments. Outlier test ROUT Q=1% was performed on Yg and Ag groups, resulting in the removal of 1 Yg sham and 2 Yg stroke samples. Outliers where removed before analysis. Unpaired analysis T-test, mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, N.S. not significant.
Flow cytometry:
A previously published brain single cell suspension protocol was used (2, 51). In brief, mice were euthanized by intraperitoneal avertin injection. Blood was drawn by cardiac puncture with heparinized needles. Red blood cell lysis was achieved by two consecutive 10-min incubations with tris–ammonium chloride (Stem Cell Technologies). Mice were transcardially perfused with 20 ml cold, sterile PBS prior to aseptic removal of brain tissues. Brain tissue was placed in complete Roswell Park Memorial Institute 1640 (Lonza) medium then mechanically and enzymatically digested in Collagenase/Dispase (1 mg/mL; Roche Diagnostics) and DNase (10 mg/mL; Roche Diagnostics) for 45 minutes at 37 °C with gentle shaking (80 RPM). The cell suspension was filtered through a 70μm cell strainer. Leukocytes were harvested from the interphase of a 70%–30% Percoll gradients for the brain tissue after a 20-minute centrifugation at room temperature with no brakes. Cells were washed and blocked with Fc receptor block (BioLegend, Lot: B298973) before staining with the following pre-conjugated fluorophores: CD45.2-eF450 (eBioscience, Cat#: 48-0451-82), CD45.1- FITC (eBioscience, Cat#: 11045385), CD11b-APC (BioLegend, Cat#: 101212), CD19-FITC (BioLegend, Cat# 101506), Ly6C-PerCP-Cy5.5 (BioLegend, Cat#: 128011), Tmem119-PE-Cy7 (eBioscience, Cat#: 25-6119-82), P2RY12-PE (BioLegend, Cat#: 848003), MHCII-APC-Fire750 (BioLegend, Cat#: 107652), IgM-PE-Cy7 (BioLegend, Cat#: 406514), IgD-PE (BioLegend Cat#: 405706), CD268-FITC (ThermoFischerScientific Cat#: 11-5943-82), CD27-PerCP-Cy5.5 (BioLegend, Cat#: 124214), CD80-FITC, CD138-APC (BioLegend, Cat#: 142506), CD73-PE-cy7 (BioLegend, Cat#: 127224), T-bet-PE (BioLegend, Cat#: 644810), TNF-alpha-APC (BioLegend Cat#: 506308), and Zombie Aqua (BioLegend, Cat#: 423102). Cell stimulation for cytokine staining was done using Cell Activation Cocktail (with Brefeldin A) (BioLegend, Cat#:423304). Cell isolation, Percoll gradient, and immunostaining steps were carried out at once for both controls and injury models to minimize experimental variability, i.e. all sham and stroke samples were processed together, and all naïve young and aged samples were processed together. Data were acquired on Cytoflex-S (Beckman Coulter) or BD FACSMelody (BD) and analyzed using FlowJo (Treestar Inc.). No less than 300,000 events were recorded for each sample. Tissue-matched and injury-matched fluorescence-minus-one and unstained controls were used to aid in positive gating strategy (Fig S1A). t-distributed stochastic neighbor embedding (tSNE) plots were generated in FlowJo using DownSample plug-in (3,000 cells per sample for each study group) followed by tSNE algorithm on all compensated parameters (except viability) at 1,000 iterations, perplexity of 30, learning rate of 5040, and Barnes-Hut gradient algorithm.
Cell sorting:
Single cell suspension and surface staining were performed as described above. After viability and single cell selections, MG gated as Live Tmem119+ (verified to be CD45intCD11b+) were sorted under an aseptic hood from the single cell suspension prepared from naïve aged male brains (full brains, n=8) stroked aged male brains (full brains, n=4) naïve young male brains (full brains, n=4) using BD FACSMelody.
After viability and single cell selections, B cells, gated as Live CD45+CD19+ were sorted under the hood from the single cell suspension prepared from naïve or stroked aged male spleens (spleen, n=14) using BD FACSMelody. Fluorescence-minus-one and unstained controls were used to aid in positive gating strategy to delineate CD11bhigh from CD11blow and CD11bneg B cells. Sorting was performed from tube directly into 96-well plate used for co-incubation experiments to preserve cell counts, and into FACs tubes for adoptive transfer experiments. Cell counts were conducted in ratios similar to what was analyzed from our post-stroke data (i.e. 1:1000 MG to CD11bhigh B cells and 1:100 MG to CD11blow B cells), to maintain physiologic relevance of our ex vivo experimental conditions. Each sorted sample was then divided (by volume) into control or co-incubated cohorts with each B cell subset for 4 hours under sterile cell-culture environment. Cells were then washed with PBS, stained for surface markers and viability after the 4-hour co-incubation and then analyzed by flow cytometry.
Adoptive B cell Transfer:
Donor mice were 18–20 months old so that they would a have sufficient number of cells to collect for transfer. Naïve or stroked mice were euthanized and immune cells collected post mortem. Using BD Melody cell sorting system, a pure cell population of approximately 50,000 CD11b expressing B lymphocytes were collected for adoptive transfer into a single recipient mouse. 1 donor mouse for every 1 recipient mouse was required in order to have an adequate number of cells. The number of cells (50,000) was determined by the physiologic amount we have detected in aged and stroked mice. Cells were resuspended in 37°C PBS, and retro-orbitally injected into anesthetized recipient using a BD Insulin Syringe with the BD Ultra-Fine™ needle 12.7mm x 30G 3/10 mL/cc.
Ex vivo co-incubation experiments:
Collection and isolation of brain monocytes was performed by optimized enzymatic digested followed by Percoll gradient protocol (2, 51). Brain tissue was harvested after cardiac perfusion with PBS to eliminate blood from the brain tissue. There is a possibility that PBS-perfused samples contain some blood-sourced immune cells prior to ex vivo co-incubation. Thus, we performed digestion and Percoll-gradient based separation of MG at which point, each individual single cell suspension of CNS mononuclear cells was divided (by equal volumes) into a two or three co-incubation conditions with different B cell subsets (Fig. 1). Co-incubated cells were kept in a sterile cell-culture environment at 37°C for 4 hours and 24 hours.
Phagocytosis Assay:
0.5μm Fluoresbrite® 641-conjugated carboxylate microspheres (1 μl stock solution/200 μl in 96-well plate) (52) were added to sorted MG or to CNS mononuclear cells enriched in MG (from brain that is post-digestion and post-Percoll gradient protocol) and incubated for 4- or 24-hours. Cell mixtures were incubated with beads for 30 minutes at 37°C. After, cells were washed twice with PBS, and then stained for flow cytometry following the previous protocol.
Microscopy:
Images were obtained on a Keyence BZ-X810 all in one fluorescence microscope at 40x and 60x magnification. Images were processed for brightness and contrast correction, cropping, and addition of scale bars with Keyence BZ-X800 Analyzer 1.1.1.8. software.
Statistical analysis:
Statistical analysis for flow cytometry data was performed using unpaired t test, One-way ANOVA, and paired one-way ANOVA with post hoc analysis with all related p values adjusted by Dunnett’s or Tukey’s methods for multiple comparisons (specified in figure legends). Statistical significance was considered at p < 0.05 and *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 convention was used in the presented figures. All statistical analyses were performed with GraphPad Prism 7.
Results
CD11bhigh B cells increase with aging and have a distinct surface phenotype, increased TNF-α production, and increased phagocytosis.
First, we provided a representative gating strategy from Cells/Singlets/Live/CD45+/CD19+ B cells in naïve aged and young mice to demonstrate the significant increase in relative frequency of CD11bhigh B cells in aged mice (Fig. 1A and Fig. S1A). The increased ratio of CD11bhigh to CD11blow B cells with aging was independent of tissue origin (spleen or brain) (Fig. 1B and Table 1). We then performed multidimensional flow cytometric phenotyping of CD11bhigh B cells. We asked if CD11bhigh B cells were predominated by mature B cells, memory B cells, or class switched B cells using expression of CD73, CD138, and CD80, respectively. Results are depicted in a tSNE map superimposed with heat maps for each surface marker (Fig. 1C). Individual gating revealed an increase in CD80 and CD73 median fluorescent intensities (MFIs) in CD45+CD19+CD11bhigh compared to CD45+CD19+CD11blow splenic B cells of young and aged naïve mice, with plasma cell marker CD138 MFI also significantly increased in CD45+CD19+CD11bhigh of young naïve mice (Fig. 1D). A significant increase in CD27 (young p=0.0002) supports a memory B cell phenotype within the CD11bhigh B cell population. To understand the functional role of CD11bhigh B cells, we examined surface phenotype markers CD268, IgD, and IgM expression (Fig. 1D) on CD45+CD19+CD11bhigh compared to CD45+CD19+CD11blow B cells isolated from splenocytes of young (n=4) and aged (n=6) naïve mice. CD45+CD19+CD11bhigh B cells showed a significant increase in CD268 (aged p=0.0011 and young p< 0.0001), a significant decrease in IgD (aged p<0.0001 and young p=0.0008), and no significant changes in IgM in young or aged B cells, compared to aged-matched CD45+CD19+CD11blow (Fig. 1D). The relative frequency of these populations varied (Fig. S2A–B). Together, these observations indicated that the CD11bhigh B cells are a mixed population of B cell subsets. We also examined CD11bhigh B cells from the spleen, blood, and skull bone marrow of young and aged stroked mice, which showed a distinct surface phenotype from CD11blow B cells (Fig. S2C–E). These observations suggest that CD11bhigh B cells increase in the periphery and in the brain with aging, in the absence of any brain injury. Further, CD11bhigh B cells are a highly activated, heterogeneous subset of B cells with distinct surface phenotype from their CD11blow B cell counterparts.
Table 1:
Absolute counts of naïve brain and spleen B cell populations for Fig. 1B
| Absolute Count of Naïve Brain and Spleen B cell Populations | ||||
|---|---|---|---|---|
| Tissue | Naïve Group | CD11bhigh B Cells (Average ± SD) | CD11blow B Cells (Average ± SD) | Sample size |
| Spleen | Aged | 28485.6 ± 5895.95 | 102279.80 ± 20288.60 | 5 |
| Young | 23032.25 ± 3647.26 | 95702.75 ± 11175.64 | 4 | |
| Brain | Aged | 96.40 ± 64.92 | 595.40 ± 441.76 | 5 |
| Young | 4.75 ± 5.19 | 166.75 ± 62.78 | 4 | |
To verify that B cells can increase their expression of CD11b under pro-inflammatory conditions, we incubated sorted CD45+CD19+ B cells with LPS for 48 hours. Indeed, our data showed that CD11b expression is increased on sorted B cells after a 48-hour LPS stimulation when compared to B cells not treated with LPS isolated from the same animal (Fig. S1B). We also examined T-bet expression levels of CD45+CD19+CD11bhigh B cells and found that their relative frequency of T-bet+ cells are significantly higher than that of their CD45+CD19+CD11blow B cell counterparts (Fig. S1C), which is consistent with their previously proposed age-associated B cell phenotype (43).
We detected differences in phagocytic activity and cytokine production of CD11bhigh B cells when compared to CD11blow B cells. A phagocytosis assay was performed using 0.5μm fluorescent beads incubated for 30 minutes with sorted CD45+CD19+CD11bhigh and CD11blow B subsets from naïve aged spleens, and then analyzed by flow cytometry. Representative images of fluorescent beads with CD11blow (left) compared to CD11bhigh (right) B cells stained with DAPI were generated to visualize the uptake of beads by the B cells (Fig. 1E). Flow cytometric analysis of B cell phagocytic activity revealed a significant increase in the uptake of fluorescent beads in CD11bhigh B cells compared to CD11blow B cells (Fig. 1G). Previously described activated B cells in mice showed increased TNF-α production (53). Next, to determine if CD11bhigh B cells produce cytokines capable of mediating pro-inflammatory activity in MG, TNF-α production was quantified in CD11bhigh and CD11blow B cells sorted as CD45+CD19+CD11bhigh cells, sorted CD45+CD19+CD11blow cells, sorted CD45+CD19+ cells, and un-sorted splenocytes isolated from naïve aged animals. This analysis revealed CD11bhigh B cells have higher capacity for TNF-α production when compared to CD11blow B cells (Fig. 1F). Importantly, CD11bhigh B cells did not depend on presence of other immune cells for this effect (Fig. 1F). These findings indicate activated state of these CD11bhigh B cells in both antigen uptake and cytokine production.
CD11bhigh B cells are increased in both young and aged brains as early as post-MCAO day 7.
To test whether CD11bhigh B cells increase in the brain after stroke, both young and aged mice underwent 60-min middle cerebral artery occlusion (MCAO) (Fig. 2A). At post-MCAO day 7, brain CD11bhigh and CD11blow B cells were compared in contralateral and ipsilateral hemispheres in stroke and sham animals. After stroke, both young and aged samples show an increase the density of immune populations, notably in CD11bhigh B cells (Fig. 2B, red island). The ipsilateral stroke hemisphere in aged animals demonstrated a significant increase in absolute CD11bhigh B cell counts (p = 0.0019) with young cells trending upwards in number (p = 0.0573). The ipsilateral stroke hemisphere in aged animals demonstrated that stroke resulted in a significant increase in the percentage of CD11bhigh B cells within the total CD45+/CD19+ B cell population (aged p < 0.0001 and young p = 0.0027) (Fig. 2C). Absolute counts of B cell populations are provided in Table 2, and a representative Kaplan-Meier curve of MCAO mortality in young and aged mice is included in Fig. 2D.
Table 2:
Absolute cell counts from right brain hemisphere in Figure 2.
| Absolute Cell Counts from Right Brain Hemisphere | ||||
|---|---|---|---|---|
| Group | CD45+/CD19+ B Cell (Average ± SEM) | CD11bhigh B Cell (Average ± SEM) | CD11blow B Cell (Average ± SEM) | |
| Aged | MCAO | 1111 ± 201.3 | 557.6 ± 113.2 | 367.7 ± 101.2 |
| Sham | 830.3 ± 251.1 | 63.31 ± 21.85 | 735.9 ± 235.9 | |
| Young | MCAO | 1321 ± 449.2 | 635 ± 176.6 | 595.2 ± 383.3 |
| sham | 63.29 ± 22.03 | 3 ± 0.55 | 54 ± 21.59 | |
Differential MG surface phenotype after stroke and after incubation with sorted CD11bhigh or CD11blow B cells.
Next, CD45, CD11b, MHC-II, P2RY12, and Tmem119 were analyzed to assess MG activation from ipsilateral hemispheres in young (Fig. 3A) and aged (Fig. 3B) mice compared to either contralateral hemisphere or sham brain samples. The gating strategy for MG is provided (Fig. S1D). Consistent with previous studies (2, 3, 54, 55), MFIs of CD45 and MHC-II were significantly increased while P2RY12 and Tmem119 were significantly decreased in young post-stroke MG (Fig. 3A). The surface profile of MG from aged mice after MCAO also revealed a significant increase in CD45 in the ipsilateral hemisphere as well as similar trends in MHC-II, P2RY12, and Tmem119 expressions after MCAO (Fig. 3B). These results confirmed activation of MG after stroke in our model, which was also associated with significantly increased presence of CD11bhigh B cells in the brain.
FIGURE 3. Differential MG surface phenotype after stroke and after incubation with sorted CD11bhigh or CD11blow B cells.
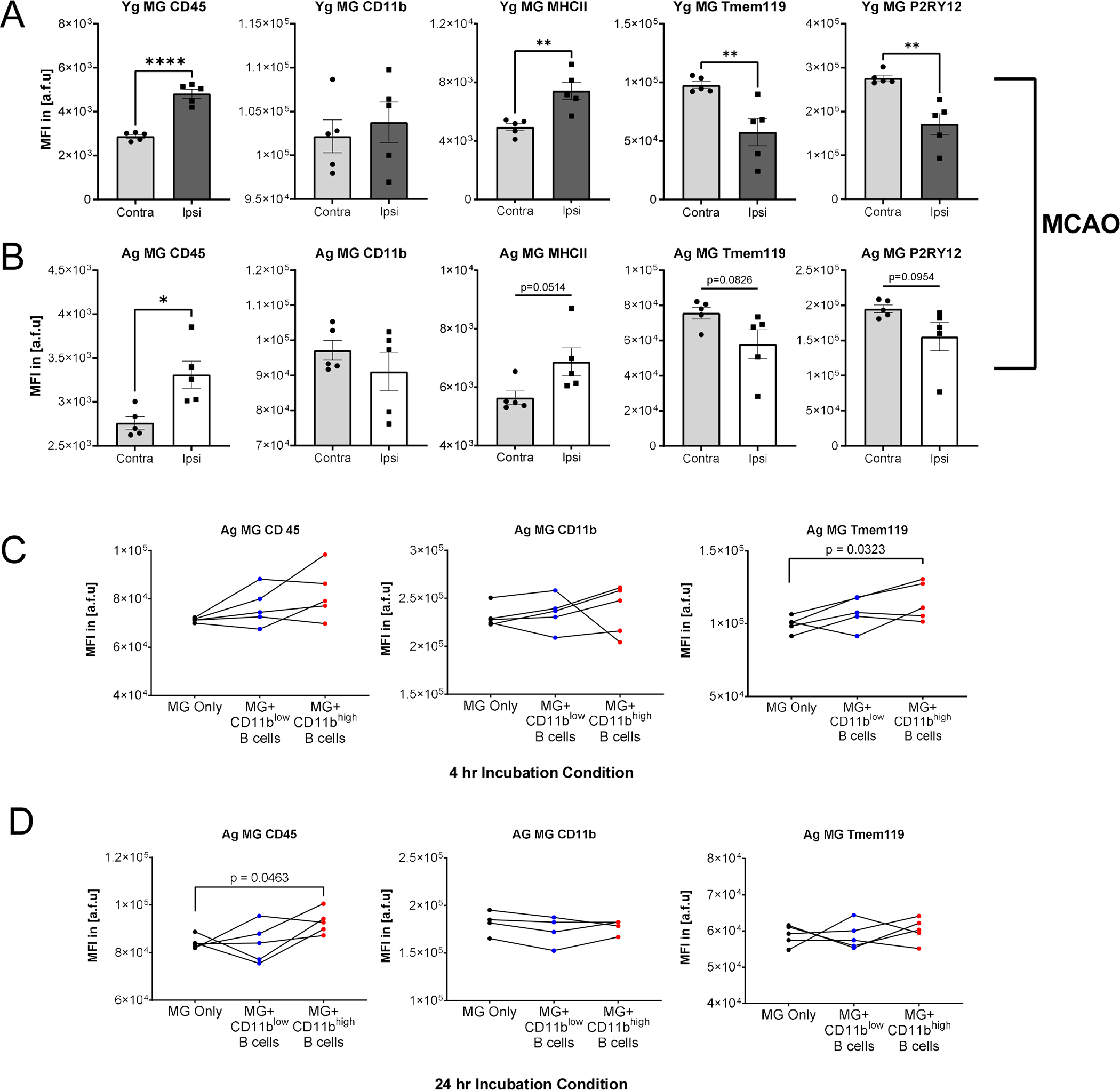
(A) Surface profile of MG from Yg mice after MCAO comparing contralateral hemisphere and ipsilateral hemispheres. CD45 MFI increased in MG in the ipsilateral hemisphere (p<0.0001), CD11b MFI in MG had no significant change in the ipsilateral hemisphere, MHCII MFI significantly increased in MG in the ipsilateral hemisphere (P=0.0048), P2RY12 MFI decreased in MG in the ipsilateral hemisphere (p=0.0026), Tmem119 MFI decreased in MG in the ipsilateral hemisphere (p=0.0098). (B) Surface profile of MG from Ag mice after MCAO comparing contralateral hemisphere and ipsilateral hemispheres. CD45 MFI increased in MG in the ipsilateral hemisphere (p=0.0119), CD11b, MHCII, Tmem119, and P2RY12 MFI in MG had no significant change in the ipsilateral hemisphere. Ag MG-enriched CNS mononuclear cells respond differently after incubation with CD11bhigh or CD11blow B cells sorted from Ag splenocytes after 4-hour and 24-hour incubations. (C) Analysis of MG derived from Ag mice and co-incubated with B cells sorted from the same respective animal. Post-percoll brain homogenate was divided across three incubation conditions for paired comparison. MG after a 4-hour co-incubation with CD11blow B cells, CD11bhigh B cells, or alone resulted in no significant changes in CD45 or CD11b MFI, and Tmem119 MFI significantly increasing (p=0.0323) in CD11bhigh B cells compared to MG alone. (D) Using the same design as C with a longer, 24-hour co-incubation with CD11blow B cells, CD11bhigh B cells, or alone resulted in CD45 MFI significantly increasing (p=0.0463), no significant changes in CD11b MFI or Tmem119 MFI in CD11bhigh B cells compared to MG alone. A and B were reproduced in two independent experiments underwent unpaired one-way ANOVA. C and D were performed once and underwent paired one-way ANOVA with Dunnett’s multiple comparisons test. mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, N.S. not significant.
To understand the role of CD11bhigh B cells in the context of neuroinflammation, we assessed the ex vivo ability of CD11bhigh B cells to induce changes to MG surface phenotype. Aged MG-enriched CNS mononuclear cell suspensions were divided across three co-incubation conditions with: sorted CD11bhigh B cells, sorted CD11blow B cells, and a control group. Sorted cells were derived from the spleen of the same animal. We hypothesized that CD11bhigh B cells are capable of inducing an activated state in MG. 4hrs of co-incubation with CD11bhigh B cells compared to the control resulted in significantly increased Tmem119 expression levels, while CD11blow B cells induced no changes (Fig. 3C). After a 24-hour co-incubation, there was a significant increase in CD45 expression in MG co-incubated with sorted CD11bhigh B cells compared to control, and the addition of CD11blow B cells had no significant differences from the control group (Fig. 3D). Our data suggests CD11bhigh B cells can regulate MG activation. The increase of CD45 and decrease of Tmem119 indicate an activation state(3, 56–58).
To directly compare the capacity of CD11bhigh and CD11blow B cells to differentially influence MG surface phenotype responses, young and aged MG-enriched CNS mononuclear cells and sorted MG underwent 4hr co-incubation using a broader panel of MG surface markers (CD45, CD11b, Tmem119, P2RY12, and MHC-II) (Fig. S3A–D). Young MG after 4hr co-incubation with CD11bhigh B cells compared to CD11blow B cells resulted in relatively decreased CD45, CD11b, Tmem119, and P2RY12 expression levels (Fig. S3A top). Analysis of aged MG co-incubated with B cells sorted from the same animals resulted in relatively decreased CD45 and Tmem119 expression (Fig. S3A bottom). Our data suggests CD11bhigh B cells can regulate MG activation unlike CD11blow B cells. Reduction of CD45 and CD11b indicate reduced activation state while decrease in Tmem119 and P2RY12 suggest an increased activation state of MG. Furthermore, aged MG appeared more resistant to changes in their surface phenotype compared to young MG.
We then evaluated surface expression of pure MG (sorted as Live Tmem119+) from both young and aged mice after a 4-hour co-incubation with sorted CD11bhigh or CD11blow B cells from aged splenocytes. Young MG after co-incubation with CD11bhigh B cells compared to CD11blow B cells resulted in a relative decrease in CD45 and CD11b, no significant changes in Tmem119 and P2RY12 expression levels, and MHC-II had a relative decrease (Fig. S3Btop). Aged MG showed no significant changes for CD45, CD11b, Tmem119, P2RY12, and MHC-II after co-incubation with CD11bhigh B cells compared to CD11blow B cells (Fig. S3Bbottom). These observations indicate that CD11bhigh B cells can, independently of other immune cells, influence young MG but not aged MG. MG have been shown to alter their surface expression in ex vivo conditions (59). Therefore, our next step in understanding the effects of CD11bhigh B cells on MG was to use an in vivo model of adoptive transfer.
Differential MG surface phenotype after adoptive transfer of CD11bhigh or CD11blow B cells into young naïve host.
Adoptive transfer can determine if increasing the number of CD11bhigh B cells alters MG activation. We used PepBoy mice with a distinguishable CD45 haplotype (i.e. CD45.1) to carry out our adoptive B cell transfer experiments. First, spleen, blood, brain and skull bone marrow underwent analysis to detect the presence of CD45.2+ cells in recipient mice 24hrs after retroorbital injection. This validated that the cells from the donor mice were successfully transferred to the recipient in all tissues, and it indicated that CD11bhigh B cells distribute throughout the host in the absence of injury (Table 3). Next, sorted CD11bhigh and CD11blow B cells from the spleens of aged wildtype mice were transferred into young PepBoy mice (Fig. 4A). The brains of the recipient underwent analysis 24hrs after injection. CD11b increased in MG in CD11bhigh B cell recipients compared to CD11blow B cell recipients (Fig. S3D). CD11b increased in MG in CD11bhigh B cell recipients compared to CD11blow B cell recipients. CD45, CD11b, and MHCII MFI had no significant difference in MG between vehicle control and CD11bhigh or CD11blow B cell recipients. Tmem119 and P2RY12 MFI increased in MG in CD11bhigh and CD11blow B cell recipients compared to vehicle control (Fig 4B). Our data suggests while there were responses in MG due to the presence of additional B cells, there was no clear pattern of MG activation based on surface phenotyping alone. Next, we investigated the phagocytotic activity in MG as a response to ex vivo and in vivo stimulation with CD11bhigh or CD11blow B cells.
Table 3:
Absolute counts of total live and donor cells (CD45.2) in recipient mouse (CD45.1) 24hrs after adoptive transfer by retroorbital injection.
| Adoptive Transfer: Absolute cell counts 24hrs after retroorbital injection | ||
|---|---|---|
| Sample: | Detectable CD45.2 cells | Number of Live cells |
| 01-Spleen-C1.fcs | 78 | 275637 |
| 01-Spleen-C2.fcs | 31 | 285155 |
| 01-Spleen-C3.fcs | 60 | 278033 |
| 01-Spleen-C4.fcs | 65 | 270406 |
| 01-Blood-E1.fcs | 661 | 23962 |
| 01-Blood-E2.fcs | 208 | 11333 |
| 01-Blood-E3.fcs | 105 | 4946 |
| 01-Blood-E4.fcs | 152 | 17447 |
| 01-Skull-G1.fcs | 347 | 27276 |
| 01-Skull-G2.fcs | 288 | 48142 |
| 01-Skull-G3.fcs | 318 | 62906 |
| 01-Skull-G4.fcs | 628 | 89692 |
| 01-Brain-A1.fcs | 234 | 10208 |
| 01-Brain-A2.fcs | 105 | 19886 |
| 01-Brain-A3.fcs | 178 | 15527 |
| 01-Brain-A4.fcs | 252 | 10378 |
| Tissue totals | Transferred immune cells | Number of live cells |
| Mouse 1 | 1320 | 337083 |
| Mouse 2 | 632 | 364516 |
| Mouse 3 | 661 | 361412 |
| Mouse 4 | 1097 | 387923 |
FIGURE 4. Differential MG surface phenotype after adoptive transfer of CD11bhigh or CD11blow B cells into young naïve host.
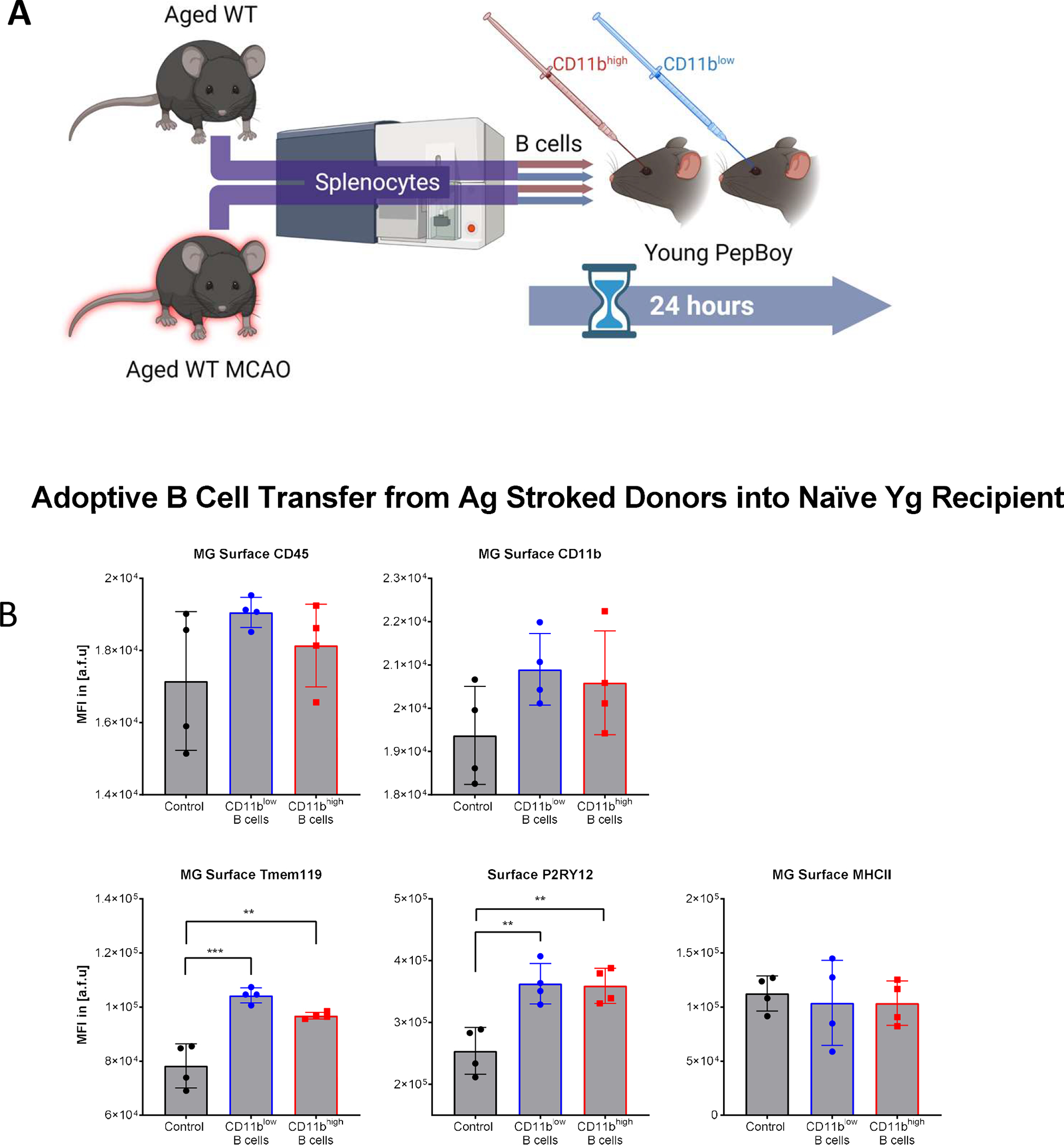
(A) Schematic. Splenocytes from naïve and stroked Ag male C57BL/6 mice underwent cell sorting to isolate CD11bhigh and CD11blow B cells. Cells were then adoptively transferred to Yg Pepboy recipient mice via retroorbital injection to contribute to modulation of MG phenotype. (B) Surface profile of MG from Yg recipient mice after adoptive transfer from Ag stroke donors. CD45 and CD11b MFI had no significant difference in MG between vehicle control and CD11bhigh or CD11blow B cell recipients. Tmem119 MFI increased in MG in CD11bhigh and CD11blow B cell recipients compared to vehicle controls (CD11bhigh p=0.0014 and CD11blow p=0.0001). P2RY12 increased in MG in CD11bhigh and CD11blow B cell recipients compared to vehicle controls (CD11bhigh p=0.0040 and CD11blow p=0.0032). MHCII MFI had no significant difference in MG between CD11bhigh and CD11blow B cell recipients and vehicle control. Experiments were performed once. Unpaired one-way ANOVA with Tukey’s multiple comparisons test, mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, N.S. not significant.
CD11bhigh B cells increase MG phagocytosis.
TNF-α has been shown to regulate MG phagocytosis (24, 26, 27, 60), thus we tested whether CD11bhigh B cells increase MG phagocytosis, given their higher capacity for TNF-α production (Fig. 1F). Phagocytotic activity of MG-enriched CNS mononuclear cells after 4 (Fig. 5A) and 24hrs (Fig. 5B–C) of co-incubation with CD11bhigh and CD11blow B cell subsets was assessed. MG showed significantly increased phagocytosis after a 4hr ex vivo co-incubation with CD11bhigh B cells compared to controls, while CD11blow B cells had no significant effect on the median fluorescent intensity of bead+ MG (Fig. 5A–C). However, MG incubated with CD11blow B cells had a greater percentage of bead+ MG (Fig. 5A–C). Taken together, MG incubated with CD11bhigh had higher phagocytosis capacity, even though the overall number of phagocytosing MG were similar in both MG incubated with CD11blow and MG incubated with CD11bhigh (Fig. 5A). Young and Aged MG significantly increased their phagocytic activity after 24hrs co-incubation with CD11bhigh B cells measured by relative frequency of bead positive MG (Fig. 5B–C).
FIGURE 5. CD11bhigh B cells increase MG phagocytosis.
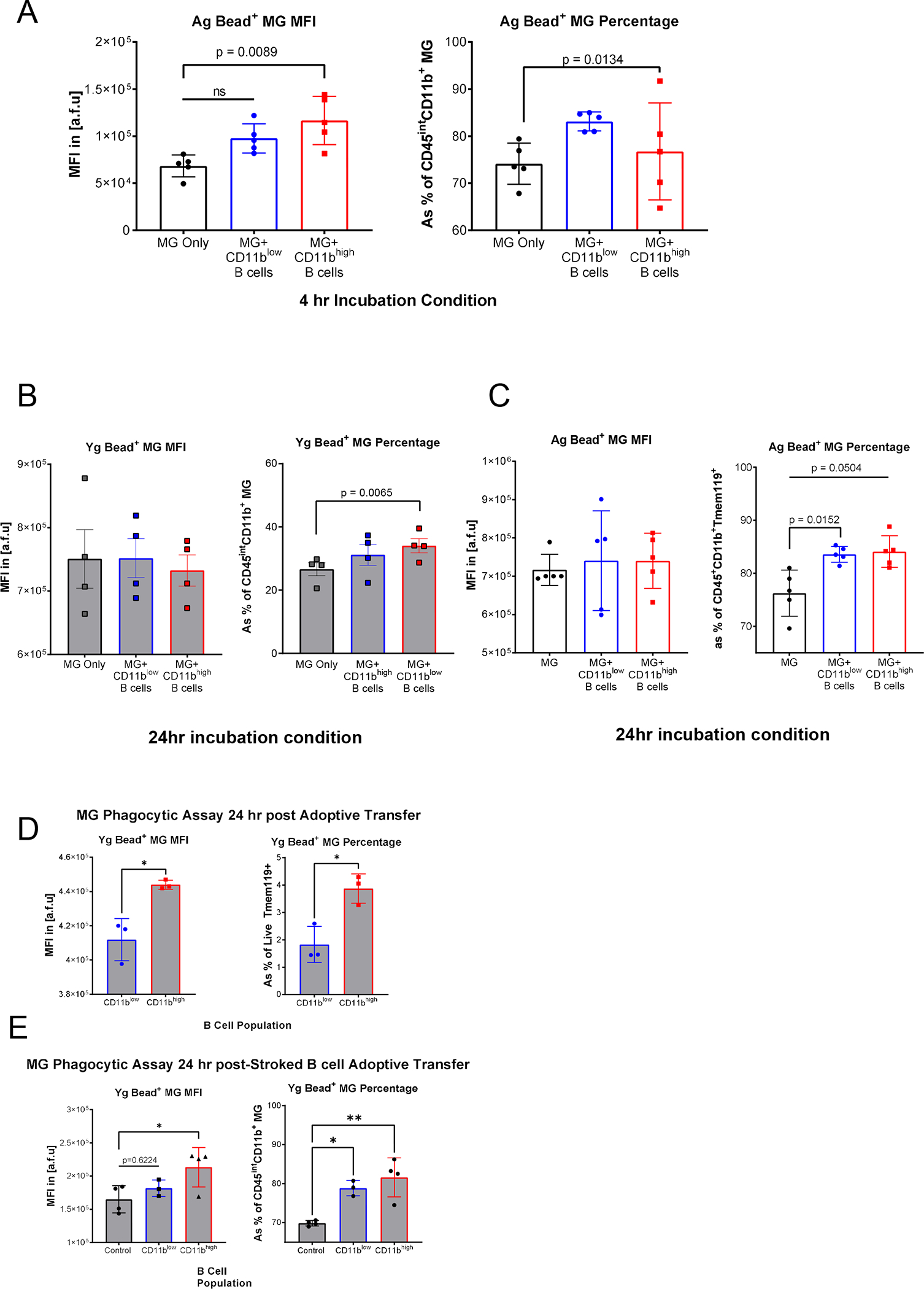
(A) Phagocytotic activity of 0.5μm fluorescent beads by Ag MG after 4-hour ex vivo co-incubation (n=5). Left measured bead MFI, with a significant increase (p=0.0089), in MG incubated with CD11bhigh B cells compared to MG alone, right show an increased relative frequency of phagocytotic activity in MG incubated with CD11blow B cells and compared to MG alone (p=0.0134). (B) Relative frequency of phagocytotic activity in Ag left and Yg right MG after 24-hours co-incubation with no significant differences in bead MFI, and a significant increased relative frequency of phagocytotic activity in MG incubated with CD11bhigh B cells compared to MG alone (p=0.0065). (n=4). (C) Ag MG had no significant differences in bead MFI, and an increased relative frequency of phagocytotic activity in MG incubated with CD11blow compared to MG alone (p=0.0504). Paired 1-way ANOVA with Dunnett’s multiple comparisons test. (D) Phagocytotic activity of Yg MG after adoptively transferred Ag CD11bhigh and CD11blow B cells 24hrs after transfer of B cell populations (n=3). Left measured bead MFI, with a significant increase in MG in mice who received CD11bhigh B cells compared and CD11blow B cells (p= 0.0116), right shows an increased relative frequency of phagocytotic activity in MG incubated with CD11bhigh and CD11blow B cells (p= 0.0140). Unpaired T-test. (E) Phagocytotic activity of Yg MG after adoptively transferred stroked Ag CD11bhigh and CD11blow B cells 24hrs after transfer of B cell populations (n=3–4). Left measured bead MFI, with a significant increase in MG incubated with CD11bhigh B cells compared to MG alone (p=0.0417), right show an increased relative frequency of phagocytotic activity in MG incubated with CD11bhigh and CD11blow B cells compared to MG alone (p=0.0023 and 0.0154 respectively). A and C were independently reproduced in two experiments. B, D, and E were performed once. One-way ANOVA post hoc Tukey’s multiple comparison, Mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, N.S. not significant.
Finally, phagocytosis assays of MG after adoptive B cell transfer from aged (Fig. 5D) and aged stroke (Fig. 5E) donors demonstrated that phagocytosis capacity of MG is significantly increased in MG from mice that received CD11bhigh B cells compared to MG from mice that received CD11blow B cells and vehicle control groups. Both ex vivo and in vivo data in our study consistently show that CD11bhigh B cells can independently increase MG phagocytosis.
Discussion
The central nervous system (CNS) has long been considered “immune privileged,” a dogma that arose from studies by Medawar (61) and others, in which transplanting heterologous tissue into the CNS parenchyma failed to induce an effective immune response (62, 63). However, accumulating evidence of neuroimmune communication in the CNS in both homeostatic and pathological conditions has shifted this paradigm (64). The CNS contains microglia (MG) which play a central role in both the acute and chronic phases of neuroinflammation. Additionally, innate and adaptive immune cells in the CNS meninges take an active part in the brain immune surveillance (65, 66). Pioneering work by Kipnis (12, 14, 67) and others revealed an extensive network of meningeal lymphatics and their role in the neuroimmune interface, shedding light on the importance of central and peripheral immune interactions.
Age-associated B cells (ABCs) are a group of functionally distinct B cells that significantly increased in aged mice and in autoimmune diseases in humans (53). The surface phenotyping of these cells vary and they contribute up to 30% of the mature B cell pool of aged mice (40–42, 53, 68). Here, we demonstrate that the CD11bhigh subset of B cells have a distinct surface phenotype in the CNS and periphery, and demonstrate increased TNF-α production and increased phagocytosis in ex vivo assays. The ABC phenotype has been associated with increased expression of T-bet (69, 70), a transcription factor essential for autoantibody production (71, 72). A significant increase in T-bet levels in the CD11bhigh B cells was also seen in this study (Fig. S1C), supporting that these CD11bhigh B cells could be a subset of ABCs. We then determined that CD11bhigh B cells have a distinct surface phenotype and are a highly activated and heterogeneous as demonstrated by higher expression of CD138 (expressed by plasma cells (73)), CD80 and CD27 (expressed by memory B cells (74, 75)), CD73 (expressed by class-switched B cells (76)), and CD268 (aka BAFFR, expressed by B cells implicated in auto-reactivity (33, 77). This conclusion is further supported by the significant decrease in IgD with no significant change in IgM (Fig. 1D), a finding that is consistent with previous phenotyping of ABCs (37, 68). These findings combined along with accumulation of CD11bhigh B cells in the brain with aging and after neurological injury suggest that these cells could be considered as a subset of ABCs.
The amount of CD11bhigh B cells increase significantly by post-MCAO day 7 in both young and aged brain and this increase is associated with expected activation of MG after stroke when assessed by higher expression of CD45 and MHC-II while lower expression of Tmem119 and P2RY12 (3, 78). The function of Tmem119 is not yet understood, however its decreased expression has been associated with activation of MG (58, 79–81). The increased expression of CD45 and MHC-II is characteristic of increased MG activation and phagocytosis (82, 83). Next, we sought to induce a stroke-like MG activation state by co-incubating sorted MG from young and aged brains with either CD11bhigh or CD11blow sorted B cells. We found that the CD11bhigh B cells can distinctly modify surface phenotype and increased MG phagocytosis when compared to their CD11blow counterparts ex vivo. Furthermore, 24hrs after adoptively transferring CD11bhigh B cells from both aged naïve and aged stroke WT mice into young PepBoy mice resulted in a significant increase in phagocytosis in MG compared to CD11blow transferred B cells Given the increasing availability of FDA-approved B cell therapies for variety of inflammatory conditions, better insights into the interaction between B cells and innate CNS immune cells is of high clinical relevance (84).
The infiltration of peripheral lymphocytes after stroke is a key feature of the progression of neuroinflammation and B cell-derived cytokine production can influence outcomes after stroke (42, 85). Studies have shown that B cells contribute to post-stroke cognitive impairment (42, 85–87). Here, we demonstrated that CD11bhigh B cells produce significantly higher amounts of TNF-α than CD11blow B cells and this increased production of TNF-α is correlated with ex vivo increased MG phagocytic activity, which is consistent with prior reports (24, 26, 27, 60, 88). By affecting MG activation and phagocytosis and possessing the chronic memory function, CD11bhigh B cells have the ability to regulate long-term neurological outcomes in aging and after cerebrovascular injury, as recently shown by a robust presence of ABCs cells in the meningeal layers (48). Therefore, we speculate that the increased activation of MG and their increased phagocytosis to be, at least in part, due to the presence of CD11bhigh B cells and their high capacity to produce TNF-α. Future mechanistic studies using TNF-receptor knockouts in MG can shed more light on the regulatory influence of TNF-α produced by CD11bhigh B cells on MG-mediated neuroinflammation.
Previous studies have shown that flow cytometric analysis exclude CD11bhigh B cells (44–47) or “infiltrating monocytes” that include CD11bhigh B cells (89). As we demonstrate here, this gating strategy would only exclude a negligible fraction of the B cell population in young mice (Fig. 1A), thus appropriate when using young uninjured animal models. However, in studies investigating B cells in brain injury or aging models, the exclusion of CD11bhigh B cells will result in the exclusion of a significant subpopulation of B cells (Fig. 1A). Not only is this a large sum of cells to exclude, but also, we demonstrated here that CD11bhigh B cells have increased cytokine production and phagocytic activity, and their exclusion may lead to skewed results in the assessment of B cell function in neuroinflammation. Therefore, careful validations should be performed when using flow cytometric identification of B cells in the context of neuroinflammation.
As both APCs and adaptive immune cells with memory function, B cells are uniquely positioned to regulate both acute and chronic phases of the post-stroke immune response, and their influence is subset-specific. Future studies are warranted to better understand the function of CD11bhigh B cells in neuroinflammation.
Supplementary Material
Key Points:
CD11bhigh B cells are a distinct subset of B cells that increase with aging.
CD11bhigh B cells are increased in both young and aged brains after stroke.
CD11bhigh B cells can regulate MG phenotypes and increase phagocytosis.
Acknowledgements:
Figures created in BioRender.com.
Funding:
This work was supported by the Center for Clinical and Translational Sciences TL1 Training Program National Institutes of Health (NIH) Grant No. TL1 TR003169 (to PH), NIH/National Institute of Neurological Disorders and Stroke (NINDS) Grant No. 1F31NS118984-01 (to PH), NIH/National Institute on Aging (NIA) and National Institute of Neurological Disorders and Stroke (NINDS): NIH/NINDS 5-R01-NS103592-02 (Detrimental Effects of Age Related Dysbiosis to LDM), NIH/NIA 1-R01-AG070934-01 (Link between early gut dysfunction and amyloid beta aggregation in Alzheimer’s Disease related dementia to BPG), NINDS Exploratory Neuroscience Research Grant 1 R21 NS114836-01A1 (Role of CD13 in Ischemic Stroke to AC), and NIH/NINDS 5-R01-NS094543-04 (Reversing Age Related Inflammation to LDM).
List of abbreviations:
- MG
Microglia
- CNS
central nervous system
- CD
cluster of differentiation
- Tmem119
trans-membrane protein 119
- P2RY12
purinergic receptor P2Y12
- MCAO
middle cerebral artery occlusion
- LPS
lipopolysaccharide
- tSNE
t-distributed stochastic neighbor embedding
- AD
Alzheimer’s disease
- EAE
experimental autoimmune encephalomyelitis
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Disclosures
Ethics approval: No human data is included in this study. Animal procedures were performed at an AAALAC accredited facility and were approved by the Animal Welfare Committee at the University of Texas Health Science Center at Houston, TX, USA.
Consent for publication: License granted to publish figures created in BioRender.com under agreement numbers KQ23P0DMSF and NE23P0D37X.
Availability of data and materials: We agree that upon request all the repeatability and reproducibility data files will be provided.
References
- 1.Roy-O’Reilly M, and McCullough LD. 2018. Age and Sex Are Critical Factors in Ischemic Stroke Pathology. Endocrinology 159: 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritzel RM, Lai Y-J, Crapser JD, Patel AR, Schrecengost A, Grenier JM, Mancini NS, Patrizz A, Jellison ER, Morales-Scheihing D, Venna VR, Kofler JK, Liu F, Verma R, and McCullough LD. 2018. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 136: 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honarpisheh P, Lee J, Banerjee A, Blasco-Conesa MP, Honarpisheh P, d’Aigle J, Mamun AA, Ritzel RM, Chauhan A, Ganesh BP, and McCullough LD. 2020. Potential caveats of putative microglia-specific markers for assessment of age-related cerebrovascular neuroinflammation. J. Neuroinflammation 17: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritzel R, He J, Li Y, Cao T, Khan N, Shim B, Sabirzhanov B, Aubrecht T, Stoica B, Faden A, Wu L, and Wu J. 2021. Proton extrusion during oxidative burst in microglia exacerbates pathological acidosis following traumatic brain injury. Glia 69: 746–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan A, Al Mamun A, Spiegel G, Harris N, Zhu L, and McCullough LD. 2018. Splenectomy protects aged mice from injury after experimental stroke. Neurobiol. Aging 61: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbaizar-Rovirosa M, Gallizioli M, Pedragosa J, Lozano JJ, Casal C, Pol A, and Planas AM. 2022. Age-dependent lipid droplet-rich microglia worsen stroke outcome in old mice. bioRxiv 2022.03.14.484305. [Google Scholar]
- 7.Manwani B, Liu F, Xu Y, Persky R, Li J, and McCullough LD. 2011. Functional recovery in aging mice after experimental stroke. Brain. Behav. Immun. 25: 1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieber MW, Claus RA, Witte OW, and Frahm C. 2011. Attenuated Inflammatory Response in Aged Mice Brains following Stroke. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritzel R, Lai Y, Crapser J, Patel A, Schrecengost A, Grenier J, Mancini N, Patrizz A, Jellison E, Morales-Scheihing D, Venna V, Kofler J, Liu F, Verma R, and McCullough L. 2018. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 136: 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manwani B, Liu F, Scranton V, Hammond M, Sansing L, and McCullough L. 2013. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 249: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, Dai X, Iyer K, Hitchens TK, Foley LM, Li S, Stolz DB, Chen K, Ding Y, Thomson AW, Leak RK, Chen J, and Hu X. 2021. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radjavi A, Smirnov I, Derecki N, and Kipnis J. 2014. Dynamics of the meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry 19: 531–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastorakos P, and McGavern D. 2019. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 4: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, Papadopoulos Z, Kanamori M, Salvador AF, Baker W, Lemieux M, Da Mesquita S, Cugurra A, Fitzpatrick J, Sviben S, Kossina R, Bayguinov P, Townsend RR, Zhang Q, Erdmann-Gilmore P, Smirnov I, Lopes M-B, Herz J, and Kipnis J. 2021. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184: 1000–1016.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candlish M, and Hefendehl JK. 2021. Microglia Phenotypes Converge in Aging and Neurodegenerative Disease. Front. Neurol. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood EK, and Brown DR. 2021. Senescent Microglia: The Key to the Ageing Brain? Int. J. Mol. Sci. 22: 4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez F, Novarino J, Mejía JE, Fazilleau N, and Aloulou M. 2021. Ageing of T-dependent B cell responses. Immunol. Lett. 233: 97–103. [DOI] [PubMed] [Google Scholar]
- 18.Xie X, Shrimpton J, Doody GM, Conaghan PG, and Ponchel F. 2021. B-cell capacity for differentiation changes with age. Aging Cell 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DM N, and JP G. 2013. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, and Glass CK. 2017. An environment-dependent transcriptional network specifies human microglia identity. Science (80-. ). 356: 1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K, Yu W, Cao R, Zhu Z, and Zhao G. 2017. Microglia-mediated BAFF-BAFFR ligation promotes neuronal survival in brain ischemia injury. Neuroscience 363: 87–96. [DOI] [PubMed] [Google Scholar]
- 22.Honarpisheh P, Blixt FW, Blasco Conesa MP, Won W, d’Aigle J, Munshi Y, Hudobenko J, Furr JW, Mobley A, Lee J, Brannick KE, Zhu L, Hazen AL, Bryan RM, McCullough LD, and Ganesh BP. 2020. Peripherally-sourced myeloid antigen presenting cells increase with advanced aging. Brain. Behav. Immun. 90: 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oishi Y, and Manabe I. 2016. Macrophages in age-related chronic inflammatory diseases. npj Aging Mech. Dis. 2016 21 2: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harms AS, Lee J-K, Nguyen TA, Chang J, Ruhn KM, Treviño I, and Tansey MG. 2012. Regulation of Microglia Effector Functions by Tumor Necrosis Factor (TNF) Signaling. Glia 60: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCoy M, and Tansey M. 2008. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lively S, and Schlichter LC. 2018. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 0: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neniskyte U, Vilalta A, and Brown GC. 2014. Tumour necrosis factor alpha-induced neuronal loss is mediated by microglial phagocytosis. Febs Lett. 588: 2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frasca D, Diaz A, Romero M, Landin AM, and Blomberg BB. 2014. High TNF-α levels in resting B cells negatively correlate with their response. Exp. Gerontol. 0: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, and Offner H. 2011. Regulatory B Cells Limit CNS Inflammation and Neurologic Deficits in Murine Experimental Stroke. J. Neurosci. 31: 8556–8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Bodhankar S, Murphy SJ, Vandenbark AA, Alkayed NJ, and Offner H. 2012. Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice. Metab. Brain Dis. 2012 274 27: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou T, Zheng Y, Sun L, Badea SR, Jin Y, Liu Y, Rolfe AJ, Sun H, Wang X, Cheng Z, Huang Z, Zhao N, Sun X, Li J, Fan J, Lee C, Megraw TL, Wu W, Wang G, and Ren Y. 2019. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 22: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planas AM, Gómez-Choco M, Urra X, Gorina R, Caballero M, and Chamorro Á. 2012. Brain-Derived Antigens in Lymphoid Tissue of Patients with Acute Stroke. J. Immunol. 188: 2156–2163. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Jiang X, Liu R, Wang L, Qian T, Zheng Y, Deng Y, Huang E, Xu F, Wang J-Y, and Chu Y. 2015. B cells expressing CD11b effectively inhibit CD4+ T-cell responses and ameliorate experimental autoimmune hepatitis in mice. Hepatology 62: 1563–1575. [DOI] [PubMed] [Google Scholar]
- 34.Schittenhelm L, Hilkens CM, and Morrison VL. 2017. β2 Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 8: 1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, Blalock EL, Scharer CD, Tipton CM, Wei C, Lim SS, Petri M, Niewold TB, Anolik JH, Gibson G, Lee FEH, Boss JM, Lund FE, and Sanz I. 2018. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 49: 725–739.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Rahman S, Zerrouki K, Hanna R, Morehouse C, Holoweckyj N, Liu H, Manna Z, Goldbach-Mansky R, Hasni S, Siegel R, Sanjuan M, Streicher K, Cancro MP, Kolbeck R, and Ettinger R. 2018. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 2018 91 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao Y, O’Neill P, Naradikian MS, Scholz JL, and Cancro MP. 2011. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118: 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, Cui Y, Angelini L, Lee K-A, McGowan SJ, Burrack AL, Wang D, Dong Q, Lu A, Sano T, O’Kelly RD, McGuckian CA, Kato JI, Bank MP, Wade EA, Pillai SPS, Klug J, Ladiges WC, Burd CE, Lewis SE, LaRusso NF, Vo NV, Wang Y, Kelley EE, Huard J, Stromnes IM, Robbins PD, and Niedernhofer LJ. 2021. An aged immune system drives senescence and ageing of solid organs. Nature 594: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindestam Arlehamn CS, Pham J, Alcalay RN, Frazier A, Shorr E, Carpenter C, Sidney J, Dhanwani R, Agin-Liebes J, Garretti F, Amara AW, Standaert DG, Phillips EJ, Mallal SA, Peters B, Sulzer D, and Sette A. 2019. Widespread Tau-Specific CD4 T Cell Reactivity in the General Population. J. Immunol. 203: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du S, Arkatkar T, Al Qureshah F, Jacobs H, Thouvenel C, Chiang K, Largent A, Li Q, Hou B, Rawlings D, and Jackson S. 2019. Functional Characterization of CD11c + Age-Associated B Cells as Memory B Cells. J. Immunol. 203: 2817–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanco E, Pérez-Andrés M, Arriba-Méndez S, Contreras-Sanfeliciano T, Criado I, Pelak O, Serra-Caetano A, Romero A, Puig N, Remesal A, Torres Canizales J, López-Granados E, Kalina T, Sousa A, van Zelm M, van der Burg M, van Dongen J, and Orfao A. 2018. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J. Allergy Clin. Immunol. 141: 2208–2219.e16. [DOI] [PubMed] [Google Scholar]
- 42.Doyle KP, and Buckwalter MS. 2020. Immunological mechanisms in poststroke dementia. Curr. Opin. Neurol. 33: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubtsova K, Rubtsov A, Cancro M, and Marrack P. 2015. Age-Associated B Cells: A T-bet-Dependent Effector with Roles in Protective and Pathogenic Immunity. J. Immunol. 195: 1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weitbrecht L, Berchtold D, Zhang T, Jagdmann S, Dames C, Winek K, Meisel C, and Meisel A. 2020. CD4+ T cells promote delayed B cell responses in the ischemic brain after experimental stroke. Brain. Behav. Immun. [DOI] [PubMed] [Google Scholar]
- 45.Harp CRP, Archambault AS, Cheung M, Williams JW, Czepielewski RS, Duncker PC, Kilgore AJ, Miller AT, Segal BM, Kim AHJ, Randolph GJ, and Wu GF. Neutrophils promote VLA-4-dependent B cell antigen presentation and accumulation within the meninges during neuroinflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, and Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu GF, Shimoda M, Linington C, Russell JH, Ferris ST, Mikesell RJ, Koni PA, Harp CRP, Archambault AS, and Sim J. 2021. Model of Multiple Sclerosis Drive Neuroinflammation in an Animal B Cell Antigen Presentation Is Sufficient To. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schafflick D, Wolbert J, Heming M, Thomas C, Hartlehnert M, Börsch A-L, Ricci A, Martín-Salamanca S, Li X, Lu I-N, Pawlak M, Minnerup J, Strecker J-K, Seidenbecher T, Meuth SG, Hidalgo A, Liesz A, Wiendl H, and zu Horste GM. 2021. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat. Neurosci. 2021 1–10. [DOI] [PubMed] [Google Scholar]
- 49.Chauhan A, Moser H, and McCullough LD. 2017. Sex differences in ischaemic stroke: potential cellular mechanisms. Clin. Sci. (Lond). 131: 533–552. [DOI] [PubMed] [Google Scholar]
- 50.Verma R, Ritzel RM, Harris NM, Lee J, Kim T, Pandi G, Vemuganti R, and McCullough LD. 2018. Inhibition of miR-141–3p Ameliorates the Negative Effects of Poststroke Social Isolation in Aged Mice. Stroke 49: 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J, d’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino J, Putluri N, Zhu L, Durgan DJ, Bryan RM, McCullough LD, and Venna VR. 2020. Gut Microbiota–Derived Short-Chain Fatty Acids Promote Poststroke Recovery in Aged Mice. Circ. Res. 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rangaraju S, Raza SA, Li NX, Betarbet R, Dammer EB, Duong D, Lah JJ, Seyfried NT, and Levey AI. 2018. Differential Phagocytic Properties of CD45low Microglia and CD45high Brain Mononuclear Phagocytes—Activation and Age-Related Effects. Front. Immunol. 0: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma S, Wang C, Mao X, and Hao Y. 2019. B Cell Dysfunction Associated With Aging and Autoimmune Diseases. Front. Immunol. 0: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kronenberg G, Uhlemann R, Richter N, Klempin F, Wegner S, Staerck L, Wolf S, Uckert W, Kettenmann H, Endres M, and Gertz K. 2017. Distinguishing features of microglia- and monocyte-derived macrophages after stroke. Acta Neuropathol. 2017 1354 135: 551–568. [DOI] [PubMed] [Google Scholar]
- 55.Rawlinson C, Jenkins S, Thei L, Dallas ML, and Chen R. 2020. Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy. Brain Sci. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Z, Harvey SS, Chiang T, Foltz AG, Lee AG, Cheng MY, and Steinberg GK. 2021. Unique subtype of microglia in degenerative thalamus after cortical stroke. Stroke 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajan WD, Wojtas B, Gielniewski B, and Gieryng A. 2018. Dissecting functional phenotypes of microglia and macrophages in the rat brain after transient cerebral ischemia. [DOI] [PubMed] [Google Scholar]
- 58.Satoh J, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T, and Saito Y. 2016. TMEM119 marks a subset of microglia in the human brain. Neuropathology 36: 39–49. [DOI] [PubMed] [Google Scholar]
- 59.Hasselmann J, Coburn MA, England W, Figueroa Velez DX, Kiani Shabestari S, Tu CH, McQuade A, Kolahdouzan M, Echeverria K, Claes C, Nakayama T, Azevedo R, Coufal NG, Han CZ, Cummings BJ, Davtyan H, Glass CK, Healy LM, Gandhi SP, Spitale RC, and Blurton-Jones M. 2019. Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo. Neuron 103: 1016–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, and O’Callaghan JP. 2006. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-α1. FASEB J. 20: 670–682. [DOI] [PubMed] [Google Scholar]
- 61.MEDAWAR PB 1948. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 29: 58–69. [PMC free article] [PubMed] [Google Scholar]
- 62.Widner H, and Brundin P. 1988. Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res. Rev. 13: 287–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy JB, and Sturm E. 1923. CONDITIONS DETERMINING THE TRANSPLANTABILITY OF TISSUES IN THE BRAIN. J. Exp. Med. 38: 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louveau A, Harris TH, and Kipnis J. 2015. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 36: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, and Becher B. 2018. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 48: 380–395.e6. [DOI] [PubMed] [Google Scholar]
- 66.Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, Aerts J, Berx G, Boeckxstaens GE, Vandenbroucke RE, Vereecke L, Moechars D, Guilliams M, Van Ginderachter JA, Saeys Y, and Movahedi K. 2019. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 2019 226 22: 1021–1035. [DOI] [PubMed] [Google Scholar]
- 67.Da Mesquita S, Fu Z, and Kipnis J. 2018. The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 100: 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, and Marrack P. 2011. Toll-like receptor 7 (TLR7)–driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood 118: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myles A, Gearhart P, and Cancro M. 2017. Signals that drive T-bet expression in B cells. Cell. Immunol. 321: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubtsova K, Rubtsov A, Cancro M, and Marrack P. 2015. Age-Associated B Cells: A T-bet-Dependent Effector with Roles in Protective and Pathogenic Immunity. J. Immunol. 195: 1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, Bengsch B, Linderman SL, Stelekati E, Spolski R, Wherry EJ, Hunter C, Hensley SE, Leonard WJ, and Cancro MP. 2016. Cutting Edge: IL-4, IL-21, and IFN-γ Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J. Immunol. 197: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng SL, Szabo SJ, and Glimcher LH. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. 99: 5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanderson RD, Lalor P, and Bernfield M. 1989. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Good-Jacobson KL, Song E, Anderson S, Sharpe AH, and Shlomchik MJ. 2012. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival and plasma cell generation. J. Immunol. 188: 4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu YCB, Kipling D, and Dunn-Walters DK. 2011. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front. Immunol. 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schneider E, Rissiek A, Winzer R, Puig B, Rissiek B, Haag F, Mittrücker H-W, Magnus T, and Tolosa E. 2019. Generation and Function of Non-cell-bound CD73 in Inflammation. Front. Immunol. 0: 1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qian T, Hong J, Wang L, Wang Z, Lu Z, Li Y, Liu R, and Chu Y. 2019. Regulation of CD11b by HIF-1α and the STAT3 signaling pathway contributes to the immunosuppressive function of B cells in inflammatory bowel disease. Mol. Immunol. 111: 162–171. [DOI] [PubMed] [Google Scholar]
- 78.Ronaldson PT, and Davis TP. 2020. Regulation of blood–brain barrier integrity by microglia in health and disease: A therapeutic opportunity: 10.1177/0271678X20951995 40: S6–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bennett M, Bennett F, Liddelow S, Ajami B, Zamanian J, Fernhoff N, Mulinyawe S, Bohlen C, Adil A, Tucker A, Weissman I, Chang E, Li G, Grant G, Hayden Gephart M, and Barres B. 2016. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U. S. A. 113: E1738–E1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner H, and Lassmann H. 2017. Loss of “homeostatic” microglia and patterns of their activation in active multiple sclerosis. Brain 140: 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohnert S, Seiffert A, Trella S, Bohnert M, Distel L, Ondruschka B, and Monoranu C. 2020. TMEM119 as a specific marker of microglia reaction in traumatic brain injury in postmortem examination. Int. J. Legal Med. 134: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen H, Sun Y, Chen C, Kuo Y, Kuan I, Tiger Li Z, Short-Miller J, Smucker M, and Kuan C. 2020. Fate mapping via CCR2-CreER mice reveals monocyte-to-microglia transition in development and neonatal stroke. Sci. Adv. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garner K, Amin R, Johnson R, Scarlett E, and Burton M. 2018. Microglia priming by interleukin-6 signaling is enhanced in aged mice. J. Neuroimmunol. 324: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iadecola C 2012. Brain-Immune Interactions and Ischemic Stroke. Arch. Neurol. 69: 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iadecola C, Buckwalter MS, and Anrather J. 2020. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Invest. 130: 2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doyle KP, Quach LN, Solé M, Axtell RC, Nguyen TVV, Soler-Llavina GJ, Jurado S, Han J, Steinman L, Longo FM, Schneider JA, Malenka RC, and Buckwalter MS. 2015. B-lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci. 35: 2133–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai AS, Berry K, Beneyto MM, Gaudilliere D, Ganio EA, Culos A, Ghaemi MS, Choisy B, Djebali K, Einhaus JF, Bertrand B, Tanada A, Stanley N, Fallahzadeh R, Baca Q, Quach LN, Osborn E, Drag L, Lansberg MG, Angst MS, Gaudilliere B, Buckwalter MS, and Aghaeepour N. 2019. A year-long immune profile of the systemic response in acute stroke survivors. Brain 142: 978–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mir M, Tolosa L, Asensio VJ, Lladó J, and Olmos G. 2008. Complementary roles of tumor necrosis factor alpha and interferon gamma in inducible microglial nitric oxide generation. J. Neuroimmunol. 204: 101–109. [DOI] [PubMed] [Google Scholar]
- 89.Monson NL, Ortega SB, Ireland SJ, Meeuwissen AJ, Chen D, Plautz EJ, Shubel E, Kong X, Li MK, Freriks LH, and Stowe AM. 2014. Repetitive hypoxic preconditioning induces an immunosuppressed B cell phenotype during endogenous protection from stroke. J. Neuroinflammation 2014 111 11: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


