Abstract
Purpose
Nitazoxanide is a broad-spectrum antiparasitic that has been tested for COVID-19 due to its anti-inflammatory effects and in vitro antiviral activity. This study synthesized the best evidence on the efficacy and safety of nitazoxanide in COVID-19.
Methods
Searches for studies were performed in peer-reviewed and grey-literature from January 1, 2020 to May 23, 2022. The following elements were used to define eligibility criteria: (1) Population: individuals with COVID-19; (2) Intervention: nitazoxanide; (3) Comparison: placebo; (4) Outcomes: primary outcome was death, and secondary outcomes were viral load, positive RT-PCR status, serum biomarkers of inflammation, composite measure of disease progression (ICU admission or invasive mechanical ventilation), and any adverse events; (5) Study type: blinded, placebo-controlled, randomized clinical trials (RCTs). Treatment effects were reported as relative risk (RR) for dichotomous variables and standardized mean difference (SMD) for continuous variables with 95% confidence intervals (CI).
Results
Five blinded, placebo-controlled RCTs were included and enrolled individuals with mild or moderate SARS-CoV-2 infection. We found no difference between nitazoxanide and placebo in reducing viral load (SMD = − 0.16; 95% CI − 0.38 to 0.05) and the frequency of positive RTP-PCR results (RR = 0.92; 95% CI 0.81 to 1.06). In addition, there was no decreased risk for disease progression (RR = 0.63; 95% CI 0.38 to 1.04) and death (RR = 0.81; 95% CI 0.36 to 1.78) among patients receiving nitazoxanide. Patients with COVID-19 treated with nitazoxanide had decreased levels of white blood cells (SMD = − 0.15; 95% − 0.29 to − 0.02), lactate dehydrogenase (LDH) (SMD − 0.32; 95% − 0.52 to − 0.13), and D-dimer (SMD − 0.49; 95% CI − 0.68 to − 0.31) compared to placebo, but the magnitude of effect was considered small to moderate.
Conclusion
This systematic review showed no evidence of clinical benefits of the use of nitazoxanide to treat patients with mild or moderate COVID-19. In addition, we found a reduction in WBC, LDH, and D-dimer levels among nitazoxanide-treated patients, but the effect size was considered small to moderate.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00228-022-03380-5.
Keywords: COVID-19, SARS-CoV-2 infection, Nitazoxanide, Meta-analysis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel single-stranded RNA virus associated with an acute pulmonary disease known as COVID-19 [1]. The binding between SARS-CoV-2 spike (S) protein and human receptor cells may lead to a dysregulated immune response with increased release of pro-inflammatory cytokines implicated in multi-organ damage and risk of death [2]. Given the lack of effective and safe antiviral agents against SARS-CoV-2, drug repurposing has played a critical role in the identification of rapidly available therapeutic solutions in treating patients with COVID-19 [3].
The tenth version of the World Health Organization (WHO) living guideline, published on July 14, 2022, contains 19 recommendations for the use of therapeutics in the treatment of COVID-19 [4], including strong recommendations to use nirmatrelvir plus ritonavir in patients with non-severe illness at the highest risk of hospitalization, and systemic corticosteroids and interleukin-6 (IL-6) blocking agents in patients with severe or critical disease. Baricitinib has been indicated as an alternative to IL-6 receptor blockers, in combination with corticosteroids, in patients with severe or critical COVID-19. There is evidence that nirmatrelvir plus ritonavir is associated with a reduced risk of progression to severe disease in non-hospitalized patients with COVID-19 [5]. In addition, it has been shown that there is a decreased risk of mortality among hospitalized patients with COVID-19 treated with systemic corticosteroids [6] and tocilizumab [7].
Other promising drugs, including antiparasitic agents, have also been tested in controlled clinical settings, but no benefits were found in preventing or treating patients with SARS-CoV-2 infection [8–10]. After a comprehensive review by Sanders and colleagues [11] in April 2020 and a letter to the editor published by our research group in July 2020 [12] calling attention to the potential antiviral effects of nitazoxanide and the need for high-quality trial evidence of this thiazolide antiparasitic drug in the treatment of SARS-CoV-2 infection, 28 interventional studies were registered on ClinicalTrials.gov, of which eight were completed or published by August 2022.
The best available evidence to assess treatment effects can be obtained through the identification, critical appraisal, and summary of results from blinded, placebo-controlled, randomized clinical trials (RCTs), considered the gold standard in clinical research. The aim of this systematic review and meta-analysis was to summarize the available evidence on the efficacy and safety of nitazoxanide as a treatment option in COVID-19 patients.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [13].
Search strategy
Searches for studies were performed in PubMed, Web of Science, Scopus, Embase, Google Scholar (first 100 hits), the website ClinicalTrials.gov, and the preprint server medRxiv from January 1, 2020 to May 23, 2022, without language restriction. On ClinicalTrials.gov, only completed studies with results were analyzed. The reference lists of all eligible studies and reviews were evaluated to identify additional studies for inclusion.
We used the following structured search strategy for each electronic database and other sources: (nitazoxanide) AND (COVID-19 OR “2019-nCoV Infection” OR “Coronavirus Disease-19” OR “2019-nCoV Disease” OR SARS-CoV-2). To expand the number of eligible studies, specific filters for RCTs were not used.
Study selection and eligibility criteria
Two reviewers (P.R.M.-F. and E.M.N.-J.) independently screened the search results and identified studies that were potentially relevant based on their title and abstract. Relevant studies were read in full and selected according to eligibility criteria. Disagreements between the two reviewers were resolved by consensus.
The following elements were used to define eligibility criteria: (1) Population: individuals with COVID-19; (2) Intervention: nitazoxanide; (3) Comparison: placebo; (4) Outcomes: primary outcome was death, and secondary outcomes were viral load, positive RT-PCR status, serum biomarkers of inflammation (white blood cells [WBC], neutrophils, lymphocytes, C-reactive protein [CRP], D-dimer, lactate dehydrogenase [LDH], IL-6, IL-8, TNF-α), composite measure of disease progression (ICU admission or invasive mechanical ventilation), and any adverse events; (5) Study type: blinded, placebo-controlled, RCTs. Eligible studies must report at least one of the outcomes of interest. Potential overlapping populations, open-label trials, and observational studies were excluded. Trials testing drug associations were also excluded.
Data extraction
Two authors (P.R.M.-F. and E.M.N.-J.) extracted the data from included studies and crosschecked them for accuracy. Using a standardized data extraction worksheet, the following information were extracted from the studies: the registry of the study protocol; demographic characteristics of study participants; pre-existing medical conditions; treatment arms; nitazoxanide protocol; concomitant medications; follow-up duration; and outcome data.
Risk of bias assessment
The study-level assessment of risk of bias was judged according to the Cochrane guidelines for RCTs [14]. The following domains were evaluated: sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), sample size calculation, power analysis, and early stopping for futility (operational bias), outcome measurements (information bias), and the authors' financial or non-financial conflicts of interest that could appear to affect the judgment of the research team when designing, conducting, or reporting a study. Studies that used real-time reverse transcription-polymerase chain reaction (RT-PCR) to detect SARS-CoV-2 or provided a diagnosis based on COVID-19-related symptoms and epidemiological data if testing was limited were considered to have a low risk of bias.
The overall risk of bias for an outcome across studies was judged as (1) low risk of bias, if most information is from studies at low risk of bias; (2) unclear risk of bias, if most information is from studies at unclear risk of bias; and (3) high risk of bias, if the proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results [15].
Data synthesis
Treatment effects were reported as relative risk (RR) for dichotomous variables (positive RT-PCR status, composite measure of disease progression, death, and any adverse events) and standardized mean difference (SMD) for continuous variables (viral load and serum biomarkers of inflammation) with 95% confidence intervals (CI). To calculate the RR, the number of events and individuals in each treatment group were extracted. To calculate SMD, means and standard deviations (SD) were obtained for each study group. If the means and SD were not directly reported in the publication, indirect methods of extracting estimates were used [16]. For viral load, we analyzed the results based on changes from baseline. A negative effect size indicated that nitazoxanide decreased viral load and levels of inflammatory biomarkers in patients with COVID-19.
Depending on the presence of heterogeneity, we used a fixed- or random-effects model to pool the results of individual studies. Statistical heterogeneity was quantified by the I2 index using the following interpretation: 0%, no between-study heterogeneity; < 50%, low heterogeneity; 50–75%, moderate heterogeneity; > 75%, high heterogeneity [17]. In the case of heterogeneity, we used the random-effects model, otherwise, the fixed-effects model was used.
Although funnel plots may be useful tools in investigating small study effects in meta-analyses, they have limited power to detect such effects when there are few studies [18]. Therefore, because we had only a small number of included studies, we did not perform a funnel plot analysis. Forest plots were used to present the effect sizes and the 95% CI, and a 2-tailed p < 0.05 was used to determine significance. Analyses were conducted using Review Manager, version 5.3 (Cochrane IMS).
Grading the strength of evidence
We graded the strength of evidence for the association between the use of nitazoxanide and the outcomes of interest as high, moderate, low, or very-low using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) rating system [19, 20]. In the GRADE system, RCTs begin as high-quality evidence but may be downrated according to the risk of bias assessment, inconsistency, indirectness, imprecision in the results, and publication bias [21]. Certainty is uprated for estimates with a large (RR > 2.0 or RR < 0.5; SMD > 0.8) magnitude of effect, in the case of plausible residual opposing confounding or for dose–response gradients.
Although the funnel plot asymmetry was not evaluated, we reduced the potential for publication bias by planning a comprehensive search including grey-literature without restrictions. For this criterion, we analyzed discrepancies in findings between studies and the influence of small trials (< 100 patients per arm) on estimated treatment effects. The influence of small trials on the pooled estimates was analyzed using a “leave-one-out” sensitivity approach [22].
Results
Study selection
The search strategy yielded 1031 potentially relevant records. After screening of titles and abstracts and evaluation of completed trials retrieved from ClinicalTrials.gov, eight full-text articles were assessed for eligibility, and five [23–27] blinded, placebo-controlled RCTs were included in the meta-analysis. A flow diagram of the study selection process and specific reasons for exclusion are detailed in the supplement (eFig. 1; supplementary information).
Study characteristics and risk of bias assessment
The studies were conducted in the USA, Puerto Rico, Brazil, and Argentina and included individuals with mild to moderate COVID-19 [24–27] or requiring supplemental oxygen therapy [23]. All trial protocols were registered on ClinicalTrials.gov, had a parallel design, and were classified as Phase 2 or Phase 3. Population characteristics, the dosage of nitazoxanide, and outcomes of interest are detailed in Table 1. Outcomes assessment was performed within the first month of follow-up. Although most trials evaluating nitazoxanide as treatment for patients with COVID-19 had a low risk of bias in most domains, the study by Silva et al. [26] was classified as having a high risk of bias for detection, attrition, reporting, and operational bias. A small sample bias was also found in the study by Blum et al. [27] (eFig. 2; supplementary information).
Table 1.
Blinded, placebo-controlled, randomized clinical trials evaluating the efficacy and safety of nitazoxanide in treating COVID-19
| Author | Country | Design (protocol/phase) | Setting | Population | Intervention | Control | Outcomes of interest and follow-up period |
|---|---|---|---|---|---|---|---|
| Rocco et al. [23] | Brazil | Double-blinded, placebo-controlled trial (NCT04561219/Phase 2) | 19 hospitals | Patients with COVID-19 requiring supplemental oxygen (median age 56 years; 39% female) | NTZ 500 mg TID for 5 days (n = 202) |
Placebo (n = 203) |
Viral load and RT-PCR status (day 7), serum biomarkers of inflammation (day 7), ICU (day 14), mortality (day 14), and any adverse events |
| Rocco et al. [25] | Brazil | Double-blinded, placebo-controlled trial (NCT04552483/Phase 2) | Five freestanding urgent care centres and two hospitals | Patients with mild COVID-19 (94.4% less than 60 years old; 53.1% female) | NTZ 500 mg TID for 5 days (n = 194) |
Placebo (n = 198) |
Viral load, RT-PCR status, serum biomarkers of inflammation, ICU, mortality, and any adverse events. All outcomes were evaluated after 5 days of therapy |
| Blum et al. [27] | Brazil | Double-blinded, placebo-controlled trial (NCT04348409/Phase 2) | Six hospitals | Patients with mild COVID-19 (median age 64 years; 70% female) | NTZ 600 mg BID for 7 days (n = 25) |
Placebo (n = 25) |
RT-PCR status, serum biomarkers of inflammation, mortality, and any adverse events. All outcomes were evaluated after 21 days of therapy |
| Rossignol et al. [24] | USA and Puerto Rico | Double-blinded, placebo-controlled trial (NCT04486313/Phase 3) | 36 outpatient medical clinics | Patients with mild or moderate COVID-19 (median age 40 year; 56.5% female) | NTZ 600 mg BID for 5 days (n = 184) |
Placebo (n = 195) |
Viral load and RT-PCR status (day 10), mortality (day 28), and any adverse events |
| Silva et al. [26] | Argentina | Single-blinded, placebo-controlled trial (NCT04463264/Phase 2–3) | Two hospitals | Patients with mild or moderate COVID-19 (age range 19–68 years; 27.8% female) |
NTZ 500 mg, 4 × per day for 14 days (n = 23) |
Placebo (n = 13) |
Viral load, RT-PCR status, mortality, and gastrointestinal adverse events. All outcomes were evaluated after 7 days of therapy |
NTZ nitazoxanide
SARS-CoV-2 viral load and RT-PCR status
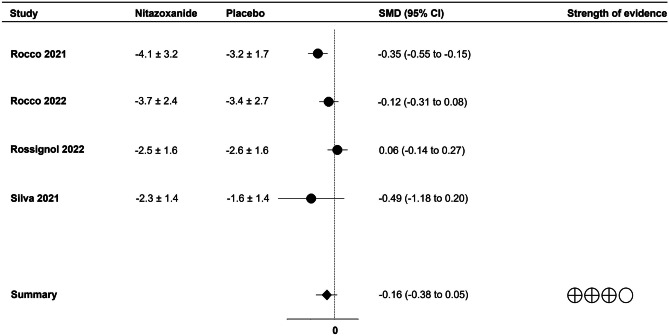
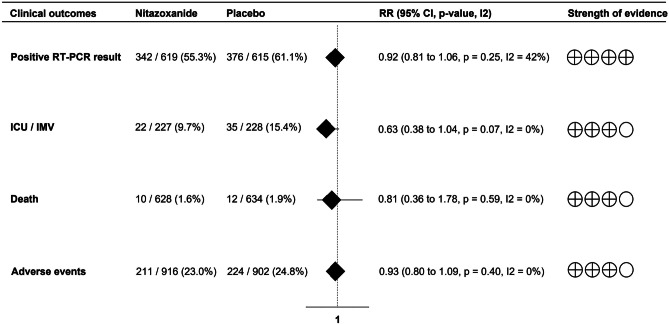
Most studies evaluated viral load and RT-PCR results during the first 10 days after treatment. Viral load in nasopharyngeal swabs was reduced over time with no difference between treatment groups (SMD = − 0.16; 95% CI -0.38 to 0.05; p = 0.14; I2 = 67%;) (Fig. 1). In addition, nitazoxanide did not decrease the frequency of positive RTP-PCR results compared to placebo (RR = 0.92; 95% CI 0.81 to 1.06; p = 0.25; I2 = 42%) (Fig. 2).
Fig. 1.
Forest plot showing the effect of nitazoxanide on viral load in patients with COVID-19
Fig. 2.
Forest plot showing the effects of nitazoxanide on RT-PCR status and clinical outcomes in patients with COVID-19
Serum biomarkers of inflammation
Patients with COVID-19 treated with nitazoxanide had decreased levels of WBC (SMD − 0.15; 95% − 0.29 to − 0.02; p = 0.03; I2 = 0%), LDH (SMD − 0.32; 95% − 0.52 to − 0.13; p < 0.01; I2 = not applicable), and D-dimer (SMD − 0.49; 95% CI − 0.68 to − 0.31; p < 0.01; I2 = 0%) compared to placebo, but the magnitude of effect was considered small to moderate. No differences were found for the other inflammatory biomarkers (Table 2).
Table 2.
Results of meta-analysis analyzing the levels of inflammatory biomarkers in patients with COVID-19 treated with nitazoxanide compared to placebo
| Laboratory findings | SMD | 95% CI | I2 | p-value | Magnitude of effect |
|---|---|---|---|---|---|
| WBC | − 0.15 | − 0.29 to − 0.02 | 0% | 0.03 | Small |
| Neutrophils | − 0.07 | − 0.21 to 0.07 | 0% | 0.32 | No effect |
| Lymphocytes | − 0.07 | − 0.21 to 0.06 | 0% | 0.29 | No effect |
| LDH | − 0.32 | − 0.52 to -0.13 | NA | < 0.01 | Small to moderate |
| IL-6 | − 0.48 | − 1.51 to 0.55 | 89% | 0.36 | No effect |
| IL-8 | − 0.25 | − 0.92 to 0.42 | 77% | 0.47 | No effect |
| TNF-α | − 0.30 | − 0.91 to 0.31 | NA | 0.33 | No effect |
| CRP | − 0.36 | − 1.00 to 0.27 | 94% | 0.26 | No effect |
| D-dimer | − 0.49 | − 0.68 to − 0.31 | 0% | < 0.01 | Small to moderate |
WBC white blood cells, LDH lactate dehydrogenase, CRP C-reactive protein, SMD standardized mean difference, NA not applicable
Clinical outcomes
There was no reduction in the risk of ICU admission or invasive mechanical ventilation (RR = 0.63; 95% CI 0.38 to 1.04; p = 0.07; I2 = 0%) or death (RR = 0.81; 95% CI 0.36 to 1.78; p = 0.59; I2 = 0%) among patients with COVID-19 receiving nitazoxanide. We also found no differences between groups regarding the frequency of individuals with any adverse events (RR = 0.93; 95% CI 0.80 to 1.09; p = 0.40; I2 = 0%) (Fig. 2). Of the 211 adverse events reported among patients using nitazoxanide, 98.6% were mild to moderate. Gastrointestinal symptoms were the most common adverse events in most studies [23–26]. The results of the meta-analyses were not influenced by studies with small samples.
Strength of evidence
The quality of evidence was graded as high for RT-PCR status; moderate for viral load, the composite measure of disease progression, death, and adverse events; and very-low to moderate for inflammatory biomarkers (eTable 1; supplementary information).
Discussion
Several drug repurposing strategies have been evaluated for the prevention and treatment of COVID-19. However, meta-analyses of clinical trials have shown that some of these promising drugs, including hydroxychloroquine [8], ivermectin [9, 28], colchicine [29], and statins [30] have no clinical benefits against the disease. This systematic review and meta-analysis synthesized the evidence on the efficacy and safety of nitazoxanide as a potential treatment of patients with COVID-19 based on the results of blinded, placebo-controlled, RCTs. Our findings confirm the inefficacy of nitazoxanide against COVID-19 in the current state of the art as established by best practices.
Nitazoxanide is a broad-spectrum antiparasitic and antiviral drug, originally approved for the treatment of parasite-mediated infectious diarrhea and enteritis, that has been tested for COVID-19 due to the anti-inflammatory effects [31], and in vitro anti-viral activity and promising clinical benefits against influenza and other viruses [32–34]. Moreover, there is in vitro evidence that nitazoxanide may induce a significant down-regulation of IL-6/JAK2/STAT3 [35] and may increase eIF2α and PKR phosphorylation, critical mediators involved in IFN-induced antiviral responses [36].
Individual studies [27] have suggested that nitazoxanide reduces the SARS-CoV-2 viral load in Vero E6 cells by 75% at a minimal dose of 0.1 μM with no cytotoxic effects. High SARS-CoV-2 viral loads were found to be associated with lymphopenia, increased markers of inflammation, and poor clinical outcomes in hospitalized patients with COVID-19 [37, 38]. Therefore, the use of nitazoxanide might accelerate viral clearance, improve clinical symptoms, and decrease the risk of hospitalization and death for patients with SARS-CoV-2 infection. Despite promising theoretical and experimental findings, this systematic review showed no evidence of clinical benefits of the use of nitazoxanide to treat patients with mild or moderate COVID-19. In addition, we found a reduction in WBC, LDH, and D-dimer levels among nitazoxanide-treated patients, but the effect size was considered small to moderate.
Because of its low cost, wide availability, and safety, nitazoxanide has been recommended for patients with COVID-19 in low- and middle-income countries. Individual results have shown that nitazoxanide can reduce the viral load during the first week of treatment [25, 26], the levels of some inflammatory mediators including CRP and D-dimer [23, 27], the time for hospital discharge [23, 27], and the time to sustained clinical response [24] in patients with mild to moderate COVID-19. However, the magnitude of the intervention effect of nitazoxanide in these studies seems to be limited, and the results need to be interpreted with caution. Furthermore, despite the potential reduction in viral load and inflammatory status, the clinical impact on critical outcomes such as ICU admission, need for mechanical ventilation, and death was insignificant. Since the quality of evidence for these outcomes was considered moderate, trials of high quality need to be designed to confirm our findings.
Despite the ineffectiveness of nitazoxanide in the treatment of COVID-19, the results of this meta-analysis showed a safety profile comparable to that of placebo. Approximately 23% of patients who received nitazoxanide experienced adverse effects, with the vast majority (98.6%) classified as mild or moderate. However, the proportion of individuals with at least one adverse effect was higher than that observed by Gamiño-Arroyo et al. [39], who evaluated the efficacy and safety of nitazoxanide in severe influenza-like illness in a population in which more than 50% of patients were children and teenagers. In addition, nitazoxanide is absorbed from the intestinal tract [33] and transient adverse effects such as diarrhea, abdominal pain, and nausea/vomiting have been reported to be the most common in patients treated with this antiprotozoal agent [40–42], as shown in the present study.
To date, there is still no optimal approach to the management of COVID-19. Symptomatic cases require supportive care with a medical evaluation, risk factor stratification for unfavorable clinical outcomes, and clinical monitoring of symptoms. In outpatients, symptomatic treatment includes analgesics and antipyretics. In the hospital setting, patients may need supplemental oxygen and adequate management of pulmonary ventilation. Moreover, the use of dexamethasone [43] and IL-6 antagonists [7, 44] has been associated with a decreased risk of death in patients with the most severe forms of the disease. Recently, open-label RCTs showed that prophylactic or therapeutic anticoagulation did not result in clinical improvement for hospitalized patients with COVID-19, except in the context of diagnosing a thromboembolic event [45, 46].
Our study has some major limitations, including trials with a high-risk of operational bias and slight differences at the time of outcomes assessment between studies, which may have led to some degree of clinical heterogeneity. Finally, the results of this study cannot be generalized to severe or critical COVID-19. Despite these limitations, available evidence based on the results of blinded, placebo-controlled, RCTs showed no clinical benefits of nitazoxanide in COVID-19.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
PRMF: conceptualization, methodology, project administration, supervision, study selection, data extraction, data analysis, risk of bias assessment, and writing. EMNJ: study selection, data extraction, risk of bias assessment, and writing. LCF, JABA and RF: methodology, literature search, and writing. All authors discussed the results and contributed to the final manuscript.
Data availability
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Institutional review board approval and informed consent were not required for this systematic review and meta-analysis.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.V’kovski P, Kratzel A, Steiner S, , et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmudpour M, Roozbeh J, Keshavarz M, et al. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heimfarth L, Serafini MR, Martins-Filho PR, et al. Drug repurposing and cytokine management in response to COVID-19: a review. Int Immunopharmacol. 2020;88:106947. doi: 10.1016/j.intimp.2020.106947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO (2022) Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4. Accessed 4 Aug 2022 [PubMed]
- 5.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19. JAMA. 2020;324:1330. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosn L, Chaimani A, Evrenoglou T et al (2021) Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev 3:CD013881. 10.1002/14651858.CD013881 [DOI] [PMC free article] [PubMed]
- 8.Martins-Filho PR, Ferreira LC, Heimfarth L, et al. Efficacy and safety of hydroxychloroquine as pre-and post-exposure prophylaxis and treatment of COVID-19: a systematic review and meta-analysis of blinded, placebo-controlled, randomized clinical trials. Lancet Reg Heal - Am. 2021;2:100062. doi: 10.1016/j.lana.2021.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izcovich A, Peiris S, Ragusa M, et al. Bias as a source of inconsistency in ivermectin trials for COVID-19: a systematic review. Ivermectin’s suggested benefits are mainly based on potentially biased results. J Clin Epidemiol. 2022;144:43–55. doi: 10.1016/j.jclinepi.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill A, Mirchandani M, Ellis L, Pilkington V. Ivermectin for the prevention of COVID-19: addressing potential bias and medical fraud. J Antimicrob Chemother. 2022;77:1413–1416. doi: 10.1093/jac/dkac052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 12.Martins-Filho PR, Barreto-Alves JA, Fakhouri R. Potential role for nitazoxanide in treating SARS-CoV-2 infection. Am J Physiol Lung Cell Mol Physiol. 2020;319:L35–L36. doi: 10.1152/ajplung.00170.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Simmonds M. Quantifying the risk of error when interpreting funnel plots. Syst Rev. 2015;4:24. doi: 10.1186/s13643-015-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meader N, King K, Llewellyn A, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst Rev. 2014;3:82. doi: 10.1186/2046-4053-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocco PRM, Silva PL, Cruz FF, et al. Nitazoxanide in patients hospitalized with COVID-19 pneumonia: a multicentre, randomized, double-blind, placebo-controlled trial. Front Med. 2022;9:844728. doi: 10.3389/fmed.2022.844728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossignol J-F, Bardin MC, Fulgencio J, et al. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. EClinicalMedicine. 2022;45:101310. doi: 10.1016/j.eclinm.2022.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocco PRM, Silva PL, Cruz FF, et al. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021;58:2003725. doi: 10.1183/13993003.03725-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva M, Espejo A, Pereyra ML et al (2021) Efficacy of nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, pilot study. medRxiv 1–17
- 27.Blum VF, Cimerman S, Hunter JR, et al. Nitazoxanide superiority to placebo to treat moderate COVID-19 - a pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine. 2021;37:100981. doi: 10.1016/j.eclinm.2021.100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcolino MS, Meira KC, Guimarães NS, et al. Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype. BMC Infect Dis. 2022;22:639. doi: 10.1186/s12879-022-07589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan S-H, Hsu C-K, Lai C-C, et al. Effect of colchicine on the outcomes of patients with COVID-19: a systematic review and meta-analysis of randomised controlled trials. Ann Med. 2022;54:1956–1965. doi: 10.1080/07853890.2022.2096919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins-Filho PR, Barreto-Filho JAS, Sousa ACS. Effects of statins on clinical outcomes in hospitalized patients with COVID-19. Eur J Intern Med. 2022 doi: 10.1016/j.ejim.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong SK, Kim HJ, Song CS, et al. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int Immunopharmacol. 2012;13:23–27. doi: 10.1016/j.intimp.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Jasenosky LD, Cadena C, Mire CE et al (2019) The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits ebola virus. iScience 19:1279–1290. 10.1016/j.isci.2019.07.003 [DOI] [PMC free article] [PubMed]
- 33.Rossignol J-F. Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haffizulla J, Hartman A, Hoppers M, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tantawy MA, El-Sherbeeny NA, Helmi N, et al. Synthetic antiprotozoal thiazolide drug induced apoptosis in colorectal cancer cells: implications of IL-6/JAK2/STAT3 and p53/caspases-dependent signaling pathways based on molecular docking and in vitro study. Mol Cell Biochem. 2020;469:143–157. doi: 10.1007/s11010-020-03736-4. [DOI] [PubMed] [Google Scholar]
- 36.Elazar M, Liu M, McKenna SA, et al. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2alpha via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009;137:1827–1835. doi: 10.1053/j.gastro.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 37.Eberhardt KA, Meyer-Schwickerath C, Heger E, et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID-19 patients. Viruses. 2020 doi: 10.3390/v12091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamiño-Arroyo AE, Guerrero ML, McCarthy S, et al. Efficacy and safety of nitazoxanide in addition to standard of care for the treatment of severe acute respiratory illness. Clin Infect Dis. 2019;69:1903–1911. doi: 10.1093/cid/ciz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stockis A, De Bruyn S, Gengler C, Rosillon D. Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d. Int J Clin Pharmacol Ther. 2002;40:221–227. doi: 10.5414/cpp40221. [DOI] [PubMed] [Google Scholar]
- 41.Guttner Y, Windsor HM, Viiala CH, et al. Nitazoxanide in treatment of Helicobacter pylori: a clinical and in vitro study. Antimicrob Agents Chemother. 2003;47:3780–3783. doi: 10.1128/AAC.47.12.3780-3783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barlow A, Landolf KM, Barlow B, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40:416–437. doi: 10.1002/phar.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO Rapid Evidence Appraisal for COVID-19 therapies (REACT) working group, Shankar-Hari M, Vale CL et al (2021) Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 326:499–518. 10.1001/jama.2021.11330 [DOI] [PMC free article] [PubMed]
- 45.Lopes RD, de Barros E, Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.INSPIRATION investigators, Sadeghipour P, Talasaz AH, , et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinic. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.