Abstract
Introduction
Clear communication of diagnostic test results and dementia diagnosis is challenging yet important to empower patients and care partners. A personalized diagnostic report could support the communication of dementia diagnostics and aid patients’ understanding of diagnosis. In this study, we aimed to design a diagnostic report in co‐creation with patients and care partners.
Methods
We used a mixed‐methods approach, combining surveys with focus groups in iteration. Phase 1 consisted of an international survey assessing needs among patients (n = 50) and care partners (n = 46), and phase 2 consisted of focus group meetings (n = 3) to co‐create the content and to hands‐on co‐design the layout of the diagnostic report with patients (n = 7) and care partners (n = 7). Phase 3 validated results from phase 2 in a survey among patients (n = 28) and care partners (n = 12), and phase 4 comprised final feedback by dementia (care) experts (n = 5). Descriptive statistics were used to report quantitative results and directed content analysis was used to analyze qualitative data.
Results
Most patients (39/50, 78%) and care partners (38/46, 83%) positively valued a diagnostic report to summarize test results. The report should be brief, straightforward, and comprise results of the diagnostic tests, including brain imaging and information on future expectations. Despite a clear preference for visual display of test results, several visualization options were deemed best and were equally comprehended.
Discussion
In this study, we developed a prototype of a personalized patient report through an iterative design process and learned that co‐creation is highly valuable to meet the specific needs of end‐users.
Keywords: brain imaging, communication, dementia, diagnosis, diagnostic testing, neuropsychology, prevention, prognosis, progression
1. BACKGROUND
The diagnostic workup of dementia requires an elaborate set of investigations, including neuropsychological testing and brain imaging, sometimes supplemented with biomarkers. 1 , 2 , 3 , 4 , 5 , 6 With increasing use of these tests, 7 a new challenge for the clinician arises: how to adequately discuss these results with the patient? Should they explain every test result or summarize the conclusion? How to stimulate understanding while preventing information overload?
Patients and care partners need to remember and understand the given information to cope with their condition and its implications. 8 A lack of understanding about dementia might lead to uncertainty about the future and suboptimal disease management. 9 However, patients and care partners frequently feel that test results and their meaning are inadequately explained. 10 , 11 This might result from patients’ recall of information, as patients generally remember only half of the provided information directly after consultation and even less in the long term. 12 Furthermore, the more information the clinician supplies, the more the patient will forget 13 —which is problematic considering the increasing number of diagnostic tests.
Several communication strategies could be used to present information in a comprehensible and orderly manner to stimulate understanding and recall in cognitively impaired patients. Health communication research has shown that tailored information is more effective for recall than standard information. 14 In other medical fields like oncology, written summaries have promoted patient understanding and recall. 15 , 16 Effective explanation of test results could also be facilitated by visual aids, for example, adding visualizations to written and spoken language increases patients’ comprehension and recall, 13 , 17 especially for those with low literacy. 18 , 19 Overall, an individualized diagnostic report containing tailored information and visualized test results could be a delicate way of presenting a patient's health data in a comprehensible manner, supporting clinician‐patient communication about test results, and stimulating information recall and understanding by patients and their care partners. 18 , 19
In this study, we aim to design a diagnostic report in an iterative design process, in co‐creation with patients and care partners, following the concept of design thinking. 20 By creating the diagnostic report in iteration with the end‐users, we aim to meet their specific needs and preferences.
RESEARCH IN CONTEXT
Systematic Review: Patients and care partners need to remember and understand all given information on diagnostic test results and dementia diagnosis to cope with their condition and its implications. The literature shows that several communication strategies could be used to present information in a comprehensible manner. A tailored diagnostic report including diagnostic test results could aid communication and stimulate understanding and recall of all given information.
Interpretation: Through several iterative steps involving patients and care partners, this study resulted in the development of a prototype of a tailored diagnostic report including information on diagnosis, visualization of diagnostic test results, brain health, practical issues, and information sources.
Future Directions: This study showed that one size does not fit all regarding the diagnostic report. Preferably, diagnostic reports should be compiled on the spot, attuned to patients’ and care partners’ informational needs, preferences, phase of the disease, and health literacy.
2. METHODS
2.1. Study design
We used an iterative, mixed‐methods study design based on the design thinking framework 20 consisting of four phases (Figure 1). Table 1 shows an overview of the study participants per phase of the study. The study was reviewed by the board of the Medical Ethics Committee of the VU University Medical Center, Amsterdam UMC. All participants gave (digital) informed consent before participation.
FIGURE 1.
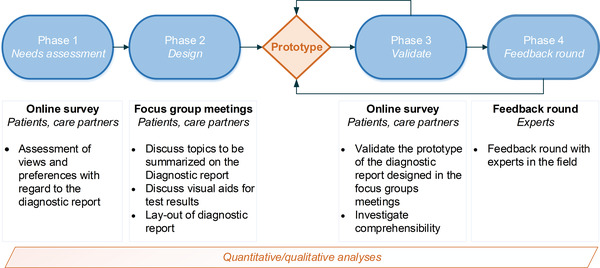
Study flowchart of developing the diagnostic report
TABLE 1.
Participant characteristics
| 1. Needs survey | 2. Focus groups | 3. Validation survey | ||||
|---|---|---|---|---|---|---|
| Phase | Patients | Care partners | Patients | Care partners | Patients | Care partners |
| N | 50 a | 46 | 7 | 7 | 28 b | 12 |
| Age, years | 73 ± 8 c | 65 ± 12 c | 68 ± 6 | 70 ± 4 | 75 ± 7 c | 69 ± 6 c |
| Female (%) | 17 (41%) c | 25 (60%) c | 4 (57%) | 4 (57%) | 5 (18%) | 3 (25%) |
| Education, years | 11.8 ± 4.1 | 12.2 ± 4.4 | 12.3 ± 2.6 | 12.3 ± 2.6 | 12.3 ± 4.4 | 11.9 ± 4.1 |
| Relation to patient, n (%) | ||||||
| Spouse | N/A | 33 (72%) | N/A | 6 (86%) | N/A | 12 (100%) |
| (Grand)child | N/A | 12 (26%) | N/A | 0 | N/A | 0 |
| Sister/brother | N/A | 1 (2%) | N/A | 0 | N/A | 0 |
| Other | N/A | 0 | N/A | 1 (14%) | N/A | 0 |
|
Diagnosis of patient, n (%) d |
||||||
| SCD | 21 (42%) | 2 (4%) | 5 (71%) | 2 (29%) | 10 (36%) | 0 |
| MCI | 16 (32%) | 8 (17%) | 2 (29%) | 2 (29%) | 11 (39%) | 1 (8%) |
| Dementia | 13 (26%) | 36 (78%) | 0 (0%) | 3 (43%) | 7 (25%) | 11 (92%) |
Note: Data represent mean ± SD or n (%). The groups of participants in phase 2 and phase 3 are subgroups of the participants from phase 1.
Abbreviations: MCI, mild cognitive impairment; N/A, not applicable; SD, standard deviation; SCD, subjective cognitive decline.
Of whom n = 20 (44%) participated together with their care partner.
Of whom n = 10 (36%) participated together with their care partner.
Pairwise comparisons indicate a group difference (P < .05).
Self‐reported data.
2.2. Phase 1—Needs assessment
2.2.1. Online survey
We performed a needs assessment using an online survey sent out between July and November 2020. The survey was part of a more extensive survey, of which the methods are described in detail in our previous study. 21 We added specific topics relevant to the present study to explore the views of patients and their care partners regarding a diagnostic report. To explore topics they wanted to include in a report, we used a predefined list of 10 topics 22 , 23 (Table 2), which they could complement with their topics.
TABLE 2.
Preferences regarding pre‐selected list of ten topics during phase 1—the needs survey
| Topics (n, %) | Total (n = 76) | Patients (n = 42) | Care partners (n = 34) | P‐value |
|---|---|---|---|---|
| Short explanation of the diagnosis | 68 (90%) | 39 (93%) | 29 (85%) | .244 |
| The test results | 63 (83%) | 37 (88%) | 26 (77%) | .151 |
| Future – What can I expect over the course of the symptoms | 63 (83%) | 35 (83%) | 28 (82%) | .574 |
| Tips for brain health | 62 (82%) | 36 (86%) | 26 (77%) | .230 |
| Diagnosis | 60 (79%) | 31 (74%) | 29 (85%) | .174 |
| The best way (for the person I care for) to cope with (their) symptoms | 58 (76%) | 28 (67%) | 30 (88%) | .025 |
| The best way to cope with the person I care for | N/A | N/A | 30 (88%) | N/A |
| Pictures of brain imaging | 45 (59%) | 25 (60%) | 20 (59%) | .568 |
| Where to find more information | 38 (50%) | 19 (45%) | 19 (45%) | .245 |
| Test results compared with other people of the same age | 29 (38%) | 19 (45%) | 10 (29%) | .120 |
Abbreviations: N/A, not applicable.
2.2.2. Participants
An international, memory clinic population from the Amsterdam Dementia Cohort (ADC), 24 , 25 the Amsterdam Ageing Cohort (AAC), 26 Alzheimer Europe and Alzheimer's Society UK, consisting of patients (n = 50) with subjective memory decline (SCD, n = 21, 42%), mild cognitive impairment (MCI, n = 16, 32%), and dementia (n = 13, 26%); and care partners (n = 46) of patients with SCD (n = 2, 4%), MCI (n = 8, 17%), or dementia (n = 36, 78% dementia) participated (Table 1).
2.2.3. Analysis
Quantitative survey results were analyzed using IBM SPSS Statistics version 26. P‐values <.05 were considered statistically significant. Frequencies and percentages of topics in the topic list were calculated, and similarities between patients and care partners were studied.
2.3. Phase 2—Design
Subsequently, we used focus group meetings (1) to further examine the most relevant topics to be summarized, using the topic list from phase 1 as input; and (2) to design the diagnostic report.
2.3.1. Focus group meetings
In November and October 2020, we conducted three focus groups, each consisting of four to six participants with a duration of 2 hours. We recruited participants from all Dutch survey respondents. Patients were eligible if they had an Mini‐Mental Status Examination (MMSE) score ≥18. All focus groups were conducted in the Alzheimer Center Amsterdam, Amsterdam UMC. Due to the coronavirus disease 2019 (COVID‐19) situation, the focus groups had a hybrid format, with some participants (n = 3) participating through a video connection and others being present live. All groups included a mix of patients and their care partners, patients alone, or care partners alone, and were heterogeneous in gender, age, and disease stage. All participants were relatively highly educated (Table 1).
2.3.2. Procedures
The focus group procedure was based on the nominal group technique (NTG) and consisted of three parts. 27 All focus groups were audio‐recorded.
First, we provided participants with topics from phase 1 and asked them to rate these on a 5‐point Likert scale (1, unimportant; 2, not very important; 3, neutral; 4 important; and 5, very important). Afterward, we asked each participant to explain which topics they had rated as important (Likert 4 or 5) and why. Results were discussed, and participants were asked if they would change their initial ratings. Then we asked participants to select five topics they considered most important.
Second, we supplied the participants with six visual options to display test results (Figure 2). The visualizations were based on original designs from Visualizing Health, a collaborative project between the University of Michigan and the Robert Wood Johnson Foundation made publicly available via Creative Commons license 28 and derived from a literature search. 18 , 19 , 29 We included visualizations with no text, a small amount of text, and only text, and we asked participants to rank the visualizations. Afterward, the rankings were discussed.
FIGURE 2.
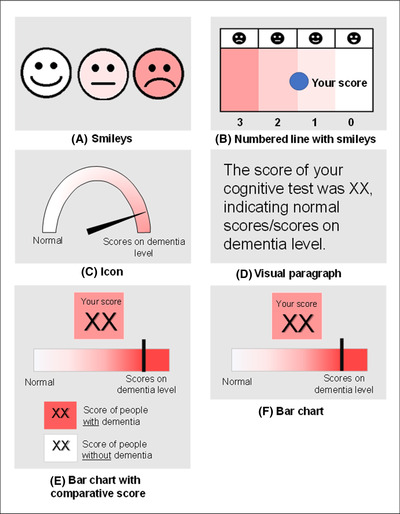
Visualizations to depict test results
Third, participants were instructed to design their preferred diagnostic report. All topics from part 1 and all visualizations from part 2 were available during this hands‐on design exercise. Participants were encouraged to write down additional information from their perspectives.
2.3.3. Analysis
One author (A.G.) transcribed the audiotapes using intelligent verbatim transcription. A second author (H.H.) checked the transcripts. The transcripts were analyzed using directed content analysis 30 using MAXQDA software. 31 One author (A.G.) generated a start list of codes. Two authors (A.G., H.H.) independently coded all audio records and the designed diagnostic reports. Any text that could not be categorized based on the start list was given a new code. Afterward, A.G. and H.H. discussed the codes until consensus was reached. To select the most important topics to be summarized and the best visualization(s) for test results, and to build the initial prototype, quantitative and qualitative analyses were combined.
2.4. Phase 3—Validation survey
According to phase 2, there was no consensus on the visualization of test results. To further investigate the ideal visualization and validate the layout of the prototype, we conducted a second survey among patients and care partners in which Dutch participants from the initial survey were invited. Patients who participated in the focus groups were excluded. In total, n = 28 patients and n = 12 care partners participated (Table 1). We provided the participants with a fictive case of a person with dementia and the diagnostic report with summarized diagnostic test results. To test the comprehensibility of the report, we asked questions about the content of the report (e.g., “what is the diagnosis of this patient?”). To test the comprehensibility of the individual visualizations, we supplied two versions of each visualization—one correctly illustrated the test result mentioned in the text and one that was not in correspondence to the written test result—and asked the participants which visual corresponded to the patients’ results as summarized in the report. In addition, we asked which type of visualization they would prefer to summarize test results.
2.5. Phase 4—Feedback round
Phase 4 emerged during the study, spurred by spontaneous written feedback from one of the care partners participating in the survey and additionally from a formal caregiver to whom a participating care partner supplied the prototype. Accordingly, we decided to present the prototype to other dementia (care) experts (two dementia care nurses and one elderly care physician) to gather more feedback, after which the final prototype was compiled.
3. RESULTS
3.1. Phase 1—Needs assessment
As part of the more extensive project, we performed a survey to assess the views of clinicians, patients, and care partners toward computer tools for support during the diagnostic process (Clinical Decision Support Systems [CDSS]). 21 If a CDSS could be used to explain test results in “layperson” language, 78% of patients and 83% of the care partners would want their doctor to use such a tool. Ninety‐two percent (71/77) of those patients and care partners would like to receive a hand‐out of this explanation on paper. Patients and care partners would use this hand‐out to re‐read it at home (72/96, 75%) or to inform family and friends about their situation (47/96, 49%). These percentages did not differ between patients and care partners (P‐values >.20), but patients were more inclined to use this summary to compare future test results (33/50, 66%) than care partners (21/46, 46%, P = .036).
Table 2 shows our list of pre‐selected topics and the proportion of patients and care partners selecting topics to be included in a diagnostic report. Nine of 11 pre‐selected topics were chosen by over half of the patients and care partners. Apart from the topic, the best way (for the person I care for) to cope with (their) symptoms (patients 67%, care partners 88%, P = .025), no differences were found in answers between patients and care partners. Suggestions for additional topics were: contact details of the memory clinic, information on facilities, services, and support groups.
3.2. Phase 2—Design
3.2.1. Focus groups—topics
Given the high percentages of favored topics in phase 1, selecting the most relevant topics was challenging. Based on phase 1 and the first focus group results, we divided the topic “test results” into (1) Lumbar puncture, and (2) Cognitive test results and added the topic “Explanation of brain imaging,” resulting in a list of 13 topics to present to the second and third focus group participants. After the first part of the focus group, all participants were asked to compose their top‐five topics of the 13‐item topic list. Next, we calculated how often topics appeared in each top‐five and defined the most important topics to be included in the diagnostic report: diagnosis (100%) including a short explanation (85%), test results (cognitive and lumbar puncture) (62%), future expectations (69%), explanation of brain imaging (54%), and how to deal with complaints (54%).
3.2.2. Focus groups—layout
3.2.2.1. Visualizations
According to the rankings, the top‐three of visualizations of test results consisted of (1) Paragraph (Figure 2D; 57%). (2) Icon (Figure 2C; 43%) and Bar chart with comparative score (Figure 2E; 43%), and (3) Smileys (Figure 2A; 36%). Thematic content analyses revealed that visualization preferences differed between patients and care partners and within patients and care partners groups. One of the participants (patient with MCI, 60 years of age) said,“[..] what phase you are in, and is it for you or your partner? That is quite a difference. Suppose you take the paragraph of text, and you are already a bit further [in the disease process]; it is quite difficult to read, and it is the clearest to show an image.” Furthermore, the participants had different opinions on whether to show their test scores compared to the test scores of other people of the same age. Most participants were positive and thought it could help them better understand their situation. On the other hand, some were more negative and did not see the point in comparing their scores to others. In addition, the visualization with the smileys was judged twofold. On the one hand, negative: “Not the smiley's, I think they do not fit this miserable [situation][…]” (partner of SCD patient, 67 years of age), and on the other hand, positive: “Well I think of course if you use this it is just very clear with all those emoticons. Everybody understands.” (MCI patient, 60 years of age).
3.2.2.2. Layout
Several important themes regarding the layout of the diagnostic report were identified. The themes were divided into two main categories: (1) Content and (2) Display. Regarding “content,” the report should contain information on where reliable information can be found and how to get formal help. It should entail (practical) tips, for example, how to get in touch with other patients and care partners, and tips for brain health, such as eating healthy and exercising regularly. Furthermore, participants wanted to include information about the future, such as follow‐up procedures and future expectations. Regarding “display,” the report must be brief and concise, that is, include the main points. One of the participants (patient with SCD, 76 years of age) said: “I have been writing [during the consultation], […] and then something goes through your head I have to ask, and a little later you forget it. So that is very nice if you know that you get the main points home.” Furthermore, the report should contain clear language, comprehensible for a layman. One of the participants (care partner, 71 years of age) mentioned: “I can look for that letter [from the general practitioner], […]; however, when I am going to read it, I do not understand it […].”
3.2.3. Focus groups—hands‐on designing the diagnostic report
At the final stage of phase 2, a total of 13 report pages were made by the participants (Figure 3). One report page was constructed by a patient‐care partner couple. Analyses of the hands‐on designing of the layout of the diagnostic report showed that diagnosis was included in 11 of 13 report pages and explanation of diagnosis in 10 of 13. Then, 10 of 13 contained future expectations. Relating this topic, two participants wrote down wanting to know about both expected symptoms and progression rates. Brain imaging was placed on 10 of 13 pages. Both cognitive test results and how to deal with complaints (of loved ones) were included on 9 of 13 pages. A slight majority of 7 of 13 pages contained the topic tips, and lumbar puncture result was placed on 6 of 9 pages. According to phases 1 and 2, a prototype was built.
FIGURE 3.

Overview of the hands‐on design session during the focus group meeting.
3.3. Phase 3—Validation
The focus group results indicated a preference but no consensus on the ideal visualization of test results. However, we could exclude two visualizations (Figure 2B,F). To keep the report comprehensible for all end‐users, we also decided to exclude the visual paragraph (Figure 2D). We presented the three remaining visualizations (Figure 2A,C,E) to a larger group in the validation survey. Most participants preferred either the icon (Figure 2C, n = 33, 38%) or the bar chart (Figure 2E, n = 37, 43%). When asked to correctly match one of two visualization versions to the written test result, we found that most participants matched the correct visualization version to the results summarized on the report page. The highest scores were found for the smiley (Figure 2A; 81% answered correctly) and the icon bar (Figure 2C; 79% answered correctly) compared to the bar chart (Figure 2E; 66% answered correctly). After combining the results from the focus groups and the validation survey, the icon (Figure 2C) was chosen as the visualization to display test results for the prototype.
3.4. Phase 4—Feedback round
After the validation survey, we received written feedback from one of the participants (care partner) and a formal caregiver involved through this care partner. They pointed out that the textual information was not clear enough, contained too many words, and was too academic. They advised us to rephrase some sentences to make the report more comprehensible. According to this feedback, we adapted the prototype and decided to present it to three other dementia (care) experts. They provided us with verbal feedback, and together we made changes to linguistics and layout. Afterward, the final prototype was constructed.
3.5. Prototype
The final prototype of the diagnostic report consisted of two pages, shown in Figure 4. The first page includes information on diagnosis and displays the results from diagnostic tests. The second page entails information on the course of the disease, tips for brain health, information on practical issues, and where to find more information.
FIGURE 4.
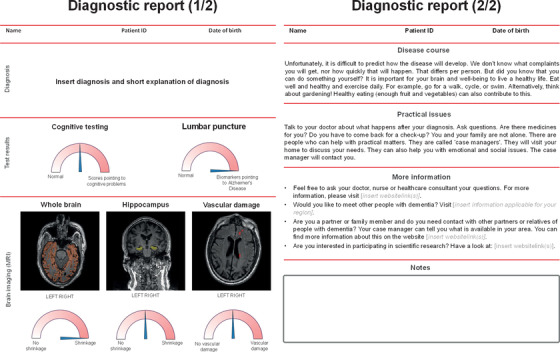
Prototype of the diagnostic report
4. DISCUSSION
This study reports the development of a diagnostic report for dementia diagnosis in co‐creation with patients and care partners. Overall, patients and care partners were enthusiastic and eager to join, and they provided constructive input. In the first phase of the study, in which we conducted a needs assessment, we found that patients and care partners positively value a diagnostic report to summarize and communicate test results. In the second and third phases, consensus was reached on the topics to be outlined, and a choice was made on the visualization to explain test results. A prototype was made, and in phase 4, we asked experts for their opinion. Subsequently, we designed the diagnostic report according to the results from all phases, in close collaboration with patients and care partners.
Our study intends a first step toward developing a personalized diagnostic report that contributes to a personalized medicine approach. 32 By portraying the needs of patients and care partners through the design thinking process, we developed an example of what a diagnostic report should entail. The diagnostic report developed in the current study can support the harmonization of clinical practice and is aimed at supporting both clinicians and patients. Clinicians might use the report as a guide to explain test results and diagnoses. For patients, the report can improve their understanding of their test results and diagnosis. They can take the report home on paper as a reference page and facilitate communication about test results with relatives.
We strived to develop an acceptable and comprehensible diagnostic report that eventually contributes to effective implementation. 33 However, the feasibility of generating and structurally using this report in clinical practice remains to be tested. In a subsequent study, we collaborate in a public‐private partnership to study the implementation of this report in various memory clinics. In that study, the report page will be generated from a CDSS that combines digital cognitive testing, magnetic resonance imaging (MRI), and lumbar puncture results in a simple machine learning method. 34 We will study how clinicians use the report page in a real‐life setting, what barriers and facilitators can be identified for successful implementation, and what requirements must be met to generate a report for all memory clinic patients. In addition, we will further examine outcomes and experiences reported by patients and care partners.
In designing the diagnostic report, we aimed to balance the opinions of patients and care partners and what is considered to be advantageous based on research and expertise. 33 For instance, as existing literature suggests that adding visual information to a written report enhances recall, we aimed to display test results visually. 13 , 14 It was difficult to get a consensus on one ideal visualization, as patients and care partners differed in their preferences. Because almost all visual options were rated with high scores, the final choice to use the icon (Figure 2C) as visualization for the diagnostic report in our study was made on the high scoring combined with our expert‐based opinion. Preferably, in the future, diagnostic reports should not be static but should leave room for adaptation to individual patients. Different visualizations could be generated (e.g., a bar chart and an icon array; see, e.g., www.ADappt.health 23 ), and the clinician and patient could decide together which visualization appeals most to the patient and should appear in the printed report page, or the clinician could switch between both.
In this study and others, 11 , 23 , 35 we found that patients want to know about their expected symptoms and progression rates. Furthermore, they indicated feeling left in ignorance after receiving the diagnosis. 8 With the ongoing developments in biomarker‐based prognosis for patients with early stages of Alzheimer's disease, there might be a future in which a personalized prediction of progression is possible and could be added to the diagnostic report. 36 , 37 Before we get there, supplying information on coping strategies, practical tips, and tips stimulating brain health can make patients and care partners feel less alone after receiving the diagnosis and enable adequate coping.
The strength of our study is the careful and iterative design, based on the proven design thinking methodology, and carried out in co‐creation with a heterogeneous group of patients and care partners. 20 As a result, we optimally understood and depicted their needs and preferences regarding a diagnostic report. The limitation of our study was that the study participants were relatively highly educated. Therefore, we do not yet know whether the diagnostic report is also suited for patients with less education. However, we strove for comprehensibility, even for lower educated or illiterate patients, by using easily understandable visualizations to depict test results. 14
5. CONCLUSION
We co‐created a diagnostic report to communicate test results in dementia diagnostics to meet the urgent patient need for more information on their diagnosis. By developing the diagnostic report with patients and care partners, we learned that not one size fits all. It is most important that all essential information is delivered in line with their preferences in a comprehensible manner. Next, we will perform a pilot study in clinical practice to study the usability and feasibility of this prototype of the diagnostic report, thereby strengthening the results of this study.
CONFLICTS OF INTEREST
HRM performs contract research for Combinostics; all funding is paid to her institution. WF performs contract research for Biogen. Research programs of WF have been funded by ZonMW, NWO, EU‐FP7, EU‐JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health‐Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes‐Strijbis fonds, stichting Equilibrio, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc., Novartis‐NL, Life‐MI, AVID, Roche BV, Fujifilm, Combinostics. WF holds the Pasman chair. WF is recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (#73305095007) and Health‐Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). WF has performed contract research for Biogen MA Inc. and Boehringer Ingelheim. WF has been an invited speaker at Boehringer Ingelheim, Biogen MA Inc., Danone, Eisai, WebMD Neurology (Medscape), Springer Healthcare. WF is consultant to Oxford Health Policy Forum CIC, Roche, and Biogen MA Inc. WF participated in advisory boards of Biogen MA Inc. and Roche; all funding is paid to her institution. WF was associate editor of Alzheimer, Research & Therapy in 2020/2021. WF is associate editor at Brain. JG is Executive Director of Alzheimer Europe which receives funding from EU research and health programmes as well as private foundations and companies. All other coauthors report no conflicts of interest.
ACKNOWLEDGMENTS
Research of the Alzheimer's Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. The Vrije Universiteit Medical Center Alzheimer Center is supported by the Stichting Alzheimer Nederland and Stichting Vrije Universiteit Medical Center Fonds. The collaboration project Dementia diagnostics using Artificial Intelligence (DAILY; project number LSHM19123‐HSGF) is co‐funded by the public–private partnership allowance made available by Health‐Holland, Top Sector Life Sciences, and Health to stimulate public‐private partnerships and Combinostics. The chair of WF is supported by the Pasman Stichting. WF and LV are recipients of ABOARD, which is a public‐private partnership receiving funding from ZonMW (number 73305095007) and Health‐Holland, Top Sector Life Sciences and Health (public‐private partnership allowance; number LSHM20106). LV is supported by a fellowship grant received from Alzheimer Nederland (WE.15‐2019‐05). HRM is recipient of the Memorabel Dementia Fellowship 2021 (ZonMw project number 10510022110004). WF, LV, and JG are recipients of the EU Joint Programme‐Neurodegenerative Disease Research (JPND) project EURO‐FINGERS (ZonMW‐Memorabel #733051102), which is supported through the following funding organizations under the aegis of Joint Programme‐Neurodegenerative Disease: Finland, Academy of Finland; Germany, Federal Ministry of Education and Research; Spain, National Institute of Health Carlos III; Luxemburg, National Research Fund; Hungary, National Research, Development, and Innovation Office; The Netherlands, Netherlands Organisation for Health Research and Development; and Sweden, Swedish Research Council (grant agreement INTER/JPND/19/BM/14012609). These funding sources were not involved in the study design, collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
van Gils AM, Visser LNC, Hendriksen HMA, Georges J, van der Flier WM, Rhodius‐Meester HFM. Development and design of a diagnostic report to support communication in dementia: Co‐creation with patients and care partners. Alzheimer's Dement. 2022;14:e12333. 10.1002/dad2.12333
REFERENCES
- 1. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43:250‐260. [DOI] [PubMed] [Google Scholar]
- 4. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurology. 2017;16:661‐676. [DOI] [PubMed] [Google Scholar]
- 8. Kunneman M, Pel‐Littel R, Bouwman FH, et al. Patients' and caregivers' views on conversations and shared decision making in diagnostic testing for Alzheimer's disease: the ABIDE project. Alzheimers Dement (N Y). 2017;3:314‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quinn C, Clare L, Pearce A, van Dijkhuizen M. The experience of providing care in the early stages of dementia: an interpretative phenomenological analysis. Aging Ment Health. 2008;12:769‐778. [DOI] [PubMed] [Google Scholar]
- 10. Bailey C, Dooley J, McCabe R. ‘How do they want to know?’ Doctors' perspectives on making and communicating a diagnosis of dementia. Dementia (London). 2019;18:3004‐3022. [DOI] [PubMed] [Google Scholar]
- 11. Fruijtier AD, Visser LNC, Bouwman F, et al. What patients want to know, and what we actually tell them: the ABIDE project. Alzheimer's & dementia (New York, N.Y.). 2020;6(1):e12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Arcy F, Yip CL, Manya K, et al. Prospective randomised controlled trial of written supplement to verbal communication of results to patients at the time of flexible cystoscopy. World J Urol. 2018;36:883‐887. [DOI] [PubMed] [Google Scholar]
- 13. Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Edu Couns. 2006;61:173‐190. [DOI] [PubMed] [Google Scholar]
- 14. van Weert JC, van Noort G, Bol N, van Dijk L, Tates K, Jansen J. Tailored information for cancer patients on the internet: effects of visual cues and language complexity on information recall and satisfaction. Patient Edu Couns. 2011;84:368‐378. [DOI] [PubMed] [Google Scholar]
- 15. Grill JD, Apostolova LG, Bullain S, et al. Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther. 2017;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damian D, Tattersall MH. Letters to patients: improving communication in cancer care. Lancet. 1991;338:923‐925. [DOI] [PubMed] [Google Scholar]
- 17. Bol N, van Weert JC, de Haes HC, Loos EF, Smets EM. The effect of modality and narration style on recall of online health information: results from a Web‐based experiment. J Med Internet Res. 2015;17:e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turchioe MR, Myers A, Isaac S, et al. A systematic review of patient‐facing visualizations of personal health data. Appl Clin Inform. 2019;10:751‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arcia A, Suero‐Tejeda N, Bales ME, et al. Sometimes more is more: iterative participatory design of infographics for engagement of community members with varying levels of health literacy. J Am Med Inform Assoc. 2016;23:174‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts JP, Fisher TR, Trowbridge MJ, Bent C. A design thinking framework for healthcare management and innovation. Healthc (Amst). 2016;4:11‐14. [DOI] [PubMed] [Google Scholar]
- 21. van Gils AM, Visser LN, Hendriksen HM, et al. Assessing the views of professionals, patients, and care partners concerning the use of computer tools in memory clinics: international survey study. JMIR Form Res. 2021;5:e31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fruijtier AD, Visser LNC, van Maurik IS, et al. ABIDE Delphi study: topics to discuss in diagnostic consultations in memory clinics. Alzheimers Res Ther. 2019;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Maurik IS, Visser LN, Pel‐Littel RE, et al. Development and Usability of ADappt: web‐based tool to support clinicians, patients, and caregivers in the diagnosis of mild cognitive impairment and Alzheimer disease. JMIR Form Res. 2019;3:e13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Flier WM, Pijnenburg YA, Prins N, et al. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41:313‐327. [DOI] [PubMed] [Google Scholar]
- 25. van der Flier WM, Scheltens P. Amsterdam Dementia Cohort: performing research to optimize care. J Alzheimers Dis. 2018;62:1091‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rhodius‐Meester HFM, van de Schraaf SAJ, Peters MJL, Kleipool EEF, Trappenburg MC, Muller M. Mortality risk and its association with geriatric domain deficits in older outpatients: the Amsterdam Ageing Cohort. Gerontology. 2021;67:194‐201. [DOI] [PubMed] [Google Scholar]
- 27. McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vizualizing Health [Internet] . The Regents of the University of Michigan and the Robert Wood Johnson Foundation; 2014. [cited 2021 November 29th]. Available from: https://www.vizhealth.org/
- 29. Spiegelhalter D, Pearson M, Short I. Visualizing uncertainty about the future. Science. 2011;333:1393‐400. [DOI] [PubMed] [Google Scholar]
- 30. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277‐1288. [DOI] [PubMed] [Google Scholar]
- 31. Software. MAXQDA Standard 2018 18.2.0, software for Qualitative Data Analysis [computer software] . Berlin, Germany: VERBI Software; 1995‐2018.
- 32. van der Flier WM, Kunneman M, Bouwman FH, Petersen RC, Smets EMA. Diagnostic dilemmas in Alzheimer's disease: room for shared decision making. Alzheimers Dement (N Y). 2017;3:301‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Altman M, Huang TTK, Breland JY. Design thinking in health care. Prev Chronic Dis. 2018;15:E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruun M, Frederiksen KS, Rhodius‐Meester HFM, et al. Impact of a clinical decision support tool on dementia diagnostics in memory clinics: the PredictND validation study. Curr Alzheimer Res. 2019;16:91‐101. [DOI] [PubMed] [Google Scholar]
- 35. Mank A, van Maurik IS, Bakker ED, et al. Identifying relevant outcomes in the progression of Alzheimer's disease; what do patients and care partners want to know about prognosis? Alzheimer's & Dementia (New York, N Y). 2021;7(1):e12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Maurik IS, Vos SJ, Bos I, Bouwman FH, et al. Biomarker‐based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurology. 2019;18:1034‐44. [DOI] [PubMed] [Google Scholar]
- 37. Rhodius‐Meester HFM, Liedes H, Koikkalainen J, et al. Computer‐assisted prediction of clinical progression in the earliest stages of AD. Alzheimers Dement (Amst). 2018;10:726‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]


