Keywords: β-arrestin, effector, G protein-coupled receptor, GPCR kinase, G protein, transducer
Abstract
G protein-coupled receptors (GPCRs) are of considerable interest due to their importance in a wide range of physiological functions and in a large number of Food and Drug Administration (FDA)-approved drugs as therapeutic entities. With continued study of their function and mechanism of action, there is a greater understanding of how effector molecules interact with a receptor to initiate downstream effector signaling. This review aims to explore the signaling pathways, dynamic structures, and physiological relevance in the cardiovascular system of the three most important GPCR signaling effectors: heterotrimeric G proteins, GPCR kinases (GRKs), and β-arrestins. We will first summarize their prominent roles in GPCR pharmacology before transitioning into less well-explored areas. As new technologies are developed and applied to studying GPCR structure and their downstream effectors, there is increasing appreciation for the elegance of the regulatory mechanisms that mediate intracellular signaling and function.
INTRODUCTION
Receptor theory was first postulated at the beginning of the 20th century by Paul Ehrlich’s study of agent selectivity (1) and John Newport Langely’s study of receptive substance (2). However, the concept of receptors as physical entities was controversial and it became clear that new technologies were needed to better understand the interaction between ligands and receptors. Successful radioligand binding on both β-adrenergic receptors (βAR) (3) and α-adrenergic receptors (αAR) (4) paved the road for the functional characterization of GPCRs and the eventual discovery of the superfamily of seven-transmembrane (TM) receptors (5).
Although it had been established that hormones could stimulate the production of cyclic AMP (cAMP) (6), precise mechanisms were unknown. Initial studies on coupling between receptors and adenyl cyclase (AC) demonstrated the necessity of guanosine triphosphate (GTP) (7) and GTPase (8). These studies were followed by a series of reconstitution experiments that led to the identification of a novel protein: the Gαs subunit, a heterotrimeric G protein complex component that coupled the receptor to its effectors (9). It is now well-known that heterotrimeric complexes are composed of α, β, and γ subunits. Gα subunit consists of four major families, including Gαs, Gαi, Gαq/11, and Gα12/13, ranging in size from 39 to 52 kDa (10) (Fig. 1).
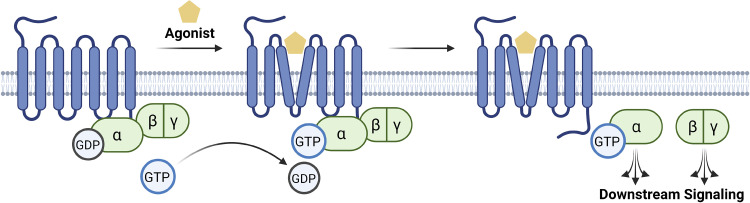
Figure 1.
G protein-coupled receptor activation. Seven-transmembrane GPCRs are a group of diverse receptors in eukaryotes. When an external agonist binds to the GPCR, the receptor will undergo a conformational change, triggering a subsequent interaction with the heterotrimeric G protein complex. The G protein is considered inactive when the α subunit is bound to GDP; however, upon an agonist stimulation, the GDP will physically be displaced by the GTP, rendering the complex active. Both the Gα and the Gβγ subunits will then go on to activate distinct downstream effectors. Figure created with BioRender and published with permission. GPCR, G protein-coupled receptor; GTP, guanosine triphosphate.
Understanding how receptors become desensitized following stimulation has also been a vigorous area of study. First observed was the light-induced phosphorylation of visual rhodopsin (11), which led to the subsequent discovery and purification of rhodopsin kinase (GRK1) as the enzyme catalyzing the phosphorylation of rhodopsin (12). In addition to the demonstration of receptor phosphorylation via the cAMP-dependent protein kinase A (PKA), another mechanism whereby agonist-occupied receptors undergo phosphorylation was identified and termed, homologous desensitization, and led to the purification of the β-adrenergic receptor kinase GRK2 (13).
Although the mechanism of receptor desensitization by GPCR kinase (GRK) phosphorylation was initially observed using crude enzyme preparations, it was realized that another factor was necessary for desensitization when highly purified GRK enzyme was used(14, 15). This factor, a functionally analogous and structurally homologous protein to visual arrestin, was identified as a regulator of the β-adrenergic receptor and named β-arrestin (16, 17). Today, they are known as the ubiquitously expressed β-arrestin-1 (arrestin-2) and β-arrestin-2 (arrestin-3).
G PROTEINS
G Protein Structure and Function
G proteins bind to both GTP and GDP, playing a central role in GPCR signaling and mediating a wide range of physiological responses (Fig. 1). G proteins differ in size, ranging from the small signaling protein Ras to the large heterotrimeric G protein complexes composed of α, β, and γ subunits.
The Gα subunit has three distinct structural components: a Ras-like GTPase domain, an α-helical domain, and an N-terminal helical domain (18). The GTPase and helical domains are conserved across all Gα subunits (19). The GTPase domain is structurally homologous to other monomeric G proteins and is the site of GTP hydrolysis. The GTPase also exhibits conformational plasticity, demonstrated by its highly dynamic state while bound to GDP as well as its rigid, locked structure while bound to GTP (20). Furthermore, the helical domain is credited for enhancing the Gα subunit affinity for guanine nucleotides (21) and for increasing the rate of GTP hydrolysis (22). In addition, changes in the carboxyl end, including single point mutations, can alter the type of the G protein (23, 24). The N-terminus serves as a critical site for Gα and the Gβγ subunit interaction (25), with myristoylation increasing the affinity between the Gα and Gβγ subunits (26), further stabilizing the N-terminal helix (27). Earlier structural studies showed that both a “switch interface” and an “N-terminal interface” of Gα are involved in heterotrimeric G protein interactions, resulting in conformational changes (18, 28). The crystal structure of an agonist-occupied monomeric β2 adrenergic receptor (β2AR) and a nucleotide-free Gs subunit revealed displacement of the helical domain relative to the Ras-like GTPase domain (29). Gα subunits may undergo modification by myristoylation, phosphorylation, and palmitoylation (30). Fatty acid modification is prevalent within the N-terminus, exemplified by the thioester bond between cysteines and the saturated 16-carbon palmitate (31).
Gβ and Gγ subunits, anchored to the plasma membrane by lipids, are obligate heterodimers and work as a single Gβγ unit. The Gβ subunit comprises a seven-bladed β-propeller that has seven WD40 repeats, and its N-terminal helix forms extensive, primarily hydrophobic, parallel interactions with the N-terminal helix of the Gγ subunit (32). The Gβ subunit may also interact with the receptor as shown by studies using a synthetic peptide derived from the third intracellular loop of the α2-adrenergic receptor (33). The Gγ subunit, composed of two α helices, is much smaller, between 7 and 8 kDa (34), and undergoes either isoprenylation or geranylgeranylation (35, 36). In the absence of Gγ, Gβ subunits are unstable and tend to form aggregates (37).
Fatty acid modification is vital for membrane localization since mutations that result in the improper fatty acid modification may lead to impaired cellular localization (38, 39). In particular, Gγ interacts with the plasma membrane and the Gβ subunit through its highly variable C-terminal posttranslational modification (40). Furthermore, studies have shown that the Gγ subunits are predominately found on the endoplasmic reticulum (ER), but upon Gα activation, are subsequently directed to the plasma membrane (41). There are multiple possible Gβγ subunit combinations, with some specificity among the different subtypes of Gβ and Gγ subunits (42). Gβγ subunits are around 36 kDa in size (34) and are frequently subjected to prenylation and phosphorylation (19).
G Protein Activation
The heterotrimeric G protein complex couples the activation of the receptor to downstream effector signaling. Early kinetic models suggest a collision coupling theory between the GPCR and the G protein, but more recent fluorescence resonance energy transfer (FRET) studies demonstrate a conformational rearrangement of a preexisting GPCR-G protein complex upon agonist binding (43, 44). Early studies using the retinal receptor rhodopsin showed that the light-activated conformational change of rhodopsin creates a critical interaction between the cytoplasmic hydrophobic cleft of the receptor and the C-terminus of the transducing Gα subunit (45), consistent with previous studies using synthetic peptides (46). Based on site-directed spin-labeling experiments, an outward movement of the receptor TM helix VI creates a binding pocket for the Gα subunit on the receptor cytoplasmic site (47).
After ligand binding, the GPCR, acting as a guanine nucleotide exchange factor, initiates exchange of GTP for GDP on the G protein. Release of GDP from the G protein occurs by the allosteric disruption of the receptor on the nucleotide-binding site, resulting in low binding affinity for GDP (48). The unoccupied binding site is then filled with GTP due to its high intracellular concentration (49). The GDP-GTP switch occurs in the Ras-like G domain of the Gα subunit (18), and linkers between the Ras-like G domain and the helical domain exhibit dramatic changes in conformation (50). Structurally, an N/TKXD motif and a highly conserved P-loop are critical for the binding specificity (51). Binding between the Gα subunit and GTP produces an unstable heterotrimeric complex resulting in the dissociation of Gβγ from the Gα subunit. Once dissociated, the Gα and the Gβγ subunits diffuse freely, interacting with downstream effector proteins to elicit cellular and physiological responses (52).
G Protein Signaling
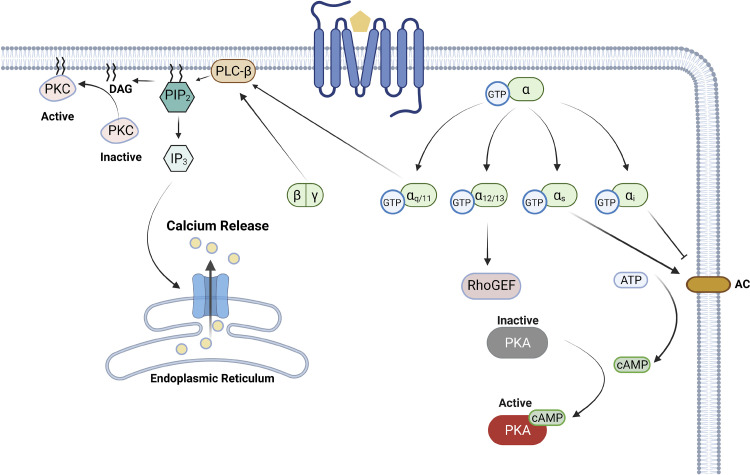
Both the Gα and the Gβγ subunits exert downstream effects on signaling and different families of Gα activate distinct downstream signaling profiles (Fig. 2). Gαs and Gαi subunits regulate adenylyl cyclase (AC) activity generating the second messenger cAMP. Gαs stimulates AC resulting in increased intracellular concentrations of cAMP, whereas coupling of the inhibitory G protein, Gαi, reduces intracellular cAMP concentration (10). Gαq/11 activates β-type phospholipase C (PLC-β) to hydrolyze membrane-bound phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol triphosphate (IP3) (53). After cleavage, DAG remains membrane-bound, whereas IP3 can diffuse throughout the cytoplasm. As second messengers, IP3 and DAG continue to exert their effects downstream, including calcium release through the calcium channel IP3 receptor from the ER and protein kinase C (PKC) activation, respectively (54). Gα12/13 can target regulator of G protein signaling homology (RH)-RhoGEFs, activating GTPase Rho (55), including proteins such as leukemia-associated RhoGEF (LARG) and p115RhosGEF (56, 57).
Figure 2.
The heterotrimeric G protein signaling. There are four major families in the α subunit: Gαs, Gαi, Gαq/11, and Gα12/13. Although Gαs activates the AC, Gαi inhibits it, both of which will regulate the intracellular concentration of cAMP. Gαq/11 activates PLC-β, hydrolyzing PIP2 into DAG and IP3. Gα12/13 also has a variety of downstream targets, including RhoGEF. AC, adenyl cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; DAG, diacylglycerol; GEF, guanine nucleotide exchange factor; GTP, guanosine triphosphate; IP3, inositol trisphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLC-β: phospholipase C-β. Figure created with BioRender and published with permission.
Early studies assumed a passive role for the Gβγ subunit; however, the molecular basis for an interaction of Gβγ with effectors was demonstrated using a mutational analysis of Gβ residues that makes contact with Gα-GDP to identify Gβγ subunits as critical regulators of signal transduction (58). With the loss of the subunit Gβγ, GDP-GTP exchange does not proceed and effector signaling is impaired (59). Gβγ interacting effectors include various isoforms of phospholipases (60), ion channels (61), Src-dependent phosphorylation of the epidermal growth factor receptor (EGFR) (62), and GRK2 (63), to name a few (64–69). Therefore, once the GPCR is activated, both the Gα and the Gβγ subunits can act as regulators of a wide range of effectors.
Many other noncanonical signaling pathways also involve G proteins. In the absence of a GPCR, a group of activators of G protein signaling (AGS) may promote GTP binding or stabilize the heterotrimeric G protein complex (70). Gαq/11 can interact with p115RhoGEF, PDZ-RhoGEF, and LARG (71), all of which are key regulators in cellular growth and differentiation that are frequently impaired in cancer and metabolic diseases (72). Heterotrimeric G proteins can also be regulated by non-GPCR entities, such as Ric-8A to promote GTP-GDP exchange (73) and AGS3 to inhibit guanine nucleotide dissociation (70).
G Protein Signal Termination
Following Gβγ dissociation, intrinsic GTPase activity of GTP-bound Gα will hydrolyze GTP back to GDP and reform the heterotrimer, which can be accelerated by GTPase-activating proteins (GAP) that are regulators of G protein signaling (RGS) (74). For example, RGS3 can bind to Gβ1γ2, limiting its ability to produce IP3 and activate Akt and PKA (75). Gβγ subunits can also terminate signaling by reassociating with Gα. Receptor-G protein interaction may also be inhibited through GRK-mediated receptor phosphorylation and subsequent arrestin binding, which will be discussed in GRK and β-arrestin sections.
G Proteins: Cardiovascular Physiological Functions and Possible Therapeutic Intervention
In the heart, overexpression of the Gαs subunit can depress heart rate variability and the baroreflex (76), blunt βAR desensitization (77), and cause dilated cardiomyopathy (78). A common synonymous polymorphism (ATT → ATC, Ile131) of exon 5 in GNAS1 has been found to be associated with hypertension (79).
Upregulation of Gαi has been observed in patients with heart failure (80), and may be cardioprotective under conditions of chronic adrenergic signaling (81). Overexpression of Gαi in the swine heart by viral gene transfer can modulate electrical currents in a model persistent atrial fibrillation (82). In contrast to Gαi, moderate Gαq overexpression induces cardiac hypertrophy, whereas a high level of overexpression results in cardiomyocyte apoptosis (83), contractile failure (84, 85), and cardiomyopathy (86). Gαq inhibition reduces the hypertrophic response induced by mechanical pressure overload along with preserved cardiac function despite the increase in wall stress (87–89). Gαq deficient mice lack response from platelet activators and have increased bleeding time (90). A noncoding polymorphism in the GNAQ promoter which codes for the Gαq subunit is also associated with cardiac hypertrophy and adverse outcomes in heart failure (91, 92).
In cultured neonatal rat ventricular cardiomyocytes, overexpression of a constitutive active Gα13 increases cell size (93). Constitutive active Gα12/13 will induce a RhoA-mediated vascular smooth muscle cell contraction in vitro (94). Although Gα12/13 deficiency is embryonic lethal (95), mice with cardiomyocyte-specific deletion of Gα13 are protected against pressure overload-induced hypertrophy and fibrosis (96).
Agonist-induced βAR desensitization requires GRK2 that is recruited to the membrane through its interaction with Gβγ subunits (97). When Gβγ subunits are sequestered through overexpression of the C-terminal of βARK1 (βARKct), there is enhanced catecholamine responsiveness (98), preserved cardiac function in a model of genetic cardiomyopathy (99), and improved survival (100). Among the βARKct transgenic animals, a linear positive correlation is further observed between the level of βARKct protein expression and fractional shortening after chronic pressure overload (101). The salutary findings from the βARKct animal model study are confirmed by M119 (102) and its analog galleon (103), a Gβγ subunit inhibitor.
Polymorphisms in the Gβγ subunit have been identified. In particular, a C825T polymorphism in Gβ3 has been shown to increase G protein signaling, reduce the risk for myocardial infarction, and increase efficacy of statin therapy to lower cholesterol (104, 105). In patients with hypertension, the role of C825T is less clear with studies showing an association with elevated blood pressure (106, 107) and others showing no increased risk for hypertension (108, 109).
G PROTEIN-COUPLED RECEPTOR KINASES
GRK Characteristics and Structure
The termination of agonist-induced GPCR signaling is primarily mediated by the phosphorylation of GRKs, a group of serine and threonine kinases (110). There are seven members in the GRK family, which are grouped into three subfamilies: GRK1 and GRK7; GRK2 and GRK3; GRK4, GRK5, and GRK6 (111), with each class of GRK having distinct N- and C-terminal characteristics (112). GRK1 and GRK7 are known as visual GRKs because they are largely restricted to retinal cells (113). GRK2 and GRK3 are also referred to as the β-AR kinases because of their close association with the βARs (114). Due to their high degree of structural similarities, GRK4, GRK5, and GRK6 are grouped together (115). GRK4 is commonly found in the testes (116), whereas GRK2, GRK3, GRK5, and GRK6 can be found throughout the human body with relatively even distribution (112, 117).
Unlike other kinases, GRKs do not require autophosphorylation for full activity (118). Although there are seven different isoforms of GRKs proteins, they share a conserved structure among its over 500 residue assembly: a protein kinase catalytic domain inserted into a loop of the regulator of G protein signaling (RGS) homology (RH) domains (119, 120). Besides interacting with the kinase domain, the RH domain has two additional domains: one that interacts with the C-terminal pleckstrin homology (PH) domain and one with the Gαq subunit (121). The PH domain interacts with membrane lipids and free Gβγ subunits (122).
GRKs also contain an N-terminal α-helical domain that has an antiparallel β-sheet with five strands and a variable C-terminus featuring multiple α-helices, which acts as the primary binding site (123). Mutation of any residue within the N-terminus can lead to a loss of function in receptor phosphorylation (124, 125). The N-terminus may play a stabilization role—its hydrophobic residues may interact with the membrane, whereas polar residues interact with the kinase domain (126, 127). When the kinase catalytic domain is stabilized in a closed conformation, the N-terminus may also interact with the C-terminus to form a docking site for the activated GPCRs (128). Similarly, mutations in the C-terminal region can inhibit receptor phosphorylation (129, 130), with the C-terminus playing a critical role in mediating cytosol membrane localization (131).
GRK1/7.
GRK1 was first crystalized in 2008 (132). A more recent cryo-electron microscopy single-particle reconstruction of rhodopsin and GRK1 shows the N-terminus forming a helix that interacts with rhodopsin’s cytoplasmic cleft and stabilizes the activated configured kinase domain (133). Thus, receptor recognition and kinase activation are closely coupled together for GRKs. Even though both GRK1 and GRK7 share a signature CaaX motif on the C-terminus that is used in membrane localization, GRK1 is farnesylated (134), whereas GRK7 is geranylgeranylated (135).
GRK2/3.
In the βARK family, the crystal structure of bovine GRK2 was first published in 2003 in complex with the Gβ1γ2 subunit and shown to simultaneously inhibit Gα and Gβγ signaling (136). The Gαq-GRK2-Gβγ complex has further demonstrated that GRK2 can serve as a scaffold protein, reorienting the subunits and allowing diverse effectors to recognize specific regions on the subunits (137). To date, GRK3 has not been crystallized. Both GRK2 and GRK3 have a Pleckstrin homology domain, critical for membrane localization (138).
GRK4/5/6.
There are four splice variants in human GRK4, namely, GRK4α, GRK4β, GRK4γ, and GRK4δ (116). One such polymorphism, GRK4α A486V, associated with hypertension, has been crystallized (139). GRK5 is a monomer and its active-site forms part of the nucleotide-binding pocket, a feature that is not observed among other GRKs (140). The crystal structure of GRK5 in complex with an inhibitor identified a localization function for the C-terminus (141). More recently, the binding interface of the β2AR-GRK5 complex has been mapped by mass spectrometry, in which a three-dimensional model was generated that confirms the importance of the cytoplasmic loops (142). The leucine and threonine on N-terminus of GRK5 are also implicated in receptor phosphorylation (126).
The crystal structure of GRK6 reveals a phospholipid-binding site near its N-terminus and other structural elements in the kinase domain that may affect GPCR access and specificity (143). Furthermore, hydrophobic residues in the N-terminus are thought to interact directly with the receptor based on the closed conformation of GRK in crystal structure (128).
GRK Cellular Physiology
Short-term receptor desensitization is divided into two mechanisms: heterologous desensitization, and refers to the involvement of cAMP and PKA in the absence of an agonist (144), and homologous desensitization, which is agonist-dependent and typically mediated by GRKs (145). As serine/threonine kinases, GRKs phosphorylate the third intracellular loop or the C-terminal tail of the activated GPCR (146) (Fig. 3). When phosphorylation sites are removed from the receptor, a delay in desensitization rate is observed (149). Recruitment of arrestins to agonist-occupied phosphorylated receptors will uncouple the heterotrimeric G protein from the receptor (150). In addition, specific anionic phospholipids work in concert with the activated GPCR to regulate GRK activity (151).
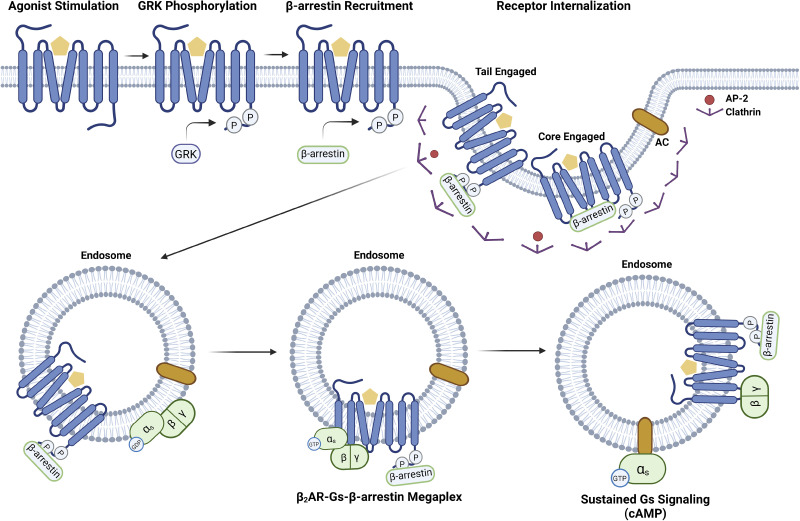
Figure 3.
GPCR desensitization and internalization. Two critical factors are involved in receptor desensitization and internalization after stimulation. The first is GRK phosphorylation of the C-terminal tail of the GPCR. The second is β-arrestin recruitment to the phosphorylated receptor followed by clathrin-dependent internalization. Depending on the GPCR-β-arrestin complex conformation, β-arrestin may either bind to the core (core conformation) or the tail (tail conformation). Because the tail conformation presumably exposes the core for further G protein interactions, it is possible to form a GPCR-G protein-β-arrestin “megaplex” that can exhibit sustained G protein signaling as demonstrated for the β2AR and cytoplasmic cAMP generation (147, 148). Figure created with BioRender and published with permission. β2AR, β2-adrenergic receptor; cAMP, cyclic AMP; GPCR, G protein-coupled receptor; GRK, GPCR kinase.
GRK1/7.
The GRK activation mechanism was first studied in the rhodopsin system. Phosphorylation of rhodopsin by GRK1 was first identified in 1972 (152). When light strikes the receptor, subsequent activation of GRK1 can phosphorylate many sites in close proximity including exogenous peptides (153). Using truncated photolyzed rhodopsin, it was noted that GRK1 binds to cytoplasmic loops of the receptor to stimulate catalytic function (153). Receptor mutagenesis has further confirmed the importance of cytoplasmic loops for GRK docking (154). Similar to GRK1, GRK7 can phosphorylate the cone photopsins and terminate light-activated signaling pathways (155).
GRK2/3.
GRK2 was initially named βAR kinase or βARK due to its ability to phosphorylate β2ARs (13, 120). Synthetic peptides that are composed of β2AR domains demonstrate the potential of multiple interaction sites between the receptor and the kinase (156). For example, the first intracellular loop plays an inhibitory role (157), whereas the second and third intracellular loop are critical for binding affinity between receptor and kinase (158, 159).
GRK2’s C-terminal proline-rich motif is involved in the recruitment of agonist-occupied receptors through its binding of Gβγ subunits on the pleckstrin homology domain (160, 161). GRK2 can also promote phosphorylation-independent desensitization through sequestration of Gαq via its RGS homology domain (162). A noncanonical role for GRK2 has been shown by its direct protein-protein interaction between the RGS domain of GRK2 and Gαq/11 (163). GRK2 can also phosphorylate non-GPCRs, exemplified by its negative regulation on the platelet-derived growth factor receptor β (PDGFRβ) (164).
Although GRK2 and GRK3 share a high degree of structural similarity (165), some key differences do exist between the two isoforms. For example, although GRK2 is the predominating isoform, the olfactory epithelium only contains GRK3 (166, 167). GRK3 can also enhance angiogenesis through its downregulation on thrombospondin-1 and plasminogen activator inhibitor-2 (168). For the chemokine receptor C-X-C chemokine receptor type 4 (CXCR4), GRK3 overexpression can restore the desensitization of the receptor and normalize chemotaxis (169).
GRK4/5/6.
Unlike other GRKs, GRK4 demonstrates a constitutive phosphorylation activity in the absence of agonist stimulation (170), achieved through palmitoylation (116). GRK4 can also directly interact with caveolin-1, preventing further phosphorylation of the dopamine 1-like (D1) receptors (171).
In addition to its canonical role in phosphorylation, GRK5 binds calmodulin and is necessary for nuclear localization and maladaptive cardiomyocyte growth (172). Due to its large array of targets, it is thought that nuclear targeting following agonist stimulation may decrease cell survival, whereas GRK5 localized to the myocyte membrane may be protective (173). Beyond nuclear accumulation, GRK5 can also phosphorylate numerous other substrates, such as class II histone deacetylase 5 (174), the platelet-derived growth factor receptor-β (175), the insulin-like growth factor-1 receptor (176), and moesin (177). GRK5 polymorphisms also exist, such as the Leu41Glu variant that may promote a cardioprotective effect (178, 179).
Similar to GRK4, palmitoylated GRK6 can only be found in the membrane (180) and its activity not only depends on membrane association (181), but also on the rate of palmitoylation (182). GRK6 can also target non-GPCR low-density lipoprotein receptor-related protein 6 (183) and the signaling protein Na+/H+ exchange regulatory factor (184). These nonreceptor roles of GRKs indicate that GRKs play a broad role in signaling and disease (185).
GRK Physiological Functions and Possible Therapeutic Intervention
Maladaptive GRK activity is frequently observed in cardiovascular pathophysiology and therefore has potential as a therapeutic target (110).
GRK2/5.
As GRK2 desensitizes β adrenergic receptors, they are pharmaceutically relevant targets for potential heart failure therapies (186). In hypertrophied hearts, high GRK2 activity leads to marked desensitization of βARs (187). In patients with heart failure, there is a threefold increase in GRK2 protein expression (188) and levels of GRK2 may be a prognostic marker for heart failure mortality (189). Treatment with β-blockers in patients with heart failure has been shown to decrease GRK2 activity (190). Although homozygous knockout of GRKs is embryonic-lethal (191), heterozygous knockout show enhanced contractility (192). Mice that overexpress GRK2 show blunted catecholamine responsiveness (98) and increased susceptibility to ischemia-reperfusion injury (193), likely involving Akt signaling (194). In contrast, targeted ablation of GRK2 has been shown to improve cardiac function in murine heart failure models (195).
Inhibition of GRK2 has been tested as a potential therapy for heart failure. Rats with chronic heart failure injected with adeno-associated virus type 6 (AAV6) vectors that express βARKct showed improved cardiac function and reversal of reverse cardiac remodeling (196). Similar results were also achieved in large animal heart failure models (197). Thus, there is a considerable interest in developing highly selective GRK inhibitors. The crystal structure of a GRK2 complex with an RNA aptamer demonstrates how a hairpin loop can mimic ATP interaction in the active site (198). Other compounds, such as balanol (199), balanol-related compounds (200), and paroxetine (201), offer structural hints about the development of more potent and selective inhibitors.
GRK5, another highly expressed GRK in the heart, is also implicated in heart failure (202, 203). Transgenic mice with elevated GRK5 expression develop more hypertrophy than its wild-type counterparts after pressure overload (174). When GRK5 is knocked-out, mice show attenuated hypertrophy and preserved cardiac function in response to pressure overload by transverse aortic constriction (204) as well as enhanced muscarinic acetylcholine receptor signaling, manifesting in an exaggeration of cholinergic responses (205). The RGS homology domain within the N-terminus (GRK5-NT) can also inhibit NF-κB transcription activity and ameliorate cardiac hypertrophy (206).
GRK1/3/4/6/7.
Because of their roles in phosphorylation within the visual system, the in vivo ablation of GRK1 (207) or GRK7 (208) will lead to an abnormal response to light. Cardiac-specific GRK3 inhibition leads to elevated systolic pressure (209), and in the renal proximal convoluted tubules, reduced GRK4 expression can alleviate the severity of hypertension (210). GRK6, a key regulator in dopaminergic signaling, has been studied as a pharmaceutical target for addiction and Parkinson’s disease (211).
β-ARRESTINS
β-Arrestin Characteristics and Structure
Arrestins are multifunctional adaptor proteins that function to regulate a wide array of receptors and cellular signaling cascades (212). First discovered in 1978 (213), four isoforms of arrestins are currently known in vertebrates: the visual arrestins (Arrestin-1 and Arrestin-4) and the nonvisual arrestins: (Arrestin-2 and Arrestin-3) also known as β-arrestin-1 and β-arrestin-2.
Arrestin-1 and 4 are restricted to ocular rod and cone cells, binding almost exclusively to rhodopsin and color opsins, respectively (214). The nonvisual arrestins, β-arrestin-1 and β-arrestin-2, are ubiquitously expressed and interact with Class A and B GPCRs (16). Class A GPCRs, such as the β2AR, are characterized by β2AR·β-arrestin complexes that dissociate at or near the plasma membrane, whereas class B GPCRs, typified by the angiotensin type I receptor (AT1R), form a stable AT1R·β-arrestin complex and remain associated in endocytic vesicles (215). Despite sharing highly similar amino acid sequences, the two β-arrestin isoforms have distinct structural and functional identities. For example, β-arrestin-2 contains a leucine-rich nuclear export signal that restricts its location to the cytoplasm, whereas β-arrestin-1 can be found in both the nucleus and cytoplasm (216).
The structure of arrestin remains largely conserved across all four isoforms with an overall architecture that contains a central polar core flanked by N- and C-domains and a C tail connecting the two domains (217, 218). Structural variation is largely restricted to the distal C-domain. The polar core is made up of charged side chains (219) and is involved in its activation (220), as well as interaction between the N- and the C-terminal domain (221). Multiple loops are also critical for the stabilization of the molecule, including the “finger loop,” “middle loop,” and “lariat loop,” which are respectively defined by residues 63–75, 129–140, and 274–300 (222). The clathrin-binding domain on β-arrestin is located between residues 376–380 (223), whereas the binding domain of c-Src is located within the proline-rich residue regions of 88–96 and 120–124 (224).
β-Arrestin Activation
Phosphorylated GPCRs are the most common activators of β-arrestin and the binding to the receptor is proposed to be a two-step process that uses a phosphate and a conformational sensor (225). Structural studies of β-arrestin-1 (222) and β-arrestin-2 (226) have shown that a lysine and arginine on the N-domain form salt bridges with phosphates on the C-terminal tail. Phosphate binding will lead to structural changes, such as physical disruption of the polar core through a 20° rotation around the central axis of the N- and C- domains and displacement of the lariat loop (222). In addition to the phosphorylated c-tail of a GPCR, β-arrestins can be activated by IP6 (226) and an arginine to glutamic acid mutation at residue 175 (227).
The “finger loop” is thought to be the active sensor on the arrestin that will insert itself into a newly emerged binding pocket (228, 229). The C-edge loop of β-arrestin contributes to formation of a stable receptor complex through its engagement with the lipid bilayer (230, 231). Thus, when in complex with its receptor, β-arrestin has also been found to undergo a series of conformational changes distinct from that in its basal inactive state.
β-arrestins and G proteins can additionally bind to the same interhelical cavity on the cytoplasmic side of GPCRs, with arrestin showing a greater binding affinity for the site (232). With the ability of β-arrestin to bind to the phosphorylated C-terminal tail of the GPCR and promote receptor internalization (233), a GPCR-β-arrestin-G protein super complex was shown to occur in endocytic vesicles following agonist stimulation that can promote sustained intracellular G protein signaling (147).
β-Arrestin in Cellular Physiology
Although bound to GPCRs, β-arrestins can fulfill a variety of cellular functions that range from receptor desensitization, receptor trafficking and internalization, and activation of downstream signaling (234). A key element in the process of β-arrestin recruitment to activated GPCRs is phosphorylation of amino acid residues along the C-terminal tail of the receptor. Distinct phosphorylation patterns on the C-tail of the receptor have led to the concept of a “barcode” of phosphorylated serine and threonine residues as a driver of β-arrestin-GPCR interaction stability (235, 236). The importance of a receptor tail phosphorylation barcode is that it stabilizes distinct active conformations of β-arrestin (237, 238), thereby directing unique signaling cascades (239), and physiological responses (240). Specific receptor phosphorylation sites by different GRKs may further define the cellular function carried out by β-arrestin. For example, β-arrestin-mediated extracellular signal-regulated kinases (ERK) activation is associated with GRK 6 sites, whereas β2AR internalization is mediated by GRK2 sites, as well as both GRK sites resulting in receptor desensitization (236). Other studies have postulated a “flute model” of receptor tail phosphorylation—β-arrestin conformation that directs selective signaling (241, 242).
β-Arrestin in Receptor Desensitization
Among the wide array of functions ascribed to β-arrestin, its role in receptor desensitization, defined as the rapid loss of GPCR responsiveness with agonist stimulation, is of critical importance. Although kinase phosphorylation results in partial attenuation of receptor signaling, β-arrestin recruitment to the phosphorylated receptor leads to complete termination of G protein signaling through G protein uncoupling (243). Although the precise mechanism by which a GPCR is desensitized depends on the receptor subtype and its cellular context, the physical steric inhibition that uncouples G protein from the receptor is commonly observed (244). Furthermore, based on its capability of scaffolding phosphodiesterase 4, β-arrestin can promote the negative regulation on the receptor through both receptor desensitization and an accelerated degradation rate of cAMP (245). β-arrestin-2 is potentially more effective in mediating GPCR desensitization compared with β-arrestin-1, emphasizing an underlying correlation between structural conformation and cellular function (246).
β-Arrestin in Receptor Trafficking
Following receptor desensitization, GPCRs are internalized and trafficked from the plasma membrane to endocytic vesicles by β-arrestin (233). GPCR internalization is contingent upon β-arrestin binding to the phosphorylated receptor followed by binding of clathrin and its adaptor protein (AP2) to by β-arrestin (247). Clathrin-dependent endocytosis is achieved through the interaction between a clathrin-binding box on β-arrestin and blades in the clathrin β-propeller domain (248, 249). Based on a dynamin-dependent mechanism (250), β-arrestin may recruit other proteins, such as ADP-ribosylation factor 6 (251) and N-ethylmaleimide-sensitive fusion protein (252).
Through GPCR internalization and the formation of a megaplex, receptors can further maintain their signaling properties in internalized compartments (147, 148) (Fig. 3). This function is believed to be native to both the tail and core binding conformations of β-arrestin and therefore is common to both Class A and Class B GPCRs (253). After GPCR internalization to endosomes, mediated by Rab proteins (254), receptors are directed either toward the lysosomal pathway for degradation, the trans-Golgi-Network for recycling, or the plasma membrane for repopulation and resensitization (255). Therefore, β-arrestin maintains an integral role in the mechanics of the G protein-coupled receptor, either by its effects on receptor signaling or trafficking.
β-Arrestin as a Signal Transducer
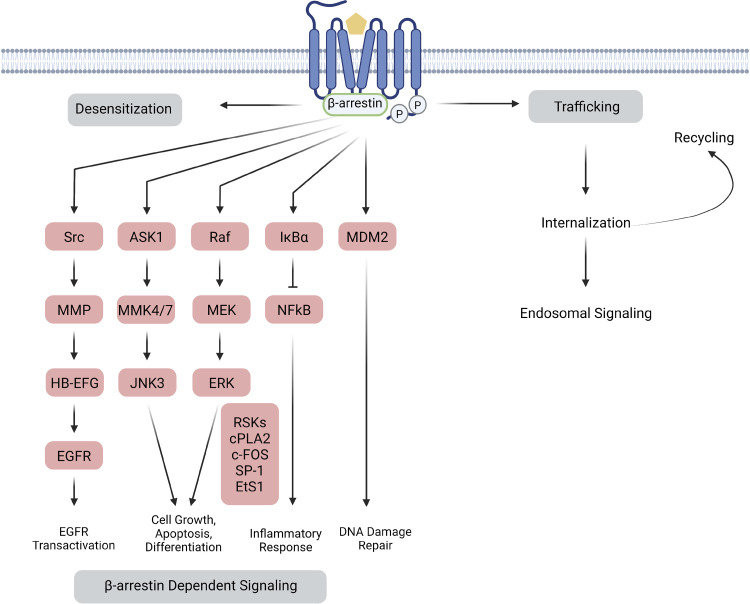
In 1999, it was discovered that the role of β-arrestin extends beyond “arresting” (224). In fact, β-arrestin maintains the ability to scaffold molecules and initiate receptor-dependent and -independent signaling cascades (212). Its vast interactome and involvement in a wide array of signaling pathways allow it to play a central role in a variety of cellular functions (Fig. 4).
Figure 4.
β-Arrestin-mediated signaling. β-arrestins have been shown to regulate a diverse set of signaling pathways involved in a variety of cellular processes. The transducer plays a crucial role in the desensitization, trafficking, internalization, and translocation of its receptor, directing crucial stages of the GPCR life span. β-arrestin has also been shown to activate many signaling cascades, particularly due to its ability to scaffold additional proteins. The wide array of signaling cascades consequently regulate diverse aspects of cellular function, including but not limited to EGFR transactivation, cell growth, apoptosis, cell differentiation, inflammatory responses, and DNA damage repair. ASK1, apoptosis signal-regulating kinase 1; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; GPCR, G protein-coupled receptor; HB-EFG, heparin-binding EGF-like epidermal growth factor; IkBα, NF-κB inhibitor; JNK3, Jun N-terminal kinase 3; MDM2, murine double minute 2 gene (negatively regulates p53); MEK, mitogen-activated protein kinase; MMK4/7, mitogen-activated protein kinase 4/7; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappa B; RaF, rapidly accelerated fibrosarcoma kinase; Src, protooncogene tyrosine-kinase Src. Figure created with BioRender and published with permission.
c-Src Recruitment and Signaling.
GRK2 phosphorylation residues along the C-terminal tail of a GPCR lead to the recruitment and binding of β-arrestin to the phosphorylated receptor, followed by the β-arrestin-mediated protooncogene tyrosine-protein kinase Src (c-Src) recruitment (224). The two main interactions between β-arrestin-1 and c-Src occur between the Src homology 3 domain and PXXP motifs at resides 121–124 as well as at the terminal catalytic SH1 Src domain and epitopes within residue 185 of the N-terminal (256). β2AR, β-arrestin-1, and c-Src then form a complex upon agonist activation of the receptor, leading to receptor-dependent activation of ERK. GPCR activation and engagement of β-arrestin with the phosphorylated receptor tail allosterically activate Src (257, 258). Src-dependent GPCR-mediated signaling effects include tyrosine phosphorylation of dynamin (256), ERK MAP kinase cascade activation (259), and EGFR transactivation (260).
GPCR transactivation.
Receptor transactivation is defined as agonist-stimulation of a receptor leading to the activation and cytosolic generation of downstream products of a second distinct receptor, particularly in the context of cell surface receptors (261). More specifically, ligand activation of βARs leads to recruitment of β-arrestin-1-Src to the receptor complex initiating matrix metalloproteinase (MMP) activation, heparin-binding epidermal growth factor (HB-EGF) shedding and EGFR binding (260). EGFR transactivation by β1-adrenergic receptor (β1AR) has also been shown to confer cardioprotective effects under conditions of sustained catecholamine stimulation (260). Interestingly, the β1AR antagonists, alprenolol and carvedilol, have been found to stimulate βAR-EGFR transactivation and EGFR-dependent ERK activation (262).
ERK activation.
As a scaffolding protein, β-arrestin may also bring two or more proteins closer together for downstream signaling cascades. For example, GPCRs can partially transduce signals via the ERK/β-arrestin signaling complex, as exemplified by β-arrestin-dependent ERK activation (263). Upon formation, the receptor complex undergoes endocytosis mediated by clathrin-coated pits, whereby activated ERK is translocated to endocytic vehicles (264). For AT1aR, there are two distinct pathways for ERK activation: a G protein-dependent pathway that is rapid, transient, and will lead to nuclear translocation, and a β-arrestin-dependent pathway that is slow and sustained (265). Furthermore, β-arrestin-activated ERK functions plays a bigger role in cellular chemotaxis, cytoskeletal rearrangements (266, 267), cell growth and proliferation (268), mobility, and apoptosis (269).
JNK3 activation.
c-Jun N-terminal kinases (JNK) signaling is involved in cellular proliferation, apoptosis, differentiation, and migration (270). β-arrestin-2 acts as a scaffold for JNK3 along with the upstream JNK activators apoptosis signal-regulating kinase 1 (ASK1) and mitogen-activated protein kinase MAPK kinase 4 (MKK4) (271). Stimulation of the AT1R results in JNK3 activation and colocalization of β-arrestin-2 and JNK3 in endocytic vesicles (271). JNK3 has been shown to interact with the β1 strand of β-arrestin-2, which requires a C-terminal truncation (272). In addition, an active JNK3 molecule can also replace an inactive JNK3 molecule in the β-arrestin-2/MKK complex leading to signal amplification (273).
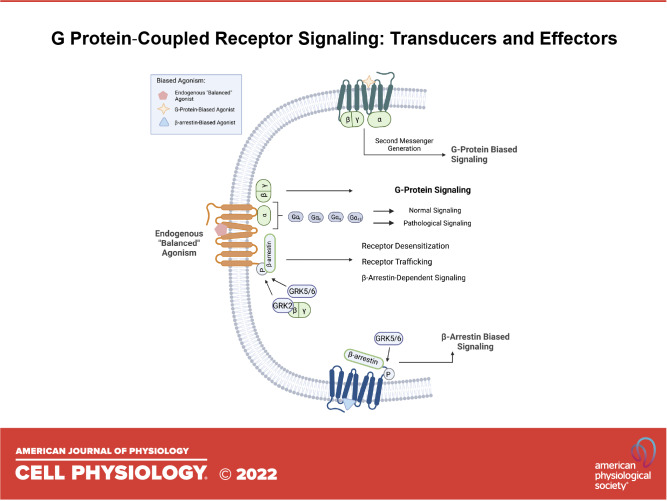
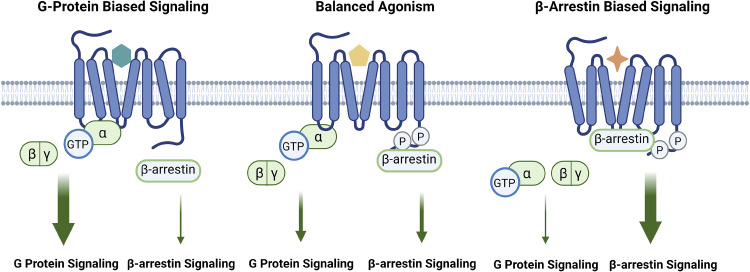
Biased Agonism
Biased agonism refers to the ability of a ligand to activate a distinct subset of a receptor’s signaling portfolio by allowing the receptor to engage distinct transducers (274–276) (Fig. 5). In the context of a GPCR, transducers can be Gα and Gβγ, GRKs, and β-arrestin resulting in “bias” toward either β-arrestin-mediated signaling or G protein-mediated signaling.
Figure 5.
Biased agonism and signaling selectivity. Biased agonism is a recently emerging concept in which a ligand preferentially activates one signaling pathway over another. In balanced agonism, binding of a ligand potentiates both G protein and β-arrestin signaling. However, when a β-arrestin-biased-ligand binds to a GPCR, conformational changes in the receptor favor β-arrestin-mediated signaling while silencing of G protein-mediated pathways. Similarly, when a G protein-biased ligand binds to the GPCR, conformational changes in the receptor favor G protein-mediated signaling while silencing β-arrestin signaling. Figure created with BioRender.com. GPCR, G protein-coupled receptor; GTP, guanosine triphosphate.
Ligand bias.
Different ligands can promote the stabilization of different conformations of the receptor-transducer complex (277). β-arrestin-biased ligands stabilize receptor conformations favoring the engagement β-arrestin as the signaling transducer resulting in distinct downstream cellular signaling (278). For the AT1R, β-arrestin-biased ligands, such as [Sar1,Ile4,Ile8]-AngII (SII) and TRV120023, can lead to β-arrestin-dependent signaling, including the ERK activation signaling cascade (275, 279, 280). βARs also exhibit ligand bias. For example, carvedilol, a βAR antagonist, can stabilize distinct receptor conformations to stimulate β-arrestin-mediated signaling (281, 282).
Allosteric modulation.
Allosteric modulators are small molecules that can preferentially bind to allosteric sites which differ from the orthosteric site at which endogenous agonists bind (283). Allosteric modulators bound to receptors may then either increase or decrease the binding affinity of orthosteric ligands, and therefore are described as positive or negative allosteric modulators, respectively (284). Because of their enhanced selectivity and intrinsic properties in modulating receptor function they hold great clinical promise (285).
Recently, it was shown that the βAR allosteric modulator Cmpd-6 positively cooperates with carvedilol to augment the properties of carvedilol by enhancing β-arrestin signaling (286, 287) and cardioprotection to ischemia reperfusion (287). Thus, GPCR allosteric modulators are of interest as novel therapeutics due to associated increases in receptor subtype specificity and potential for a decrease in side effects (276, 287).
β-Arrestin Cardiovascular Physiological Function and Possible Therapeutics
In the heart, β-arrestin-1 is the predominated form (288) and has been shown to be associated with adverse remodeling post myocardial infarction (289). In contrast, β-arrestin-2 can be cardioprotective following myocardial infarction due to its anti-apoptotic and anti-inflammatory effects (290, 291). Knockout of β-arrestin-2 in aged mice shows signs of cardiac dysfunction (292). β-arrestin activation of the AT1R through the biased ligand TRV120023 is cardioprotective in ischemia-reperfusion injury by promoting cardiomyocyte survival (293). Interestingly, β-arrestin-dependent signaling activated by the β-blocker carvedilol confers cardioprotection in experimental ischemia-reperfusion injury through a β-arrestin-dependent mechanism (287). Finally, the βAR allosteric modulator, Cmpd-6, can potentiate the prosurvival/antiapoptotic effect of carvedilol in vivo by enhancing the binding affinity of carvedilol to the βAR (287). Therefore, β-arrestin’s multifunctionality allows it to promote signaling cascades that can be protective or detrimental within the context of the cellular function or disease process. For instance, β-arrestin-1 has been implicated in the cellular process of T-cell activation (294).
CONCLUSION
With advancement in understanding of the mechanisms that drive GPCR transducer interactions, we can appreciate the magnitude and versatility of their cellular effects. With continued investigation into mechanisms of ligand–receptor interaction, effector-receptor engagement, conformational analysis, and signaling profiles should yield new approaches to the development of novel therapeutics.
GRANTS
This work was supported by the National Institutes of Health Grants HL056687 and HL075443 (to H.A.R.).
DISCLOSURES
H. A. Rockman is a scientific cofounder of Trevena Inc., a company that is developing new GPCR ligands. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
This article is part of the special collection “Advances in GPCRs: Structure, Mechanisms, Disease, and Pharmacology.” Wei Kong, MD, PhD, and Jinpeng Sun, PhD, served as Guest Editors of this collection.
AUTHOR CONTRIBUTIONS
H.J., D.G., and J.W. prepared figures; H.J., D.G., and J.W. drafted manuscript; H.J., D.G., J.W., and H.A.R. edited and revised manuscript; H.J., D.G., J.W., and H.A.R. approved final version of manuscript.
REFERENCES
- 1. Drews J. Paul Ehrlich: magister mundi. Nat Rev Drug Discov 3: 797–801, 2004. doi: 10.1038/nrd1498. [DOI] [PubMed] [Google Scholar]
- 2. Langley JN. On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J Physiol 33: 374–413, 1905. doi: 10.1113/jphysiol.1905.sp001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mukherjee C, Caron MG, Coverstone M, Lefkowitz RJ. Identification of adenylate cyclase-coupled β-adrenergic receptors in frog erythrocytes with (minus)-[3-H] alprenolol. J Biol Chem 250: 4869–4876, 1975. doi: 10.1016/s0021-9258(19)41249-0. [DOI] [PubMed] [Google Scholar]
- 4. Williams LT, Lefkowitz RJ. α-adrenergic receptor identification by (3H)dihydroergocryptine binding. Science 192: 791–793, 1976. doi: 10.1126/science.4894. [DOI] [PubMed] [Google Scholar]
- 5. Dohlman HG, Caron MG, Lefkowitz RJ. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry 26: 2657–2664, 1987. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- 6. Rall TW, Sutherland EW. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem 232: 1065–1076, 1958. doi: 10.1016/S0021-9258(19)77422-5. [DOI] [PubMed] [Google Scholar]
- 7. Rodbell M, Birnbaumer L, Pohl SL, Krans HM. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem 246: 1877–1882, 1971. doi: 10.1016/S0021-9258(18)62390-7. [DOI] [PubMed] [Google Scholar]
- 8. Cassel D, Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta 452: 538–551, 1976. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- 9. Ross EM, Howlett AC, Ferguson KM, Gilman AG. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem 253: 6401–6412, 1978. doi: 10.1016/S0021-9258(19)46947-0. [DOI] [PubMed] [Google Scholar]
- 10. Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 11. Bownds D, Dawes J, Miller J, Stahlman M. Phosphorylation of frog photoreceptor membranes induced by light. Nat New Biol 237: 125–127, 1972. doi: 10.1038/newbio237125a0. [DOI] [PubMed] [Google Scholar]
- 12. Shichi H, Somers RL. Light-dependent phosphorylation of rhodopsin. Purification and properties of rhodopsin kinase. J Biol Chem 253: 7040–7046, 1978. doi: 10.1038/321869a0. [DOI] [PubMed] [Google Scholar]
- 13. Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. β-Adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA 83: 2797–2801, 1986. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated β-adrenergic receptor by the β-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci USA 84: 8879–8882, 1987. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhn H, Wilden U. Deactivation of photoactivated rhodopsin by rhodopsin-kinase and arrestin. J Recept Res 7: 283–298, 1987. doi: 10.3109/10799898709054990. [DOI] [PubMed] [Google Scholar]
- 16. Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. β-Arrestin: a protein that regulates β-adrenergic receptor function. Science 248: 1547–1550, 1990. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 17. Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. β-Arrestin2, a novel member of the arrestin/β-arrestin gene family. J Biol Chem 267: 17882–17890, 1992. doi: 10.1016/S0021-9258(19)37125-X. [DOI] [PubMed] [Google Scholar]
- 18. Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 A crystal structure of a heterotrimeric G protein. Nature 379: 311–319, 1996. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 19. Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene 20: 1643–1652, 2001. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 20. Goricanec D, Stehle R, Egloff P, Grigoriu S, Plückthun A, Wagner G, Hagn F. Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proc Natl Acad Sci USA 113: E3629–E3638, 2016. doi: 10.1073/pnas.1604125113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warner DR, Weng G, Yu S, Matalon R, Weinstein LS. A novel mutation in the switch 3 region of Gsα in a patient with Albright hereditary osteodystrophy impairs GDP binding and receptor activation. J Biol Chem 273: 23976–23983, 1998. doi: 10.1074/jbc.273.37.23976. [DOI] [PubMed] [Google Scholar]
- 22. Markby DW, Onrust R, Bourne HR. Separate GTP binding and GTPase activating domains of a Gα subunit. Science 262: 1895–1901, 1993. doi: 10.1126/science.8266082. [DOI] [PubMed] [Google Scholar]
- 23. Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gqα to that of Giα. Nature 363: 274–276, 1993. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 24. Natochin M, Muradov KG, McEntaffer RL, Artemyev NO. Rhodopsin recognition by mutant G(s)α containing C-terminal residues of transducin. J Biol Chem 275: 2669–2675, 2000. doi: 10.1074/jbc.275.4.2669. [DOI] [PubMed] [Google Scholar]
- 25. Mazzoni MR, Malinski JA, Hamm HE. Structural analysis of rod GTP-binding protein, Gt. Limited proteolytic digestion pattern of Gt with four proteases defines monoclonal antibody epitope. J Biol Chem 266: 14072–14081, 1991. doi: 10.1016/S0021-9258(18)92811-5. [DOI] [PubMed] [Google Scholar]
- 26. Linder ME, Pang IH, Duronio RJ, Gordon JI, Sternweis PC, Gilman AG. Lipid modifications of G protein subunits. Myristoylation of Goα increases its affinity for βγ. J Biol Chem 266: 4654–4659, 1991. doi: 10.1016/S0021-9258(20)64372-1. [DOI] [PubMed] [Google Scholar]
- 27. Preininger AM, Van Eps N, Yu NJ, Medkova M, Hubbell WL, Hamm HE. The myristoylated amino terminus of Gα(i)(1) plays a critical role in the structure and function of Gα(i)(1) subunits in solution. Biochemistry 42: 7931–7941, 2003. doi: 10.1021/bi0345438. [DOI] [PubMed] [Google Scholar]
- 28. Mixon MB, Lee E, Coleman DE, Berghuis AM, Gilman AG, Sprang SR. Tertiary and quaternary structural changes in Gi α 1 induced by GTP hydrolysis. Science 270: 954–960, 1995. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 29. Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477: 549–555, 2011. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ping YQ, Mao C, Xiao P, Zhao RJ, Jiang Y, Yang Z, An WT, Shen DD, Yang F, Zhang H, Qu C, Shen Q, Tian C, Li ZJ, Li S, Wang GY, Tao X, Wen X, Zhong YN, Yang J, Yi F, Yu X, Xu HE, Zhang Y, Sun JP. Structures of the glucocorticoid-bound adhesion receptor GPR97-Go complex. Nature 589: 620–626, 2021. doi: 10.1038/s41586-020-03083-w. [DOI] [PubMed] [Google Scholar]
- 31. Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem 73: 559–587, 2004. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 32. Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein βγ dimer at 2.1A resolution. Nature 379: 369–374, 1996. [Erratum in Nature 379: 847, 1996]. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 33. Taylor JM, Jacob-Mosier GG, Lawton RG, VanDort M, Neubig RR. Receptor and membrane interaction sites on Gβ. A receptor-derived peptide binds to the carboxyl terminus. J Biol Chem 271: 3336–3339, 1996. doi: 10.1074/jbc.271.7.3336. [DOI] [PubMed] [Google Scholar]
- 34. Downes GB, Gautam N. The G protein subunit gene families. Genomics 62: 544–552, 1999. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 35. Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 65: 241–269, 1996. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JB, Casey PJ. The role of prenylation in G-protein assembly and function. Cell Signal 8: 433–437, 1996. doi: 10.1016/s0898-6568(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt CJ, Neer EJ. In vitro synthesis of G protein βγ dimers. J Biol Chem 266: 4538–4544, 1991. doi: 10.1016/S0021-9258(20)64356-3. [DOI] [PubMed] [Google Scholar]
- 38. Morales J, Fishburn CS, Wilson PT, Bourne HR. Plasma membrane localization of Gα z requires two signals. Mol Biol Cell 9: 1–14, 1998. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Degtyarev MY, Spiegel AM, Jones TL. The G protein α s subunit incorporates [3H]palmitic acid and mutation of cysteine-3 prevents this modification. Biochemistry 32: 8057–8061, 1993. doi: 10.1021/bi00083a001. [DOI] [PubMed] [Google Scholar]
- 40. Chisari M, Saini DK, Kalyanaraman V, Gautam N. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem 282: 24092–24098, 2007. doi: 10.1074/jbc.M704246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takida S, Wedegaertner PB. Heterotrimer formation, together with isoprenylation, is required for plasma membrane targeting of Gβγ. J Biol Chem 278: 17284–17290, 2003. doi: 10.1074/jbc.M213239200. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt CJ, Thomas TC, Levine MA, Neer EJ. Specificity of G protein β and γ subunit interactions. J Biol Chem 267: 13807–13810, 1992. doi: 10.1016/S0021-9258(19)49638-5. [DOI] [PubMed] [Google Scholar]
- 43. Tolkovsky AM, Levitzki A. Mode of coupling between the β-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry 17: 3795, 1978. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- 44. Galés C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol 13: 778–786, 2006. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 45. Janz JM, Farrens DL. Rhodopsin activation exposes a key hydrophobic binding site for the transducin α-subunit C terminus. J Biol Chem 279: 29767–29773, 2004. doi: 10.1074/jbc.M402567200. [DOI] [PubMed] [Google Scholar]
- 46. Hamm HE, Deretic D, Arendt A, Hargrave PA, Koenig B, Hofmann KP. Site of G protein binding to rhodopsin mapped with synthetic peptides from the α subunit. Science 241: 832–835, 1988. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- 47. Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274: 768–770, 1996. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 48. Mahoney JP, Sunahara RK. Mechanistic insights into GPCR-G protein interactions. Curr Opin Struct Biol 41: 247–254, 2016. doi: 10.1016/j.sbi.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McKee EE, Bentley AT, Smith RM Jr, Ciaccio CE. Origin of guanine nucleotides in isolated heart mitochondria. Biochem Biophys Res Commun 257: 466–472, 1999. doi: 10.1006/bbrc.1999.0489. [DOI] [PubMed] [Google Scholar]
- 50. Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mapping allosteric connections from the receptor to the nucleotide-binding pocket of heterotrimeric G proteins. Proc Natl Acad Sci USA 104: 7927–7932, 2007. doi: 10.1073/pnas.0702623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saraste M, Sibbald PR, Wittinghofer A. The P-loop – a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci 15: 430–434, 1990. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 52. McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci 62: 551–577, 2005. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science 252: 802–808, 1991. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 54. Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96: 1261–1296, 2016. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 55. Chen Z, Singer WD, Sternweis PC, Sprang SR. Structure of the p115RhoGEF rgRGS domain-Gα13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat Struct Mol Biol 12: 191–197, 2005. doi: 10.1038/nsmb888. [DOI] [PubMed] [Google Scholar]
- 56. Suzuki N, Nakamura S, Mano H, Kozasa T. Gα 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci USA 100: 733–738, 2003. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science 280: 2112–2114, 1998. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 58. Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular basis for interactions of G protein βγ subunits with effectors. Science 280: 1271–1274, 1998. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 59. Mahon MJ, Bonacci TM, Divieti P, Smrcka AV. A docking site for G protein βγ subunits on the parathyroid hormone 1 receptor supports signaling through multiple pathways. Mol Endocrinol 20: 136–146, 2006. doi: 10.1210/me.2005-0169. [DOI] [PubMed] [Google Scholar]
- 60. Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol 36: 481–509, 1996. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 61. Wickman KD, Iñiguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature 368: 255–257, 1994. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 62. Luttrell LM, Della Rocca GJ, van Biesen T, Luttrell DK, Lefkowitz RJ. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem 272: 4637–4644, 1997. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 63. Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science 257: 1264–1267, 1992. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 64. Hamm HE. The many faces of G protein signaling. J Biol Chem 273: 669–672, 1998. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 65. Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80: 249–257, 1995. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 66. Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36: 461–480, 1996. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 67. Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci 22: 267–272, 1997. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 68. Lin HC, Gilman AG. Regulation of dynamin I GTPase activity by G protein βγ subunits and phosphatidylinositol 4,5-bisphosphate. J Biol Chem 271: 27979–27982, 1996. doi: 10.1074/jbc.271.45.27979. [DOI] [PubMed] [Google Scholar]
- 69. Langhans-Rajasekaran SA, Wan Y, Huang XY. Activation of Tsk and Btk tyrosine kinases by G protein βγ subunits. Proc Natl Acad Sci USA 92: 8601–8605, 1995. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S 3rd, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem 274: 33202–33205, 1999. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- 71. Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280: 2109–2111, 1998. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 72. Kozasa T, Hajicek N, Chow CR, Suzuki N. Signalling mechanisms of RhoGTPase regulation by the heterotrimeric G proteins G12 and G13. J Biochem 150: 357–369, 2011. doi: 10.1093/jb/mvr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tall GG, Krumins AM, Gilman AG. Mammalian Ric-8A (synembryn) is a heterotrimeric Gα protein guanine nucleotide exchange factor. J Biol Chem 278: 8356–8362, 2003. doi: 10.1074/jbc.M211862200. [DOI] [PubMed] [Google Scholar]
- 74. Druey KM, Blumer KJ, Kang VH, Kehrl JH. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature 379: 742–746, 1996. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- 75. Shi CS, Lee SB, Sinnarajah S, Dessauer CW, Rhee SG, Kehrl JH. Regulator of G-protein signaling 3 (RGS3) inhibits Gβ1γ 2-induced inositol phosphate production, mitogen-activated protein kinase activation, and Akt activation. J Biol Chem 276: 24293–24300, 2001. doi: 10.1074/jbc.M100089200. [DOI] [PubMed] [Google Scholar]
- 76. Uechi M, Asai K, Osaka M, Smith A, Sato N, Wagner TE, Ishikawa Y, Hayakawa H, Vatner DE, Shannon RP, Homcy CJ, Vatner SF. Depressed heart rate variability and arterial baroreflex in conscious transgenic mice with overexpression of cardiac Gsα. Circ Res 82: 416–423, 1998. doi: 10.1161/01.RES.82.4.416. [DOI] [PubMed] [Google Scholar]
- 77. Vatner DE, Asai K, Iwase M, Ishikawa Y, Wagner TE, Shannon RP, Homcy CJ, Vatner SF. Overexpression of myocardial Gsα prevents full expression of catecholamine desensitization despite increased β-adrenergic receptor kinase. J Clin Invest 101: 1916–1922, 1998. doi: 10.1172/JCI1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Iwase M, Uechi M, Vatner DE, Asai K, Shannon RP, Kudej RK, Wagner TE, Wight DC, Patrick TA, Ishikawa Y, Homcy CJ, Vatner SF. Cardiomyopathy induced by cardiac Gsα overexpression. Am J Physiol Heart Circ Physiol 272: H585–H589, 1997. doi: 10.1152/ajpheart.1997.272.1.H585. [DOI] [PubMed] [Google Scholar]
- 79. Jia H, Hingorani AD, Sharma P, Hopper R, Dickerson C, Trutwein D, Lloyd DD, Brown MJ. Association of the G(s)α gene with essential hypertension and response to β-blockade. Hypertension 34: 8–14, 1999. doi: 10.1161/01.hyp.34.1.8. [DOI] [PubMed] [Google Scholar]
- 80. Feldman AM, Cates AE, Bristow MR, Van Dop C. Altered expression of α-subunits of G proteins in failing human hearts. J Mol Cell Cardiol 21: 359–365, 1989. doi: 10.1016/0022-2828(89)90646-9. [DOI] [PubMed] [Google Scholar]
- 81. Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through β 2-adrenoceptors. Proc Natl Acad Sci USA 100: 14475–14480, 2003. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Donahue JK, Heldman AW, Fraser H, McDonald AD, Miller JM, Rade JJ, Eschenhagen T, Marbán E. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med 6: 1395–1398, 2000. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 83. Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW 2nd.. Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA 95: 10140–10145, 1998. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW 2nd.. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci USA 94: 8121–8126, 1997. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW 2nd, Armstrong RC, Kitsis RN. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Gα(q) transgenic mice. Circulation 108: 3036–3041, 2003. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 86. Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Gαq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci USA 95: 13893–13898, 1998. doi: 10.1073/pnas.95.23.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 280: 574–577, 1998. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 88. Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 105: 85–92, 2002. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 89. Wettschureck N, Rütten H, Zywietz A, Gehring D, Wilkie TM, Chen J, Chien KR, Offermanns S. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat Med 7: 1236–1240, 2001. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 90. Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in Gα(q)-deficient mice. Nature 389: 183–186, 1997. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 91. Liggett SB, Kelly RJ, Parekh RR, Matkovich SJ, Benner BJ, Hahn HS, Syed FM, Galvez AS, Case KL, McGuire N, Odley AM, Sparks L, Kardia SL, Dorn GW 2nd.. A functional polymorphism of the Gαq (GNAQ) gene is associated with accelerated mortality in African-American heart failure. Hum Mol Genet 16: 2740–2750, 2007. doi: 10.1093/hmg/ddm229. [DOI] [PubMed] [Google Scholar]
- 92. Frey UH, Lieb W, Erdmann J, Savidou D, Heusch G, Leineweber K, Jakob H, Hense HW, Lowel H, Brockmeyer NH, Schunkert H, Siffert W. Characterization of the GNAQ promoter and association of increased Gq expression with cardiac hypertrophy in humans. Eur Heart J 29: 888–897, 2008. doi: 10.1093/eurheartj/ehm618. [DOI] [PubMed] [Google Scholar]
- 93. Finn SG, Plonk SG, Fuller SJ. Gα 13 stimulates gene expression and increases cell size in cultured neonatal rat ventricular myocytes. Cardiovasc Res 42: 140–148, 1999. doi: 10.1016/s0008-6363(98)00294-6. [DOI] [PubMed] [Google Scholar]
- 94. Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res 87: 221–227, 2000. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- 95. Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science 275: 533–536, 1997. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- 96. Takefuji M, Wirth A, Lukasova M, Takefuji S, Boettger T, Braun T, Althoff T, Offermanns S, Wettschureck N. G(13)-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure. Circulation 126: 1972–1982, 2012. doi: 10.1161/CIRCULATIONAHA.112.109256. [DOI] [PubMed] [Google Scholar]
- 97. Lefkowitz RJ. G protein-coupled receptor kinases. Cell 74: 409–412, 1993. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- 98. Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a β ARK inhibitor. Science 268: 1350–1353, 1995. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 99. Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J Jr, Lefkowitz RJ, Koch WJ. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA 95: 7000–7005, 1998. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac β ARK1 inhibition prolongs survival and augments β blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci USA 98: 5809–5814, 2001. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tachibana H, Naga Prasad SV, Lefkowitz RJ, Koch WJ, Rockman HA. Level of β-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation 111: 591–597, 2005. doi: 10.1161/01.CIR.0000142291.70954.DF. [DOI] [PubMed] [Google Scholar]
- 102. Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, Smrcka AV, Blaxall BC. Small molecule disruption of G βγ signaling inhibits the progression of heart failure. Circ Res 107: 532–539, 2010. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kamal FA, Mickelsen DM, Wegman KM, Travers JG, Moalem J, Hammes SR, Smrcka AV, Blaxall BC. Simultaneous adrenal and cardiac g-protein-coupled receptor-gβγ inhibition halts heart failure progression. J Am Coll Cardiol 63: 2549–2557, 2014. doi: 10.1016/j.jacc.2014.02.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B. Association of a human G-protein β3 subunit variant with hypertension. Nat Genet 18: 45–48, 1998. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 105. Peters BJ, Maitland-van der Zee AH, Stricker BH, van Wieren-de Wijer DB, de Boer A, Kroon AA, de Leeuw PW, Schiffers P, Janssen RG, van Duijn CM, Klungel OH. Effectiveness of statins in the reduction of the risk of myocardial infarction is modified by the GNB3 C825T variant. Pharmacogenet Genomics 18: 631–636, 2008. doi: 10.1097/FPC.0b013e3283023fb2. [DOI] [PubMed] [Google Scholar]
- 106. Schunkert H, Hense H-W, Döring A, Riegger GAJ, Siffert W. Association between a polymorphism in the G protein β3 subunit gene and lower renin and elevated diastolic blood pressure levels. Hypertension 32: 510–513, 1998. doi: 10.1161/01.HYP.32.3.510. [DOI] [PubMed] [Google Scholar]
- 107. Benjafield AV, Jeyasingam CL, Nyholt DR, Griffiths LR, Morris BJ. G-protein β3 subunit gene (GNB3) variant in causation of essential hypertension. Hypertension 32: 1094–1097, 1998. doi: 10.1161/01.hyp.32.6.1094. [DOI] [PubMed] [Google Scholar]
- 108. Brand E, Herrmann SM, Nicaud V, Ruidavets JB, Evans A, Arveiler D, Luc G, Plouin PF, Tiret L, Cambien F. The 825C/T polymorphism of the G-protein subunit β3 is not related to hypertension. Hypertension 33: 1175–1178, 1999. doi: 10.1161/01.hyp.33.5.1175. [DOI] [PubMed] [Google Scholar]
- 109. Snapir A, Heinonen P, Tuomainen TP, Lakka TA, Kauhanen J, Salonen JT, Scheinin M. G-protein β3 subunit C825T polymorphism: no association with risk for hypertension and obesity. J Hypertens 19: 2149–2155, 2001. doi: 10.1097/00004872-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 110. Pfleger J, Gresham K, Koch WJ. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol 16: 612–622, 2019. doi: 10.1038/s41569-019-0220-3. [DOI] [PubMed] [Google Scholar]
- 111. Premont RT, Macrae AD, Aparicio SA, Kendall HE, Welch JE, Lefkowitz RJ. The GRK4 subfamily of G protein-coupled receptor kinases. Alternative splicing, gene organization, and sequence conservation. J Biol Chem 274: 29381–29389, 1999. doi: 10.1074/jbc.274.41.29381. [DOI] [PubMed] [Google Scholar]
- 112. Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem 67: 653–692, 1998. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 113. Weiss ER, Ducceschi MH, Horner TJ, Li A, Craft CM, Osawa S. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J Neurosci 21: 9175–9184, 2001. doi: 10.1523/JNEUROSCI.21-23-09175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Benovic JL, Mayor F Jr, Staniszewski C, Lefkowitz RJ, Caron MG. Purification and characterization of the β-adrenergic receptor kinase. J Biol Chem 262: 9026–9032, 1987. doi: 10.1016/S0021-9258(18)48041-6. [DOI] [PubMed] [Google Scholar]
- 115. Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J 9: 175–182, 1995. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 116. Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem 271: 6403–6410, 1996. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- 117. Gurevich VV, Gurevich EV. GPCR signaling regulation: the role of GRKs and arrestins. Front Pharmacol 10: 125, 2019. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22, 2010. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 119. Siderovski DP, Hessel A, Chung S, Mak TW, Tyers M. A new family of regulators of G-protein-coupled receptors? Curr Biol 6: 211–212, 1996. doi: 10.1016/s0960-9822(02)00454-2. [DOI] [PubMed] [Google Scholar]
- 120. Benovic JL, DeBlasi A, Stone WC, Caron MG, Lefkowitz RJ. β-Adrenergic receptor kinase: primary structure delineates a multigene family. Science 246: 235–240, 1989. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- 121. Sallese M, Mariggiò S, D'Urbano E, Iacovelli L, De Blasi A. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: direct interaction of kinase N terminus with activated Gαq. Mol Pharmacol 57: 826–831, 2000. doi: 10.1124/mol.57.4.826. [DOI] [PubMed] [Google Scholar]
- 122. Homan KT, Tesmer JJ. Molecular basis for small molecule inhibition of G protein-coupled receptor kinases. ACS Chem Biol 10: 246–256, 2015. doi: 10.1021/cb5003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. McClendon CL, Kornev AP, Gilson MK, Taylor SS. Dynamic architecture of a protein kinase. Proc Natl Acad Sci USA 111: E4623–4631, 2014. [Erratum in Proc Natl Acad Sci USA 111: 16973, 2014]. doi: 10.1073/pnas.1418402111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Palczewski K, Buczyłko J, Lebioda L, Crabb JW, Polans AS. Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J Biol Chem 268: 6004–6013, 1993. doi: 10.1016/S0021-9258(18)53419-0. [DOI] [PubMed] [Google Scholar]
- 125. Huang CC, Orban T, Jastrzebska B, Palczewski K, Tesmer JJ. Activation of G protein-coupled receptor kinase 1 involves interactions between its N-terminal region and its kinase domain. Biochemistry 50: 1940–1949, 2011. doi: 10.1021/bi101606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Noble B, Kallal LA, Pausch MH, Benovic JL. Development of a yeast bioassay to characterize G protein-coupled receptor kinases. Identification of an NH2-terminal region essential for receptor phosphorylation. J Biol Chem 278: 47466–47476, 2003. doi: 10.1074/jbc.M308257200. [DOI] [PubMed] [Google Scholar]
- 127. Pao CS, Barker BL, Benovic JL. Role of the amino terminus of G protein-coupled receptor kinase 2 in receptor phosphorylation. Biochemistry 48: 7325–7333, 2009. doi: 10.1021/bi900408g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Boguth CA, Singh P, Huang CC, Tesmer JJ. Molecular basis for activation of G protein-coupled receptor kinases. EMBO J 29: 3249–3259, 2010. doi: 10.1038/emboj.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sterne-Marr R, Leahey PA, Bresee JE, Dickson HM, Ho W, Ragusa MJ, Donnelly RM, Amie SM, Krywy JA, Brookins-Danz ED, Orakwue SC, Carr MJ, Yoshino-Koh K, Li Q, Tesmer JJ. GRK2 activation by receptors: role of the kinase large lobe and carboxyl-terminal tail. Biochemistry 48: 4285–4293, 2009. doi: 10.1021/bi900151g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Huang CC, Yoshino-Koh K, Tesmer JJG. A surface of the kinase domain critical for the allosteric activation of G protein-coupled receptor kinases. J Biol Chem 284: 17206–17215, 2009. doi: 10.1074/jbc.M809544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Komolov KE, Benovic JL. G protein-coupled receptor kinases: past, present and future. Cell Signal 41: 17–24, 2018. doi: 10.1016/j.cellsig.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Singh P, Wang B, Maeda T, Palczewski K, Tesmer JJ. Structures of rhodopsin kinase in different ligand states reveal key elements involved in G protein-coupled receptor kinase activation. J Biol Chem 283: 14053–14062, 2008. doi: 10.1074/jbc.M708974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen Q, Plasencia M, Li Z, Mukherjee S, Patra D, Chen CL, Klose T, Yao XQ, Kossiakoff AA, Chang L, Andrews PC, Tesmer JJG. Structures of rhodopsin in complex with G-protein-coupled receptor kinase 1. Nature 595: 600–605, 2021. doi: 10.1038/s41586-021-03721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Inglese J, Koch WJ, Caron MG, Lefkowitz RJ. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature 359: 147–150, 1992. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- 135. Hisatomi O, Matsuda S, Satoh T, Kotaka S, Imanishi Y, Tokunaga F. A novel subtype of G-protein-coupled receptor kinase, GRK7, in teleost cone photoreceptors. FEBS Lett 424: 159–164, 1998. doi: 10.1016/s0014-5793(98)00162-8. [DOI] [PubMed] [Google Scholar]
- 136. Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300: 1256–1262, 2003. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 137. Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science 310: 1686–1690, 2005. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 138. Touhara K, Inglese J, Pitcher JA, Shaw G, Lefkowitz RJ. Binding of G protein β γ-subunits to pleckstrin homology domains. J Biol Chem 269: 10217–10220, 1994. doi: 10.1016/S0021-9258(17)34048-6. [DOI] [PubMed] [Google Scholar]
- 139. Allen SJ, Parthasarathy G, Darke PL, Diehl RE, Ford RE, Hall DL, Johnson SA, Reid JC, Rickert KW, Shipman JM, Soisson SM, Zuck P, Munshi SK, Lumb KJ. Structure and function of the hypertension variant A486V of G protein-coupled receptor kinase 4. J Biol Chem 290: 20360–20373, 2015. doi: 10.1074/jbc.M115.648907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Komolov KE, Bhardwaj A, Benovic JL. Atomic structure of GRK5 reveals distinct structural features novel for G protein-coupled receptor kinases. J Biol Chem 290: 20629–20647, 2015. doi: 10.1074/jbc.M115.647297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Homan KT, Waldschmidt HV, Glukhova A, Cannavo A, Song J, Cheung JY, Koch WJ, Larsen SD, Tesmer JJG. Crystal structure of G protein-coupled receptor kinase 5 in complex with a rationally designed inhibitor. J Biol Chem 290: 20649–20659, 2015. doi: 10.1074/jbc.M115.647370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Komolov KE, Du Y, Duc NM, Betz RM, Rodrigues JPGLM, Leib RD, Patra D, Skiniotis G, Adams CM, Dror RO, Chung KY, Kobilka BK, Benovic JL. Structural and functional analysis of a β2-adrenergic receptor complex with GRK5. Cell 169: 407–421.e16, 2017. doi: 10.1016/j.cell.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. The structure of G protein-coupled receptor kinase (GRK)-6 defines a second lineage of GRKs. J Biol Chem 281: 16785–16793, 2006. doi: 10.1074/jbc.M601327200. [DOI] [PubMed] [Google Scholar]
- 144. Sibley DR, Peters JR, Nambi P, Caron MG, Lefkowitz RJ. Desensitization of turkey erythrocyte adenylate cyclase. β-adrenergic receptor phosphorylation is correlated with attenuation of adenylate cyclase activity. J Biol Chem 259: 9742–9749, 1984. doi: 10.1016/S0021-9258(17)42762-1. [DOI] [PubMed] [Google Scholar]
- 145. Sibley DR, Strasser RH, Caron MG, Lefkowitz RJ. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the β-adrenergic receptor. J Biol Chem 260: 3883–3886, 1985. [PubMed] [Google Scholar]
- 146. Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38: 289–319, 1998. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 147. Thomsen ARB, Plouffe B, Cahill TJ 3rd, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP, Huang L, Breton B, Heydenreich FM, Sunahara RK, Skiniotis G, Bouvier M, Lefkowitz RJ. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 166: 907–919, 2016. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Nguyen AH, Thomsen ARB, Cahill TJ 3rd, Huang R, Huang LY, Marcink T, Clarke OB, Heissel S, Masoudi A, Ben-Hail D, Samaan F, Dandey VP, Tan YZ, Hong C, Mahoney JP, Triest S, Little J, Chen X, Sunahara R, Steyaert J, Molina H, Yu Z, Des Georges A, Lefkowitz RJ. Structure of an endosomal signaling GPCR-G protein-β-arrestin megacomplex. Nat Struct Mol Biol 26: 1123–1131, 2019. doi: 10.1038/s41594-019-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bouvier M, Hausdorff WP, De Blasi A, O'Dowd BF, Kobilka BK, Caron MG, Lefkowitz RJ. Removal of phosphorylation sites from the β 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature 333: 370–373, 1988. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- 150. Gurevich VV, Gurevich EV. Extensive shape shifting underlies functional versatility of arrestins. Curr Opin Cell Biol 27: 1–9, 2014. doi: 10.1016/j.ceb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Homan KT, Glukhova A, Tesmer JJ. Regulation of G protein-coupled receptor kinases by phospholipids. Curr Med Chem 20: 39–46, 2013. doi: 10.2174/0929867311302010005. [DOI] [PubMed] [Google Scholar]
- 152. Kuhn H, Dreyer WJ. Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett 20: 1–6, 1972. doi: 10.1016/0014-5793(72)80002-4. [DOI] [PubMed] [Google Scholar]
- 153. Palczewski K, Buczyłko J, Kaplan MW, Polans AS, Crabb JW. Mechanism of rhodopsin kinase activation. J Biol Chem 266: 12949–12955, 1991. doi: 10.1016/S0021-9258(18)98787-9. [DOI] [PubMed] [Google Scholar]
- 154. Shi W, Osawa S, Dickerson CD, Weiss ER. Rhodopsin mutants discriminate sites important for the activation of rhodopsin kinase and Gt. J Biol Chem 270: 2112–2119, 1995. doi: 10.1074/jbc.270.5.2112. [DOI] [PubMed] [Google Scholar]
- 155. Liu P, Osawa S, Weiss ER. M opsin phosphorylation in intact mammalian retinas. J Neurochem 93: 135–144, 2005. doi: 10.1111/j.1471-4159.2004.03003.x. [DOI] [PubMed] [Google Scholar]
- 156. Benovic JL, Onorato J, Lohse MJ, Dohlman HG, Staniszewski C, Caron MG, Lefkowitz RJ. Synthetic peptides of the hamster β 2-adrenoceptor as substrates and inhibitors of the β-adrenoceptor kinase. Br J Clin Pharmacol 30, Suppl 1: 3S–12S, 1990. doi: 10.1111/j.1365-2125.1990.tb05462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Winstel R, Ihlenfeldt HG, Jung G, Krasel C, Lohse MJ. Peptide inhibitors of G protein-coupled receptor kinases. Biochem Pharmacol 70: 1001–1008, 2005. doi: 10.1016/j.bcp.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 158. Pao CS, Benovic JL. Structure/function analysis of α2A-adrenergic receptor interaction with G protein-coupled receptor kinase 2. J Biol Chem 280: 11052–11058, 2005. doi: 10.1074/jbc.M412996200. [DOI] [PubMed] [Google Scholar]
- 159. Dhami GK, Babwah AV, Sterne-Marr R, Ferguson SS. Phosphorylation-independent regulation of metabotropic glutamate receptor 1 signaling requires g protein-coupled receptor kinase 2 binding to the second intracellular loop. J Biol Chem 280: 24420–24427, 2005. doi: 10.1074/jbc.M501650200. [DOI] [PubMed] [Google Scholar]
- 160. Koch WJ, Inglese J, Stone WC, Lefkowitz RJ. The binding site for the βγ subunits of heterotrimeric G proteins on the β-adrenergic receptor kinase. J Biol Chem 268: 8256–8260, 1993. doi: 10.1016/S0021-9258(18)53090-8. [DOI] [PubMed] [Google Scholar]
- 161. Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G βγ-mediated signaling. J Biol Chem 269: 6193–6197, 1994. doi: 10.1016/S0021-9258(17)37587-7. [DOI] [PubMed] [Google Scholar]
- 162. Day PW, Carman CV, Sterne-Marr R, Benovic JL, Wedegaertner PB. Differential interaction of GRK2 with members of the Gα q family. Biochemistry 42: 9176–9184, 2003. doi: 10.1021/bi034442+. [DOI] [PubMed] [Google Scholar]
- 163. Carman CV, Parent JL, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T. Selective regulation of Gα(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem 274: 34483–34492, 1999. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]
- 164. Hildreth KL, Wu JH, Barak LS, Exum ST, Kim LK, Peppel K, Freedman NJ. Phosphorylation of the platelet-derived growth factor receptor-β by G protein-coupled receptor kinase-2 reduces receptor signaling and interaction with the Na+/H+ exchanger regulatory factor. J Biol Chem 279: 41775–41782, 2004. doi: 10.1074/jbc.M403274200. [DOI] [PubMed] [Google Scholar]
- 165. Arriza JL, Dawson TM, Simerly RB, Martin LJ, Caron MG, Snyder SH, Lefkowitz RJ. The G-protein-coupled receptor kinases β ARK1 and β ARK2 are widely distributed at synapses in rat brain. J Neurosci 12: 4045–4055, 1992. doi: 10.1523/JNEUROSCI.12-10-04045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem 272: 25425–25428, 1997. doi: 10.1074/jbc.272.41.25425. [DOI] [PubMed] [Google Scholar]
- 167. Schleicher S, Boekhoff I, Arriza J, Lefkowitz RJ, Breer H. A β-adrenergic receptor kinase-like enzyme is involved in olfactory signal termination. Proc Natl Acad Sci USA 90: 1420–1424, 1993. doi: 10.1073/pnas.90.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Li W, Ai N, Wang S, Bhattacharya N, Vrbanac V, Collins M, Signoretti S, Hu Y, Boyce FM, Gravdal K, Halvorsen OJ, Nalwoga H, Akslen LA, Harlow E, Watnick RS. GRK3 is essential for metastatic cells and promotes prostate tumor progression. Proc Natl Acad Sci USA 111: 1521–1526, 2014. doi: 10.1073/pnas.1320638111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, Baleux F, Arenzana-Seisdedos F, Bachelerie F. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest 118: 1074–1084, 2008. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Rankin ML, Marinec PS, Cabrera DM, Wang Z, Jose PA, Sibley DR. The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol 69: 759–769, 2006. doi: 10.1124/mol.105.019901. [DOI] [PubMed] [Google Scholar]
- 171. Gildea JJ, Israel JA, Johnson AK, Zhang J, Jose PA, Felder RA. Caveolin-1 and dopamine-mediated internalization of NaKATPase in human renal proximal tubule cells. Hypertension 54: 1070–1076, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gold JI, Martini JS, Hullmann J, Gao E, Chuprun JK, Lee L, Tilley DG, Rabinowitz JE, Bossuyt J, Bers DM, Koch WJ. Nuclear translocation of cardiac G protein-coupled receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PLoS One 8: e57324, 2013. doi: 10.1371/journal.pone.0057324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hullmann J, Traynham CJ, Coleman RC, Koch WJ. The expanding GRK interactome: implications in cardiovascular disease and potential for therapeutic development. Pharmacol Res 110: 52–64, 2016. doi: 10.1016/j.phrs.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Martini JS, Raake P, Vinge LE, DeGeorge BR, DeGeorge B, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci USA 105: 12457–12462, 2008. [Erratum in Proc Natl Acad Sci USA 105: 17206, 2008]. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wu JH, Goswami R, Cai X, Exum ST, Huang X, Zhang L, Brian L, Premont RT, Peppel K, Freedman NJ. Regulation of the platelet-derived growth factor receptor-β by G protein-coupled receptor kinase-5 in vascular smooth muscle cells involves the phosphatase Shp2. J Biol Chem 281: 37758–37772, 2006. doi: 10.1074/jbc.M605756200. [DOI] [PubMed] [Google Scholar]
- 176. Zheng H, Worrall C, Shen H, Issad T, Seregard S, Girnita A, Girnita L. Selective recruitment of G protein-coupled receptor kinases (GRKs) controls signaling of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA 109: 7055–7060, 2012. doi: 10.1073/pnas.1118359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chakraborty PK, Zhang Y, Coomes AS, Kim WJ, Stupay R, Lynch LD, Atkinson T, Kim JI, Nie Z, Daaka Y. G protein-coupled receptor kinase GRK5 phosphorylates moesin and regulates metastasis in prostate cancer. Cancer Res 74: 3489–3500, 2014. doi: 10.1158/0008-5472.CAN-13-2708. [DOI] [PubMed] [Google Scholar]
- 178. Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, Spertus JA, Koch WJ, Kardia SL, Dorn GW 2nd.. A GRK5 polymorphism that inhibits β-adrenergic receptor signaling is protective in heart failure. Nat Med 14: 510–517, 2008. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Eijgelsheim M, Visser LE, Uitterlinden AG, Stricker BH. Protective effect of a GRK5 polymorphism on heart failure and its interaction with β-adrenergic receptor antagonists. Pharmacogenomics 9: 1551–1555, 2008. doi: 10.2217/14622416.9.10.1551. [DOI] [PubMed] [Google Scholar]
- 180. Stoffel RH, Randall RR, Premont RT, Lefkowitz RJ, Inglese J. Palmitoylation of G protein-coupled receptor kinase, GRK6. Lipid modification diversity in the GRK family. J Biol Chem 269: 27791–27794, 1994. doi: 10.1016/S0021-9258(18)46852-4. [DOI] [PubMed] [Google Scholar]
- 181. Loudon RP, Benovic JL. Altered activity of palmitoylation-deficient and isoprenylated forms of the G protein-coupled receptor kinase GRK6. J Biol Chem 272: 27422–27427, 1997. doi: 10.1074/jbc.272.43.27422. [DOI] [PubMed] [Google Scholar]
- 182. Stoffel RH, Inglese J, Macrae AD, Lefkowitz RJ, Premont RT. Palmitoylation increases the kinase activity of the G protein-coupled receptor kinase, GRK6. Biochemistry 37: 16053–16059, 1998. doi: 10.1021/bi981432d. [DOI] [PubMed] [Google Scholar]
- 183. Chen M, Philipp M, Wang J, Premont RT, Garrison TR, Caron MG, Lefkowitz RJ, Chen W. G Protein-coupled receptor kinases phosphorylate LRP6 in the Wnt pathway. J Biol Chem 284: 35040–35048, 2009. doi: 10.1074/jbc.M109.047456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Hall RA, Spurney RF, Premont RT, Rahman N, Blitzer JT, Pitcher JA, Lefkowitz RJ. G protein-coupled receptor kinase 6A phosphorylates the Na+/H+ exchanger regulatory factor via a PDZ domain-mediated interaction. J Biol Chem 274: 24328–24334, 1999. doi: 10.1074/jbc.274.34.24328. [DOI] [PubMed] [Google Scholar]
- 185. Hendrickx JO, van Gastel J, Leysen H, Santos-Otte P, Premont RT, Martin B, Maudsley S. GRK5 – a functional bridge between cardiovascular and neurodegenerative disorders. Front Pharmacol 9: 1484, 2018. doi: 10.3389/fphar.2018.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Belmonte SL, Blaxall BC. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ Res 109: 309–319, 2011. doi: 10.1161/CIRCRESAHA.110.231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of β-adrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J Biol Chem 272: 17223–17229, 1997. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- 188. Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β 1-adrenergic receptors in the failing human heart. Circulation 87: 454–463, 1993. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 189. Rengo G, Pagano G, Filardi PP, Femminella GD, Parisi V, Cannavo A, Liccardo D, Komici K, Gambino G, D'Amico ML, de Lucia C, Paolillo S, Trimarco B, Vitale DF, Ferrara N, Koch WJ, Leosco D. Prognostic value of lymphocyte g protein-coupled receptor kinase-2 protein levels in patients with heart failure. Circ Res 118: 1116–1124, 2016. doi: 10.1161/CIRCRESAHA.115.308207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Leineweber K, Rohe P, Beilfuss A, Wolf C, Sporkmann H, Bruck H, Jakob HG, Heusch G, Philipp T, Brodde OE. G-protein-coupled receptor kinase activity in human heart failure: effects of β-adrenoceptor blockade. Cardiovasc Res 66: 512–519, 2005. doi: 10.1016/j.cardiores.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 191. Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of β-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA 93: 12974–12979, 1996. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem 273: 18180–18184, 1998. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 193. Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res 107: 1140–1149, 2010. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Huang ZM, Gao E, Fonseca FV, Hayashi H, Shang X, Hoffman NE, Chuprun JK, Tian X, Tilley DG, Madesh M, Lefer DJ, Stamler JS, Koch WJ. Convergence of G protein-coupled receptor and S-nitrosylation signaling determines the outcome to cardiac ischemic injury. Sci Signal 6: ra95, 2013. doi: 10.1126/scisignal.2004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW 2nd, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res 103: 413–422, 2008. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-βARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation 119: 89–98, 2009. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P, Muller OJ. AAV6.βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J 34: 1437–1447, 2013. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Tesmer VM, Lennarz S, Mayer G, Tesmer JJ. Molecular mechanism for inhibition of g protein-coupled receptor kinase 2 by a selective RNA aptamer. Structure 20: 1300–1309, 2012. doi: 10.1016/j.str.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tesmer JJ, Tesmer VM, Lodowski DT, Steinhagen H, Huber J. Structure of human G protein-coupled receptor kinase 2 in complex with the kinase inhibitor balanol. J Med Chem 53: 1867–1870, 2010. doi: 10.1021/jm9017515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Thal DM, Yeow RY, Schoenau C, Huber J, Tesmer JJ. Molecular mechanism of selectivity among G protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol 80: 294–303, 2011. doi: 10.1124/mol.111.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, Sklar LA, Koch WJ, Tesmer JJ. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol 7: 1830–1839, 2012. doi: 10.1021/cb3003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol Rev 95: 377–404, 2015. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Traynham CJ, Hullmann J, Koch WJ. Canonical and non-canonical actions of GRK5 in the heart". J Mol Cell Cardiol 92: 196–202, 2016. doi: 10.1016/j.yjmcc.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Gold JI, Gao E, Shang X, Premont RT, Koch WJ. Determining the absolute requirement of G protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: short communication. Circ Res 111: 1048–1053, 2012. doi: 10.1161/CIRCRESAHA.112.273367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron 24: 1029–1036, 1999. doi: 10.1016/S0896-6273(00)81048-X. [DOI] [PubMed] [Google Scholar]
- 206. Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-κB-dependent hypertrophic gene expression. Hypertension 56: 696–704, 2010. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
- 207. Chen CK, Burns ME, Spencer M, Niemi GA, Chen J, Hurley JB, Baylor DA, Simon MI. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci USA 96: 3718–3722, 1999. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Rinner O, Makhankov YV, Biehlmaier O, Neuhauss SC. Knockdown of cone-specific kinase GRK7 in larval zebrafish leads to impaired cone response recovery and delayed dark adaptation. Neuron 47: 231–242, 2005. doi: 10.1016/j.neuron.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 209. Vinge LE, von Lueder TG, Aasum E, Qvigstad E, Gravning JA, How OJ, Edvardsen T, Bjornerheim R, Ahmed MS, Mikkelsen BW, Oie E, Attramadal T, Skomedal T, Smiseth OA, Koch WJ, Larsen TS, Attramadal H. Cardiac-restricted expression of the carboxyl-terminal fragment of GRK3 uncovers distinct functions of GRK3 in regulation of cardiac contractility and growth: GRK3 controls cardiac α1-adrenergic receptor responsiveness. J Biol Chem 283: 10601–10610, 2008. doi: 10.1074/jbc.M708912200. [DOI] [PubMed] [Google Scholar]
- 210. Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 47: 1131–1139, 2006. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
- 211. Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38: 291–303, 2003. doi: 10.1016/S0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 212. Ahn S, Shenoy SK, Luttrell LM, Lefkowitz RJ. SnapShot: β-arrestin functions. Cell 182: 1362–1362.e1, 2020. doi: 10.1016/j.cell.2020.07.034. [DOI] [PubMed] [Google Scholar]
- 213. Kuhn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry 17: 4389–4395, 1978. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- 214. Gurevich VV, Hanson SM, Song X, Vishnivetskiy SA, Gurevich EV. The functional cycle of visual arrestins in photoreceptor cells. Prog Retin Eye Res 30: 405–430, 2011. doi: 10.1016/j.preteyeres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275: 17201–17210, 2000. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 216. Scott MG, Le Rouzic E, Périanin A, Pierotti V, Enslen H, Benichou S, Marullo S, Benmerah A. Differential nucleocytoplasmic shuttling of β-arrestins. Characterization of a leucine-rich nuclear export signal in β-arrestin2. J Biol Chem 277: 37693–37701, 2002. doi: 10.1074/jbc.M207552200. [DOI] [PubMed] [Google Scholar]
- 217. Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of β-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure 9: 869–880, 2001. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 218. Gurevich VV, Chen CY, Kim CM, Benovic JL. Visual arrestin binding to rhodopsin. Intramolecular interaction between the basic N terminus and acidic C terminus of arrestin may regulate binding selectivity. J Biol Chem 269: 8721–8727, 1994. doi: 10.1016/S0021-9258(17)37028-X. [DOI] [PubMed] [Google Scholar]
- 219. Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 A crystal structure of visual arrestin: a model for arrestin's regulation. Cell 97: 257–269, 1999. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 220. Gurevich VV, Benovic JL. Visual arrestin binding to rhodopsin. Diverse functional roles of positively charged residues within the phosphorylation-recognition region of arrestin. J Biol Chem 270: 6010–6016, 1995. doi: 10.1074/jbc.270.11.6010. [DOI] [PubMed] [Google Scholar]
- 221. Kim YJ, Hofmann KP, Ernst OP, Scheerer P, Choe HW, Sommer ME. Crystal structure of pre-activated arrestin p44. Nature 497: 142–146, 2013. doi: 10.1038/nature12133. [DOI] [PubMed] [Google Scholar]
- 222. Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, Paduch M, Tripathi-Shukla P, Koide A, Koide S, Weis WI, Kossiakoff AA, Kobilka BK, Lefkowitz RJ. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497: 137–141, 2013. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Tian X, Kang DS, Benovic JL. β-Arrestins and G protein-coupled receptor trafficking. Handb Exp Pharmacol 219: 173–186, 2014. doi: 10.1007/978-3-642-41199-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283: 655–661, 1999. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 225. Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther 110: 465–502, 2006. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Chen Q, Perry NA, Vishnivetskiy SA, Berndt S, Gilbert NC, Zhuo Y, Singh PK, Tholen J, Ohi MD, Gurevich EV, Brautigam CA, Klug CS, Gurevich VV, Iverson TM. Structural basis of arrestin-3 activation and signaling. Nat Commun 8: 1427, 2017. doi: 10.1038/s41467-017-01218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Granzin J, Stadler A, Cousin A, Schlesinger R, Batra-Safferling R. Structural evidence for the role of polar core residue Arg175 in arrestin activation. Sci Rep 5: 15808, 2015. doi: 10.1038/srep15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hanson SM, Francis DJ, Vishnivetskiy SA, Kolobova EA, Hubbell WL, Klug CS, Gurevich VV. Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci USA 103: 4900–4905, 2006. doi: 10.1073/pnas.0600733103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, Qian J, Li S, Blanc A, Oleskie AN, Dosey AM, Su M, Liang CR, Gu LL, Shan JM, Chen X, Hanna R, Choi M, Yao XJ, Klink BU, Kahsai AW, Sidhu SS, Koide S, Penczek PA, Kossiakoff AA, Woods VL Jr, Kobilka BK, Skiniotis G, Lefkowitz RJ. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512: 218–222, 2014. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Staus DP, Hu H, Robertson MJ, Kleinhenz ALW, Wingler LM, Capel WD, Latorraca NR, Lefkowitz RJ, Skiniotis G. Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature 579: 297–302, 2020. doi: 10.1038/s41586-020-1954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Huang W, Masureel M, Qu Q, Janetzko J, Inoue A, Kato HE, Robertson MJ, Nguyen KC, Glenn JS, Skiniotis G, Kobilka BK. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579: 303–308, 2020. doi: 10.1038/s41586-020-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Heitzler D, Durand G, Gallay N, Rizk A, Ahn S, Kim J, Violin JD, Dupuy L, Gauthier C, Piketty V, Crépieux P, Poupon A, Clément F, Fages F, Lefkowitz RJ, Reiter E. Competing G protein-coupled receptor kinases balance G protein and β-arrestin signaling. Mol Syst Biol 8: 590, 2012. doi: 10.1038/msb.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Ferguson SS, Downey WE 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271: 363–366, 1996. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 234. Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science 308: 512–517, 2005. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 235. Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA 102: 1442–1447, 2005. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal 4: ra51, 2011. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Latorraca NR, Wang JK, Bauer B, Townshend RJL, Hollingsworth SA, Olivieri JE, Xu HE, Sommer ME, Dror RO. Molecular mechanism of GPCR-mediated arrestin activation. Nature 557: 452–456, 2018. doi: 10.1038/s41586-018-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lee MH, Appleton KM, Strungs EG, Kwon JY, Morinelli TA, Peterson YK, Laporte SA, Luttrell LM. The conformational signature of β-arrestin2 predicts its trafficking and signalling functions. Nature 531: 665–668, 2016. doi: 10.1038/nature17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Pfeiffer CT, Wang J, Paulo JA, Jiang X, Gygi SP, Rockman HA. Mapping angiotensin II type 1 receptor-biased signaling using proximity labeling and proteomics identifies diverse actions of biased agonists. J Proteome Res 20: 3256–3267, 2021. doi: 10.1021/acs.jproteome.1c00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Abraham DM, Davis RT 3rd, Warren CM, Mao L, Wolska BM, Solaro RJ, Rockman HA. β-Arrestin mediates the Frank-Starling mechanism of cardiac contractility. Proc Natl Acad Sci USA 113: 14426–14431, 2016. doi: 10.1073/pnas.1609308113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Yang F, Yu X, Liu C, Qu CX, Gong Z, Liu HD, Li FH, Wang HM, He DF, Yi F, Song C, Tian CL, Xiao KH, Wang JY, Sun JP. Phospho-selective mechanisms of arrestin conformations and functions revealed by unnatural amino acid incorporation and (19)F-NMR. Nat Commun 6: 8202, 2015. doi: 10.1038/ncomms9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. He QT, Xiao P, Huang SM, Jia YL, Zhu ZL, Lin JY, Yang F, Tao XN, Zhao RJ, Gao FY, Niu XG, Xiao KH, Wang J, Jin C, Sun JP, Yu X. Structural studies of phosphorylation-dependent interactions between the V2R receptor and arrestin-2. Nat Commun 12: 2396, 2021. doi: 10.1038/s41467-021-22731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of β-arrestin and arrestin in the β 2-adrenergic receptor and rhodopsin systems. J Biol Chem 267: 8558–8564, 1992. doi: 10.1016/S0021-9258(18)42479-9. [DOI] [PubMed] [Google Scholar]
- 244. Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 153, Suppl 1: S379–S388, 2008. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to β 2-adrenergic receptors by β-arrestins. Science 298: 834–836, 2002. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 246. Ghosh E, Dwivedi H, Baidya M, Srivastava A, Kumari P, Stepniewski T, Kim HR, Lee MH, van Gastel J, Chaturvedi M, Roy D, Pandey S, Maharana J, Guixà-González R, Luttrell LM, Chung KY, Dutta S, Selent J, Shukla AK. Conformational sensors and domain swapping reveal structural and functional differences between β-arrestin isoforms. Cell Rep 28: 3287–3299, 2019. e3286. doi: 10.1016/j.celrep.2019.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Gurevich VV, Gurevich EV. The new face of active receptor bound arrestin attracts new partners. Structure 11: 1037–1042, 2003. doi: 10.1016/s0969-2126(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 248. Kang DS, Kern RC, Puthenveedu MA, von Zastrow M, Williams JC, Benovic JL. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J Biol Chem 284: 29860–29872, 2009. doi: 10.1074/jbc.M109.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69: 451–482, 2007. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 250. Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The β2-adrenergic receptor/βarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA 96: 3712–3717, 1999. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ. β-Arrestin-mediated ADP-ribosylation factor 6 activation and β 2-adrenergic receptor endocytosis. J Biol Chem 276: 42509–42513, 2001. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- 252. McDonald PH, Cote NL, Lin FT, Premont RT, Pitcher JA, Lefkowitz RJ. Identification of NSF as a β-arrestin1-binding protein. Implications for β2-adrenergic receptor regulation. J Biol Chem 274: 10677–10680, 1999. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- 253. Cahill TJ 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai AW, Bassoni DL, Gavino BJ, Lamerdin JE, Triest S, Shukla AK, Berger B, Little J, Antar A, Blanc A, Qu CX, Chen X, Kawakami K, Inoue A, Aoki J, Steyaert J, Sun JP, Bouvier M, Skiniotis G, Lefkowitz RJ. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci SA 114: 2562–2567, 2017. doi: 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6: a022616, 2014. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Pavlos NJ, Friedman PA. GPCR signaling and trafficking: the long and short of it. Trends Endocrinol Metab 28: 213–226, 2017. doi: 10.1016/j.tem.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Miller WE, Maudsley S, Ahn S, Khan KD, Luttrell LM, Lefkowitz RJ. β-Arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC. Role of β-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J Biol Chem 275: 11312–11319, 2000. doi: 10.1074/jbc.275.15.11312. [DOI] [PubMed] [Google Scholar]
- 257. Yang F, Xiao P, Qu CX, Liu Q, Wang LY, Liu ZX, He QT, Liu C, Xu JY, Li RR, Li MJ, Li Q, Guo XZ, Yang ZY, He DF, Yi F, Ruan K, Shen YM, Yu X, Sun JP, Wang J. Allosteric mechanisms underlie GPCR signaling to SH3-domain proteins through arrestin. Nat Chem Biol 14: 876–886, 2018. doi: 10.1038/s41589-018-0115-3. [DOI] [PubMed] [Google Scholar]
- 258. Pakharukova N, Masoudi A, Pani B, Staus DP, Lefkowitz RJ. Allosteric activation of proto-oncogene kinase Src by GPCR-β-arrestin complexes. J Biol Chem 295: 16773–16784, 2020. doi: 10.1074/jbc.RA120.015400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148: 1267–1281, 2000. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. β-Arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 117: 2445–2458, 2007. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 379: 557–560, 1996. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 262. Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. β-Blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA 105: 14555–14560, 2008. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem 281: 1261–1273, 2006. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 264. Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc Natl Acad Sci USA 98: 2449–2454, 2001. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279: 35518–35525, 2004. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 266. Sun Y, Cheng Z, Ma L, Pei G. β-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem 277: 49212–49219, 2002. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 267. Hunton DL, Barnes WG, Kim J, Ren XR, Violin JD, Reiter E, Milligan G, Patel DD, Lefkowitz RJ. β-arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol Pharmacol 67: 1229–1236, 2005. doi: 10.1124/mol.104.006270. [DOI] [PubMed] [Google Scholar]
- 268. Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med 15: 75–83, 2009. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 269. Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem 284: 8855–8865, 2009. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252, 2000. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 271. McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. β-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290: 1574–1577, 2000. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 272. Park JY, Qu CX, Li RR, Yang F, Yu X, Tian ZM, Shen YM, Cai BY, Yun Y, Sun JP, Chung KY. Structural mechanism of the arrestin-3/JNK3 interaction. Structure 27: 1162–1170, 2019. e1163. doi: 10.1016/j.str.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 273. Perry NA, Kaoud TS, Ortega OO, Kaya AI, Marcus DJ, Pleinis JM, Berndt S, Chen Q, Zhan X, Dalby KN, Lopez CF, Iverson TM, Gurevich VV. Arrestin-3 scaffolding of the JNK3 cascade suggests a mechanism for signal amplification. Proc Natl Acad Sci USA 116: 810–815, 2019. doi: 10.1073/pnas.1819230116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Eiger DS, Pham U, Gardner J, Hicks C, Rajagopal S. GPCR systems pharmacology: a different perspective on the development of biased therapeutics. Am J Physiol Cell Physiol 322: C887–C895, 2022. doi: 10.1152/ajpcell.00449.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 9: 373–386, 2010. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Wang J, Gareri C, Rockman HA. G-protein-coupled receptors in heart disease. Circ Res 123: 716–735, 2018. [Erratum in Circ Res 123: e34, 2018]. doi: 10.1161/CIRCRESAHA.118.311403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA 105: 9988–9993, 2008. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Wingler LM, Elgeti M, Hilger D, Latorraca NR, Lerch MT, Staus DP, Dror RO, Kobilka BK, Hubbell WL, Lefkowitz RJ. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell 176: 468–478.e11, 2019. doi: 10.1016/j.cell.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Strachan RT, Sun JP, Rominger DH, Violin JD, Ahn S, Rojas Bie Thomsen A, Zhu X, Kleist A, Costa T, Lefkowitz RJ. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR). J Biol Chem 289: 14211–14224, 2014. doi: 10.1074/jbc.M114.548131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100: 10782–10787, 2003. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Wang J, Hanada K, Staus DP, Makara MA, Dahal GR, Chen Q, Ahles A, Engelhardt S, Rockman HA. Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat Commun 8: 1706, 2017. doi: 10.1038/s41467-017-01855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA 104: 16657–16662, 2007. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci 28: 366–373, 2007. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 284. Christopoulos A, Changeux JP, Catterall WA, Fabbro D, Burris TP, Cidlowski JA, Olsen RW, Peters JA, Neubig RR, Pin JP, Sexton PM, Kenakin TP, Ehlert FJ, Spedding M, Langmead CJ. International Union of Basic and Clinical Pharmacology. XC. multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol Rev 66: 918–947, 2014. doi: 10.1124/pr.114.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Changeux JP, Christopoulos A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Cell 166: 1084–1102, 2016. doi: 10.1016/j.cell.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 286. Pani B, Ahn S, Rambarat PK, Vege S, Kahsai AW, Liu A, Valan BN, Staus DP, Costa T, Lefkowitz RJ. Unique positive cooperativity between the β-arrestin-biased β-blocker carvedilol and a small molecule positive allosteric modulator of the β2-adrenergic receptor. Mol Pharmacol 100: 513–525, 2021. [Erratum in Mol Pharmacol 100: 597, 2021]. doi: 10.1124/molpharm.121.000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Wang J, Pani B, Gokhan I, Xiong X, Kahsai AW, Jiang H, Ahn S, Lefkowitz RJ, Rockman HA. β-Arrestin-biased allosteric modulator potentiates carvedilol-stimulated β adrenergic receptor cardioprotection. Mol Pharmacol 100: 568–579, 2021. doi: 10.1124/molpharm.121.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of β-arrestins and β-adrenergic receptor kinases in the failing human heart. Circ Res 74: 206–213, 1994. doi: 10.1161/01.RES.74.2.206. [DOI] [PubMed] [Google Scholar]
- 289. Bathgate-Siryk A, Dabul S, Pandya K, Walklett K, Rengo G, Cannavo A, De Lucia C, Liccardo D, Gao E, Leosco D, Koch WJ, Lymperopoulos A. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension 63: 404–412, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Watari K, Nakaya M, Nishida M, Kim KM, Kurose H. β-Arrestin2 in infiltrated macrophages inhibits excessive inflammation after myocardial infarction. PLoS One 8: e68351, 2013. doi: 10.1371/journal.pone.0068351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. McCrink KA, Maning J, Vu A, Jafferjee M, Marrero C, Brill A, Bathgate-Siryk A, Dabul S, Koch WJ, Lymperopoulos A. β-Arrestin2 improves post-myocardial infarction heart failure via sarco(endo)plasmic reticulum Ca2+-ATPase-dependent positive inotropy in cardiomyocytes. Hypertension 70: 972–981, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09817. [DOI] [PubMed] [Google Scholar]
- 292. Capote AE, Batra A, Warren CM, Chowdhury SAK, Wolska BM, Solaro RJ, Rosas PC. B-arrestin-2 signaling is important to preserve cardiac function during aging. Front Physiol 12: 696852, 2021. doi: 10.3389/fphys.2021.696852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Kim KS, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol 303: H1001–H1010, 2012. doi: 10.1152/ajpheart.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Fernández-Arenas E, Calleja E, Martinez-Martin N, Gharbi SI, Navajas R, García-Medel N, Penela P, Alcamí A, Mayor F Jr, Albar JP, Alarcón B. β-Arrestin-1 mediates the TCR-triggered re-routing of distal receptors to the immunological synapse by a PKC-mediated mechanism. EMBO J 33: 559–577, 2014. doi: 10.1002/embj.201386022. [DOI] [PMC free article] [PubMed] [Google Scholar]