Abstract
Context
Michelia champaca L. (Magnoliaceae) has been known since ancient times for its rich medicinal properties.
Objective
The ethanol extract of Michelia champaca leaves (EEMC) was evaluated on depression and anxiety using in vivo and in silico studies
Materials and methods
Swiss albino mice were divided into control, standard, 100 and 200 mg/kg b.w. EEMC groups and for drug administration using oral gavage. The antidepressant activity was evaluated using forced swim test (FST) and tail suspension test (TST) whereas the anxiolytic activity through elevated plus maze and light and dark tests. The in silico studies included molecular docking against human potassium channel KCSA-FAB and human serotonin transporter, and ADME/T analysis.
Results
Open arm duration and entries were comparable between 200 mg/kg b.w. group (184.45 ± 1.00 s and 6.25 ± 1.11, respectively) and that of diazepam treated group (180.02 s ± 0.40 and 6.10 ± 0.05, respectively). Time spent in the light cubicle was higher (46.86 ± 0.03%), similar to that of diazepam (44.33 ± 0.64%), suggesting its potent anxiolytic activity. A delayed onset of immobility and lowered immobility time was seen at both the treatment doses (FST: 93.7 ± 1.70 and 89.1 ± 0.40 s; TST: 35.05 ± 2.75 and 38.50 ± 4.10 s) and the standard drug imipramine (FST: 72.7 ± 3.72 and TST: 30.01 ± 2.99 s), indicative of its antidepressant ability. In silico studies predicted doripenem to induce anxiolytic and antidepressant activity by inhibiting human potassium channel KCSA-FAB and human serotonin transporter proteins, respectively.
Conclusions
EEMC is a rich source of bioactive compounds with strong antidepressant and anxiolytic properties.
Keywords: Antidepressant activity, anxiolytic activity, Magnoliaceae family, forced swim test, tail suspension test, molecular docking studies
Introduction
Depression is a condition caused by a persistent sadness and lack of interest to carry out routine activities. A person is considered clinically depressed if he shows a persistent feeling of sadness and lack of interest for at least a period of 2 weeks or more. Lower mood, difficulties in thinking, loss of interest, and disruption in the normal routine such as disrupted sleep, loss of energy, and change in sex desire are all common symptoms of depression (Porter and Meldrum 2009). Characteristics such as loss of appetite, weight, sleep disturbances, psychomotor activity, decreased energy, feeling of worthlessness, guilty and suicidal ideation are commonly seen (Fauci et al. 2008). Major depression is estimated to be around 5% among the general population while a female to male ratio prevails at 5:2. The World Health Organization (WHO) reported that about 450 million people exhibited certain amount of mental or behavioural disorder, which is often left undetected or untreated with the fear of the societal circumstances (Tsegay et al. 2020).
Anxiety is also a mental condition leading to a state of extreme trepidation and uncertainty as a result of anticipation of a future threat. When anxiety and depression coexist, it predicts poor outcomes and a higher rate of treatment resistance than when the two disorders occur separately. The presence of anxiety and depression together complicates diagnosis and makes treatment difficult. Individuals suffering from coronary heart diseases, diabetes and stroke have an increased risk of developing anxiety and depression at some point in their life. In addition, there is an increased risk of suicide rate among the depressed patients (Bhatt et al. 2020). Drugs that target the levels of monoamines, namely, noradrenaline, dopamine and serotonin are generally used to treat this condition (Reddy 2010). However, their success rate is only up to 60% and requires prolonged administration for visible improvement in the signs and symptoms. They are always associated with numerous side effects owing to their effects on the brain and neurotransmission (Kumar et al. 2004; Jawaid et al. 2011). Advancements in research have identified some of the newer drugs that are associated with fewer side effects. However, fewer than half of those affected in the world do not receive such treatments mainly due to barriers such as lack of resources, lack of trained health-care providers, and social stigma associated with mental disorders. Therefore, it becomes highly necessary to look for naturally occurring alternatives from plant sources with proven advantage and favourable benefit to risk ratio.
In addition, major depressive disorder is caused by one or more of the following namely, genetic predisposition, unregulated monoamine synthesis and regulation as well as altered brain structure (Hasler 2010). Even the environmental and socioeconomic factors contribute significantly to the development of clinical depression. Studies that have incorporated targeted treatment for the regulation of monoamine production and functions (such as tricyclic antidepressants, serotonin reuptake inhibitors) have fared better in the treatment of depression, suggesting its pivotal role in the development of depression (Fedoce et al. 2018; Greaney et al. 2019; Bhatt et al. 2020).
In this regard, plants have been a valuable source of natural products for maintaining human health for many years. Michelia champaca L. (Magnoliaceae) is a folk-medicinal plant well known for its antioxidant and antidiabetic properties. It has traditionally been used to treat diarrhoea, cough, bronchitis, hypertension, dyspepsia, fever, rheumatism, abscesses, dysmenorrhoea and inflammation. While the dried root and bark of M. champaca is used as purgative, expectorant, cardio tonic, digestive, carminative, stimulant, the flower of M. champaca is used as diuretic, diaphoretic, antipyretic, the leaves help as a colic aid and the bark is used as an astringent agent. Several studies report its antipyretic, anti-inflammatory antioxidant antimicrobial, cytotoxic, antidiabetic and analgesic activities (Nickavar et al. 2006; Hossain et al. 2009; Taprial 2015). M. champaca contains various bioactive constituents including alkaloids, palmitic acid, oleic acid, carbonyl acid and esters, oliveroline, lysicamine, nornuciferine, cyperone, ficaprenolepi-yangambin, pheophytin, aristophyll, michephyll and formylanonaine, which is likely the reason for these innumerable medicinal properties (Hoffmann et al. 1977; Khan et al. 2002; Jarald et al. 2008; Wang et al. 2010; Chandra et al. 2015).
Disease conditions such as inflammation bring about oxidative stress, which is characterized by an imbalance in the regulation of reactive oxygen species (ROS) and inability of the body to detoxify the generated redox intermediates. Plant-based medicines have an added advantage mainly by countering these ROS productions and in turn reduce inflammation caused by their action (Halliwell and Whiteman 2004; Patil, Shirahatti, et al. 2021). Various studies have reported the use of root, bark and flowers of Michelia champaca for other activities (Swantara et al. 2020). However, the perusal of literature reveals that, so far, no attempt is made to study the role of M. champaca leaves in relation to its anxiolytic and anti-depressant properties and therefore an attempt was made to elucidate its effects in depression and anxiety.
Materials and methods
Animal model
Swiss albino mice of either sex weighing 25–30 kg were procured from the Central Animal House, JSS Medical College, Mysuru, India. The animals were maintained under ambient temperature (25 °C) and humidity (45–55%). All the animals were given free access to food and water with a natural day and night cycle. The experiments were carried out after obtaining clearance from the Institutional Animal Ethics Committee of JSS Medical College, Mysuru, Karnataka, India dated 18 March 2019, proposal no. 260-2017. Table 1 provides a detailed description of the various inclusion and exclusion criteria to employ animals for the experiment.
Table 1.
Criteria for the selection of animals for the study.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Healthy Swiss Albino mice of either sex Weight: 25–30 g Age: 3–4 months Healthy mice with normal behaviour and activity. |
Unhealthy animal Obese animal Pregnant animal Animals previously used in other experiments |
Chemicals
Imipramine, diazepam (Sisco Research Laboratories, Mumbai, India) were used as a standard drug for antidepressant and anxiolytic activity. Saline and ethanol Merck (Mumbai, India) were used for plant material preparation.
Instruments
Glass cylinder (25 × 12 × 25 cm), metal lever and stop watch, oral gavage, glass tubes, sterile tuberculin and insulin syringes, glass beakers, cotton and glass rod stirrer.
Plant material and preparation of drug solution
Fresh leaves of M. champaca were collected in the month of October 2018 from Honnuru village, Mysore, Karnataka, India. The leaves were authenticated by the taxonomist (Prof. Siddaramaiah) working at the Department of Horticulture, Government of Karnataka, Mysore, India and a voucher specimen was submitted in the herbarium at the same department with a specimen number 66786 MYS. The leaves that were initially washed with 70% alcohol were then shade-dried and powdered coarsely. Further, about 500 g of this powder was mixed with ethanol and subjected to Soxhlet extraction under room temperature for a period of 24 h. This was further subjected to vacuum evaporation for the removal of the solvent. The concentrated ethanol extract of the leaves of Michelia champaca (EEMC) was used for further experiments to evaluate the antidepressant activity. For the study, a stock solution was prepared by dissolving EEMC in saline, which was then adjusted to various concentrations using saline. The experimental procedure was carried out with two dosing schedules.
High-resolution liquid chromatography and mass spectrometry analysis
EEMC was analysed using high-resolution liquid chromatography and mass spectrometry (HR-LCMS) at the advanced analytical instrument facility (SAIF), IIT-Bombay, Mumbai, India. Chemical fingerprints of the selected medicinal plant extracts were prepared by Agilent HR-LCMS model-G6550A with 0.01% mass resolution. The acquisition method was set to be MS – minimum range 150 (m/z) and maximum 1000 Da (m/z), with the scanning rate each spectrum per second. Liquid chromatography was maintained at 40 °C, with HIP sampler (1000 µL/min) and binary sampler (0.300 mL/min). Chromatographic separations were performed on a column (100 mm × 1.0 mm, particle size 1.8 μm; Waters, Milford, MA), where 10 μL of sample was injected with flush out factor 5.0 s. The solvent system used for HR-LCMS was 100% water in pump A, and 100% acetonitrile in pump B. The SAIF (IIT-Bombay, Mumbai, India) database, which has over 62,000 patterns was used to identify components in the extract and analyse them using mass spectrometry HR-LCMS. The unknown component's spectrum was compared with that of the known components recorded in the SAIF library.
Acute toxicity evaluation
The study animals were taken in two phases. In the first phase, nine mice divided into three groups were administered doses of 10, 100 and 1000 mg/kg of EEMC and monitored for seven days in order to evaluate acute toxicity. Further, in the second phase, three animals were treated with 1200, 1500 and 2000 mg/kg body weight and monitored for a week to check for behavioural changes, if any.
Experimental design
A total of 48 mice were used for this study and divided into four different groups for each experimental model. Each group consisted of six animals.
Antidepressant activity (24 mice)
Group I: Control treated with normal saline (10 mL/kg).
Group II: Standard drug for antidepressant activity – imipramine (15 mg/kg).
Group III: Ethanol extract M. champaca leaves (EEMC-100 mg/kg).
Group IV: Ethanol extract M. champaca leaves (EEMC-200 mg/kg).
Anxiolytic activity (24 mice)
Group V: Control treated with saline.
Group VI: Standard drug for anxiolytic activity – diazepam (5 mg/kg, b.w.; i.p.).
Group VII: Ethanol extract M. champaca leaves (EEMC-100 mg/kg).
Group VIII: Ethanol extract M. champaca leaves (EEMC-200 mg/kg).
All the groups were treated with vehicle, test drug EEMC (100 and 200 mg/kg) and standard drug for a period of seven days. On the 7th day, after one hour of drug treatment, animals were screened for antidepressant and anxiolytic activities.
Anxiolytic activity
Elevated plus maze test
This is one of the widely accepted tests to assess anti-anxiety properties of drug molecules in rodent models as well as to assess the underlying mechanism (Walf and Frye 2007). The study was performed on 24 mice taken in four different groups of six animals each as stated previously. The study design consisted of two open arms (50 cm long and 10 cm wide) and two closed arms (50 cm long, 10 cm wide and 38 cm height) that were placed opposite to each other in a 10 × 10 platform. The animals were exposed to the maze for 5 min, which was followed by recording of the number of entries in open and closed arms and the duration spent in both the arms (Riaz and Khan 2014).
Light and dark test
The light and dark test was performed as per the method given by Gong et al. (2006). With a span of 30 min between the tests, the same animals used in the above assay were subjected to the light and dark test. The study design comprised of a dark safe cubicle and an aversive light cubicle with a dimension of 1/3 for dark and 2/3 for light compartment and an external size of 46 × 27 × 30 cm. The mice were initially placed at the midpoint of the light cubicle facing towards the light cubicle. The time each animal spends was recorded for 5 min and then returned to their home cage by holding them with their tail. Further, the maze was sanitized using 10% ethanol and dried between tests. Anxiety was recorded as high if the mice spent less than 50% of time in the light compartment.
Acute study: The drugs were administered as a single dose 1 h prior to the observation.
Chronic study: The drugs were administered for seven days continuously. On the 8th day morning, outcome observations were obtained.
Antidepressant activity
The antidepressant activity was evaluated using experimental models of depression namely, (i) forced swim test (FST) and (ii) tail suspension test (TST). Animals were weighed and appropriate dose of the drug was administered orally to different groups.
Forced swim test
The FST was performed according to the procedure given by Porsolt et al. (1977). This is a standard test for screening of the antidepressant activity. Briefly, a cylindrical container filled with water 20 cm depth was taken and the experimental mice were forced to swim in this water for a period of 6 min. Once the mice stopped its struggle and floated on water, it was considered immovable, which was taken as the end point in the study. The duration of immobility was assayed during the last 4 min after treatment.
Tail suspension test
The TST was performed according to the method of Steru et al. (1985). Mice were trapped 1 cm from its tail such that they were suspended 75 cm above the ground. This treatment was performed for 6 min and the mice were considered immovable when it refrained from demonstrating escape-oriented behaviour. The total immobility duration was assayed for the last 4 min.
In silico studies
Molecular docking and virtual screening
Crystallographic 3D structures of human potassium channel KCSA-FAB (PDB ID: 4UUJ) and human serotonin transporter (PDB ID: 516X) proteins were retrieved from Protein Data Bank RCSB PDB (https://www.rcsb.org/). Autodock Tools 1.5.6 was used for protein and ligand preparation according to the Autodock 4.0 methodology which uses Lamarckian Genetic Algorithm (LGA) (Patil, Martiz, Ramu, Shirahatti, Prakash, Chandra, et al. 2021). Proteins were cleaned by removing water and ions. Polar hydrogens were added and non-polar hydrogens were merged into their corresponding carbons for the appropriate treatment of electrostatics, subsequently leading to the protein stabilization. The protein structures were further processed with energy minimization by adding Kollman united and Gasteiger charges, prior to assigning AD4 atom type for all the atoms of the protein structures. Alternatively, 3D chemical structures of the 69 phytochemical compounds of M. champaca were obtained from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). For docking simulation with human potassium channel KCSA-FAB, diazepam was used as the reference ligand. Alternatively, imipramine was used in case of human serotonin transporter. Ligand preparation was done using Autodock Tools 1.5.6, where the energy minimization was done by applying Kollman united and Gasteiger charges. The torsion of the ligands was set to default, as predicted by the Autodock Tools 1.5.6 software. For the conversion of protein and ligand file formats, OpenBabel 2.3.1 (Patil, Martiz, Ramu, Shirahatti, Prakash, Kumar, et al. 2021) software was used.
Binding pockets of the proteins were positioned in their respective grid boxes. For human potassium channel KCSA-FAB, the grid box of the size 42 Å×42 Å×42 Å was prepared, and was centred at the coordinates x = 33.953646, y = −25.173089 and z = −0.221643. In case of human serotonin transporter, the grid box sized 30 Å×30 Å×30 Å was centred at the coordinates x = −32.605542 Å, y = −21.385708 Å and y = 1.852667 Å. Autodock Vina 1.1.2 was used to dock the ligands to the proteins (Trott and Olson 2010). Ligand molecules were allowed with 10 degrees of freedom. During the docking process, ligands were considered to be flexible, and the protein was assumed to be rigid. Out of the 10 binding poses generated, the first binding pose with no root mean square deviation (RMSD) of atomic positions was considered to be highly valid. They also possess the most negative binding affinity, which indicates stronger binding efficiency. Based on the binding affinity, total number of non-bonding interactions, and respective hydrogen bonds, the extent of protein–ligand interaction was analysed using Biovia Discovery Studio Visualizer 2021 (Kumar et al. 2021).
Drug-likeness and toxicity analysis
Drug-likeness (a parameter that describes how ‘druglike’ a given substance is) and toxicity factors are essential for evaluating the in silico drug pharmacological potential. Any experimental chemical being investigated as a prospective drug candidate should have a high safety margin, as well as an appropriate drug-likeness and toxicological profile. Therefore, using OSIRIS property explorer (http://www.cheminfo.org/Chemistry/Cheminformatics/Property_explorer) (Patil, Maruthi, et al. 2021), the drug-likeness and toxicological properties of the selected phytoconstituents from the previous virtual screening was evaluated. All the structures were submitted to the server in SMILES format.
Statistical analysis
Statistical comparisons between the normal and treatment groups were performed by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test using SPSS Software (version 21.0; Chicago, IL). Results were expressed as mean ± SE. The results were considered statistically significant if the p values were 0.05 or less.
Results
Phytochemical screening test
Freshly prepared EEMC was subjected to phytochemical screening tests for the detection of various active constituents. The extract showed the presence of alkaloids, tannins, steroids, phenols, flavonoids, carbohydrates and glycosides.
Furthermore, the HR-LCMS results revealed the presence of a wide array of compounds that could be indicated with several biological properties. The name and molecular weight of the test materials' components were determined during HR-LCMS (Tables 2a and 2b).
Table 2a.
Chemical profile of the EEMC of M. champaca by high-resolution liquid chromatography and mass spectrometry in + electron spray ionization mode.
| S. no. | Name of the compound | Ret. time | Mass | Molecular formula | m/z | Difference (ppm) |
|---|---|---|---|---|---|---|
| 1 | Methyldopa | 0.858 | 211.085 | C10H13NO4 | 212.09 | –2.43 |
| 2 | 3-Methyldioxyindole | 1.101 | 163.0632 | C9H9NO2 | 164.07 | 0.58 |
| 3 | Lentiginosine | 1.282 | 157.1103 | C8H15NO2 | 158.11 | –0.13 |
| 4 | 1-Dehydro-9-fluoro-11-oxotestololactone | 3.69 | 332.1395 | C19H21FO4 | 355.1285 | 8.81 |
| 5 | Memantine | 6.126 | 179.17 | C12H21N | 202.15 | –14.76 |
| 6 | Betaxolol | 7.586 | 307.215 | C18H29NO3 | 308.22 | –0.25 |
| 7 | 6-(1,2,3,4-Tetrahydro-6-methoxy-2-naphthyl)-2(1H)-pyridone | 8.281 | 255.1262 | C16H17NO2 | 256.1335 | –1.01 |
| 8 | 1-Phosphatidyl-1D-myoinositol 3-phosphate | 8.38 | 470.0252 | C11H20O16P2 | 493.016 | –5.44 |
| 9 | Dihydrodeoxystreptomycin | 8.962 | 567.2897 | C21H41N7O11 | 568.2967 | –5.82 |
| 10 | trans-1,4-bis(2-Chlorobenzaminomethyl)cyclohexane | 9.06 | 390.1604 | C22H28Cl2N2 | 391.1674 | 6.5 |
| 11 | Beclomethasone | 9.07 | 408.1709 | C22H29ClO5 | 409.1779 | –1.27 |
| 12 | Veraguensin | 9.075 | 372.1939 | C22H28O5 | 373.201 | –0.68 |
| 13 | Momilactone A | 9.093 | 314.191 | C20H26O3 | 337.1803 | –8.8 |
| 14 | 3,6-Dimethoxyestra-1,3,5(10),6,8-pentaene-17beta-carboxylic acid methyl ester | 9.1 | 354.1831 | C22H26O4 | 355.1906 | –0.05 |
| 15 | Exemestane | 9.106 | 296.1798 | C20H24O2 | 319.1692 | –7.24 |
| 16 | Longistylin C | 9.107 | 278.1675 | C20H22O | 279.1748 | –1.57 |
| 17 | 1-(beta-d-Ribofuranosyl)-1,4-dihydronicotinamide | 10.117 | 256.1047 | C11H16N2O5 | 279.0939 | 4.61 |
| 18 | Carbosulfan | 10.374 | 380.2132 | C20H32N2O3S | 403.2016 | 0.33 |
| 19 | Sodium glycocholate | 10.943 | 465.3094 | C26H43NO6 | 466.3165 | –0.87 |
| 20 | Sphinganine | 11.545 | 301.2982 | C18H39NO2 | 302.3056 | –0.4 |
| 21 | Mitoxantrone | 11.722 | 444.2055 | C22H28N4O6 | 445.2127 | –10.28 |
| 22 | Butyl 2-aminobenzoate | 11.821 | 193.1108 | C11H15NO2 | 194.118 | –2.56 |
| 23 | Azafrin | 12.826 | 426.278 | C27H38O4 | 427.2842 | –2.36 |
| 24 | Fluticasone propionate | 12.967 | 500.1854 | C25H31F3O5S | 501.1928 | –1.87 |
| 25 | 1-Octen-3-yl glucoside | 12.989 | 290.1707 | C14H26O6 | 313.1602 | 7.85 |
| 26 | Austinol | 12.989 | 458.1956 | C25H30O8 | 481.1856 | –3.44 |
| 27 | Trenbolone | 12.99 | 270.1628 | C18H22O2 | 293.1539 | –2.86 |
| 28 | Imazamethabenz | 12.995 | 274.1363 | C15H18N2O3 | 275.1432 | –16.49 |
| 29 | (E,E)-1,7-Diphenyl-4,6-heptadien-3-ol | 13.001 | 264.1516 | C19H20O | 265.1591 | –0.86 |
| 30 | Panaquinquecol 1 | 13.143 | 292.2044 | C18H28O3 | 293.2118 | –1.85 |
| 31 | Kanamycin | 13.279 | 484.2397 | C18H36N4O11 | 507.2289 | –3.33 |
| 32 | Dihydrospheroidene/methoxyneurosporene | 13.36 | 570.4661 | C41H62O | 308.2223 | 24.42 |
| 33 | (9Z,11E,13E,15Z)-4-Oxo-9,11,13,15-octadecatetraenoic acid | 13.569 | 290.1887 | C18H26O3 | 291.1959 | –1.73 |
| 34 | Alangimarckine | 13.571 | 475.2846 | C29H37N3O3 | 476.2914 | –2.28 |
| 35 | Diplodiatoxin | 13.572 | 308.1994 | C18H28O4 | 309.2066 | –2.15 |
| 36 | 3-Phenylpropyl propanoate | 13.606 | 192.115 | C12H16O2 | 193.1228 | 0.16 |
| 37 | Deoxytubulosine | 13.634 | 459.2889 | C29H37N3O2 | 460.2962 | –0.7 |
| 38 | MG(22:6(4Z,7Z,10Z,13Z,16Z,1 9Z)/0:0/0:0) | 14.249 | 402.2797 | C25H38O4 | 425.2684 | –6.7 |
| 39 | Vulgarone A | 14.321 | 218.1673 | C15H22O | 219.1746 | –1.26 |
| 40 | 19-Noretiocholanolone | 14.526 | 276.2094 | C18H28O2 | 277.2167 | –1.55 |
| 41 | Hydroxyprogesterone caproate | 14.585 | 428.2933 | C27H40O4 | 429.30 | –1.55 |
| 42 | 17-Hydroxylinolenic acid | 14.601 | 294.2198 | C18H30O3 | 295.2271 | –1.1 |
| 43 | Norethindrone enanthate | 14.783 | 410.2822 | C27H38O3 | 411.2893 | –0.23 |
| 44 | Hydroflumethiazide | 15.338 | 330.9958 | C8H8F3N3O4S2 | 353.986 | –15.09 |
| 45 | Lyngbyatoxin | 15.396 | 437.3053 | C27H39N3O2 | 460.2943 | –2.52 |
| 46 | Heteratisine | 15.403 | 391.2265 | C22H33NO5 | 392.2334 | 24.02 |
| 47 | Linoleoyl ethanolamide | 15.622 | 323.283 | C20H37NO2 | 324.2903 | –1.7 |
| 48 | Pheophorbide b | 16.269 | 606.2477 | C35H34N4O6 | 607.2556 | 0.22 |
| 49 | 7-O-Acetylaustroinulin | 16.771 | 364.2616 | C22H36O4 | 365.269 | –0.67 |
| 50 | Virol B | 16.846 | 262.1934 | C17H26O2 | 263.2006 | –0.28 |
| 51 | Harderoporphyrin | 17.164 | 608.2634 | C35H36N4O6 | 609.2708 | 0.17 |
| 52 | Pheophorbide a | 17.925 | 592.2685 | C35H36N4O5 | 593.2759 | 0.02 |
| 53 | Pyropheophorbide a | 18.492 | 534.2631 | C33H34N4O3 | 535.2701 | 0.01 |
| 54 | C16 Sphinganine | 10.248 | 273.2674 | C16H35NO2 | 274.2746 | –2.14 |
Table 2b.
Chemical profile of the EEMC of M. champaca by high-resolution liquid chromatography and mass spectrometry in – electron spray ionization mode.
| S. no. | Name of the compound | Ret. time | Mass | Molecular formula | m/z | Difference (ppm) |
|---|---|---|---|---|---|---|
| 1 | 2-(Methylthiomethyl)-3-phenyl-2-propenal | 1.089 | 192.0611 | C11H12OS | 191.0536 | –0.98 |
| 2 | l-Arabinose | 1.141 | 150.0509 | C5H10O5 | 149.0437 | 13.05 |
| 3 | Allamandin | 1.15 | 308.0883 | C15H16O7 | 307.081 | 4.18 |
| 4 | Diminazene | 4.728 | 281.1388 | C14H15N7 | 340.1524 | 0.47 |
| 5 | Poncirin | 5.668 | 594.1895 | C28H34O14 | 653.2034 | 8.97 |
| 6 | Forsythiaside | 5.806 | 624.2003 | C29H36O15 | 623.1927 | 8.18 |
| 7 | 17-beta-(Acetylthio)estra-1,3,5(10)-trien-3-ol acetate | 6.282 | 372.1757 | C22H28O3S | 371.1683 | 0.6 |
| 8 | Isorhamnetin 3-O-[b-d-glucopyranosyl-(1->2)-a-l-rhamnopyranoside] | 7.118 | 624.1642 | C28H32O16 | 623.1568 | 7.77 |
| 9 | Albanol A | 7.577 | 562.1641 | C34H26O8 | 607.1625 | –2.41 |
| 10 | 5′-Butyrylphosphoinosine | 7.616 | 418.0865 | C14H19N4O9P | 477.1001 | 5.99 |
| 11 | Salviaflaside methyl ester | 12.952 | 536.1545 | C25H28O13 | 535.1496 | –2.88 |
| 12 | Doripenem | 15.103 | 420.5109 | C15H24N4O6S2 | 419.1024 | 9.38 |
| 13 | cis-Resveratrol 4′-O-glucuronide | 15.927 | 404.113 | C20H20O9 | 403.1074 | –5.67 |
| 14 | Methyl (9Z)-10′-oxo-6,10′-diapo-6-carotenoate | 17.636 | 312.1723 | C20H24O3 | 311.1665 | 0.86 |
| 15 | Broussonin C | 18.023 | 312.173 | C20H24O3 | 311.1659 | –1.42 |
Acute toxicity study
The acute toxicity study determines the therapeutic safety of the pharmacologically active molecule and provides an insight into the lethal dose for the study animal model (Rajput and Khan 2017). This study demonstrated that EEMC was safe up to the dose of 2000 mg/kg body weight. Behaviour of the animals was evaluated continuously for 3 h and then at an interval of 4 h up to 48 h. Although mild behavioural changes were observed, the treatment did not cause mortality in any mice up to the concentration of 2000 mg/kg. The results of the LD50 study performed on mice were expressed using Karber’s method.
Anxiolytic activity
Elevated plus maze test
The anxiolytic effects of EEMC in comparison with that of diazepam as observed in the elevated plus maze test are given in Table 3. The study showed that the number of entries and time spent in open arms exhibited by EEMC treated group (6.25 ± 1.11 and 184.45 ± 1.00, respectively) were remarkably greater in comparison with that of the results seen in closed arm at both the doses. Yet, no significant change in the number of entries in closed arm was seen in case of 100 mg/kg treatment group. Incidentally, diazepam treatment at 1 mg/kg (6.10 ± 0.05 and 180.02 ± 0.40) exhibited similar effects. The findings suggest that the anxiolytic activity exerted by EEMC could be comparable with that of the standard drug.
Table 3.
Anxiolytic effects of M. champaca leaves extract and diazepam in elevated plus maze test.
| Groups | Open arms |
Closed arms |
||
|---|---|---|---|---|
| Number of entries | Time spent | Number of entries | Time spent | |
| Control | 3.05 ± 0.31a | 120.04 ± 0.64a | 5.00 ± 1.20b | 166.05 ± 0.56c |
| 100 mg/kg | 4.15 ± 0.76b | 150.78 ± 2.02b | 4.78 ± 1.05b | 127.48 ± 1.04b |
| 200 mg/kg | 6.25 ± 1.11c | 184.45 ± 1.00c | 3.78 ± 0.13a | 111.09 ± 0.99a |
| Diazepam 1 mg/kg | 6.10 ± 0.05c | 180.02 ± 0.40c | 4.85 ± 3.01b | 125.07 ± 0.88b |
Values are expressed as mean ± SE. Means in the same column with distinct superscripts are significantly different (p ≤ 0.05) as separated by Duncan's multiple range test (the different alphabets indicate the statistically significant difference between the values).
Light and dark test
As recorded in Table 4, the results of the present study demonstrate notable anxiolytic effects of EEMC assessed using light and dark boxes. The study revealed that there was a significant increase in the time spent in light cubicle at both the doses. However, the treatment using 200 mg/kg body weight (46.86 ± 0.03%) fared optimal among the two doses tested and was better than that exhibited by the standard drug diazepam (44.33 ± 0.64%).
Table 4.
Anxiolytic effects of M. champaca leaves extract and diazepam based on percentage of time spent in light cubicle.
| Groups | % time spent in light cubicle |
|---|---|
| Control | 25.08 ± 1.91a |
| 100 mg/kg | 42.15 ± 0.54b |
| 200 mg/kg | 46.86 ± 0.03b |
| Diazepam 1 mg/kg | 44.33 ± 0.64b |
Values are expressed as mean ± SE. Means in the same column with distinct superscripts are significantly different (p ≤ 0.05) as separated by Duncan's multiple range test (the different alphabets indicate the statistically significant difference between the values).
Anti-depression activity
Forced swim test
There was no significant difference in the duration of immobility among the different groups tested. A significant delay in the onset of immobility was observed in the EEMC (100 and 200 mg/kg body weight) treated groups along with significantly lowered the immobility time after seven days of treatment. In comparison with the control group which was 146.5 s, the EEMC treated groups at both the doses (93.7 ± 1.70 and 89.1 ± 0.40) fared significantly better and was comparable with that of the standard drug (72.7 ± 3.72) as shown in Table 5.
Table 5.
Effects of EEMC on immobility time in mouse using forced swim test (FST).
| Groups | Treatment | Immobility time (s) |
|
|---|---|---|---|
| 1st day | 7th day | ||
| I | Control group treated with normal saline (10 mL/kg) | 154.51 ± 3.87d | 146.55 ± 3.73c |
| II | Standard drug imipramine (15 mg/kg) | 96.90 ± 9.02a | 72.70 ± 3.72a |
| III | Ethanol extract M. champaca leaves (EEMC-100 mg/kg) | 144.25 ± 0.94c | 93.77 ± 1.70b |
| IV | Ethanol extract M. champaca leaves (EEMC-200 mg/kg) | 134.20 ± 1.07b | 89.12 ± 0.40b |
Values are expressed as mean ± SE. Means in the same column with distinct superscripts are significantly different (p ≤ 0.05) as separated by Duncan's multiple range test (the different alphabets indicate the statistically significant difference between the values).
Tail suspension test
In TST, on day 1, there were no significant differences in the durations of immobility among the different groups. On day 7, all animals of the test groups showed significant improvement in their response to depression. The EEMC extract (100 and 200 mg/kg body weight) treated groups exhibited significant delay in the onset of immobility and significantly reduced the time of immobility in the TST after seven days treatment, which was statistically significant and comparable with that of the control group in which the immobility time was 81.06 s. Comparison between groups I, II, III and IV was made and there was a significant reduction in the immobility time in comparison with that of the control (81.06 ± 3.24 s). However, the results of the standard drugs (35.05 ± 2.75 s) were significantly better (38.50 ± 4.10 and 30.01 ± 2.00 s) for 100 and 200 mg/kg body, respectively on day 7 as shown in Table 6.
Table 6.
Effects of EEMC on immobility time in mouse using tail suspension test (TST).
| Groups | Treatment | Immobility time (s) |
|
|---|---|---|---|
| 1st day | 7th day | ||
| I | Control group treated with normal saline (10 mL/kg) | 75.54 ± 1.55b | 81.06 ± 3.24c |
| II | Standard drug imipramine (15 mg/kg) | 45.87 ± 2.30b | 35.05 ± 2.75b |
| III | Ethanol extract M. champaca leaves (EEMC-100 mg/kg) | 43.36 ± 1.99b | 38.50 ± 4.10b |
| IV | Ethanol extract M. champaca leaves (EEMC-200 mg/kg) | 36.00 ± 5.07a | 30.01 ± 2.00a |
Values are expressed as mean ± SE. Means in the same column with distinct superscripts are significantly different (p ≤ 0.05) as separated by Duncan's multiple range test (the different alphabets indicate the statistically significant difference between the values).
Molecular docking and virtual screening
The virtual screening of phytoconstituents based on the criteria of their binding affinity, total non-bonding interactions and hydrogen bonds are given in Tables 7 and 8 for human potassium channel KCSA-FAB (PDB ID: 4UUJ) and human serotonin transporter (PDB ID: 516X), respectively. In this investigation, the human potassium channel KCSA-FAB and human serotonin transporter proteins were used to screen anxiolytic and antidepressant docking analysis, respectively. Out of the 69 compounds, nine compounds were selected based on the above-mentioned criteria. Further, these compounds were selected for drug-likeness and toxicity analysis to select the most potent drug candidate. Diazepam was used as a reference drug for the in silico inhibition of human potassium channel KCSA-FAB, whereas imipramine was used against human serotonin transporter as the same.
Table 7.
Binding affinity, total number of non-bonding interactions, and hydrogen bonds formed by phytoconstituents of M. champaca with human potassium channel KCSA-FAB (PDB ID: 4UUJ).
| S. no. | Name of the compound | Binding affinity (kJ/mol) | Total no. non-bonding interactions | Total no. of hydrogen bonds |
|---|---|---|---|---|
| 1 | (9Z,11E,13E,15Z)-4-Oxo-9,11,13,15-octadecatetraenoic acid | –5.9 | 10 | 4 |
| 2 | (E,E)-1,7-Diphenyl-4,6-heptadien-3-ol | –6.2 | 5 | 1 |
| 3 | 1-(beta-d-Ribofuranosyl)-1,4-dihydronicotinamide | –6.6 | 7 | 6 |
| 4 | 1-Dehydro-9-fluoro-11-oxotestololactone | –8.8 | 6 | 5 |
| 5 | 1-Octen-3-yl glucoside | –6.5 | 11 | 7 |
| 6 | 1-Phosphatidyl-1D-myoinositol 3-phosphate | –7.2 | 10 | 10 |
| 7 | 2-(Methylthiomethyl)-3-phenylpropenal | –5.0 | 4 | 2 |
| 8 | 3,6-Dimethoxyestra-1,3,5(10),6,8-pentaene-17-beta-carboxylic acid methyl ester | –7.8 | 9 | 5 |
| 9 | 3-Methyldioxyindole | –6.0 | 4 | 2 |
| 10 | 3-Phenylpropyl propionate | –5.1 | 2 | 0 |
| 11 | 5′-Butyrylphosphoinosine | –9.8 | 11 | 6 |
| 12 | 6-(1,2,3,4-Tetrahydro-6-methoxy-2-naphthyl)-2(1H)-pyridone | –7.4 | 3 | 1 |
| 13 | 17-beta-(Acetylthio)estra-1,3,5(10)-trien-3-ol acetate | –8.1 | 7 | 1 |
| 14 | 7-O-Acetylaustroinulin | –10.1 | 1 | 0 |
| 15 | 17-Hydroxylinolenic acid | –5.5 | 8 | 2 |
| 16 | 19-Noretiocholanolone | –7.2 | 2 | 0 |
| 17 | Alangimarckine | –8.8 | 6 | 1 |
| 18 | Albanol A | –7.4 | 7 | 2 |
| 19 | Allamandin | –7.6 | 6 | 4 |
| 20 | Austinol | –8.6 | 1 | 1 |
| 21 | Azafrin | –7.4 | 4 | 2 |
| 22 | Beclomethasone (2) | –7.9 | 8 | 7 |
| 23 | Betaxolol | –5.5 | 9 | 4 |
| 24 | Broussonin C | –7.7 | 4 | 1 |
| 25 | Butyl 2-aminobenzoate | –5.4 | 6 | 4 |
| 26 | Carbosulfan | –6.0 | 5 | 1 |
| 27 | trans-1,4-bis(2-Chlorobenzaminomethyl)cyclohexane | –7.8 | 4 | 3 |
| 28 | cis-Resveratrol 4′-O-glucuronide | –8.2 | 5 | 3 |
| 29 | Deoxytubulosine | –8.8 | 6 | 1 |
| 30 | Dihydrodeoxystreptomycin | –8.2 | 15 | 9 |
| 31 | Dihydrospheroidene | –5.2 | 3 | 0 |
| 32 | Diminazene | –7.7 | 7 | 3 |
| 33 | Diplodiatoxin | –6.7 | 8 | 3 |
| 34 | Doripenem | –9.2 | 9 | 9 |
| 35 | Exemestane | –7.8 | 0 | 0 |
| 36 | Fluticasone propionate | –7.7 | 3 | 2 |
| 37 | Forsythiaside | –8.6 | 14 | 8 |
| 38 | Harderoporphyrin | –8.3 | 8 | 1 |
| 39 | Heteratisine | –7.4 | 4 | 1 |
| 40 | C-16 Sphinganine | –5.5 | 6 | 0 |
| 41 | Hydroflumethiazide | –6.9 | 7 | 5 |
| 42 | Hydroxyprogesterone caproate | –6.7 | 1 | 1 |
| 43 | Imazamethabenz | –6.2 | 6 | 4 |
| 44 | Isorhamnetin 3-O-[b-d-glucopyranosyl-(1-2)-a-l-rhamnopyranoside] | –11.2 | 8 | 7 |
| 45 | Kanamycin | –7.6 | 10 | 9 |
| 46 | l-Arabinose | –5.1 | 2 | 2 |
| 47 | Lentiginosine | –5.5 | 7 | 3 |
| 48 | Linoleoylethanolamide | –5.5 | 6 | 3 |
| 49 | Longistylin C | –7.1 | 5 | 2 |
| 50 | Lyngbyatoxin | –7.6 | 5 | 0 |
| 51 | Memantine | –5.2 | 2 | 2 |
| 52 | Methyl (9Z)-10′-oxo-6,10′-diapo-6-carotenoate | –5.0 | 3 | 1 |
| 53 | Methyldopa | –6.1 | 5 | 4 |
| 54 | MG(226(4Z_7Z_10Z_13Z_16Z_1 9Z)0000) | –7.1 | 5 | 3 |
| 55 | Mitoxantrone | –7.7 | 9 | 6 |
| 56 | Momilactone A | –7.9 | 3 | 1 |
| 57 | Norethindrone enanthate | –7.1 | 2 | 1 |
| 58 | Panaquinquecol 1 | –5.5 | 7 | 1 |
| 59 | Pheophorbide b | –8.9 | 6 | 1 |
| 60 | Pheophorbide a | –9.2 | 12 | 4 |
| 61 | Poncirin | –9.6 | 8 | 6 |
| 62 | Pyropheophorbide a | –9.1 | 5 | 2 |
| 63 | Salviaflaside methyl ester | –7.7 | 10 | 7 |
| 64 | Sodium glycocholate | –8.3 | 4 | 4 |
| 65 | Sphinganine | –5.6 | 3 | 2 |
| 66 | Trenbolone | –7.4 | 5 | 1 |
| 67 | Veraguensin | –7.3 | 6 | 1 |
| 68 | Virol B | –5.3 | 5 | 0 |
| 69 | Vulgarone A | –6.3 | 3 | 1 |
| 70 | Diazepam | –6.7 | 6 | 3 |
The compound names and their docking results given in bold indicate the potential hit compounds from docking simulation.
Table 8.
Binding affinity, total number of non-bonding interactions, and hydrogen bonds formed by phytoconstituents of M. champaca with human serotonin transporter (PDB ID: 516X).
| S. no. | Name of the compound | Binding affinity (kJ/mol) | Total no. of non-bonding interactions | Total no. of hydrogen bonds |
|---|---|---|---|---|
| 1 | (9Z,11E,13E,15Z)-4-Oxo-9,11,13,15-octadecatetraenoic acid | –7.1 | 11 | 1 |
| 2 | (E,E)-1,7-Diphenyl-4,6-heptadien-3-ol | –8.8 | 9 | 0 |
| 3 | 1-(beta-d-Ribofuranosyl)-1,4-dihydronicotinamide | –7.5 | 7 | 3 |
| 4 | 1-Dehydro-9-fluoro-11-oxotestololactone | –10.0 | 3 | 2 |
| 5 | 1-Octen-3-yl glucoside | –7.8 | 9 | 6 |
| 6 | 1-Phosphatidyl-1D-myoinositol-3-phosphate | –7.6 | 14 | 7 |
| 7 | 2-(Methylthiomethyl)-3-phenylpropenal | –6.3 | 9 | 0 |
| 8 | 3,6-Dimethoxyestra-1,3,5(10),6,8-pentaene-17-beta-carboxylic acid methyl ester | –8.7 | 9 | 2 |
| 9 | 3-Methyldioxyindole | –6.7 | 6 | 5 |
| 10 | 3-Phenylpropyl propionate | –6.7 | 7 | 0 |
| 11 | 5′-Butyrylphosphoinosine | –10.4 | 17 | 4 |
| 12 | 6-(1,2,3,4-Tetrahydro-6-methoxy-2-naphthyl)-2(1H)-pyridone | –9.4 | 9 | 1 |
| 13 | 17-beta-(Acetylthio)estra-1,3,5(10)-trien-3-ol acetate | –9.2 | 5 | 0 |
| 14 | 7-O-Acetylaustroinulin | –12.9 | 7 | 0 |
| 15 | 17-Hydroxylinolenic acid | –6.9 | 10 | 1 |
| 16 | 19-Noretiocholanolone | –9.3 | 6 | 1 |
| 17 | Alangimarckine | –11.3 | 11 | 0 |
| 18 | Albanol A | –9.7 | 5 | 0 |
| 19 | Allamandin | –8.3 | 1 | 0 |
| 20 | Austinol | –10.1 | 5 | 2 |
| 21 | Azafrin | –9.0 | 9 | 2 |
| 22 | Beclomethasone (2) | –8.4 | 3 | 2 |
| 23 | Betaxolol | –7.1 | 10 | 2 |
| 24 | Broussonin C | –9.6 | 13 | 1 |
| 25 | Butyl 2-aminobenzoate | –6.7 | 10 | 2 |
| 26 | Carbosulfan | –7.9 | 7 | 1 |
| 27 | trans-1,4-bis(2-Chlorobenzaminomethyl)cyclohexane | –10.3 | 5 | 3 |
| 28 | cis-Resveratrol 4′-O-glucuronide | –9.2 | 5 | 3 |
| 29 | Deoxytubulosine | –11.3 | 12 | 0 |
| 30 | Dihydrodeoxystreptomycin | –8.3 | 6 | 5 |
| 31 | Dihydrospheroidene | –8.6 | 3 | 0 |
| 32 | Diminazene | –8.5 | 9 | 4 |
| 33 | Diplodiatoxin | –8.2 | 8 | 3 |
| 34 | Doripenem | –9.8 | 13 | 11 |
| 35 | Exemestane | –9.8 | 1 | 1 |
| 36 | Fluticasone propionate | –9.6 | 8 | 5 |
| 37 | Forsythiaside | –10.8 | 13 | 6 |
| 38 | Harderoporphyrin | –4.9 | 9 | 2 |
| 39 | Heteratisine | –9.0 | 3 | 0 |
| 40 | C-16 Sphinganine | –6.1 | 8 | 1 |
| 41 | Hydroflumethiazide | –7.9 | 8 | 2 |
| 42 | Hydroxyprogesterone caproate | –9.6 | 5 | 0 |
| 43 | Imazamethabenz | –8.8 | 4 | 0 |
| 44 | Isorhamnetin 3-O-[b-d-glucopyranosyl-(1-2)-a-l-rhamnopyranoside] | –12.5 | 11 | 7 |
| 45 | Kanamycin | –7.9 | 6 | 5 |
| 46 | l-Arabinose | –5.2 | 5 | 3 |
| 47 | Lentiginosine | –6.0 | 3 | 1 |
| 48 | Linoleoylethanolamide | –7.1 | 10 | 2 |
| 49 | Longistylin C | –9.4 | 11 | 1 |
| 50 | Lyngbyatoxin | –9.6 | 8 | 0 |
| 51 | Memantine | –7.0 | 3 | 1 |
| 52 | Methyl (9Z)-10′-oxo-6,10′-diapo-6-carotenoate | –7.1 | 5 | 1 |
| 53 | Methyldopa | –6.7 | 5 | 2 |
| 54 | MG(226(4Z_7Z_10Z_13Z_16Z_1 9Z)0000) | –7.0 | 5 | 1 |
| 55 | Mitoxantrone | –7.7 | 16 | 3 |
| 56 | Momilactone A | –9.7 | 3 | 1 |
| 57 | Norethindrone enanthate | –10.2 | 10 | 2 |
| 58 | Panaquinquecol 1 | –7.7 | 10 | 0 |
| 59 | Pheophorbide b | –4.3 | 12 | 1 |
| 60 | Pheophorbide a | –4.9 | 14 | 2 |
| 61 | Poncirin | –11.3 | 15 | 7 |
| 62 | Pyropheophorbide a | –6.3 | 9 | 0 |
| 63 | Salviaflaside methyl ester | –9.8 | 12 | 7 |
| 64 | Sodium glycocholate | –9.0 | 3 | 1 |
| 65 | Sphinganine | –7.3 | 8 | 3 |
| 66 | Trenbolone | –10.3 | 9 | 2 |
| 67 | Veraguensin | –8.3 | 13 | 2 |
| 68 | Virol B | –7.5 | 9 | 2 |
| 69 | Vulgarone A | –8.6 | 5 | 0 |
| 70 | Imipramine | –8.5 | 6 | 0 |
The compound names and their docking results given in bold indicate the potential hit compounds from docking simulation.
Drug-likeness and toxicity analysis
OSIRIS property explorer uses Lipinski’s rule of five for the analysis of drug-likeness and toxicity of the compounds. Lipinski’s rule of five states that a potential drug candidate must have its molecular weight ≤500 Da, cLogP value (logarithm of its partition coefficient between n-octanol and water log(coctanol/cwater) ≤4.15, hydrogen bond acceptors ≤10, hydrogen bond donors ≤5 and rotatable bonds ≤10) (Lipinski et al. 2001). Based on these parameters, nine compounds obtained from previous virtual screening were assessed for their drug-likeness and toxicity. Out of the nine compounds, only doripenem was predicted with no violation of Lipinski’s rule of five. It was also predicted with no risk of causing mutagenic, tumorigenic, irritating and reproductive aberrations. Although reference drugs diazepam and imipramine showed no violations of Lipinski’s rule of five, their drug scores were found to be lower than doripenem. Besides, during the toxicity analysis, diazepam was predicted with a high risk showing possible mutagenicity, tumorigenicity and reproductive health aberrations. In addition, imipramine was also predicted with reproductive health aberrations. Apart from docking simulations, drug-likeness and toxicity studies also favour doripenem in comparison with the reference drugs (Table 9).
Table 9.
ADMET profile of the M. champaca phytoconstituents and standard drugs.
| Compounds | Drug-likeness parameters |
Toxicological parameters |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cLogP | Mol. weight (g/mol) | HBA | HBD | nRB | Drug- score | Mutagenicity | Tumorigenicity | Irritability | Reproductive effectivity | |
| 1-Octen-3-yl glucoside | 0.66 | 290.35 | 6 | 4 | 8 | 0.23 | No risk | High risk | Medium risk | No risk |
| 1-Phosphatidyl-1D-myoinositol 3-phosphate | –9.13 | 470.21 | 16 | 9 | 10 | 0.40 | No risk | No risk | No risk | No risk |
| 5′-Butyrylphosphoinosine | –3.28 | 418.30 | 13 | 4 | 8 | 0.43 | No risk | No risk | No risk | No risk |
| Dihydrodeoxystreptomycin | –7.65 | 583.59 | 19 | 13 | 9 | 0.62 | No risk | No risk | No risk | No risk |
| Doripenem | –3.68 | 420.51 | 10 | 5 | 6 | 0.86 | No risk | No risk | No risk | No risk |
| Forsythiaside | –0.38 | 624.59 | 15 | 9 | 11 | 0.34 | No risk | No risk | No risk | No risk |
| Isorhamnetin 3-O-[b-d-glucopyranosyl-(1-2)-a-l-rhamnopyranoside] | –0.98 | 624.55 | 16 | 9 | 7 | 0.32 | No risk | No risk | No risk | No risk |
| Poncirin | –0.47 | 594.56 | 14 | 7 | 7 | 0.43 | No risk | No risk | No risk | No risk |
| Salviaflaside methyl ester | –0.11 | 536.48 | 13 | 7 | 11 | 0.35 | No risk | No risk | No risk | No risk |
| Diazepam | 2.98 | –4.67 | 3 | 0 | 1 | 0.20 | High risk | High risk | No risk | High risk |
| Imipramine | 3.89 | 280.41 | 2 | 0 | 4 | 0.51 | No risk | No risk | No risk | High risk |
cLogP: logarithm of its partition coefficient between n-octanol and water log(coctanol/cwater); HBA: hydrogen bond acceptors; HBD: hydrogen bond donors; nRB: number of rotatable bonds.
Bold values indicate the result of selected compounds which were taken for ADMET studies.
Interaction analysis of doripenem with target proteins
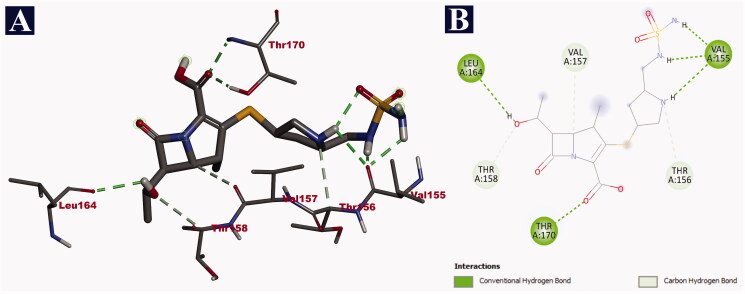
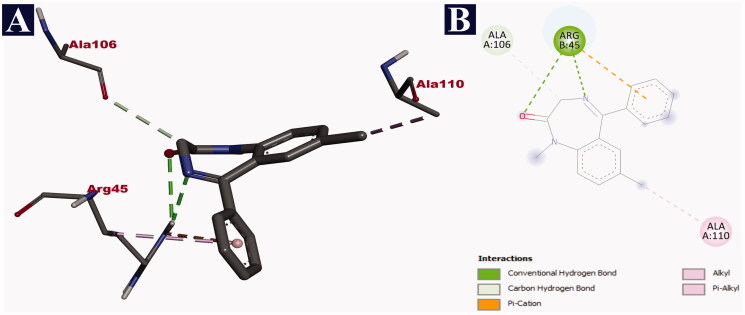
Doripenem was bound with the fragment antigen-binding (FAB) site of the human potassium channel KCSA-FAB. The phytochemical binds to the external surface of the protein within the hydrophobic binding pocket. The FAB domain remains outside the cell to facilitate the binding of the antigens. Doripenem binds through nine non-bonding interactions, all of them being hydrogen bonds with a binding affinity of −9.2 kJ/mol. It formed hydrogen bonds with THR 170 (2.16 Å), THR 170 (2.25 Å), LEU 164 (2.61 Å), VAL 155 (2.65 Å), VAL 155 (1.83 Å), VAL 155 (2.32 Å), THR 156 (3.62 Å), THR 158 (3.53 Å) and VAL 157 (3.52 Å) (Table 10). The binding interactions of doripenem with human potassium channel KCSA-FAB are given in Figure 1. Binding at this site is expected to reduce the influx of potassium ions, which reduces the neuronal excitability, ultimately resulting in a relaxed state of mind leading to the expected anxiolytic activity. In comparison with doripenem, the reference drug diazepam could form only six non-bonding interactions with a binding affinity of −6.7 kJ/mol. Diazepam formed only three hydrogen bonds with ARG 45 (2.50 Å), ARG 45 (3.05 Å) and ALA 106 (3.49 Å). It also formed a lone electrostatic bond with ARG 45 (4.40 Å), and two hydrophobic/interactions (pi-alkyl and alkyl) with ARG 45 (4.24 Å) and ALA 110 (3.74 Å), respectively (Table 10). The binding interactions of diazepam with the human potassium channel KCSA-FAB are depicted in Figure 2.
Table 10.
Binding interactions of doripenem and diazepam with human potassium channel (PDB ID: 4UUJ) along with their respective bond lengths (Å).
| Name of the compound | Binding affinity (kJ/mol) | Hydrogen bonds | Electrostatic bonds | Hydrophobic bonds |
|
|---|---|---|---|---|---|
| Pi-Alkyl | Alkyl | ||||
| Doripenem | –9.2 | THR 170 (2.16), THR 170 (2.25), LEU 164 (2.61), VAL 155 (2.65), VAL 155 (1.83), VAL 155 (2.32), THR 156 (3.62), THR 158 (3.53), VAL 157 (3.52) | – | – | – |
| Diazepam | –6.7 | ARG 45 (2.50), ARG 45 (3.05), ALA 106 (3.49) | ARG 45 (4.40) | ARG 45 (4.24) | ALA 110 (3.74) |
Figure 1.
Visualization of docking analysis of doripenem binding with human potassium channel KCSA-FAB (PDB ID: 4UUJ). (A) 3D representation. (B) 2D representation.
Figure 2.
Visualization of docking analysis of diazepam binding with human potassium channel KCSA-FAB (PDB ID: 4UUJ). (A) 3D representation. (B) 2D representation.
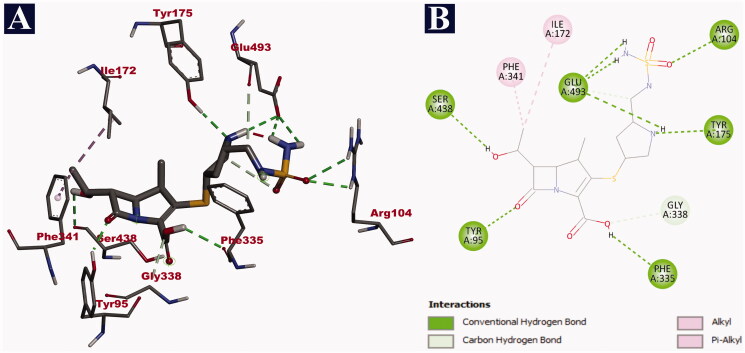
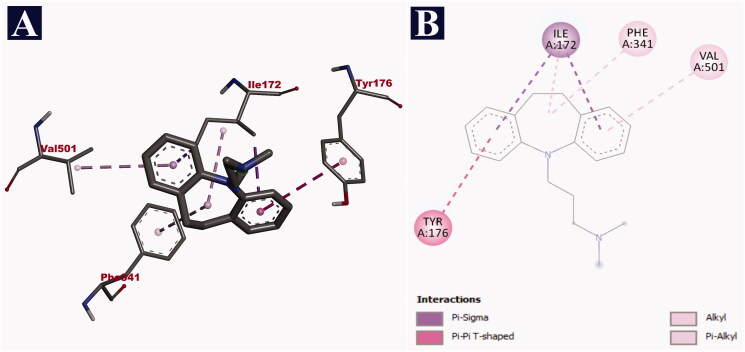
In case of human serotonin transporter, doripenem was bound to the central binding site of the hydrophobic binding pocket, in the exact site of the co-crystal ligand paroxetine with a binding affinity of −9.8 kJ/mol. The hydrophobic binding pocket was created by 10 transmembrane helices (TM) of the protein. Out of 13 non-bonding interactions, doripenem formed 11 hydrogen bonds with SER 438 (2.71 Å), PHE 335 (2.63 Å), GLU 493 (2.87 Å), GLU 493 (2.71 Å), GLU 493 (2.79 Å), TYR 95 (2.02 Å), ARG 104 (2.55 Å), ARG 104 (2.75 Å), TYR 175 (2.46 Å), GLU 493 (3.63 Å) and GLY 338 (3.69 Å). It also formed two hydrophobic interactions (pi-alkyl and alkyl) with PHE 341 (4.59 Å) and ILE 172 (3.89 Å), respectively (Table 11). The binding interactions of doripenem are given in Figure 3. In comparison with doripenem, imipramine reference drug could bind with the binding affinity of −8.5 kJ/mol. It formed a total of six non-bonding interactions, with all of them being hydrophobic bonds. It bound to the central binding site of the hydrophobic binding pocket. In the context, two pi-sigma bonds with ILE 172 (3.90 Å) and ILE 172 (3.62 Å), a single pi–pi interaction with TYR 176 (5.04 Å), two pi-alkyl bonds with PHE 341 (4.63 Å) and VAL 501 (5.00 Å), and another single alkyl bond with ILE 172 (4.56 Å) were formed by imipramine (Table 11). The details of the interaction of imipramine with human serotonin transporter are depicted in Figure 4.
Table 11.
Binding interactions of doripenem and imipramine with human serotonin transporter (PDB ID: 516X) along with their respective bond lengths (Å).
| Name of the compound | Binding affinity (kJ/mol) | Hydrogen bonds | Hydrophobic bonds |
|||
|---|---|---|---|---|---|---|
| Pi–sigma | Pi–Pi | Pi–alkyl | Alkyl | |||
| Doripenem | –9.8 | SER 438 (2.71), PHE 335 (2.63), GLU 493 (2.87), GLU 493 (2.71), GLU 493 (2.79), TYR 95 (2.02), ARG 104 (2.55), ARG 104 (2.75), TYR 175 (2.46), GLU 493 (3.63), GLY 338 (3.69) | – | – | PHE 341 (4.59) | ILE 172 (3.89) |
| Imipramine | –8.5 | – | ILE 172 (3.90), ILE 172 (3.62) | TYR 176 (5.04) | PHE 341 (4.63), VAL 501 (5.00) | ILE 172 (4.56) |
Figure 3.
Visualization of docking analysis of doripenem binding with human serotonin transporter (PDB ID: 516X). (A) 3D representation. (B) 2D representation.
Figure 4.
Visualization of docking analysis of imipramine binding with human serotonin transporter (PDB ID: 516X). (A) 3D representation. (B) 2D representation.
Discussion
Anxiety and depression are mental conditions considered among the most studied psychiatric diseases in humans. About 1/8th of the world population suffers from these conditions and at least 10% of the people suffer from it once in their entire life (Porter and Meldrum 2009). Studies have proven the beneficiary effects of flavonoids and other plant phytochemicals that have been efficacious in treating mild to moderate conditions (Gerzson et al. 2012). However, the exact mechanism of action of these drugs is less exploited and hence they cannot be established as commercial drugs for the treatment of depression and anxiety.
In our study, preliminary screening of the various phytochemicals present in the ethanol extract of M. champaca leaves was performed. It was revealed that the extract was abundant in alkaloids, tannins, steroids, phenols, flavonoids, carbohydrates and glycosides. Previous study evaluated the free radical scavenging activity of the flower extract that demonstrated compounds such as anonaine, an aporphine (isoquinoline) alkaloids present in the extract showed significant pharmacological activities such as, antibacterial, antifungal, antioxidative, anticancer and antidepressant (Li et al. 2013).
The study also evaluated the toxicity of the extract that revealed the extract was not toxic up to a dose of 2000 mg/kg body administration. In this study, the anxiolytic activity of the extract was assessed using two standard assays namely, elevated plus maze and light and dark tests. In comparison with the results of the standard drug diazepam, 100 and 200 mg/kg body weight treatment of the extract demonstrated significant activity in lowering anxiety. The number of entries and time spent in open arm increased remarkably with a corresponding decrease in the number of entries and time spent in the closed arms. Mice experience anxiety because of the fear of height when placed in the elevated plus maze design. This is represented in the motor activity and preferences shown by the mice to stay in its safe zone (Walia et al. 2018). Agents such as diazepam have proven ability to improve the motor activity in terms of the time spent in the open arms by the experimental animal (Shimizu et al. 2018). Previous study on the anxiolytic activity of Nymphaea alba L. (Nymphaeaceae) revealed a significant enhancement in time spent and number of entries in the open arm and a decrease in the duration of immobility in light box (Thippeswamy et al. 2011). The results from the present study are in agreement with this study revealing that the ability of the extract to lower anxiety induced by height in mice, which is on par with that of diazepam, the standard drug. The light and dark assay is yet another test to evaluate the anxiolytic activity of drug molecules. The increased percentage of time spent in the light compartment indicates improved anxiolytic activity. In our study, the anxiolytic activity exerted by the extract was significant in comparison with that of the standard drug diazepam at both the doses with extract treated at 200 mg/kg concentration was better than 100 mg/kg concentration. In yet another study, Cynodon dactylon (L.) Pers. (Poaceae) extract on albino mice demonstrated remarkable antidepressant and anxiolytic potential, which was indicated to be owing to the presence of high flavonoid content in the extract (Kothari 2021). Flavones present in the plant extracts are known to bind to the benzodiazepine site of the GABA adrenergic receptor that is expected to bring about the observed anxiolytic effects. Several plant flavones have been assessed based on their traditional uses to reduce anxiety (Liu et al. 2021). In a study by Malathi and Ravindran (2015), the antidepressant and anxiolytic activities of the methanolic extract of its flower were evaluated. It was observed that the extract was potential in reducing anxiety induced by noise by the stimulation of dopamine. Therefore, it can be suggested that the targeted regulation of anxiety by lowering dopamine-mediated signal transduction leads to the observed effects in the present study.
Furthermore, the present study evaluated the antidepressant activity of the extract using FST and TST. In both the tests, immobility shown by the experimental mice on the 1st and 7th day was compared. The results demonstrated that there was more reduction in the immobility on the 7th day than on the 1st day. These results indicate that the EEMC on chronic administration of higher doses has antidepressant effect similar to that of the standard drug imipramine. The two are the established methods for the assessment of depression among various animal models (Shoeb et al. 2013). In humans, a sense of hopelessness is seen that is in turn represented as the paradigm of helplessness as observed by the failure to work towards survival in rodents. The behaviour of immobility after only a short struggle is represented as a depressive behaviour in case of mice that can be extrapolated to the human depressive behaviour (Jahani et al. 2019). In our study, the improved time taken to reach immobility state shows that the extract is indeed beneficiary in bringing about antidepressant activity.
Prediction of ligand–target interactions using in silico approaches is gaining momentum in the research of natural products and their bioactivity. There is an added advantage of an insight on the probable binding mechanisms in the protein binding pocket demonstrating its interaction with the ligand. To validate the findings from the biological studies, the present study also evaluated the molecular interaction of phytochemicals from M. champaca with the two target proteins, namely human potassium channel KCSA-FAB and human serotonin transporter.
The passive flow of potassium ions in neurons and other body cells is mediated by the human potassium channel KCSA-FAB. An increased potassium influx causes neuronal excitability, which is thought to be the cause of anxiety disorders (Lenaeus et al. 2014). As a result, inhibiting the human potassium channel KCSA-FAB would result in potassium depletion and anxiolytic effects. Doripenem has been reported to interact with the outer FAB complex, which serves as an antigen binding site in this study. This would close the channel, inhibiting potassium ions from entering the cells. Despite the fact that diazepam was bound to the FAB complex, doripenem was chosen over diazepam because of its superior interaction and binding affinity. In case of drug-likeness and toxicity analyses, diazepam was predicted with probable mutagenic, tumorigenic and reproductive health aberrant properties. Alternatively, doripenem was predicted with no risk of inducing toxic effects. Besides, drug score of doripenem is higher than that of diazepam. Collectively, the superior binding interaction, lower binding affinity, higher drug-score and zero risk of inducing toxicity make doripenem a better potential drug candidate, in comparison with the conventional drug diazepam.
The regulation of serotonin levels is aided by human serotonin transporters. Serotonin regulates the functioning of the central nervous system as well as other bodily functions such as digestion, cardiovascular function and reproduction. Serotonin is released into the synaptic cleft and diffused by serotonin receptors. Re-uptake of serotonin, on the other hand, causes depression. Antidepressant medicines such as prozac, imipramine and paroxetine have been shown to impede serotonin reuptake, resulting in depression-free state (Coleman et al. 2016). As a result, blocking the human serotonin transporter may pave the way for the development of antidepressant drugs. Doripenem occupies the centre of the binding pocket created by all the transmembrane helices during the molecular docking simulation. Despite the fact that imipramine binds to the pocket's core site, its interactions and binding affinity appear to be inferior to doripenem. The blockage of serotonin reuptake would result in a depression-free state. During drug-likeness and toxicity analysis, imipramine was predicted with probable reproductive health aberrations. However, doripenem was found to be predicted with no risk with all the toxicity parameters used. Even the drug-score of doripenem was found to be more than imipramine. Therefore, doripenem could be employed as a strong inhibitor of human serotonin transporter because of the degree of interaction, lower binding affinity, higher drug-likeness and zero risk of probable toxicity, compared to imipramine.
Conclusions
Based on the biological result interpretations, M. champaca leaves extracted using ethanol as a solvent is a rich source of compounds with potential antidepressant and anxiolytic properties. In vivo findings also support their traditional importance as potent formulations in the treatment of depression and anxiety. Besides, the molecular docking study revealed that doripenem showed good interaction with both human potassium channel KSCA-FAB and human serotonin transporter proteins docking simulations, in comparison with reference drugs, thereby showing in silico anxiolytic and antidepressant activities, respectively. The drug-likeness and toxicity profile of doripenem also favours it as an effective potential drug candidate, whereas reference drugs were reported with toxicity predictions. Overall, this study paves way for future research in understanding the ethnomedicinal uses of M. champaca leaves in the treatment of anxiety and depression.
Acknowledgements
All the authors thank JSS Academy of Higher Education and Research authorities for all support and eternally extending help throughout the course of study.
Disclosure statement
The authors declare that there is no conflict of interest.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- Bhatt S, Nagappa AN, Patil CR.. 2020. Role of oxidative stress in depression. Drug Discov Today. 25(7):1270–1276. [DOI] [PubMed] [Google Scholar]
- Chandra K, Salman AS, Mohd A, Sweety R, Ali KN.. 2015. Protection against FCA induced oxidative stress induced DNA damage as a model of arthritis and in vitro anti-arthritic potential of Costus speciosus rhizome extract. Int J Pharmacogn Phytochem Res. 7:383–389. [Google Scholar]
- Coleman JA, Green EM, Gouaux E.. 2016. X-ray structures and mechanism of the human serotonin transporter. Nature. 532(7599):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson JL, Loscalzo J.. 2008. Harrison’s principles of internal medicine. New York: McGraw Hill Medical. [Google Scholar]
- Fedoce ADG, Ferreira F, Bota RG, Bonet-Costa V, Sun PY, Davies KJ.. 2018. The role of oxidative stress in anxiety disorder: cause or consequence? Free Radic Res. 52(7):737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzson MFB, Victoria FN, Radatz CS, de Gomes MG, Boeira SP, Jacob RG, Alves D, Jesse CR, Savegnago L.. 2012. In vitro antioxidant activity and in vivo antidepressant-like effect of α-(phenylselanyl) acetophenone in mice. Pharmacol Biochem Behav. 102(1):21–29. [DOI] [PubMed] [Google Scholar]
- Gong ZH, Li YF, Zhao N, Yang HJ, Su RB, Luo ZP, Li J.. 2006. Anxiolytic effect of agmatine in rats and mice. Eur J Pharmacol. 550(1–3):112–116. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Saunders EF, Santhanam L, Alexander LM.. 2019. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res. 124(4):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M.. 2004. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 142(2):231–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G. 2010. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry. 9(3):155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JJ, Torrance SJ, Widehopf RM, Cole JR.. 1977. Cytotoxic agents from Michelia champaca and Talauma ovata: parthenolide and costunolide. J Pharm Sci. 66(6):883–884. [DOI] [PubMed] [Google Scholar]
- Hossain MM, Jahangir R, Hasan SR, Akter R, Ahmed T, Islam MI, Faruque A.. 2009. Antioxidant, analgesic and cytotoxic activity of Michelia champaca Linn. leaf. Stamford. J Pharm Sci. 2(2):1–7. [Google Scholar]
- Jahani R, Khaledyan D, Jahani A, Jamshidi E, Kamalinejad M, Khoramjouy M, Faizi M.. 2019. Evaluation and comparison of the antidepressant-like activity of Artemisia dracunculus and Stachys lavandulifolia ethanolic extracts: an in vivo study. Res Pharm Sci. 14(6):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarald EE, Joshi SB, Jain DC.. 2008. Antidiabetic activity of flower buds of Michelia champaca Linn. Indian J Pharmacol. 40(6):256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaid T, Gupta R, Siddiqui ZA.. 2011. A review on herbal plants showing antidepressant activity. Int J Pharm Sci Res. 2:3051–3060. [Google Scholar]
- Khan MR, Kihara M, Omoloso AD.. 2002. Antimicrobial activity of Michelia champaca. Fitoterapia. 73(7–8):744–748. [DOI] [PubMed] [Google Scholar]
- Kothari S. 2021. Evaluation of antianxiety and antidepressant activity of aqueous extract of Cynodon dactylon (Doob grass) in Swiss albino mice. Int J Green Pharm. 15:182–187. [Google Scholar]
- Kumar A, Gupta RC, Thomas MA, Alger J, Wyckoff N, Hwang S.. 2004. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 130(2):131–140. [DOI] [PubMed] [Google Scholar]
- Kumar V, Ramu R, Shirahatti PS, Kumari VC, Sushma P, Mandal SP, Patil SM.. 2021. α-Glucosidase, α-amylase inhibition, kinetics and docking studies of novel (2-chloro-6-(trifluoromethyl) benzyloxy) arylidene) based rhodanine and rhodanine acetic acid derivatives. Chemistry Select. 6(36):9637–9644. [Google Scholar]
- Lenaeus MJ, Burdette D, Wagner T, Focia PJ, Gross A.. 2014. Structures of KcsA in complex with symmetrical quaternary ammonium compounds reveal a hydrophobic binding site. Biochemistry. 53(32):5365–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HT, Wu HM, Chen HL, Liu CM, Chen CY.. 2013. The pharmacological activities of (−)-anonaine. Molecules. 18(7):8257–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ.. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 46(1–3):3–26. [DOI] [PubMed] [Google Scholar]
- Liu Z, Silva J, Shao AS, Liang J, Wallner M, Shao XM, Li M, Olsen RW.. 2021. Flavonoid compounds isolated from Tibetan herbs, binding to GABAA receptor with anxiolytic property. J Ethnopharmacol. 267:113630. [DOI] [PubMed] [Google Scholar]
- Malathi S, Ravindran R.. 2015. Effect of Michelia champaca on locomotor activity, anxiety and depression-like behaviours in noise stress induced Wistar albino rats. World J Pharm Res. 4:1409–1426. [Google Scholar]
- Nickavar B, Kamalinejad M, Haj-Yahya M, Shafaghi B.. 2006. Comparison of the free radical scavenging activity of six Iranian Achillea species. Pharm Biol. 44(3):208–212. [Google Scholar]
- Patil SM, Martiz RM, Ramu R, Shirahatti PS, Prakash A, Chandra JS, Ranganatha LV.. 2021. In silico identification of novel benzophenone-coumarin derivatives as SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) inhibitors. J Biomol Struct Dyn. 11:1–17. [DOI] [PubMed] [Google Scholar]
- Patil SM, Martiz RM, Ramu R, Shirahatti PS, Prakash A, Kumar BRP, Kumar N.. 2021. Evaluation of flavonoids from banana pseudostem and flower (aminoguanidine and catechin) as potent inhibitors of α-glucosidase: an in silico perspective. J Biomol Struct Dyn. 6:1–15. [DOI] [PubMed] [Google Scholar]
- Patil SM, Maruthi KR, Bajpe SN, Vyshali VM, Sushmitha S, Akhila C, Ramu R.. 2021. Comparative molecular docking and simulation analysis of molnupiravir and remdesivir with SARS-CoV-2 RNA dependent RNA polymerase (RdRp). Bioinformation. 17(11):932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SM, Shirahatti PS, Chandana Kumari VB, Ramu R, Nagendra Prasad MN.. 2021. Azadirachta indica A. Juss (neem) as a contraceptive: an evidence-based review on its pharmacological efficiency. Phytomedicine. 88:153596. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre MJ.. 1977. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 229(2):327–336. [PubMed] [Google Scholar]
- Porter RJ, Meldrum BS.. 2009. Antidepressant agents. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology. New Delhi: Tata McGraw Hill; p. 521–525. [Google Scholar]
- Rajput MA, Khan RA.. 2017. Phytochemical screening, acute toxicity, anxiolytic and antidepressant activities of the Nelumbo nucifera fruit. Metab Brain Dis. 32(3):743–749. [DOI] [PubMed] [Google Scholar]
- Reddy MS. 2010. Depression: the disorder and the burden. Indian J Psychol Med. 32(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz A, Khan RA.. 2014. Effect of Punica granatum on behavior in rats. Afr J Pharm Pharmacol. 8:1118–1126. [Google Scholar]
- Shimizu T, Minami C, Mitani A.. 2018. Effect of electrical stimulation of the infralimbic and prelimbic cortices on anxiolytic-like behavior of rats during the elevated plus-maze test, with particular reference to multiunit recording of the behavior-associated neural activity. Behav Brain Res. 353:168–175. [DOI] [PubMed] [Google Scholar]
- Shoeb A, Chowta M, Pallempati G, Rai A, Singh A.. 2013. Evaluation of antidepressant activity of vanillin in mice. Indian J Pharmacol. 45(2):141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P.. 1985. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 85(3):367–370. [DOI] [PubMed] [Google Scholar]
- Swantara IMD, Bawa IGAG, Suprapta DN, Agustina KK, Temaja IGRM.. 2020. Identification Michelia alba barks extract using gas chromatography-mass spectrometry (GC–MS) and its antifungal properties to inhibit microbial growth. Biodiversitas. 21(4):1541–1550. [Google Scholar]
- Taprial S. 2015. A review on phytochemical and pharmacological properties of Michelia champaca Linn. Family: Magnoliaceae. Int J Pharmacogn. 2:430–436. [Google Scholar]
- Thippeswamy BS, Mishra B, Veerapur VP, Gupta G.. 2011. Anxiolytic activity of Nymphaea alba Linn. in mice as experimental models of anxiety. Indian J Pharmacol. 43(1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ.. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 31(2):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegay A, Damte A, Kiros A.. 2020. Determinants of suicidal ideation among patients with mental disorders visiting psychiatry outpatient unit in Mekelle town, psychiatric clinics, Tigray, Northern Ethiopia: a case-control study. Ann Gen Psychiatr. 19(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA.. 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia V, Garg C, Garg M.. 2018. Anxiolytic-like effect of pyridoxine in mice by elevated plus maze and light and dark box: evidence for the involvement of GABAergic and NO-sGC-cGMP pathway. Pharmacol Biochem Behav. 173:96–106. [DOI] [PubMed] [Google Scholar]
- Wang HM, Lo WL, Huang LY, Wang YD, Chen CY.. 2010. Chemical constituents from the leaves of Michelia alba. Nat Prod Res. 24(5):398–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.