Abstract
Objective
To determine if daily supplementation with cod liver oil, a low dose vitamin D supplement, in winter, prevents SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections in adults in Norway.
Design
Quadruple blinded, randomised placebo controlled trial.
Setting
Norway, 10 November 2020 to 2 June 2021.
Participants
34 601 adults (aged 18-75 years), not taking daily vitamin D supplements.
Intervention
5 mL/day of cod liver oil (10 µg of vitamin D, n=17 278) or placebo (n=17 323) for up to six months.
Main outcome measures
Four co-primary endpoints were predefined: the first was a positive SARS-CoV-2 test result determined by reverse transcriptase-quantitative polymerase chain reaction and the second was serious covid-19, defined as self-reported dyspnoea, admission to hospital, or death. Other acute respiratory infections were indicated by the third and fourth co-primary endpoints: a negative SARS-CoV-2 test result and self-reported symptoms. Side effects related to the supplementation were self-reported. The fallback method was used to handle multiple comparisons.
Results
Supplementation with cod liver oil was not associated with a reduced risk of any of the co-primary endpoints. Participants took the supplement (cod liver oil or placebo) for a median of 164 days, and 227 (1.31%) participants in the cod liver oil group and 228 (1.32%) participants in the placebo group had a positive SARS-CoV-2 test result (relative risk 1.00, multiple comparison adjusted confidence interval 0.82 to 1.22). Serious covid-19 was identified in 121 (0.70%) participants in the cod liver oil group and in 101 (0.58%) participants in the placebo group (1.20, 0.87 to 1.65). 8546 (49.46%) and 8565 (49.44%) participants in the cod liver oil and placebo groups, respectively, had ≥1 negative SARS-CoV-2 test results (1.00, 0.97 to 1.04). 3964 (22.94%) and 3834 (22.13%) participants in the cod liver oil and placebo groups, respectively, reported ≥1 acute respiratory infections (1.04, 0.97 to 1.11). Only low grade side effects were reported in the cod liver oil and placebo groups.
Conclusion
Supplementation with cod liver oil in the winter did not reduce the incidence of SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections compared with placebo.
Trial registration
ClinicalTrials.gov NCT04609423.
Introduction
Vitamin D has received much attention during the covid-19 pandemic for its potential role in preventing and treating covid-19.1 2 3 4 5 6 7 8 9 Preclinical studies have reported a role for vitamin D metabolites in the immune responses to respiratory viruses, although the mechanisms are not fully understood.10 Low levels of 25-hydroxyvitamin D3 (25(OH)D3) have been associated with an increased risk of acute respiratory infections.11 A recent meta-analysis, examining 46 randomised controlled trials, concluded that vitamin D supplementation (400-1000 IU/day or 10-25 µg/day) decreased the risk of acute respiratory infections compared with placebo.12
Serious covid-19 has been associated with increased inflammation with uncontrolled activation of immune cells and excessive release of proinflammatory cytokines.13 Long chained omega 3 fatty acids, particularly eicosapentaenoic acid and docosahexaenoic acid, have been reported to have anti-inflammatory effects.14 15 16 17 Ensuring adequate levels of these fatty acids and vitamin D has been proposed as a cost effective measure to prevent SARS-CoV-2 infection and serious covid-19.13
Cod liver oil is a low dose vitamin D supplement with eicosapentaenoic acid and docosahexaenoic acid. A long tradition exists in Norway of taking cod liver oil during the winter to prevent vitamin D deficiency. Therefore, we initiated the Cod Liver Oil for Covid-19 Prevention Study (CLOC), where participants were randomised to receive cod liver oil or placebo (corn oil) during the winter of 2020-21, and we examined whether cod liver oil could prevent SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections.
Methods
CLOC was a randomised, parallel group treatment, quadruple masked (participant, investigator, outcomes assessor, and data analysts), two armed trial. Supplement 1 provides details of the study protocol, changes to the protocol, and the statistical analysis plan. Ethical approval of the trial was obtained (30 September 2020), and the trial was registered in ClinicalTrials.gov (22 October 2020) before recruitment of participants. The statistical analysis plan was registered in ClinicalTrials.gov (26 November 2021) before the analysis and unblinding of the intervention.
Trial population
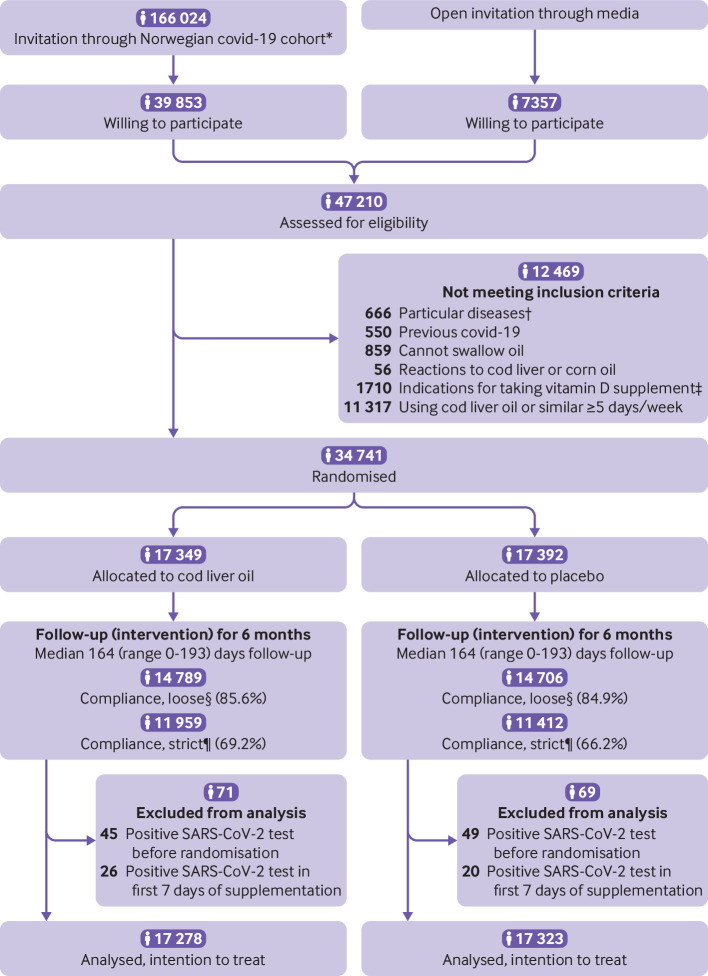
Between 10 November 2020 and 13 April 2021, adults (aged ≥18 years) with a Norwegian personal identity number and electronic access to the secure national digital governmental identification service, were invited to participate in the trial through a media campaign, and invitations were also sent to participants of the Norwegian Covid-19 Cohort Study. Excluded were people with prespecified diseases (including a history of renal failure or dialysis, hypercalcaemia, severe liver disease (cirrhosis), sarcoidosis, or other granulomatous diseases (eg, granulomatosis with polyangiitis (formerly Wegener’s granulomatosis)), and previous covid-19), those who could not swallow oil, those with reactions to fish or cod liver oil, or corn oil, and those with indications for taking vitamin D supplements (vegan, pregnant, aged >75 years). Participants already taking cod liver oil or any other supplements with vitamin D for ≥5 days/week were also excluded, except for those with dark skin when all participants were included, regardless of their use of vitamin D supplements. Figure 1 shows the numbers of eligible randomised participants and the number of participants in the final analyses.
Fig 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of Cod Liver Oil for Covid-19 Prevention Study (CLOC). *Norwegian Covid-19 Cohort Study is a population based open cohort established in March 2020. †Including history of renal failure or dialysis, hypercalcaemia, severe liver disease (cirrhosis), sarcoidosis, or other granulomatous disease (eg, granulomatosis with polyangiitis (formerly Wegener’s granulomatosis)). ‡Vegan, pregnant, ≥75 years old. §Loose compliance: reported consuming >0.1 L of cod liver oil or placebo, consuming cod liver oil or placebo for >0-1 month, or consuming cod liver oil or placebo for >1 day/week. ¶Strict compliance: reported consuming >0.5 L of cod liver oil or placebo or consuming cod liver oil or placebo for >2-3 months
Intervention
Participants were randomised in a 1:1 ratio to take cod liver oil or placebo (corn oil). Both products were oils in liquid form, and the recommended daily dosage was 5 mL. The cod liver oil was flavoured with lemon, and the same lemon aroma was added to the placebo oil. Both products were successfully blind tested by an experienced taste panel (who could not distinguish between the products) who routinely blind test each batch of cod liver oil that is produced. Participants were encouraged to take the supplement at the same time each day, with a 5 mL measurement spoon or tablespoon, according to the instructions they received with the product (a picture of a tablespoon with 5 mL of oil). For the cod liver oil, 5 mL of oil contained about 10 µg of vitamin D3 (400 IU), 1.2 g of long chained omega 3 polyunsaturated fatty acids, including 0.4 g of eicosapentaenoic acid and 0.5 g of docosahexaenoic acid, 250 µg of vitamin A, and 3 mg of vitamin E. For placebo, 5 mL of corn oil contained about 15.8 µg of vitamin A and 3.8 mg of vitamin E (analysed levels of vitamins A, D, and E in cod liver oil and placebo are presented in sTable 1, supplement 2).
Randomisation, blinding, and data storage
Randomisation was conducted at the Department of Research Support, Oslo University Hospital, by staff not involved in the trial (rounds of randomisation are listed in sTable 2, supplement 2). Randomisation was conducted as simple randomisation without blocking or stratification because of the large study population. A list of participants with their addresses and group assignment (cod liver oil or placebo group) was provided to a packaging company, which sent cod liver oil or placebo to participants. Staff at the packaging company were not involved in the trial, and no individuals involved in the trial had access to the list.
Participants and researchers involved in all phases of the trial were blinded to the group assignment of each participant. Unblinding was done when the analysis of all co-primary endpoints was completed (13 December 2021). The University of Oslo’s services for sensitive data was used to collect, store, and analyse the data.
Questionnaires
Participants completed baseline questionnaires before they were randomised to receive cod liver oil or placebo. The questionnaires covered personal data, questions related to vitamin D, and other questions. After randomisation, practical instructions were sent to participants, followed by six (monthly) reporting questionnaires with items on compliance with the intervention, infection with SARS-CoV-2, acute respiratory infections, vaccination for covid-19, open question on side effects, and other questions (sAppendix 1, supplement 2). Compliance was defined as strict if >0.5 L of cod liver oil or placebo was consumed, or cod liver oil or placebo was taken for >2-3 months. Loose compliance was defined as >0.1 L of cod liver oil or placebo consumed, cod liver oil or placebo taken for >0-1 month, or cod liver oil or placebo consumed for >1 day/week. Total participation time for each participant was estimated from the earliest reported start date of taking cod liver oil or placebo until the latest reported final date of taking cod liver oil or placebo. If no start date was reported, the start date was set as the randomisation date plus 14 days. If no final date was reported, the final date was set as the final date of the intervention period (2 June 2021). Side effects were categorised and graded according to the Common Terminology Criteria for Adverse Events (CTCAE). The first questionnaire was sent out on 21 December 2020 and the last on 2 June 2021. The University of Oslo’s web based solution Nettskjema was used to distribute information and questionnaires electronically.
Endpoints: covid-19 and other acute respiratory infections
Four co-primary endpoints were assessed. The first co-primary endpoint was the incidence of a positive SARS-CoV-2 nasopharyngeal or oropharyngeal swab test determined by reverse transcriptase-quantitative polymerase chain reaction in a Norwegian microbiology laboratory reported to the Mandatory Norwegian Surveillance System for Communicable Diseases (MSIS). The second co-primary endpoint was the incidence of serious covid-19 (MSIS confirmed covid-19) with self-reported dyspnoea, or admission to hospital or death. If data were missing for the variable serious covid-19, participants were included in the non-serious covid-19 outcome. Missing information was checked against medical records connected to the Norwegian Cause of Death Registry for information on deaths. The third co-primary endpoint was the incidence of participants with ≥1 negative SARS-CoV-2 test results recorded in MSIS. Most testing in Norway during the trial was conducted after participants showed symptoms of covid-19, and in our data >85% of negative test results were accompanied by symptoms. Thus having ≥1 negative SARS-CoV-2 test results was used as an indication of having ≥1 acute respiratory infections. The fourth co-primary endpoint was the incidence of participants reporting ≥1 acute respiratory infections. Missing information or unreported acute respiratory infections were included in the non-acute respiratory infections outcome during the intervention period.
Prespecified secondary endpoints and exploratory endpoints
Prespecified secondary endpoints (number of participants admitted to hospital for covid-19 and number of participants in the intensive care unit for covid-19) were self-reported. Exploratory endpoints included self-reported side effects, blinding of the study supplement, and change in blood levels of 25(OH)D3 and omega 3 index over the study period (from a subsample of the population).
Dried blood spot samples
To determine the effect of the intervention on levels of 25(OH)D3 and omega 3 fatty acids, capillary blood was collected at home using dried blood spot samples in a random subpopulation of participants. Samples were obtained before and during supplementation with cod liver oil or placebo and analysed by Vitas Analytical Services (Oslo). In total, 945 participants were sent kits twice, and 342 participants returned both kits. Participants received the kits by post and took a fasting blood sample from the fingertip, placing the drops of blood directly onto Whatman 903 Protein Saver dried blood spot cards. Participants then placed the dried blood spot card in a light and airproof Ziploc bag with a desiccant and posted it to Vitas Analytical services. Samples were stored at −80°C until analysed after the intervention period (2–9 months after the samples were collected).
For analyses of 25(OH)D3, dried blood spot punches (four circles, diameter 3.2 mm, with dried whole blood on certified paper) of human whole blood were diluted with water. After whole blood extraction, analytes were extracted with 2-propanol containing an internal standard. After analytes were extracted, samples were centrifuged through a filter plate for removal of whole blood debris. The eluate was injected into a Ultivo Triple Quadrupole liquid chromatography-mass spectrometer (Agilent Technologies, Santa Clara, CA), separated by a Kinetex 2.6 µm C18 100 Å liquid chromatography column 100×4.6 mm (Phenomenex, Torrance, CA) (Vitas Analytical Services). The accuracy of the 25(OH)D3 analyses was maintained by participation in the Vitamin D External Quality Assessment Scheme (DEQAS, sAppendix 2 and sTable 3, supplement 2).
For analyses of omega 3 fatty acids, two punches of human whole blood were methylated with sodium methylate. After methylation, fatty acid methyl esters were extracted with hexane. After thorough mixing and centrifugation, 3 µL of the aliquot were injected into a gas chromatography-flame ionisation detector. Gas chromatography-flame ionisation detection was performed with an Agilent HP 7890A Gas Chromatograph System (Agilent Technologies, Palo Alto, CA). The fatty acid methyl esters were separated on a TR-FRAME gas chromatography column (30 m×0.25 mm×0.25 µm film column) from Thermo Scientific (part No 260M142P) (Vitas Analytical Services).
Baseline SARS-CoV-2 antibody analysis
Randomly selected participants (n=1333) provided whole blood samples to a local laboratory for analysis of SARS-CoV-2 antibodies at baseline. A flow cytometer based method was used to identify IgG antibodies against SARS-CoV-2 derived recombinant antigens in residual sera.18 Samples with antibodies against both the receptor binding domain and the spike protein of SARS-CoV-2 were considered positive.
Statistical analyses
Power calculation was done with the fallback method, used to adjust for multiple testing.19 Sample size calculations were based on our own unpublished results from the Norwegian Covid-19 Cohort Study. A significance level (α) of 0.05 was divided between the four co-primary endpoints (0.03, 0.018, 0.001, and 0.001, respectively). The α levels were different because the four endpoints were weighted differently. The first and second (covid-19 related) co-primary endpoints were assigned an α value of 0.03 and 0.018, respectively. Based on an expected incidence of SARS-CoV-2 infection of 1%, a 20% reduction in the incidence of SARS-CoV-2 infection in the intervention group, and power of 70%, 65 000 participants were required for the first co-primary endpoint. Based on an incidence of serious covid-19 of 0.25%, a 40% reduction in serious covid-19 in the intervention group, and power of 70%, 67 000 participants were required for the second co-primary endpoint. Based on the expected frequency of acute respiratory infections of >30% and a threshold of 10% reduction in acute respiratory infections, 23 000 participants would need to be included for the third and fourth co-primary endpoints with a power of >95%. An α value of 0.001 was assigned for the third and fourth co-primary endpoints. Multiple comparison adjusted confidence intervals are reported for the four co-primary endpoints (97%, 98.2%, 99.9%, and 99.9% confidence intervals, respectively).
All four co-primary endpoints were analysed for the relative risk of acquiring a condition using cod liver oil versus placebo with the Wald test. The time to first occurrence of the co-primary endpoints was plotted with the Kaplan-Meier approach. Logistic regression and the Wald test were used in the subgroup analyses (grouped by sex, age, body mass index, skin type, exposure to sun from July to October 2020, use of vitamin D supplements, vaccinated during the study period, consumers of fatty fish, and strict compliance) to assess the effect on the co-primary endpoints. These ad hoc subgroup analyses were chosen as they could affect (or cause a known difference in) levels of 25(OH)D3 or omega 3 fatty acids, or affect the incidence of SARS-CoV-2 infection or serious covid-19.
The Wilcoxon signed rank test, the Mann-Whitney test, and linear regression were used to compare 25(OH)D3 and omega 3 index levels and changes between and within the cod liver oil and placebo groups (α<0.05). Pearson’s χ2 test was used to examine side effects and which treatment participants thought they were assigned to in the cod liver oil and placebo groups (α<0.05).
Participant and public involvement
The Norwegian Covid-19 Cohort Study had a user panel that was involved in the planning of the CLOC study, including setting the research agenda and planning of the questionnaires.
Results
Trial participants
Overall, 34 741 men and women were randomised to receive cod liver oil or placebo, and 140 participants were excluded from the analyses because of a positive SARS-CoV-2 test result during the period from consent to participation up to seven days after starting to take cod liver oil or placebo. Participants took the supplement (cod liver oil or placebo) for a median of 164 days. We included 17 278 and 17 323 participants in the cod liver oil and placebo groups, respectively, in the analyses (fig 1).
More than half of participants were women (64.5%), mean age was 44.9 years, and mean body mass index was 26.1 at baseline (table 1). Most participants (75.5%) did not use vitamin D supplements before enrolling in the trial, 61.5% consumed fatty fish, and 39.8% reported ≤30 hours of exposure to the sun from July to October 2020. Subsample analyses for SARS-CoV-2 antibodies at baseline showed that 28 of 1333 participants (2.1%) had a positive antibody test result (data not shown). During the intervention, 35.6% of participants (6233 and 6097 in the cod liver oil and placebo groups, respectively) reported receiving ≥1 doses of a SARS-CoV-2 vaccine (table 1).
Table 1.
Characteristics of participants at baseline, according to randomisation to cod liver oil or placebo group. Data are number (%) of participants unless stated otherwise
| Characteristics | Overall (n=34 601) | Cod liver oil group (n=17 278) | Placebo group (n=17 323) |
|---|---|---|---|
| Sex: | |||
| Women | 22 346 (64.6) | 11 161 (64.6) | 11 185 (64.6) |
| Men | 12 254 (35.4) | 6117 (35.4) | 6137 (35.4) |
| Mean (SD) age (years) | 44.9 (13.4) | 45.0 (13.5) | 44.9 (13.4) |
| Mean (SD) body mass index | 26.1 (4.7) | 26.1 (4.7) | 26.1 (4.7) |
| Smoking: | |||
| Never | 17 770 (51.4) | 8906 (51.5) | 8864 (51.2) |
| Past smoker | 12 116 (35.0) | 6025 (34.9) | 6091 (35.2) |
| Current smoker | 2687 (7.8) | 1340 (7.8) | 1347 (7.8) |
| Chronic disease*: | |||
| No chronic disease | 25 403 (73.4) | 12 658 (73.3) | 12 745 (73.6) |
| ≥1 chronic diseases | 7669 (22.2) | 3852 (22.3) | 3817 (22.0) |
| Parental ethnic origin: | |||
| Europe | 32 831 (94.9) | 16 441 (95.2) | 16 390 (94.6) |
| Asia | 720 (2.1) | 358 (2.1) | 362 (2.1) |
| Africa | 217 (0.6) | 108 (0.6) | 109 (0.6) |
| Other | 576 (1.7) | 268 (1.6) | 308 (1.8) |
| Skin type: | |||
| Easily burnt | 6453 (18.7) | 3270 (18.9) | 3183 (18.4) |
| Sometimes burnt to never burnt | 25 809 (74.6) | 12 895 (74.7) | 12 914 (74.6) |
| Naturally tanned | 695 (2.0) | 334 (1.9) | 361 (2.1) |
| Sun exposure from July to October 2020: | |||
| ≤30 hours | 13 752 (39.8) | 6924 (40.1) | 6828 (39.4) |
| >30 hours | 20 197 (58.4) | 10 035 (58.1) | 10 162 (58.6) |
| Vitamin D supplement use†: | |||
| No | 26 130 (75.5) | 13 092 (75.8) | 13 038 (75.3) |
| Yes | 7705 (22.3) | 3799 (22.0) | 3906 (22.5) |
| Fatty fish consumer‡: | |||
| No | 12 714 (36.7) | 6381 (36.9) | 6333 (36.6) |
| Yes | 21 285 (61.5) | 10 601 (61.4) | 10 684 (61.7) |
| Household count: | |||
| 1 | 5351 (15.5) | 2703 (15.6) | 2648 (15.3) |
| 2 | 10 981 (31.7) | 5477 (31.7) | 5504 (31.8) |
| ≥3 | 16 979 (49.0) | 8462 (48.9) | 8517 (49.1) |
| Children in household: | |||
| 0 | 19 120 (55.3) | 9549 (55.3) | 9571 (55.3) |
| 1 | 4943 (14.3) | 2507 (14.5) | 2436 (14.1) |
| ≥2 | 9190 (26.5) | 4543 (26.3) | 4647 (26.8) |
| Education: | |||
| Primary or lower secondary school§ | 895 (2.6) | 466 (2.7) | 429 (2.5) |
| Secondary school or vocational programmes¶ | 6988 (20.2) | 3469 (20.1) | 3519 (20.3) |
| Higher education | 22 529 (65.1) | 11 302 (65.4) | 11 227 (64.8) |
| Household income (NOK): | |||
| ≤1 million | 18 290 (52.9) | 9108 (52.6) | 9182 (53.0) |
| >1 million | 14 444 (41.7) | 7274 (42.1) | 7170 (41.4) |
| Occupational status: | |||
| Working or student | 28 634 (82.8) | 14 325 (82.9) | 14 309 (82.6) |
| Retired | 2566 (7.4) | 1294 (7.5) | 1272 (7.3) |
| Unemployed, sick leave, or social security | 3253 (9.4) | 1595 (9.2) | 1658 (9.6) |
| Other | 1352 (3.9) | 668 (3.9) | 684 (3.9) |
| Vaccinated during study period** | 12 330 (35.6) | 6233 (36.1) | 6097 (35.2) |
SD=standard deviation; NOK=Norwegian kroner (1 Kr; £0.09; €0.10; $0.10).
Data were missing for 1.7-5.4% of participants except for the variable education where data were missing for 11.9% of participants.
One or more of these chronic conditions: heart disease, hypertension, lung disease, asthma, diabetes, cancer, and other, or treated with immunosuppressants.
Taking vitamin D supplements (including cod liver oil) ≥5 days/week was an exclusion criterion but individuals with a lower frequency of use were included.
Consuming fatty fish ≥1-2 days/week or ≥1-3 slices of bread with fatty fish, or both.
≤10 years of school (seven years of primary school, three years of lower secondary school).
Upper secondary school or vocational programmes (usually about three years).
Reported having ≥1 SARS-CoV-2 vaccines during the intervention period.
Cod liver oil and covid-19
In total, 455 participants had a positive SARS-CoV-2 test result, with similar event rates in the cod liver oil and placebo groups (227 (1.31%) and 228 (1.32%) respectively; relative risk 1.00, 97.0% confidence interval 0.82 to 1.22, table 2). The Kaplan-Meier curve showed similar rates of positive SARS-CoV-2 test results in the cod liver oil and placebo groups (fig 2).
Table 2.
Absolute and relative risk, and confidence intervals, for first, second, third, and fourth co-primary endpoints, according to randomisation to cod liver oil or placebo group, in intention-to-treat analyses
| Co-primary endpoint | Overall (n=34 601) (No (%)) |
Cod liver oil group (n=17 278) | Placebo group (n=17 323) |
Relative risk (CI*) | P value† | |||
|---|---|---|---|---|---|---|---|---|
| No | Absolute risk (% (CI*)) |
No | Absolute risk (% (CI*)) | |||||
| Covid-19 | ||||||||
| First: SARS-CoV-2 positive test result | 455 (1.32) | 227 | 1.31 (1.13 to 1.50) | 228 | 1.32 (1.13 to 1.50) | 1.00 (0.82 to 1.22) | 0.98 | |
| Second: serious covid-19‡ | 222 (0.64) | 121 | 0.70 (0.55 to 0.85) | 101 | 0.58 (0.45 to 0.72) | 1.20 (0.87 to 1.65) | 0.17 | |
| Acute respiratory infections | ||||||||
| Third: ≥1 SARS-CoV-2 negative test results | 17 111 (49.45) | 8546 | 49.46 (48.21 to 50.71) | 8565 | 49.44 (48.19 to 50.69) | 1.00 (0.97 to 1.04) | 0.97 | |
| Fourth: ≥1 self-reported acute respiratory infections | 7798 (22.54) | 3964 | 22.94 (21.89 to 24.00) | 3834 | 22.13 (21.09 to 23.17) | 1.04 (0.97 to 1.11) | 0.07 | |
First and second co-primary endpoints (covid-19), 97.0% and 98.2% confidence interval, respectively; third and fourth co-primary endpoints (acute respiratory infections), 99.9% confidence interval.
Logistic procedure P value for difference between cod liver oil and placebo groups determined with the Wald test.
SARS-CoV-2 positive test result and self-reported dyspnoea (n=222), admission to hospital (n=17, eight in the cod liver oil group and nine in the placebo group), or death (n=0). Data were missing for n=17 (13 in the cod liver oil group and four in the placebo group) for the variable serious covid-19; these were included in the non-serious covid-19 outcome.
Fig 2.
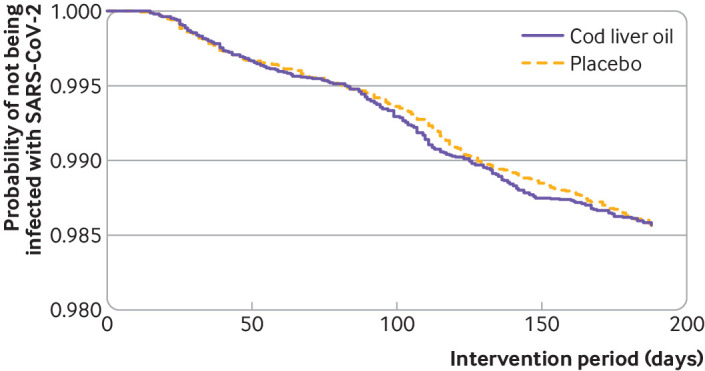
Kaplan-Meier plot of the probability of a positive SARS-CoV-2 test result for participants in the cod liver oil (n=17 278) and placebo (n=17 323) groups during the intervention period
Serious covid-19 was reported by 222 participants (121 (0.70%) in the cod liver oil group and 101 (0.58%) in the placebo group), all had dyspnoea, 17 were admitted to hospital (eight in the cod liver oil group and nine in the placebo group), and no participants died. Of those admitted to hospital for covid-19, eight participants (four in each group) were in the intensive care unit. The relative risk of serious covid-19 was 1.20 (98.2% confidence interval 0.87 to 1.65, table 2) for the cod liver oil group compared with the placebo group. Analyses stratified by sex, age, body mass index, exposure to sun from July to October 2020, use of vitamin D supplements, vaccinated during the study period, consumers of fatty fish, and strict compliance did not modify the effect of cod liver oil on having a positive SARS-CoV-2 test result or serious covid-19 (sTable 4, supplement 2).
Cod liver oil and acute respiratory infections
Our results showed that 17 111 participants had ≥1 negative SARS-CoV-2 test results, with similar event rates in the cod liver oil and placebo groups (8546 (49.46%) and 8565 (49.44%), respectively; relative risk 1.00, 99.9% confidence interval 0.97 to 1.04, table 2). One or more acute respiratory infections were reported by 7798 participants (3964 (22.94%) and 3834 (22.13%) participants in the cod liver oil and placebo groups, respectively). The relative risk of having ≥1 acute respiratory infections was 1.04 (99.9% confidence interval 0.97 to 1.11, table 2) for the cod liver oil group compared with the placebo group. Analyses stratified by sex, age, body mass index, skin type, exposure to sun from July to October 2020, use of vitamin D supplements, vaccinated during the study period, consumers of fatty fish, and strict compliance did not appear to modify the effect of cod liver oil on having ≥1 negative SARS-CoV-2 test results or ≥1 acute respiratory infections (sTable 5 and sTable 6, supplement 2).
Blood levels of 25(OH)D3 and omega 3 index
Of 945 participants who were sent a dried blood spot kit for measuring levels of 25(OH)D3 and omega 3 index before and while taking the supplements, 342 returned two complete samples (172 in the cod liver oil group and 170 in the placebo group, table 3). From the first to the second measurement, participants in the cod liver oil group had only slightly increased concentrations of 25(OH)D3 (median 4.4 nmol/L, 25th to 75th centiles −14.4-23.3, P=0.06). Cod liver oil was observed to prevent a reduction in 25(OH)D3 in winter, however, as found in the placebo group (−12.5 nmol/L, −24.1-4.1, P<0.001). In the cod liver oil group, the mean concentration of 25(OH)D3 was increased by 15.0 nmol/L (95% confidence interval 8.8 to 21.2, P<0.001) and the omega 3 index by 1.9% (1.6 to 2.2, P<0.001) compared with the placebo group. Our results showed that 295 (86.3%) participants had concentrations of 25(OH)D3 ≥50 nmol/L before the intervention period (before supplementation). During the intervention period (during supplementation), 155 (90.1%) and 123 (72.4%) participants in the cod liver oil and placebo groups, respectively, had concentrations ≥50 nmol/L.
Table 3.
Exploratory endpoints; side effects, blinding, and measured compliance according to randomisation to cod liver oil or placebo group. Data are median (25th to 75th centiles) or number (%) unless stated otherwise
| Overall (n=34 601) | Cod liver oil group (n=17 278) | Placebo group (n=17 323) | P value | |
|---|---|---|---|---|
| Measured compliance, from dried blood spots of a subsample* | ||||
| 25-hydroxyvitamin D3 (nmol/L): | ||||
| Before supplementation | 70.5 (56.7-92.3) | 66.9 (52.2-91.0) | 73.3 (59.6-92.7) | 0.04† |
| During supplementation | 67.9 (54.0-85.6) | 74.1 (60.1-88.0) | 62.8 (48.2-81.6) | <0.001† |
| Change | −3.6 (−20.9-14.4) | 4.4 (−14.4-23.3) | −12.5 (−24.1-4.1)‡‡ | <0.001† |
| Omega 3 index (%): | ||||
| Before supplementation | 4.6 (3.7-5.7) | 4.6 (3.7-5.5) | 4.6 (3.7-5.8) | 0.62† |
| During supplementation | 5.1 (3.8-6.5) | 6.2 (5.3-7.4) | 4.1 (3.4-5.0) | <0.001† |
| Change | 0.3 (−0.7-1.7) | 1.5 (0.4-2.5)‡‡ | −0.5 (−1.1-0.1)‡‡ | <0.001† |
| Side effects | ||||
| Reported ≥1 side effects | 3708 (10.7) | 1742 (10.1) | 1966 (11.3) | <0.001‡ |
| Grade§: | ||||
| 1 | 3640 (10.5) | 1721 (10.0) | 1919 (11.1) | <0.001‡ |
| 2¶ | 68 (0.2) | 21 (0.1) | 47 (0.3) | 0.002‡ |
| Most commonly self-reported: | ||||
| Nausea, vomiting | 1519 (4.4) | 694 (4.0) | 825 (4.8) | <0.001‡ |
| Regurgitation, burping | 854 (2.5) | 420 (2.4) | 434 (2.5) | 0.68‡ |
| Stomach symptoms** | 826 (2.4) | 369 (2.1) | 457 (2.6) | 0.002‡ |
| Reflux | 772 (2.2) | 384 (2.2) | 388 (2.2) | 0.94‡ |
| Response to question “What supplement did you think you were taking?”†† | ||||
| Cod liver oil | 3024 (16.9) | 1966 (21.4) | 1058 (12.2) | <0.001‡ |
| Placebo | 11 458 (64.2) | 5364 (58.4) | 6094 (70.3) | <0.001‡ |
| Do not know | 3378 (18.9) | 1856 (20.2) | 1522 (17.5) | <0.001‡ |
Subsample of 342 participants (cod liver oil group n=172, placebo group n=170) with dried blood spot samples from before randomisation (before supplementation) and during the intervention period (during supplementation).
Mann-Whitney U test, cod liver oil versus placebo group.
Pearson χ2 test, cod liver oil versus placebo group.
Graded according to the Common Terminology Criteria for Adverse Events (CTCAE).
Of the CTCAE grade 2 side effects, self-reported low vitamin D levels were n=8 in the cod liver group and n=36 in the placebo group.
Stomach symptoms included stomach pain, diarrhoea, and constipation.
Answered by 17 860 (51.6%) participants.
Significant change (Wilcoxon signed rank test) from before to during supplementation, P<0.001.
Side effects and blinding of trial participants
Overall, 10.1% and 11.3% of participants in the cod liver oil and placebo groups, respectively, reported ≥1 side effects while taking the supplements during the intervention period (table 3). The most common side effects were mild gastrointestinal symptoms, classified as CTCAE grade 1. The side effects classified as CTCAE grade 2 were more common in the placebo group than in the cod liver oil group because of a higher number of participants self-reporting low levels of vitamin D in the placebo group (n=36 v n=8, respectively). The other CTCAE grade 2 symptoms reported by participants were similarly distributed in the cod liver oil (n=13) and placebo (n=11) groups and included heart palpitations, allergic reactions, and gastrointestinal symptoms.
In the last reporting questionnaire, 17 860 (51.6%) participants responded to the question about which type of supplement they thought they had been taking. Of these, 7220 (78.6%) in the cod liver oil group and 7616 (87.8%) in the placebo group believed they had been taking a placebo or did not know, whereas 1966 (21.4%) in the cod liver oil group and 1058 (12.2%) in the placebo group thought they had been taking cod liver oil (table 3).
Discussion
In this large, randomised, primary prevention trial, supplementation with cod liver oil, a low dose vitamin D supplement, was not associated with a reduction in the incidence of SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections compared with placebo. Only low grade side effects were reported, and fewer participants reported side effects in the cod liver oil group than in the placebo group.
The null findings of supplementation with cod liver oil on the risk of SARS-CoV-2 infection and serious covid-19 in our trial are in line with the results of a mendelian randomisation study that found no association between concentrations of 25(OH)D3 and risk of SARS-CoV-2 infection or serious covid-19, and the authors concluded that vitamin D supplementation would have had no preventive effect.7 Our findings are also similar to the results of a large trial on vitamin D and covid-19 (published as a preprint), although participants had lower levels of 25(OH)D3 initially than our subsample and were given larger vitamin D doses.20 Our null findings contrast with a recent small double blind, placebo controlled trial suggesting that vitamin D supplements prevented covid-19 in people at high risk of SARS-CoV-2 infection.21 The supplementation regimen differed from ours, however, with 4000 IU of vitamin D given every day for one month, and 67% of participants had 25(OH)D3 concentrations <50 nmol/L at the start of the trial. Our null findings also contrast our own unpublished, preliminary unadjusted results (Norwegian Covid-19 Cohort Study), indicating that participants taking cod liver oil had a reduced risk of developing covid-19 and of being admitted to hospital for covid-19 than non-users. Furthermore, two large observational studies (app based European/American study and UK Biobank study) have found an association between the use of vitamin D supplements and reduced risk of covid-19.1 22
A meta-analysis described a protective effect of vitamin D supplements against acute respiratory infections, with the strongest effect for supplement doses of 400-1000 IU/day for up to a year.12 In a more recent meta-analysis (published as a preprint), however, the authors questioned these initial results because clustering was not accounted for in one of the cluster randomised controlled trials included. The authors did a secondary analysis of these data, accounting for clustering in the randomised controlled trial, and the updated meta-analysis showed no protective effect of vitamin D supplements on acute respiratory infections.23 This finding is in line with our trial of no protective effect of vitamin D supplementation against acute respiratory infections.
In our trial, 86% of participants in the subsample with dried blood spot tests had 25(OH)D3 concentrations ≥50 nmol/L before the trial started, which has been suggested as an adequate level.2 3 24 25 Thus the possibility that an effect of supplementation with vitamin D on the risk of SARS-CoV-2 infection, serious covid-19, and other acute respiratory infections was missed because of adequate concentrations of 25(OH)D3 at the start of the trial cannot be excluded. We found no difference in the relative risk for the co-primary endpoints when stratified by factors associated with levels of 25(OH)D3 before the trial started (such as exposure to sun from July to October 2020, use of vitamin D supplements, and consuming fatty fish). The proportion of participants with vitamin D deficiency (25(OH)D3 <30 nmol/L) in the trial population, however, was most likely low. Our trial had a practical and realistic approach to supplementation with vitamin D for the prevention of covid-19 and other acute respiratory infections, testing whether those not taking vitamin D supplements at the start of the trial would benefit from supplementation during the winter. Widespread testing for 25(OH)D3 levels and only providing supplements for those with low levels would require a different study design.
Few studies have examined omega 3 fatty acids and the risk of SARS-CoV-2 infection, serious covid-19, and other acute respiratory infections. The NutriNet-Santé cohort found no association between dietary intake of omega 3 fatty acids and susceptibility to covid-19.26 Conversely, others have seen an association between omega 3 supplementation and reduced risk of covid-191 and upper respiratory tract infections.27 Also, an inverse association between the omega 3 index in blood and death from covid-19 has been reported.28 The effect of vitamins A and E, also present in cod liver oil, on SARS-CoV-2 infection, serious covid-19, and other acute respiratory infections is not known. Vitamin D has been suggested to reduce the risk of severe asthma exacerbations in those with mild to moderate asthma,29 whereas a Norwegian study found an increase in the incidence of adult onset asthma with the use of cod liver oil.30 Vitamin A levels in the cod liver oil used in that study were more than three times the daily amount provided by the cod liver oil in our study, however. We believe it is unlikely that vitamin A or E would have masked any effect of vitamin D or omega-3 fatty acids on covid-19 disease or other acute respiratory infections.1 31
Only low grade side effects were reported in our trial. More side effects were reported by participants in the placebo group than in the cod liver oil group, including low levels of vitamin D.
Strengths and limitations of this trial
Limitations of our trial include self-reported data for two of the four endpoints: dyspnoea, defining serious covid-19, and self-reported acute respiratory infections. Self-experienced symptoms are important for participants, however, and the objective MSIS based endpoints corroborated these results. Compliance was self-reported, and therefore we had no data on compliance from participants not returning our questionnaires. Hence we could not distinguish between not responding to the questionnaires and being compliant with the intervention. However, the results were comparable for the strict compliant subgroup and the whole trial population.
The median intervention time was relatively short at 164 days, and we do not have data on the possible longer term effects of cod liver oil. Also, we could not distinguish between the potential effect of vitamin D and eicosapentaenoic acid or docosahexaenoic acid, or explore a dose-response relation. Concentrations of 25(OH)D3 and omega-3 index were only available for a small subsample of participants and thus we could not study how levels of 25(OH)D3 at the start of the trial were related to the risk of SARS-CoV-2 infection and other acute respiratory infections. The available 25(OH)D3 results indicated that our trial population seemed to have adequate levels of 25(OH)D3 at inclusion in the trial.
We aimed to include 80 000 participants in our trial but we could only include 34 601. Because of fewer covid-19 incident cases than expected (455 instead of 650), the trial was slightly underpowered to detect a 20% reduction in the incidence of SARS-CoV-2 infection. Serious covid-19 was more prevalent than anticipated, however, and the trial was adequately powered for the second, third and fourth co-primary endpoint despite the reduced numbers of participants enrolled in the trial.
Our trial had several strengths, including a large general adult population that did not use vitamin D supplements regularly before the start of the trial, implying that our vitamin D supplementation regimen was realistic for testing whether this population would benefit from supplements during the winter. We found good compliance with the study supplement among participants, and blinding was successful because most participants thought they had been taking a placebo or did not know. The SARS-CoV-2 swab test, analysed by reverse transcriptase-quantitative polymerase chain reaction in an accredited Norwegian microbiology laboratory, was the basis for the first and third co-primary endpoints (positive and negative tests, respectively). Also, analysis of SARS-CoV-2 antibodies in a subsample of participants at baseline confirmed that only 2.1% had been infected with SARS-CoV-2 previously.
Conclusions
Daily supplementation with cod liver oil, a low dose vitamin D, eicosapentaenoic acid, and docosahexaenoic acid supplement, for six months during the SARS-CoV-2 pandemic among Norwegian adults, did not reduce the incidence of SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections. Only low grade side effects were reported.
What is already known on this topic
Vitamin D has been suggested as having a role in the prevention of covid-19, but most studies have been observational
A recent meta-analysis of 46 randomised controlled trials showed that vitamin D supplementation decreased the risk of acute respiratory infections compared with placebo, but the effect was small
What this study adds
Of 34 601 unselected adult participants, no difference in the incidence of SARS-CoV-2 infection, serious covid-19, or acute respiratory infections was found for those randomised to daily supplements of low dose vitamin D (cod liver oil) or placebo (corn oil) during the winter
The cod liver oil and placebo group had similar side effects, and only low grade side effects were reported
Acknowledgments
We thank all participants for contributing to the trial every morning by taking the cod liver oil or placebo and completing the questionnaires; the user panel of the Norwegian Covid-19 Cohort Study for their involvement in the planning of the CLOC study; and the following contributors to the project: statistician Inge Olsen, Department of Research Support, Oslo University Hospital, for discussions on study design and conducting the randomisation; Inger Tvenning, Department of Research Support, Oslo University Hospital, for practical work with blinding and unblinding; and Kay Frode Hofstad, Bring AS, who successfully led the work of delivering the tens of thousands of bottles of cod liver oil or placebo to participants.
Web extra.
Extra material supplied by authors
Web appendix 1: Supplement 1—study protocol and data analysis plan
Web appendix 2: Supplement 2—supplemental online content
Contributors: AS, KTK, and CLS conceptualised the trial. SHB, ABN, KTK, CLS, KBH, SMU, AH, JAD, HEM, and AS designed the trial. All authors acquired, analysed, or interpreted the data. SHB, ABN, and TH curated the data and did the statistical analyses. SHB and ABN drafted the manuscript. ME-D, PH, MSI, KTK, CLS, KBH, SMU, AH, TH, FL-J, JAD, HEM, and AS critically revised the manuscript for important intellectual content. MSI provided administrative and technical support. KBH, SMU, AH, JAD, HEM, and AS supervised. All authors approved the final manuscript and work. SHB and ABN contributed equally to this article and act as guarantors. The corresponding author, AS, attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was funded by Orkla Health AS, the manufacturer of Möller’s Tran, the cod liver oil used in the trial. Orkla Health delivered cod liver oil or placebo to the trial and had the right to comment on the manuscript content. It had no role in the conduct of the trial, collection of data, management, or analysis of the data, nor preparation of the manuscript. Orkla Health also had no influence on the decision to submit the manuscript for publication or which journal the manuscript was submitted to. The trial was also funded by Oslo University Hospital and the University of Oslo; they had no role in the trial.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Orkla Health AS, Oslo University Hospital, and the University of Oslo for the submitted work. During the past three years, KBH has received research grants or personal fees from Olympic Seafood, Amgen, and Sanofi, not related to the content of this manuscript. SMU has received a research grant from Olympic Seafood during the past three years, not related to the content of this manuscript. AS and KTK are employed by Age Laboratories AS, a company developing SARS-CoV-2 diagnostics, and FL-J has received grants from Helse-SørØst for developing SARS-CoV-2 diagnostics, not related to the content of this manuscript. The authors declare; no support from any organisation for the submitted work (except from the funders); no financial relationship with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (SHB and ABN) affirm that this manuscript is an honest, accurate, and transparent account of the trial being reported; that no important aspects of the trial have been omitted; and that any discrepancies from the trial as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Participants of the CLOC study will be informed of the results through the website https://www.transtudien.no/, they will be sent details of the results in a study newsletter, and we expect interest from the media who will disseminate the results of the trial to a broader audience.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
All participants signed an electronic informed consent form. All procedures involving participants were approved by the Norwegian Regional Committee for Medical Research Ethics (REK-172796) and by the Data Protection Officer at Oslo University Hospital following the European GDPR. The trial was carried out according to the guidelines in the Declaration of Helsinki.
Data availability statement
Individual level data from the trial for the purposes outlined in the consent form can be shared with other researchers in a timely fashion. The data are regulated under the European GDPR regulative and sharing of data must be approved by the Data Protection Officer at Oslo University Hospital. The data dictionary, informed consent form, and analytic code will be made available at: https://oslo-universitetssykehus.no/kliniske-studier/forebygging-av-covid-19-med-tran at the time of publication. Data will be made available for researchers whose proposed use of the data has been approved.
References
- 1. Louca P, Murray B, Klaser K, et al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr Prev Health 2021;4:149-57. 10.1136/bmjnph-2021-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oscanoa TJ, Amado J, Vidal X, Laird E, Ghashut RA, Romero-Ortuno R. The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration - a metaanalysis. Adv Respir Med 2021;89:145-57. 10.5603/ARM.a2021.0037. [DOI] [PubMed] [Google Scholar]
- 3. Kaya MO, Pamukçu E, Yakar B. The role of vitamin D deficiency on COVID-19: a systematic review and meta-analysis of observational studies. Epidemiol Health 2021;43:e2021074. 10.4178/epih.e2021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism 2021;119:154753. 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wise J. Covid-19: Evidence is lacking to support vitamin D’s role in treatment and prevention. BMJ 2020;371:m4912. 10.1136/bmj.m4912. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Mei K, Xie L, et al. Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: a meta-analysis and GRADE assessment of cohort studies and RCTs. Nutr J 2021;20:89. 10.1186/s12937-021-00744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler-Laporte G, Nakanishi T, Mooser V, et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study. PLoS Med 2021;18:e1003605. 10.1371/journal.pmed.1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talaei M, Faustini S, Holt H, et al. Determinants of pre-vaccination antibody responses to SARS-CoV-2: a population-based longitudinal study (COVIDENCE UK). BMC Med 2022;20:87. 10.1186/s12916-022-02286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holt H, Talaei M, Greenig M, et al. Risk factors for developing COVID-19: a population-based longitudinal study (COVIDENCE UK). Thorax 2021;thoraxjnl-2021-217487. 10.1136/thoraxjnl-2021-217487. [DOI] [PubMed] [Google Scholar]
- 10. Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015;7:4240-70. 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pham H, Rahman A, Majidi A, Waterhouse M, Neale RE. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis. Int J Environ Res Public Health 2019;16:E3020. 10.3390/ijerph16173020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jolliffe DA, Camargo CA, Jr, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol 2021;9:276-92. 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 13. Story MJ. Essential sufficiency of zinc, ω-3 polyunsaturated fatty acids, vitamin D and magnesium for prevention and treatment of COVID-19, diabetes, cardiovascular diseases, lung diseases and cancer. Biochimie 2021;187:94-109. 10.1016/j.biochi.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei Y, Meng Y, Li N, Wang Q, Chen L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: a systematic review and meta-analysis. Food Funct 2021;12:30-40. 10.1039/D0FO01976C. [DOI] [PubMed] [Google Scholar]
- 15. Zárate R, El Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med 2017;6:25. 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang J, Li K, Wang F, et al. Effect of marine-derived n-3 polyunsaturated fatty acids on major eicosanoids: a systematic review and meta-analysis from 18 randomized controlled trials. PLoS One 2016;11:e0147351. 10.1371/journal.pone.0147351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun 2012;26:988-95. 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holter JC, Pischke SE, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A 2020;117:25018-25. 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CBER). Multiple Endpoints in Clinical Trials, Guidance for Industry . https://www.fda.gov/media/102657/.
- 20. Jolliffe DA, Holt H, Greenig M, et al. Vitamin D supplements for prevention of Covid-19 or other acute respiratory infections: a phase 3 randomized controlled trial (CORONAVIT). medRxiv 2022:2022.03.22.22271707. 10.1101/2022.03.22.22271707 [DOI]
- 21. Villasis-Keever MA, López-Alarcón MG, Miranda-Novales G, et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. a randomized clinical trial. Arch Med Res 2022;53:423-30. 10.1016/j.arcmed.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma H, Zhou T, Heianza Y, Qi L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank. Am J Clin Nutr 2021;113:1275-81. 10.1093/ajcn/nqaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bolland MJ, Avenell A, Grey A, et al. Vitamin D and acute respiratory infection: secondary analysis of a previous randomised controlled trial and updated meta-analyses. medRxiv 2022:2022.02.03.22270409. 10.1101/2022.02.03.22270409 [DOI]
- 24. Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D deficiency - is there really a pandemic? N Engl J Med 2016;375:1817-20. 10.1056/NEJMp1608005. [DOI] [PubMed] [Google Scholar]
- 25.Nordic Council of Ministers, Nordic Council of Ministers Secretariat. Nordic Nutrition Recommendations, 2012: Integrating nutrition and physical activity. https://www.norden.org/en/publication/nordic-nutrition-recommendations-2012.
- 26. Deschasaux-Tanguy M, Srour B, Bourhis L, et al. SAPRIS-SERO study group . Nutritional risk factors for SARS-CoV-2 infection: a prospective study within the NutriNet-Santé cohort. BMC Med 2021;19:290. 10.1186/s12916-021-02168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raposo SE, Fondell E, Ström P, et al. Intake of vitamin C, vitamin E, selenium, zinc and polyunsaturated fatty acids and upper respiratory tract infection-a prospective cohort study. Eur J Clin Nutr 2017;71:450-7. 10.1038/ejcn.2016.261. [DOI] [PubMed] [Google Scholar]
- 28. Asher A, Tintle NL, Myers M, Lockshon L, Bacareza H, Harris WS. Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot Essent Fatty Acids 2021;166:102250. 10.1016/j.plefa.2021.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martineau AR, Cates CJ, Urashima M, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev 2016;9:CD011511. 10.1002/14651858.CD011511.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mai X-M, Langhammer A, Chen Y, Camargo CA, Jr. Cod liver oil intake and incidence of asthma in Norwegian adults--the HUNT study. Thorax 2013;68:25-30. 10.1136/thoraxjnl-2012-202061. [DOI] [PubMed] [Google Scholar]
- 31. Vlieg-Boerstra B, de Jong N, Meyer R, et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy 2022;77:1373-88. 10.1111/all.15136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Supplement 1—study protocol and data analysis plan
Web appendix 2: Supplement 2—supplemental online content
Data Availability Statement
Individual level data from the trial for the purposes outlined in the consent form can be shared with other researchers in a timely fashion. The data are regulated under the European GDPR regulative and sharing of data must be approved by the Data Protection Officer at Oslo University Hospital. The data dictionary, informed consent form, and analytic code will be made available at: https://oslo-universitetssykehus.no/kliniske-studier/forebygging-av-covid-19-med-tran at the time of publication. Data will be made available for researchers whose proposed use of the data has been approved.