Abstract
The CD99 gene encodes a transmembrane protein that is involved in cell differentiation, adhesion, migration, and protein trafficking. CD99 is differentially expressed on the surface of hematopoietic cells both in the myeloid and lymphoid lineages. CD99 has two isoforms, the long and short isoforms that play different roles depending on the cellular context. There has been extensive evidence supporting the role of CD99 in myeloid and lymphoblastic leukemias. Here we review research findings related to the CD99 in malignant hematopoiesis. We also summarize the significance of CD99 as a therapeutic target in hematological malignancies.
Keywords: Hematological Malignancies, Acute Myeloid Leukemia (AML), Acute Lymphoblastic Leukemia (ALL), Chronic Myeloid Leukemia (CML), Chronic Lymphocytic Leukemia (CLL), CD99, scFv, Antibody
Introduction
CD99 was first discovered in 1979 as human thymus-leukemia antigen.1 CD99 is a highly O-glycosylated transmembrane protein encoded by the CD99 or previously known as MIC2 gene.2 CD99 is located in the pseudoautosomal region (PAR) of the Y (Yp11-Ypter) and X (Xp22.33-Xpter) chromosomes in humans.3,4 The CD99 gene encodes two distinct proteins: a wild-type full-length CD99 long isoform (CD99 L) with 185 amino acids (molecular weight of 32 kDa) and a truncated short isoform (CD99 S) with 161 amino acids as consequence of alternative splicing (28 kDa).5 The CD99 S transcript contains an 18-bp insertion between exons 8 and 9 which leads to an in-frame stop codon resulting in a truncated polypeptide. The resulting short isoform shares a similar extracellular and transmembrane domain as the long isoform but varies in the cytoplasmic domain (Figure 1).5
Figure 1.
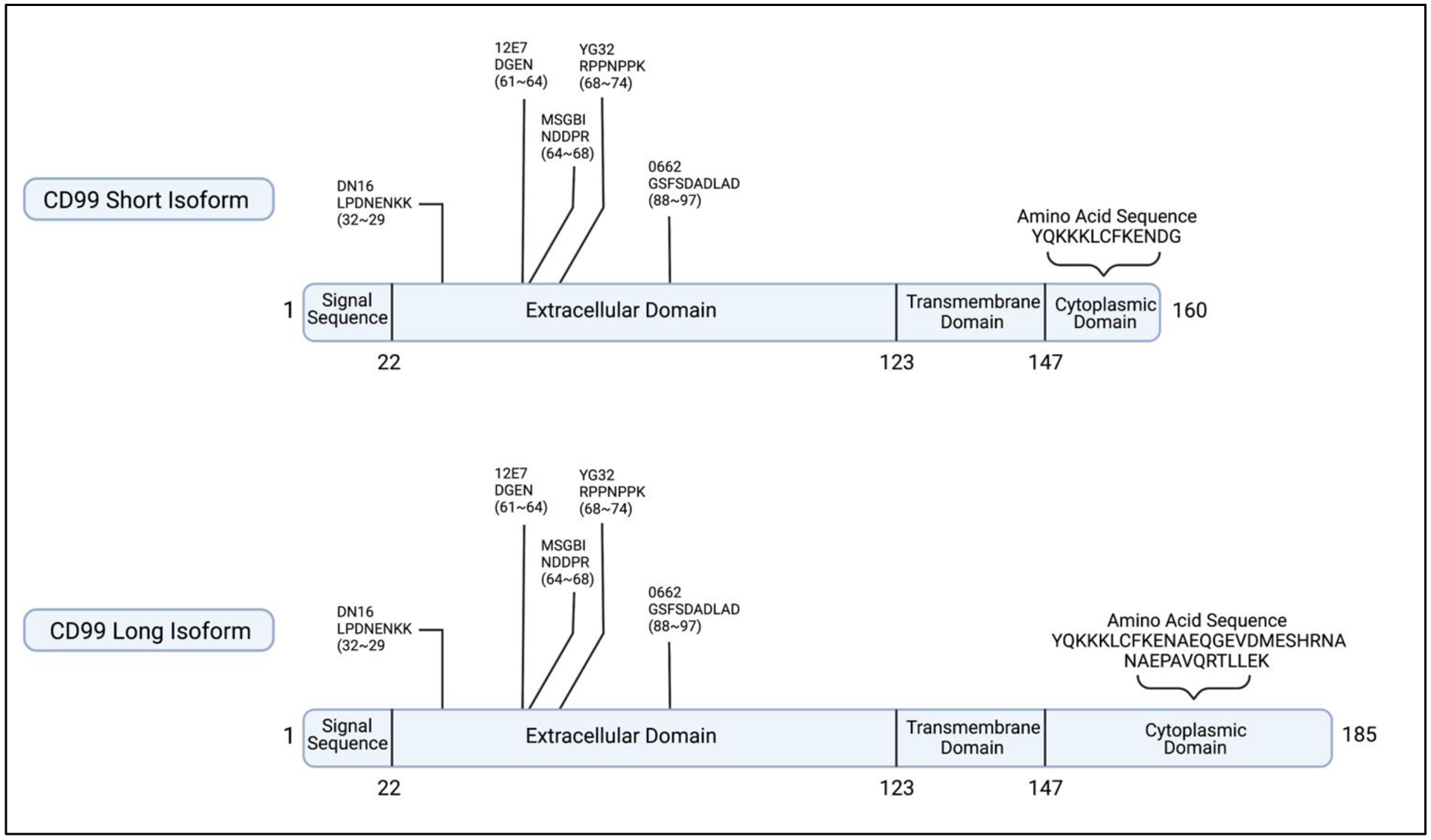
A schematic representation of both CD99 Short and CD99 Long isoforms illustrates the similarities and differences of both isoforms, with the main difference observed in the cytoplasmic domain. Epitope mapping also shows the recognition sites of different antibodies along with the sequences they recognize.28
In normal tissues, human CD99 is mostly known for its expression in T cells. It is involved in several processes that affect T cell adhesion by regulating T cell rosette formation and increasing the binding of T cells and activated peripheral blood lymphocytes to the vascular endothelial cells.6,7 It also contributes to the diapedesis of leukocytes through homotypic interaction of CD99 expressed on leukocytes and endothelial cells.8 CD99 is also known to regulate intracellular protein trafficking of MHC class 1 molecules, and plays a role in cell apoptosis and differentiation of immature thymocytes.9,10
In cancer tissues, human CD99 is mostly known for its upregulation in Ewing Sarcoma where it is considered a diagnostic marker.11,12 However, it has gained traction in recent years due to the discovery of its deregulation in various cancers including hematological malignancies. Several studies also reported different roles for CD99 in osteosarcoma13, breast cancer14,15, pancreatic adenocarcinoma16, malignant gliomas17, and epithelial cancers.18 More recently, studies have highlighted the role of CD99 and presented this protein as a potential therapeutic target in hematological malignancies such as in acute myeloid leukemia (AML) and T-lineage acute lymphoblastic leukemia (T-ALL).19–23
The function of CD99 in normal physiology and its deregulation in cancer have been covered in recent reviews. However, with new reports emerging regarding the role of CD99 in hematological malignancies and considering its expression in various hematopoietic lineages, we intend to focus on highlighting major findings related to the expression and role of CD99 in acute and chronic leukemias both in the myeloid and lymphoid lineages.
CD99 in Acute Myeloid Leukemia
Acute Myeloid Leukemia (AML) is the most common acute leukemias in adults. AML is characterized by the abnormal hematopoietic proliferation and differentiation leading to the accumulation of poorly differentiated myeloid cells called blasts.24 Studies have found that human CD99 is upregulated in AML.19,23 CD99 is particularly upregulated in leukemic stem cells (LSCs).19 CD99 expression is higher in CD34+CD38− subpopulations from AML blasts compared with normal CD34+CD38− bone marrow cells and high CD99 expression on AML blasts enriches for functional LSCs19,23. Chung et al has demonstrated that the expression of CD99 allows for separating leukemic stem cells (LSCs) from functionally normal hematopoietic stem cells (HSCs) in AML.19 In methylcellulose plating, on the contrary of CD99 positive cells, the CD99 negative cells within the CD34+CD38− population of AML cells resulted in colonies that resembled normal HSC.19 Interestingly, this subpopulation also lacked the presence of leukemia mutations that were found within the bulk AML cells. Xenograft models from these cells resulted in lympho-myeloid human engraftment that lacked the mutations as well. However, engraftment of CD99 positive CD34+CD38− AML cells led to deadly myeloid leukemia in mice.19 Association of human CD99 with the LSC may also be speculated from the high expression of CD99 in relapse AML blasts.19 Thus, CD99 expression may serve as a potential marker to identify leukemic blasts from residual normal or pre-leukemic hematopoietic cells and serve as a marker to enrich for LSCs. It is also worth noting that residual HSCs from patients with AML often carry some of the leukemia mutations.25
Patients with AML are known to have several mutations which contribute to disease progression, treatment response, and clinical outcome. Vaikari et al reported an association between elevated CD99 expression and the presence of FLT3-ITD mutations which occur in almost 30% of patients with AML.23 In addition, CD123/CD99/CD25(+) cells in CD34+ cell fraction were shown to predict FLT3-ITD mutation with high specificity and sensitivity.26 The expression of CD99 was also inversely associated with the presence of TP53 mutations in AML. CD99 expression is lower in patients with a mutated TP53 when compared with patients carrying the wild type TP53.23 Patients with an overexpression of CD99 were less frequently mutated with TP53.23
CD99 isoforms are expressed at varying levels throughout different tissue types. In AML, both isoforms are expressed, yet the CD99-S was the predominant isofrom.23 Although CD99 long and short isoforms are expressed on AML cells they have been found to contribute differently to leukemia growth. AML cells transduced with human CD99-L exhibited enhanced initial proliferation, accumulation of reactive oxygen species, enhanced DNA damage, and increased cell apoptosis. On the other hand, cells ectopically expressing human CD99-S showed very little change in their phenotype compared with control transduced cells. Whether the increased apoptosis is a result of the enhanced homotypic interaction driven by the overexpression remains to be investigated. However, it is clear that cells overexpressing CD99-L showed increased tendency to aggregate compared with cells expressing the CD99-S.23 Mechanistically, ectopic expression of the CD99 long isoform in AML cells caused a transient induction followed by a dramatic decrease in both ERK and SRC phosphorylation.23 Chung et al found that knockdown of CD99 in MOLM-13 cell induced Src family kinase (SFK) activation, while overexpressing CD99 repressed SFK activation.19
The CD99-L and CD99-S isoforms have the ability to naturally dimerize on the cells surface.27 This dimerization process is believed to begin in the Golgi apparatus and upon dimerization the transmembrane protein is sent to the cell surface where it acts as a receptor that can be stimulated and results in protein kinase c phosphorylation.28 The dimerization effect was also observed in Jurkat T cells, where co-expression of both isoforms induced cell death.27 However, overexpressing the CD99-L isoform induced cell aggregation which could drive various signaling cascades to support oncogenic stress.1 The opposite effects of CD99’s isoforms on cell migration have also been demonstrated in other tumors.5,14 In AML xenograft mouse models, the ectopic expression of the CD99-L isoform resulted in lower leukemia engraftment in the bone marrow and peripheral blood. Altogether, these studies demonstrate the link between CD99’s deregulation and AML. However, they also suggest a more complex mechanism by which CD99 contributes to leukemogenesis.
CD99 in Chronic Myeloid Leukemia
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder characterized by the presence of the BCR-ABL fusion oncogene resulting from the genetic translocation of chromosome 22 and 9.29,30 BCR-ABL encodes for a constitutively active tyrosine kinase that leads to increased cell growth and proliferation.29 Limited data are available related to the expression and the role of CD99 in CML. One study has reported that CD99 levels were lower in chronic phase CML HSC’s when compared with healthy donor bone marrow cells (Figure 2). Our analysis of Bloodspot dataset GSE1315931 has shown that CD99 expression is significantly lower (~50% less, P< 0.0001) in 76 patients with CML compared with 74 healthy samples (Figure 2). Treatment with the anti-CD99 mAb in chronic myeloid leukemia blast cell line K562 that carry the BCR-ABL translocation showed no anti-leukemia activity in vitro.19
Figure 2.
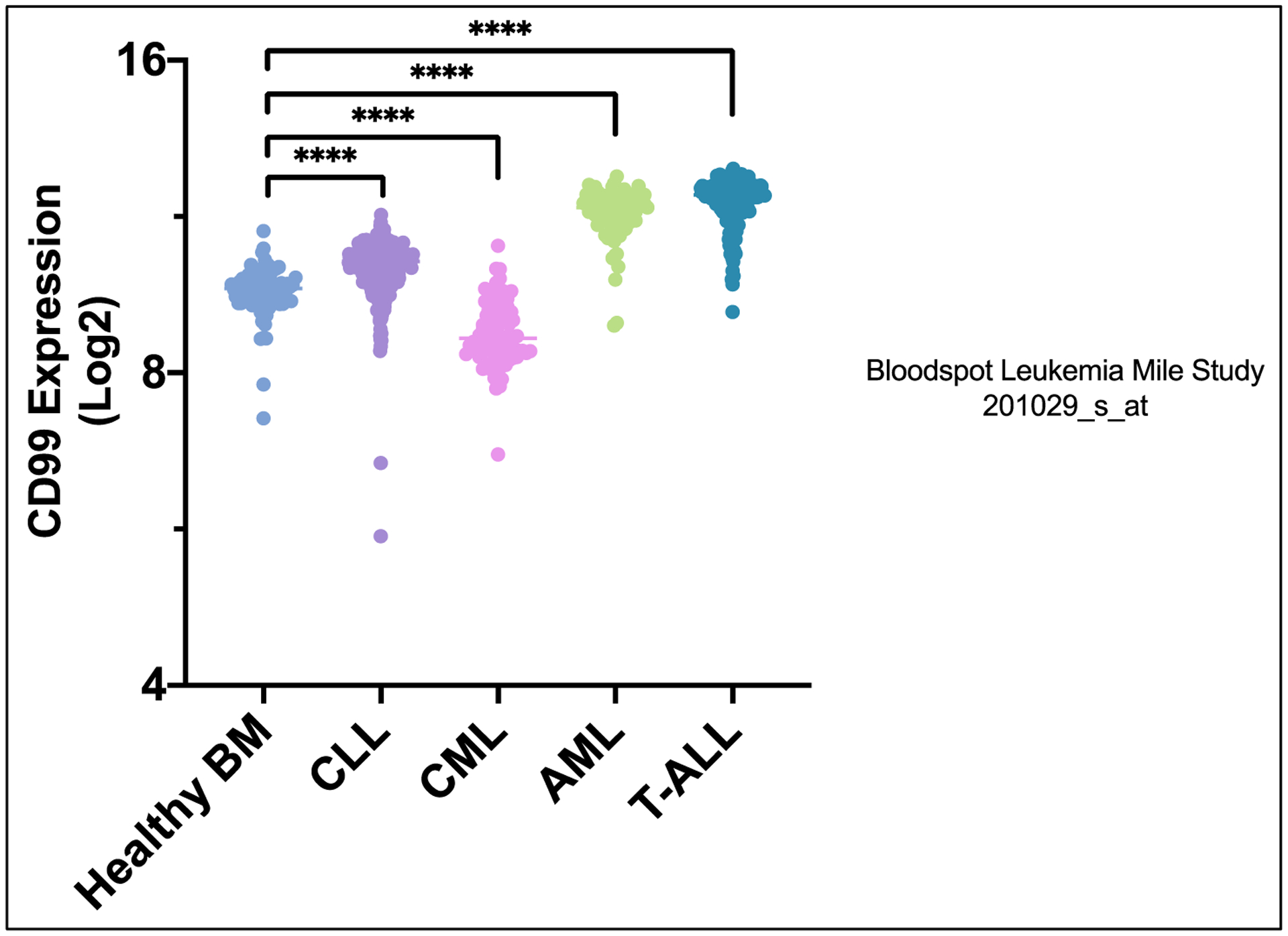
CD99 expression in Healthy Bone Marrow, CLL, CML, AML, and T-ALL. Obtained from Leukemia MILE Study 201029_s_at.
CD99 in Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL) is the most common acute leukemia in children and results from chromosomal abnormalities and genetic alterations involved in the differentiation and proliferation of lymphoid precursor cells.32,33 Cortical thymocytes and T-lineage acute lymphoblastic leukemia (T-ALL) cells strongly expressed CD99 in comparison with normal peripheral blood lymphocytes.1 Diagnostic bone marrow samples have revealed an almost eight-fold increase in the expression of CD99 than normal T lymphocytes within the same sample. It is however important to note that at least 15% of the T-ALL cases did not strongly express CD99 and this was independent of leukemic subtypes.21 This could be owing to patterns of expression changes occurring during maturation of thymocytes where CD99’s expression is downregulated with a concurrent increase in CD3 expression.34
CD99 was found to be particularly useful for the detection of T-ALL in the bone marrow and peripheral blood.21 Since CD99 is a surface marker, it is advantageous for cell surface staining methods unlike Terminal Deoxynucleotidyl Transferase (TdT), traditionally used to monitor minimal residual disease (MRD) which requires cell permeabilization.35 However, CD99’s expression should be used cautiously as a marker since there could also be a decline of expression of CD99 and TdT during induction chemotherapy leading to inconsistent results for MRD.36 Though CD99 could serve as a promising diagnostic marker for T-ALL, its role in disease initiation for T-ALL if any is not clear. Studies showed no difference in cell proliferation or leukemia propagation in vivo in CD99− or CD99+ cells and both the subpopulations were capable of self-renewal.37
CD99 is also highly expressed in immature B cell-precursors (BCP) and its expression is remarkably reduced in differentiated B cells.34 However, normal BCP cells and BCP-ALL cells have similar levels of human CD99 expression.38 Notably, only CD99 long isoform was expressed in normal BCP. In normal BCP cells the mRNA and protein expression of CD99 short isoform was highest in immature cells and expression decreased with maturation leading to a speculation that CD99 long isoform and not the short isoform in fact exclusively plays a role in BCP differentiation.38,39 There is substantial association between CD99 mRNA expression and CD99 surface expression, suggesting that CD99 can possibly be used as a biomarker using antibody based measurements.39 In addition, high CD99 is associated with high risk BCP-ALL like BCR-ABL1, CRLF2-rearranged (CRLF2Re) and a third of B-other BCP-ALL cases whereas TCF3-PBX1 BCP-ALL was associated with a decrease in CD99 expression. CD99 was also highly expressed in almost half ALL cases with ETV6-RUNX1 (TEL-AML1) or hyper-diploid. Unlike in AML, high CD99 expression is associated with poor clinical outcome in high-risk pediatric BCP-ALL patients and with an increased risk (55%) of relapse.39
CD99 in Chronic Lymphocytic Leukemia
Chronic Lymphocytic Leukemia (CLL) is the most common leukemia in developed countries. CLL is characterized by the clonal proliferation and accumulation of mature B-cells within the blood, bone marrow, lymph nodes, and spleen.40 In CLL the CD99 long isoform was found to be the most dominantly expressed CD99, while CD99 short isoform was barely present.41 We used the GSE13159 dataset to examine the differential expression of CD99 between healthy bone marrow and CLL samples; these data were downloaded from Bloodspot.31,42 In 448 patients we found that CD99 was 1.5-fold (P< 0.0001) higher in CLL samples compared with that in 74 healthy bone marrow samples (Figure 2).31 Previous studies have also shown that the CD99 long isoform supports migration of CLL cells.41 Furthermore, CD99’s long isoform also regulates integrin function in CLL by regulating CLL cell adhesion to α4β1 integrin ligands. Interestingly, it was also shown that CD99 is regulated by MMP9 and silencing of MMP9 resulted in increased CD99 surface expression in CLL.41
CD99 as a Therapeutic Target
CD99 is upregulated and plays an important role in several hematological malignancies, therefore, it presents a viable therapeutic target in leukemia. Research efforts have focused on developing therapeutic strategies that leverage this feature and some have demonstrated promising potential. One such strategy is with the use of an anti-CD99 mAb. Anti-CD99 mAbs such as HO36-1.1 have been shown to induce cytotoxicity in AML stem cells in vitro.19,23,43 Table 1 lists HO36-1.1’s effect as well as other CD99 targeting mAbs and their respective effects in different cell populations. Anti-CD99 mAbs also proved effective at inducing cytotoxicity among myeloid leukemia cell lines such as MOLM-13.19,23,43 Treatment of AML cells with anti-CD99 mAbs induces the activation of SFKs.19 Anti-CD99 mAbs at concentrations that were found toxic to AML cells, had minimal effect on HSC cells and did not show significant toxicity to endothelial cells or normal peripheral blood mononuclear cells.19,23
Table 1:
Antibody Comparisons & Characteristics in Hematological Malignancies
| Antibody Clones | T-Cell | T-ALL | B-Cells | B-ALL | CD34+ | AML | MDS | Subtype | Target Species | Epitopes |
|---|---|---|---|---|---|---|---|---|---|---|
| DN16 | Co-stimulation along with suboptimal CD3 mAb OKT3 enhanced proliferation of T-cells and stimulation of IL-2.52 | Induction of HSP70 in Jurkat cells.44 Induced homotypic cell aggregation of Jurkat T-cells and cell apoptosis.45 | Induction of cell death of undifferentiated B-cell precursors.46 | Induction of HSP70 in REH cells.44 Induces cell apoptosis of BCP-ALL.46 | IgG | Mouse (reacts with Human) | Recognizes ‘LPDNENKK’ sequence located between residues 32 and 39 towards the N-terminus.56 | |||
| YG32 | Induced homotypic aggregation of Jurkat T-cells but no cell apoptosis. However, it enhanced Fas-mediated cell death.45 | IgG | Mouse | Recognizes ‘RPPNPPK’ sequence between residues 68 and 74.56 | ||||||
| 12E7 | Does not induce cell death of thymocytes.10 | Does not induce cell apoptosis.46 | Slight protection from apoptosis, inhibits transendothelial migration of CD34+ and homing to bone marrow.53 | IgG1 | Mouse | Recognizes ‘DGEN’ sequence between residues 61~64.28 | ||||
| 0062 | Inhibit T-cell rosette formation. Induced cell death of thymocytes.10 | Induced cell death of Jurkat T-cells.10 | Induction of cell death of undifferentiated B-cell precursors.46 | Induction of HSP70 in REH cells.44 Does not induce cell apoptosis.46 | Does not induce apoptosis and has no effect on cell migration.53 | Human | Recognizes ‘GSFSDADLAD’ sequence between residues 88~97.28 | |||
| TU12 | Enhanced CD4+ cell proliferation in the presence of CD3 costimulation.54 | IgG2 | Mouse | |||||||
| Hec2 | Induction of HSP70 in REH cells.44 | Does not induce apoptosis and has no effect on cell migration.53 | ||||||||
| 3B2/TA8 | Enhanced proliferation of peripheral blood T-cells along with CD3 mAb.53 | Increased IL-2 promoter activity in Jurkat cells in the presence of CD3 mAb.53 | Does not induce cell apoptosis.46 | IgG2 | Mouse | |||||
| AD20 | Induces cell aggregation and apoptosis in Jurkat T-cells.55 | IgM | Human | |||||||
| HO36-1.1 | Induces apoptosis in Jurkat T-cells.55 | Cytotoxic to AML cell lines and myeloid blasts.19 | Cytotoxic to purified primary MDS CD34+ cells.19 | IgM | Mouse |
| Antibody Comparisons in Hematological Malignancies | |||||||
|---|---|---|---|---|---|---|---|
| Antibody Clones | T-Cell | T-ALL | B-Cells | B-ALL | CD34+ | AML | MDS |
| DN16 | Co-stimulation along with suboptimal CD3 mAb OKT3 enhanced proliferation of T-cells and stimulation of IL-2.52 | Induction of HSP70 in Jurkat cells.44 Induced homotypic cell aggregation of Jurkat T-cells and cell apoptosis.45 | Induction of cell death of undifferentiated B-cell precursors.46 | Induction of HSP70 in REH cells.44 Induces cell apoptosis of BCP-ALL.46 | |||
| YG32 | Induced homotypic aggregation of Jurkat T-cells but no cell apoptosis. However, it enhanced Fas-mediated cell death.45 | ||||||
| 12E7 | Does not induce cell death of thymocytes.10 | Does not induce cell apoptosis.46 | Slight protection from apoptosis, inhibits transendothelial migration of CD34+ and homing to bone marrow.53 | ||||
| 0062 | Inhibit T-cell rosette formation. Induced cell death of thymocytes.10 | Induced cell death of Jurkat T-cells.10 | Induction of cell death of undifferentiated B-cell precursors.46 | Induction of HSP70 in REH cells.44 Does not induce cell apoptosis.46 | Does not induce apoptosis and has no effect on cell migration.53 | ||
| TU12 | Enhanced CD4+ cell proliferation in the presence of CD3 costimulation.54 | ||||||
| Hec2 | Induction of HSP70 in REH cells.44 | Does not induce apoptosis and has no effect on cell migration.53 | |||||
| 3B2/TA8 | Enhanced proliferation of peripheral blood T-cells along with CD3 mAb.53 | Increased IL-2 promoter activity in Jurkat cells in the presence of CD3 mAb.53 | Does not induce cell apoptosis.46 | ||||
| AD20 | Induces cell aggregation and apoptosis in Jurkat T-cells.55 | ||||||
| HO36-1.1 | Induces apoptosis in Jurkat T-cells.55 | Cytotoxic to AML cell lines and myeloid blasts.19 | Cytotoxic to purified primary MDS CD 34+ cells19 | ||||
| Antibody Characteristic Comparisons | |||
|---|---|---|---|
| Antibody | Subtype | Target Species | Epitopes |
| DN16 | IgG | Mouse (reacts with Human] | Recognizes ‘LPDNENKK’ sequence located between residues 32 and 39 towards the N-terminus.56 |
| YG32 | IgG | Mouse | Recognizes ‘RPPNPPK’ sequence between residues 68 and 74.56 |
| 12E7 | IgG1 | Mouse | Recognizes ‘DGEN’ sequence between residues 61~64.28 |
| 0062 | Human | Recognizes ‘GSFSDADLAD’ sequence between residues 88~97.28 | |
| TU12 | IgG2 | Mouse | |
| 3B2/TA8 | IgG2 | Mouse | |
| AD20 | IgM | Human | |
| HO36-1.1 | IgM | Mouse | |
In vivo, HO36-1.1 proved effective at neutralizing LSC’s. IgM antibodies were also used in vivo and showed no ability to induce antibody dependent cellular cytotoxicity.19 Furthermore, studies showed a significant reduction in leukemia in both the peripheral blood and bone marrow in mice engrafted with leukemic cells.19 Interestingly when anti-CD99 mAbs were administered to mice that were engrafted with normal HSC’s, there was a minimal effect on engraftment.19 An elastin like polypeptides (ELP’s) conjugated with anti-CD99 singe chain antibody fragments (scFvs) were developed as a therapeutic strategy to target CD99.43 This approach overcomes some of the challenges associated with the generation of monoclonal antibodies and provides a clustering advantage of the antibody on the target antigen. This formulation provides a superior pharmacokinetic profile over free scFvs which are filtered out by the glomerular filtration system in the kidneys due to their small 30 kDa size.43 ELPs are peptides that are derived from human tropoelastin, a peptide found naturally in the body.43 α-CD99-A192 synthesized by fusing ELP, specifically A192 to an anti-CD99 scFv,43 demonstrated both in vitro and in vivo antileukemia activity.43 α-CD99-A192 also prolonged survival of mice in an AML xenograft model.43
Treatment with anti-CD99 antibodies resulted in upregulation of surface HSP70 expression in both T and B-ALL cells and enhanced NK-cell cytotoxicity.44 Also, binding to different epitopes of human CD99 seems to have different effects on the apoptosis pathway. For instance, in Jurkat cells, treatment with CD99 monoclonal antibody targeting DN16 epitope resulted in increased cell apoptosis whereas antibody targeting YG32 epitope did not influence cell apoptosis (Table 1). However, antibodies binding both epitopes resulted in increased cell aggregation, and activation of the MAP kinase pathway.45 Similar results were observed in induction of T cell death by antibody binding to the AD20 epitope of CD99 whereas antibody binding 0662 epitope has no effect on cell apoptosis (Table 1).
In B-ALL, cells expressing high CD99 expression were sensitive to treatment with a CD99 monoclonal antibody. TEL/AML1-positive ALL cells were the most sensitive to treatment with a CD99 monoclonal antibody (DN16 clone) and more prone to increased cell apoptosis and homotypic cell aggregation. However, these effects were delayed in the presence of stroma for support TEL/AML1 indicating that CD99 may play a role in the dependency of TEL/AML1 on the microenvironment bone marrow.46 On the other hand, though CD99 is highly expressed in hyper-diploid B-ALL, these cells were not sensitive to treatment with CD99 monoclonal antibody in stroma free condition. Noteworthy, BCP cells, which express high CD99 expression, exhibited sensitivity when treated with CD99 monoclonal antibody (DN16 clone).44 It’s also worth noting that MT99/3, an anti-CD99 mAb, exhibited antibody dependent cell cytotoxicity in malignant B-cells.47 However, this same anti-CD99 antibody did not show any cytotoxic effect on peripheral blood mononuclear cells (PBMC’s).48 Besides antibody based targeting strategies against CD99, the FDA approved purine nucleoside analogs, Clofarabine and Cladribine, were found to inhibit CD99 dimerization and its interaction with downstream signaling components in Ewing sarcoma.49,50
A fusion protein consisting of the murine Cd99 sequence and a bioengineered truncated version of bacterial thioredoxin was manufactured into a vaccine.51 This vaccine resulted in an activation of specific CD99 auto reactive B-cells.51 In mouse studies this vaccine was found to reduce tumor micro-vessel density and functionality with no side effects observed.51 The researchers in this study concluded that targeting Cd99 via vaccination can inhibit tumor growth. However, it is not clear whether these findings may extend to human Cd99, since murine Cd99 has only 46% homology with human CD99.51
Concluding Remarks and Future Directions
Although there is a vast amount of literature regarding CD99 and its role in T cells and an increase in evidence suggesting its role in hematological malignancies, several gaps remain in the understanding of its different isoforms and the potential each one has in hematological malignancies. Dissecting the different mechanistic pathways by which each isoform act may facilitated the development of better modalities to target this oncoprotein. The association between CD99 upregulation and the presence of specific molecular or cytogenetic aberrations is another area of much needed research. Whether CD99 is a suitable therapeutic target for specific subsets of patients such as patients with FLT3-ITD positive AML or TP53 wt. type patients remains to be established. While certain antibodies against CD99 have demonstrated effectiveness in AML preclinical models, whether they are also effective in ALL or CLL remains to be investigated. Different CD99 antibodies enact different responses on hematopoietic and leukemic cells, with some acting more as agonists while others as antagonists. The difference in their ability to engage different CD99 domains likely contribute to the conflicting functional and mechanistic phenotypes in both malignant and normal cells. Current strategies used to target CD99 have yielded promising results in preclinical models. How CD99 targeting approaches perform in conjunction with existing therapies and whether a synergistic effect that drives an even greater therapeutic response could be obtained with combinational approaches remains to be determined.
Highlights.
In normal tissues, human CD99 is mostly known for its expression in T cells
In cancer tissues, human CD99 is mostly known for its upregulation in Ewing Sarcoma
CD99 is particularly upregulated in leukemic stem cells (LSCs)
Anti-CD99 mAbs such as HO36-1.1 induce cytotoxicity in AML stem cells in vitro
Acknowledgments
All affiliations or financial involvement with any entity with a financial interest in the subject matter are completely disclosed and all financial and material support for this research and work are clearly identified in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: The manuscript is the original work of the authors except for material in the public domain and such excerpts from other works as may be included with the prior written permission of the copyright owners.
Publisher's Disclaimer: Neither this manuscript nor another manuscript with substantially similar content under our authorship has been published, has been accepted, or is being considered elsewhere for publication except as described in an attachment.
References
- 1.Levy R, Dilley J, Fox RI, Warnke R. A human thymus-leukemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci U S A. Dec 1979;76(12):6552–6. doi: 10.1073/pnas.76.12.6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodfellow PN, Pym B, Pritchard C, et al. MIC2: a human pseudoautosomal gene. Philos Trans R Soc Lond B Biol Sci. Dec 1 1988;322(1208):145–54. doi: 10.1098/rstb.1988.0122 [DOI] [PubMed] [Google Scholar]
- 3.Banting GS, Pym B, Darling SM, Goodfellow PN. The MIC2 gene product: epitope mapping and structural prediction analysis define an integral membrane protein. Mol Immunol. Feb 1989;26(2):181–8. doi: 10.1016/0161-5890(89)90100-4 [DOI] [PubMed] [Google Scholar]
- 4.Aubrit F, Gelin C, Pham D, Raynal B, Bernard A. The biochemical characterization of E2, a T cell surface molecule involved in rosettes. Eur J Immunol. Aug 1989;19(8):1431–6. doi: 10.1002/eji.1830190813 [DOI] [PubMed] [Google Scholar]
- 5.Hahn JH, Kim MK, Choi EY, et al. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol. Sep 1 1997;159(5):2250–8. [PubMed] [Google Scholar]
- 6.Bernard A, Aubrit F, Raynal B, Pham D, Boumsell L. A T cell surface molecule different from CD2 is involved in spontaneous rosette formation with erythrocytes. J Immunol. Mar 15 1988;140(6):1802–7. [PubMed] [Google Scholar]
- 7.Bernard G, Raimondi V, Alberti I, et al. CD99 (E2) up-regulates alpha4beta1-dependent T cell adhesion to inflamed vascular endothelium under flow conditions. Eur J Immunol. Oct 2000;30(10):3061–5. doi: [DOI] [PubMed] [Google Scholar]
- 8.Dufour EM, Deroche A, Bae Y, Muller WA. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun Adhes. Nov 2008;15(4):351–63. doi: 10.1080/15419060802442191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn HW, Shin YK, Lee IS, et al. CD99 regulates the transport of MHC class I molecules from the Golgi complex to the cell surface. J Immunol. Jan 15 2001;166(2):787–94. doi: 10.4049/jimmunol.166.2.787 [DOI] [PubMed] [Google Scholar]
- 10.Bernard G, Breittmayer JP, de Matteis M, et al. Apoptosis of immature thymocytes mediated by E2/CD99. J Immunol. Mar 15 1997;158(6):2543–50. [PubMed] [Google Scholar]
- 11.Baldauf MC, Orth MF, Dallmayer M, et al. Robust diagnosis of Ewing sarcoma by immunohistochemical detection of super-enhancer-driven EWSR1-ETS targets. Oncotarget. Jan 5 2018;9(2):1587–1601. doi: 10.18632/oncotarget.20098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocchi A, Manara MC, Sciandra M, et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J Clin Invest. Mar 2010;120(3):668–80. doi: 10.1172/jci36667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sciandra M, Marino MT, Manara MC, et al. CD99 drives terminal differentiation of osteosarcoma cells by acting as a spatial regulator of ERK 1/2. J Bone Miner Res. 2014;29(5):1295–309. doi: 10.1002/jbmr.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byun HJ, Hong IK, Kim E, et al. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. Nov 17 2006;281(46):34833–47. doi: 10.1074/jbc.M605483200 [DOI] [PubMed] [Google Scholar]
- 15.Baccar A, Ferchichi I, Troudi W, et al. CD99 and HLA-II immunostaining in breast cancer tissue and their correlation with lymph node metastasis. Dis Markers. 2013;34(5):363–71. doi: 10.3233/dma-130982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto A, Niki T, Terado Y, Fukushima J, Fukayama M. Prevalence of CD99 protein expression in pancreatic endocrine tumours (PETs). Histopathology. Oct 2004;45(4):384–92. doi: 10.1111/j.1365-2559.2004.01967.x [DOI] [PubMed] [Google Scholar]
- 17.Cardoso LC, Soares RDS, Laurentino TS, Lerario AM, Marie SKN, Oba-Shinjo SM. CD99 Expression in Glioblastoma Molecular Subtypes and Role in Migration and Invasion. Int J Mol Sci. Mar 6 2019;20(5)doi: 10.3390/ijms20051137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo SH, Han J, Kim TJ, Chung DH. Expression of CD99 in pleomorphic carcinomas of the lung. J Korean Med Sci. Feb 2005;20(1):50–5. doi: 10.3346/jkms.2005.20.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SS, Eng WS, Hu W, et al. CD99 is a therapeutic target on disease stem cells in myeloid malignancies. Sci Transl Med. Jan 25 2017;9(374)doi: 10.1126/scitranslmed.aaj2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang PJ, Barcos M, Stewart CC, Block AW, Sait S, Brooks JJ. Immunoreactivity of MIC2 (CD99) in acute myelogenous leukemia and related diseases. Mod Pathol. Apr 2000;13(4):452–8. doi: 10.1038/modpathol.3880077 [DOI] [PubMed] [Google Scholar]
- 21.Dworzak MN, Fröschl G, Printz D, et al. CD99 expression in T-lineage ALL: implications for flow cytometric detection of minimal residual disease. Leukemia. Apr 2004;18(4):703–8. doi: 10.1038/sj.leu.2403303 [DOI] [PubMed] [Google Scholar]
- 22.Enein AA, Rahman HA, Sharkawy NE, et al. Significance of CD99 expression in T-lineage acute lymphoblastic leukemia. Cancer Biomark. Mar 18 2016;17(2):117–23. doi: 10.3233/cbm-160608 [DOI] [PubMed] [Google Scholar]
- 23.Vaikari VP, Du Y, Wu S, et al. Clinical and preclinical characterization of CD99 isoforms in acute myeloid leukemia. Haematologica. Apr 2020;105(4):999–1012. doi: 10.3324/haematol.2018.207001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Kouchkovsky I, Abdul-Hay M. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J. Jul 1 2016;6(7):e441. doi: 10.1038/bcj.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jan M, Snyder TM, Corces-Zimmerman MR, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med. Aug 29 2012;4(149):149ra118. doi: 10.1126/scitranslmed.3004315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelini DF, Ottone T, Guerrera G, et al. A Leukemia-Associated CD34/CD123/CD25/CD99+ Immunophenotype Identifies FLT3-Mutated Clones in Acute Myeloid Leukemia. Clin Cancer Res. Sep 1 2015;21(17):3977–85. doi: 10.1158/1078-0432.ccr-14-3186 [DOI] [PubMed] [Google Scholar]
- 27.Alberti I, Bernard G, Rouquette-Jazdanian AK, et al. CD99 isoforms expression dictates T cell functional outcomes. Faseb j. Dec 2002;16(14):1946–8. doi: 10.1096/fj.02-0049fje [DOI] [PubMed] [Google Scholar]
- 28.Pasello M, Manara MC, Scotlandi K. CD99 at the crossroads of physiology and pathology. J Cell Commun Signal. Mar 2018;12(1):55–68. doi: 10.1007/s12079-017-0445-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granatowicz A, Piatek CI, Moschiano E, El-Hemaidi I, Armitage JD, Akhtari M. An Overview and Update of Chronic Myeloid Leukemia for Primary Care Physicians. Korean J Fam Med. Sep 2015;36(5):197–202. doi: 10.4082/kjfm.2015.36.5.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. Jun 2020;95(6):691–709. doi: 10.1002/ajh.25792 [DOI] [PubMed] [Google Scholar]
- 31.Haferlach T, Kohlmann A, Wieczorek L, et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. May 20 2010;28(15):2529–37. doi: 10.1200/jco.2009.23.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onciu M Acute lymphoblastic leukemia. Hematol Oncol Clin North Am. Aug 2009;23(4):655–74. doi: 10.1016/j.hoc.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 33.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. Jun 30 2017;7(6):e577. doi: 10.1038/bcj.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dworzak MN, Fritsch G, Buchinger P, et al. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. Jan 15 1994;83(2):415–25. [PubMed] [Google Scholar]
- 35.Della Starza I, Chiaretti S, De Propris MS, et al. Minimal Residual Disease in Acute Lymphoblastic Leukemia: Technical and Clinical Advances. Front Oncol. 2019;9:726. doi: 10.3389/fonc.2019.00726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roshal M, Fromm JR, Winter S, Dunsmore K, Wood BL. Immaturity associated antigens are lost during induction for T cell lymphoblastic leukemia: implications for minimal residual disease detection. Cytometry B Clin Cytom. May 2010;78(3):139–46. doi: 10.1002/cyto.b.20511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox CV, Diamanti P, Moppett JP, Blair A. Investigating CD99 Expression in Leukemia Propagating Cells in Childhood T Cell Acute Lymphoblastic Leukemia. PLoS One. 2016;11(10):e0165210. doi: 10.1371/journal.pone.0165210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dworzak MN, Fritsch G, Fleischer C, et al. CD99 (MIC2) expression in paediatric B-lineage leukaemia/lymphoma reflects maturation-associated patterns of normal B-lymphopoiesis. Br J Haematol. Jun 1999;105(3):690–5. doi: 10.1046/j.1365-2141.1999.01426.x [DOI] [PubMed] [Google Scholar]
- 39.Chen D, Camponeschi A, Wu Q, et al. CD99 expression is strongly associated with clinical outcome in children with B-cell precursor acute lymphoblastic leukaemia. Br J Haematol. Feb 2019;184(3):418–423. doi: 10.1111/bjh.15683 [DOI] [PubMed] [Google Scholar]
- 40.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. Apr 14 2018;391(10129):1524–1537. doi: 10.1016/s0140-6736(18)30422-7 [DOI] [PubMed] [Google Scholar]
- 41.Aguilera-Montilla N, Bailón E, Uceda-Castro R, et al. MMP-9 affects gene expression in chronic lymphocytic leukemia revealing CD99 as an MMP-9 target and a novel partner in malignant cell migration/arrest. Oncogene. Jun 2019;38(23):4605–4619. doi: 10.1038/s41388-019-0744-3 [DOI] [PubMed] [Google Scholar]
- 42.Kohlmann A, Kipps TJ, Rassenti LZ, et al. An international standardization programme towards the application of gene expression profiling in routine leukaemia diagnostics: the Microarray Innovations in LEukemia study prephase. Br J Haematol. Sep 2008;142(5):802–7. doi: 10.1111/j.1365-2141.2008.07261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaikari VP, Park M, Keossayan L, MacKay JA, Alachkar H. Anti-CD99 scFv-ELP nanoworms for the treatment of acute myeloid leukemia. Nanomedicine. Jun 12 2020;29:102236. doi: 10.1016/j.nano.2020.102236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husak Z, Dworzak MN. CD99 ligation upregulates HSP70 on acute lymphoblastic leukemia cells and concomitantly increases NK cytotoxicity. Cell Death Dis. Nov 15 2012;3(11):e425. doi: 10.1038/cddis.2012.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung KC, Kim NH, Park WS, Park SH, Bae Y. The CD99 signal enhances Fas-mediated apoptosis in the human leukemic cell line, Jurkat. FEBS Lett. Nov 20 2003;554(3):478–84. doi: 10.1016/s0014-5793(03)01224-9 [DOI] [PubMed] [Google Scholar]
- 46.Husak Z, Printz D, Schumich A, Pötschger U, Dworzak MN. Death induction by CD99 ligation in TEL/AML1-positive acute lymphoblastic leukemia and normal B cell precursors. J Leukoc Biol. Aug 2010;88(2):405–12. doi: 10.1189/jlb.0210097 [DOI] [PubMed] [Google Scholar]
- 47.Takheaw N, Sittithumcharee G, Kariya R, Kasinrerk W, Okada S. Anti-human CD99 antibody exerts potent antitumor effects in mantle cell lymphoma. Cancer Immunol Immunother. Jun 2021;70(6):1557–1567. doi: 10.1007/s00262-020-02789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laopajon W, Pata S, Takheaw N, Surinkaew S, Khummuang S, Kasinrerk W. Triggering of CD99 on monocytes by a specific monoclonal antibody regulates T cell activation. Cell Immunol. Jan 2019;335:51–58. doi: 10.1016/j.cellimm.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 49.Sevim H, Çelik H, Düşünceli L, et al. Clofarabine induces ERK/MSK/CREB activation through inhibiting CD99 on Ewing sarcoma cells. PLoS One. 2021;16(6):e0253170. doi: 10.1371/journal.pone.0253170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Çelik H, Sciandra M, Flashner B, et al. Clofarabine inhibits Ewing sarcoma growth through a novel molecular mechanism involving direct binding to CD99. Oncogene. Apr 2018;37(16):2181–2196. doi: 10.1038/s41388-017-0080-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huijbers EJM, van der Werf IM, Faber LD, et al. Targeting Tumor Vascular CD99 Inhibits Tumor Growth. Front Immunol. 2019;10:651. doi: 10.3389/fimmu.2019.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh KI, Kim BK, Ban YL, et al. CD99 activates T cells via a costimulatory function that promotes raft association of TCR complex and tyrosine phosphorylation of TCR zeta. Exp Mol Med. Apr 30 2007;39(2):176–84. doi: 10.1038/emm.2007.20 [DOI] [PubMed] [Google Scholar]
- 53.Imbert AM, Belaaloui G, Bardin F, Tonnelle C, Lopez M, Chabannon C. CD99 expressed on human mobilized peripheral blood CD34+ cells is involved in transendothelial migration. Blood. Oct 15 2006;108(8):2578–86. doi: 10.1182/blood-2005-12-010827 [DOI] [PubMed] [Google Scholar]
- 54.Wingett D, Forcier K, Nielson CP. A role for CD99 in T cell activation. Cell Immunol. Apr 10 1999;193(1):17–23. doi: 10.1006/cimm.1999.1470 [DOI] [PubMed] [Google Scholar]
- 55.Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO. CD99 signals caspase-independent T cell death. J Immunol. Apr 15 2001;166(8):4931–42. doi: 10.4049/jimmunol.166.8.4931 [DOI] [PubMed] [Google Scholar]
- 56.Gil MC, Lee MH, Seo JI, et al. Characterization and epitope mapping of two monoclonal antibodies against human CD99. Exp Mol Med. Dec 31 2002;34(6):411–8. doi: 10.1038/emm.2002.58 [DOI] [PubMed] [Google Scholar]


