Abstract
In vivo studies suggest that arrhythmia risk may be greater with less selective dipeptidyl peptidase-4 inhibitors, but evidence from population-based studies is missing. We aimed to compare saxagliptin, sitagliptin, linagliptin with regard to risk of sudden cardiac arrest (SCA)/ventricular arrhythmia (VA). We conducted high-dimensional propensity score (hdPS) matched, new-user cohort studies. We analyzed Medicaid Optum Clinformatics separately. We identified new users of saxagliptin, sitagliptin (both databases), linagliptin (Optum only). We defined SCA/VA outcomes using emergency department inpatient diagnoses. We identified then controlled for confounders via a data-adaptive, hdPS approach. We generated marginal hazard ratios (HRs) via Cox proportional hazards regression using a robust variance estimator while adjusting for calendar year. We identified the following matched comparisons: saxagliptin vs. sitagliptin (23,895 vs. 96,972) in Medicaid, saxagliptin vs. sitagliptin (48,388 vs. 117,383) in Optum, linagliptin vs. sitagliptin (36,820 vs. 78,701) in Optum. In Medicaid, use of saxagliptin (vs. sitagliptin) was associated with an increased rate of SCA/VA (adjusted HR (aHR), 2.01, 95% confidence interval (CI) 1.24–3.25). However, in Optum data, this finding was not present (aHR, 0.79, 95% CI 0.41–1.51). Further, we found no association between linagliptin (vs. sitagliptin) SCA/VA (aHR, 0.65, 95% CI 0.36–1.17). We found discordant results regarding the association between SCA/VA with saxagliptin compared with sitagliptin in two independent datasets. It remains unclear whether these findings are due to heterogeneity of treatment effect in the different populations, chance, or unmeasured confounding.
The use of dipeptidyl peptidase-4 (DPP-4) inhibitors (e.g., sitagliptin saxagliptin) has increased substantially since their approval by the US Food Drug Administration (FDA) in 2006.1 As add-on therapy in patients with type 2 diabetes, DPP-4 inhibitors have moderate glucose-lowering potency, low risk of hypoglycemia, a neutral effect on body weight.2 Despite sharing a common mechanism of action, preclinical studies suggested that the risk of adverse cardiac outcomes may be greater with less selective DPP-4 inhibitors (i.e., drugs demonstrating off-target inhibition of other DPP-4 family enzymes), such as saxagliptin.3,4 Animal studies found no association between sitagliptin or linagliptin electrocardiographic QTc interval prolongation.4–6 However, in cardiomyocytes guinea pig hearts, saxagliptin aggravated pre-existing cardiac dysfunction leading to systolic diastolic dysfunction, reduced contractile force, QTc prolongation.4 Prolongation of the QT interval can predispose patients to a potentially life-threatening ventricular arrhythmia (VA) leading to sudden cardiac arrest (SCA).7
Despite advances in diabetes treatment, SCA remains a major cause of mortality in patients with type 2 diabetes.8–11 In 2008, the FDA mated that manufacturers of new antihyperglycemic drugs conduct postmarketing cardiovascular outcomes trials. Although several cardiovascular end points have since been evaluated, none of these trials examined SCA as a st-alone end point. Further, whereas prior observational studies have evaluated differences in the risk of cardiovascular diseases heart failure among DPP-4 inhibitors, none assessed differences in the SCA risk.12–15 A recent statement by the American Heart Association emphasizes the identification of drug-induced arrhythmia to reduce cardiovascular risk to increase awareness among clinicians patients.16 An assessment of the comparative safety of DPP-4 inhibitors regarding risk of SCA using population-based data may provide evidence to inform treatment selection in clinical practice. Therefore, we aimed to assess the comparative safety of individual DPP-4 inhibitors with respect to SCA/VA.
METHODS
Data source
We used healthcare data of > 70 million beneficiaries from the US Medicaid programs of California, Florida, New York, Ohio, Pennsylvania during 2008–2012 (data from 2008 were used to ascertain baseline covariates only). The administrative database records enrollment, demographics (e.g., age race), outpatient, emergency, inpatient, long-term care encounters, nonhospital prescription drug dispensings. Because ~ 15% of Medicaid patients are dually enrolled in Medicare, we supplemented the dataset with Medicare enrollment claims of dual enrollees to more completely capture their health experience.17,18 We then linked both datasets to the US Social Security Administration Death Master File to ascertain dates of death. In addition, we used commercial data from Optum’s de-identified Data Mart Database 2008–2019 (data from 2008 were used to ascertain baseline covariates only). The administrative database includes de-identified individual-level data on enrollment, demographics, outpatient, emergency, inpatient, other encounters, nonhospital prescription drug dispensings, laboratory data (the latter for a subset of beneficiaries). The study was approved by the University of Pennsylvania Institutional Review Board. The study design analysis plan were set a priori.
Study population
In Medicaid data, we performed a new user cohort study of persons who had ≥ 1 prescription dispensing for a DPP-4 inhibitor (i.e., sitagliptin saxagliptin) from January 1, 2009, to December 31, 2012. Because saxagliptin was introduced in 2009, we excluded sitagliptin users from earlier years as potential matches to avoid using historical comparators.19 Similarly, in Optum data, we performed a new user cohort study of persons who had ≥ 1 prescription dispensing for saxagliptin vs. sitagliptin from January 1, 2009, to June 30, 2019, for linagliptin vs. sitagliptin from January 1, 2012 (linagliptin was approved in May 2011), to June 30, 2019. We were unable to examine linagliptin in Medicaid because the dataset ended in 2012. The study cohorts included new users of DPP-4 inhibitors (i.e., devoid of a prior DPP-4 dispensing during a 12-month lookback period) with at least 365 days of continuous enrollment prior to treatment initiation (i.e., lookback period), aged 30–75 years, with no diagnosis in any setting of SCA or VA during the lookback period to minimize the inclusion of recurrent events. The date of the first eligible prescription was assigned as the cohort entry date. We excluded persons younger than 30 years of age because SCA rarely occurs in this age group is unlikely to result from prescription drug exposure.20 We excluded persons older than 75 years of age to minimize concern for high-risk comorbidities associated with SCA that may serve as competing events.21
Exposure ascertainment
We identified new users of DPP-4 inhibitors using pharmacy dispensings. Persons were considered exposed as long as they continued to refill their prescriptions. Follow-up ended on the first of the following: (i) a gap between DPP-4 dispensings exceeding 15 days, because drug-induced proarrhythmic effects are expected to diminish rapidly after treatment discontinuation; (ii) date of dispensing of a drug with a known risk of torsade de pointes (TdP)22; (iii) date of dispensing of a DPP-4 inhibitor different than that upon cohort entry; (iv) an outcome of interest (SCA or VA); (v) non-outcome SCA/VA diagnosis (i.e., SCA/VA that did not meet the outcome definition listed below); (vi) date of death (available for Medicaid data only); (vii) disenrollment; or (viii) end of study period. Person-time during nonevent hospitalization was excluded to minimize the bias arising from the absence of information on medications dispensed during hospitalization.23
Outcome ascertainment
We identified outpatient-originating SCA/VA events (Table S1) using previously validated International Classification of Diseases (ICD) codes first-listed emergency department principal inpatient discharge diagnosis codes. This algorithm was validated in a Medicaid population had a positive predictive value (PPV) = 85%.24 We did not consider SCA/VA events reported on death certificates or those occurring during hospitalization because: (i) the PPV of causes of death on death certificates are particularly poor in identifying SCA25,26; (ii) inpatient drug exposure is not recorded in most claims databases.23
Statistical analysis
We used a semi-automated data-adaptive high dimensional propensity score (hdPS) method to reduce the potential for confounding by indication.27,28 We included nine data dimensions in the algorithm. Within each data dimension, we selected the top 200 most prevalent covariates, including diagnostic, procedural, or dispensed medications, along with the following predefined covariates which were forced into the model: (i) demographics; (ii) measures of intensity of healthcare utilization; (iii) risk factors or medications associated with increased risk of SCA (e.g., drugs with TdP risk4; Tables S2, S3).
Using the Bross formula, we ranked confounders based on their potential for bias by examining the prevalence of each confounder their association with exposure outcome.29 Then, we selected the top 500 empiric covariates to be included in the propensity score (PS) estimation. We used a logistic regression model to calculate PS defined as persons’ predicted probability of receiving saxagliptin vs. sitagliptin (both databases) linagliptin vs. sitagliptin (Optum only). We used 1: up to 5 matching without replacement based on hdPS within 0.2 SDs of the logit of the PS. We assessed the balance between users using absolute stardized differences.30 We plotted Kaplan-Meier curves compared their equality using a stratified log-rank test.31 We generated marginal hazard ratios (HRs) via Cox proportional hazards regression using a robust variance estimator while adjusting for calendar year.31,32 We assessed proportional hazards assumptions by including an interaction term of exposure by time.
We conducted several sensitivity analyses including: (i) limiting follow-up to 30 days; (ii) accounting for variable ratio matching using weighting; (iii) decreasing the permissible gap between contiguous dispensings; (iv) excluding persons with a history of SCA/VA; (v) excluding empiric covariates from the hdPS thought to be strong correlates of exposure but not associated with the outcome; (vi) examining dose-response relationships; (vii) omitting the censoring criterion for drugs with a “known TdP risk”; (viii) using a competing risk model with death as the competing event (Medicaid only; Table S4). Because no prior preclinical study reported an association between linagliptin SCA/VA, we examined linagliptin (vs. sitagliptin vs. saxagliptin) in a sensitivity analysis.
We also evaluated fatal events (i.e., sudden cardiac death or fatal VA) by requiring death on the same day or the day after the SCA/VA diagnosis. In subgroup analyses, using Cox proportional hazard models, we examined whether differential effects existed between individual DPP-4 inhibitors risk of SCA/VA by concomitant use of drugs that inhibit transport or hepatic CYP450-based metabolism of DPP-4 inhibitors, concomitant use of drugs with a “known risk of TdP,”22 other potentially high-risk subgroups (e.g., age heart failure). We assessed the potential for effect modification by including an interaction term of exposure each variable in the primary models. To ensure covariate balance, we performed matching again within each subgroup analysis. To account for multiple testing in subgroup analyses, we used Bonferroni adjustment, which considered results statistically significant if the corresponding P value was ≤ α (i.e., 0.05) / n where n = total number of subgroup analyses.33 We also calculated the E-value to assess the potential effect of unmeasured confounding. The E-value provides the minimum needed strength of association among an unmeasured confounder, exposure, study outcome necessary to move the observed effect estimates to the null value of 1, conditional on measured confounders included in the hdPS model (https://mmathur.shinyapps.io/evalue/).34 All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Role of the funding sources
Neither the American Diabetes Association nor the National Institutes of Health had a role in the study’s conduct or interpretation.
RESULTS
Description of study cohorts
Medicaid database (saxagliptin vs. sitagliptin).
We identified 23,902 new users of saxagliptin 150,460 new users of sitagliptin meeting inclusion criteria (Table 1). The hdPS model included a total of 564 covariates, of which 64 were prespecified 500 empirically identified (Table S5). We matched 23,895 saxagliptin users to 96,972 sitagliptin users. The distribution of PS before after matching is illustrated in Figure S1. After matching, demographics clinical characteristics were well balanced (absolute stardized differences of < 0.1 between groups) including female sex (61% vs. 61%), presence of comorbidities, such as heart failure (10% vs. 10%), kidney disease (19% vs. 19%), hypoglycemia (2% vs. 2%), baseline use of medications, such as insulin (12% vs. 12%), metformin (40% vs. 40%), sulfonylureas (27% vs. 28%; Table 2). The median follow-up time was 52 days (5th, 95th percentile, 1, 384 days) for saxagliptin users 68 days (1, 456 days) for sitagliptin users.
Table 1.
Demographics and clinical characteristics of new users of dipeptidyl peptidase-4 inhibitors in Medicaid and Optum databases, prior to high-dimensional propensity score matching
| Medicaid database |
Optum database |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Saxagliptin | Sitagliptin | SMDa | Saxagliptin | Sitagliptin | SMDa | Linagliptin | Sitagliptin | SMDa | |
|
| |||||||||
| Users, N | 23,902 | 150,460 | 49,024 | 164,906 | 43,080 | 109,635 | |||
|
| |||||||||
| Demographics, % (unless otherwise indicated) | |||||||||
|
| |||||||||
| Age in years, median (Q1–Q3) | 60.4 (51.2–68.5) | 60.0 (51.3–68.1) | 0.01 | 57.7 (49.8–65.2) | 62.6 (53.5–69.0) | 0.34 | 58.8 (50.8–66.2) | 65.6 (55.7–69.9) | 0.42 |
|
| |||||||||
| Sex, female | 61.2 | 60.3 | 0.02 | 44.5 | 47.8 | 0.07 | 45.4 | 48.8 | 0.07 |
|
| |||||||||
| Race | |||||||||
|
| |||||||||
| White | 35.5 | 34.3 | 0.03 | 57.2 | 54.0 | 0.06 | 54.3 | 51.4 | 0.06 |
|
| |||||||||
| Black | 13.5 | 17.2 | 0.10 | 15.1 | 14.0 | 0.03 | 15.3 | 14.1 | 0.03 |
|
| |||||||||
| Hispanic/Latino | 30.7 | 31.5 | 0.02 | 15.1 | 15.6 | 0.01 | 16.7 | 16.4 | 0.01 |
|
| |||||||||
| Asian | 9.1 | 9.2 | 0.00 | 4.6 | 4.5 | 0.01 | 4.9 | 4.6 | 0.01 |
|
| |||||||||
| Other/unknown | 11.2 | 7.9 | 0.11 | 7.9 | 11.8 | 0.13 | 8.8 | 13.4 | 0.15 |
|
| |||||||||
| State of residence | NA | ||||||||
|
|
|||||||||
| CA | 45.7 | 31.9 | 0.28 | ||||||
|
|
|||||||||
| FL | 16.7 | 10.3 | 0.19 | ||||||
|
|
|||||||||
| NY | 23.0 | 43.0 | 0.43 | ||||||
|
|
|||||||||
| OH | 9.7 | 9.4 | 0.01 | ||||||
|
|
|||||||||
| PA | 4.8 | 5.5 | 0.03 | ||||||
|
| |||||||||
| Census level division based on US state | NA | ||||||||
|
|
|
||||||||
| New England | 2.0 | 2.8 | 0.05 | 2.7 | 3.0 | 0.02 | |||
|
|
|
||||||||
| Middle Atlantic | 6.0 | 7.1 | 0.05 | 7.4 | 8.0 | 0.02 | |||
|
|
|
||||||||
| East North Central | 13.4 | 11.7 | 0.05 | 14.9 | 11.4 | 0.10 | |||
|
|
|
||||||||
| West North Central | 5.9 | 6.2 | 0.01 | 7.3 | 6.4 | 0.04 | |||
|
|
|
||||||||
| South Atlantic | 31.4 | 28.4 | 0.07 | 26.6 | 26.8 | 0.00 | |||
|
|
|
||||||||
| East South Central | 5.2 | 4.4 | 0.04 | 5.8 | 4.4 | 0.06 | |||
|
|
|
||||||||
| West South Central | 22.4 | 18.0 | 0.11 | 19.9 | 18.3 | 0.04 | |||
|
|
|
||||||||
| Mountain | 6.7 | 7.6 | 0.04 | 7.8 | 7.6 | 0.01 | |||
|
|
|
||||||||
| Pacific | 6.6 | 13.1 | 0.22 | 7.5 | 13.7 | 0.20 | |||
|
|
|
||||||||
| Unknown | 0.4 | 0.6 | 0.03 | 0.2 | 0.5 | 0.05 | |||
|
|
|
||||||||
| Education level | |||||||||
|
|
|
||||||||
| Less than 12th grade | 1.0 | 1.0 | 0.01 | 1.1 | 1.1 | 0.00 | |||
|
|
|
||||||||
| High school diploma | 35.2 | 34.3 | 0.02 | 36.2 | 34.9 | 0.03 | |||
|
|
|
||||||||
| Less than bachelor degree | 48.5 | 45.4 | 0.06 | 47.1 | 43.8 | 0.07 | |||
|
|
|
||||||||
| Bachelor degree plus | 9.2 | 9.3 | 0.00 | 9.2 | 8.7 | 0.02 | |||
|
|
|
||||||||
| Unknown | 6.1 | 9.9 | 0.14 | 6.4 | 11.5 | 0.18 | |||
|
|
|
||||||||
| Housing (rent/own) | |||||||||
|
|
|
||||||||
| Probable homeowner | 71.2 | 67.4 | 0.08 | 67.8 | 65.0 | 0.06 | |||
|
|
|
||||||||
| Unknown | 28.8 | 32.6 | 0.08 | 32.2 | 35.0 | 0.06 | |||
|
|
|
||||||||
| Household income | |||||||||
|
|
|
||||||||
| < $40 K | 20.7 | 22.7 | 0.05 | 22.6 | 24.3 | 0.04 | |||
|
|
|
||||||||
| $40 K–$49 K | 6.9 | 6.8 | 0.00 | 6.6 | 6.8 | 0.01 | |||
|
|
|
||||||||
| $50 K–$59 K | 7.3 | 7.2 | 0.01 | 6.8 | 7.1 | 0.01 | |||
|
|
|
||||||||
| $60 K–$74 K | 10.0 | 9.2 | 0.03 | 9.4 | 9.0 | 0.02 | |||
|
|
|
||||||||
| $75 K–$99 K | 14.0 | 12.1 | 0.05 | 12.8 | 11.3 | 0.04 | |||
|
|
|
||||||||
| $100 K+ | 21.9 | 19.0 | 0.07 | 20.7 | 16.9 | 0.10 | |||
|
|
|
||||||||
| Unknown | 19.2 | 23.0 | 0.09 | 21.1 | 24.6 | 0.08 | |||
|
|
|
||||||||
| Total net worth of the primary customer | |||||||||
|
|
|
||||||||
| < $25 K | 24.3 | 23.0 | 0.03 | 28.7 | 25.7 | 0.07 | |||
|
|
|
||||||||
| $25 K–$149 K | 21.3 | 18.0 | 0.08 | 19.7 | 17.4 | 0.06 | |||
|
|
|
||||||||
| $150 K–$249 K | 10.2 | 9.3 | 0.03 | 8.6 | 8.3 | 0.01 | |||
|
|
|
||||||||
| $250 K–$499 K | 13.2 | 13.5 | 0.01 | 10.8 | 11.6 | 0.03 | |||
|
|
|
||||||||
| $500 K+ | 12.6 | 14.5 | 0.06 | 11.5 | 13.1 | 0.05 | |||
|
|
|
||||||||
| Unknown | 18.4 | 21.7 | 0.08 | 20.7 | 24.0 | 0.08 | |||
|
| |||||||||
| Medicare enrolled | 53.4 | 53.3 | 0.00 | 26.3 | 50.6 | 0.51 | 30.2 | 62.5 | 0.68 |
|
| |||||||||
| Nursing home residence ever during baseline | 2.3 | 4.9 | 0.14 | 0.8 | 2.0 | 0.10 | 2.4 | 2.4 | 0.00 |
|
| |||||||||
| Baseline comorbidities, % | |||||||||
|
| |||||||||
| Disorders of lipid metabolism | 59.0 | 54.1 | 0.10 | 77.9 | 78.8 | 0.02 | 77.5 | 78.4 | 0.02 |
|
| |||||||||
| Rheumatic heart disease, chronic | 2.3 | 2.3 | 0.00 | 1.2 | 1.7 | 0.05 | 1.8 | 1.9 | 0.01 |
|
| |||||||||
| Hypertensive disease | 67.0 | 64.7 | 0.05 | 76.9 | 79.8 | 0.07 | 79.3 | 80.8 | 0.04 |
|
| |||||||||
| Ischemic heart disease | 21.8 | 22.9 | 0.03 | 14.5 | 19.1 | 0.12 | 17.8 | 19.8 | 0.05 |
|
| |||||||||
| Conduction disorders | 1.7 | 2.2 | 0.04 | 1.6 | 2.5 | 0.06 | 2.8 | 2.9 | 0.00 |
|
| |||||||||
| Heart failure/ cardiomyopathy | 9.7 | 11.8 | 0.07 | 5.0 | 8.2 | 0.13 | 9.0 | 9.0 | 0.00 |
|
| |||||||||
| Cardiomegaly | 4.0 | 5.2 | 0.06 | 2.8 | 4.3 | 0.08 | 4.6 | 4.7 | 0.01 |
|
| |||||||||
| Congenital anomalies of the heart, other | 1.1 | 1.2 | 0.02 | 0.3 | 0.4 | 0.01 | 0.3 | 0.3 | 0.00 |
|
| |||||||||
| Implantable cardioverter defibrillator/pacemaker use | 1.2 | 1.3 | 0.01 | 0.9 | 1.5 | 0.05 | 1.5 | 1.8 | 0.02 |
|
| |||||||||
| Kidney disease | 19.2 | 21.0 | 0.05 | 17.9 | 24.3 | 0.16 | 29.2 | 27.1 | 0.05 |
|
| |||||||||
| Liver disease | 11.6 | 11.6 | 0.00 | 9.1 | 9.1 | 0.00 | 10.2 | 9.4 | 0.03 |
|
| |||||||||
| Osteoporosis | 8.6 | 7.5 | 0.04 | 2.8 | 3.8 | 0.05 | 3.1 | 4.1 | 0.06 |
|
| |||||||||
| Depression | 30.0 | 29.1 | 0.02 | 17.0 | 20.3 | 0.08 | 20.1 | 21.8 | 0.04 |
|
| |||||||||
| Arthralgia (pain in joint) | 26.7 | 24.8 | 0.04 | 21.4 | 23.6 | 0.05 | 23.7 | 25.0 | 0.03 |
|
| |||||||||
| Bullous dermatoses | 0.0 | 0.1 | 0.02 | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.00 |
|
| |||||||||
| Pancreatic disease | 1.2 | 1.4 | 0.01 | 0.9 | 1.2 | 0.03 | 1.3 | 1.2 | 0.01 |
|
| |||||||||
| Obesity | 15.0 | 15.5 | 0.01 | 24.0 | 25.5 | 0.03 | 34.5 | 29.4 | 0.11 |
|
| |||||||||
| Tobacco use | 10.0 | 10.9 | 0.03 | 11.4 | 13.9 | 0.08 | 17.4 | 16.8 | 0.02 |
|
| |||||||||
| Alcohol abuse | 0.9 | 1.7 | 0.07 | 1.0 | 1.4 | 0.03 | 1.7 | 1.6 | 0.01 |
|
| |||||||||
| Hypoglycemia, serious | 2.0 | 2.7 | 0.05 | 0.4 | 0.6 | 0.03 | 0.4 | 0.5 | 0.01 |
|
| |||||||||
| Type 2 DMb | 97.1 | 96.8 | 0.02 | 98.7 | 98.6 | 0.01 | 98.8 | 98.8 | 0.00 |
|
| |||||||||
| Adapted diabetes complications severity index, % | |||||||||
|
| |||||||||
| 0 | 45.2 | 43.1 | 0.04 | 53.2 | 43.4 | 0.20 | 44.8 | 40.4 | 0.09 |
|
| |||||||||
| 1 | 14.8 | 14.7 | 0.00 | 18.5 | 18.9 | 0.01 | 18.3 | 19.3 | 0.02 |
|
| |||||||||
| 2 | 14.0 | 14.0 | 0.00 | 13.2 | 15.0 | 0.05 | 13.4 | 15.4 | 0.06 |
|
| |||||||||
| 3 | 8.8 | 8.8 | 0.00 | 6.4 | 8.8 | 0.09 | 8.3 | 9.6 | 0.05 |
|
| |||||||||
| 4 | 6.7 | 6.7 | 0.00 | 4.0 | 5.8 | 0.08 | 5.5 | 6.3 | 0.04 |
|
| |||||||||
| 5+ | 10.5 | 12.7 | 0.07 | 4.7 | 8.1 | 0.14 | 9.7 | 9.0 | 0.02 |
|
| |||||||||
| Drugs in the 30 days prior to cohort entry,c % | |||||||||
|
| |||||||||
| Alpha-glucosidase inhibitor | 0.6 | 0.6 | 0.01 | 0.1 | 0.2 | 0.01 | 0.1 | 0.2 | 0.01 |
|
| |||||||||
| Amylin analog | 0.1 | 0.0 | 0.01 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.01 |
|
| |||||||||
| Glucagonlike peptide 1 receptor agonist | 0.9 | 0.7 | 0.02 | 1.5 | 1.2 | 0.03 | 2.4 | 1.3 | 0.08 |
|
| |||||||||
| Insulin | 12.1 | 13.6 | 0.05 | 6.0 | 7.3 | 0.05 | 10.4 | 8.0 | 0.08 |
|
| |||||||||
| Metformin | 40.4 | 40.3 | 0.00 | 30.5 | 28.2 | 0.05 | 30.5 | 27.8 | 0.06 |
|
| |||||||||
| Meglitinides | 1.2 | 1.6 | 0.03 | 0.4 | 0.4 | 0.00 | 0.4 | 0.3 | 0.01 |
|
| |||||||||
| Sodium-glucose co-transporter 2 inhibitor | NA | 1.8 | 1.1 | 0.06 | 4.1 | 1.7 | 0.15 | ||
|
| |||||||||
| Sulfonylurea | 26.6 | 28.1 | 0.03 | 18.8 | 19.0 | 0.01 | 16.6 | 17.8 | 0.03 |
|
| |||||||||
| Thiazolidinedione | 15.0 | 14.3 | 0.02 | 4.8 | 5.3 | 0.02 | 2.7 | 2.9 | 0.02 |
|
| |||||||||
| Hepatic cytochrome P450 (CYP) 2D6 inhibitor | 23.2 | 21.7 | 0.04 | 6.4 | 7.4 | 0.04 | 6.1 | 6.6 | 0.02 |
|
| |||||||||
| CYP3A4 inhibitor | 5.4 | 5.7 | 0.01 | 4.3 | 4.4 | 0.00 | 4.9 | 4.3 | 0.03 |
|
| |||||||||
| CYP2C8 inhibitor | 20.4 | 19.6 | 0.02 | 7.8 | 8.3 | 0.02 | 6.3 | 6.2 | 0.00 |
|
| |||||||||
| CYP2D6 inducer | 0.1 | 0.1 | 0.01 | 0.1 | 0.2 | 0.01 | 0.2 | 0.2 | 0.00 |
|
| |||||||||
| CYP3A4 inducer | 18.1 | 17.5 | 0.02 | 7.4 | 8.1 | 0.03 | 6.8 | 6.7 | 0.00 |
|
| |||||||||
| CYP2C8 inducer | 0.0 | 0.0 | 0.02 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 |
|
| |||||||||
| P-glycoprotein inhibitor | 1.2 | 1.2 | 0.00 | 0.9 | 1.0 | 0.01 | 0.9 | 0.9 | 0.00 |
|
| |||||||||
| P-glycoprotein inducer | 16.4 | 18.2 | 0.05 | 9.1 | 10.8 | 0.06 | 13.8 | 11.4 | 0.07 |
|
| |||||||||
| Drug with known risk of TdPd | 12.2 | 11.4 | 0.02 | 9.5 | 10.0 | 0.02 | 11.1 | 10.4 | 0.02 |
|
| |||||||||
| Drug with known, possible, or conditional risk of TdPd | 58.4 | 56.2 | 0.04 | 38.9 | 40.6 | 0.03 | 42.4 | 41.5 | 0.02 |
|
| |||||||||
| ≥ 5 prescription dispensings for unique drugs in 30 days prior to entry | 59.8 | 58.2 | 0.03 | 26.1 | 27.1 | 0.02 | 24.2 | 24.9 | 0.02 |
|
| |||||||||
| Healthcare use intensity measures, median (Q1–Q3)e | |||||||||
|
| |||||||||
| Number of prescriptions dispensed, total | 71.0 (39.0–113) | 69.0 (37.0–112) | 0.03 | 29.0 (14.0–50.0) | 31.0 (16.0–53.0) | 0.08 | 27.0 (12.0–48.0) | 28.0 (14.0–49.0) | 0.05 |
|
| |||||||||
| Number of prescriptions dispensed, by unique drug | 16.0 (11.0–23.0) | 15.0 (10.0–22.0) | 0.07 | 8.0 (5.0–13.0) | 9.0 (6.0–13.0) | 0.11 | 8.0 (5.0–13.0) | 9.0 (5.0–13.0) | 0.07 |
|
| |||||||||
| Number of outpatient diagnosis codes, total | 38.0 (16.0–79.0) | 38.0 (17.0–85.0) | 0.06 | 42.0 (24.0–73.0) | 47.0 (26.0–85.0) | 0.13 | 51.0 (27.0–94.0) | 51.0 (28.0–92.0) | 0.04 |
|
| |||||||||
| Number of outpatient diagnosis codes, by unique code | 14.0 (7.0–24.0) | 13.0 (6.0–24.0) | 0.01 | 13.0 (8.0–20.0) | 15.0 (9.0–23.0) | 0.17 | 16.0 (10.0–25.0) | 16.0 (10.0–25.0) | 0.01 |
|
| |||||||||
| Number of outpatient CPT-4/HCPCS px codes, total | 49.0 (22.0–98.0) | 47.0 (18.0–99.0) | 0.02 | 32.0 (19.0–55.0) | 38.0 (22.0–67.0) | 0.17 | 37.0 (21.0–68.0) | 41.0 (23.0–73.0) | 0.03 |
|
| |||||||||
| Number of outpatient CPT-4/HCPCS px codes, unique code | 27.0 (13.0–47.0) | 27.0 (12.0–47.0) | 0.00 | 21.0 (13.0–32.0) | 24.0 (15.0–37.0) | 0.19 | 23.0 (14.0–37.0) | 25.0 (16.0–40.0) | 0.08 |
|
| |||||||||
| Number of laboratory LOINC codes, total | NA | 4.0 (0.0–63.0) | 2.0 (0.0–65.0) | 0.03 | 19.0 (0.0–77.0) | 10.0 (0.0–74.0) | 0.05 | ||
|
|
|
||||||||
| Number of laboratory LOINC codes, by unique code | 3.0 (0.0–49.0) | 2.0 (0.0–49.0) | 0.00 | 17.0 (0.0–53.0) | 8.0 (0.0–52.0) | 0.06 | |||
|
| |||||||||
| Laboratory values, % | |||||||||
|
| |||||||||
| Blood glucose | NA | ||||||||
|
|
|
||||||||
| No lab ever in past | 29.5 | 32.8 | 0.07 | 24.5 | 28.4 | 0.09 | |||
|
|
|
||||||||
| No lab in 1-year baseline | 27.2 | 25.2 | 0.04 | 27.2 | 26.1 | 0.03 | |||
|
|
|
||||||||
| Normal | 14.0 | 13.6 | 0.01 | 15.4 | 14.0 | 0.04 | |||
|
|
|
||||||||
| Low abnormal | 0.3 | 0.3 | 0.02 | 0.4 | 0.3 | 0.01 | |||
|
|
|
||||||||
| High abnormal | 29.1 | 28.0 | 0.02 | 32.5 | 31.2 | 0.03 | |||
|
|
|
||||||||
| Hemoglobin A1C | |||||||||
|
|
|
||||||||
| No lab ever in past | 29.5 | 32.8 | 0.07 | 24.5 | 28.4 | 0.09 | |||
|
|
|
||||||||
| No lab in 1-year baseline | 30.0 | 27.1 | 0.06 | 30.2 | 27.1 | 0.07 | |||
|
|
|
||||||||
| Normal | 9.7 | 9.9 | 0.01 | 10.8 | 10.5 | 0.01 | |||
|
|
|
||||||||
| Low abnormal | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.01 | |||
|
|
|
||||||||
| High abnormal | 30.8 | 30.2 | 0.01 | 34.5 | 34.0 | 0.01 | |||
|
|
|
||||||||
| Serum creatinine | |||||||||
|
|
|
||||||||
| No lab ever in past | 29.5 | 32.8 | 0.07 | 24.5 | 28.4 | 0.09 | |||
|
|
|
||||||||
| No lab in 1-year baseline | 26.6 | 24.6 | 0.04 | 26.3 | 25.1 | 0.03 | |||
|
|
|
||||||||
| Normal | 32.4 | 31.1 | 0.03 | 32.7 | 33.3 | 0.01 | |||
|
|
|
||||||||
| Low abnormal | 7.9 | 6.7 | 0.05 | 8.2 | 7.7 | 0.02 | |||
|
|
|
||||||||
| High abnormal | 3.6 | 4.8 | 0.06 | 8.3 | 5.5 | 0.11 | |||
|
|
|
||||||||
| Hematocrit | |||||||||
|
|
|
||||||||
| No lab ever in past | 29.5 | 32.8 | 0.07 | 24.5 | 28.4 | 0.09 | |||
|
|
|
||||||||
| No lab in 1-year baseline | 38.1 | 35.2 | 0.06 | 37.5 | 35.8 | 0.03 | |||
|
|
|
||||||||
| Normal | 29.2 | 27.8 | 0.03 | 32.6 | 31.0 | 0.03 | |||
|
|
|
||||||||
| Low abnormal | 2.8 | 3.8 | 0.06 | 4.8 | 4.4 | 0.02 | |||
|
|
|
||||||||
| High abnormal | 0.4 | 0.4 | 0.01 | 0.7 | 0.4 | 0.03 | |||
|
|
|
||||||||
| Hemoglobin | |||||||||
|
|
|
||||||||
| No lab ever in past | 29.5 | 32.8 | 0.07 | 24.5 | 28.4 | 0.09 | |||
|
|
|
||||||||
| No lab in 1-year baseline | 38.2 | 35.2 | 0.06 | 37.5 | 35.9 | 0.03 | |||
|
|
|
||||||||
| Normal | 27.9 | 26.7 | 0.03 | 31.1 | 29.5 | 0.03 | |||
|
|
|
||||||||
| Low abnormal | 3.7 | 4.8 | 0.05 | 6.0 | 5.6 | 0.02 | |||
|
|
|
||||||||
| High abnormal | 0.7 | 0.5 | 0.03 | 0.9 | 0.6 | 0.04 | |||
|
|
|
||||||||
| Hypoglycemia, alert value | |||||||||
|
|
|
||||||||
| No lab ever in past | 29.5 | 32.8 | 0.07 | 24.5 | 28.4 | 0.09 | |||
|
|
|
||||||||
| No lab in 1-year baseline | 27.2 | 25.2 | 0.04 | 27.2 | 26.1 | 0.03 | |||
|
|
|
||||||||
| No alert value | 42.5 | 40.7 | 0.04 | 46.8 | 44.1 | 0.05 | |||
|
|
|
||||||||
| Alert value | 0.9 | 1.3 | 0.04 | 1.5 | 1.5 | 0.00 | |||
|
|
|
||||||||
| Hypoglycemia of clinically significant value | |||||||||
|
|
|
||||||||
| No lab ever in past | 29.5 | 32.8 | 0.07 | 24.5 | 28.4 | 0.09 | |||
|
|
|
||||||||
| No glucose in 1-year baseline | 27.2 | 25.2 | 0.04 | 27.2 | 26.1 | 0.03 | |||
|
|
|
||||||||
| No clinically significant value | 43.1 | 41.4 | 0.03 | 47.7 | 44.9 | 0.06 | |||
|
|
|
||||||||
| Clinically significant value | 0.3 | 0.5 | 0.04 | 0.6 | 0.7 | 0.02 | |||
CA, California; CPT, Current Procedural Terminology; CYP, cytochrome P450; DM, diabetes mellitus; FL, Florida; HCPCS, Healthcare Common Procedure Coding System; ICD, International Classification of Diseases; LOINC, Logical Observation Identifiers Names and Codes; NA, not applicable; NY, New York; OH, Ohio; PA, Pennsylvania; Q, quartile; SMD, standardized mean difference; TdP, torsade de pointes.
Absolute values of standardized differences.
Defined by ratio of type 1 (e.g., ICD-9 250.X1 or 250.X3) to type 2 (e.g., ICD-9 250.X0 or 250.X2) codes ≤ 0.5, ascertained during baseline and on cohort entry date.
Antimicrobial drugs were examined within 14 (rather than 30) days prior to cohort entry since they are often prescribed for acute conditions.
Identified using CredibleMeds (AZCERT Inc., Oro Valley, AZ).
The following healthcare utilization covariates were excluded from presentation in the table, as their median values were zero for each DPP-4 inhibitors: number of inpatient ICD-9 diagnosis codes; number of unique inpatient ICD-9 diagnosis codes; number of inpatient ICD-9 procedure codes; number of unique inpatient ICD-9 procedure codes; number of inpatient CPT/HCPCS procedure codes; number of unique inpatient CPT/HCPCS procedure codes; number of outpatient ICD-9 procedure codes; number of unique outpatient ICD-9 procedure codes; number of other setting ICD-9 diagnosis codes; number of unique other setting ICD-9 diagnosis codes; number of other setting ICD-9 procedure codes; number of unique other setting ICD-9 procedure codes; number of laboratory LOINC codes (Optum only); number of unique laboratory LOINC codes (Optum only).
Table 2.
Demographics and clinical characteristics of new users of dipeptidyl peptidase-4 inhibitors in Medicaid and Optum databases, after high-dimensional propensity score matching
| Medicaid Database | Optum Database | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Saxagliptin | Sitagliptin | SMD | Saxagliptin | Sitagliptin | SMD | Linagliptin | Sitagliptin | SMD | ||
|
| ||||||||||
| Users, N | 23,895 | 96,972 | 48,388 | 117,383 | 36,820 | 78,701 | ||||
|
| ||||||||||
| Demographics, % (unless otherwise indicated) | ||||||||||
|
| ||||||||||
| Age in years, median (Q1–Q3) | 60.4 (51.2–68.5) | 60.4 (51.2–68.5) | 0.00 | 57.8 (49.9–65.4) | 59.3 (51.0–67.0) | 0.12 | 59.6 (51.5–67.2) | 62.0 (53.2–68.8) | 0.17 | |
|
| ||||||||||
| Sex, female | 61.2 | 60.8 | 0.01 | 44.6 | 45.4 | 0.02 | 45.9 | 47.2 | 0.03 | |
|
| ||||||||||
| Race | ||||||||||
|
| ||||||||||
| White | 35.5 | 36.5 | 0.02 | 57.1 | 56.6 | 0.01 | 53.6 | 52.7 | 0.02 | |
|
| ||||||||||
| Black | 13.5 | 14.3 | 0.02 | 15.1 | 14.7 | 0.01 | 15.4 | 14.9 | 0.01 | |
|
| ||||||||||
| Hispanic/Latino | 30.7 | 30.5 | 0.00 | 15.1 | 15.1 | 0.00 | 16.8 | 16.7 | 0.00 | |
|
| ||||||||||
| Asian | 9.1 | 8.9 | 0.01 | 4.6 | 4.6 | 0.00 | 4.9 | 4.8 | 0.00 | |
|
| ||||||||||
| Other/unknown | 11.2 | 9.8 | 0.05 | 8.0 | 9.0 | 0.04 | 9.3 | 10.9 | 0.05 | |
|
| ||||||||||
| State of residence | NA | |||||||||
|
|
||||||||||
| CA | 45.7 | 41.9 | 0.08 | |||||||
|
|
||||||||||
| FL | 16.7 | 14.2 | 0.07 | |||||||
|
|
||||||||||
| NY | 23.0 | 27.2 | 0.10 | |||||||
|
|
||||||||||
| OH | 9.7 | 11.0 | 0.04 | |||||||
|
|
||||||||||
| PA | 4.8 | 5.7 | 0.04 | |||||||
|
|
||||||||||
| Census level division based on US state | ||||||||||
|
|
|
|||||||||
| New England | 2.0 | 2.3 | 0.02 | 2.7 | 2.9 | 0.01 | ||||
|
|
|
|||||||||
| Middle Atlantic | 6.0 | 6.6 | 0.03 | 7.7 | 7.9 | 0.01 | ||||
|
|
|
|||||||||
| East North Central | 13.3 | 12.9 | 0.01 | 13.6 | 12.4 | 0.03 | ||||
|
|
|
|||||||||
| West North Central | 5.9 | 6.2 | 0.01 | 7.1 | 6.7 | 0.01 | ||||
|
|
|
|||||||||
| South Atlantic | 31.4 | 30.7 | 0.02 | 27.3 | 27.4 | 0.00 | ||||
|
|
|
|||||||||
| East South Central | 5.2 | 4.9 | 0.01 | 5.5 | 4.9 | 0.03 | ||||
|
|
|
|||||||||
| West South Central | 22.3 | 20.2 | 0.05 | 19.8 | 19.1 | 0.02 | ||||
|
|
|
|||||||||
| Mountain | 6.7 | 7.3 | 0.02 | 7.8 | 7.8 | 0.00 | ||||
|
|
|
|||||||||
| Pacific | 6.7 | 8.4 | 0.06 | 8.4 | 10.6 | 0.08 | ||||
|
|
|
|||||||||
| Unknown | 0.4 | 0.4 | 0.01 | 0.2 | 0.3 | 0.02 | ||||
|
|
|
|||||||||
| Education level | ||||||||||
|
|
|
|||||||||
| Less than 12th grade | 1.0 | 1.0 | 0.00 | 1.1 | 1.1 | 0.00 | ||||
|
|
|
|||||||||
| High school diploma | 35.2 | 34.9 | 0.01 | 36.1 | 35.7 | 0.01 | ||||
|
|
|
|||||||||
| Less than bachelor degree | 48.3 | 47.3 | 0.02 | 46.5 | 45.4 | 0.02 | ||||
|
|
|
|||||||||
| Bachelor degree plus | 9.3 | 9.6 | 0.01 | 9.3 | 9.1 | 0.01 | ||||
|
|
|
|||||||||
| Unknown | 6.2 | 7.2 | 0.04 | 7.0 | 8.7 | 0.06 | ||||
|
|
|
|||||||||
| Housing (rent/own) | ||||||||||
|
|
|
|||||||||
| Probable homeowner | 71.1 | 70.3 | 0.02 | 67.3 | 66.5 | 0.02 | ||||
|
|
|
|||||||||
| Unknown | 28.9 | 29.7 | 0.02 | 32.7 | 33.5 | 0.02 | ||||
|
|
|
|||||||||
| Household income | ||||||||||
|
|
|
|||||||||
| < $40 K | 20.8 | 21.5 | 0.02 | 23.1 | 23.8 | 0.02 | ||||
|
|
|
|||||||||
| $40 K–$49 K | 6.9 | 6.9 | 0.00 | 6.6 | 6.7 | 0.00 | ||||
|
|
|
|||||||||
| $50 K–$59 K | 7.3 | 7.3 | 0.00 | 6.9 | 7.0 | 0.01 | ||||
|
|
|
|||||||||
| $60 K–$74 K | 10.0 | 9.7 | 0.01 | 9.4 | 9.2 | 0.01 | ||||
|
|
|
|||||||||
| $75 K–$99 K | 13.9 | 13.2 | 0.02 | 12.4 | 12.0 | 0.01 | ||||
|
|
|
|||||||||
| $100 K+ | 21.9 | 21.2 | 0.02 | 20.0 | 18.6 | 0.03 | ||||
|
|
|
|||||||||
| Unknown | 19.2 | 20.1 | 0.02 | 21.5 | 22.6 | 0.03 | ||||
|
|
|
|||||||||
| Total net worth of the primary customer | ||||||||||
|
|
|
|||||||||
| < $25 K | 24.2 | 23.4 | 0.02 | 28.3 | 26.9 | 0.03 | ||||
|
|
|
|||||||||
| $25 K–$149 K | 21.2 | 19.8 | 0.03 | 18.9 | 18.3 | 0.01 | ||||
|
|
|
|||||||||
| $150 K–$249 K | 10.2 | 10.1 | 0.00 | 8.6 | 8.4 | 0.01 | ||||
|
|
|
|||||||||
| $250 K–$499 K | 13.3 | 13.8 | 0.02 | 11.1 | 11.6 | 0.02 | ||||
|
|
|
|||||||||
| $500 K+ | 12.7 | 13.8 | 0.03 | 12.1 | 12.6 | 0.02 | ||||
|
|
|
|||||||||
| Unknown | 18.5 | 19.1 | 0.02 | 21.1 | 22.1 | 0.02 | ||||
|
| ||||||||||
| Medicare enrolled | 53.4 | 55.3 | 0.04 | 26.7 | 38.2 | 0.25 | 34.2 | 54.0 | 0.41 | |
|
| ||||||||||
| Nursing home residence ever during baseline | 2.3 | 2.6 | 0.02 | 0.8 | 1.0 | 0.02 | 2.4 | 2.3 | 0.00 | |
|
| ||||||||||
| Baseline comorbidities, % | ||||||||||
|
| ||||||||||
| Disorders of lipid metabolism | 59.0 | 57.0 | 0.04 | 77.9 | 78.1 | 0.00 | 76.5 | 77.5 | 0.02 | |
|
| ||||||||||
| Rheumatic heart disease, chronic | 2.3 | 2.1 | 0.01 | 1.2 | 1.3 | 0.01 | 1.8 | 1.8 | 0.00 | |
|
| ||||||||||
| Hypertensive disease | 67.0 | 65.2 | 0.04 | 77.1 | 77.7 | 0.01 | 78.5 | 79.5 | 0.03 | |
|
| ||||||||||
| Ischemic heart disease | 21.8 | 21.1 | 0.02 | 14.6 | 15.9 | 0.04 | 18.0 | 18.7 | 0.02 | |
|
| ||||||||||
| Conduction disorders | 1.7 | 1.7 | 0.00 | 1.6 | 1.8 | 0.01 | 2.7 | 2.8 | 0.00 | |
|
| ||||||||||
| Heart failure/cardiomyopathy | 9.7 | 9.8 | 0.00 | 5.0 | 5.8 | 0.03 | 9.0 | 8.8 | 0.00 | |
|
| ||||||||||
| Cardiomegaly | 4.0 | 4.1 | 0.00 | 2.8 | 3.1 | 0.02 | 4.4 | 4.5 | 0.00 | |
|
| ||||||||||
| Congenital anomalies of the heart, other | 1.1 | 1.0 | 0.00 | 0.3 | 0.4 | 0.01 | 0.3 | 0.3 | 0.00 | |
|
| ||||||||||
| Implantable cardioverter defibrillator/pacemaker use | 1.2 | 1.2 | 0.01 | 0.9 | 1.1 | 0.01 | 1.5 | 1.6 | 0.01 | |
|
| ||||||||||
| Kidney disease | 19.1 | 19.1 | 0.00 | 18.0 | 19.4 | 0.04 | 28.3 | 27.4 | 0.02 | |
|
| ||||||||||
| Liver disease | 11.5 | 11.1 | 0.01 | 9.0 | 8.8 | 0.01 | 9.8 | 9.5 | 0.01 | |
|
| ||||||||||
| Osteoporosis | 8.6 | 7.9 | 0.03 | 2.8 | 3.1 | 0.02 | 3.2 | 3.7 | 0.03 | |
|
| ||||||||||
| Depression | 30.0 | 29.4 | 0.01 | 17.1 | 18.0 | 0.02 | 20.2 | 21.1 | 0.02 | |
|
| ||||||||||
| Arthralgia (pain in joint) | 26.7 | 25.3 | 0.03 | 21.4 | 21.9 | 0.01 | 23.7 | 24.2 | 0.01 | |
|
| ||||||||||
| Bullous dermatoses | 0.0 | 0.0 | 0.00 | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.01 | |
|
| ||||||||||
| Pancreatic disease | 1.2 | 1.2 | 0.00 | 0.9 | 0.9 | 0.01 | 1.3 | 1.2 | 0.00 | |
|
| ||||||||||
| Obesity | 15.0 | 15.0 | 0.00 | 23.9 | 23.3 | 0.01 | 32.4 | 30.5 | 0.04 | |
|
| ||||||||||
| Tobacco use | 10.0 | 10.1 | 0.00 | 11.3 | 11.6 | 0.01 | 16.8 | 16.5 | 0.01 | |
|
| ||||||||||
| Alcohol abuse | 0.9 | 1.0 | 0.01 | 1.0 | 1.1 | 0.01 | 1.6 | 1.6 | 0.00 | |
|
| ||||||||||
| Hypoglycemia, serious | 2.0 | 2.0 | 0.00 | 0.4 | 0.4 | 0.01 | 0.5 | 0.5 | 0.01 | |
|
| ||||||||||
| Type 2 DMc | 97.1 | 97.0 | 0.00 | 98.7 | 98.5 | 0.01 | 98.7 | 98.8 | 0.01 | |
|
| ||||||||||
| Adapted Diabetes Complications Severity Index, % | ||||||||||
|
| ||||||||||
| 0 | 45.2 | 45.8 | 0.01 | 53.0 | 50.0 | 0.06 | 44.7 | 42.9 | 0.04 | |
|
| ||||||||||
| 1 | 14.8 | 14.7 | 0.00 | 18.5 | 18.9 | 0.01 | 18.3 | 18.8 | 0.01 | |
|
| ||||||||||
| 2 | 14.0 | 13.8 | 0.01 | 13.2 | 13.9 | 0.02 | 13.6 | 14.5 | 0.03 | |
|
| ||||||||||
| 3 | 8.8 | 8.7 | 0.00 | 6.5 | 7.2 | 0.03 | 8.4 | 8.8 | 0.01 | |
|
| ||||||||||
| 4 | 6.7 | 6.5 | 0.01 | 4.1 | 4.6 | 0.02 | 5.5 | 5.9 | 0.02 | |
|
| ||||||||||
| 5+ | 10.4 | 10.6 | 0.00 | 4.7 | 5.4 | 0.03 | 9.4 | 9.0 | 0.01 | |
|
| ||||||||||
| Drugs in the 30 days prior to cohort entry,b % | ||||||||||
|
| ||||||||||
| Alpha-glucosidase inhibitor | 0.6 | 0.5 | 0.00 | 0.1 | 0.2 | 0.00 | 0.1 | 0.2 | 0.01 | |
|
| ||||||||||
| Amylin analog | 0.1 | 0.1 | 0.01 | 0.0 | 0.0 | 0.01 | 0.0 | 0.0 | 0.01 | |
|
| ||||||||||
| Glucagonlike peptide 1 receptor agonist | 0.9 | 0.8 | 0.01 | 1.5 | 1.2 | 0.02 | 2.0 | 1.6 | 0.03 | |
|
| ||||||||||
| Insulin | 12.1 | 12.4 | 0.01 | 6.0 | 6.4 | 0.01 | 9.7 | 8.8 | 0.03 | |
|
| ||||||||||
| Metformin | 40.4 | 40.7 | 0.01 | 30.4 | 29.3 | 0.02 | 29.5 | 28.6 | 0.02 | |
|
| ||||||||||
| Meglitinides | 1.2 | 1.3 | 0.01 | 0.4 | 0.4 | 0.00 | 0.4 | 0.3 | 0.00 | |
|
| ||||||||||
| Sodium-glucose co-transporter 2 inhibitor | NA | 1.6 | 1.2 | 0.03 | 3.1 | 2.1 | 0.06 | |||
|
| ||||||||||
| Sulfonylurea | 26.6 | 27.5 | 0.02 | 18.8 | 19.1 | 0.01 | 16.6 | 17.4 | 0.02 | |
|
| ||||||||||
| Thiazolidinedione | 15.0 | 14.7 | 0.01 | 4.8 | 5.2 | 0.02 | 2.7 | 2.8 | 0.01 | |
|
| ||||||||||
| Hepatic cytochrome P450 (CYP) 2D6 inhibitor | 23.2 | 22.4 | 0.02 | 6.5 | 6.8 | 0.01 | 6.3 | 6.5 | 0.01 | |
|
| ||||||||||
| CYP3A4 inhibitor | 5.4 | 5.5 | 0.00 | 4.3 | 4.3 | 0.00 | 4.7 | 4.5 | 0.01 | |
|
| ||||||||||
| CYP2C8 inhibitor | 20.4 | 20.0 | 0.01 | 7.8 | 8.1 | 0.01 | 6.2 | 6.2 | 0.00 | |
|
| ||||||||||
| CYP2D6 inducer | 0.1 | 0.1 | 0.00 | 0.1 | 0.1 | 0.00 | 0.2 | 0.2 | 0.00 | |
|
| ||||||||||
| CYP3A4 inducer | 18.1 | 17.7 | 0.01 | 7.4 | 7.7 | 0.01 | 6.7 | 6.7 | 0.00 | |
|
| ||||||||||
| CYP2C8 inducer | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 | |
|
| ||||||||||
| P-glycoprotein inhibitor | 1.2 | 1.2 | 0.00 | 0.9 | 0.9 | 0.00 | 0.9 | 0.9 | 0.00 | |
|
| ||||||||||
| P-glycoprotein inducer | 16.4 | 16.7 | 0.01 | 9.1 | 9.6 | 0.02 | 13.2 | 12.3 | 0.03 | |
|
| ||||||||||
| Drug with known risk of TdPd | 12.2 | 11.7 | 0.01 | 9.5 | 9.4 | 0.00 | 10.8 | 10.5 | 0.01 | |
|
| ||||||||||
| Drug with known, possible, or conditional risk of TdPd | 58.4 | 57.5 | 0.02 | 39.0 | 39.1 | 0.00 | 41.6 | 41.5 | 0.00 | |
|
| ||||||||||
| ≥ 5 prescription dispensings for unique drugs in 30 days prior to entry | 59.8 | 58.7 | 0.02 | 26.2 | 26.6 | 0.01 | 24.1 | 24.7 | 0.01 | |
|
| ||||||||||
| Healthcare use intensity measures, median (Q1–Q3)a | ||||||||||
|
| ||||||||||
| Number of prescriptions dispensed, total | 71.0 (39.0–113) | 68.0 (37.0–110) | 0.04 | 29.0 (14.0–50.0) | 30.0 (15.0–52.0) | 0.04 | 27.0 (13.0–48.0) | 28.0 (14.0–49.0) | 0.02 | |
|
| ||||||||||
| Number of prescriptions dispensed, by unique drug | 16.0 (11.0–23.0) | 15.0 (10.0–22.0) | 0.06 | 8.0 (5.0–13.0) | 9.0 (5.0–13.0) | 0.04 | 8.0 (5.0–13.0) | 9.0 (5.0–13.0) | 0.03 | |
|
| ||||||||||
| Number of outpatient diagnosis codes, total | 38.0 (16.0–79.0) | 36.0 (15.0–78.0) | 0.02 | 42.0 (24.0–73.0) | 43.0 (24.0–75.0) | 0.01 | 49.0 (26.0–91.0) | 49.0 (27.0–90.0) | 0.03 | |
|
| ||||||||||
| Number of outpatient diagnosis codes, by unique code | 14.0 (7.0–24.0) | 13.0 (6.0–24.0) | 0.05 | 13.0 (8.0–20.0) | 13.0 (8.0–21.0) | 0.02 | 15.0 (9.0–24.0) | 16.0 (10.0–24.0) | 0.01 | |
|
| ||||||||||
| Number of outpatient CPT-4/HCPCS px codes, total | 49.0 (22.0–98.0) | 46.0 (19.0–95.0) | 0.02 | 32.0 (19.0–55.0) | 34.0 (20.0–58.0) | 0.04 | 37.0 (21.0–68.0) | 39.0 (21.0–70.0) | 0.00 | |
|
| ||||||||||
| Number of outpatient CPT-4/HCPCS px codes, unique code | 27.0 (13.0–47.0) | 26.0 (12.0–45.0) | 0.05 | 21.0 (13.0–32.0) | 21.0 (13.0–33.0) | 0.04 | 23.0 (14.0–38.0) | 24.0 (15.0–39.0) | 0.03 | |
|
| ||||||||||
| Number of laboratory LOINC codes, total | NA | 4.0 (0.0–63.0) | 2.0 (0.0–62.0) | 0.01 | 17.0 (0.0–76.0) | 13.0 (0.0–74.0) | 0.02 | |||
|
|
|
|||||||||
| Number of laboratory LOINC codes, by unique code | 3.0 (0.0–49.0) | 2.0 (0.0–48.0) | 0.02 | 15.0 (0.0–52.0) | 11.0 (0.0–52.0) | 0.03 | ||||
|
| ||||||||||
| Laboratory values, % | ||||||||||
|
| ||||||||||
| Blood glucose | NA | |||||||||
|
|
|
|||||||||
| No lab ever in past | 29.6 | 31.4 | 0.04 | 25.0 | 26.4 | 0.03 | ||||
|
|
|
|||||||||
| No lab in 1-year baseline | 27.1 | 26.4 | 0.02 | 27.2 | 26.9 | 0.01 | ||||
|
|
|
|||||||||
| Normal | 14.1 | 13.8 | 0.01 | 15.1 | 14.5 | 0.02 | ||||
|
|
|
|||||||||
| Low abnormal | 0.3 | 0.3 | 0.00 | 0.4 | 0.4 | 0.00 | ||||
|
|
|
|||||||||
| High abnormal | 29.0 | 28.1 | 0.02 | 32.3 | 31.8 | 0.01 | ||||
|
|
|
|||||||||
| Hemoglobin A1C | ||||||||||
|
|
|
|||||||||
| No lab ever in past | 29.6 | 31.4 | 0.04 | 25.0 | 26.4 | 0.03 | ||||
|
|
|
|||||||||
| No lab in 1-year baseline | 30.0 | 28.8 | 0.03 | 29.9 | 28.3 | 0.04 | ||||
|
|
|
|||||||||
| Normal | 9.7 | 9.6 | 0.00 | 10.7 | 10.5 | 0.01 | ||||
|
|
|
|||||||||
| Low abnormal | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 | ||||
|
|
|
|||||||||
| High abnormal | 30.7 | 30.2 | 0.01 | 34.3 | 34.8 | 0.01 | ||||
|
|
|
|||||||||
| Serum creatinine | ||||||||||
|
|
|
|||||||||
| No lab ever in past | 29.6 | 31.4 | 0.04 | 25.0 | 26.4 | 0.03 | ||||
|
|
|
|||||||||
| No lab in 1-year baseline | 26.6 | 25.9 | 0.01 | 26.3 | 25.9 | 0.01 | ||||
|
|
|
|||||||||
| Normal | 32.4 | 31.6 | 0.02 | 32.8 | 33.2 | 0.01 | ||||
|
|
|
|||||||||
| Low abnormal | 7.8 | 7.1 | 0.03 | 8.1 | 7.9 | 0.01 | ||||
|
|
|
|||||||||
| High abnormal | 3.6 | 3.9 | 0.02 | 7.7 | 6.5 | 0.05 | ||||
|
|
|
|||||||||
| Hematocrit | ||||||||||
|
|
|
|||||||||
| No lab ever in past | 29.6 | 31.4 | 0.04 | 25.0 | 26.4 | 0.03 | ||||
|
|
|
|||||||||
| No lab in 1-year baseline | 38.1 | 37.2 | 0.02 | 37.6 | 37.0 | 0.01 | ||||
|
|
|
|||||||||
| Normal | 29.1 | 28.0 | 0.02 | 32.1 | 31.5 | 0.01 | ||||
|
|
|
|||||||||
| Low abnormal | 2.8 | 3.0 | 0.01 | 4.6 | 4.5 | 0.01 | ||||
|
|
|
|||||||||
| High abnormal | 0.4 | 0.4 | 0.00 | 0.6 | 0.5 | 0.02 | ||||
|
|
|
|||||||||
| Hemoglobin | ||||||||||
|
|
|
|||||||||
| No lab ever in past | 29.6 | 31.4 | 0.04 | 25.0 | 26.4 | 0.03 | ||||
|
|
|
|||||||||
| No lab in 1-year baseline | 38.1 | 37.2 | 0.02 | 37.6 | 37.1 | 0.01 | ||||
|
|
|
|||||||||
| Normal | 27.8 | 26.9 | 0.02 | 30.7 | 30.1 | 0.01 | ||||
|
|
|
|||||||||
| Low abnormal | 3.7 | 3.8 | 0.01 | 5.8 | 5.7 | 0.00 | ||||
|
|
|
|||||||||
| High abnormal | 0.7 | 0.6 | 0.01 | 0.8 | 0.7 | 0.02 | ||||
|
|
|
|||||||||
| Hypoglycemia, alert value | ||||||||||
|
|
|
|||||||||
| No lab ever in past | 29.6 | 31.4 | 0.04 | 25.0 | 26.4 | 0.03 | ||||
|
|
|
|||||||||
| No lab in 1-year baseline | 27.1 | 26.4 | 0.02 | 27.2 | 26.9 | 0.01 | ||||
|
|
|
|||||||||
| No alert value | 42.4 | 41.2 | 0.02 | 46.3 | 45.2 | 0.02 | ||||
|
|
|
|||||||||
| Alert value | 0.9 | 0.9 | 0.01 | 1.5 | 1.5 | 0.00 | ||||
|
|
|
|||||||||
| Hypoglycemia of clinically significant value | ||||||||||
|
|
|
|||||||||
| No lab ever in past | 29.6 | 31.4 | 0.04 | 25.0 | 26.4 | 0.03 | ||||
|
|
|
|||||||||
| No glucose in 1-year baseline | 27.1 | 26.4 | 0.02 | 27.2 | 26.9 | 0.01 | ||||
|
|
|
|||||||||
| No clinically significant value | 43.0 | 41.8 | 0.02 | 47.2 | 46.0 | 0.02 | ||||
|
|
|
|||||||||
| Clinically significant value | 0.3 | 0.3 | 0.01 | 0.6 | 0.6 | 0.01 | ||||
CA, California; CPT, Current Procedural Terminology; CYP, cytochrome P450; DM, diabetes mellitus; FL, Florida; HCPCS, Healthcare Common Procedure Coding System; ICD, International Classification of Diseases; LOINC, Logical Observation Identifiers Names and Codes; NA, not applicable; NY, New York; OH, Ohio; PA, Pennsylvania; Q, quartile; SMD, standardized mean difference; TdP, torsade de pointes.
The following healthcare utilization covariates were excluded from presentation in the table, as their median values were zero for each DPP-4 inhibitors: number of inpatient ICD-9 diagnosis codes; number of unique inpatient ICD-9 diagnosis codes; number of inpatient ICD-9 procedure codes; number of unique inpatient ICD-9 procedure codes; number of inpatient CPT/HCPCS procedure codes; number of unique inpatient CPT/HCPCS procedure codes; number of outpatient ICD-9 procedure codes; number of unique outpatient ICD-9 procedure codes; number of other setting ICD-9 diagnosis codes; number of unique other setting ICD-9 diagnosis codes; number of other setting ICD-9 procedure codes; number of unique other setting ICD-9 procedure codes; number of laboratory LOINC codes (Optum only); number of unique laboratory LOINC codes (Optum only).
Antimicrobial drugs were examined within 14 (rather than 30) days prior to cohort entry because they are often prescribed for acute conditions.
Defined by ratio of type 1 (e.g., ICD-9 250.X1 or 250.X3) to type 2 (e.g., ICD-9 250.X0 or 250.X2) codes ≤ 0.5, ascertained during baseline and on cohort entry date.
Identified using CredibleMeds (AZCERT Inc., Oro Valley, AZ).
Optum database (saxagliptin vs. sitagliptin).
We identified 49,024 new users of saxagliptin 164,906 new users of sitagliptin (Table 1). The hdPS model included a total of 576 covariates with 76 prespecified 500 empirically identified (Table S6). We matched 48,388 saxagliptin users to 117,383 sitagliptin users. The distribution of PS before after matching is illustrated in Figure S2. After matching, demographics clinical characteristics were well-balanced, including female sex (45% vs. 45%), presence of comorbidities, such as heart failure (5% vs. 6%), kidney disease (18% vs. 19%), hypoglycemia (0.4% vs. 0.4%), baseline use of medications, such as insulin (6% vs. 6%), metformin (31% vs. 28%), sulfonylureas (19% vs. 19%; Table 2). The median follow-up time was 79 days (8, 546 days) for saxagliptin users 76 days (6, 474 days) for sitagliptin users.
Optum database (linagliptin vs. sitagliptin).
We identified 43,080 new users of saxagliptin 109,635 new users of sitagliptin (Table 1). The hdPS model included a total of 575 covariates with 76 prespecified 499 empirically identified (Table S7). We matched 36,820 linagliptin users to 78,701 sitagliptin users. The distribution of PS before after matching is illustrated in Figure S3. After matching, persons’ demographics clinical characteristics were well-balanced, including female sex (46% vs. 47%), presence of comorbidities, such as heart failure (9% vs. 9%), kidney disease (28% vs. 27%), hypoglycemia (0.5% vs. 0.5%), baseline use of medications, such as insulin (10% vs. 9%), metformin (31% vs. 28%), sulfonylureas (17% vs. 17%). The median follow-up time was 75 days (7, 469 days) for linagliptin users 75 days (5, 428 days) for sitagliptin users.
Rate of SCA/VA with saxagliptin vs. sitagliptin
Medicaid database.
Figure 1a presents the Kaplan-Meier curve comparing the rate of SCA/VA between saxagliptin sitagliptin users in the matched cohort in Medicaid data. In the matched cohort, we identified 26 SCA/VA events among saxagliptin users (3.7 per 1,000 person-years) vs. 55 among sitagliptin users (1.6 per 1,000 person-years). In the Cox regression model, use of saxagliptin was associated with a higher rate of SCA/VA compared with sitagliptin (adjusted HR (aHR), 2.01, 95% confidence interval [CI] 1.24–3.25; Table 3).
Figure 1.
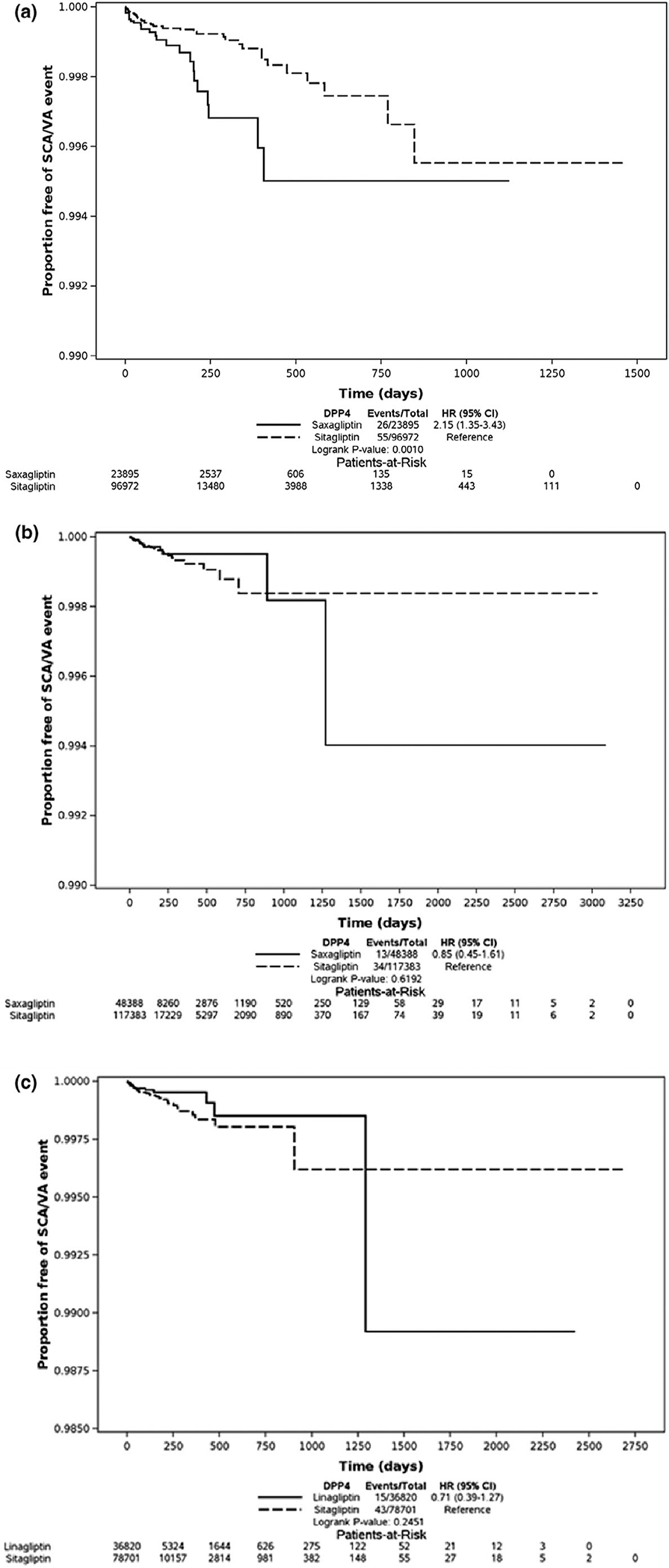
Kaplan–Meier curves depicting the proportion free of sudden cardiac arrest / ventricular arrhythmia (SCA/VA) events among new users of dipeptidyl peptidase-4 inhibitors in Medicaid Optum databases in the matched cohort. (a) Kaplan-Meier curves depicting the proportion free of SCA/VA events among new users of saxagliptin vs. sitagliptin in Medicaid database (P value based on stratified log-rank test P = 0.007). (b) Kaplan–Meier curves depicting the proportion free of SCA/VA events among new users of saxagliptin vs. sitagliptin in Optum database (P value based on stratified log-rank test P = 0.8093). (c) Kaplan–Meier curves depicting the proportion free of SCA/VA events among new users of linagliptin vs. sitagliptin in Optum database (P value based on stratified log-rank test P = 0.586). CI, confidence interval; HR, hazard ratio.
Table 3.
Rates of sudden cardiac arrest and ventricular arrhythmia among new users of saxagliptin, sitagliptin, and linagliptin in Medicaid and Optum databases, for high-dimensional propensity score matched cohorts
| Database | Drug | New users, n | Events, n | Person-years of follow-up | Incidence rate per 1,000 persons-years | Adjusted marginal HR, 95% CIa |
|---|---|---|---|---|---|---|
| Medicaid | Saxagliptin | 23,895 | 26 | 7,096 | 3.7 | 2.01 (1.24, 3.25) |
| Sitagliptin | 96,972 | 55 | 33,470 | 1.6 | Reference | |
| Optum | Saxagliptin | 48,388 | 13 | 20,239 | 0.6 | 0.79 (0.41, 1.51) |
| Sitagliptin | 117,383 | 34 | 44,247 | 0.8 | Reference | |
| Linagliptin | 36,820 | 15 | 13,707 | 1.1 | 0.65 (0.36, 1.17) | |
| Sitagliptin | 78,701 | 43 | 27,372 | 1.6 | Reference |
CI, confidence interval, HR, hazard ratio.
Adjusted for calendar year of cohort entry in the high-dimensional propensity score matched cohort.
Optum database.
Figure 1b presents the Kaplan-Meier curve comparing the rate of SCA/VA between saxagliptin sitagliptin users in the matched cohort in Optum data. In the matched cohort, we identified 13 SCA/VA events among saxagliptin users (0.6 per 1,000 person-years) compared with 34 among sitagliptin users (0.8 per 1,000 person-years). In the Cox regression model, the association between saxagliptin (vs. sitagliptin) SCA/VA was consistent with the null (aHR, 0.79, 95% CI 0.41–1.51; Table 3).
Figure 1c presents the Kaplan-Meier curve comparing the rate of SCA/VA between linagliptin sitagliptin users in the matched cohort in Optum data. In the matched cohort, we identified 15 SCA/VA events among linagliptin users (1.1 per 1,000 person-years) compared with 43 among sitagliptin users (1.6 per 1,000 person-years). In the Cox regression model, the association between linagliptin (vs. sitagliptin) SCA/VA was consistent with the null (aHR, 0.65, 95% CI 0.36–1.17; Table 3).
Sensitivity subgroup analyses
Results from sensitivity analyses in both databases were similar to primary findings, including when accounting for variable ratio matching using weighting, limiting follow-up to 30 days, changing the permissible gap between contiguous dispensings to 7 days 30 days, excluding persons with a prior SCA/VA diagnosis, excluding covariates from the hdPS thought to be strong correlates of exposure but not associated with the outcome, examining a competing risk model with death as the competing event (Medicaid only; Table S8). We did not identify any dose-response relationships. We further found null associations when examining other DPP-4 inhibitors, including linagliptin saxagliptin (vs. sitagliptin; aHR, 0.65; 95% CI 0.37–1.16), linagliptin (vs. saxagliptin; aHR, 0.92, 95% CI 0.36–2.38). None of the stratified analyses by subgroups demonstrated heterogeneity of treatment effect after adjusting for multiple testing. The E-value corresponding to the lower bound for SCA/VA in Medicaid database was 1.79 (E-value for the point estimate, 3.43; Figure S4). Thus, the observed CI for SCA/VA could be moved to include the null by an unmeasured confounder that was associated with both DPP-4 inhibitors SCA/VA by a risk ratio of 1.79-fold each.
DISCUSSION
In these population-based analyses conducted in two independent databases, we observed discordant findings for associations of individual DPP-4 inhibitors SCA/VA. Our analysis in the Medicaid population, with a smaller number of patient-years of observation (7,096 for saxagliptin vs. 33,470 for sitagliptin) suggested a higher rate of SCA/VA with saxagliptin (vs. sitagliptin; aHR, 2.01, 95% CI 1.24–3.25). However, the results in the larger Optum dataset (20,239 patient-years for saxagliptin vs. 44,247 for sitagliptin) differed (aHR, 0.79; 95% CI 0.41–1.51).
DPP-4 inhibitors are widely used as an add-on to metformin because of their neutral effect on body weight low risk of hypoglycemia.1,35,36 Their overall cardiovascular safety has been documented in specific cardiovascular outcome trials for each of the agents in the class. However, there is uncertainty as to whether saxagliptin might be associated with an increase in hospitalization for heart failure.13–15 Although studies have evaluated other cardiovascular outcomes, including stroke, myocardial infarction, cardiovascular mortality, none have assessed the risk of SCA/VA.12,15,37 SCA remains a major concern in patients with type 2 diabetes.38 Nearly 80% of SCAs occur at home, despite improvement in cardiopulmonary resuscitation procedures, the survival rate after SCA remains low (8–18%).39
Our investigation of the association between individual DPP-4 inhibitors the risk of SCA/VA was motivated by the differential effect of DPP-4 inhibitors on QTc prolongation observed in some animal model studies4,40 but not others.41 For example, in cardiomyocytes, saxagliptin impaired the Ca2+/calmodulin-dependent protein kinase II (CaMKII)-phospholamban (PLB)-sarcoplasmic reticulum Ca2+-ATPase 2a axis protein kinase C (PKC) activity leading to impairment in cardiac contractility.4,40 Additionally, unlike sitagliptin or linagliptin, in vitro ex vivo studies linked saxagliptin to cardiac dysfunction, heart failure, QTc prolongation.4,7,40 Activation of the sympathetic nervous system degradation of multiple chemokines of stromal cell-derived factor-1 (SDF-1) can adversely affect both cardiac structure function.42
There are several potential explanations for the discordant findings of saxagliptin vs. sitagliptin SCA/VA between Medicaid Optum including differences in: data availability; formulary restrictions; patient populations. For instance, laboratory values were available for a subset of beneficiaries in Optum but not Medicaid data. However, differences in data availability are unlikely to explain the discordant findings given that: (i) laboratory values were available for < 30% of beneficiaries in Optum data; (ii) Medicaid data included some information on selected demographics, such age, sex, race, geographic location; (iii) our examination of the E-value showed a risk ratio of 3.43 suggesting that our observed HRs for the risk of SCA/VA in Medicaid database could be explained by an unmeasured confounder that is associated with saxagliptin (vs. sitagliptin) the risk of SCA/VA by a risk ratio more than 3.43-fold; (iv) our examination of the results in Optum data after omitting laboratory values sociodemographic factors from the hdPS model were consistent with results when including those variables. Further, chance or differential unmeasured confounding cannot be ruled out.
There are potentially important differences between the populations under study. Medicaid is the largest government-based health insurance serving mostly lower-income populations who are often sicker have more health problems.43 Medicaid mostly provides coverage to low-income children, pregnant women, people with disabilities, economically disadvantaged older adults. Optum, on the other h, represents employees their dependents who are covered by commercial health plans. Differences between the two populations are evident in baseline comorbidities, prior medication use, intensity of healthcare utilization, follow-up time. Compared with Optum enrollees, our examination of differences in the matched cohort revealed that Medicaid patients who were newly prescribed saxagliptin or sitagliptin had a higher prevalence of ischemic heart disease (21% vs. 15%), serious hypoglycemic events (2% vs. 0.4%), use of drugs with known, possible, or conditional risk of TdP (59% vs. 39%), use of ≥ 5 drugs suggesting polypharmacy (59% vs. 26%). The intensity of healthcare utilization prior to treatment initiation in Medicaid data suggests a sicker population. For example, compared with Optum beneficiaries, Medicaid patients had a higher median number of prescriptions during the year preceding their first DPP-4 inhibitor prescription (n = 68 vs. 30). The number of person-years of follow-up of saxagliptin in the Medicaid dataset is one third that in the Optum dataset. Differences in formulary coverage between Medicaid (a governmental database) Optum (a commercial healthcare database) may help explain the discordant findings. Formulary restrictions (e.g., prior authorization, step therapy, or exclusion) may contribute to heterogeneity of treatment effect across populations. For instance, Medicaid enrollees who were newly prescribed saxagliptin or sitagliptin were twice as likely as Optum enrollees to receive insulin prior to their first DPP-4 inhibitor (12% vs. 6%) to have an adapted diabetes comorbidity score ≥ 5 (10% vs. 5%), suggesting a more advanced stage of type 2 diabetes among Medicaid enrollees.
The incidence rate of SCA/VA among saxagliptin users in Medicaid was six times as great as that in Optum database (3.7 vs. 0.6 per 1,000 person-years). There are several possible explanations including: (i) Medicaid patients are sicker have a higher prevalence of comorbidities, including risk factors for SCA; (ii) the algorithm used to define SCA/VA was validated in Medicaid not Optum data, which may have led to differential ascertainment of the study outcome. Most saxagliptin users in Medicaid data apparently discontinued treatment in less than 4 months from treatment initiation.
The current study has several strengths. First, we used semi-automated hdPS, a robust adjustment method, to minimize observed differences reduce residual bias by unmeasured confounders. Second, we used an outcome definition that was developed validated in US claims data. Third, we studied a large number of new users of DPP-4 inhibitors, permitting us to assess potential treatment effect heterogeneity in selected subpopulations. Several limitations must also be noted. First, our approach would have missed fatal outcomes not leading to hospital presentation. However, our examination of death as a competing event in Medicaid data yielded consistent findings. Second, despite the use of a robust adjustment method to control for confounding, there is the potential for residual confounding due to missing data or unmeasured confounders. Third, although the median follow-up time was relatively short, censoring due to treatment discontinuation was relatively comparable between the two groups. Additionally, this short follow-up time was unlikely to affect our ability to capture SCA/VA events as most drug-induced proarrhythmia occurs soon after drug exposure,44 the HRs did not appear to vary with duration of follow-up. Fourth, despite large sample sizes, the Optum data may have lacked sufficient power to replicate the Medicaid findings, as reflected in the wide CIs. Fifth, we lacked information on direct adherence to DPP-4 inhibitors, although results remained robust when modifying the allowable grace period between contiguous prescriptions. Sixth, there is the potential for outcome misclassification because we relied on ICD codes. Prior studies evaluating the validity of ICD codes for SCA/VA focused on ICD-9 but not ICD-10.
We identified discordant results regarding the association between saxagliptin compared with sitagliptin SCA/VA in two independent datasets. It remains unclear whether these findings are due to heterogeneity of treatment effect in very different study populations, chance, or differential unmeasured confounding.
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
With a low risk of hypoglycemia no effect on body weight, dipeptidyl peptidase-4 (DPP-4) inhibitors are a widely used antihyperglycemic drug class. In vivo studies suggest that arrhythmia risk may be greater with less selective DPP-4 inhibitors, but evidence from population-based studies is missing.
WHAT QUESTION DID THIS STUDY ADDRESS?
We aimed to compare saxagliptin, sitagliptin, linagliptin with regard to risk of sudden cardiac arrest (SCA)/ventricular arrhythmia (VA).
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Our results showed discordant findings regarding the association between SCA/VA with saxagliptin compared with sitagliptin in two independent datasets.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Future trials observational analyses evaluating DPP-4 inhibitors should consider including SCA/VA as a safety outcome.
FUNDING
This work was supported by grants from the American Diabetes Association (1–18-ICTS-097) the United States Department of Health Human Services’ National Institute on Aging (R01AG060975, R01AG025152, R01AG064589), National Institute on Drug Abuse (R01DA048001), National Institute of General Medical Sciences (T32GM075766), National Heart, Lung, Blood Institute (F32HL154519), the National Cancer Institute (P30CA008748), the Patient Centered Outcomes Research Institute (PCORI CER-2017C3–9230).
Footnotes
CONFLICT OF INTEREST
C.E.L. serves on the Executive Committee of SH directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research Training. The Center receives funds for education from Pfizer Sanofi. C.E.L. has received honoraria from the University of Florida the American College of Clinical Pharmacy Foundation. C.E.L. receives travel support from John Wiley & Sons. S.H. has consulted for the Medullary Thyroid Cancer Consortium (Novo Nordisk, AstraZeneca, Eli Lilly, GlaxoSmithKline) on matters unrelated to the topic of this paper. W.B.B. has consulted for Genentech on matters unrelated to the topic of this paper. J.H.F. has consulted for Genentech, Eli Lilly, Boehringer Ingelheim on matters unrelated to the topic of this paper. S.E.K. has consulted for GlaxoSmithKline Janssen, unrelated to the contents of this study. Z.B. has consulted for Astra Zeneca, Merck, Boehringer Ingelheim, Sanofi on matters unrelated to the topic of this paper. All other authors declared no competing interests for this work.
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
References
- 1.Montvida O, Shaw J, Atherton JJ, Stringer F & Paul SK Long-term trends in antidiabetes drug usage in the U.S.: Real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 41, 69–78 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Ahren B Dipeptidyl peptidase-4 inhibitors: Clinical data clinical implications. Diabetes Care 30, 1344–1350 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Deacon CF Corrigendum: physiology pharmacology of DPP-4 in glucose homeostasis the treatment of type 2 diabetes. Front. Endocrinol 10, 275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyani CN et al. Dipeptidyl peptidase-4 independent cardiac dysfunction links saxagliptin to heart failure. Biochem. Pharmacol. 145, 64–80 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Tang ST et al. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-k appaB/IkappaBalpha system through the activation of AMP-activated protein kinase. Int. J. Mol. Med. 37, 1558–1566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ring A, Port A, Graefe-Mody EU, Revollo I, Iovino M & Dugi KA The DPP-4 inhibitor linagliptin does not prolong the QT interval at therapeutic supratherapeutic doses. Br. J. Clin. Pharmacol. 72, 39–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straus SM et al. Prolonged QTc interval risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 47, 362–367 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Aune D, Schlesinger S, Norat T & Riboli E Diabetes mellitus the risk of sudden cardiac death: A systematic review meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 28, 543–556 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Junttila MJ et al. Sudden cardiac death after myocardial infarction in patients with type 2 diabetes. Heart Rhythm 7, 1396–1403 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Eranti A et al. Diabetes, glucose tolerance, the risk of sudden cardiac death. BMC Cardiovasc. Disord. 16, 51–55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huikuri HV, Castellanos A & Myerburg RJ Sudden death due to cardiac arrhythmias. N. Engl. J. Med 345, 1473–1482 (2001). [DOI] [PubMed] [Google Scholar]
- 12.McGuire DK et al. Association between sitagliptin use heart failure hospitalization related outcomes in type 2 diabetes mellitus: Secondary analysis of a romized clinical trial. JAMA Cardiol. 1, 126–135 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM et al. Heart failure, saxagliptin, diabetes mellitus: Observations from the SAVOR-TIMI 53 romized trial. Circulation 130, 1579–1588 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Zannad F et al. Heart failure mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, romised, double-b lind trial. Lancet 385, 2067–2076 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Green JB et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Tisdale JE et al. Drug-induced arrhythmias: A scientific statement from the American Heart Association. Circulation 142, e214–e233 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Leonard CE et al. The quality of Medicaid Medicare data obtained from CMS its contractors: Implications for pharmacoepidemiology. BMC Health Serv. Res. 17, 304–307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennessy S, Leonard CE, Palumbo CM, Newcomb C & Bilker WB Quality of Medicaid Medicare data obtained through Centers for Medicare Medicaid services (CMS). Med. Care 45, 1216–1220 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Malay S & Chung KC The choice of controls for providing validity evidence in clinical research. Plast. Reconstr. Surg. 130, 959–965 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberthson RR Sudden death from cardiac causes in children young adults. N. Engl. J. Med. 334, 1039–1044 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Chan PS et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N. Engl. J. Med. 368, 1019–1026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woosley RL, Heise CW & Romero KA QTdrugs list. (AZCERT, Inc., Catalina Foothills, AZ, 2020). www.Crediblemeds.org [Google Scholar]
- 23.Suissa S Immeasurable time bias in observational studies of drug effects on mortality. Am. J. Epidemiol. 168, 329–335 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Hennessy S et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death ventricular arrhythmia in Medicaid Medicare claims data. Pharmacoepidemiol. Drug Saf. 9, 555–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox CS et al. A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. Am. J. Cardiol. 95, 856–859 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Iribarren C, Crow RS, Hannan PJ, Jacobs DR Jr & Luepker RV Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am. J. Cardiol. 82, 50–53 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Guertin JR, Rahme E & LeLorier J Performance of the high-dimensional propensity score in adjusting for unmeasured confounders. Eur. J. Clin. Pharmacol. 72, 1497–1505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rassen JA, Glynn RJ, Brookhart MA & Schneeweiss S Covariate selection in high-dimensional propensity score analyses of treatment effects in small samples. Am. J. Epidemiol. 173, 1404–1413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bross ID Spurious effects from an extraneous variable. J. Chronic Dis. 19, 637–647 (1966). [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Kim HJ, Lonjon G & Zhu Y, written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann. Transl. Med. 7, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in romized experiments. Stat. Med. 33, 1242–1258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H & Brookhart MA High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20(4):512–522 (2009). 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble WS How does multiple testing correction work? Nat. Biotechnol. 27, 1135–1137 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VerWeele TJ & Ding P Sensitivity analysis in observational research: Introducing the E-value. Ann. Intern. Med. 167, 268–274 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Amori RE, Lau J & Pittas AG Efficacy safety of incretin therapy in type 2 diabetes: Systematic review meta-analysis. JAMA 298, 194–206 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Ahrén B Are sulfonylureas less desirable than DPP-4 inhibitors as add-on to metformin in the treatment of type 2 diabetes? Curr. Diab. Rep 11, 83–90 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Scirica BM et al. Saxagliptin cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X & Lemaitre RN Type 2 diabetes mellitus the risk of sudden cardiac arrest in the community. Rev. Endocr. Metab. Disord. 11, 53–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vreede-Swagemakers JJ et al. Out-of-hospital cardiac arrest in the 1990’s: A population-based study in the Maastricht area on incidence, characteristics survival. J. Am. Coll. Cardiol. 30, 1500–1505 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Koyani CN et al. Saxagliptin but not sitagliptin inhibits CaMKII PKC via DPP9 inhibition in cardiomyocytes. Front. Physiol 9, 1622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollack PS et al. Nonclinical clinical pharmacology evidence for cardiovascular safety of saxagliptin. Cardiovasc. Diabetol. 16, 113–116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Packer M Do DPP-4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity? Circ. Res. 122, 928–932 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Altman D & Frist WH Medicare Medicaid at 50 years: perspectives of beneficiaries, health care professionals institutions, policy makers. JAMA 314, 384–395 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Naccarelli GV, Wolbrette DL & Luck JC Proarrhythmia. Med. Clin. North Am. 85(2):503–526 (2001). 10.1016/S0025-7125(05)70324-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


