Abstract
Pathogenic Yersinia species employ type III machines to target effector Yops into the cytosol of eukaryotic cells. Yersinia tyeA mutants are thought to be defective in the targeting of YopE and YopH without affecting the injection of YopM, YopN, YopO, YopP, and YopT into the cytosol of eukaryotic cells. One model suggests that TyeA may form a tether between YopN (LcrE) and YopD on the bacterial surface, a structure that may translocate YopE and YopH across the plasma membrane of eukaryotic cells (M. Iriarte, M. P. Sory, A. Boland, A. P. Boyd, S. D. Mills, I. Lambermont, and G. R. Cornelis, EMBO J. 17:1907–1918, 1998). We have examined the injection of Yop proteins by tyeA mutant yersiniae with the digitonin fractionation technique. We find that tyeA mutant yersiniae not only secreted YopE, YopH, YopM, and YopN into the extracellular medium but also targeted these polypeptides into the cytosol of HeLa cells. Protease protection, cell fractionation, and affinity purification experiments suggest that TyeA is located intracellularly and binds to YopN or YopD. We propose a model whereby TyeA functions as a negative regulator of the type III targeting pathway in the cytoplasm of yersiniae, presumably by preventing the export of YopN.
Three pathogenic Yersinia species, Yersinia enterocolitica, Y. pseudotuberculosis, and Y. pestis, infect human and animal hosts and cause a variety of intestinal and septicemic diseases (6). To evade phagocytic killing by the host's immune system, pathogenic yersiniae employ the type III secretion machinery and export a set of virulence factors, named Yops (Yersinia outer proteins) (10, 27). During the infection of tissue culture cells, some Yops, also named effector Yops (YopE, YopH, YopM, YopN, YopO, YopP, and YopT), are injected into the eukaryotic cytosol (type III targeting) (5, 16, 19, 29, 31, 32). Other Yops are either secreted into the extracellular milieu (YopB, YopD, and YopR; type III secretion) or remain associated with the bacterial envelope (YopQ and LcrV) (13, 17, 23, 25, 30). Yop expression and secretion by the type III pathway can also be induced when yersiniae are grown at 37°C in the absence of calcium (38). Under these conditions, the type III machinery massively secretes all Yops into the culture medium, thereby slowing bacterial growth (27). The genes required for type III secretion across the bacterial envelope have been isolated as mutants that permit growth of Y. pestis at 37°C in the absence of calcium (15, 41) or abolish Yersinia Yop secretion under low-calcium conditions (26 ysc [for Yop secretion] genes) (26). Mutations in several regulatory genes cause a different phenotype, and the mutant yersiniae secrete Yops even in the presence of calcium at 37°C (calcium blind, or Lcr [for low calcium response] phenotype) (42). The lcrE (yopN), tyeA, sycN, yscB, yscM1/yscM2, lcrG, lcrH, and yopD genes are thought to regulate the bacterial type III pathway in response to low calcium induction and/or other stimuli such as contact with the eukaryotic cell (4, 12, 14, 18, 20, 35, 37, 40). All ysc, yop, and lcr genes are located on the 70-kB virulence plasmid that is essential for the pathogenesis of Yersinia infections (10).
To understand how yersiniae inject effector Yops into the eukaryotic cytosol, strains carrying mutations in genes of the type III machinery or its secretion substrates have been analyzed during the infection of tissue culture cells (31, 32). Four different mutant phenotypes have been reported thus far. Yersinia mutants that can not express type III machinery components (ysc genes) fail to target effector Yops and fail to secrete YopB, YopD, and YopR (32). Mutations that prevent the expression of some secretion substrates, for example, yopD, abrogate type III targeting of effector Yops and cause the aberrant secretion of other Yops into the extracellular milieu (25, 32). This phenotype is here referred to as Not (no type III targeting) (2). Mutations in a gene specifying another type III secretion substrate, yopN (lcrE), abolish the specificity of targeting for all effector Yops, causing these polypeptides to be located in the extracellular medium as well as in the eukaryotic cytosol (5, 23). We have named this phenotype Los (loss of type III targeting specificity) (2). Recently, a fourth phenotype has been described. Mutations that prevent expression of tyeA (targeting of Yersinia effector Yops) abolished the targeting of YopE and YopH but not that of other effector Yops such as YopM, YopO, YopP, and YopT (20).
To account for these observations, two models for effector Yop targeting have been discussed. The one-step translocation model views type III targeting as a continuous translocation of effector Yops from the bacterial cytoplasm across the double membrane envelope and the plasma membrane into the eukaryotic cytosol (23). Secretion of YopB, YopD, and YopR, as well as targeting of effector Yops, is thought to occur via the recognition of distinct export signals, which could occur either simultaneously or sequentially, in an ordered fashion (2). The two-step translocation model proposes the uniform type III secretion of all Yops across the bacterial envelope followed by the translocation of effector Yops across the plasma membrane (11). The secreted proteins YopB and YopD are thought to fulfill the role of translocators across the eukaryotic plasma membrane. YopN (LcrE) has been proposed to act on the bacterial surface as a stop valve for the type III machine. Once primed by interaction with the eukaryotic cell surface or the absence of calcium ions, YopN may permit other Yops to travel through the type III machine and the YopB/YopD translocator (11). Previous work also reported that TyeA may be a surface-exposed protein and binds to both YopN and YopD (20). Thus, TyeA could function as a tether between these proteins and the bacterial surface (9); however, it is not clear why tyeA mutations should abolish the injection of YopE and YopH without affecting the type III targeting of other effector Yops.
Our work has focused on characterizing the substrate recognition events of YopE-SycE complexes by the type III machinery (7, 8). The signal for the type III targeting of the YopE effector is located in amino acid residues 1 to 100, which, when fused to bacterial neomycin phosphotransferase, are sufficient to cause injection of the hybrid protein into the cytoplasm of HeLa cells (23, 34, 36). This type III targeting of YopE is absolutely dependent on the binding of SycE to YopE residues 1 to 100 (8, 23). SycE is thought to function as a secretion chaperone in the bacterial cytoplasm that delivers unfolded polypeptide to the type III machinery by cycling on and off export substrates (8, 39). We sought to identify factors responsible for the dissociation of YopE-SycE complexes in vitro (8). The compelling phenotype of tyeA mutant bacteria, i.e., disruption of the chaperone-mediated targeting pathway of YopE and YopH, suggested to us that TyeA may be uniquely required for substrate recognition of YopE-SycE complexes (20). We therefore tested whether purified TyeA could dissociate YopE-SycE complexes (data not shown). Failure of TyeA to catalyze this reaction prompted us to examine the phenotype of tyeA mutants. We find that Y. enterocolitica carrying nonpolar null mutations in the tyeA gene display a Los phenotype for all effector Yops examined. TyeA was found in the bacterial cytoplasm but not on the surfaces of Yersinia cells. TyeA was purified from the cytosol together with YopN or YopD. Binding of TyeA to YopN did not require the presence of YopD, and vice versa. TyeA was not required for YopN and YopD secretion or for the type III targeting of YopE and YopH. However, tyeA mutants harbored significantly less intracellular YopN than wild-type yersiniae, suggesting that TyeA could act as a negative regulator of YopN secretion.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Y. enterocolitica strains W22703 (wild-type), VTL1 (YopN−), and VTL2 (YopD−) have been described elsewhere (7, 23, 25). Y. enterocolitica strains LC6 (tyeA1) and LC7 (tyeA2) were constructed by allelic exchange using the suicide plasmid pLC28 (7). The tyeA1 allele was designed as a mutation that introduced a stop codon at position 10 of the tyeA open reading frame (ORF) followed by a −1 reading frame shift and a unique BamHI site as a fusion joint between two DNA fragments. tyeA1 was constructed from two PCR products amplified using primers Orf1XbaI (5′-AATCTAGAAAATTGTAGCGGGAGCCGC-3′) and TyeATGA1 (5′-GGATCCTCACATAAACTCAGAAAGGTCGTAA-3′) as well as TyeABam2 (5′-GGATCCGAGATATTGTCGCACTGGTT-3′) and ORF1KpnI (5′-AAGGTACCCATACTTTGTGCAACAGGTTAA-3′). The tyeA2 mutant allele was designed to replace codons 19 to 59 of tyeA with a unique BamHI site. tyeA2 was constructed from two PCR products with primers Orf1XbaI and TyeA19Bam (5′-AAGGATCCCTTGTCAACCAGTGCGACAA-3′) as well as TyeA59Bam (5′-AAGGATCCTTTAGCGATGAGGAGCAACG-3′) and Orf1KpnI. The PCR products were cut with XbaI-BamHI or BamHI-SalI, fused at the BamHI site, and cloned between the XbaI and SalI sites of pLC28. To generate pTyeA, the tyeA ORF was PCR amplified with two primers carrying abutted NdeI and BamHI restriction sites, TyeANde (5′-AACATATGGCTTACGACCTTTCTGAGTTT-3′) and TyeABam (5′-AAGGATCCATCCAACTCACTCAATTCTT-3′). The PCR product was digested with NdeI/BamHI and cloned between the NdeI and BamHI sites of the low-copy-number plasmid pDA234 (3) to generate pLC186 (pTyeA). pGst-TyeA was generated by inserting two PCR fragments, joined at a KpnI site, between the NdeI and BamHI sites of pDA234. gst sequences were amplified from pGEX-2TK template DNA with the primers Nde-N-Gst (5′-AACATATGTCCCCTATACTAGGTTATTGGA-3′) and N-Gst-Kpn (5′-AAGGTACCAACAGATGCACGACGAGATC-3′). tyeA sequences were PCR amplified with the primers TyeAKpn-C (5′-AAGGTACGGCTTACGACCTTTCTGAGTTT-3′) and TyeA-Bam. The PCR products were cut with NdeI/KpnI or KpnI/BamHI and cloned into pDA234. Expression of tyeA and gst-tyeA is under the control of the tac promoter. The lacIq allele is also cloned on the low-copy-number vector, and Y. enterocolitica transformants were induced for expression of tyeA and gst-tyeA by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). For purification of TyeA-His6, the tyeA ORF was PCR amplified with the primers that carry abutted BamHI sites (ORF1-B6H5′, 5′-AAGGATCCATGGCTTACGACCTTTCTGAG-3′, and ORF1-B6H3′, 3′-AAGGATCCATCCAACTCACTCAATTCTTCC-3′). The PCR product was digested with BamHI and cloned into pQE30 (Qiagen), thereby generating pLC182. For type III experiments, Y. enterocolitica was grown overnight in Trypticase soy broth (TSB) medium at 26°C. Cultures were diluted 1:50 into fresh TSB or TSB supplemented with 10 mM calcium, grown for 2 h at 26°C, and induced for 3 h at 37°C.
Cell fractionations.
Overnight cultures of Yersinia were diluted 1:50 into 800 ml of fresh TSB medium, grown for 2 h at 26°C, and induced at 37°C for 3 h. Cells were harvested at 6,000 × g for 15 min and suspended in 10 ml of HEPES buffer (20 mM HEPES, 100 mM potassium acetate [KOAc], 2 mM magnesium acetate [MgOAc], 1 mM dithiothreitol [DTT] [pH 7.5]). Bacteria were broken in a French pressure cell at 14,000 lb/in2, and intact cells were removed by centrifugation at 6,000 × g for 10 min. A 3-ml aliquot of crude bacterial extract was removed with the supernatant and subjected to ultracentrifugation at 100,000 × g for 30 min. The supernatant (S) was removed, and the membrane pellet was suspended in 3 ml of HEPES buffer (P). At each fractionation step, aliquots were withdrawn and mixed with an equal volume of sample buffer. Samples were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (15% polyacrylamide) and analyzed by immunoblotting.
Protease protection.
Overnight cultures of Y. enterocolitica LC7(pLC199) were diluted 1:50 into fresh TSB medium, grown for 2 h at 26°C, and induced for 3 h at 37°C. Four 1-ml aliquots of cultures were incubated with or without 10 μg of proteinase K/ml and either 1% SDS or 1 mM phenylmethylsulfonyl fluoride (PMSF) and incubated at 37°C for 30 min. Proteolysis was quenched by the addition of 1 mM PMSF to all reaction mixtures. At this time, proteinase K and SDS were added to each reaction mixture so that all samples contained the same reagents. Samples were precipitated with chloroform-methanol, dried, and solubilized in 100 μl of buffer composed of equal volumes of buffer B (described below) and sample buffer. Protease protection experiments for Y. enterocolitica LC7(pLC186) were performed similarly, except that 6 ml of cells were collected by centrifugation and suspended in 2 ml of 50 mM Tris-HCl, pH 7.5, prior to incubation with or without 30 μg of proteinase K/ml, 1% SDS, and 1 mM PMSF. Chloroform-methanol precipitates were solubilized in a volume of 200 μl of sample buffer and analyzed by immunoblotting.
Purification of TyeA.
One liter of Escherichia coli XL1-Blue(pLC182) was grown to mid-log phase at 37°C and induced with 1 mM IPTG for 3 h. Cells were harvested by centrifugation at 6,000 × g for 15 min and suspended in 20 ml of buffer A (6 M guanidine-hydrochloride, 0.1 M NaH2PO4, 0.01 M Tris-HCl [pH 8.0]). The sample was incubated with intermittent vortexing on ice for 1 h. Insoluble material was removed with two sequential centrifugation steps at 33,000 × g for 15 min. The supernatant was loaded onto a 1-ml column of Ni-nitrilotriacetic acid (NTA)-Sepharose that had been preequilibrated with 10 ml of buffer A. The column was washed with 10 ml of buffer A, 10 ml of buffer B (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris-HCl [pH 8.0]), and 20 ml of buffer C (the same as buffer B, but pH 6.3). TyeA-His6 was eluted with 4 ml of buffer E (the same as buffer B, but pH 4.5). Samples were aliquoted and stored frozen at −80°C. Purified TyeA was emulsified with Freund's adjuvant and injected into rabbits to raise polyclonal antibodies. Antiserum reactivity and specificity were examined by comparing bacterial extracts of wild-type and tyeA mutant strains with the purified antigen.
Binding of Yops to Gst-TyeA hybrid protein.
Overnight cultures of Y. enterocolitica LC7(pGst-TyeA) were diluted 1:50 into fresh TSB medium supplemented with 20 μg of chloramphenicol/ml. Bacteria were grown and induced by incubation for 2 h at 26°C and for 3 h at 37°C. Cells from 500 ml of culture were harvested by centrifugation at 6,000 × g for 15 min. The cell pellet was suspended in 10 ml of buffer F (50 mM Tris-HCl, 20% sucrose, 1 mM DTT [pH 7.0]), and bacteria were broken by a single passage through a French pressure cell at 14,000 lb/in2. Unbroken cells and debris were removed by centrifugation at 6,000 × g for 15 min. Membranes were sedimented by ultracentrifugation at 100,000 × g, and the supernatant, containing soluble cytoplasmic contents, was subjected to affinity chromatography on glutathione-Sepharose preequilibrated with buffer F. The column was washed with 30 column volumes of wash buffer (50 mM Tris-HCl, 150 mM NaCl, 15% glycerol [pH 7.5]), and proteins were eluted with 4 ml of wash buffer containing 10 mM glutathione. Eluted proteins were mixed with an equal volume of sample buffer containing 3 M urea and analyzed by SDS-PAGE and immunoblotting. Bacterial extracts of Y. enterocolitica VTL2 (yopD1) carrying pGst-TyeA and Y. enterocolitica VTL1 (yopN1) carrying pGst-TyeA were subjected to similar affinity chromatography analysis.
Xylene extraction.
Extractions were performed according to the protocol of Michiels et al. (27). Briefly, Yersinia overnight cultures were diluted 1:50 into fresh TSB medium, grown for 2 h, and induced at 37°C for 3 h. Two 800-μl culture aliquots were centrifuged at 6,000 × g for 2 min, and the culture medium was separated from the cell pellet. Bacteria were washed and suspended in 800 μl of fresh TSB, and 400 μl of p-xylene was added to each sample. After contents were mixed briefly, samples were centrifuged at 6,000 × g for 5 min. The organic phase (top layer) was discarded. The interface and extract supernatant were removed and precipitated with 10 ml of acetone. Bacterial pellets were precipitated with 1 ml of acetone. After incubation at 20°C for 1 h, acetone precipitates were collected by centrifugation at 33,000 × g for 15 min. Pellets were air dried and solubilized in 100 μl of sample buffer prior to SDS-PAGE and immunoblotting.
Microscopy.
HeLa cells were grown in Dulbecco's modified Eagle medium (DMEM)–10% fetal bovine serum (FBS) on glass coverslips in a 24-well plate for 48 h at 37°C. Coverslips were washed with phosphate-buffered saline (PBS) and incubated with DMEM prior to infection with Y. enterocolitica W22703 (wild type) or LC7 (TyeA−) with or without plasmid pDA36 (full-length yopE fused to npt) (1) for 3 h at a multiplicity of infection (MOI) of 20. After infection, coverslips were fixed with 3.7% formaldehyde for 30 min. All fixation was quenched by the addition of 0.1 M glycine for 10 min, followed by permeabilization with 0.1% Triton X-100 for 10 min. Samples were blocked with 5% goat serum in PBS for 30 min. Coverslips were then incubated with α-Npt polyclonal antibodies for 30 min. After washings with PBS, samples were incubated with anti (α)-rabbit immunoglobulin G (IgG) fluorescein isothiocyanate (FITC)-conjugated antibody (Jackson ImmunoResearch Laboratories) for 30 min. Samples were washed and viewed under a fluorescence microscope. Images were captured with a Hamamatsu charge-coupled device (CCD) camera.
RESULTS
Calcium-blind phenotype of tyeA mutant Y. enterocolitica.
Two tyeA mutants, Y. enterocolitica strains LC6 and LC7, were constructed. Y. enterocolitica LC6 (tyeA1) carries a nonsense mutation at codon 10 followed by a −1 frameshift mutation. LC7 (tyeA2) carries an in-frame deletion of codons 19 to 59. To examine the role of tyeA in type III secretion induced by temperature shift to 37°C, Yersinia cultures were grown in the presence or absence of calcium. Cultures were centrifuged to separate the extracellular medium from the bacteria. Proteins in each fraction were precipitated with trichloroacetic acid (TCA) and analyzed by SDS-PAGE and Coomassie staining (Fig. 1A). When induced by a temperature shift and a low calcium concentration, the wild-type strain W22703 secreted Yops into the extracellular medium. However, yersiniae carrying the tyeA1 or tyeA2 allele secreted Yops into the culture medium even in the presence of calcium ions (calcium-blind phenotype) (Fig. 1A; also data not shown). As expected, the yopN mutant Y. enterocolitica strain VTL1 also displayed a calcium-blind phenotype (14, 23).
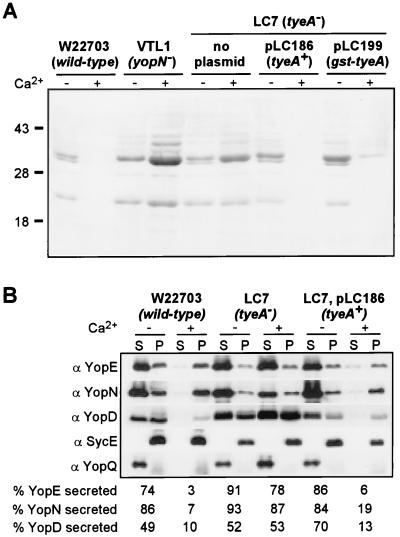
FIG. 1.
tyeA mutant yersiniae secrete Yops in the presence of calcium. The wild-type Y. enterocolitica strain W22703, yopN (VTL1) and tyeA (LC7 [tyeA2]) isogenic mutant strains, and LC7 transformed with either pLC186 (wild-type tyeA) or pLC199 (gst-tyeA) were grown at 37°C in TSB in the presence or absence of calcium. Cultures were centrifuged, and the supernatant (S) was separated from the cell pellet (P). Protein in each sample was precipitated with TCA, solubilized in sample buffer, and analyzed by SDS-PAGE. (A) Coomassie blue staining of culture supernatants. (B) Immunoblotting of culture supernatants and bacterial extracts with antisera raised against YopE, YopN, YopD, SycE, and YopQ. The wild-type Y. enterocolitica strain W22703 secretes Yops in the absence but not in the presence of calcium. In contrast, yopN and tyeA mutant strains display a calcium-blind phenotype and secrete Yops in the presence and absence of calcium. Transformation of tyeA mutant cells with either pLC186 or pLC199 restored the wild-type phenotype. Secretion is indicated as the percentage of polypeptide that is present in the culture medium divided by the total amount of polypeptide.
The tyeA mutant Yersinia strains (carrying tyeA1 or tyeA2) were observed to be temperature sensitive for growth even in the presence of calcium, like the yopN mutant strain Y. enterocolitica VTL1 (data not shown). We asked whether the tyeA2 mutant strain was defective in secreting YopN and quantified immunoreactive signals of protein samples from fractionated Yersinia cultures (Fig. 1B). Under low calcium conditions, YopN secretion was not diminished, as 87% of this polypeptide was found in the extracellular medium of Y. enterocolitica tyeA2 cultures (86% secretion in wild-type yersiniae). However, in the presence of calcium, the tyeA2 mutant secreted 78% of YopE, 87% of YopN, and 53% of YopD, whereas wild-type cells secreted only 3% of YopE, 7% of YopN, and 10% of YopD (Fig. 1B). Thus, TyeA is dispensable for YopN secretion but is required to shut down type III secretion when bacteria are grown at 37°C in the presence of calcium. These data corroborate previous observations on the calcium-blind phenotype of tyeA mutant strains (20).
When transformed with plasmid-encoded wild-type tyeA and analyzed by growth at 37°C in the presence of calcium, Y. enterocolitica LC7(pTyeA) neither synthesized nor secreted large amounts of YopE, YopN, or YopQ (Fig. 1B). Thus, pTyeA complemented the calcium-blind phenotype of strain LC7, indicating that the mutation in tyeA did not exert a polar effect on other genes in the yopNtyeAsycN operon. The tyeA1 mutant allele also caused a calcium-blind phenotype, which was complemented in trans by wild-type tyeA (data not shown). A gst-tyeA fusion was constructed on a low-copy-number plasmid and expressed from the tac promoter. After transformation of pGst-TyeA into the tyeA2 mutant, Y. enterocolitica LC7(pGst-TyeA) did not display a calcium-blind phenotype, indicating that Gst-TyeA is fully functional and complements the regulatory defect of tyeA mutants (Fig. 1A).
tyeA mutants display a Los phenotype during Yersinia infection of HeLa cells.
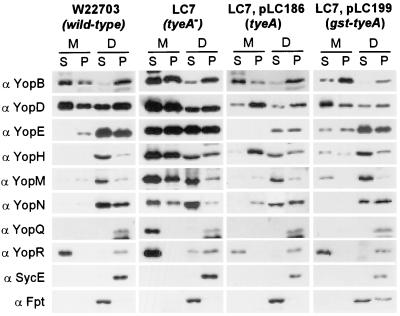
To examine the role of tyeA in type III secretion and type III targeting, HeLa cells were infected with the wild-type Y. enterocolitica strain W22703 or the tyeA mutant strain LC7. Infected HeLa cells were fractionated by decanting and centrifuging the medium, thereby separating nonadherent bacteria (P) from the extracellular medium (S). HeLa cells and adherent bacteria were extracted with digitonin, a detergent known to disrupt the cholesterol-containing plasma membrane of HeLa cells but not the bacterial envelope (23). Digitonin extracts were centrifuged to sediment bacteria as well as HeLa cell debris (P), while the soluble contents of the eukaryotic cytosol remained in the supernatant (S) (24). Proteins in all fractions were precipitated with chloroform-methanol and analyzed by immunoblotting with antisera raised against purified proteins (Fig. 2). As controls for proper fractionation, digitonin extraction released farnesyl protein transferase (Fpt) from the eukaryotic cytosol (digitonin soluble [S]). SycE, a protein located in the Yersinia cytoplasm, was not released and sedimented with the bacteria (digitonin pellet). The wild-type Yersinia strain specifically targeted YopE, YopH, YopM, and YopN into the cytosol of HeLa cells. The tyeA2 mutant strain secreted effector Yops into the extracellular medium (S); however, significant amounts of YopE, YopH, YopM, and YopN were also observed in the supernatant of digitonin-extracted HeLa cells (Fig. 2). These results suggest that tyeA2 mutant cells are defective in the specificity of type III targeting without completely abolishing this pathway (Los phenotype) (Fig. 2 and Table 1). YopQ, a protein that remained associated with wild-type yersiniae, was found secreted into the extracellular medium. Y. enterocolitica LC7 was not affected for the type III secretion of YopB, YopD, and YopR during HeLa cell infections. When transformed with plasmid-encoded wild-type tyeA, Y. enterocolitica LC7(pTyeA) injected effector Yops in a manner similar to wild-type yersiniae. Complementation was also observed when strain LC7 was transformed with pGst-TyeA. Y. enterocolitica LC6 (tyeA1) displayed the same Los phenotype as strain LC7, and this defect was also complemented by plasmid-encoded wild-type tyeA or gst-tyeA (data not shown).
FIG. 2.
tyeA mutant yersiniae display a Los phenotype and secrete effector Yops into the extracellular medium during the infection of HeLa cells. HeLa cells were infected with wild-type Y. enterocolitica W22703, the tyeA isogenic mutant strain LC7 (tyeA2), LC7(pLC186) (expressing wild-type tyeA), or LC7(pLC199) (expressing gst-tyeA). After incubation for 3 h at 37°C, the tissue culture medium (M) was decanted and centrifuged to separate secreted proteins from those present within nonadherent bacteria. HeLa cells as well as adherent yersiniae were extracted with digitonin (D), a detergent that solubilizes the eukaryotic plasma membrane but not the bacterial envelope. Extracts were centrifuged to separate proteins solubilized from the HeLa cytoplasm from those that sediment with the bacteria. Proteins in each fraction were precipitated with chloroform-methanol and analyzed by SDS-PAGE and immunoblotting with antibodies directed against YopB, YopD, YopE, YopH, YopM, YopN, YopQ, YopR, SycE, and Fpt. The wild-type Y. enterocolitica strain W22703 targeted YopE, YopH, YopM, and YopN into the cytosol of HeLa cells (digitonin supernatant). In contrast, the tyeA2 mutant strain LC7 displayed a loss of targeting specificity phenotype (Los) and secreted large amounts of YopE, YopH, YopM, and YopN into the culture medium. The Los phenotype was complemented by transforming LC7 cells with plasmid pLC186 or pLC199.
TABLE 1.
Type III secretion and targeting of Y. enterocolitica strains W22703 and LC7 during the infection of cultured HeLa cellsa
| Type III export substrate |
Y. enterocoliticaW22703 (wild type)
|
Y. enterocolitica LC7 (tyeA2)
|
||
|---|---|---|---|---|
| Digitonin extracted (%)b | Secreted (%)c | Digitonin extracted (%) | Secreted (%) | |
| YopE | 71 | 3 | 24 | 27 |
| YopN | 64 | 0 | 68 | 27 |
| YopM | 90 | 7 | 30 | 52 |
| YopH | 62 | 6 | 19 | 54 |
Tissue flasks with confluent HeLa cell cultures were infected with Yersinia strains for 3 h. The medium was decanted and centrifuged to separate the extracellular medium from the bacterial sediment. HeLa cells were extracted with digitonin and centrifuged to separate the soluble cytosolic contents from the sediment of bacteria adhering to the plasma membrane. Protein in all fractions was precipitated with chloroform-methanol and analyzed by immunoblotting (see Fig. 2).
Amount of immunoreactive polypeptide in the supernatant of centrifuged tissue culture medium divided by the total amount present in all fractions (medium supernatant and pellet as well as digitonin extract supernatant and pellet).
Amount of immunoreactive polypeptide in the supernatant of digitonin extracts divided by the total amount present in all fractions (media supernatant and pellet as well as digitonin extract supernatant and pellet).
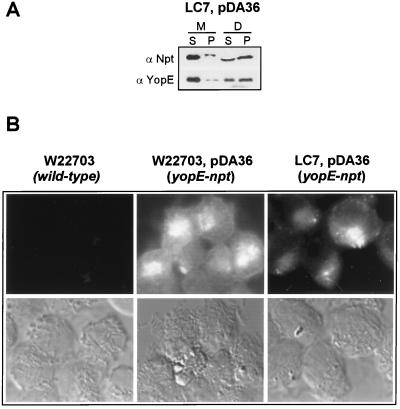
Previous work measured the injection of YopE fusions to Bordetella pertussis adenylate cyclase into the cytosol of HeLa cells (20). To test whether fusion of the neomycin phosphotransferase reporter interfered with the type III targeting of YopE, we infected HeLa cultures with yersiniae expressing the YopE-Npt hybrid (23). Tissue culture cells were fractionated by the digitonin technique and analyzed by immunoblotting (Fig. 3A). When they were infected with Y. entercolitica LC7(pDA36), similar amounts of YopE-Npt and YopE were found in the supernatant of digitonin-extracted HeLa cells, indicating that reporter fusions were also targeted by tyeA2 mutant yersiniae. To further examine whether the tyeA2 mutant strain LC7 was able to inject YopE into the cytosol of eukaryotic cells, infected HeLa tissue cultures were stained with α-Npt for immunofluorescent detection of the YopE-Npt fusion protein (Fig. 3B). A YopE-Npt-specific signal could be detected in the cytosol of HeLa cells infected with tyeA2 mutant yersiniae. As a control, HeLa cells infected with wild-type yersiniae expressing YopE-Npt [W22703(pDA36)] generated a cytoplasmic fluorescent signal, whereas tissue culture cells infected with the wild-type strain alone (W22703) did not. Further, a lcrD mutant strain expressing the YopE-Npt fusion did not generate an immunofluorescent signal in the cytosol of infected HeLa cells (25). Thus, the tyeA2 mutation confers a Los phenotype onto the mutant Yersinia strain LC7 without abolishing its ability to target effector Yops into the cytosol of HeLa cells. These observations corroborate the results obtained by digitonin fractionation, suggesting that tyeA mutants target YopE, YopH, YopM, and YopN into HeLa cells.
FIG. 3.
Y. enterocolitica LC7 (tyeA2) targets YopE-Npt into the cytosol of HeLa cells. (A) HeLa cell cultures were infected with Y. enterocolitica LC7 (tyeA2) carrying pDA36, expressing the YopE-Npt fusion protein (23), fractionated by the digitonin technique (see the legend to Fig. 2), and analyzed by immunoblotting. α-Npt measures the distribution of YopE-Npt in various fractions, whereas α-YopE measures the distribution of YopE. (B) Immunofluorescence microscopy of HeLa cells infected with Y. enterocolitica W22703, W22703(pDA36), or LC7(pDA36). Samples were fixed with formaldehyde and stained with α-Npt antibodies followed with an α-rabbit IgG-FITC conjugate. YopE-Npt staining was detected in the cytosol of HeLa cells infected with W22703(pDA36) and LC7(pDA36) but not in HeLa cells that were infected with strain W22703.
Subcellular location of TyeA and Gst-TyeA.
To investigate the subcellular location of TyeA, Yersinia cultures were grown and induced for type III secretion by a temperature shift and low calcium concentrations. Cultures of wild-type yersiniae, the tyeA2 strain LC7, and strain LC7 carrying plasmid pTyeA or pGst-TyeA were centrifuged, and the culture medium was separated from bacterial cells. Bacteria were lysed in a French pressure cell. Unbroken cells were removed by centrifugation at 6,000 × g, and the supernatant was subjected to ultracentrifugation at 100,000 × g, thereby sedimenting bacterial membranes (Fig. 4A). Soluble proteins (S) were separated from the membrane pellet (P), and both fractions were analyzed by SDS-PAGE and immunoblotting. As expected, the cytosolic SycE chaperone was found soluble in the supernatant, whereas LcrD, a type III machinery component located in the inner membrane, sedimented with the membranes (8) (Fig. 4A). TyeA was found in the soluble fraction, suggesting that TyeA is not tethered to the membrane envelope of Y. enterocolitica but resides in the bacterial cytoplasm. In contrast, small amounts of YopN were found in the pellet fraction of wild-type extracts. These species likely represent transport intermediates of the type III pathway, consistent with the notion that yersiniae growing under inducing conditions export YopN but not TyeA. Although the distribution of soluble and insoluble YopN was not altered in tyeA mutant extracts, this sample contained much less YopN than wild-type extracts (10% [Fig. 4A]). As the total amount of YopN in wild-type and tyeA mutant cultures is similar, these data suggest that YopN may be depleted from the cytoplasm of tyeA cells (Fig. 1B).
FIG. 4.
Subcellular location of TyeA. (A) Cell fractionation of Yersinia strains. Y. enterocolitica W22703 (wild type), the tyeA2 mutant strain LC7 (tyeA2), LC7(pLC186), and LC7(pLC199) were grown at 37°C and induced for type III secretion by the chelation of calcium ions. Bacteria were lysed in a French pressure cell. Unbroken cells were removed by low-speed centrifugation, and crude cell extracts were subjected to ultracentrifugation at 100,000 × g. The supernatant (S), containing soluble cytoplasmic contents, was separated from the membrane pellet (P). Samples were analyzed by separating proteins on SDS-PAGE gels and immunoblotting with antibodies raised against TyeA, Gst, LcrD, SycE, and YopN. α-TyeA recognized both wild-type TyeA and Gst-TyeA. The α-TyeA immunoblot of Gst-TyeA samples did not reveal an immunoreactive signal at the same mobility as wild-type TyeA, consistent with the notion that Gst-TyeA is not cleaved to generate native TyeA. The amount of YopN present in the supernatant fraction was quantified and calculated as the YopN/SycE ratio: we observed ratios of 2.9 for Y. enterocolitica W22703 (wild type) and 0.28 for strain LC7 (tyeA2). Small amounts of YopN were found in the pellet fraction, consistent with the notion that these species may represent transport intermediates. (B) Protease protection assay. Y. enterocolitica LC7(pLC199) were grown at 37°C and induced for type III secretion by the chelation of calcium ions. Four 6-ml culture aliquots (108 CFU/ml) were incubated at 37°C for 30 min with or without 10 μg of proteinase K/ml, alone or with 1% SDS or 1 mM PMSF. Lane 1, control reaction; lane 2, sensitivity to extracellular protease; lane 3, protease sensitivity of cytoplasmic proteins; lane 4, control for the inhibition of proteinase K by PMSF. Following incubation at 37°C, all samples were placed on ice and 1 mM PMSF was added to quench all proteolysis. Proteinase K (reactions 1 and 2) and SDS (reaction 2) were added to control for any interference of reagents during precipitation and solubilization. Samples were precipitated with chloroform-methanol, dried, and solubilized in sample buffer. Proteins were analyzed by separation on SDS-PAGE gels followed by immunoblotting. (C) Experiments similar to that for which results are shown in panel B, using cultures of Y. enterocolitica strain LC7(pLC186). TyeA and Gst-TyeA were protected from extracellular proteinase K unless the double membrane envelope of yersinae was dissolved with SDS.
If TyeA is positioned on the bacterial surface, the addition of proteinase K to Yersinia cultures may digest this polypeptide (Fig. 4B and C). A similar experiment has been performed previously, reporting TyeA sensitivity to extracellular proteinase K in combination with xylene extraction (20). To investigate the sensitivity of TyeA to extracellular protease, Yersinia cultures were induced for type III secretion by growth at 37°C in the absence of calcium. Initial experiments sought to identify proteinase K concentrations that permitted digestion of extracellular proteins (YopM), while cytoplasmic proteins (SycE) were protected by the integrity of the bacterial membrane envelope. At lower proteinase K concentrations, SycE was protected; however, increasing proteinase K concentrations above 500 μg/ml caused disintegration of the bacterial envelope and digestion of SycE and other cytoplasmic proteins (data not shown). Cultures were incubated in the presence or absence of 10 μg of proteinase K/ml. Proteolysis was quenched by the addition of PMSF, and proteins were precipitated with chloroform-methanol. Precipitates were boiled in sample buffer, separated on SDS-PAGE gels, and analyzed by immunoblotting. Proteinase K digested extracellular YopM, whereas cytoplasmic SycE and chloramphenicol acetyltransferase (CAT) were protected from proteolysis (Fig. 4B, lanes 1 and 2). TyeA (Fig. 4C) and Gst-TyeA (Fig. 4B) were protected from extracellular protease. The addition of SDS to Yersinia cultures caused disintegration of the bacterial envelope and protease sensitivity of all polypeptides examined. The simultaneous addition of proteinase K and its inhibitor PMSF prevented proteolytic cleavage in the presence of SDS. Together these data suggest that TyeA and Gst-TyeA are located intracellularly, protected from extracellular protease by the bacterial double membrane envelope.
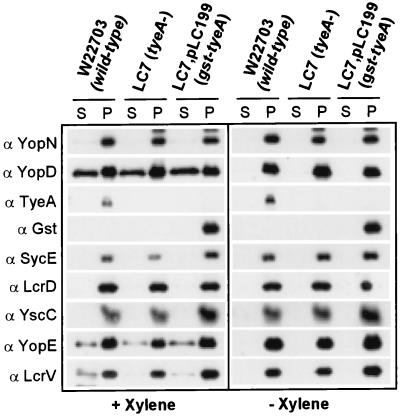
Xylene extraction has been used to examine the display of proteins on the surfaces of cells of Y. enterocolitica and other gram-negative enterobacteria (27). This organic solvent is thought to displace polypeptides that are loosely associated with the bacterial surface (27). To test this assumption, we performed a series of xylene extractions using Y. enterocolitica W22703 (Fig. 5). Samples were centrifuged, and proteins in the extract supernatant and pellet fractions were precipitated with acetone and analyzed by SDS-PAGE and immunoblotting. Xylene extraction released small amounts of YopD, YopE, and LcrV from Y. enterocolitica cells, whereas YopN, TyeA, Gst, SycE, and LcrD sedimented with the bacteria into the pellet fraction (Fig. 5). Extraction of YopD, YopE, and LcrV required xylene and was not due to the type III secretion of yersiniae during the experimental procedure, as control extractions with TSB medium alone did not release these polypeptides. The outer membrane protein YscC (22), a member of the secretin family associating into a dodecameric ring structure (28, 33), was not extracted by treatment with xylene. These results are in disagreement with the previous finding that small amounts of YopN and TyeA can be extracted with xylene from the surfaces of Y. enterocolitica W22703 cells (20).
FIG. 5.
Xylene extraction of yersiniae. Y. enterocolitica W22703 was grown at 37°C and induced for type III secretion by the chelation of calcium ions. Culture aliquots were centrifuged, and the bacterial sediment was washed and suspended in TSB. After extraction with xylene and centrifugation, the extract supernatant (S) and pellet (P) were separated, precipitated with acetone, and analyzed by immunoblotting. Small amounts of YopD, YopE, and LcrV, known type III secretion substrates, were extracted with xylene. The cytoplasmic protein SycE, the cytoplasmic membrane protein LcrD, and the outer membrane protein YscC were used as controls. YopN, another type III secretion substrate, Gst-TyeA, and TyeA were not released from yersiniae by treatment with xylene.
Copurification of Gst-TyeA with YopN and YopD.
Previous work found that YopN (LcrE) interacts with three proteins, YscB, SycN, and TyeA (12, 20, 21). We examined the ability of TyeA to form a complex with YopN by subjecting Gst-TyeA-containing Yersinia extracts to affinity chromatography on glutathione-Sepharose. Briefly, yersiniae were lysed in a French pressure cell, and unbroken cells and were removed by centrifugation at 6,000 × g. After membranes were sedimented via ultracentrifugation, the soluble supernatant (cytosolic contents) was applied to affinity chromatography. Gst-TyeA was eluted with glutathione, and samples were analyzed by SDS-PAGE and Coomassie staining or by immunoblotting. As reported previously (20), YopN and YopD copurified with Gst-TyeA (Fig. 6). While YopN was retained efficiently on the Gst-TyeA column, only small amounts of YopD bound to Gst-TyeA. Gst-TyeA binding was specific, as neither YopN nor YopD was retained on glutathione-Sepharose charged with Gst alone. Under the conditions used, YscB and SycN, presumed chaperones of YopN (12), did not bind to Gst-TyeA–YopN. Coomassie staining of SDS-PAGE gels and immunoblotting with several additional antibodies did not reveal the presence of other polypeptides that coeluted with Gst-TyeA. Because both YopN and YopD co-eluted with TyeA, we asked whether their binding to TyeA depended on the presence of all three polypeptides. This was tested, and Yersinia extracts lacking YopN or YopD were subjected to affinity chromatography. Extracts lacking YopN did not interfere with the binding of YopD to Gst-TyeA (Fig. 7). Similarly, the absence of YopD did not affect YopN binding to Gst-TyeA (Fig. 7). In summary, Gst-TyeA binds to both YopN and YopD but not to YscB or SycN.
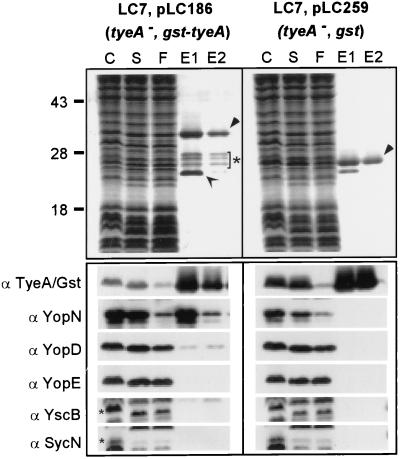
FIG. 6.
Binding of Gst-TyeA to YopN and YopD. Y. enterocolitica LC7(pLC199) and Y. enterocolitica LC7(pLC100) were grown at 37°C and induced for type III secretion by the chelation of calcium ions. Expression of the Gst and the Gst-TyeA proteins was induced by the addition of 1 mM IPTG to the culture medium. Cells (1012 CFU) were harvested by centrifugation, suspended in buffer, and lysed in a French pressure cell to generate the crude extract (C). Unbroken cells were removed by centrifugation at 6,000 × g. Membranes were sedimented by ultracentrifugation at 100,000 × g, and the supernatant (S), containing soluble cytosolic contents, was subjected to affinity chromatography on glutathione-Sepharose. Flowthrough (F) and eluate (E) fractions after the addition of 10 mM glutathione were collected and analyzed by SDS-PAGE and Coomassie staining. The migrations of full-length Gst-TyeA and Gst are marked by filled arrowheads. Gst-TyeA species within the bracket marked with a star represent degradation products that were observed in large-scale purifications but not during immunoblotting of TCA-precipitated cultures. The open arrow represents an unknown glutathione-Sepharose binding protein of Y. enterocolitica. Samples were analyzed by immunoblotting with antisera raised against Gst-TyeA, YopN, YopD, YopB, YscB, and SycN. YopN and YopD copurified with Gst-TyeA but not with Gst. Small star, migration of YscB and SycN on an SDS-PAGE gel.
FIG. 7.
YopN and YopD bind independently to Gst-TyeA. The experimental protocol described in Fig. 6 was used with Y. enterocolitica VTL1 (YopN−) carrying pLC199 and Y. enterocolitica VTL2 (YopD−) carrying pLC199. YopN copurified with Gst-TyeA in the absence of YopD, and YopD copurified with Gst-TyeA in the absence of YopN. See the legend to Fig. 6 for details.
DISCUSSION
Previous work examined the role of tyeA in type III targeting of pathogenic Yersinia species and reported that TyeA is specifically required for the injection of YopE and YopH but not for the injection of other effector Yops (20). This study employed hybrid reporter proteins to measure type III targeting. Once injected into the eukaryotic cytosol, Yop fusions to B. pertussis adenylate cyclase (Cya) are activated via binding to calmodulin, thereby causing an increase in the cytosolic concentration of cyclic AMP (cAMP). On the other hand, if the Cya fusion protein is secreted into the extracellular milieu, its activity can be measured by adding calmodulin and ATP. Iriarte et al. observed 0.2 and 0.7 nmol of cAMP/mg of protein for YopE130-Cya and YopH99-Cya fusion expressed by tyeA mutant yersiniae, whereas wild-type yersiniae caused 6.9 and 5.1 nmol of cAMP/mg of protein, respectively (20). As controls, yopB mutant yersiniae expressing YopE130-Cya and YopH99-Cya elicited 0.2 and 0.6 nmol of cAMP/mg of protein (5, 20). Reporter fusions to YopM, YopM100-Cya, expressed in tyeA mutant yersiniae caused an increase in cAMP in the eukaryotic cytosol to the same level as in wild-type yersiniae. Similar results were obtained for Yersinia strains engineered to produce increased amounts of YopO and YopP. The authors concluded that the tyeA mutant strain was specifically defective in the type III targeting of YopE and YopH (20).
We have developed digitonin fractionation as an assay for type III targeting (23). As the concentration of polypeptides in various fractions is measured by immunoblotting, this assay permits detection of as little as 5 to 10% Yops in the eukaryotic cytosol (25). When Yops were analyzed in this manner, we observed that yopB mutant yersiniae injected effector Yops, albeit at a reduced level (25). Furthermore, the fractionation assay also allows detection of Yops that are secreted into the extracellular medium. We find that tyeA mutant strains secrete all Yops into the extracellular medium. However, significant amounts of YopE, YopH, YopM, and YopN are also detected in the supernatant of digitonin extracts, suggesting that the type III targeting of YopE and YopH is not abolished in tyeA mutants. The observed phenotype is identical to that reported for yopN mutant yersiniae (23).
Cell fractionation and protease protection in combination with xylene extraction have been employed to examine the subcellular location of TyeA (20, 27). Although most of TyeA has been observed to be soluble in bacterial-cell extracts, small amounts of TyeA sedimented with the membranes (25,000 × g) in a manner such that they could not be extracted with Triton X-100. Small amounts of TyeA could, however, be extracted from whole cells with xylene, a solvent that is thought to dissociate proteins that are loosely attached to the bacterial surface (27). Proteinase K treatment followed by xylene extraction of bacteria prevented the release of TyeA (20). These results suggested that small amounts of TyeA may be located on the bacterial surface. We chose not to combine protease sensitivity experiments with xylene extraction. Our protease protection experiments employ whole cells and assay for extracellular and cytoplasmic control proteins in the presence or absence of a membrane-disrupting detergent. Such controls are not available in a combined protocol that focuses on xylene extraction. We find that TyeA is not accessible to extracellular proteinase K unless the bacterial membrane envelope is disrupted by the addition of detergent. Furthermore, TyeA appears soluble in the bacterial cytoplasm after centrifugation at 100,000 × g, even when the protein is overexpressed, suggesting that TyeA is located intracellularly. In our hands, xylene extraction of yersiniae released neither TyeA nor YopN from the bacterial surface. Furthermore, xylene extraction did not dislodge the outer membrane protein YscC. Thus, we find that xylene extraction can not distinguish between outer membrane surface proteins and polypeptides that are located elsewhere in the bacterial envelope or cytoplasm.
One model for type III targeting suggests that TyeA may form a tether with YopN and YopD on the bacterial surface (9, 10). Yersiniae are presumed to assemble a large surface structure, called an injectisome, that may be responsible for sensing calcium ions (YopN and TyeA) or eukaryotic cells (LcrG) and providing for the translocation of effector Yops across the eukaryotic plasma membrane (YopB, and YopD, and LcrV) (10). We suggest an alternative model whereby TyeA functions in the bacterial cytoplasm. Secretion of YopN appears to be a key regulatory step for the type III pathway that leads to the targeting of effector Yops into eukaryotic cells. We suggest that TyeA binding to YopN in the bacterial cytoplasm may prevent the targeting of both YopN and effector Yops (YopEHMNOPT) until yersiniae are properly cued, presumably via the attachment to eukaryotic cells. Yersiniae lacking tyeA indiscriminately export effector Yops, whether the bacteria are attached to eukaryotic cells or not, thereby generating the observed Los phenotype. This model is supported by findings that TyeA may be located exclusively in the bacterial cytoplasm and that tyeA mutant yersiniae contain reduced amounts of intracellular YopN.
ACKNOWLEDGMENTS
We thank D. Anderson, E. Cambronne, K. DeBord, V. Lee, K. Ramamurthi, and C. Tam for critical reading of the manuscript.
L.W.C. was supported by Microbial Pathogenesis Training Grant AI07323 from the Public Health Service to the Department of Microbiology and Immunology at the UCLA School of Medicine.
This work was supported by United States Public Health Service grant AI42797 (NIH-NIAID Infectious Diseases Branch).
REFERENCES
- 1.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D M, Schneewind O. Type III machines of Gram-negative pathogens: injecting virulence factors into host cells and more. Curr Opin Microbiol. 1999;2:18–24. doi: 10.1016/s1369-5274(99)80003-4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D M, Schneewind O. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol Microbiol. 1999;31:1139–1148. doi: 10.1046/j.1365-2958.1999.01254.x. [DOI] [PubMed] [Google Scholar]
- 4.Bergman T, Håkansson S, Forsberg A, Norlander L, Macellaro A, Bäckman A, Bölin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 6.Butler T. Yersinia species. In: Mandell G L, Douglas R G, Bennett J E, editors. Infectious diseases. 5th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 1748–1756. [Google Scholar]
- 7.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L W, Schneewind O. Yersinia enterocolitica type III secretion: on the role of SycE in targeting YopE into HeLa cells. J Biol Chem. 1999;274:22102–22108. doi: 10.1074/jbc.274.31.22102. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 12.Day J B, Plano G V. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol Microbiol. 1998;30:777–789. doi: 10.1046/j.1365-2958.1998.01110.x. [DOI] [PubMed] [Google Scholar]
- 13.Fields K A, Nilles M L, Cowan C, Straley S C. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun. 1999;67:5395–5408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg A, Viitanen A-M, Skunik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 15.Goguen J D, Yother J, Straley S C. Genetic analysis of the low calcium response in Yersinia pestis Mu d1(Ap lac) insertion mutants. J Bacteriol. 1984;160:842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakånsson S, Gaylov E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 17.Holmström A, Petterson J, Rosqvist R, Hakånsson S, Tafazoli F, Fällman M, Magnusson K-E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 18.Iriarte M, Cornelis G R. Identification of SycN, YscX, and YscY, three new elements of the Yersinia yop virulon. J Bacteriol. 1999;181:675–680. doi: 10.1128/jb.181.2.675-680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iriarte M, Cornelis G R. YopT, a new Yersinia effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 20.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson M W, Day J B, Plano G V. YscB of Yersinia pestis functions as a specific chaperone for YopN. J Bacteriol. 1998;180:4912–4921. doi: 10.1128/jb.180.18.4912-4921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee V T, Schneewind O. Type III secretion machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol Rev. 1999;168:241–255. doi: 10.1111/j.1600-065x.1999.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 26.Michiels T, Cornelis G R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991;173:1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson C, Nordfelth R, Holmström A, Hakånsson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 30.Petterson J, Holmström A, Hill J, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg A, Wolf-Watz H. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russel M. Phage assembly: a paradigm for bacterial virulence factor export? Science. 1994;265:612–614. doi: 10.1126/science.8036510. [DOI] [PubMed] [Google Scholar]
- 34.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sory M-P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stainier I, Iriarte M, Cornelis G R. YscM1 and YscM2, two Yersinia enterocolitica proteins causing downregulation of yop transcription. Mol Microbiol. 1997;26:833–843. doi: 10.1046/j.1365-2958.1997.6281995.x. [DOI] [PubMed] [Google Scholar]
- 38.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 39.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 40.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yother J, Chamness T W, Goguen J D. Temperature controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986;165:443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]