Abstract
Objectives
This study aims to assess the effect of ultralow dose 0.005% estriol vaginal gel in women with genitourinary syndrome of menopause (GSM).
Methods
In this prospective and multicenter single-arm study, efficacy was assessed by the evaluation of the epithelial maturation value (MV), vaginal pH, symptoms and signs of vulvovaginal atrophy. Tolerability, acceptability, and the effect on intimate relationships were also evaluated.
Results
We included 35 postmenopausal women with moderate-to-severe vaginal dryness. The most bothering symptom reported was vaginal dryness. The mean increase in the MV after 7 and 14 days of treatment were 22.1 (P < 0.001) and 39.9 (P < 0.001) points, with an increase in the superficial cells of 17.7 percentage points (pp) (95% confidence interval [CI], 7.9–27.4; P < 0.001) and 41.4 pp (95% CI, 28.2–54.6; P < 0.001) observed at the timepoints. Additionally, the pH decreased by 0.6 ± 0.7 (mean ± SD) at 7 days (P < 0.0001) and by 1.1± 0.8 at 14 days (P < 0.0001) from a baseline mean value of 6.3 ± 0.8. The severity of vaginal dryness (range, 0 [none] to 3 [severe]) was significantly reduced by a mean of 1.4 points (P < 0.0001) at 7 days and 2 points (P < 0.0001) at 14 days.
Conclusions
Ultralow dose 0.005% estriol vaginal gel produced a rapid improvement of most relevant symptoms and signs of GSM. This clinically meaningful response was observed from the initial days of treatment, confirming a fast onset and a progressive action.
Keywords: Atrophy, Estriol, Gels, Postmenopause, Vagina
INTRODUCTION
Genitourinary syndrome of menopause (GSM) is a chronic and progressive condition that can have many medical consequences that may affect relationships, daily activities, quality of life and enjoyment of sex [1]. Despite being highly prevalent it is frequently underdiagnosed, and thus remains untreated [2].
This chronic condition is often managed with intermittent courses of estrogen treatment. Cost of interventions, especially those without reimbursement, may result in an extra burden for certain women which, together with a lack of prompt response, may lead to treatment discontinuation (which is more likely to occur initially) [3]. Therefore, choosing therapies that offer a fast and effective relief may be decisive in achieving treatment success and meeting patients’ expectations [4].
Ultralow dose 0.005% estriol vaginal gel has consistently shown to be effective in reversing vulvovaginal symptoms and signs in healthy postmenopausal women [5], in women affected with common pathologies of the menopause such as prolapse [6] or overactive bladder syndrome [7], or in breast cancer women under treatment with non-steroidal aromatase inhibitors [8]. These studies evaluated efficacy after 3 and 12 weeks but there are no data reporting on its earlier action.
Therefore, we designed an interventional study to assess the effect of ultra-low dose 0.005% estriol vaginal gel over symptoms and signs of postmenopausal women with GSM within the first two weeks of treatment.
MATERIALS AND METHODS
Design
This is a prospective, multicenter, single-arm study. The protocol was approved by Regulatory Authorities and the Ethics Committee of Puerta del Hierro Majadahonda University Hospital (study code: RES-2026-C12, the EudraCT: 2019-001435-31). All participants signed the written informed consent before starting the investigation. Participants were recruited between July and December 2019 from the gynecology departments of two Spanish hospitals and from two gynecology centers.
Patients
Women aged 45 to 75 years with at least 12 months of either natural or surgical amenorrhea were included if they presented vaginal dryness of moderate to severe intensity [9] exclusively or in addition to other symptoms and signs of GSM. Exclusion criteria comprised women who presented vaginal bleeding of unknown etiology; women with a history of malignant or premalignant lesions in the breast or endometrium or with venous or arterial thromboembolic disorders; women with grade ≥ II uterovaginal prolapse or with an endometrial thickness equal to or greater than 4 mm; women receiving vulvovaginal treatment in the 15 days prior to the start of the study, phytoestrogens one month before or hormonal therapy three months before the beginning of the study; women under treatment of estrogens or progestogens, tibolone or selective estrogen receptor modulators, antiepileptics and urinary antiseptics; and women receiving long-term antibiotics or antiepileptic, antibiotic, or anti-infective treatment.
Intervention and follow-up
The intervention consisted in the administration of 1 g of 0.005% estriol vaginal gel (Blissel®/Gelistrol®; Intendis Manufacturing SpA, Segrate, Italy) daily, providing 50 µg of estriol per application, administered vaginally with an applicator. The treatment was administered at night over 14 days.
Three visits were scheduled, one at baseline and two after 7 ± 1 and 14 ± 1 days. In addition, a phone call for safety was programmed 15 days after completing the treatment.
At baseline visit, a physical and gynecological examination was performed to all patients, the use of medication and the existence of previous and concomitant diseases were recorded, symptoms and signs of vulvovaginal atrophy were assessed, and a vaginal cytology and pH determination were performed. At follow-up visits, cytology, vaginal pH and vulvovaginal symptoms and signs were evaluated.
Outcomes
Efficacy was assessed by the evaluation of the maturation value (MV) of the vaginal epithelium, the vaginal pH and the symptoms and signs of vulvovaginal atrophy. The primary outcome was the change in the MV from baseline to the second week of treatment. Secondary outcomes were changes in the MV after one week, and in the vaginal pH and vulvovaginal symptoms and signs after one and two weeks of treatment.
The MV was calculated from the percentage of superficial, intermediate and parabasal cells obtained in the vaginal smears and analyzed at a central laboratory (Centro Anatomopatológico S.L., Madrid, Spain). The MV was determined from the average percentages of each type of cell calculated twice on 100 consecutive cells as follows: [1 × (% superficial cells)] + [0.6 × (% intermediate cells)] + [0.2 × (% parabasal cells)] (range, 20–100) [10]. The investigators were given MColorphast® test strips (range, 2.0–9.0; resolution, 0.5) (Merck Millipore, Darmstadt, Germany) to measure the pH of the vaginal mucosa and the strips were discarded once the pH had been recorded.
Symptoms (vaginal dryness, dyspareunia, pruritus, dysuria, and lack of lubrication) were registered by the patient according to a 4-point Likert scale (0 = absent; 1 = mild: the symptom is present and does not interfere with daily activities; 2 = moderate: the symptom is present, disturbing and affects daily activities; and 3 = severe: the symptom is present, is very disturbing and greatly affects daily activities) on a daily basis. Evaluation of dyspareunia was only applicable to patients who had sexual activity within the 15 days prior to the baseline visit and, once the treatment had started, in those who had sexual activity within the 24 hours prior to the daily record. Signs (dryness or dehydration, fragility, paleness and flattening of vaginal folds) were assessed at 7 and 14 days by the investigators using the previously described Likert scale. Overall scores were obtained for signs (0–12) and symptoms (0–15) by adding individual intensity scores. Additionally, women were asked about the effectiveness, acceptability and tolerability of the medication. Adverse events were registered over treatment and up to 15 days after completion of the intervention.
Data analysis
To determine the sample size, a change of 15 points in the vaginal MV was established [11]. Assuming a SD similar to that observed in previous studies (25 points) and considering an alpha value of 0.05 and a beta value of 0.1, the number of subjects necessary to observe a difference in the MV of 15 points according to the two-tailed contrast was established in 30 patients. Considering a possible dropout of up to 15%, the sample size was finally established in 35 patients.
Demographic and clinical characteristics were summarized descriptively. Categorical variables were described by frequencies and percentages. Continuous variables were described using the mean, median, SD, confidence intervals (CI), quartiles, and percentiles. To contrast the hypotheses under study, t test for paired samples or its corresponding non-parametric Wilcoxon test and McNemar for categorical variables were used. For between-subgroups comparison, chi-square or Fisher’s exact test were used for categorical data and Mann–Whitney U test for continuous and ordinal variables. The change in intensity of every symptom after one and two weeks of intervention was compared using non-parametric paired tests. The last observation carried forward method was used to avoid a possible attrition bias due to dropouts during follow-up. All analyses were performed with the SAS V9.3 program (SAS Institute, Cary, NC, USA).
RESULTS
Thirty-five women were included in the study. One patient only attended the baseline visit, so data from 34 patients were reported. Table 1 summarizes their baseline characteristics.
Table 1. Demographics and baseline characteristics.
| Characteristics | Value | |
|---|---|---|
| Age (y) | 56.6 ± 6.0 | |
| Weight (kg) | 62.3 ± 8.2 | |
| Height (m) | 1.6 ± 0.1 | |
| Body mass index (kg/m2) | 23.6 ± 3.2 | |
| Years of menopausea | 7.6 ± 6.4 | |
| Age of menopause (y) | 49.1 ± 3.5 | |
| Cause of amenorrhea | ||
| Natural process | 31 (91.2) | |
| Surgical intervention | 3 (8.8) | |
Data are presented as mean ± SD or number (%).
aThirteen participants did not recall the month of the last period: in these cases, only the year has been considered for the calculation.
The most bothering symptoms reported was vaginal dryness followed by dyspareunia in 20 (58.8%) and 11 (32.4%) women, respectively (Supplementary Fig. 1, available online).
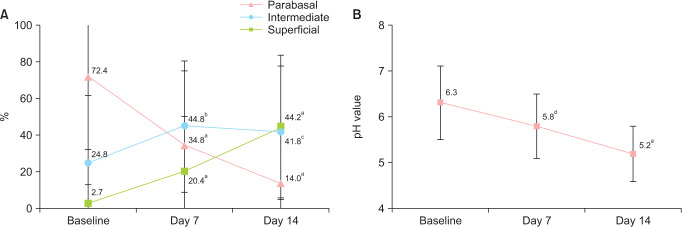
The mean and SD increase from baseline in the MV was 22.1 ± 23.6 points (P < 0.001) after one week and 39.9 ± 25.1 points (P < 0.001) after two weeks of treatment. The mean and SD MV observed was 54.2 ± 24.4 and 72.0 ± 23.6 after one and two weeks, respectively, improving from a baseline value of 32.1 ± 18.1 (Fig. 1, Table 2).
Fig. 1. Mean vaginal maturation value (± SD) at baseline, day 7 and day 14. P values were obtained by comparing with baseline. aP < 0.0001, Student’s t test for paired samples and clinically relevant > 15 points.
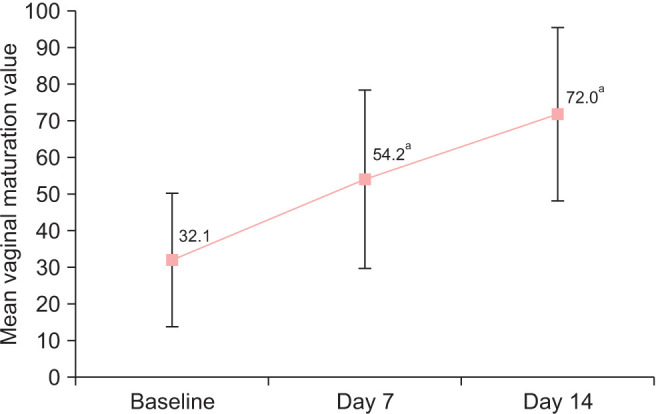
Table 2. Baseline scores for signs and symptoms.
| Signs and symptoms | Baseline | Day 7 | Day 14 | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P value vs. baselinec | Mean ± SD | P value vs. baselinec | ||
| Maturation value | 32.1 ± 18.1 | 54.2 ± 24.4 | < 0.0001 | 72.0 ± 23.6 | < 0.0001 | |
| Vaginal pHa | 6.3 ± 0.8 | 5.8 ± 0.72 | 0.0002 | 5.2 ± 0.6 | < 0.0001 | |
| Superficial cells (%) | 2.7 ± 10.3 | 20.4 ± 29.8 | < 0.0001 | 44.2 ± 39.3 | < 0.0001 | |
| Parabasal cells (%) | 72.4 ± 39.9 | 34.8 ± 40.0 | < 0.0001 | 14.0 ± 28.8 | < 0.0001 | |
| Intermediate cells (%) | 24.8 ± 36.7 | 44.8 ± 35.6 | 0.0161 | 41.8 ± 35.8 | 0.0467 | |
| Baseline global symptom score | 8.3 ± 3.0 | 3.3 ± 2.9 | < 0.0001 | 1.2 ± 1.8 | < 0.0001 | |
| Vaginal dryness | 2.5 ± 0.6 | 1.1 ± 1.0 | < 0.0001 | 0.5 ± 0.7 | < 0.0001 | |
| Itching, stinging, or burning of the vulva or vagina | 1.3 ± 1.0 | 0.6 ± 0.8 | 0.0001 | 0.1 ± 0.4 | < 0.0001 | |
| Painful urination (dysuria) | 0.3 ± 0.6 | 0.2 ± 0.7 | 0.7969 | 0.1 ± 0.2 | 0.0625 | |
| Lack of lubrication | 2.4 ± 0.7 | 1.0 ± 0.9 | < 0.0001 | 0.4 ± 0.7 | < 0.0001 | |
| Pain or discomfort with sexual activity (dyspareunia)b | 2.2 ± 1.0 | 0.8 ± 0.8 | 0.005 | 0.4 ± 0.9 | 0.001 | |
| Baseline global signs score | 8.7 ± 2.6 | 6.7 ± 2.5 | < 0.0001 | 4.2 ± 2.7 | < 0.0001 | |
| Dry or dehydrated vaginal mucosa | 2.4 ± 0.5 | 1.8 ± 0.7 | < 0.0001 | 1.1 ± 0.8 | < 0.0001 | |
| Fragility of the vaginal mucosa | 2.0 ± 0.9 | 1.5 ± 0.8 | 0.0003 | 0.9 ± 0.8 | < 0.0001 | |
| Paleness of the vaginal mucosa | 2.3 ± 0.6 | 1.7 ± 0.7 | < 0.0001 | 1.0 ± 0.7 | < 0.0001 | |
| Flattening of vaginal mucosa folds | 2.0 ± 0.9 | 1.7 ± 0.8 | 0.0034 | 1.1 ± 0.7 | < 0.0001 | |
aVaginal pH was measured in 26 women.
bDyspareunia was assessed in patients that have had intimate intercourse with vaginal penetration (29 patients at baseline, 13 patients at day 7 and 14 patients at day 14).
cChange from baseline (Wilcoxon test).
Baseline percentage of superficial cells was 2.7%. Significant increases of 17.7 percentual points (pp) (95% CI, 7.9–27.4; P < 0.001) were observed after one week (Fig. 2A) and 41.5 pp (95% CI, 28.2–54.6; P < 0.001) after two weeks. Additionally, the pH significantly decreased by 0.5 ± 0.7 at 7 days, and by 1.1 ± 0.8 at 14 days from a baseline mean value of 6.3 ± 0.8 (Fig. 2B).
Fig. 2. Mean values (± SD) at all time intervals in (A) percentage of superficial, intermediate and parabasal cells and (B) vaginal pH. P values were obtained by comparing with baseline. aP < 0.0001, Wilcoxon test. bP = 0.0161, Student’s t test. cP = 0.0467, Student’s t test. dP = 0.0002, Wilcoxon test.
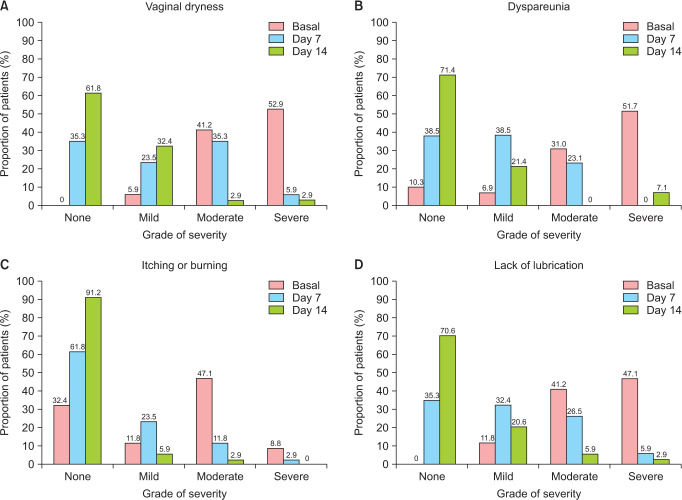
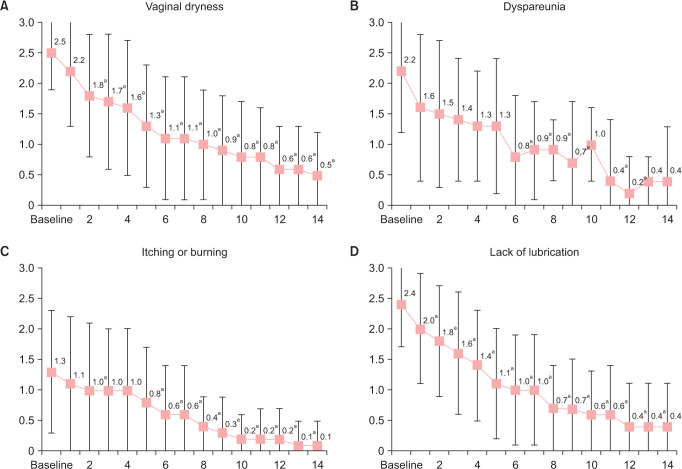
Vaginal dryness was present in all the patients at baseline with a mean score of 2.5 ± 0.6. The severity of this symptom was significantly reduced by a mean of 1.4 points (P < 0.0001) and 2.0 points (P < 0.0001) at 7 and 14 days, respectively (Table 2). While 94% of the patients reported moderate-severe dryness at baseline, the proportion of women that reported mild or absent dryness was 58.8% after 7 days of treatment, and this proportion increased to 94.2% after 14 days (Fig. 3A). The results show a significant reduction from baseline in the presence of vaginal dryness from the third day to the end of the treatment period (P < 0.05) (Fig. 4A).
Fig. 3. Proportion of patients at baseline, day 7 and day 14 reporting to have none, mild, moderate or severe symptomatology in the following symptoms: (A) vaginal dryness, (B) dyspareunia or discomfort during intercourse, (C) pruritus, stinging or burning of the vulva or vagina, and (D) lack of lubrication.
Fig. 4. Daily evolution (mean ± SD) of the self-assessed severity of the symptomatology. (A) Vaginal drynes, (B) dyspareunia or discomfort with sexual activity, (C) pruritus, stinging or burning of the vulva or vagina, and (D) lack of lubrication. Intensity of each symptoms was assessed and reported by patients according to a 4-point Likert scale (0 = absent, 1 = mild, 2 = moderate, and 3 = severe). P values were obtained by comparing with baseline. aP < 0.05, Student’s t test for paired samples.
Twenty-six (89.7%) of the 29 women who reported having sexual intercourse at baseline manifested dyspareunia, with a moderate to severe intensity in 82.7% of the cases. After 2 weeks, dyspareunia became mild or absent in 92.8% of the patients and 71.4% reported absence of discomfort or pain during intercourse (Fig. 3B). Accordingly, the intensity of the symptom was reduced by 1.4 (P = 0.005) and 1.8 (P = 0.001) after 7 and 14 days, from a mean baseline score of 2.2 (Table 2, Fig. 4B).
Lack of lubrication was present in all the participants at baseline, and 88.3% of them reported moderate to severe intensity (Fig. 3D). From a mean baseline score of 2.4, the score was significantly reduced by 1.4 points (P < 0.0001) after 1 week and by 2.0 points (P < 0.0001) after 2 weeks of treatment (Table 2, Fig. 4D). Pruritus was present in 67.7% of the sample at baseline and changed from a severity score of 1.3 at baseline to 0.6 in the first week of intervention and to 0.1 in the second week (Table 2, Fig. 4C).
Although dysuria was only present in 7 patients at baseline, significant reductions in the presence of this symptom were found on days 12 and 13 (P = 0.0143) when compared to baseline.
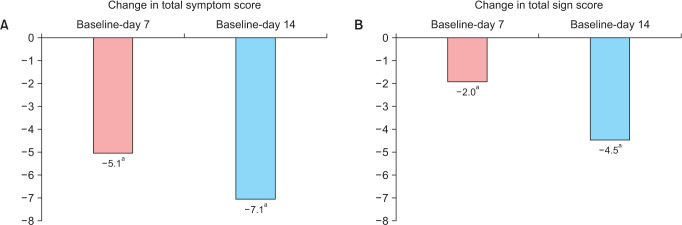
Baseline total symptom score was 8.3 and decreased to 5.1 (P < 0.001) after 7 days and to 7.1 (P < 0.001) after 14 days (mean reductions available on Fig. 5A). Significant differences in the total symptoms score were found from the first day onwards when compared to baseline (P < 0.0001). Absence of genitourinary symptoms (total symptom score of 0) was achieved in 23.5% of the patients at 7 days and in 50% at 14 days.
Fig. 5. Mean change from baseline in the total score of (A) symptoms and (B) signs at days 7 and 14. P values were obtained by comparing with baseline. aP < 0.05, Student’s t test for paired samples.
Likewise, significant changes in the total signs score were found (Fig. 5B). Table 2 summarizes the results on the evolution of each specific symptom and sign (further information on signs in Supplementary Fig. 2, available online).
Regarding the subjective assessments at the end of the study, 81.8% of the patients considered that the treatment provided a rapid relief of their symptoms, and 84.6% of the sexually active women reported an improvement in their intimate relationships—66.7% of them manifesting benefits from the first week—(Supplementary Fig. 3, available online). Of the women, 93.9% rated the moisturizing effect of the intervention as good, very good, or excellent (Supplementary Fig. 4 available online).
Thirty-one out of 34 (91.2%) patients achieved a very good therapeutic compliance (≥ 90% of daily applications), and all the patients rated tolerability from good to excellent. Only one patient reported an adverse event: a case of mild vaginal candidiasis which resolved after 8 days without any action required.
DISCUSSION
This study provides evidence on the early effect of the 0.005% vaginal gel during the first two weeks of use, demonstrating a significant effect on the primary endpoint, the change in the MV of the vaginal epithelium, after 1 and 2 weeks of treatment. This rapid effect is also reported in the patient-perceived efficacy outcomes, in each sign and symptom analyzed and, accordingly, in the overall symptomatology. The prompt and progressive resolution of the vaginal atrophy as well as the properties of the formulation itself, designed as a highly moisturizing mucoadhesive gel, may explain the fast response of 0.005% estriol vaginal gel on the symptomatology.
Interventions with this medication have proven to be effective in the change of the MV of the vaginal epithelium, according to the conclusions of high-quality controlled trials conducted in women with postmenopausal vaginal atrophy [5]. This response is obtained following the recommendations of using the lowest clinically efficacious doses with the least estrogenic exposure [12], since estriol is the estrogen with the lowest estrogenic activity and 0.005% vaginal gel is formulated in notably low concentrations and administered in very low doses [13]. Now, the results of this research have provided knowledge on the fast therapeutic effect of this intervention on symptoms and signs of vulvovaginal atrophy, which was observed from the first week and progressively increased up to the end of the treatment. This, along with the daily assessment of symptoms from the very first day of treatment, have evidenced an early therapeutic effect that has not been demonstrated previously. Other studies have been designed with longer follow-up cut-offs, as it is the case for local estrogens (first data evaluated at two weeks for estradiol and at 3 weeks for estriol) [6,14] and non-estrogenic interventions (earliest efficacy data with selective estrogen receptor modulators (SERMs) and prasterone have been reported after four and two weeks of treatment, respectively) [15,16]. Thus, as far as we are aware, this study provides the earliest efficacy data in the context of the GSM.
Vaginal atrophy is shown by changes in the cellular composition of the epithelium from MV that can be measured reliably. In our study, the baseline range of severity in relation to the MV of the vaginal epithelium was higher than that seen in previous studies with the same intervention [5]. The percentage of superficial cells increased almost 10 times from baseline after 7 days of intervention, and it had a more than two-fold increase from day 7 to day 14 of treatment: indicative of a rapid, marked, and progressive trophism of the epithelium. Importantly, these objective cytological changes cannot be attributed to the placebo effect which, in previous clinical trials, has produced barely discernible responses [5,8].
In addition to the estrogenic effect produced by estriol, essential for the maturation of the epithelium, one of the reasons that may contribute to the rapid effect of 0.005% estriol vaginal gel observed in our study is its formulation (presented as a gel with hydrating and mucoadhesive properties due to the polycarbophilic components), that synergizes with the effect of the estriol by providing a moisturizing effect per se and by increasing the retention and penetration of the hormone (in contrast to non-mucoadhesive products that may present shorter vaginal contact times and may produce leakage) [17]. This coadjuvant activity can help to further understand the immediate and progressive relief, from the first days of treatment, shown in our study.
The vaginal pH tends to increase in atrophic conditions as a consequence of the reduction in the upper layers of the epithelium, which causes a decrease in glycogen levels and, therefore, prevents the proliferation of lactobacilli. Thus, it is considered an adequate indicator to assess the therapeutic efficacy of interventions for vulvovaginal atrophy, but it is one of the parameters which takes longer to correct [18]. In our study it was observed how the baseline value was reduced significantly even after one week of intervention by more than half a point, and this reduction was practically doubled after two weeks of treatment. The results of this study show an important reduction of the pH in the very short term, consistent with previous studies of 0.005% vaginal gel, and comparable to or slightly higher than the reductions achieved by other therapies after longer treatment periods [14,15,19].
Vaginal dryness and dyspareunia are the most common and troublesome symptoms in the majority of women [20]. Accordingly, in our study, these were reported as the most bothering symptoms.
Dryness was present at baseline in the entire sample and was considered the most bothering symptom by most of the study participants. Ultralow dose 0.005% estriol vaginal gel showed an early and remarkable effect beginning with the first application that became significant from the third day of treatment. After one week, this symptom was no longer present in more than a third of the study population. In contrast, studies with prasterone and SERMs showed smaller reductions when compared to baseline after 2 and 4 weeks of treatment [15,16].
Dyspareunia is a symptom with a particular awareness for being disabling and one of the main reasons for doctor consultation, since it affects sexual intimacy in both the patient and her partner [21]. Our results show a rapid improvement of the dyspareunia from the very initial days which increased progressively up to the second week, when only four women reported pain during intercourse. Other local or systemic alternatives have proven to be effective but after longer periods of treatment [15,16,22]. Importantly, the early and progressive improvements observed in our study in both dyspareunia and lubrication are likely to have motivated the positive results in sexual intimacy revealed in the questionnaire.
The rapid improvement in the most prevalent and bothering symptoms of vulvovaginal atrophy is considered to be of high clinical value. This is a chronic condition that affects a significant amount of postmenopausal women substantially influencing their quality of life and sexual sphere [23]. As treatments are often approached intermittently, it is essential that they provide a prompt effect so therapy is not discontinued and symptoms are solved successfully [3,4]. This would be also of particular benefit in women with estrogen dependent cancer in which these therapies are sometimes used in clinical practice.
The main limitation of our trial could be considered its design as a single intervention group that performed evaluations at different time-points and comparing them with baseline values. However, the marked positive evolution consistently observed in all the outcomes within the first and the second week of treatment are indicative of a therapeutic activity beyond the placebo effect. The response to the treatment was evidenced in a progressive manner and maintained over time, was consistent for most evaluated symptoms and effective in patients with severe vaginal atrophy. Importantly, a clear effect was shown in objective cytological signs and parameters dependent on the estrogenic activity in which the placebo fails to produce a response [5,8]. This is further reinforced by the fact that the comparisons of the pre-post data of the 0.005% estriol vaginal gel group of previous controlled studies showed similar trends.
In summary, the intervention with 0.005% estriol gel has shown to be effective in the maturation of the vaginal epithelium after one and two weeks of intervention, revealing a prompt onset of action and a clinically meaningful response for the majority of the assessed outcomes from the very initial days of treatment.
ACKNOWLEDGMENTS
We would like to thank Javier Leal Martínez-Bujanda for his help in writing the manuscript and Maria Palma Santisteban for her participation in the design and development.
This work was supported by ITF Research Pharma, S.L.U., Spain.
Footnotes
CONFLICT of INTEREST: J.L.-C.F., M.C.G., and J.L.D.M. report no conflicts of interest. S.G.R. reports grants for research activities, for attending conferences, as a consultant, and/or for lecturing in scientific meetings from Isdin, Pfizer, Servier, Amgen, MSD, Kern, Casen-Recordati, Sandoz, Procare Health, Bayer, Lacer, Shionogi, GSK, Bioiberica, Theramex, Gedeon Ritcher, Effik, Italfarmaco, Iprad, Seid, Ordesa, and Zambon. C.C.C. and C.N.M. are full time employees of ITF Research Pharma.
SUPPLEMENTARY MATERIALS
Most bothersome symptom as reported by the patients.
Mean change from baseline in individual signs scores at days 7 and 14. P values were obtained by comparing with baseline. *P < 0.05, Student’s t test for paired samples.
Percentage of patients that reported to have improved their sexual relationships after 7 and 14 days of treatment with 0.005% estriol vaginal gel.
Evaluation of the product moisturizing effect as perceived by patients at the end of the study.
References
- 1.Nappi RE, Palacios S, Bruyniks N, Particco M, Panay N. The burden of vulvovaginal atrophy on women's daily living: implications on quality of life from a face-to-face real-life survey. Menopause. 2019;26:485–491. doi: 10.1097/GME.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 2.Moral E, Delgado JL, Carmona F, Caballero B, Guillán C, González PM, et al. Genitourinary syndrome of menopause. Prevalence and quality of life in Spanish postmenopausal women. The GENISSE study. Climacteric. 2018;21:167–173. doi: 10.1080/13697137.2017.1421921. [DOI] [PubMed] [Google Scholar]
- 3.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10:1790–1799. doi: 10.1111/jsm.12190. [DOI] [PubMed] [Google Scholar]
- 4.Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413–424. doi: 10.1016/j.jsxm.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Cano A, Estévez J, Usandizaga R, Gallo JL, Guinot M, Delgado JL, et al. The therapeutic effect of a new ultra low concentration estriol gel formulation (0.005% estriol vaginal gel) on symptoms and signs of postmenopausal vaginal atrophy: results from a pivotal phase III study. Menopause. 2012;19:1130–1139. doi: 10.1097/gme.0b013e3182518e9a. [DOI] [PubMed] [Google Scholar]
- 6.Caruso S, Cianci S, Vitale SG, Matarazzo MG, Amore FF, Cianci A. Effects of ultralow topical estriol dose on vaginal health and quality of life in postmenopausal women who underwent surgical treatment for pelvic organ prolapse. Menopause. 2017;24:900–907. doi: 10.1097/GME.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 7.Matarazzo MG, Caruso S, Giunta G, Valenti G, Sarpietro G, Cianci A. Does vaginal estriol make urodynamic changes in women with overactive bladder syndrome and genitourinary syndrome of menopause? Eur J Obstet Gynecol Reprod Biol. 2018;222:75–79. doi: 10.1016/j.ejogrb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Hirschberg AL, Sánchez-Rovira P, Presa-Lorite J, Campos-Delgado M, Gil-Gil M, Lidbrink E, et al. Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: a phase II, randomized, double-blind, placebo-controlled trial. Menopause. 2020;27:526–534. doi: 10.1097/GME.0000000000001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration (FDA) Guidance for industry. Estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms-recommendations for clinical evaluation. Silver Spring: FDA; 2003. [cited 2021 Dec 20]. Available from: https://www.fda.gov/media/71359/download . [Google Scholar]
- 10.Hustin J, Van den Eynde JP. Cytologic evaluation of the effect of various estrogens given in postmenopause. Acta Cytol. 1977;21:225–228. [PubMed] [Google Scholar]
- 11.Casper F, Petri E. Local treatment of urogenital atrophy with an estradiol-releasing vaginal ring: a comparative and a placebo-controlled multicenter study. Vaginal Ring Study Group. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:171–176. doi: 10.1007/s001920050040. [DOI] [PubMed] [Google Scholar]
- 12.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Rovira P, Hirschberg AL, Gil-Gil M, Bermejo-De Las Heras B, Nieto-Magro C. A phase II prospective, randomized, double-blind, placebo-controlled and multicenter clinical trial to assess the safety of 0.005% estriol vaginal gel in hormone receptor-positive postmenopausal women with early stage breast cancer in treatment with aromatase inhibitor in the adjuvant setting. Oncologist. 2020;25:e1846–e1854. doi: 10.1634/theoncologist.2020-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol. 2008;112:1053–1060. doi: 10.1097/AOG.0b013e31818aa7c3. Erratum in: Obstet Gynecol 2008; 112: 1392. [DOI] [PubMed] [Google Scholar]
- 15.Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907–922. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 16.Archer DF, Goldstein SR, Simon JA, Waldbaum AS, Sussman SA, Altomare C, et al. Efficacy and safety of ospemifene in postmenopausal women with moderate-to-severe vaginal dryness: a phase 3, randomized, double-blind, placebo-controlled, multicenter trial. Menopause. 2019;26:611–621. doi: 10.1097/GME.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso S, Cianci S, Amore FF, Ventura B, Bambili E, Spadola S, et al. Quality of life and sexual function of naturally postmenopausal women on an ultralow-concentration estriol vaginal gel. Menopause. 2016;23:47–54. doi: 10.1097/GME.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol. 2008;111:67–76. doi: 10.1097/01.AOG.0000296714.12226.0f. [DOI] [PubMed] [Google Scholar]
- 19.Labrie F, Archer DF, Koltun W, Vachon A, Young D, Frenette L, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23:243–256. doi: 10.1097/GME.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 20.Sturdee DW, Panay N International Menopause Society Writing Group. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13:509–522. doi: 10.3109/13697137.2010.522875. [DOI] [PubMed] [Google Scholar]
- 21.Kao A, Binik YM, Kapuscinski A, Khalife S. Dyspareunia in postmenopausal women: a critical review. Pain Res Manag. 2008;13:243–254. doi: 10.1155/2008/269571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Constantine G, Millheiser LS, Kaunitz AM, Parish SJ, Graham S, Bernick B, et al. Early onset of action with a 17β-estradiol, softgel, vaginal insert for treating vulvar and vaginal atrophy and moderate to severe dyspareunia. Menopause. 2019;26:1259–1264. doi: 10.1097/GME.0000000000001394. [DOI] [PubMed] [Google Scholar]
- 23.Panay N, Palacios S, Bruyniks N, Particco M, Nappi RE. Symptom severity and quality of life in the management of vulvovaginal atrophy in postmenopausal women. Maturitas. 2019;124:55–61. doi: 10.1016/j.maturitas.2019.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Most bothersome symptom as reported by the patients.
Mean change from baseline in individual signs scores at days 7 and 14. P values were obtained by comparing with baseline. *P < 0.05, Student’s t test for paired samples.
Percentage of patients that reported to have improved their sexual relationships after 7 and 14 days of treatment with 0.005% estriol vaginal gel.
Evaluation of the product moisturizing effect as perceived by patients at the end of the study.