Abstract
Introduction: The incidence and mortality of gastric cancer ranks among the highest, and the 5-year survival rate of advanced gastric cancer (AGC) is less than 10%. Currently, chemotherapy is the main treatment for AGC, and oxaliplatin is an important part of the commonly used chemotherapy regimen for AGC. A large number of RCTs have shown that Chinese herbal medicine (CHM) combined with oxaliplatin-based chemotherapy can improve objective response rate (ORR) and disease control rate (DCR), reduce the toxic and side effects of chemotherapy. There is currently a lack of systematic evaluation of the evidence to account for the efficacy and safety of CHM combined with oxaliplatin-based chemotherapy in AGC. Therefore, we carried out this study and conducted the sensitivity analysis on the herbal composition to explore the potential anti-tumor efficacy.
Methods: Databases of PubMed, EMBASE, CENTRAL, Web of Science, the Chinese Biomedical Literature Database, the China National Knowledge Infrastructure, the Wanfang database, and the Chinese Scientific Journals Database were searched from their inception to April 2022. RCTs evaluating the efficacy of CHM combined with oxaliplatin-based chemotherapy on AGC were included. Stata 16 was used for data synthesis, RoB 2 for quality evaluation of included RCTs, and GRADE for quality of synthesized evidence. Additional sensitivity analysis was performed to explore the potential anti-tumor effects of single herbs and combination of herbs.
Results: Forty trials involving 3,029 participants were included. Most included RCTs were assessed as “Some concerns” of risk of bias. Meta-analyses showed that compare to oxaliplatin-based chemotherapy alone, that CHM combined with oxaliplatin-based chemotherapy could increase the objective response rate (ORR) by 35% [risk ratio (RR) = 1.35, 95% confidence intervals (CI) (1.25, 1.45)], and disease control rate (DCR) by 12% [RR = 1.12, 95% CI (1.08, 1.16)]. Subgroup analysis showed that compare to SOX, FOLFOX, and XELOX regimens alone, CHM plus SOX, CHM plus FOLFOX, and CHM plus XELOX could significantly increase the ORR and DCR. Sensitivity analysis identified seven herbs of Astragalus, Liquorice, Poria, Largehead Atractylodes, Chinese Angelica, Codonopsis, and Tangerine Peel with potentials to improve tumor response of oxaliplatin-based chemotherapy in AGC.
Conclusion: Synthesized evidence showed moderate certainty that CHM plus oxaliplatin-based chemotherapy may promote improvement in tumor response in AGC. CHM treatment is safe for AGC. Due to the poor quality of included RCTs and small samplesizes, the quality of synthesized evidence was not high. Specific combinations of herbs appeared to produce higher contributions to ORR than the herb individually. Each of this seven above mentioned herbs has been shown in experimental studies to potentially contribute to the improvement of tumor response. To support this conclusion, these seven herbs are worthy of further clinical research.
Systematic Review Registration: [http://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=262595], identifier [CRD42022262595].
Keywords: advanced gastric cancer, Chinese herbal medicine, oxaliplatin, meta-analysis, efficacy, tumor response, synergistic action
1 Introduction
According to Global Cancer Statistics 2020, there were 1,089,000 new cases and 769,000 mortality cases of gastric cancer (GC) globally, ranked second of incidence and mortality rate of all malignant tumors of digestive system (Sung et al., 2021). The number of GC cases in China accounts for 43.9% of the global total. About 50% of GC patients were in advanced stage at the initial diagnosis. Advanced gastric cancer (AGC) has a poor prognosis, with a median overall survival (OS) of 10–12 months (Digklia et al., 2016), and the 5-year survival rate is no more than 10% (Song et al., 2017). AGC generally have distant metastasis and local infiltration, and 50% of recurrent patients were local lymph node positive (Peng et al., 2010). It is reported that the OS of patients with metastatic GC after palliative chemotherapy is only 7–15 months, and the 5-year survival rate is only 2% (Leong, 2005; Ajani et al., 2007). In recent years, the incidence and death of GC are on the rise. Therefore, AGC has become one of the main diseases endangering human life and health (Johnston et al., 2019).
Chemotherapy is the standard first-line treatment for AGC patients, and palliative chemotherapy has a statistically significant advantage over best supportive care in improving survival in AGC patients (Wagner et al., 2006). The NCCN guidelines recommend that combined chemotherapy containing platinum and fluorouracil is preferred for AGC patients (Ajani et al., 2022). Oxaliplatin is a third-generation platinum-type anticancer drug. Clinical studies have proved that it has a significant inhibitory effect on locally AGC or AGC, and its efficacy is no less than that of cisplatin (Ajani et al., 2016; Smyth et al., 2016; Yoshino et al., 2018). The median progression-free survical (mPFS) of first-line chemotherapy is 4–6 months and median overall survival (mOS) is 10–15 months (Van Cutsem et al., 2006; Cunningham et al., 2008; Koizumi et al., 2008; Kang et al., 2009). Moreover, oxaliplatin is better tolerated than cisplatin and has a better synergistic effect with fluorouracil (Al-Batran et al., 2008). Based on the above advantages, oxaliplatin has become the main platinum drug in AGC chemotherapy, which is used to form SOX, XELOX and FOLFOX regimens. However, peripheral neurotoxicity is the main side effect of oxaliplatin, which incidence of peripheral neurotoxicity is higher than that of cisplatin (63% vs. 22%), especially grade 3 or 4 neuropathy (Al-Batran et al., 2008; Cunningham et al., 2008). The targeted drugs approved for the treatment of AGC are mainly anti-angiogenic drugs, including trastuzumab and ramucirumab. The mOS of trastuzumab in the treatment of AGC patients with positive HER-2 is 7.9 months, but the incidence of anemia and visceral bleeding, two kinds of serious adverse events (AEs), is about 19% (Thuss-Patience et al., 2017). In addition, HER-2 overexpression only accounts for 15%–20% of AGC (Joshi et al., 2021), and the benefit in OS of other drugs is unclear, and the selection of targeted drugs is limited. There is no evidence supporting survival benefit of immunotherapy alone in patients with AGC (Shitara et al., 2018; Bang et al., 2019; Van Cutsem et al., 2021). Therefore, despite the variety of treatment options for AGC, chemotherapy is still the best choice for AGC, and chemotherapy drugs are constantly updated and iterated. However, obstacles such as serious AEs of chemotherapy, poor quality of life (QoL) and short survival of patients with AGC have not been well solved, and the treatment of AGC is still a major challenge.
CHM has been widely used in east Asia to fight against tumors for a long time. In particular, CHM combined with chemotherapy has advantages of synergistic efficacy and toxicity reduction, improving QoL and enhancing immune function (Cheng et al., 2021). A meta-analysis of 2,670 patients with AGC found that patients with astragalus-containing Chinese medicine combined with platinum-containing chemotherapy had better objective response rate (ORR) [risk ratio (RR) = 1.24, 95% confidence interval (CI): 1.15–1.34) ] and disease control rate (DCR) (RR = 1.10, 95% CI: 1.06–1.14), the AEs caused by chemotherapy were significantly reduced, and the QoL was significantly improved (Cheng et al., 2021). There was no previous meta-analysis of oxaliplatin-based chemotherapy regimen plus CHM, but some high quality clinical studies have demonstrated the important role of CHM combined with oxaliplatin. Yiqi Huoxue Jiedu formula combined with XELOX regimen could improve DCR (60.78% vs. 41.67%) (Hu, 2011). Shenlian capsules combined with SOX chemotherapy intervention in 157 patients with AGC, the ORR and DCR of the treatment group were 78.1% and 92.7%, respectively, while those of the control group were 66.4% and 79.9%, besides, which significantly reduced the incidence of neurotoxicity and other toxic and side effects (52.2% vs. 94.6%) and prolonged OS about 2.7 months (Diao et al., 2018). The ORR and DCR in the treatment group of Weifu formula combined with FOLFOX6 were significantly improved (ORR, 70.0% vs. 26.7%; DCR, 83.3% vs. 56.7%), and the KPS score was also improved (83.3% vs. 60.0%) (Fan et al., 2015). However, these individual studies are not enough to explain the clinical role of traditional Chinese medicine (TCM) in AGC. Evidence-based medicine should be used to comprehensively analyze existing clinical studies and to evaluate available evidence. Due to the complexity and diversity of Chinese medicine prescriptions, it is impossible to determine which CHM herbs play an important role to synergistic action with chemotherapy.
With the further development of basic research, the role and mechanism of TCM in inhibiting the division and proliferation of tumor cells, promoting the apoptosis of tumor cells, inhibiting the metastasis of tumor cells, and enhancing the curative effect with chemotherapy have been gradually revealed, laying a foundation for the effective application of anti-tumor (Zhou et al., 2016; Liu et al., 2020; Ren et al., 2020; Xiang et al., 2020; Zhang L. et al., 2021). Available experimental studies indicate that TCM compounds do have anti-tumor activity, but the more appropriate and accurate drug selection is still unknown. Therefore, future research will focus on finding individual herbs with special contributions to chemotherapy efficacy of AGC, and to improve survival benefit of AGC and precise selection of TCM.
There are several RCTs evaluated the efficacy and safety of CHM in AGC, but these clinical evidence were not systematically evaluated. Furthermore, whether CHM have a synergistic effect with oxaliplatin-based chemotherapy, the key component of the first-line treatment of AGC, also need further evaluation. The objective of this study was to systematically evaluate the available evidence of tumor response and safety of CHM combined with oxaliplatin-based chemotherapy in AGC. Furthermore, we performed sensitivity analysis of single herb and combination of herbs, to explore the potential anti-tumor effects of these herbs.
2 Methods
This study was performed by the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and checklist (Moher et al., 2009), PRISMA checklist is available in Supplementary Material S1. This study was registered on PROSPERO (No. CRD42022262595).
2.1 Eligibility criteria
2.1.1 Type of studies
This study included RCTs with or without the blinded method, observational studies and quasi-RCTs were excluded. Trials did not describe the randomization process in details were considered as non-RCTs, and were excluded. Animal studies were also excluded.
2.1.2 Types of participants
RCTs which participants diagnosed with AGC through cytological or pathological tests were included.
2.1.3 Types of intervention and control
The intervention of CHM combined with oxaliplatin-based chemotherapy, and control of oxaliplatin-based chemotherapy were included in this study. We only included CHM formulas or oral patented drugs as the treatment of intervention, Chinese medicine injections and plant extracts were excluded.
2.1.4 Types of outcomes
RCTs reporting outcomes of tumor response and safety of CHM in GC treatment were included in this study. Trials reported other efficacy outcomes were excluded. Given the strong correlation between the two anti-tumor treatment response evaluation criteria, WHO criteria, and RECIST criteria, the outcomes reported by these two criteria were considered homogeneous (Aras et al., 2016).
2.2 Search strategy
We searched PubMed, EMBASE, CENTRAL, Web of Science, the Chinese Biomedical Literature Database (CBM), the China National Knowledge Infrastructure (CNKI), the Wanfang database, and the Chinese Scientific Journals Database (VIP database). Searches were performed from the databases initiation to April 2022. The language restriction was English and Chinese. The search strategy was based on the combination of controlled vocabulary (MeSH terms and Emtree terms) and free-text terms. The terms of “Stomach Neoplasms,” “Oxaliplatin,” “Antineoplastic Combined Chemotherapy Protocols,” “Herbal Medicine,” “Medicine, Chinese Traditional,” and “Drugs, Chinese Herbal” were used to develop the search strategy for PubMed, which is shown in Supplementary Material S2. Modifications to the search strategy were used with other databases.
2.3 Screening and selection
Search results were imported to EndNote 20. The titles and abstracts of retrievals were screened after duplicates removal, then full articles of potential trials were assessed for their eligibility. Screening and selection were independently and in duplicate performed by the review authors (YT and HW). RCTs that met the inclusion criteria were included. The process was summarized using a PRISMA flow diagram.
2.4 Data extraction
The following data were extracted from the included studies: 1) identification information (first author, year of publication); 2) general information (study setting, sample size, and duration of follow-up); 3) participants (clinical stage, age, and sex); 4) intervention details (name of CHM intervention, compositions, and duration); 5) comparison details (chemotherapy regimen, dose, frequency, and duration of treatment), and 6) outcomes details.
2.5 Quality assessment
The Risk of Bias 2 (RoB-2) tool was used to assess the methodological quality of included studies (Sterne et al., 2019). We evaluated included studies of quality of the randomization process, deviation from intended intervention, missing outcome data, outcome measurement, and selection of the reported result. The overall quality of RCTs were evaluated as low, some concerns or high RoB.
2.6 Evidence synthesis for RCTs
Stata 16 was used in data synthesis to perform a meta-analysis. The RR for dichotomous data with 95% CIs were evaluated. The random-effects model was used when synthesizing data for the meta-analysis. As for the outcomes reported with zero event, the Mantel-Haenszel methods were adopted. We quantified inconsistency by applying the I2 statistic; a value of I2 > 40% was considered important heterogeneity, and I2 > 75% was considerable heterogeneity (Higgins et al., 2019). Subgroup analysis were performed according to the different regimens of chemotherapy that patients received, and to explore the source of heterogeneity if substantial heterogeneity existed. Publication bias of the cumulative evidence among individual studies was evaluated using a graphical method of funnel plot (Egger et al., 1997).
2.7 Sensitivity analysis
We performed sensitivity analysis to investigate the potential contributions of specific herbs to tumor response. Previous studies proposed that if a particular herb possessed anti-tumor effects, they would be reflected in the pooled effect estimates of the studies which interventions containing this herb (Chen et al., 2016a; Chen et al., 2016b). Sensitivity analysis of ORR will be performed for studies on herbs used in AGC, herbs, or combinations of herbs presented in two or more studies, and the following principles will be applied:
1) Studies containing the same herb or combination of herbs will be treated as one, and the pooled RR (95% CI) and I2 will be calculated; 2) herbs or combinations of herbs will be excluded if there is no significant effect in the pooled results (95% CIs of RR overlap 1.0) and/or important heterogeneity exists between studies (I2 ≥ 40%); 3) the RR results will be listed in ascending order with 95% CI, the number of studies and I2 values; 4) the combination of herbs will be excluded when they have lower RRs than herbs alone; and 5) when herb combinations have higher RRs than herbs alone, they will be identified as potential examples of synergistic effects.
2.8 Quality of evidence
The quality of the cumulative evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. Study limitations, inconsistency, indirectness, imprecision, and publication bias were evaluated. Quality of evidence was classified as high, moderate, low, or very low quality (Guyatt et al., 2008). We presented our findings in a Summary of Finding table.
3 Results
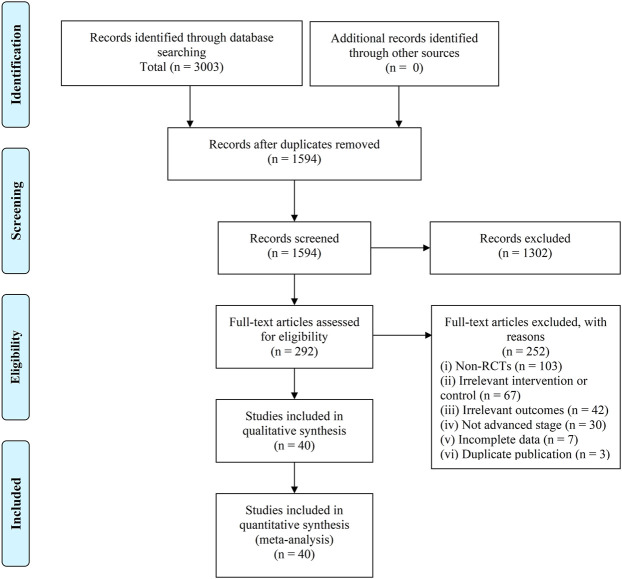
There were 3,003 retrievals exported from databases searches, and after the selection process, 40 trials involving 3,029 participants were included in this SR (Chen et al., 2007; Chi et al., 2010; Hu, 2011; Yuan, 2011; Zhao, 2011; Zhu et al., 2011; Qin, 2012; Guo, 2014; Huang, 2014; Liu et al., 2015; Li et al., 2016; Zhao et al., 2016; Chu et al., 2017; Feng, 2017; Huang et al., 2017; Wang N. et al., 2018; Yang et al., 2018; Yuan, 2018; Cai et al., 2019; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Xie, 2019; Yu et al., 2019; Zhai, 2019; Zhang, 2019; Zhong et al., 2019; Bao, 2020; Gong, 2020; Li D. H. et al., 2020; Sun et al., 2020; Zhang et al., 2020; Zhang, 2021a; Zhang, 2021b; Feng et al., 2021; Jiang et al., 2021; Long, 2021; Zhang H. O. et al., 2021; Zhao, 2021; Zhong et al., 2021). The selection process was summarized as a flowchart shown in Figure 1.
FIGURE 1.
Flowchart of records screening and selection.
3.1 Details of included trials
All these 40 trials are non-blinded RCTs that conducted in single-center. The sample sizes of included trials ranged from 50 to 124. Among these included trials, one published in an English journal (Li Y. et al., 2020), and other 39 trials were published in Chinese (Chen et al., 2007; Chi et al., 2010; Hu, 2011; Yuan, 2011; Zhao, 2011; Zhu et al., 2011; Qin, 2012; Guo, 2014; Huang, 2014; Liu et al., 2015; Li et al., 2016; Zhao et al., 2016; Chu et al., 2017; Feng, 2017; Huang et al., 2017; Wang Y. et al., 2018; Yang et al., 2018; Yuan, 2018; Cai et al., 2019; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Xie, 2019; Yu et al., 2019; Zhai, 2019; Zhang, 2019; Zhong et al., 2019; Bao, 2020; Gong, 2020; Sun et al., 2020; Zhang et al., 2020; Zhang, 2021a; Zhang, 2021b; Feng et al., 2021; Jiang et al., 2021; Long, 2021; Zhang L. et al., 2021; Zhao, 2021; Zhong et al., 2021). The characteristics of included RCTs were shown in Table 1.
TABLE 1.
Study characteristics of included RCTs.
| Study | Study Design | Sample Size | Age | Sex (Male/Female) | Stage | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | |||
| Bao (2020) | Single center | 34 | 34 | 44–86 (66.42 ± 7.48) | 45–87 (65.73 ± 7.21) | 21/13 | 22/12 | III-IV | III-IV | ①②③ |
| Cai et al. (2019) | Single center | 60 | 60 | 42–74 (61.5 ± 7.9) | 41–73 (61.2 ± 7.6) | 35/25 | 38/22 | III-IV | III-IV | ①②③ |
| Chen et al. (2007) | Single center | 27 | 23 | 34–78 (median: 58) | 32/18 | IV | IV | ①③ | ||
| Chi et al. (2010) | Single center | 30 | 30 | 40–70 (median: 57) | 38/22 | IV | IV | ①②③ | ||
| Chu et al. (2017) | Single center | 30 | 30 | 42–68 (56.3 ± 4.2) | 40–75 (55.6 ± 4.5) | 20/10 | 22/8 | IIIb-IV | IIIb-IV | ①②③ |
| Feng et al. (2021) | Single center | 41 | 41 | 35–67 (52.29 ± 4.71) | 34–69 (53.84 ± 4.91) | 23/18 | 21/20 | III-Ⅳ | III-Ⅳ | ①③ |
| Feng (2017) | Single center | 30 | 30 | >60: 19, ≤60: 11 | >60: 21, ≤60: 9 | 18/12 | 22/8 | III-IV | III-IV | ①②③ |
| Gong (2020) | Single center | 32 | 32 | 66.08 ± 9.52 | (65.56 ± 7.65 | 19/13 | 17/15 | III-IV | III-IV | ①③ |
| Gu et al. (2019) | Single center | 35 | 35 | 27–76 (48.3 ± 5.2) | 28–77 (47.5 ± 4.8) | 19/16 | 21/14 | III-IV | III-IV | ① |
| Guo (2014) | Single center | 30 | 30 | 57.10 ± 6.65 | 56.26 ± 7.51 | 22/8 | 21/9 | IIIb-IV | IIIb-IV | ①②③ |
| Hu et al. (2012) | Single center | 51 | 48 | 35–74 (57.65 ± 9.42) | 32–75 (59.09 ± 10.62) | 35/16 | 34/14 | IV | IV | ①②③ |
| Huang et al. (2017) | Single center | 34 | 34 | 41–70 (57.9 ± 61.2) | 40–69 (58.9 ± 6.6) | 19/15 | 20/14 | III-IV | III-IV | ①②③ |
| Huang (2014) | Single center | 32 | 32 | 55.13 ± 10.676 | 53.97 ± 10.304 | 17/15 | 16/16 | IV | IV | ①②③ |
| Jiang et al. (2021) | Single center | 51 | 51 | 55.78 ± 6.85 | 55.92 ± 6.49 | 32/19 | 30/21 | III-IV | III-IV | ①③ |
| Jiao (2019) | Single center | 52 | 52 | 32–68 (54.23 ± 8.67) | 33–70 (56.32 ± 8.40) | 27/25 | 29/23 | IV | IV | ① |
| Li et al. (2016) | Single center | 34 | 34 | 46.35 ± 6.21 | 46.97 ± 6.31 | 20/14 | 22/12 | T3-T4 | T3-T4 | ①②③ |
| Li D. H. et al. (2020) | Single center | 29 | 28 | 45–73 (57.36 ± 8.87) | 44–75 (58.25 ± 9.64) | 17/12 | 16/12 | IIIb-IV | IIIb-IV | ①②③ |
| Liu et al. (2015) | Single center | 62 | 62 | 61.8 ± 11.6 | 60.5 ± 10.8 | 43/19 | 46/16 | IIIb-IV | IIIb-IV | ①②③ |
| Liu et al. (2019) | Single center | 48 | 48 | 54.37 ± 8.10 | 55.09 ± 8.05 | 26/22 | 25/23 | IV | IV | ①②③ |
| Long (2021) | Single center | 32 | 32 | 59.03 ± 8.32 | 57.81 ± 7.49 | 23/19 | 20/12 | IV | IV | ②③ |
| Qin (2012) | Single center | 27 | 26 | 59.04 ± 8.716 | 57.73 ± 10.724 | 19/8 | 20/6 | III-IV | III-IV | ①②③ |
| Sun et al. (2020) | Single center | 40 | 40 | 35–74 (63.1 ± 8.6) | 32–75 (2.7 ± 8.9) | 27/13 | 26/14 | III-IV | III-IV | ①②③ |
| Wang N. et al. (2018) | Single center | 60 | 60 | 55–78 (64.16 ± 1.17) | 61–79 (68/58 ± 1.25) | 34/26 | 31/29 | III-IV | III-IV | ①②③ |
| Xie (2019) | Single center | 33 | 31 | 62.73 ± 8.769 | 59.52 ± 9.095 | 18/15 | 15/10 | III-IV | III-IV | ①②③ |
| Yang et al. (2018) | Single center | 40 | 40 | 59.41 ± 13.59 | 59.41 ± 13.59 | 21/19 | 23/17 | III-IV | III-IV | ①②③ |
| Yu et al. (2019) | Single center | 41 | 41 | 42–78 (55.17 ± 5.86) | 43–79 (56.30 ± 6.28) | 23/18 | 25/16 | IIIb-IV | IIIb-IV | ①②③ |
| Yuan (2011) | Single center | 26 | 25 | 18–75 | 18–75 | 18/8 | 20/5 | IV | IV | ①②③ |
| Yuan (2018) | Single center | 30 | 30 | 58.93 ± 7.056 | 61.43 ± 7.142 | 23/7 | 20/10 | IIIb-IV | IIIb-IV | ①②③ |
| Zhai (2019) | Single center | 32 | 32 | 18–70 | 18–70 | 24/8 | 20/12 | IIIb | IIIb | ①②③ |
| Zhang H. O. et al. (2021) | Single center | 30 | 30 | 66.66 ± 5.912 | 67.21 ± 6.425 | 21/9 | 21/9 | IIIb-IV | IIIb-IV | ①②③ |
| Zhang H. O. et al. (2021) | Single center | 30 | 30 | 62.16 ± 9.53 | 64.30 ± 8.92 | 13/17 | 11/19 | III-IV | III-IV | ①②③ |
| Zhang L. et al. (2021) | Single center | 28 | 27 | 65.57 ± 6.06 | 66.56 ± 5.57 | 20/8 | 18/9 | IIIb-IV | IIIb-IV | ①②③ |
| Zhang et al. (2020) | Single center | 43 | 43 | 33–80 (50.41 ± 8.16) | 35–78 (49.06 ± 7.34) | 24/19 | 26/17 | III-IV | III-IV | ①③ |
| Zhang (2019) | Single center | 50 | 50 | 65.24 ± 5.27 | 68.46 ± 5.94 | 30/16 | 26/11 | III-IV | III-IV | ①②③ |
| Zhao (2011) | Single center | 30 | 24 | 57.60 ± 11.34 | 57.47 ± 12.06 | 19/11 | 11/7 | IIIb-IV | IIIb-IV | ①②③ |
| Zhao et al. (2016) | Single center | 39 | 39 | 38–70 (57.03 ± 9.47) | 41–69 (58.31 ± 10.23) | 28/11 | 26/13 | III-IV | III-IV | ①②③ |
| Zhao (2021) | Single center | 49 | 49 | 48–77 (57.21 ± 4.58) | 47–76 (56.87 ± 4.62) | 26/23 | 25/24 | IIIb-IV | IIIb-IV | ①③ |
| Zhong et al. (2021) | Single center | 41 | 41 | 36.70 (58.8 ± 9.2) | 35.70 (58.3 ± 9.5) | 24/17 | 22/19 | IIIb-IV | IIIb-IV | ①③ |
| Zhong et al. (2019) | Single center | 41 | 41 | 33–76 (54.86 ± 3.77) | 32–78 (55.04 ± 3.14) | 22/19 | 20/21 | IV | IV | ①②③ |
| Zhu et al. (2011) | Single center | 40 | 40 | 35–86 (54.5 ± 4.5) | 51/29 | III-IV | III-IV | ①② | ||
T Treatment group, C Control group.
Outcomes: ①Tumor Response, ②Quality of Life, ③Adverse Events.
3.1.1 Intervention details
Among these 40 trials, three trials adopted TCM syndrome differentiation treatment, and patients in these three trials received more than one core prescription, other 37 trials used single formula as core prescription. As for the chemotherapy, SOX was the most frequent regimen that adopted by 16 trials (Qin, 2012; Guo, 2014; Liu et al., 2015; Zhao et al., 2016; Feng, 2017; Wang N. et al., 2018; Yuan, 2018; Zhai, 2019; Zhang, 2019; Bao, 2020; Gong, 2020; Li Y. et al., 2020; Zhang et al., 2020; Zhang, 2021a; Jiang et al., 2021; Zhao, 2021), FOLFOX regimen was adopted in 14 trials (Chen et al., 2007; Chi et al., 2010; Zhao, 2011; Zhu et al., 2011; Huang, 2014; Li et al., 2016; Chu et al., 2017; Huang et al., 2017; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Zhong et al., 2019; Sun et al., 2020; Long, 2021), and XELOX regimen in 10 trials (Hu, 2011; Yuan, 2011; Yang et al., 2018; Cai et al., 2019; Xie, 2019; Yu et al., 2019; Zhang, 2021b; Feng et al., 2021; Zhang H. O. et al., 2021; Zhong et al., 2021). Intervention details of included RCTs were shown in Table 2. The Chinese phonetic transcription, scientific name, Latin drug name, and English name of herbs adopted in the prescriptions of included trials were shown in Table 3.
TABLE 2.
Intervention details of included RCTs.
| Study | Treatment in intervention group | Treatment in intervention group |
|---|---|---|
| Bao (2020) | Yiqi Huoxue Formula combined with SOX regimen chemotherapy. Formula composition: Acruginous turmeric, Chinese angelica, Finger citron, Villous amomum, Sanchi, Codonopsis, Astragalus | SOX regimen for 4 cycles: S-1 Capsules 40 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Cai et al. (2019) | Jianpi Yiqi Formula combined with XELOX regimen chemotherapy. Formula composition: Codonopsis, Poria, Largehead atractylodes, Perilla frutescens leaf, Coxi seed, Pinellia, Inula, Ruddle, Villous amomum, Liquorice | XELOX regimen for 3 cycles: Capetabine 1,000 mg/m2, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Chen et al. (2007) | TCM syndrome differentiation treatment combined with FOLFOX regimen chemotherapy | FOLFOX regimen chemotherapy: Oxaliplatin injection 130 mg/m2 intra venous drip on day 1; CF 200 mg intra venous drip on day 1–3; 5-Fu 400 mg/m2 on day 1, and a infusion (2,000 mg/m2) for 70 consecutive hours, one cycle for 21 days |
| Chi et al. (2010) | Yiqi Huoxue Formula combined with FOLFOX regimen chemotherapy. Formula composition: Astragalus, Pseudostellaria, Suberect spatholobus, Largehead atractylodes, Poria, Wolfberry, Ligustrum, Cuscuta, Red paeony, Coxi seed, Actinidia root | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1–2; 5-Fu 400 mg/m2 on day 1–2, and a 20-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Chu et al. (2017) | Zhangshi Yiwei Decoction combined with FOLFOX regimen chemotherapy. Formula composition: Largehead atractylodes, Poria, Codonopsis, Coptis, Tangerine peel, Thunberbg fritillary, Aucklandia, Pinellia, Villous amomum, Dandelion, Silktree bark, Bletilla, Liquorice | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 130 mg/m2 intra venous drip on day 1; CF 100 mg/m2 intra venous drip on day 1–5; 5-Fu 300 mg/m2 on day 1–5, one cycle for 21 days |
| Feng et al. (2021) | Xuezheng Decoction combined with XELOX regimen chemotherapy. Formula composition: Acruginous turmeric, Hedyotis diffusa, Smilax, Chinese angelica, Red paeony, Sparganii, Cassia twig, Frankincense, Myrrh | XELOX regimen for 2 cycles: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 85 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Feng (2017) | Gancao Xiexin Decoction combined with SOX regimen chemotherapy. Formula composition: Liquorice, Scutellaria, Coptis, Pinellia, Dried ginger, Ginseng, Red date | SOX regimen: S-1 Capsules 40–60 mg, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Gong (2020) | Wenyang Sanjie Decoction combined with SOX regimen chemotherapy. Formula composition: Astragalus, Yam, Chinese clematis, Coxi seed, Scutellaria barbata, White paeony, Ruddle, Largehead atractylodes, Psoralea, Cuscuta, Barley sprout, Dandelion, Wolfberry, Acruginous turmeric, Membrane of chickens gizzard, Inula, Pinellia, Gekko, Cassia twig, Tangerine peel, Dried ginger | SOX regimen: S-1 Capsules 40–60 mg, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Gu et al. (2019) | Jianwei Yiai Powder combined with FOLFOX regimen chemotherapy. Formula composition: Nightshade, Vietnamese sophora root, Scutellaria barbata, Hedyotis diffusa, Scolopendra, Pinellia, Arcae concha, Aucklandia, Poria, Suberect spatholobus, Codonopsis, Largehead atractylodes, Liquorice | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1–2; 5-Fu 400 mg/m2 on day 1, and a 22-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Guo (2014) | Wenyang Sanjie Decoction combined with SOX regimen chemotherapy. Formula composition: Codonopsis, Astragalus, Largehead atractylodes, Poria, Ligustrum, Pinellia, Hedyotis diffusa, Cremastra, Tangerine peel, Actinidia root, Membrane of chickens gizzard, Liquorice | SOX regimen: S-1 Capsules 40 mg, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Hu (2011) | Yiqi Huoxue Jiedu Formula combined with XELOX regimen chemotherapy. Formula composition: Astragalus, Codonopsis, Pseudostellaria, Largehead atractylodes, Poria, Wolfberry, Ligustrum Cuscuta, Suberect spatholobus, Red paeony, Acruginous turmeric, Paris polyphylla, Hedyotis diffusa, Actinidia root | XELOX regimen for 3 cycles: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 85 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Huang (2017) | Jianpi Yangwei Formula combined with FOLFOX4 regimen chemotherapy. Formula composition: Hedyotis chrysotricha, Smilax, Coxi seed, Yam, Codonopsis, Largehead atractylodes, Poria, Aucklandia, Chinese angelica, White paeony, Liquorice | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1–2; 5-Fu 400 mg/m2 on day 1, and a 22-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Huang (2014) | Jianpi Huayu Decoction combined with FOLFOX4 regimen chemotherapy. Formula composition: Codonopsis, Largehead atractylodes, Dandelion, Perilla frutescens stem, Nardostachys, Snakegourd seed, Pinellia, Gekko, Acruginous turmeric, Crataegi, Rhei, Magnolia bark, Membrane of chickens gizzard | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1–2; 5-Fu 400 mg/m2 on day 1, and a 22-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Jiang et al. (2021) | Jiedu Sanjie Formula combined with SOX regimen chemotherapy. Formula composition: Dandelion, Prunella, Honeysuckle, Forsythia, Chinese angelica, Figwort, Isatis, Stiff silkworm, Myrrh, Scorpion, Gleditsia sinensis | SOX regimen: S-1 Capsules 40–60 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Jiao (2019) | Modified Xuezheng Decoction combined with FOLFOX4 regimen chemotherapy. Formula composition: Sparganii, Acruginous turmeric, Hedyotis diffusa, Smilax, Chinese angelica, Red paeony, Frankincense, Myrrh, Cassia twig | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1–2; 5-Fu 400 mg/m2 on day 1, and a 22-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Li (2016) | Fuzheng Kangai Formula combined with FOLFOX4 regimen chemotherapy. Formula composition: Largehead atractylodes, Morinda, Wolfberry, Drynaria Rehmannia glutinosa, Epimedium, Cornus, Ginseng, Eucommia, Psoralea, Cassia bark, Chinese angelica, Curculigo | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1–2; 5-Fu 400 mg/m2 on day 1, and a 22-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Li Y. et al. (2020) | Shunqi Yiwei Decoction combined with SOX regimen chemotherapy. Formula composition: Bupleurum, Aurantii fructus, White paeony, Liquorice, Poria, Atractylodis, Codonopsis | SOX regimen: S-1 Capsules 40 mg, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Liu et al. (2015) | Jianpi Xiaozheng Formula combined with SOX regimen chemotherapy. Formula composition: Astragalus, Smilax, Hedyotis chrysotricha, Coxi seed, Codonopsis, Poria, Yam, Sparganii, Acruginous turmeric, Largehead atractylodes, Aucklandia, Tangerine peel, Chinese angelica, White paeony | SOX regimen: S-1 Capsules 40–60 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Liu et al. (2019) | Jianpi Huayu Formula combined with FOLFOX4 regimen chemotherapy. Formula composition: Largehead atractylodes, Dandelion, Perilla frutescens stem, Nardostachys, Snakegourd seed, Pinellia, Gekko, Acruginous turmeric, Crataegi, Rhei, Magnolia bark, Membrane of chickens gizzard | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1–2; 5-Fu 400 mg/m2 on day 1, and a 22-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Long (2021) | Yiqi Fuyuan Formula combined with mFOLFOX6 regimen chemotherapy. Formula composition: Astragalus, Codonopsis, Largehead atractylodes, Aucklandia, Tangerine peel, Poria, Chinese angelica, Spine date seed, Yam, Raw ginger, Lotus seed, Liquorice | mFOLFOX6 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 400 mg/m2 intra venous drip on day 1; 5-Fu 400 mg/m2 on day 1, and a infusion (2,400 mg/m2) for 46 consecutive hours, one cycle for 21 days |
| Qin (2012) | Modified Buzhong Yiqi Decoction combined with SOX regimen chemotherapy. Formula composition: Astragalus, Codonopsis, Largehead atractylodes, Liquorice, Chinese angelica, Sichuan lovase, Tangerine peel, Acruginous turmeric, Hedyotis diffusa, Coxi seed | SOX regimen: S-1 Capsules 80 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Sun et al. (2020) | Fuzheng Kangai Formula combined with FOLFOX6 regimen chemotherapy. Formula composition: Codonopsis, Largehead atractylodes, Bupleurum, White paeony, Poria, Tangerine peel, Pinellia, Sichuan lovase, Aurantii fructus, Ligustrum, Ganoderma, Barley sprout, Millet sprout, Paris polyphylla, Scutellaria barbata, Hedyotis diffusa, Liquorice | FOLFOX6 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 400 mg/m2 intra venous drip on day 1; 5-Fu 400 mg/m2 on day 1, and a infusion (2,400 mg/m2) for 46 consecutive hours, one cycle for 21 days |
| Wang Y. et al. (2018) | Guishao Liujunzi Decoction combined with SOX regimen chemotherapy. Formula composition: Chinese angelica, White paeony, Codonopsis, Poria, Largehead atractylodes, Yam, Coxi seed, Tangerine peel, Pinellia, Hedyotis diffusa, Scutellaria barbata, Sparganii, Acruginous turmeric, Liquorice | SOX regimen: S-1 Capsules 50 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Xie (2019) | Wendan Decoction combined with XELOX regimen chemotherapy. Formula composition: Pinellia, Bambusae caulis, Aurantii fructus Immaturus, Tangerine peel, Poria, Liquorice, Raw ginger, Red date | XELOX regimen for 3 cycles: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 85 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Yang et al. (2018) | Shenyu Yangwei Decoction combined with XELOX regimen chemotherapy. Formula composition: Ginseng, Cornus, Dendrobium, Salviae | XELOX regimen for 2 cycles: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Yu et al. (2019) | Modified Shengyang Yiwei Decoction combined with XELOX regimen chemotherapy. Formula composition: Ginseng, Cornus, Dendrobium, Salviae | XELOX regimen for 2 cycles: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Yuan (2011) | Modified Shengyang Yiwei Decoction combined with XELOX regimen chemotherapy. Formula composition: Codonopsis, Largehead atractylodes, Poria, Coxi seed, Pinellia, Tangerine peel, Agaric Yam, Millet sprout, Barley sprout, Poria with hostwood, Loquat leaf, Liquorice | XELOX regimen for 2 cycles: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 2 h on day 1, one cycle for 21 days |
| Yuan (2018) | Yiqi Jiedu Formula combined with SOX regimen chemotherapy. Formula composition: Ginseng, Gekko, Paris polyphylla, Pinellia, Tangerine peel, Magnolia bark, Liquorice | SOX regimen: S-1 Capsules 40 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Zhai (2019) | Huayu Jiedu Formula combined with SOX regimen chemotherapy. Formula composition: Scorpion, Gekko, Sanchi, Nitroum, Scutellaria barbata, Membrane of chickens gizzard | SOX regimen: S-1 Capsules 40–60 mg, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 3 h on day 1, one cycle for 21 days |
| Zhang (2021a) | Modified Shiquan Dabu Decoction combined with XELOX regimen chemotherapy. Formula composition: Astragalus, Coxi seed, Largehead atractylodes, Poria, Chinese angelica, Pinellia, White paeony, Rehmannia glutinosa, Sparganii, Acruginous turmeric, Sichuan lovase, Ginseng, Cassia bark, Villous amomum, Liquorice | XELOX regimen: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 2 h on day 1, one cycle for 21 days |
| Zhang (2021a) | TCM syndrome differentiation treatment combined with XELOX regimen chemotherapy | XELOX regimen for 3 cycles: Capetabine 1,000 mg/m2, twice daily, once after breakfast and dinner respectively, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for 2 h on day 1, one cycle for 21 days |
| Zhang (2021b) | Shugan Yangwei Decoction combined with SOX regimen chemotherapy. Formula composition: Bupleurum, White paeony, Scutellaria, Aurantii fructus Immaturus, Coptis, Dried ginger, Pinellia, Aucklandia, Rhei, Salviae, Toosendan Corydalis Codonopsis, Katsumada galangal, Liquorice | SOX regimen: S-1 Capsules 40∼60 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Zhang et al. (2020) | Shengyang Yiwei Decoction combined with SOX regimen chemotherapy. Formula composition: Astragalus, Pinellia, Ginseng, Liquorice, Angelicae tuhuo, Divaricate saposhniovia, White paeony, Notopterygium, Tangerine peel, Poria, Bupleurum, Alisma orientale, Largehead atractylodes, Coptis | SOX regimen: S-1 Capsules 60 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Zhang (2019) | Erteng Sanjie Capsule combined with SOX regimen chemotherapy. Formula composition: Pseudostellaria, Largehead atractylodes, Coxi seed, Pinellia, Tangerine peel, Villous amomum, Gekko, Sargentgloryvine Smilax, Amur grape vines, Actinidia root, Prunella, Ostreae concha, Acruginous turmeric, Areca, Liquorice | SOX regimen: S-1 Capsules 40∼60 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Zhao (2011) | Kun Shen Granule combined with FOLFOX regimen chemotherapy. Formula composition: Laminaria, Actinidia root, Agrimony, Ginseng | FOLFOX regimen chemotherapy for 2 cycles: Oxaliplatin injection 200 mg intra venous drip on day 1; CF 300 mg intra venous drip on day 1–5; 5-Fu 750 mg on day 1–5, one cycle for 21 days |
| Zhao (2016) | Jianpi Huayu Formula combined with SOX regimen chemotherapy. Formula composition: Pseudostellaria, Largehead atractylodes, Paris polyphylla, Salviae, Scutellaria barbata, Salvia chinensis, Hedyotis diffusa, Poria, Nightshade, Dendrobium, Yam | SOX regimen: S-1 Capsules 60∼80 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 65 mg/m2 intra venous drip for 3 h on day 1 and day 8, one cycle for 21 days |
| Zhao (2021) | Wenyang Jianpi Decoction combined with SOX regimen chemotherapy. Formula composition: Pseudostellaria, Hedyotis diffusa, Largehead atractylodes, Acruginous turmeric, Nightshade, Millet sprout, Barley sprout, Pinellia, Poria, Tangerine peel, Aurantii fructus, Cassia twig, Dried ginger, Liquorice | SOX regimen: S-1 Capsules 40 mg, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip on day 1, one cycle for 21 days |
| Zhong et al. (2021) | Jianpi Fuzheng Xiaoliu Formula combined with XELOX regimen chemotherapy. Formula composition: Pseudostellaria, Coxi seed, Largehead atractylodes, Hedyotis diffusa, Smilax, Scutellaria barbata, Sparganii, Acruginous turmeric, Poria, Yam, Chinese angelica, Crataegi, Liquorice, Membrane of chickens gizzard | XELOX regimen for 4 cycles: Capetabine 1,000 mg/m2, twice daily, taking it for 14 days, stopping for 7 days; Oxaliplatin injection 130 mg/m2 intra venous drip for on day 1, one cycle for 21 days |
| Zhong et al. (2019) | Modified Shenling Baizhu Decoction combined with FOLFOX4 regimen chemotherapy. Formula composition: Tangerine peel, Cimicifuga, Bupleurum, Platycodon, Liquorice, Lotus seed, Villous amomum, Chinese angelica, Largehead atractylodes, Dolichos, Poria, Yam, Codonopsis, Astragalus, Coxi seed | FOLFOX4 regimen chemotherapy: Oxaliplatin injection 85 mg/m2 intra venous drip on day 1; CF 200 mg/m2 intra venous drip on day 1; 5-Fu 400 mg/m2 on day 1, and a 22-h infusion (600 mg/m2/d) for 2 consecutive days, one cycle for 21 days |
| Zhu (2011) | Jianpi Yiqi Decoction combined with FOLFOX regimen chemotherapy. Formula composition: Astragalus, Crataegi, Scorch-fried medicated leaven, Barley sprout, Adenophorae, Glehnia, Solomonseal, Tangerine peel, Pinellia, Finger citron, Magnolia bark, Membrane of chickens gizzard, Villous amomum, Whitefruit amomim, Liquorice | FOLFOX regimen chemotherapy for 2 cycles: Oxaliplatin injection 135 mg/m2 intra venous drip on day 1; CF 100 mg/m2 intra venous drip on day 1–5; 5-Fu 500 mg/m2 on day 1–5, one cycle for 21 days |
TABLE 3.
The names of Herbs.
| Phonetic transcription | Scientific name | Latin drug name | English name |
|---|---|---|---|
| Bajitian | Morinda officinalis How | Morindae Officinalis Radix | Morinda |
| Baqia | Smilax china L. | Smilacis Chinae Rhizoma | Smilax |
| Baibiandou | Dolichos lablab L. | Lablab Semen Album | Dolichos |
| Baidoukou | Amomum kravanh Pierre ex Gagnep. | Amomi Fructus Rotundus | Whitefruit Amomim |
| Baihuasheshecao | Hedyotis diffusa Willd. | Hedyotis Diffusa Herba | Hedyotis diffusa |
| Baiji | Bletilla striata (Thunb.) Reichb.f. | Bletillae Rhizoma | Bletilla |
| Baishao | Paeonia lactiflora Pall. | Paeoniae Radix Alba | White Paeony |
| Baizhu | Atractylodes macrocephala Koidz. | Atractylodis Macrocephalae Rhizoma | Largehead Atractylodes |
| Banlangen | Isatis indigotica Fort. | Isatidis Radix | Isatis |
| Banxia | Pinellia ternata (Thunb.) Makino. | Pinelliae Rhizoma | Pinellia |
| Banzhilian | Scutellaria barbata D.Don | Scutellariae Barbatae Herba | Scutellaria barbata |
| Beishanshen | Glehnia littoralis Fr. Schmidtex Miq. | Glehniae Radix | Glehnia |
| Bihu | Gekko swinhonis Guenther | Gekko Swinhonis | Gekko |
| Binlang | Areca catechu L. | Arecae Semen | Areca |
| Buguzhi | Psoralea corylifolia L. | Psoraleae Fructus | Psoralea |
| Caodoukou | Alpinia katsumadai Hayata | Alpiniae Katsumadai Semen | Katsumada Galangal |
| Chaihu | Bupleurum chinense DC. | Bupleuri Radix | Bupleurum |
| Chenpi | Citrus reticulata Blanco | Citri Reticulatae Pericarpium | Tangerine Peel |
| Chishao | Paeonia lactiflora Pall. | Paeoniae Radix Rubra | Red paeony |
| Chuanlianzi | Melia toosendan Sieb.et Zucc. | Toosendan Fructus | Toosendan |
| Chuanxiong | Ligusticum chuanxiong Hort. | Chuanxiong Rhizoma | Sichuan lovase |
| Dahuang | Rheum officinale Baill. | Rhei Radix et Rhizoma | Rhei |
| Daxueteng | Sargentodoxa cuneata (Oliv.) Rehd. et Wils. | Sargentodoxae Caulis | Sargentgloryvine |
| Dazao | Ziziphus jujuba Mill. | Jujubae Fructus | Red date |
| Dansheng | Salvia miltiorrhiza Bunge | Salviae Miltiorrhizae Radix et Rhizoma | Salviae |
| Danggui | Angelica sinensis (Oliv.) Diels | Angelicae Sinensis Radix | Chinese Angelica |
| Dangshen | Codonopsis pilosula (Franch.) Nannf. | Codonopsis Radix | Codonopsis |
| Duhuo | Angelica pubescens Maxim.f. biserrata Shan et Yuan | Angelicae Pubescentis Radix | Angelicae tuhuo |
| Duzhong | Eucommia ulmoides Oliv. | Eucommiae Cortex | Eucommia |
| Ezhu | Curcuma phaeocaulis Val. | Curcumae Rhizoma | Acruginous Turmeric |
| Fangfeng | Saposhnikovia divaricata (Turcz.) Schischk. | Saposhnikoviae Radix | Divaricate Saposhniovia |
| Fangji | Stephania tetrandra S.Moore | Stephaniae Tetrandrae Radix | Fourstamen Stephania |
| Foshou | Citrus medica L. var. sarco- dactylis Swingle | Citri Sarcodactylis Fructus | Finger Citron |
| Fuling | Poria cocos (Schw.) Wolf | Poria | Poria |
| Fushen | Poria cocos (Schw.) Wolf | Poria Cum Radix Pini | Poria with hostwood |
| Gancao | Glycyrrhiza uralensis Fisch. | Glycyrrhizae Radix et Rhizoma Praeparata Cum Melle | Liquorice |
| Gansong | Nardostachys jatamansi DC. | Nardostachyos Radix et Rhizoma | Nardostachys |
| Ganjiang | Zingiber officinale Roscoe | Zingiberis Rhizoma | Dried Ginger |
| Gouqi | Lycium barbarum L. | Lycii Fructus | Wolfberry |
| Guya | Setaria italica (L.) Beauv. | Setariae Fructus Germinatus | Millet sprout |
| Gusuibu | Drynaria fortunei (Kunze) J.Sm. | Drynariae Rhizoma | Drynaria |
| Gualou | Trichosanthes kirilowii Maxim. | Trichosanthis Semen | Snakegourd seed |
| Guizhi | Neolitsea cassia (L.) Kosterm. | Cinnamomi Ramulus | Cassia twig |
| Hehuanpi | Albizia julibrissin Durazz. | Albiziae Cortex | Silktree bark |
| Houpu | Magnolia officinalis Rehder & E.H.Wilson | Magnoliae Officinalis Cortex | Magnolia bark |
| Huangjing | Polygonatum kingianum Coll.et Hemsl. | Polygonati Rhizoma | Solomonseal |
| Huanglian | Coptis chinensis Franch. | Coptidis Rhizoma | Coptis |
| Huangqi | Astragalus mongholicus Bunge | Astragali Radix | Astragalus |
| Huangqin | Scutellaria baicalensis Georgi | Scutellariae Radix | Scutellaria |
| Jineijin | — | Galli Gigerii Endothelium Corneum | Membrane of Chickens Gizzard |
| Jixueteng | Spatholobus suberectus Dunn | Spatholobi Caulis | Suberect Spatholobus |
| Jiangcan | — | Bombyx Batryticatus | Stiff Silkworm |
| Jiaoshenqu | — | — | Scorch-fried medicated leaven |
| Jinyinhua | Lonicera japonica Thunb. | Lonicerae Japonicae Flos | Honeysuckle |
| Jiegeng | Platycodon grandiflorus (Jacq.) A.DC. | Platycodonis Radix | Platycodon |
| Kushen | Sophora flavescens Ait. | Sophorae Flavescentis Radix | Lightyellow Sophora |
| Kuxingren | Prunus armeniaca L. | Armeniacae Semen Amarum | Bitter Apricot Seed |
| Lianqiao | Forsythia suspensa (Thunb.) Vahl | Forsythiae Fructus | Forsythia |
| Lianzi | Nelumbo nucifera Gaertn. | Nelumbinis Semen | Lotus seed |
| Lingzhi | Ganoderma lucidum (Leyss.ex Fr.) Karst. | Ganoderma | Ganoderma |
| Longkui | Solanum nigrum L. | Solani Nigri Herba | Nightshade |
| Maiya | Hordeum vulgare L. | Hordei Fructus Germinatus | Barley Sprout |
| Mangxiao | — | Natrii Sulfas | Mirabilite |
| Moyao | Commiphora myrrha Engl. | Myrrha | Myrrh |
| Muli | Ostrea gigas Thunberg | Ostreae Concha | Ostreae Concha |
| Muxiang | Aucklandia lappa Decne. | Aucklandiae Radix | Aucklandia |
| Nanshashen | Adenophora stricta Miq. | Adenophorae Radix | Adenophorae |
| Nvzhenzi | Ligustrum lucidum Ait. | Ligustri Lucidi Fructus | Ligustrum |
| Pipaye | Eriobotrya japonica (Thunb.) Lindl. | Eriobotryae Folium | Loquat Leaf |
| Pugongying | Taraxacum mongolicum Hand. -Mazz. | Taraxaci Herba | Dandelion |
| Qianghuo | Notopterygium incisum Ting ex H. T. Chang | Notopterygii Rhizoma et Radix | Notopterygium |
| Quanxie | Buthus martensii Karsch | Scorpio | Scorpion |
| Renshen | Panax ginseng C. A. Mey. | Ginseng Radix et Rhizoma | Ginseng |
| Rougui | Cinnamomum cassia Presl | Cinnamomi Cortex | Cassia Bark |
| Ruxiang | Boswellia carterii Birdw. | Olibanum | Frankincense |
| Sanleng | Sparganium stoloniferum Buch.-Ham. | Sparganii Rhizoma | Sparganii |
| Sanqi | Panax notoginseng (Burk.) F. H. Chen | Notoginseng Radix et Rhizoma | Sanchi |
| Sharen | Amomum villosum Lour. | Amomi Fructus | villous amomum |
| Shangcigu | Cremastra appendiculata (D.Don) Makino | Cremastrae Pseudobulbus Pleiones Pseudobulbus | Cremastra |
| Shandougen | Sophora tonkinensis Gagnep. | Sophorae Tonkinensis Radix et Rhizoma | Vietnamese Sophora Root |
| Shanyao | Dioscorea opposita Thunb. | Dioscoreae Rhizoma | Common Yam |
| Shanzha | Crataegus pinnatifida Bge. | Crataegi Fructus | Crataegi |
| Shanzhuyu | Cornus officinalis Sieb. et Zucc. | Corni Fructus | Cornus |
| Shengma | Cimicifuga heracleifolia Kom. | Cimicifugae Rhizoma | Cimicifuga |
| Shengjiang | Zingiber officinale Roscoe | Zingiberis Rhizoma Recens | Raw Ginger |
| Shidachuan | Hedyotis chrysotricha (Palib.) Merr. | Hedyotis Chrysotrichae Herba | Hedyotis Chrysotricha |
| Shihu | Dendrobium nobile Lindl. | Dendrobii Caulis | Dendrobium |
| Shudihuang | Rehmannia glutinosa Libosch. | Rehmanniae Radix Praeparata | Rehmannia Glutinosa |
| Suanzaoren | Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou | Ziziphi Spinosae Semen | Spine Date Seed |
| Taizishen | Pseudostellaria heterophylla (Miq.) Pax | Pseudostellariae Radix | Pseudostellaria |
| Taoren | Prunus persica (L.) Batsch | Persicae Semen | Peach kernel |
| Tengligen | Actinidia chinensis Planch. var. hispida C.F.Liang | Actinidiae Chinensis Radix | Actinidia root |
| Tusizi | Cuscuta chinensis Lam. | Cuscutae Semen | Cuscuta |
| Walengzi | Arca subcrenata Lischke | Arcae Concha | Arcae concha |
| Weilingxian | Clematis chinensis Osbeck | Clematidis Radix et Rhizoma | Chinese Clematis |
| Wumei | Prunus mume (Siebold) Siebold et Zucc. | Mume Fructus | Dark plum |
| Wugong | Scolopendra subspinipes mutilans L. Koch | Scolopendra | Scolopendra |
| Xiakucao | Prunella vulgaris L. | Prunellae Spica | Prunella |
| Xianmao | Curculigo orchioides Gaertn. | Curculiginis Rhizoma | Curculigo |
| Xuanshen | Scrophularia ningpoensis Hemsl. | Scrophulariae Radix | Figwort |
| Xuanfuhua | Inula japonica Thunb. | Inulae Flos | Inula |
| Yeputaoteng | Vitis amurensis Rupr. | Vitis Amurensis Caulis | Amur grape vines |
| Yiyiren | Coix lacryma-jobi L. | Coicis Semen | Coxi seed |
| Yinyanghuo | Epimedium brevicornu Maxim. | Epimedii Folium | Epimedium |
| Yanhusuo | Corydalis yanhusuo W.T.Wang | Corydalis Rhizoma | Corydalis |
| Zaojiaoci | Gleditsia sinensis Lam. | Gleditsiae Spina | Gleditsia sinensis |
| Zexie | Alisma plantago-aquatica subsp. orientale (Sam.) Sam. | Alismatis Rhizoma | Alisma orientale |
| Zhebeimu | Fritillaria thunbergii Miq. | Fritillariae Thunbergii Bulbus | Thunberbg fritillary |
| Zhiqiao | Citrus × aurantium L. | Aurantii Fructus | Aurantii Fructus |
| Zhishi | Citrus × aurantium L. | Fructus Aurantii Immaturus | Aurantii Fructus Immaturus |
| Chonglou | Paris polyphylla Smith var.yunnanensis(Franch.)Hand.-Mazz. | Paridis Rhizoma | Paris Polyphylla |
| Zhuling | Polyporus umbellatus (Pers.) Fries | Polyporus | Agaric |
| Zhuru | Bambusa tuldoides Munro | Bambusae Caulis in Taenias | Bambusae Caulis |
| Zisugeng | Perilla frutescens (L.) Britt. | Perillae Caulis | Perilla frutescens stem |
3.1.2 Risk of bias of included trials
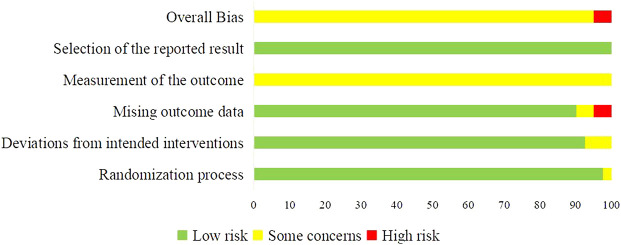
RoB 2 was used to assess the quality of 40 included trials. Two trials were assessed as “High” risk of bias (Zhao, 2011; Zhang, 2019), and 38 trials were assessed as “Some concerns” (Chen et al., 2007; Chi et al., 2010; Hu, 2011; Yuan, 2011; Zhu et al., 2011; Qin, 2012; Guo, 2014; Huang, 2014; Liu et al., 2015; Li et al., 2016; Zhao et al., 2016; Chu et al., 2017; Feng, 2017; Huang et al., 2017; Wang Y. et al., 2018; Yang et al., 2018; Yuan, 2018; Cai et al., 2019; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Xie, 2019; Yu et al., 2019; Zhai, 2019; Zhong et al., 2019; Bao, 2020; Gong, 2020; Li D. H. et al., 2020; Sun et al., 2020; Zhang et al., 2020; Zhang, 2021a; Zhang, 2021b; Feng et al., 2021; Jiang et al., 2021; Long, 2021; Zhang L. et al., 2021; Zhao, 2021; Zhong et al., 2021). Most concerns were caused by the measurement of the outcomes, since the assessment of outcomes could be influenced by knowledge of interventions patients received. The imbalanced missing data in two trials lead to the “High” risk in domain of missing outcome data, and in overall bias. The summary of RoB was shown in Figure 2.
FIGURE 2.
Summary of risk of bias of included trials.
3.2 The tumor response of CHM combined with oxaliplatin-based chemotherapy in AGC
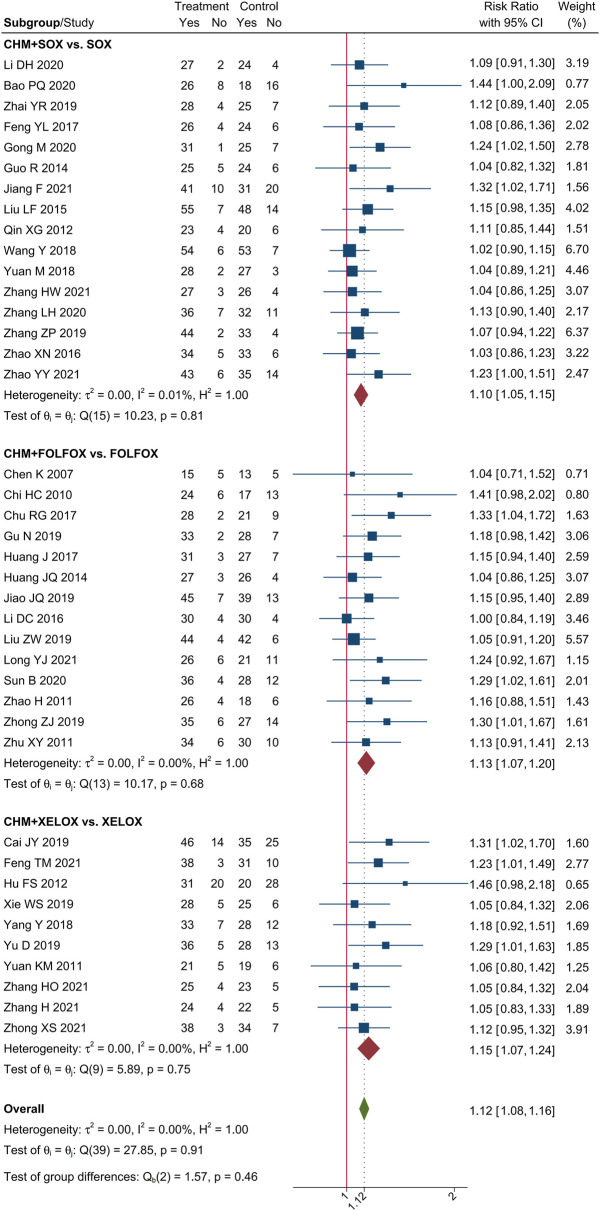
Meta-analysis of 40 trials showed that CHM combined with oxaliplatin-based chemotherapy could increase the ORR by 35% [RR = 1.35, 95% CI (1.25, 1.45)]. The value of I2 = 0 indicates that there was no statistical heterogeneity among these trials. The forest plot of ORR was shown in Figure 3. Meta-analysis of 40 trials showed that CHM combined with oxaliplatin-based chemotherapy could increase the DCR by 12% [RR = 1.12, 95% CI (1.08, 1.16)]. I2 = 0 indicates that there was no statistical heterogeneity among these trials. The forest plot of DCR was shown in Figure 4.
FIGURE 3.
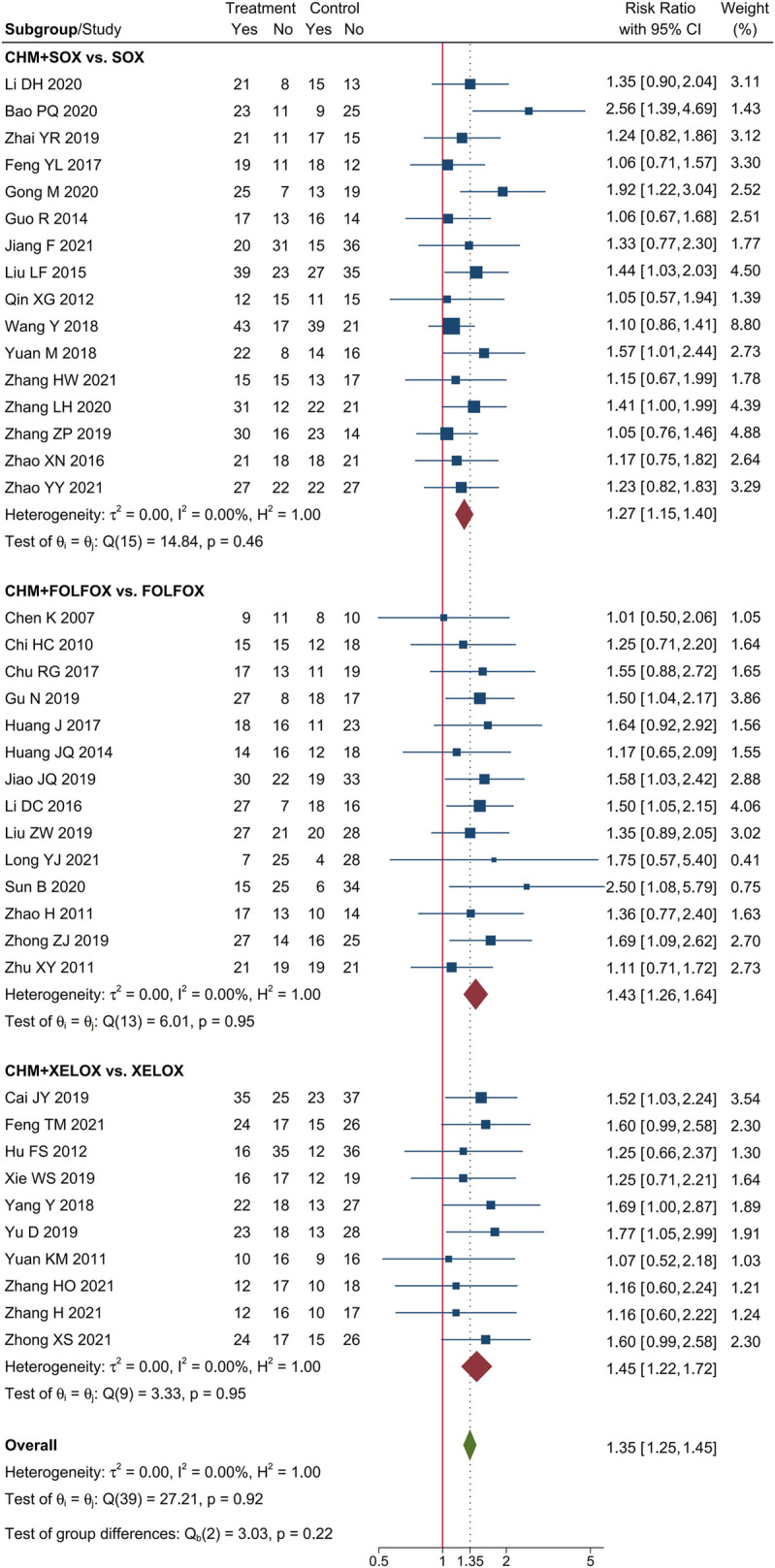
Forest plot of ORR.
FIGURE 4.
Forest plot of DCR.
3.3 The safety of CHM combined with oxaliplatin-based chemotherapy in AGC
Safety outcomes were reported in 37 trials. We evaluated the incidence of AEs of blood system, gastrointestinal reaction, hepatorenal toxicity, and peripheral neurotoxicity.
3.3.1 AEs of blood system
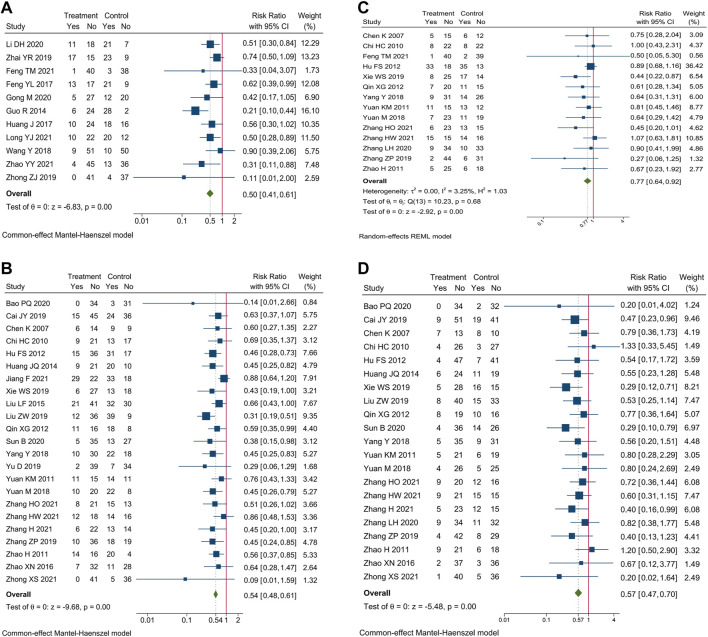
Incidence of AEs of blood system were reported in 35 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of myelosuppression by 50% [RR = 0.50, 95% CI (0.41, 0.61)], leucopenia by 46% [RR = 0.54, 95% CI (0.48, 0.61)], anemia by 23% [RR = 0.77, 95% CI (0.64, 0.92)], and thrombocytopenia by 43% [RR = 0.57, 95% CI (0.47, 0.70)]. Forest plots of incidence of AEs of blood system were shown in Figure 5.
FIGURE 5.
Forest plot of incidence of AEs of blood system. Incidence of (A) myelosuppression, (B) leucopenia, (C) anemia, and (D) thrombocytopenia.
3.3.2 Gastrointestinal reaction
Incidence of gastrointestinal reaction were reported in 34 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of gastrointestinal reaction by 45% [RR = 0.55, 95% CI (0.47, 0.64)], nausea and vomiting by 39% [RR = 0.61, 95% CI (0.51, 0.73)], and diarrhea by 46% [RR = 0.54, 95% CI (0.42, 0.69)]. Forest plots of incidence of gastrointestinal reaction were shown in Figure 6.
FIGURE 6.
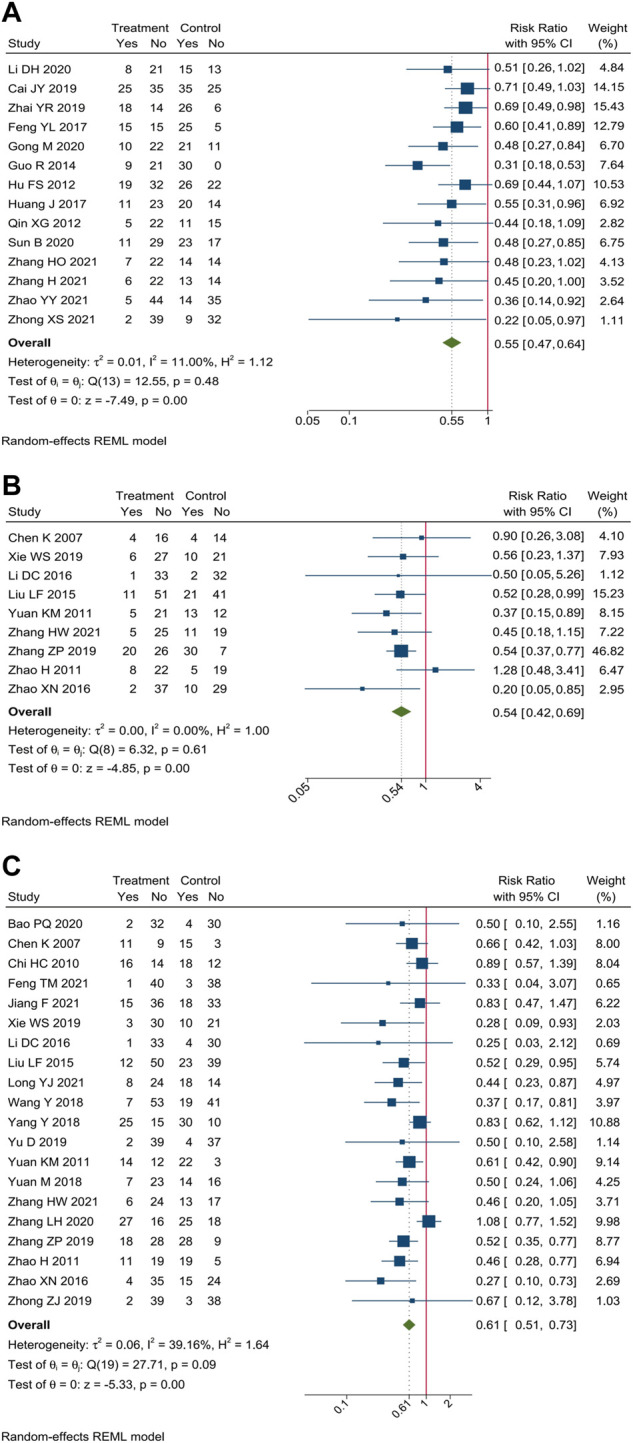
Forest plot of incidence of gastrointestinal reaction. Incidence of (A) gastrointestinal reaction, (B) diarrhea, and (C) nausea and vomiting.
3.3.3 Hepatorenal toxicity
Incidence of hepatorenal toxicity were reported in 29 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of hepatorenal toxicity by 29% [RR = 0.71, 95% CI (0.56, 0.89)], hepatotoxicity by 35% [RR = 0.65, 95% CI (0.52, 0.81)], and renal toxicity by 45% [RR = 0.55, 95% CI (0.40, 0.77)]. Forest plots of incidence of hepatorenal toxicity were shown in Figure 7.
FIGURE 7.
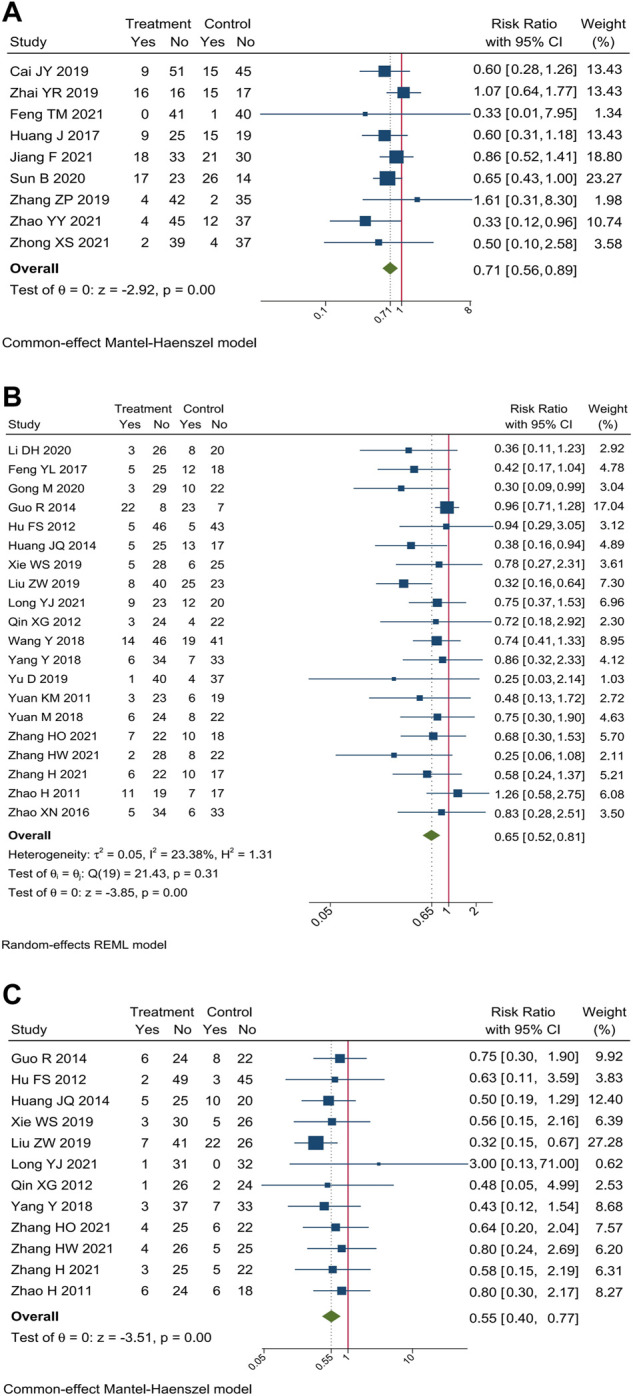
Forest plot of incidence of hepatorenal toxicity. Incidence of (A) hepatorenal toxicity, (B) hepatotoxicity, and (C) renal toxicity.
3.3.4 Peripheral neurotoxicity
Incidences of peripheral neurotoxicity were reported in 27 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of peripheral neurotoxicity by 30% [RR = 0.70, 95% CI (0.61, 0.80)]. Forest plots of incidence of hepatorenal toxicity were shown in Figure 8.
FIGURE 8.
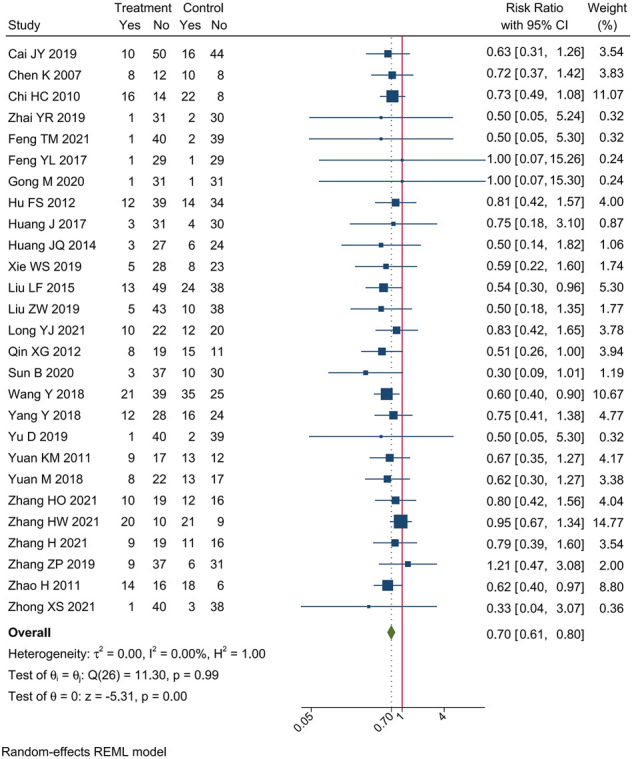
Forest plot of incidence of peripheral neurotoxicity.
3.4 Subgroup analysis
We performed subgroup analysis of the outcomes of tumor-response, according to the different regimen of chemotherapy that patients received. Compare to SOX regimen alone, CHM combined with SOX regimen chemotherapy could increase the ORR by 27% [RR = 1.27, 95% CI (1.15, 1.40)], and DCR by 10% [RR = 1.10, 95% CI (1.05, 1.15)]. Compare to FOLFOX regimen alone, CHM combined with FOLFOX regimen chemotherapy could increase the ORR by 43% [RR = 1.43, 95% CI (1.26, 1.64)], and DCR by 14% [RR = 1.13, 95% CI (1.07, 1.20)]. Compare to XELOX regimen alone, CHM combined with XELOX regimen chemotherapy could increase the ORR by 27% [RR = 1.35, 95% CI (1.26, 1.45)], and DCR by 12% [RR = 1.12, 95% CI (1.09, 1.16)]. The details were shown in Figures 3, 4.
3.5 Sensitivity analysis
We performed sensitivity analysis to investigate the potential contributions of specific herbs to tumor response. Three trials which adopted multiple core prescriptions were excluded from sensitivity analysis, and we analyzed the composition of core prescriptions in other 37 trials that adopted single core prescription. There are 114 herbs in the formulas of included trials, 47 of these herbs were used only in one trial and were excluded from the sensitivity analysis. Among 67 herbs analyzed, 43 herbs were only used with other herbs as a combination, which means that when herb was used in several formulas, there was always an herb in the same formulas. RRs of the group of trials that included each specific herb or herb combination were calculated. Among 380 sensitivity analyses performed, 21 single herbs and 129 herb combinations were located, and 3 single herbs along with 227 herb combinations were excluded according to the predetermined principle.
3.5.1 Tier 1: Single herbs
The five most frequently used single herbs were Largehead Atractylodes (n = 26), Liquorice (n = 23), Poria (n = 21), Pinellia (n = 20), and Codonopsis Pilosula (n = 18). The single herbs with the five highest RR were Dandelion [RR = 1.47, 95% CI (1.17, 1.83), n = 5], Paris Polyphylla [RR = 1.47, 95% CI (1.07, 2.02), n = 3], Red Paeony [RR = 1.46, 95% CI (1.13, 1.88) n = 4], Chinese Angelica [RR = 1.44, 95% CI (1.27, 1.64), n = 14], and Astragalus [RR = 1.43, 95% CI (1.27, 1.64), n = 13]. Other details of the contributions of single herbs were shown in Table 4.
TABLE 4.
Sensitivity analysis of specific contributions of herbs.
| Tier | Composition | No. of RCTs | Effect estimates (EE) | 95% CI of EE | I2 statistics (%) |
|---|---|---|---|---|---|
| 1 | Dandelion | 5 | 1.47 | 1.17, 1.83 | 0.00 |
| 1 | Paris Polyphylla | 3 | 1.47 | 1.07, 2.02 | 10.95 |
| 1 | Red paeony | 4 | 1.46 | 1.13, 1.88 | 0.00 |
| 1 | Chinese Angelica | 14 | 1.44 | 1.27, 1.64 | 9.33 |
| 1 | Astragalus | 13 | 1.43 | 1.25, 1.64 | 0.00 |
| 1 | Scutellaria Barbata | 8 | 1.41 | 1.18, 1.69 | 28.11 |
| 1 | Villous Amomum | 7 | 1.40 | 1.13, 1.73 | 33.15 |
| 1 | Smilax | 6 | 1.40 | 1.17, 1.67 | 8.17 |
| 1 | White Paeony | 10 | 1.39 | 1.20, 1.61 | 14.62 |
| 1 | Ginseng | 6 | 1.39 | 1.17, 1.65 | 0.00 |
| 1 | Codonopsis | 18 | 1.38 | 1.23, 1.55 | 5.92 |
| 1 | Acruginous Turmeric | 15 | 1.36 | 1.20, 1.54 | 16.12 |
| 1 | Poria | 21 | 1.35 | 1.23,1.49 | 0.00 |
| 1 | Largehead Atractylodes | 26 | 1.34 | 1.23,1.47 | 0.00 |
| 1 | Gekko | 6 | 1.33 | 1.10, 1.60 | 14.72 |
| 1 | Tangerine Peel | 17 | 1.32 | 1.18, 1.47 | 6.29 |
| 1 | Hedyotis Diffusa | 12 | 1.32 | 1.16, 1.50 | 3.56 |
| 1 | Membrane of Chickens Gizzard | 7 | 1.32 | 1.11, 1.57 | 0.00 |
| 1 | Salviae | 4 | 1.32 | 1.04, 1.67 | 0.00 |
| 1 | Liquorice | 23 | 1.30 | 1.18, 1.42 | 0.00 |
| 1 | Pinellia | 20 | 1.28 | 1.17, 1.41 | 0.00 |
| 2 | Codonopsis + Villous Amomum | 4 | 1.70 | 1.34, 2.16 | 0.00 |
| 2 | Ginseng + Cornus | 2 | 1.56 | 1.16, 2.10 | 0.00 |
| 2 | Acruginous Turmeric + Astragalus | 7 | 1.55 | 1.28, 1.88 | 0.00 |
| 2 | Chinese Angelica + Astragalus | 7 | 1.55 | 1.28, 1.89 | 0.00 |
| 2 | Chinese Angelica + Smilax | 5 | 1.55 | 1.27, 1.88 | 0.00 |
| 2 | Acruginous Turmeric + Cassia Twig | 4 | 1.54 | 1.23, 1.91 | 0.00 |
| 2 | Scutellaria Barbata + Membrane of Chickens Gizzard | 3 | 1.53 | 1.18, 1.98 | 0.00 |
| 2 | Chinese Angelica + Myrrh | 3 | 1.52 | 1.15, 2.00 | 0.00 |
| 2 | Largehead Atractylodes + Wolfberry | 4 | 1.51 | 1.22, 1.89 | 0.00 |
| 2 | Astragalus + Villous Amomum | 4 | 1.50 | 1.11, 2.03 | 25.93 |
| 2 | Codonopsis + Astragalus | 8 | 1.47 | 1.23, 1.76 | 0.00 |
| 2 | Liquorice + Bupleurum | 5 | 1.46 | 1.19, 1.79 | 0.00 |
| 2 | Codonopsis + Chinese Angelica | 8 | 1.46 | 1.20, 1.77 | 27.05 |
| 2 | Codonopsis + Aucklandia | 6 | 1.46 | 1.20, 1.77 | 0.00 |
| 2 | Largehead Atractylodes + Scutellaria Barbata | 7 | 1.44 | 1.19, 1.74 | 27.24 |
| 2 | Largehead Atractylodes + White Paeony | 9 | 1.41 | 1.21, 1.63 | 12.42 |
| 2 | Largehead Atractylodes + Ginseng | 3 | 1.41 | 1.21, 1.78 | 0.00 |
| 2 | Largehead Atractylodes + Membrane of Chickens Gizzard | 5 | 1.40 | 1.14, 1.73 | 0.00 |
| 2 | Acruginous Turmeric + Hedyotis Diffusa | 8 | 1.40 | 1.16, 1.70 | 21.43 |
| 2 | Membrane of Chickens Gizzard + Gekko | 4 | 1.40 | 1.12, 1.76 | 0.00 |
| 2 | Largehead Atractylodes + Yam | 9 | 1.38 | 1.18, 1.62 | 13.56 |
| 2 | Liquorice + Poria | 17 | 1.36 | 1.22, 1.52 | 0.00 |
| 2 | Pinellia + Gekko | 5 | 1.35 | 1.10, 1.65 | 14.01 |
| 2 | Pinellia + Magnolia Bark | 4 | 1.30 | 1.04, 1.64 | 0.00 |
| 2 | Pinellia + Dried Ginger | 4 | 1.29 | 1.03, 1.61 | 1.47 |
| 3 | Chinese Angelica + Astragalus + Villous Amomum | 3 | 1.73 | 1.26, 2.36 | 0.00 |
| 3 | Largehead Atractylodes + Wolfberry + Psoralea | 2 | 1.65 | 1.24, 2.19 | 0.00 |
| 3 | Codonopsis + Chinese Angelica + Astragalus | 6 | 1.60 | 1.30, 1.97 | 0.00 |
| 3 | Liquorice + Codonopsis + Bupleurum | 3 | 1.57 | 1.14, 2.15 | 0.00 |
| 3 | Pinellia + White Paeony + Dried Ginger | 2 | 1.56 | 1.10, 2.21 | 0.00 |
| 3 | Codonopsis + Acruginous Turmeric + Astragalus | 5 | 1.53 | 1.22, 1.91 | 0.00 |
| 3 | Largehead Atractylodes + Astragalus + White Paeony | 5 | 1.52 | 1.26, 1.84 | 0.00 |
| 3 | Largehead Atractylodes + Poria + Aucklandia | 5 | 1.51 | 1.22, 1.86 | 0.00 |
| 3 | Largehead Atractylodes + Acruginous Turmeric + Membrane of Chickens Gizzard | 4 | 1.51 | 1.19, 1.91 | 0.00 |
| 3 | Acruginous Turmeric + Chinese Angelica + Astragalus | 5 | 1.51 | 1.21, 1.89 | 0.00 |
| 3 | Acruginous Turmeric + Hedyotis Diffusa + Red paeony | 3 | 1.51 | 1.14, 2.01 | 0.00 |
| 3 | Scutellaria Barbata + Membrane of Chickens Gizzard + Gekko | 2 | 1.51 | 1.11, 2.04 | 0.00 |
| 3 | Largehead Atractylodes + Pinellia + Scutellaria Barbata | 5 | 1.50 | 1.18, 1.92 | 39.55 |
| 3 | Largehead Atractylodes + Astragalus + Villous Amomum | 2 | 1.50 | 1.04, 2.16 | 0.00 |
| 3 | Largehead Atractylodes + Pinellia + Dandelion | 4 | 1.49 | 1.17, 1.91 | 0.00 |
| 3 | Largehead Atractylodes + Acruginous Turmeric + Astragalus | 6 | 1.47 | 1.20, 1.80 | 0.00 |
| 3 | Liquorice + Codonopsis + Aucklandia | 5 | 1.47 | 1.15, 1.86 | 0.00 |
| 3 | Largehead Atractylodes + Acruginous Turmeric + Scutellaria Barbata | 4 | 1.46 | 1.12, 1.89 | 39.92 |
| 3 | Largehead Atractylodes + Chinese Angelica + Astragalus | 6 | 1.46 | 1.19, 1.80 | 0.00 |
| 3 | Largehead Atractylodes + Astragalus + Coxi Seed | 6 | 1.46 | 1.21, 1.78 | 0.00 |
| 3 | Largehead Atractylodes + Pinellia + Astragalus | 5 | 1.44 | 1.17, 1.77 | 0.00 |
| 3 | Largehead Atractylodes + Tangerine Peel + White Paeony | 6 | 1.44 | 1.19, 1.74 | 27.78 |
| 3 | Acruginous Turmeric + Hedyotis Diffusa + Cassia Twig | 3 | 1.44 | 1.12, 1.84 | 0.00 |
| 3 | Largehead Atractylodes + Pinellia + White Paeony | 6 | 1.43 | 1.15, 1.77 | 29.02 |
| 3 | Codonopsis + White Paeony + Aucklandia | 3 | 1.41 | 1.09, 1.82 | 0.00 |
| 3 | Liquorice + White Paeony + Bupleurum | 4 | 1.40 | 1.11, 1.76 | 0.00 |
| 3 | Largehead Atractylodes + Poria + Suberect Spatholobus | 3 | 1.39 | 1.05, 1.83 | 0.00 |
| 3 | Pinellia + Tangerine Peel + Barley Sprout | 5 | 1.39 | 1.10, 1.75 | 4.55 |
| 3 | Pinellia + Gekko + Magnolia Bark | 3 | 1.39 | 1.06, 1.81 | 0.00 |
| 3 | Largehead Atractylodes + Tangerine Peel + Yam | 6 | 1.38 | 1.13, 1.69 | 22.74 |
| 3 | Largehead Atractylodes + Liquorice + Poria | 16 | 1.37 | 1.22, 1.53 | 0.00 |
| 3 | Largehead Atractylodes + Pinellia + Membrane of Chickens Gizzard | 4 | 1.36 | 1.08, 1.72 | 0.00 |
| 4 | Codonopsis + Chinese Angelica + Astragalus + Villous Amomum | 2 | 1.95 | 1.36, 2.78 | 0.00 |
| 4 | Largehead Atractylodes + Pinellia + Tangerine Peel + Dandelion | 2 | 1.76 | 1.24, 2.52 | 0.00 |
| 4 | Acruginous Turmeric + Chinese Angelica + Astragalus + Villous Amomum | 2 | 1.75 | 1.01, 3.03 | 34.11 |
| 4 | Liquorice + Pinellia + Tangerine Peel + Paris Polyphylla | 2 | 1.74 | 1.18, 2.56 | 0.00 |
| 4 | Largehead Atractylodes + Tangerine Peel + Astragalus + Yam | 4 | 1.63 | 1.30, 2.05 | 0.00 |
| 4 | Codonopsis + Acruginous Turmeric + Chinese Angelica + Astragalus | 4 | 1.57 | 1.24, 1.99 | 0.00 |
| 4 | Largehead Atractylodes + Liquorice + Poria + Aucklandia | 4 | 1.55 | 1.19, 2.02 | 0.00 |
| 4 | Largehead Atractylodes + Tangerine Peel + Acruginous Turmeric + Astragalus | 4 | 1.54 | 1.23, 1.93 | 0.00 |
| 4 | Largehead Atractylodes + Poria + Chinese Angelica + Astragalus | 5 | 1.53 | 1.23, 1.91 | 0.00 |
| 4 | Largehead Atractylodes + Astragalus + Wolfberry + Cuscuta | 3 | 1.53 | 1.12, 2.08 | 0.00 |
| 4 | Acruginous Turmeric + Chinese Angelica + Sparganii + Smilax | 4 | 1.53 | 1.24, 1.89 | 0.00 |
| 4 | Largehead Atractylodes + Liquorice + Poria + Villous Amomum | 4 | 1.52 | 1.19, 1.93 | 0.00 |
| 4 | Largehead Atractylodes + Liquorice + Poria + Bupleurum | 4 | 1.51 | 1.22, 1.89 | 0.00 |
| 4 | Largehead Atractylodes + Poria + Codonopsis + Aucklandia | 5 | 1.51 | 1.22, 1.86 | 0.00 |
| 4 | Largehead Atractylodes + Pinellia + Tangerine Peel + Barley Sprout | 4 | 1.50 | 1.15, 1.97 | 3.91 |
| 4 | Largehead Atractylodes + Codonopsis + Chinese Angelica + Astragalus | 5 | 1.50 | 1.21, 1.87 | 0.00 |
| 4 | Largehead Atractylodes + Liquorice + Chinese Angelica + Astragalus | 5 | 1.48 | 1.14, 1.02 | 0.00 |
| 4 | Largehead Atractylodes + Pinellia + Tangerine Peel + Astragalus | 4 | 1.47 | 1.19, 1.83 | 0.00 |
| 4 | Liquorice + Pinellia + Tangerine Peel + Ginseng | 2 | 1.47 | 1.12, 1.93 | 0.00 |
| 4 | Largehead Atractylodes + Poria + Tangerine Peel + Astragalus | 6 | 1.45 | 1.21, 1.73 | 0.00 |
| 4 | Largehead Atractylodes + Poria + Codonopsis + Astragalus | 6 | 1.44 | 1.18, 1.75 | 0.00 |
| 4 | Liquorice + Pinellia + Codonopsis + Aucklandia | 3 | 1.42 | 1.08, 1.85 | 0.00 |
| 4 | Liquorice + Pinellia + White Paeony + Bupleurum | 3 | 1.42 | 1.08, 1.88 | 0.00 |
| 4 | Largehead Atractylodes + Poria + Codonopsis + White Paeony | 5 | 1.40 | 1.13, 1.73 | 23.16 |
| 4 | Largehead Atractylodes + Acruginous Turmeric + Coxi Seed + Yam | 4 | 1.39 | 1.12, 1.72 | 30.94 |
| 4 | Largehead Atractylodes + Liquorice + Poria + Aurantii Fructus | 3 | 1.38 | 1.05, 1.81 | 0.00 |
| 4 | Largehead Atractylodes + Acruginous Turmeric + Membrane of Chickens Gizzard + Crataegi | 3 | 1.38 | 1.05, 1.82 | 0.00 |
| 5 | Largehead Atractylodes + Pinellia + Acruginous Turmeric + Astragalus + White Paeony | 3 | 1.67 | 1.23, 2.27 | 0.00 |
| 5 | Largehead Atractylodes + Acruginous Turmeric + Astragalus + Wolfberry + Cuscuta | 2 | 1.66 | 1.15, 2.41 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Chinese Angelica + Astragalus | 4 | 1.59 | 1.19, 2.13 | 0.00 |
| 5 | Acruginous Turmeric + Chinese Angelica + Hedyotis Diffusa + Sparganii + Smilax | 3 | 1.59 | 1.22, 2.08 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Codonopsis + Villous Amomum | 3 | 1.58 | 1.22, 2.05 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Tangerine Peel + Bupleurum | 3 | 1.58 | 1.22, 2.05 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Codonopsis + Aucklandia | 4 | 1.55 | 1.19, 2.02 | 0.00 |
| 5 | Largehead Atractylodes + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus | 5 | 1.50 | 1.21, 1.87 | 0.00 |
| 5 | Largehead Atractylodes + Poria + Codonopsis + Tangerine Peel + Aucklandia | 3 | 1.49 | 1.12, 1.97 | 0.00 |
| 5 | Largehead Atractylodes + Poria + Codonopsis + Acruginous Turmeric + Astragalus | 3 | 1.48 | 1.14, 1.93 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + White Paeony + Bupleurum | 3 | 1.46 | 1.13, 1.88 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Pinellia + Coptis | 2 | 1.45 | 1.08, 1.94 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Tangerine Peel + Astragalus | 5 | 1.45 | 1.17, 1.78 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Codonopsis + White Paeony | 4 | 1.44 | 1.07, 1.93 | 33.23 |
| 5 | Largehead Atractylodes + Poria + Codonopsis + Hedyotis Diffusa + Suberect Spatholobus | 2 | 1.43 | 1.04, 1.97 | 0.00 |
| 5 | Largehead Atractylodes + Liquorice + Poria + Hedyotis Diffusa + Scutellaria Barbata | 5 | 1.42 | 1.14, 1.77 | 26.53 |
| 6 | Largehead Atractylodes + Pinellia + Tangerine Peel + White Paeony + Scutellaria Barbata + Barley Sprout | 2 | 2.04 | 1.37, 3.05 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Bupleurum | 2 | 1.84 | 1.24, 2.72 | 0.00 |
| 6 | Largehead Atractylodes + Acruginous Turmeric + Coxi Seed + Yam + Scutellaria Barbata + Membrane of Chickens Gizzard | 2 | 1.76 | 1.27, 2.45 | 0.00 |
| 6 | Largehead Atractylodes + Pinellia + Coxi Seed + Inula + Haematitum | 2 | 1.68 | 1.25, 2.25 | 0.00 |
| 6 | Largehead Atractylodes + Poria + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus | 4 | 1.59 | 1.25, 2.01 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus | 4 | 1.55 | 1.16, 2.06 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Poria + Pinellia + Codonopsis + Villous Amomum | 2 | 1.53 | 1.11, 2.10 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Poria + White Paeony + Bupleurum + Aurantii Fructus | 2 | 1.52 | 1.05, 2.20 | 0.00 |
| 6 | Largehead Atractylodes + Poria + Chinese Angelica + Coxi Seed + Yam + Smilax | 3 | 1.52 | 1.18, 1.95 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Poria + Pinellia + Codonopsis + Aucklandia | 2 | 1.51 | 1.11, 2.06 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Poria + Tangerine Peel + Astragalus + Bupleurum | 2 | 1.51 | 1.15, 1.98 | 0.00 |
| 6 | Largehead Atractylodes + Poria + Codonopsis + Chinese Angelica + Yam + Aucklandia | 3 | 1.51 | 1.13, 2.00 | 0.00 |
| 6 | Largehead Atractylodes + Tangerine Peel + Acruginous Turmeric + Barley Sprout + Cassia Twig + Dried Ginger | 2 | 1.49 | 1.10, 2.03 | 3.12 |
| 6 | Largehead Atractylodes + Pinellia + Acruginous Turmeric + Membrane of Chickens Gizzard + Gekko + Dandelion | 3 | 1.48 | 1.13, 1.95 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Astragalus | 4 | 1.47 | 1.13, 1.91 | 0.00 |
| 6 | Largehead Atractylodes + Poria + Acruginous Turmeric + Chinese Angelica + Astragalus + White Paeony | 3 | 1.47 | 1.13, 1.91 | 0.00 |
| 6 | Largehead Atractylodes + Liquorice + Poria + Pinellia + Tangerine Peel + Coptis | 2 | 1.45 | 1.08, 1.94 | 0.00 |
| 7 | Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus | 3 | 1.75 | 1.25, 2.38 | 0.00 |
| 7 | Largehead Atractylodes + Liquorice + Poria + Pinellia + Tangerine Peel + White Paeony + Bupleurum | 2 | 1.53 | 1.11, 2.11 | 0.00 |
| 7 | Largehead Atractylodes + Liquorice + Poria + Chinese Angelica + Coxi Seed + Yam + Smilax | 2 | 1.61 | 1.12, 2.34 | 0.00 |
| 7 | Largehead Atractylodes + Pinellia + Tangerine Peel + Acruginous Turmeric + Astragalus + White Paeony + Scutellaria Barbata | 2 | 1.86 | 1.31, 2.62 | 0.00 |
| 8 | Largehead Atractylodes + Liquorice + Poria + Pinellia + Tangerine Peel + Astragalus + White Paeony + Alisma Orientale | 2 | 1.51 | 1.13, 2.02 | 0.00 |
| 8 | Largehead Atractylodes + Poria + Codonopsis + Tangerine Peel + Acruginous Turmeric + Chinese Angelica + Astragalus + White Paeony | 2 | 1.53 | 1.15, 2.04 | 0.00 |
| 8 | Largehead Atractylodes + Poria + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus + Yam + Aucklandia | 2 | 1.47 | 1.06, 2.04 | 0.00 |
| 8 | Largehead Atractylodes + Poria + Acruginous Turmeric + Chinese Angelica + Coxi Seed + Yam + Sparganii + Smilax | 2 | 1.50 | 1.13, 1.97 | 0.00 |
| 9 | Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus + Yam + Lotus Seed | 2 | 1.70 | 1.12, 2.56 | 0.00 |
| 9 | Acruginous Turmeric + Chinese Angelica + Hedyotis Diffusa + Sparganii + Smilax + Red paeony + Cassia Twig + Myrrh + Frankincense | 2 | 1.59 | 1.15, 2.18 | 0.00 |
| 10 | Largehead Atractylodes + Poria + Codonopsis + Chinese Angelica + Coxi Seed + White Paeony + Yam + Aucklandia + Smilax + Hedyotis Chrysotricha | 2 | 1.49 | 1.11, 2.00 | 0.00 |
3.5.2 Tier 2: Combination of 2 herbs
The three most frequently used combination of 2 herbs were: Liquorice + Poria (n = 17), Largehead Atractylodes + White Paeony (n = 9), and Largehead Atractylodes + Yam (n = 9). The combination of 2 herbs with the five highest RR were Codonopsis Pilosula + Villous Amomum [RR = 1.70, 95% CI (1.34, 2.16), n = 4], Cornus + Ginseng [RR = 1.56, 95% CI (1.16,2.10), n = 2], Acruginous Turmeric + Astragalus [RR = 1.55, 95% CI (1.28, 1.88), n = 7], Chinese Angelica + Astragalus [RR = 1.55, 95% CI (1.28, 1.89), n = 7], and Chinese Angelica + Smilax [RR = 1.55, 95% CI (1.27, 1.88), n = 5]. Other sensitivity analysis of combination of 2 herbs were shown in Table 4.
3.5.3 Tier 3: Combination of 3 herbs
The combination of 3 herbs with the five highest RR were Chinese Angelica + Astragalus + Villous Amomum [RR = 1.73, 95% CI (1.26, 2.36), n = 3], Largehead Atractylodes + Wolfberry + Psoralea [RR = 1.65, 95% CI (1.24, 2.19), n = 2], Codonopsis + Chinese Angelica + Astragalus [RR = 1.60, 95% CI (1.30, 1.97), n = 6], Liquorice + Codonopsis + Bupleurum [RR = 1.57, 95% CI (1.14, 2.15), n = 3], and Pinellia + White Paeony + Dried Ginger [RR = 1.56, 95% CI (1.10, 2.21), n = 2]. Other sensitivity analysis of combination of 3 herbs were shown in Table 4.
3.5.4 Tier 4: Combination of 4 herbs
Two combination of 4 herbs were used more than five times: Largehead Atractylodes + Poria + Tangerine Peel + Astragalus (n = 6), and Largehead Atractylodes + Poria + Codonopsis + Astragalus (n = 6). The combination of 3 herbs with the five highest RR were Codonopsis + Chinese Angelica + Astragalus + Villous Amomum [RR = 1.95, 95% CI (1.36, 2.78), n = 2], Largehead Atractylodes + Pinellia + Tangerine Peel + Dandelion [RR = 1.76, 95% CI (1.24, 2.52), n = 2], Acruginous Turmeric + Chinese Angelica + Astragalus + Villous Amomum [RR = 1.75, 95% CI (1.01, 3.03), n = 2], Liquorice + Pinellia + Tangerine Peel + Paris Polyphylla [RR = 1.74, 95% CI (1.18, 2.56), n = 2], and Largehead Atractylodes + Tangerine Peel + Astragalus + Yam [RR = 1.63, 95% CI (1.30, 2.05), n = 4]. Other sensitivity analyses were shown in Table 4.
3.5.5 Tier 5: Combination of 5 herbs
The three most frequently used combination of 5 herbs were: Largehead Atractylodes + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus (n = 5), Largehead Atractylodes + Liquorice + Poria + Tangerine Peel + Astragalus (n = 5), and Largehead Atractylodes + Liquorice + Poria + Hedyotis Diffusa + Scutellaria Barbata (n = 5). The combination of 5 herbs with the two highest RR were Largehead Atractylodes + Pinellia + Acruginous Turmeric + Astragalus + White Paeony [RR = 1.67, 95% CI (1.23, 2.27), n = 3] and Largehead Atractylodes + Acruginous Turmeric + Astragalus + Wolfberry + Cuscuta [RR = 1.66, 95% CI (1.15, 2.41), n = 2]. Other sensitivity analyses were shown in Table 4.
3.5.6 Tier 6: Combination of 6 herbs or above
The three most frequently used combination of 6 or more herbs were Largehead Atractylodes + Poria + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus (n = 4), Largehead Atractylodes + Liquorice + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus (n = 4), and Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Astragalus (n = 4). Other combination of 6 or more herbs only used 2 or 3 times in the formulas. The combination of 6 or more herbs with the three highest RR were Largehead Atractylodes + Pinellia + Tangerine Peel + White Paeony + Scutellaria Barbata + Barley Sprout [RR = 2.04, 95% CI (1.37, 3.05), n = 2], Largehead Atractylodes + Pinellia + Tangerine Peel + Acruginous Turmeric + Astragalus + White Paeony + Scutellaria Barbata [RR = 1.86, 95% CI (1.31, 2.62), n = 2], and Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Bupleurum [RR = 1.84, 95% CI (1.24, 2.72), n = 2]. Other sensitivity analyses were shown in Table 4.
3.6 Publication bias
We assessed the publication bias with a funnel plot and Egger test. The funnel plot was shown in Figure 9A p-value = 0.1197 in Egger test indicated that no serious publication bias was observed.
FIGURE 9.
Funnel plot of publication bias.
3.7 Quality of evidence
We assessed the quality of synthesized evidence of tumor response with GRADE approach. The results in subgroup analysis were adopted, since the subgroup of different chemotherapy regimen minimize the clinical heterogeneity. We got moderate to low quality of evidence, and the reasons to downgrade were that the majority of the evidence was from the trials with moderate methodological quality, and small sample size. The summary of findings was shown in Table 5.
TABLE 5.
Summary of findings.
| Outcome No. of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects (95% CI) | Certainty | ||
|---|---|---|---|---|---|
| Oxaliplatin-based chemotherapy alone (%) | CHM combined with oxaliplatin-based chemotherapy | Risk difference | |||
| ORR (CHM + SOX vs. SOX) of participants: 1,237 (16 RCTs) | RR 1.27 (1.15–1.40) | 47.6 | 60.5% (54.8–66.7) | 12.9% more (7.1 more to 19.1 more) | ⊕⊕⊕○ Moderate a |
| ORR (CHM + FOLFOX vs. FOLFOX) No. of participants: 1,064 (15 RCTs) | RR 1.45 (1.27–1.66) | 36.2 | 52.5% (45.9–60) | 16.3% more (9.8 more to 23.9 more) | ⊕⊕⊕○ Moderate a |
| ORR (CHM + XELOX vs. XELOX) No. of participants: 772 (10 RCTs) | RR 1.45 (1.22–1.72) | 34.6 | 50.1% (42.2–59.4) | 15.5% more (7.6 more to 24.9 more) | ⊕⊕○○ Low a , b |
| DCR (CHM + SOX vs. SOX) No. of participants: 1,237 (16 RCTs) | RR 1.10 (1.05–1.15) | 78.0 | 85.8% (81.9–89.7) | 7.8% more (3.9 more to 11.7 more) | ⊕⊕⊕○ Moderate a |
| DCR (CHM + FOLFOX vs. FOLFOX) No. of participants: 1,064 (15 RCTs) | RR 1.14 (1.08–1.21) | 72.9 | 83.1% (78.8–88.2) | 10.2% more (5.8 more to 15.3 more) | ⊕⊕⊕○ Moderate a |
| ORR (CHM + XELOX vs. XELOX) No. of participants: 772 (10 RCTs) | RR 1.12 (1.09–1.16) | 69.4 | 77.7% (73.7–78.4) | 8.3% more (6.1 more to 10.8 more) | ⊕⊕⊕○ Moderate a |
Most information is from studies at moderate (Some concerns) risk of bias.
Small sample size, the number of events is less than 200.
CI, confidence interval; RR, risk ratio.
Explanations.
The meaning of the bold is to highlighted the clinical value of the outcomes.
4 Discussion
4.1 Meta-analysis of trial results
Our study showed with a moderate certainty that compare with SOX, FOLFOX or XELOX regimen alone, CHM combined with SOX, FOLFOX or XELOX could significantly improve the ORR without heterogeneity, which has a practical guiding value for the clinical application of CHM synergistic chemotherapy regimen. In addition, we also found that although the relative effect of SOX regimen was lower than that of FOLFOX and XELOX, which was due to the higher anticipated absolute effects of SOX regimen alone than that of FOLFOX and XELOX, so the improvement was not particularly significant. When SOX combined with CHM, the anticipated absolute effects had increased to 60.5%, which was higher than about 50% of other regimens. The results of our study were consistent with another previously published meta-analysis of CHM combined with paclitaxel-based chemotherapy in the treatment of AGC (Li Y. et al., 2020). Compared with paclitaxel-based chemotherapy, TCM combined with paclitaxel-based chemotherapy could also significantly improve the ORR [RR = 1.39, 95% CI (1.24–1.57), I2 = 12%]. But the prescriptions of CHM included in the above studies are not consistent, which brings more uncertain factors to the comparison.
In reducing the incidence of AEs, CHM combined with oxaliplatin-based chemotherapy showed consistent efficacy in leukopenia, thrombocytopenia, nausea and vomiting with paclitaxel-based chemotherapy, but had more improvement advantages in anemia, gastrointestinal reactions, diarrhea, hepatorenal toxicity and peripheral neurotoxicity. It is worth noting that both paclitaxel-based and oxaliplatin-based chemotherapy are clinically useful chemotherapy regimens that easily lead to peripheral neurotoxicities (Yamashita et al., 2021). However, inconsistent clinical outcomes occurred between these regimens. CHM prescriptions in our study were effective in reducing the incidence of peripheral neurotoxicity, indicating that different herbal combinations may have different effects in reducing the incidence of AEs. Therefore, it is important to excavate the effects of herbs and their combinations which may affect the efficacy to guide clinical application. In addition, this study also found that the interventions of CHM was safe in AGC patients, and the participation of CHM did not increase the occurrence of AEs.
Furthermore, in order to explore the effect of CHM on the survival time of AGC, we reviewed the following studies and hope that will provide a useful reference, which is also the focus for future studies. A cohort study that 1:1 matched in Taiwan found that CHM could improve the OS rate of GC and reduce the risk of death by 45% (Hung et al., 2017). Afterward, using propensity score matching according to 1:3 also found that regardless of short-term or long-term application of TCM, compared with non-users, it could effectively reduce the risk of death by 41% or 59%, and pointed out that the most frequently used single herbal medicine was Astragalus (11.8%), and the most frequently used formula was Xiangsha Liujunzi Decoction (15.5%) [Codonopsis pilosula, Largehead Atractylodes, Poria, Liquorice, Muxiang, Amomum villosum] (Shih et al., 2021). Interestingly, these single herbs or formulas were generally consistent with the listed herbs in our study. In addition, a meta-analysis evaluating the efficacy of Astragalus-containing TCM combined with platinum-based chemotherapy for AGC suggested that compare with platinum-based chemotherapy alone, chemotherapy combined with Astragalus-containing TCM could improve ORR, DCR, 1 or 2-year survival rate, QoL and lower incidence of AEs (Cheng et al., 2021). These above studies strongly corroborated the credibility of the herbal compositions obtained in this study.
4.2 Analysis of TCM contribution
It is well-known that CHM formulas were prescriptions composed of varieties of herbs, which plays a complex and multiple effect role in clinical efficacy. Based on the characteristics of syndrome differentiation treatment, the CHM prescriptions issued by different physicians for the same disease may be different from others. Similarly, this unique treatment characteristic is significantly highlighted in this study: the specific herbs of interventions is inconsistent in clinical studies of different CHM treatments for AGC. It should be emphasized that the same herbs may be used in different studies, which provides an important basis for findings the special contribution of different herbs.
However, these herbs are not only focused on improving ORR, and perhaps some herbs pay more attention to the reduction of the occurrence of AEs to chemotherapy, or the improvement of QoL. Therefore, to eliminate the interference of other herbs, we applied sensitivity analysis to explore the effect of individual herbs on ORR in AGC one by one firstly. Then analyze the multi-herb combination containing the same herbs to further evaluate which specific herbs and their combinations are most likely to improve the ORR of chemotherapy for AGC.
Inferred to previous studies (Chen et al., 2016a; Chen et al., 2016b; Chen MH. et al., 2016), to analyze the results more cautiously, our selected herbs and compositions must have more significant differences, no heterogeneity, and equal or higher RRs within each level. Based on the above criteria, from the results of multiple sensitivity analyses, we identified seven herbs as potential effect of synergistic action with chemotherapy for AGC: Astragalus (n = 13), Liquorice (n = 23), Poria (n = 21), Largehead Atractylodes (n = 26), Chinese Angelica (n = 14), Codonopsis (n = 18), and Tangerine Peel (n = 17). Among them, Dandelion and Paris Polyphylla possessed the highest effect estimate (RR = 1.47) in individual herb analysis. In addition, this study also analyzed the combination of herbs. It is reported that the composition of Codonopsis and Liquorice, and the composition of Codonopsis, Liquorice, Largehead Atractylodes and Poria both significantly improved the ORR of TCM combined with paclitaxel-based chemotherapy, which also provided supporting evidence for our study (Li Y. et al., 2020).
Surprisingly, some relatively high-frequency herbs such as Pinellia (n = 20), Acruginous Turmeric (n = 15), or some herbs with relatively high RR such as Dandelion [1.47 (1.17, 1.83)], Paris Polyphylla [1.47 (1.07, 2.02)], and Red Paeony [1.46 (1.13, 1.88)] in the individual herb analysis were not included in the final combination. We found that compared to the ORR under multi-herb combinations, the above potential herbs may not show a consistent effect with other herbs under multi-herb combinations. Nevertheless, these herbs also have the potential to contribute to ORR, and we found that these individual herbs with relatively high RR were mostly combined with the above 7 herbs in multi-herb combinations showing higher ORR value. Therefore, these herbs can also be considered for subsequent development.
In addition, we also excluded some highly heterogeneous herbs, such as Panax notoginseng (I2 = 73.56%), although commonly used in AGC, which seems to significantly reduce ORR [1.71, (0.84, 3.48)], but only two studies were evaluated. Notably, no negative RRs emerged for any of the herbs or combinations, suggesting that these herbs did not impair the effects of chemotherapy.
4.3 Strength and limitations
To our knowledge, this is the first meta-analysis evaluating the efficacy and safety of CHM combined with oxaliplatin-based chemotherapy in AGC. Our study suggests that the synergistic treatment of CHM may be more TRR-improving on FOLFOX and XELOX regimens, and has high safety for clinical application. In addition, this study complied strict inclusion and exclusion criteria to exclude studies without a clear randomization method, which reduces the RoB in studies and improves the reliability of results. Therefore, quality of only one outcome was classified as low, and other qualities of evidence were moderate. There was also no serious publication bias.
At the same time, our study also has several limitations. Firstly, lacks multi-center, large-sample RCTs and some of the original study sample sizes are small, which causes bias in the results, which requires the publication of more high-quality clinical studies. Secondly, the included studies had an insufficient assessment of long-term survival indicators (OS, PFS), and our study showed that CHM could improve TRR in AGC, but whether it could translate into a benefit in long-term outcomes, also requires reassessment. In addition, CHMs are a part of TCM preparations, including Chinese patent medicines and TCM injections, and these preparation types should also be widely evaluated. Even if there have been studies on the anti-tumor efficacy of TCM preparations such as cinobufotalin (Sun et al., 2019) and brucea javanica oil injection (Wang et al., 2021) for AGC. A more comprehensive evaluation of CHM is needed to obtain more real, effective and safe evidence.
In addition, an obvious innovation point lies in exploring special herbs that have a synergistic action with chemotherapy on the ORR for AGC. The advantage of this methodology is based on the contribution of each herb to the contribution of ORR and is not simply based on the total frequency of herb emergence. A sensitivity analysis was chosen to identify the contribution of each herb in the study to ORR without missing it at a lower frequency. In summary, one cannot simply choose according to the overall frequency or the order of effect sizes of herbs in the dataset, but rather needs to consider the overall effect sizes at multiple levels simultaneously. For the selection process of final results, these herbs do not show improvement in ORR in only one clinical intervention study. In contrast, these herbs have shown consistent or higher effects in multiple studies and multiple combinations. Another limitation is that all possible combinations cannot be evaluated, and as the number of multi-herb combinations increases, the number of combinations with a significant difference and no heterogeneity is smaller, the number of corresponding clinical studies and their sample size is also reduced, and the interpretation of the results should be more cautious. And there is no clear clinical trial to evaluate the clinical efficacy of this formula, and the corresponding RCT should be carried out for evaluation in the future.
4.4 Review of anti-gastric cancer mechanisms of seven selected herbs
Similarly, we cannot clarify that the listed herbs are all for improving the efficacy of chemotherapy, however, seven herbs have been used for AGC treatment in clinical in China (Cao et al., 2009). Among them, four herbs constitute a typical formula in China, Sijunzi decoction, which has been shown to reduce the nuclear accumulation and DNA-binding activity of β-catenin, thereby repressing cell growth and inducing apoptosis in human GC MKN74 and MKN45 cells (Li et al., 2021). Network pharmacology and experimental validation suggested that Sijunzi decoction could inhibit tumor proliferation and angiogenesis by down-regulating the expression of VEGFA, iNOS, COX-2, and Bax/Bcl2 proteins in NCG-bearing mice with human gastric adenocarcinoma cell NUGC-4, regulate the PI3K/AKT pathway, and induce apoptosis (Ding et al., 2022). To further explore how seven herbs improve the short-term efficacy of platinum-based chemotherapy for AGC, we will review each herb to assess the mechanistic evidence of the specific anti-tumor activity. We found that the number of published mechanistic studies of these herbs was uneven, with Astragalus and Liquorice being more intensively studied.
4.4.1 Astragalus
Previous study has shown that Astragalus dose-dependently stimulates dendritic cells (DCs) to express Toll-like receptor 4 (TLR4), thereby enhancing cellular immune function, and inhibiting IκB-α protein expression and regulating NF-kB signaling pathways (Tian et al., 2014). Secondly, after co-culture with MKN45 in vitro, MTT showed that Astragalus could reduce cell viability and induce apoptosis, and significantly reduce the number of cells. In vitro experiments also confirmed that Astragalus could significantly inhibit tumor diameter and weight, with a tumor inhibition rate of 57.1%. Another study also found that the aqueous extract of Astragalus could mediate antigen-presenting cells (APCs) to stimulate CXCR5 + Tfh-like cells to highly express IL-21, enhance humoral immunity and regulate CD8 + T cell activity (Dong et al., 2021).
In addition, the pharmacologically active components of Astragalus include polysaccharides, saponins and flavonoids, of which Astragalus polysaccharide (APS) is the most widely studied (Ma et al., 2002). In one study evaluating the anti-tumor activity of APS combined with adriamycin in human GC cell SGC-7901 and SGC-7901/ADR, the results showed that APS reduced cell viability and enhanced the rate of apoptosis in a time-dosage dependent manner, up-regulated p-AMPK and caspase-3 expression levels, induced apoptosis through the AMPK pathway, and improved the sensitivity of GC cells to adriamycin, suggesting that APS can be used as a chemosensitizer (Song et al., 2020). This paper speculated that the effect may be related to the decreased expression of MDR1, and the overexpression of tumor suppressor genes such as SEMA3F, P21WAF1/CIP1 and FBXW7. Similarly, APS can also inhibit the expression of phosphorylated AKT (p-AKT) and MMP-9, and then inhibit the proliferation, migration and invasion of GC AGS cells through the AKT pathway, and also induce autophagy (Wu et al., 2018). In addition, APS also has a variety of immunoregulatory activities, such as regulating B lymphocytes and cytokines, especially inducing the activation and differentiation of splenic DCs, followed by the completion of Th2 to Th1 transition and enhancing the immune function of T lymphocytes (Liu et al., 2011).
4.4.2 Liquorice
A large number of bioactive components can be isolated from Liquorice, including triterpenoid saponins, flavonoids, isoflavones and chalcone (Asl et al., 2008). Glycyrrhizic acid (GA) is usually considered to be the main active component. The study has shown that GA can induce G1/S phase arrest of cell cycle and apoptosis by down-regulating the expression of cyclin D1, D2, D3, E1, E2, Bcl-2, survivin and p65, up-regulating the protein levels of Bax, PARP and pro-caspase-3, -8, -9. The proliferation of human GC cells (MGC-803, BGC-823, SGC-7901) has a time- and dose-dependent inhibitory effect by down-regulating PI3K/AKT signaling pathway (Wang et al., 2020).
Isoliquiritigenin (ISL) is a flavonoid extracted from Liquorice. Studies have shown that ISL can down-regulate Bcl-2 protein and p62, up-regulate Bax protein, caspase-3, Beclin 1 and LC3II/LC3I ratio, mediate apoptosis and autophagy of human GC MKN28 cells by inhibiting PI3K/AKT/mTOR signaling pathway, thereby inhibiting cell proliferation, and reducing invasion and migration (Zhang et al., 2018). ISL can also induce apoptosis in human GC MGC-803 cells by increasing free calcium concentration and decreasing mitochondrial transmembrane potential (Ma et al., 2001). However, licochalcone A (LCA), as a cytotoxic flavonoid, alone or in combination with 5-FU, increased the expression levels of Bax, PARP, tumor proteins 21, 27, 53 and caspase 3, and decreased the expression levels of Bcl-2, cyclin A, cyclin B and MDM2, which in turn blocked G 2/M cell cycle progression and induced apoptosis, inhibiting SGC7901 and MKN-45 cell proliferation in a time-dependent manner (Xiao et al., 2011; Lin et al., 2017). In addition, LCA can also increase the level of reactive oxygen species (ROS) and induce oxidative stress and apoptosis of BGC-823 cells through PI3K/AKT/mTOR and MAPK signaling cascades (Hao et al., 2015).
In addition, glycyrrhetinic acid (GA) can inhibit the viability of GC cells in a dose- and time-dependent manner, but its toxic and side effects limit its wide application, thus making many beneficial explorations on related derivatives (Xu et al., 2017). For example, 11-deoxy glycyrrhetinic acid can effectively inhibit GC formation by up-regulating p21 and down-regulating cdc2 and cyclin B1, mediating BID translocation from the nucleus to mitochondria, thereby inducing apoptosis and G2 phase arrest in GC cell (Lin et al., 2014). For another example, 18β-glycyrrhetinic acid (18β-GA) can inhibit MMP-2 and MMP-9 activities in a dose-dependent manner, up-regulate E-cadherin expression and down-regulate vimentin expression, and reduce the metastatic potential of human GC cell line SGC-7901 cells through ROS/PKC-α/ERK signaling pathway and inhibition of EMT (Cai et al., 2018). In addition, new isoliquiritigenin (ISL) analogues (Huang et al., 2020), licochalcone A derivatives (LCA) (Shibata, 1994) can also provide more options for anti-GC drug research and development.
4.4.3 Poria
In vitro studies confirmed that Poria combined with oxaliplatin significantly decreased Snail, Twist, vimentin, and N-cadherin mRNA and protein expression, significantly increased E-cadherin mRNA and protein expression, inhibited the EMT process, and decreased the invasion and migration of SGC7901 (Wang N. et al., 2018). This study also found that Poria could reduce the tumor volume, and improve the morphological parameters of GC cells. In addition, both dehydroebriconic acid and dehydrotrametenonic acid found from Poria sclerotia inhibited the growth of GC cells by arresting the G1 phase of the cell cycle, with LD (50) values of 63.6 and 38.4 microM, respectively (Mizushina et al., 2004).
4.4.4 Largehead Atractylodes
Atractylenolide (AT) is the main anticancer active ingredient of Largehead Atractylodes. Atractylenolide I (AT-I) has been found to inactivate the Notch signaling pathway by down-regulating the protein and mRNA expression of Notch1, Jagged1, Hes1, Hey1 and CD44, inhibiting the sphere formation ability and cell viability of HGC-27, MGC-803 and MKN-45, thereby inhibiting the self-renewal ability and cell proliferation of GC stem-like cells (GCSC) and inducing apoptosis (Ma et al., 2014). A randomized controlled trial also verified the therapeutic effect of AT-1 on GC cachexia (Liu et al., 2008). Similarly, for Atractylenolide II (AT-II), Bax expression could also be up-regulated, and B-cell lymphoma 2 (Bcl-2), phosphorylated protein kinase B (p-Akt), and phosphorylated ERK (p-ERK) expression could be down-regulated, which induced apoptosis and inhibited proliferation and migration of HGC-27 and AGS in a concentration- and time-dependent manner by inactivating Ras/ERK and PI3K/Akt signaling pathways (Tian et al., 2017). AT-1 and AT-2 also inhibit Akt/IκBα/NF-κB signaling pathway to play a role, thereby inhibiting gastritis to GC transformation (Amin et al., 2022). In addition, the aqueous extract of Largehead Atractylodes could inhibit the proliferation of BGC-823 and SGC-7901, decrease the mitochondrial transmembrane potential, and induce apoptosis and cell cycle arrest in a dose- and time-dependent manner (Zhao et al., 2014).
4.4.5 Chinese Angelica
Decursin is one of the active components of Chinese Angelica, which has been confirmed to down-regulate CXC chemokine receptor 7 (CXCR7) and Bcl-2 expression in a dose-dependent manner, mediate STAT3/c-Myc signaling pathway, induce apoptosis, and inhibit the proliferation, migration, and invasion of SNU484 and SNU216 (Kim et al., 2019). Another study by the same team also confirmed that Decursin could reduce cell viability, inhibited cell growth and induce G0/G1 arrest in vitro in a dose- and time-dependent manner (Kim et al., 2021). And by promoting the accumulation of LC3 and SQSTM1, inhibiting CTSC and E2F3 expression, and reducing the activity of lysosomal protein cathepsin C (CTSC), thereby inducing autophagic flux disorders. While in vivo studies, Decursin decreased the growth of tumor spheroids and patient-derived gastric organoids, and regulated the expression of CTSC and autophagy-related proteins, which in turn validated the in vivo experimental results.
In addition, a clinical data from Taiwan verified that Chinese Angelica could prolong the survival rate of GC patients [adjusted hazard ratio (HR) 0.72 [95% CI, 0.57–0.92] (p = 0.009)], and experimental studies also found that N-butylidenephthalide (BP), the active component of Chinese Angelica, could induce increased REDD1 expression, inhibit mTOR signaling, activate apoptosis through the mitochondrial pathway, and inhibit the proliferation and EMT of AGS, NCI-N87, and TSGH-9201 cells (Liao et al., 2018).
4.4.6 Codonopsis
Lobetyolin (LBT) and Lobetyol are the essential components of Codonopsis. The anti-GC activity of LBT mainly depends on the regulation of glutamine metabolism, the decrease of mRNA and protein expression of amino acid transporter alanine-serine-cysteine transporter 2 (ASCT2), the induction of apoptosis and the inhibition of GC cell proliferation (Bailly, 2021). Lobetyol induced apoptosis and G1/S phase cell cycle arrest in MKN45 cells by mediating MAPK signaling pathway in a time- and dose-dependent manner (Shen et al., 2016). Both can be further considered as potential anti-GC active ingredients. In addition, the Chinese medicine formula “Weikang Keli”, containing Codonopsis and Largehead Atractylodes, can induce autophagic cell death in SGC-7901 cels. In vitro experiments showed that compared with the positive control of 5-FU, “Weikang Keli” aqueous extract could reduce the tumor volume in the GC model. Although the difference was not statistically significant, the motility, response sensitivity and food intake were increased (Huo et al., 2013).
4.4.7 Tangerine Peel
Nobiletin (Nob), a poly methoxy flavonoid extracted from Tangerine Peel, has been shown to inhibit MMP-9 activity in a concentration-dependent manner and to significantly reduce the total weight and number of disseminated nodules in a SCID mouse model (0.07g vs. 0.78g, p = 0.0059; 7.5 vs. 69.3/body, p = 0.0001), thereby inhibiting the proliferation of TMK-1 cell with peritoneal disseminated nodule formation, which is beneficial in preventing GC metastasis (Minagawa et al., 2001). In addition, it could also up-regulate E-cadherin protein expression and down-regulate vimentin, fibronectin, MMP-9 protein and p-STAT3 expression in SGC-7901 cell, by inhibiting the STAT3 pathway, followed by inhibiting EMT (Yang et al., 2020).
In addition, Nob-induced apoptosis is mediated by activating ER stress, decreasing phosphorylated Akt and mTOR, and mediating protective autophagy, thereby inhibiting GC proliferation in SNU-16 cells (Moon et al., 2016). And it increased Bax/Bcl-2 ratio, caspase-3/9 proteolytic activation with degradation of poly (ADP-ribose) polymerase (PARP) protein, induced apoptosis and had a synergistic anticancer effect with 5-fluorouracil in p53-mutant SNU-16 cell (Moon et al., 2013).
5 Conclusion
Overall, CHM has a positive effect on improving oxaliplatin-based chemotherapy for AGC, which can improve short-term efficacy and reduce the incidence of AEs. In the sensitivity analysis of herbal medicines, it was found that herbal medicines such as Astragalus, Liquorice, Poria, Largehead Atractylodes, Chinese Angelica, Codonopsis, and Tangerine Peel had more advantages in increasing ORR. Previous mechanistic studies also confirmed their anti-GC activity, and the results of our study will provide beneficial evidence support for the combination therapy of CHM. Considering factors such as low quality of literature and insufficient sample size for clinical trials corresponding to shortlisted herbal medicines, quality of evidence was not high, definite conclusions cannot be drawn. More rigorously designed, large-sample, multi-center RCTs of herbal synergistic chemotherapy are needed in the future to better validate the action characteristics of CHM and its potential herbal medicines.
Author contributions
YT conceived this study. YT and HW registered the protocol and performed the search, screen, inclusion, and quality assessment of the included trials. HW and BX performed the evidence synthesis. YT, HW, and BX drafted the first version of this manuscript. XZ, GZ, YG, and TL provided critical revisions and edited the manuscript. JL and RG revised the manuscript. All authors read and approved the final manuscript for submission.
Funding
This work was supported by Scientific and technological innovation project of China Academy of Chinese Medical Sciences (grant number CI2021A01802).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SC declared a shared parent affifiliation with the authors BX, GZ, YG, and TL at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.977708/full#supplementary-material
References
- Ajani J. A., D'Amico T. A., Almhanna K., Bentrem D. J., Chao J., Das P., et al. (2016). Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 10, 1286–1312. 10.6004/jnccn.2016.0137 [DOI] [PubMed] [Google Scholar]
- Ajani J. A., D'Amico T. A., Bentrem D. J., Chao J., Cooke D., Corvera C., et al. (2022). Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 20, 167–192. 10.6004/jnccn.2022.0008 [DOI] [PubMed] [Google Scholar]
- Ajani J. A., Moiseyenko V. M., Tjulandin S., Majlis A., Constenla M., Boni C., et al. (2007). Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: The V-325 study group. J. Clin. Oncol. 22, 3205–3209. 10.1200/JCO.2006.10.4968 [DOI] [PubMed] [Google Scholar]
- Al-Batran S. E., Hartmann J. T., Probst S., Schmalenberg H., Hollerbach S., Hofheinz R., et al. (2008). Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J. Clin. Oncol. 9, 1435–1442. 10.1200/JCO.2007.13.9378 [DOI] [PubMed] [Google Scholar]
- Amin A., Hossen M. J., Fu X. Q., Chou J. Y., Wu J. Y., Wang X. Q., et al. (2022). Inhibition of the Akt/NF-κB pathway is involved in the anti-gastritis effects of an ethanolic extract of the rhizome of Atractylodes macrocephala. J. Ethnopharmacol. 293, 115251. 10.1016/j.jep.2022.115251 [DOI] [PubMed] [Google Scholar]
- Asl M. N., Hosseinzadeh H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 6, 709–724. 10.1002/ptr.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C. (2021). Anticancer properties of lobetyolin, an essential component of radix Codonopsis (dangshen). Nat. Prod. Bioprospect. 2, 143–153. 10.1007/s13659-020-00283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang Y. J., Kang Y. K., Catenacci D. V., Muro K., Fuchs C. S., Geva R., et al. (2019). Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 4, 828–837. 10.1007/s10120-018-00909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao P. Q. (2020). Effect of Yiqi Huoxue Recipe combined with SOX chemotherapy on serum tumor markers, T lymphocyte subsets and quality of life in patients with stage III-IV gastric cancer. Int. Med. Health Guid. 2, 241–243+248. 10.3760/cma.j.issn.1007-1245.2020.02.028 [DOI] [Google Scholar]
- Cai H., Chen X., Zhang J., Wang J. (2018). 18β-glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-α/ERK pathway. J. Nat. Med. 1, 252–259. 10.1007/s11418-017-1145-y [DOI] [PubMed] [Google Scholar]
- Cai J. Y., Jin Y., Lin H., Gan J. W., Yue S. B., Chen Q. T. (2019). Efficacy of tumor response and quality of life of traditional Chinese medicine of strengthening spleen and reinforcing qi in the treatment of advanced gastric cancer. Mod. J. Integr. Traditional Chin. West. Med. 33, 3711–3714. 10.3969/j.issn.1008-8849.2019.33.016 [DOI] [Google Scholar]
- Cao W., Zhao A. G. (2009). Prescription rules of Chinese herbal medicines in treatment of gastric cancer. Zhong Xi Yi Jie He Xue Bao 1, 1–8. 10.3736/jcim20090101 [DOI] [PubMed] [Google Scholar]
- Chen K., Wang Q. C., Yin H., Duan W. M., Gao Y. (2007). Clinical observation of chemotherapy combined with TCM syndrome differentiation treatment in advanced gastric cancer. Chin. J. Clin. Healthc. 3, 275–277. 10.3969/j.issn.1672-6790.2007.03.021 [DOI] [Google Scholar]
- Chen M., May B. H., Zhou I. W., Sze D. M., Xue C. C., Zhang A. L. (2016a). Oxaliplatin-based chemotherapy combined with traditional medicines for neutropenia in colorectal cancer: A meta-analysis of the contributions of specific plants. Crit. Rev. Oncol. Hematol. 105, 18–34. 10.1016/j.critrevonc.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Chen M., May B. H., Zhou I. W., Xue C. C., Zhang A. L. (2016b). Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: Contributions of specific plants to tumor response. Integr. Cancer Ther. 1, 40–59. 10.1177/1534735415596424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. H., May B. H., Zhou I. W., Zhang A. L., Xue C. C. (2016c). Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: A systematic review and meta-analysis. Phytother. Res. 5, 741–753. 10.1002/ptr.5586 [DOI] [PubMed] [Google Scholar]
- Cheng M. Q., Hu J. Q., Zhao Y., Jiang W. J. L., Qi R. Z., Chen S. T., et al. (2021). Efficacy and safety of astragalus-containing traditional Chinese medicine combined with platinum-based chemotherapy in advanced gastric cancer: A systematic review and meta-analysis. Front. Oncol. 11, 632168. 10.3389/fonc.2021.632168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. C., Zhao W. S., Yang Z., Hu F. S., Zhang Q. (2010). Effective observation on treating advanced gastric cancer by Yiqi Huoxue plus FOLFOX4. Clin. J. Chin. Med. 11, 77–79. 10.3969/j.issn.1674-7860.2010.11.047 [DOI] [Google Scholar]
- Chu R. G., Guo H. F., Xie H., Wu L., Wu H. Y., Shi M., et al. (2017). Effect of zhangshi yiwei decoction on T-lymphocyte subsets of advanced gastric cancer. Guangming J. Chin. Med. 4, 499–502. 10.3969/j.issn.1003-8914.2017.04.018 [DOI] [Google Scholar]
- Cunningham D., Starling N., Rao S., Iveson T., Nicolson M., Coxon F., et al. (2008). Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 1, 36–46. 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- Diao Z., Wang C., Liu X. C., Tang Y. H. (2018). The clinic effects of Shenlian capsule, trastuzumab combined with SOX regimen in the treatment for HER-2 positive advanced gastric carcinoma. Int. J. Trad. Chin. Med. 11, 1020–1024. 10.13288/j.11-2166/r.2015.02.009 [DOI] [Google Scholar]
- Digklia A., Wagner A. D. (2016). Advanced gastric cancer: Current treatment landscape and future perspectives. World J. Gastroenterol. 8, 2403–2414. 10.3748/wjg.v22.i8.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding P., Guo Y., Wang C., Chen J., Guo C., Liu H., et al. (2022). A network pharmacology approach for uncovering the antitumor effects and potential mechanisms of the Sijunzi decoction for the treatment of gastric cancer. Evid. Based. Complement. Altern. Med. 2022, 9364313. 10.1155/2022/9364313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q., Pu J., Du T., Xu S., Liu W., Liu L., et al. (2021). Astragalus-mediated stimulation on antigen-presenting cells could result in higher IL-21 production from CXCR5+ Tfh-like cells and better IL-21-mediated effector functions. Hum. Immunol. 6, 429–437. 10.1016/j.humimm.2021.03.012 [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G. D., Schneider M., Minder C. (1997). Bias in meta analysis detected by a simple, graphical test. BMJ 315, 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. L., Li D. F., Jiao J., Tang J. Y., Hou F. F., Hu Y. (2015). Effect of combined treatment of Weifu formula and chemotherapy on the quality of life in advanced gastric carcinoma patients with spleen deficiency and toxin accumulation syndrome. J. Tradit. Chin. Med. 2, 120–123. 10.13288/j.11-2166/r.2015.02.009 [DOI] [Google Scholar]
- Feng T. M., Wang E. Q., Wu D. P. (2021). Effects of modified xuezhen decoction combined with western medicine on serum CEA and CA199 in patients with advanced gastric cancer. West. J. Traditional Chin. Med. 8, 126–129. 10.12174/j.issn.2096-9600.2021.08.29 [DOI] [Google Scholar]
- Feng Y. L. (2017). Clinical observation of gancao xiexin decoction combined with SOX chemotherapy in treating gastric adenocarcinoma (syndrome of dampness-heat due to spleen deficiency). Chendu: Chendu University of Traditional Chinese Medicine. [Google Scholar]
- Gong M. (2020). Efficacy and safety of wenyang sanjie decoction combined with chemotherapy in the treatment of advanced gastric adenocarcinoma. Chongqing Yixue S02, 248–250. [Google Scholar]
- Gu N., Wang Z. X., Li Z. G. (2019). Clinical efficacy of chemotherapy combined with traditional Chinese medicine in advanced gastric cancer. Henan Med. Res. 18, 3383–3385. 10.3969/j.issn.1004-437X.2019.18.066 [DOI] [Google Scholar]
- Guo R. (2014). The clinical research of jianpi xiaoji decoction combined with S-1 combined oxaliplatin chemotherapy in the treatment of advanced middle-late gastric cancer. Zhengzhou: Zhengzhou University. [Google Scholar]
- Guyatt G. H., Oxman A. D., Vist G. E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 7650, 924–926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao W., Yuan X., Yu L., Gao C., Sun X., Wang D., et al. (2015). Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 5, 10336. 10.1038/srep10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F. S. (2011). Summary of professor Yu rencun’s academic thought and clinical experience and clinical observation of yiqi huoxue Jiedu recipe combined with chemotherapy in the treatment of advanced gastric cancer. Beijing: Beijing University of Chinese Medicine. [Google Scholar]
- Hu F., Li C., Yang G., Yang Z., Zhao W., Zhang Q. (2012). Clinical observation of yiqi huoxue jiedu formula combined with chemotherapy on advanced gastric cancer. Int. J. Trad. Chin. Med. 34, 1024–1026. 10.3760/cma.j.issn.1673-4246.2012.11.023 [DOI] [Google Scholar]
- Huang F., Wang J., Xu Y., Zhang Y., Xu N., Yin L. (2020). Discovery of novel isoliquiritigenin analogue ISL-17 as a potential anti-gastric cancer agent. Biosci. Rep. 6, BSR20201199. 10.1042/BSR20201199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Hu K. (2017). Clinical observation of jianpi yangwei prescription combined with FOLFOX4 regimen for advanced gastric cancer. J. New Chin. Med. 4, 121–124. 10.13457/j.cnki.jncm.2017.04.043 [DOI] [Google Scholar]
- Huang J. Q. (2014). Clinical observation of jianpi huayu decoction combined with chemotherapy for advanced gastric cancer of spleen deficiency and phlegm stasis syndrome. Changsha: Hunan University of Chinese Medicine. [Google Scholar]
- Hung K. F., Hsu C. P., Chiang J. H., Lin H. J., Kuo Y. T., Sun M. F., et al. (2017). Complementary Chinese herbal medicine therapy improves survival of patients with gastric cancer in taiwan: A nationwide retrospective matched-cohort study. J. Ethnopharmacol. 199, 168–174. 10.1016/j.jep.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Huo J., Qin F., Cai X., Ju J., Hu C., Wang Z., et al. (2013). Chinese medicine formula "Weikang Keli" induces autophagic cell death on human gastric cancer cell line SGC-7901. Phytomedicine. 2, 159–165. 10.1016/j.phymed.2012.10.001 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., et al. Editors (2019). Cochrane handbook for systematic reviews of interventions version 6.0 (updated july 2019) (London, United Kingdom: Cochrane; ). Available at: http://www.training.cochrane.org/handbook . [Google Scholar]
- Jiang F., Liu X. F., Gao Y. W., Sun X. H., Jiang S. Q. (2021). Effect of Jiedu sanjie recipe combined with chemotherapy on patients with advanced gastric cancer and the effect of serum VEGF and VEGFR-1. Shaanxi J. Traditional Chin. Med. 5, 582–585. 10.3969/j.issn.1000-7369.2021.05.009 [DOI] [Google Scholar]
- Jiao J. Q. (2019). Application of dialectical addition and subtraction of xuezheng decoction combined with chemotherapy in patients with advanced gastric cancer and its effects on serum tumor markers and immune status. J. Sichuan Traditional Chin. Med. 12, 99–101. [Google Scholar]
- Johnston F. M., Beckman M. (2019). Updates on management of gastric cancer. Curr. Oncol. Rep. 21, 67. 10.1007/s11912-019-0820-4 [DOI] [PubMed] [Google Scholar]
- Joshi S. S., Badgwell B. D. (2021). Current treatment and recent progress in gastric cancer. Ca. Cancer J. Clin. 3, 264–279. 10.3322/caac.21657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. K., Kang W. K., Shin D. B., Chen J., Xiong J., Wang J., et al. (2009). Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann. Oncol. 4, 666–673. 10.1093/annonc/mdn717 [DOI] [PubMed] [Google Scholar]
- Kim S., Kim J. E., Kim N., Joo M., Lee M. W., Jeon H. J., et al. (2019). Decursin inhibits tumor growth, migration, and invasion in gastric cancer by down-regulating CXCR7 expression. Am. J. Cancer Res. 9, 2007–2018. [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee S. I., Kim N., Joo M., Lee K. H., Lee M. W., et al. (2021). Decursin inhibits cell growth and autophagic flux in gastric cancer via suppression of cathepsin C. Am. J. Cancer Res. 4, 1304–1320. [PMC free article] [PubMed] [Google Scholar]
- Koizumi W., Narahara H., Hara T., Takagane A., Akiya T., Takagi M., et al. (2008). S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet. Oncol. 3, 215–221. 10.1016/S1470-2045(08)70035-4 [DOI] [PubMed] [Google Scholar]
- Leong T. (2005). Chemotherapy and radiotherapy in the management of gastric cancer. Curr. Opin. Gastroenterol. 6, 673–678. 10.1097/01.mog.0000179833.28158.b7 [DOI] [PubMed] [Google Scholar]
- Li D. C., Xiong Y. J. (2016). Fuzheng kang'ai prescription combined with mFOLFOX4 chemotherapy in treatment of advanced gastric cancer. Acta Chin. Med. 9, 1253–1257. 10.16368/j.issn.1674-8999.2016.09.352 [DOI] [Google Scholar]
- Li D. H., Su Y. F., Sun C. X., Fan H. F. (2020). Effect of shunqi yiwei decoction combined with sox chemotherapy on advanced gastric cancer. Acta Medica Mediterr. 36, 2313. 10.19193/0393-6384_2020_4_360 [DOI] [Google Scholar]
- Li Y., Sui X., Su Z., Yu C., Shi X., Johnson N. L., et al. (2020). Meta-analysis of paclitaxel-based chemotherapy combined with traditional Chinese medicines for gastric cancer treatment. Front. Pharmacol. 11, 132. 10.3389/fphar.2020.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. J., Liao L. L., Liu P., Tang P., Wang H., Peng Q. H. (2021). Sijunzi decoction inhibits stemness by suppressing β-catenin transcriptional activity in gastric cancer cells. Chin. J. Integr. Med. 28, 702–710. 10.1007/s11655-021-3314-9 [DOI] [PubMed] [Google Scholar]
- Liao K. F., Chiu T. L., Huang S. Y., Hsieh T. F., Chang S. F., Ruan J. W., et al. (2018). Anti-cancer effects of radix Angelica sinensis (danggui) and N-butylidenephthalide on gastric cancer: Implications for REDD1 activation and mTOR inhibition. Cell. Physiol. biochem. 6, 2231–2246. 10.1159/000492641 [DOI] [PubMed] [Google Scholar]
- Lin D., Zhong W., Li J., Zhang B., Song G., Hu T. (2014). Involvement of BID translocation in glycyrrhetinic acid and 11-deoxy glycyrrhetinic acid-induced attenuation of gastric cancer growth. Nutr. Cancer 3, 463–473. 10.1080/01635581.2013.877498 [DOI] [PubMed] [Google Scholar]
- Lin X., Tian L., Wang L., Li W., Xu Q., Xiao X. (2017). Antitumor effects and the underlying mechanism of licochalcone A combined with 5-fluorouracil in gastric cancer cells. Oncol. Lett. 3, 1695–1701. 10.3892/ol.2017.5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Liu Z. R., Cheng Z. R., Wang C. X. (2015). Jianpi-Xiaozheng recipe combined with chemotherapy for late gastric cancer. Int. J. Traditional Chin. Med. 2, 126–129. 10.3760/cma.j.issn.1673-4246.2015.02.008 [DOI] [Google Scholar]
- Liu Q. Y., Yao Y. M., Zhang S. W., Sheng Z. Y. (2011). Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro . J. Ethnopharmacol. 3, 457–464. 10.1016/j.jep.2010.06.041 [DOI] [PubMed] [Google Scholar]
- Liu Y., Jia Z., Dong L., Wang R., Qiu G. (2008). A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid. Based. Complement. Altern. Med. 3, 337–344. 10.1093/ecam/nem031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. M., Yang X. L., Jiang F., Pan Y. C., Zhang L. (2020). Matrine involves in the progression of gastric cancer through inhibiting miR-93-5p and upregulating the expression of target gene AHNAK. J. Cell. Biochem. 3, 2467–2477. 10.1002/jcb.29469 [DOI] [PubMed] [Google Scholar]
- Liu Z. W., Wang D. L., Zhao X. F., Zhao P. (2019). Effect of jianpi huayu decoction on immune function and toxicity for patients with gastric cancer before and after chemotherapy. J. Sichuan Traditional Chin. Med. 10, 92–96. [Google Scholar]
- Long Y. J. (2021). Clinical observation of yiqi fuyuan paste combined with chemotherapy in the treatment of advanced gastric cancer of spleen qi deficiency syndrome. Changsha: Hunan University of Chinese Medicine. [Google Scholar]
- Ma J., Fu N. Y., Pang D. B., Wu W. Y., Xu A. L. (2001). Apoptosis induced by isoliquiritigenin in human gastric cancer MGC-803 cells. Planta Med. 8, 754–757. 10.1055/s-2001-18361 [DOI] [PubMed] [Google Scholar]
- Ma L., Mao R., Shen K., Zheng Y., Li Y., Liu J., et al. (2014). Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 1, 353–359. 10.1016/j.bbrc.2014.05.110 [DOI] [PubMed] [Google Scholar]
- Ma X. Q., Shi Q., Duan J. A., Dong T. T., Tsim K. W. (2002). Chemical analysis of radix astragali (huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 17, 4861–4866. 10.1021/jf0202279 [DOI] [PubMed] [Google Scholar]
- Minagawa A., Otani Y., Kubota T., Wada N., Furukawa T., Kumai K., et al. (2001). The citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Jpn. J. Cancer Res. 12, 1322–1328. 10.1111/j.1349-7006.2001.tb02156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushina Y., Akihisa T., Ukiya M., Murakami C., Kuriyama I., Xu X., et al. (2004). A novel DNA topoisomerase inhibitor: Dehydroebriconic acid, one of the lanostane-type triterpene acids from Poria cocos. Cancer Sci. 4, 354–360. 10.1111/j.1349-7006.2004.tb03215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. Prisma Group (2009). Preferred reporting Items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 7, e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J. Y., Cho M., Ahn K. S., Cho S. K. (2013). Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr. Cancer 2, 286–295. 10.1080/01635581.2013.756529 [DOI] [PubMed] [Google Scholar]
- Moon J. Y., Cho S. K. (2016). Nobiletin induces protective autophagy accompanied by ER-stress mediated apoptosis in human gastric cancer SNU-16 cells. Molecules 7, 914. 10.3390/molecules21070914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W. H., Tien Y. C., Huang C. Y., Huang T. H., Liao J. C., Kuo C. L., et al. (2010). Fraxinus rhynchophylla ethanol extract attenuates carbon tetrachloride-induced liver fibrosis in rats via down-regulating the expressions of uPA, MMP-2, MMP-9 and TIMP-1. J. Ethnopharmacol. 3, 606–613. 10.1016/j.jep.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Qin X. G. (2012). Clinical observation of buzhong yiqi decoction combined with SOX therapy on the patient with advanced gastric cancer. J. Pract. Traditional Chin. Intern. Med. 10, 39–41. 10.3969/j.issn.1671-7813.2012.10.24 [DOI] [Google Scholar]
- Ren L. Q., Li Q., Zhang Y. (2020). Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. Biomed. Res. Int. 2020, 9396512. 10.1155/2020/9396512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Lu X., Du W., Zhou J., Qiu H., Chen J., et al. (2016). Lobetyol activate MAPK pathways associated with G1/S cell cycle arrest and apoptosis in MKN45 cells in vitro and in vivo . Biomed. Pharmacother. 81, 120–127. 10.1016/j.biopha.2016.03.046 [DOI] [PubMed] [Google Scholar]
- Shibata S. (1994). Anti-tumorigenic chalcones. Stem Cells 1, 44–52. 10.1002/stem.5530120109 [DOI] [PubMed] [Google Scholar]
- Shih W. T., Yang P. R., Shen Y. C., Yang Y. H., Wu C. Y. (2021). Traditional Chinese medicine enhances survival in patients with gastric cancer after surgery and adjuvant chemotherapy in taiwan: A nationwide matched cohort study. Evid. Based. Complement. Altern. Med. 2021, 7584631. 10.1155/2021/7584631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara K., Özgüroğlu M., Bang Y. J., Di Bartolomeo M., Mandalà M., Ryu M. H., et al. (2018). Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 10142, 123–133. 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- Smyth E. C., Verheij M., Allum W., Cunningham D., Cervantes A., Arnold D., et al. (2016). Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (5), v38–v49. 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- Song J., Chen Y., He D., Tan W., Lv F., Liang B., et al. (2020). Astragalus polysaccharide promotes adriamycin-induced apoptosis in gastric cancer cells. Cancer Manag. Res. 12, 2405–2414. 10.2147/CMAR.S237146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Wu Y., Yang J., Yang D., Fang X. (2017). Progress in the treatment of advanced gastric cancer. Tumour Biol. 7, 1010428317714626. 10.1177/1010428317714626 [DOI] [PubMed] [Google Scholar]
- Sterne J. A. C., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in RandomisedTrials. BMJ 366, l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Sun B., Zhou L., Wang X. L., Zhou D. Y., Li Z. Q. (2020). Clinical study of self-made fuzheng kang'ai decoction combined with FOLFOX6 chemotherapy on symptoms, signs and quality of life for patients with stage III-IV gastric cancer. J. Sichuan Traditional Chin. Med. 12, 101–104. [Google Scholar]
- Sun H., Wang W., Bai M., Liu D. (2019). Cinobufotalin as an effective adjuvant therapy for advanced gastric cancer: A meta-analysis of randomized controlled trials. Onco. Targets. Ther. 12, 3139–3160. 10.2147/OTT.S196684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 3, 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Thuss-Patience P. C., Shah M. A., Ohtsu A., Van Cutsem E., Ajani J. A., Castro H., et al. (2017). Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet. Oncol. 5, 640–653. 10.1016/S1470-2045(17)30111-0 [DOI] [PubMed] [Google Scholar]
- Tian S., Yu H. (2017). Atractylenolide II inhibits proliferation, motility and induces apoptosis in human gastric carcinoma cell lines HGC-27 and AGS. Molecules 11, 1886. 10.3390/molecules22111886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Li X., Li H., Lu Q., Sun G., Chen H. (2014). Astragalus mongholicus regulate the Toll-like-receptor 4 meditated signal transduction of dendritic cells to restrain stomach cancer cells. Afr. J. Tradit. Complement. Altern. Med. 3, 92–96. 10.4314/ajtcam.v11i3.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E., Moiseyenko V. M., Tjulandin S., Majlis A., Constenla M., Boni C., et al. (2006). Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J. Clin. Oncol. 31, 4991–4997. 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Valderrama A., Bang Y. J., Fuchs C. S., Shitara K., Janjigian Y. Y., et al. (2021). Quality of life with first-line pembrolizumab for PD-L1-positive advanced gastric/gastroesophageal junction adenocarcinoma: Results from the randomised phase III KEYNOTE-062 study. ESMO Open 4, 100189. 10.1016/j.esmoop.2021.100189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. D., Grothe W., Haerting J., Kleber G., Grothey A., Fleig W. E. (2006). Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 18, 2903–2909. 10.1200/JCO.2005.05.0245 [DOI] [PubMed] [Google Scholar]
- Wang H., Ge X., Qu H., Wang N., Zhou J., Xu W., et al. (2020). Glycyrrhizic acid inhibits proliferation of gastric cancer cells by inducing cell cycle arrest and apoptosis. Cancer Manag. Res. 12, 2853–2861. 10.2147/CMAR.S244481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Liu D., Guo J., Sun Y., Guo T., Zhu X. (2018). Molecular mechanism of Poria cocos combined with oxaliplatin on the inhibition of epithelial-mesenchymal transition in gastric cancer cells. Biomed. Pharmacother. 102, 865–873. 10.1016/j.biopha.2018.03.134 [DOI] [PubMed] [Google Scholar]
- Wang X., Wang H., Cao L., Wu J., Lu T., Li S., et al. (2021). Efficacy and safety of brucea javanica oil emulsion injection in the treatment of gastric cancer: A systematic review and meta-analysis. Front. Nutr. 8, 784164. 10.3389/fnut.2021.784164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Z. P., Qian W. T., Jia Z. J., Li W. B., He Y. M., et al. (2018). Treated HER2 negative advanced metastatic gastric cancer by combining guishao Liujunzi decoction with SOX regimen. J. Anhui Sci. Technol. Univ. 5, 48–52. [Google Scholar]
- Wu J., Yu J., Wang J., Zhang C., Shang K., Yao X., et al. (2018). Astragalus polysaccharide enhanced antitumor effects of Apatinib in gastric cancer AGS cells by inhibiting AKT signalling pathway. Biomed. Pharmacother. 100, 176–183. 10.1016/j.biopha.2018.01.140 [DOI] [PubMed] [Google Scholar]
- Xiang S., Zhao Z., Zhang T., Zhang B., Meng M., Cao Z., et al. (2020). Triptonide effectively suppresses gastric tumor growth and metastasis through inhibition of the oncogenic Notch1 and NF-κB signaling pathways. Toxicol. Appl. Pharmacol. 388, 114870. 10.1016/j.taap.2019.114870 [DOI] [PubMed] [Google Scholar]
- Xiao X. Y., Hao M., Yang X. Y., Ba Q., Li M., Ni S. J., et al. (2011). Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 1, 69–75. 10.1016/j.canlet.2010.12.016 [DOI] [PubMed] [Google Scholar]
- Xie W. S. (2019). The clinical observation of wendan decoction combined with chemotherapy in the treatment of advanced gastric cancer with the type of phlegm-damp coagulation. Taiyuan: Shanxi University of Chinese Medicine. [Google Scholar]
- Xu B., Wu G. R., Zhang X. Y., Yan M. M., Zhao R., Xue N. N., et al. (2017). An overview of structurally modified glycyrrhetinic acid derivatives as antitumor agents. Molecules 6, 924. 10.3390/molecules22060924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Hosoda K., Niihara M., Hiki N. (2021). History and emerging trends in chemotherapy for gastric cancer. Ann. Gastroenterol. Surg. 4, 446–456. 10.1002/ags3.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. Z., Zhang H., Cui X. Y., Su P. Z., Li Z. T. (2020). Study on the mechanism of nobiletin inhibiting the invasiveness of SGC-7901 gastric cancer cells. Mod. Oncol. 18, 3099–3104. 10.3969/j.issn.1672-4992.2020.18.001 [DOI] [Google Scholar]
- Yang Y., Ma J., Zhang H. Y. (2018). Clinical study on 40 cases of advanced gastric cancer treated with shenyu yangwei decoction combined with capeox chemotherapy. Jiangsu J. Traditional Chin. Med. 4, 40–43. 10.3969/j.issn.1672-397X.2018.04.017 [DOI] [Google Scholar]
- Yoshino T., Arnold D., Taniguchi H., Pentheroudakis G., Yamazaki K., Xu R. H., et al. (2018). Pan-asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 1, 44–70. 10.1093/annonc/mdx738 [DOI] [PubMed] [Google Scholar]
- Yu D., Huang L. P., Shao L. L., Tian Y. Z. (2019). Effect of shengyang yiwei decoction combined with chemotherapy on advanced gastric cancer and its effect on immune function. Chin. J. Traditional Med. Sci. Technol. 4, 556–557. [Google Scholar]
- Yuan K. M. (2011). The clinical research of effect of WD-3 combined with XELOX scheme on the quality of life in treating patients with Ⅳ period gastric carcinoma. Nanjing: Nanjing University of Chinese Medicine. [Google Scholar]
- Yuan M. (2018). Clinical observation of YiQi Jiedu decoction combined with chemotherapy for gastric cancer in stage ⅢB-Ⅳ. Zhengzhou: Henan University of Chinese Medicine. [Google Scholar]
- Zhai Y. R. (2019). Clinical observation on treatment of advanced GastricCancer (syndrome of intermin-gled phlegm and bloodstasis) by huayu Jiedu method. Zhengzhou: Henan University of Chinese Medicine. [Google Scholar]
- Zhang H. O., Chen W. J., Yang S. W., Huang H. Q., Huang M. H., Tang S. H., et al. (2021). Evaluation of curative effect of traditional Chinese medicine treatment for advanced gastric cancer. Fujian J. Traditional Chin. Med. 11, 15–17. 10.3969/j.issn.1000-338X.2021.11.004 [DOI] [Google Scholar]
- Zhang H. (2021b2021). The clinical observation of all nourishing decoction combined with chemotherapy in the treatment of advanced gastric cancer with qi blood deficiency and stasis syndrome. Fuzhou: Fujian University of Traditional Chinese Medicine. [Google Scholar]
- Zhang H. W. (2021a2021). Pei's shugan yangwei decoction combined with SOX regimen chemotherapy under treatment clinical observation of advanced gastric cancer. Lanzhou: Gansu University of Chinese Medicine. [Google Scholar]
- Zhang L., Xiao Y., Yang R., Wang S., Ma S., Liu J., et al. (2021). Systems pharmacology to reveal multi-scale mechanisms of traditional Chinese medicine for gastric cancer. Sci. Rep. 1, 22149. 10.1038/s41598-021-01535-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. H., Zhao C. J., Wu H., Dang Y. Y., Zheng J. (2020). Exploration on the effect of shengyang yiwei decoction plus reduction in chemotherapy for advanced gastric cancer patients with wet resistance syndrome of Yang deficiency. World Chin. Med. 24, 3792–3796. 10.3969/j.issn.1673-7202.2020.24.012 [DOI] [Google Scholar]
- Zhang X. R., Wang S. Y., Sun W., Wei C. (2018). Isoliquiritigenin inhibits proliferation and metastasis of MKN28 gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 3, 3429–3436. 10.3892/mmr.2018.9318 [DOI] [PubMed] [Google Scholar]
- Zhang Z. P. (2019). Clinical observation of erteng sanjie capsule combined with SOX regimen in the treatment of advanced gastric cancer with spleen deficiency and phlegm stasis syndrome. Taiyuan: Shanxi Provincial Institute of Tradtional Chinese Medicine. [Google Scholar]
- Zhao H. (2011). Experimental research on effect of kunshen granule to antiangiogenesis factors and clinical research on it treating advanced gastric cancer. Jinan: Shandong University of Traditional Chinese Medicine. [Google Scholar]
- Zhao M., Wang Q., Ouyang Z., Han B., Wang W., Wei Y., et al. (2014). Selective fraction of Atractylodes lancea (Thunb.) DC. and its growth inhibitory effect on human gastric cancer cells. Cytotechnology 2, 201–208. 10.1007/s10616-013-9559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. N., Xue W. H. (2016). Effect of jianpi huayu decoction combined with chemotherapy on advanced gastric cancer and its impact on quality of life. Acta Chin. Med. Pharmacol. 3, 105–108. 10.19664/j.cnki.1002-2392.2016.03.034 [DOI] [Google Scholar]
- Zhao Y. Y. (2021). Clinical efficacy of wenyang jianpi decoction supplemented with SOX regimen in the treatment of patients with advanced gastric cancer. Acta Med. Sin. 4, 63–67. 10.19296/j.cnki.1008-2409.2021-04-016 [DOI] [Google Scholar]
- Zhong X. S., Shen G. Z., Qin H. H., Liang W. J., Zhou Z. L. (2021). Clinical effect of weijixiao combined with chemotherapy in advanced gastric cancer and its effect on MDSCs, treg cells and immune factors. Mod. J. Integr. Traditional Chin. West. Med. 9, 974–978. 10.3969/j.issn.1008-8849.2021.09.015 [DOI] [Google Scholar]
- Zhong Z. J., Hu C., Liu J. Y. (2019). Efficacy of shenling baizhu san plus FOLFOX4 on advanced gastric cancer. Clin. J. Chin. Med. 31, 84–86. 10.3969/j.issn.1674-7860.2019.31.029 [DOI] [Google Scholar]
- Zhou X., Wang W., Li P., Zheng Z., Tu Y., Zhang Y., et al. (2016). Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo . Oncol. Res. 1-2, 29–34. 10.3727/096504015X14452563486011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. Y., Chen B. S. (2011). Clinical research of traditional Chinese medicine combined with chemotherapy following palliative gastrectomy for gastric cancer. Chin. J. Prim. Med. Pharm. 2, 208–209. 10.3760/cma.j.issn.1008-6706.2011.02.033 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.