Abstract
After more than four decades of hepatitis B virus (HBV) vaccine implementation, its safety and efficacy in preventing HBV infection have been proven and several milestones have been achieved. Most countries have included HBV immunization schedules in their health policies and progress has been made regarding universalization of the first HBV vaccine dose at birth. All of these actions have significantly contributed to reducing both the incidence of HBV infection and its related complications. However, there are still many drawbacks to overcome. The main concerns are the deficient coverage rate of the dose at birth and the large adult population that has not been reached timely by universal immunization. Additionally, the current most widely used second-generation vaccines do not induce protective immunity in 5% to 10% of the population, particularly in people over 40-years-old, obese (body mass index > 25 kg/m2), heavy smokers, and patients undergoing dialysis or infection with human immunodeficiency virus. Recently developed and approved novel vaccine formulations using more potent adjuvants or multiple antigens have shown better performance, particularly in difficult settings. These advances re-launch the expectations of achieving the World Health Organization’s objective of completing hepatitis control by 2030.
Keywords: Hepatitis B virus, Vaccine, Immune response, Antibodies, Neutralizing
Core Tip: Second-generation vaccines induce the production of anti-hepatitis B surface antibodies (anti-HBs). Anti-HBs levels ≥ 10 mIU/mL prevent against infection. More than 90% of immunized persons achieve protective anti-HBs levels 1 mo after completing the three-dose vaccination schedule. Although antibody titers significantly drop during the 1st years after vaccination, memory immunity is sufficient to prevent infection regardless of the antibody levels. In some specific settings showing lower immune response rates, schemes with larger or additional doses and novel vaccine formulations are recommended.
INTRODUCTION
Hepatitis B infection has been a major public health concern for a long time. Identification of the hepatitis B virus (HBV) in the 1960s and the subsequent development of a safe and effective vaccine were the kickoff to begin to retrace the path and achieve the desired control of this health problem.
First-generation vaccines based on heat-treated plasma derived from hepatitis B surface antigen (HBsAg)-positive donors raised concerns about its safety and availability to meet the vaccine manufacturer’s needs. Shortly afterwards, second-generation DNA vaccines prepared in yeast transfected with recombinant plasmids encoding small HBV surface proteins (SHBs) were developed and approved in 1986[1]. Lastly, third-generation vaccines have been produced in mammalian cells that express and secrete SHBs and middle pre-S2 proteins (MHBs) or the three HBV envelope proteins (SHBs, MHBs, and large HBs).
It is worth noting that several studies have compared the immune response to plasma-derived first-generation vaccines to the recombinant second-generation ones. Most of the studies showed that the lowering rate of anti-HBs was higher in people receiving the recombinant HBV vaccine. However, plasma-derived vaccines were replaced by the recombinant ones due to safety concerns about human blood-derived products[2-5]. In addition, it has been shown that third-generation vaccines containing the pre-S2 and pre-S1 antigens would induce a higher anti-HBs response than second-generation ones, particularly in people ≥ 45-years-old[6-8]. Moreover, compared to plasma-derived vaccines, it has been observed that the HBsAg seropositive rate drops by about 71% and that the anti-HBc seropositive rate decreases by approximately 65% when recombinant HBV vaccines are used, supporting their higher effectiveness[4].
The HBV vaccine has been introduced progressively in the national vaccination calendars. Currently, second-generation HBV vaccines have been widely implemented for newborns in most countries. The pentavalent vaccine formulation protecting against diphtheria, pertussis, tetanus, hepatitis B, and Haemophilus influenzae type B is administered in three doses, 4 wk apart (recommended dosing at 6, 10, and 14 postnatal wk), and aims to lessen horizontal transmission. In addition, a monovalent single dose of the HBV vaccine (HepB-BD) administered within 24 h after delivery is also recommended to reduce mother-to-child transmission (Figure 1). The advised immunization schedule for the adult population includes three vaccine doses (HepB3) according to the individuals’ age. Immunocompromised adults or patients on dialysis treatment require higher or additional doses of HBV vaccines. Recently, a novel vaccine (Heplisav-B) with a different adjuvant was approved for adult immunization with a recommended schedule of two doses 1 mo apart.
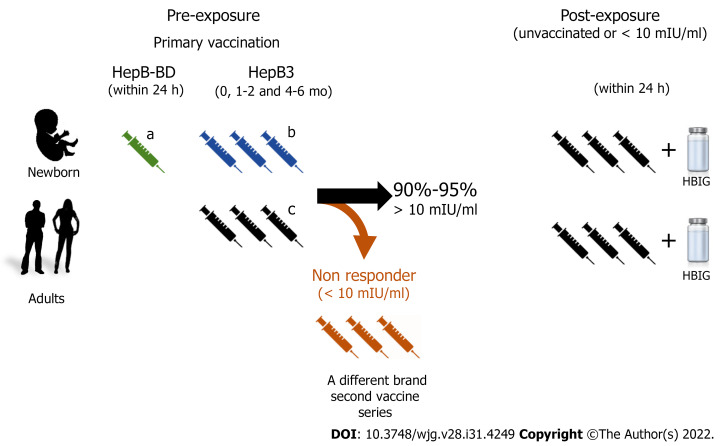
Figure 1.
Recommended hepatitis B virus vaccination schemes. The hepatitis B immunization schedule is flexible, but minimal intervals and ages need to be observed. The recommended dose varies (5-40 μg of hepatitis B surface antigen protein/mL) depending on the individuals’ age and the vaccine brand. aMonovalent hepatitis B vaccine 0.5 mL must be used for the at birth immunization (HepB-BD). Immunocompromised adults or patients under dialysis require larger or additional doses of the hepatitis B vaccine; bCombined hepatitis B, diphtheria, tetanus, adsorbed acellular pertussis, inactivated poliovirus vaccine. This vaccine cannot be administered at birth, before 6 postnatal weeks, or at age ≥ 7 years; cHeplisav-B is a vaccine recently approved for adults; it has a novel adjuvant and its recommended schedule is two doses 1 mo apart. HepB3: Three doses of hepatitis B vaccine; HepB-BD: Monovalent single dose of the hepatitis B virus vaccine; HBIG: Hepatitis B Immunoglobulin.
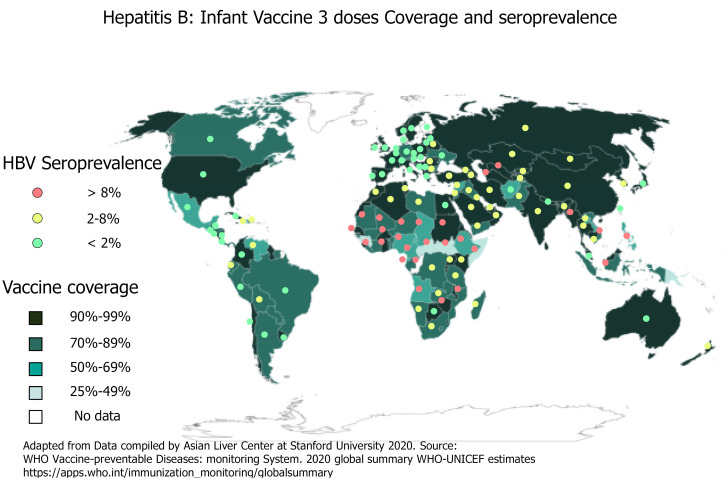
Since 1990, the proportion of children receiving all three doses of the HBV vaccine has increased globally from 1% to 85%. By 2020, the HBV vaccine was introduced to 190 countries with 83% of three-dose coverage rate. Few countries with very low endemicity that consider HBV infection as a limited public health problem provide the HBV vaccine to only well-defined risk groups. Likewise, a dose of HepB-BD has been introduced in the national calendar of 113 countries. Figure 2 shows the HBV worldwide three-dose infant vaccine coverage and the HBsAg seroprevalence[2,9]. However, the at birth dose coverage rate is poor (estimated at 43%) with remarkable disparities according to region and development level[10-12].
Figure 2.
Hepatitis B three doses of infant vaccine coverage and seroprevalence.Hepatitis B virus seroprevalence data is from Polaris Observatory Collaborators: Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol Hepatol 2018; 3: 383–403. HBV: Hepatitis B virus.
Universal vaccination is the most effective strategy to prevent and control HBV infection. In 2016, the World Health Organization (WHO) set the goal of controlling HBV by 2030. The proposed targets include the 90% global coverage of three-dose infant vaccination by 2020, birth-dose vaccination of 50% of infants by 2020, and of 90% of them by 2030[13].
MILESTONES ACHIEVED WITH THE HBV VACCINE
One of the goals of the strategy to achieve HBV control by 2030 is to reduce HBsAg prevalence to 0.1% in 5-year-old children and many countries are already on track to that milestone[14].
The global implementation of the HBV vaccine as part of the national health policies has contributed to directly reducing the global burden of infection, and indirectly, the HBV-related mortality. After the inclusion of HBV vaccination schedules, several surveillance studies have shown an overtime global HBsAg prevalence decrease in most countries[15], either in hyperendemic ones or in those with low or medium HBV infection prevalence[16-20].
Taiwan was the first country to implement a mass vaccination program against HBV in 1984 and it is the paradigm of its impact on the control of hepatitis. After 30 years of sustained immunization programs, the prevalence of HBsAg has decreased from 9.8% in the pre-vaccination period to 0.5% in the cohort reached by HBV vaccination protocols[21]. The main reason for Taiwan’s success was its high three-dose hepatitis infant vaccine coverage rate, which increased from 88.9% in 1985[22,23] to 98.1% in 2018[21].
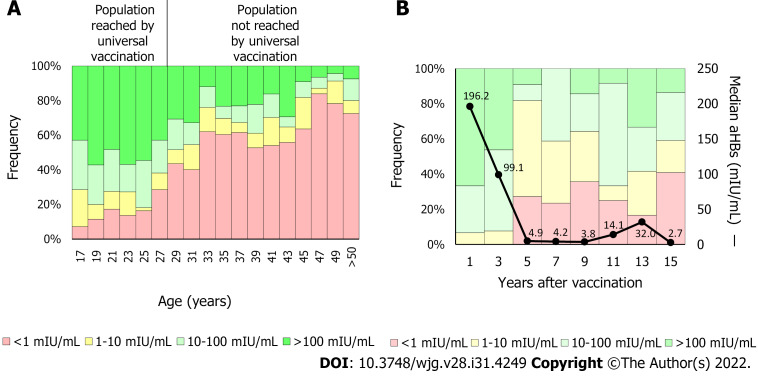
In the United States, since first HBV vaccine recommendations, the infection incidence has decreased by approximately 90%, from 9.6/100000 cases in 1982 to 1.0/100000 cases in 2018[24]. Similarly, in China, where the coverage of the three-dose vaccine schedule has increased from 30.0% to 93.4% and the at birth dose increased from 22.2% to 82.6%, the HBsAg prevalence decreased from 5.5% to 0.9% between 1992 and 2005[25]. In Argentina, a country with low HBV endemicity, the HBV vaccine was included in newborns’ schedules in 2000 and, later in 2003, the catch-up strategy was implemented in 11-year-old adolescents. Currently, the coverage rate of protective antibodies is significantly higher in persons born after 1992 than in those born previously (Figure 3A)[26,27]. In fact, new infections generally occur in the population over 20-years-old not reached by vaccination[28]. These results emphasize the need to raise awareness among people not reached by universal HBV immunization programs and to focus vaccination campaigns on this group.
Figure 3.
Anti-hepatitis B surface antibodies titers by age. A: The anti-hepatitis B surface antibodies (anti-HBs) titer was determined in 765 blood donors. In 2000, vaccination against hepatitis B virus was included in the Argentine newborns’ National Vaccination Calendar. In 2003, the catch-up strategy for 11-year-old children was implemented. Therefore, individuals under 28-years-old are reached currently by the universal vaccine implementation. On average, protective levels of anti-HBs (> 10 mIU/mL) were detected in 75.2% of the population reached by universal vaccination (< 28-years-old) and in 32.2% of the not reached population (> 28-years-old); B: Anti-HBs kinetics. The anti-HBs titer was determined in 132 children born after 2000. In the first 2 years, the median anti-HBs titer fell from 196.2 mIU/mL to less than 10 mIU/mL (black line). Five years post-vaccination, about 20% of the population showed anti-HBs levels below 10 mIU/mL. aHBs: Anti-hepatitis B surface antibodies.
However, the most outstanding HBV preventive action impact has been detected in regions that were hyperendemic before introduction of the vaccine. In a Southern Italian area, where the vaccine was introduced in 1991, the HBsAg prevalence dropped from 13.4% in 1978 to 0.91% in 2006[29]. Likewise, in Alaska, where one of the highest HBV infection incidences has been reported, universal childhood vaccination was implemented for newborns in 1993. The HBsAg prevalence dropped from 13% detected before the HBV vaccination program, to 0% HBsAg-positive children less than 10-years-old[30].
Although slowly and delayed, a significant reduction in the hepatocellular carcinoma annual average incidence has been observed concomitantly with the HBV infection incidence decline, particularly in those countries where early vaccine protocols have been introduced[31-33].
IMMUNITY
HBV vaccine-induced protective immunity
Several studies have shown that HBV vaccines induced both humoral and cellular immunity providing long-term protection[34,35]. On the one hand, neutralizing antibodies are elicited and two types of them have been identified. The first type targets the “a” determinant and neutralizes cell viral penetration by blocking the interaction with heparan sulfate[36], required by the virus at an early stage of hepatocyte entrance[37]. The second type targets the high-affinity receptor-binding site of the HBV pre-S1 domain and blocks the binding to the Na+-taurocholate cotransporting polypeptide receptor preventing the infection of hepatocytes[38,39]. On the other hand, immune memory cells are generated, which upon contact with the HBV can be activated to expand rapidly. This response has been well demonstrated in studies that administered a booster dose to previously vaccinated persons whose antibody titers had fallen below protective titers[16,40-42].
Pre-exposure: Efficacy and effectiveness studies carried out in animal models first and then in human beings have shown that the HBV vaccine induces the production of neutralizing antibodies against HBV surface antigen (anti-HBs)[43]. Two main questions raised suddenly once the HBV vaccine was developed: What are the levels of antibodies that protect against infection and how long does immunity last? Soon after the release of the vaccine, several studies have shown that anti-HBs levels ≥ 10 mIU/mL, determined 1 to 3 mo after the complete three-dose vaccination scheme administration, were a surrogate marker for vaccine-induced protective immunity[44-46].
The overall response rate to the HBV vaccine, defined as individuals achieving anti-HBs levels > 10 mIU/mL, is 90% to 95% of immunized persons. Different factors such as host genetics, age, body weight, smoking, and concomitant disease have been shown to affect the response rate to the vaccine[47]. These variations probably rely on the strength of the cellular immune response. Velu et al[48] have characterized the cellular immune response and the cytokine profile of vaccine responders and non-responders to investigate the immunization outcome underlying mechanisms. The authors reported that HBsAg-specific interferon gamma, interleukin 10, and tumor necrosis factor alpha secretion correlated with the HBV vaccine-induced humoral immune response. Likewise, non-responders had lower levels of T helper type 1 (Th1) and Th2 cytokines. In addition, Körber et al[49] observed a higher frequency of regulatory B cells in HBV vaccine non-responders. Regulatory B cells suppress immunopathology by skewing T-cell differentiation. Overall, these results suggest that impaired lymphocyte activation is associated with a weak or no response to HBV vaccination.
Notably, although the HBV vaccine induces protective immunity against infection it would not be sterilizing. Consequently, vaccinated people can become infected although episodes are usually asymptomatic and self-limited[50-52]. These benign infection results are possibly due to long-lasting HBV cellular immunity induced by the vaccine despite antibody loss against HBV surface antigens[53].
Post-exposure: Immunization with HBV vaccines combined with different injection sites of HBV immunoglobulin administration, within 12 h after birth, showed a greater than 85% efficacy in preventing infection in infants born to HBsAg-positive mothers[54]. In adult persons, post-exposure prophylaxis is also recommended depending on the individual’s vaccination and anti-HBs status. In unvaccinated subjects or with non-protective levels of anti-HBs, the HBV vaccine has shown high efficacy in preventing infection when administered within 24 h after percutaneous or mucosal exposure to HBV-positive blood. Additional post-exposure immunoprophylaxis is not suggested in individuals who achieved anti-HBs protective levels after vaccination.
Although, as previously mentioned, the massive implementation of the HBV vaccine substantially reduced the incidence and prevalence of the infection, few works have addressed the effectiveness of the vaccine and most of them have been carried out in high endemic countries. In general, a 70% to 94% effectiveness range has been reported, depending mainly on the follow-up time, the exposure risk rate (HBsAg prevalence of the population), and the studied cohort age[18,23,55]. A recent study showed an approximately 58% effectiveness in a birth cohort (mean age, 12 years) and 85% in participants at least 20-years-old[56]. The lower efficacy observed in the birth cohort could be a consequence of a lower level of exposure.
HOW LONG DO THE ANTIBODIES LAST?
Protective antibodies levels tend to decrease over time, especially during the 1st years after vaccination[57]. In a study carried out by our group, including 132 children born after infants’ vaccine implementation, we observed that anti-HBs titers were significantly higher 1 year post-vaccination compared to the 2 years and 3 years earliest vaccinated population (Figure 3B). In addition, approximately 20% of the 5-year post-vaccination cohort showed anti-HBs levels lower than 10 mIU/mL[27]. These results are in line with a study conducted in Germany, where anti-HBs levels were determined in 106 teenagers, mean age 13.7-years-old, after primary vaccination. Forty percent of cases had anti-HBs levels < 10 mIU/mL. However, almost all (97%) teenagers who received a booster vaccine achieved anti-HBs levels ≥ 100 mIU/mL regardless of their pre-booster levels[58]. Besides time elapsed since primary vaccination, a systematic meta-analysis including 46 studies analyzed the anti-HBs levels from 5 years to 20 years after the primary vaccination and identified the vaccination dose and the less than 6-mo interval between the last dose and the previous one as the main factors associated with anti-HBs titer loss[59]. On the other hand, the duration of antibody levels is directly correlated with the titers reached when completing the vaccination scheme[60].
Another interesting issue regarding antibodies duration refers to the subjects’ age at the time of vaccination. Numerous studies have shown that vaccination in adolescence generates higher and long-lasting titers compared to children vaccinated at birth[61-64], being the age at the time of vaccination an independent variable associated with an anti-HBs titer < 10 mIU/mL. However, childhood HBV vaccination, together with other vaccines, guarantees a higher coverage rate.
Overall, it is widely accepted that a large proportion of vaccinated individuals, particularly those immunized during childhood, rapidly lose their anti-HBs titers below protective levels[40]. However, it has also been extensively described that individuals who achieved anti-HBs protective titers at the time of vaccination, show a rapid anamnestic response when boosted[41,42], suggesting that memory immunity plays a decisive role in the protection against clinical disease and the development of a carrier state regardless of anti-HBs antibody levels[35]. In this regard, it has been observed that even in the lack of anti-HBs, a significant amount of HBsAg-specific memory T and B cells are detected in vaccine responders. For this reason, although it remains a controversial issue, vaccine booster doses are not recommended currently for children and adults with normal immune status, despite the overtime drop of anti-HBs antibody titers[35,65]. Nonetheless, the anti-HBs titer decline could represent a problem for high-risk groups.
MANAGEMENT OF SPECIAL POPULATIONS
Adult persons with increased risk factors for infection are one of the WHO identified obstacles to HBV elimination as a public health problem[66,67]. These groups mainly include health care providers, illicit injected drug users, sexually active individuals (more than 1 partner in the past 6 mo), persons with diabetes, dialysis patients, and people living with human immunodeficiency virus (HIV). The last two groups, in addition to showing a higher risk of HBV infection compared to the general population[68-70] due to the frequent use of percutaneous materials and the common route of HBV and HIV transmission, have shown suboptimal responses to HBV immunization[71,72]. Patients on dialysis have also shown a diminished response to the HBV vaccine probably due to a uremic-associated suppression of the immune system that leads to a significant progressive reduction of the percentage and count of lymphocytes CD3+, CD4+, and CD8+ and a disturbance of antigen-presenting cells that results in an inability to sustain a satisfactory antibody titer over time[73-76]. In fact, in this population subset, the rate of seroprotection level ranges from 33.3% to 86%[77].
It has been reported that patients living with HIV present a poor initial HBV immunization response, lower seroconversion rates, and difficulty in maintaining immunity over time, mainly due to B-cell dysfunction[67,71,78,79]. For this group, the efficacy of the standard vaccine scheme in the era of the highly active antiretroviral therapy ranges from 17.5% to 71%[80-83].
Consequently, for patients on dialysis and/or living with HIV, other approaches are recommended to enhance the HBV vaccine immune response. For patients undergoing dialysis therapy, alternative strategies include the use of adjuvants, additional vaccination cycles, different vaccine formulations, greater number and concentration of doses, greater frequency of doses, dual vaccination, alternative administration routes, and/or use of booster vaccines[67,72,84-86]. On the other hand, for HIV-infected individuals with negative or < 10 mIU/mL anti-HBs levels after a primary vaccine series, a second HBV vaccine series using larger or additional doses is recommended[71,87]. Furthermore, revaccination should be attempted after HIV viral load suppression and CD4 cell count improvement[83].
RATE OF RESPONSE TO HBV VACCINE
As mentioned above, the average response rate to the HBV vaccine is greater than 90% with 5% to 10% vaccinated persons failing to mount a protective immunity level once the vaccination schedule is completed. The response to the vaccine ultimately relies on the individual immune system; however, different factors impairing the response rate have been identified. Response rates can drop drastically when more than one of these factors are present.
Host genetics
Several studies have addressed, through different experimental approaches, the role of genetic polymorphisms in the response to the HBV vaccine. Single nucleotide polymorphisms in HLA loci[88-92], ILs (with a key role in the cellular and humoral response interplay)[93,94], or even other genes have been associated with the response rate to HBV vaccination[90,95,96]. However, these findings have not been widely validated in different cohorts and should be considered with caution.
Age
One of the most recognized consequences of aging is the declination of the immune function and the concomitant vaccination response reduction. The HBV vaccine response rate decreases in people 40-years-old and even more in people older than 60 years[97,98]. This highlights the need to vaccinate the population not covered by health policies before they reach 40-years-old, which will result in important cost-benefit profits.
Body weight
Overweight and obesity are a growing public health problem worldwide that affects all age groups[99]. They are caused by the deposition of lipids into the adipose tissue and are defined as a body mass index ≥ 25 kg/m.
Shortly after the HBV vaccine was developed and implemented, obesity was found to be a factor impairing the strength of the immune response[100]. This finding has been widely validated in subsequent studies[101,102]. This drawback is not only attributed to the HBV vaccine but has also been described for other vaccines[103,104]. Adipose tissue has a role in modulating the immune system through different pathways, inducing a chronic pro-inflammatory state[105,106], which in the end is associated with immune system dysfunction. This includes the chronic activation of cells of the innate immune system and consequent local and systemic inflammation[107]. In addition, Frasca et al[108] described a percentage decrease of switched memory and transitional B cells and an increase of late/exhausted memory B cells with the consequent impaired response to the vaccine.
Smoking
As described for obesity, the link between tobacco smoking and impaired vaccine response has been proposed to be mediated by inflammation[109]. However, data from different studies are less robust. Some studies have reported lower responses to vaccination while others showed no association[110]. This controversy could be based on the level of daily cigarette intake. A recent study reported that subjects in the non-responder group were almost exclusively ‘heavy smokers’ defined as consumers of ≥ 10 cigarettes per day[111]. The development of new vaccine formulations including either additional antigens or more potent adjuvants could represent a solution to improve the response rate of individuals affected by these factors as well as for dialyzed or immunosuppressed patients.
NEW FORMULATIONS
As previously mentioned, one of the main drawbacks of the second-generation vaccines is the poor induction of immune response in 5% to 10% of the general population and individuals presenting detrimental factors that impair vaccine response. Therefore, efforts have been made to find more effective formulations to overcome this limitation. Two advances have been reported in recent years. One of them is the development and evaluation of new and more powerful adjuvants to enhance immunogenicity[112,113].
Heplisav-B (HepB-CpG), a single-antigen vaccine with a novel immunostimulatory adjuvant, has been approved for its use in people at least 18-years-old. This vaccine is administered in two doses, 1 mo apart[114]. The new adjuvant is a small synthetic cytidine-phosphate-guanosine oligodeoxynucleotide containing non-methylated CpG patterns, similar to those present in microbial DNA. This structure acts as an agonist of the toll-like receptor 9 that enhances the immune response. Several studies have shown higher response rates to other second-generation vaccines both in general population[115-117] and in persons with detrimental factors for vaccination response[118-120]. In addition, the two doses-1 mo apart-simplified schedule could help increase patient compliance and raise the coverage rate.
Alternatively, third-generation vaccines derived from mammalian cells, containing the medium and large HBV envelope proteins have been developed. The advantage of this approach is that antigens display the same in vivo post-translational modifications and protein folding.
In 2021, Sci-B-Vac was licensed, and phase III trials showed faster seroprotection and higher response rates than the second-generation vaccines[8,121]. These data making turn Sci-B-Vac of particular interest for its use in people with poor or no response. Particularly, Sci-B-Vac has shown greater efficacy in HIV-infected individuals’ immunization and in the prevention of vertical infection transmission[122,123]. Furthermore, the multiple antigen display of the third-generation vaccines would protect against HBV vaccine breakthrough infections caused by the HBV S gene mutants widely described[124-126]. The results obtained through novel vaccine formulation approaches suppose a contribution to the prophylaxis of HBV infection and represent a promising future.
CURRENT CHALLENGE
Beyond significant advances in the prevention of HBV infection, several pitfalls have been identified that need to be overcome in order to eliminate HBV as a health problem[127]. Particularly, in developing countries, sustainable financial mechanisms are required to scale up screening interventions and ensure access to vaccines.
Regarding the at birth dose, out-of-hospital deliveries, shortage of monovalent vaccine formulation in some regions, insufficient training of health care providers, weak monitoring and reporting systems, and low government commitment impair its implementation. On the other hand, in the adult population not covered by universal vaccination, promoting information, raising consciousness about risk, and finally focusing and promoting vaccination campaigns should improve immunization strategies for this group.
CONCLUSION
The worldwide application of HBV vaccines has led to a significant decrease in HBV infection incidence and its related death rates. As a general strategy, surveys are necessary to identify local constraints (in regions or countries) in order to achieve the implementation of WHO guidelines, both at the prophylaxis and diagnostic levels. Increasing efforts to improve vaccination coverage and raise awareness among populations not reached by universal vaccination will contribute significantly to achieving the WHO goals by 2030. Although HBV vaccines induce protective immunity in more than 90% of immunized people, there are particular settings where the efficacy is lower. In recent years, new formulations containing new adjuvants or other HBV antigens in addition to HBs, that could overcome the limitations of current presentations.
ACKNOWLEDGEMENTS
To Silvina Heisecke, from CEMIC-CONICET, for the copyediting of the manuscript.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 16, 2022
First decision: May 10, 2022
Article in press: July 24, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: JAN CFJ, Taiwan; Kumar R, India; Maslennikov R, Russia S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
Contributor Information
Federico Alejandro Di Lello, Microbiology, Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Instituto de Investigaciones en Bacteriología y Virología Molecular, Buenos Aires C1113AAD, Argentina; Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires C1425FQB, Argentina.
Alfredo Pedro Martínez, Virology Section, Centro de Educación Médica e Investigaciones Clínicas Norberto Quirno “CEMIC”, Buenos Aires C1431FWO, Argentina.
Diego Martín Flichman, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires C1425FQB, Argentina; Microbiology, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y Síndrome de Inmunodeficiencia Adquirida, Buenos Aires C1121ABG, Argentina. dflichman@ffyb.uba.ar.
References
- 1.Beasley RP. Development of hepatitis B vaccine. JAMA. 2009;302:322–324. doi: 10.1001/jama.2009.1024. [DOI] [PubMed] [Google Scholar]
- 2.Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine. 2009;27:1858–1862. doi: 10.1016/j.vaccine.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Kim YJ, Li P, Hong JM, Ryu KH, Nam E, Chang MS. A Single Center Analysis of the Positivity of Hepatitis B Antibody after Neonatal Vaccination Program in Korea. J Korean Med Sci. 2017;32:810–816. doi: 10.3346/jkms.2017.32.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu SH, Chih AH, Lee YC, Huang KC, Jan CF. Higher disappearance rate of anti-HBs in Taiwanese freshers neonatally vaccinated with recombinant yeast hepatitis B vaccine. Liver Int. 2017;37:1780–1787. doi: 10.1111/liv.13437. [DOI] [PubMed] [Google Scholar]
- 5.Hu YC, Yeh CC, Chen RY, Su CT, Wang WC, Bai CH, Chan CF, Su FH. Seroprevalence of hepatitis B virus in Taiwan 30 years after the commencement of the national vaccination program. PeerJ. 2018;6:e4297. doi: 10.7717/peerj.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shouval D, Roggendorf H, Roggendorf M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Med Microbiol Immunol. 2015;204:57–68. doi: 10.1007/s00430-014-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krawczyk A, Ludwig C, Jochum C, Fiedler M, Heinemann FM, Shouval D, Roggendorf M, Roggendorf H, Lindemann M. Induction of a robust T- and B-cell immune response in non- and low-responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine. 2014;32:5077–5082. doi: 10.1016/j.vaccine.2014.06.076. [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Langley JM, Segall N, Ward BJ, Cooper C, Poliquin G, Smith B, Gantt S, McElhaney JE, Dionne M, van Damme P, Leroux-Roels I, Leroux-Roels G, Machluf N, Spaans JN, Yassin-Rajkumar B, Anderson DE, Popovic V, Diaz-Mitoma F PROTECT Study Group. Immunogenicity and safety of a tri-antigenic vs a mono-antigenic hepatitis B vaccine in adults (PROTECT): a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2021;21:1271–1281. doi: 10.1016/S1473-3099(20)30780-5. [DOI] [PubMed] [Google Scholar]
- 9.Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Hepatitis B Control Through Immunization: A Reference Guide. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: http://iris.wpro.who.int/bitstream/handle/10665.1/10820/9789290616696_eng.pdf;jsessionid=FEDDD4672274909D7E6998B86BED45C8?sequence=3%0Ahttp://www.who.int/immunization/sage/meetings/2015/october/8_WPRO_Hepatitis_B_Prevention_Through_Immunization_Regional_R .
- 11.de Villiers MJ, Nayagam S, Hallett TB. The impact of the timely birth dose vaccine on the global elimination of hepatitis B. Nat Commun. 2021;12:6223. doi: 10.1038/s41467-021-26475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Progress Towards Global Immunization Goals – 2019. Summary presentations of key indicators, Updated July 2020. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://cdn.who.int/media/docs/default-source/immunization/global_monitoring/slidesglobalimmunization.pdf?sfvrsn=25385c3b_7 .
- 13.World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief (World Health Organization, Geneva, 2016). [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/combating-hepatitis-b-and-c-to-reach-elimination-by-2030 .
- 14.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/WHO-HIV-2016.06 .
- 15.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–6273. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 16.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390–1396. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- 17.Mohaghegh Shelmani H, Karayiannis P, Ashtari S, Mahmanzar MA, Khanabadi B, Modami N, Gholipour F, Zare F, Zali MR. Demographic changes of hepatitis B virus infection in Iran for the last two decades. Gastroenterol Hepatol Bed Bench. 2017;10:S38–S43. [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia D, Porras A, Rico Mendoza A, Alvis N, Navas MC, De La Hoz F, De Neira M, Osorio E, Valderrama JF. Hepatitis B infection control in Colombian Amazon after 15 years of hepatitis B vaccination. Effectiveness of birth dose and current prevalence. Vaccine. 2018;36:2721–2726. doi: 10.1016/j.vaccine.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Stroffolini T, Guadagnino V, Rapicetta M, Menniti Ippolito F, Caroleo B, De Sarro G, Focà A, Liberto MC, Giancotti A, Barreca GS, Marascio N, Lombardo F, Staltari O Sersale's Study Collaborating Group. The impact of a vaccination campaign against hepatitis B on the further decrease of hepatitis B virus infection in a southern Italian town over 14 years. Eur J Intern Med. 2012;23:e190–e192. doi: 10.1016/j.ejim.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Huynh C, Minuk GY, Uhanova J, Baikie M, Wong T, Osiowy C. Serological and molecular epidemiological outcomes after two decades of universal infant hepatitis B virus (HBV) vaccination in Nunavut, Canada. Vaccine. 2017;35:4515–4522. doi: 10.1016/j.vaccine.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Lu FT, Ni YH. Elimination of Mother-to-Infant Transmission of Hepatitis B Virus: 35 Years of Experience. Pediatr Gastroenterol Hepatol Nutr. 2020;23:311–318. doi: 10.5223/pghn.2020.23.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gust ID. Immunisation against hepatitis B in Taiwan. Gut. 1996;38 Suppl 2:S67–S68. doi: 10.1136/gut.38.suppl_2.s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien YC, Jan CF, Kuo HS, Chen CJ. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28:126–135. doi: 10.1093/epirev/mxj010. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Diseases Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, Chapter 10: Hepatitis B. [cited 5 February 2021]. Available From: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html .
- 25.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 26.Stecher D, Katz N, Vizzotti C. Hepatitis B en Argentina. Situación actual y estrategia de vacunación universal para su control y eliminación. Actualizaciones en Sida e Infectología. 2014;22:18–21. [Google Scholar]
- 27.Di Lello FA, Blejer J, Alter A, Bartoli S, Vargas F, Ruiz R, Galli C, Blanco S, Gallego S, Fernández R, Martínez AP, Flichman DM. Hepatitis B surface antibodies seroprevalence among people born before and after implementation of universal HBV vaccination. Vaccine. 2020;38:2678–2682. doi: 10.1016/j.vaccine.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Ministerio de Salud y desarrollo Social. Boletín sobre las Hepatitis Virales en Argentina, N 1, AÑO 1, Octubre 2019. [cited 5 February 2021]. Available from: https://bancos.salud.gob.ar/sites/default/files/2020-01/0000001592cnt-2019-10_boletin-hepatitis.pdf .
- 29.Da Villa G, Romanò L, Sepe A, Iorio R, Paribello N, Zappa A, Zanetti AR. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine. 2007;25:3133–3136. doi: 10.1016/j.vaccine.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Harpaz R, McMahon BJ, Margolis HS, Shapiro CN, Havron D, Carpenter G, Bulkow LR, Wainwright RB. Elimination of new chronic hepatitis B virus infections: results of the Alaska immunization program. J Infect Dis. 2000;181:413–418. doi: 10.1086/315259. [DOI] [PubMed] [Google Scholar]
- 31.Lin CL, Kao JH. Hepatitis B: Immunization and Impact on Natural History and Cancer Incidence. Gastroenterol Clin North Am. 2020;49:201–214. doi: 10.1016/j.gtc.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 33.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 34.Said ZN, Abdelwahab KS. Induced immunity against hepatitis B virus. World J Hepatol. 2015;7:1660–1670. doi: 10.4254/wjh.v7.i12.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Damme P, Dionne M, Leroux-Roels G, Van Der Meeren O, Di Paolo E, Salaun B, Surya Kiran P, Folschweiller N. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J Viral Hepat. 2019;26:1066–1075. doi: 10.1111/jvh.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sureau C, Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology. 2013;57:985–994. doi: 10.1002/hep.26125. [DOI] [PubMed] [Google Scholar]
- 37.Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- 38.Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48–64. doi: 10.1053/j.gastro.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, Lee CY. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis. 2008;197:1419–1426. doi: 10.1086/587695. [DOI] [PubMed] [Google Scholar]
- 41.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, Spradling PR, Baum R, Hennessy T, McMahon BJ. Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J Infect Dis. 2016;214:16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 42.Wang ZZ, Gao YH, Lu W, Jin CD, Zeng Y, Yan L, Ding F, Li T, Liu XE, Zhuang H. Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum Vaccin Immunother. 2017;13:909–915. doi: 10.1080/21645515.2016.1250990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bitter GA, Egan KM, Burnette WN, Samal B, Fieschko JC, Peterson DL, Downing MR, Wypych J, Langley KE. Hepatitis B vaccine produced in yeast. J Med Virol. 1988;25:123–140. doi: 10.1002/jmv.1890250202. [DOI] [PubMed] [Google Scholar]
- 44.Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–492. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 45.Hall AJ. Hepatitis B vaccination: protection for how long and against what? BMJ. 1993;307:276–277. doi: 10.1136/bmj.307.6899.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Immunisation against hepatitis B. Lancet. 1988;1:875–876. [PubMed] [Google Scholar]
- 47.Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, Xu K, Ren J, Yao J, Li Y, Cao Q, Chen P, Xie T, Wang C, Wang B, Mao C, Ruan B, Jiang T, Li L. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6:27251. doi: 10.1038/srep27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velu V, Saravanan S, Nandakumar S, Shankar EM, Vengatesan A, Jadhav SS, Kulkarni PS, Thyagarajan SP. Relationship between T-lymphocyte cytokine levels and sero-response to hepatitis B vaccines. World J Gastroenterol. 2008;14:3534–3540. doi: 10.3748/wjg.14.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Körber N, Pohl L, Weinberger B, Grubeck-Loebenstein B, Wawer A, Knolle PA, Roggendorf H, Protzer U, Bauer T. Hepatitis B Vaccine Non-Responders Show Higher Frequencies of CD24highCD38high Regulatory B Cells and Lower Levels of IL-10 Expression Compared to Responders. Front Immunol. 2021;12:713351. doi: 10.3389/fimmu.2021.713351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werner JM, Abdalla A, Gara N, Ghany MG, Rehermann B. The hepatitis B vaccine protects re-exposed health care workers, but does not provide sterilizing immunity. Gastroenterology. 2013;145:1026–1034. doi: 10.1053/j.gastro.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su TH, Chen PJ. Emerging hepatitis B virus infection in vaccinated populations: a rising concern? Emerg Microbes Infect. 2012;1:e27. doi: 10.1038/emi.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Kuriyakose S, Leyssen M, Jacquet JM. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat. 2011;18:369–375. doi: 10.1111/j.1365-2893.2010.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simons BC, Spradling PR, Bruden DJ, Zanis C, Case S, Choromanski TL, Apodaca M, Brogdon HD, Dwyer G, Snowball M, Negus S, Bruce MG, Morishima C, Knall C, McMahon BJ. A Longitudinal Hepatitis B Vaccine Cohort Demonstrates Long-lasting Hepatitis B Virus (HBV) Cellular Immunity Despite Loss of Antibody Against HBV Surface Antigen. J Infect Dis. 2016;214:273–280. doi: 10.1093/infdis/jiw142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu AS, Cheung RC, Keeffe EB. Hepatitis B vaccines. Clin Liver Dis. 2004;8:283–300. doi: 10.1016/j.cld.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Peto TJ, Mendy ME, Lowe Y, Webb EL, Whittle HC, Hall AJ. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986-90) and in the nationwide immunisation program. BMC Infect Dis. 2014;14:7. doi: 10.1186/1471-2334-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He WQ, Guo GN, Li C. The impact of hepatitis B vaccination in the United States, 1999-2018. Hepatology. 2022;75:1566–1578. doi: 10.1002/hep.32265. [DOI] [PubMed] [Google Scholar]
- 57.Jilg W, Schmidt M, Deinhardt F. Four-year experience with a recombinant hepatitis B vaccine. Infection. 1989;17:70–76. doi: 10.1007/BF01646879. [DOI] [PubMed] [Google Scholar]
- 58.Anderson CL, Remschmidt C, Drobnitzky FP, Falkenhorst G, Zimmermann R, Wichmann O, Harder T. Hepatitis B immune status in adolescents vaccinated during infancy: A retrospective cohort study from a pediatric practice in Germany. Hum Vaccin Immunother. 2016;12:779–784. doi: 10.1080/21645515.2015.1105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schönberger K, Riedel C, Rückinger S, Mansmann U, Jilg W, Kries RV. Determinants of Long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32:307–313. doi: 10.1097/INF.0b013e31827bd1b0. [DOI] [PubMed] [Google Scholar]
- 60.Mendy M, Peterson I, Hossin S, Peto T, Jobarteh ML, Jeng-Barry A, Sidibeh M, Jatta A, Moore SE, Hall AJ, Whittle H. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One. 2013;8:e58029. doi: 10.1371/journal.pone.0058029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coppola N, Corvino AR, De Pascalis S, Signoriello G, Di Fiore E, Nienhaus A, Sagnelli E, Lamberti M. The long-term immunogenicity of recombinant hepatitis B virus (HBV) vaccine: contribution of universal HBV vaccination in Italy. BMC Infect Dis. 2015;15:149. doi: 10.1186/s12879-015-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trevisan A, Mason P, Nicolli A, Maso S, Fonzo M, Scarpa B, Bertoncello C. Future Healthcare Workers and Hepatitis B Vaccination: A New Generation. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18157783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35:6302–6307. doi: 10.1016/j.vaccine.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 64.Stefanati A, Bolognesi N, Sandri F, Dini G, Massa E, Montecucco A, Lupi S, Gabutti G. Long-term persistency of hepatitis B immunity: an observational cross-sectional study on medical students and resident doctors. J Prev Med Hyg. 2019;60:E184–E190. doi: 10.15167/2421-4248/jpmh2019.60.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24:572–577. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization. Hepatitis B vaccines: WHO position paper – July 2017. [cited 5 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/immunization/policy/position_papers/hepatitis_b/en/
- 67.Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for Use of a Hepatitis B Vaccine with a Novel Adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455–458. doi: 10.15585/mmwr.mm6715a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burdick RA, Bragg-Gresham JL, Woods JD, Hedderwick SA, Kurokawa K, Combe C, Saito A, LaBrecque J, Port FK, Young EW. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2003;63:2222–2229. doi: 10.1046/j.1523-1755.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 69.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138–S145. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 70.Pereson MJ, Martínez AP, Isaac K, Laham G, Ridruejo E, Garcia GH, Flichman DM, Di Lello FA. Seroprevalence of hepatitis B, hepatitis C and HIV infection among patients undergoing haemodialysis in Buenos Aires, Argentina. J Med Microbiol. 2021;70 doi: 10.1099/jmm.0.001278. [DOI] [PubMed] [Google Scholar]
- 71.Whitaker JA, Rouphael NG, Edupuganti S, Lai L, Mulligan MJ. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect Dis. 2012;12:966–976. doi: 10.1016/S1473-3099(12)70243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabrizi F, Cerutti R, Alfieri CM, Ridruejo E. An Update on Hepatocellular Carcinoma in Chronic Kidney Disease. Cancers (Basel) 2021;13 doi: 10.3390/cancers13143617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fabrizi F, Martin P. Hepatitis B virus infection in dialysis patients. Am J Nephrol. 2000;20:1–11. doi: 10.1159/000013548. [DOI] [PubMed] [Google Scholar]
- 74.Cohen G, Hörl WH. Immune dysfunction in uremia—an update. Toxins (Basel) 2012;4:962–990. doi: 10.3390/toxins4110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sampani E, Vagiotas L, Daikidou DV, Nikolaidou V, Xochelli A, Kasimatis E, Lioulios G, Dimitriadis C, Fylaktou A, Papagianni A, Stangou M. End stage renal disease has an early and continuous detrimental effect on regulatory T cells. Nephrology (Carlton) 2022;27:281–287. doi: 10.1111/nep.13996. [DOI] [PubMed] [Google Scholar]
- 76.Sampani E, Stangou M, Daikidou DV, Nikolaidou V, Asouchidou D, Dimitriadis C, Lioulios G, Xochelli A, Fylaktou A, Papagianni A. Influence of end stage renal disease on CD28 expression and T-cell immunity. Nephrology (Carlton) 2021;26:185–196. doi: 10.1111/nep.13784. [DOI] [PubMed] [Google Scholar]
- 77.Fabrizi F, Lunghi G, Martin P. Treatment of HBV-related liver disease in the dialysis population: reality and promises. Int J Artif Organs. 2001;24:598–605. [PubMed] [Google Scholar]
- 78.Kernéis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boëlle PY. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis. 2014;58:1130–1139. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moretto F, Catherine FX, Esteve C, Blot M, Piroth L. Isolated Anti-HBc: Significance and Management. J Clin Med. 2020;9 doi: 10.3390/jcm9010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, Wendling MJ, Vetter D, Nicolle M, Kempf-Durepaire G, Lang JM. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161–1165. doi: 10.1016/s0264-410x(99)00389-8. [DOI] [PubMed] [Google Scholar]
- 81.Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41:1045–1048. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]
- 82.Paitoonpong L, Suankratay C. Immunological response to hepatitis B vaccination in patients with AIDS and virological response to highly active antiretroviral therapy. Scand J Infect Dis. 2008;40:54–58. doi: 10.1080/00365540701522975. [DOI] [PubMed] [Google Scholar]
- 83.Landrum ML, Huppler Hullsiek K, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, Peel S, Agan BK. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27:4731–4738. doi: 10.1016/j.vaccine.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janus N, Vacher LV, Karie S, Ledneva E, Deray G. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23:800–807. doi: 10.1093/ndt/gfm851. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W, Du X, Zhao G, Jin H, Kang Y, Xiao C, Liu M, Wang B. Levamisole is a potential facilitator for the activation of Th1 responses of the subunit HBV vaccination. Vaccine. 2009;27:4938–4946. doi: 10.1016/j.vaccine.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 86.Haddiya I. Current Knowledge of Vaccinations in Chronic Kidney Disease Patients. Int J Nephrol Renovasc Dis. 2020;13:179–185. doi: 10.2147/IJNRD.S231142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vargas JI, Jensen D, Martínez F, Sarmiento V, Peirano F, Acuña P, Provoste F, Bustos V, Cornejo F, Fuster A, Acuña M, Fuster F, Soto S, Estay D, Jensen W, Ahumada R, Arab JP, Soza A. Comparative Efficacy of a High-Dose vs Standard-Dose Hepatitis B Revaccination Schedule Among Patients With HIV: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2120929. doi: 10.1001/jamanetworkopen.2021.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39:978–988. doi: 10.1002/hep.20142. [DOI] [PubMed] [Google Scholar]
- 89.Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010;11:232–238. doi: 10.1038/gene.2010.1. [DOI] [PubMed] [Google Scholar]
- 90.Pan LP, Zhang W, Zhang L, Wu XP, Zhu XL, Yan BY, Li JY, Xu AQ, Liu Y, Li H. CD3Z genetic polymorphism in immune response to hepatitis B vaccination in two independent Chinese populations. PLoS One. 2012;7:e35303. doi: 10.1371/journal.pone.0035303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan L, Zhang L, Zhang W, Wu X, Li Y, Yan B, Zhu X, Liu X, Yang C, Xu J, Zhou G, Xu A, Li H, Liu Y. A genome-wide association study identifies polymorphisms in the HLA-DR region associated with non-response to hepatitis B vaccination in Chinese Han populations. Hum Mol Genet. 2014;23:2210–2219. doi: 10.1093/hmg/ddt586. [DOI] [PubMed] [Google Scholar]
- 92.Ou G, Liu X, Jiang Y. HLA-DPB1 alleles in hepatitis B vaccine response: A meta-analysis. Medicine (Baltimore) 2021;100:e24904. doi: 10.1097/MD.0000000000024904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui W, Sun CM, Deng BC, Liu P. Association of polymorphisms in the interleukin-4 gene with response to hepatitis B vaccine and susceptibility to hepatitis B virus infection: a meta-analysis. Gene. 2013;525:35–40. doi: 10.1016/j.gene.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 94.Höhler T, Reuss E, Freitag CM, Schneider PM. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology. 2005;42:72–76. doi: 10.1002/hep.20740. [DOI] [PubMed] [Google Scholar]
- 95.Duan Z, Chen X, Liang Z, Zeng Y, Zhu F, Long L, McCrae MA, Zhuang H, Shen T, Lu F. Genetic polymorphisms of CXCR5 and CXCL13 are associated with non-responsiveness to the hepatitis B vaccine. Vaccine. 2014;32:5316–5322. doi: 10.1016/j.vaccine.2014.07.064. [DOI] [PubMed] [Google Scholar]
- 96.Höhler T, Reuss E, Evers N, Dietrich E, Rittner C, Freitag CM, Vollmar J, Schneider PM, Fimmers R. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: a vaccination study in twins. Lancet. 2002;360:991–995. doi: 10.1016/S0140-6736(02)11083-X. [DOI] [PubMed] [Google Scholar]
- 97.Van Der Meeren O, Crasta P, Cheuvart B, De Ridder M. Characterization of an age-response relationship to GSK's recombinant hepatitis B vaccine in healthy adults: An integrated analysis. Hum Vaccin Immunother. 2015;11:1726–1729. doi: 10.1080/21645515.2015.1039758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weinberger B, Haks MC, de Paus RA, Ottenhoff THM, Bauer T, Grubeck-Loebenstein B. Impaired Immune Response to Primary but Not to Booster Vaccination Against Hepatitis B in Older Adults. Front Immunol. 2018;9:1035. doi: 10.3389/fimmu.2018.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985;254:3187–3189. [PubMed] [Google Scholar]
- 101.Young KM, Gray CM, Bekker LG. Is obesity a risk factor for vaccine non-responsiveness? PLoS One. 2013;8:e82779. doi: 10.1371/journal.pone.0082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu F, Guo Z, Dong C. Influences of obesity on the immunogenicity of Hepatitis B vaccine. Hum Vaccin Immunother. 2017;13:1014–1017. doi: 10.1080/21645515.2016.1274475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33:4422–4429. doi: 10.1016/j.vaccine.2015.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tagliabue C, Principi N, Giavoli C, Esposito S. Obesity: impact of infections and response to vaccines. Eur J Clin Microbiol Infect Dis. 2016;35:325–331. doi: 10.1007/s10096-015-2558-8. [DOI] [PubMed] [Google Scholar]
- 105.Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 107.Thomas AL, Alarcon PC, Divanovic S, Chougne CA, Hildeman DA, Moreno-Fernandez ME. Implications of Inflammatory States on Dysfunctional Immune Responses in Aging and Obesity. Front Aging. 2021;2:732414. doi: 10.3389/fragi.2021.732414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) 2016;24:615–625. doi: 10.1002/oby.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Younas M, Carrat F, Desaint C, Launay O, Corbeau P ANRS HB03 VIHVAC-B Trial Group. Immune activation, smoking, and vaccine response. AIDS. 2017;31:171–173. doi: 10.1097/QAD.0000000000001311. [DOI] [PubMed] [Google Scholar]
- 110.Zimmermann P, Curtis N. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev. 2019;32 doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meier MA, Berger CT. A simple clinical score to identify likely hepatitis B vaccination non-responders - data from a retrospective single center study. BMC Infect Dis. 2020;20:891. doi: 10.1186/s12879-020-05634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leroux-Roels G. Old and new adjuvants for hepatitis B vaccines. Med Microbiol Immunol. 2015;204:69–78. doi: 10.1007/s00430-014-0375-9. [DOI] [PubMed] [Google Scholar]
- 113.Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15:51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Food and Drug Administration. Product approval information: package insert. Heplisav-B. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2018. [cited 5 February 2021]. Available from: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm584752.htm .
- 115.Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, Shockey G, Akella L, Erby K, Heyward WL, Janssen RS HBV-23 Study Group. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36:668–674. doi: 10.1016/j.vaccine.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 116.Splawn LM, Bailey CA, Medina JP, Cho JC. Heplisav-B vaccination for the prevention of hepatitis B virus infection in adults in the United States. Drugs Today (Barc) 2018;54:399–405. doi: 10.1358/dot.2018.54.7.2833984. [DOI] [PubMed] [Google Scholar]
- 117.Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, Embree J, McGeer A, Zickler P, Moltz KH, Martz R, Meyer I, McNeil S, Langley JM, Martins E, Heyward WL, Martin JT. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine. 2012;30:2556–2563. doi: 10.1016/j.vaccine.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 118.Heyward WL, Kyle M, Blumenau J, Davis M, Reisinger K, Kabongo ML, Bennett S, Janssen RS, Namini H, Martin JT. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine. 2013;31:5300–5305. doi: 10.1016/j.vaccine.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 119.Halperin SA, Ward BJ, Dionne M, Langley JM, McNeil SA, Smith B, Mackinnon-Cameron D, Heyward WL, Martin JT. Immunogenicity of an investigational hepatitis B vaccine (hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide) in nonresponders to licensed hepatitis B vaccine. Hum Vaccin Immunother. 2013;9:1438–1444. doi: 10.4161/hv.24256. [DOI] [PubMed] [Google Scholar]
- 120.Champion CR. Heplisav-B: A Hepatitis B Vaccine With a Novel Adjuvant. Ann Pharmacother. 2021;55:783–791. doi: 10.1177/1060028020962050. [DOI] [PubMed] [Google Scholar]
- 121.Vesikari T, Finn A, van Damme P, Leroux-Roels I, Leroux-Roels G, Segall N, Toma A, Vallieres G, Aronson R, Reich D, Arora S, Ruane PJ, Cone CL, Manns M, Cosgrove C, Faust SN, Ramasamy MN, Machluf N, Spaans JN, Yassin-Rajkumar B, Anderson D, Popovic V, Diaz-Mitoma F CONSTANT Study Group. Immunogenicity and Safety of a 3-Antigen Hepatitis B Vaccine vs a Single-Antigen Hepatitis B Vaccine: A Phase 3 Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2128652. doi: 10.1001/jamanetworkopen.2021.28652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alon D, Stein GY, Hadas-Golan V, Tau L, Brosh T, Turner D. Immunogenicity of Sci-B-Vac (a Third-Generation Hepatitis B Vaccine) in HIV-Positive Adults. Isr Med Assoc J. 2017;19:143–146. [PubMed] [Google Scholar]
- 123.Safadi R, Khoury T, Saed N, Hakim M, Jamalia J, Nijim Y, Farah N, Nuser T, Natur N, Mahamid M, Amer J, Roppert PL, Gerlich WH, Glebe D. Efficacy of Birth Dose Vaccination in Preventing Mother-to-Child Transmission of Hepatitis B: A Randomized Controlled Trial Comparing Engerix-B and Sci-B-Vac. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Lello FA, Ridruejo E, Martínez AP, Pérez PS, Campos RH, Flichman DM. Molecular epidemiology of hepatitis B virus mutants associated with vaccine escape, drug resistance and diagnosis failure. J Viral Hepat. 2019;26:552–560. doi: 10.1111/jvh.13052. [DOI] [PubMed] [Google Scholar]
- 125.Campos-Valdez M, Monroy-Ramírez HC, Armendáriz-Borunda J, Sánchez-Orozco LV. Molecular Mechanisms during Hepatitis B Infection and the Effects of the Virus Variability. Viruses. 2021;13 doi: 10.3390/v13061167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qin Y, Liao P. Hepatitis B virus vaccine breakthrough infection: surveillance of S gene mutants of HBV. Acta Virol. 2018;62:115–121. doi: 10.4149/av_2018_210. [DOI] [PubMed] [Google Scholar]
- 127.Ropero Alvarez AM, Vilajeliu A, Magariños M, Jauregui B, Guzmán L, Whittembury A, Cain E, Garcia O, Montesanos R, Ruiz Matus C PAHO MNI working group. Enablers and barriers of maternal and neonatal immunization programs in Latin America. Vaccine. 2021;39 Suppl 2:B34–B43. doi: 10.1016/j.vaccine.2020.07.051. [DOI] [PubMed] [Google Scholar]