Abstract
Although people with HIV are living longer, as they age they remain disproportionately burdened with multimorbidity that is exacerbated in resource-poor settings. The geroscience hypothesis postulates that a discrete set of between five and ten hallmarks of biological ageing drive multimorbidity, but these processes have not been systematically examined in the context of people with HIV. We examine four major hallmarks of ageing (macromolecular damage, senescence, inflammation, and stem-cell dysfunction) as gerodrivers in the context of people with HIV. As a counterbalance, we introduce healthy ageing, physiological reserve, intrinsic capacity, and resilience as promoters of geroprotection that counteract gerodrivers. We discuss emerging geroscience-based diagnostic biomarkers and therapeutic strategies, and provide examples based on recent advances in cellular senescence, and other, non-pharmacological approaches. Finally, we present a conceptual model of biological ageing in the general population and in people with HIV that integrates gerodrivers and geroprotectors as modulators of homoeostatic reserves and organ function over the lifecourse.
Introduction
Advances in antiretroviral therapy (ART) have enabled people with HIV to live longer and healthier lives than individuals diagnosed in the earlier years of the HIV pandemic.1 However, life expectancy and disease-free years of people with HIV variably differ compared with people without HIV, for reasons that include earlier treatment and traditional risk factors across cohorts.2,3 The Centers for Disease Control and Prevention estimate that 50% of people with HIV are older than 50 years, and account for 70% of total deaths among people with HIV.4 The primary causes of death for people with HIV who have access to combination ART are non-communicable diseases such as cancer, cardiovascular disease, and neurocognitive impairment. Older people with HIV also have deficits in physical function, frailty-related syndromes, and many other non-communicable diseases that limit their quality of life.5 Notably, the prevalence of age-related comorbidities has been found to be considerably higher in people with HIV than in people without HIV across a range of cohorts, including those in Brazil,6 Italy,7 and the USA.3 Efforts to explain this disproportionate comorbid burden in people with HIV have proposed that HIV infection accelerates or accentuates ageing independent of traditional risk factors, without specifying an association between mechanisms of ageing and disease susceptibility. Regardless of whether lifespan (ie, a person’s length of time alive) can be increased through evidence-based interventions, most health-care spending occurs at the end of life owing to a high prevalence of age-related conditions. Improving healthspan (ie, the part of a person’s life during which they are generally in good health) is therefore proposed to have a greater effect on wellbeing and economic indicators for a given population.8
The geroscience hypothesis postulates that a discrete set of biological processes creates a global susceptibility to diseases that leads to multimorbidity.9 Geroscience emerged from key advances in the biology of ageing, including the discoveries that biological ageing is influenced by evolutionarily conserved pathways that are modifiable, and that a discrete set of biochemical mechanisms (termed pillars or hallmarks) drive multiple age-related conditions.10–14 Of the five to ten overlapping processes that have been described extensively in the literature,10–14 given the constraints of space and scope we have focused on four in this Series paper—macro-molecular damage, senescence, inflammation, and stem-cell dysfunction—in the context of people with HIV. Innovations in measuring the rate of biological ageing in relation to chronological age include biomarkers for age-correlated changes in immune function,11 DNA methylation patterns,15,16 and the plasma proteome.17 Because of the disproportionate burden of comorbidities in people with HIV, and the framing of the biology of ageing as a condition of increasing multimorbid risk owing to loss in homoeostatic control of key biological processes, in this Series paper we explore whether advances in the biology of ageing and geroscience inform pathogenesis and provide insight into the management of people with HIV, and, reciprocally, how understanding HIV infection dynamics has informed the science of ageing.
Macromolecular damage
DNA damage and genomic instability
The maintenance of genome stability is continuously challenged by extrinsic and intrinsic destabilising factors that contribute to genomic instability (eg, point mutations, insertions and deletions, or rearrangements of large genomic fragments). Characteristic features of genomic instability are telomere dysfunction, epigenetic alterations, proteostatic stress, and compromised mitochondrial function.18
Several mechanisms related to HIV infection can lead to DNA damage and genomic instability. For example, a deficiency in DNA damage response has been observed both in latently infected cells and from exposure to nucleoside reverse transcriptase inhibitors.19 Long-term HIV-related psychosocial and behavioural stressors, through excessive stimulation of glucocorticoid signalling or sympathetic nervous system (adrenergic) signalling, increase oxidative stress and inflammation, which, in turn, promote DNA damage and genomic instability.20
Although there is good evidence for genomic instability in people with HIV, the link between genomic instability and age-related outcomes in people with HIV is less clear. Although newer technologies (eg, next-generation sequencing technology) offer the opportunity to survey human genomes, including ageing-related and infection-related genetic damage and genomic instability, the benefits of these technologies are currently limited by an incomplete understanding of the link between ageing-related genetic mutations and clinical outcomes.
Telomere attrition
Analysis of telomere length across multiple tissue types indicates that a shortening of telomere length is generally positively correlated with age in most tissues and in blood cells.21 Telomere attrition has been associated with multiple disease conditions.22 However, although knowledge of telomere attrition derived primarily from in-vitro and in-vivo studies suggests that telomere length is a hallmark of ageing, data from epidemiological and clinical studies show such strong linkage between telomere length and characteristics of ageing that causation and correlation cannot yet be differentiated.23 In the context of chronic, treated HIV infection, shorter telomere length has been documented in the blood cells of people with HIV than in people without HIV,24–26 and shorter blood telomere length has been linked to increased cardiovascular risk in people with HIV.27 Telomere length is also shorter in the lung epithelia of people with HIV than in people without HIV, possibly increasing risk of lung disease.28
Although there is evidence for excessive loss of telomere length in people with HIV, and associations of telomere attrition with disease progression, whether incremental declines in telomere length disproportionately increase the risk of disease onset, disease severity, and multimorbidity is unclear. In addition, whether the dynamics of the shortening of telomere length across tissue types in people with HIV differ from age-related declines in people without HIV is also not clear. Finally, more research is needed on the mechanistic links between telomere attrition and physical and neurocognitive function in people with HIV.
Loss of proteostasis
Under normal and stress conditions, protein homoeostasis (the equilibrium between protein synthesis and degradation) is maintained by two primary protein-clearance mechanisms: the ubiquitin–proteasome system and autophagy-mediated proteolysis.29 Proteostasis declines with ageing,30 and, conversely, the maintenance of proteostasis increases lifespan.31 Various non-communicable diseases are associated with impaired protein homoeostasis.32 For example, altered proteostasis and proteotoxicity are linked to diabetes, neurodegenerative conditions,33 cardiovascular disease,34 and sarcopenia.35 Notably, chaperone-mediated autophagy declines with ageing and brain pathology,36 and, by contrast, mice with preserved chaperone-mediated autophagy have increased lifespan and reduced frailty. Evidence is also emerging that the loss of regulated autophagy affects other hallmarks of ageing, including senescence37,38 and stem-cell function.39
HIV infection and viral proteins disrupt the autophagy process in various cell types, including macrophages, T cells, microglia, and astrocytes.40–42 Notably, HIV-associated neurocognitive disorder is increasing in prevalence among people with HIV despite treatment, and could be due to accentuated brain ageing,43 or alerted chaperone-mediated autophagy that prematurely degrades essential cellular machinery in neurons leading to neurodegeneration, or both.44 Also, mitophagy is impaired in primary neurons when exposed to the HIV proteins gp120 and Tat.45
Although there is clear evidence for deficits in proteostasis in many age-related diseases that disproportionately affect people with HIV, more data are needed that link proteostasis to health-related outcomes, quality of life, multimorbidity, and resilience, by comparing carefully matched people ageing with HIV to people ageing without HIV.
Mitochondrial dysfunction
Human ageing is linked to a progressive decline in mitochondrial function, which is characterised by the accumulation of mutations and deletions in mitochondrial DNA (mtDNA), mitophagy, reduced cellular energy production, and a mitochondrial-dysfunction-associated senescence phenotype.46 Mitochondrial dysfunction preferentially affects tissues with high energy demands, such as the brain, skeletal muscle, and heart, adversely affecting healthspan. Perturbations to this energetic pathway appear to be amplified by inflammation and reactive oxygen species47 and linked to age-related non-communicable disease.
Earlier nucleoside reverse transcriptase inhibitors were known inhibitors of mtDNA polymerase γ. The consequent accumulation of mtDNA mutations in skeletal muscle and decreased oxidative phosphorylation are well established and represent the earliest evidence of advanced ageing in people with HIV.48 Evidence from current nucleoside reverse transcriptase inhibitors shows that mitochondrial function might be affected in other cell types, such as renal cells.49 Abacavir and tenofovir have been shown to decrease the quantity of mtDNA in adipocytes, but only tenofovir decreased oxidative phosphorylation activity.50 Despite scarce information on other current ART, mitochondrial dysfunction shows the need for research into ageing that specifically focuses on people with HIV, so that the additive effects of ART can be considered.51
Mitochondrial dysfunction in people with HIV adversely affects physiological determinants of healthspan. There is accumulating evidence for deficits in skeletal muscle bioenergetics based on deficits in PGC-1α in muscle52 and reduced skeletal muscle oxidative phosphorylation, which correlates with low cardiorespiratory fitness.53 Cognitive dysfunction manifested as HIV-associated neurocognitive disorder provides evidence for the direct effects of HIV on mitochondrial function in people with HIV in whom a plasma HIV RNA load is undetectable but virally encoded proteins are expressed in the brain and linked to mtDNA mutations and oxidative damage.54 Similarly, in people with HIV who have not received ART, numerous virally encoded proteins are implicated in mitochondrial-facilitated apoptosis of CD4 lymphocytes and disrupted mitochondrial biogenesis.51
Cellular senescence and inflammation
The accumulation of senescent cells with ageing55 occurs asymmetrically across organ systems56 and contributes to age-related diseases.57 Clearance of senescent cells increases lifespan in mouse models.58 Notably, multiple hallmarks of ageing are influenced by cellular senescence, including inflammaging, mitochondrial dysfunction, and stem-cell exhaustion.59 Inflammaging, which was originally considered to be a non-infection, age-related, low-grade inflammation,60 might both be driven in part by senescence and be a component of the senescence-associated secretory phenotype.61 Inflammaging is exacerbated by multiple factors, including dysbiosis (imbalance of the intestinal microbiota composition) and the associated release of microbial products into the circulation (microbial translocation), and various other stressors that increase risk for many age-related diseases.60,62–64
The prevalence of age-related comorbidities remains higher in people with HIV than in the general population, which could be due partly to cellular senescence and consequent inflammation.65 Although there are no universal biomarkers for senescence, a composite score for cellular biomarkers has been associated with multimorbidity in a cohort of people with HIV.66 Notably, chronic exposure to pathogenic antigens (ie, cytomegalovirus or HIV) drives expansion of T cells into a state of replicative senescence (eg, elevated CD28 and decline in CD57)67 that is similar to, but distinct from, cellular senescence.68 People with HIV continue to have chronic low-grade inflammation compared with people without HIV, despite controlled viremia; for example, people with HIV who are on effective ART show persistent elevations in interleukin-6 (IL-6), TNF, soluble CD14, soluble CD163, C-reactive protein, and MCP-1 concentrations in peripheral blood compared with people without HIV.69–71 This observed HIV-associated inflammation is linked to adverse functional outcomes.72,73 The contribution of age-related inflammation in compartments and tissues other than peripheral blood remains unclear, as is how inflammaging pathways and their associated morbidity risks differ between people ageing with and without HIV.74
People with HIV have more exaggerated loss in thymic function than do people without HIV of similar age,75 and thymic function has been linked to geriatric syndromes such as frailty.76 However, the mechanistic links between age-related functional decline in lymphoid organs, the emergence and spread of senescent immune cells, and consequent multimorbidity in people with HIV remains unclear.
Stem-cell exhaustion
Stem cells reside in most tissues and range from unipotent (eg, muscle stem cells) to multipotent (eg, haematopoietic stem cells), with lineage and cell differentiation outcomes largely influenced by the epigenome, external stimuli, and stress response (eg, to injury). The regenerative potential of stem cells is generally associated with tissue turnover rates77 and markedly declines with ageing,77,78 with characteristic loss in lineage specificity, loss of self-renewal capacity, onset of cellular senescence, and accumulation of damage (eg, proteotoxicity).79 For example, with ageing, stem-cell function in blood skews towards the myeloid lineage, skeletal muscle repair declines, and neurogenesis in the brain declines.80 Chromatin modifications that limit stem-cell function have been observed in brain and muscle.81
In the context of HIV infection, loss in stem-cell functionality is evident across the range of stem-cell regenerative potentials. For example, loss of haematopoietic progenitors (CD34) and naive T cells is observed,82–86 resulting in outcomes that are possibly linked to systemic immune activation and inflammaging. Impaired neurogenesis has been linked to cognitive impairment in transgenic mice.87 Evidence for loss of intermediate stem-cell regenerative potential is indirect. For example, there is evidence for premature expression of an ageing signature in skeletal muscle88 and deficits in bioenergetic capacity52 that reflect ageing profiles in skeletal muscle.89,90 The prevalence of inflammation-related clonal haematopoiesis of indeterminate potential was shown to be higher in people with HIV than in a control group.91 Finally, mesenchymal stem cells that drive bone formation show evidence of impairment in HIV infection.92 Whether these losses resemble ageing-related losses in uninfected people will require carefully conducted comparative studies.
We have summarised the relevance of geroscience hallmarks to HIV, and biomarkers for measuring changes in these hallmarks (table 1).
Table 1:
Hallmarks for biological ageing, associated biomarkers, and relevance for HIV infection
| Biomarkers and approaches | HIV-specific references | |
|---|---|---|
| Genetic damage and genomic instability | Genome stability (next-generation sequencing) | DNA damage response in latent infection19 and psychosocial effect on DNA integrity20 |
| Telomere attrition | Telomere loss (telomere length and telomerase activity, and telomere-associated foci) | Telomere length in blood,25,26 association of telomere loss with cardiovascular risk27 and lung disease28 |
| Loss of proteostasis | Proteostasis (autophagy markers and flux, chaperone proteins, protein aggregates) | Disruption of autophagy in multiple cell types,40–42 neurodegeneration,44 and mitophagy in primary neurons45 |
| Mitochondrial dysfunction | Mitochondrial dysfunction (mitochondrial number and volume, markers of biogenesis, and mitochondria DNA copy number, NAD(H) concentrations) | Deficits in PGC-1α in skeletal muscle52 and reduced oxidative phosphorylation,53 brain mitochondrial DNA mutations and oxidative damage,54 and disrupted mitochondrial biogenesis51 and deficits in intervention studies93 in patients who were not treated with ART |
| Epigenetic changes | Epigenetic changes (methylation, histone acetylation, and clocks) | Aberrant methylation in immune modulation,94,95 accelerated epigenetic age,96,97 and influence of ART98,99 |
| Senescence | Cellular senescence (circulating SASP factors, senescence-associated β-galactosidase, p16, p21, mtDNA, MIDAS, CD4/CD8 ratio, and CD3T with p16) | Multimorbidity and senescence,65,66 and replicative senescence67 |
| Inflammation | SASP, inflammaging, IL-6, TNF, soluble CD14, soluble CD163, CRP, and MCP-1 | Persistent elevation in IL-6, TNF, soluble CD14, soluble CD163, CRP, and MCP-1;69–71 and association with adverse functional outcomes72,73 |
| Stem-cell exhaustion | Stem-cell dysfunction (proliferative, differentiation, and regenerative potential) | Loss in regenerative potential of haematopoietic progenitors (CD34+) and loss of naive T cells,82–86 impaired neurogenesis,87 indirect evidence for loss in stem-cell regenerative potential in skeletal muscle,52,88–90 clonality in haematopoetic stem cells,91 and impaired mesenchymal stem cells92 |
MIDAS=mitochondrial dysfunction-associated senescence. SASP=senescence-associated secretory phenotype.
Can the geroscience hypothesis be reframed to include both gerodrivers and geroprotectors in the general population and in people with HIV?
In the previous sections of this Series paper, we have summarised several discrete biochemical mechanisms of ageing that represent hallmarks of the geroscience hypothesis and can be considered as gerodrivers (figure). The clinical phenotype of ageing in the context of chronic HIV infection is a higher prevalence of traditional risk factors for cardiovascular, neurocognitive, malignant, metabolic, liver, and kidney diseases. There are also unique host–environmental interactions for people with HIV that can alter the typical clinical manifestations that are associated with age-related diseases. Notably, the loss in immunological homoeostasis, a broadly recognised feature of HIV infection, ultimately leads to changes in the intestinal mucosa, persistent systemic inflammation, tissue fibrosis, and disruption of neoplastic surveillance mechanisms, thereby providing a fertile landscape for inflammatory conditions, malignancies, and autoimmune conditions. Loss of crosstalk between multiple other organs (figure B) is also likely to promote asynchronous ageing. There are two prominent hypotheses that provide explanations for differences in the ageing trajectory between individuals that are potentially relevant for people with HIV. The first hypothesis, compression of morbidity, is based on the observation that individuals without HIV who reach the age of 100 years (in the absence of HIV infection) have longer lifespans and a later onset and compressed period of comorbidity before death.100,101 This observation contrasts with individuals who do not reach this age, who have variable onset of comorbidities and progressive decline in health before death. The second model, the decelerated ageing hypothesis, proposes that the ageing process begins early for all individuals, but that the rate of canonical ageing processes differs for each person.102 Both models of ageing could be relevant to people with HIV. As discussed in previous sections and reviews,103 people with HIV have an accelerated onset and increased age-adjusted burden of multimorbidity, making them ideal populations to comparatively analyse ageing mechanisms that prevent compression of morbidity and decelerated ageing in future studies.
Figure: Cascade and crosstalk conceptual model.
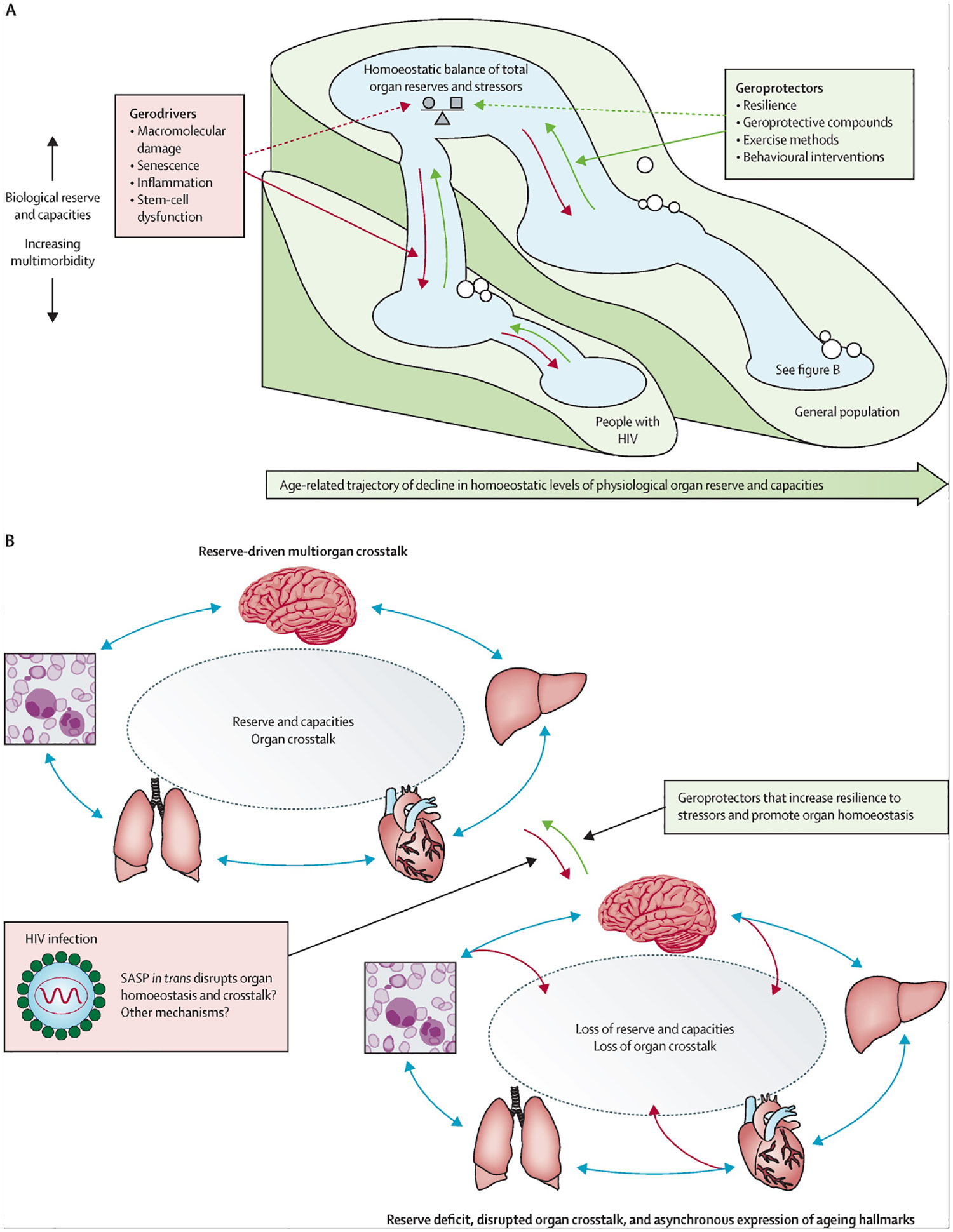
(A) Cascade model. During biological ageing, total organ physiological function (the size of the pool) declines over the lifecourse as a result of exposure to stressors (eg, injury, chronic high blood pressure, oxidative stress, environmental toxicity, and structural determinants such as stigma), concomitant with an increase in the multimorbidity burden. Gerodrivers accelerate this process, whereas geroprotectors are postulated to attenuate and ideally reverse the process. (B) Organ crosstalk model. HIV and age-related decline in reserve and capacities that counteract stressors result in a loss of organ crosstalk and homoeostasis. As an illustrative example, the expansion of a senescence phenotype with the deposition of senescent cells in distal organs could in trans result in dysfunction and disruption of interorgan communication and loss of homoeostasis. Geroprotectors are postulated to increase resilience in response to stressors and maintain homoeostatic pools. SASP=senescence-associated secretory phenotype.
A crucial counterbalance to physiological decline with ageing under the geroscience hypothesis is the notion of healthy ageing, which is defined as a composite of reserves and capacities that promote recovery, adaptation, and psychosocial growth over the lifecourse.104 Physiological reserves are conceptualised as the energy available for metabolic homoeostasis and other energy-consuming activities such as physical activity and health maintenance105 and organ homoeostasis.106 Efforts to operationalise healthy ageing, although ongoing, have gained traction with the introduction of two related constructs, resilience and intrinsic capacity, which measure multiple functional domains including mobility, cognition, psychology, vitality, and sensory capacities.107 The interplay between resilience and intrinsic capacity as a counterbalance to age-related deficit accumulation and vulnerability to stressors is an understudied area of research in geroscience that is advancing as a result of studies of geriatric conditions in people with HIV.108
Hallmarks of ageing are inter-related and can be modifiable, which raises the possibility of delaying ageing at a person-centred level and increasing healthspan in people with HIV. For example, environmental protective factors, lifestyle changes, and pharmacological interventions can change trajectories of ageing12 and could be termed geroprotectors (figure). Successful ageing, a biopsychosocial model that integrates biological, psychological, and social determinants of functional independence,109 has been associated with several resilience factors such as a strong self-concept of cognition, strong perception of social relationships, well developed emotional regulation, and positive coping skills among middle-aged people living with HIV infection.110 Lifestyle changes (eg, smoking cessation, physical activity, dietary changes, healthy sleep, and alcohol abstinence) increase healthspan through multiple ageing mechanisms. Smoking cessation substantially reverses smoking-associated DNA methylation markers,111 and could change the outcomes of smoking-associated comorbidities. Physical activity appears to preserve telomere length and to favourably modulate ageing.112
Integrative and comprehensive measures of biological ageing under the geroscience hypothesis have recently attracted substantial attention. However, few studies have applied biological ageing as a person-centred holistic measure in uninfected people or in people with HIV. Furthermore, future studies are needed to develop and operationalise strategies that promote resilience and capacity, to identify HIV-specific ageing protective factors and behaviours, to apply ageing-attenuating (gero-protective) medication treatments that are calibrated for ageing in people with HIV, and to gain a better understanding of how and which interventions can address the apparent balance of gerodrivers and geroprotectors. A summary of current limitations in geroscience and opportunities in HIV research is presented in table 2.
Table 2:
Limitations of and opportunities for geroscience in HIV research
| Limitations and opportunities | |
|---|---|
| Overall limitations | Geroscience does not yet fully incorporate strategies for accessing reserve, measures of resilience, intrinsic capacity, stress response adaptive mechanisms, from cell function to holistic person-centered response; it does not provide a disease-specific roadmap of inter-relations between hallmarks; and progressive loss in physiological integrity as a hallmark is ill-defined and lacks testable insight |
| Specific geroscience hallmarks | |
| Genome instability | Identify genomic markers associated with HIV infection and ageing-related outcomes in people with HIV |
| Telomere attrition | Understand the mechanisms of cell-type-specific telomere alteration because of HIV infection |
| Loss of proteostasis | Understand the link between proteostasis decline and HIV comorbidities, such as HIV-associated neurocognitive disorder |
| Mitochondrial dysfunction | Link age-related mitochondrial dysfunction with HIV treatment, polypharmacy, and the onset of comorbidities |
| Cellular senescence and inflammation | Better understand how the mechanisms of inflammation in chronic HIV infection contribute to immune-cell senescence |
| Stem-cell exhaustion | Investigate whether ART affects stem-cell decline in specific organs |
| Additional mechanistic limitations | |
| Transcriptional drift | Transcription becomes instable and drifts with ageing, with increased transcriptional noise and accumulation of genetic errors |
| Segmental progeria | Inadequate inclusion of segmental progeria phenotypes in which ageing pathways are engaged asynchronously |
| Other relevant ageing factors | |
| Healthy ageing constructs | |
| Physical and cognitive reserve, resilience, and intrinsic capacity | Identify hallmarks of healthy ageing, operational measures of reserve, resilience, and intrinsic capacity in people with HIV, compared with uninfected people |
| Environmental stress and resilience | |
| Substance use and misuse | Understand the effects of substance misuse (eg, tobacco, cocaine) on ageing in people with HIV |
| Psychological and physiological stress | Understand the role of hypothalamic–pituitary–adrenal dysfunction in people with HIV under physical and psychological stress conditions |
| Nutrition and physical activity | Understand the effects of nutrition and physical activity on geoscience hallmarks and derive interventions tailored to people with HIV that change the trajectories of ageing |
| Subpopulations of people with HIV | |
| Co-infection of HIV with hepatitis C virus, hepatitis B virus, and SARS-CoV-2 | Models for understanding multimorbidity and chronic inflammation |
| Elite control of HIV infection, longevity with HIV infection | Models for understanding successful immune ageing and consequent increases in lifespan |
Can we leverage insights from diagnostic and therapeutic approaches based on geroscience to identify new approaches in HIV care?
Regarding diagnostics, although there are biomarkers in use or being tested for each hallmark of ageing, there is currently no composite score or biological signature that captures all the relevant biological processes to provide a holistic understanding of biological ageing either in the general population or in the nested population of ageing people with HIV. Nevertheless, taken individually, there is substantial utility in biomarker discovery (table 1). Various epigenetic changes, including DNA methylation, histone modifications, and chromatin remodelling, are associated with the ageing process and are linked to other hallmarks of ageing.113–115 For example, members of the sirtuin family of NAD-dependent deacetylases have been evaluated in relation to epigenetic reprogramming and biological ageing.116–118 Methylation alterations associated with HIV infection have been linked to genes involved in immune modulation.94,95 Horvath and Levine96 reported an increase in epigenetic age of approximately 5 years in blood and 7 years in brain in people with HIV compared with uninfected people. Subsequently, Esteban-Cantos and colleagues98 reported a smaller age acceleration. The role of ART in defining epigenetic age among people with HIV has also been investigated. Several studies have shown that differences in epigenetic age between people with HIV and uninfected people are reduced with ART.97,99 A better understanding of how mechanisms of ageing are linked to epigenetic signatures would facilitate the use of these measures scientifically and clinically. There is also a need to evaluate the effects of ART on the homoeostatic balance of gerodriver and geroprotective mechanisms that affect ageing trajectories in people with HIV.119,120
Composite scores for ageing that attempt to assess multiple domains (ie, both deficits and resilience factors) include the comprehensive geriatric assessment (CGA),121 physiological and deficit-based scores for frailty as a phenotype,122,123 multimorbidity,124 and intrinsic capacity.107,125 Apart from the CGA, the frailty phenotype,126 and the Veterans Aging Cohort Study Index,127 these approaches are yet to be applied to people with HIV and adapted or calibrated for healthy ageing trajectories among people with HIV. There is some progress in the clinical use of the CGA, which often includes geriatric referral and geriatric syndrome assessments.128,129 Newer approaches that incorporate artificial intelligence and machine learning to omic and dataset methods in infection and integrative medicine, although early in development, appear promising.130
None of the composite scores currently capture all the relevant biological processes to provide a holistic understanding of biological ageing either in people with HIV or in the general population. Also, although there is some progress in the use of comprehensive assessments in people with HIV, there remains a need for composite measures that capture distinct trajectories of functional ability and reserves, intrinsic capacities, and resilience with ageing in people with HIV and in the general population (figure).
There is an increasing number of therapeutic strategies based on geroscience that could be useful in the context of HIV and ageing. These include dietary, exercise, and biobehavioural (a conceptual model that includes behavioural and biomedical components) interventions, senolytics, and senomorphics. Discussing each approach is outside the scope of this Series paper, but as an illustration we will discuss current approaches based on senescence (senolytic and senomorphic). Senolytic approaches promote the clearance of senescent cells through apoptosis by B-cell lymphoma 2 (Bcl-2) inhibitors or Bcl-2 homology 3 (BH3) mimetics, signalling-pathway inhibitors (such as heat-shock proteins, p53, histone deacetylase, and kinases), and mitochondria targeting (tamoxifen). For example, when quercetin (a plant flavanol with senolytic activity that inhibits the Bcl-2 pro-survival pathway) was combined with dasatinib (a tyrosine kinase inhibitor) in people with idiopathic pulmonary fibrosis it was found to be safe and showed some improvement in physical function.131 Senomorphic approaches do not clear senescent cells but block the proliferation of a senescence-associated secretory phenotype,61 and include compounds with mTOR or JAK1 and JAK2 inhibitory activity.
A distinct advantage of senolytic compounds for people with HIV might be the potential effect on the HIV viral reservoir, which can contribute to residual chronic inflammation. As a result, several of these agents have been explored as curative therapy for HIV infection. Venetoclax is a Bcl-2 antagonist that, through selective elimination of HIV-infected cells by reversing the intrinsic ability of HIV to block apoptotic pathways, has shown a reduction of the HIV viral reservoir in vitro following reactivation132 and homoeostatic proliferation.133 Several histone deacetylase inhibitors have been tested in people with HIV to reverse latency, with the expectation that such treatment would lead to a reservoir decline, known as the kick-and-kill strategy. Although some studies have shown a transient increase in viral RNA, no effect on the reservoir has been observed despite bearing the cost of an increase in T-cell activation.134 Fimepinostat (Curis, Lexington, MA, USA) is a newer histone deacetylase inhibitor that induces potent latency reversal but decreases T-cell activation ex vivo.135 Panobinostat has also been shown to reduce concentrations of C-reactive protein and IL-6.136
The use of senomorphic compounds as an approach to reduce senescence-associated secretory phenotype concentrations in people with HIV is another major area under investigation. In addition to lowering lipid concentrations, statins can reduce autophagy through the mTOR pathway and block the upregulation and binding of intercellular adhesion molecules, among other effects. As an example, treatment with rosuvastatin for 1 year resulted in a reduction in D-dimer, IL-8, and IL-12 concentrations. Based on these and other data, REPRIEVE (NCT02344290), the largest randomised trial to date in HIV research, is examining the cardiovascular and anti-inflammatory benefits of pitavastatin for people with HIV who would not otherwise receive a statin. Results from this study are expected in 2023.137 Additionally, the mTOR inhibitor sirolimus reduced the percentages of cycling Ki67, CD4, and CD8 T cells, PD-1 and CD8+ T cells, and CCR5 and CD8 T cells but did not affect the viral reservoir.138 Janus kinase inhibitors have been studied in vitro and ex vivo and shown to reduce inflammation and the HIV reservoir.139 In the randomised trial ACTG A5336,140 concentrations of IL-6 were not reduced, but concentrations of other biomarkers of inflammation, immune activation, and microbial translocation decreased significantly for people with HIV receiving ruxolitinib, a Janus kinase inhibitor approved for myelofibrosis. Incidentally, Bcl-2 concentrations were also significantly reduced for patients on the study drug, suggesting combined senomorphic and senolytic activity. In LILAC,141 a single-arm study of metformin for people with HIV but not diabetes, there was a reduction in CD4 T-cell infiltration in the colon, and mTOR activation and phosphorylation. Finally, the targeting ageing with metformin (TAME) trial142 will be the first trial with age-related multimorbidity as an outcome in people uninfected with HIV, and should provide insights for future trials in people with HIV.
Other non-pharmacological interventions have shown variable effects on inflammation, ageing, and frailty. Exercise and physical activity have been studied extensively in the general population and in people with HIV. Beyond the obvious benefits of increasing muscle mass, cardiovascular endurance, and strength, there have been mixed effects on inflammatory biomarkers.143–145 Although some data are available,93 further studies are needed to evaluate mitochondrial function and ageing in people with HIV in different contexts (eg, with interventions such as calorie restriction, stress reduction, and exercise). Approaches modifying the diet and microbiome that involve probiotics might lower concentrations of D-dimer but did not affect the microbiome.146 Nutritional interventions, although scarce, appear to be the most effective at reducing frailty when combined with exercise,72 although few studies have examined the effects of micronutrient replacement or calorie restriction on inflammation in people with HIV. Mindfulness techniques and meditation interventions are actively being explored as methods to reduce psychological stress, improve mental wellbeing, and reduce biomarkers of inflammation. To date, studies have been positive regarding mental health improvement, but only a few have shown changes in immunological parameters.147,148,149
How has the HIV pandemic driven advances in ageing research and what gaps remain in geroscience-guided approaches?
Advances in geroscience are converging on the need to focus on reducing multimorbidity and improving healthspan. The disproportionate burden of multimorbidity for ageing people with HIV has been recognised as a key challenge in HIV management for over a decade. Although there has been substantial focus on improving the quality of life of people with HIV, our geroscience model supports future work to improve healthspan by increasing healthy ageing reserves and capacities that promote resilience.150,151
For 40 years, efforts to characterise the pathophysiology of HIV infection have driven key advances in understanding inflammation associated with elevated CD38 concentrations152 and a loss of immune homoeostasis (as we discussed), with the eventual recognition of the transcription factor NF-κB as a major driver of inflammation and biological ageing,63,153 of gut dysbiosis and persistent inflammation,69 and of links between inflammation, immune activation, and cognitive stress.147,154 Measuring changes in inflammatory profiles with geroscience-driven interventions for people with HIV could be an effective cohort approach to quantifying improvements in healthspan and lifespan.155 An inflammation ageing clock that predicts multimorbidity, immune senescence, frailty, and cardiovascular ageing156 could be informative in intervention studies that enrol people with HIV at earlier ages.
We acknowledge that a single model cannot encompass ageing as a process, and that the crucial relationships and interactions across primary ageing (biological), secondary ageing (lifestyle), and tertiary ageing (comorbidity and coinfection) require integration. As a next step, we suggest that characterising the interaction of biology with geography, ancestry, sex, and structural and psychosocial factors (eg, socioeconomic status, stigma, and access to care) will be crucial to improve both health and health-care delivery (eg, adherence and access to ART, and its off-target effects) and to understand how these interacting factors contribute to the increased risk of adverse outcomes and potential long-term complications (eg, chronic fatigue) in HIV infection, and of co-infection.157 Notably, in SARS-CoV-2 infection, older people are at increased risk of adverse outcomes owing to a dysregulation of the inflammatory response.158,159
In conclusion, we provide a conceptual model of biological ageing with HIV, informed by geroscience, that attempts to integrate hallmarks from the literature (figure). As shown in figure A, the model is reminiscent of a cascade waterfall with a series of descending water pools that decrease in size as homoeostatic levels (ie, capacity) of organ function and reserve become diminished in response to a life history of exposure to stressors, concomitant with increases in multimorbid burden. Gerodrivers that represent the major hallmarks of ageing accelerate this process by driving a loss in protective mechanisms, whereas geroprotectors are postulated to attenuate and ideally reverse this process by promoting mechanisms that favour homoeostatic reserves and organ function (as we discussed before; figure A). Furthermore, HIV and age-related declines in reserve and capacities that promote resilience to stressors drive loss in homoeostatic organ crosstalk and compromised homoeostatic levels (ie, capacity) that result in asynchronous organ and physiological ageing. Notably, the study by Lee and colleagues160 supports a broad role for immune system resilience as a homoeostatic geroprotector. To illustrate the dynamic role of gerodrivers and geroprotectors in biological ageing, we provide an example wherein we speculate that the expansion of a senescence phenotype with deposition of senescent cells in trans results in dysfunction and disruption of interorgan communication, reducing reserve, resilience, and physiological homoeostasis (figure B). Although asynchronous senescence-driven ageing has been shown in mice,56,161 a similar process in people with HIV has not yet been established.
Going forward, carefully conducted clinical studies, which are designed to ensure equal access to care and treatment for all and which interrogate geroprotector and gerodriver pathways with targeted interventions, will be crucial to inform pathogenesis, diagnosis, and therapeutic management for people with HIV in resource-poor settings, and indeed in the general population. As mused in Joni Mitchell’s song, “Both Sides Now”,162 she notes that with life, “well something’s lost, but something’s gained, in living every day”. The time has come for geroscience to advance our understanding beyond a focus on decline to include those gains that are key to successful ageing, and in so doing, leverage the profound progress made in our efforts to combat HIV/AIDS.
Key messages.
Large gaps exist in our understanding of the effect of HIV on the ageing process, even though age-related conditions seem to be more common, and occur at an earlier age, in people with HIV than in people without HIV infection.
Although many hallmarks of biological ageing appear to be dysregulated in people with HIV, there is a need to precisely define the effect of HIV infection and therapy on the molecular processes defined by geroscience as gerodrivers and geroprotectors.
Models of ageing that integrate and better define measures of reserve and resilience are needed to holistically define the ageing process and to counterbalance and leverage advances in identifying drivers of ageing.
Search strategy and selection criteria.
We used both Medical Subject Headings terms (controlled language) and free-text terms to search PubMed and Google Scholar. The search queries included terms related to geroscience and HIV infection—such as biological ageing, hallmarks, chronic infection, and comorbidity—and various specific combinations. The search was last done on July 29, 2021. We did not restrict the search by language, date, or publication status.
Acknowledgments
MM acknowledges support from the Boston Claude D Pepper Older Americans Independence Center (P30 AG031679-10 6777) and the Harvard University Center for AIDS Research (P30AI060354-17 7331). VCM received support from the Emory University Center for AIDS Research (P30AI050409). We thank Kimberly Birkett (Salem Veterans Affairs Medical Center) for assistance with figure design.
Declaration of interests
VCM receives research support from Lilly, Gilead Sciences, and ViiV Healthcare. All other authors declare no competing interests.
Footnotes
This is the first in a Series of four papers about ageing with HIV (papers 3 and 4 appear in The Lancet HIV)
References
- 1.Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4: e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open 2020; 3: e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV surveillance report, 2018 updated edition, volume 31. May, 2020. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed July 29, 2021). [Google Scholar]
- 5.Montano M, Bhasin S, D’Aquila RT, et al. Harvard HIV and Aging workshop: perspectives and priorities from Claude D. AIDS Res Hum Retroviruses 2019; 35: 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: a cross-sectional study. Int J Infect Dis 2018; 70: 30–35. [DOI] [PubMed] [Google Scholar]
- 7.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53: 1120–26. [DOI] [PubMed] [Google Scholar]
- 8.Crimmins EM. Lifespan and healthspan: past, present, and promise. Gerontologist 2015; 55: 901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience 2017; 39: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 2014; 69 (suppl 1): S1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019; 25: 1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell 2014; 159: 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenyon C A conserved regulatory system for aging. Cell 2001; 105: 165–68. [DOI] [PubMed] [Google Scholar]
- 14.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153: 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath S DNA methylation age of human tissues and cell types. Genome Biol 2013; 14: R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopalan S, Carja O, Fagny M, et al. Trends in DNA methylation with age replicate across diverse human populations. Genetics 2017; 206: 1659–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Biancotto A, Moaddel R, et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018; 17: e12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher B, Pothof J, Vijg J, Hoeijmakers JHJ. The central role of DNA damage in the ageing process. Nature 2021; 592: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piekna-Przybylska D, Sharma G, Maggirwar SB, Bambara RA. Deficiency in DNA damage response, a new characteristic of cells infected with latent HIV-1. Cell Cycle 2017; 16: 968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gidron Y, Russ K, Tissarchondou H, Warner J. The relation between psychological factors and DNA-damage: a critical review. Biol Psychol 2006; 72: 291–304. [DOI] [PubMed] [Google Scholar]
- 21.Demanelis K, Jasmine F, Chen LS, et al. Determinants of telomere length across human tissues. Science 2020; 369: eaaz6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann M, Pusceddu I, März W, Herrmann W. Telomere biology and age-related diseases. Clin Chem Lab Med 2018; 56: 1210–22. [DOI] [PubMed] [Google Scholar]
- 23.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 2013; 35: 112–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 1996; 10: F17–22. [DOI] [PubMed] [Google Scholar]
- 25.Zanet DL, Thorne A, Singer J, et al. Association between short leukocyte telomere length and HIV infection in a cohort study: no evidence of a relationship with antiretroviral therapy. Clin Infect Dis 2014; 58: 1322–32. [DOI] [PubMed] [Google Scholar]
- 26.Cobos Jiménez V, Wit FW, Joerink M, et al. T-cell activation independently associates with immune senescence in HIV-infected recipients of long-term antiretroviral treatment. J Infect Dis 2016; 214: 216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel T, Raffenberg M, Schoepf IC, et al. Telomere length, traditional risk factors, factors related to human immunodeficiency virus (HIV) and coronary artery disease events in Swiss persons living with HIV. Clin Infect Dis 2020; 73: e2070–76. [DOI] [PubMed] [Google Scholar]
- 28.Xu S, Vucic EA, Shaipanich T, et al. Decreased telomere length in the small airway epithelium suggests accelerated aging in the lungs of persons living with human immunodeficiency virus (HIV). Respir Res 2018; 19: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong E, Cuervo AM. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol 2010; 2: a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell 2011; 146: 682–95. [DOI] [PubMed] [Google Scholar]
- 31.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med 2015; 21: 1406–15. [DOI] [PubMed] [Google Scholar]
- 32.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem 2015; 84: 435–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol 2018; 217: 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christians ES, Ishiwata T, Benjamin IJ. Small heat shock proteins in redox metabolism: implications for cardiovascular diseases. Int J Biochem Cell Biol 2012; 44: 1632–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinciguerra M, Musaro A, Rosenthal N. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol 2010; 694: 211–33. [DOI] [PubMed] [Google Scholar]
- 36.Bourdenx M, Gavathiotis E, Cuervo AM. Chaperone-mediated autophagy: a gatekeeper of neuronal proteostasis. Autophagy 2021; 17: 2040–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuervo AM, Macian F. Autophagy and the immune function in aging. Curr Opin Immunol 2014; 29: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Puleston DJ, Simon AK. Autophagy and immune senescence. Trends Mol Med 2016; 22: 671–86. [DOI] [PubMed] [Google Scholar]
- 39.Dong S, Wang Q, Kao YR, et al. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature 2021; 591: 117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinkins C, Pilli M, Kehrl JH. Roles of autophagy in HIV infection. Immunol Cell Biol 2015; 93: 11–17. [DOI] [PubMed] [Google Scholar]
- 41.Leymarie O, Lepont L, Berlioz-Torrent C. Canonical and non-canonical autophagy in HIV-1 replication cycle. Viruses 2017; 9: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nardacci R, Ciccosanti F, Marsella C, Ippolito G, Piacentini M, Fimia GM. Role of autophagy in HIV infection and pathogenesis. J Intern Med 2017; 281: 422–32. [DOI] [PubMed] [Google Scholar]
- 43.Cole JH, Underwood J, Caan MW, et al. Increased brain-predicted aging in treated HIV disease. Neurology 2017; 88: 1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fields J, Dumaop W, Eleuteri S, et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci 2015; 35: 1921–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teodorof-Diedrich C, Spector SA. Human immunodeficiency virus type 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J Virol 2018; 92: e00993–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiley CD, Velarde MC, Lecot P, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 2016; 23: 303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zampino M, Brennan NA, Kuo PL, et al. Poor mitochondrial health and systemic inflammation? Test of a classic hypothesis in the Baltimore Longitudinal Study of Aging. Geroscience 2020; 42: 1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payne BA, Wilson IJ, Hateley CA, et al. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet 2011; 43: 806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt M, Payne BAI. Mitochondria and ageing with HIV. Curr Opin HIV AIDS 2020; 15: 101–09. [DOI] [PubMed] [Google Scholar]
- 50.McComsey GA, Daar ES, O’Riordan M, et al. Changes in fat mitochondrial DNA and function in subjects randomized to abacavir-lamivudine or tenofovir DF-emtricitabine with atazanavir-ritonavir or efavirenz: AIDS Clinical Trials Group study A5224s, substudy of A5202. J Infect Dis 2013; 207: 604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schank M, Zhao J, Moorman JP, Yao ZQ. The Impact of HIV- and ART-induced mitochondrial dysfunction in cellular senescence and aging. Cells 2021; 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran T, Guardigni V, Pencina KM, et al. Atypical skeletal muscle profiles in human immunodeficiency virus-infected asymptomatic middle-aged adults. Clin Infect Dis 2018; 66: 1918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortmeyer HK, Ryan AS, Hafer-Macko C, Oursler KK. Skeletal muscle cellular metabolism in older HIV-infected men. Physiol Rep 2016; 4: e12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozzi SJ, Avdoshina V, Fields JA, et al. Human immunodeficiency virus promotes mitochondrial toxicity. Neurotox Res 2017; 32: 723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burd CE, Sorrentino JA, Clark KS, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 2013; 152: 340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009; 8: 311–23. [DOI] [PubMed] [Google Scholar]
- 57.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479: 232–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 2016; 530: 184–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL. Reducing senescent cell burden in aging and disease. Trends Mol Med 2020; 26: 630–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018; 15: 505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coppé JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008; 6: 2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnes PJ. Senescence in COPD and its comorbidities. Annu Rev Physiol 2017; 79: 517–39. [DOI] [PubMed] [Google Scholar]
- 63.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014; 69 (suppl 1): S4–9. [DOI] [PubMed] [Google Scholar]
- 64.Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019; 11: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen J, Torres C. HIV-associated cellular senescence: a contributor to accelerated aging. Ageing Res Rev 2017; 36: 117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duffau P, Ozanne A, Bonnet F, et al. Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS 2018; 32: 1651–60. [DOI] [PubMed] [Google Scholar]
- 67.Effros RB. The silent war of CMV in aging and HIV infection. Mech Ageing Dev 2016; 158: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24: 501–06. [DOI] [PubMed] [Google Scholar]
- 69.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39: 633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gandhi RT, McMahon DK, Bosch RJ, et al. Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13: e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Margolick JB, Bream JH, Martínez-Maza O, et al. Frailty and circulating markers of inflammation in HIV+ and HIV− men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 2017; 74: 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erlandson KM, Piggott DA. Frailty and HIV: moving from characterization to intervention. Curr HIV/AIDS Rep 2021; 18: 157–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Premeaux TA, Javandel S, Hosaka KRJ, et al. Associations between plasma immunomodulatory and inflammatory mediators with VACS Index scores among older HIV-infected adults on antiretroviral therapy. Front Immunol 2020; 11: 1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210: 1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998; 396: 690–95. [DOI] [PubMed] [Google Scholar]
- 76.Guaraldi G, Franconi I, Milic J, et al. Thymus imaging detection and size is inversely associated with metabolic syndrome and frailty in people with HIV. Open Forum Infect Dis 2019; 6: ofz435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rando TA. Stem cells, ageing and the quest for immortality. Nature 2006; 441: 1080–86. [DOI] [PubMed] [Google Scholar]
- 78.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell 2013; 12: 152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol 2011; 193: 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 2007; 8: 703–13. [DOI] [PubMed] [Google Scholar]
- 81.Brunet A, Rando TA. Interaction between epigenetic and metabolism in aging stem cells. Curr Opin Cell Biol 2017; 45: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Appay V, Fastenackels S, Katlama C, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 2011; 25: 1813–22. [DOI] [PubMed] [Google Scholar]
- 83.Fastenackels S, Sauce D, Vigouroux C, et al. HIV-mediated immune aging in young adults infected perinatally or during childhood. AIDS 2019; 33: 1705–10. [DOI] [PubMed] [Google Scholar]
- 84.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest 1995; 95: 2061–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sauce D, Larsen M, Fastenackels S, et al. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood 2011; 117: 5142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsukamoto T Hematopoietic stem/progenitor cells and the pathogenesis of HIV/AIDS. Front Cell Infect Microbiol 2020; 10: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Putatunda R, Zhang Y, Li F, et al. Sex-specific neurogenic deficits and neurocognitive disorders in middle-aged HIV-1 Tg26 transgenic mice. Brain Behav Immun 2019; 80: 488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kusko RL, Banerjee C, Long KK, et al. Premature expression of a muscle fibrosis axis in chronic HIV infection. Skelet Muscle 2012; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res 2012; 2012: 194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tumasian RA 3rd, Harish A, Kundu G, et al. Skeletal muscle transcriptome in healthy aging. Nat Commun 2021; 12: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bick AG, Popadin K, Thorball CW, et al. Increased CHIP prevalence amongst people living with HIV. medRxiv 2020; published online Nov 7. 10.1101/2020.11.06.20225607 (preprint). [DOI] [Google Scholar]
- 92.Delpino MV, Quarleri J. Influence of HIV infection and antiretroviral therapy on bone homeostasis. Front Endocrinol 2020; 11: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jankowski CM, Wilson MP, MaWhinney S, et al. Blunted muscle mitochondrial responses to exercise training in older adults with HIV. J Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bogoi RN, de Pablo A, Valencia E, et al. Expression profiling of chromatin-modifying enzymes and global DNA methylation in CD4+ T cells from patients with chronic HIV infection at different HIV control and progression states. Clin Epigenetics 2018; 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Justice AC, Hu Y, et al. Epigenome-wide differential DNA methylation between HIV-infected and uninfected individuals. Epigenetics 2016; 11: 750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis 2015; 212: 1563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nelson KN, Hui Q, Rimland D, et al. Identification of HIV infection-related DNA methylation sites and advanced epigenetic aging in HIV-positive, treatment-naive U.S. veterans. AIDS 2017; 31: 571–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Esteban-Cantos A, Rodríguez-Centeno J, Barruz P, et al. Epigenetic age acceleration changes 2 years after antiretroviral therapy initiation in adults with HIV: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV 2021; 8: e197–205. [DOI] [PubMed] [Google Scholar]
- 99.Sehl ME, Rickabaugh TM, Shih R, et al. The effects of anti-retroviral therapy on epigenetic age acceleration observed in HIV-1-infected adults. Pathog Immun 2020; 5: 291–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med 1980; 303: 130–35. [DOI] [PubMed] [Google Scholar]
- 101.Perls TT. Centenarians prove the compression of morbidity hypothesis, but what about the rest of us who are genetically less fortunate? Med Hypotheses 1997; 49: 405–07. [DOI] [PubMed] [Google Scholar]
- 102.Franceschi C, Garagnani P, Morsiani C, et al. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med 2018; 5: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2014; 69: 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.WHO. World report on ageing and health. Geneva: World Health Organization, 2015. [Google Scholar]
- 105.Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc 2010; 58 (suppl 2): S329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Atamna H, Tenore A, Lui F, Dhahbi JM. Organ reserve, excess metabolic capacity, and aging. Biogerontology 2018; 19: 171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci 2018; 73: 1653–60. [DOI] [PubMed] [Google Scholar]
- 108.Guaraldi G, Milic J. The interplay between frailty and intrinsic capacity in aging and HIV infection. AIDS Res Hum Retroviruses 2019; 35: 1013–22. [DOI] [PubMed] [Google Scholar]
- 109.Anton SD, Woods AJ, Ashizawa T, et al. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev 2015; 24: 304–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fumaz CR, Ayestaran A, Perez-Alvarez N, et al. Resilience, ageing, and quality of life in long-term diagnosed HIV-infected patients. AIDS Care 2015; 27: 1396–403. [DOI] [PubMed] [Google Scholar]
- 111.Dugué PA, Jung CH, Joo JE, et al. Smoking and blood DNA methylation: an epigenome-wide association study and assessment of reversibility. Epigenetics 2020; 15: 358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Semeraro MD, Smith C, Kaiser M, et al. Physical activity, a modulator of aging through effects on telomere biology. Aging 2020; 12: 13803–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet 2016; 17: 487–500. [DOI] [PubMed] [Google Scholar]
- 114.Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol 2019; 20: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sen P, Shah PP, Nativio R, Berger SL. Epigenetic mechanisms of longevity and aging. Cell 2016; 166: 822–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 2021; 22: 119–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kane AE, Sinclair DA. Epigenetic changes during aging and their reprogramming potential. Crit Rev Biochem Mol Biol 2019; 54: 61–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab 2018; 27: 529–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guaraldi G, De Francesco D, Milic J, et al. The interplay between age and frailty in people living with HIV: results from an 11-year follow-up observational study. Open Forum Infect Dis 2019; 6: ofz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guaraldi G, Zona S, Brothers TD, et al. Aging with HIV vs. HIV seroconversion at older age: a diverse population with distinct comorbidity profiles. PLoS One 2015; 10: e0118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown AS, Brummel-Smith K, Burgess L, et al. National Institutes of Health Consensus Development Conference Statement: geriatric assessment methods for clinical decision-making. J Am Geriatr Soc 1988; 36: 342–47. [DOI] [PubMed] [Google Scholar]
- 122.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 123.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–27. [DOI] [PubMed] [Google Scholar]
- 124.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 125.Fontes AP, Neri AL. Resilience in aging: literature review. Cien Saude Colet 2015; 20: 1475–95. [DOI] [PubMed] [Google Scholar]
- 126.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007; 62: 1279–86. [DOI] [PubMed] [Google Scholar]
- 127.Justice AC, Tate JP. Strengths and limitations of the Veterans Aging Cohort Study Index as a measure of physiologic frailty. AIDS Res Hum Retroviruses 2019; 35: 1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Greene M, Myers J, Tan JY, et al. The Golden Compass Program: overview of the initial implementation of a comprehensive program for older adults living with HIV. J Int Assoc Provid AIDS Care 2020; 19: 2325958220935267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siegler EL, Burchett CO, Glesby MJ. Older people with HIV are an essential part of the continuum of HIV care. J Int AIDS Soc 2018; 21: e25188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen LK. Machine learning improves analysis of multi-omics data in aging research and geroscience. Arch Gerontol Geriatr 2021; 93: 104360. [DOI] [PubMed] [Google Scholar]
- 131.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 2019; 40: 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cummins NW, Sainski AM, Dai H, et al. Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol 2016; 90: 4032–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cummins NW, Sainski-Nguyen AM, Natesampillai S, Aboulnasr F, Kaufmann S, Badley AD. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J Virol 2017; 91: e00012–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rosás-Umbert M, Ruiz-Riol M, Fernández MA, et al. In vivo effects of romidepsin on T-cell activation, apoptosis and function in the BCN02 HIV-1 kick&kill clinical trial. Front Immunol 2020; 11: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gunst JD, Kjær K, Olesen R, et al. Fimepinostat, a novel dual inhibitor of HDAC and PI3K, effectively reverses HIV-1 latency ex vivo without T cell activation. J Virus Erad 2019; 5: 133–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Høgh Kølbæk Kjær AS, Brinkmann CR, Dinarello CA, et al. The histone deacetylase inhibitor panobinostat lowers biomarkers of cardiovascular risk and inflammation in HIV patients. AIDS 2015; 29: 1195–200. [DOI] [PubMed] [Google Scholar]
- 137.Hoffmann U, Lu MT, Olalere D, et al. Rationale and design of the Mechanistic Substudy of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019; 212: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Henrich TJ, Bosch R, Godfrey C, et al. Sirolimus reduces T-cell cycling and immune checkpoint marker expression, ACTG A5337. Conference on Retroviruses and Opportunistic Infections; March 4–7, 2019 (abstr 131). [Google Scholar]
- 139.Gavegnano C, Brehm JH, Dupuy FP, et al. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog 2017; 13: e1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marconi VC, Moser C, Gavegnano C, et al. Randomized trial of ruxolitinib in antiretroviral-treated adults with HIV. Clin Infect Dis 2021; published online March 6. 10.1093/cid/ciab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Planas D, Pagliuzza A, Ponte R, et al. LILAC pilot study: effects of metformin on mTOR activation and HIV reservoir persistence during antiretroviral therapy. EBioMedicine 2021; 65: 103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Justice JN, Ferrucci L, Newman AB, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. Geroscience 2018; 40: 419–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bonato M, Galli L, Passeri L, et al. A pilot study of brisk walking in sedentary combination antiretroviral treatement (cART)-treated patients: benefit on soluble and cell inflammatory markers. BMC Infect Dis 2017; 17: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cutrono SE, Lewis JE, Perry A, Signorile J, Tiozzo E, Jacobs KA. The effect of a community-based exercise program on inflammation, metabolic risk, and fitness levels among persons living with HIV/AIDS. AIDS Behav 2016; 20: 1123–31. [DOI] [PubMed] [Google Scholar]
- 145.Erlandson KM, Wilson MP, MaWhinney S, et al. The impact of moderate or high-intensity combined exercise on systemic inflammation among older persons with and without HIV. J Infect Dis 2021; 223: 1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stiksrud B, Nowak P, Nwosu FC, et al. Reduced levels of D-dimer and changes in gut microbiota composition after probiotic intervention in HIV-infected individuals on stable ART. J Acquir Immune Defic Syndr 2015; 70: 329–37. [DOI] [PubMed] [Google Scholar]
- 147.Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: a small randomized controlled trial. Brain Behav Immun 2009; 23: 184–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scott-Sheldon LAJ, Balletto BL, Donahue ML, et al. Mindfulness-based interventions for adults living with HIV/AIDS: a systematic review and meta-analysis. AIDS Behav 2019; 23: 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Titanji BK, Tejani M, Farber EW, et al. Cognitively-based compassion training for HIV immune non-responders—an attention-placebo randomized controlled trial. J Acquir Immune Defic Syndr 2021; published online Dec 7. 10.1097/QAI.0000000000002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guaraldi G, Milic J, Wu AW. What is the measure of success in HIV? The fourth 90: quality of life or healthy aging? Eur Geriatr Med 2019; 10: 267–74. [DOI] [PubMed] [Google Scholar]
- 151.Levin J, Montano M. What aging with HIV means in the year 2019. AIDS Res Hum Retroviruses 2019; 35: 982–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 1981; 305: 1425–31. [DOI] [PubMed] [Google Scholar]
- 153.Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol 2017; 102: 977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cole SW. The conserved transcriptional response to adversity. Curr Opin Behav Sci 2019; 28: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60 (suppl 1): S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sayed N, Huang Y, Nguyen K, et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nature Aging 2021; 1, 598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Montano M Pressing questions and challenges in the HIV-1 and SARS-CoV-2 syndemic. AIDS Res Hum Retroviruses 2021; 37: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020; 383: 1757–66. [DOI] [PubMed] [Google Scholar]
- 159.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020; 20: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee GC, Restrepo IM, Harper N, et al. Immunological resilience and COVID-19 survival advantage. J Allergy Clin Immunol 2021; 148: 1176–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yousefzadeh MJ, Flores RR, Zhu Y, et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021; 594: 100–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mitchell J Both Sides Now. On: Clouds. Hollywood, CA: A&M, 1969. [Google Scholar]


