Abstract
The metabolic syndrome (MetS) has become a global crisis and is believed to affect almost one-quarter of the world's population. Its prevalence has been rising, especially in the younger age group. The interactions of the skin and MetS are myriad. Physiological functions of the skin may confer a protective role, whereas cutaneous diseases may play the role of MetS initiator or amplifier. Cutaneous signs may be some of the earliest manifestations of insulin resistance, the basic pathophysiology behind MetS. Skin changes are also prominent in type 2 diabetes mellitus, the consequence of MetS. Drugs used in dermatological disorders can lead to metabolic dysfunction. Awareness about the risk factors and early lifestyle interventions can help delay or even prevent the life-threatening complications of this syndrome. Dermatologists are in a unique position to predict and prevent MetS or its complications, a long time before the patient visits a physician for systemic problems. To write this review, an internet search was made focusing on articles on skin problems associated with MetS and its components, its risk factors, pathogenesis, and ways to prevent it. Information relevant to dermatological practice was compiled.
KEY WORDS: Cutaneous markers of metabolic syndrome, predicting and preventing metabolic syndrome
The doctor of the future will give no medicine, but will instruct his patient in the care of the human frame, in diet and in the cause and prevention of disease.
Thomas Edison
Introduction
The metabolic syndrome (MetS), previously known as Syndrome X, Reaven Syndrome, or insulin resistance (IR) syndrome, is a constellation of risk factors that increases the risk of atherosclerotic cardiovascular diseases, type 2 diabetes mellitus, and stroke. MetS is also associated with a large number of other diseases like nonalcoholic fatty liver, polycystic ovarian syndrome, obstructive sleep apnoea, gout, cancers, neurodegenerative disorders etc. It is a complex pathophysiologic state that originates primarily from an imbalance of calorie intake, storage and energy expenditure causing visceral adiposity. MetS is estimated to affect one-quarter of the world population.
Risk Factors
Energy excess and modern malnutrition: High-calorie, low-fibre, processed and fast food is driving the epidemic of the MetS. Obesity is now the most prevalent form of malnutrition. This is in part driven by marketing and ease of availability of processed and non-home-cooked food. Excess intraabdominal fat, also called visceral fat or ectopic fat as opposed to subcutaneous fat, is typically linked with MetS [Figures 1a and b, and 2a and b].
Physical inactivity: Use of modern technology for transportation, work, household chores and recreation has reduced avenues for energy expenditure. In India, more than 50% of the population can be considered physically inactive. Physical inactivity is higher in urban compared to rural populations and in females compared to males [Figure 3a].[1]
Age: With passing decades, the incidence of various components of MetS increases. However, a notable feature is the prevalence of MetS cases in the under 40 years of age group. Obesity prevalence among school-age children is reaching worrisome levels. Young adults are exposed to lifestyle modifications, there is upgradation of socioeconomic status and increasing physical inactivity.[2]
Genetic/epigenetic factors: Patients with a family history of MetS, even if otherwise healthy, are at high risk of developing MetS. The concept of thrifty genotype and phenotype tries to explain evolutionary or early life influences on metabolic traits. Evolutionary exposure to famines leads to selection of thrifty genotype that has highly efficient storage during brief periods of food availability, which is probably why certain ethnicities like Indians and Chinese are at greater risk of MetS. Thrifty phenotype describes the epigenetic metabolic adaptations adopted as a survival strategy by a malnourished fetus; changes that may also be inappropriate to deal with a later life of affluence.[3]
Vitamin D deficiency: Various studies have reported that vitamin D insufficiency or deficiency is linked with MetS risk.[4]
Stressful life events: Life events perceived as stressful, particularly those related to finance and work, maybe a signal for poor metabolic health. A hypothalamic-pituitary-adrenocortical axis abnormality, autonomic nervous system changes and inflammatory activity, changes in lifestyle, through smoking, alcohol use, and physical inactivity, sleep pattern and depression may be the mechanisms [Figure 3b].[5]
Chronodisruption: Late nights, light exposure at night, inadequate night time sleep, nocturnal feeding, shift work at nights and travel across time zones disrupt the circadian rhythm (biological or body clock) impairing carbohydrate and lipid metabolism [Figure 3a].[6]
Gut dysbiosis: High-fat, low-fibre diet induces intestinal dysbiosis, resulting in aberrant metabolite concentrations that disrupt tight junction integrity. This loss of integrity, the so called 'leaky gut' makes the gut epithelium more permissive to microbial lipopolysaccharide, trimethylamine, and other metabolites entering the circulation and contributing to the chronic inflammation of liver and adipose tissue that is associated with the MetS.[7]
Xenobiotics: Our exposure to foreign chemicals like pollutants, insecticides, food additives, cosmetics and drugs has exploded after industrialization. Numerous meta-analyses of the association between DDT and DDE indicate that there is a positive association between these chemicals and risk of obesity, type 2 diabetes, and hypertension.[8]
Figure 1.

(a) Eight-year-old boy with new onset psoriasis and abdominal obesity. He had a history of eating chocolates daily and rarely played outdoors. Investigation showed severe vitamin D deficiency. (b) Fifty-four-year-old sweet shop owner with psoriasis for 15 years and abdominal obesity. He was on treatment for hypertension and dyslipidemia
Figure 2.

Forty-year-old female with androgenetic alopecia. (a) She had history of weight gain after moving to a western country. She had a waist circumference of 110 cm (b) and BMI of 47.8. She was diagnosed with type 2 diabetes mellitus, dyslipidemia and vitamin D deficiency
Figure 3.

(a) Twenty-eight-year-old software engineer with acne keloidalis nuchae, acanthosis nigricans and acrochordons. He worked night shifts, had a sedentary lifestyle and was a smoker. He was diagnosed with type 2 diabetes mellitus, hypertension, dyslipidemia, fatty liver, hypothyroidism and vitamin D deficiency. (b) Thirty-year-old female with acne which occurred after starting clonazepam, amitriptyline and propranolol for anxiety-depression. She was diagnosed with fasting hyperglycemia and vitamin D deficiency. (c) Twenty-year-old female with hidradenitis suppurativa and hirsutism diagnosed with insulin resistance, hyperandrogenemia, polycystic ovaries and vitamin D deficiency
Pathophysiology
The most widely accepted theory for the pathophysiology of the MetS is IR. A subnormal biologic tissue response to normal insulin concentrations has been called IR (i.e., the body produces insulin, usually in higher concentrations than in normal subjects, but does not use it effectively)[9] The main regulator of insulin secretion is plasma glucose concentration. The main insulin-responsive tissues related to glucose metabolism are the liver, skeletal muscle and adipose tissue. Under fasting conditions, hepatic glucose production is regulated by basal insulin levels, whereas muscle uptake of glucose from the plasma is low and adipose tissue provides free fatty acids (FFAs) via lipolysis as an energy source. In post-prandial conditions, that is when insulin levels are elevated, hepatic glucose production and adipose lipolysis are suppressed, whereas muscle glucose uptake is increased. Insulin sensitivity differs between several insulin-responsive organs. If there is IR in skeletal muscle, greater insulin concentrations will be necessary to induce muscle glucose uptake. If hepatic IR is present (i.e., resistance in the insulin signal transduction pathway regulating gluconeogenesis), greater basal insulin concentrations will be necessary to maintain normal fasting glucose levels. Both examples, which usually occur concurrently to some degree, result in relative hyperinsulinemia to which all tissues and organs will be exposed and may respond. For example, kidneys of insulin-resistant individuals have an impaired natriuretic response to increased sodium intake leading to increased intravascular volume and potentially increased blood pressure. Exposure of specific brain nuclei to hyperinsulinemia results in an increased sympathetic discharge, manifesting similarly in elevated blood pressure. In the ovaries hyperinsulinemia induces androgen production in theca cells resulting in hyperandrogenism [Figure 3c]. In the liver, whereas elevated insulin concentrations maybe needed to regulate hepatic glucose production, hepatic, insulin-responsive lipogenesis mechanisms have no resistance and are hyper-activated, resulting in increased very low density lipoprotein (VLDL) and reduced high-density lipoprotein (HDL) particle production, manifesting as increased plasma triglycerides and low HDL-cholesterol concentration. Thus, multiple manifestations of the IR syndrome are the result of a normal response of metabolic pathways to increased insulin concentrations that are induced in order to maintain normal glucose metabolism.[10] Overnutrition elevates the levels of glucose and FFAs in blood which induce metabolic stress in β-cells of pancreatic islets and insulin sensitive tissues notably adipocytes. The metabolic stress induced in these tissues activates the release of various pro-inflammatory cytokines. As a result, immune cells are recruited which contribute to the tissue-specific inflammation.[11]
The reasons for development of IR in insulin responsive tissues are multiple and complex. The common paradigm of this process suggests that accumulation of intracellular lipid induces inhibition of specific components of the insulin signal transduction pathways related to glucose metabolism in liver and muscle. Increased adipose tissue plays a major role in the development of IR. Visceral obesity increases the amount of FFAs in the body, and FFAs decrease insulin-mediated glucose uptake at the muscle-cell level. As a result, blood glucose levels increase requiring higher levels of insulin secretion. Beta cells of the pancreas initially try to produce more insulin to achieve euglycaemia. Progressively, pancreas fails to keep up with the increased demand for insulin and excess glucose builds up in the bloodstream, that is, type 2 diabetes mellitus.
Insulin itself has a lipolytic effect – thus a vicious circle develops where increased levels of insulin lead to increased lipolysis, in turn increasing FFA levels and further promoting IR and stimulation of its production and secretion.[12]
There is no uniform definition of insulin sensitivity/resistance. The reason for this is that there is still no standardized assay for measurement of plasma insulin, no standardized cut off values for diagnosis and ethnic differences between insulin susceptibility and insulin response.[13] The hyperinsulinemic, euglycaemic clamp, a gold standard for measuring insulin sensitivity is not possible outside research settings. In practice, clinical findings are corroborated with laboratory tests for glucose and lipids. Surrogate markers for IR, like HOMA-IR or QUICKI can be used.
Skin as predictor, initiator or amplifier of MetS
Interaction of the skin and MetS is a two-way street. Any pathophysiologic dysfunction that results in a loss of metabolic control in the body can result in cutaneous disease. There is the notion that a cascade of reactions happens when there is a single hormonal imbalance as it is the case with insulin, and this affects other organ systems, including the skin.[14] The two main target cell types in skin diseases, keratinocytes and fibroblasts, both have insulin receptors and insulin-like growth factor (IGF) receptors. Insulin has been reported to cross the dermal-epidermal junction to affect keratinocytes.[15] Hyperinsulinemia stimulates the IGF-1 receptor, which has a hyperproliferative effect. This growth promoting effect leads to acanthosis nigricans and acrochordons. It influences the pilosebaceous unit and apocrine glands, directly as well as through formation of androgens, leading to acne,[16] hidradenitis suppurativa and early onset androgenetic alopecia. These lesions have a predictive value for the MetS.
Conversely, skin disorders may act as initiators of IR and MetS. MetS is known to be a chronic inflammatory state. Inflammatory skin diseases, such as atopic dermatitis and psoriasis, produce a wide range of proinflammatory cytokines and chemokines not only in the lesional skin, but also in the circulation, causing systemic inflammation, the so-called inflammatory skin march, which play a role in developing comorbidities.[17] In fact, the histological features and cytokine profiles of psoriasis skin lesions and atherosclerotic vascular lesions are very similar, which has led to the hypothesis 'two plaques for one syndrome'.[18] Whether the skin is an initiator or amplifier has implications on prognosis, for example, the risk of developing severe vascular events is higher when psoriasis acts as a disease amplifier (i.e., when metabolic disorder precedes psoriasis), compared to when it acts as the disease initiator.[19]
Biochemical communication between the immunological and metabolic pathways, the so called immune – metabolic cross talk, may explain the association between autoimmune and connective tissue disorders with MetS.[20]
Broadly speaking, skin disorders with known proliferative or inflammatory mechanisms, may be considered potential early markers for the development of IR.
Advanced glycation end products (AGEs) are formed in the skin due to hyperglycaemia or are ingested in diet. They affect collagen flexibility and solubility and are associated with accelerated skin ageing and disorders of collagen degeneration like necrobiosis lipoidica. Patients with severe psoriasis have accumulation of skin and serum AGEs which may facilitate the emergence of cardiovascular diseases in psoriasis patients.[21] Skin autofluorescence, a noninvasive biomarker for AGEs, is associated with the MetS and its individual components. The idea of a simple skin test that could in the future give information about the metabolic status of a patient is appealing.[22]
'Antimetabolic syndrome' role of intact, healthy skin
Newer research suggests that the skin may have a protective 'antimetabolic syndrome' role owing to its excretory and antioxidant function.[23] Secretion of sebaceous lipids has been suggested to play a role in whole-body lipid clearance in response to diet.[24] Isotretinoin reduces sebum production and is related to increased levels of cholesterol and triglycerides in the body. Skin plays a role in elimination of reactive oxygen species (ROS) via enzymes and sebum. An imbalance between ROS and antioxidants is a major contributor to the pathology of disease.[25] The effect of ambient temperature on the skin may be responsible for the seasonal variation of MetS, for example, increased blood pressure and cholesterol in winter.[26] A profound hypermetabolic response to burn injury is associated with IR and hyperglycaemia. These responses are present in all trauma, surgical, or critically ill patients, but the severity, length, and magnitude is unique for burn patients.[27] Hence, the role of an intact, healthy skin in preventing the MetS needs further evaluation.
Dermatotherapeutics and MetS
Glucocorticosteroids, oral contraceptives, cyclosporine, retinoids, niacin, protease inhibitors, thiazide diuretics, beta blockers, antipsychotics, antidepressants and antiepileptics[28] can increase risk of MetS. Compared to age and gender matched controls; prescription H1 antihistamine users have significantly greater weight, BMI, waist circumference, and insulin levels.[29]
Use of local corticosteroids is associated with MetS, especially in women in the general population. Among local use, inhaled corticosteroids cause more metabolic disturbances, compared to topical use because a large percentage is swallowed via oropharynx.[30] Hyperglycemia and the unmasking of latent diabetes mellitus can occur after prolonged application and high percutaneous absorption of topical steroids.[31]
Glucocorticoids are well recognized for their diabetogenic potential, risk of elevated blood pressure with long-term use, and effect on lipid changes. Altered glucose control with glucocorticoid steroid use is related to several mechanisms. These include increased hepatic gluconeogenesis, the direct suppression of insulin secretion from pancreatic cells, and increasing body weight and, thus, IR with impaired glucose uptake.[32] For dermatoses requiring long term glucocorticoid use, we must keep the requirement for continuing systemic steroid treatment under constant review, and titrate the dose against therapeutic response.[33] Steroid sparing agents, immunomodulators and disease modifying agents should be used appropriately, to minimize dose and duration of corticosteroids. All patients on large surface area topical steroids and long-term systemic steroids must be regularly assessed for development of MetS.
While selecting systemic therapies for skin conditions associated with the MetS, like psoriasis, choice of therapy can affect the MetS. Acitretin, cyclosporin and TNF α inhibitors can have a negative effect on components of the MetS.
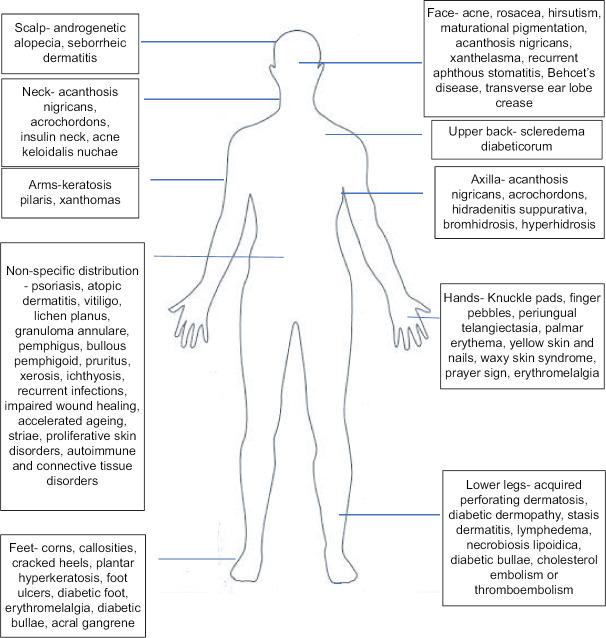
Dermatological manifestations associated with insulin resistance, metabolic syndrome or its components [Figure 4]
Figure 4.
Dermatological manifestations of insulin resistance, metabolic syndrome or its components with usual sites of presentation
Evidence for association of various skin disorders with MetS varies from very strong, for example, psoriasis, to possible, for example, rosacea, to weak or anecdotal, for example, scleredema.[34] A study of 750 patients with diabetes found that any skin disorder was present in 79.2% of diabetics, the most common being cutaneous infections (47.5%), xerosis (26.4%), and inflammatory skin diseases (20.7%).[35] Individuals with type 2 diabetes are more likely than those with type 1 diabetes to develop cutaneous manifestations,[36] which highlights the contribution of IR in their pathophysiology. The type of skin manifestation also reflects the timeline of IR, and gives us clues to look for internal organ involvement. Acanthosis nigricans is seen early in the course of the disease and reflects the proliferative effect of high levels of insulin with mostly normal or mildly elevated blood sugar. Skin changes offer insights into patients' glycaemic control.[37] Infections and impaired wound healing reflect high blood sugar. Diabetic bullae, diabetic dermopathy and scleredema diabeticorum are more commonly seen in longstanding diabetes. Foot ulcers are seen in long-standing diabetic neuropathy and vasculopathy. Patients presenting with diabetic dermopathy should be examined for other diabetic microangiopathies including retinopathy and nephropathy.[38] Certain skin signs are a result of obesity-induced mechanical factors like striae and corns. For disorders requiring use of systemic steroids, like SLE or pemphigus, the disease as well as treatment contribute to MetS.
MetS has aptly been described as the 'underbelly of dermatology'.[39]
Diagnosis of MetS
The definition and criteria for this syndrome have undergone repeated modifications as our understanding of its biology has evolved with time.
Table 1 shows the latest available criteria for diagnosis popularly used in the community setting.[40]
Table 1.
Criteria for diagnosis of metabolic syndrome
| IDF and AHA/NHLBI Harmonized criteria 2009 (any 3 or more risk factors) | |
|---|---|
| Fasting glucose | >=5.6 mmol/L (100 mg/dl) or diagnosed diabetes |
| HDL cholesterol* | <1.0 mmol/L (40 mg/dl) in men, <1.3 mmol/L (50 mg/dl) in women or drug treatment for low HDL-C |
| Triglycerides | >=1.7 mmol/L (150 mg/dl) or drug treatment for elevated triglycerides |
| Blood pressure | Blood pressure >=130/85 mmHg or drug treatment for hypertension |
| Anthropometric measurement for abdominal obesity† | Population, ethnicity or country specific criteria should be used. Asians: waist circumference >=90 cm (men) or >=80 cm (women) Europids/Caucasians >=94 cm (men) or >=80 cm (women) |
*high LDL or high total cholesterol are not components of the metabolic syndrome. In fact, LDL is often below average in patients with the metabolic syndrome.[41] † some criteria use BMI instead of waist circumference, although it is not considered an ideal representative of abdominal obesity.
Role of dermatologist in predicting and preventing MetS
Although MetS is not a dermatological diagnosis, its skin manifestations have garnered growing attention. It takes months or years for MetS or its sequelae to develop after the occurrence of IR. In just three decades, the number of school-going children and adolescents with obesity has increased by 10-fold. About one-third of urban-Asian children have IR.[42] The prevalence of MetS increases with age, but it is rising dramatically in children, adolescents and young adults. Type 2 diabetes in childhood is now not rare.
All patients, especially those with new-onset inflammatory or proliferative lesions, even in childhood or young adulthood, should be critically assessed with regards to weight gain, dietary habits and lifestyle. A history of diabetes and cardiovascular disease in first-degree relatives should be inquired. Patients' weight, waist circumference, BMI and blood pressure can be measured in the dermatologist's office. Basic investigations for blood sugar and lipids can be advised. Referrals can be made when needed.
Lifestyle intervention is the most effective prevention strategy for the MetS. Simple advice can have long term, positive implications. Diet, physical activity, sleep, emotion control, peer support, and avoidance of tobacco, moderation of alcohol, and other drugs/medications that alter satiety or body weight are key targets of any healthy lifestyle program.[43] The last treatment target is the control of co-morbidities. Effective drug therapies are available for treating hyperglycaemia, arterial hypertension, and dyslipidemia. Metformin, statins, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers are among the most frequent drugs used to treat co-morbidities.[44]
A goal-based weight loss program should be aimed to reduce body weight by at least 7% at the rate of 500 g to 1 kg per week and to do physical activity to burn at least 700 calories per week with exercise. This translates into 150 mins of moderate physical activity similar in intensity to brisk walking.[45] Even multiple short bouts of physical activity for at least 10 minutes at a time spread out throughout the day and week can be accumulated to reach the goal. Daytime outdoor physical activity can help overcome vitamin D deficiency as well. An obese patient should aim for weight loss of approximately 10% of baseline weight in six months. The best evidence of the prominent role of weight loss in MetS treatment is bariatric surgery, in which a 30 to 40% weight loss is capable of reversing the majority of the MetS abnormalities.[46]
Various dietary modifications recommended[47,48,49,50,51,52] are provided in Table 2.
Table 2.
Dietary recommendations for MetS
| Energy restricted diet (reduction of 500-600 kilocalories per day) for overweight or obese patients |
| Low glycemic index diet (limiting “ready-to-eat processed foods” including sweetened beverages, soft drinks, cookies, cakes, candy, juice drinks, and other foods which contain high amounts of added sugars, artificial sweeteners or High Fructose Corn Syrup) |
| Diet rich in unsaturated fatty acids, especially omega 3 fatty acids, dietary fibre, and low in saturated and trans-fat. Trans fat is present in fast food, snack food, baked and fried goods. Hard margarine, vanaspathi (vegetable ghee used in Indian cooking) and reusing of cooking oils are other sources. |
| Diet rich in polyphenols, vitamins, anti- oxidants (fruit and vegetable consumption of minimum 400 g per day excluding potatoes and starchy tubers, cooking with spices to maintain flavour while reducing salt), Mediterranean diet |
| Moderate–high protein diet, but low in branched chain amino acids |
| Heavy breakfast, early dinner, eating main meal of the day before 3 pm, time restricted feeding, intermittent fasting |
| Home cooked food instead of food prepared away from home |
| Cooking methods to lower AGEs, for example, Moist low heat methods like steaming, stewing, boiling and brewing compared to dry high heat methods like frying, searing or broiling. |
Skin is a mirror of our internal health, providing visual clues to a process that may remain hidden till it is too late. Cutaneous signs may provide the earliest hints to the risk of developing either the MetS or its complications. It is possible to identify risk even as early as the first decade of life. MetS takes years or decades to develop [Figure 5]. Dermatologists are in a unique position to predict and diagnose MetS, before a patient visits a physician for systemic problems. Detailed guidance about early interventions can stall or even reverse the pathology. Primary management of MetS is dietary calorie restriction combined with increased physical activity. By proposing these early lifestyle interventions, we may help delay or even prevent the occurrence of its lifethreatening consequences.
Figure 5.
Timeline of development of MetS
Abbreviations
MetS = metabolic syndrome
IR = insulin resistance
HDL = high-density lipoproteins
VLDL = very low density lipoproteins
LDL = low-density lipoproteins
FFA = free fatty acids
HOMA-IR = homeostatic model assessment for insulin resistance
QUICKI = quantitative insulin sensitivity check index
AGEs = advanced glycation end products
DDT = dichlorodiphenyltrichloroethane
DDE = dichlorodiphenyldichloroethylene
ROS = reactive oxygen species
BMI = body mass index
IDF = International Diabetes Federation
AHA = American Heart Association
NHLBI = National Heart, Lung and Blood Institute.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Anjana RM, Pradeepa R, Das AK, Deepa M, Bhansali A, Joshi SR, et al. Physical activity and inactivity patterns in India - Results from the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-5] Int J Behav Nutr Phys Act. 2014;11:26. doi: 10.1186/1479-5868-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan Y, Lalchandani A, Gupta AC, Khadanga S, Kumar S. Prevalence of metabolic syndrome crossing 40% in Northern India: Time to act fast before it runs out of proportions. J Family Med Prim Care. 2018;7:118–23. doi: 10.4103/jfmpc.jfmpc_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prentice AM, Rayco-Solon P, Moore SE. Insights from the developing world: Thrifty genotypes and thrifty phenotypes. Proc Nutr Soc. 2005;64:153–61. doi: 10.1079/pns2005421. [DOI] [PubMed] [Google Scholar]
- 4.Park JE, Pichiah PBT, Cha YS. Vitamin D and metabolic diseases: Growing roles of Vitamin D. J Obes Metab Syndr. 2018;27:223–32. doi: 10.7570/jomes.2018.27.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyykkönen AJ, Räikkönen K, Tuomi T, Eriksson JG, Groop L, Isomaa B. Stressful life events and the metabolic syndrome: The prevalence, prediction and prevention of diabetes (PPP)-Botnia Study. Diabetes Care. 2010;33:378–84. doi: 10.2337/dc09-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erren TC, Reiter RJ. Defining chronodisruption. J Pineal Res. 2009;46:245–7. doi: 10.1111/j.1600-079X.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- 7.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050–7. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendrick DL, Diehl AM, Topor LS, Dietert RR, Will Y, La Merrill MA, et al. Metabolic Syndrome and Associated Diseases: From the Bench to the Clinic. Toxicol Sci. 2018;162:36–42. doi: 10.1093/toxsci/kfx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moller DE, Flier JS. Insulin resistance – mechanisms, syndromes, and implications. N Engl J Med. 1991;325:938–48. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 10.Ighbariya A, Weiss R. Insulin resistance, prediabetes, Metabolic syndrome: What should every pediatrician know? J Clin Res Pediatr Endocrinol. 2017;9:49–57. doi: 10.4274/jcrpe.2017.S005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J Biomed Sci. 2016;23:87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroith D. Pathophysiology of the metabolic syndrome: Implications for the cardiometabolic risks associated with type 2 diabetes. Am J Med Sci. 2012;343:13–6. doi: 10.1097/MAJ.0b013e31823ea214. [DOI] [PubMed] [Google Scholar]
- 13.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care. 2013;36:1789–96. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanadi EC, Dimitrakakis G, Antoniou CK, Challoumas D, Punjabi N, Dimitrakaki IA, et al. 2018. Diabetol Metab Syndr 2018;10:9; p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: Proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98:82S–5S. doi: 10.1111/1523-1747.ep12462293. [DOI] [PubMed] [Google Scholar]
- 16.Mehta-Ambalal S. Clinical, biochemical, and hormonal associations in female patients with acne: A study and literature review. J Clin Aesthet Dermatol. 2017;10:18–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Furue M, Kadono T. “Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm Res. 2017;66:833–42. doi: 10.1007/s00011-017-1065-z. [DOI] [PubMed] [Google Scholar]
- 18.Flammer AJ, Ruschitzka F. Psoriasis and atherosclerosis: Two plaques, one syndrome? Eur Heart J. 2012;33:1989–91. doi: 10.1093/eurheartj/ehr425. [DOI] [PubMed] [Google Scholar]
- 19.Su YS, Yu HS, Li WC, Ko YC, Chen GS, Wu CS, et al. Psoriasis as initiator or amplifier of the systemic inflammatory march: Impact on development of severe vascular events and implications for treatment strategy. J Eur Acad Dermatol Venereol. 2013;27:876–83. doi: 10.1111/j.1468-3083.2012.04599.x. [DOI] [PubMed] [Google Scholar]
- 20.Medina G, Vera-Lastra O, Peralta-Amaro AL, Jiménez-Arellano MP, Saavedra MA, Cruz-Domínguez MP, et al. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol Res. 2018;133:277–88. doi: 10.1016/j.phrs.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Ergun T, Yazici V, Yavuz D, Seckin-Gencosmanoglu D, Ozen G, Salman A, et al. Advanced glycation end products, a potential link between psoriasis and cardiovascular disease: A case-control study. Indian J Dermatol. 2019;64:201–6. doi: 10.4103/ijd.IJD_396_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Waateringe RP, Slagter SN, van Beek AP, van Beek AP, van der Klauw MM, van Vliet-Ostaptchouk JV, et al. Skin autofluorescence, a non-invasive biomarker for advanced glycation end products, is associated with the metabolic syndrome and its individual components. Diabetol Metab Syndr. 2017;9:42. doi: 10.1186/s13098-017-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou SS, Li D, Zhou YM, Cao JM. The skin function: A factor of anti-metabolic syndrome. Diabetol Metab Syndr. 2012;4:15. doi: 10.1186/1758-5996-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumas SN, Ntambi JM. A discussion on the relationship between skin lipid metabolism and whole-body glucose and lipid metabolism: Systematic review. J Cell Signal. 2008;3:191. doi: 10.4172/2576-1471.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–12. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Kamezaki F, Sonoda S, Tomotsune Y, Yunaka H, Otsuji Y. Seasonal variation in metabolic syndrome prevalence. Hypertens Res. 2010;33:568–72. doi: 10.1038/hr.2010.32. [DOI] [PubMed] [Google Scholar]
- 27.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: Underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008;29:683–94. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wofford MR, King DS, Harrell TK. Drug-induced metabolic syndrome. J Clin Hypertens (Greenwich) 2006;8:114–9. doi: 10.1111/j.1524-6175.2006.04751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratliff JC, Barber JA, Palmese LB, Reutenauer EL, Tek C. Association of prescription H1 antihistamine use with obesity: Results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring) 2010;18:2398–400. doi: 10.1038/oby.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savas M, Muka T, Wester VL, van den Akker ELT, Visser JA, Braunstahl GJ, et al. Associations between systemic and local corticosteroid use with metabolic syndrome and body mass index. J Clin Endocrinol Metab. 2017;102:3765–74. doi: 10.1210/jc.2017-01133. [DOI] [PubMed] [Google Scholar]
- 31.Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol Online J. 2014;5:416–25. doi: 10.4103/2229-5178.142483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, et al. Pancreatic β cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100:2094–8. doi: 10.1172/JCI119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duru N, van der Goes MC, Jacobs JW, Andrews T, Boers M, Buttgereit F, et al. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2013;72:1905–13. doi: 10.1136/annrheumdis-2013-203249. [DOI] [PubMed] [Google Scholar]
- 34.Seremet S, Mehmet SG. Miscellaneous skin disease and the metabolic syndrome. Clin Dermatol. 2018;36:94–100. doi: 10.1016/j.clindermatol.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Demirseren DD, Emre S, Akoglu G, Arpacı D, Arman A, Metin A, et al. Relationship between skin diseases and extracutaneous complications of diabetes mellitus: Clinical analysis of 750 patients. Am J Clin Dermatol. 2014;15:65–70. doi: 10.1007/s40257-013-0048-2. [DOI] [PubMed] [Google Scholar]
- 36.Duff M, Demidova O, Blackburn S, Shubrook J. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40–8. doi: 10.2337/diaclin.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen J, Yosipovitch G. Skin manifestations of diabetes mellitus. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, et al., editors. Endotext. [Internet] South Dartmouth (MA): MDText.com, Inc.; 2000. pp. 1–82. [Google Scholar]
- 38.Salman Bustan R, Wasim D, Yderstræde KB, Bygum A. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state: A systematic review. Dan Med J. 2017;64:A5316. [PubMed] [Google Scholar]
- 39.Agarwal K, Das S. Metabolic syndrome- The underbelly of dermatology. Gulf J Dermatol Venereol. 2019;26:1–10. [Google Scholar]
- 40.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 41.Blaton V. How is the metabolic syndrome related to the dyslipidemia? EJIFCC. 2007;18:15–22. [PMC free article] [PubMed] [Google Scholar]
- 42.Sashindran VK, Dudeja P. Obesity in school children in India. In: Aungwom EE, editor. Public Health in Developing Countries- Challenges and Opportunities. London: Intech Open; 2020. pp. 1–27. [Google Scholar]
- 43.Aspry KE, Van Horn L, Carson JAS, Wylie-Rosett J, Kushner RF, Lichtenstein AH, et al. Medical nutrition education, training, and competencies to advance guideline-based diet counseling by physicians: A science advisory from the American Heart Association. Circulation. 2018;137:e821–41. doi: 10.1161/CIR.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 44.Aguilar-Salinas CA, Viveros-Ruiz T. Recent advances in managing/understanding the metabolic syndrome. F1000Res. 2019;8 doi: 10.12688/f1000research.17122.1. Faculty Rev-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25:2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Toro-Martín J, Arsenault BJ, Després JP, Vohl MC. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients. 2017;9:913. doi: 10.3390/nu9080913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoyas I, Leon-Sanz M. Nutritional challenges in metabolic syndrome. J Clin Med. 2019;8:1301. doi: 10.3390/jcm8091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Iglesia R, Loria-Kohen V, Zulet MA, Martinez JA, Reglero G, Ramirez de Molina A. Dietary strategies implicated in the prevention and treatment of metabolic syndrome. Int J Mol Sci. 2016;17:1877. doi: 10.3390/ijms17111877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Martínez P, Mikhailidis DP, Athyros VG, Bullo M, Couture P, Covas MI, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr Rev. 2017;75:307–26. doi: 10.1093/nutrit/nux014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129:557–70. doi: 10.1542/peds.2011-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-Restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–21. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr Diab Rep. 2014;14:453. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]