Abstract
Synechocystis strain PCC 6803 exhibits similar levels of cyclic AMP (cAMP) and cyclic GMP (cGMP). A thorough analysis of its genome showed that Cya2 (Sll0646) has all the sequence determinants required in terms of activity and purine specificity for being a guanylyl cyclase. Insertional mutagenesis of cya2 caused a marked reduction in cGMP content without altering the cAMP content. Thus, Cya2 represents the first example of a prokaryotic guanylyl cyclase.
Cyclic nucleotides (cyclic AMP [cAMP] and cyclic GMP [cGMP]) are important signaling molecules that mediate responses of living organisms to their environment (4, 8, 33). Cyanobacteria contain high levels of cGMP in comparison to other bacteria (12). Under standard growth conditions, the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 contains similar intracellular levels of cAMP and cGMP (12) which have to be synthesized by adenylyl-cyclases (ACs) (EC 4.6.1.1) or guanylyl-cyclases (GCs) (EC 4.6.1.2), respectively. However, no GC has been annotated as such in the complete genome of Synechocystis strain PCC 6803.
A comparative analysis of ACs and GCs from a variety of organisms led Danchin (7) to classify purine nucleotide cyclases into three phylogenetically separate classes: (i) the enterobacterial class of ACs, or class I; (ii) the calmodulin-dependent toxic class of ACs, or class II; and iii) the universal class, or class III, comprising the GCs and the remaining ACs whether of eukaryotic or prokaryotic origin. Two new phylogenetically unrelated AC types, class IV and V, have recently been identified in hyperthermophilic archaebacteria (25) and in a ruminal anaerobic bacterium (6), respectively. At present, only class III ACs have been identified and characterized in cyanobacteria (14, 15, 27).
Genomic survey for purine nucleotide cyclases.
Using BLAST similarity searches (1, 2) for each of the purine nucleotide cyclase classes against the Synechocystis strain PCC 6803 genome, only class III homologues were found: Cya1 (Slr1991), Cya2 (Sll0646), and Cya3 (Sll1161), each with a different domain organization (Fig. 1A). Cya1 has just been reported to be a true AC, responsible for the synthesis of 96% of the intracellular content of cAMP (27). In the Cyanobase (21) and GeneQuiz (23) databases, Cya2 is annotated as a putative AC/GC, and Cya3 is annotated as a hypothetical protein, whereas in the SMART database (24) the latter is recognized as a putative AC/GC. Cya1 and Cya2 have homologues in the cyanobacterium Anabaena strain PCC 7120: CyaD and CyaA, respectively (15, 27).
FIG. 1.
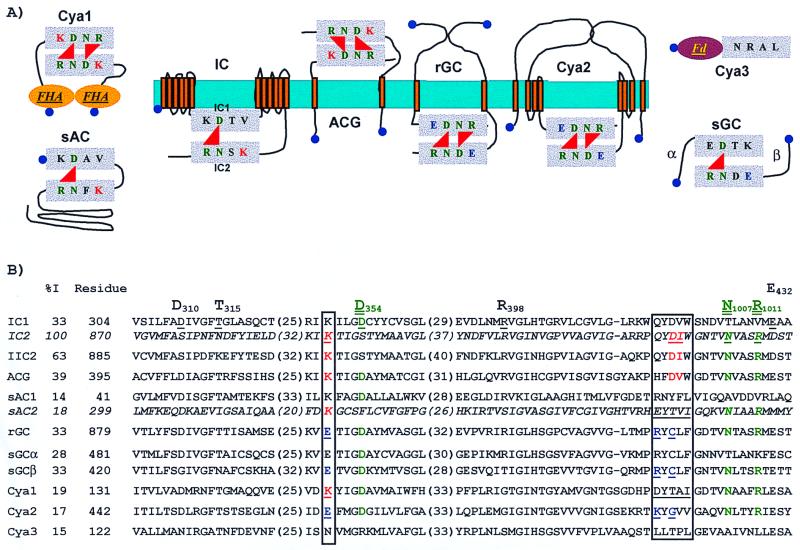
(A) Domain architectures of class III purine nucleotide cyclases representative of eukaryotes (IC, ACG, rGC, sGC and sAC) and of their homologues in Synechocystis strain PCC 6803 (Cya1, Cya2, and Cya3 [PID1652963, PID1652044, and PID1652908, respectively]). Purine nucleotide cyclase catalytic domains are drawn as grey boxes. Red triangles indicate the reaction centres that contain all the potential determinants for catalysis (D, N, and R) and specificity (K for ACs and E for GCs). The colors of the amino acids refer to those that appear in the alignment shown in panel B. ACs are on the right, and GCs are on the left. The forkhead associated (FHA) and ferredoxin-like (Fd) domains are also indicated. The membrane is drawn in blue, and transmembrane helices are represented by orange barrels. A blue dot indicates the position of the N terminus. (B) Clustal W alignment of the AC/GC catalytic domains. The sequences correspond to a membrane mammalian AC (IC, PID117785) with its two domains (IC1 and IC2), the domain of a rat AC (IIC2, PID117786) whose three-dimensional structure has been determined, a homodimeric membrane AC from D. discoideum (ACG, PID399320), a soluble mammalian AC with its two domains (sAC1 and sAC2, PID4140400), a homodimeric membrane retinal GC (rGC), and the α and β subunits of a heterodimeric soluble GC (sGCα and sGCβ [PID118059 and PID118056, respectively]). Sequences are preceded by the percentage of identity with respect to IC2 and the position of the first residue in the corresponding sequence used for the alignment. When the two domains required for the building up of the catalytic centers are part of the same polypeptide chain, the sequence of the second domain is italicized. Boxes show the potential determinants for nucleotide specificity. The amino acids essential for catalysis are in green and double underlined. Numbering of the key residues refer to their position in the IC sequence, IC1 or IC2 depending of the domain that carries them. The residues discussed in the text are underlined. Numbers in parentheses correspond to residues not shown in the alignment. The topology and architecture of the eukaryotic proteins were obtained from GenBank. The FHA and AC/GC catalytic domains of the cyanobacterial polypeptides were detected using SMART. The Fd domain was identified using PSI-BLAST, and the prediction of transmembrane helices and topology of Cya2 were carried out using HMMTOP.
Class III enzymes catalyze stereochemically analogous reactions and have homologous catalytic domains (7). The crystal structure recently solved for a class III rat AC (IIC2) (34) provided a model for the active site of eukaryotic ACs and GCs (18). The residues likely to be essential for catalysis and the determinants that confer specificity for either adenine or guanine nucleotides were deduced by modeling. Mutational analyses of the presumptive catalytic residues supported these models (3, 18, 30), providing sequence-function data for the characterization of class III purine nucleotide cyclases.
To determine which residues in Cya1, Cya2, and Cya3 could be involved in the cyclase activity and purine specificity, we aligned them, using Clustal W (29), with the domains of the eukaryotic ACs and GCs that have already been crystallized and/or modeled (18, 34). These proteins are representative examples of membrane or soluble purine nucleotide cyclases. The catalytic center of these proteins is formed either by the interaction of two domains within the same polypeptide (IC) or by the formation of homodimers (ACG, rGC) or heterodimers (sGC) (Fig. 1A). We also included in the alignment two domains (sAC1 and sAC2) of a soluble mammalian AC, which are more closely related to cyanobacterial ACs than to their eukaryotic counterparts (5). In spite of the low overall sequence similarity among the catalytic domains of some of the purine nucleotide cyclases (Fig. 1B), this alignment allowed us to recognize in the cyanobacterial homologues (i) the three residues that are essential to form the active site of class III ACs and GCs (Asp, Asn, and Arg [D354, N1007, and R1011 in IC]) (13); (ii) the two acidic ligands (D310 and E432 in IC1) that bridge the nucleotide triphosphate via two Mg2+ or Mn2+ ions (18, 28), (iii) the residue that interacts with the ribose but is not implicated in the catalytic mechanism (T315 in IC1), and (iv) the residues that confer the specificity for either adenine (K923, D1000, and I1001 in IC) or guanine (E925, R995, and C997 in rGC) (26, 30). This alignment demonstrates that the residues thought to interact with the ribose and phosphate moieties of the nucleotide triphosphates are present in Cya2.
According to the purine selectivity model (30), Cya2 could well be a GC, as it possesses (i) the Glu residue (E488), common to all GCs, that interacts with N-1 and N-2 of the guanine ring; (ii) a Lys residue (K560) that could ensure the interaction by its amino group with the O-6 of the guanine moiety, a function assigned to R995 in rGC; and (iii) a Gly residue (G562) that may be a substitute for C997 of rGC, i.e., a small residue that does not produce any unfavorable electrostatic repulsion of the guanine ring. Our hypothesis of Cya2 being a GC is supported by the fact that a Cya2− strain of this cyanobacterium exhibits wild-type levels of cAMP (27). Cya1 would be more specific than Cya2 for ATP since its determinants for specificity (K177 and the sequence DYTAI, boxed in Fig. 1B) are similar to those of sAC2.
Class III purine nucleotide cyclases are usually dimeric (13, 18). They require two AC/GC domains to form an active center. As Cya1 and Cya2 only have one AC/GC domain per polypeptide and each domain contains all the determinants required to form an active center, dimerization would result in a protein carrying two catalytic sites. HMMTOP (31) detected the presence of four putative transmembrane helices in Cya2, indicating that this protein could be located in a membrane (Fig. 1A). By homology to the membrane receptor GCs (10), the large putative periplasmic domain of 289 residues that connects helices 1 and 2 of Cya2 could correspond to the sensor domain that controls the catalytic activity.
Targeted inactivation of cya2.
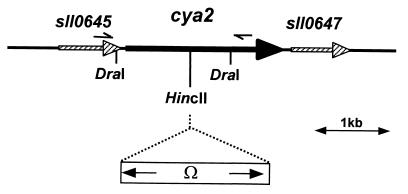
Inactivation of cya2 was carried out as follows. A 1.5-kb DraI fragment encoding the N-terminal part of cya2 was amplified by PCR from genomic DNA using 5′-GCAGGAATTTCTGGAGTCGG-3′ and 5′-ATGACATCGGCCATTTTGCC-3′ as forward and reverse primers, respectively. The PCR product was digested with DraI and ligated into the HincII site of pUC19, generating pCYA. The omega cassette (aadA gene conferring resistance to spectinomycin and streptomycin [22]) was inserted into the unique HincII site (codon 315 of cya2) of pCYA to produce pCYAΩ. This plasmid was used to transform Synechocystis strain PCC 6803 (32) grown in a modified BG11 medium (11). Transformants were selected on BG11 plates containing spectinomycin (20 μg/ml) and streptomycin (5 μg/ml). Antibiotic resistance might only result from plasmid integration into the chromosome since pUC19 could not replicate in the cyanobacterium. Two independent colonies were further studied. Gene inactivation by integration of the cassette into cya2 as a result of a double crossover and complete segregation of the transformants were ascertained by DNA blot analyses (results not shown). The resulting construct is shown in Fig. 2.
FIG. 2.
Physical map of the region that surrounds cya2 (sll0646) on the Synechocystis strain PCC 6803 genome. The large arrows represent open reading frames, and the locations of the primers used for the PCR are indicated above the genes. The restriction sites used for the construction of the cya2 mutant are drawn. The omega cassette was inserted into the HincII site.
Cyclic nucleotides content.
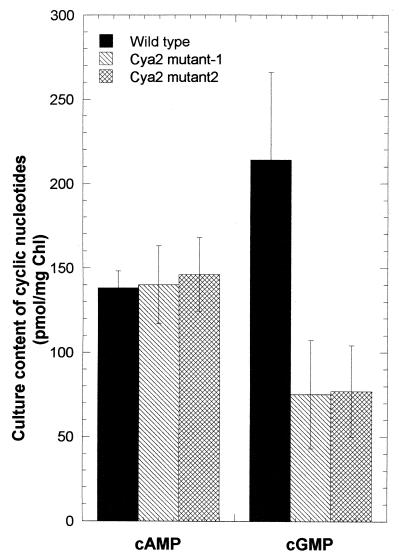
The wild-type and cya2 mutant strains were grown in 100 ml of BG11 medium supplemented with 10 mM NaHCO3, bubbled with wet air (3 liters min−1) containing 1% CO2 at 32°C and 37 μmol of photons m−2 s−1 under a continuous illumination provided by fluorescent lamps. Both cya2 mutants and the wild type showed similar growth rates and identical visible absorption spectra (data not shown). Cyclic nucleotides were extracted and analyzed as previously described (12), with slight modifications. One milliliter of exponential-phase cultures (14 to 16 μg of chlorophyll ml−1) were thoroughly mixed with 60 μl of 100% trichloroacetic acid (TCA) and kept on ice for 2 h. TCA-insoluble material was removed by centrifugation (15,000 × g for 5 min). TCA was extracted by seven successive washings of the supernatant with 3 volumes of diethylether. The final aqueous phase was evaporated to dryness overnight in a vacuum drier (SpeedVac; Savant). Dried samples were analyzed within 2 weeks using a commercially available immunoassay (EIA system; Amersham-Pharmacia, Orsay, France). Chlorophyll concentrations were estimated using cell suspensions (32). Mutant and wild-type strains showed similar levels of cAMP, whereas the cGMP content was severely reduced in the cya2 strains (Fig. 3). This result demonstrates that cya2 is expressed and its function is correlated with cGMP accumulation. Since Cya2 showed an overall homology to purine nucleotide cyclases and possessed in the catalytic pocket the three amino acids which determine specificity for GTP, we propose that Cya2 is a GC. The residual cGMP detected in the cya2 mutant strains could result either from interference in the immunoassay of some cellular component extracted by TCA (control BG11 medium did not produced such an interference), from cGMP synthesized by the Cya1 AC, from synthesis of a truncated Cya2 polypeptide that would have kept some biosynthetic activity, or from the presence of a yet unidentified GC.
FIG. 3.
Cyclic nucleotide levels in cultures of Synechocystis strain PCC 6803 and two independent cya2 mutants. Data (averages ± standard deviations) correspond to three independent cultures of each strain measured in triplicate.
Conclusions.
GCs have classically been divided into two groups, receptor GCs and soluble GCs. Receptor GCs are integral membrane proteins such as the retinal GCs and the atrial natriuretic peptide receptors (20), and Cya2 likely belongs to this group. The identification of a prokaryotic GC raised the question of the phylogenetic origin of GCs. Currently described GCs occur in metazoa and protozoa (7, 17, 20). Protozoan GCs evolved from an ancestral AC independently of the mammalian GCs (17). The catalytic domain of CyaA of Anabaena strain PCC 7120, the whole primary structure of which is similar to Cya2, is closely related to bacterial ACs and to the two presumptive catalytic domains of the mammalian sAC (5). In agreement, BLAST searches for Cya2 homologues showed that this protein is more similar to bacterial ACs and the mammalian sAC than to its eukaryotic GC counterparts. Hence, our analysis suggests that (i) Cya2 could predate the phylogenetic separation between eukaryotes and prokaryotes and (ii) GCs have a polyphyletic origin, which would be consistent with the apparent facility to change the nucleotide specificity of class III purine nucleotide cyclases (3, 26, 30).
The occurrence of cGMP and cAMP in plants has been demonstrated by various mass spectrometry techniques (9, 16). However, in contrast to animals, genes encoding purine nucleotide cyclases or the phosphodiesterases which are responsible for cyclic nucleotide degradation have not yet been identified in plants. Cyanobacteria are nowadays considered as the ancestors of the eukaryotic chloroplasts, and there is abundant evidence for the relocation of plastid genes to the nucleus (19). If GCs exist in plants, they could originate from a primitive cyanobacterial GC. The analysis presented in this contribution would help in the identification of GCs in other photosynthetic organisms.
Acknowledgments
We are grateful to M.-I. Tapia for careful reading of the manuscript, to J.-P. Roux for computer assistance, and to I. Perewoska for technical assistance. We thank A.-L. Etienne for her encouraging support.
This work was supported by grants from the Centre National de la Recherche Scientifique to the UMR8543 and to J. A. G. Ochoa de Alda.
REFERENCES
- 1.Altschul S F, Koonin E V. Iterated profile searches with PSI-BLAST--a tool for discovery in protein databases. Trends Biochem Sci. 1998;23:444–447. doi: 10.1016/s0968-0004(98)01298-5. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuve A, Krin E, Danchin A. Rhizobium meliloti adenylate cyclase: probing of a NTP-binding site common to cyclases and cation transporters. CR Acad Sci III. 1993;316:553–559. [PubMed] [Google Scholar]
- 4.Botsford J L, Harman J G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck J, Sinclair M L, Schapal L, Cann M J, Levin L R. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotta M A, Whitehead T R, Wheeler M B. Identification of a novel adenylate cyclase in the ruminal anaerobe, Prevotella ruminicola D31d. FEMS Microbiol Lett. 1998;164:257–260. doi: 10.1111/j.1574-6968.1998.tb13095.x. [DOI] [PubMed] [Google Scholar]
- 7.Danchin A. Phylogeny of adenylyl cyclases. In: Shenolikar S, Nairn A C, editors. Advances in second messenger and phosphoprotein research. Vol. 27. New York, N.Y: Raven Press Ltd.; 1993. pp. 109–162. [PubMed] [Google Scholar]
- 8.Daniel P B, Walker W H, Habener J F. Cyclic AMP signaling and gene regulation. Annu Rev Nutr. 1998;18:353–383. doi: 10.1146/annurev.nutr.18.1.353. [DOI] [PubMed] [Google Scholar]
- 9.Ehsan H, Reichheld J P, Roef L, Witters E, Lardon F, Van Bockstaele D, Van Montagu M, Inze D, Van Onckelen H. Effect of indomethacin on cell cycle dependent cyclic AMP fluxes in tobacco BY-2 cells. FEBS Lett. 1998;422:165–169. doi: 10.1016/s0014-5793(97)01610-4. [DOI] [PubMed] [Google Scholar]
- 10.Foster D C, Wedel B J, Robinson S W, Garbers D L. Mechanisms of regulation and functions of guanylyl cyclases. Rev Physiol Biochem Pharmacol. 1999;135:1–39. doi: 10.1007/BFb0033668. [DOI] [PubMed] [Google Scholar]
- 11.Herdman M, Delaney S F, Carr N G. A new medium for the isolation and growth of auxotrophic mutants of the blue-green alga Anacystis nidulans. J Gen Microbiol. 1973;79:233–237. [Google Scholar]
- 12.Herdman M, Elmorjani K. Cyclic nucleotides. Methods Enzymol. 1988;167:584–591. [Google Scholar]
- 13.Hurley J H. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem. 1999;274:7599–7602. doi: 10.1074/jbc.274.12.7599. [DOI] [PubMed] [Google Scholar]
- 14.Kasahara M, Ohmori M. Activation of a cyanobacterial adenylate cyclase, CyaC, by autophosphorylation and a subsequent phosphotransfer reaction. J Biol Chem. 1999;274:15167–15172. doi: 10.1074/jbc.274.21.15167. [DOI] [PubMed] [Google Scholar]
- 15.Katayama M, Ohmori M. Isolation and characterization of multiple adenylate cyclase genes from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:3588–3593. doi: 10.1128/jb.179.11.3588-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingston E E, Beynon J H, Newton R P. The identification of cyclic nucleotides from living systems using collision-induced dissociation of ions generated by fast atom bombardment mass spectrometry. Biomed Mass Spectrom. 1984;11:367–374. doi: 10.1002/bms.1200110709. [DOI] [PubMed] [Google Scholar]
- 17.Linder J U, Engel P, Reimer A, Kruger T, Plattner H, Schultz A, Schultz J E. Guanylyl cyclases with the topology of mammalian adenylyl cyclases and an N-terminal P-type ATPase-like domain in Paramecium, Tetrahymena and Plasmodium. EMBO J. 1999;18:4222–4232. doi: 10.1093/emboj/18.15.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Ruoho A E, Rao V D, Hurley J H. Catalytic mechanism of the adenylyl and guanylyl cyclases: modeling and mutational analysis. Proc Natl Acad Sci USA. 1997;94:13414–13419. doi: 10.1073/pnas.94.25.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin W, Stoebe B, Goremykin V, Hansmann S, Hasegawa M, Kowallik K V. Gene transfer to the nucleus and the evolution of chloroplasts. Nature. 1998;393:162–165. doi: 10.1038/30234. [DOI] [PubMed] [Google Scholar]
- 20.Morton D B, Hudson M L, Waters E, O'Shea M. Soluble guanylyl cyclases in Caenorhabditis elegans: NO is not the answer. Curr Biol. 1999;9:R546. doi: 10.1016/s0960-9822(99)80349-2. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Kaneko T, Hirosawa M, Miyajima N, Tabata S. CyanoBase, a www database containing the complete nucleotide sequence of the genome of Synechocystis sp. strain PCC 6803. Nucleic Acids Res. 1998;26:63–67. doi: 10.1093/nar/26.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 23.Scharf M, Schneider R, Casari G, Bork P, Valencia A, Ouzounis C, Sander C. GeneQuiz: a workbench for sequence analysis. Ismb. 1994;2:348–353. [PubMed] [Google Scholar]
- 24.Schultz J, Milpetz F, Bork P, Ponting C P. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sismeiro O, Trotot P, Biville F, Vivares C, Danchin A. Aeromonas hydrophila adenylyl cyclase 2: a new class of adenylyl cyclases with thermophilic properties and sequence similarities to proteins from hyperthermophilic archaebacteria. J Bacteriol. 1998;180:3334–3349. doi: 10.1128/jb.180.13.3339-3344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunahara R K, Beuve A, Tesmer J J, Sprang S R, Garbers D L, Gilman A G. Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem. 1998;273:16332–16338. doi: 10.1074/jbc.273.26.16332. [DOI] [PubMed] [Google Scholar]
- 27.Terauchi K, Ohmori M. An adenylate cyclase, Cya1, regulates cell motility in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1999;40:248–251. doi: 10.1093/oxfordjournals.pcp.a029534. [DOI] [PubMed] [Google Scholar]
- 28.Tesmer J J, Sunahara R K, Johnson R A, Gosselin G, Gilman A G, Sprang S R. Two-metal-ion catalysis in adenylyl cyclase. Science. 1999;285:756–760. doi: 10.1126/science.285.5428.756. [DOI] [PubMed] [Google Scholar]
- 29.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker C L, Hurley J H, Miller T R, Hurley J B. Two amino acid substitutions convert a guanylyl cyclase, RetGC-1, into an adenylyl cyclase. Proc Natl Acad Sci USA. 1998;95:5993–5997. doi: 10.1073/pnas.95.11.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tusnady G E, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998;283:489–506. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- 32.Williams J G K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988;167:766–778. [Google Scholar]
- 33.Zagotta W N, Siegelbaum S A. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 34.Zhang G, Liu Y, Ruoho A E, Hurley J H. Structure of the adenylyl cyclase catalytic core. Nature. 1997;386:247–253. doi: 10.1038/386247a0. [DOI] [PubMed] [Google Scholar]