Abstract
Background:
Uromodulin regulates activity of the sodium-potassium-two-chloride transporter in the loop of Henle. In adults, higher urine uromodulin levels are associated with greater rise in blood pressure (BP) in response to salt intake. We hypothesized that higher urine uromodulin levels would be associated with higher BP in children with CKD and that there would be an interaction of dietary sodium on this association.
Methods:
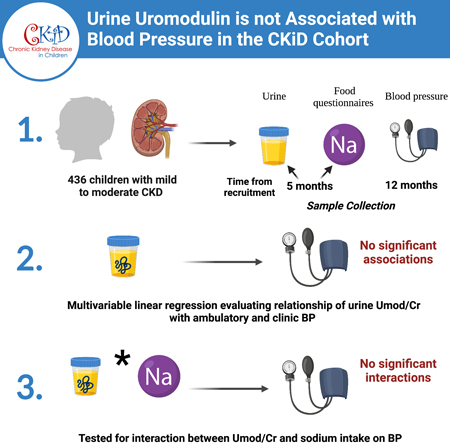
In the CKD in Children (CKiD) Cohort, we utilized univariable and multivariable linear regression models to evaluate the relationship between baseline spot urine uromodulin levels indexed to urine creatinine (Umod/Cr mg/g) and 24-hour mean systolic and diastolic BP, as well as baseline clinic BP. We also tested whether sodium intake (g/day) modified these relationships.
Results:
Among 436 participants, the median age was 12.4 years [8.9–15.2], median eGFR was 50 ml/min/1.73 m2 [36–62] and median 24-hour mean systolic BP was 112 mmHg [104–119]. The etiology of CKD was glomerular disease in 27%. In univariable models, each two-fold higher Umod/Cr ratio was associated with a 1.66 mmHg (95% CI −2.31, −1.00) lower 24-hour mean systolic and a 1.71 mmHg (−2.45, −0.97) lower clinic systolic BP. However, there was no statistically significant association between Umod/Cr and either 24-hour or clinic BP in multivariable models. We did not find a significant interaction between uromodulin and sodium intake in their effect on BP (p > 0.05 in all models).
Conclusions:
Urine uromodulin levels are not associated with BP in the CKiD cohort. Further studies are needed to confirm this finding in healthy pediatric cohorts.
Graphical Abstract
Introduction:
In healthy individuals, uromodulin, or Tamm Horsfall protein, is the most abundant protein in the urine and has a number of functional roles1. In addition to protecting against urinary tract infection and kidney stone formation, more recently, uromodulin has been found to regulate the activity of the sodium-potassium-two-chloride transporter (NKCC2) in the thick ascending limb of the loop of Henle1. Overexpression of the uromodulin gene (UMOD) in transgenic mice induces salt-sensitive hypertension, with an enhanced blood pressure (BP) response to loop diuretics2. On the contrary, UMOD-knockout mice have lower BP and are resistant to salt-induced hypertension3. In a study of community-living adults, higher urine uromodulin levels were associated with greater rise in BP in response to salt intake4. Additionally, furosemide significantly enhanced natriuresis and reduced BP in hypertensive individuals homozygous for the uromodulin increasing allele2. Hence, we hypothesized that higher urine uromodulin levels would be associated with higher BP in children with chronic kidney disease (CKD) and that there would be an interaction between urine uromodulin and dietary sodium intake.
Despite improvements in care, progression of CKD to end-stage kidney disease (ESKD) in childhood is associated with survival of 79% at 10 years5. Hypertension is an established modifiable risk factor for progression of CKD in children, and strict BP control is highly important in management of disease6. Thus, exploring potential biomarkers for optimizing hypertension management in this population is important. Identifying an association between uromodulin and BP would be a critical step in providing a physiology-based approach to treatment of hypertension using salt restriction and diuretics as initial strategies, and guided by urine uromodulin concentrations.
Methods:
Study population and design:
We conducted an ancillary study using data from a subset of participants from the CKD in Children (CKiD) cohort. The details of the CKiD study design have been previously described7. Briefly, CKiD is a multi-center, prospective, observational study that recruited children ages 6 months to 16 years with Schwartz eGFR of 30 to 90 ml/min/1.73 m2 7. Participants were recruited from 59 different centers across North America7. Participants were excluded if they had a history of dialysis within the three months prior, a history of bone marrow, solid organ, or stem cell transplantation, or structural heart disease among others. Informed consent and age-appropriate assent were obtained for all participants across CKiD study sites. The study protocol was approved by the Institutional Review Board (IRB) of each participating CKiD center and conducted in adherence with the tenets of the Declaration of Helsinki. Within our ancillary study, baseline urine uromodulin (from Visit 1b) was successfully measured in 677 participants of a total of 891 from within the CKiD cohort. Of the 677 participants with measured urine uromodulin, 436 had a successful ambulatory BP monitoring study performed at 12 months from recruitment (Visit 2). We included these 436 participants in our ancillary study. The median time elapsed between Visit 1b and visit 2 is 7 months [IQR 6–9]. Deidentified data were derived and analyzed at the CKiD Data Coordinating Center.
Uromodulin measurement:
The primary exposure was urine uromodulin level indexed to urine creatinine. We indexed urine uromodulin to urine creatinine to account for urine tonicity. This was calculated by dividing urine uromodulin concentration (pg/ml) by the urine creatinine concentration (mg/dl). Units were converted to produce a ratio of uromodulin to creatinine in mg/g. Uromodulin and creatinine were measured in spot urine specimens collected at a median of 5 months [interquartile range (IQR):4–7] after study enrollment. Specimens were stored at −80°C until time of measurement. Urine uromodulin was measured in duplicate using a Meso Scale Discovery assay and results were averaged. Sample measurement was repeated if the intra-assay coefficient of variation was > 15%. The final intra- and inter-assay coefficients of variation were 5.5% and 6.4%, respectively. The assays were performed at the Brigham and Women’s Hospital Central Biomarker Consortium Laboratory.
Ambulatory blood pressure monitoring:
SpaceLabs 90217 monitors (Issaquah, WA) were utilized for ambulatory BP monitoring. All ambulatory BP monitoring studies were read and analyzed centrally, as has been previously described8. Over the course of a 24-hour period, BP was measured every 20 minutes at a bleed step of 8 mmHg8. An ambulatory BP monitoring study was defined as successful/adequate if 1) it was worn for ≥21 hours with ≥1 valid BP measured per hour for at least 18 hours, and 2) it had ≥1 successful BP recording in ≥75% of wake hours and ≥75% in sleep hours8.
Clinic blood pressure measurement:
Clinic BP was measured at 12 months after study enrollment, and the detailed protocol for clinic BP measurement in CKiD has been previously described9. Briefly, trained study personnel measured auscultatory BP using an aneroid sphygmomanometer (Mabis MedicKit 5, Mabis Healthcare, Waukegan, IL)9. After a rest period of five minutes, BP was measured three times consecutively, with 30 second intervals in between9. The mean of these three measurements was then calculated.
Dietary sodium assessment:
To assess sodium intake, the Child Harvard Service Food Frequency Questionnaire was utilized10, 11. The questionnaires were performed a median of 5 months after enrollment (Visit 1b), at the same time as urine uromodulin measurement. Within the CKiD study, three different versions were used for children ages 2–5, 6–13, and 14–18 years. Either the study coordinator administered the questionnaire in an interview setting, or it was self-administered by the child or their parent10. This information was then used to extract the daily nutrient intake using the 2013 Nutrition Data System Software Estimation10.
Other variables:
As part of the CKiD protocol other variables were collected at a median of 5 months after enrollment (Visit 1b) including age, sex, race, ethnicity, birthweight (kg), body mass index (BMI) z-score, and etiology of CKD (glomerular vs. non-glomerular). BMI z-score was utilized, to account for differences by age in a broad pediatric cohort. The use of anti-hypertensive medications (including different classes) was obtained at 12 months from enrollment (Visit 2). Serum creatinine and cystatin C were also measured at 12 months from baseline visit. These measurements, as well as urine creatinine, were performed at the CKiD central laboratory at the University of Rochester (Rochester, NY). Estimated glomerular filtration rate (eGFR) was calculated using the CKiDU25 equation12.
Statistical Analysis:
Descriptive statistics were calculated and reported by urine uromodulin to creatinine (Umod/Cr mg/g) quartiles. Continuous variables are described using median and IQR, and categorical variables are described using frequencies and percentages. We used univariable and multivariable linear regression models to evaluate the relationship between Umod/Cr (mg/g) and 24-hour mean systolic and diastolic BP. Using the same method, we evaluated the relationship between Umod/Cr and clinic systolic and diastolic BP. We log2-transformed Umod/Cr (mg/g) given its skewed distribution. Variables that were deemed important to include as covariates in the model a priori included: age, sex, race, ethnicity, BMI z-score, birthweight (kg), eGFR, etiology of CKD, anti-hypertensive use, and sodium intake (g/day). We tested whether sodium intake (g/day) modified the relationship between uromodulin and BP. Additionally, in sensitivity analyses, we stratified by age group (age < 13 years or ≥ 13 years), etiology of CKD (glomerular vs. non-glomerular) and anti-hypertensive use (yes or no) to determine if the relationship between uromodulin and BP differed by these factors. All statistical analyses were conducted using SAS Version 9.4 (Cary, NC).
Results:
Among 436 CKiD participants, the median [IQR] age was 12.4 years [8.9–15.2], and the median eGFR was 50 ml/min/1.73 m2 [36–62]. The median of 24-hour mean systolic BP was 112 mmHg [104–119], and median clinic systolic BP was 107 mmHg [100–116]. The median clinic systolic BP z-score was 0.33 [−0.39–1.08]. Overall, 41% were female, 17% were Black, 27% had glomerular disease, and 68% were taking anti-hypertensive medications. There were 2% receiving loop diuretic medications (n=9). The median Umod/Cr level was 0.118 mg/g [0.048, 0.225]. In Table 1, we present the baseline characteristics of the cohort by Umod/Cr quartiles. The lowest quartile, had a median age of 14.3 years [12.0–16.0] whereas the highest quartile, had a mean age of 8.8 years [5.6–10.8]. Within the lowest quartile, 39% had glomerular disease, as compared to 16% within the highest quartile. Median sodium intake was 3.1 g/day in both the lowest and highest quartiles. Anti-hypertensive medications were used in 69% of both the lowest and highest quartiles. The lowest Umod/Cr quartile had the highest median clinic systolic BP at 111 mmHg [103–118]; however, this finding did not hold for clinic systolic BP z-score. Similarly, the highest Umod/Cr quartile had the lowest 24-hour mean systolic BP at 115 mmHg (Table 1).
Table 1.
Baseline characteristics of the CKiD Cohort by urine uromodulin to creatinine (mg/g) quartiles.
| Characteristic | Median [IQR] or n (%) | |||
|---|---|---|---|---|
| Uromodulin, mg/g Cr | <0.048 (n=109) |
[0.048, 0.118) (n=109) |
[0.118, 0.225) (n=109) |
≥0.225 (n=109) |
| Age, years | 14.3 [12.0, 16.0] | 13.9 [11.4, 15.9] | 11.8 [8.9, 14.5] | 8.8 [5.6, 10.8] |
| Male sex | 65 (60%) | 61 (56%) | 65 (60%) | 66 (61%) |
| Black race | 22 (20%) | 17 (16%) | 14 (13%) | 22 (20%) |
| Hispanic ethnicity | 12 (11%) | 15 (14%) | 18 (17%) | 18 (17%) |
| Glomerular diagnosis | 42 (39%) | 37 (34%) | 23 (21%) | 17 (16%) |
| Duration of CKD, years | 9.7 [4.1, 14.2] | 11.4 [6.6, 14.6] | 9.6 [6.0, 12.7] | 6.9 [4.3, 9.6] |
| U25eGFR, ml/min|1.73m2 | 53 [35, 71] | 45 [31, 61] | 49 [36, 59] | 51 [41, 64] |
| Weight, kg | 52 [39, 66] | 50 [34, 65] | 40 [29, 53] | 28 [19, 39] |
| Weight z-score | 0.2 [−0.6, 1.1] | 0.3 [−0.6, 1.0] | 0.1 [−0.8, 0.9] | −0.1 [−0.9, 1.0] |
| Height, cm | 159 [145, 167] | 156 [141, 167] | 143 [129, 157] | 127 [112, 141] |
| Height z-score | −0.5 [−1.3, 0.3] | −0.3 [−1.1, 0.4] | −0.8 [−1.4, 0.0] | −0.7 [−1.4, 0.0] |
| BMI | 20 [18, 25] | 20 [18, 24] | 19 [16, 23] | 17 [16, 20] |
| BMI z-score | 0.6 [−0.4, 1.4] | 0.4 [−0.2, 1.2] | 0.5 [−0.3, 1.4] | 0.4 [−0.2, 1.3] |
| Birth weight, kg | 3.2 [2.8, 3.6] | 3.2 [2.7, 3.6] | 3.2 [2.8, 3.6] | 3.2 [2.7, 3.6] |
| Low birth weight | 15 (15%) | 19 (19%) | 15 (15%) | 21 (21%) |
| Hypertension | 20 (19%) | 21 (20%) | 24 (23%) | 25 (24%) |
| Antihypertensive meds | 75 (69%) | 79 (72%) | 69 (63%) | 75 (69%) |
| Diuretic meds | 7 (6%) | 11 (10%) | 6 (6%) | 8 (7%) |
| Loop diuretics | 4 (4%) | 3 (3%) | 0 (0%) | 2 (2%) |
| Sodium intake, g/day | 3.1 [2.4, 4.2] | 3.4 [2.3, 5.0] | 2.9 [2.2, 4.0] | 3.1 [2.2, 3.8] |
| Clinic BP | ||||
| Systolic BP (mmHg) | 111 [103, 118] | 108 [100, 120] | 106 [98, 116] | 104 [97, 111] |
| Systolic BP z-score | 0.2 [−0.6, 0.8] | 0.2 [−0.4, 0.8] | 0.2 [−0.4, 1.2] | 0.6 [0.0, 1.3] |
| Diastolic BP (mmHg) | 68 [61, 75] | 63 [57, 73] | 63 [59, 73] | 64 [59, 71] |
| Diastolic BP z-score | 0.4 [−0.3, 1.2] | 0.0 [−0.7, 0.7] | 0.2 [−0.3, 1.1] | 0.6 [−0.1, 1.3] |
| ABPM | ||||
| 24-hour mean systolic BP (mmHg) | 115 [108, 122] | 113 [107, 121] | 111 [103, 118] | 109 [100, 116] |
| Systolic percent dipping | 11.8 [8.5, 14.6] | 11.8 [9.5, 15.1] | 11.4 [7.9, 14.3] | 11.6 [7.9, 15.2] |
| 24-hour mean diastolic BP (mmHg) | 67 [62, 72] | 67 [61, 73] | 66 [61, 72] | 65 [61, 71] |
| Diastolic percent dipping | 18.0 [14.1, 22.6] | 17.9 [14.4, 22.7] | 17.7 [13.5, 20.2] | 17.9 [12.0, 21.0] |
| Inadequate dipping (Systolic or Diastolic dip < 10%) | 39 (36%) | 40 (37%) | 45 (41%) | 46 (42%) |
| BP category | ||||
| Normotensive | 44 (42%) | 41 (39%) | 40 (38%) | 34 (32%) |
| Masked hypertension | 42 (40%) | 42 (40%) | 40 (38%) | 46 (44%) |
| White coat hypertension | 1 (1%) | 4 (4%) | 4 (4%) | 5 (5%) |
| Confirmed hypertension | 19 (18%) | 17 (16%) | 20 (19%) | 20 (19%) |
Abbreviations: IQR, interquartile range; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; BMI, body mass index; BP, blood pressure; ABPM, ambulatory blood pressure monitoring.
The Relationship between Urine Uromodulin and Blood Pressure
In univariate regression analyses, each two-fold higher Umod/Cr ratio was associated with a 1.66 mmHg (95% CI −2.31, −1.00) lower 24-hour mean systolic and a 0.49 mmHg (−0.97, −0.01) lower 24-hour mean diastolic BP (p<0.0001 and p = 0.05 respectively). Additionally, each two-fold higher Umod/Cr ratio was associated with a 1.71 mmHg (−2.45, −0.97) lower clinic systolic and a 0.90 mmHg (−1.59, −0.22) lower clinic diastolic BP (p<0.0001, p=0.01 respectively). In multivariable linear regression analyses, Umod/Cr ratio was not significantly associated either 24-hour mean systolic or diastolic BP (Table 2). Similarly, Umod/Cr ratio was not significantly associated with either clinic systolic or diastolic BP (Table 3). We found that inclusion of age in particular, was responsible for much of the confounding influence, as its inclusion alone rendered the association between Umod/Cr and BP no longer statistically significant. We also did not find a significant interaction between uromodulin levels and sodium intake in their effect on BP (p > 0.05 in all models). In sensitivity analyses, we stratified the cohort by age (< 13 years or ≥ 13 years), etiology of CKD (glomerular vs. non-glomerular), and anti-hypertensive use. We did not identify an association between urine uromodulin and clinic or ambulatory systolic BP in either age group (Tables 4, 5). Similarly, we did not find a significant relationship between urine uromodulin and clinic or ambulatory BP in either those with or without glomerular disease (supp. Tables S1, S2) or when stratified by anti-hypertensive use (supp. Tables S3, S4).
Table 2.
Multivariable linear regression models evaluating the relationship of baseline urine uromodulin levels, indexed to creatinine, with 24-hour mean systolic and diastolic blood pressure.
| Predictor | Outcome: 24-hour Systolic Mean BP |
Outcome: 24-hour Diastolic Mean BP |
||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Umod/Cr, per doubling | −0.30 (−1.08, 0.48) | 0.45 | −0.06 (−0.66, 0.55) | 0.86 |
| Age, per year | 1.08 (0.76, 1.39) | <0.0001 | 0.20 (−0.05, 0.44) | 0.12 |
| Male sex | 3.40 (1.21, 5.59) | 0.002 | 0.03 (−1.68, 1.74) | 0.97 |
| BMI z-score | 0.74 (−0.24, 1.72) | 0.14 | −0.92 (−1.69, −0.15) | 0.02 |
| Black racea | 3.86 (0.99, 6.73) | 0.008 | 2.54 (0.30, 4.78) | 0.03 |
| Hispanic ethnicityb | −1.10 (−4.24, 2.04) | 0.49 | −0.67 (−3.13, 1.78) | 0.59 |
| Glomerular diagnosis | −0.68 (−3.42, 2.06) | 0.63 | 1.31 (−0.83, 3.45) | 0.23 |
| Birth weight, per kg | −0.16 (−1.72, 1.39) | 0.84 | 0.78 (−0.43, 2.00) | 0.21 |
| eGFR, per 10% decrease | 0.06 (−0.22, 0.33) | 0.69 | 0.11 (−0.11, 0.32) | 0.32 |
| Antihypertensive use | −0.47 (−2.84, 1.91) | 0.70 | −1.81 (−3.67, 0.05) | 0.06 |
| Sodium intake, per g/day | −0.04 (−0.41, 0.32) | 0.81 | −0.15 (−0.43, 0.14) | 0.31 |
Abbreviations: BP, blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Reference group for race is non-Black: including White, Asian, Pacific Islander.
Reference group for ethnicity is non-Hispanic.
Table 3.
Multivariable linear regression models evaluating the relationship of baseline urine uromodulin levels, indexed to creatinine, with clinic systolic and diastolic blood pressure.
| Predictor | Outcome: Systolic Clinic BP |
Outcome: Diastolic Clinic BP |
||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Umod/Cr, per doubling | −0.17 (−1.05, 0.70) | 0.70 | −0.21 (−1.09, 0.68) | 0.65 |
| Age, per year | 1.09 (0.74, 1.45) | <0.0001 | 0.54 (0.18, 0.90) | 0.003 |
| Male sex | 4.05 (1.60, 6.51) | 0.001 | 0.56 (−1.90, 3.03) | 0.65 |
| BMI z-score | 1.98 (0.88, 3.08) | 0.0005 | 0.06 (−1.05, 1.16) | 0.92 |
| Black racea | 2.14 (−1.10, 5.38) | 0.19 | 3.72 (0.46, 6.97) | 0.03 |
| Hispanic ethnicityb | 2.11 (−1.43, 5.65) | 0.24 | 1.06 (−2.50, 4.61) | 0.56 |
| Glomerular diagnosis | 0.33 (−2.73, 3.40) | 0.83 | −0.49 (−3.56, 2.59) | 0.76 |
| Birth weight, per kg | −1.00 (−2.75, 0.75) | 0.26 | 1.21 (−0.54, 2.96) | 0.18 |
| eGFR, per 10% decrease | 0.37 (0.06, 0.68) | 0.02 | 0.31 (−0.00, 0.62) | 0.052 |
| Antihypertensive use | 0.11 (−2.57, 2.79) | 0.94 | −2.13 (−4.83, 0.56) | 0.12 |
| Sodium intake, per g/day | 0.12 (−0.28, 0.53) | 0.55 | 0.03 (−0.38, 0.43) | 0.90 |
Abbreviations: BP, blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Reference group for race is non-Black (including White, Asian, Pacific Islander).
Reference group for ethnicity is non-Hispanic.
Table 4.
Multivariable linear regression models evaluating the relationship of baseline urine uromodulin levels, indexed to creatinine, with 24-hour mean systolic blood pressure, stratified by age.
| Predictor | Age < 13 (n=242) |
Age ≥ 13 (n=194) |
||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Umod/Cr, per doubling | −0.37 (−1.44, 0.69) | 0.49 | −0.21 (−1.38, 0.95) | 0.72 |
| Age, per year | 1.00 (0.45, 1.55) | 0.0004 | 1.80 (0.51, 3.09) | 0.007 |
| Male sex | 1.06 (−1.85, 3.97) | 0.47 | 6.47 (3.05, 9.89) | 0.0003 |
| BMI z-score | −0.11 (−1.43, 1.21) | 0.87 | 1.36 (−0.18, 2.90) | 0.08 |
| Black racea | 3.05 (−0.55, 6.65) | 0.10 | 4.81 (−0.05, 9.67) | 0.053 |
| Hispanic ethnicityb | −1.10 (−5.30, 3.10) | 0.61 | −1.39 (−6.30, 3.52) | 0.58 |
| Glomerular diagnosis | −1.74 (−5.87, 2.40) | 0.41 | 0.35 (−3.47, 4.16) | 0.86 |
| Birth weight, per kg | 1.36 (−0.69, 3.40) | 0.19 | −1.90 (−4.35, 0.55) | 0.13 |
| eGFR, per 10% decrease | 0.06 (−0.34, 0.47) | 0.75 | 0.08 (−0.31, 0.47) | 0.67 |
| Antihypertensive use | 0.45 (−2.49, 3.40) | 0.76 | −2.51 (−6.57, 1.56) | 0.22 |
| Sodium intake, per g/day | 0.20 (−0.29, 0.68) | 0.42 | −0.40 (−0.97, 0.16) | 0.16 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate.
Reference group for race is non-Black (including White, Asian, Pacific Islander).
Reference group for ethnicity is non-Hispanic.
Table 5.
Multivariable linear regression models evaluating the relationship of baseline urine uromodulin levels, indexed to creatinine, with clinic systolic blood pressure, stratified by age.
| Predictor | Age < 13 (n=242) |
Age ≥ 13 (n=194) |
||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Umod/Cr, per doubling | 0.00 (−1.13, 1.12) | 0.99 | −0.59 (−1.98, 0.81) | 0.41 |
| Age, per year | 0.86 (0.28, 1.44) | 0.004 | 2.36 (0.81, 3.92) | 0.003 |
| Male sex | 1.67 (−1.38, 4.73) | 0.28 | 6.70 (2.61, 10.80) | 0.002 |
| BMI z-score | 1.05 (−0.34, 2.43) | 0.14 | 3.24 (1.39, 5.10) | 0.0007 |
| Black racea | 0.44 (−3.40, 4.29) | 0.82 | 4.17 (−1.61, 9.95) | 0.16 |
| Hispanic ethnicityb | −0.67 (−5.15, 3.82) | 0.77 | 5.80 (−0.04, 11.63) | 0.052 |
| Glomerular diagnosis | 2.59 (−1.74, 6.93) | 0.24 | −1.93 (−6.49, 2.63) | 0.40 |
| Birth weight, per kg | 0.23 (−1.92, 2.38) | 0.83 | −2.09 (−5.05, 0.88) | 0.17 |
| eGFR, per 10% decrease | 0.39 (−0.04, 0.82) | 0.07 | 0.53 (0.06, 1.00) | 0.03 |
| Antihypertensive use | −0.22 (−3.33, 2.90) | 0.89 | 0.14 (−4.72, 5.00) | 0.96 |
| Sodium intake, per g/day | 0.15 (−0.36, 0.66) | 0.56 | 0.12 (−0.55, 0.79) | 0.73 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate.
Reference group for race is non-Black (including White, Asian, Pacific Islander).
Reference group for ethnicity is non-Hispanic.
Discussion:
Among a diverse cohort of children with CKD, we did not identify a significant relationship between urine uromodulin levels and ambulatory or clinic BP patterns, after multivariable adjustment. This finding was consistent in sub-group analyses, in which we stratified by age, etiology of CKD, and anti-hypertensive use. While urine uromodulin levels were significantly associated with lower BP in the univariate analyses, this effect was attenuated with the inclusion of age as a covariate.
The absence of an association between urine uromodulin and BP in children with CKD is contrary to our a priori hypothesis and distinct from what has been observed in healthy adults. There may be a few reasons for the lack of an association. First, uromodulin, in addition to modulating BP response to salt, is a robust biomarker of tubular health and nephron mass1, 13, 14. Within a CKD population, uromodulin level may vary more by extent of tubular disease, and this may confound our ability to interpret its relationship with BP. Second, BP in CKiD participants could reflect the etiology and severity of their kidney disease, and uromodulin levels decline as CKD progresses. In adults with CKD, higher urine uromodulin levels have been associated with slower eGFR decline15, 16. Thus, the relationship between uromodulin and BP may differ in children with CKD as compared with other healthy pediatric populations. Finally, certain biomarkers of kidney injury and function change with age, growth, and puberty in children17–20. In our study, we identified that the urine uromodulin levels appeared inversely correlated with age, as those in the highest quartile of Umod/Cr were significantly younger than those in the lowest quartile (8.8 vs. 14.3 years).
Our study has important limitations. First, uromodulin was measured on spot urine samples, given the challenges of collecting 24-hour specimens in children. Second, sodium intake was estimated by food frequency questionnaires, rather than by urine sodium measurement. Future studies are needed to confirm our results using 24-hour urine studies in children and adolescents with CKD. Additionally, given our study was done within a pediatric CKD cohort without healthy controls, further investigations are needed to explore whether the relationship between uromodulin and BP may differ in healthy pediatric populations. Third, the measures we analyzed were not all performed simultaneously. Urine uromodulin was measured at Visit 1b and ambulatory BP monitoring performed at Visit 2, with a median time of 7 months [IQR 6–9] between the visits. Finally, we do not have data on UMOD genotype within this cohort, precluding our ability to analyze the relationship of genetic polymorphisms with BP patterns.
Conclusions:
Within a large cohort of children with mild to moderate CKD, we did not identify a significant relationship between urine uromodulin and ambulatory or clinic BP in multivariable models. Higher urine uromodulin was significantly associated with lower BP in univariable models; however, this relationship was rendered insignificant the with inclusion of age. Thus, further studies are needed to more closely evaluate the relationship between urine uromodulin and age in children.
Perspectives:
Higher urine uromodulin levels have been associated with greater BP rise in response to sodium intake in adults. However, the relationship of uromodulin with BP has not yet been evaluated in a pediatric population. In this ancillary study of children from within the CKiD study, the largest cohort of children with CKD in North America, baseline urine uromodulin levels were not significantly associated with either ambulatory or clinic BP patterns, after multivariable adjustment. There was no interaction between urine uromodulin and reported dietary sodium intake in their effect on BP. Future studies are needed to explore this relationship within healthy pediatric cohorts.
Supplementary Material
Novelty and Relevance:
What is new?
In adults, higher urine uromodulin levels have been associated with greater rise in BP in response to salt intake. This relationship has not yet been explored in pediatric populations. Here, within the CKiD cohort, the largest study of children with CKD in North America, we evaluated the relationship of baseline urine uromodulin levels with ambulatory and clinic BP patterns and tested whether there is an interaction between uromodulin and sodium intake on BP. In univariable models, higher urine uromodulin levels were associated with lower BP. However, this effect was attenuated with the inclusion of age in the multivariable models. There was no significant interaction between uromodulin and sodium intake in their effect on BP.
What is relevant?
Hypertension is a modifiable risk factor for CKD progression in children, and as such, exploring potential biomarkers for optimizing hypertension management in this population is important. In this cross-sectional analysis within the CKiD study, we did not identify a relationship between urine uromodulin and BP patterns. This finding was consistent in all sub-group analyses, in which we stratified by age, etiology of CKD, and anti-hypertensive use.
Clinical/Pathophysiological significance?
While urine uromodulin was not associated with BP within the CKiD cohort, future studies are needed to determine whether this may be different within healthy pediatric populations. Additionally, given that inclusion of age attenuated the association between uromodulin and BP, further investigations are needed to explore how urine uromodulin levels change across childhood and adolescence.
Acknowledgements
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD website is located at https://statepi.jhsph.edu/ckid and a list of CKiD collaborators can be found at https://statepi.jhsph.edu/ckid/site-investigators/.
Graphical abstract was created with BioRender.com.
Sources of Funding
The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01DK66143, U01DK66174, U24DK082194, and U24DK066116). The work for this ancillary study was supported by the NIDDK through grant K23 DK129836 (CYB), K24 DK110427 (JHI), and K23 DK114556 (PSG). This work was also supported by American Heart Association Grant # 857722 (CYB).
Nonstandard Abbreviations and Acronyms:
- BP
blood pressure
- UMOD
uromodulin gene
- CKiD
Chronic Kidney Disease in Children
- Umod/Cr
uromodulin to creatinine
Footnotes
Disclosures
None.
References
- 1.Devuyst O, Olinger E, Rampoldi L. Uromodulin: From physiology to rare and complex kidney disorders. Nature Reviews Nephrology. 2017;13:525–544 [DOI] [PubMed] [Google Scholar]
- 2.Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, Citterio L, Demaretz S, Trevisani F, Ristagno G, Glaudemans B, Laghmani K, Dell’Antonio G, Bochud M, Burnier M, Devuyst O, Martin P-Y, Mohaupt M, Paccaud F, Pechère-Bertschi A, Vogt B, Ackermann D, Ehret G, Guessous I, Ponte B, Pruijm M, Loffing J, Rastaldi MP, Manunta P, Devuyst O, Rampoldi L, the Swiss Kidney Project on Genes in Hypertension t. Common noncoding umod gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nature Medicine. 2013;19:1655–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham Lesley A, Padmanabhan S, Fraser Niall J, Kumar S, Bates James M, Raffi Hajamohideen S, Welsh P, Beattie W, Hao S, Leh S, Hultstrom M, Ferreri Nicholas R, Dominiczak Anna F, Graham D, McBride Martin W. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension. 2014;63:551–558 [DOI] [PubMed] [Google Scholar]
- 4.Ponte B, Pruijm M, Ackermann D, Olinger E, Youhanna S, Vogt B, Burnier M, Pechere-Bertschi A, Bochud M, Devuyst O. Uromodulin, salt, and 24-hour blood pressure in the general population. Clinical Journal of the American Society of Nephrology. 2021;16:787–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald SP, Craig JC, Australian, New Zealand Paediatric Nephrology A. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662 [DOI] [PubMed] [Google Scholar]
- 6.Strict blood-pressure control and progression of renal failure in children. New England Journal of Medicine. 2009;361:1639–1650 [DOI] [PubMed] [Google Scholar]
- 7.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA. Design and methods of the chronic kidney disease in children (ckid) prospective cohort study. Clinical Journal of the American Society of Nephrology. 2006;1:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA, Chronic Kidney Disease in Children Study G. Blood pressure in children with chronic kidney disease: A report from the chronic kidney disease in children study. Hypertension. 2008;52:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Ducharme-Smith K, Davis L, Hui WF, Warady BA, Furth SL, Abraham AG, Betoko A. Dietary sources of energy and nutrient intake among children and adolescents with chronic kidney disease. Pediatr Nephrol. 2017;32:1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui WF, Betoko A, Savant JD, Abraham AG, Greenbaum LA, Warady B, Moxey-Mims MM, Furth SL. Assessment of dietary intake of children with chronic kidney disease. Pediatric nephrology (Berlin, Germany). 2017;32:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pivin E, Ponte B, de Seigneux S, Ackermann D, Guessous I, Ehret G, Pechère-Bertschi A, Olinger E, Mohaupt M, Vogt B, Martin P-Y, Burnier M, Bochud M, Devuyst O, Pruijm M. Uromodulin and nephron mass. Clinical Journal of the American Society of Nephrology. 2018;13:1556–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechère-Bertschi A, Vogt B, Mohaupt MG, Martin PY, Youhanna SC, Nägele N, Vollenweider P, Waeber G, Burnier M, Devuyst O, Bochud M. Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin J Am Soc Nephrol. 2016;11:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jotwani V, Garimella PS, Katz R, Malhotra R, Bates J, Cheung AK, Chonchol M, Drawz PE, Freedman BI, Haley WE, Killeen AA, Punzi H, Sarnak MJ, Segal MS, Shlipak MG, Ix JH. Tubular biomarkers and chronic kidney disease progression in sprint participants. Am J Nephrol. 2020;51:797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Kemmner S, Bachmann Q, Angermann S, Wen M, Heemann U, Renders L, Garimella PS, Scherberich J. Urinary uromodulin independently predicts end-stage renal disease and rapid kidney function decline in a cohort of chronic kidney disease patients. Medicine. 2019;98:e15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg JH, Parikh CR. Biomarkers for diagnosis and prognosis of aki in children: One size does not fit all. Clin J Am Soc Nephrol. 2017;12:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol. 2015;30:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westhoff JH, Tönshoff B, Waldherr S, Pöschl J, Teufel U, Westhoff TH, Fichtner A. Urinary tissue inhibitor of metalloproteinase-2 (timp-2) • insulin-like growth factor-binding protein 7 (igfbp7) predicts adverse outcome in pediatric acute kidney injury. PLoS One. 2015;10:e0143628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cangemi G, Storti S, Cantinotti M, Fortunato A, Emdin M, Bruschettini M, Bugnone D, Melioli G, Clerico A. Reference values for urinary neutrophil gelatinase-associated lipocalin (ngal) in pediatric age measured with a fully automated chemiluminescent platform. Clin Chem Lab Med. 2013;51:1101–1105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


