Abstract
Introduction
We evaluated the prevalence of dementia and mild cognitive impairment (MCI) in indigenous Tsimane and Moseten, who lead a subsistence lifestyle.
Methods
Participants from population‐based samples ≥ 60 years of age (n = 623) were assessed using adapted versions of the Modified Mini‐Mental State Examination, informant interview, longitudinal cognitive testing and brain computed tomography (CT) scans.
Results
Tsimane exhibited five cases of dementia (among n = 435; crude prevalence = 1.2%, 95% confidence interval [CI]: 0.4, 2.7); Moseten exhibited one case (among n = 169; crude prevalence = 0.6%, 95% CI: 0.0, 3.2), all age ≥ 80 years. Age‐standardized MCI prevalence was 7.7% (95% CI: 5.2, 10.3) in Tsimane and 9.8% (95% CI: 4.9, 14.6) in Moseten. Cognitive impairment was associated with visuospatial impairments, parkinsonian symptoms, and vascular calcification in the basal ganglia.
Discussion
The prevalence of dementia in this cohort is among the lowest in the world. Widespread intracranial medial arterial calcifications suggest a previously unrecognized, non‐Alzheimer's disease (AD) dementia phenotype.
Keywords: cognitive dysfunction, dementia, mental status and dementia tests, Moseten, Tsimane
1. INTRODUCTION
Of individuals 65 years of age and older living in high‐income countries, 8% to 11% have a dementing illness. 1 , 2 Prevalence increases exponentially with age. The leading cause of dementia is Alzheimer's disease (AD). The most prominent genetic risk factor for AD is the apolipoprotein E (APOE) ε4 allele. 1
We report the prevalence of dementia and mild cognitive impairment (MCI) in Tsimane and Moseten Amerindians of the Bolivian Amazon. The Tsimane are an indigenous population of ≈17,000 who live a physically demanding subsistence lifestyle in small communities located mostly along the Maniqui River, an Amazon tributary. They fish, hunt, and farm with hand tools and gather food from the forest. There is minimal access to electricity, clean water, sewage treatment, or medications 3 (see Figure S1). The Moseten (population ≈3000), although genetically and linguistically related to the Tsimane, are more acculturated into Bolivian society. They live in closer residential proximity to the market economy, have greater Spanish fluency, schooling, and access to clean water, store‐bought foods and medical services. Nevertheless, Moseten still reside in rural villages and engage in high subsistence work effort, mainly agricultural.
Studies of other indigenous and rural low‐literacy populations have found widely varying prevalence of dementia. A systematic review of 15 studies of indigenous populations in Australia, North America, Guam, and Brazil found dementia prevalence ranging from 0.5% to 20% for age ≥ 60 or 65 years. 4 Where comparator non‐indigenous populations were available, indigenous rates tended to be higher than non‐indigenous, which the authors attributed to low education levels and a higher prevalence of other risk factors including HIV/AIDS, diabetes, hypertension, alcohol abuse, obesity, cardiovascular disease and mental health disorders. 4
Evidence is converging as to the major modifiable risk factors for dementia and AD. 5 , 6 These include low formal education 7 ; vascular factors, including midlife hypertension and diabetes 8 ; cardiovascular disease other than stroke 9 ; physical inactivity 10 ; and—a recently recognized addition—air pollution. 6 Higher coronary artery calcium (CAC) scores—a marker of atherosclerosis—is related to increased risk of dementia. 11 Evidence‐based dietary recommendations for reducing risk of dementia and AD include regular consumption of fresh vegetables, fruits, and fish. 12 In addition, several microbial pathogens have been associated with AD. 13 The antimicrobial protection hypothesis describes how neuroinflammatory pathways may help fight infection while contributing to AD pathology. 14
On the basis of this literature, we hypothesized a low prevalence of AD and related dementias among Tsimane and Moseten, due to their low prevalence of CAC 15 and atrial fibrillation 16 ; low rates of hypertension 17 , type 2 diabetes, 18 obesity, and smoking 15 ; high levels of physical activity 19 ; and a diet low in processed carbohydrates and fat. 20 In addition, as most dementia is found among individuals ≥ 75 years of age, 2 the pyramid‐shaped age structure of the Tsimane and Moseten populations means that the crude prevalence would be expected to be low. On the other hand, these indigenous populations are subject to high infectious disease burden 21 and systemic inflammation, and the mode with respect to formal schooling for older Tsimane is zero years. 3 Relatively low exposure to traffic and industrial sources of environmental pollution are offset to an unknown extent by cooking fires 22 and biomass burning. 23
1.1. Aim of study
To test our hypothesis that Tsimane and Moseten populations will show a low prevalence of AD and related dementias, we undertook a comprehensive assessment of dementia and cognitive impairment among Tsimane and Moseten who were 60 years of age and older. We calculated the prevalence of dementia and MCI. Furthermore, to characterize observed cases, we compared cognitively impaired to not cognitively impaired on neurological symptoms, cognitive test scores, and computed tomography (CT) brain scan images, using a matched case‐control sub‐sample for the CT analyses.
2. METHODS
2.1. Participants
The Tsimane Health and Life History Project (THLHP) has been studying the Tsimane population since 2002, focusing on health and aging, and in 2011 expanded its coverage to ≈100 Tsimane villages, where residents were enumerated and tracked. All individuals ≥60 years of age were invited to enroll; individuals age 40 to 59 were recruited by a random sample stratified by community. 15 Beginning in 2015, a total of 10 Moseten villages were added to the project. Ongoing assessment by THLHP physicians and Tsimane anthropologists includes medical evaluations with brief neurological exams, hearing and vision tests, blood panels, and cognitive testing, at ≈2‐year intervals. THLHP anthropologists update village censuses biennially to identify anyone who had moved from the village or had died and to establish cause of death based on indirect reports and medical histories. The census has 95% coverage among participating villages.
HIGHLIGHTS
Indigenous Tsimane and Moseten have a low prevalence of Alzheimer's disease (AD) and related dementias.
The subsistence lifestyle is physically demanding, with a diet low in saturated fats.
Low dementia prevalence parallels low prevalence of coronary artery calcification (CAC).
Mild cognitive impairment (MCI) has prevalence comparable to other populations.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed literature on dementia in indigenous, rural, and low‐literacy populations using traditional (eg, PubMed) sources and meeting abstracts and presentations. There are almost no other reports on dementia in a subsistence‐based population; we cite the most relevant prior studies.
Interpretation: Our data indicated that the prevalence of dementia, especially Alzheimer's disease (AD), is among the lowest in the world, although we observed prevalent mild cognitive impairment (MCI) similar to other populations. Cases of cognitive impairment among Tsimane were characterized by visuospatial impairments, parkinsonian symptoms, and vascular calcification of the lenticulostriate arteries supplying the basal ganglia.
Future Directions: Findings are consistent with the Tsimane having the lowest reported levels of coronary artery calcification (CAC) of any population recorded to date. An incidence phase will clarify the role of mortality in creating low prevalence rates and will enable testing predictors of disease risk and protection.
The sample for the present study was drawn in 2017, comprising every living THLHP participant ≥60 years of age in the participating Tsimane and Moseten territorial village censuses. Participants were visited for a dementia assessment between July 2017 and December 2019. This visit constitutes the baseline dementia evaluation, although we had the advantage of prior waves of cognitive tests as part of earlier THLHP data collection.
All phases of the study were approved by the ethics committee of the San Simon University School of Medicine (Cochabamba, Bolivia), the institutional review board (IRB) of the University of New Mexico Health Sciences Center (which serves as the designated IRB) and the University of California, Santa Barbara. The Tsimane and Moseten governments, village leaders, and study participants approved all protocols. All participants provided informed consent in their native language. When incidental findings arose during brain and chest CT scans, participants were advised and supported to receive medical treatment.
2.2. Procedures
Dementia assessments were performed by an experienced Bolivian physician (RQG) who had repeatedly examined each participant in the past as part of the ongoing THLHP. Tsimane were interviewed in the Tsimane language; Moseten were interviewed in Spanish.
In designing our protocols, we drew on the Kimberly Indigenous Cognitive Assessment (KICA) 24 ; the Indo‐US Cross‐National Dementia Epidemiology Study 25 ; and the Indianapolis‐Ibadan Dementia Project. 26
2.2.1. Clinical interview
The clinical interview began with self‐reported evaluation of vision, hearing, memory, and thinking abilities. Next, we administered the Modified Mini‐Mental State Examination (3MS) 27 that we further modified for illiteracy and lack of ability to count by substituting tasks from the same domain (see Supplement A.) We iteratively consulted with Tsimane anthropologists on the team and piloted adjustments. Training and quality control included direct observation of Bolivian physicians in the field and by video. The 3MS score ranges from 0 to 100. A subset of the 3MS items also provide a score for the Mini‐Mental State Examination (MMSE). 28
2.2.2. Informant interview
A family informant was interviewed for all participants. The informant interview combines a modified caregiver interview from the KICA tool and questions from the Peruvian Spanish translation of the Clinical Dementia Rating scale 29 (see Supplement B.) The interview provides scores for both the KICA 30 and the Blessed Dementia Scale. 31
2.2.3. Cognitive battery
The cognitive battery is largely adapted from the Mexican Health and Aging Study. 32 The battery includes Visual Scan (searching for a target symbol amongst distractor symbols), Digit Span Forward, Immediate and Delayed Word Recall, Semantic Fluency (naming animals and fish), Spatial Span (a variation of the Corsi block tapping task), and Stick Design Test 33 (a measure of visuo‐constructional ability). For all tasks, we derived population‐specific norms for age ≥60 years using cognitive scores from 2008 with cut‐offs corresponding to −1 SD, −1.5 SD, and −2 SD from the mean. 34 Longitudinal cognitive profiles with two to four times of testing separated by ≈2 years between assessments were available for over 75% of participants, enabling us to determine whether there had been cognitive decline.
2.2.4. Neurological evaluation
A brief neurological assessment evaluated signs associated with parkinsonism or stroke. The Bolivian THLHP physicians received training and supervision at a neurology clinic in the United States and remotely by video.
2.2.5. Brain CT scan
Between 2015 and 2018, individuals who consented and were able to travel were transported to the German Busch Hospital in Trinidad, Bolivia, for chest and brain CT scans. CT scans were performed by a licensed radiological technician using a 16‐detector row multi‐slice CT (GE Brightspeed, Milwaukee, WI, USA) under the supervision of project clinicians, using a 0.625‐mm slice thickness. Project radiologists (EML, GB, MLS, JDS)—blinded to age, sex, or any clinical information—reviewed CT scans for focal brain lesions (eg, major infarcts, masses, trauma) and visually rated for global cortical atrophy (Pasquier GCA scale), medial temporal atrophy (Scheltens scale), white matter disease/leukoariosis (Fazekas scale), and number of infarcts (see 35 ), as well as vascular calcification 36 and basal ganglia calcification. 37 Separately from the diagnostic process, brain volumes were obtained by segmenting CT scans using a probabilistic tissue classification method, 38 with those carrying out the segmentation and quantitative assessment blinded to clinical or demographic information.
2.2.6. Clinical diagnosis of dementia and MCI
Independent diagnoses were made at separate case conferences by two Bolivian physicians with 15 or more years of experience performing medical exams of Tsimane and Moseten populations and by a USC‐based team composed of a clinical psychologist, neurologist, and neuroradiologists. First, each team applied Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) criteria for major and mild neurocognitive disorder, 39 corresponding to dementia and MCI, using the clinical interview including 3MS and MMSE scores; informant interview; and longitudinal cognitive scores from prior data collection occasions by the THLHP. Second, toward specifying an etiologic subtype, if the individual was judged to be demented, each team then applied National Institute on Aging (NIA)/Alzheimer's Association criteria for dementia caused by AD, 40 for which step the USC team viewed the brain CT scans with the preliminary visual ratings. Third, for individuals judged to meet criteria for MCI, we noted which cognitive domains were affected: predominantly or only memory, predominantly one domain other than memory, or multiple domains with none predominant. Once Bolivian and USC teams completed independent diagnoses, disagreements were resolved by discussion to arrive at a final consensus clinical diagnosis.
2.3. Data analysis
Dementia and MCI prevalence were estimated by population and age groups (60‐64, 65‐69, 70‐74, and ≥75). Denominators for each population and age stratum were the number assessed, minus the number who died during the prevalence period. Age‐stratified prevalence estimates are provided with exact binomial confidence limits. To compare age ≥60 years prevalence between the Tsimane and Moseten populations, directly age‐standardized prevalence estimates with confidence limits were computed, using the age distribution of the combined Tsimane plus Moseten population. To account for the different age structure of Tsimane and Moseten compared to Western populations, Tsimane and Moseten age ≥60 prevalence estimates were further directly age‐ and sex‐standardized to the US age ≥60 population structure, using US Census 2002, 41 because it was the year used to calculate the US published rate. 42
To analyze associations of prevalent cognitive status with CT measures, we constructed a prevalent case‐control sample (n = 155) comprising a subset of participants. We individually matched all prevalent cases where CT was available (4 dementia and 39 MCI cases) with up to three controls per case, matched on population, sex, and age (within 3 years). Controls were individuals who had completed the diagnostic protocol and received a consensus diagnosis of cognitively normal. The matched sample optimized statistical power without requiring CT rater assessments of all unimpaired individuals. Raters blind to case ascertainment viewed and rated the brain CT images for this sample. Population estimates of severity of brain calcification from brain CT were obtained using inverse‐weighted sampling probabilities among cases and controls. Matched cases and controls were compared on semi‐quantitative CT measures using conditional logistic regression, specifying matched case‐control sets. Associations of cognitive test scores, neurologic measures, and brain volume from CT were evaluated with logistic regression, using the entire available sample, controlling for age and sex. All association analyses were performed within Tsimane or Moseten population, respectively.
3. RESULTS
3.1. Demographic and other characteristics
Visits were completed for 451 Tsimane 60 to 93 years of age and 172 Moseten 60 to 86 years of age (total n = 623; 78.6% of the census population), of whom 534 (85.7%) also participated in CT. See Figure 1 for STROBE sample flow chart showing the sample visited (n = 623) and the sample included in prevalence calculations (n = 604). Table 1 shows the distribution of participants by age and sex. The proportion of men and women did not differ by population. Further description of the population with respect to education, biomarkers, and lifestyle can be found in Table S2,
FIGURE 1.
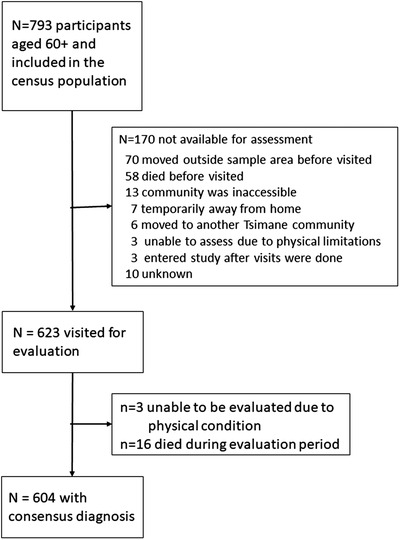
Sample flow chart. Notes: Of the 793 individuals identified from the population census as potentially eligible, 170 were not available for assessment, primarily due to moving outside of the area or to death. The absence of refusals reflects the fact that these individuals were already part of the THLHP. Of 623 visited for evaluation, 3 were unable to be assessed due to physical limitations; 16 were excluded from prevalence calculations due to death during the prevalence period. Table S1 compares those who were able to be visited for a dementia evaluation to those who were identified as eligible from the census, but who were unable to participate in a dementia evaluation. Those who did not participate were on average 2 years older, but did not differ from participants on prior waves of cognitive testing
TABLE 1.
Prevalence of dementia and mild cognitive impairment by population
| Age group (in years) | 60‐64 | 65‐69 | 70‐74 | ≥75 | Age‐standardized |
|---|---|---|---|---|---|
| TSIMANE | |||||
| N | 135 | 139 | 82 | 79 | 435 |
| % Men | 44.4% | 54.7% | 57.3% | 48.1% | 50.8% |
| Dementia | |||||
| N cases | 0 | 0 | 0 | 5 | 5 |
| Prevalence % (95% CI) | – | – | – | 6.3 (2.1, 14.2) | 1.2 (0.1, 2.0) |
| MCI | |||||
| N cases | 1 | 13 | 7 | 14 | 35 |
| Prevalence % (95% CI) | 0.7 (0.0, 4.1) | 9.4 (5.1,15.5) | 8.5 (3.5, 16.8) | 17.7 (10.0, 27.9) | 7.7 (5.2, 10.3) |
| MOSETEN | |||||
| N | 68 | 50 | 28 | 23 | 169 |
| % Men | 52.9% | 54.0% | 50.0% | 56.5% | 53.2% |
| Dementia | |||||
| N cases | 0 | 0 | 0 | 1 | 1 |
| Prevalence % (95% CI) | – | – | – | 4.4 (0.1,22.0) | 0.7 (0.0, 2.2) |
| MCI | |||||
| N cases | 5 | 4 | 4 | 3 | 16 |
| Prevalence % (95% CI) | 7.4 (2.4, 16.3) | 8.0 (2.2, 19.2) | 14.3 (4.0, 32.7) | 13.0 (2.8, 35.6) | 9.8 (4.9, 14.6) |
Note: 95% Confidence intervals (CIs) for age‐specific estimates are exact binomial. 95% CI for age‐standardized estimates are based on normal distribution. If those who died during the prevalence period are not removed, crude prevalence is 1.1% for Tsimane and 1.2% for Moseten (vs crude prevalence of 1.2% and 0.7% with adjustment for deaths during the prevalence period). One Moseten dementia case was among those who died during the prevalence period.
3.2. Prevalence
Initial interrater agreement for dementia/MCI/normal cognition was kappa = 0.66. Analyses used the final consensus clinical diagnoses. Table 1 shows crude prevalence rates by age group within each population for consensus clinical diagnoses for dementia and for MCI, and also age‐standardized rates for each population standardized to age ≥60 based on a combined Tsimane and Moseten population age distribution. Crude prevalence of dementia for age ≥60 years was 1.2% (exact 95% confidence interval [CI]: 0.4, 2.7) in Tsimane and 0.6% (95% CI: 0.0, 3.2) in Moseten (P = .73 for age‐standardized population difference). All dementia cases were mild and all cases were ≥80 of age when assessed. CT was available for three of the five Tsimane dementia cases; two of these indicated predominantly vascular causes and one followed an AD pattern. When Tsimane and Moseten prevalence was age‐standardized to the US 2002 population structure, for Tsimane, dementia prevalence was 2.7% (exact 95% CI: 0.1, 5.4); for Moseten it was 0.9% (95% CI: 0.0, 2.6).
MCI age‐standardized prevalence estimates for age ≥60 years were comparable for the two populations: 7.7% (95% CI: 5.2,10.3) in Tsimane and 9.8% (95% CI: 4.9, 14.6) in Moseten (P = .44 for population difference). For Tsimane, 5 of the 35 MCI cases had memory as the predominant cognitive domain affected; 30 cases were either predominantly affected in another domain (eg, executive) or in multiple domains. For Moseten, 9 of the 16 MCI cases predominantly involved memory.
Across populations, the number of dementia cases was the same for men as for women (three in each). MCI was significantly more prevalent among women than among men. In Tsimane, using the combined Tsimane/Moseten population for standardization, the age‐standardized prevalence of MCI was 11.1% (95% CI: 6.6, 15.6) in women and 4.7% (95% CI: 1.9, 7.5) in men (P = .02 for sex difference). MCI prevalence in Moseten was 10.7 (95% CI: 3.2, 18.3) in women and 8.9% (95% CI: 2.7, 15.1) in men (P = .71).
3.3. Brain calcification
Median interrater reliability of visual CT ratings was kappa = 0.88. Prevalence of intracranial vascular calcifications in both large and small arteries was notable, regardless of cognitive status. Weighting the case‐control sample results (which disproportionately represent older participants) to match the entire population using inverse weighting by sampling probability, we estimate that 95.2% (95% CI: 88.7, 98.6) of Tsimane ≥60 years of age have intracranial internal carotid artery (ICA) calcification, 98.2% (95% CI: 90.4, 100.0) have intracranial vertebral artery calcification, and 74.4% (95% CI: 62.2, 84.3) have vascular calcification involving the lenticulostriate arteries (LSAs), representing terminal vessels supplying the basal ganglia and posterior limb of the internal capsule. Calcifications in the ICA appeared in the majority of cases as a continuous rim in the media (rather than the intima) of the arterial wall, in a pattern known as medial arterial calcification. 34 Complete population prevalence figures for all visual rating scales for Tsimane and Moseten are shown in Table S3.
In the subsample of Tsimane comprising cases with cognitive impairment (dementia or MCI) and matched controls, shown in Table 2, there is a general pattern of cases having higher scores than controls on indices of brain calcification, with these differences most notable for calcification of the LSAs.
TABLE 2.
Semi‐quantitative ratings of CT for Tsimane cases and controls
| Rating scale | Interrater reliability (kappa) | Percentage in cases (n = 35) | Percentage in controls (n = 89) | Association with cognitive impairment OR (95% CI) | |
|---|---|---|---|---|---|
| Global cortical atrophy (simplified Pasquier) | 0 a | .83 | 25.7 | 46.1 | 3.70 (0.88, 15.6) |
| 1 a | 57.1 | 48.3 | |||
| 2 | 17.1 | 5.6 | |||
| 3 | 0.0 | 0.0 | |||
| Medial temporal atrophy (Scheltens) | 0 a | .85 | 37.1 | 59.6 | 8.89 (1.85, 42.9) |
| 1 a | 40.0 | 36.0 | |||
| 2 | 20.0 | 3.4 | |||
| 3 | 2.9 | 1.1 | |||
| 4 | 0.0 | 0.0 | |||
| Internal carotid artery (ICA) calcification extent (Babiarz/ Kockelkoren) | Absent a | .87 | 0.0 | 1.1 | 4.06 (0.77, infinity) |
| Dots a | 0.0 | 6.7 | |||
| <90o | 2.9 | 4.5 | |||
| 90‐270o | 42.9 | 38.3 | |||
| 270‐360o | 54.3 | 49.4 | |||
| ICA calcification morphology | Indistinguishable b | .77 | 0.0 | 1.1 | 6.10 (1.23, 30.4) |
| Irregular/patchy a | 14.3 | 32.6 | |||
| Continuous | 85.7 | 66.3 | |||
| Basal ganglia (BG) calcification | Absent a | .97 | 0.0 | 10.1 | 5.27 (1.05, infinity) |
| Vascular only | 74.3 | 70.8 | |||
| Parenchymal only | 0.0 | 0.0 | |||
| Both | 25.7 | 19.1 | |||
| Lenticulostriate arteries (LSA) calcification | Absent a | .93 | 0.0 | 10.1 | 4.77 (1.04, 22.0) |
| Dots a | 5.7 | 12.4 | |||
| 1‐2 LSA | 57.1 | 51.7 | |||
| Multiple LSA | 37.1 | 25.8 | |||
| LSA calcification diameter | Mean mm | r = .90 | Mean = 1.4 SD = 0.3 | Mean = 1.2 SD = 0.5 | 2.36 (0.94, 5.92) |
| LSA calcification density | Maximum Hounsfield units | r = .98 | Mean = 124.7 SD = 34.8 | Mean = 103.3 SD = 49.2 | 1.11 (1.01, 1.23) |
| Non‐ICA vascular calcification | None b | .92 | 0.0 | 1.1 | 4.58 (0.57, 36.6) |
| LSA only b | 0.0 | 0.0 | |||
| Vertebral artery only a | 2.9 | 10.1 | |||
| Both | 97.1 | 88.8 | |||
| Temporal artery calcification | Absent a | .85 | 77.1 | 79.8 | 1.18 (0.42, 3.36) |
| Present | 22.9 | 20.2 | |||
| Deep white matter disease (WMD) (Fazekas) | 0 a | .91 | 82.9 | 89.9 | 1.62 (0.57, 4.65) |
| 1 | 2.9 | 7.9 | |||
| 2 | 11.4 | 1.1 | |||
| 3 | 2.9 | 1.1 | |||
| Periventricular WMD | 0 a | .86 | 52.2 | 74.2 | 2.63 (0.98, 7.05) |
| 1 | 26.1 | 15.7 | |||
| 2 | 21.7 | 9.0 | |||
| 3 | 0.00 | 1.1 | |||
| Infarcts | Absent a | .76 | 62.9 | 75.3 | 1.60 (0.70, 3.66) |
| Lacunar | 28.6 | 22.5 | |||
| Cortical | 8.6 | 2.2 |
Notes: Semi‐quantitative ratings summarized as percentages in each scoring category. Quantitative scores shown as means and standard deviations. Reliability was evaluated for semi‐quantitative variables by kappa (or weighted kappa where there were multiple ordinal categories) or by Pearson correlation where there was a quantitative scale. Association with cognitive impairment was evaluated by conditional logistic regression, using the matched case‐control sample (where age and sex were matching variables). Case (combining dementia and mild cognitive impairment) versus control status (cognitively non‐impaired) is the dichotomous outcome and each rating scale is tested as the predictor. Rating scales were binarized based on neuroanatomical meaningfulness, either whether there was pathology present, or the location of the pathology.
Indicates the codes included in the reference group.
Indicates codes that were excluded from the analysis. Results are reported as an odds ratio (OR) and 95% confidence interval (CI), with exact OR for internal carotid artery (ICA) calcification extent and basal ganglia (BG) calcification. OR >1.00 indicate that cases had greater atrophy, intracranial calcification, or white matter density than controls.
3.4. Characterizing observed cases
Table 3 shows APOE genotype, brain volumes, cognitive scores, and neurological symptoms for all impaired (dementia and MCI cases) compared to all non‐impaired individuals in Tsimane and Moseten. The number of APOE ε4 alleles did not significantly distinguish impaired from non‐impaired Tsimane or Moseten. However, carrying two APOE ε4 alleles was associated with significantly greater odds of cognitive impairment. Total brain volume and white matter volume, but not gray matter volume, were significantly lower for impaired than non‐impaired Tsimane (by 3.9% for total brain volume and 9.8% for white matter volume), but did not differ for Moseten. Cognitive test scores were significantly lower for impaired than non‐impaired Tsimane. Results were similar for Moseten. Among neurological symptoms, bradykinesia and rigidity were common in these populations, more so for Tsimane than Moseten. Both rigidity and gait abnormalities distinguished impaired from non‐impaired Tsimane. Tables S4 and S5 show that poorer cognition was associated with severity of intracranial vascular calcification, greater severity of atrophy, and reduced brain volumes, especially white matter volume.
TABLE 3.
Associations of cognitive impairment with brain volume, APOE, cognitive test scores, and neurological symptoms
| TSIMANE | MOSETEN | |||||
|---|---|---|---|---|---|---|
| Predictor | Impaired | Non‐impaired | Impaired | Non‐impaired | ||
| Mean (SD) or % | (n = 41) | (n = 407) | OR (95% CI) | (n = 18) | (n = 154) | OR (95% CI) |
| Volumes, % of intracranial volume | ||||||
| Total brain volume | 80.7 (4.9) | 84.0 (3.9) | 1.15 (1.04, 1.27) | 81.0 (3.5) | 82.7 (4.4) | 1.06 (0.94, 1.20) |
| Gray matter volume | 46.9 (5.2) | 46.6 (6.7) | 0.96 (0.90, 1.02) | 45.9 (7.5) | 46.0 (6.8) | 1.00 (0.93, 1.08) |
| White matter volume | 33.7 (7.7) | 37.4 (7.3) | 1.08 (1.02, 1.14) | 35.2 (8.4) | 36.6 (6.9) | 1.02 (0.94, 1.10) |
| APOE | ||||||
| No ε4 | 75.8% (57.7, 88.9) | 80.4% (75.7, 84.5) | Overall effect 1.65 (0.79, 3.44) | 60.0% (26.2, 87.8) | 69.9% (59.5, 79.0) | Overall effect 2.24 (0.78, 6.40) |
| One ε4 | 15.2% (5.1, 31.9) | 18.8% (14.7, 23.3) | 0 versus 1 allele 0.86 (0.30, 2.51) | 20.0% (2.5, 55.6) | 28.0% (19.1, 38.2) | 0 versus 1 allele 0.81 (0.14, 4.67) |
| Two ε4 | 9.1% (1.9, 24.3) | 0.9% (0.2, 2.6) | 0 versus 2 alleles 10.7 (1.47, 78.6) | 20.0% (2.5, 55.6) | 2.2% (0.3, 7.6) | 0 versus 2 alleles 11.5 (1.28, 102.8) |
| Cognitive test | ||||||
| Visual scan | 15.1 (6.8) | 21.7 (8.8) | 1.09 (1.03,1.16) | 16.5 (9.1) | 21.8 (12.2) | 1.04 (0.99,1.10) |
| Digit span forward | 1.5 (1.0) | 2.7 (1.0) | 1.99 (1.45,2.72) | 2.9 (1.2) | 3.6 (0.9) | 1.96 (1.11, 3.44) |
| Immediate recall | 2.9 (0.8) | 4.2 (0.9) | 6.81 (3.76,12.4) | 3.2 (0.6) | 3.9 (0.8) | 3.26 (1.50, 7.10) |
| Delayed recall | 1.4 (1.5) | 3.8 (1.9) | 1.82 (1.48,2.24) | 0.9 (1.3) | 3.0 (1.8) | 2.00 (1.41, 2.83) |
| Verbal fluency | 8.2 (2.2) | 11.6 (2.4) | 1.91 (1.55, 2.35) | 8.6 (2.5) | 10.8 (2.6) | 1.44 (1.14, 1.83) |
| Spatial span | 0.9 (1.1) | 2.6 (1.2) | 2.77 (1.98,3.86) | 2.8 (0.9) | 3.3 (1.0) | 1.57 (0.96, 2.57) |
| Stick design test | 5.1 (3.0) | 8.7 (2.6) | 1.47 (1.27, 1.70) | 8.4 (3.0) | 10.4 (1.9) | 1.63 (1.14, 2.32) |
| Neurological evaluation | ||||||
| Tremor at rest | 19.4% | 8.2% | 1.99 (0.67,5.86) | 20.0% | 2.5% | 9.16 (1.47, 57.1) |
| Bradykinesia | 71.0% | 47.0% | 1.59 (0.65,3.85) | 53.3% | 24.8% | 3.58 (1.07, 12.0) |
| Facial masking | 35.5% | 9.5% | 2.25 (0.87, 5.83) | 0.0% | 1.6% | 3.32 (0.00, 29.2) |
| Rigidity | 64.5% | 35.1% | 2.35 (1.02, 5.38) | 50.0% | 21.5% | 3.39 (1.06, 10.8) |
| Abnormal gait | 45.4% | 9.0% | 4.85 (1.80, 13.1) | 0.0% | 8.6% | 0.42 (0.00, 2.38) |
Note: Cognitively impaired includes both those diagnosed with dementia and MCI. Sample sizes: Brain volume N = 357 Tsimane; 150 Moseten. APOE N = 369 Tsimane; 103 Moseten. Visual scan N = 392 Tsimane; 152 Moseten. Digit span N = 420 Tsimane; 169 Moseten. Immediate and delayed recall and verbal fluency N = 441 Tsimane; 170 Moseten. Spatial span N = 432 Tsimane; 166 Moseten. Stick design test N = 423 Tsimane; 81 Moseten. Tremor at rest N = 349 Tsimane; 136 Moseten. Bradykinesia and facial masking N = 346 Tsimane; 136 Moseten. Rigidity N = 347 Tsimane; 135 Moseten. Abnormal gait N = 322 Tsimane; 130 Moseten. 95% CIs are shown for APOE allele frequencies based on exact binomial method. Associations with cognitive impairment were evaluated by logistic regression, adjusting for sex and age at visit. Odds ratios (ORs) are per unit of the continuous variables. Brain volume is shown as percentage of intracranial volume. APOE = apolipoprotein E. Number of APOE ε4 alleles is shown as 0, 1, or 2. Logistic regression tests the overall effect of number of ε4 alleles, and then compares one or two ε4 alleles to a reference group of no ε4 alleles. For cognitive tests, the unit of measurement is points scored on the respective test. Digits forward was administered in Tsimane for Tsimane, in Spanish for Moseten. Immediate recall is the average of three learning trials. Neurological evaluation is shown as percent manifesting each symptom. Logistic regression tests having the symptom relative to absence of the neurological symptom. OR >1.00 indicates that cognitively impaired individuals scored worse (lower brain volume, presence of ε4, lower cognitive scores, more likely to show neurological symptoms).
Post hoc analyses explored the relationship between intracranial and CAC. Although in general, CAC was low and intracranial calcifications were extensive, CAC scores tended to be higher with greater extent of ICA calcification and LSA calcification (linear regressions predicting intracranial calcification by CAC score controlling for age, sex and population, P = .08 for ICA, P = .015 for LSA).
4. DISCUSSION
Our results show the expected low prevalence of dementia in Tsimane and Moseten Amerindians. There were no significant overall differences between these two related populations in rates of dementia and cognitive impairment.
We contextualize the results by comparing to published rates of dementia for the United States 42 and Europe 2 (Figure 2A, yellow bars). We also compared Tsimane and Moseten to published prevalence rates in similar groups, including indigenous and illiterate rural populations, selecting studies that applied clinical diagnostic criteria and were not based only on medical record review or mental status screening (Figure 2A, green bars). Rates for Tsimane and Moseten are closest to the lowest rates included in a systematic review of indigenous populations. 4 Highest age‐standardized prevalence rates were among Australian Aboriginals 43 and Chamorros on Guam. 44 Rates for Tsimane and Moseten were most analogous to earlier reports from a rural Indian agrarian population 45 and a Cree native population in Manitoba. 46 AD accounted for only 12.5% of Cree dementia cases. Similarly, within Tsimane, the proportion of dementia cases that were clearly AD was low.
FIGURE 2.
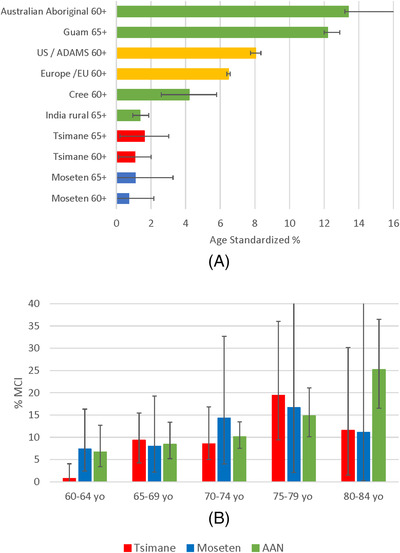
(A) age‐standardized dementia prevalence and (B) age‐specific mild cognitive impairment (MCI) rates among Tsimane and Moseten compared to other populations. Notes: (A) Australian Aboriginal 43 ; Guam 44 ; US /ADAMS 42 ; Europe 2 ; Cree 46 ; India rural 45 (B) AAN = American Academy of Neurology meta‐analysis 47
We compared Tsimane and Moseten MCI prevalence within age groups to rates of MCI in a published meta‐analysis from the American Academy of Neurology (AAN), 47 largely including high‐income countries (Figure 2B). The prevalence of MCI for Tsimane and Moseten is generally within the CIs of MCI prevalence in persons 60 to 80 years of age from high‐income countries. 47 Rates in the meta‐analysis continued to increase after age 80, while rates among Tsimane and Moseten did not.
Low prevalence of dementia in Tsimane and Moseten occurs in populations with a physically active subsistence lifestyle and low rates of cardiovascular disease, diabetes, and obesity, which may protect brain health despite a high load of parasitic and bacterial infections. 15 , 18 , 19 , 20 , 21 In contrast, indigenous populations with high rates of dementia do not practice a subsistence lifestyle and are prone to these other conditions. 4
Furthermore, we were struck by an unusual phenotype in dementia and MCI cases, associated with prominent medial arterial calcifications affecting the intracranial internal carotid, vertebral, and lenticulostriate arteries. It is notable that greater severity of vascular intracranial calcification was associated with smaller brain volumes and greater risk of cognitive impairment. Consistent with the pattern of calcifications that we observed, dementia and MCI participants frequently displayed parkinsonian symptoms on neurological examination and cognitive deficits in attention, visuospatial, and executive domains. 48
Although calcifications were more common among the cognitively impaired, we observed high rates of intracranial vascular calcification in both cases and controls. Tsimane rates of calcification of 95.7% in internal carotid and 98.2% in vertebral arteries can be compared to 79.0% intracranial carotid artery calcification and 16.9% vertebrobasilar artery calcification in a population‐based European sample ≥60 years of age. 49 Although intracranial vascular calcification was correlated with CAC, the high prevalence of intracranial vascular calcification contrasts with the very low prevalence of CAC found in these populations. 15 Currently, the pathogenic mechanism leading to the observed medial arterial calcification is not known. Future research will investigate not only vascular factors but also infectious and inflammatory disorders—highly prevalent in these populations—as well as metabolic, toxic, dietary, and familial risk factors. 50
Limitations of this work include the lack of a separate validation for protocols, low power and large confidence intervals resulting from low numbers of cases, and not yet having biomarkers for amyloid beta and tau. We adapted cognitive assessments to minimize biases related to illiteracy, innumeracy, and how the culture understands time. To optimize diagnostic accuracy, we made diagnostic determinations by two independent teams before arriving at a consensus. Finally, because cognitive test scores were used in establishing cognitive decline, they are thus not independent of the classification of impaired versus non‐impaired.
There are three classes of explanation for low disease prevalence: failure to locate cases; selective mortality (both selective survival of those less at risk for dementia and high mortality among cases); and a favorable risk factor profile. Because of the long‐term research presence of the THLHP staff and doctors in the Tsimane communities, there are low rates of failure to locate participants. Although the diagnostic protocols required considerable adaptation, the administration of a complete clinical interview and complete informant interview to all participants and family members during a single visit, plus a separate cognitive test battery, makes it unlikely that cognitive impairment was missed.
An incidence phase currently in progress will enable us to identify MCI cases that have progressed sufficiently to meet diagnostic criteria for dementia, to tease apart the contribution of mortality to low prevalence, and to analyze specific risk and protective factors for incident cases. Beyond the exogenous and endogenous exposome, as part of the risk/protection factor profile, we will consider the potential role of protection from AD associated with AmerIndian ancestry. 51 We will also pursue correlates of intracranial calcification in order to further characterize the unusual phenotype of vascular cognitive impairment with medial arterial calcification that we observed.
CONFLICT OF INTERESTS
Margaret Gatz, Wendy J. Mack, Helena C. Chui, E. Meng Law, Giuseppe Barisano, M. Linda Sutherland, James D. Sutherland, Daniel Eid Rodriguez, Raul Quispe Gutierrez, Juan Copajira Adrian, Amy R. Borenstein, Ellen E. Walters, Andrei Irimia, Christopher J. Rowan, Edmond Seabright, Angela R. Garcia, Paul L. Hooper, Thomas S. Kraft, Caleb E. Finch, Gregory S. Thomas, Jonathan Stieglitz, Benjamin C. Trumble, Michael D. Gurven, and Hillard Kaplan received support from the National Institutes of Health (NIH) Grant No. RF1 AG054442, through the main grant (to Chapman University), a subcontract from the main grant to their institution, or a contract to them personally from one of the participating institutions. Outside of the submitted work, E. Meng Law, M. Linda Sutherland, James D. Sutherland, Raul Quispe Gutierrez, Juan Copajira Adrian, Jesus Bani Cuata, L. Samuel Wann, Adel H. Allam, David E. Michalik, Daniel K. Cummings, Edmond Seabright, Paul L. Hooper, Thomas S. Kraft, Benjamin C. Trumble, Michael D. Gurven, and Hillard Kaplan report no disclosures. Margaret Gatz receives research grant support from NIH. Wendy J. Mack receives research grant support from NIH and payments from four Data Safety and Monitoring Boards for clinical trials. Helena C. Chui receives research grant support from the NIH and consulting fees as an external advisory committee member for Oregon Health Sciences University, and is a Board Member of Alzheimer Los Angeles (no payment). Giuseppe Barisano receives research grant support from the NIH and received a royalty from Elsevier. Daniel Eid Rodriguez received funding from Swedish International Development Cooperation Agency for travel related to research training. Amy R. Borenstein received royalties from Academic Press. Ellen E. Walters is an independent contractor on NIH research grants. Andrei Irimia receives research grant support from the NIH. Christopher J. Rowan has a relationship with Zoll for LifeVest and a patent for software for flying. Randall C. Thompson is President: American Society of Nuclear Cardiology (payment to institution) and Chair: Coding Task Force; American College of Cardiology (no payment). Michael I. Miyamoto has a relationship with Hegeler and Anderson, LLP, and is a board member of Vallum Corp (no payment). Angela R. Garcia received an NIH Butler‐Williams Scholarship (no payment). Caleb E. Finch receives research grant support from the NIH and US DoD; consults for Cure Alz Fund; holds stock in Acumen Pharmaceuticals; and receives royalties from Elsevier and University of Chicago Press. Gregory S. Thomas received Astellas Pharma speaker's bureau fees and royalties from Oxford University Press. Jonathan Stieglitz holds a research grant from the National Science Foundation (NSF) (no payment); receives consulting fees as a member of an advisory board for a project funded by Templeton; and is a board member for One Pencil Project (no payment).
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
This work was financially supported by the National Institutes of Health Grant No. RF1 AG054442. Jonathan Stieglitz acknowledges the Institute for Advanced Study in Toulouse (IAST) funding from the French National Research Agency (ANR) under grant ANR‐17‐EURE‐0010 (Investissements d'Avenir program). The authors thank the Tsimane and Moseten participants, the Gran Consejo Tsimane (GCT), the Consejo Regional Tsimane Moseten (CRTM) and the Organización del Pueblo Indígena Mosetén (OPIM), the Tsimane Health and Life History Project staff in San Borja, Bolivia, and the Hospital Presidente German Busch of Trinidad, Bolivia.
Gatz M, Mack WJ, Chui HC, et al. Prevalence of dementia and mild cognitive impairment in indigenous Bolivian forager‐horticulturalists. Alzheimer's Dement. 2023;19:44–55. 10.1002/alz.12626
REFERENCES
- 1. Alzheimer's Association. 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327‐406. 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 2. Alzheimer Europe . Dementia in Europe Yearbook 2019: estimating the prevalence of dementia in Europe. 2019. Accessed March 9, 2021 https://www.alzheimer‐europe.org/Publications/Dementia‐in‐Europe‐Yearbooks
- 3. Gurven M, Stieglitz J, Trumble B, et al. The Tsimane Health and Life History Project: integrating anthropology and biomedicine. Evol Anthropol. 2017;26(2):54‐73. 10.1002/evan.21515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warren LA, Shi Q, Young K, Borenstein A, Martiniuk A. Prevalence and incidence of dementia among indigenous populations: a systematic review. Int Psychogeriatr. 2015;27(12):1959‐1970. 10.1017/S1041610215000861 [DOI] [PubMed] [Google Scholar]
- 5. Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A systematic review of meta‐analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. 2019;70(s1):S165‐S186. 10.3233/JAD-190181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brayne C, Ince PG, Keage HA, et al. Education, the brain and dementia: Neuroprotection or compensation? Brain. 2010;133(Pt 8):2210‐2216. 10.1093/brain/awq185 [DOI] [PubMed] [Google Scholar]
- 8. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eriksson UK, Bennet AM, Gatz M, Dickman PW, Pedersen NL. Nonstroke cardiovascular disease and risk of Alzheimer disease and dementia. Alzheimer Dis Assoc Disord. 2010;24(3):213‐219. 10.1097/WAD.0b013e3181d1b99b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stephen R, Hongisto K, Solomon A, Lönnroos E. Physical activity and Alzheimer's disease: a systematic review. J Gerontol A Biol Sci Med Sci. 2017 Jun 1;72(6):733‐739. 10.1093/gerona/glw251 [DOI] [PubMed] [Google Scholar]
- 11. Fujiyoshi A, Jacobs DR Jr, Fitzpatrick AL, et al. Coronary artery calcium and risk of dementia in MESA (Multi‐Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2017;10(5):e005349. 10.1161/CIRCIMAGING.116.005349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Brink AC, Brouwer‐Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean‐DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer's disease‐A review. Adv Nutr. 2019;10(6):1040‐1065. 10.1093/advances/nmz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, Lukiw WJ. Pathogenic microbes, the microbiome, and Alzheimer's disease (AD). Front Aging Neurosci. 2014;16;6:127. 10.3389/fnagi.2014.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimers Dement. 2018;14(12):1602‐1614. 10.1016/j.jalz.2018.06.3040 [DOI] [PubMed] [Google Scholar]
- 15. Kaplan H, Thompson RC, Trumble BC, et al. Coronary atherosclerosis in indigenous South American Tsimane: a cross‐sectional cohort study. Lancet. 2017;389(10080):1730‐1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rowan CJ, Eskander MA, Seabright E, et al. Very low prevalence and incidence of atrial fibrillation among Bolivian forager‐farmers. Ann Glob Health. 2021;87(1):18. 10.5334/aogh.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age? Longitudinal evidence among forager‐horticulturalists. Hypertension. 2012;60(1):25‐33. 10.1161/HYPERTENSIONAHA.111.189100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurven MD, Trumble BC, Stieglitz J, et al. Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths? Evol Med Public Health. 2016, (1):338‐357. 10.1093/emph/eow028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gurven M, Jaeggi AV, Kaplan H, Cummings D. Physical activity and modernization among Bolivian Amerindians. PLoS One. 2013;8(1):e55679. 10.1371/journal.pone.0055679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kraft TS, Stieglitz J, Trumble BC, Martin M, Kaplan H, Gurven M. Nutrition transition in 2 lowland Bolivian subsistence populations. Am J Clin Nutr. 2018;108(6):1183‐1195. 10.1093/ajcn/nqy250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blackwell AD, Trumble BC, Maldonado Suarez I, et al. Immune function in Amazonian horticulturalists, Ann Hum Biol 2016; 43(4):382‐396. 10.1080/03014460.2016.1189963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saenz JL, Adar SD, Zhang YS, et al. Household use of polluting cooking fuels and late‐life cognitive function: a harmonized analysis of India, Mexico, and China. Environ Int. 2021;156:106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urrutia‐Pereira M, Rizzo LV, Chong‐Neto HJ, Solé D. Impact of exposure to smoke from biomass burning in the Amazon rain forest on human health. J Bras Pneumol. 2021;47(5):e20210219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LoGiudice D, Smith K, Thomas J, et al. Kimberley Indigenous Cognitive Assessment tool (KICA): development of a cognitive assessment tool for older indigenous Australians. Int Psychogeriatr. 2006;18(2):269‐280. 10.1017/S1041610205002681 [DOI] [PubMed] [Google Scholar]
- 25. Ganguli, M. , Ratcliff, G. , Chandra, V. , et al. A Hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatry. 1995;10:367‐377. [Google Scholar]
- 26. Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152(10):1485‐1492. 10.1176/ajp.152.10.1485 [DOI] [PubMed] [Google Scholar]
- 27. Teng EL, Chui HC. The Modified Mini‐Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314‐318 [PubMed] [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 29. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993. ;43(11):2412‐4. [DOI] [PubMed] [Google Scholar]
- 30. Smith K, Flicker L, Atkinson D, et al. . The KICA Carer: Informant information to enhance the Kimberley Indigenous Cognitive Assessment. Int Psychogeriatr. 2016. ;28(1):101‐107. 10.1017/S1041610215001283 [DOI] [PubMed] [Google Scholar]
- 31. Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797‐811. 10.1192/bjp.114.512.797 [DOI] [PubMed] [Google Scholar]
- 32. Mejía‐Arango S, Wong R, Michaels‐Obregón A. Normative and standardized data for cognitive measures in the Mexican Health and Aging Study. Salud Publica Mex. 2015;57 (1[0 1]):S90‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baiyewu O, Unverzagt FW, Lane KA, et al. The Stick Design test: a new measure of visuoconstructional ability. J Int Neuropsychol Soc. 2005;11(5):598‐605. 10.1017/S135561770505071X [DOI] [PubMed] [Google Scholar]
- 34. Gurven M, Fuerstenberg E, Trumble B, et al. Cognitive performance across the life course of Bolivian forager‐farmers with limited schooling. Dev Psychol. 2017;53(1):160‐176. 10.1037/dev0000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wahlund LO, Westman E, van Westen D, et al. From the Imaging Cognitive Impairment Network (ICINET). Imaging biomarkers of dementia: recommended visual rating scales with teaching cases. Insights Imaging. 2017;8(1):79‐90. 10.1007/s13244-016-0521-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kockelkoren R, Vos A, Van Hecke W, et al. Computed tomographic distinction of intimal and medial calcification in the intracranial internal carotid artery. PLoS One. 2017;12(1):e0168360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Brouwer EJM, Kockelkoren R, De Vis JB, et al. Dutch acute stroke study investigators (DUST). Prevalence and vascular risk factors of basal ganglia calcifications in patients at risk for cerebrovascular disease. J Neuroradiol. 2020;47(5):337‐342. 10.1016/j.neurad.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 38. Irimia A, Chaudhari NN, Robles DJ, et al. The indigenous South American Tsimane exhibit relatively modest decrease in brain volume with age despite high systemic inflammation. J Gerontol A Biol Sci Med Sci. 2021;glab138. 10.1093/gerona/glab138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed. American Psychiatric Association, 2013. [Google Scholar]
- 40. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. U.S. Census Bureau . Statistical abstract of the United States: 2003 (123th Ed). 2003. Accessed April 14, 2021. https://www.census.gov/library/publications/2003/compendia/statab/123ed.html
- 42. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1‐2):125‐32. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith K, Flicker L, Lautenschlager NT, et al. High prevalence of dementia and cognitive impairment in Indigenous Australians. Neurology. 2008;71(19):1470‐1473. 10.1212/01.wnl.0000320508.11013.4f [DOI] [PubMed] [Google Scholar]
- 44. Galasko D, Salmon D, Gamst A, et al. . Prevalence of dementia in Chamorros on Guam: relationship to age, gender, education, and APOE. Neurology. 2007;68(21):1772‐1781. 10.1212/01.wnl.0000262028.16738.64 [DOI] [PubMed] [Google Scholar]
- 45. Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer's disease and other dementias in rural India: the Indo‐US study. Neurology. 1998;51(4):1000‐1008. 10.1212/wnl.51.4.1000 [DOI] [PubMed] [Google Scholar]
- 46. Hendrie HC, Hall KS, Pillay N,et al. . Alzheimer's disease is rare in Cree. Int Psychogeriatr. 1993. Spring;5(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 47. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126‐135. 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Oliveira JRM, de Oliveira MF. Basal ganglia calcification as a putative cause for cognitive decline. Dement Neuropsychol. 2013;7(2):151‐154. 10.1590/S1980-57642013DN70200003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vinke EJ, Yilmaz P, van der Toorn JE, et al. Intracranial arteriosclerosis is related to cerebral small vessel disease: a prospective cohort study. Neurobiol Aging. 2021;105:16‐24. 10.1016/j.neurobiolaging.2021.04.005 [DOI] [PubMed] [Google Scholar]
- 50. Saade C, Najem E, Asmar K, Salman R, El Achkar B, Naffaa L. Intracranial calcifications on CT: an updated review. J Radiol Case Rep. 2019;13(8):1‐18. 10.3941/jrcr.v13i8.3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Horimoto ARVR, Xue D, Thornton TA, Blue EE. Admixture mapping reveals the association between Native American ancestry at 3q13.11 and reduced risk of Alzheimer's disease in Caribbean Hispanics. Alzheimers Res Ther. 2021;13(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION


