Abstract
Osteoporosis is a systemic degenerative bone disease characterized by low bone mass and damage to bone microarchitecture, which increases bone fragility and susceptibility to fracture. The risk of osteoporosis increases with age; with the aging of the global population, osteoporosis is becoming more prevalent, adding to the societal healthcare burden. Histone modifications such as methylation, acetylation, ubiquitination, and ADP-ribosylation are closely related to the occurrence and development of osteoporosis. This article reviews recent studies on the role of histone modifications in osteoporosis. The existing evidence indicates that therapeutic targeting of these modifications to promote osteogenic differentiation and bone formation may be an effective treatment for this disease.
Keywords: osteoporosis, histone modification, osteoblast, osteoclast, differentiation
Introduction
Osteoporosis is a common skeletal disease characterized by a decrease in bone mass, changes in bone microarchitecture, and increased bone fragility and risk of fracture. Pain, fractures, and other complications of osteoporosis are associated with high rates of death and disability. Bone homeostasis (1), which is maintained under physiologic conditions by a balance between bone formation and resorption during bone remodeling, is critical for ensuring the long-term stability of bone morphology and strength (2). Although the pathogenesis of osteoporosis is not fully understood, an imbalance in bone homeostasis during bone reconstruction whereby bone resorption exceeds bone formation is a major cause (1). The function of osteoblasts and osteoclasts is regulated and influenced by many factors (3–5), and epigenetic studies have provided evidence for the role of histone modifications in the development of osteoporosis (6–8).
Histones H1, H2A, H2B, H3, and H4 are small proteins enriched in positively charged basic amino acids (arginine [R] and lysine [K]) that interact with the negatively charged phosphate groups in DNA and are enveloped by DNA to form nucleosomes, the basic structural unit of chromatin. Histone N-terminal R and K undergo covalent post-transcriptional modifications such as methylation, acetylation, ubiquitination, and ADP-ribosylation that affect histone binding to DNA and alter the structure and state (open vs. closed) of chromatin (9), and also affect the binding of transcription factors at gene promoters to influence gene regulation (10, 11). Histone modifications occur at every stage of development, growth, and aging and are a key aspect of epigenetic regulation that has been linked to the development and progression of multiple diseases (12).
There is increasing evidence that dysregulation of histone modification (methylation, acetylation, ubiquitination, and ADP-ribosylation) and impaired function of related enzymes contribute to the development of osteoporosis. However, molecular-level details of the relationship between these modifications and disease pathogenesis are lacking, and the full clinical significance of histone modifications in osteoporosis remains to be determined (13, 14). Nonetheless, histone modifications may be important for the diagnosis, treatment, and prognosis of osteoporosis and are potential therapeutic targets (15).
In this review, we summarize the current state of knowledge on histone modifications in osteoporosis. Many studies have demonstrated that regulators of histone modifications and their targets function in a complex regulatory network in cells. We discuss the evidence for targeting the regulation of histone modifications as a treatment for osteoporosis, as well as the potential utility of these modifications as disease markers.
Histone methylation
Histone methylation usually occurs at R and K residues at the N terminus of histones. K residues can be mono-, di- or trimethylated and R residues can be mono- or dimethylated. Histone methylation positively and negatively regulates gene expression: H3K4me1, H3K4me3, H3K36me3, and H3K79me2 are associated with the activation of gene transcription whereas H3K27me3 and H3K9me3 are associated with transcriptional repression (16). Histone methylation is regulated by histone methyltransferases (HMTs) and histone demethylases (HMDs) (17). Thus, the expression of genes related to bone homeostasis and osteoporosis can be regulated by altering the level of histone methylation ( Table 1 ).
Table 1.
Histone methyltransferases, histone demethylases, target histone sites, and their roles in the occurrence and development of osteoporosis.
| HMTs and HMDs | Target histone sites | Target genes | Function |
|---|---|---|---|
| PRMT1/PRMT4 (CARM1) | H4R3me2a, H3R17me2a | CYP24A1 | Activates CYP24A1 gene in osteoblasts (18). |
| PRMT5 | H4R3me2s (19), H3R8me2s (20) | ISG ( 19), CXCL10 and RSAD2 (20) | Regulates osteogenic differentiation of BMSCs (19). Inhibits PRMT5 from suppressing osteoclast differentiation (20). |
| PADI4 | Runx2 | Promotes osteoblast mineralization (21). | |
| KMT1A (Suv39h1) | H3K9me2/3 | Runx2 | Delays osteoblast differentiation (22). |
| KMT1C (G9a, EHMT2) | H3K9me2 (23), H3K27me1 (24) | Runx2 (23), MMP-9 (24) | Regulates proliferation and differentiation of cranial bone cells (23) Induces expression of osteoclastogenesis-related genes and promotes osteoclast differentiation (24). |
| KMT1D (EHMT1) | H3K9me2 | Runx2 | Suppresses osteogenic differentiation of mesenchymal stem cells (25). |
| KMT1E (ESET,SETDB1) | H3K9me3 | Regulates osteoblast differentiation of MSCs (26). | |
| KMT6 (EZH2) | H3K27me3 | Wnt4, Foxc1 | Enhances both osteogenesis and osteoclastogenesis (27). |
| Mll-COMPASS complexes | H3K4me3 | Runx2, p57 | Promotes Runx2, p57 gene transcription (28). |
| KMT2D (MLL4) | H3K4me1 | Runx2 | Promotes osteoblast differentiation (29). |
| KMT4 (DOT1L) | H3K9me2 | CD9, MMP-9 | Inhibits osteoclastogenesis and protects against osteoporosis (30). |
| KDM1 (LSD1) | H3K4me1 (31), H3K4me2 (29) | Runx2 (31), Wnt7b, BMP2 (29) | Inhibits osteoblast differentiation of C2C12 cells (31). Inhibits osteogenic differentiation of BMSCs (29). |
| KDM4A (JMJD2A) | H3K9me3 | Sfrp4, C/ebpα | Promotes adipogenic differentiation and inhibits osteogenic differentiation (32). |
| KDM4B (JMJD2B) | H3K9me3 | Runx2, Ccnd1 | Promotes osteogenic differentiation of BMSCs and maintains bone–fat balance (33, 34). |
| KDM5A (JARID1A, RBP2) | H3K4me3 | Runx2 | Inhibits BMP2-induced osteogenesis of MSCs (35). Inhibits osteogenic differentiation of human adipose-derived stromal cells (36). |
| KDM5B (JARID1B, PLU-1) | H3K4me3 | Runx2 | Enhances osteoblast differentiation (37). |
| KDM6A (UTX) | H3K27me3 | Inhibits adipogenic differentiation and promotes osteogenic differentiation of BMSCs (38). | |
| KDM6B (JMJD3) | H3K27me3 | Runx2, Osx (39), NFATc1 (40) | Regulates osteoblast differentiation (39).Promotes osteoclast differentiation (40). |
HDM, histone demethylase; HMT, histone methyltransferase.
PRMT, Protein arginine methyltransferase; CARM1, Coactivator associated arginine methyltransferase 1; CYP24A1, Cytochrome P450 family 24 subfamily A member 1; ISG, Interferon-stimulated gene; CXCL10, C-X-C motif chemokine ligand 10; RSAD2, Radical S-adenosyl methionine domain containing 2; PADI4, Peptidyl arginine deiminase 4; Runx2, Runt-related transcription factor 2; Suv39h1, Suppressor of variegation 3-9 homolog 1; KMT, Calmodulin-Lysine N-methyltransferase; EHMT, Euchromatic histone lysine methyltransferase; MMP, Matrix metallopeptidase; ESET,SETDB1, SET domain bifurcated histone lysine methyltransferase; EZH2, Enhancer of zeste 2 polycomb Repressive Complex 2 Subunit; Foxc1, Forkhead box C1; DOT1L, DOT1 Like Histone Lysine Methyltransferase; KDM, LSD, Lysine demethylase; BMP2, Bone morphogenetic protein 2; JMJD, JmjC domain-containing histone demethylation protein; Sfrp4, Secreted frizzled related protein 4; C/ebpα, CCAAT enhancer binding protein alpha; Ccnd1, Cyclin D1; RBP2, Retinol binding protein 2; UTX, Utx histone demethylase; Osx, Osterix; NFATc1, Nuclear factor of activated T cells 1.
Several genes are regulated by HMTs or HMDs during the differentiation and maturation of osteoblasts and osteoclasts (6, 14, 15). Suv39h1 is an H3K9 methyltransferase that can modify H3K9 with two or three methyl groups. H3K9me2 and H3K9me3 bind to the promoter of Runx2 ( 22)—a key gene involved in osteoblast differentiation and maturation (41)—and suppress gene transcription, thereby delaying osteoblast differentiation, which may be relevant to the pathogenesis of osteoporosis. EZH2 is a trimethyltransferase of H3K27; H3K27me3 activates transcription of the Wnt4 gene in osteoblasts to promote osteogenic differentiation (27). H3K27me3 also activates the transcription of Foxc1 in osteoclasts and promotes osteoclast differentiation (27). Thus, histone methylation plays opposing regulatory roles in the pathogenesis of osteoporosis. However, some of the evidence regarding the function of histone methylation in bone homeostasis is controversial. For instance, JMJD2A and JMJD2B are H3K9me3 demethylases; in one study, demethylation of H3K9me3 by JMJD2A promoted adipogenic differentiation and inhibited osteogenic differentiation (32), whereas another study found that H3K9me3 demethylation by JMJD2B promoted osteogenic differentiation of bone marrow-derived stem cells (BMSCs) and maintained bone–fat balance (33).
Histone acetylation
Histone acetyltransferases and histone deacetylases
Acetylation was one of the first histone modifications found to affect transcriptional regulation and is therefore the most widely studied. Acetylation causes K residues in the N-terminal histone tails protruding from nucleosomes to become negatively charged, which repels negatively charged DNA and leads to relaxation of the chromatin structure. The open chromatin conformation allows transcription factors to bind more easily, resulting in an increase in gene expression (42, 43). Thus, histone acetylation is mainly associated with gene activation; it is known to be involved in cell cycle regulation, cell proliferation, and apoptosis, cellular differentiation, DNA replication and repair, nuclear import, and neuronal inhibition (44, 45), whereas dysregulation of histone acetylation has been implicated in osteoporosis progression (46–48).
Histone acetylation is regulated by HATs and HDACs ( Table 2 ). The HAT family includes GNAT (HAT1, GCN5, PCAF) and MYST (Tip60, MOF, MOZ, MORF, HBO1) as well as CREB-binding protein (CBP)/p300, which share very high sequence similarity in the bromodomain, cysteine-histidine-rich region, and HAT structural domain and specifically bind phosphorylated CREB to enhance its transcription of cAMP-responsive genes.
Table 2.
Histone acetyltransferases, histone deacetylases, and their roles in the occurrence and development of osteoporosis.
| HATs and HDACs | Target genes | Function |
|---|---|---|
| KAT2A (GCN5) | Wnt, NF-κB | Enhances osteogenic differentiation ability of BMSCs (49–51). |
| KAT2B (PCAF) | BMP, Runx2 (52), CXCL12 (53) NFATc1 (54) | Promotes osteogenic differentiation of MSCs (52, 53). Promotes osteoclast differentiation (54) |
| CBP/p300 | Runx2 (55), NFATc1 (54) | Promotes osteoblast differentiation (55). Promotes osteoclast differentiation (54). |
| HDAC1 | IGF-1 | Prevents achievement of peak bone mass by inhibiting IGF-1 expression in the liver and IGF-1 signaling in bone (56). |
| HDAC2 | SP7 (57), AKT, FoxO1 (58) | Inhibits osteogenic differentiation of MSCs (57). Activates Akt and thereby suppresses FoxO1 transcription, resulting in enhanced osteoclastogenesis (58). |
| HDAC3 | NF-κB | Controls bone remodeling by suppressing the responsiveness of osteoclast lineage cell to RANKL (59). |
| HDAC4 | MEF2C, MMP13 (60), Runx2 (61) | HDAC4 interacts with MEF2C at the MMP13 promoter and inhibits MMP13 gene transcription (60). Deacetylates and degrades Runx2, leading to reduced osteoblast function (61). |
| HDAC5 | MEF2C (62), NFATc1 (54) | HDAC5 binds and inhibits the function of MEF2C and decreases SOST expression in osteocytes (62). Reduces RANKL- or PCAF-mediated NFATc1 acetylation, stability, and transactivation activity and suppresses osteoclast differentiation (54) |
| HDAC6 | Runx2 | Inactivates Runx2 promoter to block osteogenesis of BMSCs (48). |
| HDAC7 | Mitf | Represses Mitf function and inhibits osteoclast differentiation (63). Represses Runx2 expression and suppresses osteoblast maturation (64). |
| HDAC8 | Runx2 | Suppresses osteogenic differentiation of BMSCs by inhibiting H3K9ac and Runx2 activity (65). |
| HDAC11 | 11β-HSD2 | Suppresses osteogenic differentiation of BMSCs by downregulating H3K9ac and 11β-HSD2 expression (66). |
HAT, histone acetyltransferases; HDAC, histone deacetylase.
KAT2A(GCN5), Lysine acetyltransferase 2A; NF-κB, Nuclear factor kappa B; KAT2B(PCAF), Lysine acetyltransferase 2B; CXCL12, C-X-C motif chemokine ligand 12; CBP, CREB binding protein, IGF-1, Insulin like growth factor 1; SP7, Sp7 transcription factor; Akt, AKT serine/threonine kinase; RANKL, NF-κB ligand-receptor activator; MEF2C, Myocyte enhancer factor 2C; SOST, Sclerostin; Mitf, Melanocyte inducing transcription factor; 11β-HSD2, Hydroxysteroid 11-beta dehydrogenase 2.
CBP/p300 has a regulatory role in bone formation, targeting transcription factors such as Runx2 during osteoblast differentiation. During parathyroid hormone-induced osteoblast differentiation, phosphorylated HDAC4 dissociates from Runx2, which interacts with CBP/P300 (67). Transforming growth factor beta-1(TGF-β1) and BMP2 stimulate ERK-mediated phosphorylation of Runx2 to promote its interaction with CBP/p300 (68). BMP2 activates SMAD1/5, leading to CBP/p300-mediated acetylation of Runx2, which enhances the expression of osteogenic genes such as alkaline phosphatase(ALP) and collagen type I (COL-I) (69, 70).
Runx2 (41), Sp7 (71), and FoxO1 (72) are important transcription factors for osteoblast differentiation and maturation that induce the transcription of downstream osteogenesis-related genes such as ALP, osteocalcin(OCN), osteopontin(OPN), and COL-1 and promote the maturation and mineralization of osteoblasts. As Runx2 transcription is initiated, PCAF and CBP/p300 acetylate histone H3 to promote osteoblast differentiation and maturation (52, 55). However, the transcription of Runx2, SP7, and FoxO1 was shown to be inhibited after HDAC reduced the histone acetylation level, leading to suppression of osteoblast differentiation and maturation (48, 57, 58, 61, 65) ( Table 2 ). The differentiation and maturation of osteoclasts are controlled by the transcription factors NFATc1 (73) and NF-κB (74), among others. PCAF and CBP/p300 acetylate histone H3 to promote osteoclast differentiation, whereas differentiation is inhibited by HDAC-mediated H3 deacetylation (52, 55) ( Table 2 ). These findings suggest that the balance between the activities of HATs and HDACs is critical for the regulation of transcription factors involved in osteoblast and osteoclast differentiation.
Sirtuins
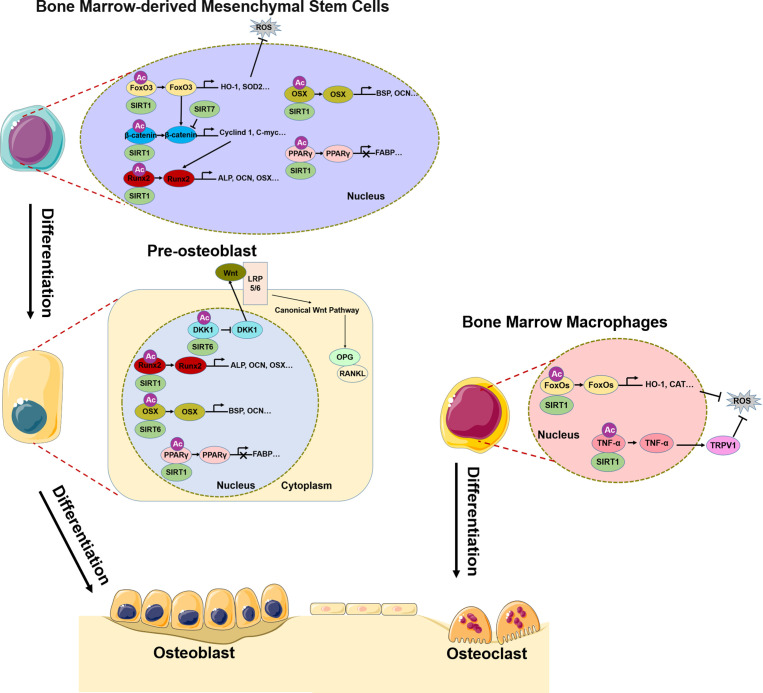
SIRTs are highly conserved HATs that transfer the acetyl group of a substrate to the ADP-ribosyl moiety of nicotinamide adenine dinucleotide (NAD+), NAD+ dependent protein deacetylation consumes NAD+, transfers the acetyl group from the lysine to ADP-ribose to form 2’-O-acetyl-ADPR, nicotinamide, and a deacetylated lysine. The SIRT family comprises SIRT1–7, of which SIRT1, SIRT6, and SIRT7 are mainly localized in the nucleus (75, 76). It should be noted that, most of the discussed Sirtuin effects do not exhibit their function by direct deacetylation of histones, but by deacetylation of other targets. SIRT1 deacetylates H3K9 and regulates of a variety of physiologic processes including metabolism, immune response, and aging (77), and has been linked to osteoporosis (78). The Wnt/β-catenin signaling pathway plays a central role in the differentiation of BMSCs into osteoblasts (79–82); SIRT1 deacetylates K49R or K345R of β-catenin, promoting its entry into the nucleus where it induces the transcription of osteogenic differentiation-related genes such as Cyclin D1 and C-myc and promotes the expression of Runx2 (83). SIRT1 also directly deacetylates Runx2, which in turn induces the transcription of genes that promote osteogenic differentiation of BMSCs (84). peroxisome proliferators-activated receptors gamma(PPARγ) is an important transcription factor for the adipogenic differentiation of BMSCs (85), which inhibits osteogenic differentiation and disrupts bone–fat balance; this is restored by reduction of PPARγ acetylation level by SIRT1 and consequent suppression of the adipogenic differentiation of BMSCs (86, 87) ( Figure 1 ). In pre-oisteoblasts, SIRT1 also down-regulates PPARγ to promote osteogenic differentiation (88). Excessive reactive oxygen species (ROS) production in BMSCs under oxidative stress affects osteogenic differentiation (72). SIRT1 reduces the acetylation level of FoxO3, a transcription factor involved in the cellular response to oxidative stress, leading to FoxO3 transcription and the expression of antioxidant enzymes such as heme oxygenase 1(HO-1) and superoxide dismutase 2(SOD2) (89, 90). The subsequent removal of excess ROS in BMSCs restores their osteogenic differentiation capacity. FoxO3 transcription also promotes the expression of β-catenin, resulting in osteogenic differentiation (90) ( Figure 1 ). Bone resorption depends on ROS produced in osteoclasts; SIRT1 was shown to reduce FoxO acetylation level in bone marrow macrophages(BMMs) and promote the expression of antioxidant enzymes that clear ROS (91). At the same time, SIRT1 reduced TNF-α acetylation level, thereby increasing the expression of transient receptor potential cation channel subfamily V member 1(TRPV1), ROS scavenging, and inhibiting bone resorption (92) ( Figure 1 ).
Figure 1.
Role of SIRT1/6/7 in bone remodeling. In BMSCs, SIRT1, SIRT6, and SIRT7 promote osteogenic differentiation by regulating transcription factors FoxO3, β-catenin, Runx2, Osx, and PPARγ. In pre-osteoblasts, SIRT6 not only regulates Runx2 and Osx transcription, but also inhibits DKK1 transcription, activates canonical Wnt signaling, and promotes osteogenic differentiation. In BMMs, SIRT1 inhibits osteoclast differentiation by regulating FoxO and TNF-α transcription, which results in ROS scavenging.
SIRT6 and SIRT7 are involved in chromatin regulation and play multiple roles in metabolism, aging, and disease. In osteoblasts, SIRT6 reduces H3K9 acetylation level and promotes the transcription of osteogenic transcription factors Runx2 and Osx and the expression of osteogenic genes (93). Additionally, SIRT6 negatively regulates the expression of dickkopf1 (DKK1) (93), a secreted protein that binds to the Wnt receptor LRP5/6; this induces rapid endocytosis and reduces LRP5/6 in the cell membrane (94), thereby blocking the canonical Wnt signaling cascade. Blocking the Wnt pathway leads to a reduction in the synthesis of osteoprotegerin (OPG), which competitively binds to RANKL to inhibit osteoblast differentiation (95). Thus, reducing DKK1 expression facilitates the nuclear entry of β-catenin, which initiates the transcription of target genes that promote osteoblast differentiation while inhibiting those involved in osteoblast differentiation. Additionally, SIRT7 has the effect of reducing β-catenin level in BMSCs (96), although the significance of this observation in the context of bone homeostasis and osteoporosis remains unclear.
Histone ubiquitination
All histones can be ubiquitinated, with H2A and H2B being the most frequent targets. Histone ubiquitination plays a central role in the DNA damage response. Monoubiquitination of H2A, H2B, and H2AX has been observed at DNA double-strand break sites; the most common forms are monoubiquitination of K119 on H2A and K123 (yeast)/K120 (vertebrate) on H2B. H2A and H2B monoubiquitination was shown to be associated with gene silencing and transcriptional activation, respectively (97, 98).
Ring finger protein 40 (RNF40), an E3 ubiquitin ligase that monoubiquitinates H2B (99), was found to regulate the transcription of the osteogenic genes bone gamma-carboxyglutamate protein (BGLAP), ALP, and glucose-6-phosphate dehydrogenase (G6PD) to induce osteogenic differentiation of human BMSCs (100). The Tnfsf11 gene (encoding RANKL) is a target gene of H2Bub1 (101). It was reported that H2Bub1, whose expression was induced by RNF40, was required in the early stages of osteoblast differentiation and modulated osteoblast function by regulating VDR-induced Tnfsf11 expression in crosstalk between osteoblasts. The long noncoding RNA ODIR1 was significantly downregulated during osteogenic differentiation of human umbilical cord mesenchymal stem cells (hUC-MSCs); the interaction of ODIR1 with F-box protein 25 (FBXO25) increased monoubiquitination of H2BK120 (H2BK120ub), promoted the trimethylation of H3K4 (H3K4me3), and induced the expression of the transcription factor Osx, thereby enhancing the expression of the osteoblast markers OCN, OPN, and ALP. Thus, ODIR1 negatively regulates the osteogenic differentiation of hUC-MSCs via the FBXO25/H2BK120ub/H3K4me3/Osx axis (102).
Myb like, SWIRM and MPN domains 1(Mysm1) is an H2A deubiquitinating enzyme that regulates osteoblast differentiation and maturation by promoting Runx2 expression in osteoblasts. Mysm1−/− mice exhibit significant skeletal deformation and osteoporosis; however, osteogenic differentiation capacity was not significantly affected in MSCs lacking Mysm1. In p53−/−Mysm1−/− double knockout mice, p53 deletion rescued the skeletal defects and bone loss caused by Mysm1 deficiency. On the other hand, loss of p53 did not restore Runx2 expression in Mysm1−/− osteoblasts although MSCs proliferation and osteogenic differentiation was enhanced (103).
Histone ADP-ribosylation
Poly (ADP-Ribose) polymerases (PARPs) (also known as ARTDs), are a family of 17 proteins, some of PARPs are mono-ADP-ribosyl transferases and some poly. PARPs uses NAD+ as substrate to transfer single or straight or branched ADP-ribose to itself or other target proteins, thus regulating various cellular responses. The most widely studied member of the PARPs family is PARP1, which is thought to play a role in DNA repair (104). PARP1 binds to DNA damage sites and catalyzes its own ADP-ribosylation reactions and the trans-modification of local substrate proteins, including DNA repair proteins, histones and other chromatin-associated proteins, to promote the repair of DNA lesions, influence chromatin structure and gene transcription (105, 106).
Induction of NFATc1 by macrophage colony-stimulating factor (M-CSF) and RANKL is essential for macrophage differentiation into osteoblasts (73). ADP-ribosylation of H2B at serine 7 by PARP1 reduced the occupancy of this histone at the NFATc1 promoter, reducing NFATc1 expression and osteoclast formation (107). Moreover, PARP1 inhibited the expression of osteoclast-promoting genes via regulation of histone ADP-ribosylation at the IL-1β promoter, which increased IL-1β expression (108). M-CSF induced PARP1 self–ADP-ribosylation in macrophages, resulting in PARP1 cleavage at D214 and its subsequent degradation; this stimulated RANKL-induced osteoclast differentiation and osteoclast maturation, whereas osteoclastogenesis was inhibited by expression of the cleavage-resistant D214N mutant form of PARP1 (109). The PARP inhibitor olaparib decreased ALP activity in preosteoblastic MC3T3-E1 cells and inhibited the formation of the mineralized nodules that characterize osteoblasts (110). In contrast, inhibiting the enzymatic activity of poly(ADP-Ribose) glycohydrolase (PARG) using the inhibitor PDD00017273 enhanced Runx2 PARylation (ribosylation) and osteoblast formation while having no effect on PARG mRNA expression.
Summary and prospects
Osteoporosis is a complex disease whose pathogenesis remains unclear, although an imbalance in bone homeostasis during bone reconstruction is thought to contribute. Accumulating evidence indicates that histone modifications play an essential role in various diseases including osteoporosis. Specifically, changes in the levels of histone modification and related enzymes can lead to altered expression of genes involved in bone formation or bone resorption, resulting in an imbalance in bone homeostasis and abnormal bone reconstruction.
According to this review, it can be proved that histone modifications play important role in osteoporosis, and related research results have been applied to the diagnosis and treatment of osteoporosis. Identifying specific biomarkers associated with osteoporosis will significantly improve the clinical diagnosis and treatment of this disease. In recent years, drugs targeting the activity of histone-modifying enzymes have been evaluated in studies focused on osteoporosis treatment. As an example, SOST—which negatively regulates bone formation—is regulated by a class I HDAC (111); meanwhile, the class I HDAC inhibitor MS-275 was shown to promote bone formation (112).
There have been a limited number of studies on the effects of histone modification on bone homeostasis, leaving many open questions. For example, the relationship between abnormal regulation of histone modifications in genes related to bone homeostasis and the development of osteoporosis requires clarification. It is unclear whether histone modifications are a primary cause of osteoporosis or a secondary effect, although it is clear that the effects of histone modifications are associated with the pathogenesis of osteoporosis. More in-depth mechanistic studies are expected to provide a better understanding of the effect of histone modification on individual bone cell type by using tissue-specific deletion and transgenic animal models. Further studies in this area can provide insight not only into disease pathogenesis, but also novel diagnostic biomarkers and therapeutic targets.
Author contributions
PS and TH performed the literature review and wrote the manuscript. CH consulted and summarized the literature. YW provided supervision and advisory during the writing process. DT reviewed the literature and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by the National Natural Science Foundation of China (81730107, 81973883), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202202), the National Key R&D Program of China (2018YFC1704300), the Shanghai Scientific Research Project (19ZR1458000), the Shanghai Traditional Chinese Medicine Medical Center of Chronic Disease (2017ZZ01010), and the Innovation Team of the Ministry of Education (IRT1270).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Kenkre JS, Bassett J. The bone remodelling cycle. Ann Clin Biochem (2018) 55(3):308–27. doi: 10.1177/0004563218759371 [DOI] [PubMed] [Google Scholar]
- 2. Intemann J, De Gorter DJJ, Naylor AJ, Dankbar B, Wehmeyer C. Importance of osteocyte-mediated regulation of bone remodelling in inflammatory bone disease. Swiss Med Weekly (2020) 150:w20187. doi: 10.4414/smw.2020.20187 [DOI] [PubMed] [Google Scholar]
- 3. Fang H, Deng Z, Liu J, Chen S, Deng Z, Li W. The mechanism of bone remodeling after bone aging. Clin Interv Aging (2022) 17:405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seely KD, Kotelko CA, Douglas H, Bealer B, Brooks AE. The human gut microbiota: A key mediator of osteoporosis and osteogenesis. Int J Mol Sci (2021) 22(17):9452. doi: 10.3390/ijms22179452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song S, Guo Y, Yang Y, Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther (2022) 237:108168. doi: 10.1016/j.pharmthera.2022.108168 [DOI] [PubMed] [Google Scholar]
- 6. Xu F, Li W, Yang X, Na L, Chen L, Liu G. The roles of epigenetics regulation in bone metabolism and osteoporosis. Front Cell Dev Biol (2020) 8:619301. doi: 10.3389/fcell.2020.619301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avilkina V, Chauveau C, Ghali Mhenni O. Sirtuin function and metabolism: Role in pancreas, liver, and adipose tissue and their crosstalk impacting bone homeostasis. Bone (2022) 154:116232. doi: 10.1016/j.bone.2021.116232 [DOI] [PubMed] [Google Scholar]
- 8. Zhao L, Yin B, Ma Q, Shi Y, Wang C, Ye L. The involvement of histone methylation in the osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther (2021) 16(8):915–23. doi: 10.2174/1574888X16666210203110354 [DOI] [PubMed] [Google Scholar]
- 9. Liu CF, Abnousi A, Bazeley P, Ni Y, Morley M, Moravec CS, et al. Global analysis of histone modifications and long-range chromatin interactions revealed the differential cistrome changes and novel transcriptional players in human dilated cardiomyopathy. J Mol Cell Cardiol (2020) 145:30–42. doi: 10.1016/j.yjmcc.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan MAJ, Shilatifard A. Reevaluating the roles of histone-modifying enzymes and their associated chromatin modifications in transcriptional regulation. Nat Genet (2020) 52(12):1271–81. doi: 10.1038/s41588-020-00736-4 [DOI] [PubMed] [Google Scholar]
- 11. Venkatesh S, Workman JL. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol (2015) 16(3):178–89. doi: 10.1002/wdev.109 [DOI] [PubMed] [Google Scholar]
- 12. Saul D, Kosinsky RL. Epigenetics of aging and aging-associated diseases. Int J Mol Sci (2021) 22(1):401. doi: 10.3390/ijms22010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghayor C, Weber FE. Epigenetic regulation of bone remodeling and its impacts in osteoporosis. Int J Mol Sci (2016) 17(9):1446. doi: 10.3390/ijms17091446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vrtacnik P, Marc J, Ostanek B. Epigenetic mechanisms in bone. Clin Chem Lab Med (2014) 52(5):589–608. doi: 10.1515/cclm-2013-0770 [DOI] [PubMed] [Google Scholar]
- 15. Holroyd C, Harvey N, Dennison E, Cooper C. Epigenetic influences in the developmental origins of osteoporosis. Osteoporos Int (2012) 23(2):401–10. doi: 10.1007/s00198-011-1671-5 [DOI] [PubMed] [Google Scholar]
- 16. Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol (2007) 8(12):983–94. doi: 10.1038/nrm2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet (2012) 13(5):343–57. doi: 10.1038/nrg3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moena D, Merino P, Lian JB, Stein GS, Stein JL, Montecino M. Switches in histone modifications epigenetically control vitamin D3-dependent transcriptional upregulation of the CYP24A1 gene in osteoblastic cells. J Cell Physiol (2020) 235(6):5328–39. doi: 10.1002/jcp.29420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kota SK, Roening C, Patel N, Kota SB, Baron R. PRMT5 inhibition promotes osteogenic differentiation of mesenchymal stromal cells and represses basal interferon stimulated gene expression. Bone (2018) 117:37–46. doi: 10.1016/j.bone.2018.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong Y, Song C, Wang Y, Lei Z, Xu F, Guan H, et al. Inhibition of PRMT5 suppresses osteoclast differentiation and partially protects against ovariectomy-induced bone loss through downregulation of CXCL10 and RSAD2. Cell Signal (2017) 34:55–65. doi: 10.1016/j.cellsig.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 21. Zhai Q, Zhao Y, Wang L, Dai Y, Zhao P, Xiang X, et al. CircRNA hsa_circ_0008500 acts as a miR-1301-3p sponge to promote osteoblast mineralization by upregulating PADI4. Front Cell Dev Biol (2020) 8:602731. doi: 10.3389/fcell.2020.602731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HJ, Park JW, Lee KH, Yoon H, Shin DH, Ju UI, et al. Plant homeodomain finger protein 2 promotes bone formation by demethylating and activating Runx2 for osteoblast differentiation. Cell Res (2014) 24(10):1231–49. doi: 10.1038/cr.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim K, Shin Y, Kim J, Ulmer TS, An W. H3K27me1 is essential for MMP-9-dependent H3N-terminal tail proteolysis during osteoclastogenesis. Epigenet Chromatin (2018) 11(1):23. doi: 10.1186/s13072-018-0193-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ideno H, Nakashima K, Komatsu K, Araki R, Abe M, Arai Y, et al. G9a is involved in the regulation of cranial bone formation through activation of Runx2 function during development. Bone (2020) 137:115332. doi: 10.1016/j.bone.2020.115332 [DOI] [PubMed] [Google Scholar]
- 25. Huang H, Dou L, Song J, Luo J. CBFA2T2 is required for BMP-2-induced osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun (2018) 496(4):1095–101. doi: 10.1016/j.bbrc.2018.01.144 [DOI] [PubMed] [Google Scholar]
- 26. Lawson KA, Teteak CJ, Gao J, Li N, Hacquebord J, Ghatan A, et al. ESET histone methyltransferase regulates osteoblastic differentiation of mesenchymal stem cells during postnatal bone development. FEBS Lett (2013) 587(24):3961–7. doi: 10.1016/j.febslet.2013.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao X, He W, Rong K, Xu S, Chen Z, Liang Y, et al. DZNep promotes mouse bone defect healing via enhancing both osteogenesis and osteoclastogenesis. Stem Cell Res Ther (2021) 12(1):605. doi: 10.1186/s13287-021-02670-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rojas A, Sepulveda H, Henriquez B, Aguilar R, Opazo T, Nardocci G, et al. Mll-COMPASS complexes mediate H3K4me3 enrichment and transcription of the osteoblast master gene Runx2/p57 in osteoblasts. J Cell Physiol (2019) 234(5):6244–53. doi: 10.1002/jcp.27355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Munehira Y, Yang Z, Gozani O. Systematic analysis of known and candidate lysine demethylases in the regulation of myoblast differentiation. J Mol Biol (2017) 429(13):2055–65. doi: 10.1016/j.jmb.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao Y, Ge W. The histone methyltransferase DOT1L inhibits osteoclastogenesis and protects against osteoporosis. Cell Death Dis (2018) 9(2):33. doi: 10.1038/s41419-017-0040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun J, Ermann J, Niu N, Yan G, Yang Y, Shi Y, et al. Histone demethylase LSD1 regulates bone mass by controlling WNT7B and BMP2 signaling in osteoblasts. Bone Res (2018) 6:14. doi: 10.1038/s41413-018-0015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qi Q, Wang Y, Wang X, Yang J, Xie Y, Zhou J, et al. Histone demethylase KDM4A regulates adipogenic and osteogenic differentiation via epigenetic regulation of C/EBPalpha and canonical wnt signaling. Cell Mol Life Sci (2020) 77(12):2407–21. doi: 10.1007/s00018-019-03289-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng P, Yuan Q, Cheng Y, Li J, Liu Z, Liu Y, et al. Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging. Cell Stem Cell (2021) 28(6):1057–73 e7. doi: 10.1016/j.stem.2021.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, et al. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell (2012) 11(1):50–61. doi: 10.1016/j.stem.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang C, Wang J, Li J, Hu G, Shan S, Li Q, et al. KDM5A controls bone morphogenic protein 2-induced osteogenic differentiation of bone mesenchymal stem cells during osteoporosis. Cell Death Dis (2016) 7(8):e2335. doi: 10.1038/cddis.2016.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ge W, Shi L, Zhou Y, Liu Y, Ma GE, Jiang Y, et al. Inhibition of osteogenic differentiation of human adipose-derived stromal cells by retinoblastoma binding protein 2 repression of RUNX2-activated transcription. Stem Cells (2011) 29(7):1112–25. doi: 10.1002/stem.663 [DOI] [PubMed] [Google Scholar]
- 37. Rojas A, Aguilar R, Henriquez B, Lian JB, Stein JL, Stein GS, et al. Epigenetic control of the bone-master Runx2 gene during osteoblast-lineage commitment by the histone demethylase JARID1B/KDM5B. J Biol Chem (2015) 290(47):28329–42. doi: 10.1074/jbc.M115.657825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hemming S, Cakouros D, Isenmann S, Cooper L, Menicanin D, Zannettino A, et al. EZH2 and KDM6A act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells (2014) 32(3):802–15. doi: 10.1002/stem.1573 [DOI] [PubMed] [Google Scholar]
- 39. Yang D, Okamura H, Nakashima Y, Haneji T. Histone demethylase Jmjd3 regulates osteoblast differentiation via transcription factors Runx2 and osterix. J Biol Chem (2013) 288(47):33530–41. doi: 10.1074/jbc.M113.497040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yasui T, Hirose J, Tsutsumi S, Nakamura K, Aburatani H, Tanaka S. Epigenetic regulation of osteoclast differentiation: possible involvement of Jmjd3 in the histone demethylation of Nfatc1. J Bone Miner Res (2011) 26(11):2665–71. doi: 10.1002/jbmr.464 [DOI] [PubMed] [Google Scholar]
- 41. Komori T. Whole aspect of Runx2 functions in skeletal development. Int J Mol Sci (2022) 23(10):5776. doi: 10.3390/ijms23105776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verdone L, Agricola E, Caserta M, Di Mauro E. Histone acetylation in gene regulation. Brief Funct Genomic Proteomic (2006) 5(3):209–21. doi: 10.1093/bfgp/ell028 [DOI] [PubMed] [Google Scholar]
- 43. Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol (2014) 15(11):703–8. doi: 10.1038/nrm3890 [DOI] [PubMed] [Google Scholar]
- 44. Peleg S, Feller C, Ladurner AG, Imhof A. The metabolic impact on histone acetylation and transcription in ageing. Trends Biochem Sci (2016) 41(8):700–11. doi: 10.1016/j.tibs.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 45. Arora I, Sharma M, Sun LY, Tollefsbol TO. The epigenetic link between polyphenols, aging and age-related diseases. Genes (2020) 11(9):1094. doi: 10.3390/genes11091094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo Q, Kang H, Wang J, Dong Y, Peng R, Zhao H, et al. Inhibition of ACLY leads to suppression of osteoclast differentiation and function Via regulation of histone acetylation. J Bone Miner Res (2021) 36(10):2065–80. doi: 10.1002/jbmr.4399 [DOI] [PubMed] [Google Scholar]
- 47. Hu M, Xing L, Zhang L, Liu F, Wang S, Xie Y, et al. NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation. Aging Cell (2022) 21(2):e13551. doi: 10.1111/acel.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma C, Gao J, Liang J, Dai W, Wang Z, Xia M, et al. HDAC6 inactivates Runx2 promoter to block osteogenesis of bone marrow stromal cells in age-related bone loss of mice. Stem Cell Res Ther (2021) 12(1):484. doi: 10.1186/s13287-021-02545-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jing H, Su X, Gao B, Shuai Y, Chen J, Deng Z, et al. Epigenetic inhibition of wnt pathway suppresses osteogenic differentiation of BMSCs during osteoporosis. Cell Death Dis (2018) 9(2):176. doi: 10.1038/s41419-017-0231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y, Cheng W, Zhao Y, Gao L, Chang Y, Tong Z, et al. Cyclic mechanical strain regulates osteoblastic differentiation of mesenchymal stem cells on TiO2 nanotubes through GCN5 and wnt/beta-catenin. Front Bioeng Biotechnol (2021) 9:735949. doi: 10.3389/fbioe.2021.735949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang P, Liu Y, Jin C, Zhang M, Tang F, Zhou Y. Histone acetyltransferase GCN5 regulates osteogenic differentiation of mesenchymal stem cells by inhibiting NF-kappaB. J Bone Miner Res (2016) 31(2):391–402. doi: 10.1002/jbmr.2704 [DOI] [PubMed] [Google Scholar]
- 52. Zhang P, Liu Y, Jin C, Zhang M, Lv L, Zhang X, et al. Histone H3K9 acetyltransferase PCAF is essential for osteogenic differentiation through bone morphogenetic protein signaling and may be involved in osteoporosis. Stem Cells (2016) 34(9):2332–41. doi: 10.1002/stem.2424 [DOI] [PubMed] [Google Scholar]
- 53. Lian WS, Ko JY, Chen YS, Ke HJ, Hsieh CK, Kuo CW, et al. MicroRNA-29a represses osteoclast formation and protects against osteoporosis by regulating PCAF-mediated RANKL and CXCL12. Cell Death Dis (2019) 10(10):705. doi: 10.1038/s41419-019-1942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim JH, Kim K, Youn BU, Jin HM, Kim JY, Moon JB, et al. RANKL induces NFATc1 acetylation and stability via histone acetyltransferases during osteoclast differentiation. Biochem J (2011) 436(2):253–62. doi: 10.1042/BJ20110062 [DOI] [PubMed] [Google Scholar]
- 55. Krishnan RH, Sadu L, Das UR, Satishkumar S, Pranav Adithya S, Saranya I, et al. Role of p300, a histone acetyltransferase enzyme, in osteoblast differentiation. Differentiation (2022) 124:43–51. doi: 10.1016/j.diff.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 56. Bachagol D, Joseph GS, Ellur G, Patel K, Aruna P, Mittal M, et al. Stimulation of liver IGF-1 expression promotes peak bone mass achievement in growing rats: a study with pomegranate seed oil. J Nutr Biochem (2018) 52:18–26. doi: 10.1016/j.jnutbio.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 57. Zhu X, Yu J, Du J, Zhong G, Qiao L, Lin J. LncRNA HOXA-AS2 positively regulates osteogenesis of mesenchymal stem cells through inactivating NF-kappaB signalling. J Cell Mol Med (2019) 23(2):1325–32. doi: 10.1111/jcmm.14034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dou C, Li N, Ding N, Liu C, Yang X, Kang F, et al. HDAC2 regulates FoxO1 during RANKL-induced osteoclastogenesis. Am J Physiol Cell Physiol (2016) 310(10):C780–C7. doi: 10.1152/ajpcell.00351.2015 [DOI] [PubMed] [Google Scholar]
- 59. Molstad DHH, Mattson AM, Begun DL, Westendorf JJ, Bradley EW. Hdac3 regulates bone modeling by suppressing osteoclast responsiveness to RANKL. J Biol Chem (2020) 295(51):17713–23. doi: 10.1074/jbc.RA120.013573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nakatani T, Partridge NC. MEF2C interacts with c-FOS in PTH-stimulated Mmp13 gene expression in osteoblastic cells. Endocrinology (2017) 158(11):3778–91. doi: 10.1210/en.2017-00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li J, Liu C, Li Y, Zheng Q, Xu Y, Liu B, et al. TMCO1-mediated Ca(2+) leak underlies osteoblast functions via CaMKII signaling. Nat Commun (2019) 10(1):1589. doi: 10.1038/s41467-019-09653-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, et al. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J Bone Miner Res (2015) 30(3):400–11. doi: 10.1002/jbmr.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pham L, Kaiser B, Romsa A, Schwarz T, Gopalakrishnan R, Jensen ED, et al. HDAC3 and HDAC7 have opposite effects on osteoclast differentiation. J Biol Chem (2011) 286(14):12056–65. doi: 10.1074/jbc.M110.216853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jensen ED, Schroeder TM, Bailey J, Gopalakrishnan R, Westendorf JJ. Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. J Bone Miner Res (2008) 23(3):361–72. doi: 10.1359/jbmr.071104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fu Y, Zhang P, Ge J, Cheng J, Dong W, Yuan H, et al. Histone deacetylase 8 suppresses osteogenic differentiation of bone marrow stromal cells by inhibiting histone H3K9 acetylation and RUNX2 activity. Int J Biochem Cell Biol (2014) 54:68–77. doi: 10.1016/j.biocel.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 66. Xiao H, Wu Z, Li B, Shangguan Y, Stoltz JF, Magdalou J, et al. The low-expression programming of 11beta-HSD2 mediates osteoporosis susceptibility induced by prenatal caffeine exposure in male offspring rats. Br J Pharmacol (2020) 177(20):4683–700. doi: 10.1111/bph.15225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shimizu E, Nakatani T, He Z, Partridge NC. Parathyroid hormone regulates histone deacetylase (HDAC) 4 through protein kinase a-mediated phosphorylation and dephosphorylation in osteoblastic cells. J Biol Chem (2014) 289(31):21340–50. doi: 10.1074/jbc.M114.550699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gomathi K, Rohini M, Partridge NC, Selvamurugan N. Regulation of transforming growth factor-beta1-stimulation of Runx2 acetylation for matrix metalloproteinase 13 expression in osteoblastic cells. Biol Chem (2022) 403(3):305–15. doi: 10.1515/hsz-2021-0292 [DOI] [PubMed] [Google Scholar]
- 69. Jeon EJ, Lee KY, Choi NS, Lee MH, Kim HN, Jin YH, et al. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem (2006) 281(24):16502–11. doi: 10.1074/jbc.M512494200 [DOI] [PubMed] [Google Scholar]
- 70. Jun JH, Yoon WJ, Seo SB, Woo KM, Kim GS, Ryoo HM, et al. BMP2-activated Erk/MAP kinase stabilizes Runx2 by increasing p300 levels and histone acetyltransferase activity. J Biol Chem (2010) 285(47):36410–9. doi: 10.1074/jbc.M110.142307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hojo H, Ohba S. Sp7 action in the skeleton: Its mode of action, functions, and relevance to skeletal diseases. Int J Mol Sci (2022) 23(10):5647. doi: 10.3390/ijms23105647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ma X, Su P, Yin C, Lin X, Wang X, Gao Y, et al. The roles of FoxO transcription factors in regulation of bone cells function. Int J Mol Sci (2020) 21(3):692. doi: 10.3390/ijms21030692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kang JY, Kang N, Yang Y-M, Hong JH, Shin DM. The role of Ca2+-NFATc1 signaling and its modulation on osteoclastogenesis. Int J Mol Sci (2020) 21(10):3646. doi: 10.3390/ijms21103646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yao Z, Getting SJ, Locke IC. Regulation of TNF-induced osteoclast differentiation. Cells (2021) 11(1):132. doi: 10.3390/cells11010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang Y, He J, Liao M, Hu M, Li W, Ouyang H, et al. An overview of sirtuins as potential therapeutic target: Structure, function and modulators. Eur J Med Chem (2019) 161:48–77. doi: 10.3390/cells11010132 [DOI] [PubMed] [Google Scholar]
- 76. Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun (2016) 7:12235. doi: 10.1038/ncomms12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol (2010) 5:253–95. doi: 10.1146/annurev.pathol.4.110807.092250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Q, Cheng JC, Jiang Q, Lee WY. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell (2021) 20(2):e13301. doi: 10.1111/acel.13301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, et al. A wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat Commun (2020) 11(1):332. doi: 10.1038/s41467-019-14029-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu S, Zhu K, Lai Y, Zhao Z, Fan J, Im HJ, et al. atf4 promotes beta-catenin expression and osteoblastic differentiation of bone marrow mesenchymal stem cells. Int J Biol Sci (2013) 9(3):256–66. doi: 10.7150/ijbs.5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. eLife (2020) 9:e52779. doi: 10.7554/eLife.52779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhu Y, Wang Y, Jia Y, Xu J, Chai Y. Catalpol promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the wnt/β-catenin pathway. Stem Cell Res Ther (2019) 10(1):37. doi: 10.1186/s13287-019-1143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, et al. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol Med (2013) 5(3):430–40. doi: 10.1002/emmm.201201606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C, Lueders C, et al. Resveratrol mediated modulation of sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PloS One (2012) 7(4):e35712. doi: 10.1371/journal.pone.0035712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Evseeva MN, Balashova MS, Kulebyakin KY, Rubtsov YP. Adipocyte biology from the perspective of in vivo research: Review of key transcription factors. Int J Mol Sci (2021) 23(1):322. doi: 10.3390/ijms23010322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T. SIRT1 is regulated by a PPARγ–SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res (2010) 38(21):7458–71. doi: 10.1093/nar/gkq609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bäckesjö C-M, Li Y, Lindgren U, Haldosén L-A. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Mineral Res (2006) 21(7):993–1002. doi: 10.1359/jbmr.060415 [DOI] [PubMed] [Google Scholar]
- 88. Qu B, Ma Y, Yan M, Gong K, Liang F, Deng S, et al. Sirtuin1 promotes osteogenic differentiation through downregulation of peroxisome proliferator-activated receptor gamma in MC3T3-E1 cells. Biochem Biophys Res Commun (2016) 478(1):439–45. doi: 10.1016/j.bbrc.2016.06.154 [DOI] [PubMed] [Google Scholar]
- 89. Sun W, Qiao W, Zhou B, Hu Z, Yan Q, Wu J, et al. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism (2018) 88:61–71. doi: 10.1016/j.metabol.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 90. Iyer S, Han L, Bartell SM, Kim HN, Gubrij I, de Cabo R, et al. Sirtuin1 (Sirt1) promotes cortical bone formation by preventing beta-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J Biol Chem (2014) 289(35):24069–78. doi: 10.1074/jbc.M114.561803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim H-N, Han L, Iyer S, de Cabo R, Zhao H, O'Brien CA, et al. Sirtuin1 suppresses osteoclastogenesis by deacetylating FoxOs. Mol Endocrinol (2015) 29(10):1498–509. doi: 10.1210/me.2015-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yan S, Miao L, Lu Y, Wang L. Sirtuin 1 inhibits TNF-alpha-mediated osteoclastogenesis of bone marrow-derived macrophages through both ROS generation and TRPV1 activation. Mol Cell Biochem (2019) 455(1-2):135–45. doi: 10.1007/s11010-018-3477-7 [DOI] [PubMed] [Google Scholar]
- 93. Sugatani T, Agapova O, Malluche HH, Hruska KA. SIRT6 deficiency culminates in low-turnover osteopenia. Bone (2015) 81:168–77. doi: 10.1016/j.bone.2015.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Glantschnig H, Scott K, Hampton R, Wei N, McCracken P, Nantermet P, et al. A rate-limiting role for dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J Pharmacol Exp Ther (2011) 338(2):568–78. doi: 10.1124/jpet.111.181404 [DOI] [PubMed] [Google Scholar]
- 95. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med (2007) 13(2):156–63. doi: 10.1038/nm1538 [DOI] [PubMed] [Google Scholar]
- 96. Chen EEM, Zhang W, Ye CCY, Gao X, Jiang LLJ, Zhao TTF, et al. Knockdown of SIRT7 enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly via activation of the wnt/beta-catenin signaling pathway. Cell Death Dis (2017) 8(9):e3042. doi: 10.1038/cddis.2017.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen Z, Djekidel MN, Zhang Y. Distinct dynamics and functions of H2AK119ub1 and H3K27me3 in mouse preimplantation embryos. Nat Genet (2021) 53(4):551–63. doi: 10.1038/s41588-021-00821-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang Y, Yang L, Zhang X, Cui W, Liu Y, Sun QR, et al. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep (2019) 20(7):e47563. doi: 10.15252/embr.201847563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Budhidarmo R, Nakatani Y, Day CL. RINGs hold the key to ubiquitin transfer. Trends Biochem Sci (2012) 37(2):58–65. doi: 10.1016/j.tibs.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 100. Karpiuk O, Najafova Z, Kramer F, Hennion M, Galonska C, Konig A, et al. The histone H2B monoubiquitination regulatory pathway is required for differentiation of multipotent stem cells. Mol Cell (2012) 46(5):705–13. doi: 10.1016/j.molcel.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 101. Najafova Z, Liu P, Wegwitz F, Ahmad M, Tamon L, Kosinsky RL, et al. RNF40 exerts stage-dependent functions in differentiating osteoblasts and is essential for bone cell crosstalk. Cell Death Differ (2021) 28(2):700–14. doi: 10.1038/s41418-020-00614-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. He S, Yang S, Zhang Y, Li X, Gao D, Zhong Y, et al. LncRNA ODIR1 inhibits osteogenic differentiation of hUC-MSCs through the FBXO25/H2BK120ub/H3K4me3/OSX axis. Cell Death Dis (2019) 10(12):947. doi: 10.1038/s41419-019-2148-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Haffner-Luntzer M, Kovtun A, Fischer V, Prystaz K, Hainzl A, Kroeger CM, et al. Loss of p53 compensates osteopenia in murine Mysm1 deficiency. FASEB J (2018) 32(4):1957–68. doi: 10.1096/fj.201700871R [DOI] [PubMed] [Google Scholar]
- 104. Wang M, Wu W, Wu W, Rosidi B, Zhang L, Wang H, et al. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res (2006) 34(21):6170–82. doi: 10.1093/nar/gkl840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Langelier MF, Zandarashvili L, Aguiar PM, Black BE, Pascal JM. NAD(+) analog reveals PARP-1 substrate-blocking mechanism and allosteric communication from catalytic center to DNA-binding domains. Nat Commun (2018) 9(1):844. doi: 10.1038/s41467-018-03234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kraus WL, Lis JT. PARP goes transcription. Cell (2003) 113(6):677–83. doi: 10.1016/s0092-8674(03)00433-1 [DOI] [PubMed] [Google Scholar]
- 107. Wang C, Xiao J, Nowak K, Gunasekera K, Alippe Y, Speckman S, et al. PARP1 hinders histone H2B occupancy at the NFATc1 promoter to restrain osteoclast differentiation. J Bone Miner Res (2020) 35(4):776–88. doi: 10.1002/jbmr.3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Robaszkiewicz A, Qu C, Wisnik E, Ploszaj T, Mirsaidi A, Kunze FA, et al. ARTD1 regulates osteoclastogenesis and bone homeostasis by dampening NF-κB-dependent transcription of IL-1β. Sci Rep (2016) 6(1):21131. doi: 10.1038/srep21131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang C, Qu C, Alippe Y, Bonar SL, Civitelli R, Abu-Amer Y, et al. Poly-ADP-ribosylation-mediated degradation of ARTD1 by the NLRP3 inflammasome is a prerequisite for osteoclast maturation. Cell Death Dis (2016) 7:e2153. doi: 10.1038/cddis.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sasaki Y, Nakatsuka R, Inouchi T, Masutani M, Nozaki T. Inhibition of poly (ADP-ribose) glycohydrolase accelerates osteoblast differentiation in preosteoblastic MC3T3-E1 cells. Int J Mol Sci (2022) 23(9):5041. doi: 10.3390/ijms23095041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baertschi S, Baur N, Lueders-Lefevre V, Voshol J, Keller H. Class I and IIa histone deacetylases have opposite effects on sclerostin gene regulation. J Biol Chem (2014) 289(36):24995–5009. doi: 10.1074/jbc.M114.564997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim H-N, Lee J-H, Bae S-C, Ryoo H-M, Kim H-H, Ha H, et al. Histone deacetylase inhibitor MS-275 stimulates bone formation in part by enhancing Dhx36-mediated TNAP transcription. J Bone Mineral Res (2011) 26(9):2161–73. doi: 10.1002/jbmr.426 [DOI] [PubMed] [Google Scholar]