Abstract
Oligonucleotide therapeutics, drugs consisting of 10–50 nucleotide‐long single‐ or double‐stranded DNA or RNA molecules that can bind to specific DNA or RNA sequences or proteins, include antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), microRNAs (miRNAs), aptamers, and decoys. These oligonucleotide therapeutics could potentially become the third pillar of drug development. In particular, ASOs and siRNAs are advanced tools that are widely used to silence gene expression. They are used in clinical trials, as they have high specificity for target mRNAs and non‐coding RNAs and limited toxicity. However, their clinical application remains challenging. Although chemotherapy has benefits, it has severe adverse effects in many patients. Therefore, new modalities for targeted molecular therapy against tumors, including oligonucleotide therapeutics, are required, and they should be compatible with diagnosis using next‐generation sequencing. This review provides an overview of the therapeutic uses of ASOs, siRNAs, and miRNAs in clinical studies on malignant tumors. Understanding previous research and development will help in developing novel oligonucleotide therapeutics against malignant tumors.
Keywords: antisense oligonucleotide, drug delivery, microRNA, oligonucleotide therapeutics, small interfering RNA
Antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) are widely‐used advanced tools for silencing gene expression, and even suitable for targets that are not druggable via other therapeutic modalities. However, their application in clinical studies is limited because of the off‐target effects, and poor accumulation at their target sites and cells. Drug delivery systems (DDSs) for oligonucleotides play an important role in overcoming these difficulties.
Abbreviations
- ASO
antisense oligonucleotide
- AGO2
argonaute 2
- DDS
drug delivery system
- HCC
hepatocellular carcinoma
- LNP
lipid nanoparticle
- miRNA
microRNA
- NSCLC
non‐small cell lung cancer
- PDAC
pancreatic ductal adenocarcinoma
- PLGA
poly (lactic‐co‐glycolic) acid
- RISC
RNA‐induced silencing complex
- SSO
splice switching oligonucleotide
- VEGF
vascular endothelial growth factor
- SNALP
stable nucleic acid lipid particle
1. INTRODUCTION
Oligonucleotide therapy involves the use of drugs consisting of single‐stranded DNA or RNA that can bind to specific sequences of DNA, RNA, or protein and inhibit gene expression or intercept protein functions. Oligonucleotide therapeutics include antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), microRNAs (miRNAs), aptamers, and decoys. Antisense oligonucleotides and siRNAs are widely used advanced tools to silence gene expression because of their high specificity and limited toxicity. Oligonucleotides are even suitable for targets that are not druggable via other therapeutic modalities; thus, oligonucleotide therapeutics have the potential to become the third pillar of drug development.
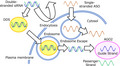
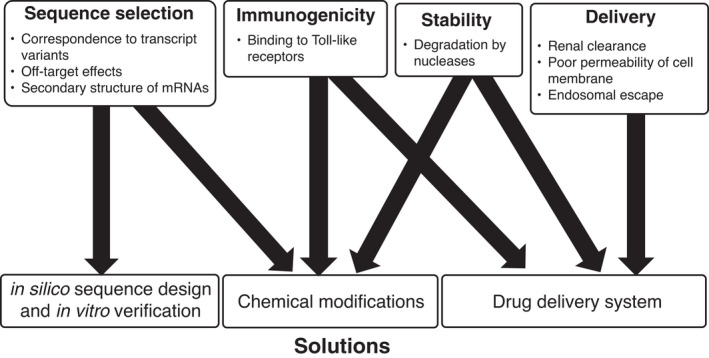
The advantages of oligonucleotide therapeutics include the simple design of the drug constructs based only on the genomic sequences of target genes, and they require less time for development. However, their applications in clinical studies are limited because of the off‐target effects of ASO and siRNA sequences and poor stability due to the degradation by ribonucleases when injected systemically. They also activate the innate immune system via Toll‐like receptors. ASO–mRNA interactions depending on mRNA structure, thermodynamic stability, and hybridization site position have been well understood; however, design methods of ASO sequence have not been established. Design methods for the siRNA sequence are important to reduce potential off‐target effects in siRNA, and the homology of the seed region of siRNA should be minimized to the 3' UTR of non‐target mRNA. Chemical modifications, such as the introduction of phosphorothioate backbones and sugar‐modified nucleic acids, including 2‐O‐methyl, 2‐O‐methoxyethyl, 2‐flouro, locked nucleic acid (LNA), and bridged nucleic acids (BNA), increase not only resistance to nuclease degradation and avoid immunologic reactions but also prevent toxicity. 1 Therapeutic oligonucleotides are filtered by the kidneys because of their small size, resulting in poor accumulation in their target sites. Furthermore, ASOs of high concentrations can penetrate the lipophilic cell membranes. However, siRNAs cannot completely diffuse across them. Drug delivery systems (DDSs) for oligonucleotides play an important role in overcoming these difficulties (Figures 1 and 2). After the penetration of ASOs and siRNAs into the cell membrane, they must escape from endosomes to reach their target RNAs in the cytosol. siRNAs are loaded onto Argonaute2 (Ago2), which is a component of RNA‐induced silencing complexes (RISCs) (Figure 2). 1 , 2 Although ASOs and siRNAs possess considerable therapeutic potential, complete remission cannot be achieved with only a single ASO or siRNA, as cancers and tumors are heterogeneous.
FIGURE 1.
Strategies to overcome difficulties associated with oligonucleotide therapeutics
FIGURE 2.
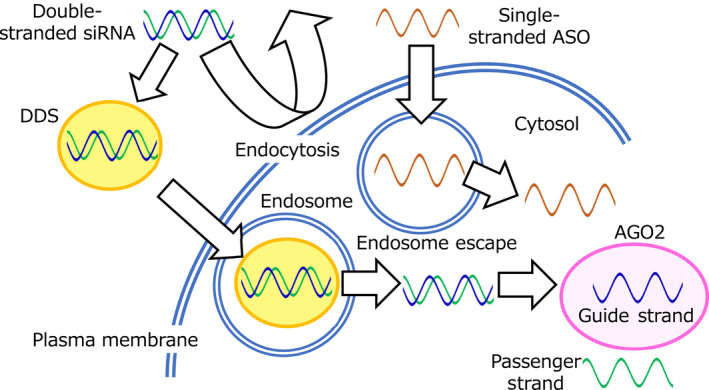
Drug delivery system (DDS) for oligonucleotide therapeutics
Herein, we focused on ASO and siRNA drugs currently used to treat malignant tumors, mainly in clinical trials. Understanding oligonucleotide therapeutics will help develop novel therapeutic strategies against tumors.
2. ANTITUMORAL ANTISENSE OLIGONUCLEOTIDES
Antisense oligonucleotides are 12–25 nucleotide‐long single‐stranded DNA molecules. They modulate the function of their RNA targets via several mechanisms, such as RNase H‐mediated degradation, mRNA modification, and miRNA inhibition. 3
Antisense oligonucleotides are classified by their mechanisms of action into (a) gapmers, which are composed of DNA antisense with chemically modified RNA segments on both sides of the sequence, and act as substrates for RNase H (Figure 3A); (b) RNase H‐independent splice switching oligonucleotides (SSOs), which bind to pre‐mRNAs and disrupt their recognition by splicing factors 4 ; and (c) modified ASOs, which inhibit miRNA function. 5 The ASO drugs used to treat malignant tumors in clinical trials are as follows (Table 1).
FIGURE 3.
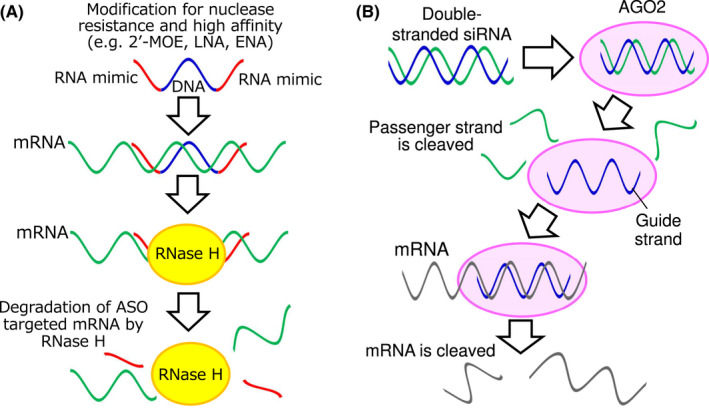
Modes of action of antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs). (A) Gapmer‐type ASO binds to the target RNA and forms the RNA/DNA heteroduplex in the central gap region, and RNase H cleaves the RNA strand of the heteroduplex. (B) Effect of siRNA. siRNA is composed of two complementary strands, the passenger strand and the guide strand. The latter binds to AGO2, inducing the degradation of complementary mRNA
TABLE 1.
List of antisense oligonucleotides (ASOs) in clinical trials
| Development product name | Target | Application | Stage | Development company/University | NCT# |
|---|---|---|---|---|---|
| Ongoing clinical trials | |||||
| AZD4785/IONIS‐KRAS‐2.5Rx | KRAS | Advanced solid tumors | Phase I | AstraZeneca | NCT03101839 |
| AZD5312/IONIS‐AR‐2.5RX/ARRx | Androgen receptor (AR) | Cancer – prostatic | Phase I/II | University of Michigan Rogel Cancer Center | NCT03300505 NCT02144051 |
| BP1001/Prexigebersen | GRB‐2 | Cancer | Phase I/II | Bio‐Path Holdings | NCT04196257 |
| NCT02923986 | |||||
| NCT02781883 | |||||
| NCT01159028 | |||||
| BP1002 | Bcl‐2 | Advanced lymphoid malignancy | Phase I | Bio‐Path Holdings | NCT04072458 |
| EZN‐2968/Anti‐HIF‐1a/LNA AS ODN | HIF‐1a | Solid tumor | Phase I | National Cancer Institute (NCI) | NCT01120288 |
| NCT00466583 | |||||
| NCT02564614 | |||||
| EZN‐4176 | AR exon 4 | Prostatic neoplasm | Phase I | Enzon Pharmaceuticals | NCT01337518 |
| G4460/C‐MYB asODN | C‐Myb | Hematological malignancy | Phase II | Abramson Cancer Center of the University of Pennsylvania | NCT00002592 |
| GRN163L/Imetelstat® | RNA component of telomerase | Cancer | Phase II/III | Geron Corporation | NCT02598661 |
| GTI‐2040 | R2 component of R2 | Cancer | Phase II | Aptose Biosciences | NCT00565058 |
| NCT00068588 | |||||
| NCT00087165 | |||||
| IGV‐001/IGF‐1R/AS ODN | IGF‐1R | Glioblastoma | Phase I Phase II | David Andrews, Thomas Jefferson University | NCT02507583 |
| NCT01550523 NCT04485949 | |||||
| IONIS‐STAT3RX/AZD9150/Danvatirsen | STAT3 | Cancer | Phase I/II | Ionis Pharmaceuticals | NCT01563302 NCT02417753 |
| ISIS 5132/CGP69846A | C‐Raf‐1 | Ovarian/breast cancer | Phase II | Eastern Cooperative Oncology Group | NCT00003236 |
| NCT00003892 | |||||
| LErafAON‐ETU | C‐Raf‐1 | Cancer | Phase I | INSYS Therapeutics | NCT00100672 |
| LY900003/ISIS 3521 | PKC‐α | Cancer | Phase III | Ionis Pharmaceuticals | NCT00017407 |
| NCT00034268 | |||||
| MRG‐106/Cobomarsen | miR‐155 | Cutaneous T‐cell lymphoma/mycosis fungoides | Phase I/II | miRagen Therapeutics | NCT02580552 |
| NCT03713320 | |||||
| NCT03837457 | |||||
| OGX‐011/Custirsen | Clusterin | Cancer | Phase III | Achieve Life Sciences | NCT01188187 |
| NCT01578655 | |||||
| OGX‐427/Apatorsen | HSP27 | Cancer | Phase II | British Columbia Cancer Agency | NCT01120470 |
| NCT02423590 | |||||
| NCT01829113 NCT01780545 | |||||
| NCT01454089 | |||||
| RO7070179 | HIF‐1a | Hepatocellular carcinoma | Phase I | Hoffmann‐La Roche | NCT02564614 |
| SPC2996 | Bcl‐2 | Chronic lymphocytic leukemia | Phase I/II | Santaris Pharma A/S | NCT00285103 |
| Discontinued and terminated clinical trials | |||||
| AEG35156 | XIAP | Cancer | Phase II | Aegera Therapeutics | NCT00363974 |
| Multiple studies terminated | NCT00882869 NCT00558545 NCT00557596 | ||||
| EL625/Cenersen/Aezeaa | P53 | Myelodysplastic syndromes/Leukemia/Lymphoma | Phase II, Terminated | Eleos | NCT00074737 |
| G3139/Oblimersen/Genasenseb | Bcl‐2 | Cancer/Waldenstroms Macroglobulinemia | Phase II/III, Terminated | M.D. Anderson Cancer Center | NCT00030641 NCT00017602 NCT00016263 NCT00024440 NCT01200342 |
| NCT00543205 | |||||
| VEGF‐AS/Veglinb | VEGF | Mesothelioma | Phase I/II, Terminated | University of Southern California | NCT00668499 |
Note: Termination reasons are (a) lack of funding and (b) withdrawn from business.
AZD9150 (danvatirsen) is an STAT3 ASO. In heavily pretreated patients with diffuse large B‐cell lymphoma (DLBCL), AZD9150 was well tolerated and demonstrated efficacy. 6 In a phase 1b trial of patients with relapsed/refractory DLBCL, AZD9150 plus durvalumab helped achieve the primary endpoint and was well tolerated; however, its antitumor activity was limited. 7
BP1001 is an ASO for liposomal growth factor receptor‐bound protein 2 (Grb2). In a phase 1/1b trial of patients with relapsed/refractory hematological malignancies, BP1001 exhibited antitumor activity in combination with low‐dose cytarabine. 8
EZN‐4176 binds to the hinge region (exon 4) of androgen receptor mRNA. In a phase 1 study of patients with castration‐resistant prostate cancer (CRPC), EZN‐4176 activity was minimal. 9
GRN163L (imetelstat) is a telomerase inhibitor targeting the template region of functional telomerase RNA subunits. In a phase 2 study of patients with intermediate‐2 or high‐risk myelofibrosis relapsed/refractory to Janus‐associated kinase inhibitors, GRN163L improved the symptom response rate and bone marrow fibrosis. 10 In a phase 2 trial of patients with low‐risk myelodysplastic syndromes, GRN163L resulted in a meaningful and durable transfusion independence rate. 11 In a phase 2 study of patients with advanced non‐small cell lung cancer (NSCLC), GRN163N did not improve progression‐free survival (PFS). However, there was a trend toward improvement in the median PFS and overall survival (OS) in patients with short telomeres. 12 In a phase 2 study of children with recurrent central nervous system tumors, GRN163N demonstrated intratumoral and peripheral blood mononuclear cell target inhibition, although the regimen was toxic. 13
GTI‐2040 is an ASO of the R2 subunit of ribonucleotide reductase (RNR). The prostate‐specific antigen (PSA) response rate in a phase 2 trial of GTI‐2040 plus docetaxel/prednisone for patients with CRPC met the minimum phase 2 criteria for further enrolment. 14 In another phase 2 trial of patients with previously treated NSCLC, the activity of GTI‐2040 plus docetaxel was not superior to that of docetaxel alone. 15 In a phase 1 study of patients with advanced solid tumors, although GTI‐2040 plus gemcitabine showed no clear evidence of antitumor activity, several patients had prolonged stable disease (SD). 16
IMV‐001 is an ASO against type‐1 insulin‐like growth factor receptor (IGF‐1R). Both IGF ligands and their receptor IGF‐1R are overexpressed in several tumors. IGFs stimulate the proliferation and prevent the apoptosis of cancer cells. Thus, IGF signaling is also critical in tumor dissemination in addition to carcinogensis. Previously, we reported that anti‐IGF‐1R therapies may be a potential treatment strategy for several cancers. 17 , 18 In a phase 1 trial of patients with newly diagnosed glioblastoma, IGV‐001, which combines autologous tumor cells and IMV‐001, was well tolerated. The PFS of patients on IGV‐001 was longer than that of patients in the standard of care arms, and the immune system was stimulated by IGV‐001. 19
ISIS 3521 inhibits the expression of protein kinase C‐alpha (PKCα), which presents increased expression in tumor tissues and is implicated in malignant transformation, proliferation, and anti‐apoptosis. Inhibition of PKCα has been reported to arrest the growth of several tumors. ISIS 3521 has demonstrated anti‐tumor activity in a phase 2 study of patients with relapsed low‐grade NHL. 20 ISIS 3521 plus cisplatin/gemcitabine exhibited antitumor activity in a phase 2 trial of patients with advanced NSCLC. 21 However, neither ISIS 3521 nor ISIS 5132, which inhibits the expression of PKC‐alpha and Raf‐1, showed clinically significant single‐agent antitumor activity in a phase 2 trial of patients with chemotherapy‐naive CRPC 22 or those with untreated colorectal cancer (CRC). 23
OGX011 (custirsen) inhibits the production of clusterin, the secretory isoform of which protects the cancer cells from apoptosis induced by cellular stress, such as chemotherapy, radiotherapy, or androgen/estrogen depletion. Researchers have revealed that clusterin promotes cell survival via the inhibition of BAX and activation of the PI3K pathway or ERK 1/2 signaling in several cancers. Inhibition of clusterin in cancer cells induces a significant reduction in cell growth and apoptosis; additionally, it increases the effectiveness of chemo drugs via p53 activation. In a phase 3 trial of patients with metastatic CRPC, OGX011 plus cabazitaxel/prednisone provided no survival benefits. 24 In a phase 1/2 trial of patients with untreated advanced NSCLC, 31% patients on OGX011 plus gemcitabine/platinum showed an overall response, and the 2‐year survival rate of the patients was 30%. OGX011 decreased the serum clusterin level. Patients with low clusterin levels presented longer median survival. 25 In a phase 2 trial of patients with metastatic breast cancer, OGX011 plus docetaxel was well tolerated and clinical activity was observed, although the number of responses to meet the criteria for the next stage was insufficient. 26
OGX‐427 (apatorsen) is an ASO for heat shock protein 27 (HSP27). In a phase 2 study of patients with CRPC, OGX‐427 plus prednisone failed to alter the proportion of patients without disease progression compared with prednisone alone but significantly decreased PSA. 27 In another phase 2 trial of patients with metastatic pancreatic ductal adenocarcinoma (PDAC) in the first‐line setting, OGX‐427 plus chemotherapy did not improve outcomes, although a trend toward prolonged PFS and OS in patients with high baseline serum HSP27 level was observed. 28 In a phase 2 trial for patients with untreated metastatic non‐squamous NSCLC, OGX‐427 plus carboplatin/pemetrexed, although well tolerated, did not improve outcomes. 29
3. ANTITUMORAL SMALL INTERFERING RNAS
RNA interference is a conserved biological response that causes sequence‐specific gene silencing via 21‐bp dsRNAs known as siRNAs. siRNAs are composed of two complementary strands, passenger (sense) and guide (antisense) strands. The guide strand binds to AGO2 and is activated through the RISC, leading to the degradation of complementary mRNA 4 (Figure 3B). The siRNA drugs used to treat malignant tumors, mainly in clinical phases, are as follows (Table 2).
TABLE 2.
List of siRNAs in clinical trials
| Development product name | Target | DDS | Application | Stage | Development company/hospital | NCT# and others |
|---|---|---|---|---|---|---|
| Ongoing clinical trials | ||||||
| ALN‐VSP02 | KSP/VEGF | SNALP | Hepatocellular carcinoma | Phase I | Alnylam Pharmaceuticals | NCT00882180 |
| NCT01158079 | ||||||
| APN 401 | Cbl‐b | none (in vitro electroporation) | Solid tumors | Phase I | APEIRON Biologic | NCT03087591 |
| ARO‐HIF2 | HIF2α | Undisclosed (TRiM™ platform) | Clear cell renal cell carcinoma | Phase I | Arrowhead Pharmaceuticals | NCT04169711 |
| Atu027 | PKN3 | AtuPLEX | Pancreatic carcinoma | Phase Ib/IIa | Silence Therapeutics | NCT00938574 |
| NCT01808638 | ||||||
| EPHARNA | EphA2 | DOPC | Solid tumors | Phase I | M.D. Anderson Cancer Center | NCT01591356 |
| iExosomes | KRASG12D | Exosome | Pancreatic Cancer | Phase I | M.D. Anderson Cancer Center | NCT03608631 |
| NBF‐006 | GST‐π | LNP | NSCLC | Phase I | Nitto BioPharma | NCT03819387 |
| NU‐0129 | BCL2L12 | Gold core SNP | Recurrent glioblastoma/gliosarcoma | Early Phase I | Northwestern University | NCT03020017 |
| siG12D‐LODER | KRASG12D | LODER (bio‐degradable matrix) | Pancreatic cancer/pancreatic ductal carcinoma | Phase II | Silenseed | NCT01676259 |
| SRN‐14 | PRDM14 | PEG‐PLO | Breast cancer | Phase I | The Cancer Institute Hospital Of JFCR | (jRCT2031190181) |
| STNM01 | CHST15 | ‐ | Pancreatic cancer | Phase IIa | Keio University | (jRCT2031190055) |
| STP705 | TGF‐β1/COX‐2 | Polypeptide nanoparticle (PNP) | Hepatocellular carcinoma /isSCC, Bowen's disease | Phase I/II | Sirnaomics | NCT04676633 |
| NCT04293679 | ||||||
| NCT04844983 | ||||||
| SXL01 | AR | Undisclosed | Prostate cancer | Phase I | Institut Claudius Regaud | NCT02866916 |
| TKM‐080301 | PLK1 | SNALP | Hepatocellular carcinoma | Phase I/II | Arbutus Biopharma | NCT02191878 |
| NCT01262235 | ||||||
| Discontinued and terminated clinical trials | ||||||
| Development product name | Target | DDS | Application | Stage | Development company/hospital | NCT# and others |
| CALAA‐01 | RRM2 | CAL101/AD‐PEG/AD‐PEG‐Tf | Solid tumors | Phase I, terminated | Calando Pharmaceuticals | NCT00689065 |
| DCR‐MYCa | c‐Myc | LNP | Hepatocellular carcinoma | Phase Ib/2, terminated | Dicerna Pharmaceuticals | NCT02314052 |
| Nek2 siRNA | Nek2 | Atelocollagen | Pancreatic Cancer | Phase I, terminated | Nagoya University | (UMIN000016330) |
| TDM‐812b | RPN2 | A6K | Breast cancer | Phase I, terminated | St. Luke’s International Hospital | (jRCT2031200057) |
Note: Termination reasons are (a) withdrawn from business and (b) COVID‐19 pandemic.
ALN‐VSP02 is a lipid nanoparticle formulation containing two chemically modified siRNAs against kinesin spindle protein and vascular endothelial growth factor (VEGF), with stable nucleic acid lipid particles (SNALPs) as DDSs and is introduced intravenously. In a phase 1 trial for treating hepatocellular carcinoma (HCC) and other tumors with liver involvement, 8.3% of patients receiving doses ≤0.4 mg/kg presented SD for at least 2 months and 46.6% of patients receiving doses ≥0.7 mg/kg presented SD or PR. In particular, human tissue samples showed RNAi‐mediated target mRNA cleavage. 30
ARO‐HIF2 is composed of HIF2 siRNA targeting HIF2α and uses a proprietary targeted‐RNAi molecule (TRiM) delivery platform, which comprises targeting ligands, such as RGD motifs designed to transport siRNA to cancer cells. ARO‐HIF2 use resulted in HIF2α mRNA knockdown, tumor growth inhibition, and OS improvement in a xenograft model of clear cell renal cell carcinoma, frequently involving the inactivation of the von Hippel–Lindau tumor suppressor, leading to the accumulation of HIFs. 31 Arrowhead Pharmaceuticals reported that seven of nine tumor samples had a low HIF2α level, and one patient achieved partial response with tumor shrinkage of approximately 65% in a phase 1 study. 32
Atu027 is a liposomal siRNA formulation targeting human PKN3, which acts as a Rho effector downstream of phosphoinositide 3‐kinase signaling, with AtuPLEX comprising three types of lipids. 33 In a phase 1 study of patients with advanced solid tumors, 41% patients had SD for at least 8 weeks. 34 Combination of Atu027 with gemcitabine for the treatment of advanced PDAC in a phase 1b/2a study was safe, and twice‐weekly Atu027 dosing was observably superior to the once‐weekly regimen. 35
EPHARNA, an EphA2 siRNA incorporated into 1,2‐dioleoyl‐sn‐glycero‐3‐phosphocholine (DOPC) nanoliposomes, has been highly effective in reducing the EphA2 level in ovarian cancer cells in vivo. 36 Patients with advanced recurrent solid tumors were enrolled and treated in the dose‐escalation phase (Table 2). 37
iExosomes, exosomes expressing CD47, purified from human fibroblast cultures and electroporated to introduce the siRNA targeting KRASG12D, most prevalent in PDAC, were used in a preclinical trial. 38 , 39 In a phase 1 trial, participants with metastatic PDAC with KRASG12D were treated with mesenchymal stromal cell‐derived exosomes with iExosomes (Table 2).
NBF‐006 is an lipid nanoparticle (LNP) formulation delivering an encapsulated siRNA that inhibits the expression of glutathione‐S‐transferase P (GST‐π). GST‐π weakens the efficacy of chemotherapeutic drugs by promoting their in vitro extrusion and functions as an MAPK‐pathway inhibitor to prevent the apoptosis of cells with KRAS and BRAF mutations. NBF‐006 significantly inhibits tumors in KRAS‐mutant animal models of NSCLC. 40 Patients with progressive/metastatic NSCLC, PDAC, or CRC will be prescribed dose escalation, and those with previously treated KRAS‐mutated NSCLC under a high dose will be subjected to dose expansion in a clinical trial (Table 2).
NU‐0129 is a gold‐base spherical nucleic acid nanoconjugate siRNA against BCL2‐like protein 12 (BCL2L12), an anti‐apoptotic factor expressed in glioma cells. NU‐0129 can permeate the blood–brain and blood–tumor barriers and reach glioblastoma cells. In an early phase 1 study of patients with recurrent glioblastoma, followed by tumor resection, NU‐0129 uptake into glioma cells correlated with the reduction in tumor‐associated BCL2L12 expression. 41
siG12D‐LODER, a miniature biodegradable matrix, is a copolymer of high‐molecular weight poly (lactic‐co‐glycolic) acid (PLGA) encompassing a novel siRNA targeting KRASG12D and all additional G12 mutations. 42 It is placed in PDAC using an endoscopic ultrasound biopsy procedure. In an open‐label phase 1/2a study, siG12D‐LODER plus gemcitabine or modified FOLFIRINOX was well tolerated and safe, and it demonstrated potential efficacy in patients with locally advanced PDAC. 43
SRN‐14 is a PRDM14‐specific double‐stranded RNA/DNA chimera combined with a novel branched PEGylated polyaminoacid‐based intravenous DDS. 44 PRDM14 is expressed at considerable levels in several cancers but is not expressed in normal tissues; it confers stem cell‐like properties to cancer cells. 45 , 46 The use of PEGylated poly‐L‐ornithine, a novel DDS, leads to the accumulation of the siRNA in target cancer tissues, rather than the liver and spleen, owing to its narrow and monodisperse size distribution. This siRNA drug reduced the size of inoculated tumors, suppressed distant metastasis, relieved chemo‐drug resistance, and improved prognosis in nude mice. 44 Our package of pre‐clinical tests and production of an investigational new drug using GMP‐grade active pharmaceutical ingredients has been approved by PMDA. A physician‐initiated phase 1 trial of patients with triple‐negative breast cancer has been started (Table 2).
STNM01 selectively inhibits the expression of carbohydrate sulfotransferase 15, an enzyme that promotes tumor invasion and correlates with a poor prognosis in PDAC. 47 Repeated endoscopic ultrasonography‐guided fine‐needle injection of STNM01 in PDAC as a second‐line treatment was safe and feasible in a phase 1/2a study. The 6‐month survival rate of patients treated with 10,000 nM STNM01 was 83.3%. 48
STP705 is composed of siRNA oligonucleotides targeting TGFB1 and COX‐2 mRNAs formulated with nanoparticles containing a unique histidine‐lysine copolymer peptide. 49 , 50 Clinical trials for STP705 with intravenous or intralesional administration in patients with advanced/metastatic solid tumors in phase 1 or cutaneous squamous cell carcinoma (in situ) in phase 2 are currently underway.
TKM‐080301 is an SNALP formulation against Polo‐like kinase 1, a serine/threonine kinase associated with poor prognoses. Phase 1/2 studies for adrenocortical cancer and advanced HCC have been conducted; preliminary antitumor efficacy has been observed. 51 , 52
4. CONCLUDING REMARKS
Antisense oligonucleotides and siRNAs are not commercially available for cancer treatment, and several problems discussed in the review impede their use in oligonucleotide therapy.
Oligonucleotide therapeutics must be based on biological engineering, such as nucleic acid chemistry, especially related to stability in vivo, and avoidance of off‐target effects, focusing on DDS. Due to space limitations, we referred to recent advances and problems of DDS in nucleic acid medicine with an actual case of siRNA drugs for patients with hereditary transthyretin amyloidosis developed by Alnylam Pharmaceuticals. LNPs have been used as a DDS of patisiran targeting transthyretin mRNA via intravenous administration once every 2 weeks. LNPs accumulate in the liver in an apolipoprotein E‐dependent manner. Later, vutrisiran was developed using enhanced stabilization chemistry technology for the modification of siRNA‐conjugated GalNAc via subcutaneous administration once every 3 months. GalNAc binds to the liver‐expressed asialoglycoprotein receptor 1 with high affinity. Therefore, drug efficacy of ligand conjugation with chemically modified siRNA is more effective than that of LNPs. However, the accumulation of siRNA drugs in target lesions except the liver has not been successful. Ligand‐conjugated siRNAs or new types of DDS, such as PEGylated poly‐L‐ornithine, have the potential to solve this difficulty. Consequently, researchers will be able to overcome the existing problems regarding the application of oligonucleotide therapeutics in the clinical context.
AUTHOR CONTRIBUTIONS
H.T. is a supervisor, contributed to the concept, and wrote and edited the manuscript. Y.A. contributed to the concept and wrote and edited the manuscript. Y.S edited the figures and tables. K.I. is a supervisor and edited the manuscript. All authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
Hiroaki Taniguchi has received research funding from NanoCarrier Co., Ltd. The other authors declare no potential conflicts of interest.
ACKNOWLEDGMENTS
We thank Editage (www.editage.jp) for English language editing.
Taniguchi H, Suzuki Y, Imai K, Adachi Y. Antitumoral RNA‐targeted oligonucleotide therapeutics: The third pillar after small molecule inhibitors and antibodies. Cancer Sci. 2022;113:2952‐2961. doi: 10.1111/cas.15461
Funding information
This work was partially supported by the Japan Agency for Medical Research and Development (AMED; 21ae0121031h0001), the Japanese Society of Gastroenterology, Sendai Kousei Hospital, the Japan Society for the Promotion of Science KAKENHI Grant (21 K08801), and the Princess Takamatsu Cancer Research Fund.
REFERENCES
- 1. Adachi H, Hengesbach M, Yu YT, Morais P. From antisense RNA to RNA modification: therapeutic potential of RNA‐based technologies. Biomedicine. 2021;9(5):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35(3):222‐229. [DOI] [PubMed] [Google Scholar]
- 3. Hagedorn PH, Hansen BR, Koch T, Lindow M. Managing the sequence‐specificity of antisense oligonucleotides in drug discovery. Nucleic Acids Res. 2017;45(5):2262‐2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46(4):1584‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ariyoshi J, Momokawa D, Eimori N, Kobori A, Murakami A, Yamayoshi A. Development of novel antisense oligonucleotides for the functional regulation of RNA‐induced silencing complex (RISC) by promoting the release of microRNA from RISC. Bioconjug Chem. 2015;26(12):2454‐2460. [DOI] [PubMed] [Google Scholar]
- 6. Reilley MJ, McCoon P, Cook C, et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J Immunother Cancer. 2018;6(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ribrag V, Lee ST, Rizzieri D, et al. A phase 1b study to evaluate the safety and efficacy of durvalumab in combination with tremelimumab or danvatirsen in patients with relapsed or refractory diffuse large B‐cell lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(5):309‐317. e3. [DOI] [PubMed] [Google Scholar]
- 8. Ohanian M, Tari Ashizawa A, Garcia‐Manero G, et al. Liposomal Grb2 antisense oligodeoxynucleotide (BP1001) in patients with refractory or relapsed haematological malignancies: a single‐centre, open‐label, dose‐escalation, phase 1/1b trial. Lancet Haematol. 2018;5(4):e136‐e146. [DOI] [PubMed] [Google Scholar]
- 9. Bianchini D, Omlin A, Pezaro C, et al. First‐in‐human phase I study of EZN‐4176, a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen receptor mRNA in patients with castration‐resistant prostate cancer. Br J Cancer. 2013;109(10):2579‐2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mascarenhas J, Komrokji RS, Palandri F, et al. Randomized, single‐blind, multicenter phase II study of two doses of imetelstat in relapsed or refractory myelofibrosis. J Clin Oncol. 2021;39(26):2881‐2892. [DOI] [PubMed] [Google Scholar]
- 11. Steensma DP, Fenaux P, Van Eygen K, et al. Imetelstat achieves meaningful and durable transfusion independence in high transfusion‐burden patients with lower‐risk myelodysplastic syndromes in a phase II study. J Clin Oncol. 2021;39(1):48‐56. [DOI] [PubMed] [Google Scholar]
- 12. Chiappori AA, Kolevska T, Spigel DR, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non‐small‐cell lung cancer. Ann Oncol. 2015;26(2):354‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salloum R, Hummel TR, Kumar SS, et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J Neurooncol. 2016;129(3):443‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sridhar SS, Canil CM, Chi KN, et al. A phase II study of the antisense oligonucleotide GTI‐2040 plus docetaxel and prednisone as first‐line treatment in castration‐resistant prostate cancer. Cancer Chemother Pharmacol. 2011;67(4):927‐933. [DOI] [PubMed] [Google Scholar]
- 15. Leighl NB, Laurie SA, Chen XE, et al. A phase I/II study of GTI‐2040 plus docetaxel as second‐line treatment in advanced non‐small cell lung cancer: a study of the PMH phase II consortium. J Thorac Oncol. 2009;4(9):1163‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malik L, Zwiebel A, Cooper J. A phase I pharmacokinetic and pharmacodynamic study of GTI‐2040 in combination with gemcitabine in patients with solid tumors. Cancer Chemother Pharmacol. 2018;82(3):533‐539. [DOI] [PubMed] [Google Scholar]
- 17. Adachi Y, Lee CT, Coffee K, et al. Effects of genetic blockade of the insulin‐like growth factor receptor in human colon cancer cell lines. Gastroenterology. 2002;123(4):1191‐1204. [DOI] [PubMed] [Google Scholar]
- 18. Matsunaga Y, Adachi Y, Sasaki Y, et al. The effect of forced expression of mutated K‐RAS gene on gastrointestinal cancer cell lines and the IGF‐1R targeting therapy. Mol Carcinog. 2017;56(2):515‐526. [DOI] [PubMed] [Google Scholar]
- 19. Andrews DW, Judy KD, Scott CB, et al. Phase Ib clinical trial of IGV‐001 for patients with newly diagnosed glioblastoma. Clin Cancer Res. 2021;27(7):1912‐1922. [DOI] [PubMed] [Google Scholar]
- 20. Rao S, Watkins D, Cunningham D, et al. Phase II study of ISIS 3521, an antisense oligodeoxynucleotide to protein kinase C alpha, in patients with previously treated low‐grade non‐Hodgkin's lymphoma. Ann Oncol. 2004;15(9):1413‐1418. [DOI] [PubMed] [Google Scholar]
- 21. Villalona‐Calero MA, Ritch P, Figueroa JA, et al. A phase I/II study of LY900003, an antisense inhibitor of protein kinase C‐alpha, in combination with cisplatin and gemcitabine in patients with advanced non‐small cell lung cancer. Clin Cancer Res. 2004;10:6086‐6093. [DOI] [PubMed] [Google Scholar]
- 22. Tolcher AW, Reyno L, Venner PM, et al. A randomized phase II and pharmacokinetic study of the antisense oligonucleotides ISIS 3521 and ISIS 5132 in patients with hormone‐refractory prostate cancer. Clin Cancer Res. 2002;8(8):2530‐2535. [PubMed] [Google Scholar]
- 23. Cripps MC, Figueredo AT, Oza AM, et al. Phase II randomized study of ISIS 3521 and ISIS 5132 in patients with locally advanced or metastatic CRC: a National Cancer Institute of Canada clinical trials group study. Clin Cancer Res. 2002;8(7):2188‐2192. [PubMed] [Google Scholar]
- 24. Beer TM, Hotte SJ, Saad F, et al. Custirsen (OGX‐011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration‐resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open‐label, international, phase 3 trial. Lancet Oncol. 2017;18(11):1532‐1542. [DOI] [PubMed] [Google Scholar]
- 25. Laskin JJ, Nicholas G, Lee C, et al. Phase I/II trial of custirsen (OGX‐011), an inhibitor of clusterin, in combination with a gemcitabine and platinum regimen in patients with previously untreated advanced non‐small cell lung cancer. J Thorac Oncol. 2012;7(3):579‐586. [DOI] [PubMed] [Google Scholar]
- 26. Chia S, Dent S, Ellard S, et al. Phase II trial of OGX‐011 in combination with docetaxel in metastatic breast cancer. Clin Cancer Res. 2009;15(2):708‐713. [DOI] [PubMed] [Google Scholar]
- 27. Yu EY, Ellard SL, Hotte SJ, et al. A randomized phase 2 study of a HSP27 targeting antisense, apatorsen with prednisone versus prednisone alone, in patients with metastatic castration resistant prostate cancer. Invest New Drugs. 2018;36(2):278‐287. [DOI] [PubMed] [Google Scholar]
- 28. Ko AH, Murphy PB, Peyton JD, et al. A randomized, double‐blinded, phase II trial of gemcitabine and nab‐paclitaxel plus apatorsen or placebo in patients with metastatic pancreatic cancer: the RAINIER trial. Oncologist. 2017;22(12):1427‐e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spigel DR, Shipley DL, Waterhouse DM, et al. A randomized, double‐blinded, phase II trial of carboplatin and pemetrexed with or without apatorsen (OGX‐427) in patients with previously untreated stage IV non‐squamous‐non‐small‐cell lung cancer: the SPRUCE trial. Oncologist. 2019;24(12):e1409‐e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tabernero J, Shapiro GI, LoRusso PM, et al. First‐in‐humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406‐417. [DOI] [PubMed] [Google Scholar]
- 31. Wong SC, Nicholas A, Carlson J, et al. Optimizing the potency and dosing design for ARO‐HIF2: an RNAi therapeutic for clear cell renal cell carcinoma. Cancer Res. 2019;79(13):4775. [Google Scholar]
- 32. Arrowhead Pharmaceuticals, Inc. Arrowhead announces positive interim results from Phase 1b Study of ARO‐HIF2 for Treatment of Clear Cell Renal Cell Carcinoma. Accessed Febrary 4, 2022. https://ir.arrowheadpharma.com/news‐releases/news‐release‐details/arrowhead‐announces‐positive‐interim‐results‐phase‐1b‐study‐aro
- 33. Aleku M, Schulz P, Keil O, et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68(23):9788‐9798. [DOI] [PubMed] [Google Scholar]
- 34. Schultheis B, Strumberg D, Santel A, et al. First‐in‐human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32(36):4141‐4148. [DOI] [PubMed] [Google Scholar]
- 35. Schultheis B, Strumberg D, Kuhlmann J, et al. Safety, efficacy and pharcacokinetics of targeted therapy with the liposomal RNA interference therapeutic Atu027 combined with gemcitabine in patients with pancreatic adenocarcinoma. A randomized phase Ib/IIa study. Cancers (Basel). 2020;12(11):3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Landen CN Jr, Chavez‐Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910‐6918. [DOI] [PubMed] [Google Scholar]
- 37. Wagner MJ, Mitra R, McArthur MJ, et al. Preclinical mammalian safety studies of EPHARNA (DOPC Nanoliposomal EphA2‐targeted siRNA). Mol Cancer Ther. 2017;16(6):1114‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mendt M, Kamerkar S, Sugimoto H, et al. Generation and testing of clinical‐grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8):e99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Brien Z, Wang L, Majeti B, et al. A novel lipid nanoparticle (NBF‐006) encapsulating glutathione S‐transferase P (GSTP) siRNA for the treatment of KRAS‐driven non‐small cell lung cancer. Cancer Res. 2018;78(13 Suppl):5917.30154151 [Google Scholar]
- 41. Kumthekar P, Ko CH, Paunesku T, et al. A first‐in‐human phase 0 clinical study of RNA interference‐based spherical nucleic acids in patients with recurrent glioblastoma. Sci Transl Med. 2021;13(584):eabb3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zorde Khvalevsky E, Gabai R, Rachmut IH, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci USA. 2013;110(51):20723‐20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Golan T, Khvalevsky EZ, Hubert A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6(27):24560‐24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taniguchi H, Natori Y, Miyagi Y, et al. Treatment of primary and metastatic breast and pancreatic tumors upon intravenous delivery of a PRDM14‐specific chimeric siRNA/nanocarrier complex. Int J Cancer. 2021;149(3):646‐656. [DOI] [PubMed] [Google Scholar]
- 45. Taniguchi H, Hoshino D, Moriya C, et al. Silencing PRDM14 expression by an innovative RNAi therapy inhibits stemness, tumorigenicity, and metastasis of breast cancer. Oncotarget. 2017;8(29):46856‐46874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moriya C, Taniguchi H, Miyata K, Nishiyama N, Kataoka K, Imai K. Inhibition of PRDM14 expression in pancreatic cancer suppresses cancer stem‐like properties and liver metastasis in mice. Carcinogenesis. 2017;38(6):638‐648. [DOI] [PubMed] [Google Scholar]
- 47. Matsuda Y, Fujii Y, Matsukawa M, Ishiwata T, Nishimura M, Arai T. Overexpression of carbohydrate sulfotransferase 15 in pancreatic cancer stroma is associated with worse prognosis. Oncol Lett. 2019;18(4):4100‐4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nishimura M, Matsukawa M, Fujii Y, et al. Effects of EUS‐guided intratumoral injection of oligonucleotide STNM01 on tumor growth, histology, and overall survival in patients with unresectable pancreatic cancer. Gastrointest Endosc. 2018;87(4):1126‐1131. [DOI] [PubMed] [Google Scholar]
- 49. Molyneaux M, Xu J, Evans DM, Lu P. Effect on tumor growth by TGF‐beta 1/COX‐2 siRNA combination product (STP705) in a human cholangiocarcinoma (HuCCT‐1) xenograft tumor model in nude mice. J Clin Oncol. 2019;37(15 Suppl):e14652. [Google Scholar]
- 50. Molyneaux M, Berman B, Xu J, Evans DM, Lu PY. Effect of TGF‐beta 1/COX‐2 small interfering RNA combination product (STP705) on cell viability and tumor growth in a human squamous carcinoma xenograft tumor model in nude mice. J Am Acad Dermatol. 2020;83(6):AB156. [Google Scholar]
- 51. Northfelt DW, Hamburg SI, Borad MJ, et al. A phase I dose‐escalation study of TKM‐080301, a RNAi therapeutic directed against polo‐like kinase 1 (PLK1), in patients with advanced solid tumors: expansion cohort evaluation of biopsy samples for evidence of pharmacodynamic effects of PLK1 inhibition. J Clin Oncol. 2013;31(15 Suppl):TPS2621. [Google Scholar]
- 52. El Dika I, Lim HY, Yong WP, et al. An open‐label, multicenter, phase I, dose escalation study with phase II expansion cohort to determine the safety, pharmacokinetics, and preliminary antitumor activity of intravenous TKM‐080301 in subjects with advanced hepatocellular carcinoma. Oncologist. 2019;24(6):747‐e218. [DOI] [PMC free article] [PubMed] [Google Scholar]


