Abstract
Rhabdomyosarcoma (RMS) exhibits varying degrees of clinical manifestations, and one of the determining factors is its primary site of origin. Ocular proptosis is an infrequent presentation of parameningeal RMS. The growing tumor in spacious environments such as air-filled sinuses can obscure its early detection, leading to late disease intervention. Among the four subtypes (embryonal, leiomyomatous, sclerosing and spindle cell, and alveolar), the predominant type of RMS in the paranasal sinuses is alveolar. The incidence in adult-onset RMS is relatively low compared with those of children. We herein present a rare case of a 23-year-old man with an unusual presentation of bilateral proptosis from alveolar RMS of the ethmoid sinus. In contrast to our patient, most reported cases of ocular involvement in RMS turned out to be unilateral and responded poorly to treatment. Despite the aggressive behavior of the adult-onset alveolar subtype in comparable reports, our case shows an excellent outcome. Negative FOXO1 fusion status has been recognized in recent studies as a molecular feature inclined toward a favorable outcome in alveolar RMS. The integration of molecular prognostic factors to risk stratification could be advantageous in determining different prognoses and proper management for an individual patient.
Keywords: Proptosis, Alveolar-type rhabdomyosarcoma, Ethmoid sinus, Adult
Introduction
Rhabdomyosarcoma (RMS) is a rare soft-tissue sarcoma originating from pluripotent mesenchymal cells. Most patients with RMS are diagnosed before the first decade of life; RMS is a relatively rare disease in adults, constituting less than 1% of all cancers in adults. Any anatomic location in the body can be affected by RMS. The primary tumor in pediatric RMS is most often present in the head and neck area (40%), and the incidence of ocular involvement is 25%; this presentation is extremely rare in adults. In contrast, the extremities are the most common predilection sites in adult RMS. Clinical manifestations of RMS can be variable and multifactorial. Determinant factors include the site of origin, histopathology of RMS, tumor size, and patient demographics such as age and sex [1]. Parameningeal RMS produces nasal or sinus obstruction with or without mucopurulent discharge, whereas proptosis is an uncommon presentation of RMS in this location. Furthermore, RMS involving the head and neck is most commonly of the embryonal subtype and rarely involves the lymph nodes.
The four broad histologic subtypes of RMS, namely embryonal, leiomyomatous, sclerosing and spindle cell, and alveolar, are imperative for prognosis prediction and treatment optimization. Information regarding treatment for adults with RMS is still limited. Some reports have described unfavorable outcomes in adults when using the same principles as established in children [2]. In this report, we present a rare case of alveolar RMS of the ethmoid sinus with an unusual presentation of bilateral proptosis in a 23-year-old man with a favorable treatment response.
Case Report
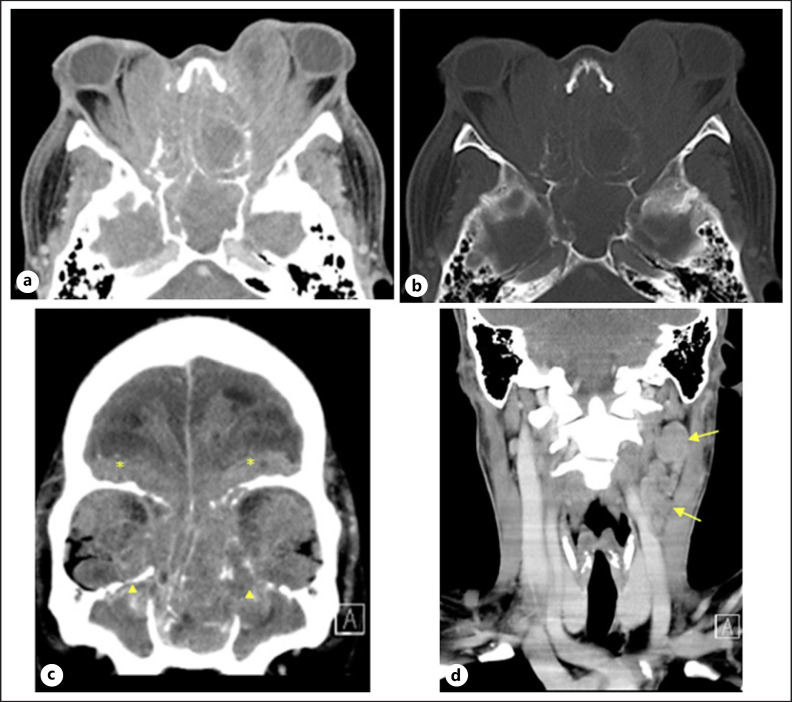
A 23-year-old previously healthy Thai man initially presented with a 5-month history of a decreased sense of smell along with nasal congestion. He had also experienced progressive and painful bilateral ocular proptosis with blurry vision. In August 2016, he was referred to our hospital for full multidisciplinary diagnosis and therapy. On presentation, his vital signs were stable, but he had complete loss of vision. Ocular examination revealed marked proptosis with lagophthalmos (incomplete closure of the eyelids). Slit-lamp examination revealed severe chemosis, keratinized conjunctiva, and total opacity of cornea. The other ocular structures were obscured. The eye movements were limited in all directions because of severe proptosis and pain. Sinuscopy of the left nasal cavity demonstrated a large irregular mass at the inferior meatus, and the scope could not be further advanced. An irregular mass was present at the middle meatus in the right nasal cavity, and the entire nasal mucosa was swollen. A 2-cm lymph node with hard consistency was palpable in the left submental region. Computed tomography of the head and neck showed an infiltrative mass in the bilateral ethmoid and sphenoid sinuses with associated bony destruction. The tumor had extended into the bilateral retrobulbar spaces and encased the bilateral optic nerves. Proptosis and deformed contours of both orbital globes were noted. Matted nodes were also present at left cervical lymph node levels II and III (Fig. 1).
Fig. 1.
Axial contrast-enhanced computed tomography scan in soft tissue (a) and bone windows (b) showed an infiltrative mass in the bilateral ethmoid and sphenoid sinuses with associated bony destruction. The tumor had extended into the bilateral retrobulbar spaces and encased the bilateral optic nerves. Note the proptosis and deformed contour of both orbital globes.cCoronal contrast-enhanced CT scan showed intracranial extension of the infiltrative tumor to the bilateral frontonasal regions (*). The tumor also extended into the bilateral maxillary sinuses (▲).dCoronal contrast-enhanced CT scan showed matted nodes at left cervical lymph node levels II and III (arrows).
Biopsy of the masses in both nasal cavities was performed. The biopsied specimens showed crushed blue round neoplastic cells in fibrous tissue (Fig. 2). Immunohistochemical analysis revealed the following staining pattern: desmin (+), CD99 (weak +), CD56 (+), myogenin (+), AE1/AE3 (−), FLI-1 (−), and S-100 (−). The patient was diagnosed with alveolar-type RMS of the ethmoid sinus with phthisis bulbi. Without evidence of distant metastasis, his disease was classified as stage III (parameningeal head and neck) and T2b N1 M0, consistent with the intermediate risk classification according to the Intergroup Rhabdomyosarcoma Study Group (IRSG).
Fig. 2.
Histopathologic examination of the ethmoidal sinus mass showed crushed small blue cells in the subepithelial layer. These neoplastic cells contained round basophilic nuclei, a convoluted nuclear membrane, and usually nuclear molding with clear neoplasm under optical microscopy at (a) ×20 and (b) ×40.
The consensus decision was to treat the patient nonsurgically by multiple chemotherapy (VAC regimen: vincristine, adriamycin/dactinomycin, and cyclophosphamide) and definitive local radiation therapy after four cycles of chemotherapy according to the IRSG protocol. Supportive Chinese acupuncture for pain control and dental care were also provided.
A response evaluation was routinely carried out every 3 weeks following each VAC cycle. A significant regression in the ocular proptosis was noted by the end of the first VAC cycle, and the visual acuity was considerably improved. After complete treatment with 40 cycles of VAC, brain magnetic resonance imaging revealed significant improvements in the tumor size and adjacent extensions, as shown in Figure 3. There was no evidence of disease recurrence at the 5-year follow-up.
Fig. 3.
aAxial and coronal contrast-enhanced T1-weighted magnetic resonance images with fat suppression (b) showed a significant decrease in the size of the heterogeneously enhancing infiltrative tumor until it mainly involved the destroyed left ethmoid sinus (*), leptomeninges, and dura of the bilateral inferior frontal sulci. The tumor was still extending into the medial aspect of the left orbit (arrows) with encasement of the left extraocular muscles. Resolution of the bilateral proptosis was also noted. The patient had cystic encephalomalacic change in the left inferior frontal lobe without tumor involvement (▲).
Discussion/Conclusion
In 1854, the first case of RMS was described in the tongue of a 21-year-old man [3]. Cases of RMS in this age-group are uncommon and have been scarcely reported to date. In addition to the rarity of RMS in adults, bilateral exophthalmos is an infrequent clinical presentation. The differential diagnosis of bilateral proptosis is, in fact, fairly broad. Pathologies affecting both eyes are more often associated with infection, inflammation, and vascular causes than with neoplasia despite the absence of a lymphatic system in the eyes. Moreover, most intraocular tumors arise from distant metastases via hematogenous spread, mainly from breast cancer. However, RMS should be considered as a possible diagnosis of a rapidly growing intraocular malignancy among other several differential diagnoses, including neuroblastoma, Ewing sarcoma, leukemia-associated chloroma, poorly differentiated carcinoma, and invasion of a tumor from adjacent structures such as the paranasal sinuses [4].
Our patient developed bilateral proptosis due to secondary extension of RMS from the left ethmoid sinus. Tumors arising in the paranasal sinuses can grow silently for a long time because of the air-filled spaces. Patients may present with ophthalmologic symptoms as the first signs of a developing tumor. A literature search revealed only a few cases of RMS arising from the paranasal sinuses with orbital extension in adults. Notably, these patients exhibited only unilateral ocular disease [5, 6, 7, 8, 9, 10, 11, 12] (Table 1). In contrast, the presentation of bilateral protrusion of the eyes is usually caused by the primary orbital RMS itself, although such cases are rare. Gupta and Tomar [13] reported a similar case of an 11-year-old Hindu boy who exhibited signs of bilateral proptosis with conjunctival chemosis and keratinization. In that case, however, the immunohistochemical analysis demonstrated embryonal characteristics, which is consistent with most cases of primary orbital RMS. Although a few cases show the embryonal subtype, the predominant type of RMS in the paranasal sinuses is alveolar. This rationale suggests that our patient developed the primary alveolar-subtype ethmoidal sinus RMS.
Table 1.
Summary of case reports of adult-onset alveolar-type RMS of ethmoid sinus
| Authors, year | Age, years | Sex | Clinical presentation | Site | Therapy | Outcome |
|---|---|---|---|---|---|---|
| Muto et al. [5], 2005 | 69 | Female | Impaired bilateral vision and hyposmia | Nasal and left paranasal sinuses extending to orbital cavity and anterior skull base | Chemoradiation with VAC for 3 cycles | Complete disappearance of tumor from nasal and paranasal sinuses; all initial symptoms except left visual loss improved Develop bone metastasis, then rapidly spread to multiple sites and death occurred after 12 months (no local recurrence before death] |
| Moon et al. [6], 2006 | 48 | Male | Unilateral proptosis (left) | Left ethmoid sinus extending to left orbit | Surgery followed by chemoradiation with adriamycin | Progressive metastasis to the spine; death 7 months after diagnosis |
| Sepúlveda et al. [7], 2014 | 42 | Male | Unilateral exophthalmos with nasal congestion | Left nasoethmoidal region invading left frontal sinus, left maxillary sinus, and left orbit | Chemoradiation with VAC regimen | Progression of the lesion after 4 cycles of VAC; referred for pain control and palliation |
| Parikh et al. [8], 2014 | 40 | Male | Left-sided epiphora with nasal congestion | Left maxillary sinus extending to other sinuses (including bilateral ethmoids, frontal sinuses, and left sphenoid sinus) and left orbit | Chemotherapy with VAC regimen | Not available |
| Torres-Peña et al. [9], 2015 | 24 | Male | Unilateral proptosis (right), epistaxis, and nasal congestion | Right nasal cavity extending to right ethmoid sinus and right orbit | Chemotherapy and radiotherapy | Death 2 years after treatment due to systemic complication associated with invasion |
| Torres-Peña et al. [9], 2015 | 26 | Male | Decreased right eye visual acuity and nasal congestion with epistaxis | Right nasal cavity extending to ethmoid and frontal sinuses and right orbit | Chemotherapy and radiotherapy | Death 5 months after treatment due to systemic complication associated with invasion |
| Eshraghi et al. [10], 2016 | 23 | Male | Anosmia and mild proptosis of left eye | Left ethmoid region extending into maxillary sinus and left orbit | Chemotherapy (VAC, IE) followed by radiotherapy | Recurrence at 1-year follow-up followed by retreatment with chemoradiation, then recurrence-free for 2 years |
| Joo et al. [11], 2019 | 21 | Female | Unilateral proptosis (right) and nasal congestion | Right maxillary sinus extending to right nasal cavity and right orbit | Surgical debulking, chemotherapy (6 cycles of VAC), and radiation therapy | No local recurrence of tumors for total follow-up period of 1.5 years, then lost to follow-up |
| Bernaola-Paredes et al. [12], 2021 | 20 | Male | Unilateral proptosis with painful, rapid growth of left hemimaxilla mass | Left maxillary sinus/left osteomeatal complex, extending to the periorbital and orbital region with left proptosis and extra-axial intracranial invasion | Induction chemotherapy (IE) followed by concurrent chemoradiation (vincristine, ifosfamide) | Death caused by brain metastasis 4 months after diagnosis |
| Current case | 23 | Male | Anosmia and bilateral proptosis | Bilateral ethmoid and sphenoid sinuses | Chemoradiation with VAC regimen | Disease-free for 5 years |
VAC, vincristine, adriamycin/dactinomycin, and cyclophosphamide; IE, ifosfamide and etoposide.
Among the four major subtypes, alveolar RMS exhibits highly aggressive behavior, especially rapid dissemination; it also shows the poorest response to chemotherapy [14]. Therefore, it is logical that the rapid spread of the primary tumor in our patient led to aggressive bilateral ocular invasion. According to prior studies of adult patients, alveolar RMS is associated with substantially lower 5-year overall survival rates than the embryonic subtype. These survival rates might also be affected by statistically proven confounding factors, such as stage at presentation, fusion genes, local extension, primary tumor size, and metastases. Alveolar RMS is associated with higher lymph node involvement and tends to present at parameningeal sites, which are recognized as unfavorable primary locations as demonstrated in patients under 18 years of age. Several studies have also revealed a poorer prognosis of alveolar RMS because of the rapid metastasis and progressive extent of the tumor at presentation. Aggressive distant metastases have also been described in adults of more advanced age. A 48-year-old man who had alveolar RMS in the ethmoid sinus with orbital extension and distant spinal dissemination survived only 7 months from the diagnosis [6]. In a comparable case, a 69-year-old man survived for only 12 months because of metastatic bone disease [5].
Compared with previous reports of adult-onset alveolar-subtype RMS with ethmoid sinus involvement (Table 1), our patient had an excellent outcome in terms of his 5-year disease-free status. Recent studies have demonstrated that the FOXO1 fusion status is the most important molecular feature affecting the prognosis of RMS. Patients with alveolar histology and a negative FOXO1 fusion status have favorable survival outcomes similar to those of patients with embryonal histology and a negative FOXO1 fusion status [15]. However, we did not perform FOXO1 fusion analysis in our patient.
Multidisciplinary treatment typically includes surgical resection, radiation, and multi-agent therapy; however, intensive chemotherapy is still the standard treatment for RMS. The risk stratification of RMS is based on clinicopathologic features, which dictates the treatment and prognosis for each patient. Although survival rates have improved, they still exhibit substantial variation among different risk groups. Incorporation of molecular prognostic factors may help to identify which patients will benefit from intensive therapy and/or novel agents [15].
In conclusion, we have herein reported an uncommon presentation of a young man with ethmoid sinus RMS presenting with bilateral proptosis, which has not been previously described. Paranasal sinus RMS can be recognized as one etiology of bilateral proptosis. Despite the poor prognosis of adult-onset alveolar RMS, our patient had an excellent outcome under the IRSG risk stratification protocol. Utilizing molecular genetics for risk stratification could help to identify patients with different prognoses and tailor their treatment.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and accompanying images. The study protocol was approved by the Chulabhorn Research Institute Ethics Committee (No. 200/2564).
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
Funding Sources
No funding was received for this study.
Author Contributions
Natcha Watanapokasin drafted the manuscript. Thanita Limsiri and Praeploy Tungyingyong reviewed the patient's imaging data. Nanthida Phattraprayoon reviewed and edited the manuscript. Teerapat Ungtrakul contributed to the design, supervision, review, and editing of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Skapek SX, Ferrari A, Gupta AA, Lupo PJ, Butler E, Shipley J, et al. Rhabdomyosarcoma. Nat Rev Dis Primers. 2019 Jan;5((1)):1. doi: 10.1038/s41572-018-0051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009 Jul;27((20)):3391–7. doi: 10.1200/JCO.2008.19.7483. [DOI] [PubMed] [Google Scholar]
- 3.Weber CO, Virchow R. Anatomische Untersuchung einer hypertrophischen Zunge nebst Bemerkungen über die Neubildung quergestreifter Muskelfasern. Archiv für Pathol Anat. 1854;7((1)):115–25. [Google Scholar]
- 4.Topilow NJ, Tran AQ, Koo EB, Alabiad CR. Etiologies of proptosis: a review. Intern Med Rev. 2020 Mar;6((3)):10. doi: 10.18103/imr.v6i3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muto E, Shioyama Y, Nakamura K, Ohga S, Nomoto S, Toba T, et al. Adult rhabdomyosarcoma in the nasal and paranasal sinuses showing complete local response to a combination of chemotherapy and radiotherapy using 3D-CRT and IMRT. Fukuoka Igaku Zasshi. 2005 Oct;96((10)):363–9. [PubMed] [Google Scholar]
- 6.Moon HS, Kwon SW, Lee JH. A case of alveolar rhabdomyosarcoma of the ethmoid sinus invading the orbit in an adult. Korean J Ophthalmol. 2006 Mar;20((1)):70–5. doi: 10.3341/kjo.2006.20.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepúlveda I, Spencer ML, Cabezas C, Platino MO, Schorwer M, Ortega P, et al. Orbito-ethmoidal rhabdomyosarcoma in an adult patient: a case report and review of the literature. Case Rep Oncol. 2014 Jul;7((2)):513–21. doi: 10.1159/000365547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh D, Spindle J, Linden C, Bardarov S, Shinder R. Adult rhabdomyosarcoma of the maxillary sinus with orbital extension. Orbit. 2014 Aug;33((4)):302–4. doi: 10.3109/01676830.2014.902480. [DOI] [PubMed] [Google Scholar]
- 9.Torres-Peña JL, Ramos Castrillo AI, Mencía-Gutiérrez E, Gutiérrez-Díaz E, Rodríguez-Peralto JL, Bengoa-González Á. Nasal cavity or alveolar paranasal sinus rhabdomyosarcoma with orbital extension in adults: 2 cases. Plast Reconstr Surg Glob Open. 2015 Jul;3((6)):e414. doi: 10.1097/GOX.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshraghi B, Ameli K, Anvari P. Adult rhabdomyosarcoma of ethmoid sinus recurring as an orbital mass. J Clin Diagn Res. 2016 Apr;10((4)):ND06–7. doi: 10.7860/JCDR/2016/17661.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joo JH, Han JS, Choi SM, Park IK, Shin JH. One-year survivor of adult alveolar rhabdomyosarcoma of the maxillary sinus with orbital extension: case reportErratum in: Medicine. Medicine. 2018 Aug;97((35)):e11866. doi: 10.1097/MD.0000000000011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernaola-Paredes WE, Favareto SL, Filho VB, Filippetti EP, Bellotto WP, Veronese HRM, et al. An atypical presentation of sinonasal tract alveolar rhabdomyosarcoma in a young male patient submitted to multimodality treatment. Case Rep Oncol Med. 2021 Oct;2021:1–8. doi: 10.1155/2021/8401755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta BK, Tomar S. A rare case in an 11-year old child with bilateral primary orbital rhabdomyosarcoma with rapid onset of bilateral proptosis and temporal lobe abscess secondary to chronic suppurative otitis media. Int J Res Med Sci. 2020 Nov;8((12)):4515–7. [Google Scholar]
- 14.Stepan K, Konuthula N, Khan M, Parasher A, Del Signore A, Govindaraj S, et al. Outcomes in adult sinonasal rhabdomyosarcoma. Otolaryngol Head Neck Surg. 2017 Jul;157((1)):135–41. doi: 10.1177/0194599817696287. [DOI] [PubMed] [Google Scholar]
- 15.Haduong JH, Heske CM, Allen-Rhoades W, Xue W, Teot LA, Rodeberg DA, et al. An update on rhabdomyosarcoma risk stratification and the rationale for current and future Children's Oncology Group clinical trials. Pediatr Blood Cancer. 2022 Apr;69((4)):e29511. doi: 10.1002/pbc.29511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.