Abstract
Cardiovascular disease (CVD) and cancer are the first and second leading causes of death worldwide, respectively. Epidemiological evidence has demonstrated that the incidence of cancer is elevated in patients with CVD and vice versa. However, these conditions are usually regarded as separate events despite the presence of shared risk factors between both conditions, such as metabolic abnormalities and lifestyle. Cohort studies suggested that controlling for CVD risk factors may have an impact on cancer incidence. Therefore, it could be concluded that interventions that improve CVD and cancer shared risk factors may potentially be effective in preventing and treating both diseases. The ketogenic diet (KD), a low-carbohydrate and high-fat diet, has been widely prescribed in weight loss programs for metabolic abnormalities. Furthermore, recent research has investigated the effects of KD on the treatment of numerous diseases, including CVD and cancer, due to its role in promoting ketolysis, ketogenesis, and modifying many other metabolic pathways with potential favorable health effects. However, there is still great debate regarding prescribing KD in patients either with CVD or cancer. Considering the number of studies on this topic, there is a clear need to summarize potential mechanisms through which KD can improve cardiovascular health and control cell proliferation. In this review, we explained the history of KD, its types, and physiological effects and discussed how it could play a role in CVD and cancer treatment and prevention.
Keywords: ketogenic diet, cardiovascular disease, cancer, hypertension, oxidation, inflammation, diabetes, obesity
1. Introduction
Cardiovascular disease (CVD) and cancer are the first and second leading causes of death worldwide, respectively [1]. CVD accounted for approximately 17.8 million deaths, followed by cancer with 9.56 million deaths in 2017 [1]. These conditions are usually treated as separate events. However, they share several risk factors, such as obesity, diabetes, hypertension, hyperlipidemia, diet, and lifestyle, suggesting some shared biology [2,3]. Among the pathophysiological mechanisms, many processes in common between CVD and cancer have been investigated, including inflammation, oxidative stress, resistance to cell death, cellular proliferation, neurohormonal stress, angiogenesis, and genomic instability [2,4]. Epidemiological evidence has demonstrated that the incidence of cancer was elevated in patients with CVDs, such as heart failure (HF). The opposite was also true, as an increased occurrence of HF in cancer survivors may be related to chemotherapy, radiotherapy, and immunotherapy, often combined [3].
Cohort studies suggested that controlling for CVD risk factors may also have an impact on cancer incidence and its outcomes. In the European Prospective Investigation into Cancer and Nutrition (EPIC) study, a large cohort of 23,153 individuals aged 35 to 65 years, adherence to a healthy lifestyle (no smoking, body mass index (BMI) < 30, physical activity > 3.5 h weekly, and healthy diet) resulted in a hazard ratio (HR) of 0.19 (95% confidence interval (CI), 0.07–0.53) for myocardial infarction; 0.50 (95% CI, 0.21–1.18) for stroke; and 0.64 (95% CI, 0.43–0.95) for cancer after 7.8 years of follow-up [5]. The Atherosclerosis Risk in Communities Study (ARIC), with 13,253 participants aged 45 to 64 years between 1987 to 2006, demonstrated that adhering to six of the seven ideal health metrics reduced the risk of incident cancer compared to subjects meeting zero ideal health metrics by 51% [6]. Therefore, interventions that improve CVD and cancer shared risk factors may play a role in the development of a combined prevention and treatment strategy for both diseases.
Several nutritional interventions have been tested to prevent and treat CVD and cancer [7,8,9,10]. The ketogenic diet (KD), which consists of a low-carbohydrate and high-fat diet, was developed in 1920 for the treatment of refractory epilepsy with successful outcomes and became widely known in the 1970s when used for weight loss purposes. KD has been recently investigated for the treatment of numerous diseases, including CVD and cancer, due to its role in promoting ketolysis, ketogenesis, and modifying many other metabolic pathways that might lead to beneficial health effects [11]. Considering the number of studies on this topic, there is a clear need to summarize the evidence on the potential therapeutic mechanisms of KD on CVD and cancer. This review presented a wide range of studies that examined the impact of KD on CVD and cancers and their shared risk factors and the potential mechanisms involved.
2. Overview of Ketogenic Diet Metabolism and Its Physiological Effects
2.1. History
Over the centuries, fasting has been used as the primary treatment for epilepsy [12]. In the early twentieth century, Guepa and Maria used fasting to treat epilepsy in France [13]. Ten years later, in 1921, Wilder suggested that this treatment for epilepsy may be due to body ketones [14] and hence introduced KD, which produced similar biochemical changes as fasting [15]. In the same year, Woodiat stated that in conditions of starvation or low carbohydrates, ketone bodies appeared in the blood [16]. KD consists of a significant amount of fats, low carbohydrates, and limited protein, which leads to altered energy metabolism and an increase in body ketones in the blood, which eventually forces the body to use them to produce energy [17,18]. The recommended ratio of fat to protein plus carbohydrates varies between 4:1 and 2:1 by weight [15]. Later, Wilder and Teperman described this diet as 1 g and 10–15 g per kilogram of body weight of protein and carbohydrates, respectively, and the rest of the required energy from fat [16]. Table 1 shows different versions of KD.
Table 1.
Different types of ketogenic diet (KD).
| KD Type | Macronutrient Proportion (% of Total Energy) | General Characteristics | ||
|---|---|---|---|---|
| Carbohydrate | Fat | Protein | ||
| Classic ketogenic diet | 4 | 90 | 6 | Developed for epilepsy treatment |
| Medium-chain-triglyceride (MCT) ketogenic diet | 17 | 73 (30–60% MCT) | 10 | MCT supplements should be incorporated into all meals and snacks |
| The modified Atkins diet (MAD) | 5 (10–20 g/day) | 65 | 30 | No restriction on energy content, fluid, or protein |
| The modified ketogenic diet (MKD) | 5 (30 g/day) | 65–80 | 20–25 | No restriction on energy |
| Very low-calorie ketogenic diet (VLCKD) | 13 (usually <30 g/day) | 44 | 43 (1.2–1.5 g/kg of ideal body weight) | Total energy intake of <800 kcal/day |
| Ketogenic Mediterranean diet/modified Mediterranean ketogenic diet | <30–50 g/day | 45–50 | 30–35 | With an emphasis on lean meats, fish, olive oil, walnuts, and salad |
2.2. Classification
In general, four types of KD have been characterized. The first type is the classical or traditional type, with a 4:1 ratio of fat to protein plus carbohydrates. Thus, in this diet, fat intake provides 90% of the energy, which may be difficult and intolerant to the public [19]. The second type contains medium-chain-triglyceride (MCT) like caprylic acid, capric acid, caproic acid, and lauric acid [20], with 70% of energy uptake from fat including 10% long chain triglyceride (LCT) fat and 60% MCT fat, 20% from carbohydrate, and 10% from protein. Due to MCT’s faster membrane diffusion, the absorption process occurs earlier. Since the production rate of ketone bodies is higher than the previous type, for its equal formulation, less fat intake is required [21]. The third type of KD with a 1.1:1 ratio of 65% fat, 10% carbohydrate, and 25% protein was developed in 1970 by Dr. Robert Atkins based on carbohydrate restriction for weight loss. It is characterized by a higher carbohydrate restriction and high-fat intake with no restrictions on calories and protein. This diet was called the Atkins modified diet [22]. The fourth type is called the low glycemic index (GI) diet, which recommends food with a GI lower than 50 with fat to carbohydrate ratio of 6:1 [23].
2.3. Physiology and Metabolism
A drop in blood glucose levels through fasting or starvation stimulates the liver to produce glucose by breaking down glycogen stores and through gluconeogenesis [24]. Therefore, persistent low blood glucose leads to the preferential breakdown of fat, with a major contribution to increased lipolysis due to lowered insulin levels. However, under these conditions, energy metabolism can hardly continue beyond the production of acetyl-coenzyme A (CoA). This is due to the limited availability of oxaloacetate for oxidation in the tricarboxylic acid (TCA) cycle in hepatocytes. This way, the liver produces ketone bodies as metabolic energy for extrahepatic tissues. Ketosis is caused by the preferential breakdown of fats for energy production due to an insufficient amount of carbohydrates, causing low systemic insulin levels [25].
This process of ketone production is controlled by the sensitive lipase hormone, acetyl-CoA carboxylase, and 3-hydroxy-3-methylglutaryl (HMG) CoA synthase, all of which are regulated by three hormones: adrenaline, insulin, and glucagon [26]. By acting on lipase-sensitive hormones, glucagon leads to the production of fatty acids and thus increases ketogenesis while inhibiting acetyl-CoA carboxylase, which prevents fatty acids from entering the liver mitochondria and increases ketogenesis. In the process of ketogenesis, glucagon accelerates ketogenesis by stimulating HMG CoA synthase [27,28]. Unlike glucagon, insulin has an inhibitory effect on ketogenesis by inhibiting lipolysis and increasing lipogenesis, leading to a reduction in available fatty acids [27].
The amount of ketone bodies in KD is between 0.5 and 3 mmoL, which is called nutritional or physiological ketosis [29,30]. Reducing carbohydrate intake to less than 50 g per day and suppressing subsequent insulin secretion leads to reduced glucose and fat storage, increases the triacylglycerol lysis, and the formed fatty acids are subsequently converted to ketone bodies [29]. The glycogen of the liver and skeletal muscle is depleted during carbohydrate restriction for 24 h and one week, respectively, in the ketogenesis process to supply energy to the mitochondrial matrix of the liver [31,32]. The condensation of β-oxidation-derived acetyl-coenzyme (acetyl-CoA) into acetoacetate (AcAc) is initiated by the sequential need of mitochondrial enzymes 3-hydroxy-3-methylglutaryl CoA synthase and 3-hydroxy-3-methylglutaryl CoA lyase. Acetoacetate forms acetone through spontaneous decarboxylation, which is a volatile substance. By reducing the ketone fractions, it becomes beta-hydroxybutyrate, which is the circulating form of ketone bodies in the human body [33,34].
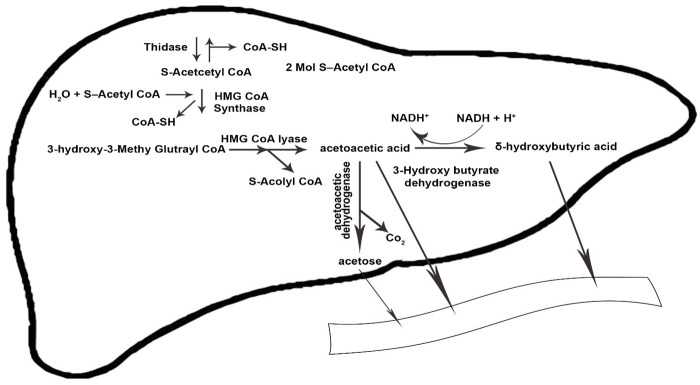
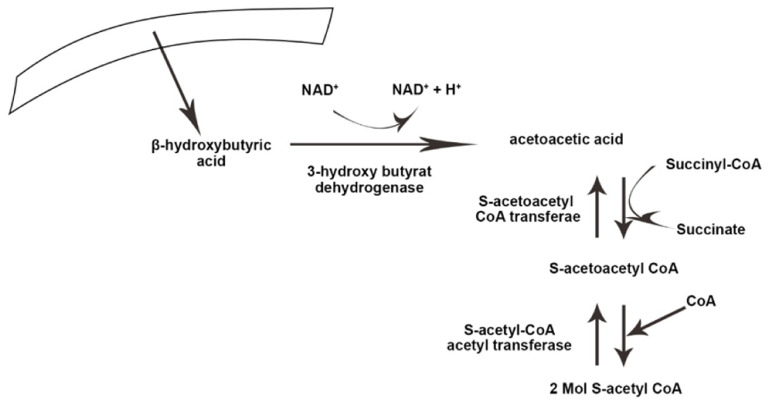
Ketolysis occurs in almost all extrahepatic tissues [35]. Once in the bloodstream, monocarboxylic acid carriers transport ketone bodies [36] and are absorbed by the brain and other tissues. The key enzyme in the ketolysis process is succinyl CoA transferase, which is the most active in the heart, muscle, kidneys, nervous system, and skeletal muscles [37]. First, β-hydroxybutyrate (BOHB) is converted back to AcAc by NAD+ oxidation. It is finally converted to acetyl-CoA by BDH1, succinyl-CoA:3-oxoacid CoA transferase (SCOT), and mitochondrial thiolase, which are used in the Krebs cycle [12]. Figure 1 and Figure 2 illustrate the process of ketogenesis and ketolysis in the ketogenic diet.
Figure 1.
The ketogenesis process in the body. CoA-SH, coenzyme A-SH; CoA, coenzyme A; H2O, water; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; NADH+, nicotinamide adenine dinucleotide + hydrogen; H+, Hydrogen; Co2, carbon dioxide. The arrows indicate the direction of changes in the process, as well substrates and products.
Figure 2.
The process of ketolysis in the body. NAD+, Nicotinamide adenine dinucleotide. The arrows indicate the direct ion of changes in the process, as well substrates and products.
3. Ketogenic Diet and Cardiovascular Disease
3.1. Ketogenic Diet and Cardiovascular Disease Function
The effect of dietary intake on the cardiovascular system has been studied more than other physiological functions [38]. In recent studies, the therapeutic effect of KD in CVD has been investigated, especially in the case of heart failure with a reduced ejection fraction [39]. Many animal and human studies, both observational and experimental, indicated the effect of KD on cardiovascular health. A meta-analysis on 1141 obese patients demonstrated that KD had a beneficial effect on cardiovascular health [40]. Another randomized clinical trial (RCT) by Polito et al. [41] showed that the heart rate decreased following weight loss due to KD. Several mechanisms proposed the impact of KD on cardiovascular function.
3.2. Ketogenic Diet and Energy Inducing in Heart
Ketone bodies have cardio-protection effects in patients with heart failure with a reduced ejection fraction from improvements in the cardiac metabolic state and may specifically increase cardiac efficiency [42]. However, a few animal studies have indicated that increasing BOHB by hyperketonemia had no effect on improving cardiac efficiency in diabetic mice [43].
Mobilization of fatty acids, glucose, lactate, ketones, and amino acids provides a cardiac energy source. Recent studies showed the role of ketone bodies as an important fuel source for the heart [42]. Ketone bodies are a source of energy during fasting, starvation, uncontrolled diabetes, heart failure, prolonged exercise as well as following KD consumption. Another potential mechanism for the beneficial effect of KD on CVD is the relationship between carbohydrate intake and CVD. A recent meta-analysis of 19 cohort studies including 15,663,111 participants suggested that higher carbohydrate intake was directly associated with increased CVD and stroke risk, while no association was found for coronary heart disease (CHD) or CVD mortality [44]. However, some studies showed conflicting findings. Lagiou et al. found that low carbohydrate–high protein diets, used on a regular basis and without consideration of the nature of the carbohydrates or source of proteins, were associated with an increased risk of cardiovascular events in women [45].
3.3. Ketogenic Diet and Endothelial Function
A ketone body of 1,3-butanediol (BD) has some advantages in experimental models of hypoxia or ischemia. BD is transformed to BOHB, which is an influential endothelium-dependent vasodilator through increasing nitric oxide (NO) synthase and hence may have a beneficial role in CVD as KD-fed animals had increased endothelial NO synthase protein expressions [46]. Myocardial blood flow was enhanced by BOHB infusion by about 75% and induced vasodilatation [42]. Cellular senescence is a process that eventually leads to an irreversible state of growth arrest. In addition, there is also much evidence that shows old cells have adverse effects on the body like tissue regeneration, aging process, vascular diseases, and endothelial dysfunction cells [47]. Since aging is a CVD risk factor, delaying or preventing it can play a main role in cardiovascular dysfunction. Caloric restriction with the production of ketones can prevent the aging of vascular cells [48]. Improvement in progressive dilation of the heart and cardiac contractile function was observed in a study following KD in mice [39]. Studies have demonstrated that myocardial function may be improved under the influence of ketone bodies following KD [49]. It has been suggested that a low level of ketone bodies through KD exerts beneficial effects on inflammatory status, senescence, and metabolism of endothelial cells. However, hyperketonemia in patients with diabetes-induced the inflammatory status, which led to the adhesion of monocytes to endothelial cells and endothelial dysfunction that consequently increased CVD risk [49].
3.4. Ketogenic Diet and Mitochondrial Function
One potential mechanism related to the beneficial role of KD on CVD is its effect on mitochondrial function. It has been suggested that mitochondrial dynamics play an essential role in heart function. Although diabetes decreases the mitochondrial respiratory control ratio and adenosine triphosphate (ATP) content, which can break the mitochondrial membrane and reduce their size, KD improved mitochondrial dynamics by preventing mitochondrial fission and hence raised its function in mice [50]. The mitochondria played an important role in controlling energy levels in cells and ATP production through oxidative phosphorylation and electron transport chain (ETC) activity in an in vitro study [51]. Thus, KD may protect against heart damage by regulating the heart’s energy metabolism and mitochondrial function.
3.5. Ketogenic Diet and Inflammation
In most vascular diseases, atherogenesis is a common underlying cause, and inflammation plays a role in the atherogenesis process. It is possible that the low level of ketone bodies can help in reducing inflammation in the vessels of individuals with CVD [49]. BOHB has an anti-inflammatory effect on the heart by suppressing the Nod-like receptor protein 3’s (NLRP3’s) inflammasome expression. This multiprotein complex has an important role in cardiac inflammation, as seen in myocardium dysfunction in rats [52]. In addition, BOHB inhibits pro-inflammatory cytokine production [52]. Thus, the anti-inflammatory action of ketone bodies is a potential beneficial cardio-protective mechanism of KD.
3.6. Ketogenic Diet and Oxidative Stress
Reactive oxygen species (ROS) or reactive nitrogen species (RNS) play a crucial role in the cell signaling pathways and regulate cellular function [53]. However, an imbalanced redox state, mitochondrial dysfunction, and inefficient antioxidant system can lead to overproduction of ROS or RNS. Elevated levels of these factors contribute to the pathogenesis of various diseases, which is mediated through their possible damaging effects on the cell membrane, protein, and DNA [53]. ROS is a by-product of normal metabolism in the mitochondrial respiratory chain [54]. Therefore, in cells with high mitochondria density, like cardiac myocytes, higher oxygen consumption predisposes them to oxidative stress. Increments of ketone body oxidation through BOHB de-hydrogenases 1 overexpression increase the antioxidant superoxide dismutase production and reduces the oxidative stress following transaortic constriction in rats [55]. However, owing to few human studies and a rather low number of studies in animal models, the beneficial effects of nutritional ketosis on the recovery of ischemia cannot be concluded [39,56].
3.7. Ketogenic Diet and Carotid Intima-Media Thickness
Carotid intima-media thickness (cIMT) has been used as an indicator of cardiovascular disease risk. Several studies have demonstrated that there was no significant change in cardiac indexes, including cIMT and elastic properties of the carotid artery, aortic strain, the stiffness index, and distensibility after short- and long-term KD in epileptic children [57,58]. Kapetanakis et al. reported that one year of KD treatment decreased the carotid artery distensibility. However, there was no improvement in cIMT and carotid artery compliance. In addition, the beneficial changes of KD on the cardiovascular system did not remain after two years [40].
In conclusion, although KD has controversial effects on the cardiovascular system, many studies have revealed a potential role of KD in CVD prevention, treatment, and disease reversal through improvements in energy induction, endothelial function, mitochondrial function, inflammation state, and antioxidant effect.
4. Proposed Mechanism of Action of Ketogenic Diet in Cancer
4.1. Glucose Dependence on Cancer Cells
Various mechanisms have been proposed for the inhibitory effects of KD on the growth of tumors and cancer cells. Part of these inhibitory effects is through KD’s effect on glucose metabolism in cancer cells. Elevated glucose uptake is a frequent feature of cancer cells, which is used to produce mediators of lipid, protein, and nucleic acids synthesis [59]. Hyperglycemia and hyperinsulinemia have been identified as stimuli for tumor growth in several cancers, such as breast, pancreatic, and colorectal [60]. In fact, cancer cells use glycolysis as their main pathway of energy production to have more access to glucose and speed up glucose utilization, even in the presence of adequate amounts of oxygen, resulting in the accumulation of lactate, a process called the Warburg effect [61]. For this reason, it seems that slowing down the Warburg effect and reducing access to glucose by administering low-carbohydrate diets and KDs to cancer patients may have positive effects on slowing the growth of cancer cells [62]. It has been reported in several pre-clinical studies that KD administration led to a significant reduction in serum glucose levels. For example, Woolf et al., in an animal study of 21 mice models of glioma, demonstrated that the administration of a high-fat, low-carbohydrate, adequate protein ketogenic diet led to a significant reduction in blood glucose, both 7- and 14-days post-implantation [63]. Lussier et al., in another animal study of ten mice models of malignant glioma, demonstrated that mice fed a therapeutic KD for 7 days had a significant reduction in blood glucose [64]. Another study that was conducted on mice models of medulloblastoma showed that serum insulin and glucose levels were significantly reduced in mice fed KD for 7 days [65]. Moreover, Caso et al., in an animal study of 160 male SCID mice (mice with severe combined immunodeficiency), showed that mice fed a no-carbohydrate ketogenic diet (NCKD) for 14 days had significantly lower glucose levels [66]. KD not only reduced the serum concentration of glucose but also reduced the serum levels of insulin and insulin-like growth factor 1 (IGF1), which are involved in tumorigenesis, and this can have a synergistic effect [62]. Tsujimoto et al., in a population-based study on 9778 participants aged over 20 years, showed that insulinemia was an important risk factor for cancer death in individuals with and without obesity [67]. Carbohydrate intake restriction, followed by decreased insulin levels in patients on low-carbohydrate diets such as the KD, can partially suppress these pathways in cancer cells [68].
Fine et al., in a pilot clinical trial of 10 patients with advanced cancer, showed a significant inverse correlation between ketone bodies serum levels and insulin and IGF1 concentration [69]. Similar results were reported in another human study of 73 women with ovarian cancer [70]. Activation of insulin receptors following hyperglycemia-induced the activity of some enzymatic pathways and caused cascades such as the RAS-mitogen activated protein (RAS-MAP) pathway that plays an important role in the growth and proliferation of cancer cells. In fact, activating the RAS-MAP pathway stimulated mitogenic effects of insulin and also induced cell survival by protein kinase B (Akt) and mechanistic target of rapamycin (mTOR), and nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) which activated antiapoptotic and pro-inflammatory pathways [71]. In addition, high serum insulin levels have been shown to stimulate the gene expression of vascular endothelial growth factor (VEGF), which is an essential component of vascular angiogenesis [72].
4.2. Mitochondrial Metabolism and Cancer
Another mechanism involved in the beneficial effects of KD on cancer cells through its effect on mitochondrial metabolism.
Numerous experimental studies have shown that in some cancers, such as cancers of the head and neck [73], liver, breast, colon, ovary, and prostate, mutations occur in mitochondrial DNA and impair normal mitochondrial function. One of the main reasons for this mutation is the increase in the level of ROS and oxidative stress in cancer patients, which causes mitochondrial damage and mutations [74,75]. Since, in cancer cells, the oxidative phosphorylation (OXPHOS) of mitochondria is not performed well, mitochondrial mass decreases, and its function is impaired, cancer cells use aerobic fermentation to supply their own energy. Replacing ketone bodies with carbohydrates as an energy source in cancer cells requires the use of efficient mitochondria to provide the energy needed for these cells to grow and proliferate. Considering the low efficiency of mitochondria in these cells, the presence of ketone bodies cannot stimulate the growth of cancer cells like carbohydrates [76].
4.3. Oxidative Stress and Cancer Cells
It has been reported in a review study that ROS can activate metabolic adaptations, the phosphoinositide 3-kinase (PI3K) and the hypoxia-inducible factor (HIF) pathways and consequently change bioenergetic metabolisms and cause tumorigenicity [77].
Another proposed mechanism of KD on cancer cells is increasing the level of oxidative stress in these cells. Firstly, KD reduces the amount of glucose available to produce glucose 6-phosphate, pyruvate, and NADPH production, which is necessary for reducing hydroperoxides. On the other hand, cancer cells do not have efficient mitochondria, and shifting the energy supply pathway to mitochondrial metabolism and disrupting the ETCs function resulted in increased one-electron reductions of O2 leading to ROS production, and is exposed to cancer cells under conditions of oxidative stress. In fact, when glucose is restricted in the diet of cancer patients, cancer cells are expected to be exposed to more oxidative stress, which in turn leads to more apoptosis of cancer cells and slower growth and proliferation [18,78]. The difference between high-fat and high-protein diets is that although high-protein diets also shift energy to mitochondrial metabolism, some amino acids contribute to NADPH production by entering the citric acid cycle and cannot increase oxidative stress levels in the tumor environment as much as high-fat diets such as KD. The effectiveness of KD on the level of oxidative stress in cancer cells has been investigated by various experimental studies [62,79]. Allen et al., in an animal study, found that KD administration 2 days before radio-chemotherapy in a mouse model of lung cancer improved the effects of radio-chemotherapy responses by enhancing oxidative stress [80].
4.4. Ketogenic Diet and Systemic Inflammation
One of the main factors involved in the etiology and progression of various cancers is systemic inflammation [81]. Systemic inflammation reduces the feeling of hunger, exacerbates cytokine cascades, activates catabolic pathways, induces lean body mass loss, induces cancer-induced wasting syndrome (cachexia) in cancer patients, and reduces the quality of life [82,83]. On the other hand, obesity, one of the main risk factors for cancer, has also been shown to increase the risk of the disease by exacerbating systemic inflammation [84]. Under normal circumstances, regulatory mechanisms in the body control the amount of inflammation. One of the important regulatory mechanisms in immune cells is mediated by proteins called inflammasomes, which are classified into two groups based on structural features: nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) and absent melanoma 2 (AIM2)-like receptors (ALRs) [85]. One of these important proteins (a mutation in this protein is associated with inflammatory diseases) is NLRP3. Some of the previous review studies have shown that therapeutic approaches that inhibit the expression of this protein can reduce the growth of cancer cells and prolong survival [86,87].
It has been reported that ketone metabolites, specifically β-hydroxybutyrate, can directly suppress NLRP3 and its related cytokine cascades [88]. Other experimental studies have shown that the administration of KD in animal models inhibited NLRP3 [50,89,90]. In fact, KD stimulates the production of β-hydroxybutyrate and AcAc in the liver, which inhibits some of the factors involved in the development of cancer cells, such as NLRP3. Moreover, it has been found that KD has anti-inflammatory, anti-angiogenic, and anti-invasive functions that could kill cancer cells by pro-apoptotic and anti-inflammatory mechanisms [68].
4.5. Ketogenic Diet in Combination with Chemotherapy and Radiotherapy
Schmidt et al. designed the first study, which evaluated a low carbohydrate ketogenic diet (LCKD) during chemotherapy in 2011. In this study, sixteen patients with advanced metastatic tumors and no conventional therapeutic options with adherence to LCKD (less than 70 g/day of carbohydrates) were evaluated in terms of their quality of life. It was found that LCKD during chemotherapy for three months was suitable, and there were no severe side effects and even some improvements in quality of life [91]. It has additionally been reported that KD was safe and effective in patients with high-grade glioma during treatment [92]. An animal study of neuroblastoma cases revealed that KD plus chemotherapy exerted anti-tumor effects, suppressing growth and causing a significant reduction of tumor blood-vessel density and intra-tumoral hemorrhage, accompanied by activation of AMP-activated protein kinase in neuroblastoma cells [93]. Allen et al. evaluated the effects of KD plus radiation as well as conventionally fractionated radiation combined with carboplatin in lung cancer xenografts. They found that KD plus radiotherapy reduced tumor growth by a mechanism that may involve increased oxidative stress [80]. Moreover, Yang et al., in an animal study, reported that KD administered for 30 days in mice models with pancreatic cancer increased the sensitivity of cancer cells to chemotherapy and disrupted pancreatic cancer cell metabolism and growth [94]. A prospective clinical trial from Japan evaluated the effects of KD with fluorouracil-based chemotherapy compared to a control group among patients with rectal cancer. They found that patients who had adhered to KD had a higher response rate to chemotherapy than the control group (60% in the intervention vs. 21% in the control group) [95]. Moreover, the results of a clinical study in patients with pancreatic ductal carcinoma showed that patients who have been treated with gemcitabine or FOLFIRINOX chemotherapy revealed that those patients in the KD group experienced a median overall survival of 15.8 months (95% CI 10.5–21.1) compared to the group that received only chemotherapy and hyperthermia only [96].
Despite the positive results observed, the results of some human studies are contradictory. Woodhouse et al., in a clinical study with 29 patients, evaluated the effects of KD plus temozolomide and radiation on tumor progression six months after the completion of radiotherapy on magnetic resonance image (MRI) scans. They did not find any significant correlation between β-hydroxybutyrate and tumor progression [97]. In addition, in another clinical study in eleven patients with glioblastoma, it was reported that the overall survival in patients ranged between 9.8 and 19.0 months, which was not a significant improvement. Quality of life, neurological functioning, and Karnofsky Performance Scale outcomes did not change substantially during the study [98].
4.6. Indication and Contraindication of Ketogenic Diet
Over the past few decades, the effectiveness of KD on cancer cells has been evaluated in both pre-clinical and clinical studies. One of the types of cancers that has been associated with positive results in pre-clinical studies of KD is brain cancer. Morscher et al., in a pre-clinical study on 11 mice models with neuroblastoma tumors, showed that KD administered for three weeks with or without calorie restriction led to a significant reduction in tumor growth and prolonged survival [99]. Aminzadeh-Gohari et al., in another study, found significant positive results after the administration of KD with medium-chain triglycerides for 40 days on neuroblastoma xenografts in a CD1-nu mouse model [93]. Glioblastoma is another type of cancer that has been studied extensively in relation to KD. Champ et al., in a retrospective review of patients with high-grade glioma, found that KD was well tolerated in most of the patients and led to a significant reduction in serum glucose levels and increased survival [100]. In a case report study of women aged 65 years, it was reported that two months of adherence to KD led to a 20% reduction in body weight, but no brain tumor tissue was detected [100]. Like the pre-clinical studies, most of the human studies on KD were conducted on patients with brain tumors. In contrast to the safe application of KD reported in various cancer models, there were some concerns about KD use in some cancer types. Vidali et al., in an animal study on 10 CD-1 nu/nu mice models of renal cell carcinoma with features of Stauffer’s syndrome, showed that KD administered for two weeks led to severe weight loss and liver dysfunction [101]. Liśkiewicz et al., in an animal study that evaluated the effects of long-term KD treatment on kidney cancer, showed a pro-tumor effect of this diet that aggravated the disease [102]. Another worrying result was seen in models of mice with BRAF V600E-positive melanoma whose KD exacerbated tumor growth. In fact, they found that acetoacetate as a ketone metabolite could induce BRAF V600E mutant-dependent mitogen-activated protein kinase 1 (MEK1) activation in melanoma cells, and KD might play a pathogenic effect in this cancer [103].
Furthermore, some short-term side effects (such as hypercholesterolemia, dehydration, constipation, acidosis, lethargy, and gastrointestinal distress) [104,105] and long-term side effects (such as kidney problems, cardiomyopathy, hyperlipidemia, and bone mineral loss) following the administration of KD in cancer patients were reported [68]. It is worth mentioning that KD may cause deficiencies in some micronutrients in patients, and patients who are exposed to nutritional deficiencies may exacerbate their nutrition status if they adhere to such diets [106].
5. Effects of Ketogenic Diet on Shared Cardiovascular and Cancer Aspects: Pre-Clinical and Clinical Studies
5.1. Ketogenic Diet and Oxidative Stress
Despite several studies exploring the effects of KD on oxidative stress, findings are still controversial. In a rat model study, KD reduced oxidative damage (protein nitration, 4-Hydroxynonenal (4-HNE) adducts, and 8-hydroxydeoxyguanosine (8-OHdG)), but it increased antioxidant defenses (glutathione peroxidase (GPx) and superoxide dismutase (SOD)) activity [107]. It is assumed that KD increases mitochondrial biogenesis, which, in turn, induces cellular resistance to oxidative stress at both genetic and mitochondrial levels [108]. In contrast, KD might exert anti-tumor effects through increasing intratumor oxidative stress and inducing apoptosis against tumor cells [80,109]. These effects were mainly attributed to lipid peroxidation [110]. In addition, while KD diminished plasma SOD levels in Wistar rats after two months [111], it might have improved mitochondrial respiratory complex activity and increased the production of some antioxidants such as glutathione and detoxification enzymes [55,112]. There is evidence that BOHB, as a ketone body, mitigates ROS production and improves mitochondrial respiration [113]. However, there is no consensus in this context, and further studies are required to demonstrate how ketone bodies affect redox signaling molecules and pathways.
5.2. Ketogenic Diet and Inflammation
The effect of KD on inflammatory biomarkers is inconsistent and may differ from one tissue to another [114]. For example, 4 weeks’ consumption of KD in mice induced inflammation and macrophage accumulation in the liver while decreasing them in white adipose tissue [114]. In a human model study, a 4-week isocaloric KD, containing 80% energy from fat, increased C-reactive proteins but not interleukin-6 in comparison with a control diet [115]. These findings were in line with other studies [116,117] but not all [118]. Nevertheless, in diabetic patients, a low carbohydrate diet (20% energy from carbohydrates) may be preferred over a low-fat diet in terms of subclinical inflammatory improvement, though both diets were similarly effective in weight reduction [118]. A recent systematic review of 63 studies assessing the impact of KD on inflammatory biomarkers showed that in approximately three-quarters of these studies [119], inflammatory biomarkers were reduced after KD. KD might exert an anti-inflammatory property via various mechanisms, including inhibiting activation of the nuclear factor kappa-light-chain-enhancer of activated B cells and the inflammatory nucleotide-binding, leucine-rich-containing family, pyrin domain-containing-3, and histone deacetylases [113].
5.3. Mitochondrial Function of Ketogenic Diet
Mitochondrial bioenergetics dysregulation and elevated ROS production are associated with different metabolic diseases. There is mounting evidence suggesting that KD can affect these processes. KD stimulates mitochondrial biogenesis and increases uncoupling proteins in the hippocampus of animals [112,120]. ROS production is regulated by uncoupling proteins. Furthermore, the reduced effect of β-hydroxybutyrate and AcAc on ROS production may be another reason for modified mitochondrial functions in KD. Rats fed KD for 3 weeks had higher mitochondrial reduced glutathione to oxidized glutathione ratio (GSH:GSSG) compared with the control group, indicating an improvement in GSH production. Lower production of H2O2 in KD-fed rats elicited an improvement in the mitochondrial functions [112]. KD increased mitochondrial density by regulating the deacetylation of various mitochondrial proteins [121]. The KD diet may benefit mitochondrial function via enhancing antioxidant activity, reducing ROS production, and ROS-induced damages [122].
5.4. Microbiota and Epigenetics: Therapeutic Approaches in Ketogenic Diet
Gut microbiota, as a “hidden metabolic organ” or a “virtual endocrine organ” [123], interacts with diet and has an enormous influence on the host metabolic pathways and health status. To date, little is known about the effect of KD on gut microbiota, and most of the information comes from trials on patients with neurological or mental disorders. It has been shown that the source and the proportion of dietary fat [124] and protein [125] have diverse effects on gut microbiota. For example, in patients with obesity, KD containing whey or vegetable protein, in comparison with an animal-based KD, led to a greater decline in the Firmicutes to Bacteroidetes ratio [125]. This ratio goes up in individuals with and is associated with various comorbidities [126]. Notably, KD reduced microbiota diversity, but its associated weight loss can increase microbiota diversity in the long term [127,128]. Other changes in gut microbiota following KD include a growth in the abundance of Alistipes and Parabacteroides, which are negatively correlated with waist circumference, and a decline in the abundance of Orodibacter splanchnicus and Lactobacillus [129]. In a 6-month pre- and post-intervention on refractory epilepsy patients, KD decreased the richness of gut microbiota, Firmicutes, and Actinobacteria but increased Bacteroidetes [130]. Further research to clarify the long-term effects of KD, with different macronutrient compositions and sources, is warranted.
5.5. Effect of Ketogenic Diet on Cardiovascular and Cancer Shared Risk Factors
Overall, owing to poor adherence to any restrictive diet [131], the long-term effects of KD on various metabolic risk factors are not well-established. In addition, KD composition varies from one study to another. For instance, restricted-carbohydrate diets might be either high in fat or protein and differ in their fatty acids and protein sources. In addition, the type of saturated fatty acid (SFA) might be a determinant of health outcomes [132,133], and the ratio of different dietary fatty acids can potentially affect health consequences [134,135]. Meta-analyses reported no significant association between SFA and CVD [136], type 2 diabetes [137], or lipid profile [138,139]. Therefore, these factors should be considered when interpreting the effects of KD. Table 2 shows the summary of studies that assessed the shared mechanisms between cancer and cardiovascular diseases.
Table 2.
Summary of studies that assessed shared mechanisms between cancer and cardiovascular diseases [70,115,125,127,130,140,141,142,143].
| First Author, Year, Country | Population | Study Design | Follow Up | Intervention | Comparator | Outcomes |
|---|---|---|---|---|---|---|
| Wolver S, 2021, United States | 85 T2DM who initially presented on insulin the age 56.1 ± 9.9 years 70% Female |
One arm intervention | 1 year | LCKD (20 g of total carbohydrates per day from non-starchy vegetables) | - | ↓ insulin dose, HbA1c, and weight |
| Li S, 2022, China | 60 overweight or obese patients newly diagnosed with T2DM | RCT | 12 weeks | KD (the main foods for the diet were olive oil, butter, fried eggs, double-fried pork, pan-fried salmon, pacific saury, sardines, broccoli, avocado, etc.; daily limits for ingredients were as follows: carbohydrate 30–50 g, protein 60 g, fat 130 g, and total calories 1500 kcal + 2000 mL water/day | Routine diet for diabetes (carbohydrate 250–280 g, protein 60 g, fat 20 g), total calories (1500 kcal, without no limitation on foods) + 2000 mL water/day | Greater ↓ in weight, BMI, waist, TG, TC, LDL-C, HDL-C, FBG, FIN, and HbA1c in the KD group compared with the control group |
| Dashti HM, 2006, Kuwait | 66 healthy obese patients (BMI ≥ 30 kg/m2) with a high cholesterol level (group I; n = 35) and normal cholesterol level (group II; n = 31) | Non-randomized clinical trial | 56 weeks | KD (less than 20 g of carbohydrates in the form of green vegetables and salad, and 80–100 g of proteins in the form of meat, fish, fowl, eggs, shellfish, and cheese. PUFA and MUFA (5 tablespoons olive oil) were included in the diet. | KD (less than 20 g of carbohydrates in the form of green vegetables and salad and 80–100 g of proteins in the form of meat, fish, fowl, eggs, shellfish, and cheese. PUFA and MUFA (5 tablespoons olive oil) were included in the diet. | ↓ weight, BMI, TC, LDL-C, TG, FBG, and ↑ HDL-C in both groups. KD was safe and beneficial in both groups. |
| Volek JS, 2009, USA | 40 subjects with atherogenic dyslipidemia | RCT | 12 weeks | Low carbohydrate diet % carbohydrate: fat: protein = 12:59:28 | Low-fat diet (56:24:20) | Greater ↓ in glucose and insulin levels, insulin sensitivity, weight, adiposity, and more favorable TG, HDL-C, and TC/HDL-C ratio in the low carbohydrate diet group compared with the low-fat diet group |
| Zhang Y, 2018, China | 20 patients (14 males, 6 females) | Single arm trial | 6 months | KD non-fasting diet with a classic 4:1 ratio KD fat:protein plus carbohydrates. Fat from pork, oils such as olive oil, coconut oil, and other sources. Plant fat accounted for about 70% of the amount of fat. At least 1 g of protein per kg body weight from animal sources (e.g., eggs, meat, poultry, and fish). Carbohydrate-containing foods such as fruits and vegetables were added. |
- | ↓ alpha diversity in fecal microbiota ↓ abundance of Firmicutes ↑ levels of Bacteroidetes |
| Gutiérrez-Repiso C, 2019, Spain | 33 obese patients | RCT | 2 months | VLCKD + synbiotics | Low-calorie diet | ↔ microbial diversity ↑ short-chain fatty acid-producing bacteria ↑ Odoribacter and Lachnospira |
| Basciani S, 2020, Italy | 48 patients with obesity (19 males and 29 females, HOMA index ≥ 2.5, aged 56.2 ± 6.1 years, BMI 35.9 ± 4.1 kg/m2 | RCT | 45 days | VLCKD regimens (≤800 kcal/day) containing whey, plant, or animal protein | Greater ↓ in relative abundance of Firmicutes and greater ↑ in Bacteroidetes in whey and plant protein compared with animal protein group. | |
| Rosenbaum M, 2019, USA | 17 men (BMI: 25–35 kg/m2) | Single arm trial | 4 weeks | Isocaloric KD (15% protein, 5% carbohydrate, 80% fat) | Baseline diet (15% protein, 50% carbohydrate, 35% fat) | ↑ free fatty acids, TC, LDL-C, and CRP ↓ Fasting insulin, C-peptides, TG, and fibroblast growth factor 21 |
| Cohen CW, 2018, Birmingham | women with ovarian or endometrial cancer (age: ≥19 years; BMI ≥ 18.5 kg/m2) | RCT | 12 weeks | KD (70:25:5 energy from fat, protein, and carbohydrate) | The American Cancer Society diet (high-fiber, low-fat) | Greater ↓ in total and android fat mass, visceral fat, and fasting serum insulin in KD compared with control ↔ lean mass |
T2DM: diabetes mellitus type II; LCKD: low-carbohydrate ketogenic diet; HbA1c: hemoglobin A1c; RCT: randomized clinical trial; KD: ketogenic diet; BMI: body mass index; TG: triglyceride; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; FBG: fasting blood glucose; FIN: fasting insulin; PUFA: polyunsaturated fatty acid; MUFA: mono-unsaturated fatty acid; VLCKD: very-low-calorie ketogenic diet; HOMA: homeostatic model assessment; CRP: C-reactive protein; ↓: decrease; ↑: increase; ↔: no change.
5.5.1. Hypertension
Previous meta-analyses have shown that hypertension is a risk factor for different cancers, such as breast, prostate, colorectal, and renal [144,145,146]. Mutual pathophysiological pathways mediated by adipose tissue, such as elevated inflammatory biomarkers, can increase the risk of hypertension, cancers, and CVD. However, hypertension may block apoptosis and disrupt cell turnover [145]. Hypertension modifies the activity of the sympathetic nervous system (SNS) and thereby affects the risk of both cancer and CVD through androgen-mediated pathways [146]. Besides the beneficial effects of KD on reducing adipose tissue [147,148], KD can regulate SNS activity by reducing salivary cortisol and the hypothalamus-pituitary-adrenal (HPA) axis [149]. In addition, β-hydroxybutyrate suppresses SNS activity by antagonizing G protein-coupled receptor 41 (GPR41) [150] and reducing heart rate as an indicator of SNS activity [41].
5.5.2. Dyslipidemia
The contributory role of dyslipidemia in CVD and some specific cancers is well documented [151,152,153]. Dyslipidemia increases cancer and CVD risk through some shared mechanisms, such as their effects on insulin resistance, inflammation, and oxidative stress [154,155,156,157]. Therefore, improving lipid profile can be a viable approach to reducing both CVD and cancer risks. A recent meta-analysis suggested that KD decreased serum levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglyceride and increased high-density lipoprotein cholesterol (HDL-C) in patients with type 2 diabetes [158]. However, another meta-analysis on overweight and obese individuals showed a reduction only in triglyceride and total cholesterol following KD consumption [147]. The follow-up duration of these studies ranged from 1 to 56 weeks in diabetic patients [158] and 3 to 104 weeks in overweight and obese individuals [147]. Another meta-analysis of long-term (>1 year) effects of KD, in comparison with a low-fat diet, showed a greater reduction in triglyceride but increased LDL-C and HDL-C [148]. Differences in the composition of KD can influence its effects. Furthermore, it has been shown that KD increases the particle size of LDL-C, which has lower atherogenicity compared with smaller ones [159]. Therefore, improvement of the lipid profile because of KD might be associated with a lower risk of cardiomyocyte dysfunction and impede carcinogenicity processes. However, the fatty acid composition of KD is a relevant factor that can modify KD’s effect on the lipid profile. Traditional KD is high in saturated fats, which come from cream (up to 50% of fat intake), butter, bacon, and other proteins high in SFA [160]. Hyperlipidemia is a common consequence of traditional KD, while substituting SFA with unsaturated fatty acids can prevent hyperlipidemia even with fat consumption of up to 90% of daily energy intake [160]. A KD with an appropriate composition of protein, mono-unsaturated and saturated fatty acids can increase HDL-C and reduce triglycerides, LDL-C, body fat mass, and inflammatory biomarkers [106,140].
5.5.3. Obesity
Obesity, especially abdominal obesity, is a well-known risk factor for both CVD and cancer [161]. According to the results of a meta-analysis, longer adherence to the KD leads to more weight loss, which can be kept off even two years after follow-up [147]. In comparison with a low-fat diet, KD is also associated with a greater than 0.9 kg weight reduction in the long term [148]. Castellana et al., in a systematic review and meta-analysis study, showed that very-low-calorie KD (VLCKD) with less than 800 kcal energy intake and 50 g carbohydrate administered to overweight and obese individuals led to a significant reduction in BMI and waist circumference [147]. In a RCT study among patients with ovarian or endometrial cancer, it was reported that after 12 weeks, patients in the KD group, compared to the control group, had lower fat mass, body weight, and fasting serum insulin. Also, they found a significant inverse association between the changes in serum β-hydroxybutyrate and insulin-like growth factor 1 (IGF-1) concentrations [70]. Increased levels of IGF-1 were associated with several common cancer types because IGF-1 has mitogenic and antiapoptotic effects [162]. Similar findings were reported for waist circumference. In overweight and obese individuals, KD could decrease waist circumference by 12.6 cm [147]. Excess body fat, in particular abdominal adiposity, is linked to higher inflammation and oxidative stress, which play a crucial role in the pathogenesis of both cancer and CVD [53]. Gut microbiota might be another link between obesity and cancer. On the other hand, obesity is associated with an increased abundance of mollicutes, a subclass of Firmicutes. The higher fecal mollicutes content, the higher the production of lactate, acetate, and butyrate. This, in turn, promotes fat storage and causes obesity. Gut microbiota also contributes to nutrient metabolism and may metabolize them into mutagens, such as N-nitroso compounds and heterocyclic amines [163]. Indeed, altered gut microbiota in obese patients may affect tumor initiation and development as well as change inflammatory processes [163]. There is evidence suggesting that KD can decrease Firmicutes and increase Bacteroidetes [164]. However, it is interesting to note that gut flora activity is also influenced by environmental factors such as diet. In a murine model study, transmitted bacteria derived from lean twins to mice were less protective against obesity when they were fed with a high saturated fatty acids diet compared with a low-SFA diet [163]. These findings highlight the relevance of a KD composition to obtain benefits.
5.5.4. Diabetes Mellitus
Hyperinsulinemia and hyperglycemia are associated with an increased risk of CVD and cancer [2]. Insulin affects lipid metabolism pathways such as lipid uptake, lipolysis, and lipogenesis and is potentially associated with obesity [165]. It also affects the neoplastic process through its stimulating effects on mitogenesis, proliferation, invasion, and metastasis [11]. Increased levels of free IGF-1 because of hyperinsulinemia have mitogenic and antiapoptotic properties, which can enhance cancer promotion and progression [11]. In addition, a U-shaped association has been reported between IGF-1 and nonfatal CVD events in elderly men [166]. A meta-analysis of RCTs indicated beneficial effects of a KD on glycemic control, with a greater improvement in diabetic patients [167]. Improvement in glycemic parameters was obtained after 2 weeks [168] and continued throughout the long term (56 weeks) [141], which is probably mediated by the inverse association between circulating levels of ketone bodies and hepatic glucose output [11]. Given that hyperinsulinemia mediates the adverse effects of diabetes on both cancer and CVD, improving insulin resistance can be an advantageous strategy to prevent their progression. In addition, weight and fat mass loss are closely correlated with insulin sensitivity. An RCT on newly diagnosed patients with type 2 diabetes showed that a periodic KD could reduce fasting insulin more than a healthy diet [142]. In another trial, depending on the adherence rate, 70–90% of patients with diabetes who initially presented on insulin and followed a low-calorie diet with carbohydrate restrictions were no longer taking insulin after one year [143].
6. Conclusions
Several potential mechanisms have been proposed to explain the potential effects of KD on cancer and CVD. KD might suppress oxidation through increasing mitochondrial biogenesis, exert an anti-inflammatory effect and consequently reduce cancer and CVD risk, and improve various shared risk factors of cancer and CVD (e.g., hypertension, obesity, diabetes mellitus, and dyslipidemia). However, it has been well-established that the effects of KD might vary by its composition and type of macronutrients. Although available evidence has shown that KD can improve shared CVD and cancer risk factors over a short period of time, there are only a few long-term trials and this limits the strength of recommendations for KD. Moreover, there is no study that has exclusively examined KD effects on patients with both medical conditions, and therefore the interplay between KD and various pathophysiological pathways involved in both conditions cannot be exactly known. In addition, adherence to such a restrictive diet is difficult for children and older adults, and even adults for the long term. Therefore, an alternative diet might be a more appropriate approach to managing metabolic abnormalities. It was suggested that future studies examine KD effects in people from different age groups, follow participants and their adherence rate for a longer period, and compare the efficacy of KD with other healthy diets (e.g., Dietary Approaches to Stop Hypertension (DASH) or Mediterranean diet) to improve various risk factors.
Author Contributions
Conceptualization, N.S., N.M. and E.A.S.; methodology, N.M., F.H. and E.A.S.; writing—original draft preparation, N.M., F.H., M.R., A.P.S.R., M.K.G. and P.O.; writing—review and editing, C.d.O., E.A.S. and N.S.; supervision, F.H., N.M., E.A.S. and N.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The Economic and Social Research Council supported this work (grant number ES/T008822/1).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Boer R.A., Meijers W.C., van der Meer P., van Veldhuisen D.J. Cancer and heart disease: Associations and relations. Eur. J. Heart Fail. 2019;21:1515–1525. doi: 10.1002/ejhf.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan V., Thompson E.W., Demissei B., Ho J.E., Januzzi J.L., Jr., Ky B. Mechanistic Biomarkers Informative of Both Cancer and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020;75:2726–2737. doi: 10.1016/j.jacc.2020.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nöthlings U., Ford E.S., Kröger J., Boeing H. Lifestyle factors and mortality among adults with diabetes: Findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam study. J. Diabetes. 2010;2:112–117. doi: 10.1111/j.1753-0407.2010.00069.x. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen-Torvik L.J., Shay C.M., Abramson J.G., Friedrich C.A., Nettleton J.A., Prizment A.E., Folsom A.R. Ideal cardiovascular health is inversely associated with incident cancer: The Atherosclerosis Risk in Communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandhorst S., Longo V.D. Dietary Restrictions and Nutrition in the Prevention and Treatment of Cardiovascular Disease. Circ Res. 2019;124:952–965. doi: 10.1161/CIRCRESAHA.118.313352. [DOI] [PubMed] [Google Scholar]
- 8.Bowen K.J., Sullivan V.K., Kris-Etherton P.M., Petersen K.S. Nutrition and Cardiovascular Disease-an Update. Curr. Atheroscler. Rep. 2018;20:8. doi: 10.1007/s11883-018-0704-3. [DOI] [PubMed] [Google Scholar]
- 9.Logan J., Bourassa M.W. The rationale for a role for diet and nutrition in the prevention and treatment of cancer. Eur. J. Cancer Prev. 2018;27:406–410. doi: 10.1097/CEJ.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 10.Talib W.H., Mahmod A.I., Kamal A., Rashid H.M., Alashqar A.M.D., Khater S., Jamal D., Waly M. Ketogenic Diet in Cancer Prevention and Therapy: Molecular Targets and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2021;43:558–589. doi: 10.3390/cimb43020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paoli A., Rubini A., Volek J.S., Grimaldi K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013;67:789–796. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams M.S., Turos E. The Chemistry of the Ketogenic Diet: Updates and Opportunities in Organic Synthesis. Int. J. Mol. Sci. 2021;22:5230. doi: 10.3390/ijms22105230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guelpa G. La lutte contre l’epiepsie par la desintoxication et par la reeducation alimentaire. Rev. Ther. Med. Chir. 1911;78:8. doi: 10.1111/j.1528-1157.1911.tb03003.x. [DOI] [Google Scholar]
- 14.Wilder R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921;2:307–308. [Google Scholar]
- 15.Sinha S.R., Kossoff E.H. The ketogenic diet. Neurology. 2005;11:161–170. doi: 10.1097/01.nrl.0000160818.58821.d2. [DOI] [PubMed] [Google Scholar]
- 16.Wheless J.W. History of the ketogenic diet. Epilepsia. 2008;49((Suppl. S8)):3–5. doi: 10.1111/j.1528-1167.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 17.Lima P.A., Sampaio L.P., Damasceno N.R. Neurobiochemical mechanisms of a ketogenic diet in refractory epilepsy. Clinics. 2014;69:699–705. doi: 10.6061/clinics/2014(10)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen B.G., Bhatia S.K., Anderson C.M., Eichenberger-Gilmore J.M., Sibenaller Z.A., Mapuskar K.A., Schoenfeld J.D., Buatti J.M., Spitz D.R., Fath F.A. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:963–970. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kossoff E.H., Zupec-Kania B.A., Auvin S., Ballaban-Gil K.R., Christina Bergqvist A.G., Blackford R., Buchhalter J.R., Caraballo R.H., Cross J.H., Dahlin M.G., et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3:175–192. doi: 10.1002/epi4.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira L., Lisenko K., Barros B., Zangeronimo M., Pereira L., Sousa R. Influence of medium-chain triglycerides on consumption and weight gain in rats: A systematic review. J. Anim. Physiol. Anim. Nutr. 2014;98:1–8. doi: 10.1111/jpn.12030. [DOI] [PubMed] [Google Scholar]
- 21.Giordano C., Marchiò M., Timofeeva E., Biagini G. Neuroactive peptides as putative mediators of antiepileptic ketogenic diets. Front. Neurol. 2014;5:63. doi: 10.3389/fneur.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kossoff E.H., Dorward J.L. The modified Atkins diet. Epilepsia. 2008;49((Suppl. S8)):37–41. doi: 10.1111/j.1528-1167.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 23.Miranda M.J., Turner Z., Magrath G. Alternative diets to the classical ketogenic diet—Can we be more liberal? Epilepsy Res. 2012;100:278–285. doi: 10.1016/j.eplepsyres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Rui L. Energy metabolism in the liver. Compr. Physiol. 2014;4:177. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson A.M., Williamson D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980;60:143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- 26.Leonard T. The physiology of ketosis and the ketogenic diet. South. Afr. J. Anaesth. Analg. 2020;26:S94–S97. doi: 10.36303/SAJAA.2020.26.6.S3.2547. [DOI] [Google Scholar]
- 27.Laffel L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/Metab. Res. Rev. 1999;15:412–426. doi: 10.1002/(SICI)1520-7560(199911/12)15:6<412::AID-DMRR72>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.McGarry J.D., Woeltje K.F., Kuwajima M., Foster D.W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes/Metab. Rev. 1989;5:271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- 29.Arnold A., Ali A., Kaka N., Kakodkar P. Ketogenic Diet: Biochemistry, Weight Loss and Clinical Applications. Nutri. Food Sci. Int. J. 2020;10:555782. doi: 10.19080/NFSIJ.2020.10.555782. [DOI] [Google Scholar]
- 30.Krebs H. The regulation of the release of ketone bodies by the liver. Adv. Enzym. Regul. 1966;4:339–353. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- 31.Bilsborough S.A., Crowe T. Low carbohydrate diets: What are the potential short and long term health implications? Asia Pac. J. Clin. Nutr. 2003;12:397–404. [PubMed] [Google Scholar]
- 32.Adam-Perrot A., Clifton P., Brouns F. Low-carbohydrate diets: Nutritional and physiological aspects. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2006;7:49–58. doi: 10.1111/j.1467-789X.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 33.Laeger T., Metges C.C., Kuhla B. Role of β-hydroxybutyric acid in the central regulation of energy balance. Appetite. 2010;54:450–455. doi: 10.1016/j.appet.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Glew H.R. You can get there from here: Acetone, anionic ketones and even-carbon fatty acids can provide substrates for gluconeogenesis. Niger. J. Physiol. Sci. 2010;25:2–4. [PubMed] [Google Scholar]
- 35.McGarry J., Foster D. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 36.Masino S.A., Rho J.M. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4th ed. National Center for Biotechnology Information (US); Bethesda, MD, USA: 2012. Mechanisms of ketogenic diet action. [PubMed] [Google Scholar]
- 37.Grabacka M., Pierzchalska M., Dean M., Reiss K. Regulation of ketone body metabolism and the role of PPARα. Int. J. Mol. Sci. 2016;17:2093. doi: 10.3390/ijms17122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iacovides S., Meiring R.M. The effect of a ketogenic diet versus a high-carbohydrate, low-fat diet on sleep, cognition, thyroid function, and cardiovascular health independent of weight loss: Study protocol for a randomized controlled trial. Trials. 2018;19:1–9. doi: 10.1186/s13063-018-2462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luong T.V., Abild C.B., Bangshaab M., Gormsen L.C., Søndergaard E. Ketogenic Diet and Cardiac Substrate Metabolism. Nutrients. 2022;14:1322. doi: 10.3390/nu14071322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos F.L., Esteves S.S., da Costa P.A., Yancy W.S., Jr., Nunes J.P. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes. Rev. 2012;13:1048–1066. doi: 10.1111/j.1467-789X.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 41.Polito R., Valenzano A., Monda V., Cibelli G., Monda M., Messina G., Villano I., Messina A. Heart Rate Variability and Sympathetic Activity Is Modulated by Very Low-Calorie Ketogenic Diet. Int. J. Environ. Res. Public Health. 2022;19:2253. doi: 10.3390/ijerph19042253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen R., Møller N., Gormsen L.C., Tolbod L.P., Hansson N.H., Sorensen J., Harms H.J., Frøkiær J., Eiskjaer H., Jespersen N.R., et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation. 2019;139:2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho K.L., Karwi Q.G., Wagg C., Zhang L., Vo K., Altamimi T., Uddin G.M., Ussher J.R., Lopaschuk G.D. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc. Res. 2021;117:1178–1187. doi: 10.1093/cvr/cvaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohammadifard N., Mansourian M., Firouzi S., Taheri M., Haghighatdoost F. Longitudinal association of dietary carbohydrate and the risk cardiovascular disease: A dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2022;62:6277–6292. doi: 10.1080/10408398.2021.1900057. [DOI] [PubMed] [Google Scholar]
- 45.Lagiou P., Sandin S., Lof M., Trichopoulos D., Adami H.O., Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: Prospective cohort study. BMJ (Clin. Res. Ed.) 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yurista S.R., Chong C.R., Badimon J.J., Kelly D.P., de Boer R.A., Westenbrink B.D. Therapeutic Potential of Ketone Bodies for Patients With Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021;77:1660–1669. doi: 10.1016/j.jacc.2020.12.065. [DOI] [PubMed] [Google Scholar]
- 47.Donato A.J., Machin D.R., Lesniewski L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han Y.M., Bedarida T., Ding Y., Somba B.K., Lu Q., Wang Q., Song P., Zhou M.-H. β-Hydroxybutyrate Prevents Vascular Senescence through hnRNP A1-Mediated Upregulation of Oct4. Mol. Cell. 2018;71:1064–1078. doi: 10.1016/j.molcel.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasser S., Vialichka V., Biesiekierska M., Balcerczyk A., Pirola L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes. 2020;11:584. doi: 10.4239/wjd.v11.i12.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo M., Wang X., Zhao Y., Yang Q., Ding H., Dong Q., Chen X., Cui M. Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing Drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front. Mol. Neurosci. 2018;11:86. doi: 10.3389/fnmol.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brand M.D., Orr A.L., Perevoshchikova I.V., Quinlan C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013;169((Suppl. S2)):1–8. doi: 10.1111/bjd.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamanashi T., Iwata M., Kamiya N., Tsunetomi K., Kajitani N., Wada N., Iitsuka T., Yamauchi T., Miura A., Pu S., et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 2017;7:7677. doi: 10.1038/s41598-017-08055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He F., Zuo L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015;16:27770–27780. doi: 10.3390/ijms161126059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greco T., Glenn T.C., Hovda D.A., Prins M.L. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 2016;36:1603–1613. doi: 10.1177/0271678X15610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolwicz S.C., Jr. Ketone Body Metabolism in the Ischemic Heart. Front. Cardiovasc. Med. 2021;8:789458. doi: 10.3389/fcvm.2021.789458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Özdemir R., Güzel O., Küçük M., Karadeniz C., Katipoglu N., Yılmaz Ü., Yılmazer M.M., Meşe T. The Effect of the Ketogenic Diet on the Vascular Structure and Functions in Children With Intractable Epilepsy. Pediatric Neurol. 2016;56:30–34. doi: 10.1016/j.pediatrneurol.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Doksöz Ö., Güzel O., Yılmaz Ü., İşgüder R., Çeleğen K., Meşe T., Uysal U. The Short-Term Effect of Ketogenic Diet on Carotid Intima-Media Thickness and Elastic Properties of the Carotid Artery and the Aorta in Epileptic Children. J. Child Neurol. 2015;30:1646–1650. doi: 10.1177/0883073815576793. [DOI] [PubMed] [Google Scholar]
- 59.Frezza C. Metabolism and Cancer: The Future Is Now. Nature Publishing Group; Berlin, Germany: 2020. pp. 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belfiore A., Malaguarnera R. Insulin receptor and cancer. Endocr.-Relat. Cancer. 2011;18:R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 61.Hay N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber D.D., Aminzadeh-Gohari S., Tulipan J., Catalano L., Feichtinger R.G., Kofler B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020;33:102–121. doi: 10.1016/j.molmet.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woolf E.C., Curley K.L., Liu Q., Turner G.H., Charlton J.A., Preul M.C., Scheck A.C. The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angiogenesis, invasive potential and vascular permeability in a mouse glioma model. PLoS ONE. 2015;10:e0130357. doi: 10.1371/journal.pone.0130357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lussier D.M., Woolf E.C., Johnson J.L., Brooks K.S., Blattman J.N., Scheck A.C. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16:1–10. doi: 10.1186/s12885-016-2337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dang M.T., Wehrli S., Dang C.V., Curran T. The ketogenic diet does not affect growth of hedgehog pathway medulloblastoma in mice. PLoS ONE. 2015;10:e0133633. doi: 10.1371/journal.pone.0133633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caso J., Masko E.M., Ii J.A.T., Poulton S.H., Dewhirst M., Pizzo S.V., Freedland S.J. The effect of carbohydrate restriction on prostate cancer tumor growth in a castrate mouse xenograft model. Prostate. 2013;73:449–454. doi: 10.1002/pros.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsujimoto T., Kajio H., Sugiyama T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. Int. J. Cancer. 2017;141:102–111. doi: 10.1002/ijc.30729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrea L., Caprio M., Tuccinardi D., Moriconi E., Di Renzo L., Muscogiuri G., Colao A., Savastano S., on behalf of the Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group Could ketogenic diet “starve” cancer? Emerging evidence. Crit. Rev. Food Sci. Nutr. 2022;62:1800–1821. doi: 10.1080/10408398.2020.1847030. [DOI] [PubMed] [Google Scholar]
- 69.Fine E.J., Segal-Isaacson C., Feinman R.D., Herszkopf S., Romano M.C., Tomuta N., Bontempo A.F., Negassa A., Sparano J.A. Targeting insulin inhibition as a metabolic therapy in advanced cancer: A pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28:1028–1035. doi: 10.1016/j.nut.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Cohen C.W., Fontaine K.R., Arend R.C., Alvarez R.D., Leath C.A., III, Huh W.K., Bevis K., Kim K.H., Straughn J.M., Jr., Gower B.A. A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer. J. Nutr. 2018;148:1253–1260. doi: 10.1093/jn/nxy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang J., Slingerland J.M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:336–342. doi: 10.4161/cc.2.4.433. [DOI] [PubMed] [Google Scholar]
- 72.Saboory E., Gholizadeh-Ghaleh Aziz S., Samadi M., Biabanghard A., Chodari L. Exercise and insulin-like growth factor 1 supplementation improve angiogenesis and angiogenic cytokines in a rat model of diabetes-induced neuropathy. Exp. Physiol. 2020;105:783–792. doi: 10.1113/EP088069. [DOI] [PubMed] [Google Scholar]
- 73.Zhou S., Kachhap S., Sun W., Wu G., Chuang A., Poeta L., Grumbine L., Mithani S.K., Chatterjee A., Koch W., et al. Frequency and phenotypic implications of mitochondrial DNA mutations in human squamous cell cancers of the head and neck. Proc. Natl. Acad. Sci. USA. 2007;104:7540–7545. doi: 10.1073/pnas.0610818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chatterjee A., Mambo E., Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 75.Gammage P.A., Frezza C. Mitochondrial DNA: The overlooked oncogenome? BMC Biol. 2019;17:1–10. doi: 10.1186/s12915-019-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klement R.J. The emerging role of ketogenic diets in cancer treatment. Curr. Opin. Clin. Nutr. Metab. Care. 2019;22:129–134. doi: 10.1097/MCO.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan L.B., Chandel N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khodabakhshi A., Akbari M.E., Mirzaei H.R., Mehrad-Majd H., Kalamian M., Davoodi S.H. Feasibility, safety, and beneficial effects of MCT-based ketogenic diet for breast cancer treatment: A randomized controlled trial study. Nutr. Cancer. 2020;72:627–634. doi: 10.1080/01635581.2019.1650942. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka K., Abe M., Sato Y. Roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the signal transduction of basic fibroblast growth factor in endothelial cells during angiogenesis. Jpn. J. Cancer Res. 1999;90:647–654. doi: 10.1111/j.1349-7006.1999.tb00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen B.G., Bhatia S.K., Buatti J.M., Brandt K.E., Lindholm K.E., Button A.M., Szweda L.I., Smith B.J., Spitz D.R., Fath M.A. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin. Cancer Res. 2013;19:3905–3913. doi: 10.1158/1078-0432.CCR-12-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diakos C.I., Charles K.A., McMillan D.C., Clarke S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 82.Baracos V.E. Skeletal muscle anabolism in patients with advanced cancer. Lancet Oncol. 2014;16:13–14. doi: 10.1016/S1470-2045(14)71185-4. [DOI] [PubMed] [Google Scholar]
- 83.Fearon K., Borland W., Preston T., Tisdale M.J., Shenkin A., Calman K.C. Cancer cachexia: Influence of systemic ketosis on substrate levels and nitrogen metabolism. Am. J. Clin. Nutr. 1988;47:42–48. doi: 10.1093/ajcn/47.1.42. [DOI] [PubMed] [Google Scholar]
- 84.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, inflammation, and cancer. Annu. Rev. Pathol. Mech. Dis. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 85.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 86.Moossavi M., Parsamanesh N., Bahrami A., Atkin S.L., Sahebkar A. Role of the NLRP3 inflammasome in cancer. Mol. Cancer. 2018;17:1–13. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamarsheh S., Zeiser R. NLRP3 inflammasome activation in cancer: A double-edged sword. Front. Immunol. 2020;11:1444. doi: 10.3389/fimmu.2020.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Youm Y.-H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T.D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seyfried T., Sanderson T., El-Abbadi M., McGowan R., Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br. J. Cancer. 2003;89:1375–1382. doi: 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakamura K., Tonouchi H., Sasayama A., Ashida K. A ketogenic formula prevents tumor progression and cancer cachexia by attenuating systemic inflammation in colon 26 tumor-bearing mice. Nutrients. 2018;10:206. doi: 10.3390/nu10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt M., Pfetzer N., Schwab M., Strauss I., Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr. Metab. 2011;8:54. doi: 10.1186/1743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weber D.D., Aminazdeh-Gohari S., Kofler B. Ketogenic diet in cancer therapy. Aging. 2018;10:164–165. doi: 10.18632/aging.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aminzadeh-Gohari S., Feichtinger R.G., Vidali S., Locker F., Rutherford T., O’Donnel M., Stöger-Kleiber A., Mayr J.A., Sperl W., Kofler B. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget. 2017;8:64728. doi: 10.18632/oncotarget.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang L., TeSlaa T., Ng S., Nofal M., Wang L., Lan T., Zeng X., Cowan A., McBride M., Lu W., et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 2022;3:119–136. doi: 10.1016/j.medj.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klement R.J., Koebrunner P.S., Meyer D., Kanzler S., Sweeney R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: IV. Final results of the KETOCOMP study for rectal cancer patients. Clin. Nutr. 2021;40:4674–4684. doi: 10.1016/j.clnu.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 96.Iyikesici M.S. Long-term survival outcomes of metabolically supported chemotherapy with gemcitabine-based or FOLFIRINOX regimen combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy in metastatic pancreatic cancer. Complement. Med. Res. 2020;27:31–39. doi: 10.1159/000502135. [DOI] [PubMed] [Google Scholar]
- 97.Woodhouse C., Ward T., Gaskill-Shipley M., Chaudhary R. Feasibility of a modified Atkins diet in glioma patients during radiation and its effect on radiation sensitization. Curr. Oncol. 2019;26:433–438. doi: 10.3747/co.26.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van der Louw E.J., Olieman J.F., van den Bemt P.M., Bromberg J.E., Oomen-de Hoop E., Neuteboom R.F., Catsman-Berrevoets C.E., Vincent A.J.P.E. Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: A feasibility and safety study. Ther. Adv. Med. Oncol. 2019;11:1758835919853958. doi: 10.1177/1758835919853958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morscher R.J., Aminzadeh-Gohari S., Feichtinger R.G., Mayr J.A., Lang R., Neureiter D., Sperl W., Kofler B. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-Nu mouse model. PLoS ONE. 2015;10:e0129802. doi: 10.1371/journal.pone.0129802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Champ C.E., Palmer J.D., Volek J.S., Werner-Wasik M., Andrews D.W., Evans J.J., Glass J., Kim L., Shi W. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J. Neuro-Oncol. 2014;117:125–131. doi: 10.1007/s11060-014-1362-0. [DOI] [PubMed] [Google Scholar]
- 101.Vidali S., Aminzadeh-Gohari S., Feichtinger R.G., Vatrinet R., Koller A., Locker F., Rutherford T., O’Donnell M., Stöger-Kleiber A., Lambert B., et al. The ketogenic diet is not feasible as a therapy in a CD-1 nu/nu mouse model of renal cell carcinoma with features of Stauffer’s syndrome. Oncotarget. 2017;8:57201–57215. doi: 10.18632/oncotarget.19306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liśkiewicz A.D., Kasprowska D., Wojakowska A., Polański K., Lewin-Kowalik J., Kotulska K., Jędrzejowska-Szypułka H. Long-term High Fat Ketogenic Diet Promotes Renal Tumor Growth in a Rat Model of Tuberous Sclerosis. Sci. Rep. 2016;6:21807. doi: 10.1038/srep21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia S., Lin R., Jin L., Zhao L., Kang H.B., Pan Y., Liu S., Qian G., Qian Z., Konstantakou E., et al. Prevention of Dietary-Fat-Fueled Ketogenesis Attenuates BRAF V600E Tumor Growth. Cell Metab. 2017;25:358–373. doi: 10.1016/j.cmet.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kossoff E.H., Hartman A.L. Ketogenic diets: New advances for metabolism-based therapies. Curr. Opin. Neurol. 2012;25:173. doi: 10.1097/WCO.0b013e3283515e4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kwiterovich P.O., Jr., Vining E.P., Pyzik P., Skolasky R., Jr., Freeman J.M. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA. 2003;290:912–920. doi: 10.1001/jama.290.7.912. [DOI] [PubMed] [Google Scholar]
- 106.Caprio M., Infante M., Moriconi E., Armani A., Fabbri A., Mantovani G., Mariani S., Lubrano C., Poggiogalle E., Migliaccio S., et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE) J. Endocrinol. Investig. 2019;42:1365–1386. doi: 10.1007/s40618-019-01061-2. [DOI] [PubMed] [Google Scholar]
- 107.Rojas-Morales P., León-Contreras J.C., Sánchez-Tapia M., Silva-Palacios A., Cano-Martínez A., González-Reyes S., Jiménez-Osorio A.S., Hernández-Pando R., Osorio-Alonso H., Sánchez-Lozada L.G., et al. A ketogenic diet attenuates acute and chronic ischemic kidney injury and reduces markers of oxidative stress and inflammation. Life Sci. 2022;289:120227. doi: 10.1016/j.lfs.2021.120227. [DOI] [PubMed] [Google Scholar]
- 108.Ricci A., Idzikowski M.A., Soares C.N., Brietzke E. Exploring the mechanisms of action of the antidepressant effect of the ketogenic diet. Rev. Neurosci. 2020;31:637–648. doi: 10.1515/revneuro-2019-0073. [DOI] [PubMed] [Google Scholar]
- 109.Zhang N., Liu C., Jin L., Zhang R., Wang T., Wang Q., Chen J., Yang F., Siebert H.-S., Zheng X. Ketogenic diet elicits antitumor properties through inducing oxidative stress, inhibiting MMP-9 expression, and rebalancing M1/M2 tumor-associated macrophage phenotype in a mouse model of colon cancer. J. Agric. Food Chem. 2020;68:11182–11196. doi: 10.1021/acs.jafc.0c04041. [DOI] [PubMed] [Google Scholar]
- 110.Jain S.K., McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes. 1999;48:1850–1855. doi: 10.2337/diabetes.48.9.1850. [DOI] [PubMed] [Google Scholar]
- 111.Arsyad A., Idris I., Rasyid A.A., Usman R.A., Faradillah K.R., Latif W.O.U., Lubis Z.I., Aminuddin A., Yustisia I., Djabir Y.Y. Long-term ketogenic diet induces metabolic acidosis, anemia, and oxidative stress in healthy wistar rats. J. Nutr. Metab. 2020;2020:3642035. doi: 10.1155/2020/3642035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Milder J., Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012;100:295–303. doi: 10.1016/j.eplepsyres.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barrea L., Caprio M., Watanabe M., Cammarata G., Feraco A., Muscogiuri G., Verde L., Colao A., Savastano S., on behalf of Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence. Crit. Rev. Food Sci. Nutr. 2022:1–17. doi: 10.1080/10408398.2022.2054935. [DOI] [PubMed] [Google Scholar]
- 114.Asrih M., Altirriba J., Rohner-Jeanrenaud F., Jornayvaz F.R. Ketogenic diet impairs FGF21 signaling and promotes differential inflammatory responses in the liver and white adipose tissue. PLoS ONE. 2015;10:e0126364. doi: 10.1371/journal.pone.0126364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenbaum M., Hall K.D., Guo J., Ravussin E., Mayer L.S., Reitman M.L., Smith S.R., Walsh B.T., Leibel R.L. Glucose and Lipid Homeostasis and Inflammation in Humans Following an Isocaloric Ketogenic Diet. Obesity. 2019;27:971–981. doi: 10.1002/oby.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ebbeling C.B., Swain J.F., Feldman H.A., Wong W.W., Hachey D.L., Garcia-Lago E., Ludwig D.S. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song X., Kestin M., Schwarz Y., Yang P., Hu X., Lampe J.W., Kratz M. A low-fat high-carbohydrate diet reduces plasma total adiponectin concentrations compared to a moderate-fat diet with no impact on biomarkers of systemic inflammation in a randomized controlled feeding study. Eur. J. Nutr. 2016;55:237–246. doi: 10.1007/s00394-015-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jonasson L., Guldbrand H., Lundberg A.K., Nystrom F.H. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann. Med. 2014;46:182–187. doi: 10.3109/07853890.2014.894286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Field R., Field T., Pourkazemi F., Rooney K. Low-carbohydrate and ketogenic diets: A scoping review of neurological and inflammatory outcomes in human studies and their relevance to chronic pain. Nutr. Res. Rev. 2022:1–71. doi: 10.1017/S0954422422000087. [DOI] [PubMed] [Google Scholar]
- 120.Hasan-Olive M.M., Lauritzen K.H., Ali M., Rasmussen L.J., Storm-Mathisen J., Bergersen L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem. Res. 2019;44:22–37. doi: 10.1007/s11064-018-2588-6. [DOI] [PubMed] [Google Scholar]
- 121.Chung J.Y., Kim O.Y., Song J. Role of ketone bodies in diabetes-induced dementia: Sirtuins, insulin resistance, synaptic plasticity, mitochondrial dysfunction, and neurotransmitter. Nutr. Rev. 2022;80:774–785. doi: 10.1093/nutrit/nuab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gano L.B., Patel M., Rho J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014;55:2211–2228. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y., Yang X., Zhang J., Jiang T., Zhang Z., Wang Z., Gong M., Zhao L., Zhang C. Ketogenic diets induced glucose intolerance and lipid accumulation in mice with alterations in gut microbiota and metabolites. MBio. 2021;12:e03601-20. doi: 10.1128/mBio.03601-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Basciani S., Camajani E., Contini S., Persichetti A., Risi R., Bertoldi L., Strigari L., Prossomariti G., Watanabe M., Mariani S., et al. Very-Low-Calorie Ketogenic Diets With Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020;105:2939–2949. doi: 10.1210/clinem/dgaa336. [DOI] [PubMed] [Google Scholar]
- 126.Di Rosa C., Lattanzi G., Taylor S.F., Manfrini S., Khazrai Y.M., Practice C. Very low calorie ketogenic diets in overweight and obesity treatment: Effects on anthropometric parameters, body composition, satiety, lipid profile and microbiota. Obes. Res. Clin. Pract. 2020;14:491–503. doi: 10.1016/j.orcp.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 127.Gutiérrez-Repiso C., Hernández-García C., García-Almeida J.M., Bellido D., Martín-Núñez G.M., Sánchez-Alcoholado L., Alcaide-Torres J., Sajoux I., Tinahones F.J., Moreno-Indias I. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol. Nutr. Food Res. 2019;63:e1900167. doi: 10.1002/mnfr.201900167. [DOI] [PubMed] [Google Scholar]
- 128.Reddel S., Putignani L., Del Chierico F. The Impact of Low-FODMAPs, Gluten-Free, and Ketogenic Diets on Gut Microbiota Modulation in Pathological Conditions. Nutrients. 2019;11:373. doi: 10.3390/nu11020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rondanelli M., Gasparri C., Peroni G., Faliva M.A., Naso M., Perna S., Bazire P., Sajuox I., Maugeri R., Rigon C. The potential roles of very low calorie, very low calorie ketogenic diets and very low carbohydrate diets on the gut microbiota composition. Front. Endocrinol. 2021;12:518. doi: 10.3389/fendo.2021.662591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y., Zhou S., Zhou Y., Yu L., Zhang L., Wang Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018;145:163–168. doi: 10.1016/j.eplepsyres.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 131.Ye F., Li X.J., Jiang W.L., Sun H.B., Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: A meta-analysis. J. Clin. Neurol. 2015;11:26–31. doi: 10.3988/jcn.2015.11.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sellem L., Flourakis M., Jackson K.G., Joris P.J., Lumley J., Lohner S., Mensink R.P., Soedamah-Muthu S.S., Lovegrove J.A. Impact of Individual Dietary Saturated Fatty Acid Replacement on Circulating Lipids and Other Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-analysis of RCTs in Humans. Adv. Nutr. 2021;13:1220–1225. doi: 10.1093/advances/nmab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu M., Zuo L.-S.-Y., Sun T.-Y., Wu Y.-Y., Liu Y.-P., Zeng F.-F., Chen Y.-M. Circulating Very-Long-Chain Saturated Fatty Acids Were Inversely Associated with Cardiovascular Health: A Prospective Cohort Study and Meta-Analysis. Nutrients. 2020;12:2709. doi: 10.3390/nu12092709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Asrih M., Jornayvaz F.R. Diets and nonalcoholic fatty liver disease: The good and the bad. Clin. Nutr. 2014;33:186–190. doi: 10.1016/j.clnu.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 135.Fechner E., Smeets E., Schrauwen P., Mensink R.P. The Effects of Different Degrees of Carbohydrate Restriction and Carbohydrate Replacement on Cardiometabolic Risk Markers in Humans-A Systematic Review and Meta-Analysis. Nutrients. 2020;12:991. doi: 10.3390/nu12040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu Y., Bo Y., Liu Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019;18:91. doi: 10.1186/s12944-019-1035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang L., Lin J.S., Aris I.M., Yang G., Chen W.Q., Li L.J. Circulating Saturated Fatty Acids and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2019;11:998. doi: 10.3390/nu11050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Panth N., Abbott K.A., Dias C.B., Wynne K., Garg M.L. Differential effects of medium- and long-chain saturated fatty acids on blood lipid profile: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018;108:675–687. doi: 10.1093/ajcn/nqy167. [DOI] [PubMed] [Google Scholar]
- 139.Hannon B.A., Thompson S.V., An R., Teran-Garcia M. Clinical outcomes of dietary replacement of saturated fatty acids with unsaturated fat sources in adults with overweight and obesity: A systematic review and meta-analysis of randomized control trials. Ann. Nutr. Metab. 2017;71:107–117. doi: 10.1159/000477216. [DOI] [PubMed] [Google Scholar]
- 140.Volek J.S., Phinney S.D., Forsythe C.E., Quann E.E., Wood R.J., Puglisi M.J., Kraemer W.J., Bibus D.M., Fernandez M.L., Feinman R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44:297–309. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- 141.Dashti H.M., Al-Zaid N.S., Mathew T.C., Al-Mousawi M., Talib H., Asfar S.K., Behbahani A.I. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol. Cell. Biochem. 2006;286:1–9. doi: 10.1007/s11010-005-9001-x. [DOI] [PubMed] [Google Scholar]
- 142.Li S., Lin G., Chen J., Chen Z., Xu F., Zhu F., Zhang J., Yuan S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022;22:34. doi: 10.1186/s12902-022-00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wolver S., Fadel K., Fieger E., Aburish Z., O’Rourke B., Chandler T.M., Shimotani D., Clingempeel N., Jain S., Jain A., et al. Clinical Use of a Real-World Low Carbohydrate Diet Resulting in Reduction of Insulin Dose, Hemoglobin A1c, and Weight. Front. Nutr. 2021;8:690855. doi: 10.3389/fnut.2021.690855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Radišauskas R., Kuzmickienė I., Milinavičienė E., Everatt R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina. 2016;52:89–98. doi: 10.1016/j.medici.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Han H., Guo W., Shi W., Yu Y., Zhang Y., Ye X., He J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017;7:44877. doi: 10.1038/srep44877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liang Z., Xie B., Li J., Wang X., Wang S., Meng S., Ji A., Zhu Y., Xu X., Zheng X., et al. Hypertension and risk of prostate cancer: A systematic review and meta-analysis. Sci. Rep. 2016;6:31358. doi: 10.1038/srep31358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Castellana M., Conte E., Cignarelli A., Perrini S., Giustina A., Giovanella L., Giorgino F., Trimboli P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020;21:5–16. doi: 10.1007/s11154-019-09514-y. [DOI] [PubMed] [Google Scholar]
- 148.Bueno N.B., de Melo I.S., de Oliveira S.L., da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013;110:1178–1187. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 149.Polito R., Messina G., Valenzano A., Scarinci A., Villano I., Monda M., Cibelli G., Porro C., Pisanelli D., Monda V., et al. The Role of Very Low Calorie Ketogenic Diet in Sympathetic Activation through Cortisol Secretion in Male Obese Population. J. Clin. Med. 2021;10:4230. doi: 10.3390/jcm10184230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A., Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc. Natl. Acad. Sci. USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Esposito K., Chiodini P., Capuano A., Bellastella G., Maiorino M.I., Rafaniello C., Giugliano D. Metabolic syndrome and postmenopausal breast cancer: Systematic review and meta-analysis. Menopause. 2013;20:1301–1309. doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 152.Esposito K., Chiodini P., Capuano A., Bellastella G., Maiorino M.I., Parretta E., Lenzi E., Giugliano D. Effect of metabolic syndrome and its components on prostate cancer risk: Meta-analysis. J. Endocrinol. Investig. 2013;36:132–139. doi: 10.1007/BF03346748. [DOI] [PubMed] [Google Scholar]
- 153.Yao X., Tian Z. Dyslipidemia and colorectal cancer risk: A meta-analysis of prospective studies. Cancer Causes Control. 2015;26:257–268. doi: 10.1007/s10552-014-0507-y. [DOI] [PubMed] [Google Scholar]
- 154.Esteve E., Ricart W., Fernández-Real J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005;24:16–31. doi: 10.1016/j.clnu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 155.Kim S., Keku T.O., Martin C., Galanko J., Woosley J.T., Schroeder J.C., Satia J.A., Halabi S., Sandler R.S. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vekic J., Kotur-Stevuljevic J., Jelic-Ivanovic Z., Spasic S., Spasojevic-Kalimanovska V., Topic A., Zeljkovic A., Stefanovic A., Zunic G. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. European journal of clinical investigation. Eur. J. Clin. Investig. 2007;37:715–723. doi: 10.1111/j.1365-2362.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 157.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes. 2015;6:456. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yuan X., Wang J., Yang S., Gao M., Cao L., Li X., Hong D., Tian S., Sun C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Volek J.S., Sharman M.J., Forsythe C.E. Modification of lipoproteins by very low-carbohydrate diets. J. Nutr. 2005;135:1339–1342. doi: 10.1093/jn/135.6.1339. [DOI] [PubMed] [Google Scholar]
- 160.Bergqvist A.G. Long-term monitoring of the ketogenic diet: Do’s and Don’ts. Epilepsy Res. 2012;100:261–266. doi: 10.1016/j.eplepsyres.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 161.Pi-Sunyer X. The medical risks of obesity. Postgrad. Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaaks R., editor. Biology of IGF-1 Novartis Foundation Symposium. John Wiley; New York, NY, USA: 2005. Nutrition, insulin, IGF-1 metabolism and cancer risk: A summary of epidemiological evidence. [PubMed] [Google Scholar]
- 163.Berger N.A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paoli A., Mancin L., Bianco A., Thomas E., Mota J.F., Piccini F. Ketogenic diet and microbiota: Friends or enemies? Genes. 2019;10:534. doi: 10.3390/genes10070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Templeman N.M., Skovsø S., Page M.M., Lim G.E., Johnson J.D. A causal role for hyperinsulinemia in obesity. J. Endocrinol. 2017;232:R173–R183. doi: 10.1530/JOE-16-0449. [DOI] [PubMed] [Google Scholar]
- 166.Carlzon D., Svensson J., Petzold M., Karlsson M.K., Ljunggren Ö., Tivesten Å., Mellström D., Ohlsson C. Both low and high serum IGF-1 levels associate with increased risk of cardiovascular events in elderly men. J. Clin. Endocrinol. Metab. 2014;99:E2308–E2316. doi: 10.1210/jc.2014-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Choi Y.J., Jeon S.M., Shin S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boden G., Sargrad K., Homko C., Mozzoli M., Stein T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005;142:403–411. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.