Abstract
Pectin is a dietary fiber, and its health effects have been described extensively. Although there are limited clinical studies, there is a growing body of evidence from in vitro studies investigating the effect of pectin on human gut microbiota. This comprehensive review summarizes the findings of gut microbiota modulation in vitro as assessed by 16S rRNA gene-based technologies and elucidates the potential structure-activity relationships. Generally, pectic substrates are slowly but completely fermented, with a greater production of acetate compared with other fibers. Their fermentation, either directly or by cross-feeding interactions, results in the increased abundances of gut bacterial communities such as the family of Ruminococcaceae, the Bacteroides and Lachnospira genera, and species such as Lachnospira eligens and Faecalibacterium prausnitzii, where the specific stimulation of Lachnospira and L. eligens is unique to pectic substrates. Furthermore, the degree of methyl esterification, the homogalacturonan-to-rhamnogalacturonan ratio, and the molecular weight are the most influential structural factors on the gut microbiota. The latter particularly influences the growth of Bifidobacterium spp. The prebiotic potential of pectin targeting specific gut bacteria beneficial for human health and well-being still needs to be confirmed in humans, including the relationship between its structural features and activity.
Keywords: pectin, microbiota, human, prebiotic, dietary fiber, gut health
1. Introduction
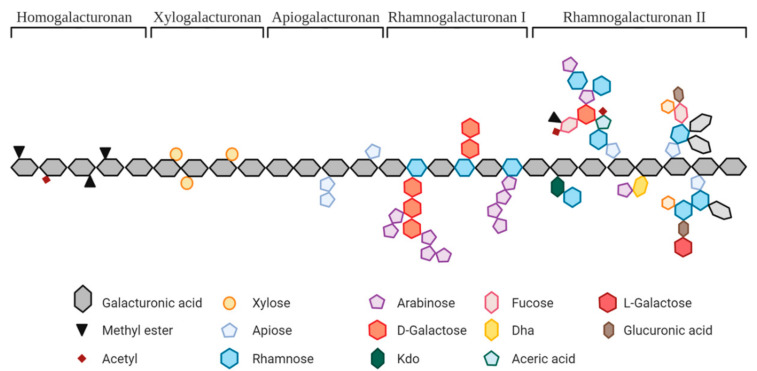
Pectin constitutes a family of complex heteropolysaccharides present in the primary cell walls and middle lamella of higher plants (Figure 1). Pectin is widely used as a food additive, and its chemical structure depends on its botanical origin, part of the plant used for extraction, and extraction method. Structurally, pectin polysaccharides share some similar features. Homogalacturonan (HG) or ‘smooth region’ is the most abundant domain (approx. 60%) which is primarily composed of a homopolymer of partially methyl-esterified α-1,4-D-galacturonic acid (GalA) units. Rhamnogalacturonan I (RGI) or ‘hairy’ region consists of a backbone of repeating disaccharides based on GalA and rhamnose units. RGI regions account for about 15–20% of pectin molecules and are highly branched structures with neutral sugars (mainly arabinose and galactose) and side chains (arabinans, galactans and arabinogalactans) attached to the rhamnose units. RGI is highly present in carrots, okra, tomatoes, and potatoes, where its side chains principally contain arabinan in apples and sugar beet, and galactan in potatoes. Other pectin domains include rhamnogalacturonan II (RGII) and xylogalacturonan, the former being an overly complex branched structure composed of a HG backbone branched with L-rhamnose, D-galactose and other minor sugars. RGII typically accounts for 10% of pectin, and it has been described as the most complex polysaccharide known [1,2].
Figure 1.
Overview of the pectin structure [3]. Copyright: Creative Commons—Attribution 4.0 International—CC BY 4.0.
As a food additive, pectin on the current market is commonly obtained from citrus, apple, and sugar beet, containing a minimum of 65% of GalA. The technical classification of pectin is based on its degree of methyl esterification (DM), (i.e., the percentage of GalA units esterified with methanol). High-methoxy (HM) pectin has over 50% of its carboxyl groups esterified with methanol whereas low-methoxy (LM) pectin has less than 50% [2]. Aside from DM, other structural features govern the suitability of pectin for specific applications, including the molecular weight, GalA content, neutral sugars content, and proportion of HG:RG regions [4].
Pectin qualifies as a dietary fiber, since it is neither digested in the stomach nor the small intestine, but largely fermented in the large intestine [5]. Fiber-associated health benefits have been shown with pectin in vitro such as enhanced antihypertensive effect in fermented food products [6], as well as in vivo, such as a reduction in postprandial glycemic response and the maintenance of blood cholesterol in a normal range [7,8]. Dietary fibers can modulate the gut microbiome and specific bacterial groups, and their variety might be key to supporting it through cross-feeding interactions [9]. It could then be questioned if pectic substrates can be classified as prebiotics, a term defined as a “substrate that is selectively utilized by host microorganisms conferring a health benefit” [10]. Several human clinical studies have shown the potential benefits of pectin on gut health [11,12] (e.g., by reducing digestive symptoms such as regurgitation in infants, and the alleviation of diarrhea or intolerance symptoms in adults fed enteral nutrition) [13,14]. Overall, it is unclear how these beneficial effects are related to the fermentation of pectin in the large intestine. Until now, human clinical studies that have studied the effect of the dietary supplementation of pectin on gut microbiota composition are still scarce [15,16].
In vitro gut models have been widely used to study the impact of diet on the gut microbiota since they allow gaining insight into the fermentation processes mediated by the gut microbiota [17]. In vitro gut models vary in design, from simple batch incubations to more complex semi-continuous or multi-compartmental continuous models representing distinct parts of the human colon [18]. These fermentation models coupled with recent advances in high-throughput sequencing techniques and culture-independent methodologies, such as sequencing of the 16S rRNA gene have allowed the extensive investigation of the diversity, function, and dynamics of the gut microbial communities. Earlier (and widely employed) cultivation-independent 16S rRNA-based methods such as quantitative (real-time) PCR and fluorescent in situ hybridization (FISH) target specific bacterial groups and exhibit an overall good taxonomic resolution and sensitivity. High-throughput methods including next-generation sequencing techniques provide sequencing of PCR amplicons of the 16S rRNA gene or fragmented total (meta)genomic DNA from the whole community (e.g., Illumina, PacBio, ion semiconductor sequencing (Ion Torrent), and Nanopore sequencing, among others). Although 16S rRNA gene-based technologies pose different advantages and limitations, their adaptation allows superior monitoring of changes in the overall microbial community diversity due to fiber consumption [19].
Most of the research investigating the effects of pectin and pectin-derived substrates on the human gut microbiota has been performed in vitro, and to this date, this evidence has not been systematically reviewed [20,21]. Therefore, this review aims to be the first to systematically evaluate in vitro fermentation studies using human fecal samples to (1) determine the state of evidence of the potential effects of pectic substrates on the gut microbiota composition and their fermentative activities, and (2) clarify the potential structure-function relationships based on the complexity of the pectin molecular structure. This knowledge could aid in the better design of human clinical studies, and the development of pectin-derived ingredients with the greatest prebiotic potential.
2. Materials and Methods
This systematic review was conducted to elucidate the available evidence on the effects of pectin and pectin-derived substrates on the human gut microbiota using an in vitro fermentation setup. This review was conducted in line with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22].
2.1. Search Strategy and Eligibility Criteria
A literature search was performed in the electronic databases PubMed and Scopus on 29 January 2021. The detailed search strategies used for each database are described in Supplementary Table S1. Peer-reviewed publications were also identified by searching the reference lists of other papers, and they were identified after the search date and until submission. To be included in the systematic review, the following eligibility criteria were used: (1) an in vitro fermentation system was used with human gut microbiota as inoculum (fecal and from ileostomy), (2) pectic substrates (pectin and/or pectin-derived substrates extracted from different raw materials, and in some cases further modified by different treatments) were tested individually (not in a blend), (3) the gut microbiota composition was assessed using comprehensive molecular biological methodologies, (4) the gut microbiota composition and fermentation activity (if also studied) were included as results of the study, (5) the articles were published in the English language, and (6) the articles were published after 1 January 2010, to evaluate the most recent data available.
2.2. Study Screening
Two authors (F.G. and N.P.) performed the primary screening (i.e., title and abstract). The articles were assessed for their eligibility, and disagreements were resolved through consensus with a third author (F.R.). Supplementary literature searches involved examining the reference lists of all relevant studies and review articles to identify articles that were not captured in the initial search. Additional articles were selected for inclusion when deemed necessary. The full text of all potential eligible articles was retrieved, and consensus between the three investigators determined the final eligibility of each reference. Data from each eligible article were extracted by two authors (N.P. and F.R.) and included the author, subject (number, health status, and age), fecal inoculum (pooled and from a single donor), test product description and comparators included in the study, concentration of the ingredient used, pH (controlled and non-controlled), sampling time (hours and days), method of analysis of the gut microbiota, and main outcomes in terms of microbiota composition and fermentative activities.
3. Results
3.1. Study Selection and Characteristics
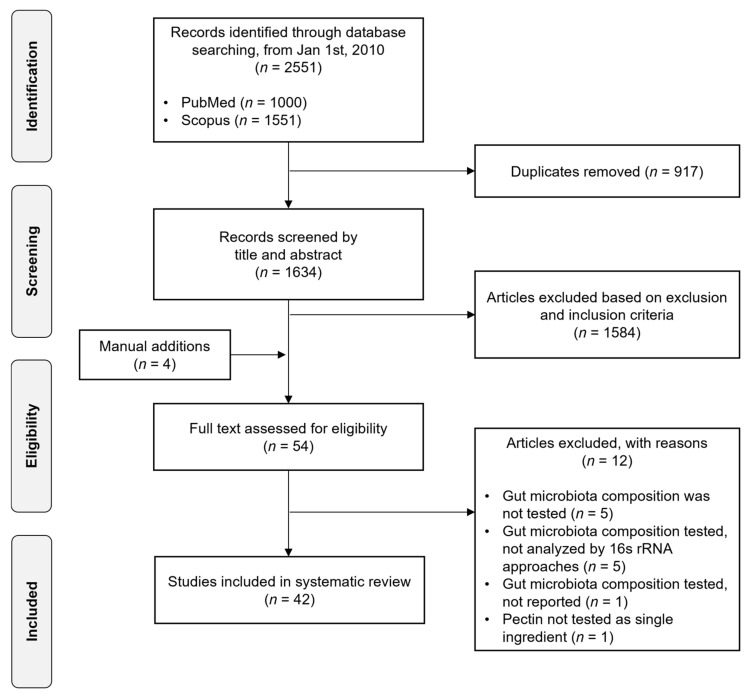
The study identification and selection are detailed in the PRISMA flow chart (Figure 2). The initial electronic search generated 1634 unduplicated records across 2 databases. Based on the inclusion and exclusion criteria applied to the titles and abstracts of all studies, 50 publications along with 4 manually added articles were selected for a full-text review, and of those, 42 articles were included in this systematic review.
Figure 2.
PRISMA flow diagram of studies evaluated in the systematic review.
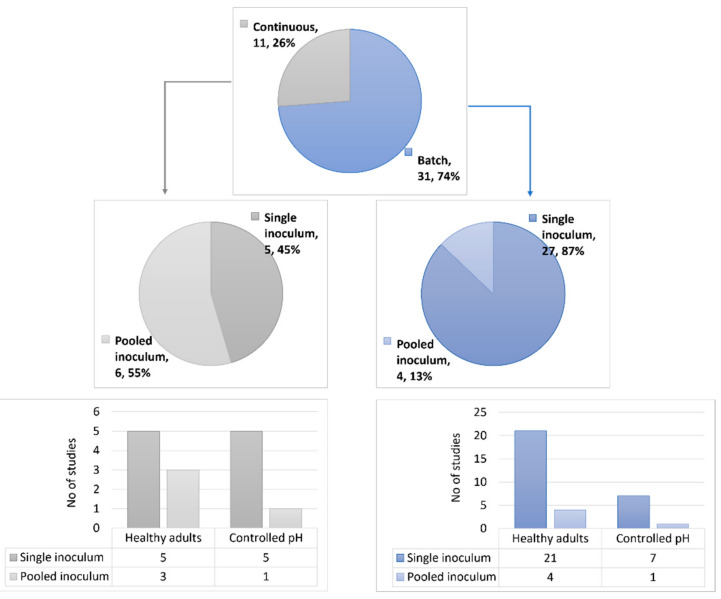
All 42 studies investigated the effect of pectic substrates on the human gut microbiota via in vitro fermentations, and of those, 5 studies also investigated their fermentation in upper gastrointestinal tract conditions. Overall, 31 studies were performed using batch fermentations and 11 studies used continuous fermenters, such as the Simulator of Human Intestinal Microbial Ecosystem (SHIME®) and the TNO in vitro colon model (TIM-2). Differences were found regarding the fecal inoculum of single donor vs. pooled, where most batch fermentations were performed using fecal samples from single donors (n = 1–17 donors). In contrast, approximately half of the continuous fermenter studies were performed with pooled fecal inocula (n = 3–8 donors) (Figure 3). Another difference found between the fermentation systems was the duration of the fermentation process. Batch fermentations were commonly performed for between 10 and 72 h, while continuous processes were mostly carried out in from 3 days to 21 days. Furthermore, pH differences were found among the batch fermentation and continuous fermenter studies, and interestingly, most batch fermentations (23 studies) were not pH-controlled. From the studies that were pH-controlled, 6 studies used a pH range resembling distal colon (DC) conditions (i.e., pH = 6.7–7.0), 1 study used a pH range of 5.8–6.3, and 1 study used 2 different conditions (i.e., pH = 5.5 and 6.5). In contrast, continuous fermenters (all pH-controlled) were either performed at a specific pH (6 studies, pH = 5.8 or 6.2) or at a pH that gradually changed during fermentation, resembling the different parts of the luminal colon (5 studies, pH = 5.6–6.9). Regarding the approach used in the technologies for analysis of the gut microbiota composition, targeted and non-targeted high-throughput technologies were used in the batch fermentation and continuous fermenter studies. Non-targeted approaches were more commonly used (18 batch fermentation studies and 9 continuous fermenter studies) than targeted approaches, and among the latter, qPCR was the most-used technology.
Figure 3.
Overview of in vitro studies included in this systematic review according to their experimental design.
Thirty-three studies were conducted with fecal samples from healthy adults with a normal range for their body mass index (BMI, kg/m2) who did not receive antibiotics for the last 2-6 months (3 months in general). One study was performed with samples from ileostomy and not fecal samples. One study was conducted with fecal samples from the elderly. Three studies evaluated the impact of pectin on the gut microbiota from overweight or obese subjects, and two of them compared lean and obese subjects in their experiments. Two studies were conducted using fecal samples from patients with ulcerative colitis (UC), one study was conducted with fecal samples from patients (condition not defined) with no gastrointestinal disorders, and one study was conducted using fecal samples from patients with cirrhosis.
3.2. Tested Products
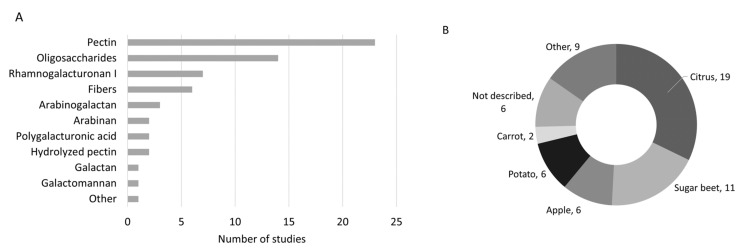
The type of pectic substrates tested varied among the studies based on raw material and structural differences (Table 1). In terms of structure, pectin was the most tested substrate, followed by oligosaccharides, RGI, and other fibers rich in pectin (Figure 4). In terms of raw material, most studies included pectic substrates from citrus and sugar beet, followed by apple and potato.
Table 1.
Description of the various structures of pectic substrates used in the studies.
| Pectic Substrates |
Origin | Molecular Structure and Main Linkages |
Other Parameters Described in the Studies |
|---|---|---|---|
| Pectin | Citrus, apple, sugar beet, soy, sunflower, artichoke, and prune | (GalA)n and/or (GalA-Rha)n and/or (GalA-Gal)n; α(1,4); α(1,2) |
GalA: 32–88% DE: 2–79% |
| Hydrolyzed pectin | Citrus, sunflower, and artichoke |
GalA: 56–79% DE: 5–17% MW: 9.2–300 kDa |
|
| OS from pectin | Methylated citrus pectin, orange or lemon peel, and apple |
GalA: 42–96%; DE: 29–62%; DP 1–10 or MW > 23 kDa |
|
| Sugar beet | GalA: <2–78%; Ara: 10–85%; DP 2–10 or MW: <1–12 kDa | ||
| Polygalacturonic acid | Citrus pectin | α(1,4)GalAn | GalA: >90% |
| OS from PolyGalA |
Polygalacturonic acid | GalA: 91–98% DP 1–23 |
|
| RG1- enriched |
Okra, carrot, A. thaliana seed mucilage, prune, lime, and potato |
α-(1,2)(Rha)n and α-(1,4)(GalA)n and β-(1,4)(Gal)n (potato only), and α-Ara and β-D-Gal residues of different sizes |
GalA: 10–25%; Ara > 48%; Potato: Gal 61%, 34 kDa |
| OS from RG1 | A. thaliana seed mucilage; Potato | Potato: >70% Gal; DP 2–70 | |
| Arabinan | Sugar beet | α-(1,5)(Ara)n and possible Ara residues or short side chains |
MW: 18 kDa, debranched, Ara:Gal:Rha = 71:26:3 |
| OS from Arabinan | DP 1–11, Ara: 93.4% | ||
| Arabinogalactan | Acacia fiber and larch tree | AGI: β-(1,4)-D-(Gal)n and occasional β-(1,3), and α-Ara/Fuc/GlucA AGII: β-(1,3)-D-(Gal)n and β-(1,6)-D-Gal/Ara |
MW: 300–800 kDa |
| Galactan | Potato | β-(1,4)(Gal)n and may contain Ara/Rha/GalA | MW: ~100 kDa |
| OS from Galactan |
Gal: 95%, DP 1–10 | ||
| Galactomannan | Carob tree and guar plant | Man(β-1,4)[Gal(α-1,6)]β-Man | MW: 1.07 × 105–0.67 × 106 kDa |
| Fibers rich in pectin | Potato | α-(1,2)(Rha)n and α-(1,4)(GalA)n and β-(1,4)(Gal)n side chains | 65% fiber; GalA: 13% |
| Chicory root pulp | Pectin fraction: (GalA)n and/or (GalA-Rha)n; α(1,4); α(1,2). Inulin fraction: β-(2-1)(Fru)n |
62% pectin, uronic acids 38% | |
| Apple | α-(1,2)(Rha)n and α-(1,4)(GalA)n and α-(1,4)(Ara)n, β-(1,4)(Gal)n | GalA: 23%, 60% total sugars (45% glucose) |
|
| Citrus fiber | (GalA)n and/or (GalA-Rha)n; α(1,4); α(1,2) | 42% pectin, 25% cellulose and hemicellulose |
Legend: Ara: arabinose; AG: arabinogalactan; AOS: arabino-oligosaccharides; DE: degree of methyl esterification; DP: degree of polymerization; Fuc: fucose; Fru: fructose; Gal: galactose; GalA: galacturonic acid; GlucA: glucuronic acid; GOS: galactooligosaccharides; HG: homogalacturonan; Man: mannose; MW: molecular weight; RG: rhamnogalacturonan; Rha: rhamnose.
Figure 4.
Overview of the pectic substrates tested in the studies reviewed based on (A) structure and (B) botanical origin (number of studies).
3.3. Effects on Gut Microbiota Composition and SCFA Production
The fermentation of pectic substrates was relatively slow (18–30 h) but complete, regardless of their structure, compared with other fibers and prebiotics. The pectic substrates were able to modulate specific populations of fecal microbiota, promoting the production of SCFA (Table 2 and Table 3). In most studies, the composition of the gut microbiota after fermentation of the pectic substrates was remarkably different compared with other prebiotics, and this specific effect was evidenced by an increased abundance at the genus, species, or even strain level.
In the phylum Bacteroidetes, the relative abundances of the genus Bacteroides and the Bacteroides-Prevotella group were increased in fermentations with pectin extracted from different raw materials or with polygalacturonic acid (18 studies), as well as in fermentations with RGI (4 studies). Furthermore, two studies evidenced the specific effect of pectin on the relative abundance at the species level, where increases in Bacteroides vulgatus, Bacteroides stercoris and Bacteroides dorei were found.
In the phylum Firmicutes, the Ruminococcaceae family was promoted in fermentations with native pectins (11 studies). At the genus level, Lachnospira was commonly increased in fermentations of pectic substrates with a variety of structures (10 studies). Lachnospira eligens, previously known as Eubacterium eligens [23], is particularly increased in pectin fermentations (four studies). In six studies, the genus Roseburia was promoted in fermentations with different pectic substrates, and in some of them, increased abundances of R. intestinalis and R. hominis were observed. The genus Faecalibacterium was commonly increased in fermentations with pectic substrates (14 studies), and of those, pectin particularly promoted an increased abundance of F. prausnitzii (5 studies).
A structure function relationship can be observed, and some bacteria will preferentially use a sub-part of the pectin structure (linear or branched) or a pectin with either a high or low DM. Fermentation of the pectic substrates resulted in moderate SCFA levels, with acetate being the highest and most abundant, as evidenced in 24 studies, followed by lower levels of propionate and butyrate. Furthermore, fermentation of the pectic substrates resulted in higher acetate levels (15 studies) compared with fructans. Propionate production was either low or moderate, depending on the substrate tested, and butyrate production was generally lower compared with other fibers or prebiotics and fructans in particular.
Table 2.
Batch fermentation studies with human gut microbiota; (n = 31; 27 single donor and 4 with pooled samples (healthy vs. specific population).
| Ref. | Subjects (Age, Years) | Test Products 1 | Comparators | Methods 2 | Main Outcomes, Including Changes in Gut Microbiota Composition and SCFA Production Linked to Pectic Substrates |
|---|---|---|---|---|---|
| SINGLE DONOR (n = 27) | |||||
| HEALTHY ADULTS (n = 21) | |||||
| Non-controlled pH (n = 15) | |||||
| Cantu-Jungles, 2021 [24] | 10 (26–42 y) |
Citrus pectin (GalA 3 74%, >6.7% methoxy group, Sigma, St. Louis, MO, USA) |
Blank, FOS from chicory (>95%, Sigma, USA), RS2 from potato (Bob’s Red Mill, Clackamas, OR, USA), and insoluble β-glucan |
50 mg/50 mL; 0 and 24 h |
|
| Wu, 2021 [25] | 4 (18–30 y) |
RGI-enriched fraction (MW 1.93 × 105 Da, polydispersity 1.63, Rha:GalA:Gal = 1:0.8:18) from Okra fruit (Abelmoschus esculentus, harvested from Chengdu, Sichuan, China) | Basal medium, FOS (Sigma, St. Louis, MO, USA) |
1% w/v; 0, 6, 12, 24, and 48 h |
|
| Yu, 2020 [26] | 9 (25–40 y) |
Pectin (ND) | No fiber, inulin (ND), andcellulose (ND) |
5 g/L pectin, 10 g/L inulin, 20 g/L cellulose; 0 and 24 h |
|
| Cui, 2020 [27] | 4 (age ND) |
Orange or grapefruit pectin: P2 (acidic, pH 2, DE 71%), P10 (alkali, pH 10, DE 2%), C (cellulase, DE 69%), P2 + C (acid +cellulase, DE 65%), and P10 + C (alkali + cellulase, more RG1, DE 15%) |
Baseline | 1% w/v; 0, 4, 8, 12, 24, 48, and 72 h |
|
| Bang, 2018 [28] | 3 (29–30 y) |
Citrus pectin (GalA > 74%, Sigma, St. Louis, MO, USA) | Baseline | 1%; 0, 6, 12, 18, 24, 36, and 48 h |
|
| Tuncil, 2017 [29] | 3 donors (age ND) | PGalA from citrus pectin (Megazyme, Wicklow, Ireland) | FOS (Sigma, St. Louis, MO, USA), galactomannan (Carob) and Xyloglucan (Tamarind) (Megazyme, Wicklow, Ireland), and Arabinoxylan |
50 mg/5 mL; 0, 2, 4, 6, 8, 10, 12, and 24 h |
|
| Min, 2015 [30] | 4 (23–28 y) |
High methoxy pectin (HMP, DM 76%, DP492, Tic Gums, Belcamp, MD, USA), SBP (DM 21%, DP3729, Herbstreith & Fox (Elmsford, N.Y., USA), pectin from soy (DM 23%, DP1510) |
FOS (95% purity, DP 3–5, Ingredion, USA) | Unclear concentration; 0, 6, 12, 24, and 30 h |
|
| Van den Abbeele, 2020 [31] | 1 (26 y) |
RGI from carrot (min. 80% purity; Nutrileads, Wageningen, The Netherlands) | Blank and inulin (average DP > 23, Beneo, Mannheim, Germany) |
5 g/L; 0, 6, 24, and 48 h; targeted bacterial groups. |
|
| Gómez, 2016 [32] | 3 (age ND) |
SBP, SBPOS (DM 50%, DA 37%, mostly AOS, and pH 1.8), Lemon pectin (LP), lemon POS (LPOS, DM 62%, DA 4.6%, more oligogalacturonides) |
FOS from chicory (Sigma, St. Louis, MO, USA) | 10 g/L; 0, 5, 10, and 24 h; targeted bacterial groups. |
|
| Sulek, 2014 [33] | 6 (41 ± 9 y) |
Sugar beet AOS (Danisco A/S, Nakskov, Denmark), base solution (BA), LA fraction (<1 kDa), and HA fraction (>1 kDa) |
No CHO in media; FOS from chicory (>95%, Beneo, Tienen, Belgium) | 5 g/L; 0 and 24 h; targeted bacterial groups. |
|
| Gómez, 2014 [34] | 3 donors(age ND) | Orange pectin and orange POS (≈90% oligomers, 53.4% OGalA, 25.3% AOS, and 16.5% GOS) | No fiber in media; FOS (>95% purity, Sigma, St. Louis, MO, USA) | 10 g/L; 0, 5, 10, and 24 h; targeted bacterial groups. |
|
| Gullón, 2011 [35] | 1 (age ND) |
Apple-derived oligosaccharides: GLOS, AOS, GOS, OGalA, and XOS; total oligomers (OS) | No CHO in culture media | 10 g/L; 0, 7, 10, 12, 24, 32, and 48 h; Targeted bacterial groups. |
|
| Holck, 2011 [36] | 6 (41 ± 9 y) |
Sugar beet AOS (Danisco A/S, Nakskov, Denmark): small (mostly DP 2–5), small and feruloylated; long (mostly DP 5–10), and long and feruloylated |
FOS from chicory (>95%, DP 2–8, Beneo, Tienen, Belgium) | 5 g/L; 0 and 24 h; targeted phyla (2) and genera (2) |
|
| Thomassen 2011 [37] | 3 (43 ± 10 y) |
Destarched potato pulp (DNE, no enzyme), destarched potato pulp (DPP, enzyme treated), crude potato pulp (CNE, no enzyme), crude potato pulp (CPP, enzyme treated), CCP fractions: CPP < 10 kDa, CPP 10–100 kDa, and CPP > 100 kDa. |
FOS from chicory (DP 2–8, Beneo, Tienen, Belgium) | 5 g/L; 0 and 24 h. |
|
| Adamberg, 2018 [9] |
5 (28–48 y) |
Arabino-galactan from larch tree (AG, DP > 23, Sigma, USA), and citrus pectin (GalA >74%, Sigma, St. Louis, MO, USA); |
Culture medium without CHO, mucin from porcine stomach (type III, Sigma, St. Louis, MO, USA), GOS (DP 2–10, Friesland Campina, Wolvega, The Netherlands), inulin (HSI, DP 2–8), and lnulin (HP, DP > 23% (Beneo, Oreye, Belgium), Levan (DP > 100), RS (Cargill, Malchin, Germany) xylan and chitin from shrimp cells (Sigma, St. Louis, MO, USA) |
5 g/L; 0, 24 and 48 h |
|
| Specific pH range (n =6) | |||||
| Johnson, 2015 [38] | 3 (age ND) |
Pectin (ND) | Control medium (low fibers and inulin (ND) | 1.5 g; pH 6.7–6.9; 0, 5, 10, 24, 30, and 48 h. |
|
| Reichardt, 2018 [39] | 3 (age ND) |
RGI from potato (Megazyme, Bray Ireland), and apple pectin (Sigma, UK) |
FOS (95%, DP 2–8) and Inulin (99%, DP > 23) (Beneo, Tienen, Belgium), arabinoxylan (Megazyme, Bray, Ireland), barley β-glucan (PolyCell Technologies, Crookston, USA), RS2 and RS3 (National Starch and Chemical Comp., Bridgewater, USA), FiberSol (Matsutani, Itami-City, Japan) |
0.2% w/v; pH 5.5 and 6.5; 0, 6, and 24 h |
|
| Di, 2017 [40] | 5 (30 ± 7 y) |
POS1 (MW 72.8 × 103, DM 40%, Gal:Rha 3.14), POS2 (MW 811 × 103, DM 42%, Gal:Rha 1.97), MCP1 (MW 9.2 × 103, DM 5%, Gal:Rha 2.92), and MCP2 (MW 17.7 × 103, Gal:Rha 4.47, DM 3%) from orange peels (EcoNugenics Inc., Santa Rosa, CA, USA) |
Inulin (99%, Beneo, Tienen, Belgium) | 1% w/v; pH 6.7–6.9;0, 10, 24, 36, and 48 h; targeted bacterial groups. |
|
| Moon, 2015 [41] | 3 (age ND) |
Debranched sugar beet arabinan (LAR, average MW 18 kDa, Megazyme, Wicklow, Ireland) and sugar beet linear AOS (LAOS, 50% DP3, 29% DP2, 20% DP4, and 1% DP5). |
FOS (DP 3–5, Wako, Osaka, Japan) | 1% w/v; pH 6.8; 0, 12, and 24 h; targeted bacterial groups. |
|
| Onumpai, 2011 [42] | 4 (30 ± 4 y) |
PGalA (Sigma, St. Louis, MO, USA); OGalA DP5 (DP 1–10), OGalA DP 9 (DP 4–23), methylated citrus pectin (MPec, DM 34.5%, Danisco A/S, Copenhagen, Denmark), methylated OGalA (MOGalA, DP 1–10), RGI (A. thaliana seed mucilage), oligorhamnogalacturonides (Orham, DP 2–19), potato galactan and beet arabinan (British Sugar, Peterborough, UK), oligogalactosides (PGOS, DP 1–10), oligoarabinosides (OAr, DP 1–11) |
Inulin (>97%, Beneo ST, Orafti, Tienen, Belgium) | 1% w/v; pH 6.7–6.9;0, 12, 24, and 36 h; targeted bacterial groups. |
|
| Ferreira-Lazarte, 2018 [43] | 5 (31± 4 y) |
Sunflower pectin (DM 45.7%, 800–100 kDa), sunflower MP (DM 17%, 12.5 kDa), Artichoke pectin (DM 8.9%, >500 kDa), artichoke MP (DM 8.5%), citrus pectin (Ceamsa, Pontevedra, Spain, DM 70.7%), and citrus MP (DM 14.2%) | Negative: no CHO. Positive: FOS (ND) and inulin (ND). |
1% w/v; pH 6.7–6.9;0, 10, 24, 36, and 48 h; targeted bacterial groups. |
|
| SPECIFIC POPULATIONS (n = 6) | |||||
| Non-controlled pH (n = 5) | |||||
| Van Trijp, 2020 [44] | 5 ileostomy subjects (30–75 y) | Lemon pectin (DM 67%, CP Kelco, Lille Skensved, Denmark) | Inulin and FOS (DP 2–60, Sensus, Roosendaal, the Netherlands), GOS (69%, DP 2–6, Friesland Campina, Wageningen, the Netherlands), and potato IMMP (92% α-1-6, average DP 50, Avebe, Veendam, Belgium) |
10 g/L; 0, 5 ,7 ,9, and 24 h |
|
| Yang, 2013 [45] | 15 adult patients (age ND) | Pectin (TIC gums, White Marsh, MD, USA): 35% polymeric uronic acid residues, DM 72%, MW peak at 9.4 × 105, and 38% free glucose; botanical origin ND | Guar gum (TIC gums, White Marsh, MD, USA), agave inulin (Ciranda, Hudson, WI, USA), corn RS2 (70% high amylose, Cargill, Cedar Rapids, IA, USA), oat β-glucan (Quaker, Chicago, IL, USA), corn arabinoxylan (AX, Bunge Milling, Danville, IL, USA) |
1% w/v; 0 and 12 h. |
|
| Vigsnæs, 2011 [46] | 12 UC patients with 6 healthy adults (41 ± 9 y) | Sugar beet AOS (DP 2–10, Danisco A/S, Nakskov, Denmark) and arabinose moiety (85 mol%, 125 mg/g free sugars, ferulic acid 36 µg/g) | No substrate; FOS (95%, Beneo, DP 2–8, Tienen, Belgium) |
5 g/L; 0 and 24 h; targeted bacterial groups. |
|
| Holck, 2011 [47] | 3 UC remission (36 ± 5 y); 3 UC relapse (44 ± 6 y); 3 healthy (43 ± 10 y) | HG oligosaccharides (DP4 and DP5) from SBP (Danisco A/S, Nakskov, Denmark) | Baseline | 5 g/L; 0 and 24 h; targeted phyla (n = 2). |
|
| Jin, 2019 [48] | 17 patients with cirrhosis and 17 healthy (18–80 y) |
Citrus pectin (Unipectine™, Cargill Inc., Wayzata, MN, USA) | Baseline, RS type 4 (Fibersym® RW, MGP Ingredients, Atchison, KS, USA), lactulose (LL, Sigma, St. Louis, MO, USA), arabinoxylan (AX, Corn Biofiber Gum Agrifiber Holdings LLC, (Mundelein, IL, USA) |
2%; 0 and 14 h |
|
| Specific pH range (n = 1) | |||||
| Adamberg, 2018 [49] | 7 OW (7–14 y) with 6 healthy NW (4–15 y) |
Apple pectin (AP, Sigma, St. Louis, MO, USA) | Arabinogalactan (AG) | From 0.2 L/h to 0.06 L/h; pH 7; 0 and 10 h |
|
| POOLED (ALL HEALTHY DONORS) (n = 4) | |||||
| Non-controlled pH (n = 3) | |||||
| Perez-Burillo, 2019 [50] | 3 (mean BMI 21.3, age ND) | Citrus fiber (42% pectin and 25% cellulose and hemicellulose; Fiberstars, USA) | Control salami (no fiber), inulin (99.5%, Beneo, Belgium), acacia gum (Arabinogalactan, Nexira, France) |
2% in salami; 0 and 24 h |
↑ Dorea and Clostridium cluster XIVb with citrus and acacia fiber. ↓ Escherichia/Shigella with citrus and acacia fiber.↑ SCFA (total and individuals) vs. control salami for all fiber-salami. |
| Cantu-Jungles, 2019 [51] | 3 (age ND) |
Isolated highly-branched RGI (AGI), HG, and AGI (uronic acid/(Ara + Gal): 1.3, HG − DM 79%). Xyloglucan (XYG, tucumã pulp). |
FOS (95%, Sigma, St. Louis, MO, USA) | 1% w/v; 0, 4, 8, 12, and 24 h |
|
| Leijdekkers, 2014 [52] | 10 (44 ± 7 y) |
SBPOS (90%, 15% average DP5, GalA 43%; Cosun, Breda, the Netherlands) | FOS (95%, Sensus, Roosendaal, the Netherlands) | 1% w/v; 0, 3, 6, 9, 12, and 24 h |
|
| Specific pH range (n = 1) | |||||
| Ramasamy, 2014 [53] | 8 (25–45 y) |
Chicory root pulp (62% pectin and 38% uronic acid; Sensus, Roosendaal, the Netherlands) | Baseline | 1% w/v; pH 5.8–6.3;0, 2, 6, 8, 12, and 24 h. |
|
Legend: Upward arrows indicate an increased bacterial abundance, and downward arrows indicate a decreased bacterial abundance. 1 Test products were described as in the studies. They were produced under laboratory conditions when no supplier is mentioned. 2 Concentration, sampling time, and microbiota determination. All studies used a non-targeted bacterial group determination approach within their methodology unless specified. 3 ACE: acetic acid; Ara: arabinan; AOS: arabino-oligosaccharides; BUT: butyric acid; CHO: carbohydrates; DE: degree of esterification; DM: degree of methylation; DP; degree of polymerization; FOS: fructo-oligosaccharides; Gal: galactose; GalA: galacturonic acid; GOS: galacto-oligosaccharides; GLOS: gluco-oligosaccharides; HG: homogalacturonan; IMMP: isomalto/malto-polysaccharides; ISOBUT: isobutyric acid; ISOVAL: isovaleric acid; LAC: lactate; MW: molecular weight; MP: modified pectin; OGalA: oligogalacturonides; PGalA: polygalacturonic acid; POS: pectic oligosaccharides; PRO: propionic acid; Rha: rhamnose; RG: rhamnogalacturonan; RS: resistant starch; SBP: sugar beet pectin; SCFA: short-chain fatty acids; SBPOS: sugar beet pectic oligosaccharides; SD: significant differences; SUC: succinate; UC: ulcerative colitis; VAL: valeric acid; XOS: xylo-oligosaccharides. 4 Lachnospira eligens previously known as Eubacterium eligens [23].
Table 3.
In vitro continuous fermenter studies with human gut microbiota; n = 11: 5 single donor; 6 with pooled samples (healthy vs. specific population).
| Ref | Subjects (Age, Years) | Product Tested 1 | Comparator | Methods 2 | Main Outcomes, Including Changes in Gut Microbiota Composition and SCFA Production Linked to Pectic Substrates |
|---|---|---|---|---|---|
| SINGLE DONOR (ALL HEALTHY DONORS) (n = 5) | |||||
| Chung, 2019 [54] | 2 (53–64 y) |
Apple pectin (Unipectin, Cargill, Belgium) | Inulin, AXOS 3, mixture 1 (all), and mixture 2 (all and RS, galactomannan, and β-glucan) |
4.2 g/L (for single substrate); pH 6.1 ± 0.1; 20 days; single-stage anaerobic |
|
| Chung, 2016 [55] | 3 (age ND) |
Apple pectin (Sigma, St. Louis, MO, USA) | Inulin DP < 10 (Oligo-Fiber DS2, Cargill) | 0.5% w/v; pH 5.5–6.9; 12 days; single-stage anaerobic |
|
| Ferreira, 2019 [4] | 1 (age ND) |
Citrus pectin, (DM 70%, average MW 350 kDa, GalA 66%, Ceamsa, Pontevedra, Spain) | Baseline | 3% w/v; pH 5.6 (AC), 6.3 (TC), and 6.8 (DC); 14 days; SIMGI; targeted bacterial groups |
|
| Van den Abbeele, 2021 [56] | 4 (29–33 y) |
RGI (80%, carrot, Nutrileads, Wageningen, the Netherlands) | Baseline | 3 g/d;pH 5.7–5.9 (PC) and 6.6–6.9 (DC);21 days;SHIME® |
|
| Khodaei, 2016 [57] | 1 (age ND) |
RGI from potato (90.8% polysaccharides, 6.5% DP 2–70, and 2.7% DP1; Megazyme, Wicklow, Ireland); oligo-RGI (51% DP 2–12 and 6.3% DP1; 73% Gal), Oligo-RGI (GOS, no polysaccharides, 51% DP 13–70, and 6.1% DP1; 70% Gal). |
FOS (>95%, DP 2–8, Beneo, Belgium); 3.2 g/L CHO as negative control | 9.7 g/L; pH 6.2; 4 days; BIOSTAT®; targeted bacterial groups |
|
| POOLED (n = 6) | |||||
| HEALTHY ADULTS (n = 3) | |||||
| Larsen, 2019 [58] | 8 (25–42 y) |
Potato fiber (FiberBind, KMC, Brande, Denmark, 65% dietary fiber, containing pectin, cellulose, and hemicellulose). Pectin fraction consisted of GalA (13.1%) and rhamnose (0.5%) | Baseline, native potato starch (NS), and potato cross-linked resistant starch (RS) |
7.5 g/d; pH 5.8; 0, 24, 48, 56, and 72 h TIM-2 |
|
| Larsen, 2019 [59] | 8 (25–42 y) |
Citrus pectins with various DM and extraction processes (P1–P3, P5–P8; CP Kelco, Lille Skensved, Denmark), SBP (P4), and RGI (P10). |
Baseline | 7.5 g/d; pH 5.8; 0, 24, 48, 56, and 72 h; TIM-2 |
|
| Bianchi, 2019 [60] | 3 (age ND) |
Lemon pectin (harshly extracted, LM, CP Kelco, Lille Skensved, Denmark) and probiotic strain B. longum BB-46 (Chr. Hansen, Hørsholm, Denmark) | Probiotic strain only | 2% w/v (8 g/d); pH 5.6–5.9 (AC), 6.1–6.9 (TC), and 6.6–6.9 (DC); 7 days for each treatment; SHIME®. |
|
| SPECIFIC POPULATIONS (n = 3) | |||||
| Aguirre, 2014 [17] | 4 lean healthy adults (BMI 23) and 4 obese adults (BMI 33) (age ND) | Apple fiber (23% uronic acid; CSM, Bingen, Germany) and SBP (GENU pectin, DE 53%, and 58% uronic acid; CP Kelco, CPKelco, Nijmegen, the Netherlands). | SIEM (control), GOS (97%, DP 2–6; Friesland Campina, Beilen, the Netherlands), lactulose (98%, Sigma, Zwijndrecht, the Netherlands) |
7.5 g/d; pH 5.8; 0 and 72 h; TIM-2 (PC conditions) |
|
| Bianchi, 2018 [61] | 3 obese adults (BMI > 30 Kg/m2, age ND) | Lemon pectin (harshly extracted, DM 36%, CP Kelco, Lille Skensved, Denmark) | Baseline | 2% (w/v); pH 5.6–5.9 (AC), 6.1–6.4 (TC), 6.6–6.9 (DC); 7 days; SHIME® |
|
| Míguez, 2020 [62] | 6 elderly subjects (60–83 y) | POS mixtures (OGs 44.4%, AOS 16.9%, and GOS 11.6%) | Baseline and FOS from chicory (Sigma, Madrid, Spain) |
6.5 g/d; pH 5.8; 0, 24, 48, and 72 h; TIM-2 |
|
Legend: Upward arrows indicate an increased bacterial abundance, and downward arrows indicate a decreased bacterial abundance. 1 Test product were described as in the studies, and they were produced under laboratory conditions when no supplier was mentioned. 2 All studies used a non-targeted bacterial group determination approach within their methodology unless specified. 3 AC: ascending colon; ACE: acetic acid; AOS: arabino-oligosaccharides; AXOS: arabino-xylo-oligosaccharides; BIOSTAT®: stirred-glass bioreactor; BMI: body mass index; BCFA: branched-chain fatty acids; BUT: butyric acid; DB: degree of branching; DC: distal colon; DE: degree of esterification; DM: degree of methylation; DP: degree of polymerization; FOS: fructo-oligosaccharides; Gal: galactose; GalA: galacturonic acid; GOS: galacto-oligosaccharides; LAC: lactate; MW: molecular weight; NH4+: ammonium; ND: not described; OGs: oligogalacturonides; PC: proximal colon; POS: pectic oligosaccharides; PRO: propionic acid; RG: rhamnogalacturonan; SBP: sugar beet pectin; SCFA: short-chain fatty acids; SD: significant differences; SHIME®: Simulator of Human Intestinal Microbial Ecosystem; SIEM: simulated ileal efflux medium; TC: transverse colon; TIM-2: TNO in vitro model of the colon; WC: waist circumference. 4 Lachnospira eligens previously known as Eubacterium eligens [23].
4. Discussion
To our knowledge, this is the first systematic review aiming to elucidate the prebiotic effect of pectic substrates investigated in vitro using human gut microbiota. Pectin and other pectin-derived substrates such as hydrolyzed pectins, RG1, and oligosaccharides have been widely studied, and recent studies even indicate that many of the structural characteristics of pectin have been shown to influence the utilization of these substrates by the gut microbiota. This systematic review demonstrates that fermentation of pectic substrates with human gut microbiota appears to have beneficial effects in terms of specific gut microbiota modulation and SCFA production.
4.1. Influence of the Methodology on the Fermentation of Pectic Substrates
Simple fermentation models such as batch fermentations and more complex continuous fermenter models can capture the complex microbial interactions driven by dietary substrates [18]. As of today, no standardized in vitro methodology exists for studying the gut microbiome. Differences in the methodology of these fermentation systems can influence the microbial abundances and metabolite production. The main differences observed between the fermentation systems were pH control, DNA-based techniques for gut microbiota analysis, and fecal inoculum.
The pH conditions play a key role in the competition between bacteria from different phyla or families that share the ability to utilize the same substrate. In pectin fermentations, a slightly acidic pH (pH < 6) suppresses the growth of Bacteroides spp. [55]. Even though most batch fermentations and continuous fermenters can detect an increase in this genus with different pectic substrates regardless of the pH, the effect is more obvious in pH-controlled studies [4,38,39,40,42,43,49,54,55,56]. Furthermore, growth inhibition of F. prausnitzii in fermentations with apple pectin at a mildly acidic pH has been reported to be strain-dependent [63]. However, the effect on this species in fermentations of pectic substrates has been detected regardless of pH control in both batch [34,39] and continuous fermenters [4,55].
In terms of differences in the DNA-based techniques for gut microbiota analysis used in the studies, most studies using a non-targeted approach detected an increased abundance of the genus Lachnospira [9,24,29,51,52,53,58,59,62]. The fact that the genus Lachnospira was not investigated in studies targeting specific bacterial groups could be a possible explanation for its non-detection in these studies. On the contrary, studies using a targeted-approach detected the increased abundances of the genus Lactobacillus and the Lactobacillus-Enterococcus group [32,33,34,35,40,43,46,57], as well as of C. histolyticum [32,34,35,40,42]. Changes in Bifidobacterium abundances were observed in most studies regardless of the DNA-based technique used for gut microbiota analysis. However, this finding is more obvious in studies using a targeted approach, suggesting that this method might be more accurate for detecting the change in this genus [4,31,32,33,34,35,39,40,41,42,43,46,57].
Using a pooled fecal inoculum rather than the microbiota from a single individual remains controversial, particularly with the concern of how representative such an inoculum is in regard to the colonic ecosystem, considering the abundance and the variety of bacterial species [17,64]. Overall, no major differences were observed between the studies performed with a fecal inocula from single donors compared with pooling. One study recently compared both ways of preparing a fecal inoculum (pooled and single) in an in vitro system monitoring the composition and activity of the gut microbiota under a standard TIM-2 fermentation [17]. Despite some differences observed in certain groups of bacteria in the single-donor fermentations, no major differences were obtained in terms of diversity in the gut microbiota. The majority of the operational taxonomic units (OTUs) were shared among both types of inocula (e.g., Oscillibacter, Alistipes, Bacteroides and Dorea genera were detected to have similar levels under both conditions). Furthermore, a similar metabolic activity in terms of SCFA production in the experiments using fecal inocula prepared from individuals and a pool of these was observed, indicating that the metabolic response of the gut microbiota present in both types of inocula was similar under both conditions.
4.2. Common Features for Pectic Substrates in Terms of Fermentation Rate, Gut Microbiota Composition, and SCFA Production
In comparison with other fibers and prebiotics, (e.g., fructans and galacto-oligosaccharides), microbiota fermentation of pectic substrates is slower (within 18-30h) [38,44,51]. The gradual fermentation is also observed when comparing pectic oligosaccharides (POS) to other oligosaccharides, such as fructo-oligosaccharides (FOS) with a similar degree of polymerization (DP) [41]. This phenomenon may be accentuated when pectic substrates are fed to the human gut microbiota in mixtures with other fibers (which may resemble the common human diet) rather than individually [29]. The available literature in humans confirm the slow and complete fermentation of pectic substrates in the large intestine [5], being non-detectable in the feces of adults during intervention studies even at daily doses of up to 30–40 g [65]. Gas production during fermentation (including breath hydrogen, H2) is commonly lower with pectin than with other fibers (e.g., wheat bran, fructans, and lactulose) in both in vitro [26] and human clinical studies [66,67]. Commonly used rapidly fermented prebiotics may lead to discomfort and flatulence in humans, especially at higher doses [68]. In contrast, non-digestible carbohydrates that are slowly fermented may reach the distal part of the colon and promote the growth of beneficial gut microflora, resulting in beneficial metabolites involved in the prevention of intestinal or metabolic diseases [41,69].
4.2.1. The Effects of Pectic Substrates on the Gut Microbiota Composition
Despite slower fermentation, due to the complex molecular structure of pectic substrates, drastic changes to the gut microbiota composition can be induced which are stronger than FOS or type-2 resistant starch for instance [1,24]. These changes are not observed at the phylum or family level (except for Ruminococcaceae), but rather at the genus, species, and strain level [49,55]. Bacteroidetes, commonly regarded as dominant plant polysaccharides degraders in the human gastrointestinal tract [70], are decreased in some in vitro fermentations with pectic substrates [27,36,46,47], and they increased in three studies using different RGI fractions [25,31,51]. Among Bacteroidetes, the Bacteroidaceae family is generally equipped with several enzymes (40–50) able to degrade pectin (e.g., polysaccharide lyases, glycoside hydrolases, and carbohydrates esterases) [1,71]. Utilization of pectic substrates, and pectin in particular, is relatively common among Bacteroides spp., which seem to respond rapidly and specifically to the presence of polysaccharides in their environment [72]. In particular, B. vulgatus [49,54], B. dorei [31,54,56], and B. stercoris [54,55] are significantly stimulated during in vitro fermentation, especially at more neutral pH levels such as those in the descending colon [49,55].
The phylum Firmicutes is variably influenced by pectin during in vitro fermentation. Within this phylum, the family Ruminococcaceae, mostly promoted by pectin in continuous fermenters, represents abundant members of the normal microbiome, reaching approximately 10–20% of abundance in healthy humans, where they break down indigestible carbohydrates and produce SCFA [73]. F. prausnitzii, a member of the Ruminococcaceae family, is one of the dominant butyrate producers in the human gut, with a prevalence of approx. 99% [74]. However, its relative abundance is reduced in case of ulcerative colitis or inflammatory bowel disease [75,76], and metabolic disorders [74,77]. Interestingly, F. prausnitzii is promoted in pectin fermentations with fecal samples from healthy subjects [34,39], but also with citrus pectin rich in RGI [27], RGII [39], as well as with oligosaccharides from citrus [34] or sugar beet [52]. In contrast, one study found that the abundance of F. prausnitzii varied in pectin fermentations based on its structural differences [59]. A plausible reason for these differences could be that substrate utilization by this species may be strain dependent, as previously reported [63]. The authors also confirm the ability of intestinal isolates of F. prausnitzii to utilize pectin from apple (but not citrus) in culture growth experiments and that it possesses small repertoires of carbohydrate-active enzymes (CAZyme) encoding genes involved in pectin degradation.
The Lachnospiraceae family presents enzymatic capabilities to degrade pectic substrates among its members [71]. Particularly, L. eligens possesses various enzymes able to degrade pectin, at least two glycoside hydrolases, two polysaccharide lyases, and two carbohydrate esterases. These enzymatic capabilities are also represented in Lachnospira spp. and Roseburia intestinalis, but are absent in the genera Coprococcus and Anaerostipes. This variation in enzymatic capabilities to degrade pectin could justify why the modulation of the Lachnospiraceae family varied among studies. A significant increase in the relative abundance of the genus Lachnospira in the gut microbiota of healthy subjects was observed with citrus pectic substrates and various DM [24,28,59], polygalacturonic acid from citrus pectin [29], RGI [51], as well as potato fiber (rich in pectin) [58]. Additionally, L. eligens can also be stimulated by apple pectin (with no clear effect of a pH level between 5.5 and 6.5–6.9) [39,55]. In these studies, the specific stimulation of L. eligens or Lachnospira was quite unique to pectic substrates and not observed with fructans (FOS or inulin) or resistant starch [24,58]. Lachnospira is not stimulated in microbiota from obese subjects after fermentation with citrus pectin, possibly due to its very low initial concentration [61]. Interestingly, one study demonstrated that the effect is less visible when polygalacturonic acid is fed to the gut microbiota within a blend of three or six fibers, than when presented alone [29].
The selective modulation of these bacterial groups by pectic substrates can be beneficial. For example, the ability of pectin to stimulate L. eligens could be particularly important from a health perspective, since this bacterium is known to exert strong anti-inflammatory effects in vitro to an even greater extent than F. prausnitzii [71]. L. eligens, which is around 90–92% prevalent with a relative abundance between 1–2% [74,78], was recently identified as one of the top 30 bacterial species strongly correlated with the healthy eating index [74], or the Mediterranean diet [79], similar to F. prausnitzii. In these studies, the L. eligens species was particularly negatively correlated with visceral fat, blood triglycerides, and very low-density lipoprotein (VLDL) at 6 h but also with fasting and post-prandial glycoprotein acetylation, a biomarker of inflammation. In another clinical study conducted in type 2 diabetes patients, L. eligens was selectively increased by a high dietary fiber diet and negatively associated with postprandial glucose and insulin, body weight, and waist circumference [80].
4.2.2. The Effects of Pectic Substrates on the Production of SCFA
The amount and type of fiber consumed can have dramatic effects on the composition of the intestinal microbiota, and consequently, on the type and amount of SCFA produced [81]. Overall, total SCFA production is generally moderate, but significant, during the fermentation of pectic substrates by the human gut microbiota. In the first hours of fermentation, the total SCFA levels were lower for pectic substrates compared with fructans. Furthermore, pectic substrates induce a larger amount of acetate produced by the gut microbiota from healthy adults and the elderly (60–83 years), regardless of their botanical source or structure when compared with other fibers [24,30,42,62]. One study showed that the SCFA levels that resulted from pectin fermentation (including acetate) were lower in the obese compared with the lean subjects [17]. Accordingly, two clinical studies showed higher acetate levels (in feces or blood) after dietary supplementation of 20–25 g of pectin, or citrus fiber containing pectin [82,83]. The large amounts of acetate commonly produced in the fermentations of pectic substrates may be in alignment with studies showing an increase in the relative abundance of Lachnospira, since some species of Lachnospira are known to produce acetate [84], and its level is negatively correlated with propionate in two in vitro studies [24,58].
Although more variable, the propionate and butyrate levels are lower compared with the fructans [26,29,38]. This finding is particularly observed in butyrate production in fermentations of different pectic substrates [26,34], hydrolyzed pectins and oligomers [40].
In terms of other metabolites, a limited amount of lactate was observed in pectin fermentations aligned with a limited increase of Bifidobacterium and Lactobacillus, which suggests that it could be further used by other bacteria via cross-feeding interactions [31,34,35]. Only five studies measured ammonia (NH4+) production during pectin fermentations, and its levels were either reduced [4,60,61] or unchanged [17,31].
4.2.3. The Impact on Fermentative Activities Based on Donor Health Status
The effect of pectic substrates on the gut microbiota of overweight or obese subjects [17,49,61] is slightly different compared with healthy or normal weight subjects. For instance, B. vulgatus was stimulated by apple pectin and arabinogalactan only in the gut microbiota of normal weight children but not in overweight ones [49]. An increase in the relative abundances of Bacteroides spp., Bifidobacterium, Catenibacterium, Clostridium cluster XIVb, and Parasutterella were only observed with the gut microbiota from lean subjects with apple fiber and/or sugar beet pectin [17]. The authors argue whether the gut microbiota from overweight subjects might have lost their capacity to degrade the pectic structures due to depletion or a decrease in some bacterial species (e.g., B. vulgatus is reduced in overweight children compared with normal weight children) [49]. Interestingly, LM pectin promotes an increase in bacteria with potential anti-inflammatory effects (Succinivibrionaceae members) and SCFA levels, and a decrease in Lachnospiraceae in obese microbiota [61].
Two studies compared the effect of pectic substrates on the human gut microbiota from UC patients (in remission or relapse) with healthy adults [46,47]. In both studies, a treatment with arabino-oligosaccharides from sugar beet (DP 2–10) resulted in no significant differences in gut microbiota composition between the populations. However, slightly different effects were found between the studies. While both showed a reduction of Bacteroidetes, one study reported an increased relative abundance of Firmicutes (for oligosaccharides with a DP4 only) [47], and the other one reported a decrease in the same phylum [46]. Interestingly, a limited SCFA production was obtained in comparison with other studies, and the common production of acetate was only seen with microbiota from relapse subjects [46,47].
4.3. Structure-Function Relationship of Pectic Substrates
4.3.1. Degree of Methyl-Esterification
The effect of DM of pectic substrates on the modulation of the gut microbiota was investigated in fives studies [27,30,32,40,59]. LM pectins were previously reported to be fermented faster than HM pectins [85]. Similar findings were observed in two studies comparing pectins with different botanical origins, production processes and molecular weights [27,30]. In contrast, HM pectins can induce a slightly higher production of SCFA, especially propionate [59]. Other authors also reported that DM does not influence the fermentability of pectic oligosaccharides or hydrolyzed pectin, as reflected by a similar SCFA yield and profile [40]. These contradictory findings may suggest that DM either has an overall moderate effect on gut microbiota modulation or that it might depend on the initial gut microbiota composition. The species Prevotella copri, and F. prausnitzii and the family Ruminococcaceae are positively correlated with DM, contrary to many genera such as Coprococcus, Oscillospira, Prevotella, Ruminococcus, and Lachnospira [59]. Regardless of whether these bacteria are considered fast or slow growers, they can impact fermentation kinetics and induce different results between LM and HM pectins in terms of the rate of SCFA production.
4.3.2. Composition of Neutral Sugars
The nature of neutral sugars and the types of linkages in the pectin structure may influence the composition and activity of the human gut microbiota. In this regard, gluco-oligosaccharides and galacto-oligosaccharides are fermented faster than arabino-oligosaccharides, and even faster than oligogalacturonides, for which there is a lag time of 5–7 h in vitro [32,34,35]. Galactan molecules are degraded by glycoside hydrolases (GHs). For example, GH2 is present in the genome of many genera in the human gut, such as Bacteroides, Bifidobacterium, and Ruminococcus, and species such as F. prausnitzii, R. intestinalis, Akkermansia muciniphila and Escherichia coli. The genera Bifidobacterium and Lactobacillus possess enzymatic capabilities to degrade β-1,4-galactan chains as a carbon source in potato pulp [37]. The widespread presence of these enzymes in the gut commensals may explain why these molecules are quickly fermented. Furthermore, enzymes such as the GH families that can degrade arabinans (e.g., GH51, GH43, GH27, and GH127), are also largely present in Bacteroides spp. and several species of Bifidobacterium (e.g., B. longum subsp. longum or B. adolescentis). On the contrary, enzymes such as glycoside hydrolases and polysaccharide lyases (PLs) that can degrade the RGI backbone (e.g., PL11) are only present in some Bacteroides spp., and only the GH type appears to be present in F. prausnitzii, R. intestinalis, Klebsiella oxytoca, Enterobacter cloacae, and A. muciniphila [1].
Bifidobacterium spp. (at least B. adolescentis and B. breve) show a poor capacity to degrade pectin or the RGI backbone contrary to Bacteroides [71], but it seems to be more adapted to degrading side chains made of arabinan and galactan [1,37]. This is in agreement with a pure culture experiment showing that Bifidobacterium spp. can partially metabolize distinct types of apple pectin-derived oligosaccharides, except oligogalacturonides [35]. Similarly, Bifidobacterium exhibits a preference for oligosaccharides rich in galactose and arabinose, as shown by several in vitro studies [33,42,43]. Bifidobacterium further develops with alkali-extracted pectin containing the highest RGI ratio (≈60%) than with pectin obtained by acid extraction presenting a lower RGI ratio (≈26%) [27]. Previous genomic and in vitro studies have shown that Bifidobacterium preferentially utilizes arabinan (sugar beet) and arabinogalactan (potato) rather than polymers or oligomers of GalA [42]. Finally, this genus also increases in fermentations with sugar beet arabino-oligosaccharides [33,36,41], and apple-derived oligosaccharides [35]. This preference for RGI certainly because of its side chains has been confirmed in humans, where a dietary supplementation with 15 g of potato fiber did not induce a significant modification of fecal microbiota. However, RGI derived from the same fiber could increase the relative abundance of Bifidobacterium [86]. Additionally, in infants, the consumption of formulae containing citrus pectin-derived oligosaccharides could stimulate the growth of Bifidobacterium [87].
The presence of arabinose (and arabinan side chains) also seems to be positively correlated with higher counts of Prevotella, (and P. copri in particular) but negatively correlated with Lachnospira, Bacteroides ovatus, and members of Coprococcus [59]. In most studies, Prevotella is investigated jointly with other bacterial genera such as Bacteroides, but not on its own, thus not allowing the confirmation of a strong effect of pectic substrates on its growth. Furthermore, the low abundance of Prevotella could also be due to the lower degree of branching and/or arabinose content of the pectic substrates tested in these studies compared which RGI and sugar beet pectin. Since P. copri is generally associated with high plant fiber diets and favorable postprandial glucose metabolism [74], further studies are needed to provide evidence of its fermentative activities.
Interestingly, these structural differences in terms of the neutral sugar composition of pectic substrates, as described above, may result in different fermentation patterns and activities by the gut bacteria. In particular, galactan and arabinan and their respective oligosaccharides, may promote higher total SCFA levels compared with fermentations with polygalacturonic acid and pectin [42].
4.3.3. Distribution of HG and RG Fractions
The distributions of the linear and branched structural regions of pectin have been shown to influence its fermentation by the human gut microbiota. The Ruminococcaceae family and especially F. prausnitzii positively correlated with GalA and DM [4,17,59]. This suggests that different microorganisms may exhibit specific preferences for defined substrates, which may be the case for F. prausnitzii that preferentially utilized the HG backbone compared with RG, and HM compared with LM pectins. Previous research has demonstrated the enzymatic capabilities of F. prausnitzii to degrade pectin and RGI-backbone [1], as well as GalA [88]. The utilization of the latter in particular was also confirmed for several strains of F. prausnitzii [63]. These strains are able to compete with B. thetaiotaomicron and L. eligens for pectin degradation, especially in mildly acidic pH conditions. Interestingly, the ability of the specific strains of F. prausnitzii to function both as a consumer and a producer of acetate, depending on the conditions, has been previously reported, and in particular, a higher uptake of GalA as carbon source is facilitated by acetate production by the bacteria [88].
4.3.4. Degree of Branching
The degree and distribution of neutral sugar branches attached to the RGI region may influence the molecular conformation of pectins [89], and further influence their fermentability [42]. Pectins derived from sugar beet or apple are usually characterized by a higher degree of branching compared with those of a citrus origin [89]. Sugar beet-derived pectin and POS may be fermented more slowly, and not completely, than from apple or citrus [32]. Furthermore, the genus Lachnospira, particularly promoted in pectin fermentations, is positively correlated with the degree of branching [59]. The same study also showed a similar finding regarding the genus Coproccocus, contrary to the species P. copri.
4.3.5. Molecular Weight
The molecular structure, and even nature of monosaccharide units, may influence the kinetics and extent of SCFA production. POS are generally fermented faster and to a greater extent (resulting in the greater production of SCFA and pH reduction) than pectin polysaccharides from the same botanical origin [32,34,42]. A minor difference in the degree of polymerization (four vs. five) from the same type of substrate induces a different effect on the gut microbiota. While a decrease in the Bacteroidetes phylum was observed for both oligosaccharides, a significant increase in Firmicutes was only seen with the substrate with DP4 [47]. Furthermore, Bifidobacterium was more stimulated by oligosaccharides from potato-derived RG1 (without a difference between DP 2–12 and DP 13–70) than by polysaccharides [57]. A similar effect was observed in fermentations with modified pectins from citrus and artichoke compared with their parent pectins [43]. The genus Bifidobacterium exhibits a well-known preference for shorter molecules (e.g., the fermentation of fructan-derived substrates), as was recently reviewed [90], even though this was not observed in a previous study with different pectic substrates [42]. In contrast to Bifidobacterium, the effect of the DP on F. prausnitzii may be strain-dependent. Certain F. prausnitzii strains possess some ability to utilize apple pectin, and in common with L. eligens, they can utilize the galacturonide oligosaccharides with DP4 and DP5 derived from sugar beet pectin [71].
4.3.6. Other Structural Characteristics
One study investigated the possible impact of the presence of amide groups in the pectin structure on the gut microbiota [59]. The authors reported that amidated citrus pectins had a similar effect on the gut microbiota in comparison to non-amidated pectins from citrus or sugar beet. However, both pectins shared a similar (low) DM, and this parameter might have had a stronger influence on the modulation of the gut microbiota than amidation. Another study investigated the effect of feruloyl substitution of short (DP 2–10) and long-chain (DP 7–14) arabino-oligosaccharides on the gut microbiota [36]. In this study, Bifidobacterium was similarly and selectively stimulated by both the feruloylated and non-feruloylated arabino-oligosaccharides. The potential specific effect of this structural difference on the gut microbiota may have been hidden by a greater effect of different DPs between the substrates.
5. Conclusions
Based on this comprehensive review, we found evidence to support the potential prebiotic effect of pectic substrates based on in vitro studies, as shown by a specific fermentation profile at the genus, species, and even strain levels. It is evident that most pectic substrates can stimulate the growth of Bacteroides and Lachnospira genera as well as species such as F. prausnitzii and L. eligens, and increase the production of SCFA (acetate in particular). The structural characteristics of pectic substrates have been shown to influence their utilization by the gut microbiota. Bifidobacterium in particular, shows a preference for the fermentation of shorter molecules, especially arabinose-rich side chains such as from RGI. F. prausnitzii prefers HG to RG, but its preferences are less clearly defined and might be determined at the strain rather than species level.
Even though these findings are promising, they should be carefully interpreted since direct extrapolation to humans cannot be performed. A recent clinical trial where a dietary intervention of 15 g/d of sugar beet pectin for 4 weeks resulted in no significant changes in gut microbiota composition or SCFA production [15]. Furthermore, the fermentation profile of pectic substrates by the human gut microbiota varies depending on whether the fiber is provided alone or in a blend, which is more representative of the typical human diet, as was previously reported [29]. Therefore, well-designed clinical trials are needed to prove the specific effect of these substrates on human gut microbiota and the potential health effects derived from it.
Acknowledgments
The authors would like to thank Jan Larsen, Jimmy Sejberg and Beinta Marr for interesting discussions on development of the methodology and ways to describe pectic substrates, and Maryann Fabian for English editing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14173629/s1, Table S1: The search strategy used for identification of the relevant studies included in the systematic review.
Author Contributions
Conceptualization: F.R.; methodology: F.G., N.P., L.J., N.L. and F.R.; Validation: F.G., N.P., L.J., N.L. and F.R.; investigation: F.G., N.P. and F.R.; writing—original draft preparation: N.P. and F.R.; writing—review and editing, F.G., N.P., L.J., N.L. and F.R.; supervision, F.R. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
All authors declare no competing interests.
Funding Statement
This project was supported by the Innovation Fund Denmark (Case Number 0154-00018A). F.G., N.P. and F.R. are employees of CP Kelco.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ndeh D., Gilbert H.J. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol. Rev. 2018;42:146–164. doi: 10.1093/femsre/fuy002. [DOI] [PubMed] [Google Scholar]
- 2.Gawkowska D., Cybulska J., Zdunek A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers. 2018;10:762. doi: 10.3390/polym10070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beukema M., Faas M.M., de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020;52:1364–1376. doi: 10.1038/s12276-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira-Lazarte A., Moreno F.J., Cueva C., Gil-Sánchez I., Villamiel M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®) Carbohydr. Polym. 2019;207:382–390. doi: 10.1016/j.carbpol.2018.11.088. [DOI] [PubMed] [Google Scholar]
- 5.Holloway W.D., Tasman-Jones C., Maher K. Pectin digestion in humans. Am. J. Clin. Nutr. 1983;37:253–255. doi: 10.1093/ajcn/37.2.253. [DOI] [PubMed] [Google Scholar]
- 6.Yeo S.-K., Liong M.-T. Angiotensin I-converting enzyme inhibitory activity and bioconversion of isoflavones by probiotics in soymilk supplemented with prebiotics. Int. J. Food Sci. Nutr. 2010;61:161–181. doi: 10.3109/09637480903348122. [DOI] [PubMed] [Google Scholar]
- 7.Panel E.N. Scientific opinion on the substantiation of health claims related to pectins and reduction of post-prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1747. [Google Scholar]
- 8.FDA Review of the Scientific Evidence on the Physiological Effects of Certain Non-Digestible Carbohydrates. [(accessed on 29 January 2021)]; Available online: www.fda.gov.
- 9.Adamberg K., Kolk K., Jaagura M., Vilu R., Adamberg S. The composition and metabolism of faecal microbiota is specifically modulated by different dietary polysaccharides and mucin: An isothermal microcalorimetry study. Benef. Microbes. 2018;9:21–34. doi: 10.3920/BM2016.0198. [DOI] [PubMed] [Google Scholar]
- 10.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 11.Dupont C., Kalach N., Soulaines P., Bradatan E., Lachaux A., Payot F., De Blay F., Guénard-Bilbault L., Hatahet R., Mulier S., et al. Safety of a New Amino Acid Formula in Infants Allergic to Cow’s Milk and Intolerant to Hydrolysates. J. Pediatr. Gastroenterol. Nutr. 2015;61:456–463. doi: 10.1097/MPG.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 12.Rabbani G.H., Teka T., Zaman B., Majid N., Khatun M., Fuchs G.J. Clinical studies in persistent diarrhea: Dietary management with green banana or pectin in Bangladeshi children. Gastroenterology. 2001;121:554–560. doi: 10.1053/gast.2001.27178. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K., Inokuchi R., Fukushima K., Naraba H., Takahashi Y., Sonoo T., Hashimoto H., Doi K., Morimura N. Pectin-containing liquid enteral nutrition for critical care: A historical control and propensity score matched study. Asia Pac. J. Clin. Nutr. 2019;28:57–63. doi: 10.6133/apjcn.201903_28(1).0009. [DOI] [PubMed] [Google Scholar]
- 14.Tabei I., Tsuchida S., Akashi T., Ookubo K., Hosoda S., Furukawa Y., Tanabe Y., Tamura Y. Effects of a novel method for enteral nutrition infusion involving a viscosity-regulating pectin solution: A multicenter randomized controlled trial. Clin. Nutr. ESPEN. 2018;23:34–40. doi: 10.1016/j.clnesp.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 15.An R., Wilms E., Smolinska A., Hermes G.D.A., Masclee A.A.M., de Vos P., Schols H.A., van Schooten F.J., Smidt H., Jonkers D., et al. Sugar Beet Pectin Supplementation Did Not Alter Profiles of Fecal Microbiota and Exhaled Breath in Healthy Young Adults and Healthy Elderly. Nutrients. 2019;11:2193. doi: 10.3390/nu11092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.So D., Whelan K., Rossi M., Morrison M., Holtmann G., Kelly J.T., Shanahan E.R., Staudacher H.M., Campbell K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018;107:965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 17.Aguirre M., Jonkers D.M., Troost F.J., Roeselers G., Venema K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS ONE. 2014;9:e113864. doi: 10.1371/journal.pone.0113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venema K., van den Abbeele P. Experimental models of the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2013;27:115–126. doi: 10.1016/j.bpg.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Swanson K.S., de Vos W.M., Martens E.C., Gilbert J.A., Menon R.S., Soto-Vaca A., Hautvast J., Meyer P.D., Borewicz K., Vaughan E.E., et al. Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: A review. Benef. Microbes. 2020;11:101–129. doi: 10.3920/BM2019.0082. [DOI] [PubMed] [Google Scholar]
- 20.Ashaolu T.J., Ashaolu J.O., Adeyeye S.A.O. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021;130:677–687. doi: 10.1111/jam.14843. [DOI] [PubMed] [Google Scholar]
- 21.Elshahed M.S., Miron A., Aprotosoaie A.C., Farag M.A. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021;255:117388. doi: 10.1016/j.carbpol.2020.117388. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oren A., Garrity G. List of new names and new combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol. 2020;70:4043–4049. doi: 10.1099/ijsem.0.004244. [DOI] [PubMed] [Google Scholar]
- 24.Cantu-Jungles T.M., Bulut N., Chambry E., Ruthes A., Iacomini M., Keshavarzian A., Johnson T.A., Hamaker B.R. Dietary Fiber Hierarchical Specificity: The Missing Link for Predictable and Strong Shifts in Gut Bacterial Communities. mBio. 2021;12:e0102821. doi: 10.1128/mBio.01028-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D.-T., Nie X., Gan R.-Y., Guo H., Fu Y., Yuan Q., Zhang Q., Qin W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021;114:106577. doi: 10.1016/j.foodhyd.2020.106577. [DOI] [Google Scholar]
- 26.Yu X., Gurry T., Nguyen L.T.T., Richardson H.S., Alm E.J. Prebiotics and Community Composition Influence Gas Production of the Human Gut Microbiota. mBio. 2020;11:e00217-20. doi: 10.1128/mBio.00217-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui J., Zhao C., Zhao S., Tian G., Wang F., Li C., Wang F., Zheng J. Alkali+ cellulase-extracted citrus pectins exhibit compact conformation and good fermentation properties. Food Hydrocoll. 2020;108:106079. doi: 10.1016/j.foodhyd.2020.106079. [DOI] [Google Scholar]
- 28.Bang S.J., Kim G., Lim M.Y., Song E.J., Jung D.H., Kum J.S., Nam Y.D., Park C.S., Seo D.H. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express. 2018;8:98. doi: 10.1186/s13568-018-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuncil Y.E., Nakatsu C.H., Kazem A.E., Arioglu-Tuncil S., Reuhs B., Martens E., Hamaker B.R. Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J. Funct. Foods. 2017;32:347–357. doi: 10.1016/j.jff.2017.03.001. [DOI] [Google Scholar]
- 30.Min B., Koo O.K., Park S.H., Jarvis N., Ricke S.C., Crandall P.G., Lee S.-O. Fermentation patterns of various pectin sources by human fecal microbiota. Food Nutr. Sci. 2015;6:1103–1114. doi: 10.4236/fns.2015.612115. [DOI] [Google Scholar]
- 31.Van den Abbeele P., Verstrepen L., Ghyselinck J., Albers R., Marzorati M., Mercenier A. A Novel Non-Digestible, Carrot-Derived Polysaccharide (cRG-I) Selectively Modulates the Human Gut Microbiota while Promoting Gut Barrier Integrity: An Integrated in vitro Approach. Nutrients. 2020;12:1917. doi: 10.3390/nu12071917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez B., Gullón B., Yanez R., Schols H.A., Alonso J.L. Prebiotic potential of pectins and pecti oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods. 2016;20:108–121. doi: 10.1016/j.jff.2015.10.029. [DOI] [Google Scholar]
- 33.Sulek K., Vigsnaes L.K., Schmidt L.R., Holck J., Frandsen H.L., Smedsgaard J., Skov T.H., Meyer A.S., Licht T.R. A combined metabolomic and phylogenetic study reveals putatively prebiotic effects of high molecular weight arabino-oligosaccharides when assessed by in vitro fermentation in bacterial communities derived from humans. Anaerobe. 2014;28:68–77. doi: 10.1016/j.anaerobe.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Gómez B., Gullón B., Remoroza C., Schols H.A., Parajó J.C., Alonso J.L. Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. J. Agric. Food Chem. 2014;62:9769–9782. doi: 10.1021/jf503475b. [DOI] [PubMed] [Google Scholar]
- 35.Gullon B., Gullon P., Sanz Y., Alonso J.L., Parajó J.C. Prebiotic potential of a refined product containing pectic oligosaccharides. LWT Food Sci. Technol. 2011;44:1687–1696. doi: 10.1016/j.lwt.2011.03.006. [DOI] [Google Scholar]
- 36.Holck J., Lorentzen A., Vigsnæs L.K., Licht T.R., Mikkelsen J.D., Meyer A.S. Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. J. Agric. Food Chem. 2011;59:6511–6519. doi: 10.1021/jf200996h. [DOI] [PubMed] [Google Scholar]
- 37.Thomassen L.V., Vigsnæs L.K., Licht T.R., Mikkelsen J.D., Meyer A.S. Maximal release of highly bifidogenic soluble dietary fibers from industrial potato pulp by minimal enzymatic treatment. Appl. Microbiol. Biotechnol. 2011;90:873–884. doi: 10.1007/s00253-011-3092-y. [DOI] [PubMed] [Google Scholar]
- 38.Johnson L.P., Walton G.E., Psichas A., Frost G.S., Gibson G.R., Barraclough T.G. Prebiotics Modulate the Effects of Antibiotics on Gut Microbial Diversity and Functioning in vitro. Nutrients. 2015;7:4480–4497. doi: 10.3390/nu7064480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichardt N., Vollmer M., Holtrop G., Farquharson F.M., Wefers D., Bunzel M., Duncan S.H., Drew J.E., Williams L.M., Milligan G., et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018;12:610–622. doi: 10.1038/ismej.2017.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di R., Vakkalanka M.S., Onumpai C., Chau H.K., White A., Rastall R.A., Yam K., Hotchkiss A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017;227:245–254. doi: 10.1016/j.foodchem.2017.01.100. [DOI] [PubMed] [Google Scholar]
- 41.Moon J.S., Shin S.Y., Choi H.S., Joo W., Cho S.K., Li L., Kang J.H., Kim T.J., Han N.S. In vitro digestion and fermentation properties of linear sugar-beet arabinan and its oligosaccharides. Carbohydr. Polym. 2015;131:50–56. doi: 10.1016/j.carbpol.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Onumpai C., Kolida S., Bonnin E., Rastall R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011;77:5747–5754. doi: 10.1128/AEM.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira-Lazarte A., Kachrimanidou V., Villamiel M., Rastall R.A., Moreno F.J. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr. Polym. 2018;199:482–491. doi: 10.1016/j.carbpol.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 44.Van Trijp M.P.H., Rösch C., An R., Keshtkar S., Logtenberg M.J., Hermes G.D.A., Zoetendal E.G., Schols H.A., Hooiveld G. Fermentation Kinetics of Selected Dietary Fibers by Human Small Intestinal Microbiota Depend on the Type of Fiber and Subject. Mol. Nutr. Food Res. 2020;64:e2000455. doi: 10.1002/mnfr.202000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J., Martínez I., Walter J., Keshavarzian A., Rose D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe. 2013;23:74–81. doi: 10.1016/j.anaerobe.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Vigsnæs L.K., Holck J., Meyer A.S., Licht T.R. In vitro fermentation of sugar beet arabino-oligosaccharides by fecal microbiota obtained from patients with ulcerative colitis to selectively stimulate the growth of Bifidobacterium spp. and Lactobacillus spp. Appl. Environ. Microbiol. 2011;77:8336–8344. doi: 10.1128/AEM.05895-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holck J., Hjerno K., Lorentzen A., Vigsnæs L.K., Hemmingsen L., Licht T.R., Mikkelsen J.D., Meyer A.S. Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length difference on human faecal microbiota composition after in vitro fermentation. Process. Biochem. 2011;46:1039–1049. doi: 10.1016/j.procbio.2011.01.013. [DOI] [Google Scholar]
- 48.Jin M., Kalainy S., Baskota N., Chiang D., Deehan E.C., McDougall C., Tandon P., Martínez I., Cervera C., Walter J., et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019;39:1437–1447. doi: 10.1111/liv.14106. [DOI] [PubMed] [Google Scholar]
- 49.Adamberg K., Adamberg S. Selection of fast and slow growing bacteria from fecal microbiota using continuous culture with changing dilution rate. Microb. Ecol. Health Dis. 2018;29:1549922. doi: 10.1080/16512235.2018.1549922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Burillo S., Mehta T., Pastoriza S., Kramer D., Paliy O., Rufian-Henares J. Potential probiotic salami with dietary fiber modulates antioxidant capacity, short chain fatty acid production and gut microbiota community structure. LWT Food Sci. Technol. 2019;105:355–362. doi: 10.1016/j.lwt.2019.02.006. [DOI] [Google Scholar]
- 51.Cantu-Jungles T.M., do Nascimento G.E., Zhang X., Iacomini M., Cordeiro L.M.C., Hamaker B.R. Soluble xyloglucan generates bigger bacterial community shifts than pectic polymers during in vitro fecal fermentation. Carbohydr. Polym. 2019;206:389–395. doi: 10.1016/j.carbpol.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Leijdekkers A.G., Aguirre M., Venema K., Bosch G., Gruppen H., Schols H.A. In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula. J. Agric. Food Chem. 2014;62:1079–1087. doi: 10.1021/jf4049676. [DOI] [PubMed] [Google Scholar]
- 53.Ramasamy U.S., Venema K., Schols H.A., Gruppen H. Effect of soluble and insoluble fibers within the in vitro fermentation of chicory root pulp by human gut bacteria. J. Agric. Food Chem. 2014;62:6794–6802. doi: 10.1021/jf501254z. [DOI] [PubMed] [Google Scholar]
- 54.Chung W.S.F., Walker A.W., Vermeiren J., Sheridan P.O., Bosscher D., Garcia-Campayo V., Parkhill J., Flint H.J., Duncan S.H. Impact of carbohydrate substrate complexity on the diversity of the human colonic microbiota. FEMS Microbiol. Ecol. 2019;95:fiy201. doi: 10.1093/femsec/fiy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung W.S., Walker A.W., Louis P., Parkhill J., Vermeiren J., Bosscher D., Duncan S.H., Flint H.J. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016;14:3. doi: 10.1186/s12915-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Abbeele P., Duysburgh C., Cleenwerck I., Albers R., Marzorati M., Mercenier A. Consistent Prebiotic Effects of Carrot RG-I on the Gut Microbiota of Four Human Adult Donors in the SHIME(®) Model despite Baseline Individual Variability. Microorganisms. 2021;9:2142. doi: 10.3390/microorganisms9102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khodaei N., Fernandez B., Fliss I., Karboune S. Digestibility and prebiotic properties of potato rhamnogalacturonan I polysaccharide and its galactose-rich oligosaccharides/oligomers. Carbohydr. Polym. 2016;136:1074–1084. doi: 10.1016/j.carbpol.2015.09.106. [DOI] [PubMed] [Google Scholar]
- 58.Larsen N., de Souza C.B., Krych L., Kot W., Leser T.D., Sørensen O.B., Blennow A., Venema K., Jespersen L. Effect of potato fiber on survival of Lactobacillus species at simulated gastric conditions and composition of the gut microbiota in vitro. Food Res. Int. 2019;125:108644. doi: 10.1016/j.foodres.2019.108644. [DOI] [PubMed] [Google Scholar]
- 59.Larsen N., Bussolo de Souza C., Krych L., Barbosa Cahú T., Wiese M., Kot W., Hansen K.M., Blennow A., Venema K., Jespersen L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019;10:223. doi: 10.3389/fmicb.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bianchi F., Larsen N., Tieghi T.M., Adorno M.A.T., Saad S.M.I., Jespersen L., Sivieri K. In vitro modulation of human gut microbiota composition and metabolites by Bifidobacterium longum BB-46 and a citric pectin. Food Res. Int. 2019;120:595–602. doi: 10.1016/j.foodres.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Bianchi F., Larsen N., de Mello Tieghi T., Adorno M.A.T., Kot W., Saad S.M.I., Jespersen L., Sivieri K. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with Bifidobacterium longum BB-46. Appl. Microbiol. Biotechnol. 2018;102:8827–8840. doi: 10.1007/s00253-018-9234-8. [DOI] [PubMed] [Google Scholar]
- 62.Míguez B., Vila C., Venema K., Parajó J.C., Alonso J.L. Prebiotic effects of pectooligosaccharides obtained from lemon peel on the microbiota from elderly donors using an in vitro continuous colon model (TIM-2) Food Funct. 2020;11:9984–9999. doi: 10.1039/D0FO01848A. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Siles M., Khan T.M., Duncan S.H., Harmsen H.J., Garcia-Gil L.J., Flint H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012;78:420–428. doi: 10.1128/AEM.06858-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ladirat S.E., Schols H.A., Nauta A., Schoterman M.H., Keijser B.J., Montijn R.C., Gruppen H., Schuren F.H. High-throughput analysis of the impact of antibiotics on the human intestinal microbiota composition. J. Microbiol. Methods. 2013;92:387–397. doi: 10.1016/j.mimet.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Cummings J.H., Southgate D.A., Branch W.J., Wiggins H.S., Houston H., Jenkins D.J., Jivraj T., Hill M.J. The digestion of pectin in the human gut and its effect on calcium absorption and large bowel function. Br. J. Nutr. 1979;41:477–485. doi: 10.1079/BJN19790062. [DOI] [PubMed] [Google Scholar]
- 66.Hamberg O., Rumessen J.J., Gudmand-Høyer E. Inhibition of starch absorption by dietary fibre. A comparative study of wheat bran, sugar-beet fibre, and pea fibre. Scand. J Gastroenterol. 1989;24:103–109. doi: 10.3109/00365528909092246. [DOI] [PubMed] [Google Scholar]
- 67.Christl S.U., Murgatroyd P.R., Gibson G.R., Cummings J.H. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269–1277. doi: 10.1016/0016-5085(92)90765-Q. [DOI] [PubMed] [Google Scholar]
- 68.Grabitske H.A., Slavin J.L. Gastrointestinal effects of low-digestible carbohydrates. Crit. Rev. Food Sci. Nutr. 2009;49:327–360. doi: 10.1080/10408390802067126. [DOI] [PubMed] [Google Scholar]
- 69.Müller M., Hermes G.D.A., Canfora E.E., Smidt H., Masclee A.A.M., Zoetendal E.G., Blaak E.E. Distal colonic transit is linked to gut microbiota diversity and microbial fermentation in humans with slow colonic transit. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;318:G361–G369. doi: 10.1152/ajpgi.00283.2019. [DOI] [PubMed] [Google Scholar]
- 70.El Kaoutari A., Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 71.Chung W.S.F., Meijerink M., Zeuner B., Holck J., Louis P., Meyer A.S., Wells J.M., Flint H.J., Duncan S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017;93:fix127. doi: 10.1093/femsec/fix127. [DOI] [PubMed] [Google Scholar]
- 72.Martens E.C., Lowe E.C., Chiang H., Pudlo N.A., Wu M., McNulty N.P., Abbott D.W., Henrissat B., Gilbert H.J., Bolam D.N., et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pryde S.E., Duncan S.H., Hold G.L., Stewart C.S., Flint H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 74.Asnicar F., Berry S.E., Valdes A.M., Nguyen L.H., Piccinno G., Drew D.A., Leeming E., Gibson R., Le Roy C., Khatib H.A., et al. Microbiome connections with host metabolism and habitual diet from 1098 deeply phenotyped individuals. Nat. Med. 2021;27:321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sokol H., Seksik P., Furet J.P., Firmesse O., Nion-Larmurier I., Beaugerie L., Cosnes J., Corthier G., Marteau P., Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 76.Zhao H., Xu H., Chen S., He J., Zhou Y., Nie Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021;36:320–328. doi: 10.1111/jgh.15222. [DOI] [PubMed] [Google Scholar]
- 77.Lecronier M., Tashk P., Tamzali Y., Tenaillon O., Denamur E., Barrou B., Aron-Wisnewsky J., Tourret J. Gut microbiota composition alterations are associated with the onset of diabetes in kidney transplant recipients. PLoS ONE. 2020;15:e0227373. doi: 10.1371/journal.pone.0227373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruengsomwong S., La-Ongkham O., Jiang J., Wannissorn B., Nakayama J., Nitisinprasert S. Microbial Community of Healthy Thai Vegetarians and Non-Vegetarians, Their Core Gut Microbiota, and Pathogen Risk. J. Microbiol. Biotechnol. 2016;26:1723–1735. doi: 10.4014/jmb.1603.03057. [DOI] [PubMed] [Google Scholar]
- 79.Ghosh T.S., Rampelli S., Jeffery I.B., Santoro A., Neto M., Capri M., Giampieri E., Jennings A., Candela M., Turroni S., et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69:1218–1228. doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao L., Zhang F., Ding X., Wu G., Lam Y.Y., Wang X., Fu H., Xue X., Lu C., Ma J., et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 81.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fechner A., Kiehntopf M., Jahreis G. The formation of short-chain fatty acids is positively associated with the blood lipid-lowering effect of lupin kernel fiber in moderately hypercholesterolemic adults. J. Nutr. 2014;144:599–607. doi: 10.3945/jn.113.186858. [DOI] [PubMed] [Google Scholar]
- 83.Pomare E.W., Branch W.J., Cummings J.H. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J. Clin. Investig. 1985;75:1448–1454. doi: 10.1172/JCI111847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dusková D., Marounek M. Fermentation of pectin and glucose, and activity of pectin-degrading enzymes in the rumen bacterium Lachnospira multiparus. Lett. Appl. Microbiol. 2001;33:159–163. doi: 10.1046/j.1472-765x.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 85.Olano-Martin E., Gibson G.R., Rastell R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002;93:505–511. doi: 10.1046/j.1365-2672.2002.01719.x. [DOI] [PubMed] [Google Scholar]
- 86.Thorning T.K., Bertolt C.J., Nielsen M.S., Ritz C., Astrup A., Raben A. Potato Fibers Have Positive Effects on Subjective Appetite Sensations in Healthy Men, but Not on Fecal Fat Excretion: A Randomized Controlled Single-Blind Crossover Trial. Nutrients. 2020;12:3496. doi: 10.3390/nu12113496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magne F., Hachelaf W., Suau A., Boudraa G., Bouziane-Nedjadi K., Rigottier-Gois L., Touhami M., Desjeux J.F., Pochart P. Effects on faecal microbiota of dietary and acidic oligosaccharides in children during partial formula feeding. J. Pediatr. Gastroenterol. Nutr. 2008;46:580–588. doi: 10.1097/MPG.0b013e318164d920. [DOI] [PubMed] [Google Scholar]
- 88.Heinken A., Khan M.T., Paglia G., Rodionov D.A., Harmsen H.J., Thiele I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014;196:3289–3302. doi: 10.1128/JB.01780-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zdunek A., Pieczywek P.M., Cybulska J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021;20:1101–1117. doi: 10.1111/1541-4337.12689. [DOI] [PubMed] [Google Scholar]
- 90.Rastall R.A., Diez-Municio M., Forssten S., Hamaker B.R., Meyer A.S., Moreno F.J., Respondek F., Stahl B., Venema K., Wiese M. Structure and function of non-digestible carbohydrates in the gut microbiome. Beneficial. Microbes. 2022;13:95–108. doi: 10.3920/BM2021.0090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.