Abstract
Objectives
To understand the recent prevalence and time trends of Helicobacter pylori infection rates in the Japanese population.
Design
Repeated cross-sectional study.
Participants
A total of 22 120 workers (age: 35–65 years) from one Japanese company, who underwent serum H. pylori antibody tests in a health check-up between 2008 and 2018.
Measures
H. pylori infection rates among participants aged 35 years from 2008 to 2018, and participants aged 35, 40, 45, and 50–65 years in 2018, based on the results of serum antibody tests, were analysed. In the 2018 analysis, in addition to the antibody test results, all participants who had undergone eradication treatment for H. pylori were considered as infected. Trends were examined using joinpoint analysis.
Results
H. pylori was detected in 1100 of 7586 male and 190 of 1739 female participants aged 35 years. Annual infection rates among those aged 35 years showed linear downward trends as follows: men, 17.5% in 2008 to 10.1% in 2018 (slope: −0.66); women, 12.3% in 2008 to 9.2% in 2018 (slope: −0.51) without joinpoints. In the 2018 analysis, 2432 of 9580 men and 431 of 1854 women were H. pylori positive. Infection rates tended to increase with older age (men: 11.0% (35 years) to 47.7% (65 years); women: 10.0% (35 years) to 40.0% (65 years)), and showed joinpoints in both sexes (men: 54 years; women: 45 years). Although both the first and second trends were upward, the second trend for both men and women was steeper than the first trend (p<0.05).
Conclusions
Our study demonstrated that in the previous 11 years, infection rates of H. pylori in 35-year-old male and female Japanese workers have constantly decreased, and furthermore, analysis of various age groups showed joinpoints around 50 years, suggesting a consistent declining trend in H. pylori infection rates in Japan.
Keywords: Gastrointestinal infections, Gastrointestinal tumours, HEALTH ECONOMICS, Health policy, Epidemiology, PREVENTIVE MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study presents a recent 11-year time trend of Helicobacter pylori infection rates based on H. pylori serum antibody test results of 35-year-old workers from one large company with many branches around Japan, using joinpoint trend analysis.
The 2018 data compared infection rates by age group (35, 40, 45 and 50–65 years), taking into account the history of H. pylori eradication treatment obtained by a questionnaire, in addition to antibody testing.
The main limitation of this study is the generalisability of the results to the general Japanese population because the study subjects were company employees.
Introduction
Helicobacter pylori is a gram-negative bacterium that is often found in the human stomach. H. pylori infection is known to be closely associated with chronic gastritis, duodenal ulcers, gastric ulcers, gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer.1–4 The International Agency for Research on Cancer Working group, which is part of the WHO, classified H. pylori as a group 1 carcinogen for gastric cancer in 1994.5 Moreover, the WHO has stated that ‘H. pylori screening and treatment strategies would be cost-effective’ for asymptomatic populations to prevent gastric cancer, and has recommended that ‘countries explore the possibility of introducing population-based H. pylori screening and treatment programmes’.6
H. pylori test-and-treat strategy for preventing gastric cancer is considered more effective in regions with a high incidence of gastric cancer.7 The incidence and mortality rates of gastric cancer are relatively high in Japan compared with other countries. Moreover, H. pylori infection is thought to be involved in more than 90% of gastric cancer cases in Japan.8 9 In addition, the infection rate of H. pylori in Japan is higher than that in other developed countries.10 Accordingly, the ‘test-and-treat’ strategy for H. pylori could be a good measure for preventing gastric cancer in Japan. In 2013, the national health insurance scheme covered the H. pylori eradication therapy for chronic gastritis in Japan. Several groups in Japan, including company health insurance societies and local municipalities, have introduced H. pylori screening tests for asymptomatic people during medical check-ups for the prophylactic intervention of gastric cancer.
Decreasing the H. pylori infection rate could reduce the incidence rate of gastric cancer11 and reduce the positive predictive value of H. pylori screening. Therefore, decreasing the H. pylori infection rate in the population may negatively affect the cost-effectiveness of the ‘test-and-treat’ strategy for asymptomatic groups. It is important to elucidate the current prevalence of H. pylori infection and its trends over time, to predict future infection rates and plan test strategies for the future. There has been a decrease in the worldwide prevalence of H. pylori,10 with several Japanese studies reporting similar results.12 However, most of these studies had small sample sizes or included specific participants, including hospital visitors. To our knowledge, there have been no recent large-scale studies on the prevalence and time trend of infection rates of H. pylori in Japan.12–14 In addition, several studies have reported that the H. pylori infection rates become steady at approximately 10% in several low-prevalence regions, including European countries.10 15 16 Watanabe et al analysed the prevalence of H. pylori infection by birth year among first-visit outpatients between 2005 and 2013 in Nagoya, Japan. The results showed three trends: the birth year per cent change (BPC)=–1.15% in patients born between 1927 and 1949, BPC=–4.59% in patients born between 1949 and 1961, and BPC=–2.04% in patients born between 1961 and 1988, indicating that after a rapid decrease in infection rates in those born between 1949 and 1961, the rate of decrease has slowed down.14 Our present study aimed to elucidate the recent trends in the infection rates of H. pylori, including the rates after 2013, which is the year that the health insurance system in Japan began to cover H. pylori eradication therapy for chronic gastritis, and whether they showed significant changes with time.
Using data from health check-ups in a company health insurance society, this repeated cross-sectional study aimed to clarify the recent 11-year trend of H. pylori infection rate in patients aged 35 years old and H. pylori infection rates in 2018 according to age, stratified by sex.
Materials and methods
Japanese law requires all citizens to have some type of health insurance. T company is one of the largest companies in Japan, with many branches. All workers of this company, which include a wide variety of people, such as office workers, manual labourers and people with disabilities, belong to the company’s health insurance society. Members of the T company health insurance society undergo serum anti-H. pylori IgG antibody tests. This test was conducted annually on members aged 35 years during their health check-ups (approximately 600–1100 people per year). However, in 2018, the health insurance society offered this test to participants aged 35, 40, 45 and >50 years. We included members who had undergone serum H. pylori antibody tests at their annual health check-ups from 1 April 2008 to 31 March 2019, at the age of 35–65 years. Participants’ blood samples were taken at their health check-ups. Serum was isolated from the samples and stored at –80°C until use. Serum anti-H. pylori IgG was measured using an ELISA with ‘E-Plate Eiken H. pylori antibody’ or ‘E-Plate II Eiken H. pylori antibody’ (Eiken Chemical Co, Tokyo, Japan). The cut-off level was set at 10 U/mL,17 with values above this being classified as positive. Anonymised participants’ data, including medical questionnaires and blood test results, were obtained from the annual health check-up database of the health insurance society. We excluded data from individuals who refused academic use of their data.
Statistical analysis
First, data obtained from 35-year-old participants between 2008 and 2018 were analysed to determine time trends in H. pylori infection rates (‘35-year-old participant analysis’) stratified by sex. Positive results of serum antibody tests for H. pylori were defined as H. pylori infection. Annual infection rates were calculated based on the antibody test results. Subsequently, we analysed the time trend of the rates.
Second, we analysed data from participants aged 35, 40, 45 and 50–65 years old obtained in 2018 according to age stratified by sex to determine generational differences in the infection rates (‘2018 analysis’). In the 2018 analysis, participants who tested positive for antibodies were considered as infected. Further, to reduce the influence of eradication treatment on the infection rate, all participants with a history of eradication treatment of H. pylori infection were defined as positive regardless of their test results for H. pylori antibody, with the assumption that a history of eradication treatment indicates a previous infection.
We performed joinpoint trend analysis18 to identify trends in the infection rate and their changes over time using Joinpoint Regression Program V.4.9.0.0.19 We used the permutation test to select the optimal number of joinpoints in the 35-year-old participant analysis, whereas we used the Bayesian Information Criterion in the 2018 analysis. Linear model was selected in the analyses. Statistical significance was set at p<0.05. Data with missing or ambiguous figures were excluded from the analysis.
Patient and public involvement
After discussions with representatives of the health insurance society about this study, the health insurance society acknowledged the importance of our study, and permitted us to collect and use participant data from its database. The results of this study are published as a report.
Results
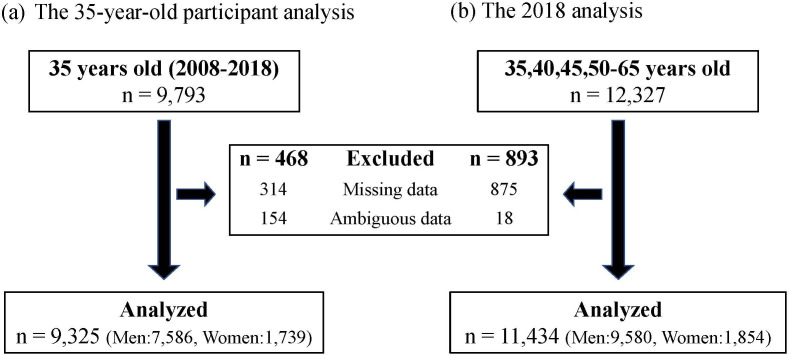
There were 9793 and 12 327 participants in the 35-year-old participant analysis and 2018 analyses, respectively, with 592 participants overlapping in both analyses. In the 35-year-old participant analysis, 468 participants were excluded for having missing (n=314) or ambiguous data (n=154) in the records, with 9325 participants (7586 men; 1739 women) being included in the final analysis. In the 2018 analysis, 893 participants were excluded for having missing (n=875) or ambiguous data (n=18) in the records, with 11 434 participants (9580 men; 1854 women) being included in the final analysis (figure 1).
Figure 1.
Numbers of participants. (A) The 35-year-old participant analysis: participants aged 35 years from 2008 to 2018. (B) The 2018 analysis: participants aged 35–65 years in 2018.
The 35-year-old participant analysis
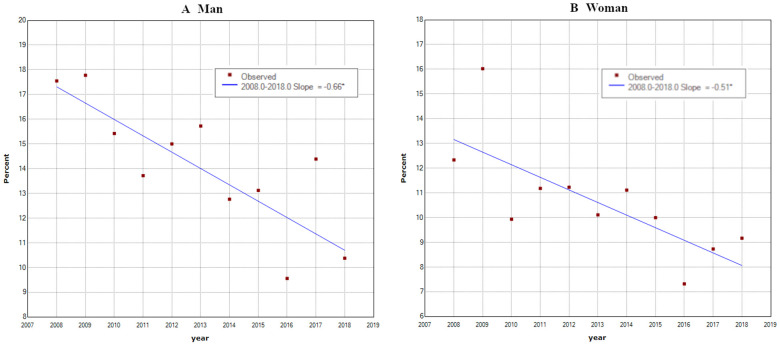
In the 35-year-old participant analysis, 1100 out of 7586 male participants and 190 out of 1739 female participants were H. pylori infected. In joinpoint analysis, infection rates showed linear downward trends in both men and women with increasing years (men: 17.5% in 2008 to 10.1% in 2018 (slope –0.66); women: 12.3% in 2008 to 9.2% in 2018 (slope –0.51) (p<0.05)). These trends lacked joinpoints at which the trend significantly changed (figure 2).
Figure 2.
Infection rates of Helicobacter pylori at 35 years old from 2008 to 2018 (men: n=7586; women: n=1739). Infection rates at 35 years linearly decreased by years. (A) Men: 17.5% in 2008 to 10.1% in 2018 (slope: −0.65). (B) Women: 12.3% in 2008 to 9.2% in 2018 (slope: −0.51). There were no joinpoints. ‘*’ in the graph legend indicates a significant difference in the slope from 0 at the alpha=0.05 level.
The 2018 analysis
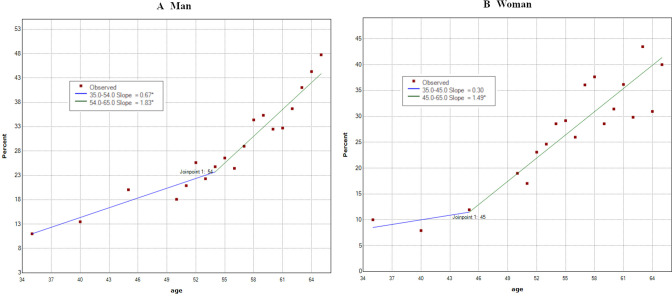
In the 2018 analysis, 2432 out of 9580 male participants and 431 out of 1854 female participants were infected with H. pylori. The infection rates showed trends of increasing positive rates with advanced age in both men and women (men: 11.0% at 35 years to 47.7% at 65 years; women: 10.0% at 35 years to 40.0% at 65 years). These trends had joinpoints at the age of 54 years in men (95% CI: 45 to 58) and at the age of 45 years in women (95% CI: 45 to 51), with two different trends in the slope before and after the point. Specifically, the first and second trends were 35–54 years (slope=0.67) and 54–65 years (slope=1.83) in men, and 35–45 years (slope=0.30) and 45–65 years (slope=1.49) in women. Both first and second trends showed a linear increase with age, with the second trend being significantly steeper than the first trend (p<0.05) (figure 3).
Figure 3.
Infection rates of Helicobacter pylori according to age in 2018 (men: n=9580; women: n=1854). Infection rates increased in two trends. (A) Men: first trend: 35–54 years (slope=0.67); second trend: 54–65 years (slope=1.83). (B) Female: first trend: 35–45 years (slope=0.30); second trend: 45–65 years (slope=1.49). ‘*’ in the graph legend indicates a significant difference in the slope from 0 at the alpha=0.05 level.
Discussion
This study investigated the 11-year time trend from 2008 to 2018, and the trend by age in 2018 regarding the prevalence of H. pylori infection in Japanese workers stratified by sex based on large-scale health check-up data. In the 35-year-old participant analysis, the infection rate showed a linear declining trend from 2008 to 2018 both in men and women. This provided a good estimate of the H. pylori infection trends in Japan, as the subjects of this study were workers in a large company, including workers in the branch offices.
In the 35-year-old participant analysis, the infection rate decreased linearly to approximately 10% both in men and women; further, this downward trend did not significantly change during the observation period. We assumed that the participants in this analysis, who were all 35 years old, were less affected by eradication treatment than older participants; and therefore, the results may closely reflect the actual trend of the incidence rate of H. pylori infections in Japan. If the downward trends (slope=–0.65 in men and slope=–0.51 in women) observed in the 35-year-old participant analysis continue, the infection rate is expected to reach nearly 0 by about 2035. In contrast, recent studies on junior high school students (aged: 12–15 years) in Japan have demonstrated that the infection rate of this generation when they reach 35 years at about 2035 will be approximately 3%–5%,20–23 which is higher than our prediction. This inconsistency suggests that the decrease in the infection rate may have slowed down.
In the 2018 analysis, the infection rates in both sexes increased with advanced age. This also indicated declining trends in the prevalence rate of H. pylori over the years. Furthermore, there were joinpoints around the age of 50 years (54 years in men (95% CI: 45 to 58) and 45 years in women (95% CI: 45 to 51)), which implies the existence of some type of change affecting the H. pylori infection rate in people of this generation (figure 3). Chronic H. pylori infection is mostly established in the human stomach during childhood.24 25 Drinking water and family members are among the sources of H. pylori infection.26 From the late 1960s to the 1970s, which is when people aged 50 years in 2018 spent their childhood, Japan experienced rapid economic growth and urbanisation. Accordingly, there was an accelerated increase in water supply and a decrease in the average number of households.27 28 These fast environmental changes may have influenced the establishment of H. pylori infection; consequently, there was a sharp decrease in the prevalence of this bacterial infection during this era. Watanabe et al revealed changes in the declining trend of H. pylori infection rate and indicated an effect of environmental changes on the infection rate,14 which is consistent with our findings.
Participants in the 35-year-old participant analysis were born after 1973; therefore, they may have not experienced the rapid environmental changes that those who were born during the period of high economic growth (1955–1972) had experienced. The 35-year-old participant analysis revealed a recent gradual decrease in the H. pylori infection rate. This suggests that factors other than hygiene and family structure may influence infection establishment. As aforementioned, H. pylori infections are likely to be established during childhood through parent-to-child transmission.25 In addition to hygienic and environmental improvements, the spread of ready-made baby food after around 1970 may have contributed to the decreased H. pylori infection rate.29 With the recently increasing recognition of H. pylori in the general population and coverage of eradication treatment through national insurance, there has been an increase in the number of H. pylori eradication treatments in Japan.30 If treatment decreases the infection rate in childrearing generation, it could accelerate the declining speed of the infection rates in the next generations.
When conducting H. pylori screening tests for prophylactic purposes, the prevalence in a target group should be considered to evaluate the effectiveness of the strategy. We observed a decreasing infection rate of H. pylori in Japan. In the future, the infection rate may reach 0; accordingly, there would be a decreased importance of screening tests for this bacterium in asymptomatic people. A study on the cost-effectiveness of the test-and-treatment strategy for H. pylori revealed that it remained effective even with a low infection rate of approximately 5%.31 Our findings could inform future public health strategies. Moreover, considering the decrease in H. pylori prevalence, H. pylori infection-negative gastric cancer has lately been receiving attention.32–34 Research for risk factors of gastric cancer other than H. pylori is also needed.
Limitations
This study has several limitations. First, there might have been selection bias, including age, sex, occupation, region and nationality (possibly including several workers who were born and raised in countries other than Japan). Several studies have analysed differences in the infection rates according to sex, occupation and region, with a study reporting a higher infection rate in men; however, there remains no consensus regarding these biases.35–37 Our present study included participants of an unequal number of each sex. This was one reason why we conducted separate analyses for each sex. A recent study showed that employees in a large company are in better health than those in a small one.38 As the target group of this study were employees of a large company, there is the possibility of such bias. Heterogeneity of age gaps between age groups and limitation of the target ages may have weakened analyses, especially in younger age groups. However, the present study targeted all health insurance society members working in a large company operating throughout Japan, and therefore, we were able to include subjects from various age groups and regions throughout Japan. Second, the history of H. pylori eradication may have influenced our findings. In 2008, T company health insurance society performed serum H. pylori antibody screening tests for all its members. Therefore, there may have been a higher proportion of post-eradication cases in our study than in the general population. We attempted to reduce the influence of eradication treatment by classifying patients who underwent eradication treatment as infected. However, history information acquired through a self-reported questionnaire could have contained inaccuracies, including recall bias.39 In the 35-year-old participant analysis, we could not obtain information regarding previous eradication treatment of the participants. We hence analysed the data based on the assumption that the rate of eradication treatment among participants in the 35-year-old participant analysis was low, due to their younger age, and therefore would not significantly affect the infection rate. The health insurance society collected and reported, as part of their health services, that the rates of 35-year-old participants who had previously undergone eradication treatment (both men and women) were 0.9%, 2.1%, and 1.4% in 2018, 2019, and 2020, respectively. Although this supports our assumption, it indicates the possibility of errors of 1%–2% from eradication treatment in the infection rates of the 35-year-old participant analysis. In addition, medical history, medications and measurement biases, including test characteristics and threshold application in the test (including high-negative issues), could have influenced our results.40 41 The limitations mentioned above may hinder the generalisation of our findings to the Japanese general population.
However, the present study collected data of participants aged 35 years old from 2009 to 2018, as well as data of various age groups from 2018, and this information was analysed using a robust statistical method called joinpoint analysis, demonstrating important findings for considering future H. pylori eradication therapy targets.
Conclusion
Our study showed a constant decreasing time trend in the infection rate of H. pylori among 35-year-old workers in Japan from 2008 to 2018.42 This time trend indicates that the infection rate of H. pylori may continue to decrease in the future. Trends in the infection rate by age in 2018 indicated the possibility of a slowing down of the rate of decrease in the prevalence of H. pylori in Japan. In populations with few H. pylori-positive individuals, the efficiency of measures to routinely test for antibodies is low, and therefore, it would be difficult to rely solely on the H. pylori test and treatment strategy to achieve gastric cancer prevention. We believe that the data regarding changes in the prevalence of H. pylori over the years observed in Japan could be useful for other countries with a high incidence of H. pylori infection, in planning future eradication strategies.
Supplementary Material
Acknowledgments
We would like to acknowledge the T company health insurance society. We would also like to thank the Center for International Education and Research of Tokyo Medical University for English language editing.
Footnotes
Contributors: SA designed the study, analysed the data and drafted the manuscript. YHi contributed to the study design and performed general supervision of the whole study as the guarantor. JO, YHa and TTo assisted in conducting the study, interpreting the results and revising the manuscript. KK and TK contributed to data collection and provided advice and opinions from an expert's perspective. TTa contributed to data analysis and revising the manuscript for statistical and public health perspectives. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. Figshare (https://doi.org/10.6084/m9.figshare.14594571.v2), CC BY 4.0.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study protocol was reviewed and approved by the Institutional Review Board of Tokyo Medical University (T2019-0044). Following the Ethical Guidelines for Medical and Health Research Involving Human Subjects,43 the study information was shown on the websites of the institutions where the researchers or participants belonged. Participants’ consent for using data was obtained through an opt-out option.
References
- 1.Wang F, Meng W, Wang B, et al. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202. 10.1016/j.canlet.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 2.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet 2002;359:14–22. 10.1016/S0140-6736(02)07273-2 [DOI] [PubMed] [Google Scholar]
- 3.Eck M, Schmausser B, Haas R, et al. Malt-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 1997;112:1482–6. 10.1016/S0016-5085(97)70028-3 [DOI] [PubMed] [Google Scholar]
- 4.Sasazuki S, Inoue M, Iwasaki M, et al. Effect of Helicobacter pylori infection combined with cagA and pepsinogen status on gastric cancer development among Japanese men and women: a nested case-control study. Cancer Epidemiol Biomarkers Prev 2006;15:1341–7. 10.1158/1055-9965.EPI-05-0901 [DOI] [PubMed] [Google Scholar]
- 5.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Schistosomes, liver flukes and Helicobacter pylori. Lyon (FR): International Agency for Research on Cancer, 1994. [PMC free article] [PubMed] [Google Scholar]
- 6.IARC Helicobacter pylori Working Group . Helicobacter pylori eradication as a strategy for preventing gastric cancer: international agency for research on cancer; 2014.
- 7.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. 10.1136/bmj.g3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center for Cancer Control and Information Services, National Cancer Center . CANCER STATISTICS IN JAPAN '19, 2020. Available: https://ganjoho.jp/en/professional/statistics/brochure/2019_en.html [Accessed 22 Dec 2020].
- 9.Matsuo T, Ito M, Takata S, et al. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter 2011;16:415–9. 10.1111/j.1523-5378.2011.00889.x [DOI] [PubMed] [Google Scholar]
- 10.Peleteiro B, Bastos A, Ferro A, et al. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci 2014;59:1698–709. 10.1007/s10620-014-3063-0 [DOI] [PubMed] [Google Scholar]
- 11.Tsuda M, Asaka M, Kato M, et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter 2017;22. 10.1111/hel.12415. [Epub ahead of print: 03 08 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Nishiyama T, Kikuchi S, et al. Changing trends in the prevalence of H. pylori infection in Japan (1908-2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep 2017;7:15491. 10.1038/s41598-017-15490-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer 2017;20:3–7. 10.1007/s10120-016-0658-5 [DOI] [PubMed] [Google Scholar]
- 14.Watanabe M, Ito H, Hosono S, et al. Declining trends in prevalence of Helicobacter pylori infection by birth-year in a Japanese population. Cancer Sci 2015;106:1738–43. 10.1111/cas.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmack SW, Genta RM. Helicobacter pylori seroprevalence in symptomatic veterans: a study of 7310 patients over 11 years. Helicobacter 2009;14:298–302. 10.1111/j.1523-5378.2009.00693.x [DOI] [PubMed] [Google Scholar]
- 16.den Hoed CM, Vila AJ, Holster IL, et al. Helicobacter pylori and the birth cohort effect: evidence for stabilized colonization rates in childhood. Helicobacter 2011;16:405–9. 10.1111/j.1523-5378.2011.00854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsu Y, Sugiyama T, Asaka M. Helicobacter pylori kansenshindan ni okeru nihonjinkabu wo siyou sita ELISA kit " E-plate eiken H.pylori koutai" no yuyousei no kentou [Study of effectiveness of E-plate eiken which is ELAISA kit using Japanese strain in diagnose of Helicobacter pylori infection]. The Journal of Clinical Laboratory Instruments and Reagents 2001;24:331–5 https://jglobal.jst.go.jp/en,J-GLOBALID:200902134517528860 [Google Scholar]
- 18.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 19.Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute . Joinpoint Regression Program [program] Version 4.9.0.0 version; 2021.
- 20.Kusano C, Gotoda T, Ishikawa H, et al. The administrative project of Helicobacter pylori infection screening among junior high school students in an area of Japan with a high incidence of gastric cancer. Gastric Cancer 2017;20:16–19. 10.1007/s10120-017-0688-7 [DOI] [PubMed] [Google Scholar]
- 21.Honma H, Nakayama Y, Kato S, et al. Clinical features of Helicobacter pylori antibody-positive junior high school students in Nagano Prefecture, Japan. Helicobacter 2019;24:e12559. 10.1111/hel.12559 [DOI] [PubMed] [Google Scholar]
- 22.Nakayama Y, Lin Y, Hongo M, et al. Helicobacter pylori infection and its related factors in junior high school students in Nagano Prefecture, Japan. Helicobacter 2017;22. 10.1111/hel.12363. [Epub ahead of print: 27 10 2016]. [DOI] [PubMed] [Google Scholar]
- 23.Kakiuchi T, Matsuo M, Endo H, et al. A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in SAGA Prefecture: a preliminary report. J Gastroenterol 2019;54:699–707. 10.1007/s00535-019-01559-9 [DOI] [PubMed] [Google Scholar]
- 24.Banatvala N, Mayo K, Megraud F, et al. The cohort effect and Helicobacter pylori. J Infect Dis 1993;168:219–21. 10.1093/infdis/168.1.219 [DOI] [PubMed] [Google Scholar]
- 25.O'Ryan ML, Lucero Y, Rabello M, et al. Persistent and transient Helicobacter pylori infections in early childhood. Clin Infect Dis 2015;61:211–8. 10.1093/cid/civ256 [DOI] [PubMed] [Google Scholar]
- 26.Ueda M, Kikuchi S, Kasugai T, et al. Helicobacter pylori risk associated with childhood home environment. Cancer Sci 2003;94:914–8. 10.1111/j.1349-7006.2003.tb01375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.JAPAN WATER WORKS ASSOCIATION . Water supply in Japan, 2017. Available: http://www.jwwa.or.jp/jigyou/kaigai_file/2017WaterSupplyInJapan.pdf [Accessed 22 Dec 2020].
- 28.Ministry of Health, Labour and Welfare . General welfare and labour Japan. Available: https://www.mhlw.go.jp/english/wp/wp-hw9/dl/01e.pdf [Accessed 01 Oct 2020].
- 29.Council JBF . Seisan Tokei [Production statistics] Japan. Available: https://www.baby-food.jp/link/ayumi-jikei.html [Accessed 11 Dec 2020].
- 30.Hiroi S, Sugano K, Tanaka S, et al. Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japan: retrospective observational study and simulation study based on real-world data. BMJ Open 2017;7:e015855. 10.1136/bmjopen-2017-015855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowada A. Cost-effectiveness of Helicobacter pylori screening followed by eradication treatment for employees in Japan. Epidemiol Infect 2018;146:1834–40. 10.1017/S095026881800208X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato S, Matsukura N, Tsukada K, et al. Helicobacter pylori infection-negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci 2007;98:790–4. 10.1111/j.1349-7006.2007.00478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto Y, Fujisaki J, Omae M, et al. Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings. Dig Endosc 2015;27:551–61. 10.1111/den.12471 [DOI] [PubMed] [Google Scholar]
- 34.Takita M, Ohata K, Inamoto R, et al. Endoscopic and histological features of Helicobacter pylori-negative differentiated gastric adenocarcinoma arising in the antrum. JGH Open 2021;5:470–7. 10.1002/jgh3.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kheyre H, Morais S, Ferro A, et al. The occupational risk of Helicobacter pylori infection: a systematic review. Int Arch Occup Environ Health 2018;91:657–74. 10.1007/s00420-018-1315-6 [DOI] [PubMed] [Google Scholar]
- 36.Ueda J, Gosho M, Inui Y, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter 2014;19:105–10. 10.1111/hel.12110 [DOI] [PubMed] [Google Scholar]
- 37.Ferro A, Morais S, Pelucchi C, et al. Sex differences in the prevalence of Helicobacter pylori infection: an individual participant data pooled analysis (stop project). Eur J Gastroenterol Hepatol 2019;31:593–8. 10.1097/MEG.0000000000001389 [DOI] [PubMed] [Google Scholar]
- 38.Kanamori S, Tsuji T, Takamiya T, et al. Size of company of the longest-held job and mortality in older Japanese adults: a 6-year follow-up study from the Japan Gerontological evaluation study. J Occup Health 2020;62:e12115. 10.1002/1348-9585.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol 1990;43:87–91. 10.1016/0895-4356(90)90060-3 [DOI] [PubMed] [Google Scholar]
- 40.Miftahussurur M, Yamaoka Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: critical importance of indirect test validation. Biomed Res Int 2016;2016:1–14. 10.1155/2016/4819423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue M, Sawada N, Goto A, et al. High-negative Anti-Helicobacter pylori IgG Antibody Titers and long-term risk of gastric cancer: results from a large-scale population-based Cohort Study in Japan. Cancer Epidemiol Biomarkers Prev 2020;29:420–6. 10.1158/1055-9965.EPI-19-0993 [DOI] [PubMed] [Google Scholar]
- 42. Abiko S. Data from: changes in prevalence of Helicobacter pylori in Japan from 2008 to 2018: a repeated cross-sectional study. Figshare 2022. 10.6084/m9.figshare.14594571.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. Figshare (https://doi.org/10.6084/m9.figshare.14594571.v2), CC BY 4.0.