Abstract
VirB6 from Agrobacterium tumefaciens is an essential component of the type IV secretion machinery for T pilus formation and genetic transformation of plants. Due to its predicted topology as a polytopic inner membrane protein, it was proposed to form the transport pore for cell-to-cell transfer of genetic material and proteinaceous virulence factors. Here, we show that the absence of VirB6 leads to reduced cellular levels of VirB5 and VirB3, which were proposed to assist T pilus formation as minor component(s) or assembly factor(s), respectively. Overexpression of virB6 in trans restored levels of cell-bound and T pilus-associated VirB5 to wild type but did not restore VirB3 levels. Thus, VirB6 has a stabilizing effect on VirB5 accumulation, thereby regulating T pilus assembly. In the absence of VirB6, cell-bound VirB7 monomers and VirB7-VirB9 heterodimers were reduced and VirB7 homodimer formation was abolished. This effect could not be restored by expression of VirB6 in trans. Expression of TraD, a component of the transfer machinery of the IncN plasmid pKM101, with significant sequence similarity to VirB6, restored neither protein levels nor bacterial virulence but partly permitted T pilus formation in a virB6 deletion strain. VirB6 may therefore regulate T pilus formation by direct interaction with VirB5, and wild-type levels of VirB3 and VirB7 homodimers are not required.
Transport of macromolecules across the cell envelope of gram-negative bacteria is mediated by different secretion machineries, classified as type I to type IV based on sequence similarities of their essential protein components (23, 36). Type IV secretion systems mediate cell-to-cell transfer of virulence factors from gram-negative pathogens such as Brucella suis, Helicobacter pylori, and Legionella pneumophila, secretion of pertussis toxin from Bordetella pertussis, as well as spread of broad-host-range plasmids and genetic transformation of plant cells by Agrobacterium tumefaciens (11, 28, 33, 40, 43). Twelve protein components of the A. tumefaciens system form the most developed model for studies on the mechanism of type IV secretion (10, 44). The 11 VirB proteins and VirD4 assemble into a complex spanning both membranes and the murein layer, and similar topology occurs in related secretion systems. The outer membrane lipoprotein VirB7 forms a heterodimeric complex with VirB9 that likely serves as nucleating center stabilizing the transmembrane complex in the periplasm (1, 4, 18, 41). Genetic and biochemical studies suggest this stabilization may involve direct interaction(s) with the periplasmic domains of VirB8 and VirB10, which form high-molecular mass complexes in a VirB9-dependent manner (5, 14). The ATPases VirB4 and VirB11 associate with the cytoplasmic side of the inner membrane and may energize macromolecular transfer (12, 35). However, a recent study suggests that VirB4 may facilitate assembly of the type IV transporter by protein-protein interactions independent of its nucleotide-hydrolyzing activity (13). In addition to macromolecular transfer, the VirB proteins mediate assembly of the T pilus, which may initiate contact formation with the plant cell (20). VirB2, the major T pilus component, undergoes several processing reactions leading to the formation of a cyclic peptide (17, 27). VirB5 cofractionates with T pili during several purification steps and may therefore be a minor T pilus component (38). Alternatively, VirB5 may constitute a T pilus assembly factor, and VirB3, which preferentially localizes to the outer membrane in a VirB4-dependent manner, may play a similar role (25). VirB1 undergoes processing, and the C-terminal domain is partly secreted into the supernatant, where it may play a role in the interaction with plant cells (3). The N-terminal domain of VirB1, which shows significant sequence similarity to lytic transglycosylases, was proposed to facilitate assembly of the type IV transporter by localized lysis of the peptidoglycan layer (16, 31).
Despite the increasing knowledge about individual VirB proteins and their interactions, however, an overall picture of the mechanism of macromolecular transfer has not emerged, and components involved in key functions have not been identified. For example, cell exit of folded macromolecules requires formation of a transmembrane pore, and in the case of pullulanase and phage f1 secretion systems, assembly of PulD and its homolog pIV to an outer membrane pore was recently demonstrated (30, 32). So far, there is no direct evidence for a pore in type IV secretion systems. However, the polytopic membrane protein VirB6 was proposed as an inner membrane pore-forming component of the type IV secretion machinery required for transfer of virulence factors into plant cells (10, 15). Deletion of virB6 affects the steady-state levels of some VirB proteins (7), but these experiments were performed with cultures grown in liquid culture at 28°C—conditions which were subsequently shown to inhibit the type IV machinery and to be unsuitable for T pilus assembly (2, 20, 21).
Here, we monitored all components of the type IV secretion apparatus (VirB1 to VirB11 and VirD4) and the transported proteinaceous substrates VirD2 and VirE2. Agrobacteria were grown on acidic AB minimal medium plates at 20°C (growth conditions optimized for Vir protein stability and T pilus formation), in liquid cultures at 20°C, and in liquid cultures at 28°C. Deletion of virB6 destabilized the type IV secretion machinery and negatively affected many VirB proteins in cells grown in liquid culture at 28°C. In contrast, the absence of VirB6 negatively affected only a limited number of VirB proteins involved in T pilus assembly in cells grown under optimized growth conditions, indicating that previously used conditions for induction are not suitable for analysis of the A. tumefaciens VirB machinery. Complementation experiments suggested direct stabilization of VirB5 by VirB6, implicating VirB6 as a key regulator of T pilus assembly.
MATERIALS AND METHODS
Bacterial growth conditions.
Escherichia coli strains used for cloning procedures were grown in Luria-Bertani media following established procedures. Agrobacteria were routinely propagated on rich YEB media and subjected to virulence gene induction in liquid AB minimal medium at 20 or 28°C for 18 h or on AB minimal medium agar plates incubated for 3 days at 20°C in the presence of acetosyringone (AS; 200 μM) and isopropyl-β-d-thiogalactopyranoside (IPTG; 0.5 mM) as described elsewhere (38).
DNA modification procedures.
Standard protocols were used for DNA manipulations using enzymes from MBI Fermentas and New England Biolabs. Sequencing was performed on an ABI Prism 377 sequencer (29).
An in-frame deletion of virB6 was introduced into the Ti plasmid of nopaline strain C58 essentially as described elsewhere (39), using the oligonucleotides dB6-1 (5′-CAAGGTCAGGTCCAAACGATGAAATATTGCCTGCTGTGCC-3′) and dB6-2 (5′-GGCACAGCAGGCAATATTTCATCGTTTGGACCTGACCTTG-3′), giving strain CB1006.
For complementation, the nopaline Ti plasmid virB6 gene and the traD gene from the IncN plasmid pKM101 were PCR amplified as described elsewhere (39), using oligonucleotide pairs B65 (5′-GGGGCCATGGCTTTCACGATCCCGGC-3′)-B63 (5′-GAAAGTACTAACGACGATCGACC-3′) and TraD5 (5′-GGGGCCATGGCATTCACCCTGG-3′)-TraD3 (5′-GAAAGTACTATGCAGCCTTCTTCCC-3′), respectively. After cleavage with enzymes NcoI and ScaI (underlined), the fragments were ligated with NcoI/SmaI-cleaved pTrc200 (39), resulting in pTrcB6 and pTrcTraD.
Subcellular fractionations.
Cell lysis, separation of subcellular fractions, and purification of T pilus-containing surface structures were performed as described elsewhere (38, 39).
Protein analysis.
To avoid the formation of high-molecular-mass aggregates of VirB6 and VirD4, cells were incubated in lysis solution (50 mM glucose, 25 mM Tris-HCl [pH 8], 10 mM EDTA, 1 mg of lysozyme/ml) for 30 min on ice, followed by addition of 1 volume of Laemmli sample buffer and incubation at 37°C for another 30 min. Lysates for analyses of other Vir proteins were generated by boiling of cells in Laemmli sample buffer (26). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (26) or Schägger and von Jagow (37), using various amounts of acrylamide depending on the molecular mass of the Vir protein under analysis. Western blotting and detection with Vir protein-specific antisera used rabbit- or mouse-specific secondary horseradish peroxidase-coupled antibodies (Bio-Rad) and a chemoluminescence detection system (NEN).
The production of most antisera was described previously (22, 42). VirB6- and VirD4-specific antisera were generated by immunization of mice with mouse serum albumin-coupled peptides corresponding to the C-terminal amino acids of the protein, and N-terminal cysteine residues were introduced for coupling (VirB6-peptide, CTQSANSLYRRFAQVDRR; VirD4-peptide, CALQQRYGPASSHSVK).
Image processing.
Photographs, gels, and chemoluminographs were recorded with a UMAX UC840 MaxVision scanner; images were further processed on a Power Macintosh G3 computer using Adobe Photoshop 5 or Canvas 6 software and printed on an Epson Stylus Photo printer.
RESULTS
Deletion of virB6 and complementation analysis.
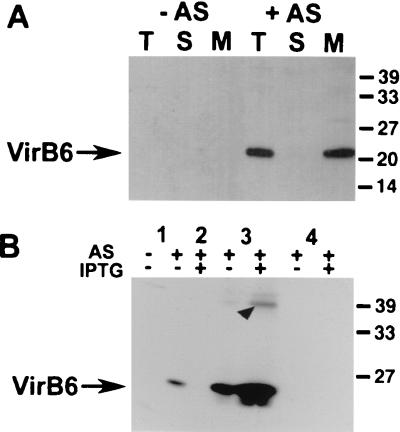
VirB6-specific antisera have not been reported so far, possibly because the overall hydrophobicity of the protein makes overproduction and purification procedures extremely tedious. To avoid this problem, a specific antiserum was generated by immunization of mice with a mouse serum albumin-coupled peptide corresponding to the hydrophilic C-terminal 17 amino acids of VirB6. According to the predicted membrane topology of VirB6, these amino acids may constitute the cytoplasmic tail of the protein (6, 15). A. tumefaciens strain C58 was virulence gene induced, and subcellular fractions were subjected to SDS-PAGE and Western blotting. The antiserum detected a 22-kDa protein exclusively in the membrane fraction of AS-induced cells when samples were treated with Laemmli sample buffer at temperatures below 50°C (Fig. 1A). Analysis of boiled samples did not lead to specific signals; instead, high-molecular-mass aggregates were detected in the stacking gel (not shown).
FIG. 1.
Expression of VirB6 in wild-type strain C58 and virB6 deletion strain CB1006. (A) Analysis of subcellular fractions from virulence gene-induced (+AS) or uninduced (−AS) strain C58 after SDS-PAGE and Western blotting with VirB6-specific antiserum. T, total cell lysate; S, soluble fraction; M, membrane proteins. (B) Analysis of cell lysates from wild-type C58 (lanes 1) and virB6 deletion strain CB1006 carrying cloning vector pTrc200 (lane 2), pTrcB6 (lanes 3), or pTrcTraD (lanes 4) grown in the absence of induction, in the presence of AS for virulence gene induction, or in the presence of AS and IPTG for simultaneous induction of the trc promoter. Cell lysates were subjected to SDS-PAGE followed by Western blotting and detection with VirB6-specific antiserum. Arrowhead indicates aggregates of VirB6 detected in overexpressing cells. Numbers on the right are molecular masses of reference proteins in kilodaltons.
To confirm the identity of the detected protein, an in-frame deletion of virB6 was introduced into the Ti plasmid of nopaline strain C58, resulting in strain CB1006. The nopaline Ti plasmid virB6 gene and the traD gene from the IncN plasmid pKM101, coding for a protein with significant sequence similarity to VirB6 (34), were PCR amplified and cloned in the broad-host-range plasmid pTrc200, allowing IPTG-inducible gene expression. The resulting plasmids pTrcB6 and pTrcTraD were introduced in strain CB1006, and synthesis of VirB6 was monitored (Fig. 1B). Compared to the wild type, CB1006/pTrcB6 synthesized increased amounts of the 22-kDa protein even without addition of IPTG, presumably due to the leakiness of the trc promoter in A. tumefaciens. Addition of IPTG further increased production of the 22-kDa protein, and aggregates approximately twice its molecular mass were detected, which were not dissociated by SDS treatment (Fig. 1B).
Optimized growth conditions for analysis of the effects of a virB6 deletion.
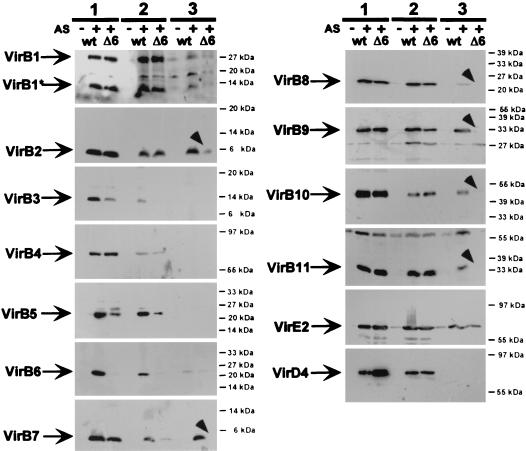
Deletion of virB6 was reported to cause reduced levels of several VirB proteins, suggesting that it may constitute a core element necessary for stabilization of the type IV secretion apparatus. Virulence gene induction was conducted in liquid culture at 28°C in these studies. Since these conditions are now considered unfavorable for functionality of the VirB complex, we compared the effects of optimized growth conditions (AB medium plates incubated at 20°C) to those previously used (AB liquid medium at 28°C). To exclude effects of growth on plate versus liquid culture, cells were also analyzed after induction in liquid culture at 20°C. Wild-type strain C58 and virB6 deletion strain CB1006 were virulence gene induced under different conditions, and Vir protein levels were analyzed (Fig. 2). Compared to cells grown at 20°C on plate or in liquid culture, analysis of cells grown in liquid culture at 28°C revealed reduced levels of VirB1, VirB6, VirB8, VirB10, and VirB11. Analysis for content of VirB3, VirB4, VirB5, and VirD4 showed reduction below the level of detection. Growth at 28°C thus negatively affected levels of several VirB proteins in wild-type strain C58, but levels of VirB2, VirB7, VirB9, and VirE2 were not reduced. When levels of Vir proteins in strains C58 and CB1006 were compared after growth under different conditions, a marked difference of the effects of the virB6 deletion was observed. In accord with previously published results, reduced levels of several VirB proteins were detected in CB1006 grown at 28°C, including those not negatively affected by growth under unfavorable conditions (VirB2, VirB7, and VirB9). In contrast, after induction using optimized conditions on AB medium plates at 20°C, levels of only three proteins (VirB3, VirB5, and VirB7) were reduced. Comparison with liquid-grown cells at 20°C showed that except in case of VirB7, this cannot be attributed to cell growth on plate versus liquid culture. Since growth in liquid at 28°C reduced levels of several VirB proteins, indicating instability of the type IV transporter, these conditions are probably not suited to assess the effect of VirB6. To analyze the role of VirB6, we therefore conducted further studies using optimized growth conditions.
FIG. 2.
Induction conditions affect levels of cell-bound virulence proteins. Wild-type strain C58 (wt) and virB6 deletion mutant CB1006 (Δ6) were grown on AB minimal medium plates at 20°C (lanes 1), in liquid culture at 20°C (lanes 2), or in liquid culture at 28°C (lanes 3). Cell lysates were subjected to SDS-PAGE followed by Western blotting and detection with specific antisera as indicated. Arrowheads indicate VirB proteins that accumulate to reduced levels in CB1006 compared to wild type only in liquid culture at 28°C. Numbers on the right are molecular masses of reference proteins.
Differential expression of virB6 affects steady-state levels of VirB proteins.
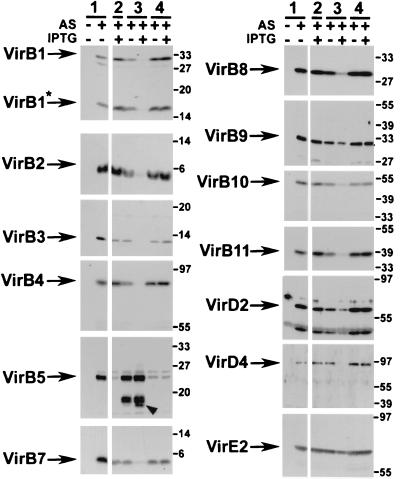
Modulation of the level of one component in a protein complex often affects the stability of others, which is suggestive of protein-protein interactions. Since VirB6 was hypothesized to play a role as a core component of the type IV secretion machinery, the effects of various levels of VirB6 on the other components of the virulence system were determined to assess its stabilizing function. As shown above, comparison of the levels of cell-bound Vir proteins in virulence gene-induced wild-type C58 and CB1006 carrying cloning vector pTrc200 revealed that the levels of most Vir proteins (VirB1, VirB2, VirB4, VirB8, VirB9, VirB10, VirB11, VirD4, VirD2, and VirE2) were virtually unaffected (Fig. 3). Levels of VirB3 and VirB7 were reduced, and VirB5 was almost undetectable in CB1006. Introduction of pTrcB6 restored the level of cell-bound VirB5 to wild type but did not restore those of VirB3 and VirB7. Surprisingly, moderate overexpression of virB6 from the leaky trc promoter led to reduced amounts of VirB1, VirB2, VirB4, VirB10, and VirB11. This effect was greatly enhanced in CB1006/pTrcB6 grown in the presence of IPTG. Under these conditions, strong decreases in the levels of cell-bound VirB1, VirB2, VirB3, VirB4, VirB7, VirB8, VirB10, VirB11, and VirD4 were observed, and cell growth was markedly retarded.
FIG. 3.
Effects of differential expression of VirB6 on other Vir proteins in cell lysates from wild-type strain C58 (lanes 1) and virB6 deletion strain CB1006 carrying cloning vector pTrc200 (lane 2), pTrcB6 (lanes 3), or pTrcTraD (lanes 4) grown on AB minimal medium plates at 20°C in the absence of induction, in the presence of AS for virulence gene induction, or in the presence of AS and IPTG for simultaneous induction of the trc promoter. Cell lysates were subjected to SDS-PAGE followed by Western blotting and detection with specific antisera as indicated. Arrowhead indicates putative degradation products of VirB5 detected in VirB6-overexpressing cells. Numbers on the right are molecular masses of reference proteins in kilodaltons.
Strong overproduction of VirB6 negatively affected cell growth, which was also indicated by reduced amounts of cell-bound VirB9 and of the transported substrates VirD2 and VirE2. These substrates are not assumed to be constituents of the type IV transporter, and its destabilization should therefore not affect their stability. The level of VirB5, however, reached wild-type levels despite overproduction of VirB6. In addition, the antiserum detected cross-reacting proteins of lower molecular mass, which may correspond to degradation products. In contrast to pTrcB6, introduction of pTrcTraD did not change the abundance of any Vir protein or the growth rate of the cells, even in the presence of IPTG.
Cross-linking analysis was used to study alterations of the protein-protein interactions in CB1006 versus C58. To this end, virulence gene-induced cells were incubated in the presence of cross-linking agents bis(sulfosuccinimidyl)suberate (BS3) and formaldehyde, which led to the formation of higher-molecular-mass multiple complexes of VirB1, VirB5, VirB8, VirB9, and VirB10 in wild-type cells (2, 3, 5; C. Baron and N. Domke, unpublished data). The cross-linking patterns observed in strains C58 and CB1006 were virtually indistinguishable (not shown), indicating that major changes in the structure of the type IV transporter did not occur.
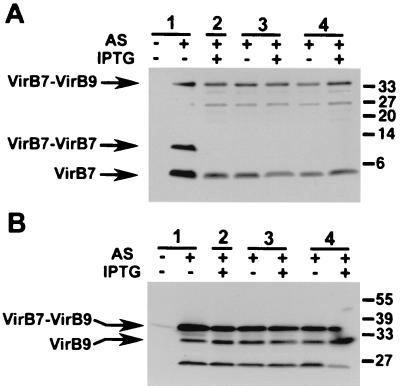
VirB6 is required for homodimer formation of VirB7.
The reduction of cellular levels of VirB7 in CB1006 prompted a further analysis of its association with VirB9 and the formation of homo- and heterodimers via disulfide bridge formation. Cell lysates were subjected to nonreducing SDS-PAGE, and analyses with VirB7- and VirB9-specific antisera showed that levels of cell-bound VirB7 monomer and VirB7-VirB9 heterodimer were not strongly affected by deficiency of VirB6 (Fig. 4). Overexpression of VirB6 was relatively moderate in this compared to other experiments (Fig. 3), and growth retardation and decrease of cell-bound Vir proteins were not as pronounced. However, in contrast to VirB7-VirB9 heterodimers and VirB7 monomers, VirB7-VirB7 homodimers were not detected in virulence gene-induced CB1006, and introduction of pTrcB6 or pTrcTraD did not restore homodimer formation (Fig. 4). Thus, VirB6 is not required for the formation of the VirB7-VirB9 heterodimer, which likely is essential for stabilization of the type IV transporter in wild-type A. tumefaciens (41).
FIG. 4.
Complex formation of VirB7 and VirB9 in cell lysates wild-type strain C58 (lanes 1) and virB6 deletion strain CB1006 carrying cloning vector pTrc200 (lane 2), pTrcB6 (lanes 3), or pTrcTraD (lanes 4) grown on AB minimal medium plates at 20°C in the absence of induction, in the presence of AS for virulence gene induction, or in the presence of AS and IPTG for simultaneous induction of the trc promoter. Cell lysates were subjected to SDS-PAGE under nonreducing conditions followed by Western blotting and detection with VirB7- and VirB9-specific antisera. Numbers on the right are molecular masses of reference proteins in kilodaltons.
Complementation of T pilus assembly and virulence.
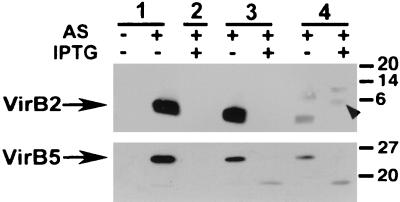
To analyze the effects of various levels of cell-bound VirB6 on the functionality of the type IV transporter, T pilus formation and tumor induction after infection of wounded plants were monitored. First, C58 wild-type and mutant strains as above were virulence gene induced on agar plates, and T pili were prepared by shearing and high-speed centrifugation of cell-free supernatants. T pilus-containing fractions from C58 contained major subunit VirB2 and pilus-associated protein VirB5 (Fig. 5). T pili were not obtained from CB1006, as expected from similar analyses performed on virB6 deletion octopine strain PC1006 (20). AS-induced CB1006 pTrcB6 synthesized T pili almost at wild-type levels, demonstrating that moderate overexpression of VirB6, which leads to reduced levels of some VirB proteins, does not negatively affect pilus assembly. In contrast, strong overproduction of VirB6 in the presence of IPTG not only decreased levels of several Vir proteins (see above) but virtually abolished T pilus formation. Introduction of pTrcTraD into strain CB1006 did not revert any of the effects of the virB6 deletion on the levels of cell-bound VirB proteins but partly restored T pilus formation (Fig. 5). This is reminiscent of the partial complementation of T pilus formation observed in CB1005(ΔvirB5) expressing TraC from pKM101, which may be a pilus-associated protein like VirB5 (39). Results varied between different independent experiments, but we often observed VirB2-cross-reactive proteins migrating with higher apparent molecular masses than mature T pilin, which may indicate an aberrant processing reaction.
FIG. 5.
Complementation of T pilus formation of strain CB1006. Extracellular T pili were isolated from wild-type strain C58 (lanes 1) and virB6 deletion strain CB1006 carrying cloning vector pTrc200 (lane 2), pTrcB6 (lanes 3), or pTrcTraD (lanes 4) grown on AB minimal medium plates at 20°C in the absence of induction, in the presence of AS for virulence gene induction, or in the presence of AS and IPTG for simultaneous induction of the trc promoter. T pilus fractions were subjected to SDS-PAGE, Western blotting, and detection with VirB2- and VirB5-specific antisera. Arrowhead indicates aberrantly migrating VirB2 in extracellular high-molecular mass structures isolated from TraD-expressing cells. Numbers on the right are molecular masses of reference proteins in kilodaltons.
As a second assay for functionality of the type IV transporter, tumor formation was monitored after infection of wounded Kalanchoë diagremontiana leaves. Nopaline as well as octopine wild-type (C58 and A348) and virB6 deletion (CB1006 and PC1006) strains were analyzed, and complementation was assessed. Deletion of virB6 caused avirulence as expected, and transformation with pTrcB6 restored virulence in nopaline as well as octopine strains (not shown). Expression of traD, however, did not restore the ability of CB1006 and PC1006 to incite tumors. Simultaneous addition of IPTG during infection to increase expression of traD did not change this, but reduced virulence of CB1006 pTrcB6, presumably due to the production of increased amounts of VirB6.
DISCUSSION
VirB6 localizes to the cell membrane as predicted and migrates with an apparent molecular mass of 22 kDa in SDS-PAGE. This deviation from the predicted 32 kDa may be due to its high content of hydrophobic amino acids, which is in accord with similar observations made for other membrane proteins. Some membrane proteins form high-molecular mass aggregates after boiling, and similar results were obtained for VirB6; cell lysates were therefore treated in sample buffer at 37°C prior to SDS-PAGE. Interestingly, VirD4, which contains only a small hydrophobic segment at its N terminus (15), shows a similar behavior.
Using a complete set of antisera specific for all components of the type IV secretion machinery (VirB1 to VirB11 and VirD4) and the transported substrates VirD2 and VirE2, the effects of different expression levels of VirB6 were investigated. In the absence of VirB6, the virulence gene-induced strain CB1006 (ΔvirB6) accumulated reduced levels of cell-bound VirB3 and VirB7, and VirB5 was almost not detectable. In contrast to a previous study, which reported decreases in the levels of all analyzed VirB proteins (VirB4, VirB9, VirB10, and VirB11) in the octopine virB6 deletion derivative PC1006 (7), changes in the levels of all other components were never observed in CB1006. These results may indicate differences in the role of VirB6 in octopine and nopaline strains. However, these earlier observations can probably be explained by the conditions used for virulence gene induction, i.e., 28°C in liquid culture, which are now known to destabilize VirB proteins and prevent formation of T pili. The type IV transporter transfers pRSF1010 derivatives efficiently at 20°C, but transfer efficiency decreases above 25°C and no transfer is detected above 28°C (21). The reduced transfer efficiency supports previous observations showing strongly reduced tumor formation after plant infection at 29°C (9). Virulence gene expression is not negatively affected by incubation at temperatures up to 30°C (21, 24). Instead, the functionality and/or stability of the type IV transporter may be affected by elevated temperatures. A previous study of Vir protein levels and cross-linking patterns revealed that VirB10 and VirB11 stability is decreased after induction at 28 versus 19°C (2). Our own work extends this analysis to show that several additional Vir proteins are diminished by growth in liquid culture at 28°C, suggesting that the type IV transporter is inherently unstable under these conditions. The absence of VirB6 in CB1006 leads to further reduced levels of several VirB proteins at 28°C, including the temperature-insensitive ones. Since we and others provided conclusive evidence for the instability of the complex at 28°C, the additional destabilization of VirB proteins in the absence of VirB6 does not necessarily reflect a role as core component. Previous results should therefore be carefully reevaluated. Our analysis using optimized growth conditions for T pilus assembly shows that the absence of VirB6 affects only a limited number of VirB proteins.
Deletion of virB6 causes avirulence, and T pili are no longer formed. Also, levels of VirB proteins implicated in T pilus assembly such as VirB5 and VirB3 are reduced, but the cell-bound major T pilus component VirB2 is not affected. This suggests that VirB6 may act in concert with VirB5 and VirB3 to mediate extracellular assembly of VirB2 from the membrane-bound pool of pilin subunits. Lower levels of cell-bound VirB7, which constitutes a major stabilizing element of the type IV transporter in complex with VirB9, are also observed in the virB6 deletion strain. In addition to its interaction with VirB9, VirB7 forms homodimers, suggested to be intermediates in heterodimer formation (41). Examination of the different forms of VirB7 revealed that deletion of virB6 affected neither the level of VirB7 monomer nor that of VirB7-VirB9 heterodimer. In contrast, the VirB7-VirB7 homodimer was not detected, suggesting a role of VirB6 in its formation. This result suggests that it is unlikely that VirB7-VirB7 serves as an intermediate in the formation of VirB7-VirB9.
Transformation of CB1006 with a virB6-overexpressing plasmid restored virulence, T pilus formation, and cell-bound VirB5 to wild-type levels. Surprisingly, wild-type levels of VirB3 and VirB7-VirB7 were not restored; two alternative explanations are possible. Expression from the trc promoter does not provide coordinate expression with the other virB genes, but synthesis of VirB6 after expression in cis may be required for formation of VirB7-VirB7 homodimers, ordered assembly of the transmembrane structure, and stabilization of its components by protein-protein interactions. Alternatively, assembly of the transmembrane structure may require a specific stoichiometric ratio between different VirB proteins, but trc promoter-driven expression leads to strongly elevated levels in the cell. Thus, VirB6 may sequester components of the type IV transporter so that its increased expression leads to misassembly of the complex, making other components, such as VirB3, more sensitive to proteolytic degradation. Levels of some VirB proteins are reduced after only moderately increased synthesis of VirB6, which may also be explained by the altered stoichiometry as above. Further increased expression retards cell growth, possibly due to toxic effects of the membrane insertion of large amounts of VirB6. Under these conditions, levels of almost all Vir proteins are strongly reduced, which may be explained by generally retarded growth. In different experiments, we observed some variations of the retardation of cell growth and the decrease of other Vir proteins. However, this strictly correlated with the degree of overexpression of VirB6, which is likely due to plasmid loss favored by the toxicity of VirB6. However, it is striking that even growth-retarded cells still accumulate wild-type levels of VirB5, with little or none of most other VirB proteins. The presence of lower-molecular mass cross-reacting products, probably degradation products, indicates rapid turnover. The stability of cell-bound VirB5 has been shown to be strictly dependent on virulence gene induction, most likely via association of one or more Vir proteins (38). VirB6-overexpressing cells are devoid of most Vir proteins, so that the observed stabilization of VirB5 likely reflects a direct interaction. Alternatively, the stabilizing effect may be mediated via binding of VirB6 to an unidentified protein in the periplasm that protects VirB5 from degradation.
Taken together, the experiments reported here do not support the hypothesis that VirB6 functions as core component of the type IV transporter. If VirB6 does indeed function as a complex-stabilizing core component, its absence should cause major changes in complex structure. However, the levels of most VirB proteins were not affected, and the cross-linking patterns did not reveal any differences from the wild type. Our data therefore suggest that the overall structure of the complex is intact. The absence of VirB6 predominantly affects components implicated in T pilus assembly, such as the putative minor T pilus component VirB5 and the outer membrane-associated protein VirB3. We suggest that VirB6 is a key regulator of T pilus assembly, via a direct stabilizing interaction with VirB5. Alternatively, in accord with previous suggestions, VirB6 may constitute a channel for transmembrane passage of the T complex or the T pilus components, which does not exert a general stabilizing function.
Studies on VirB-mediated transfer of pRSF1010 derivatives give further insight in the role(s) of VirB5 and VirB6. VirB2 through VirB11 and VirD4 are absolutely required for conjugative transfer of pRSF1010 from A. tumefaciens as a donor, and in the absence of VirB1, transfer efficiency is markedly reduced (8, 19). Virulence gene expression in the recipient strain greatly stimulates VirB-mediated transfer of pRSF1010 from the donor, suggesting that the core components of the type IV secretion machinery facilitate cell-to-cell transfer of plasmid DNA (8). However, VirB5, VirB6, and VirB11 are not required for the increased recipient capability of A. tumefaciens. Since these proteins are essential for T pilus formation, extracellular assembly of VirB2 is not required as well (8). These data together indicate that VirB6 and VirB5 function as T pilus assembly factors and not as core-stabilizing components of the type IV transporter.
ACKNOWLEDGMENTS
We thank David King and David Vogel for help with the generation of antisera and August Böck for support and discussions.
This study was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) to C.B. (BA 1416/2-2) and the National Science Foundation (IBN-9507782) to P.C.Z.
REFERENCES
- 1.Anderson L B, Vogel Hertzel A, Das A. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc Natl Acad Sci USA. 1996;93:8889–8894. doi: 10.1073/pnas.93.17.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banta L M, Bohne J, Lovejoy S D, Dostal K. Stability of the Agrobacterium tumefaciens VirB10 protein is modulated by growth temperature and periplasmic osmoadaption. J Bacteriol. 1998;180:6597–6606. doi: 10.1128/jb.180.24.6597-6606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron C, Llosa M, Zhou S, Zambryski P C. C-terminal processing and cellular localization of VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1203–1210. doi: 10.1128/jb.179.4.1203-1210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron C, Thorstenson Y R, Zambryski P C. Biochemical analysis of the complex between the lipoprotein VirB7 and VirB9 in the membranes of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1211–1218. doi: 10.1128/jb.179.4.1211-1218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaupre C E, Bohne J, Dale E M, Binns A N. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1997;179:78–89. doi: 10.1128/jb.179.1.78-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beijersbergen A, Smith S J, Hooykaas P J J. Localization and topology of VirB proteins of Agrobacterium tumefaciens. Plasmid. 1994;32:212–218. doi: 10.1006/plas.1994.1057. [DOI] [PubMed] [Google Scholar]
- 7.Berger B R, Christie P J. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohne J, Yim A, Binns A N. The Ti plasmid increases the efficiency of Agrobacterium tumefaciens as a recipient in virB-mediated conjugal transfer of an IncQ plasmid. Proc Natl Acad Sci USA. 1998;95:7057–7062. doi: 10.1073/pnas.95.12.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun A C, Mandle R J. Studies on the inactivation of the tumor inducing principle in crown gall. Growth. 1948;12:255–269. [PubMed] [Google Scholar]
- 10.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 12.Dang T A, Christie P J. The VirB4 ATPase of Agrobacterium tumefaciens is a cytoplasmic membrane protein exposed at the periplasmic surface. J Bacteriol. 1997;179:453–462. doi: 10.1128/jb.179.2.453-462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang T A, Zhou X-R, Graf B, Christie P J. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter. Mol Microbiol. 1999;32:1239–1253. doi: 10.1046/j.1365-2958.1999.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Xie Y-H. The Agrobacterium T-DNA transport pore proteins VirB8, VirB9, and VirB10 interact with one another. J Bacteriol. 2000;182:758–763. doi: 10.1128/jb.182.3.758-763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das A, Xie Y-H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol. 1998;27:405–414. doi: 10.1046/j.1365-2958.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 16.Dijkstra A J, Keck W. Peptidoglycan as a barrier to transenvelope transport. J Bacteriol. 1996;178:5555–5562. doi: 10.1128/jb.178.19.5555-5562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenbrandt R, Kalkum M, Lai E M, Lurz R, Kado C I, Lanka E. Conjugative pili of IncP plasmids, and the Ti plasmid T pilus are composed of cyclic subunits. J Biol Chem. 1999;274:22548–22555. doi: 10.1074/jbc.274.32.22548. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez D, Spudich G M, Zhou X-R, Christie P J. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J Bacteriol. 1996;178:3168–3176. doi: 10.1128/jb.178.11.3168-3176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullner K J. Role of Agrobacterium virB genes in transfer of T complexes and RSF1010. J Bacteriol. 1998;180:430–434. doi: 10.1128/jb.180.2.430-434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fullner K J, Lara J L, Nester E W. Pilus assembly by Agrobacterium T-DNA transfer genes. Science. 1996;273:1107–1109. doi: 10.1126/science.273.5278.1107. [DOI] [PubMed] [Google Scholar]
- 21.Fullner K J, Nester E W. Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J Bacteriol. 1996;178:1498–1504. doi: 10.1128/jb.178.6.1498-1504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard E A, Windsor B A, de Vos G, Zambryski P C. Activation of the T-DNA transfer process in Agrobacterium results in the generation of a T-strand-protein complex: tight association of VirD2 with the 5′ ends of T-strands. Proc Natl Acad Sci USA. 1989;86:4017–4021. doi: 10.1073/pnas.86.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hueck C J. Type III secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin S, Song Y-N, Deng W-Y, Gordon M, Nester E W. The regulatory VirA protein of Agrobacterium tumefaciens does not function at elevated temperatures. J Bacteriol. 1993;175:6830–6835. doi: 10.1128/jb.175.21.6830-6835.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones A L, Shirasu K, Kado C I. The product of the virB4 gene of Agrobacterium tumefaciens promotes accumulation of VirB3 protein. J Bacteriol. 1994;176:5255–5261. doi: 10.1128/jb.176.17.5255-5261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lai E-M, Kado C I. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J Bacteriol. 1998;180:2711–2717. doi: 10.1128/jb.180.10.2711-2717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lessl M, Balzer D, Pansegrau W, Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Biol Chem. 1992;267:20471–20480. [PubMed] [Google Scholar]
- 29.Maniatis T A, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 30.Marciano D K, Russel M, Simon S M. An aqueous channel for filamentous phage export. Science. 1999;284:1516–1519. doi: 10.1126/science.284.5419.1516. [DOI] [PubMed] [Google Scholar]
- 31.Mushegian A R, Fullner K J, Koonin E V, Nester E W. A family of lysozyme-like virulence factors in bacterial pathogens. Proc Natl Acad Sci USA. 1996;93:7321–7326. doi: 10.1073/pnas.93.14.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli M L, Bourg G, Foulongne V, Frutus P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 34.Pohlman R F, Genetti H D, Winans S C. Common ancestry between IncN conjugal transfer genes and macromolecular export systems of plant and animal pathogens. Mol Microbiol. 1994;14:655–668. doi: 10.1111/j.1365-2958.1994.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 35.Rashkova S, Spudich G M, Christie P J. Characterization of membrane and protein interaction determinants of the Agrobacterium tumefaciens VirB11 ATPase. J Bacteriol. 1997;79:583–591. doi: 10.1128/jb.179.3.583-591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salmond G P C. Secretion of extracellular virulence factors by plant pathogenic bacteria. Annu Rev Phytopathol. 1994;32:181–200. [Google Scholar]
- 37.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range of 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt-Eisenlohr H, Domke N, Angerer C, Wanner G, Zambryski P C, Baron C. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J Bacteriol. 1999;181:7485–7492. doi: 10.1128/jb.181.24.7485-7492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt-Eisenlohr H, Domke N, Baron C. TraC of IncN plasmid pKM101 associates with membranes and extracellular high-molecular-weight structures in Escherichia coli. J Bacteriol. 1999;181:5563–5571. doi: 10.1128/jb.181.18.5563-5571.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segal G, Russo J J, Shuman H A. Relationships between a new type IV secretion system and the icm/dot virulence system of Legionella pneumophila. Mol Microbiol. 1999;34:799–809. doi: 10.1046/j.1365-2958.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 41.Spudich G M, Fernandez D, Zhou X-R, Christie P J. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc Natl Acad Sci USA. 1996;93:7512–7515. doi: 10.1073/pnas.93.15.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorstenson Y R, Kuldau G A, Zambryski P C. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J Bacteriol. 1993;175:5233–5241. doi: 10.1128/jb.175.16.5233-5241.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zupan J R, Ward D, Zambryski P C. Assembly of the VirB transport complex for DNA transfer from Agrobacterium tumefaciens to plant cells. Curr Opin Microbiol. 1998;1:649–655. doi: 10.1016/s1369-5274(98)80110-0. [DOI] [PubMed] [Google Scholar]