Summary
The complexity of the depressive symptoms observed in humans makes modeling depressive behavior in rodents challenging. Here, we present a highly reproducible protocol to generate mouse models that mimic several aspects of depression, namely anhedonia and loss of motivation. We describe acclimatization of animals and baseline determination, followed by the chronic unpredictable stress (CUS) protocol to induce anhedonic and resilient behaviors. The protocol can generate anhedonic and resilient mice at roughly equal frequencies, providing a reliable model for translational research.
For complete details on the use and execution of this protocol, please refer to Baczynska et al. (2022), Bijata et al. (2022), and Krzystyniak et al. (2019).
Subject areas: Behavior, Model organisms, Neuroscience
Graphical abstract
Highlights
-
•
Modeling of depressive-like behaviors in mice
-
•
Mimicking some aspects of human depression including anhedonia and loss of motivation
-
•
Generation of two subpopulations of mice—anhedonic and resilient mice
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The complexity of the depressive symptoms observed in humans makes modeling depressive behavior in rodents challenging. Here, we present a highly reproducible protocol to generate mouse models that mimic several aspects of depression, namely anhedonia and loss of motivation. We describe acclimatization of animals and baseline determination, followed by the chronic unpredictable stress (CUS) protocol to induce anhedonic and resilient behaviors. The protocol can generate anhedonic and resilient mice at roughly equal frequencies, providing a reliable model for translational research.
Before you begin
Preparation of cylinders for predator stress
Timing: 3 min per jar
-
1.
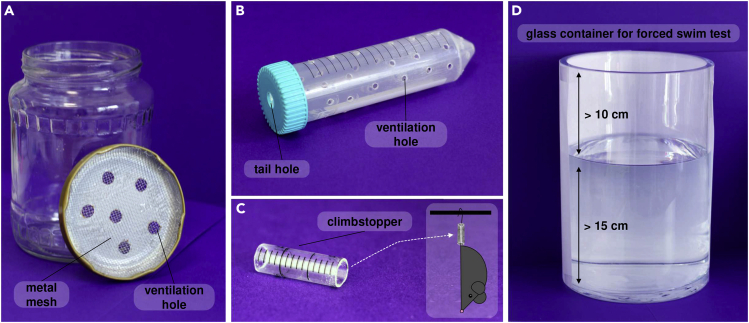
Obtain transparent glass jars (15 cm high, 8 cm in diameter) and drill holes in the metal lid (Figure 1A).
-
2.
Upholster the inner portion of the lid with a dense metal mesh to prevent the direct contact of animals through the drilled holes (Figure 1A).
Figure 1.
Accessories required for procedures
(A–D) (A) cylinder for predator stress, (B) transparent plastic tube for restraint stress, (C) climbstopper for tail suspension stress, (D) glass container for forced swim test.
Preparation of transparent plastic tubes for restraint stress
Timing: 5 min per tube
-
3.
Drill holes into plastic 50 mL tubes (26 mm internal diameter) to enable ventilation. Consider an additional hole in the cap for the tail. If you see sharp edges, smooth them with a polisher so that the mouse does not get scratched (Figure 1B).
Preparation of climbstoppers
Timing: 15 min
-
4.
Prepare 3–4 cm long transparent cylinders with 0.5–1 cm in diameter. You can use drinking tubes or serological pipette for this purpose (Figure 1C).
Preparation of rooms for animals
-
5.
Book three or four experimental rooms (for control, for stressed animals, for rats and for FST performance) and one working room next to each other with minimal access for at least 31 days.
-
6.
Keep rats, CD1 mice and control mice in an enriched environment. Place paper rolls, wood pieces and nesting material in the cage.
Note: The experimental rooms should have reversed light/dark phases of the 12/12 cycle (dark 8:00–20:00, lit 20:00–8:00). All procedures in the dark phase (stress protocol, behavioral evaluation) perform under red light.
CRITICAL: The experimental rooms should be isolated to avoid noise and odors. Control the conditions in the experimental rooms daily (humidity 40%–50%, temperature 19°C–22°C). Due to the sensitivity of behavioral tests, the entrance into experimental rooms during the whole CUS procedure should be restricted to the experimenters.
Institutional permissions
All animal experiments must be performed in accordance with guidelines approved by the Local Ethical Committee on Animal Research.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| C57BL/6J mice - male, 9±2 weeks old | Medical University of Bialystok, Poland | N/A |
| CD1 mice (SWISS RjOrl:SWISS (CD-1)) – male, ca. 12 weeks old | JANVIER LABS, France and AnimaLab EU, Poland | N/A |
| Wistar rats – male, ca. 12 weeks old | Mossakowski Research Center PAS, Warsaw, Poland | N/A |
| Other | ||
| Bottles 280 mL | Tecniplast | #CatACBT0252 |
| Caps | Tecniplast | #CatACCP2522 |
| 50 mL tube | Corning | #CatCLS430828 |
| Mason jar | N/A | N/A |
| Metal wire mesh | N/A | N/A |
| Open glass container | N/A | N/A |
| Serological pipette | Thermo Fisher Scientific | #Cat170372N |
| Cylindrical glass containers (approximately 20 cm × 40 cm) | N/A | N/A |
| 70% isopropyl alcohol | Sigma-Aldrich | #Cat59304 |
| Standard detergents to clean materials | N/A | N/A |
| Sucrose 99% | BioShop Life Science | #CatSUC600.1 |
Alternatives: The animals of the strains mentioned in the table can be obtained from other sources.
CRITICAL: The age and sex of the animals in the protocol are very important for the reproducibility of the obtained results. To obtain an anhedonic to resilient mouse ratio of 1:1, male C57BL/6J mice should be 9±2 weeks old at the start of the protocol (i.e., on the first day of adaptation). It is possible to perform the protocol on animals of different ages; however, this may result in a different ratio of anhedonic animals to resilient animals. Importantly, the age of the mice participating in one set of the experiment should be the same at the beginning of the procedure. The best age for male CD1 mice and male Wistar rats, which are used as stressors, is 12 weeks. Older animals may be less efficient in inducing stress in C57BL/6 mice.
Alternatives: You can use bottles and caps from another company.
CRITICAL: The bottles must not leak.
Step-by-step method details
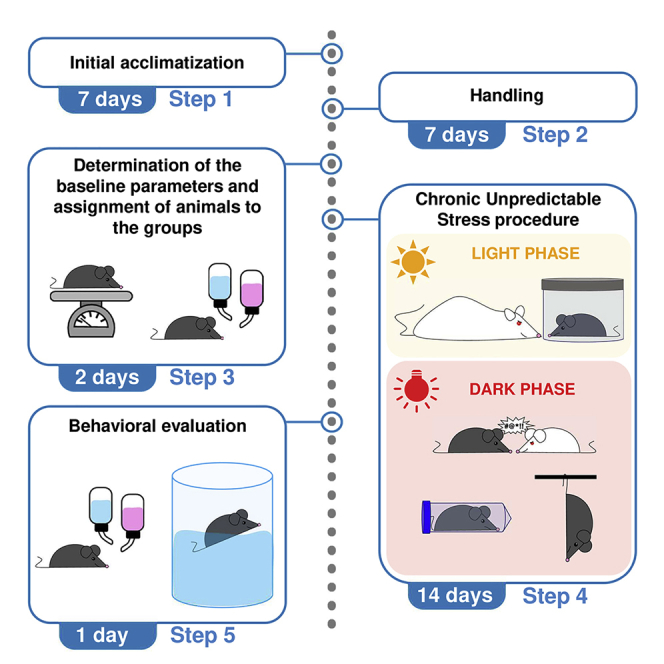
Initial acclimatization
Timing: 7 days
This section describes how to acclimatize the animals to the experimental conditions.
Place the mice in individual cages and keep them under a reverse 12 h/12 h light/dark cycle. Left them undisturbed for a week. The contact of the experimenter and the mice should be kept at a minimum to provide the mice with food and water ad libitum. It is best if all mice are housed in the same room until the CUS protocol is started.
Note: In order to receive reproducibility, mice ordered from not breeding in–house should be acclimatized for up to 2 weeks in the dedicated experimental room with reversed light/dark cycle.
Handling
Timing: 7 days
This section describes the handling procedure which aims to accustom the mice to the experimenter and to avoid additional stress during the CUS protocol and behavioral tests.
After acclimatization to the experimental room, apply daily handling for 7 consecutive days in the dark phase of the dark/light cycle under red light. Handling should be performed by all persons who will carry out further procedures on the animals. To achieve the most consistent results, it is best the number of experimenters will be limited to 2–3.
-
1.
Wear nitrile gloves and a lab coat that was contaminated with a mouse scent. Use the same lab coat during the whole handling procedure.
-
2.
On the first day, grasp the mice by the base of the tail with your thumb and forefinger and then place them on top of the cage for 2 min.
-
3.
On the second and third days, place the mice on the sleeve of the laboratory coat for 2 min.
-
4.
From the fourth day to the seventh day, put animals directly into your open hands for 2 min before being returned to their home cages.
Note: Only C57BL/6 mice (not CD1 mice and Wistar rats) require handling.
Determination of baseline parameters and assignment of animals to experimental groups
Timing: 2 days
This section describes how to measure baseline parameters and assign animals to experimental groups.
Measure baseline sucrose preference and weight before starting the chronic stress protocol. Based on the obtained results, mice will be assigned to 2 groups: the control and stressed groups.
-
5.
One day after the seventh day of handling provide a 2.5% sucrose solution (dissolved in standard drinking water) for 2 h to prevent the possible confounding effects of taste neophobia. Do not give the mice access to standard water during this time.
-
6.Give animals fresh food directly into the cage (not only in the feeder).
 CRITICAL: Do not change the location of cages in the room before the test.
CRITICAL: Do not change the location of cages in the room before the test. CRITICAL: Do not change the bedding immediately prior to testing. It is best to do this for the last time 5–6 days before the test.
CRITICAL: Do not change the bedding immediately prior to testing. It is best to do this for the last time 5–6 days before the test. -
7.The next day, perform the sucrose preference test as previously described (Bijata et al., 2022; Krzystyniak et al., 2019; Strekalova and Steinbusch, 2010; Strekalova et al., 2004). Perform all procedures during the dark phase of the dark/light cycle. Briefly turn on the red light while inserting the bottles.
 CRITICAL: If more than one room is used for the test, the humidity in both rooms should be the same. This affects both fluid viscosity and total fluid intake in mice.
CRITICAL: If more than one room is used for the test, the humidity in both rooms should be the same. This affects both fluid viscosity and total fluid intake in mice. CRITICAL: Place the water for the preparation of the sucrose solution into the experimental room at least one day beforehand to bring the solutions to room temperature. Prepare the sucrose solution from the same batch of water that will be placed in the water bottles. Make the sucrose solution fresh immediately before the test.
CRITICAL: Place the water for the preparation of the sucrose solution into the experimental room at least one day beforehand to bring the solutions to room temperature. Prepare the sucrose solution from the same batch of water that will be placed in the water bottles. Make the sucrose solution fresh immediately before the test. CRITICAL: Fill the bottles with water and the same volume of sucrose solution. This has an effect on the weight of the water droplets. When using Tecniplast 280 mL bottles and caps, filling them to 150 mL, results in the drop of water usually being approx. 0.1 g. If you notice that a drop has fallen while inserting or switching the bottles, note it and include it in your final results.
CRITICAL: Fill the bottles with water and the same volume of sucrose solution. This has an effect on the weight of the water droplets. When using Tecniplast 280 mL bottles and caps, filling them to 150 mL, results in the drop of water usually being approx. 0.1 g. If you notice that a drop has fallen while inserting or switching the bottles, note it and include it in your final results.-
a.Pour into bottles 1% sucrose solution and water (1 bottle with water and 1 bottle with sucrose solution per cage) and then weigh bottles. This measurement will later be referred to as “sucrose_t0” and “water_t0”.
-
b.Label the bottles with the mouse number and type of solutions.
-
c.Give the mice a free choice to drink 1% sucrose solution and water provided in identical transparent bottles for 8 h.
-
d.To eliminate the possible effects of side preference, switch the positions of the bottles after 4 h of testing.
-
e.Weigh the bottles after 8 h to estimate the consumption of water and sucrose solution. This measurement will later be referred to as “sucrose_t1” and “water_t1”.
-
f.Calculate sucrose preference as follows:sucrose preference = [”sucrose_t1”-“sucrose_t0” / (”sucrose_t1”-“sucrose_t0” + ”water_t1”-“water_t0”)] × 100%.Note: It is better to use the volume of fluids consumed by the animals when calculating sucrose preferences. However, accurate measurement of such a small volume (approx. 0.5–10 mL) in bottles can be problematic. Therefore, due to the small difference in densities (0.4%, water density 998 kg / m³, 1% sucrose solution density 1,002 kg/m3 at 20°C), the weight of the drunk liquids is a sufficient parameter and the most optimal approach.Troubleshooting 1: Baseline sucrose preference below 70%.
-
a.
-
8.
Weigh each mouse.
CRITICAL: Do not weigh the mice before performing the sucrose preference test. Weighing is an additional stress for the mice and may disturb the sucrose preference test results.
-
9.
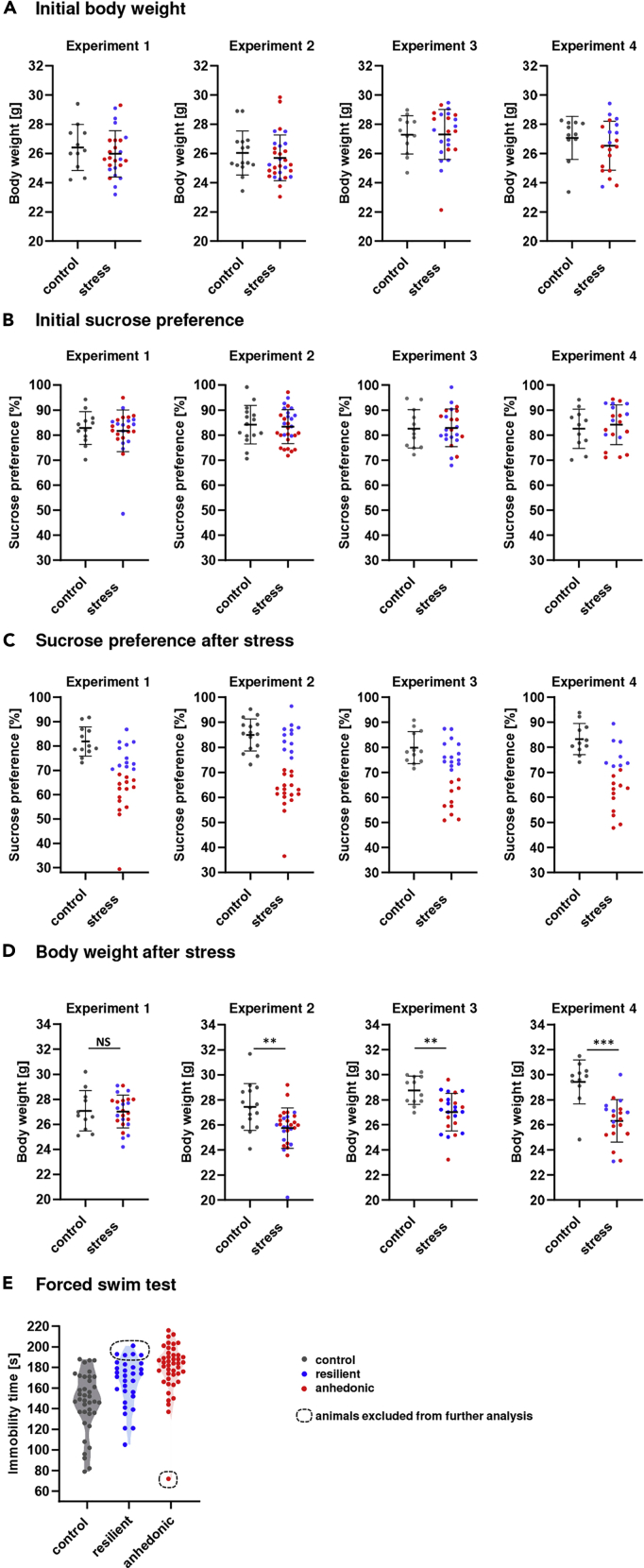
Based on the results of sucrose preference and body weight measurements, assign the animals to the control and stressed groups (Figures 2A and 2B).
Note: In the stressed group, approximately 50% of the animals develop anhedonic behavior and approximately 50% display resilient behavior. If the size of the groups at the end of the experiment is the same, assign twice as many mice to the stress group (e.g., 10 control animals and 20 stressed mice).
CRITICAL: Select mice in the control and stressed groups in such a manner that both groups have similar means (confirmed by Student t-test) and standard deviations (confirmed by Fisher's F test) of sucrose preference and body weight measurements (Figures 2A and 2B).
Figure 2.
Behavioral evaluation and body weight measurements in the CUS procedure
(A and B) Body weight (A) and sucrose preference (B) before the CUS protocol.
(C) On average, 50% of the animals developed anhedonia measured by the sucrose preference test as a result of CUS.
(D) Most stressed animals have less body weight gain than control animals.
(E) In most animals, the level of anhedonic behavior correlates with the level of escape motivation measured with the forced swim test. Experiments named 1–4 represent four independent CUS experiments performed in the same animal facility.
The data are presented as the mean ± SD; ∗∗p < 0.01, ∗∗∗p < 0.001.
Chronic unpredictable stress (CUS) protocol
Timing: 14 days
This section describes how to implement a chronic unpredictable stress protocol to induce anhedonic and resilient behaviors.
To induce anhedonic and resilient behaviors, animals assigned to the stressed group are subjected to chronic unpredictable stress (CUS). The CUS protocol involves 2 weeks of daily exposure to two out of three different stressors selected in a semi-random manner. Stressors are applied during the dark phase of the dark/light cycle under red light in the following order: social defeat for 30 min, restraint stress for 2 h, and tail suspension for 40 min with an intersession interval of at least 3 h. During the light phase of the dark/light cycle, the mice are exposed to predator stress by being placed in the vicinity of a rat for 12 h (Table 1).
-
10.
Move the animals that will be subjected to stress to a separate room. The control animals remain in the current room.
Note: If it is not possible to provide a separate room for the stressed mice, the mice may be kept in the same room as the CD1 mice and rats used for predator stress.
CRITICAL: Keep the control C57BL/6 mice in a separate room. During the stress protocol performed on the stressed group, handle the control mice on average once every four days. If handling control mice, do so before any contact with stressed mice and rats.
-
11.The stress protocol begins with exposure to a predator.
-
a.Place the mice individually in transparent, well-ventilated cylinders (as described in “Preparation of cylinders for predator stress”) with standard food and bedding.Note: We don't provide water source to mice during their exposure to a predator.
-
b.Place the cylinders in the home cage of a male Wistar rat for 12 h.Note: Label the cylinder with the mouse number. This way you will avoid confusing the mice and will be able to compare the measurement results after the CUS protocol with the baseline results.
-
a.
-
12.After a minimum of 3 h, subject the mice to social defeat stress.
-
a.For each 30-min social defeat session, place one aggressive CD1 mouse into each home cage of the C57BL/6J mouse.
-
b.Observe if the C57BL/6J mice exhibit signs of social defeat stress, such as a fight response, submissive posture, and vocalization.
-
a.
CRITICAL: Prevent the mice from fighting, which may lead to injury. Every time that social defeat stress is applied, carefully check the mice for any injuries. This is best done during the light phase when placing the mice in the cylinder (point 11). Unnoticed injury may lead to infection and additional stress in mice.
CRITICAL: Social defeat stress is also exhausting for the aggressor. One CD1 mouse should participate in a maximum of two 30 min consecutive social stressing sessions in one day. If another stress session is necessary on the same day, allow the mice to rest for at least one hour.
Table 1.
Chronic unpredictable stress protocol schedule
| Dark phase (red light) |
Light phase |
|||
|---|---|---|---|---|
| ∼ 8 am | ∼ 12 pm | ∼ 4 pm | ∼ 8 pm | |
| DAY 1 | INITIAL ACCLIMATIZATION | |||
| DAY 2 | INITIAL ACCLIMATIZATION | |||
| DAY 3 | INITIAL ACCLIMATIZATION | |||
| DAY 4 | INITIAL ACCLIMATIZATION | |||
| DAY 5 | INITIAL ACCLIMATIZATION | |||
| DAY 6 | INITIAL ACCLIMATIZATION | |||
| DAY 7 | INITIAL ACCLIMATIZATION | |||
| DAY 8 | HANDLING | |||
| DAY 9 | HANDLING | |||
| DAY 10 | HANDLING | |||
| DAY 11 | HANDLING | |||
| DAY 12 | HANDLING | |||
| DAY 13 | HANDLING | |||
| DAY 14 | HANDLING | |||
| DAY 15 | SPT pretest | |||
| DAY 16 | SPT | SPT BS | BW | RAT |
| DAY 17 | SS | RS | RAT | |
| DAY 18 | TSS | SS | RAT | |
| DAY 19 | RS | TSS | RAT | |
| DAY 20 | SS | RS | RAT | |
| DAY 21 | TSS | SS | RAT | |
| DAY 22 | RS | TSS | RAT | |
| DAY 23 | SS | RS | RAT | |
| DAY 24 | TSS | SS | RAT | |
| DAY 25 | RS | TSS | RAT | |
| DAY 26 | SS | RS | RAT | |
| DAY 27 | TSS | SS | RAT | |
| DAY 28 | RS | TSS | RAT | |
| DAY 29 | SS | RS (∼ 3 pm) | ||
| DAY 30 | SPT | SPT BS | FST, BW | |
| DAY 31 | ISOLATION OF THE TISSUES FOR ANALYSIS | |||
SPT – sucrose preference test, SPT BS - bottles switch sides after 4 h of testing, SS – social defeat stress, RS - restraint stress, RAT - exposure to a predator (rat), TS - tail suspension stress, FST – forced swim test, BW - body weight measurement.
Troubleshooting 2 Mice do not display signs of social defeat stress.
Troubleshooting 3 Interactions between mice are too aggressive.
Troubleshooting 4 Injury in mice.
-
13.After a minimum of 3 h, subject the mice to restraint stress.
-
a.Place the mice inside a transparent plastic tube (as described in “Preparation of transparent plastic tube for the restraint stress”) for 2 h.
-
b.After the mice are removed, thoroughly wash each tube, disinfect with 70% ethanol, and allow to dry.
-
a.
-
14.
After a minimum of 3 h, repeat the exposure to a predator as described in point 11.
-
15.
After a minimum of 3 h, subject the mice to tail suspension stress. Hang the mice by their tails using adhesive tape 50 cm above the floor for 40 min (standard stable low desk may be used).
Note: To prevent the mice from climbing their tails, place plastic cylinders at the base of their tails (as described in “Preparation of climbstoppers”).
Note: When suspending the mouse, attach the identifier of the mouse next to it. This way you will avoid confusing the mice and will be able to compare the measurement results (i.e., body weight and sucrose preference) after the CUS protocol with the baseline results.
CRITICAL: Always ensure that the mouse is suspended correctly and will not fall. At the same time, check that the tail is not strongly bent because this may lead to tail ischemia and necrosis.
-
16.
After a minimum of 3 h, subject the mice to the next stressors according to the sequence described in Table 1.
Note: During the first days of the protocol, stressed animals may need more frequent changes in bedding.
Note: During the CUS protocol, cages with mice are often opened. Always be sure that the water bottles are not leaking and are placed in the correct position when closing the cage. A leak from the bottle may cause additional stress due to wet bedding.
CRITICAL: Start the last stressor (i.e., restraint stress) at the latest at 3 pm to stabilize blood levels of stress hormones prior to behavioral evaluation.
Troubleshooting 5 Atypical apathetic behavior in mice.
-
17.
Move CD1 mice and Wistar rats to a separate room.
Behavioral evaluation
Timing: 1 day
Behavioral evaluation aims to identify anhedonic and resilient animals. Two tests are performed: the sucrose preference test and the forced swim test. Based on the results of the sucrose preference test after CUS protocol, animals are assigned to the group of anhedonic or resilient phenotypes. The cut off value for anhedonia is defined by the mean value minus 2×SD of the sucrose preference found in the control mice. In our experiments, this value is between 68% and 72%. However, we recommend to establish the threshold de novo for each set of experiments. The forced swim test is intended to provide additional confirmation of depressive phenotype (Can et al., 2012; Porsolt et al., 1977).
-
18.
Approximately 16 h after the last stressor in CUS protocol, perform an 8-h sucrose preference test in the same way as described in point 7 but be aware of the additional critical points below.
CRITICAL: Perform procedures first on control mice and then on stressed mice. This is of particular importance, especially when the stressed mice were placed in the same room as the Wistar rats. It is important to change clothes and take a shower before recontact with the control mice (during switching or weighing bottles) due to possible odorant communication in mice (Arakawa et al., 2008; Puścian et al., 2016). This can also be done by another person participating in the CUS experiment.
-
19.After completion of the SPT, subject the animals to the forced swim test (FST).
-
a.Keep animals for 1 h in the room where FST will be performed.
-
b.Fill cylindrical glass containers (approximately 40 cm high, 20 cm in diameter) with warm water (25°C–27°C) to a depth of minimum 15 cm (Figure 1D).
-
c.Place each mouse in the water for 6 min for a single swim session. Record a video of each swimming session for offline analysis.
-
d.Wipe each mouse with a paper towel after the swimming session.
-
e.Weigh each mouse when it is dry.
-
f.Offline analysis of FST: manually assess recorded video for the total amount of time spent immobile during the last 4 min. We recommend noting all time points at which the mouse changes its activity.
-
a.
Note: In order to determine body weight gain or loss, weigh the mice after the CUS procedure. The changes in body weight measurements are the physiological parameter resulting from exposure to chronic stress. Avoid to measure weight before the FST performance because in particular for control mice is a stressor that might affect FST results.
Troubleshooting 6 Inconsistent results between behavioral tests (SPT and FST).
Expected outcomes
The chronic unpredictable stress (CUS) protocol leads to the emergence of two subpopulations of mice: mice susceptible to stress and mice resilient to stress. Between 40% and 60% of the mice are susceptible, hence they develop anhedonic behavior (Figure 2C). Mice are considered anhedonic if they exhibit a sucrose preference below the cut off value for anhedonia after CUS. In contrast, mice that show a sucrose preference above the cut off value for anhedonia are considered stress resilient mice. The cut off value for anhedonia is defined by the mean value minus 2×SD of the sucrose preference found in the control mice. In our experiments, this value is between 68% and 72%. However, we recommend establishing the threshold de novo for each set of experiments. In most anhedonic mice, a decreased motivation in the forced swim test (understood as an increase in the time of immobility in the forced swim test) was also observed (Figure 2E).
Notwithstanding, in less than 10% of the mice, the results of the behavioral tests may be inconclusive. Long periods of immobility in the FST were observed in resilient mice, and short periods of immobility in the FST were observed in anhedonic mice (Figure 2E). This may be because of unnoticeable disturbances during behavioral testing. However, anhedonic and resilient animals exhibit unique molecular differences in the brain that may be used to confirm anhedonia or resilience (Baczynska et al., 2022; Bijata et al., 2022; Gorinski et al., 2019). Due to the high repeatability of the method and the ease of performing the test, in our opinion, the best assay confirming anhedonia is gel zymography allowing to determine the activity of MMP-9 in the hippocampus. It is worth noting that measuring the level of corticosterone in the blood is not a good solution to distinguish between anhedonic and resilient animals due to the fact that both behavioral groups have elevated levels of corticosterone in the blood (Couch et al., 2013).
Although there is no correlation between initial weight or sucrose preference and the type of stress response (resilient or anhedonic behavior) (Figures 2A and 2B), it should be taken into account that in mice that show low sucrose preference prior to CUS protocol, low sucrose preference after the CUS protocol may not unequivocally be a manifestation of anhedonic behavior. If no additional tests (apart from the forced swim test) are planned to confirm anhedonia (e.g., measuring MMP-9 activity in the hippocampus), we suggest that these mice be excluded from the analysis.
Typically, stressed animals have less body weight gain than control animals. Less frequently, however, there may also be no difference in body weight gain between control and stressed animals, and this does not indicate protocol failure (Figure 2D).
Limitations
The protocol can be successfully applied in C57BL/6J male mice to model resilient and anhedonic behavior. However, we did not test it in other mouse strains; therefore, we do not know if there will be challenges with translating the model to other strains (de Sá-Calçada et al., 2015). The main limitation in CUS protocol is the need to apply social defeat stress, which requires the use of more aggressive mouse strains (hence our use of the CD1 strain) than experimental mice. In contrast, for mice more susceptible to stress than C57BL/6J, e.g., C57BL/6N, we expect that carrying out the protocol described by us may lead to a different ratio of anhedonic to resilient mice with predominance of anhedonic mice (Sturm et al., 2015).
The use of social defeat stress also limits the applicability of the protocol to males only. Despite the fact that in recent years the number of reports on aggression in social interactions in females has been growing, this issue is still poorly understood (de Sá-Calçada et al., 2015; Will et al., 2017). Female social stress protocols have been described; however, their use is much more complicated than in males and can be difficult to implement it as a stressor in the protocols we present here. The most frequently used stressors for female mice are the following: social crowding, rat odor, new partner, wet bedding, cage rotation, stroboscope, isolation, cold, cage tilt, restraint, and food deprivation. However, we do not know how replacing social stress with another stressor would affect the effectiveness of inducing anhedonia and resilience.
The application of the protocol requires a minimum of 2 separate rooms accessible to animals 24 h a day for 30 days.
Currently, we do not know any biomarkers that would allow mice to be assigned to the appropriate behavioral group based on blood parameters. The standard test based on the determination of blood corticosterone level is not applicable in this protocol. Stressed animals are subjected to severe stress, which leads to increased levels of corticosterone in the blood in both behavioral groups (Couch et al., 2013).
Troubleshooting
Problem 1
Determination of baseline parameters and assignment of animals to experimental groups, step 7.
Baseline sucrose preference below 70%.
Potential solution
Occasionally, mice have a low baseline sucrose preference. This may be an individual feature of a given mouse. We recommend excluding such individuals from the procedure for the reasons previously discussed. However, it is possible that the uncommonly low sucrose preference appears only on one particular day. We recommend the use of an additional biochemical test to confirm anhedonia, e.g., to measure MMP-9 activity in the hippocampus. If no additional tests are planned, we suggest to exclude these mice from the analysis.
Problem 2
Chronic unpredictable stress (CUS) protocol, step 12.
Mice do not display signs of social defeat stress.
Potential solution
Replace the CD1 mouse with another CD1 mouse.
Problem 3
Chronic unpredictable stress (CUS) protocol, step 12.
Interactions between mice are too aggressive.
Potential solution
Separate mice or replace the aggressive CD1 mouse with a less aggressive one.
Problem 4
Chronic unpredictable stress (CUS) protocol, step 12.
Injury in mice.
Potential solution
The mouse should be checked by a vet. If it is necessary to implement medications, we recommend that such a mouse be excluded from further procedures. Remember that some medications can affect the behavior of animals.
Problem 5
Chronic unpredictable stress (CUS) protocol, steps 11–16.
Atypical apathetic behavior in mice.
Potential solution
Apathy and submissive behavior is a sign of experiencing stress. Therefore, this is not a sufficient reason to exclude the mice from the experiment. In a situation where the mice would become mobility impaired, hardly moving, not eating, very thin, not grooming, not responding to the presence of CD1 mice, consult your veterinarian. If the mouse requires rest, we suggest that you exclude it from the experiment. However, in our experiments such situations have not happened so far.
Problem 6
Behavioral evaluation, step 19.
Inconsistent results between behavioral tests (SPT and FST).
Potential solution
In less than 10% of the mice, the results of the behavioral tests may be inconclusive. This may be due to technical limitations of the behavioral tests used. In the case of large discrepancies in the results of both behavioral tests (SPT and FST), we recommend the use of a biochemical test to confirm anhedonia or resilience, e.g., measurement of MMP-9 activity in the hippocampus (Baczynska et al., 2022; Bijata et al., 2022; Gorinski et al., 2019). If no additional tests are planned, we suggest that such mice should be excluded from further analyses (Figure 2E). In line with our previous experiments (Baczynska et al., 2022; Bijata et al., 2022), a rule of thumb is to exclude animals classified as resilient that exhibit FST immobility time above the values observed in control mice. Maintaining the ‘purity’ of the population of resilient animals is particularly important in obtaining reliable and repeatable results of subsequent analyses, e.g., biochemical analyses. When identifying anhedonic animals, such strict exclusion criteria do not have to be applied. The sucrose preference test usually gives very reliable information about the depressive state of the animal. In anhedonic animals, sometimes the FST immobility time may be similar to that observed in control animals (however, most often these are values above the mean value observed in controls). This phenomenon is related to the complexity of depressive behavior and the fact that the proposed tests examine a different type of symptoms, i.e., anhedonia (SPT) and loss of motivation (FST). Therefore, we suggest to exclude animals from the anhedonic group only on the basis of the three-sigma rule of thumb, i.e., animals with values not within three standard deviations of the mean.
Resource availability
Lead contact
Further information should be directed to and will be fulfilled by the lead contact, Monika Bijata (m.bijata@nencki.edu.pl).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by grants from the Foundation for Polish Science (POIR.04.04.00-00-43BC/17-00) and National Science Centre (UMO-2017/26/E/NZ4/00637 and UMO-2019/35/D/NZ4/02042). We would like to thank Dr. Alicja Puścian, Dr. Bartomiej Pochwat, and Dr. Bernadeta Szewczyk for fruitful discussions during the preparation of the manuscript.
Author contributions
Conceptualization, data analysis, and writing of the original draft, M.B.; review and editing, E.B. and J.W.; conducting the experiments and data analysis, E.B. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate datasets and code.
References
- Arakawa H., Blanchard D.C., Arakawa K., Dunlap C., Blanchard R.J. Scent marking behavior as an odorant communication in mice. Neurosci. Biobehav. Rev. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczynska E., Zareba-Koziol M., Ruszczycki B., Krzystyniak A., Wojtowicz T., Bijata K., Pochwat B., Magnowska M., Roszkowska M., Figiel I., et al. The molecular fingerprint of stress resilience. biorxiv. 2022 doi: 10.1101/2022.05.19.492644. [DOI] [Google Scholar]
- Bijata M., Bączyńska E., Müller F.E., Bijata K., Masternak J., Krzystyniak A., Szewczyk B., Siwiec M., Antoniuk S., Roszkowska M., et al. Activation of the 5-HT7 receptor and MMP-9 signaling module in the hippocampal CA1 region is necessary for the development of depressive-like behavior. Cell Rep. 2022;38:110532. doi: 10.1016/j.celrep.2022.110532. [DOI] [PubMed] [Google Scholar]
- Can A., Dao D.T., Arad M., Terrillion C.E., Piantadosi S.C., Gould T.D. The mouse forced swim test. J. Vis. Exp. 2012:3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y., Anthony D.C., Dolgov O., Revischin A., Festoff B., Santos A.I., Steinbusch H.W., Strekalova T. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013;29:136–146. doi: 10.1016/j.bbi.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Gorinski N., Bijata M., Prasad S., Wirth A., Abdel Galil D., Zeug A., Bazovkina D., Kondaurova E., Kulikova E., Ilchibaeva T., et al. Attenuated palmitoylation of serotonin receptor 5-HT1A affects receptor function and contributes to depression-like behaviors. Nat. Commun. 2019;10:3924–4014. doi: 10.1038/s41467-019-11876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzystyniak A., Baczynska E., Magnowska M., Antoniuk S., Roszkowska M., Zareba-Koziol M., Das N., Basu S., Pikula M., Wlodarczyk J. Prophylactic ketamine treatment promotes resilience to chronic stress and accelerates recovery: correlation with changes in synaptic plasticity in the CA3 subregion of the Hippocampus. Int. J. Mol. Sci. 2019;20:E1726. doi: 10.3390/ijms20071726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Puścian A., Łęski S., Kasprowicz G., Winiarski M., Borowska J., Nikolaev T., Boguszewski P.M., Lipp H.-P., Knapska E. Eco-HAB as a fully automated and ecologically relevant assessment of social impairments in mouse models of autism. Elife. 2016;5:e19532. doi: 10.7554/eLife.19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sá-Calçada D., Roque S., Branco C., Monteiro S., Cerqueira-Rodrigues B., Sousa N., Palha J.A., Correia-Neves M. Exploring female mice interstrain differences relevant for models of depression. Front. Behav. Neurosci. 2015;9:335. doi: 10.3389/fnbeh.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T., Steinbusch H.W.M. Measuring behavior in mice with chronic stress depression paradigm. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:348–361. doi: 10.1016/j.pnpbp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Strekalova T., Spanagel R., Bartsch D., Henn F.A., Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Sturm M., Becker A., Schroeder A., Bilkei-Gorzo A., Zimmer A. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav. 2015;14:292–300. doi: 10.1111/gbb.12208. [DOI] [PubMed] [Google Scholar]
- Will T.R., Proaño S.B., Thomas A.M., Kunz L.M., Thompson K.C., Ginnari L.A., Jones C.H., Lucas S.-C., Reavis E.M., Dorris D.M., et al. Problems and progress regarding sex bias and omission in neuroscience research. ENeuro. 2017;4 doi: 10.1523/ENEURO.0278-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate datasets and code.