Abstract
Background
The impact of race and its related social determinants of health on cardiovascular disease outcomes has been well documented. However, limited data exist regarding the association of race with in-hospital outcomes in patients admitted for sinus node dysfunction (SND).
Objective
To evaluate whether racial disparities exist in outcomes for patients hospitalized with a primary diagnosis of SND.
Methods
The National Inpatient Sample was queried from 2011 to 2018 for relevant ICD-9 and ICD-10 diagnosis and procedure codes. Baseline characteristics and in-hospital outcomes in patients with a primary diagnosis of SND were compared among White and non-White patients. A multivariate logistic regression model was used to adjust for potential confounding factors and statistically significant comorbidities between both cohorts.
Results
We identified 655,139 persons admitted with a primary diagnosis of SND, 520,926 (79.5%) of whom were White. Non-White patients had significantly higher all-cause mortality, length of stay, and total hospital cost. There were lower odds of pacemaker insertion (adjusted odds ratio [aOR] 1.13 [95% confidence interval (CI) 1.11–1.15]), temporary transvenous pacing (aOR 1.15 [95% CI 1.11–1.22]), and cardioversion (aOR 1.50 [95% CI 1.42–1.58]) in non-White patients. A subgroup analysis was performed and non-Hispanic Black race was predictive of a decreased odds of pacemaker insertion, cardioversion/defibrillation, and temporary transvenous pacing.
Conclusion
Significant differences of in-hospital outcomes exist between White and non-White patients with SND. These findings appeared to be primarily driven by disparities in non-Hispanic Black patients. Increased recognition and focused efforts to mitigate these disparities will improve the care of underrepresented populations treated for SND.
Keywords: Sinus node dysfunction, Racial disparities, Sick sinus syndrome, Pacemaker, Cardioversion, Defibrillation, Arrhythmia
Key Findings.
-
▪
Non-White patients admitted with a primary diagnosis of sinus node dysfunction had higher rates of all-cause in-hospital mortality, longer length of stay, and higher total hospital cost compared to White patients.
-
▪
Non-White patients had lower odds of permanent pacemaker implantation, temporary transvenous pacing, and cardioversion/defibrillation despite indications for these interventions.
-
▪
Differences between outcomes in White and non-White patients were primarily driven by disparities in non-Hispanic Black and Hispanic patients.
Introduction
Sinus node dysfunction (SND) has a prevalence of approximately 1 per 1000 person-years in the United States, with a significant increase after age 65.1 The complications of SND require early recognition and include symptomatic bradycardia, sinus pause/arrest, chronotropic incompetence, and tachycardia-bradycardia syndromes.2 The management of SND and its complications involves a stepwise approach, often beginning with acute medical therapies, temporary pacing, cardioversion/defibrillation, and ultimately, permanent pacemaker (PPM) implantation.3 In fact, SND is the primary indication for implantation in approximately 50% of patients receiving PPM.4,5 According to the Center for Medicare and Medicaid Services, the average hospital charge for PPM implantation in the United States ranges from $20,753 to $82,024, with reimbursement rates ranging from $11,411 to $19,577, reflecting the financial burden on insurance payers, hospitals, and patients when managing SND.6
Previous studies investigating the epidemiology of SND have found that non-Hispanic Black patients have a lower incidence of hospitalization for SND than White individuals.1,7 Whether this finding is due to a lower prevalence of SND, less access to emergency care, or historical mistrust in the healthcare system leading to delayed presentations remains unclear.8 According to the Centers for Disease Control and Prevention, while overall rates of heart disease in non-Hispanic White patients decreased from 2000 to 2014, rates in African American, Hispanic, and Asian or Pacific Islander populations have increased.9 The Journal of the American College of Cardiology recently published a series of studies highlighting race-based disparities in cardiovascular disease outcomes, in line with a call to improve health outcomes in underrepresented populations.10
In lieu of the aforementioned reasons, we sought to investigate the association of race-based differences with in-hospital outcomes in patients admitted with a primary diagnosis of SND.
Methods
We performed a retrospective analysis using the National Inpatient Sample (NIS) database from January 1, 2011, to December 31, 2018. The NIS is a publicly accessible database of all payers, approximating a 20% stratified sample of discharges from US community hospitals participating in the Healthcare Cost and Utilization Project. The data includes information pertaining to each hospitalization, including primary and secondary diagnosis, patient demographics, primary payer, information regarding comorbidities, length of stay, and mortality. We queried the NIS database to identify all hospitalizations during the specified time period with a primary diagnosis of SND using ICD-9-CM diagnostic code 427.81 and ICD-10-CM diagnostic code I49.5. Institutional review board approval was not needed, as all patient information is de-identified within the NIS. A detailed overview of the Healthcare Cost and Utilization Project NIS is available at https://www.hcup-us.ahrq.gov/nisoverview.jsp.
Our primary outcomes of interest included all-cause in-hospital mortality, total hospital charges, and length of stay. Secondary outcomes of interest included additional arrhythmic conditions such as atrial fibrillation, complete heart block, second-degree atrioventricular block, and supraventricular tachycardia, as well as the incidence of cardiogenic shock, permanent pacemaker insertion, temporary transvenous pacing, and cardioversion/defibrillation. All outcomes were stratified by race/ethnicity, as White and non-White (this included NHB, Hispanic, Asian or Pacific Islander, Native American, or Other as classified by the NIS description of data elements) for simplicity. As recommended by the Agency for Healthcare and Research and Quality, weighted data were used for all statistical analyses. We used descriptive statistics to summarize continuous and categorical variables. The mean and standard deviation were used for continuous variables and percentages were used for categorical variables.
We used univariate analyses for between-group comparisons, Pearson χ2 testing for categorical variables, and independent samples t testing for continuous variables, with a P value of <.001 considered statistically significant. Multivariate logistic regression was used to estimate adjusted odds ratios (aORs) and 95% confidence intervals (95% CI) to determine the association between race and measured clinical outcomes in patients with SND. All multivariate logistic regression models used a P value of <.05 for statistical significance. Our models were adjusted for age, sex, elective vs nonelective admission, insurance status, patient location, median household income, and statistically significant comorbidities (hypertension, diabetes mellitus, tobacco use, heart failure, hyperlipidemia, obesity, coronary artery disease, chronic kidney disease, and chronic obstructive pulmonary disease) between groups. All statistical analyses were performed using SPSS (IBM SPSS Statistics for MAC, Version 26.0; IBM Corporation, Armonk, NY).
Results
Baseline characteristics
We identified 520,926 admissions in White patients and 134,213 in non-White patients with a primary diagnosis of SND. Baseline characteristics are shown in Table 1. Non-White patients with SND were found to be younger (74.77 ± 13.11 years vs 77.91 ± 11.19 years), were more often female (57.7% vs 52.3%), and were less likely to undergo elective admission (11.3% vs 14.6%, P < .001 for all). Further, non-White patients were more likely to have Medicaid (10.9% vs 2.0%) or self-pay (1.9% vs 0.7%) as their primary payer and were much more likely to fall within the 0–25th percentile for household income (39.7% vs 24.7%, P < .001 for all).
Table 1.
Baseline characteristics of patients with primary diagnosis of sinus node dysfunction by race
| White (N = 520,926) | Non-White (N = 134,213) | P value | |
|---|---|---|---|
| Age (years), mean ± SD | 77.91 ± 11.19 | 74.77 ± 13.11 | <.001 |
| Female | 272,336 (52.3) | 77,474 (57.7) | <.001 |
| Elective vs nonelective admission | 75,864 (14.6) | 15,058 (11.3) | <.001 |
| Insurance payer | <.001 | ||
| Medicare | 447,288 (86.0) | 102,965 (76.8) | |
| Medicaid | 10,193 (2.0) | 11,487 (8.6) | |
| Private insurance | 52,498 (10.1) | 14,672 (10.9) | |
| Self-pay | 3,532 (0.7) | 2,526 (1.9) | |
| Household income | <.001 | ||
| 0–25th percentile | 126,370 (24.7) | 52, 356 (39.7) | |
| 26th–50th percentile | 140,214 (27.4) | 29,392 (22.3) | |
| 51st–75th percentile | 129,097 (25.2) | 26,761 (20.3) | |
| 76th–100th percentile | 116,853 (22.8) | 23,235 (17.6) | |
| Comorbidities | |||
| Hypertension | 409,537 (81.7) | 112,408 (85.8) | <.001 |
| Diabetes mellitus | 152,729 (29.7) | 55,625 (41.6) | <.001 |
| Hyperlipidemia | 280,276 (55.8) | 68,093 (52.7) | <.001 |
| Tobacco | 143,525 (27.6) | 30,780 (22.9) | <.001 |
| Obesity | 57,819 (11.1) | 17,041 (12.7) | <.001 |
| Heart failure | 191,522 (36.8) | 51,570 (38.5) | <.001 |
| CAD | 228,587 (44.0) | 52,915 (39.6) | <.001 |
| CKD | 134,198 (25.8) | 46,396 (34.6) | <.001 |
| COPD | 98,786 (19.3) | 18,909 (14.4) | <.001 |
| Patient location | <.001 | ||
| “Central” counties of metro areas of ≥1million population | 78,040 (21.3) | 46,280 (48.4%) | |
| “Fringe” counties of metro areas of ≥1 million population | 99,495 (27.2) | 19,065 (19.9) | |
| Counties in metro areas of 250,000–999,999 population | 76,095 (20.8) | 16,170 (16.9) | |
| Counties in metro areas of 50,000–249,999 population | 41,345 (11.3) | 5610 (5.9) | |
| Micropolitan counties | 40,770 (11.2) | 5110 (5.3) | |
| Not metropolitan or micropolitan counties | 29,840 (8.2) | 3360 (3.5) |
Values are n (%) unless indicated otherwise.
CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease.
Comorbidities
We found that non-White patients were more often found to have a history of hypertension (85.8% vs 81.7), diabetes mellitus (41.6% vs 29.7%), obesity (12.7% vs 11.1%), heart failure (38.5% vs 36.8%), and chronic kidney disease (34.6% vs 25.8%) when compared to White patients. However, White patients were found to have a higher incidence of hyperlipidemia (55.8% vs 52.7%), tobacco use (27.6% vs 22.9%), coronary artery disease (44.0% vs 39.6%), and chronic obstructive pulmonary disease (19.3% vs 14.4%, P < .001 for all).
Primary outcomes
Primary outcomes for our study population are shown in Table 2. We found that White patients had lower in-hospital mortality compared to non-Whites (aOR 0.91 [95% CI 0.85–0.97], P = .003). Non-White patients also had longer length of stay (5.3 ± 4.9 days vs 4.8 ± 4.5 days, P < .001) and higher total hospital charges (78,945 ± 79,839 vs 66,056 ± 64,264; mean US$, P < .001). Figure 1 shows the rates of in-hospital mortality stratified by race. Hispanic ethnicity was associated with higher in-hospital mortality (aOR 1.17 [95% CI 1.06–1.29], P = .002), while non-Hispanic Black (aOR 1.08 [95% CI 0.98–1.18], P = .111), Asian (aOR 1.12 [95% CI 0.97–1.29], P = 0.113), and Native American (aOR 0.90 [95% CI 0.58–1.41], P = .646] race did not achieve statistical significance.
Table 2.
Clinical outcomes in patients with sinus node dysfunction by race
| White (N = 520,926) | Non-White (N = 134,213) | aOR (95% CI)† | P value | |
|---|---|---|---|---|
| Primary outcomes | ||||
| In-hospital mortality, n (%) | 7584 (1.5) | 2148 (1.6) | 0.91 (0.85–0.97) | .003∗ |
| Length of stay (days), mean ± SD | 4.8 ± 4.5 | 5.3 ± 4.9 | <.001∗ | |
| THC (US$), mean ± SD | 66,056 ± 64,264 | 78,945 ± 79,839 | <.001∗ | |
| Secondary outcomes | ||||
| Pacemaker insertion, n (%) | 285,242 (54.8) | 68,650 (51.2) | 1.13 (1.11–1.15) | <.001∗ |
| Atrial fibrillation, n (%) | 308,263 (59.2) | 63,583 (47.4) | 1.51 (1.48–1.53) | <.001∗ |
| Complete heart block, n (%) | 30,476 (5.9) | 8314 (6.2) | 0.94 (0.91–0.97) | <.001 |
| Second-degree atrioventricular block, n (%) | 18,552 (3.6) | 5733 (4.3) | 0.80 (0.77–0.83) | <.001 |
| Supraventricular tachycardia, n (%) | 15,178 (2.9) | 4256 (3.2) | 0.92 (0.88–0.96) | <.001 |
| Cardiogenic shock, n (%) | 3867 (0.7) | 1384 (1.0) | 0.81 (0.74–0.87) | <.001∗ |
| Temporary transvenous pacing, n (%) | 11,020 (2.1) | 2743 (2.0) | 1.15 (1.11–1.22) | <.001∗ |
| Cardioversion/defibrillation (%) | 15,810 (3.0) | 2729 (2.0) | 1.50 (1.42–1.58) | <.001∗ |
Asterisk (∗) indicates statistically significant P values.
aOR = adjusted odds ratio; CI = confidence interval; THC = total hospital charges.
Adjusted for sex, elective vs nonelective admission, age, primary payer, location, median household income, hypertension, diabetes mellitus, tobacco use, heart failure, hyperlipidemia, obesity, coronary artery disease, chronic kidney disease, and chronic obstructive pulmonary disease.
Figure 1.
In-hospital mortality in patients according to race.
Secondary outcomes
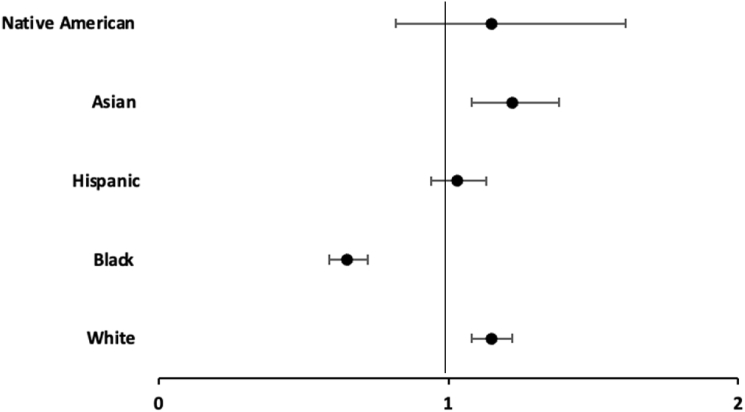
Secondary outcomes for our study are shown in Table 2. White patients had higher odds of PPM implantation (aOR 1.13 [95% CI 1.11–1.15]), atrial fibrillation (aOR 1.51 [95% CI 1.48–1.53]), temporary transvenous pacing (aOR 1.15 [95% CI 1.11–1.22]), and cardioversion/defibrillation (aOR 1.50 [95% CI 1.42–1.58], P < .001 for all). In addition, they had lower odds of complete heart block (aOR 0.94 [95% CI 0.91–0.97]), second-degree atrioventricular block (aOR 0.80 [95% CI 0.77–0.83]), supraventricular tachycardia (aOR 0.92 [95% CI 0.88–0.96]), and cardiogenic shock (aOR 0.81 [95% CI 0.74–0.87], P < .001 for all). Figure 1, Figure 2, Figure 3, Figure 4 further stratified outcomes according to race. Odds of pacemaker insertion (aOR 0.71 [95% CI 0.69–0.73]), temporary transvenous pacing (aOR 0.65 [95% CI 0.59–0.72]), and cardioversion/defibrillation (aOR 0.64 [95% CI 0.59–0.69], P < .001 for all) were all significantly lower in non-Hispanic Black patients. Odds to get cardioversion/defibrillation was also lower in Hispanic (aOR 0.71 [95% CI 0.65–0.77]) and Asian (aOR 0.79 [95% CI 0.69–0.89], P < .001 for all) patients. Supplemental Tables 1 and 2 show the incidence of procedural interventions in patients admitted with SND and additional arrhythmic complications stratified by race. Among patients admitted with SND and second-degree heart block or complete heart block, white patients had higher odds of PPM insertion (aOR 1.25 [95% CI 1.14–1.38]) and aOR 1.24 [95% CI 1.15–1.34], respectively) (Supplemental Table 1). In addition, among those admitted for SND who developed tachyarrhythmia complications, the odds of cardioversion/defibrillation was higher for White patients who developed atrial fibrillation (aOR 1.31 [95% CI 1.23–1.38]) (Supplemental Table 2).
Figure 2.
Pacemaker insertion in patients according to race.
Figure 3.
Temporary transvenous pacing in patients according to race.
Figure 4.
Incidence of cardioversion/defibrillation in patients according to race.
Discussion
In this retrospective analysis, we observed that non-White patients had higher rates of all-cause in-hospital mortality, longer length of stay, and higher total hospital cost compared to White patients among patients with a primary admission diagnosis of SND. In addition, non-White patients had lower odds of PPM implantation, temporary transvenous pacing, and cardioversion/defibrillation. A subgroup analysis showed differences in these outcomes were most evident among non-Hispanic Black and Hispanic patients. This is one of the largest studies evaluating the association between race and in-hospital outcomes in patients with SND. Underrepresented groups, in particular non-Hispanic Black patients, have been reported to have worse cardiovascular disease outcomes when compared to their White counterparts.8 According to the American Heart Association 2022 Update on Heart Disease and Stroke statistics, African American and Hispanic individuals are more likely to have obesity, worse blood pressure control, and more uncontrolled diabetes mellitus.11 These findings were similar in our study, in which we found a higher incidence of hypertension, diabetes mellitus, and obesity in non-White patients. However, after controlling for these comorbidities, non-White patients had an increased all-cause in-hospital mortality when compared to White individuals.
Other factors that are not inherent to the data used in our analysis may be contributing to the higher mortality rates seen in these patients. These factors may include socioeconomic status, education, access to care and resources, mistrust in the medical system, or implicit biases among healthcare providers.12 For example, in 2020, 17% of Hispanic and 19.5% of African American persons were living at or below the poverty line, compared to 8.2% of White individuals.13 Lower household income in the United States is associated with a higher prevalence of comorbid conditions, and according to data from the 2020 Commonwealth Fund International Health Policy Survey, lower-income Americans are also more likely to miss doctor’s appointments, forgo necessary testing and treatment, or choose not to fill prescription medications owing to cost.14 Residential segregation may also play a role in the higher mortality seen in these patients, as underrepresented populations are more likely to live in lower-income neighborhoods with more affordable housing.15, 16, 17
Another factor that may contribute to the disproportionate outcomes seen in White vs non-White patients is mistrust in the healthcare system. This mistrust is multifactorial and deeply rooted in historical mistreatment of non-Hispanic Black patients.18 The possibility that minority groups avoid seeking care in the hospital resulting in later and more advanced presentations of SND, with greater complications, is possible. Lastly, implicit bias among healthcare providers may play a role in worsened outcomes for underrepresented groups. A systematic review of 35 studies to evaluate the impact of implicit racial bias on healthcare outcomes found 25 of those studies reported that most healthcare providers carried some level of pro-White or anti–non-Hispanic Black implicit bias.19 Three of these studies used ethnicity implicit bias testing (comparing bias against Hispanic vs White patients) and found that approximately 51% of providers had moderate/strong pro-White and anti-Hispanic bias.19 Combating the role implicit bias plays in in-hospital outcomes involves, as a first step, frequent education and training/retraining of healthcare providers.
Non-White patients were found to have lower rates of PPM implantation, temporary transvenous pacing, and cardioversion/defibrillation. We propose that the disproportionate rates of therapeutic intervention may have been primarily driven by the majority prevalence of non-Hispanic Black and Hispanic patients in the non-White cohort. Previous studies have reported lower rates of invasive cardiac procedures in non-Hispanic Black compared to White patients.20,21 The overall prevalence of additional arrhythmias, including second- or third-degree heart block and tachyarrhythmias, in our analysis was low. Similar to the main findings of our study, when stratified by race, White patients were more likely to undergo PPM implantation for second- or third-degree heart block and more likely to undergo cardioversion for atrial fibrillation. Therefore, we hypothesize that similar factors related to socioeconomic status, patient education, mistrust, and implicit bias may play a role. Increasing representation of historically marginalized groups among healthcare providers, within clinical trials, and within research groups may help bridge the gap in race-based outcomes seen.22,23 The findings reported in this study necessitate a recognition that further steps to explore and explain the disparities seen between and White and non-White patients admitted for SND are needed.
There are several strengths and limitations of the current study. First, the NIS database allows us the opportunity to evaluate a large sample size of patients admitted with SND in an 8-year period (weighted estimate of 655,139 admissions). However, the NIS database is an administrative database that uses ICD-9 and ICD-10 diagnosis and procedure codes. These codes are not adjudicated and may be subject to error or omission. Additionally, each record in the NIS represents hospitalizations, not individual patients. Therefore, it is possible that patients admitted for SND may appear more than once in the dataset. Additionally, using data from an administrative database does not allow for us to take into account patient-level decision-making with regard to procedural interventions. Although the findings in the present study demonstrate important and statistically significant disparities with regard to outcomes, the margin of significance is small, and therefore the clinical significance of these findings are less clear. Lastly, outcomes are only reported during hospitalization, without subsequent follow-up. Thus, we are unable to determine whether increased in-hospital complications among non-Hispanic Black, Hispanic, Asian, and Native American populations led to increased hospital readmission, out-of-hospital major adverse events, or death.
Conclusion
Significant differences of in-hospital outcomes exist between White and non-White patients with SND, including increased mortality and decreased odds of pacemaker insertion, temporary transvenous pacing, and cardioversion/defibrillation. These findings appeared to be primarily driven by disparities in non-Hispanic Black patients. Increased recognition and focused efforts to mitigate these disparities will improve the care of underrepresented populations treated for SND.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Informed consent was not required, as all patient information is de-identified within the National Inpatient Sample (NIS) database.
Ethics Statement
Institutional review board approval for this retrospective analysis was not needed, as the NIS is a publicly accessible database and all patient information is de-identified.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2022.05.010.
Appendix. Supplementary data
References
- 1.Jensen P.N., Gronroos N.N., Chen L.Y., et al. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64:531–538. doi: 10.1016/j.jacc.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John R.M., Kumar S. Sinus node and atrial arrhythmias. Circulation. 2016;133:1892–1900. doi: 10.1161/CIRCULATIONAHA.116.018011. [DOI] [PubMed] [Google Scholar]
- 3.Kusumoto F.M., Schoenfeld M.H., Barrett C., et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [published correction appears in Circulation 2019;140:e506–e508] Circulation. 2019;140:e382–e482. doi: 10.1161/CIR.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein A.D., Parsonnet V. Survey of cardiac pacing and defibrillation in the United States in 1993. Am J Cardiol. 1996;78:187–196. [PubMed] [Google Scholar]
- 5.Bernstein A.D., Parsonnet V. Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol. 2001;24:842–855. doi: 10.1046/j.1460-9592.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- 6.MedicareHelp Permanent Cardiac Pacemaker Implant No Complications. http://www.medicarehelp.org/cost-of-medicare/procedure/permanent-cardiac-pacemaker-implant-no-complications
- 7.Baine W.B., Yu W., Weis K.A. Trends and outcomes in the hospitalization of older Americans for cardiac conduction disorders or arrhythmias, 1991-1998. J Am Geriatr Soc. 2001;49:763–770. doi: 10.1046/j.1532-5415.2001.49153.x. [DOI] [PubMed] [Google Scholar]
- 8.Carnethon M.R., Pu J., Howard G., et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 9.Heron M., Anderson R.N. Changes in the leading cause of death: recent patterns in heart disease and cancer mortality. NCHS Data Brief. 2016;254:1–8. [PubMed] [Google Scholar]
- 10.Mensah G.A., Fuster V. Race, ethnicity, and cardiovascular disease: JACC Focus Seminar Series. J Am Coll Cardiol. 2021;78:2457–2459. doi: 10.1016/j.jacc.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 12.Schneider E.C., Chin M.H., Graham G.N., et al. Increasing equity while improving the quality of care: JACC Focus Seminar 9/9. J Am Coll Cardiol. 2021;78:2599–2611. doi: 10.1016/j.jacc.2021.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrider E, Kollar M, Chen F, Semega J. Income and poverty in the United States: 2020: United States Census Bureau, 2021. Accessed February 14, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- 14.Doty M.M., Tikkanen R.S., FitzGerald M., Fields K., Williams R.D., 2nd Income-related inequality in affordability and access to primary care in eleven high-income countries. Health Aff (Millwood) 2021;40:113–120. doi: 10.1377/hlthaff.2020.01566. [DOI] [PubMed] [Google Scholar]
- 15.Bailey Z.D., Krieger N., Agénor M., Graves J., Linos N., Bassett M.T. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 16.Bravo M.A., Anthopolos R., Bell M.L., Miranda M.L. Racial isolation and exposure to airborne particulate matter and ozone in understudied US populations: environmental justice applications of downscaled numerical model output. Environ Int. 2016;92–93:247–255. doi: 10.1016/j.envint.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 17.White K., Haas J.S., Williams D.R. Elucidating the role of place in health care disparities: the example of racial/ethnic residential segregation. Health Serv Res. 2012;47:1278–1299. doi: 10.1111/j.1475-6773.2012.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsan M., Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407–455. doi: 10.1093/qje/qjx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maina I.W., Belton T.D., Ginzberg S., Singh A., Johnson T.J. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc Sci Med. 2018;199:219–229. doi: 10.1016/j.socscimed.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Lamprea-Montealegre J.A., Oyetunji S., Bagur R., Otto C.M. Valvular heart disease in relation to race and ethnicity: JACC Focus Seminar 4/9. J Am Coll Cardiol. 2021;78:2493–2504. doi: 10.1016/j.jacc.2021.04.109. [DOI] [PubMed] [Google Scholar]
- 21.Patlolla S.H., Shankar A., Sundaragiri P.R., Cheungpasitporn W., Doshi R.P., Vallabhajosyula S. Racial and ethnic disparities in the management and outcomes of cardiogenic shock complicating acute myocardial infarction. Am J Emerg Med. 2022;51:202–209. doi: 10.1016/j.ajem.2021.10.051. [DOI] [PubMed] [Google Scholar]
- 22.Ortega R.F., Yancy C.W., Mehran R., Batchelor W. Overcoming lack of diversity in cardiovascular clinical trials: a new challenge and strategies for success. Circulation. 2019;140:1690–1692. doi: 10.1161/CIRCULATIONAHA.119.041728. [DOI] [PubMed] [Google Scholar]
- 23.Food and Drug Administration (FDA) 2015-2016 Global Participation in Clinical Trials Report. 2017. https://www.fda.gov/downloads/Drugs/InformationOnDrugs/UCM570195.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.