Abstract
Purpose
Emerging evidence from rodent studies suggests that high-fat-diet (HFD)-induced obesity is characterized by increased oxidative damage in sperm and testis. However, interventions using micronutrient supplementation to mitigate oxidative damage in obesity have not been extensively studied. This study aimed to investigate the effect of an antioxidant-based micronutrient supplement (added folate, vitamin B6, choline, betaine, and zinc) on sperm and testicular oxidative damage in HFD-fed male Sprague Dawley rats.
Methods
Rats (3-weeks-old, 12/group) were weaned onto control (C) or HFD (H) or these diets with micronutrient supplement (CS; HS); sperm and testis were harvested at 30.5 weeks. To assess oxidative stress and antioxidant capacity in testis, levels of malondialdehyde (MDA), glutathione (GSH), folate and susceptibility index (SI) of pro-oxidative damage, mRNA expression of Nrf2, NFκB-p65, IL-6, IL-10 and TNF-α, in addition to superoxide-dismutase (SOD), catalase and glutathione-peroxidase (GPx) activities were measured. 8-hydroxy-2-deoxyguanosine (8-OHdG) were assessed in both sperm and testis.
Results
HFD-fed rats had significantly increased 8-OHdG content in sperm and testis, increased testicular SI, decreased testicular weight, SOD and GPx activity compared to control. Strikingly, supplementation of HFD appeared to significantly reduce 8-OHdG in sperm and testis (22% and 24.3%, respectively), reduce testicular SI and MDA content (28% and 40%, respectively), increase testicular weight (24%), SOD and GPX activity (30% and 70%, respectively) and GSH content (19%). Moreover, supplementation had significant impact to increase testicular folate content regardless of diet. Furthermore, an overall effect of supplementation to increase testicular mRNA expression of Nrf2 was observed across groups. Interestingly, testicular SI was positively correlated with sperm and testicular 8-OHdG and MDA content, suggesting a critical role of testicular antioxidant activity to combat oxidative damage in sperm and testis.
Conclusion
Our findings suggest that antioxidant-based micronutrient supplement has the potential to interrupt HFD-induced sperm and testicular oxidative damage by improving testicular antioxidant capacity.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-022-02917-9.
Keywords: Antioxidants, Sperm, Testis, Micronutrients, Obesity, Oxidative stress
Introduction
The prevalence of obesity, a major public health challenge across the world, has substantially increased in adults over the last 40 years [1]. In developed countries including USA, UK and Australia, males are more affected by obesity than females [2]. Obesity in male rodents is closely linked with sperm and testicular oxidative damage, characterized by increased lipid peroxidation [3, 4], reactive oxygen species (ROS) production and oxidative DNA damage [3, 5, 6]. Sperm oxidative DNA damage is increasingly being considered as one of the potential mechanisms underpinning paternal programming of offspring health in both human and rodents [7, 8]. Thus, reduction of oxidative stress by interventions could be a potential approach to mitigate obesity-induced sperm and testicular oxidative damage and associated programming.
Micronutrients are generally consumed in small amounts in the diet; however, they are essential and play critical roles as antioxidants [9]. A growing list of studies suggest that obesity is associated with decreased systemic levels of micronutrients having antioxidant property particularly folate, zinc, choline, betaine and vitamin B6 [10–14]. Such deficiencies may lead to increased oxidative stress [15], development of comorbidities [16] and decreased sperm quality [17]. Moreover, obesity-induced impaired antioxidant capacity can further accelerate testicular and sperm oxidative damage as demonstrated in rodent studies [18, 19]. On the other hand, micronutrient supplements have been found to not only improve conventional sperm parameters (e.g., sperm count, motility, morphology) but also to reduce ROS production, DNA damage, increase antioxidant capacity and improve DNA integrity of sperm in sub-fertile, and infertile men [20–25] as well as infertile [26] and undernourished [27] rodents. However, evidence supporting micronutrient interventions to attenuate obesity-induced oxidative stress in the male reproductive system is limited. To date, there has been only one study in rodents investigating the potential impacts of micronutrient supplement in ameliorating obesity-induced oxidative DNA damage in sperm [5]. Moreover, no study in rodents has investigated the effects of micronutrient supplement on obesity-induced oxidative DNA damage in testis. Given the limited information in the context of obesity-induced sperm/testicular oxidative damage, such micronutrient intervention requires further attention.
Therefore, this study aimed to investigate whether micronutrient supplementation in Sprague Dawley male rats could ameliorate HFD-induced oxidative damage in testis and sperm. The metabolic assessment of these animals was reported recently [28]. Semisynthetic control or HFD were supplemented with a unique formulation comprising five micronutrients: folate, vitamin B6, choline, betaine, and zinc (ZnSO4) as previously described [28]. These components participate in one carbon metabolism either as a substrate or a cofactor [29], and can also act as antioxidants [30]. One carbon-metabolism is a set of pathways where one-carbon moieties are transferred from donors to intermediate carriers and finally used for critical cellular process including the production of the antioxidant glutathione [31]. The addition of vitamin B6 in this formulation was intended to increase glutathione (a major intracellular antioxidant) production via the trans sulphuration pathway [32] with a view to combat oxidative attack more efficiently. Very little is known about the benefits of supplementing multiple micronutrients that participate in one-carbon metabolism. As far we are aware, only folate and zinc (out of the five components of our micronutrient formulation) have been tested previously in rodents to target HFD-induced oxidative damage in sperm [5]. Moreover, to the best of our knowledge, the impact of increased vitamin B6, combined with other micronutrients used here in the context of HFD-induced sperm and testicular oxidative damage is unknown. We hypothesized that improving testicular antioxidant content/activity through micronutrient supplementation could counteract testicular and sperm oxidative stress in HFD-fed rats. Interestingly significant beneficial effects of micronutrient supplement on sperm and testicular oxidative stress were seen.
Materials and methods
Animals, diets and micronutrient supplement
Male Sprague Dawley rat pups generated in the Biological Resources Centre, UNSW SYDNEY (Founders sourced from ARC, Perth, WA) were maintained at 21 ± 2 °C; 12:12 h light/dark condition. The use of animals and all experimental procedures were approved by the Animal Ethics Committee of UNSW SYDNEY (Ethics ID: 17/27A). From 3 weeks of age, animals were housed 2–3 rats per cage and randomly allocated to four diet groups (N = 12) until cull. The experimental design is depicted in Fig. 1. The diets (Table 1) used were control diet (denoted by C), control diet containing micronutrient supplement (denoted by CS), HFD (denoted by H) and HFD-containing micronutrient supplement (denoted by HS). To maintain a body weight difference between control and HFD-fed animals, we limited the energy intake of the C and CS groups from 7 weeks of age (from 4 weeks of diet) to 407 kJ (32 g/rat/day of diet), which corresponds to the daily recommended intake for rats. Our extensive experience with adult SD male rats suggests this energy intake is sufficient for normal growth [33]. The metabolic and liver outcomes from these animals was published recently [28].
Fig. 1.
Experimental design and timeline. Male Sprague Dawley rats were fed control or HFD with or without supplement from weaning till 30.5 weeks of age. At cull (30.5 weeks), sperm was harvested and analysed for sperm count and 8-OHdG content and testis was analysed for 8-OHdG, MDA, folate and GSH content, the activity of SOD, catalase and GPx, susceptibility index of pro-oxidative damage and mRNA expression of Nrf2, NFκB-p65, IL-6, IL-10 and TNF-α. 8-OHdG 8 hydroxy-2-deoxyguanosine, MDA malondialdehyde, SOD superoxide-dismutase, GPx glutathione-peroxidase, GSH glutathione, Nrf2 nuclear factor erythroid 2-related factor 2, NFκB-p65 nuclear factor kappa-light-chain-enhancer of activated B cells, subunit p65, IL-6 interleukin-6, IL-10 interleukin-10, TNF-α tumor necrosis factor alpha
Table 1.
Chemical composition per kilogram of Control (C,) HFD (H), Control supplemented (CS) and HFD supplemented (HS) diets
| Ingredients (g/kg) | C | H | CS | HS |
|---|---|---|---|---|
| Casein | 140 | 140 | 140 | 140 |
| Sucrose | 100 | 405 | 100 | 405 |
| Canola Oil | 40 | 50 | 40 | 50 |
| Lard | 190 | 190 | ||
| Cellulose | 50 | 50 | 50 | 50 |
| Corn starch | 620 | 115 | 620 | 115 |
| dl Methionine | 3 | 3 | 3 | 3 |
| Mineral mix (AIN93M) | 35 | 35 | 35 | 35 |
| Vitamin mix (AIN93) | 10 | 10 | 10 | 10 |
| Choline bitartratea | 4.1 | 4.1 | 36.5 | 36.5 |
| Betainea | 15 | 15 | ||
| Folic acida | 0.013 | 0.013 | ||
| Pyridoxine HCl (Vit B6)a | 0.0295 | 0.0295 | ||
| ZnSO4.H2Oa | 0.296 | 0.296 |
The supplemented diets had additional Choline, Betaine, Folic acid, Vitamin B6 and Zinc
aAdditional to AIN93M vitamin mix
The diets were prepared in-house using commercial ingredients, and the micronutrient supplements were added during preparation. The composition of both C and HFD were similar except for sucrose, canola oil, lard, corn starch and supplemented micronutrients content as published recently [28]. Control diet and HFD were prepared based on American Institute of Nutrition 93 G/M (AIN 93 G/M) formulation [34], and SF03-020 (Specialty Feeds, Western Australia), containing 23% total fat and 20 MJ/kg digestible energy, respectively. The energy content of our control and control supplemented diet was 12,720 kJ/kg diet, and that of HFD and HFD supplemented diet was 20,439 kJ/kg diet. The micronutrient formulation for the supplementation was designed based on the Wolff 3SMZ diet used in mice except the increase in vitamin B12 was replaced by additional B6 and methionine was given at normal levels [35].
Testis, sperm collection and sperm count
Rats were euthanised at 30.5 weeks of age to collect sperm and testis samples for further assays and all samples were stored at −80 ºC until analysed. Testis was powdered under liquid nitrogen for use in assays described below. Sperm was collected from both cauda of male rats using a swim out method. Several pores were made in the cauda (using 23 G needle) kept in Biggers, Whitter and Whittingham (BWW) buffer with underlying hydrated mineral oil in a petri dish to collect motile sperm. The plate was incubated for 10 min at 37 ºC. After that, the BWW containing sperm was aspirated and aliquoted into two tubes and snap frozen for further experiment. Sperm count was determined by placing 10 µl of 1 in 20 diluted sample in a hemocytometer, placing under a microscope (Olympus CH-2, Japan, 100X magnification); number of sperm counted in five squares were then multiplied by 106 to get counts per ml.
Oxidative DNA damage (8-OHdG) measurement in sperm and testis
At first, DNA was extracted from sperm and powdered testis following Qiagen DNeasy Blood and Tissue kit protocol (Cat No: 69504). 1–2 μg DNA was then treated with nuclease P1 and alkaline phosphatase following New England BioLabs protocols (Cat: M0660S and Cat: M0371S, respectively) to convert DNA samples into single nucleosides. 8-OHdG level in the extracted single nucleoside samples was determined using 8-hydroxy 2 deoxyguanosine ELISA kit as per the manufacturer’s instructions (Abcam Cat ID: ab201734). The 8-OHdG concentration in each sample was calculated by five-parameter logistic regression interpolated from the standard curve using elisaanalysis.com software. The intra- and inter-assay precision CVs for this assay in sperm and testis were ≤ 5% and ≤ 6%, respectively.
Testicular folate and lipid peroxidation measurement
50–100 mg of powdered testis was homogenized in phosphate buffered saline (PBS) to measure folate and lipid peroxidation content. In the supernatant, folate concentration and lipid peroxidation (Malondialdehyde, MDA) content were determined by an ELISA (Monobind Inc., Cat: 7825-300B, CA, USA) and colorimetric assay (OxiSelect™ TBARS Assay Kit, Cat: STA-330, Cell Biolab Inc), respectively, as per the manufacturer’s protocols. Values were normalized to the amount of tissue extracted. The intra- and inter-assay precision CVs for folate and lipid peroxidation assay in testis were ≤ 7% and ≤ 6%, respectively.
Testicular glutathione content measurement
To measure total glutathione content, 50–100 mg of powdered testis was homogenized using 5% sulphosalicylic acid (SSA). Total glutathione concentration was estimated in the supernatant by kinetic assay using Ellman’s reagent kit (Sigma-Aldrich, Cat: EIAGHSC, St. Louis, MO, USA) following the manufacturer’s protocol. The value was normalized to the amount of tissue used to prepare extract. The intra- and inter-assay precision CVs for this assay were ≤ 8%.
Testicular superoxide-dismutase (SOD) activity measurement
Powdered testis (~ 100 mg) was homogenised in 1 mL cold 20 mM HEPES buffer (pH 7.2 containing 1 mM EGTA, 210 mM mannitol and 70 mM sucrose). SOD activity was then measured in the collected supernatant following SOD assay kit protocol as per the manufacturer’s instructions (Cayman Chemical, Cat: 706002, MI, USA). The intra- and inter-assay precision CVs for this assay were ≤ 5%.
Testicular catalase activity measurement
Powdered testis (~ 100 mg) was homogenised in 1 mL cold 50 mM potassium phosphate (pH 7.0 containing 1 mM EDTA). Catalase activity was then measured in the collected supernatant following a Catalase assay kit protocol (Cayman Chemical, Cat: 707,002, MI, USA); intra- and inter-assay precision CVs were ≤ 8%.
Testicular glutathione-peroxidase (GPx) activity measurement
One mL cold 50 mM Tris–HCl (pH 7.5, 5 mM EDTA and 1 mM DTT) was used to homogenize 100 mg powdered testis. The collected supernatant was then assayed for GPx activity using Glutathione peroxidase assay kit protocol (Cayman Chemical, Cat: 703102, MI, USA) as per the manufacturer’s protocol. The intra- and inter-assay precision CVs for this assay were ≤ 6%.
Susceptibility index of pro-oxidative damage
Susceptibility index of pro-oxidative damage in testis was calculated from the ratio of SOD to catalase plus GPx activity as described elsewhere [36]. The amount of hydrogen peroxide (H2O2) produced upon dismutation of superoxide by SOD needs to be converted to a non-toxic product by catalase and GPx antioxidant. If the catalase and GPx activity is reduced, the produced H2O2 starts accumulating and leads to further oxidative damage in biological systems [37]. Therefore, a higher susceptibility index reflects increased oxidative damage.
Testicular RNA extraction and measurement of gene expression
Total RNA was extracted from testis, and cDNA was synthesized as described previously [38]. Real-time qPCR was performed on the QuantStudio 12K Flex (Life Technologies, CA, USA) as described previously [38], and in compliance with the MIQE guidelines [39]. The stability of each reference gene was analysed using a web based comprehensive tool RefFinder (https://www.heartcure.com.au/reffinder/), and the best combination of genes (Gapdh and TBP) was selected. Expression of genes involved in oxidative stress (Nrf2) and inflammation (NFκB-p65, IL6, IL10 and TNF-α) and the reference genes was measured using TaqMan RT-PCR. Relative gene expression was calculated using the 2−ΔΔCT method, normalised against two reference genes and an external calibrator using pooled sample [40]. Genes selected for TaqMan RT-PCR and the corresponding hydrolysis probe references (Life Technologies, CA, USA) are listed in Supplementary Table 1.
Statistical analysis
Statistical analyses were performed using IBM SPSS v 23.0 software. Normally distributed data (analysed by Shapiro–Wilk normality test) were expressed as mean ± SEM. Data were analysed by two-way ANOVA. The main effects followed by Tukey HSD post hoc were considered if there were no interaction effects. In case of interaction between independent factors, simple main effect analysis was performed, and the resulting post hoc from interaction effect was considered. Associations of testicular susceptibility index with other oxidative stress parameters in sperm and testis were determined by Pearson correlation where appropriate.
Overall HFD and supplement effect are denoted by ‘$’, and ‘#’, respectively, where required. Interactions between HFD and supplement are noted when significant. Differences were considered significant at p < 0.05.
Results
Effect of HFD and supplementation on body weight, fat mass, testis weight and sperm count at cull (30.5 weeks)
The overall growth and energy intake including the metabolic outcomes of these animals has been reported previously [28]. In summary, the weekly energy intake of H and HS groups were significantly higher than C and CS groups (p < 0.001 for both comparisons), respectively. There were no differences in energy intake among H and HS-fed animals. At weaning, body weight across the groups was similar. As expected, HFD promoted weight gain (p < 0.001, H vs C), and supplemented HFD-fed rats had significantly reduced body weight (p < 0.001, HS vs H) despite their similar energy intake throughout the study [28]. At cull, a significant interaction effect of HFD and supplement was observed on body weight, absolute and relative retroperitoneal fat mass and relative testis weight indicating that HFD-fed rats had increased body weight (H vs C, ~ 21%, p < 0.001), absolute fat mass (H vs C, 139%, p < 0.001), relative fat mass (H vs C, 96%, p < 0.001; HS vs CS, ~ 48%, p < 0.05) and decreased relative testicular weight (H vs C, ~ 17%, p < 0.001) compared to C group (Table 2). The supplement appeared to normalise those parameters in the HS group (HS < H, 21.4%, p < 0.001 for body weight; HS < H; ~ 49%, p < 0.001 for absolute fat mass; HS < H, 35.2%, p < 0.001 for relative fat mass and HS > H, ~ 24%, p < 0.001 for relative testis weight) as measured by simple main effect (Table 2). Feeding HFD and supplement had no significant impact on the sperm count (Table 2).
Table 2.
Body weight, fat mass, testicular weight and sperm count at cull
| Parameters | C | CS | H | HS | HFD effect | Supplement effect | Interaction effect |
|---|---|---|---|---|---|---|---|
| Body weight (g) | 632.5 ± 15.1 | 613.0 ± 8.7 | 765.0 ± 16.6a | 601.4 ± 12.9.d | < 0.001 | < 0.001 | < 0.001 |
| RpWAT (g) | 6.77 ± 0.28 | 5.64 ± 0.41 | 16.20 ± 1.28a | 8.32 ± 0.93.d | < 0.001 | < 0.001 | < 0.001 |
| RpWAT (% body weight) | 1.07 ± 0.04 | 0.92 ± 0.06 | 2.10 ± 0.14a | 1.36 ± 0.13b,d | < 0.001 | < 0.001 | < 0.001 |
| Testis weight (% body weight) | 0.60 ± 0.02 | 0.64 ± 0.01 | 0.50 ± 0.01a | 0.62 ± 0.02.d | 0.001 | < 0.001 | 0.011 |
| Sperm count (million/mL) | 17.5 ± 1.3 | 18.1 ± 2.9 | 16.5 ± 2.5 | 17.6 ± 1.5 | 0.745 | 0.726 | 0.925 |
Data are shown as mean ± SEM (n = 11–12 per group). Effects of HFD and supplement were assessed by simple main effect analysis, and the resulting post hoc was considered. Post hoc HFD effect ‘a’ (H vs C), ‘b’ (HS vs CS) and supplement effect ‘d’ (HS vs H). Differences were considered significant at p < 0.05 (Bolded)
C control diet, CS control diet with supplement, H HFD, HS HFD with supplement
Effect of HFD and supplementation on oxidative DNA damage in sperm and testis
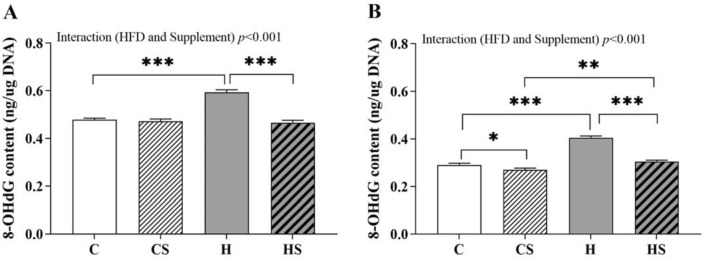
Both HFD and supplementation had overall significant effect (p < 0.001) on sperm and testicular oxidative DNA damage (8-OHdG) along with a significant interaction effect (p < 0.001) (Fig. 2A, B). HFD-fed rats had 24% increased 8-OHdG content in their sperm (H vs C, p < 0.001; Fig. 2A) and 13–40% increased 8-OHdG content in testis (H vs C, ~ 40%, p < 0.001; HS vs CS, ~ 13.3%, p < 0.01; Fig. 2B) as analysed by simple main effect. This effect was normalized in both sperm (HS < H, ~ 22%, p < 0.001; Fig. 2A) and testis (HS < H, ~ 25%, p < 0.001; Fig. 2B) by supplementation in HS rats. Supplementation combined with control diet significantly reduced 7.2% 8-OHdG content in testis (CS vs C, p < 0.05; Fig. 2B) but not in sperm (CS < C; ~ 1.5%, p > 0.05; Fig. 2A).
Fig. 2.
A Sperm and B testicular 8-OHdG content. Data are shown as mean ± SEM (n = 11–12 per group). Effects of HFD and supplement were assessed by simple main effect analysis, and the resulting post hoc was considered. HFD and supplement interaction effect p < 0.001. C Control diet, CS control diet with supplement, H HFD, HS HFD with supplement, 8-OHdG 8 hydroxy-2-deoxyguanosine. *p < 0.05, **p < 0.01 and ***p < 0.001
Effect of HFD and supplementation on testicular lipid peroxidation and folate content
In testis, HFD intake did not significantly affect lipid peroxidation (manifested as MDA, Fig. 3A) and folate content (Fig. 3B). Interestingly, supplementation in HFD-fed rats had an impact to significantly decrease testicular MDA content by 40% compared to H group (p = 0.02, Fig. 3A). Supplement when combined with control diet did not appear to affect testicular MDA content (CS < C, ~ 21%, p = 0.3; Fig. 3A). An overall significant effect (p < 0.001) of supplement on testicular folate content demonstrated that supplemented groups regardless of their diet had increased folate content compared to non-supplemented groups (CS vs C, HS vs H, ~ 22%, p = 0.001; Fig. 3B).
Fig. 3.
Testicular A MDA and B folate level. Data are shown as mean ± SEM (n = 11–12 per group). Effects of HFD and supplement were assessed by two-way ANOVA followed by Tukey HSD post hoc (Folate) or LSD post hoc (MDA). Overall HFD effect p > 0.05 (MDA and folate), supplement effect p = 0.027 (MDA) and p < 0.001 (folate). C control diet, CS control diet with supplement, H HFD, HS HFD with supplement, MDA malondialdehyde. *p < 0.05 and **p < 0.01
Effect of HFD and supplementation on testicular antioxidant capacity
Feeding HFD reduced testicular SOD activity by 21% when compared to C group (p = 0.003; Fig. 4A). Supplemented HFD-fed rats had 30% increase in testicular SOD activity as compared to H group (p = 0.001; Fig. 4A). Feeding supplemented HFD did not significantly affect testicular catalase activity as shown in Fig. 4B. A significant interaction effect of HFD and supplement (p < 0.001) was observed on testicular GPx activity suggesting that HFD-fed rats had 44% reduced GPx activity compared to CD-fed rats (p < 0.001; Fig. 4C) as analysed by simple main effect. This effect was normalized by supplementation in HS rats (HS > H, ~ 70%, p < 0.001; Fig. 4C). Supplementation in CD did not impact testicular GPx activity (Fig. 4C). Testicular glutathione content was not significantly affected by HFD intake (Fig. 4D). An overall significant effect of supplement (p = 0.013) on testicular GSH content indicating that supplementation in HFD appeared to increase testicular glutathione level by 19% when compared to HFD-fed rats (p = 0.015, Fig. 4D). Supplementation of control diet did not significantly affect the testicular glutathione level (CS vs C p = 0.6, Fig. 4D).
Fig. 4.
Testicular activity of A SOD, B catalase, C GPx and D GSH content. Data are shown as mean ± SEM (n = 11–12 per group). Effects of HFD and supplement were assessed by two-way ANOVA followed by Tukey HSD post hoc (SOD) or LSD post hoc (GSH) or by simple main effect analysis, and the resulting post hoc was considered (GPx). Overall HFD effect p = 0.001 and supplement effect p < 0.001 (SOD), p > 0.05 (catalase), overall supplement effect p = 0.013 (GSH), HFD and supplement interaction effect p < 0.001 (GPx). C control diet, CS control diet with supplement, H HFD, HS HFD with supplement, SOD superoxide-dismutase, GPx glutathione-peroxidase, GSH glutathione. *p < 0.05, **p < 0.01 and ***p < 0.001
Effect of HFD and supplementation on testicular susceptibility index of pro-oxidative damage
The testicular susceptibility index of pro-oxidative damage was calculated from the enzymatic activity of SOD, catalase and GPx as desribed in the method section. A significant interaction effect of HFD and supplement was observed on testicular susceptibility index of pro-oxidative damage indicating that HFD-fed rats had 42% increase in susceptibility index of pro-oxidative index compared to CD-fed rats (p = 0.003; Fig. 5). Supplemented HFD-fed rats had 28% reduced susceptibility index when compared to HFD-fed rats (p = 0.006; Fig. 5). No difference in the susceptibility index was observed in CS rats (CS v C; p > 0.05) (Fig. 5).
Fig. 5.
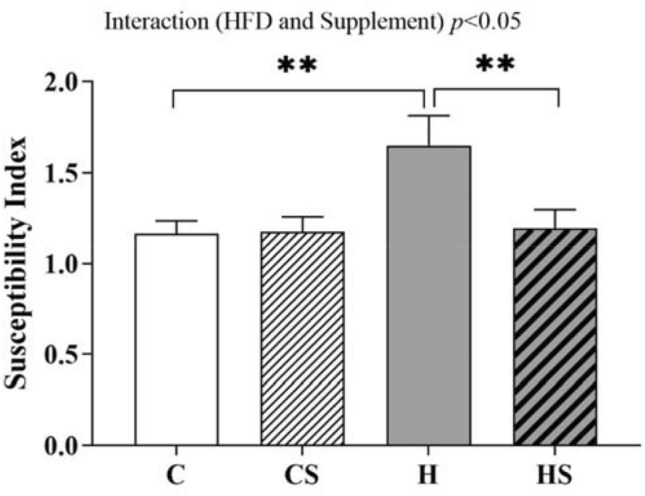
Testicular susceptibility index of pro-oxidative damage. Data are shown as mean ± SEM (n = 11–12 per group). Effects of HFD and supplement were assessed by simple main effect analysis, and the resulting post hoc was considered. HFD and supplement interaction effect p < 0.05. C control diet, CS control diet with supplement, H HFD, HS HFD with supplement. **p < 0.01
Correlation of testicular susceptibility index with sperm and testicular oxidative stress parameters
To further confirm how the testicular susceptibility index reflects other oxidative stress markers of sperm and testis, we correlated the index with sperm and testicular oxidative stress parameters. Correlational analyses (Table 3) indicated that the testicular susceptibility index correlated significantly with the measures of sperm and testicular oxidative damage. Specifically, testicular susceptibility index positively correlated with sperm oxidative DNA damage (r = 0.55, p < 0.0001, Table 3), testicular oxidative DNA damage (r = 0.31, p = 0.03, Table 3) and oxidative lipid damage (r = 0.31, p = 0.03, Table 3). A weak negative correlation was also observed between susceptibility index and GSH content in testis (r = −0.2, p = 0.07).
Table 3.
Correlations between testicular susceptibility index (SI) and oxidative stress parameters in sperm and testis
| Testicular SI | ||
|---|---|---|
| r | p | |
| Sperm 8-OHdG content | 0.55 | < 0.0001 |
| Testis 8-OHdG content | 0.31 | 0.03 |
| Testis MDA content | 0.31 | 0.03 |
Data are presented as scatterplots of individual values and analysed by Pearson correlations, n = 47
Correlation of folate content with GSH content in testis
To investigate how micronutrient supplement impacts testicular GSH content, we correlated the testicular folate content (an important component of micronutrient supplement) with testicular GSH content. Correlational analysis indicated that the testicular folate content positively correlated with testicular GSH content (r = 0.42, p < 0.01, Supp Fig. 2).
Effect of HFD and supplementation on testicular gene expression involved in oxidative stress
Feeding HFD did not significantly alter mRNA expression of Nrf2, NFκB-p65, IL-6, IL-10 and TNF-α in testis (Table 4). No significant effect of supplementation was observed on mRNA expression of NFκB-p65, IL-6, IL-10 and TNF-α. Interestingly, an overall significant effect of supplementation to increase expression of Nrf2 gene was found, however, no between group differences were found to be significant in the post hoc analysis (Table 4).
Table 4.
Effect of HFD and supplement on testicular mRNA expression of genes associated with oxidative stress in rats
| Relative expression | C | CS | H | HS | HFD effect | Supplement effect | Interaction effect |
|---|---|---|---|---|---|---|---|
| Nrf2 | 1.0 ± 0.04 | 1.10 ± 0.05 | 0.94 ± 0.05 | 1.06 ± 0.04 | 0.6 | 0.018 | 0.556 |
| NFκB-p65 | 1.0 ± 0.04 | 0.96 ± 0.04 | 1.02 ± 0.05 | 1.0 ± 0.05 | 0.448 | 0.471 | 0.864 |
| IL-6 | 1.0 ± 0.11 | 0.96 ± 0.04 | 1.07 ± 0.11 | 0.99 ± 0.07 | 0.678 | 0.608 | 0.725 |
| IL-10 | 1.0 ± 0.14 | 1.07 ± 0.13 | 0.92 ± 0.14 | 1.04 ± 0.09 | 0.691 | 0.468 | 0.849 |
| TNF-α | 1.0 ± 0.11 | 1.0 ± 0.09 | 1.17 ± 0.07 | 1.02 ± 0.06 | 0.280 | 0.389 | 0.362 |
Data are shown as mean ± SEM (n = 11–12 per group). Relative gene expression was calculated using the 2−ΔΔCT method, normalised against two reference genes (Gapdh and TBP) and an external calibrator using pooled sample. Effects of HFD and supplement were assessed by two-way ANOVA. Differences were considered significant at p < 0.05 (Bolded)
C control diet, CS control diet with supplement, H HFD, HS HFD with supplement, Nrf2 nuclear factor erythroid 2-related factor 2, NFκB-p65 nuclear factor kappa-light-chain-enhancer of activated B cells, subunit p65, IL-6 interleukin-6, IL-10 interleukin-10, TNF-α tumor necrosis factor alpha, Gapdh glyceraldehyde 3-phosphate dehydrogenase, TBP TATA-box-binding protein
Discussion
In this study, rats fed HFD for 27.5 weeks had 21% increase in total body weight (96% increase in retroperitoneal fat) and 17% decrease in relative testicular weight compared to control fed rats. The HFD-fed rats had a significant increase of 24% and 40% 8-OHdG content in their sperm and testis, respectively. This oxidative DNA damage was also associated with 21% decrease in SOD activity and 44% decrease in GPx activity in HFD-fed rat testis. Furthermore, when we calculated the susceptibility index of pro-oxidative damage as described by Peltola et al. [36], HFD-consuming rats appeared to be more vulnerable to oxidative damage. It is likely that the unaltered sperm count observed in our HFD-fed rats did not exclude further possible detrimental impact of energy dense food on sperm and testis in agreement with a recent rodent study, where mice consuming HFD for 200 days developed irreversible deteriorated sperm motility, viability, morphology and testicular metabolites related to antioxidant activity without affecting sperm count [41]. Strikingly, our micronutrient supplement containing folic acid, zinc, vitamin B6, choline and betaine, when combined with obesogenic diet, was able to normalise body weight (and adiposity), testis weight, sperm and testicular oxidative DNA damage, testicular susceptibility index in association with SOD and GPx activity. In addition, micronutrient supplementation had an impact to increase testicular glutathione and folate content, Nrf2 gene expression and reduce lipid peroxidation. To our knowledge, this is the first intervention study where antioxidant-based micronutrient supplementation in rodents was able to ameliorate HFD-induced oxidative damage in both sperm and testis.
Several rodent studies reported similar sperm and/or testicular oxidative damage as well as testicular weight upon feeding HFD [3, 5, 6, 42–46] as we have demonstrated here. However, limited work has examined the effects of supplementation with the micronutrients used in this study (single or combined) on HFD-induced oxidative damage in both sperm and testis. In a recent rodent study by McPherson et al., mice were fed HFD for 10 weeks followed by 12 days micronutrient intervention containing folic acid 1.5 mg/kg, zinc 61 mg/kg, vitamin C 700 mg/kg, vitamin E 78 mg/kg, lycopene 0.3 mg/kg, selenium 0.44 mg/kg and green tea extract 0.95 mg/kg. The supplemented HFD-fed mice had reduced ROS and 8-OHdG concentration in sperm suggesting that micronutrient supplementation only for 12 days was able to reduce oxidative damage in the sperm of obese mice. However, they did not investigate the potential effects of their supplement on oxidative damage in mouse testis [5]. The 12-day intervention designed by McPherson et al., was intended to target sperm oxidative damage during epididymal transit [5], however, this is not adequate exposure to cover a full cycle of spermatogenesis (35 days) in mice [47]. In males, one might expect intervening before conception over at least a full cycle of spermatogenesis would be required to reduce sperm oxidative damage or to facilitate repair from previous damage. On the other hand, in our study, rats were supplemented from weaning throughout their life span in line with the availability of high caloric and nutrient poor food as a key contributor of global obesity [48] and the resulting prevalence of childhood obesity [49]. Therefore, increasing the duration of an intervention could be an ideal approach to target obesity-induced oxidative damage throughout the spermatogenesis process. Considering the full cycle of spermatogenesis (54 days) in rats, an intervention study was conducted using a different micronutrient supplement from our formulation. In that study (48), micronutrient supplementation (vitamin E 2 g/kg, vitamin C 2 g/kg and astaxanthin 0.6 g/kg) for 12 weeks in HFD-fed Wistar rats did not appear to improve histological changes in testis. However, the potential effects of this supplementation on sperm and testicular oxidative DNA damage were not investigated [50].
Testicular cells and sperm are vulnerable to ROS attack, lipid peroxidation and oxidative DNA damage due to high content of polyunsaturated fatty acid in plasma membrane of testicular cells and sperm [51], a limited amount of cytoplasm (the reservoir of antioxidant) in sperm [52] and the presence of ROS producing enzymes in both sperm and testis [53–55]. To combat this oxidative attack, the testicular cells possesses a wide range of enzymatic and nonenzymatic antioxidants including SOD, catalase, GPx, and GSH [56]. SOD, catalase and GPx are considered first-line enzymatic antioxidants as they are the first to neutralize superoxide radicals [57]. Superoxide radical is the most readily formed free radical due to high consumption of mitochondrial oxygen during spermatogenesis in testis [51] or phagocytosis of germ cell debris in testis by testicular somatic cells, producing superoxide radical by-products [58]. The superoxide radical is rapidly converted to H2O2 in the presence of SOD. However, the accumulated H2O2 can induce oxidative damage to lipids, proteins and DNA [37]. In addition, this accumulated H2O2 in the presence of Fe2+ can be converted to another toxic free radical, hydroxyl radical (*OH) through Fenton reaction [57]. Therefore, to protect biological systems from H2O2-induced oxidative damage, H2O2 needs to be eliminated by either catalase or predominantly by glutathione-dependent enzyme, GPx in testis [36]. In a rat study, it has been shown that epididymal sperm and the developing sperm in testis has high SOD activity associated with low glutathione and glutathione-dependent enzymatic antioxidant activity particularly GPx [56]. This difference between SOD and GPx activity may lead to saturation of the protective systems against H2O2 which may further induce oxidative damage as described using susceptibility index of pro-oxidative damage. Moreover, the positive correlation of testicular susceptibility index with sperm 8-OHdG content, testicular 8-OHdG and MDA content observed in our study suggest that impaired first-line enzymatic antioxidant activity in testis could reflect oxidative DNA damage in both sperm and testis. Therefore, it is critical to improve testicular first-line antioxidant defence systems to target obesity-induced oxidative damage in male reproductive system. Our study is the first to investigate the beneficial effects of micronutrient supplement to mitigate HFD-induced impaired testicular first-line antioxidant activity.
Testicular MDA, and glutathione content, catalase activity and expression of genes involved in oxidative stress were not significantly affected by the HFD feeding protocol in the current study. This is in contrast to previous studies reporting increased MDA content, expression of NFκB-p65, IL6 and TNF-α gene, decreased glutathione content, catalase activity and expression of Nrf2 and IL10 gene in testis of HFD-fed rodents [3, 4, 42–44]. However, the investigation of mRNA and protein expression of Nrf2, NFκB-p65, IL6, IL10 and TNF-α in seminal plasma would be of future interest given the altered cytokine levels in seminal plasma induced by HFD in a recent study [59]. Nrf2 and NFκB-p65 are the two key transcription factors regulating cellular responses to oxidative stress. Under normal physiological conditions, both transcription factors remain in harmony [60]. Nrf2 binds to antioxidant responsive elements (ARE) in the promoters of its target genes and upregulates the gene expression of antioxidants including SOD, catalase, GPx, GSH which effectively neutralizes free radicals and inactivates NF-κB-p65 [60, 61]. However, in the event of excess ROS production leading to oxidative stress, NF-κB-p65 activation further increases the production of ROS and inflammatory cytokines including IL-1b, IL-6, TNF-α and downregulates Nrf2 [60, 61]. It is well established that increased body weight is associated with impaired sperm quality and increased oxidative damage in reproductive system [62]. Therefore, the lack of difference observed in our study may be due to a modest 21% body weight difference between obese and lean animals whereas other studies reported 30–50% body weight difference was associated with increased oxidative stress and decreased antioxidant capacity in male reproductive system [4, 42].
No significant effect of HFD was also seen in our study on testicular folate content. The effect of HFD-induced obesity on testicular folate content is relatively unknown. No previous study has investigated the effects of HFD on testicular folate content in rodents. On the other hand, in this study, an increased testicular level of folate (one of the key micronutrients in our supplement) was observed in supplemented rats regardless of their diet. It is evident that folate has potential to protect biological systems from oxidative damage [63]. Therefore, high level of testicular folate upon supplementation is likely to improve its antioxidant capacity. Interestingly, our supplement had an impact to increase testicular glutathione level, expression of the Nrf2 gene and decrease MDA content. Being a major cellular antioxidant, glutathione plays a key role in protecting cells against oxidative damage [64]. No study has previously reported the effects of supplementation containing our micronutrients (single or combined) to improve testicular glutathione level in HFD-fed rodents. Moreover, a significant positive association between testicular folate and glutathione level was seen in our study. This suggests that increased testicular folate level resulting from our supplementation has an impact to drive the one-carbon intermediate, homocysteine towards trans-sulphuration pathway leading to glutathione formation and thus promotes associated antioxidant activity.
Interestingly, our micronutrient supplement did not appear to reduce sperm oxidative DNA damage in rats consuming control diet. Similarly, the study by McPherson et al., where CD-fed mice were given micronutrient containing folic acid, zinc, vit C, vit E, lycopene, selenium and green tea extract for 12 days did not show any change in sperm oxidative DNA damage [5]. It could be conceivable that micronutrient supplement may be of most benefit to individuals who are micronutrient deficient and thus counteract the complications associated with micronutrient deficiencies. However, in the context of testicular oxidative damage and folate content, we observed that supplemented control fed rats had reduced testicular 8-OHdG and increased folate content suggesting some beneficial effects of our supplementation in lean animals that are not challenged by HFD. Overall, given the increased prevalence of male obesity and potential micronutrient deficiency, this study strongly supports the beneficial effect of our micronutrient supplement in interrupting obesity-induced sperm and testicular oxidative damage.
Conclusion
Our study is the first to demonstrate that HFD-induced impairment of testis first line of antioxidant defence, particularly SOD and GPx activity, had a significant impact to increase sperm and testicular oxidative DNA damage in rodents. Our antioxidant property-based micronutrient supplement has potential to interrupt HFD-induced oxidative DNA damage in both sperm and testis by improving testicular expression of Nrf2 gene, glutathione content and associated antioxidant activity. This is the first work assessing the impact of a unique micronutrient supplement combining folate, vitamin B6, choline, betaine, and zinc on testicular and sperm oxidative stress in rats. Such findings could provide an opportunity to utilize micronutrient-based supplement for ameliorating obesity-induced paternal programming of offspring health. Further study is warranted to decipher the mechanism(s) underpinning the beneficial effects of our micronutrient supplement.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work, including V.L. salary was supported by NHMRC Australia project grant (RG140356) held by M.J.M., and C.A.M. M.M.B. was supported by UNSW Scientia PhD scholarship and S.K. was supported by a UNSW international scholarship and School of Medical Sciences (SoMS), UNSW top up scholarship. We also thank Professor John Aitken, The University of Newcastle, Australia for providing the protocol of sperm collection. Image (Graphical abstract and Fig. 1) was created by BioRender.com. All other figures were created by using GraphPad Prism v9.
Abbreviations
- ARE
Antioxidant responsive elements
- BWW
Biggers, Whitter and Whittingham
- CD
Control diet
- CS
Control diet containing micronutrient supplement
- CVs
Coefficient of variations
- GPx
Glutathione-peroxidase
- GSH
Glutathione
- HFD
High-fat-diet
- H2O2
Hydrogen peroxide
- HS
HFD-containing micronutrient supplement
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- MDA
Malondialdehyde
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NFκB-p65
Nuclear factor kappa-light-chain-enhancer of activated B cells, subunit p65
- SI
Susceptibility index
- SOD
Superoxide-dismutase
- TNF-α
Tumor necrosis factor alpha
- ROS
Reactive oxygen species
- 8-OHdG
8-Hydroxy-2-deoxyguanosine
Author contributions
VL, MJM and CAM designed the study. SK and VL prepared the rodent diet and raised the animal. SK, CAM and MMB generated the samples. MMB performed all experiments, while VL, MJM, and CAM guided the experimental procedure and advised on the experimental plan. MMB drafted the manuscript, and all authors contributed to editing. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo Q, Li Y, Huang C, Cheng D, Ma W, et al. Soy isoflavones improve the spermatogenic defects in diet-induced obesity rats through Nrf2/HO-1 pathway. Molecules (Basel, Switzerland) 2019;24:2966. doi: 10.3390/molecules24162966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi X, Tang D, Cao S, Li T, Gao H, et al. Effect of different exercise loads on testicular oxidative stress and reproductive function in obese male mice. Oxid Med Cell Longev. 2020;2020:3071658. doi: 10.1155/2020/3071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McPherson NO, Shehadeh H, Fullston T, Zander-Fox DL, Lane M (2019) Dietary micronutrient supplementation for 12 days in obese male mice restores sperm oxidative stress. Nutrients 11 [DOI] [PMC free article] [PubMed]
- 6.Fullston T, Palmer NO, Owens JA, Mitchell M, Bakos HW, Lane M. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod (Oxford, England) 2012;27:1391–1400. doi: 10.1093/humrep/des030. [DOI] [PubMed] [Google Scholar]
- 7.Lane M, McPherson NO, Fullston T, Spillane M, Sandeman L, Kang WX, Zander-Fox DL. Oxidative stress in mouse sperm impairs embryo development, fetal growth and alters adiposity and glucose regulation in female offspring. PLoS ONE. 2014;9:e100832. doi: 10.1371/journal.pone.0100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Men's Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey RL, West KP, Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl 2):22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Randell E, Zhou H, Sun G. Higher serum choline and betaine levels are associated with better body composition in male but not female population. PLoS ONE. 2018;13:e0193114–e0193114. doi: 10.1371/journal.pone.0193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKay J, Ho S, Jane M, Pal S. Overweight & obese Australian adults and micronutrient deficiency. BMC Nutr. 2020;6:12. doi: 10.1186/s40795-020-00336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aasheim ET, Hofso D, Hjelmesaeth J, Birkeland KI, Bohmer T. Vitamin status in morbidly obese patients: a cross-sectional study. Am J Clin Nutr. 2008;87:362–369. doi: 10.1093/ajcn/87.2.362. [DOI] [PubMed] [Google Scholar]
- 13.Kimmons JE, Blanck HM, Tohill BC, Zhang J, Khan LK. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed Medsc General Med. 2006;8:59–59. [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini B, Saedisomeolia A, Allman-Farinelli M. Association between antioxidant intake/status and obesity: a systematic review of observational studies. Biol Trace Elem Res. 2017;175:287–297. doi: 10.1007/s12011-016-0785-1. [DOI] [PubMed] [Google Scholar]
- 15.Shenkin A. Micronutrients in health and disease. Postgrad Med J. 2006;82:559–567. doi: 10.1136/pgmj.2006.047670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. 2009;67:559–572. doi: 10.1111/j.1753-4887.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- 17.Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- 18.Chen XL, Gong LZ, Xu JX. Antioxidative activity and protective effect of probiotics against high-fat diet-induced sperm damage in rats. Animal Int J Anim Biosci. 2013;7:287–292. doi: 10.1017/S1751731112001528. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi Roushandeh A, Salehi I, Mortazavi M. Protective effects of restricted diet and antioxidants on testis tissue in rats fed with high-fat diet. Iran Biomed J. 2015;19:96–101. doi: 10.6091/ibj.1398.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebisch IM, Pierik FH, Thomas CM, Steegers-Theunissen RP. Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men? Int J Androl. 2006;29:339–345. doi: 10.1111/j.1365-2605.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 21.Irani M, Amirian M, Sadeghi R, Lez JL, Latifnejad Roudsari R. The effect of folate and folate plus zinc supplementation on endocrine parameters and sperm characteristics in sub-fertile men: a systematic review and meta-analysis. Urol J. 2017;14:4069–4078. [PubMed] [Google Scholar]
- 22.Barik G, Chaturvedula L, Bobby Z. Role of oxidative stress and antioxidants in male infertility: an interventional study. J Hum Reprod Sci. 2019;12:204–209. doi: 10.4103/jhrs.JHRS_135_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Dong X, Hu X, Long Z, Wang L, et al. Zinc levels in seminal plasma and their correlation with male infertility: a systematic review and meta-analysis. Sci Rep. 2016;6:22386. doi: 10.1038/srep22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2009;18:761–768. doi: 10.1016/S1472-6483(10)60024-7. [DOI] [PubMed] [Google Scholar]
- 25.Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrinol. 2019;17:24. doi: 10.1186/s12958-019-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharagozloo P, Gutiérrez-Adán A, Champroux A, Noblanc A, Kocer A, et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: promising preclinical evidence from animal models. Hum Reprod (Oxford, England) 2016;31:252–262. doi: 10.1093/humrep/dev302. [DOI] [PubMed] [Google Scholar]
- 27.McPherson NO, Fullston T, Kang WX, Sandeman LY, Corbett MA, Owens JA, Lane M. Paternal under-nutrition programs metabolic syndrome in offspring which can be reversed by antioxidant/vitamin food fortification in fathers. Sci Rep. 2016;6:27010. doi: 10.1038/srep27010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatiwada S, Lecomte V, Fenech MF, Morris MJ, Maloney CA. Effects of micronutrient supplementation on glucose and hepatic lipid metabolism in a rat model of diet induced obesity. Cells. 2021;10:1751. doi: 10.3390/cells10071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci. 2019;7:263–287. doi: 10.1146/annurev-animal-020518-115206. [DOI] [PubMed] [Google Scholar]
- 30.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 33.Martire SI, Holmes N, Westbrook RF, Morris MJ. Altered feeding patterns in rats exposed to a palatable cafeteria diet: increased snacking and its implications for development of obesity. PLoS ONE. 2013;8:e60407. doi: 10.1371/journal.pone.0060407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 35.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. doi: 10.1096/fasebj.12.11.949. [DOI] [PubMed] [Google Scholar]
- 36.Peltola V, Huhtaniemi I, Ahotupa M. Antioxidant enzyme activity in the maturing rat testis. J Androl. 1992;13:450–455. [PubMed] [Google Scholar]
- 37.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 38.Lecomte V, Maloney CA, Wang KW, Morris MJ. Effects of paternal obesity on growth and adiposity of male rat offspring. Am J Physiol Endocrinol Metab. 2017;312:E117–e125. doi: 10.1152/ajpendo.00262.2016. [DOI] [PubMed] [Google Scholar]
- 39.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Crisóstomo L, Rato L, Jarak I, Silva BM, Raposo JF, et al. A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction (Cambridge, England) 2019;158:377–387. doi: 10.1530/REP-19-0259. [DOI] [PubMed] [Google Scholar]
- 42.Wang EH, Yu ZL, Bu YJ, Xu PW, Xi JY, Liang HY. Grape seed proanthocyanidin extract alleviates high-fat diet induced testicular toxicity in rats. RSC Adv. 2019;9:11842–11850. doi: 10.1039/C9RA01017C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gujjala S, Putakala M, Gangarapu V, Nukala S, Bellamkonda R, Ramaswamy R, Desireddy S. Protective effect of Caralluma fimbriata against high-fat diet induced testicular oxidative stress in rats. Biomed Pharmacother. 2016;83:167–176. doi: 10.1016/j.biopha.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Migliaccio V, Sica R, Scudiero R, Simoniello P, Putti R, Lionetti L. Physiological adaptation to simultaneous chronic exposure to high-fat diet and dichlorodipheniletylhene (DDE) in Wistar rat testis. Cells. 2019;8:443. doi: 10.3390/cells8050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McPherson NO, Lane M. Metformin treatment of high-fat diet-fed obese male mice restores sperm function and fetal growth, without requiring weight loss. Asian J Androl. 2020;22:560. doi: 10.4103/aja.aja_141_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suleiman JB, Nna VU, Zakaria Z, Othman ZA, Eleazu CO, et al. Protective effects of bee bread on testicular oxidative stress, NF-κB-mediated inflammation, apoptosis and lactate transport decline in obese male rats. Biomed Pharmacother. 2020;131:110781. doi: 10.1016/j.biopha.2020.110781. [DOI] [PubMed] [Google Scholar]
- 47.Perrard M-H, Sereni N, Schluth-Bolard C, Blondet A, d′Estaing SG, et al. Complete human and rat ex vivo spermatogenesis from fresh or frozen testicular tissue1. Biol Reprod. 2016;95:89. doi: 10.1095/biolreprod.116.142802. [DOI] [PubMed] [Google Scholar]
- 48.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet (London, England) 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 49.Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17:212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohrabi M, Hosseini M, Inan S, Alizadeh Z, Vahabian M, Vahidinia AA, Lahoutian H. Effect of antioxidants on testicular iNOS and eNOS after high-fat diet in rat. Pak J Biol Sci (PJBS) 2017;20:289–297. doi: 10.3923/pjbs.2017.289.297. [DOI] [PubMed] [Google Scholar]
- 51.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23:737–752. [PubMed] [Google Scholar]
- 53.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 54.Kumagai A, Kodama H, Kumagai J, Fukuda J, Kawamura K, et al. Xanthine oxidase inhibitors suppress testicular germ cell apoptosis induced by experimental cryptorchidism. Mol Hum Reprod. 2002;8:118–123. doi: 10.1093/molehr/8.2.118. [DOI] [PubMed] [Google Scholar]
- 55.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Bauché F, Fouchard M-H, Jégou B. Antioxidant system in rat testicular cells. FEBS Lett. 1994;349:392–396. doi: 10.1016/0014-5793(94)00709-8. [DOI] [PubMed] [Google Scholar]
- 57.Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med. 2018;54:287–293. [Google Scholar]
- 58.Yefimova MG, Messaddeq N, Meunier A-C, Cantereau A, Jegou B, Bourmeyster N (2018) Phagocytosis by sertoli cells: analysis of main phagocytosis steps by confocal and electron microscopy. In: Alves MG, Oliveira PF (eds) Sertoli cells: methods and protocols, pp 85–101. Springer, New York [DOI] [PubMed]
- 59.Schjenken JE, Moldenhauer LM, Sharkey DJ, Chan HY, Chin PY, et al. High-fat diet alters male seminal plasma composition to impair female immune adaptation for pregnancy in mice. Endocrinology. 2021;162:bqab123. doi: 10.1210/endocr/bqab123. [DOI] [PubMed] [Google Scholar]
- 60.Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W, Khor TO, Xu C, Shen G, Jeong W-S, Yu S, Kong A-N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leisegang K, Sengupta P, Agarwal A, Henkel R. Obesity and male infertility: Mechanisms and management. Andrologia. 2021;53:e13617. doi: 10.1111/and.13617. [DOI] [PubMed] [Google Scholar]
- 63.Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radical Biol Med. 2001;30:1390–1399. doi: 10.1016/S0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 64.Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.