Abstract
Introduction
To identify patient preference drivers related to the management of wet age-related macular degeneration (wet AMD).
Methods
In this cross-sectional study, a self-explicated ‘conjoint analysis’ survey was administered online to eligible patients with wet AMD (receiving anti-vascular endothelial growth factor [VEGF] treatment for at least 12 months) from the USA, Canada, UK, France, Spain, Germany, Italy, Japan, Taiwan, and Australia. The survey consisted of six domains with 21 attributes, which were selected on the basis of a literature review, social media listening, and tele-interviews/discussions with patients, clinical experts, and patient groups. Utility and relative importance scores were generated for each attribute and utility difference significance testing was performed using ‘unequal variances t tests’. The Patient Activation Measure (PAM-13) questionnaire was administered to assess patients’ knowledge, skill, and confidence in self-management.
Results
A total of 466 patients (mean age, 68 years; women, 54%; binocular wet AMD, 28%) with an average anti-VEGF treatment duration of 3.9 years completed the survey. The most important preference domains were ‘treatment effects on vision’ (non-significant) and ‘vision-related symptom burdens’ (p < 0.001), followed by ‘treatment risk’ (p < 0.05), ‘impact on daily activities’ (p < 0.05), ‘burden of clinic/hospital visits’ (p < 0.001), and ‘impact on psychological well-being’. The five most important attributes in order of importance were clarity of vision, treatment effect on symptoms, quality of vision, time to treatment effect, and time to re-administration. The two most important attributes globally were also in the top three attributes across countries. The majority of participants in the study were level 3 or level 4 of the PAM-13 questionnaire.
Conclusions
This study identified the most important disease and treatment attributes to patients using patient-centred methods. The data showed the degree of harmonization of preferences across geographies and that participants actively adopt behaviours required for improved treatment outcomes. The identified preference drivers may inform future clinical development.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02248-5.
Keywords: Conjoint survey, Neovascular age-related macular degeneration, Patient activation measure (PAM), Patient preference, Preference drivers, Treatment adherence
Key Summary Points
| Why carry out this study? |
| Patients with wet age-related macular degeneration (AMD) often experience compromised quality of life (QoL) due to visual impairment, treatment burden, and excessive dependence on caregiver support. Understanding patient preferences on disease and treatment attributes from a patient perspective may support shared treatment decision-making that can optimize patient outcomes, treatment adherence, and treatment satisfaction |
| In this context, we conducted (1) a conjoint analysis which aimed to evaluate patient preferences and (2) assessed patient activation using the patient activation measure (PAM)-13 in wet AMD across the USA, Canada, UK, France, Spain, Germany, Italy, Japan, Taiwan, and Australia |
| What was learnt from the study? |
| Results of this study have identified the most important/relevant disease and treatment attributes to patients using patient-centred methods. Attributes identified to be significant by participants included treatment effects on symptoms, clarity of vision, frequency of clinic/hospital visits, risk of eye damage due to the treatment, and impact of wet AMD on work and daily living |
| PAM-13 scores provided an insight into the participants’ level of activation. Results revealed that participants from most of the countries (except Japan) were able to play an active role in the management of wet AMD. Most participants showed level 3 or level 4 PAM scores, which was surprisingly high and may reflect a selective cohort that is already engaged in self-management |
Introduction
Age-related macular degeneration (AMD) is a vision-threatening disease with a global prevalence of over 196 million individuals in 2020, which is expected to reach 288 million individuals by 2040 [1]. If untreated, wet AMD may result in severe visual impairment or even blindness [2, 3]. Although wet AMD represents only 10–15% of all AMD cases, it is responsible for 80–90% of cases of severe vision loss in all individuals with AMD [4]. Epidemiological data suggest that over 10% of individuals who are at least 65 years of age and over 25% of individuals who are at least 75 years of age may develop wet AMD and lose their vision as a result [5, 6].
Anti-vascular endothelial growth factor (anti-VEGF) agents—ranibizumab, aflibercept, and brolucizumab—are the standard of care for patients with wet AMD, with several landmark clinical trials having demonstrated their therapeutic benefit [7–15]. Off-label usage of bevacizumab as an intravitreal agent for wet AMD has been reported in a few countries [16, 17]. Therapy with most anti-VEGF drugs is initiated with three loading doses administered monthly, typically followed by an individualised treatment protocol [18, 19]. Although anti-VEGF agents have shown substantial vision benefits in a large number of patients with wet AMD, the degree of vision gains reported in real-world studies is often less than that reported in clinical trials. Suboptimal vision improvements in real-world patients have been attributed, in part, to low adherence to therapy caused by several contributory factors, including infrequent visits to the clinic, out-of-pocket expenses, and other barriers to care (e.g. other comorbidities) [20].
Patients with wet AMD experience a negative impact on their quality of life (QoL) measures such as mobility, ability to use a computer, performance of daily activities, reading, driving, and even self-care [21]. In addition, medical factors such as duration of therapy, route of administration, number of hospital visits, and safety aspects have been shown to have an impact on patients’ QoL [22]. The use of patient preference studies in shared decision-making is increasingly being recognized by health technology assessment (HTA) bodies, and evidence from these studies may also play a role in clinical decision-making by helping to understand attributes related to benefits, risks, and administration [23, 24].
Recent evidence has shown that optimal patient activation can result in improvements in treatment adherence, patient outcomes, and care experience. Patient activation primarily focusses on evaluating the knowledge, skills, and confidence of a patient to self-manage health symptoms, engage in activities that maintain/enhance functioning, and to be an active participant in their own healthcare [25]. However, patient activation is an under-researched area in the medical management of wet AMD [25].
This study aimed to identify patient preference drivers (measured using preference scores on various attributes and levels) with respect to various aspects of wet AMD treatment via conjoint analysis across the USA, Canada, UK, France, Spain, Germany, Italy, Japan, Taiwan, and Australia. The secondary outcome was to estimate the proportion of patients with wet AMD and with different levels of patient activation using a 13-item patient activation measure (PAM), a unidimensional scale that provides an opportunity to reliably predict future healthcare professional (HCP) visits, hospital admissions and re-admissions, medication adherence, and to tailor the treatment and assess changes in treatment pattern [26].
Methods
Study Characteristics
This cross-sectional, non-interventional study employed a self-explicated conjoint survey and PAM-13 in the study participants to understand patient preferences for the treatment of wet AMD across the USA, Canada, UK, France, Spain, Germany, Italy, Japan, Taiwan, and Australia. Data were collected for the survey between 26 August 2019 and 4 September 2020.
Only participants who provided consent were included. Maintenance of data confidentiality was ensured and all participants were free to withdraw from the study at any point. This study was reviewed and approved by the Salus institutional review board (IRB) for all research countries, with the exception of Canada, where Veritas IRB provided ethics approval. For Taiwan, the study was exempted from ethics committee approval by the Research Ethics Committee of National Taiwan University. Additional local ethics approvals were received for research in Japan. This study was conducted in accordance with the declaration of Helsinki of 1964.
Eligibility Criteria
Participants who were 50 years of age or older, had a self-reported diagnosis of wet AMD at least 12 months prior to the study, and had received at least one intravitreal anti-VEGF agent for wet AMD in the past 6–12 months were included in the study. Participants who had received anti-VEGF therapy for diabetic retinopathy, diabetic macular edema, retinal vein occlusion, or myopic choroidal neovascularization were excluded. All participants who were direct employees of any pharmaceutical company or healthcare products manufacturer, or had family members who were currently direct employees of any pharmaceutical company or healthcare products manufacturer were excluded from the study.
Survey
Participants were selected by agencies specializing in patient recruitment, using their own patient panels or referral process. Participants were provided with detailed information regarding the survey before study initiation. A period of 6 weeks was provided to the participants to complete the survey. Approximately 60 min was estimated to be required for completion of the survey; however, the participants were not restricted to complete the task within 60 min. Participants were sent a reminder to complete the survey after 24 h of inactivity.
The study was conducted using an online platform. Recruitment agencies shared a link to the online questionnaire with all potentially eligible participants. Demographic characteristics, including age and gender, were collected through the survey questionnaire. In addition, laterality of the disease (one or both eyes), treatments received for wet AMD, and treatment setting related data were collected.
Qualitative Analyses: Significant Domains, Attributes, and Levels
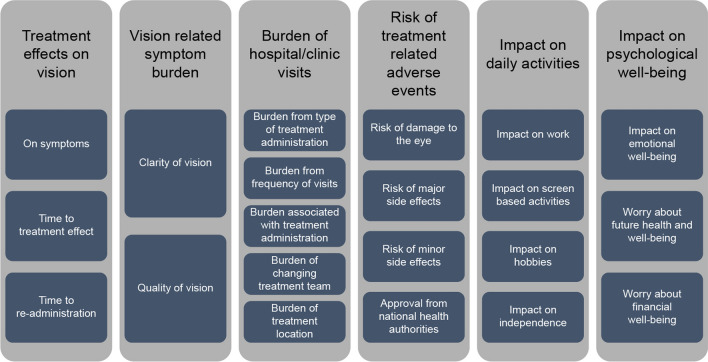
This self-explicated survey included six domains: (1) treatment effects, (2) vision-related symptom burden, (3) burden related to the treatment and clinic/hospital visits, (4) risk of treatment-related safety and tolerability issues, (5) impact of wet AMD on daily activities, and (6) impact of wet AMD on psychological well-being. These domains consisted of 21 attributes in total (Fig. 1). The domains and attributes were ranked on the basis of utility scores under each domain. The impacts of each domain and attribute under every domain were further analysed at a global level, and stratified by country, gender, and laterality. In addition, the most important attribute was identified by comparison within each domain. Further, each attribute consisted of up to six levels (level 1 representing the highest impact on activities and level 6 representing the least impact on activities). Participants were asked to select the most important level, and rank the others respective to it.
Fig. 1.
Domains and attributes
Domains were determined on the basis of a targeted literature review, social media listening, and discussions with clinical experts and patient group representatives. Confirmation of key domains/areas of importance driving patient treatment preferences was explored using telephone in-depth interviews with three participants per country including Australia, Canada, USA, and UK.
Statistical Analysis
The significance of differences in preference scores for domains, attributes, and attribute levels was tested using the “unequal variances t test”. Aggregation of individual participant preference data collected through the online survey was performed using Microsoft Excel® for calculation of preference scores and testing the significance in preference differences between attribute levels, attributes, and domains. Self-explicated conjoint analysis has lower standard errors at equivalent sample sizes to choice-based conjoint analysis (this is because indirect estimation of utilities is not required in the self-explicated approach). For a sample size of greater than 20, the standard error is estimated to be less than 5% using the self-explicated conjoint approach. However, a higher target sample size of at least 40 patients per country was used to provide added confidence in results.
Results
Study Participants
Overall, 466 participants were included: participants had a mean age of 68 years and over half of the participants were female (n = 250, 54%). In most participants (n = 335, 72%), unilateral vision was affected.
Treatment for Wet AMD
Overall, participants were receiving intravitreal wet AMD therapy for a mean (standard deviation [SD]) of 3.9 (10) years. Participants received ranibizumab (37%), aflibercept (33%), bevacizumab (23%), or other anti-VEGF agents (7%), and most participants received their treatment in an outpatient setting consisting of a large practice with at least six ophthalmologists (62%, Table 1).
Table 1.
Treatment received by patients with wet AMD and treatment settings
| Participants n = 466 (%) | |
|---|---|
| Treatment name | |
| Bevacizumab | 23 |
| Aflibercept | 33 |
| Ranibizumab | 37 |
| Other anti-VEGF agents | 7 |
| Treatment setting | |
| Outpatient–large practice with ≥ 6 ophthalmologists | 62 |
| Outpatient–small practice with ≤ 5 ophthalmologists | 18 |
| Outpatient clinic with 1 ophthalmologist | 5 |
| Mobile ophthalmology clinics | < 1 |
| Hospital/academic/university centre | 14 |
Preference Drivers of Patients with Wet AMD
Aggregated Overall and Country Results
Of all the domains included in the study, treatment effects, vision-related symptom burden, and risk of treatment-related issues were the most important domains, globally and in all included countries. In Germany, psychological burdens were considered significant; however, in the USA and France, impact on daily activities was one of the most important attributes in addition to vision-related symptom burden and treatment effects among the participants with wet AMD. In Canada and Australia, burden of hospital visits was a significant attribute. A similar trend was observed in participants of both genders and laterality.
Treatment Effect
The three attributes within the domain “treatment effect” were treatment effect on symptoms, time to treatment effect, and time to re-administration. Treatment effect on symptoms was the most important attribute followed by time to treatment effect and time to re-administration, across all the countries, both genders, and laterality. Treatment effect on symptoms was not statistically significant as compared with time to treatment effect in participants across Australia, France, and the UK. In Japan and the USA, treatment effect on symptoms was statistically significant compared with time to treatment effect. Time to re-administration was a significant attribute across all the included countries (Fig. 2 and Fig. S1 in the supplementary material).
Fig. 2.
Global and country-specific treatment effect. ns non-significant
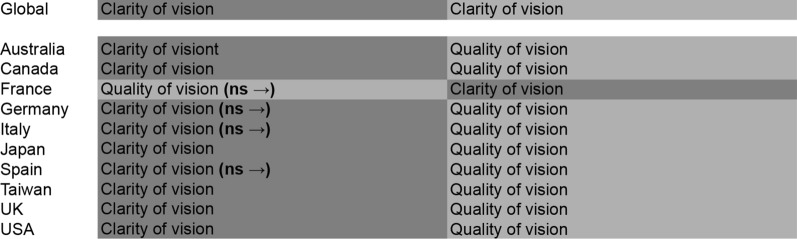
Vision-Related Symptom Burden
Clarity of vision and quality of vision were the attributes included in this domain (vision-related symptom burden). Clarity of vision was a significant attribute in Australia, Canada, Japan, Taiwan, the UK, and the USA, both genders, and laterality, whereas quality of vision was a significant attribute across all countries (Fig. 3 and Fig. S2 in the supplementary material).
Fig. 3.
Global and country-specific vision-related symptom burden. ns non-significant
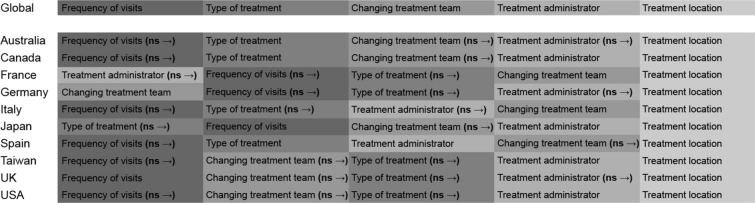
Burden of Clinic or Hospital Visits
Attributes included within the domain “burden of clinic or hospital visits” were frequency of visits, type of treatment, changing treatment team, treatment provider (i.e. nurse or doctor at the treatment facility), and treatment location. Participants across all the countries ranked the treatment location to be the most important attribute within this domain. Frequency of visits to clinics/hospitals was one of the most significant attributes within this domain across most included countries (except Germany and the UK), both genders, and laterality. Among participants from Japan, type of treatment administration was the most important attribute, whereas in Germany, burden of changing the treatment team was a significant attribute to the participants. In France, the burden associated with treatment provider was considered significant (Fig. 4 and Fig. S3 in the supplementary material).
Fig. 4.
Global and country-specific burden of clinic or hospital visits. ns non-significant
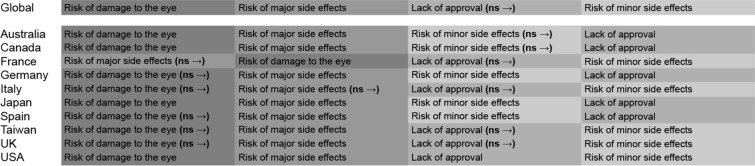
Risk of Treatment-Related Safety and Tolerability Issues
Attributes included in the domain “risk of treatment-related safety and tolerability issues” were risk of damage to the treated eye, risk of major side effects immediately after treatment (requiring hospitalization), risk of minor side effects immediately during/after treatment (not requiring hospitalization), and lack of approval from national health authorities for treating wet AMD. Among participants in all the countries except France, risk of eye damage was the most troublesome attribute within this domain. Participants in France considered risk of major side effects immediately after treatment as the most important attribute. Participants in Japan, Germany, and Spain perceived the risk of minor side effects immediately during/after treatment to be more significant than lack of approval from national authorities for treating wet AMD, whereas participants in all other countries perceived these two effects to be similar in burden and the differences were not statistically significant (Fig. 5). Of the four attributes in this domain, risk of damage to the treated eye was the most important attribute, irrespective of the gender and laterality (Fig. S4 and in the supplementary material).
Fig. 5.
Global and country-specific risk of treatment-related safety and tolerability issues or events. ns non-significant
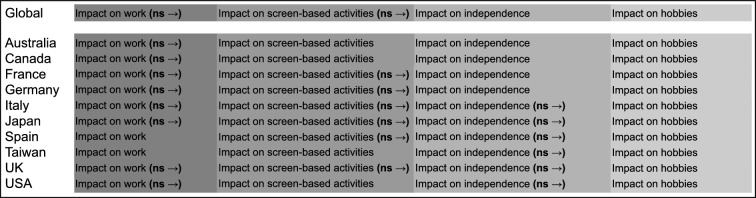
Impact of Wet AMD on Daily Activities
In the domain “impact of wet AMD on daily activities”, the four attributes included were impact on work, impact on screen-based activities, impact on hobbies, and impact on independence. Participants across all the countries, both genders, and laterality ranked impact on work as the most significant attribute followed by impact on screen activities, impact on independence, and lastly impact on hobbies. The difference in impact on work and impact on screen-based activities was not significant across most included countries; similarly, difference between impact on screen activities and impact on independence was not significant in 60% of the countries (Fig. 6 and Fig. S5 in the supplementary material).
Fig. 6.
Global and country-specific impact on daily activities. ns non-significant
Impact of Wet AMD on Psychological Well-Being
The three attributes included in the domain “impact of wet AMD on psychological well-being” were worry about future health and well-being, impact of wet AMD on emotional well-being, and worry about financial well-being. Worry about future health and well-being was the most important attribute globally, and across both genders, and laterality (Fig. 7 and Fig. S6 in the supplementary material).
Fig. 7.
Global and country-specific impact on psychological well-being. ns non-significant
Patient Activation Measure (PAM)
PAM-13 analysis of patient activation showed the proportion of patients with level 1 (n = 116, 25%), level 2 (n = 78, 17%), level 3 (n = 217, 47%), and level 4 (n = 46, 10%) PAM globally. The majority of participants from Taiwan (n = 45, 75%), USA (n = 43, 62%), the UK (n = 22, 56%), Canada (n = 23, 51%), and Australia (n = 21, 51%) were level 3 PAM (Table 2). However, a significant proportion of participants included in the study from Japan (n = 31, 70%) were in level 1 PAM.
Table 2.
Global and country-specific Patient Activation Measure-13 (PAM) results
| Levels | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| Total (%) | ||||
| Overall n | 116 (25.44) | 78 (17.11) | 217 (47.59) | 46 (10.09) |
| Female n | 51 (24.76) | 31 (15.05) | 103 (50.00) | 22 (10.68) |
| Male n | 65 (26.00) | 47 (18.80) | 114 (45.60) | 24 (9.60) |
| Australia (%) | ||||
| Overall n | 11 (26.83) | 5 (12.20) | 21 (51.22) | 4 (9.76) |
| Female n | 4 (19.05) | 1 (4.76) | 12 (57.14) | 4 (19.05) |
| Male n | 7 (35.00) | 4 (20.00) | 9 (45.00) | 0 |
| Canada (%) | ||||
| Overall n | 5 (11.11) | 9 (20.00) | 23 (51.11) | 8 (17.78) |
| Female n | 2 (6.90) | 8 (27.59) | 14 (48.28) | 5 (17.24) |
| Male n | 3 (18.75) | 1 (6.25) | 9 (56.25) | 3 (18.75) |
| Germany (%) | ||||
| Overall n | 12 (29.27) | 4 (9.76) | 19 (46.34) | 6 (14.63) |
| Female n | 5 (26.32) | 0 | 11 (57.89) | 3 (15.79) |
| Male n | 7 (31.82) | 4 (18.18) | 8 (36.36) | 3 (13.64) |
| Spain % | ||||
| Overall n | 14 (35.00) | 9 (22.50) | 11 (27.50) | 6 (15.00) |
| Female n | 5 (35.71) | 4 (28.57) | 2 (14.29) | 3 (21.43) |
| Male n | 9 (34.62) | 5 (19.23) | 9 (34.62) | 3 (11.54) |
| France (%) | ||||
| Overall n | 15 (37.50) | 8 (20.00) | 15 (37.50) | 2 (5.00) |
| Female n | 4 (26.67) | 2 (13.33) | 8 (53.33) | 1 (6.67) |
| Male n | 11 (44.00) | 6 (24.00) | 7 (28.00) | 1 (4.00) |
| Italy (%) | ||||
| Overall n | 14 (36.84) | 10 (26.32) | 12 (31.58) | 2 (5.26) |
| Female n | 9 (50.00) | 3 (16.67) | 6 (33.33) | 0 |
| Male n | 5 (25.00) | 7 (35.00) | 6 (30.00) | 2 (10.00) |
| Japan (%) | ||||
| Overall n | 31 (70.45) | 7 (15.91) | 6 (13.64) | 0 |
| Female n | 20 (68.97) | 5 (17.24) | 4 (13.79) | 0 |
| Male n | 11 (73.33) | 2 (13.33) | 2 (13.33) | 0 |
| Taiwan (%) | ||||
| Overall n | 1 (1.67) | 9 (15.00) | 45 (75.00) | 5 (8.33) |
| Female n | 0 | 4 (13.79) | 24 (82.76) | 1 (3.45) |
| Male n | 1 (3.23) | 5 (16.13) | 21 (67.74) | 4 (12.90) |
| United Kingdom (%) | ||||
| Overall n | 9 (23.08) | 4 (10.26) | 22 (56.41) | 4 (10.26) |
| Female n | 2 (15.38) | 1 (7.69) | 8 (61.54) | 2 (15.38) |
| Male n | 7 (26.92) | 3 (11.54) | 14 (53.85) | 2 (7.69) |
| USA (%) | ||||
| Overall n | 4 (5.80) | 13 (18.84) | 43 (62.32) | 9 (13.04) |
| Female n | 0 | 3 (15.00) | 14 (70.00) | 3 (15.00) |
| Male n | 4 (8.16) | 10 (20.41) | 29 (59.18) | 6 (12.24) |
Level 1, strongly disagree; level 4, strongly agree
Discussion
An understanding of patient preferences has been shown to improve quality of care [27]. However, identifying and defining the relevant patient preferences is often complex. The modifiability of preferences varies with patient characteristics and health conditions [28]. Patient preference studies conducted in wet AMD have suggested that preferences varied in patients according to their overall health status; however, a better acceptance of therapy and improved treatment adherence have been observed when patients were better informed about their therapeutic options [29–31]. This conjoint survey assessed preferences in patients with wet AMD and measured the level of patient activation in the self-management of wet AMD using PAM-13. Patient preferences across the ten countries included in the study did not vary significantly. Results showed that participants were willing to make trade-offs for improved treatment outcomes and engage themselves in shared decision-making for improved QoL.
Participants ranked the treatment effect on symptoms of wet AMD as the most important attribute, followed by time to treatment effect, and time to re-administration in the domain “treatment effects on vision”. Symptoms of wet AMD severely impact vision, making symptom alleviation one of the most important attributes among patients [32]. Most participants in this study were concerned about the frequency of clinic/hospital visits. Baxter et al. reported similar preferences in German patients with wet AMD, where participants preferred fewer visits to the hospital [29]. Similar to the preference expressed by participants of this study, Baxter et al. reported that participants were interested in being informed about the type of treatment they received and the treatment administrator [29].
Similar to the results of this study, other patient preference studies have reported long-term treatment outcomes and reduction in the frequency of clinic/hospital visits as important attributes among patients being treated for wet AMD [30, 31]. In most patients with wet AMD, compromised vision-related functioning, high dependency on caregivers, and diminished QoL increase the psychological burden and negatively impact patients’ emotional well-being [30, 33, 34]. Participants included in this study were worried about future health and well-being, impact of wet AMD on emotional well-being, and their financial well-being.
The PAM-13 questionnaire revealed that the majority of participants included in the study were level 3 or level 4 of the PAM, indicating that participants were taking action and pushing further to take charge of their health. The participant samples from the countries included in this study were therefore efficient in self-management, which was in contrast to a prior study of patient activation levels in frail older adults [35]. However, compared with other countries in this survey, Japan had the highest proportion of participants with level 1 PAM (70.45%), implying that participants from Japan were not as engaged in self-management of wet AMD, possibly as a result of cultural differences [26, 36]. Self-rated health and PAM have a significant linear relationship, specifically in patients with chronic conditions [37, 38]. In addition to tailoring treatment for patients, a higher self-reported PAM score is associated with medication adherence, self-management approach of the patients, and improved QoL [39]. Patient activation allows patients to self-manage their condition and promotes better quality and effectiveness of healthcare [40].
Limitations
This study had several limitations. The study sample majorly represented those individuals who had access to a computer and the internet; however, this limitation was adequately acknowledged in the study. Most participants included in the study were elderly (mean age 68 years); nevertheless, the participants included in this study were younger than typically observed in other studies on patients with wet AMD. Remillard and his colleagues reported that the elderly were becoming more comfortable participating in online surveys [41], which likely lessened the impact of this limitation.
The study included only those participants who were willing to participate; thus, the perceptions of those who were not willing to participate were not captured. Use of convenience sampling via patient group or doctoral referral may have presented selection bias, also in relation to patient activation status. Participants with high impairment in both eyes may have been less likely to participate and complete the survey, inferring that the study might possibly have included participants with mild to moderate visual impairment only. Although the study relied on self-reported diagnosis from participants recruited via patient panels rather than cross-validation of patient charts, use of comprehensive screening questions and recruitment through doctor referrals reduced the impact of this limitation.
Conclusion
Management of wet AMD has a significant impact on patients and caregivers. Optimizing wet AMD treatment according to patient preference may bring an improvement in treatment outcomes and overall adherence to therapy. The results of this study have identified the most important/relevant treatment attributes to patients using patient-centred methods. Participants considered treatment effect on symptoms, clarity of vision, treatment location, frequency of visits, and risk of eye damage as important attributes. Further, most participants were worried about future health and well-being and the impact of wet AMD on work. The use of relevant preference drivers identified through robust qualitative methods provided a clear understanding regarding the attributes important to participants included in the study. Although abundant evidence of patient preference studies is available, this study estimated both PAM and patient preference. Participants from most included countries showed PAM scores of level 3 or 4, implying that they actively engage in the management of their health, adopting behaviours required for improved treatment outcomes. The results of this study may further help inform future clinical trial development and discussions with health technology assessment (HTA) agencies on wet AMD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by Novartis Pharma AG, Basel, Switzerland. Novartis has also funded the journal’s Rapid Service and Open Access Fees.
Medical Writing Assistance
The authors acknowledge Gurpreet Kaur Virya for medical writing assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Adrian Skelly, Nicholas Taylor, Christina Fasser, Jean-Pierre Malkowski, Pushpendra Goswami, and Louise Downey. Acquisition, analysis, or interpretation of data: Adrian Skelly, Nicholas Taylor, Christina Fasser, Jean-Pierre Malkowski, Pushpendra Goswami, and Louise Downey. Drafting of the manuscript and review: Adrian Skelly, Nicholas Taylor, Christina Fasser, Jean-Pierre Malkowski, Pushpendra Goswami, and Louise Downey. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosures
Adrian Skelly, Jean-Pierre Malkowski, and Pushpendra Goswami are employees of Novartis Pharma AG, Basel, Switzerland. Nicolas Taylor reports participation in Inpharmation, Stokenchurch, UK. Christina Fasser reports participation in Retinal International, Zurich, Switzerland. Louise Downey reports participation in Hull Royal Infirmary, Hull, UK.
Prior Presentation
This manuscript is based on the data that was presented at ISPOR, 2022 (Skelly, A et al. POSB431 Patient Preferences in the Management of Wet Age-Related Macular Degeneration: A Conjoint Analysis. Value in Health, Volume 25, Issue 1, S235–S236).
Compliance with Ethics Guidelines
This study was reviewed and approved by the Salus institutional review board (IRB) for all research countries, with the exception of Canada, where Veritas IRB provided ethics approval. For Taiwan, the study was exempted from ethics committee approval by the Research Ethics Committee of National Taiwan University. Additional local ethics approvals were received for research in Japan. This study was conducted in accordance with the declaration of Helsinki of 1964.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
The original online version of this article was revised due to update in fourth author name.
Change history
11/30/2022
A Correction to this paper has been published: 10.1007/s12325-022-02381-1
References
- 1.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017;6:493–497. doi: 10.22608/APO.2017251. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mekjavić PJ, Balčiūnienė VJ, Ćeklić L, et al. The burden of macular diseases in central and Eastern Europe-Øimplications for healthcare systems. Value Health Reg Issues. 2019;19:1–6. doi: 10.1016/j.vhri.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Pedrosa AC, Sousa T, Pinheiro-Costa J, et al. Treatment of neovascular age-related macular degeneration with anti-VEGF agents: predictive factors of long-term visual outcomes. J Ophthalmol. 2017;2017:4263017. doi: 10.1155/2017/4263017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The incidence and progression of age-related macular degeneration over 15 years: the blue mountains eye study. Ophthalmology. 2015;122:2482–9. doi: 10.1016/j.ophtha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Stahl A. The diagnosis and treatment of age-related macular degeneration. Dtsch Arztebl Int. 2020;117:513–520. doi: 10.3238/arztebl.2020.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(57–65):e5. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Shah SM, Browning DJ, et al. A phase I study of intravitreal vascular endothelial growth factor trap-eye in patients with neovascular age-related macular degeneration. Ophthalmology. 2009;116(2141–8):e1. doi: 10.1016/j.ophtha.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Heier JS, Ciulla T, et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology. 2011;118:1089–97. doi: 10.1016/j.ophtha.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Aggarwal K, Gupta V. Management of neovascular age-related macular degeneration: a review on landmark randomized controlled trials. Middle East Afr J Ophthalmol. 2016;23:27–37. doi: 10.4103/0974-9233.173133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128:89–99. doi: 10.1016/j.ophtha.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Costa RA, Jorge R, Calucci D, Cardillo JA, Melo LAS, Jr, Scott IU. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–78. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- 17.Tufail A, Patel PJ, Egan C, et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ. 2010;340:c2459. doi: 10.1136/bmj.c2459. [DOI] [PubMed] [Google Scholar]
- 18.Holekamp NM. Review of neovascular age-related macular degeneration treatment options. Am J Manag Care. 2019;25:S172–S181. [PubMed] [Google Scholar]
- 19.Kovach JL, Schwartz SG, Flynn HW, Jr, Scott IU. Anti-VEGF treatment strategies for wet AMD. J Ophthalmol. 2012;2012:786870. doi: 10.1155/2012/786870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu J, Regillo CD. Poorer outcomes in real-world studies of anti–vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Ophthalmology. 2020;127:1189–1190. doi: 10.1016/j.ophtha.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Taylor DJ, Hobby AE, Binns AM, Crabb DP. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open. 2016;6:e011504. doi: 10.1136/bmjopen-2016-011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatziralli I, Mitropoulos P, Parikakis E, Niakas D, Labiris G. Risk factors for poor quality of life among patients with age-related macular degeneration. Semin Ophthalmol. 2017;32:772–80. doi: 10.1080/08820538.2016.1181192. [DOI] [PubMed] [Google Scholar]
- 23.van Overbeeke E, Janssens R, Whichello C. Design, conduct, and use of patient preference studies in the medical product life cycle: a multi-method study. Front Pharmacol. 2019;10:1395. doi: 10.3389/fphar.2019.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouvy JC, Cowie L, Lovett R, Morrison D, Livingstone H, Crabb N. Use of patient preference studies in HTA decision making: a nice perspective. Patient. 2020;13:145–9. doi: 10.1007/s40271-019-00408-4. [DOI] [PubMed] [Google Scholar]
- 25.Morse AR, Seiple W. Activation in individuals with vision loss. J Health Psychol. 2020:1359105320922303. [DOI] [PubMed]
- 26.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–26. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Say RE, Thomson R. The importance of patient preferences in treatment decisions–challenges for doctors. BMJ. 2003;327:542–545. doi: 10.1136/bmj.327.7414.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Street RL Jr, Elwyn G, Epstein RM. Patient preferences and healthcare outcomes: an ecological perspective. Expert Rev Pharmacoecon Outcomes Res. 2012;12:167–80. [DOI] [PubMed]
- 29.Baxter JM, Fotheringham AJ, Foss AJ. Determining patient preferences in the management of neovascular age-related macular degeneration: a conjoint analysis. Eye (Lond) 2016;30:698–704. doi: 10.1038/eye.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joko T, Nagai Y, Mori R. Patient preferences for anti-vascular endothelial growth factor treatment for wet age-related macular degeneration in japan: a discrete choice experiment. Patient Prefer Adherence. 2020;14:553–67. doi: 10.2147/PPA.S228890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller S, Agostini H, Ehlken C, Bauer-Steinhusen U, Hasanbasic Z, Wilke T. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016;123:876–83. doi: 10.1016/j.ophtha.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Skelly A, Taylor N, Banhazi J, Okede C. PSS62 - Treatment preference drivers of patients with neovascular age-related macular degeneration. Value Health. 2018;21:S433.
- 33.Soubrane G, Cruess A, Lotery A, et al. Burden and health care resource utilization in neovascular age-related macular degeneration: findings of a multicountry study. Arch Ophthalmol. 2007;125:1249–54. doi: 10.1001/archopht.125.9.1249. [DOI] [PubMed] [Google Scholar]
- 34.Cimarolli VR, Casten RJ, Rovner BW, Heyl V, Sörensen S, Horowitz A. Anxiety and depression in patients with advanced macular degeneration: current perspectives. Clin Ophthalmol. 2016;10:55–63. doi: 10.2147/OPTH.S80489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera-Almaraz A, Manrique-Espinoza B, Ávila-Funes JA. Disability, quality of life and all-cause mortality in older Mexican adults: association with multimorbidity and frailty. BMC Geriatr. 2018;18:236. doi: 10.1186/s12877-018-0928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gimbel R, Shi L, Williams JE, Dye CJ, et al. Enhancing mHealth technology in the patient-centered medical home environment to activate patients with type 2 diabetes: a multisite feasibility study protocol. JMIR Res Protoc. 2017;6:e38. doi: 10.2196/resprot.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tusa N, Kautiainen H, Elfving P, Sinikallio S, Mäntyselkä P. Relationship between patient activation measurement and self-rated health in patients with chronic diseases. BMC Fam Pract. 2020;21:225. doi: 10.1186/s12875-020-01301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinney RL, Lemon SC, Person SD, Pagoto SL, Saczynski JS. The association between patient activation and medication adherence, hospitalization, and emergency room utilization in patients with chronic illnesses: a systematic review. Patient Educ Couns. 2015;98:545–52. doi: 10.1016/j.pec.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Gimbel RW, Rennert LM, Crawford P, et al. Enhancing patient activation and self-management activities in patients with type 2 diabetes using the US department of defense mobile health care environment: feasibility study. J Med Internet Res. 2020;22:e17968. doi: 10.2196/17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graffigna G, Barello S, Bonanomi A. The role of Patient Health Engagement Model (PHE-model) in affecting patient activation and medication adherence: a structural equation model. PLoS ONE. 2017;12:e0179865. doi: 10.1371/journal.pone.0179865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remillard ML, Mazor KM, Cutrona SL, Gurwitz JH, Tjia J. Systematic review of the use of online questionnaires of older adults. J Am Geriatr Soc. 2014;62:696–705. doi: 10.1111/jgs.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.