Summary
Fifteen percent of couples of reproductive age suffer from infertility globally and the burden of infertility disproportionately impacts residents of developing countries. Assisted reproductive technologies (ARTs), including in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), have been successful in overcoming various reasons for infertility including borderline and severe male factor infertility which consists of 20%–30% of all infertile cases. Approximately half of male infertility cases stem from suboptimal sperm parameters. Therefore, healthy/normal sperm enrichment and sorting remains crucial in advancing reproductive medicine. Microfluidic technologies have emerged as promising tools to develop in-home rapid fertility tests and point-of-care (POC) diagnostic tools. Here, we review advancements in fabrication methods for paper-based microfluidic devices and their emerging fertility testing applications assessing sperm concentration, sperm motility, sperm DNA analysis, and other sperm functionalities, and provide a glimpse into future directions for paper-based fertility microfluidic systems.
Subject areas: Medical device in health technology, Biotechnology, Biodevices
Graphical abstract

Highlights
-
•
Paper-based technologies are emerging to develop in-home rapid fertility tests
-
•
Fabrication methods for paper-based microfluidic devices are presented
-
•
Emerging disposable paper-based fertility testing applications are reviewed
Medical device in health technology; Biotechnology; Biodevices;
Introduction
It is estimated that infertility affects more than 186 million people or 8%–12% of couples globally, differentially impacting residents of developing countries (Vander Borght and Wyns, 2018; Kumar and Singh, 2015). Moreover, female infertility has increased 15% and male infertility has increased 8.2% globally between 1990 and 2017 (Sun et al., 2019). Infertility has been attributed to several mechanisms including ovulatory dysfunction, tubal and uterine abnormalities, and congenital or acquired conditions impacting sperm (Santiago Brugo-Olmedo et al., 2001). Delayed childbearing age, sexually transmitted diseases (STDs), pregnancy related-infections or unsafe abortions, increasing rate of obesity, exposure to environmental contaminants, alterations in semen quality due to preventable factors, and lifestyle choices such as smoking and alcohol consumption could account for the increase in infertility over the past three decades (Thoma et al., 2021; Broughton and Moley, 2017).
Significant advancements in assisted reproductive technologies (ARTs) have been achieved and over eight million babies have been born using ARTs such as in vitro fertilization (IVF), intracytoplasmic sperm injections (ICSI), embryo cryopreservation, as well as oocyte-freezing (Maryam Okhovati et al., 2015; Crawford and Ledger, 2019). Preimplantation genetic testing (PGT) for aneuploidy (PGT-A), monogenic disease (PGT-M), and translocations (PGT-SR) has provided couples the opportunity to determine and overcome the genetic causes of infertility. IVF and ICSI have also been used to overcome tubal factor infertility, caused by fallopian tube complications, as well as severe male factor infertility, caused by low sperm motility, quality, or quantity (Thapa and Heo, 2019). Furthermore, it is predicted that suboptimal sperm parameters account for 40%–50% of all male infertility cases, indicating that healthy sperm sorting is crucial to further advance ARTs and reproductive medicine (Kumar and Singh, 2015). According to the World Health Organization (WHO), semen analysis is conducted based on seven parameters: volume, sperm concentration, total sperm number, morphology, vitality, progressive motility, and total motility (in both aspects of progressive and nonprogressive) (Kumar and Singh, 2015). While conventional methods for semen analysis, including chamber counting, computer-assisted sperm analysis (CASA), and survival tests, provide accurate results, they remain time-consuming, expensive, and dependent on trained healthcare workers (Nosrati et al., 2017). The use of microfluidic technologies for sperm sorting has therefore emerged as a cost-effective alternative to traditional methods (Knowlton et al., 2015; Chiu et al., 2017; Dabbagh et al., 2020). Owing to the versatile applications and ability to perform efficient analysis using small sample volumes, microfluidic technologies have also been utilized to develop in-home rapid fertility tests and point-of-care (POC) diagnostic tools (Sarabi et al., 2021a, 2021b, 2022; Rahmani Dabbagh et al., 2022; Rezapour Sarabi et al., 2022b; Tasoglu, 2022; Tasoglu et al., 2015; Luo et al., 2015; Amin et al., 2016).
Paper has appeared as a promising material for fabrication of microfluidics due to its high availability along with its unique properties including high surface-to-volume ratio, hydrophilicity, biological inertness, fiber-network, and ability to allow for capillary action (Cate et al., 2015; Temirel et al., 2021; Dabbagh et al., 2021a; Ghaderinezhad et al., 2020). Through selective processing of paper and generating hydrophilic and hydrophobic sections or channels using various fabrication techniques, including cutting (Li et al., 2008), photolithography (Lu et al., 2010), printing (Lu et al., 2009), and etching (Henares et al., 2017), paper-based microfluidic devices have manipulated small volumes of fluid and provided high-resolution detection and sorting, ease of mass production, simplified application, or high speed (Nishat et al., 2021). These devices have therefore recently emerged as rapid, POC diagnostics for male fertility. Moreover, to provide efficient, accurate, and affordable alternatives, several tests have been engineered to detect sperm concentration and assess sperm DNA analysis as well as other sperm functionalities. The widespread use of paper-based microfluidic tests in healthcare and diagnostics however has been challenged by a number of limiting factors. The lack of uniformity in paper has remained as a drawback for its use in paper-based devices since it can lead to indeterminate errors, lower precision, and/or variable distribution of reactants involved (Nishat et al., 2021). Inadequate and inefficient flow control in paper-based microfluidic devices causes another limitation for their rapid commercialization which often requires expensive equipment (Akyazi et al., 2018), or rather complicated techniques such as stacking papers together with optimization of gap between layers along with changing fluid strip dimensions (Trinh et al., 2022). Despite limitations in commercialization, paper-based microfluidics remain promising and offer significant advantages over traditional fabrication materials as these systems are low-cost, disposable, biodegradable, and simple to use (Li et al., 2012; Amin et al., 2017; Ghaderinezhad et al., 2017; Lepowsky et al., 2017). Here, we review materials and fabrication methods used in the development of paper-based microfluidic devices and their emerging applications in male fertility testing (Figure 1). The functionality of paper-based microfluidic setups for fertility diagnosis can be based on sperm concentration and motility, sperm DNA analysis, and other sperm functionalities, which are reviewed in detail. Finally, the future direction for paper-based fertility microfluidic setups is discussed, illuminating what next studies can focus on.
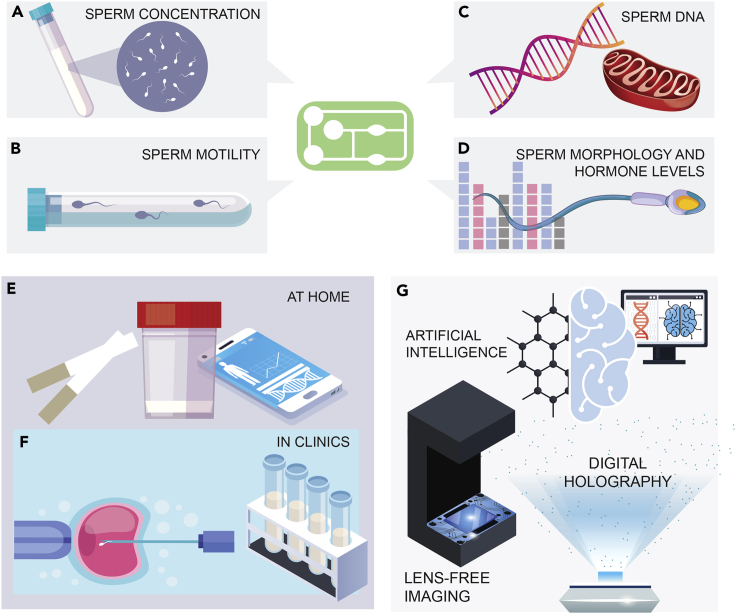
Figure 1.
An overview of disposable microfluidics for fertility testing
Applications of paper-based microfluidic devices in male fertility can be enabled by tests based on (A) sperm concentration, (B) sperm motility, (C) sperm DNA or sperm mitochondrial DNA analysis, and (D) sperm morphology and fertility-related hormones.
(E and D) Use of paper-based microfluidic technologies has emerged at home or in clinics for male fertility testing and/or sperm sorting.
(E) Convenient, inexpensive, effective in-home male fertility testing using small sample volumes. Ease of application through fast analysis, potentially using smartphones.
(F) Use of microfluidics for sperm sorting for better outcomes in assisted reproductive technologies (ARTs) such as intra-cytoplasmic sperm injections (ICSI) and/or point-of-care male sperm fertility testing with small sample volumes.
(G) Potential integration of artificial intelligence (AI), lens-free imaging, or digital holography in future applications to achieve higher quality and accuracy in microfluidics-based fertility tests.
Materials and fabrication methods
Materials
The first introduction of paper as a potential material for microfluidics was in 1949 (Müller and Clegg, 1949), when a heated dye was utilized to impregnate a filter paper with a wax barrier, aiming to create a restricted flowing channel to minimize the amount of used sample. However, it was not until 2007 that the basic terms for paper-based microfluidics were coined by proposing a paper-based protein-glucose assay patterned using lithography (Martinez et al., 2007). Since then, paper-based microfluidics have attracted much interest for the many advantages paper offers compared to traditional microfluidic tests such as creeping flow (Bandalusena et al., 2010), inertial microfluidics (Di Carlo, 2009), and droplet microfluidics (Sohrabi et al., 2020). The advantageous unique properties of paper emerge from its porous and fibrous structure, allowing capillary action that enables sample flow without the need for any external pumping force (Noh and Phillips, 2010; Yetisen et al., 2013; Lu et al., 2010). As a hydrophilic material, patterning paper with hydrophobic materials allows the formation of confined channels and chambers, which enables guided and controllable sample flow (Akyazi et al., 2018). The fibrous nature of paper prevents bubble formation, a problem seen often in traditional microfluidic channels (Osborn et al., 2010; Kokalj et al., 2014; Cummins et al., 2017). The hydrophilicity of paper allows storage of reagents for long periods. In addition, the large surface area of paper compared to its volume allows storage of reagents and uses only a small amount of the sample, such as a drop of blood or saliva, which reduces the cost significantly. The large area of paper also serves in reducing the assay time since more reagents can be immobilized (Akyazi et al., 2018). Furthermore, the network structure of paper enables sample filtration (Jagadeesan et al., 2012) and its good distribution is advantageous, empowering users to perform different assays simultaneously or different copies of the same assay for accuracy validation (Yetisen et al., 2013; Lu et al., 2010). In addition, the disposability of paper eliminates hazardous waste accumulation. Paper also provides thermal and chemical stability, since cellulose is insoluble in organic solvents. Moreover, paper manufacturing is cost-efficient as its raw materials are highly abundant in nature (Haigler, 1990).
In addition to being biodegradable, cellulose, the main component of paper, is insoluble in most solvents, especially water (Hillscher et al., 2021). However, one of its limitations is observed in assays that involve protein immobilization, where cellulose does not offer the required locomotion. Therefore, a synthesized derivative of cellulose called nitrocellulose was introduced to overcome this limitation. Nitrocellulose is made of cellulose nitrate, which offers high protein binding, and has a unique 3D structure with pores that facilitate protein immobilization directly (Lu et al., 2010), with no extra steps for substrate modification. Nitrocellulose has been utilized in dot enzyme-linked immunosorbent assay (Dot-ELISA) (Lazarovits et al., 1987), gold nanoparticles assays (Lisa et al., 2009), and Western blotting (Han et al., 2008) to name a few. Since the structure of nitrocellulose contains small uniform pores, the penetration of wax in the baking stage of manufacturing is slowed down, providing more time to control the pattern in comparison with pure cellulose, as well as allowing fabrication of high-resolution patterns with microchannels of 0.1 mm width. Even though pores in nitrocellulose are small, they are still effective in the filtration of contaminants. Nitrocellulose also allows better sample flow due to its high smoothness and uniform pore distribution (Lu et al., 2010). Compared to traditional hydrophilic paper, nitrocellulose is hydrophobic, requiring deposition of surfactants in order to be utilized in microfluidic applications. Other alternative materials in this regard include polyvinylidene fluoride, nylon, and Whatman Fusion 5; however, due to their less cost efficiency, special manufacturing requirements, and lower signal-to-noise ratio, nitrocellulose has secured its place as the most favorable material for microfluidic applications (Yetisen et al., 2013; Einav et al., 2008).
Fabrication methods
The fabrication techniques of paper microfluidics involve making parts of the paper hydrophilic for the sample to flow smoothly, while other parts are made hydrophobic to form the walls of the channels. In general, fabrication methods are divided into two categories: chemical methods in which the hydrophilic paper is treated with hydrophobic materials to form the required channels, and physical methods where the paper is cut with a variety of tools such as knives or lasers to create the channel pattern. Based on the materials and tools used for fabrication, each method has its advantages and disadvantages in terms of resolution, cost, time required to fabricate, and suitability for mass production. The fabrication methods are organized in Table 1, along with the materials and tools required for each of them, as well as their advantages and disadvantages.
Table 1.
Comparison of fabrication methods of paper microfluidics
| Fabrication Method | Required Equipment and Materials | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Photolithography | UV light, hot plate, photomask, SU8 photoresist, propanol, propylene glycol monomethyl ether acetate, oxygen plasma. | Provides a high resolution (about 200 μm) | Requires multiple steps and expensive equipment. Materials can cause the channels to be contaminated. | (Martinez et al., 2007; Martinez et al., 2008) |
| Wax Printing | Wax printer, hot plate, wax. | Cost-efficient, suitable for mass production, requires few steps. | Provides low resolution (550 μm), cannot withstand high temperatures. | (Lu et al., 2009) (Younas et al., 2019; Songjaroen et al., 2011) |
| Screen Printing | Laser printer, metallic mask, transparency film, hot plate, wax. | Cost-efficient, simple process. | Provides low resolution, requires the production of new screens for different patterns. | (Dungchai et al., 2011) |
| Plotting | Modified X-Y plotter, polydimethylsiloxane (PDMS) | Cost-efficient, rapid, allows formation of 3D structures due to flexibility. | Provides low resolution (about 1 mm) | (Bruzewicz et al., 2008) |
| Inkjet Printing | Modified inkjet printer, hot plate, alkyl ketene dimer (AKD), heptane. | Cost-efficient. | Requires multiple steps, uses hazardous organic solvents. | (Yang et al., 2017; Abe et al., 2010; Li et al., 2010; Wang et al., 2014; Manoharan et al., 2021; Henares et al., 2017) |
| Inkjet Etching | Modified inkjet printer, polystyrene, toluene. | Requires only one device to be fabricated. | Provides low resolution (550 μm), requires multiple steps, not suitable for mass production. | (Abe et al., 2008) |
| Plasma treatment | Metal mask, oven, Plasma, AKD, heptane | Cost-efficient. | Requires the production of new masks for different patterns. | (Li et al., 2008) |
| Flexographic Printing | Flexographic printer, toluene, or xylene. | High printing speed, suitable for mass production. | Printing quality is affected by the paper smoothness, printing should be repeated at least twice for the same paper. | (Olkkonen et al., 2010) |
| Knife Cutting | X-Y plotter, knife. | Provides sharp details. | Wastes raw materials as residues after cutting. | (Sadri et al., 2018; Fenton et al., 2008) |
| Laser Cutting | CO2 laser, micro silica particles. | Provides high resolution (62 μm). | Requires polymer films to protect the nitrocellulose from being damaged. | (Chitnis et al., 2011; Spicar-Mihalic et al., 2013) |
Photolithography
One popular method for fabrication of paper microfluidics is photolithography, in which the paper is made entirely hydrophobic, followed by applying UV light to create the hydrophilic patterns through a photomask (Lu et al., 2010). The first report to fabricate a paper-based microfluidic device with photolithography dates back to 2007 (Martinez et al., 2007), where the main aim was to produce a multiplexed device, i.e., a device that can perform multiple assays simultaneously using the same paper. In this method, SU8 photoresist is first applied on a chromatography paper, followed by heating for cyclopentanone removal. UV light is applied on a patterned photomask covering the paper to transfer the pattern. Then, post-baking at 95°C yields crosslinking, followed by a cleaning stage using propanol and propylene glycol monomethyl ether acetate. Finally, oxygen plasma is utilized for making the paper hydrophilic. Although this method offers good resolution, it requires multiple steps and equipment of a cleanroom, which is expensive and inconvenient when multiple devices are to be produced (Akyazi et al., 2018). In addition, in photolithography method, the channels of paper can undergo contamination, decreasing the quality of the assay significantly. To overcome these challenges, a simpler, faster, and more cost-effective approach was developed, named as fast lithographic activation of sheets (FLASH) (Martinez et al., 2008). In FLASH method, a hot plate and a UV light are used for quick and straightforward fabrication without the need for cleanroom settings.
Wax printing, screen printing, and plotting
An inexpensive alternative method for fabrication of paper microfluidics is wax printing, suitable for both prototyping and mass production of microfluidic papers (Lu et al., 2009; Younas et al., 2019). This method is more cost- and time-efficient in comparison with photolithography. In this technique, the wax pattern is printed on the paper either using a pen or using a wax printer (Lu et al., 2009), followed by heating in a hot plate or an oven to let the wax meltdown and penetrate the paper, creating hydrophobic barriers and hydrophilic channels (Lu et al., 2009). Requiring only two steps, this method is rather swift and allows fabricating multiple devices in less than 5 min. However, the channel width fabricated with wax printing can go down to 550 μm, which is large compared to channels created with photolithography (Carrilho et al., 2009). To protect the channels from contamination, the top and bottom surfaces of the paper can be printed with a toner, preventing any loss in the reagents and maintaining their optimal concentrations (Schilling et al., 2012). Masking can also be used to transfer the desired pattern from a metallic mask to the paper instead of printing the wax directly (Songjaroen et al., 2011). Wax is also used for screen printing, where a mask is designed and printed on a transparent film to create a screen with the desired pattern. The wax is then rubbed against the paper through the mask to transfer the wax into the paper, which is then heated at 100°C to melt the wax and form the channels (Songjaroen et al., 2011). A similar technique to wax printing uses a modified X-Y plotter that deposits polydimethylsiloxane (PDMS), a hydrophobic polymer, onto a filter paper, and the pattern is created as the PDMS penetrates the paper to form confined channels. This method provides lower resolution compared to wax printing, with a minimum channel size of about 1 mm, yet it is useful for rapid and cost-efficient prototyping of paper-based microfluidic assays. Moreover, papers patterned with PDMS are flexible, allowing them to be bent to form 3D structures of paper microfluidics (Bruzewicz et al., 2008).
Inkjet printing and inkjet etching
Although wax printing has the advantages of fast and cost-efficient production, it is not mature enough to be a significant method to fabricate commercial devices due to its low resolution and poor resistance to high temperatures. On the other hand, another printing method, inkjet printing is already used for inexpensive mass production (Yang et al., 2017). In this method, a regular inkjet printer with a modified nozzle and ink for microfluidic devices is used (Abe et al., 2010). One example of ink utilized in this method is alkyl ketene dimer (AKD), an organic material with similar behavior to wax, able to make specific portions of the paper hydrophobic, based on the printed pattern (Li et al., 2010). This ink is first mixed with heptane as a solvent, and then printed on the paper and heated at 100°C, transferring the barrier-channel pattern to the paper. In this method, the printer can be damaged due to the use of hazardous organic solvents. However, other safe alternatives such as hydrophobic sol-gels, including methyl silsesquioxane (Wang et al., 2014), tetraethyl-orthosilicate (Manoharan et al., 2021), and UV-curable acrylate ink where the paper is cured with UV after patterning are available for use as ink (Henares et al., 2017). In addition to the described methods which were additive, meaning that the hydrophobic walls are added to the hydrophilic paper, inkjet etching is another alternative option that operates in a subtractive way. In inkjet etching, a filter paper is soaked in polystyrene for 2 h and then allowed to be dried for 15 min, making the whole paper hydrophobic. Next, an inkjet printer is used to etch the pattern and expose specific regions of the paper by ejecting toluene droplets (Abe et al., 2008). In comparison to wax printing, fabrication of microfluidic devices with inkjet printing takes more time in general since multiple steps are involved in this method.
Plasma treatment
AKD can be used in the fabrication method of plasma treatment in addition to inkjet printing. In plasma treatment, after dissolving AKD in heptane, the filter paper is soaked in the resulting AKD-heptane solution, followed by instant removal of filter paper to allow heptane vaporization. Next, the AKD on the filter paper is cured by heating up to 100°C in an oven for 45 min. After treatment, the resulting paper is highly hydrophobic. To create the hydrophilic channels, metal masks with the desired patterns are fabricated using mechanical cutting of stainless-steel sheets, and the treated paper is then sandwiched between the stainless-steel masks, and their alignment is secured using four screws. Plasma treatment is subsequently applied on the treated paper through the masks to remove the cured AKD where channels are to be formed, creating hydrophilic areas, which can be confirmed by their high wettability by aqueous solutions (Li et al., 2008). Since AKD is mainly used in this method, it is considered as a cost-efficient method. However, a new mask must be designed and fabricated for each different pattern design.
Flexographic printing
Another printing approach that can be utilized to transfer patterns into the paper is flexographic printing. In this method, the paper is run along four different rollers: the fountain roller that transfers ink to the anilox roller, which in turn transfers the ink with a specific thickness to the plate roller that has the printing pattern engraved on it. The paper passes between the plate roller and the impression roller that applies pressure on the plate roller to print the pattern on the paper. To produce a microfluidic paper using this system, a filter paper is placed on the impression roller. Instead of an ink, a hydrophobic material, such as xylene and toluene, is used for printing. In addition, an extra component called the doctor blade is used to remove any excess material that may accumulate during transfer from one roller to another. A specific printing speed in which the anilox roller rotates four times, while the impression roller and plate roller rotate once, is set, resulting in the transfer of the hydrophobic material to the paper. The pressure applied by the impression roller as well as printing speed can be fine-tuned to improve the printing quality in terms of material penetration of the paper (Olkkonen et al., 2010). Unlike other methods, flexographic printing does not require any heat treatment for hydrophobic material penetration. However, this makes the printing quality highly dependent on paper smoothness. In addition, the printing process should be repeated at least twice to ensure complete penetration for a single assay.
Physical methods: Knife cutting and laser cutting
The eight aforementioned methods are all chemical, since specific substrates are used to transfer the pattern to the paper. On the other hand, physical patterning is also achievable via cutting paper with different tools, such as knives and lasers. Knife plotting or cutting involves a motorized X-Y platform used to control the motion of a blade with the aid of a computer. By controlling the rotation, cutting force, and angle of the knife, it is possible to achieve precise cutting of different patterns and small details (Sadri et al., 2018). To prevent the paper from being damaged during the cutting process, three sequential overlapping cuts are required (Fenton et al., 2008). The simplicity of this method makes it an appropriate candidate for rapid manufacturing. However, this method can waste raw materials due to the residues remaining after cutting (Yetisen et al., 2013). In CO2 laser cutting, hydrophobic paper is treated with laser to craft the microfluidic channels, followed by a treatment with micro silica particles to create the hydrophilic barriers (Chitnis et al., 2011). Another variation of laser cutting uses a polyester-backed nitrocellulose membrane sandwiched between two thin polymer films to protect the nitrocellulose from being damaged by the laser beam (Spicar-Mihalic et al., 2013). Although laser cutting can produce small channels down to 62 μm and it is as simple as knife cutting, the same production volume will be more expensive for laser cutting when compared to knife cutting due to the high costs of using multiple laser systems simultaneously (Chitnis et al., 2011).
Emerging fertility testing applications
Tests based on sperm concentration
Paper-based assays offer several analytical capabilities (Figure 2) which can be used for fertility tests as well. For the aim of facilitating fertility testing in developing countries, where infertility prevalence is reported to be higher than in developed countries (Gerrits and Shaw, 2010), a cost-efficient paper-based device was proposed, eliminating the requirement for operating experts or healthcare workers (Matsuura et al., 2014). The device was fabricated using paper-based wax printing with heating up to 135°C and provided two pieces of information simultaneously: sperm concentration in semen and sperm motility. Two enzyme-based assays were performed on the device: First, a thiazine assay (MB assay) for sperm concentration measurement, and second, a tetrazolium-based colorimetric assay (MTT assay) for sperm motility estimation, which was prepared by applying MTT solution on the hydrophilic part of the wax paper, and then drying it out. The MTT assay was performed by applying 5 μL of semen in the middle of the hydrophilic region, and a digital camera was used to record the color signal pattern after drying the solution out. The difference between sperm diffusion and dye molecules diffusion was determined upon staining sperm samples in the MB assays and used to calculate the sperm concentration. MTT assay utilized image processing to evaluate sperm motility. Reference values of concentration and motility were adopted from the values provided by the World Health Organization (WHO), which are 2 × 107 Cells/mL and 50%, respectively. The relationship between measured (CON) and prepared sperm concentrations was analyzed using different concentrations of sperm using dilution and the assay was found to provide accurate results. For motility evaluation, three spots on the paper pattern were chosen, and the pixel intensities in their ranges were determined after capturing an image with a camera and digitally processing it as a grayscale image. Two values of 0% and 50% of motile sperm were used, and the images for both cases were compared by summing up pixel intensities at the three chosen spots. It was demonstrated that the pixel intensity in the middle spot for the 50% sample was higher than the 0% sample, representing accurately calculated motility.
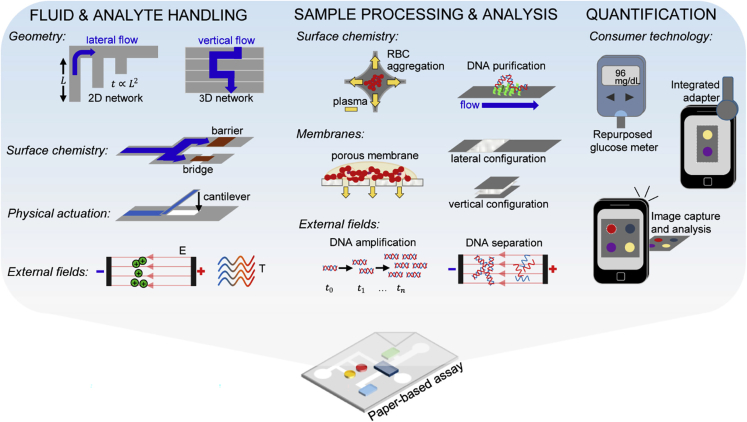
Figure 2.
A general view of analytical capabilities employed in paper-based assays
Analytical capabilities employed in paper-based assays include fluid and analyte handling (investigating the effects of the geometry, surface chemistry, physical actuation, and external fields), sample processing and analysis (investigating the effects of the surface chemistry, membranes, and external fields), and quantification (investigating the user interface and consumer technology).
Adapted from ref. (Gong and Sinton, 2017) with permission from American Chemical Society.
Similar to the previously mentioned devices, another disposable microfluidics-based chip (MFC) was developed to perform fertility testing via testing the concentration and motility of the sperm cells by allowing them to swim in the microfluidic structure of the chip for 5–10 min while being incubated at 37°C (Kim and Chun, 2020). In order to overcome the challenge of fluid evaporation while performing the assay, because of inconsistent flow of sperm cells along the microchannels, the chip had a unique design of three layers containing two main zones: sperm injection and counting zones and a sorting zone to separate cells based on their motility. Computer-aided sperm analysis (CASA) was used to count the total number of sperms in the counting zone upon their injection. Microchannels at the inlet of the device had a radial design, where eight channels of equal length converge into a common area. Unlike conventional microfluidics-based devices, the proposed device did not include an outlet. Instead, it had a single inlet in which the sample is loaded, and a feedback channel that drives the sample back to the inlet. Typically, such single inlet designs result in the entrapment of air inside the channels and prevent loading the sample at the inlet, but the feedback channel was able to solve this problem, which made the inlet act as an outlet temporarily until the flowing sample returns to the inlet. This unique design was intended to minimize the evaporation of the sample during the assay. To evaluate the ability to minimize evaporation, microbeads and sperms were injected together. 15 min after the injection, the microbeads were not found in the channels, while sperms were observed in the channels or in the sorting zone. Even if some evaporation of fluid occurred, the microbeads remained at the site of injection, indicating that there was no unwanted flow due to evaporation, and all flow was microfluidic. The performance of the MFC was compared to the performance of Leja-10, a commercial disposable slide used for quick sperm analysis. By comparing measurements of concentration and motility using the MFC and Leja-10 slide with 40 samples, a good agreement was found for both parameters using the Passing–Bablok regression analysis, which estimates agreement of two analytical methods based on continuously distributed data, along with approximation of possible systematic bias between them (Bilić-Zulle, 2011). The results indicated that the MFC can be used as an alternative of the Leja slide.
In order to facilitate performing fertility testing at home, a lateral flow diagnostic device named SpermCheck was developed, which aimed to count the number of sperms in semen samples, and report normozoospermia (normal sperm count), oligozoospermia (low sperm count), or severe oligozoospermia (Coppola et al., 2010). The working basis of the device was solid-phase chromatographic immunoassay. The design included a plastic housing that contained two strips, each one having a different concentration threshold, with 2 × 107 sperm/mL (based on WHO definition for low sperm concentration) for the first one and 5 × 106 sperm/mL (based on male fertility-related literature) for the second, reporting the cases of oligozoospermia and severe oligozoospermia, respectively. The device was able to achieve high accuracies, where over 96% of the samples were classified correctly. The study also investigated the user-friendliness of the device, where untrained users and trained professionals performed the assays, and their interpretations of the results were compared. Untrained users and trained professionals agreed on 95.1% of the results (58 out of 61), while over 93% of the survey responses were correct, demonstrating that the device does not require special training to be used at home. In addition, a consumer user survey was conducted, which was taken by 134 participants who tested their semen, tested prepared semen samples, or performed the test at home. The participants showed high agreement for all questions, with a score of correct answers ranging between 93% and 100%. These scores indicated that end-users were able to follow the instructions in order to acquire acceptable results and interpret those results using SpermCheck Fertility device. Another result of the consumer user survey was that sperm count was low in about 11.2% of the participants who did not express any knowledge of low fertility or did not show high interest in the test. This indicates that using a home fertility test can be elucidating for those who do not suspect having low fertility and do not realize the need to use such tests.
Tests based on sperm motility
In addition to evaluating sperm concentration to assess male infertility, studies have also focused on separating and sorting motile and immotile sperm. A study was performed to develop a paper-based device for quantification of the three parameters related to male fertility, which are motile and live sperm concentration, as well as sperm motility (Nosrati et al., 2016a, 2016b). The main focus of this study was to improve sensitivity and diagnosis time over existing similar assays. The assay was composed of 3 layers: 2 patterned paper layers, and a laminate layer underneath. Patterning was done using wax. Two 4 mm holes were punched in the first layer to allow the sample to access the reaction spots of the second layer in which MTT assay was used for the quantification of concentrations, generating a colorimetric signal that can be optically scanned for analysis. The motility was calculated as the ratio between the concentration of motile sperms and live sperms. The robustness of the device was also assessed by examining two storage conditions: humidity and time. The results showed that motile sperm concentration, live sperm concentration, and sperm motility determined by the proposed assay were correlated with those determined using conventional laboratory methods, such as computer-assisted sperm analysis (CASA) and dye exclusion vitality assay. Robustness assessment showed that the device can still be functional under high humidity conditions after eight weeks of storage. However, extending the storage time furthermore decreased the color intensity at the reaction spots, which was solved by including a desiccant with the device’s package. The device was able to outperform similar types of paper-based assays, with an assay time of only 10 min and 8.46 and 15.18 million/mL detection limits.
One of the early disposable, point-of-care fertility testing setups combined microfluidics with fluorescence labeling and used a micro fluorometer to measure the number of motile sperms out of the number of total sperms, calculating the motile sperm concentration (Mccormack et al., 2006). The device consisted of a semen reservoir connected to the 50 nL analytical detection microcuvette with a microfluidic channel. Upon placing 10 μL semen in the reservoir, labeled motile sperms passed into the microfluidic channel, and continued their flow to reach detection cuvette. Fluorescent molecules in the labeled sperms were excited using light and the emitted signal was measured and processed using analog electronics to be analyzed by computer. When the assay was performed, counting labeled sperms that entered the detection cuvette was possible within the first few moments using bright field microscopy. During the assay time (∼50 min), the collected data showed continuous, monotonic increase in the fluorescence signal over time. Assessment of the microfluidic device was done by comparing its results to computer-assisted semen analysis (CASA) and four parameters were measured: motility, sperm concentration, and total and progressive motile sperms’ concentration. The correlation between the fluorescence signal and the concentration of progressive and total motile sperms were 0.80 and 0.79, respectively. A fertility threshold was calculated based on the measured data and was chosen to optimize the sensitivity and specificity of the system, and that threshold was 300 AFU, which was found be correlated with the WHO standards of the normal concentration of motile sperms.
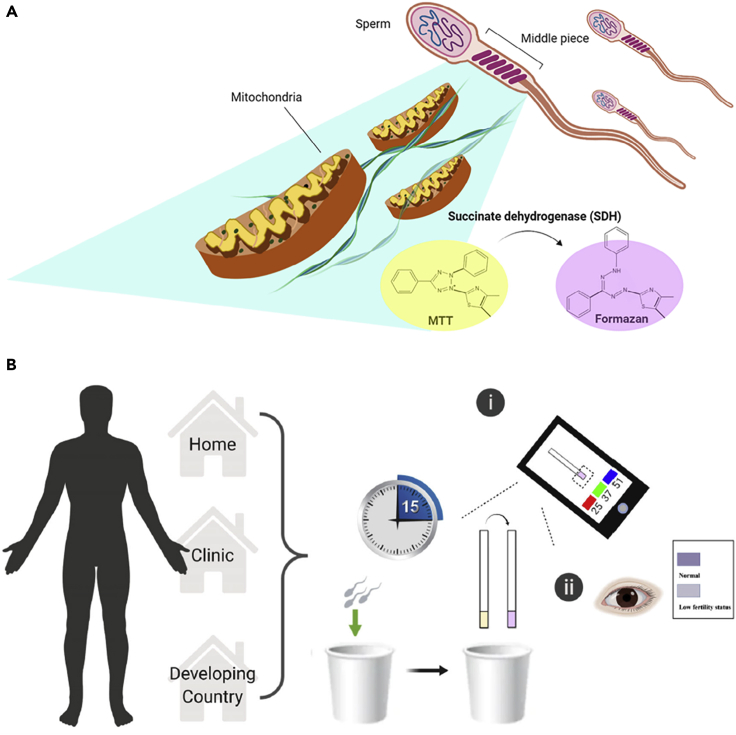
To enable point-of-care (POC) testing of male fertility with a cost-efficient, easy-to-use, and portable method, a smartphone-based colorimetric device was developed to detect low or normal total motile sperm concentration (TMSC) (Tsao et al., 2020). The device consisted of a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) strip, and determined the mitochondrial activity in the sperm cells. The mitochondria of the cell could change the yellow MTT into purple formazan. This color change occurs due to the abundance of mitochondria in the sperm cell, and succinate dehydrogenase (SDH) found on the mitochondria induces a chemical reaction that turns the yellow MTT into purple formazan (Figure 3A). Therefore, a purple color with high intensity indicated normal TMSC. The change in the color could either be detected by the naked eye or by using a smartphone camera and the app developed to measure the purple signal intensity on the captured photos of the strip. Detection by the naked eye resulted in an accuracy of 67%, sensitivity of 41%, and specificity of 95%. The smartphone-based detection, on the other hand, had an accuracy of 80.9%, sensitivity of 96%, and specificity of 65%, demonstrating that the smartphone-based approach yielded higher accuracy. Furthermore, the results of the MTT strip method were compared to the results of standard semen analysis using a microscope, and a high correlation between the two methods was found. The whole test from sample preparation to color change analysis took less than one hour, with image analysis taking about 15 min (Figure 3B). The cost of a single test was reported to be $0.03, making it a very cost-efficient candidate for fertility tests.
Figure 3.
Mechanism of and use for point-of-care, smartphone- and colorimetric-paper-based semen analysis
(A) The mechanism of color change of yellow MTT into purple formazan used to assess mitochondria content in sperm.
(B) Potential applications of the MTT test strip, which can be used either at home, at clinics, or in resource-limited developing countries. Upon collecting a semen sample, men can easily determine results using a smartphone or color indicator chart (within 15 min).
Adapted from ref. (Tsao et al., 2020) in accordance with the Creative Commons 4.0 International (CC BY 4.0) license.
Sorting and isolating motile sperm from non-motile sperm and other cells and particles was achieved using a device called microscale integrated sperm sorter (MISS), which was intended to be disposable, small, easy-to-use, and self-contained (Cho et al., 2003). In this device, a novel pumping system was introduced to passively drive the fluid flow during the assay, eliminating the need for an external power supply. The sorting mechanism of the MISS device utilized the active motion of sperms, where non-motile sperms and larger particles followed a straight path as they flowed from the inlet to the outlet, while motile sperms deviated from their original path and exited from another outlet since they are faster than non-motile sperms and smaller than cell debris. The pumping system was specially designed to tackle the gravity-driven pumping problem, in which the flow rate decreases with the decrease of fluid volume in the inlet reservoir. Here, this challenge was solved by introducing four horizontal reservoirs that serve as inlets, outlets, or pumps. Those four reservoirs were designed to have specific size, geometry, and orientation to counteract the effects of gravity, channel resistance, and surface tension, and to provide a consistent flow of the sample over the course of the assay. As a result, motile sperms were successfully separated, with a measured velocity of 20 μm/s, while non-motile sperms and other particles diffused much slower. Such devices remain promising as they can be used in clinics or at home and combined with colorimetric detection for sperm counting.
Tests based on sperm DNA analysis
To assess male fertility, studies mentioned in the previous sections have engineered microfluidic devices to determine sperm concentration and sperm motility. In addition to these parameters, examining the DNA content of sperms is essential to evaluate male fertility, since DNA damage can be inherited and affect fertility in future generations. Accumulation of mutations in mitochondrial DNA (mtDNA) (Otten and Smeets, 2015; Vaught and Dowling, 2018) as well as sperm DNA fragmentation, which could occur as a result of increased levels of cellular reactive oxygen species (ROS), protamination process failures, or apoptosis, has been associated with male infertility and reduced reproductive outcomes (Hamilton and Assumpcao, 2020).
In this regard, a DNA integrity analysis, including quantification of DNA fragmentation and DNA packaging, was done by developing a paper-based device (Nosrati et al., 2016a, 2016b). The paper was patterned with wax, creating inlet and outlet buffer reservoirs connected by a channel. The goal of the study was to quantify DNA fragmentation index (%DFI) and high DNA stainability (%HDS), two independent parameters from other fertility indicators, which can be relevantly maintained under certain thresholds to report positive fertility. The performance of the device was assessed by testing semen from seven healthy individuals and patients and comparing it with sperm chromatin structure assay (SCSA), which is considered the gold standard for DNA integrity clinical testing. %DFI measurement by the device and SCSA was correlated between 3.7% and 96.9%, proposing a ∼30% threshold for male fertility. A good correlation between the experimental and clinical testing of %HDS was also demonstrated between 1.3% and 8.1%, proposing a ∼15% threshold for fertility. Both %DFI and %HDS determined by the device were within 15% of the SCSA results, and the values calculated by the two different methods were 100% identical, in which %HDS values were below the threshold, while %DFI results were higher and indicated low DNA integrity.
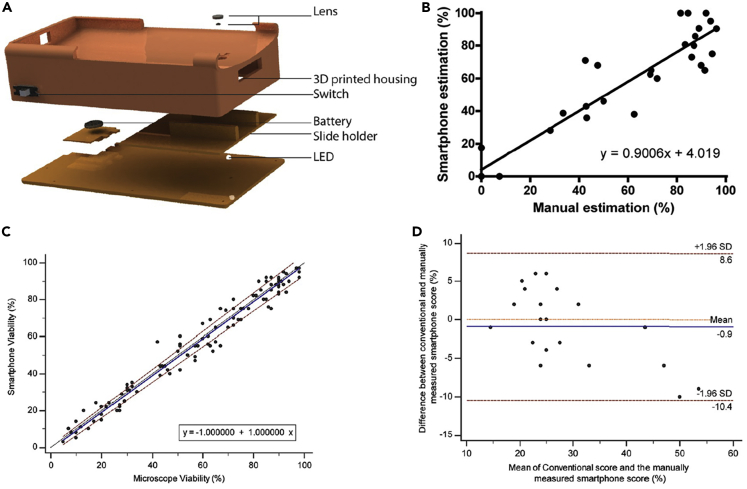
Utilizing smartphones for POC testing setups is a trending field of interest as it can provide an easier and more accessible user experience for the patients. As such, a smartphone-based male fertility testing microfluidic device, that was engineered using poly(methyl methacrylate), double-sided adhesive sheets, and a laser cutter, was developed to be used at home by the patient (Dimitriadis et al., 2019). This device measured three different markers: Hyaluronan Binding Assay (HBA) score, which describes sperm fertilization and maturity, sperm viability, in which the integrity of sperm membrane is assessed, and DNA fragmentation that evaluates damage in sperm DNA. An optical attachment was designed to be compatible with smartphones, consisting of a set of two magnification lenses, a battery, an illuminating LED, and a 3D-printed case to house the components and the smartphone together (Figure 4A). Additionally, a customized Android application was developed to provide automatic analysis of each of the measured three markers, by capturing videos or images and processing them digitally. Upon separating motile and immotile sperms using a mixture of Gaussian’s background algorithm, a sound gate was imposed on captured videos/sections to reduce background noise and HBA scores were estimated using obtained motile sperm counts. To evaluate sperm fragmentation, sperm cell images were isolated using an adaptive threshold algorithm and the size of sperm heads was analyzed. While large sperm heads exhibited haloing and no DNA fragmentation, smaller sperm heads exhibited no haloing and DNA fragmentation. By comparing the results of the smartphone-based and manual analysis, an appropriate agreement was observed between the two methods, with the smartphone-based system demonstrating a sensitivity of 100% and a specificity of 69.23%. Specifically, for the HBA score, the correlation between smartphone-based and manual methods perceived was R2 = 0.87 (Figure 4B). In addition, sperm viability testing showed similar results when compared to bright-field microscope results, showing linearity (n = 31) in the relationship between the two methods (Figure 4C). Similar results were achieved after DNA fragmentation testing. The Bland-Altman method of analysis (n = 20), which is a method of data plotting that is used for estimation of bias and agreement intervals between two methods (Giavarina, 2015), was performed and showed low mean bias of 0.9% when the smartphone-based and the conventional method on the sample set comparing the DNA fragmentation scores obtained through conventional analysis and the scores obtained manually through the use of a smartphone-based approach (Figure 4D).
Figure 4.
Smartphone-based semen analysis
(A) Illustration of smartphone-based semen analysis system displaying lens, 3D printed housing, switch, battery, slide holder, and LED.
(B–D) Comparison of manual conventional method of analysis and smartphone estimation.
(B) Linear regression analysis showed agreement (n = 31).
(C) Passing-Bablok analysis on sperm viability assessments showed strong linear relationship (n = 103).
(D) Band-Altman analysis (n = 20) on DNA fragmentation scores.
Adapted from ref. (Dimitriadis et al., 2019) in accordance with the Creative Commons 4.0 International (CC BY 4.0) license.
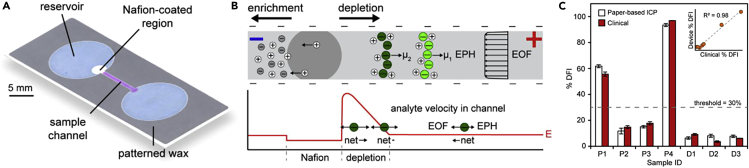
Similarly, to perform direct DNA analysis on different types of cells including sperm cells, a paper-based device capable of performing direct DNA analysis was introduced (Gong et al., 2015). The device was used for measuring the percent DNA fragmentation index (%DFI) of sperm cells using an ion concentration polarization (ICP) technique, an electrokinetic process that results from ion transportation through nonporous structures. The device consisted of a nitrocellulose paper with two reservoirs connected by a channel created by pattering wax. A small region of one of the reservoirs where ICP was designed to be carried out, was coated with cation-selective nanoporous Nafion (Figure 5A). To perform the test, the applied voltage upon loading the sample into the channel caused cations to migrate through the nanoporous region toward the cathode while anions migrated toward the anode. The electrokinetic force exerted on ions depended on the applied voltage and the ion concentration, which determined the velocity of migration, allowing their separation (Figure 5B). This method was applied to single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA) of sperm cells in order to measure their concentrations. Both dsDNA and ssDNA were separated over 15 min at 150 V/cm, where dsDNA concentrated at the depletion boundary and ssDNA concentrated downstream. Direct quantification of %DFI of sperm cells in raw semen samples from patients and donors (n = 7) showed some overlap of dsDNA and ssDNA (SR ∼ 0.5%). The results of the test were compared to results obtained using SCSA, a clinical standard test to assess DNA integrity, and a correlation of R2 = 0.98 for %DFI results was observed between the two methods (Figure 5C).
Figure 5.
Paper-based device operating with ion concentration polarization (ICP) technique
(A) Paper-based ICP device design illustrating reservoir, sample channel, and Nafion-coated region.
(B) Diagram representing ICP enrichment and depletion upon voltage application. As a result of local electric field strength, E, net movements of analytes are determined by electrophoretic migration (EPH) and electroosmotic flow (EOF).
(C) %DFI results were compared between paper-based ICP and clinical results for patients and donors, producing a correlation of R2 = 0.98. Threshold of 30% for %DFI was used to determine clinical outcome. Error bars for the device reflect one standard deviation of normal fits and error bars for clinical results, average of two measurements, represent one standard deviation.
Adapted from ref. (Gong et al., 2015) with permission from American Chemical Society.
Testing with other functionalities
In addition to sperm concentration, sperm motility, and accumulation of DNA damage or mutations, sperm morphology and hormone levels are essential factors that impact fertility. Furthermore, sperm quality significantly impacts success rate of ICSI (Loutradi et al., 2006). Therefore, a disposable microfluidics-based device was developed to analyze and select normal individual sperms based on their morphology and content, with the aid of interferometric phase microscopy (IPM), which enables imaging of cells without using any stains (Eravuchira et al., 2018). IPM was capable of analyzing small quantities (1 μL) of sample containing on average 3000 ± 200 sperm cells more efficiently than other techniques such as Zernike’s phase contrast and differential interference contrast, resulting in a hologram of the imaged cells that can be converted into a 2D image and digitally processed, providing information about optical thickness, or optical path delay. In addition, the analysis results were validated by an experienced clinical embryologist. The device allowed individual sperm cells to flow into the microchannel, where sperm cell morphology was analyzed using IPM and a dedicated algorithm, and then directed into one of two outlets based on their quality, where the second outlet contained healthy sperm cells. Results showed that the device had a high selectivity of 89.5 ± 3.5%. Additionally, no abnormal sperm cells were classified as normal, eliminating the risk of false positives. Moreover, a precision of 100% and a sensitivity of 67% were achieved. The device was able to analyze 40 to 45 cells and detect at least one healthy sperm cell in 6 min, presenting a promising tool for clinical fertility tests.
Another point-of-care device was developed to detect different fertility markers using a multiplexed immunoassay, which is a microfluidics-based platform that embeds multiple polyethylene glycol-based hydrogel sensors into a chip of a single layer (Kalme et al., 2019). Such sensors have 3D porous geometries consisting of an extruded 2D shape, and are manufactured prior to being embedded into the chip, saving the cost of using an extra amount of reagent that would typically be required to polymerize the sensors. Multiplexing was made more efficient by distributing the sensors on different sites of the chip. A custom adapter loaded with the required buffers and reagents was developed to perform the steps of the fluorescence-based immunoassay completely, including the sequential sample flow, addition of reagents used for detection and the wash buffer. The reagents were dried and stored on the chip to make the device suitable for point-of-care applications. A fluorescent scanner that has both pumping and dispensing capabilities was used to provide the final readout. To validate the performance of the device, four fertility markers, which are follicle-stimulating hormone, beta-human chorionic gonadotropin, prolactin, and luteinizing hormone, were tested, and related parameters were reported. The results showed high accuracy (R2 value of more than 0.95) and high precision (CV value less than 10%), in addition to acceptable linearity.
Challenges and future perspectives
Due to their many advantages, microfluidics are promising technologies for commercialization (Volpatti and Yetisen, 2014; Shields et al., 2017; Chin et al., 2012; Sarabi et al., 2021b; Sarabi et al., 2021a; Rezapour Sarabi et al., 2021; Dabbagh et al., 2021b), as well as for use in healthcare centers, especially for rapid fertility testing. To achieve this, further development is required to enhance the quality and accuracy of results produced by such devices. Novel materials and methods, such as nanoparticles, electrical stimuli, microvalves, and micropumps can be implemented to advance microfluidics technologies. Moreover, different approaches can be combined in a single assay, such as chemical attraction and acoustic waves, which may be applied simultaneously to provide multiple sorting systems and achieve better flow manipulation, consequently improving overall performance. The analysis of individual nanoparticles was proposed by integrating stochastic sensing into microfluidic paper-based analytical systems using nanoparticles of different sizes (10, 20, and 40 nm). Furthermore, nanoparticle impacts were recorded after silver nanoparticles were flushed along a microchannel (Weiss et al., 2022). Alternatively, integration of a cellphone-based colorimetric detection system and an in-air sampling of unmanned aerial vehicle has allowed for the quantification of metals in airborne particulate through an on-site multiaxial pollution sampling approach (Sun et al., 2018). As such, customized algorithms can be developed for mobile or personal computer applications. Furthermore, integration of artificial intelligence (AI), utilizing lens-free imaging, or digital holography are other promising directions that could ensure higher precision and accuracy in male fertility testing. While employing such complex systems would require rethinking the design of portable microfluidic platforms, which may lead to additional cost of extra materials and tools, test results would be highly accurate in return. Further improvement in this field should be directed into minimizing the time needed to prepare and manipulate the sample by combining multiple preparation steps together and automating the overall process as much as possible (Khodamoradi et al., 2021; Rezapour Sarabi et al., 2022a).
Innovative strategies to improve microfluidics for fertility testing
Translating recent developments in microfluidics to paper-based systems can help improve efficiency, precision, and accuracy of paper-based male fertility tests and sperm sorting applications. A surface-modified glass substrate was fabricated using nanodiamond particles and the velocity of sperm cells in contact with the modified surface was improved 12%–52% compared to those in contact with untreated surfaces (Lesani et al., 2022). Additionally, a 14% decrease in ROS was observed as a result of the use of nanodiamond particles. Similarly, to improve sperm sorting applications for ARTs, a microfluidic device that mimics the female reproductive tract and separates sperm cells based on rheotaxis response to flow environments was created (Sarbandi et al., 2021).
Acoustic-based platforms have been used to overcome challenges associated with capillary-driven passive mixing fabrication techniques. Drawbacks of traditional techniques include the lack of uniformity in fabrication, and this occurs as a result of the inherent variability of fiber alignment in paper. Such limitations were eliminated using surface acoustic waves during mixing to create a convective actuation mechanism for a paper-based microfluidic device (Rezk et al., 2012). Furthermore, it was demonstrated that acoustic-based platforms could be used as effective external actuation systems for paper-based platforms by providing uniform and efficient fabrication. Vapor phase polymer deposition is another fabrication technique that could improve capabilities of paper-based microfluidics devices. By depositing functional polymer coatings onto paper-based microfluidic devices, a UV-responsive switch was integrated to allow for a higher degree of control over fluid manipulation (Kwong and Gupta, 2012). Analyte separation from multicomponent mixtures was achieved and it was suggested that vapor phase polymer deposition could lead to the development of more complex diagnostic tools. Similarly, chemical vapor deposition has also been used as a method to expand applications of paper-based microfluidic devices. Vaporized tricholorsilane was deposited on chromatography paper forming strong hydrophobic layers (Lam et al., 2017). Glucose and immunoassays were performed using ∼5uL sample volumes and results demonstrated clinically relevant LODs. Owing to its adaptability, chemical vapor deposition could also offer novel ways to improve paper-based diagnostics.
Artificial intelligence (AI) integration
As the use of smartphones to analyze fertility test results has grown, a need for advanced algorithms to improve the readout accuracy has emerged. For this purpose, a portable system, called Bemaner, was developed and equipped with cloud computing and AI capabilities, dedicated to be used with all types of smartphones (Tsai et al., 2020). For the Bemaner device, microscopy and microfluidics technology were combined to perform fertility testing based on three parameters: total sperm concentration, motile sperm concentration, and motility percentage. After performing the microfluidic assay, a smartphone was fitted into the device and used to capture videos of the sperms in the assay. The videos were uploaded to the cloud with the help of a dedicated smartphone app. AI algorithms were then used to analyze the videos using image recognition, and show the results on the smartphone app. The captured videos were also evaluated by an expert, who classified them into six grades from 0 to 5 based on the number of motile sperms in the field of view, where grade 0 represented no motility of sperms and grade 6 represented a field of view comprised almost completely of motile sperms. Results of the Bemaner platform and expert analysis were compared using Pearson product-moment correlation coefficients (p < 0.001), which were r = 0.65 for total sperm concentration, r = 0.84 for motile sperm concentration, and r = 0.90 for motility percentage. High correlation was observed for the latter two parameters and the immobility of some sperms could account for the moderate correlation between expert classification and algorithm classification of the total sperm concentration parameter.
In a similar manner, to advance accuracy and precision in fertility testing, a deep learning algorithm was used in combination with interferometric imaging that did not require chemical staining, where the deep learning model was trained for fertility parameters evaluation, achieving 93.1% accuracy and 90.9% precision (Ben-Yehuda et al., 2021). Such advanced technologies have also been extended to enable utilizing smartphones for fertility testing. In another study, microfluidics, optical components, and a smartphone were integrated to design a portable, fast, and highly accurate device that can evaluate sperm motility and concentration with raw semen samples in less than 5 s and with an accuracy of about 98%, which demonstrated that the device could be highly effective for POC testing (Kanakasabapathy et al., 2017). Furthermore, an artificial neural network was created to quantify sperm concentration using a machine learning-based spectrophotometry approach. 41 human sperm samples were analyzed under 390–1100 nm light spectra to develop the neural network and the full spectrum neural network model produced results with 93% accuracy and 100% agreement with clinical assessments (Lesani et al., 2020).
Lens-free imaging
Other novel techniques have also emerged recently for fertility testing and sperm analysis, allowing the diagnosis of additional parameters of male fertility. One of these techniques is lens-free on-chip imaging, which is a compact and low-cost method, making it suitable for POC testing, and can result in high-resolution, 3D images in a microscopic scale (Kim et al., 2012). In lens-free on-chip imaging, the sample is placed very close to the digital sensor used for capturing the image, illuminated by an incoherent or partially coherent light. This allows the sensor to record the diffraction patterns formed by the shadows of the sample, achieving an effective and wide field of view.
An application of lens-free on-chip imaging in sperm analysis was done by illuminating the sperm sample with two partially coherent light sources of different wavelengths, placing each one at 45° to the other, which resulted in a depth of field of ∼0.5 to 1 mm and field of view of 17 mm (Su et al., 2012). This setup enabled 3D tracking of trajectories of more than 1500 sperms, finding that only 4%–5% of motile sperms followed well-defined helical trajectories as they swam, a percentage that can effectively be minimized when sperms swim in seminal plasma. Different physical characteristics of the helical sperms, such as helical rotation speed (3–20 rotations/sec), helix radius (0.5–3 μm), and linear speed (20–100 μm/s), were defined after processing using lens-free imaging with micro-precision. Additional optical components can be used to optimize the setup. For example, a diffuser was placed between the sensor and the sample, to allow wavelength modulation of a single light source, eliminating the need of additional illumination (Jiang et al., 2020). The setup was able to utilize the whole sensor area (6.4 mm x 4.6 mm) to represent the field of view. In addition, up-sampling was used to achieve a half-pitch resolution, increasing the image resolution significantly, compared to the initial resolution of the sensor.
Digital holography
Another novel technique used for sperm tracking and analysis is digital holography, in which a partially or fully coherent light source, such as a laser, was used for illumination (Di Caprio et al., 2015). Furthermore, a single beam was split into a reference beam and a beam that illuminated the sample and scattered to form the object beam. By measuring the interference of the two beams, the phase of the object beams along with its magnitude, which had already been captured by the imaging sensor, could be obtained. This enabled the reconstruction of a 3D view of the sample as a hologram. In a similar study, holographic imaging was used for 4D tracking of human sperm, and the motion of sperms over time was visualized in 3D (Di Caprio et al., 2014). This representation of sperms allowed for the extraction of motility parameters of the semen sample, including straight-line velocity, curvilinear velocity, and average path velocity. In addition to the charge-coupled device, camera sensor and the partially spatial coherent laser beam, a combination of other optics was used for imaging, including a rotation ground glass, two objective lenses, a collimating lens, three mirrors, two beam splitters, a focusing lens, and a refocusing lens. Digital holography was also applied in another study for fertility testing and it was used to measure parameters such as sperm motility, morphology, and DNA fragmentation (Ben-Yehuda et al., 2021).
Concluding remarks
While significant advancements have been made in the field of reproductive medicine, infertility continues to impact couples worldwide. Semen and sperm analysis tests along with sperm sorting methods for use in ARTs remain expensive, time-consuming, and inconvenient. To overcome limitations of conventional methods, microfluidics have been used to engineer rapid, cost-efficient, convenient in-home fertility tests accurately measuring sperm concentration in semen, sperm motility, sperm DNA, and other sperm functionalities as well as to engineer POC diagnostic tools and effective sperm sorting methods for use in clinical settings. Combining different approaches with microfluidics technologies, using artificial intelligence, cloud, or machine learning, utilizing lens-free imaging, and generating digital holography have emerged as promising directions to improve accuracy and precision of microfluidics devices for use in fertility testing (Nosrati et al., 2017; You et al., 2021).
Acknowledgments
S.T. acknowledges Tubitak 2232 International Fellowship for Outstanding Researchers Award (118C391), Alexander von Humboldt Research Fellowship for Experienced Researchers, Marie Skłodowska-Curie Individual Fellowship (101003361), and Royal Academy Newton-Katip Çelebi Transforming Systems Through Partnership award (120N019) for financial support of this research. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the TÜBİTAK. This work was partially supported by Science Academy’s Young Scientist Awards Program (BAGEP), Outstanding Young Scientists Awards (GEBİP), and Bilim Kahramanlari Dernegi The Young Scientist Award. This study was conducted using the service and infrastructure of Koç University Translational Medicine Research Center (KUTTAM). Some elements in Figure 1 were designed using resources from freepik.com.
Author contributions
Writing–Original Draft, M.R.S., D.Y., and M.M.A.; Writing–Review & Editing, S.T., B.A.M., B.A., and C.H.; Supervision, S.T.; Funding Acquisition, S.T. All authors revised and approved the final version of the manuscript.
Declaration of interests
The authors declare the following competing financial interest(s): S.T. is a co-founder of and has equity interest in GetDeHealth, a company that is developing microfluidic technologies for point-of-care diagnostic and wellness solutions. The interests of S.T. were viewed and managed in accordance with the conflict of interest policies. Author S.T. is a member of the iScience Editorial Board. B.A. is a member of ART Fertility Clinics, a company working on human reproductive medicine. The interests of B.A. were viewed and managed in accordance with the conflict of interest policies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Abe K., Suzuki K., Citterio D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 2008;80:6928–6934. doi: 10.1021/ac800604v. [DOI] [PubMed] [Google Scholar]
- Abe K., Kotera K., Suzuki K., Citterio D. Inkjet-printed paperfluidic immuno-chemical sensing device. Anal. Bioanal. Chem. 2010;398:885–893. doi: 10.1007/s00216-010-4011-2. [DOI] [PubMed] [Google Scholar]
- Akyazi T., Basabe-Desmonts L., Benito-Lopez F. Review on microfluidic paper-based analytical devices towards commercialisation. Anal. Chim. Acta. 2018;1001:1–17. doi: 10.1016/j.aca.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Amin R., Ghaderinezhad F., Li L., Lepowsky E., Yenilmez B., Knowlton S., Tasoglu S. Continuous-ink, multiplexed pen-plotter approach for low-cost, high-throughput fabrication of paper-based microfluidics. Anal. Chem. 2017;89:6351–6357. doi: 10.1021/acs.analchem.7b01418. [DOI] [PubMed] [Google Scholar]
- Amin R., Knowlton S., Yenilmez B., Hart A., Joshi A., Tasoglu S. Smart-phone attachable, flow-assisted magnetic focusing device. RSC Adv. 2016;6:93922–93931. [Google Scholar]
- Bandalusena H.H., Zimmerman W.B., Rees J.M. Creeping flow analysis of an integrated microfluidic device for rheometry. J. Non-Newtonian Fluid Mech. 2010;165:1302–1308. [Google Scholar]
- Ben-Yehuda K., Mirsky S.K., Levi M., Barnea I., Meshulach I., Kontente S., Benvaish D., Cur-Cycowicz R., Nygate Y.N., Shaked N.T. Simultaneous morphology, motility, and fragmentation analysis of live individual sperm cells for male fertility evaluation. Advanced Intelligent Systems. 2021;4:2100200. [Google Scholar]
- Bilić-Zulle L. Comparison of methods: passing and bablok regression. Biochem. Med. 2011;21:49–52. doi: 10.11613/bm.2011.010. [DOI] [PubMed] [Google Scholar]
- Bruzewicz D.A., Reches M., Whitesides G.M. Low-cost printing of poly(dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008;80:3387–3392. doi: 10.1021/ac702605a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton D.E., Moley K.H. Obesity and female infertility: potential mediators of obesity's impact. Fertil. Steril. 2017;107:840–847. doi: 10.1016/j.fertnstert.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Brugo-Olmedo S., Chillik C., Kopelman S. Definition and causes of infertility. Reprod. Biomed. Online. 2001;2:41–53. doi: 10.1016/s1472-6483(10)62187-6. [DOI] [PubMed] [Google Scholar]
- Cummins B.M., Chinthapatla R., Lenin B., Ligler F.S., Walker G.M. Modular pumps as programmable hydraulic batteries for microfluidic devices. Technology. 2017;05:21–30. [Google Scholar]
- Carrilho E., Martinez A.W., Whitesides G.M. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009;81:7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- Cate D.M., Adkins J.A., Mettakoonpitak J., Henry C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015;87:19–41. doi: 10.1021/ac503968p. [DOI] [PubMed] [Google Scholar]
- Cho B.S., Schuster T.G., Zhu X., Chang D., Smith G.D., Takayama S. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 2003;75:1671–1675. doi: 10.1021/ac020579e. [DOI] [PubMed] [Google Scholar]
- Chin C.D., Linder V., Sia S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip. 2012;12:2118–2134. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- Chitnis G., Ding Z., Chang C.-L., Savran C.A., Ziaie B. Laser-treated hydrophobic paper: an inexpensive microfluidic platform. Lab Chip. 2011;11:1161–1165. doi: 10.1039/c0lc00512f. [DOI] [PubMed] [Google Scholar]
- Chiu D.T., Demello A.J., Di Carlo D., Doyle P.S., Hansen C., Maceiczyk R.M., Wootton R.C. Small but perfectly formed? Successes, challenges, and opportunities for microfluidics in the chemical and biological sciences. Chem. 2017;2:201–223. [Google Scholar]
- Coppola M.A., Klotz K.L., Kim K.A., Cho H.Y., Kang J., Shetty J., Howards S.S., Flickinger C.J., Herr J.C. SpermCheck Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum. Reprod. 2010;25:853–861. doi: 10.1093/humrep/dep413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford G.E., Ledger W.L. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG. 2019;126:237–243. doi: 10.1111/1471-0528.15526. [DOI] [PubMed] [Google Scholar]
- Dabbagh S.R., Becher E., Ghaderinezhad F., Havlucu H., Ozcan O., Ozkan M., Yetisen A.K., Tasoglu S. Increasing the packing density of assays in paper-based microfluidic devices. Biomicrofluidics. 2021;15:011502. doi: 10.1063/5.0042816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbagh S.R., Rabbi F., Doğan Z., Yetisen A.K., Tasoglu S. Machine learning-enabled multiplexed microfluidic sensors. Biomicrofluidics. 2020;14:061506. doi: 10.1063/5.0025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbagh S.R., Sarabi M.R., Rahbarghazi R., Sokullu E., Yetisen A.K., Tasoglu S. 3D-printed microneedles in biomedical applications. iScience. 2021;24:102012. doi: 10.1016/j.isci.2020.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo D. Inertial microfluidics. Lab Chip. 2009;9:3038–3046. doi: 10.1039/b912547g. [DOI] [PubMed] [Google Scholar]
- Di Caprio G., El Mallahi A., Ferraro P., Dale R., Coppola G., Dale B., Coppola G., Dubois F. 4D tracking of clinical seminal samples for quantitative characterization of motility parameters. Biomedical Optics Express. 2014;5:690–700. doi: 10.1364/BOE.5.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Caprio G., Ferrara M.A., Miccio L., Merola F., Memmolo P., Ferraro P., Coppola G. Holographic imaging of unlabelled sperm cells for semen analysis: a review. J. Biophotonics. 2015;8:779–789. doi: 10.1002/jbio.201400093. [DOI] [PubMed] [Google Scholar]
- Dimitriadis I., L Bormann C., Kanakasabapathy M.K., Thirumalaraju P., Kandula H., Yogesh V., Gudipati N., Natarajan V., C Petrozza J., Shafiee H. Automated smartphone-based system for measuring sperm viability, DNA fragmentation, and hyaluronic binding assay score. PLoS One. 2019;14:e0212562. doi: 10.1371/journal.pone.0212562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungchai W., Chailapakul O., Henry C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst. 2011;136:77–82. doi: 10.1039/c0an00406e. [DOI] [PubMed] [Google Scholar]
- Einav S., Gerber D., Bryson P.D., Sklan E.H., Elazar M., Maerkl S.J., Glenn J.S., Quake S.R. Discovery of a hepatitis C target and its pharmacological inhibitors by microfluidic affinity analysis. Nat. Biotechnol. 2008;26:1019–1027. doi: 10.1038/nbt.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eravuchira P.J., Mirsky S.K., Barnea I., Levi M., Balberg M., Shaked N.T. Individual sperm selection by microfluidics integrated with interferometric phase microscopy. Methods. 2018;136:152–159. doi: 10.1016/j.ymeth.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Fenton E.M., Mascarenas M.R., López G.P., Sibbett S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces. 2008;1:124–129. doi: 10.1021/am800043z. [DOI] [PubMed] [Google Scholar]
- Gerrits T., Shaw M. Biomedical infertility care in sub-Saharan Africa: a social science-- review of current practices, experiences and view points. Facts Views Vis. Obgyn. 2010;2:194–207. [PMC free article] [PubMed] [Google Scholar]
- Ghaderinezhad F., Amin R., Temirel M., Yenilmez B., Wentworth A., Tasoglu S. High-throughput rapid-prototyping of low-cost paper-based microfluidics. Sci. Rep. 2017;7:3553–3559. doi: 10.1038/s41598-017-02931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderinezhad F., Ceylan Koydemir H., Tseng D., Karinca D., Liang K., Ozcan A., Tasoglu S. Sensing of electrolytes in urine using a miniaturized paper-based device. Sci. Rep. 2020;10:13620–13629. doi: 10.1038/s41598-020-70456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavarina D. Understanding bland altman analysis. Biochem. Med. 2015;25:141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M.M., Sinton D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 2017;117:8447–8480. doi: 10.1021/acs.chemrev.7b00024. [DOI] [PubMed] [Google Scholar]
- Gong M.M., Nosrati R., San Gabriel M.C., Zini A., Sinton D. Direct DNA analysis with paper-based ion concentration polarization. J. Am. Chem. Soc. 2015;137:13913–13919. doi: 10.1021/jacs.5b08523. [DOI] [PubMed] [Google Scholar]
- Haigler C.H. Dekker; 1990. Biosynthesis and Biodegradation of Cellulose. [Google Scholar]
- Hamilton T.R.D.S., Assumpção M.E.O.D. Sperm DNA fragmentation: causes and identification. Zygote. 2020;28:1–8. doi: 10.1017/S0967199419000595. [DOI] [PubMed] [Google Scholar]
- Han X.X., Jia H.Y., Wang Y.F., Lu Z.C., Wang C.X., Xu W.Q., Zhao B., Ozaki Y. Analytical technique for label-free multi-protein detection based on Western blot and surface-enhanced Raman scattering. Anal. Chem. 2008;80:2799–2804. doi: 10.1021/ac702390u. [DOI] [PubMed] [Google Scholar]
- Henares T.G., Yamada K., Takaki S., Suzuki K., Citterio D. “Drop-slip” bulk sample flow on fully inkjet-printed microfluidic paper-based analytical device. Sensor. Actuator. B Chem. 2017;244:1129–1137. [Google Scholar]
- Hillscher L.M., Liebich V.J., Avrutina O., Biesalski M., Kolmar H. Functional paper-based materials for diagnostics. Chemtexts. 2021;7:14. doi: 10.1007/s40828-021-00139-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeesan K.K., Kumar S., Sumana G. Application of conducting paper for selective detection of troponin. Electrochem. Commun. 2012;20:71–74. [Google Scholar]
- Jiang S., Zhu J., Song P., Guo C., Bian Z., Wang R., Huang Y., Wang S., Zhang H., Zheng G. Wide-field, high-resolution lensless on-chip microscopy via near-field blind ptychographic modulation. Lab Chip. 2020;20:1058–1065. doi: 10.1039/c9lc01027k. [DOI] [PubMed] [Google Scholar]
- Kanakasabapathy M.K., Sadasivam M., Singh A., Preston C., Thirumalaraju P., Venkataraman M., Bormann C.L., Draz M.S., JC P., H S., et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci. Transl. Med. 2017;9:eaai7863. doi: 10.1126/scitranslmed.aai7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalme S., Kandaswamy S., Chandrasekharmath A., Katiyar R., Rajamanickam G.P., Kumar S., Dendukuri D. A hydrogel sensor-based microfluidic platform for the quantitative and multiplexed detection of fertility markers for point-of-care immunoassays. Anal. Methods. 2019;11:1639–1650. [Google Scholar]
- Khodamoradi M., Rafizadeh Tafti S., Mousavi Shaegh S.A., Aflatoonian B., Azimzadeh M., Khashayar P. Recent microfluidic innovations for sperm sorting. Chemosensors. 2021;9:126. [Google Scholar]
- Kim S.B., Bae H., Koo K.-I., Dokmeci M.R., Ozcan A., Khademhosseini A. Lens-free imaging for biological applications. J. Lab. Autom. 2012;17:43–49. doi: 10.1177/2211068211426695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Chun K. New disposable microfluidic chip without evaporation effect for semen analysis in clinics and homes. Microsyst. Technol. 2020;26:647–655. [Google Scholar]
- Knowlton S.M., Sadasivam M., Tasoglu S. Microfluidics for sperm research. Trends Biotechnol. 2015;33:221–229. doi: 10.1016/j.tibtech.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Kokalj T., Park Y., Vencelj M., Jenko M., Lee L.P. Self-powered imbibing microfluidic pump by liquid encapsulation. Lab Chip. 2014;14:4329–4333. doi: 10.1039/c4lc00920g. [DOI] [PubMed] [Google Scholar]
- Kumar N., Singh A.K. Trends of male factor infertility, an important cause of infertility: a review of literature. J. Hum. Reprod. Sci. 2015;8:191–196. doi: 10.4103/0974-1208.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong P., Gupta M. Vapor phase deposition of functional polymers onto paper-based microfluidic devices for advanced unit operations. Anal. Chem. 2012;84:10129–10135. doi: 10.1021/ac302861v. [DOI] [PubMed] [Google Scholar]
- Lam T., Devadhasan J.P., Howse R., Kim J. A chemically patterned microfluidic paper-based analytical device (C-microPAD) for point-of-care diagnostics. Sci. Rep. 2017;7:1188. doi: 10.1038/s41598-017-01343-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits G., Zutra D., Bar-Joseph M. Enzyme-linked immunosorbent assay on nitrocellulose membranes (dot-ELISA) in the serodiagnosis of plant pathogenic bacteria. Can. J. Microbiol. 1987;33:98–103. doi: 10.1139/m87-017. [DOI] [PubMed] [Google Scholar]
- Lepowsky E., Ghaderinezhad F., Knowlton S., Tasoglu S. Paper-based assays for urine analysis. Biomicrofluidics. 2017;11:051501. doi: 10.1063/1.4996768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesani A., Kazemnejad S., Moghimi Zand M., Azadi M., Jafari H., Mofrad M.R.K., Nosrati R. Quantification of human sperm concentration using machine learning-based spectrophotometry. Comput. Biol. Med. 2020;127:104061. doi: 10.1016/j.compbiomed.2020.104061. [DOI] [PubMed] [Google Scholar]
- Lesani A., Ramazani Sarbandi I., Mousavi H., Kazemnejad S., Moghimi Zand M. Lower reactive oxygen species production and faster swimming speed of human sperm cells on nanodiamond spin-coated glass substrates. J. Biomed. Mater. Res. B Appl. Biomater. 2022;110:1391–1399. doi: 10.1002/jbm.b.35007. [DOI] [PubMed] [Google Scholar]
- Li X., Ballerini D.R., Shen W. A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics. 2012;6:11301–1130113. doi: 10.1063/1.3687398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutradi K.E., Tarlatzis B.C., Goulis D.G., Zepiridis L., Pagou T., Chatziioannou E., Grimbizis G.F., Papadimas I., Bontis I. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2006;23:69–74. doi: 10.1007/s10815-006-9022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Güven S., Gozen I., Chen P., Tasoglu S., Anchan R.M., Bai B., Demirci U. Deformation of a single mouse oocyte in a constricted microfluidic channel. Microfluid. Nanofluidics. 2015;19:883–890. doi: 10.1007/s10404-015-1614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Shi W., Qin J., Lin B. Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal. Chem. 2010;82:329–335. doi: 10.1021/ac9020193. [DOI] [PubMed] [Google Scholar]
- Lu Y., Shi W., Jiang L., Qin J., Lin B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis. 2009;30:1497–1500. doi: 10.1002/elps.200800563. [DOI] [PubMed] [Google Scholar]
- Li X., Tian J., Garnier G., Shen W. Fabrication of paper-based microfluidic sensors by printing. Colloids and surfaces Biointerfaces. 2010;76:564–570. doi: 10.1016/j.colsurfb.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Li X., Tian J., Nguyen T., Shen W. Paper-based microfluidic devices by plasma treatment. Anal. Chem. 2008;80:9131–9134. doi: 10.1021/ac801729t. [DOI] [PubMed] [Google Scholar]
- Lisa M., Chouhan R.S., Vinayaka A.C., Manonmani H.K., Thakur M.S. Gold nanoparticles based dipstick immunoassay for the rapid detection of dichlorodiphenyltrichloroethane: an organochlorine pesticide. Biosens. Bioelectron. 2009;25 doi: 10.1016/j.bios.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Manoharan K., Anwar M.T., Bhattacharya S. Development of hydrophobic paper substrates using silane and sol–gel based processes and deriving the best coating technique using machine learning strategies. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-90855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A.W., Phillips S.T., Butte M.J., Whitesides G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angewandte. Chemie. 2007;46:1340–1342. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A.W., Phillips S.T., Wiley B.J., Gupta M., Whitesides G.M. FLASH: a rapid method for prototyping paper-based microfluidic devices. Lab Chip. 2008;8:2146–2150. doi: 10.1039/b811135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryam Okhovati M.Z., Fatemeh Z., Maliheh S.B., Azam B. Trends in global assisted reproductive technologies research: a scientometrics study. Electron. Physician. 2015;7:1597. doi: 10.19082/1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K., Chen K.-H., Tsai C.-H., Li W., Asano Y., Naruse K., Cheng C.-M. Paper-based diagnostic devices for evaluating the quality of human sperm. Microfluid. Nanofluidics. 2014;16:857–867. [Google Scholar]
- Mccormack M.C., Mccallum S., Behr B. A novel microfluidic device for male subfertility screening. J. Urol. 2006;175:2223–2227. doi: 10.1016/S0022-5347(06)00276-X. [DOI] [PubMed] [Google Scholar]
- Müller R.H., Clegg D.L. Automatic paper chromatography. Anal. Chem. 1949;21:1123–1125. [Google Scholar]
- Nishat S., Jafry A.T., Martinez A.W., Awan F.R. Paper-based microfluidics: simplified fabrication and assay methods. Sensor. Actuator. B Chem. 2021;336:129681. [Google Scholar]
- Nosrati R., Gong M.M., San Gabriel M.C., Zini A., Sinton D. Paper-based sperm DNA integrity analysis. Anal. Methods. 2016;8:6260–6264. [Google Scholar]
- Nosrati R., Graham P.J., Zhang B., Riordon J., Lagunov A., Hannam T.G., Escobedo C., Jarvi K., Sinton D. Microfluidics for sperm analysis and selection. Nat. Rev. Urol. 2017;14:707–730. doi: 10.1038/nrurol.2017.175. [DOI] [PubMed] [Google Scholar]
- Nosrati R., Gong M.M., San Gabriel M.C., Zini A., Sinton D. Paper-based quantification of male fertility potential. Clin. Chem. 2016;62:458–465. doi: 10.1373/clinchem.2015.250282. [DOI] [PubMed] [Google Scholar]
- Noh H., Phillips S.T. Metering the capillary-driven flow of fluids in paper-based microfluidic devices. Anal. Chem. 2010;82:4181–4187. doi: 10.1021/ac100431y. [DOI] [PubMed] [Google Scholar]
- Osborn J.L., Lutz B., Fu E., Kauffman P., Stevens D.Y., Yager P. Microfluidics without pumps: reinventing the T-sensor and H-filter in paper networks. Lab Chip. 2010;10:2659–2665. doi: 10.1039/c004821f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen J., Lehtinen K., Erho T. Flexographically printed fluidic structures in paper. Anal. Chem. 2010;82:10246–10250. doi: 10.1021/ac1027066. [DOI] [PubMed] [Google Scholar]
- Otten A.B.C., Smeets H.J.M. Evolutionary defined role of the mitochondrial DNA in fertility, disease and ageing. Hum. Reprod. Update. 2015;21:671–689. doi: 10.1093/humupd/dmv024. [DOI] [PubMed] [Google Scholar]
- Rahmani Dabbagh S., Rezapour Sarabi M., Birtek M.T., Mustafaoglu N., Zhang Y.S., Tasoglu S. 3D bioprinted organ-on-chips. Aggregate. 2022;2022:e197. [Google Scholar]
- Rezapour Sarabi M., Alseed M.M., Karagoz A.A., Tasoglu S. Machine learning-enabled prediction of 3D-printed microneedle features. Biosensors. 2022;12:491. doi: 10.3390/bios12070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezapour Sarabi M., Jiang N., Ozturk E., Yetisen A.K., Tasoglu S. Biomedical optical fibers. Lab Chip. 2021;21:627–640. doi: 10.1039/d0lc01155j. [DOI] [PubMed] [Google Scholar]
- Rezapour Sarabi M., Nakhjavani S.A., Tasoglu S. 3D-Printed microneedles for point-of-care biosensing applications. Micromachines. 2022;13:1099. doi: 10.3390/mi13071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk A.R., Qi A., Friend J.R., Li W.H., Yeo L.Y. Uniform mixing in paper-based microfluidic systems using surface acoustic waves. Lab Chip. 2012;12:773–779. doi: 10.1039/c2lc21065g. [DOI] [PubMed] [Google Scholar]
- Sarabi M.R., Ahmadpour A., Yetisen A.K., Tasoglu S. Finger-actuated microneedle array for sampling body fluids. Appl. Sci. 2021;11:5329. [Google Scholar]
- Sarabi M.R., Bediz B., Falo L.D., Korkmaz E., Tasoglu S. 3D printing of microneedle arrays: challenges towards clinical translation. J. 3D Printing Med. 2021;5:65–70. [Google Scholar]
- Sarabi M.R., Yetisen A.K., Tasoglu S. Magnetic levitation for space exploration. Trends Biotechnol. 2022;40:915–917. doi: 10.1016/j.tibtech.2022.03.010. [DOI] [PubMed] [Google Scholar]
- Sarbandi I.R., Lesani A., Moghimi Zand M., Nosrati R. Rheotaxis-based sperm separation using a biomimicry microfluidic device. Sci. Rep. 2021;11:18327. doi: 10.1038/s41598-021-97602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri B., Goswami D., Martinez R.V. Rapid fabrication of epidermal paper-based electronic devices using razor printing. Micromachines. 2018;9:E420. doi: 10.3390/mi9090420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K.M., Lepore A.L., Kurian J.A., Martinez A.W. Fully enclosed microfluidic paper-based analytical devices. Anal. Chem. 2012;84:1579–1585. doi: 10.1021/ac202837s. [DOI] [PubMed] [Google Scholar]
- Shields C.W., 4th, Ohiri K.A., Szott L.M., López G.P. Translating microfluidics: cell separation technologies and their barriers to commercialization. Cytometry B Clin. Cytom. 2017;92:115–125. doi: 10.1002/cyto.b.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songjaroen T., Dungchai W., Chailapakul O., Laiwattanapaisal W. Novel, simple and low-cost alternative method for fabrication of paper-based microfluidics by wax dipping. Talanta. 2011;85:2587–2593. doi: 10.1016/j.talanta.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Sohrabi S., Kassir N., Keshavarz Moraveji M. Droplet microfluidics: fundamentals and its advanced applications. RSC Adv. 2020;10:27560–27574. doi: 10.1039/d0ra04566g. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Spicar-Mihalic P., Toley B., Houghtaling J., Liang T., Yager P., Fu E. CO2 laser cutting and ablative etching for the fabrication of paper-based devices - IOPscience. J. Micromech. Microeng. 2013;23:067003. [Google Scholar]
- Su T.W., Xue L., Ozcan A. High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories. Proc. Natl. Acad. Sci. USA. 2012;109:16018–16022. doi: 10.1073/pnas.1212506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Gong T., Jiang Y., Zhang S., Zhao Y., Wu Q. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: results from a global burden of disease study, 2017. Aging. 2019:10952–10991. doi: 10.18632/aging.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Jia Y., Dong H., Fan L., Zheng J. Multiplex quantification of metals in airborne particulate matter via smartphone and paper-based microfluidics. Anal. Chim. Acta. 2018;1044:110–118. doi: 10.1016/j.aca.2018.07.053. [DOI] [PubMed] [Google Scholar]
- Tsai V.F., Zhuang B., Pong Y.H., Hsieh J.T., Chang H.C. Web- and artificial intelligence-based image recognition for sperm motility analysis: verification study. JMIR Med. Inform. 2020;8:e20031. doi: 10.2196/20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasoglu S. Toilet-based continuous health monitoring using urine. Nat. Rev. Urol. 2022;19:219–230. doi: 10.1038/s41585-021-00558-x. [DOI] [PubMed] [Google Scholar]
- Tasoglu S., Cumhur Tekin H., Inci F., Knowlton S., Wang S.Q., Wang-Johanning F., Johanning G., Colevas D., Demirci U. Advances in nanotechnology and microfluidics for human papillomavirus diagnostics. Proc. IEEE. 2015;103:161–178. [Google Scholar]
- Tsao Y., Yang C., Wen Y., Chang T., Matsuura K., Chen Y., Cheng C. Point-of-care semen analysis of patients with infertility via smartphone and colorimetric paper-based diagnostic device. Bioeng. Transl. Med. 2020;6:e10176. doi: 10.1002/btm2.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temirel M., Dabbagh S.R., Tasoglu S. Hemp-based microfluidics. Micromachines. 2021;12:182. doi: 10.3390/mi12020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K.T.L., Chae W.R., Lee N.Y. Recent advances in the fabrication strategies of paper-based microfluidic devices for rapid detection of bacteria and viruses. Microchem. J. 2022;180:107548. [Google Scholar]
- Thoma M., Fledderjohann J., Cox C., Kantum Adageba R. Oxford Research Encyclopedia of Global Public Health. 2021. Biological and social aspects of human infertility: a global perspective. [Google Scholar]
- Thapa S., Heo Y.S. Microfluidic technology for in vitro fertilization (IVF) JMST Adv. 2019;1:11. [Google Scholar]
- Volpatti L.R., Yetisen A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014;32:347–350. doi: 10.1016/j.tibtech.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Wang J., Monton M.R.N., Zhang X., Filipe C.D.M., Pelton R., JD B., Brennan J.D. Hydrophobic sol-gel channel patterning strategies for paper-based microfluidics. Lab Chip. 2014;14:691–695. doi: 10.1039/c3lc51313k. [DOI] [PubMed] [Google Scholar]
- Weiß L.J.K., Lubins G., Music E., Rinklin P., Banzet M., Peng H., Terkan K., Mayer D., Wolfrum B. Single-impact electrochemistry in paper-based microfluidics. ACS Sens. 2022;7:884–892. doi: 10.1021/acssensors.1c02703. [DOI] [PubMed] [Google Scholar]
- Vander Borght M., Wyns C. Fertility and infertility: definition and epidemiology. Clin. Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Vaught R.C., Dowling D.K. Maternal inheritance of mitochondria: implications for male fertility? Reproduction. 2018;155:R159–R168. doi: 10.1530/REP-17-0600. [DOI] [PubMed] [Google Scholar]
- Yang M., Zhang W., Zheng W., Cao F., Jiang X. Inkjet-printed barcodes for a rapid and multiplexed paper-based assay compatible with mobile devices. Lab Chip. 2017;17:3874–3882. doi: 10.1039/c7lc00780a. [DOI] [PubMed] [Google Scholar]
- Yetisen A.K., Akram M.S., Lowe C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip. 2013;13:2210–2251. doi: 10.1039/c3lc50169h. [DOI] [PubMed] [Google Scholar]
- You J.B., Mccallum C., Wang Y., Riordon J., Nosrati R., Sinton D. Machine learning for sperm selection. Nat. Rev. Urol. 2021;18:387–403. doi: 10.1038/s41585-021-00465-1. [DOI] [PubMed] [Google Scholar]
- Younas M., Maryam A., Khan M., Nawaz A.A., Jaffery S.H.I., Anwar M.N., Ali L. Parametric analysis of wax printing technique for fabricating microfluidic paper-based analytic devices (μPAD) for milk adulteration analysis. Microfluid. Nanofluidics. 2019;23:38. [Google Scholar]