Abstract
Much of the normal high sensitivity of wild-type Helicobacter pylori to metronidazole (Mtz) depends on rdxA (HP0954), a gene encoding a novel nitroreductase that catalyzes the conversion of Mtz from a harmless prodrug to a bactericidal agent. Here we report that levels of Mtz that partially inhibit growth stimulate forward mutation to rifampin resistance in rdxA+ (Mtzs) and also in rdxA (Mtzr) H. pylori strains, and that expression of rdxA in Escherichia coli results in equivalent Mtz-induced mutation. A reversion test using defined lac tester strains of E. coli carrying rdxA+ indicated that CG-to-GC transversions and AT-to-GC transitions are induced more frequently than other base substitutions. Alkaline gel electrophoretic tests showed that Mtz concentrations near or higher than the MIC for growth also caused DNA breakage in H. pylori and in E. coli carrying rdxA+, suggesting that this damage may account for most of the bactericidal action of Mtz. Coculture of Mtzs H. pylori with E. coli (highly resistant to Mtz) in the presence of Mtz did not stimulate forward mutation in E. coli, indicating that the mutagenic and bactericidal products of Mtz metabolism do not diffuse significantly to neighboring (bystander) cells. Our results suggest that the widespread use of Mtz against other pathogens in people chronically infected with H. pylori may stimulate mutation and recombination in H. pylori, thereby speeding host-specific adaptation, the evolution of virulence, and the emergence of resistance against Mtz and other clinically useful antimicrobials.
Metronidazole (Mtz) [1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole] and related 5-nitroimidazoles are redox-active prodrugs that are often used to treat infections caused by anaerobic bacteria and protozoa (7, 18, 28). They are also a key component of combination therapies that are used to eradicate Helicobacter pylori, the microaerophilic bacterium which chronically infects the stomachs of more than half of all people worldwide and is the major cause of peptic ulcer disease and an early risk factor for gastric cancer (3, 14, 20, 30). In anaerobes, redox-active enzymes such as pyruvate/ketoacid oxidoreductases and hydrogenase, active with the low-redox carriers (ferredoxin and flavodoxin), reduce 5-nitroimidazoles to mutagenic products that also cause DNA helix destabilization and single- and double-strand DNA breakage (7, 18, 19, 28). High-level Mtz resistance is rare in anaerobes, because the activating enzymes are essential components of core metabolic pathways and because these microbes generally contain cytoplasmic components of very low redox potential that can spontaneously activate the drug (7, 19, 23, 28). In contrast, moderate to high-level resistance to nitroimidazoles is common among H. pylori clinical isolates, with frequencies ranging from ∼10 to 90% or more of strains, depending on geographic region (12). These frequencies generally reflect the incidence of Mtz usage against other (parasitic or anaerobic bacterial) infections in particular societies (6, 10).
We have found that (i) nearly all Mtz-resistant (Mtzr) H. pylori clinical isolates contain loss-of-function mutations in rdxA (HP0954 in reference 27), an H. pylori gene that encodes a nonessential oxygen-insensitive NADPH nitroreductase (RdxA); (ii) mutational inactivation of rdxA is both necessary and sufficient to confer an Mtzr phenotype; and (iii) expression of the H. pylori rdxA+ gene in E. coli renders this normally Mtzr bacterium susceptible to Mtz (5, 9, 17). Several other studies have confirmed an association between loss-of-function mutations in rdxA and Mtz resistance (15, 26). RdxA, a homolog of the classical nitroreductases of enteric bacteria, exhibits NADPH-dependent Mtz reductase activity and a substrate preference for Mtz that may be related to its unique cysteine-rich nature and alkaline pI (9). The stoichiometry of the RdxA-catalyzed reduction of Mtz indicates an anaerobic two-step (four-electron) reduction of the 5-nitro group of Mtz to mutagenic and DNA-damaging nitroso and hydroxylamine products. Although the DNA sequences of rdxA genes from unrelated strains typically differed at some 5% of nucleotide positions (as is quite typical of housekeeping genes in H. pylori), the rdxA genes from related Mtzr and Mtzs strains recovered from infections that were mixed (Mtzr, Mtzs), but uniform in overall DNA fingerprint type, differed by only one or a few base pairs. This indicated that Mtz resistance tends to result from de novo mutation in the resident rdxA gene, rather than from lateral transfer of mutant rdxA (or other) genes from unrelated but Mtzr strains. Equivalent results were obtained with a dozen patients from France and North Africa (26). These findings suggested that Mtz-based therapies might be mutagenic, inducing as well as selecting for Mtz resistance in H. pylori (9).
Here we show directly that products of Mtz activation are mutagenic and DNA damaging in H. pylori and in Escherichia coli containing a cloned rdxA+ gene. These findings have implications for the emergence of resistance to various antibacterial agents and more generally to the evolution of virulence and the adaptation of H. pylori to individual hosts.
MATERIALS AND METHODS
Bacterial strains and DNA manipulations.
H. pylori Mtzs strains 950 and 1134 and Mtzr strain 1134R have been described elsewhere (9). Strain 26695 (27) and its Mtzr (rdxA deletion mutant) derivatives 16R and 64R (carrying null mutations in rdxA and in rdxA and frxA; resistant to Mtz at 16 and 64 μg/ml, respectively) are described elsewhere (17). All H. pylori strains were cultured under microaerobic conditions on either Brucella agar supplemented with 7.5% newborn calf serum (NCS; Sigma) (9, 13) or brain heart infusion (BHI) agar with horse blood (17). E. coli strains CC101, CC103, CC104, CC105, and CC106 [araΔ(lac proB)/F′ lacI lacZ proAB+) contain mutations in lacZ that render them unable to ferment lactose (4). The rdxA gene from H. pylori strain 950 was amplified by PCR using previously described rdxA oligonucleotide primers (9) and cloned into the pBluescript (pBSK) cloning vector plasmid, creating pGS950. E. coli strains expressing RdxA nitroreductase are susceptible to Mtz (10 to 30 μg/ml) when tested on Luria-Bertani (LB) medium (9).
Mtz-induced mutation in H. pylori: Rifr.
New mutations to rifampin resistance (Rifr) which result from changes in rpoB (11) were quantified as a measure of Mtz-induced mutation in H. pylori. Frozen cultures of isogenic Mtzs and Mtzr strains of H. pylori (26695, 16R, and 64R) were streaked onto BHI blood agar plates and incubated for 3 days. Individual colonies were respread on fresh medium without Mtz and incubated for 3 days; then 107 to 108 bacterial cells were spread onto BHI blood agar plates containing various concentration of Mtz (0, 1, 2, and 3 μg/ml for 26695 (wild type); 0, 3, 8, 16, 25, and 32 μg/ml for 16R; 0, 8, 16, 32, and 90 μg/ml for 64R). Following 3 days of incubation, bacterial cells were suspended in phosphate-buffered saline, and spread on BHI blood agar with rifampin (5 μg/ml); the cells were titered by spotting aliquots of serial 10-fold dilutions on rifampin-free medium. Each test was carried out with six separate single-test cell clones. The data were normalized to 108 CFU and expressed as the mean, with P values determined by the general linear model (25).
Mtz-induced mutation in E. coli: Rifr.
New mutations to Rifr were quantified as a measure of Mtz-induced mutation in E. coli tester strains harboring pGS950 (rdxA+) or the parent pBSK vector. E. coli strains were grown for 12 h in LB broth containing Mtz at concentrations of 0, 5, 10, and 15 μg/ml, and aliquots were spread on LB agar containing rifampin (25 μg/ml) and on rifampin-free LB agar. The data were normalized to 108 bacteria and expressed as the mean of triplicate platings with an error of ±20%.
Mtz-induced mutation in E. coli: lac papillation assay of specificity.
E. coli tester strains (CC101, CC103, CC104, CC105, and CC106) harboring either pGS950 (rdxA+) or pBSK control plasmid were grown overnight in LB broth containing ampicillin (100 μg/ml; Boehringer Mannheim) at 37°C. The cultures were standardized to an optical density at 600 nm (OD600) of 1.0, and decimal dilutions were prepared in phosphate-buffered saline. Dilutions were plated onto minimal A (MinA) agar medium containing 0.2% glucose, 1 mM MgSO4 · 7H2O, 500 μg of phenyl-β-d-galactoside (Sigma) per ml, and 100 μg of ampicillin per ml (1), supplemented with Mtz at concentrations of 0, 5, 10, and 15 μg/ml. Immediately before use, the plates were spread with 40 μl of X-Gal (5-bromo-4-chloro-3-indolyl β-d-galactoside; American Biorganics). Total counts were determined by dilution and spreading on MinA (1% glucose) agar plates, which were then incubated for 24 to 36 h. All experiments were repeated three times, all determinations were performed in triplicate in each experiment, and the means and standard deviations were computed. The papillation frequency was determined as the number of blue (β-galactosidase-positive) papillae per total viable count.
Bystander effect.
To test for Mtz-induced mutation of bystander cells, a mixture of 108 cells of H. pylori strain 26695 or its rdxA deletion (Mtzr) derivative 16R plus 108 cells of E. coli tester strain CC103 at ratios of 1:1, 2:1, and 10:1 was spread on papillation assay plates containing various concentrations of Mtz (0, 5, 10, 15, 32, and 64 μg/ml) as detailed above, except that the plates were incubated under microaerobic conditions. Mtz-induced killing of E. coli was determined under microaerobic conditions by dilution at each Mtz concentration (presence or absence of H. pylori) by viable count on MinA medium supplemented with 1% glucose. The papillation medium does not support growth of H. pylori. In a parallel test for Mtz-induced bystander killing, 108 cells of an Mtzs H. pylori strain were mixed with 1,000 E. coli cells, and the mixture was spread on a 4-cm2 area of appropriate agar media (L agar and BHI with serum, plus 0, 10, or 20 μg of Mtz per ml). Incubations were carried out under microaerobic conditions.
Alkaline gel DNA analysis.
H. pylori strains 26695 (wild type) and 1134 (Mtzs) and rdxA mutant (Mtzr) derivatives 16R and 1134R, respectively, were grown overnight on Brucella agar plates containing 7.5% NCS, collected by centrifugation (7,000 × g), suspended in Brucella broth, and standardized to an OD600 of 0.25. One milliliter of suspension was added to each 500-ml sidearm flask containing 100 ml of Brucella broth and NCS. A defined amount of Mtz was then placed in the side arm of the flask. After being incubated statically in a microaerobic incubator for 6 h, the flasks were sealed and placed on a gyratory shaker at 100 rpm for 16 to 20 h at 37°C. Mtz was then tipped from the side arm into the broth, which was incubated for 30 min with shaking. Aliquots (1 ml) of the cultures were then harvested and prepared for assessment of DNA fragmentation. As a control, 100 μl of cell suspension was treated with 20 mM hydrogen peroxide at 37°C for 15 min to fragment DNA.
E. coli tester strain CC104 harboring either pBSK or its rdxA+ derivative pGS950 was grown overnight in MinA broth containing ampicillin (100 μg/ml; Boehringer Mannheim) and 0.5% glucose (1) to a standard OD600 of 0.8. One-milliliter aliquots of culture were treated for 30 min at 37°C with Mtz at 0, 10, 20, 40, or 100 μg/ml (final concentration).
Agarose plugs were prepared and treated as described elsewhere (8), with modifications. Briefly, lysozyme (Sigma) and proteinase K (Promega) were added to 100 μl of washed and resuspended cells at a final concentration of 1 mg/ml. Following incubation for 15 min at 37°C, 1.2% molten low-melting-point agarose (Eclipse Molecular Biologicals) and 10% sodium dodecyl sulfate (Sigma) were added to final concentrations of 0.5 and 0.6%, respectively. The mixture was immediately pipetted into plug molds (Bio-Rad Laboratories) and allowed to solidify for 5 to 10 min at 4°C. The agarose plugs were then incubated at 55°C for 16 to 22 h in 2 ml of ESP buffer (pH 9.0), containing 0.5 M EDTA (BDH), 1% (wt/vol) sodium lauryl sarcosine (Sigma), and 1 mg of Proteinase per ml. Following removal of ESP buffer, the plugs were washed 10 min at 55°C with sterile distilled water and four times for 15 min each at 55°C with TE buffer, pH 8.0 (10 mM Tris-HCl, 1 mM EDTA), and then allowed to cool to 4°C.
Alkaline agarose gel electrophoresis generally followed the protocols in reference 31. Briefly, the agarose plugs were placed into the preformed wells of a 0.8% agarose (Vector Biosystems) gel prepared under alkaline conditions (30 mM NaOH, 10 μM EDTA), the wells were sealed in with molten agarose, and then the gel was subjected to electrophoresis for 6 h at 25 mV. The gel was then neutralized for 1 h in 30 mM NaCl–50 mM Tris-HCl (pH 6.0), stained for 1 h with ethidium bromide (0.5 μg/ml; Sigma), destained in distilled water (2 h), and visualized under UV light.
RESULTS
Mtz-induced mutation in H. pylori.
The ability of Mtz to induce mutation in Mtzs and in Mtzr H. pylori was scored by measuring frequencies of Rifr mutants in populations of the normally Rifs H. pylori after 3 days growth on plates containing low, partially inhibitory levels of Mtz. The efficiencies of plating (EOP) of wild-type strain 26695 on medium with 2 and 3 μg of Mtz per ml were about 10−2 and 10−4, respectively, of that on Mtz-free medium (EOP = 1.0). Growth at these partially lethal Mtz concentrations increased the Rifr frequencies among survivors about 6- and 12-fold, respectively (Table 1). In contrast, no significant stimulation of mutation was detected during an equivalent growth period on medium with Mtz at 1 μg/ml, a concentration that did not have any obvious effect on the EOP or colony size of this strain.
TABLE 1.
Mutagenic action of sublethal doses of Mtz on Mtzr and Mtzs H. pylori
| Straina | Mtz dose (μg/ml) | Survivalb | Rifr frequency (108)c | Fold stimulation | Pd |
|---|---|---|---|---|---|
| 26695 | 0 | 1 | 12 | ||
| 1.5R | 1 | 1 | 14 | 1.2 | NSe |
| 2 | 10−2 | 69 | 6 | 0.006 | |
| 3 | 10−4 | 144 | 12 | <0.001 | |
| 16R | 0 | 1 | 8 | ||
| 3 | 1 | 5 | 0.6 | NS | |
| 8 | 1 | 12 | 1.5 | NS | |
| 16 | 1 | 10 | 1.3 | NS | |
| 25 | 10−2 | 213 | 27 | 0.0001 | |
| 32 | 10−4 | 1,328 | 166 | 0.0001 | |
| 64R | 0 | 1 | 30 | ||
| 8 | 1 | 36 | 1.2 | NS | |
| 16 | 1 | 20 | 0.7 | NS | |
| 32 | 1 | 32 | 1.1 | NS | |
| 90 | 10−2 | 302 | 10 | 0.0002 |
The strains used were 26695 (wild type), which is Mtzs (tolerates up to 1.5 μg of Mtz per ml in medium without lethality; 16R, a derivative of 26695 containing an rdxAΔ allele in the rdxA gene (tolerates up to 16 μg/ml); and 64R, a derivative of 16R containing a point mutation in frxA and an additional resistance-enhancing mutation at an unknown locus (tolerates up to 64 μg/ml).
Determined by EOP as described in the text.
Mean frequency of rifampin-resistant mutants in H. pylori populations after 3 days of growth on medium containing the indicated concentration of Mtz. Each value is average of six repetitions, each with a different single cell clone.
Calculated using the general linear model (25) by Bill Shannon.
NS, not significantly different from control (growth in Mtz-free medium).
The Mtzr strain 16R (an rdxA null deletion mutant derivative of 26695), although fully resistant to 16 μg/ml (EOP = 1.0), was partially killed by higher Mtz concentrations (e.g., EOP = 10−2 and 10−4 at 25 and 32 μg/ml, respectively). These Mtz concentrations were found to stimulate mutation to Rifr about 27- and 166-fold, respectively (i.e., even more strongly than had been seen with the wild-type parent grown with Mtz concentrations that were partially lethal to it). In contrast, no such induced mutation was detected in cells treated with lower (subinhibitory) concentrations of Mtz (3, 8, or 16 μg/ml) (Table 1).
Mutant derivatives of strain 16R that are resistant to even higher concentrations of Mtz (e.g., 64 but not 128 μg/ml [strain 64R]) can be obtained by forward mutation at frxA (HP0642 in reference 27; an rdxA paralog) and other loci (17). Although the basis of this heightened Mtz resistance and the residual Mtz susceptibility of these hyperresistant strains are not fully understood, it is significant that the growth of such strains on medium with Mtz at a partially inhibitory concentration (90 μg/ml) was also mutagenic. Again, no stimulation of mutation was detected after growth with the concentrations of Mtz that were fully tolerated by the strain (8, 16, or 32 μg/ml) (Table 1).
Mtz-induced mutation in E. coli expressing H. pylori rdxA.
The ability of Mtz to induce mutation in E. coli K-12 that had been rendered Mtzs by an expressed H. pylori rdxA+ gene (pGS950) was scored by measuring frequencies of Rifr mutants after 12 h of growth in broth containing low levels of Mtz. Table 2 shows that growth of rdxA+-containing E. coli with Mtz at 10 and 15 μg/ml in broth culture decreased viability by 10−2 and 10−4, respectively, and increased the Rifr mutant frequencies ∼100- and ∼340-fold, respectively. These Mtz concentrations had no effect on viability or Rifr frequencies in isogenic control strains that did not carry rdxA+. Thus, the H. pylori RdxA nitroreductase renders E. coli highly mutable by Mtz in a dose-dependent fashion.
TABLE 2.
Frequency of Rifr mutants in E. coli tester strains
| Mtz concn (μg/ml) | Viabilitya | Mutation frequency (no. of Rifr colonies/108 CFU)b
|
Mean ± SD | Fold increase | ||||
|---|---|---|---|---|---|---|---|---|
| CC101 | CC103 | CC104 | CC105 | CC106 | ||||
| 0 | 1 | 0.47 | 0.23 | 0.75 | 0.94 | 0.64 | 0.61 ± 0.27 | |
| 10 | 10−2 | 83 | 88 | 12 | 96 | 52 | 66 ± 34 | ∼100 |
| 15 | 10−4 | 178 | 383 | 118 | 238 | 137 | 210 ± 106 | ∼344 |
Determined by EOP as described in the text.
Mean of three determinations. All strains contained pGS950 (rdxA+), and the data were normalized against data from strains containing pBSK and treated in parallel with Mtz. The bacteria were challenged in LB broth for 12 h in the presence of Mtz as indicated.
Specificity of mutation induced by products of Mtz activation.
To assess the specificity of mutagenesis following Mtz activation, we scored reversion to Lac+ of a series of isogenic Lac− strains that differed only in the codon for a critical residue in the active site of β-galactosidase (essentially as in reference 4). Prior studies had indicated that phenotypic reversion from Lac− to Lac+ occurs by restoration of wild-type sequence in these mutants, not by suppressor mutations elsewhere in lacZ or in the genome as a whole. Table 3 shows that growth of these E. coli lac strains carrying the cloned rdxA+ gene on medium devised for quantitative scoring of Lac+ papillae (limiting glucose plus lactose) that was also supplemented with sublethal concentrations of Mtz (5 to 15 μg/ml) resulted in dramatic increases in Lac+ revertant frequencies, whereas no such stimulation was seen with control E. coli strains grown in parallel. Certain mutations (CG-to-GC transversions and AT-to-GC transitions) were more strongly stimulated than others (CG to AT and TA to AT). Although reversion of even the least responsive allele (TA to GC) seemed to be stimulated somewhat at the highest usable Mtz concentration (15 μg/ml), this concentration was extremely bactericidal to the E. coli (pGS950) tester strains (EOP = 10−4). In consequence, the observed increase in Lac+ papillae in this particular case can be attributed to the effects of bacterial density and the longer period of growth before exhaustion of the limiting glucose, and thus more time for Mtz-induced mutation, rather than a direct mutagenic effect per se on this particular sequence.
TABLE 3.
Base specificity of Mtz-induced Lac+ reversion
| Strain | Base Sub. lacZb | Papillation frequencya
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| pBSK
|
pGS950 (rdxA+)
|
||||||||
| 0 | 5 | 10 | 15 | 0 | 5 | 10 | 15 | ||
| E. coli (lacZ+) | GGG AAT GAG TCA GGC | ||||||||
| Glu | |||||||||
| CC101 | GGG AAT TAG TCA GGC | 1.1 | 1.2 | 1.1 | 1.6 | 2.5 | 6 (2.4) | 12 (4.8) | 115 (46) |
| G | |||||||||
| CC103 | GGG AAT CAG TCA GGC | 4.4 | 5.5 | 8.5 | 12.6 | 4.5 | 80 (18) | 7,407 (1,650) | 47,600 (10,578) |
| G | |||||||||
| CC104 | GGG AAT GCG TCA GGC | 7.7 | 5.7 | 4.4 | 3.5 | 9.5 | 45 (5) | 276 (29) | 4,555 (479) |
| A | |||||||||
| CC105 | GGG AAT GTG TCA GGC | 2.0 | 1.5 | 1.5 | 0.9 | 1.6 | 10 (6.3) | 77 (48) | 2,599 (1,624) |
| A | |||||||||
| CC106 | GGG AAT AAG TCA GGC | 2.6 | 1.0 | 5.4 | 6.3 | 9.0 | 107 (12) | 1,832 (204) | 3,222 (358) |
| G | |||||||||
Expressed as the number of Lac+ revertant papillae per 108 bacteria plated. The data represent the means of three determinations with an error of <20%. Fold increase is indicated in parentheses for pGS950 results; 0, 5, 10, and 15 indicate Mtz concentration in micrograms per milliliter.
The base substitution required for reversion in each of the LacZ− tester strains is depicted in bold.
Lack of effects on neighboring cells (bystanders).
To test whether the mutagenic products of Mtz metabolism by H. pylori strains might affect surrounding cells (bystander effect), forward mutation to Lac+ was scored in E. coli tester strain CC103 in mixed culture experiments (mixing ratios of H. pylori to E. coli of 1:1 to 10:1; results at 10:1 presented in Table 4). No Mtz-induced increase in Lac+ reversion frequency or killing of CC103 grown in mixed cultures with either Mtzs or Mtzr H. pylori was detected. Similarly, under conditions more permissive for the growth of H. pylori, there was no detectable Mtz-induced killing of E. coli bystanders cocultured with a 105-fold excess of Mtzs H. pylori (data not presented). Thus, the mutagenic and bactericidal action of the products of Mtz metabolism seem to be confined primarily to cells in which they are produced, and without impact on neighboring cells.
TABLE 4.
Bystander effect papillation assaya
| Mtz concn (μg/ml) | Viability (108) | Papillation frequency
|
||
|---|---|---|---|---|
| E. coli | E. coli + 26695 | E. coli + 16R | ||
| 0 | 1.20 ± 0.10 | 2.2 ± 1.3 (1) | 4.2 ± 1.6 (1) | 1.39 ± 0.9 (1) |
| 5 | 1.23 ± 0.05 | 3.8 ± 1.7 (1.7) | 7.1 ± 0.9 (1.6) | 6.5 ± 0.8 (4.6) |
| 10 | 1.23 ± 0.11 | 18 ± 6.3 (9) | 11 ± 4.9 (2.6) | 16 ± 1.2 (11) |
| 15 | 1.21 ± 0.12 | 22 ± 2.2 (10) | 15 ± 1.7 (3.6) | 21 ± 8 (14) |
| 32 | 1.10 ± 0.08 | 37 ± 9.7 (17) | 45 ± 14 (11) | 48 ± 12 (34) |
| 64 | 1.25 ± 0.14 | 54 ± 7.3 (25) | 62 ± 5.8 (15) | 53 ± 9.9 (38) |
Suspensions of E. coli CC103 and either H. pylori 26695 or its rdxA-null mutant derivative 16R were mixed 1:10 and spread on papillation test agar as described in the text. Similar results were obtained with other ratios (e.g., 1:1 and 2:1). Means and standard deviations are from triplicate platings. The reversion to Lac+ was scored per 108 E. coli bacteria plated. Viability was determined by dilution of E. coli on medium containing each Mtz concentration. The values in parentheses represent the fold increase over that with no Mtz in the medium.
A control in the bystander mutagenesis experiments, E. coli CC103 grown microaerobically without H. pylori cells, exhibited Mtz induction of Lac+ reversion in a dose-dependent manner (up to ∼30-fold [Table 4]) that was not observed in experiments conducted under aerobic conditions. There was also no decrease in viable counts at any of the Mtz concentrations tested. These findings suggest that under low oxygen tensions, nitroreductases or other redox-active enzymes of E. coli activate Mtz, raising the mutation frequency.
DNA fragmentation as a measure of other genetic damage resulting from Mtz activation.
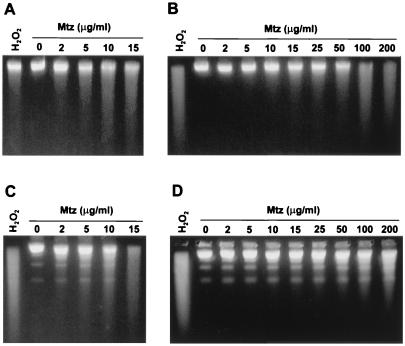
To test for DNA damage that might be distinct from Mtz-induced premutagenic lesions, isogenic Mtzs and Mtzr H. pylori strains were treated for 30 min in Brucella broth with different concentrations of Mtz or, for comparison, with 20 mM H2O2, an oxidant that causes DNA fragmentation (31). Figure 1A shows that treatment of the Mtzs strain 26695 with increasing concentrations of Mtz (5 to 15 μg/ml) caused dose-dependent DNA fragmentation. A similar result was obtained with Mtzs strain 1134, except that DNA fragmentation was evident at lower Mtz concentrations (Fig. 1C). Analysis of DNA fragmentation on nondenaturing agarose gels indicated that most of the DNA fragmentation observed using alkaline (denaturing) gels resulted from single- rather than double-strand breakage (data not presented). The Mtzr strains 16R (Fig. 1B) and 1134R (Fig. 1D) exhibited DNA breakage only at the higher Mtz concentrations that were at least partially lethal to them (e.g., ≥10 μg/ml for 16R and ≥25 μg/ml for 1134R). Since 16R and 1134R contain loss-of-function mutations in rdxA (9, 17), additional redox-active enzymes must also act on Mtz at high concentrations, generating products that also contribute to the observed DNA breakage and lethality.
FIG. 1.
Mtz-induced DNA fragmentation of MtzR and MtzS strains of H. pylori. Mtzs and Mtzr strains of H. pylori were challenged with various concentrations of Mtz for 30 min as described in the text. The bacteria were suspended and lysed in agarose plugs, and agarose gels were run under alkaline conditions to display the extent of DNA fragmentation of denatured genomic DNA. Bacteria were treated with hydrogen peroxide (20 mM) for 15 min (positive controls). Mtz was used at 0, 2, 5, 10, 15, 25, 50, 100, and 200 μg/ml. (A) H. pylori strain 26695 (Mtzs); (B) its rdxA deletion derivative strain 16R (Mtzr); (C) strain 1134 (Mtzs); (D) strain 1134R (Mtzr).
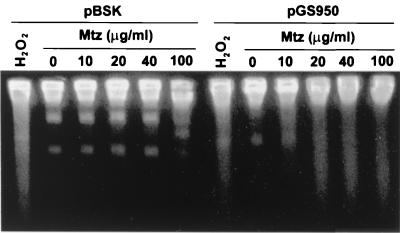
Further demonstration that Mtz activation causes DNA fragmentation was obtained with E. coli tester strains carrying a cloned rdxA+ gene. Figure 2 shows that Mtz (5 to 100 μg/ml) caused significant DNA fragmentation when rdxA+ was present (pGS950), whereas no such fragmentation was observed in E. coli lacking rdxA+, a strain fully resistant to Mtz.
FIG. 2.
Mtz-induced DNA fragmentation of E. coli strains carrying rdxA of H. pylori. E. coli strain CC104 containing either pBSK (control) or pGS950 (rdxA+) was grown in the presence of Mtz; bacteria were suspended and lysed in agarose plugs and electrophoresed as described in the legend to Fig. 1 and detailed in the text. Hydrogen peroxide was added at 20 mM as a positive control. The distinct bands noted in the various lanes are of plasmid DNA.
DISCUSSION
Here we have shown that the potent antimicrobial drug Mtz can be highly mutagenic for H. pylori and for E. coli strains carrying rdxA+, the H. pylori gene whose product is needed for efficient Mtz activation. Mtz is also mutagenic for Mtzr strains of H. pylori containing null mutations in rdxA when the drug is used at concentrations that are considered therapeutic for Mtzs strains (i.e., in combination therapies) but only partially toxic for Mtzr strains. Products of Mtz activation primarily induce single-strand DNA breakage, with extensive fragmentation occurring at Mtz levels near or higher than the MIC. Reversion tests with E. coli rdxA+-carrying tester strains revealed mostly CG-to-GC transversions and AT-to-GC transitions (with other base substitutions at lower frequencies) with the alleles studied. This suggests the participation of nitro, nitroso, and hydroxylamine intermediates of Mtz, perhaps each differing in reactivity or base specificity (7, 9, 18). Taken together, these studies indicate that Mtz therapy probably induces, as well as selects for, the loss-of-function mutations in rdxA that are characteristic of every Mtzr clinical isolate tested to date (5, 9, 15–17, 26).
It is striking that Mtz was more mutagenic for Mtzr than Mtzs H. pylori strains (27- to 166-fold versus 6- to 12-fold, respectively) when used at levels that inhibit growth to similar extents. The more efficient mutagenesis in Mtzr strains might reflect (i) the high specific activity of RdxA for Mtz (e.g., mutagenic and lethal concentrations are similar), (ii) lower specific activities (substrate specificity for Mtz) of other as yet unknown Mtz-activating enzymes, (iii) changes in the metabolic capacity in response to Mtz (13), and/or (iv) changes in balances of enzymes affecting the fidelity of DNA replication or the efficiencies of repair. That mutagenesis and cell killing were observed in simple rdxA mutants illustrates that Mtz can be activated by other cellular enzymes, albeit less efficiently than by RdxA. One such enzyme is FrxA, a flavin nitroreductase of H. pylori (9), whose inactivation together with that of RdxA results in increased Mtz resistance (17). Our finding that Mtz is also mutagenic for hyperresistant (rdxA frxA) strains indicates that additional enzymes must also participate to some extent. These additional redox-active enzymes may be quite widespread, since Mtz in high concentration (>150 μg/ml) is toxic for many bacterial species that are considered resistant clinically (7). In general, Mtz toxicity is increased for most bacteria and mammalian cells under hypoxic conditions where nitroreductases, ferredoxins, flavodoxins, cytochrome P450, and nonspecific redox-active components contribute more efficiently to Mtz activation (7, 13, 28). Mutational inactivation of genes encoding these additional Mtz activating enzymes most likely accounts for the stepwise appearance of hyper-Mtzr mutants of H. pylori, although mutations affecting other functions such as DNA repair may also contribute to some extent.
The different efficiencies of Mtz-induced DNA breakage in Mtzs and Mtzr strains of H. pylori can also be attributed to loss of function of the RdxA nitroreductase in Mtzr strains. Findings that Mtz-induced DNA breakage and mutation were greatest at levels near the MIC in E. coli strains expressing rdxA+ support this interpretation. In the absence of RdxA activity, E. coli is extremely resistant to Mtz (>250 μg/ml), indicating that its own nitroreductases do not contribute appreciably to Mtz activation during growth at normal atmosphere in the concentration range used. However, under microaerobic conditions (6% oxygen), Mtz is more mutagenic for E. coli (Tables 3 and 4), suggesting that its nitroreductases or other redox-active enzymes can metabolize this drug. These Mtz-activating enzymes may be induced to higher levels in response to oxygen-limiting conditions (metabolic transition to a more fermentative capacity) or may participate more substantially in removal of reducing equivalents to alternate electron acceptors such as Mtz. Our studies further showed that in hyper-Mtzr H. pylori strains, which contain mutations in a gene(s) in addition to rdxA and frxA, the efficiency of DNA breakage requires much higher Mtz concentrations. We have shown a correlation between MIC of Mtz for a particular strain and the efficiency of DNA breakage after exposure to Mtz. In this regard, DNA fragmentation was seen at lower Mtz concentrations for strain 26695 16R (rdxAΔ), which tolerates 16 μg/ml, than for strain 1134R, which tolerates twice that level (32 μg/ml).
In vitro studies of the interactions of reduced products of Mtz with DNA have indicated that DNA fragmentation is favored over base modification (7). In vivo, Mtz is said to cause more base pair substitution than DNA fragmentation in enteric bacteria (2) but more DNA fragmentation in highly susceptible anaerobic bacteria (7, 28, 29). In one study of selected gene targets, Mtz induced exclusively GC-to-CG transversion mutations in Bacteroides fragilis (29). Here we found CG-to-GC transversions and AT-to-GC transitions to be most common in E. coli tester strains expressing rdxA+, although Mtz induced other substitutions at concentrations that would also cause DNA fragmentation. These base substitution differences are not due to variation among tester strains since these strains showed similar frequencies of mutation to Rifr. The high frequency of mutation seen at the highest concentrations of Mtz in each of the E. coli tester strains may be due to activation of the error-prone SOS repair system, a repair mechanism that is lacking in H. pylori (27).
We suggest that Mtz-induced DNA strand breakage, like base substitution mutagenesis, is significant biologically: DNA breakage should stimulate recombination between duplicate and divergent sequences (of which there are many in H. pylori) within a given genome and perhaps recombination between different strains as well. Repair of Mtz-induced DNA breaks should also promote the metastable turning on and off of contingency genes, identified by their distinctive repetitive sequences (24), and more generally induce frameshift mutations (21) such as we and others have found after Mtz treatment (9, 15). DNA lesions not involving DNA breakage may be responsible for most of the base substitutions scored here. Mutation and genetic recombination each contribute to the genome diversity that can facilitate adaptation to new and changing conditions in human hosts, the development of resistance to clinically useful drugs, and the evolution of virulence. The emergence of resistance to Mtz, selected and possibly induced by this drug during H. pylori infection, has been documented in an animal infection model (16). NH2-containing therapies, in addition to contributing to Mtz hyperresistance, may also induce resistance to other clinically useful drugs (secondary antibiotic resistance). By extrapolation, Mtz therapies might also be mutagenic for the resident intestinal flora (which also live in microaerobic and anaerobic environments) and thereby similarly speed the emergence of drug resistance and other evolutionary changes in them.
The biologically active products of Mtz metabolism are considered to be short-lived and thus perhaps unlikely to act on cells other than those in which they are produced (7). It was therefore comforting from a public health perspective that no Mtz-induced killing or mutagenesis of adjacent bystander E. coli cells was detected in these experiments. Thus, the products of Mtz metabolism by H. pylori probably do not interact synergistically with reactive oxygen and nitrogen metabolites generated in the host inflammatory response that are suspected of contributing to gastric pathologies and cancer (14, 22). As counterpoint, we wish to consider the possibility that a generic function for the nitroreductases studied here is in the catabolism (detoxification) of nitrated aromatic amino acids and other reactive metabolites that are generated during inflammation, and thus of differences between Mtzr and Mtzs strains in virulence or in speed of adaptation to host defenses.
ACKNOWLEDGMENTS
We thank Jeffrey H. Miller for providing tester strains, Rob Bethune for technical assistance with DNA fragmentation studies, Bill Shannon for carrying out statistical analyses, an anonymous reviewer for suggesting the bystander experiment, and Hans Kusters and Jetta Bijlsma for helpful discussions.
This work was supported by grants from MRC (R-14292), from AstraZeneca Canada, and from Romark Laboratories to P.S.H. and by Public Health Service (NIH) grants AI38166 and DK53727 to D.E.B.
REFERENCES
- 1.Ausubel F M, et al., editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: Wiley Interscience; 1998. p. 1.1.1. [Google Scholar]
- 2.Chin J B, Sheinin D M K, Rauth A M. Screening for the mutagenicity of nitro-group containing hypoxic cell radiosensitizers using Salmonella typhimurium strains TA100 and TA98. Mutat Res. 1978;58:1–10. [PubMed] [Google Scholar]
- 3.Correa P. Helicobacter pylori and gastric cancer: state of the art. Epidemiol Biomarkers Prev. 1996;5:477–481. [PubMed] [Google Scholar]
- 4.Cupples C G, Miller J H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc Natl Acad Sci USA. 1989;86:5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debets-Ossenkopp Y J, Pot R G, van Westerloo D J, Goodwin A, Vandenbroucke-Grauls C M, Berg D E, Hoffman P S, Kusters J G. Insertion of mini-IS605 and deletion of adjacent sequences in the nitroreductase (rdxA) gene cause metronidazole resistance in Helicobacter pylori NCTC11637. Antimicrob Agents Chemother. 1999;43:2657–2662. doi: 10.1128/aac.43.11.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards D I. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Gauton R K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol. 1997;35:2977–2980. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten S J O, Berg D E, Hoffman P S. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28:383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 10.Glupczynski Y, Burette A. Drug therapy for Helicobacter pylori infection: problems and pitfalls. Am J Gastroenterol. 1990;85:1545–1551. [PubMed] [Google Scholar]
- 11.Heep M, Beck D, Bayerdorffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:1497–1499. doi: 10.1128/aac.43.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman P S. Antibiotic resistance mechanisms of Helicobacter pylori. Can J Gastroenterol. 1999;13:243–249. doi: 10.1155/1999/838072. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman P S, Goodwin A, Johnsen J, Magee K, Veldhuyzen van Zanten S J O. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J Bacteriol. 1996;178:4822–4829. doi: 10.1128/jb.178.16.4822-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaacson P G. Gastric MALT lymphoma: from concept to cure. Ann Oncol. 1999;10:637–645. doi: 10.1023/a:1008396618983. [DOI] [PubMed] [Google Scholar]
- 15.Jenks P J, Ferrero R L, Labinge A. The role of the rdxA gene in the evolution of metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:753–758. doi: 10.1093/jac/43.6.753. [DOI] [PubMed] [Google Scholar]
- 16.Jenks P J, Labigne A, Ferrero R L. Exposure to metronidazole in vivo readily induces resistance in Helicobacter pylori and reduces the efficacy of eradication therapy in mice. Antimicrob Agents Chemother. 1999;43:777–781. doi: 10.1128/aac.43.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong J-Y, Mukhopadhyay A K, Dailidiene D, Wang Y, Velapatiño B, Gilman R H, Parkinson A J, Nair G B, Wong B C Y, Lam S K, Mistry R, Segal I, Yuan Y, Gao H, Alarcon T, Brea M L, Ito Y, Kersulyte D, Lee H-K, Gong Y, Goodwin A, Hoffman P S, Berg D E. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori. J Bacteriol. 2000;182:5082–5090. doi: 10.1128/jb.182.18.5082-5090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindmark D G, Muller M. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob Agents Chemother. 1976;10:476–482. doi: 10.1128/aac.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockerby D L, Rabin H R, Laishley E J. Role of the phosphoroclastic reaction of Clostridium pasteurianum in the reduction of metronidazole. Antimicrob Agents Chemother. 1985;27:863–867. doi: 10.1128/aac.27.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malfertheiner P, Megraud F, O'Morain C. Current European concepts in the management of Helicobacter pylori infection. The Maastricht consensus report. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie G J, Harris R S, Lee P L, Rosenberg S M. The SOS response regulates adaptive mutation. Proc Natl Acad Sci USA. 2000;97:6646–6651. doi: 10.1073/pnas.120161797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami K, Fujioka T, Okimoto T, Mitsuishi Y, Oda T, Nishizono A, Nasu M. Analysis of p53 gene mutations in Helicobacter pylori-associated gastritis mucosa in endoscopic biopsy specimens. Scand J Gastroenterol. 1999;34:474–477. doi: 10.1080/003655299750026191. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen B A, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24:S110–S120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 24.Saunders N J, Peden J F, Hood D W, Moxon E R. Simple sequence repeats in the Helicobacter pylori genome. Mol Microbiol. 1998;27:1091–1098. doi: 10.1046/j.1365-2958.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- 25.Searle S R. Linear models. J. New York, N.Y: Wiley & Sons; 1971. [Google Scholar]
- 26.Tankovic J, Lamarque D, Delchier J-C, Soussy C-J, Labinge A, Jenks P J. Frequent association between alteration of the rdxA gene and metronidazole resistance in French and North African isolates of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:608–613. doi: 10.1128/aac.44.3.608-613.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K, Klenk H, Gill S, Dougherty B, Nelson K, Quackenbush J, Zhou L, Kirkness E, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H, Glodek A, McKenney K, Fitzegerald L, Lee N, Adams M, Hickey E, Berg D, Gocayne J, Utterback T, Peterson J, Kelley J, Cotton M, Weidman J, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W, Borodovsky M, Karp P, Smith H, Fraser C, Venter J. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 28.Townson S M, Boreham P F L, Upcroft P, Upcroft J A. Resistance to the nitroheterocyclic drugs. Acta Trop. 1994;56:173–194. doi: 10.1016/0001-706x(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 29.Trinh S, Reysset G. Mutagenic action of 5-nitroimidazoles: in vivo induction of GC→CG transversion in two Bacteroides fragilis reporter genes. Mutat Res. 1998;398:55–65. doi: 10.1016/s0027-5107(97)00240-6. [DOI] [PubMed] [Google Scholar]
- 30.Veldhuyzen van Zanten S J O, Sherman P M, Hunt R H. Helicobacter pylori: new developments and treatments. Can Med Assoc J. 1997;156:1565–1574. [PMC free article] [PubMed] [Google Scholar]
- 31.Zirkle R E, Krieg N R. Development of a method based on alkaline gel electrophoresis for estimation of oxidative damage to DNA in Escherichia coli. J Appl Bacteriol. 1996;81:133–138. doi: 10.1111/j.1365-2672.1996.tb04490.x. [DOI] [PubMed] [Google Scholar]