Abstract
Background:
The long coronavirus disease 2019 (COVID-19) syndrome includes a group of patients who, after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exhibit lingering mild-to-moderate symptoms and develop medical complications that can have lasting health problems.
Objective:
The purpose of this report was to examine the current body of evidence that deals with the relationship of COVID-19 infection with the long COVID syndrome to define the possible immunologic mechanisms involved in the pathogenesis of long COVID and to describe potential strategies for the diagnosis and clinical management of the condition.
Methods:
Extensive research was conducted in medical literature data bases by applying terms such as long COVID, post–COVID-19 condition, pathogenesis of long COVID, management of the long COVID syndrome.
Results:
The post-COVID conditions, a more recent and less anxiety-inducing term for the patient than long COVID or “long haul,” is an umbrella term for a wide range of physical and mental health symptoms similar to those seen in patients with the myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), experienced by some patients and are present ≥ 4 weeks after SARS-CoV-2 infection. Although the precise reason why long COVID develops is unknown, one of the major causes is thought to be related to chronic inflammation with overproduction of inflammatory cytokines responsible for the symptoms of the disorder.
Conclusion:
Long COVID is a growing burden for millions of patients, health-care providers, and global health-care systems, and is a particular challenge for the allergist/immunologist. Many survivors of COVID-19 struggle with multiple symptoms, increased disability, reduced function, and poor quality of life. The allergist/immunologist can assist the total health-care team's efforts in providing a comprehensive and coordinated approach to the management of these patients by promoting comprehensive vaccination and rehabilitation and social services that focus on improving physical, mental, and social well-being, and by establishing partnerships with specialists and other health-care professionals who can provide behavioral, lifestyle, and integrative approaches that may have much to offer in helping patients cope with their symptoms.
Keywords: COVID-19, cytokines, Long COVID, Long Haul, inflammation, post-COVID conditions
As of June 10, 2022, >85.2 million confirmed cases of COVID-19 and >1 million deaths have been reported in the United States. Despite these numbers, the natural history, clinical course, and long-term consequences of this disease are still incompletely understood. Most patients with coronavirus disease of 2019 (COVID-19) generally show complete recovery within 3–4 weeks and return to their baseline state of health after acute infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but as many as 30%, or more, continue to report lingering effects after 3 months and develop medical complications that can have lasting and debilitating health problems.1
This prolonged condition is referred to by a variety of designations, which include long COVID, long haul, or the post–COVID-19 condition, terms that are as perplexing to the patient as they are to the health care practitioner. Long COVID, therefore, is a form of chronic COVID-19 infection characterized by persistent symptoms after SARS-CoV-2 infection, which include fatigue, shortness of breath, cough, joint pain, and chest pain, in addition to other symptoms that include problems with thought processes, difficulty concentrating, forgetfulness, depression, muscle pain, headache, rapid heartbeat, and fever.1 The purpose of the present report was to examine the current body of evidence that deals with the relationship of COVID-19 to long COVID to define the possible immunologic mechanisms involved in the pathogenesis of long COVID and to describe potential strategies for the diagnosis and clinical management of the condition.
DEFINITION OF LONG COVID
Over the course of the pandemic, several definitions of the long COVID condition have been proposed.2 The absence of both a generally accepted single terminology as well as a well-defined clinical case definition can contribute impediments in epidemiologic reporting, research, policy making, and clinical management of patients afflicted with the post–COVID-19 condition. In response to these deficiencies, Soriano et al.2 performed a World Health Organization (WHO) led Delphi process based study that identified domains and variables to be included in a clinical case definition of the post–COVID-19 condition in which an international panel of 265 patients, clinicians, researchers, and WHO staff were engaged. Based on these parameters, a clinical case definition was created and the name proposed for the condition in September 2020 by the WHO was the post–COVID-19 condition with an International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code of U09.3 In the United States, the 2022 ICD-10, Clinical Modification (CM) diagnosis code U09.9 was proposed as a billable and specific ICD-10-CM code that can be used to indicate a diagnosis of the condition for reimbursement purposes and became effective on October 1, 2021.4
POSSIBLE MECHANISMS INVOLVED IN THE PATHOGENESIS OF LONG COVID
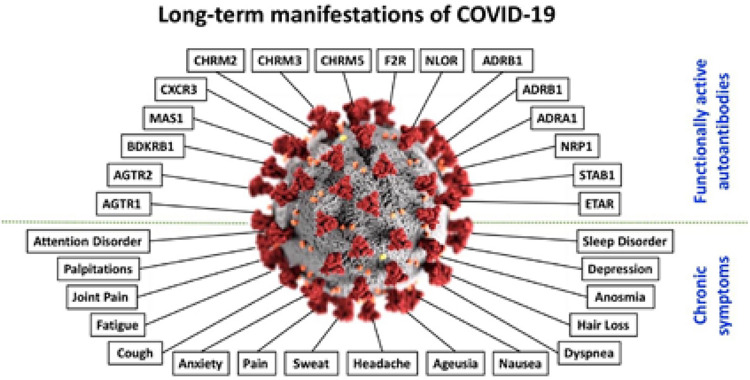
Although the precise etiology of long COVID is uncertain, there exists an emerging literature that describes various mechanisms to explain the pathogenesis of the condition. These include multiple underlying processes, e.g., immune system dysregulation, hyperinflammatory states, oxidative stress, autoimmunity, and autonomic nervous system dysfunction.5–8 The report by Dotan et al.8 raises an interesting etiologic possibilty that the autoimmune features may be involved in the induction of the autonomic nervous system dysfunction. Shown in Fig. 1 is a schematic representation of some of the functionally active autoantibodies linked to COVID-19.
Figure 1.
Schematic representation of some of the functionally active autoantibodies linked to COVID-19. In the center is shown the SARS-CoV-2; around it, at the upper part of the figure, are the autoantibodies; at the bottom part of the figure appear some of the chronic symptoms associated with COVID-19. COVID-19 = Coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Reproduced with permission from Ref. 8.
In a recent publication1, the authors proposed a hypothesis for the pathophysiology of the long COVID presentation based on increased proinflammatory cytokine production, which results from the persistence of the SARS-CoV-2 or one of its molecular components, i.e., the spike (S) protein. In support of the hypothesis are several recent publications that demonstrate the persistence of SARS-CoV-2 S1 protein in CD16+ monocytes of patients with COVID-19 infection up to 15 months after infection9 and other publications that suggest a causal relationship of the persistence of the S protein in the pathogenesis of the long-COVID syndrome.10,11
CLINICAL PRESENTATION OF LONG COVID
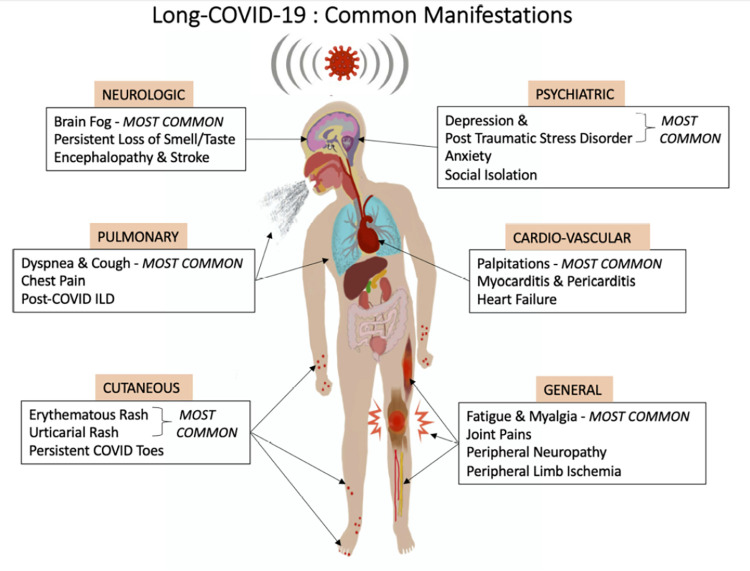
The most common signs and symptoms of long COVID in adults and in children include fatigue, headache, dyspnea, cognitive impairment, numbness, depression, altered perception of smell and taste, poor appetite, chronic cough, joint and chest pain, postural orthostatic tachycardia, autonomic dysregulation, thermoregulation abnormalities, skin eruptions, and gastrointestinal disorders.1 Shown in Fig. 2 is a schematic representation of the common clinical manifestations observed in long COVID-19. Many of the symptoms of the post–COVID-19 resemble those of the myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)12 and include problems with thought processes, difficulty concentrating, forgetfulness, depression, and muscle pain.13
Figure 2.
Schematic representation of the common clinical manifestations observed in long COVID-19. COVID-19 = Coronavirus disease 2019. Reproduced with permission from Ref. 19.
Because long COVID is demonstrated to be a similar chronic medical illness with overlapping clinical features and symptomatology, it may be conjectured that the existing knowledge on ME/CFS may benefit patients with long COVID. Several recent publications focus on the role of persistence of the SARS-CoV-2 or one of its molecular components, i.e., the S protein, which is hypothesized to trigger a dysregulated immune system with a subsequent heightened release of proinflammatory cytokines that lead to chronic low-grade inflammation with multiorgan involvement and autoimmune-mediated autonomic nervous system dysfunction symptomatology.1,9,10, 13
COVID ANTIBODY RESPONSES AFTER COVID INFECTION AND/OR AFTER IMMUNIZATION
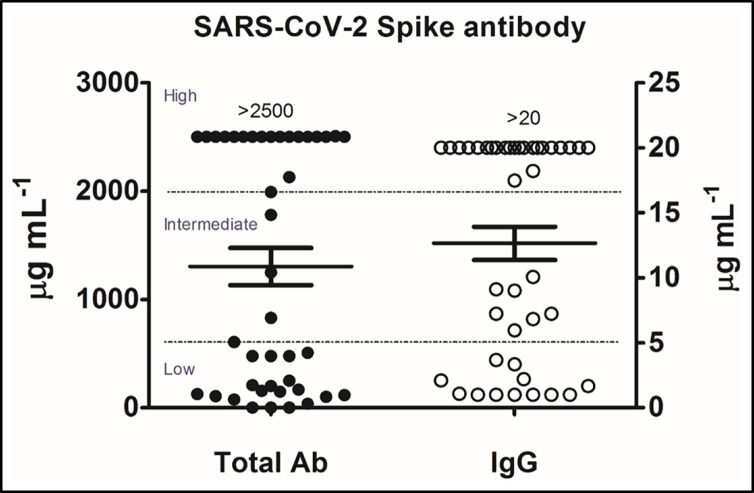
In a recent publication by Li et al.,14 the authors describe SARS-CoV-2 serum and salivary immunoglobulin A (IgA) and IgG antibody responses, found in a total of 52 paired saliva and serum samples collected from 26 study participants, that developed after infection with COVID-19 or after immunization with COVID-19 vaccines and offer some clinically related observations that have relevance to the long COVID syndrome. In this study, a comparison of semiquantitative results of serum total and IgG S-specific antibody responses performed by a commercially available SARS-CoV-2 enzyme immunoassay (EIA) were compared with those performed by an enzyme-linked immunosorbent assay (ELISA) developed in the authors′ laboratory that quantitatively measures IgA and IgG antibody responses to the S1 antigen in both serum and saliva. Analysis of these results revealed three groups of antibody responses: (1) low, (2) intermediate, and (3) high (Fig. 3).
Figure 3.
Three groups of serum total and IgG SARS-CoV-2 spike antibody responses were observed by SARS-CoV-2: (1) low, (2) intermediate, and (3) high. The cutoff points for the low group total ab level ranged from 0 to 600 μg/mL and, for the IgG level, from 0 to 5μg/mL; the cutoff points for the intermediate group total ab level ranged from 601 to 2000 μg/mL and for the IgG from 5 to 17 μg/mL; the cutoff points for the high group total ab level ranged from 2001 to >2500 μg/mL and, for the IgG level, from 17.1 to >20 μg/mL. IgG = Immunoglobulin G; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; ab = antibody. Reproduced with permission from Ref. 14.
One of the most interesting findings in this study was the wide range of variation in antibody responses seen in our study population, which is consistent with the immense diversity of human immune responses that exists in the general population.15 These findings are also supported by the recent study of Wei et al.,16 which documented a variability of anti-S antibody response to natural SARS-CoV-2 infection in the general population and by the report by Steensels et al.,17 which described the heterogeneity of antibody responses in recipients of the two U.S. authorized messenger mRNA vaccines.
CURRENT U.S. FDA AND CDC RECOMMENDATIONS
Added to the conundrum of the long COVID syndrome are the inconsistencies in COVID-19 vaccine recommendations delivered by federal health agencies, the news, and social media as well as the rapid changes in vaccine recommendations, owing to the meteoric increase of new information that occurs daily at breathtaking speed. Many patients and health-care providers are confused about if and when a second COVID-19 vaccine booster should be administered. A useful reference to a source of vaccine information that will be continuously updated is https://www.cdc.gov/coronavirus/2019-ncov/vaccines/your-vaccination.html.
ADVERSE REACTIONS TO COVID-19 VACCINES
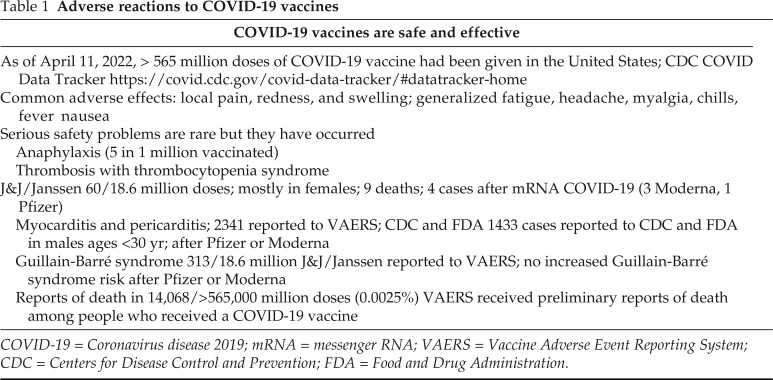
COVID-19 vaccines are safe and effective, and, although adverse effects are rare, both local and systemic reactions do occur (Table 1). As of April 25, 2022, >572 million doses of COVID-19 vaccines have been given in the United States. The most common adverse effects of these vaccines are local and consist of pain, redness, and swelling at the injection site but less commonly with generalized symptoms of fatigue, nausea, headache, myalgia chills, and fever. These last only a few days. Serious adverse effects safety problems are rare but they do occur. Anaphylaxis after COVID-19 vaccination has occurred at a rate of approximately five cases per 1 million doses of vaccine administered. Thrombosis with thrombocytopenia syndrome has been seen primarily after J&J Janssen (Janssen Biotech, Inc., Horsham, PA 19044) COVID-19 vaccination in approximately four cases per 1 million doses administered. It is a rare but serious adverse event that causes blood clots in large blood vessels and is associated with thrombocytopenia.
Table 1.
Adverse reactions to COVID-19 vaccines
COVID-19 = Coronavirus disease 2019; mRNA = messenger RNA; VAERS = Vaccine Adverse Event Reporting System; CDC = Centers for Disease Control and Prevention; FDA = Food and Drug Administration.
A review of reports by the CDC indicates a causal relationship between the J&J Janssen COVID-19 vaccine and thrombosis with thrombocytopenia syndrome.23 Cases of myocarditis and pericarditis after COVID-19 vaccination have also occurred, and a review of vaccine safety data from the Vaccine Adverse Event Reporting System (VAERS) from December 2020 to August 2021 found a small but increased risk of myocarditis after mRNA COVID-19 based on the 350 million doses of mRNA vaccines administered during this period. Rates of myocarditis were highest after the second dose of an mRNA vaccine among males in the following age groups: 12 to 15 years (70.7 cases for 1 million doses of Pfizer vaccine, Pfizer-BioNTech, 235 East 42nd Street, New York City, NY), 16 to 17 years (105.9 cases per 1 million doses of Pfizer vaccine), and 18 to 24 years (52.4 cases and 56.3 cases per million doses of Pfizer and Moderna vaccines (Global Headquarters, 200 Technology Square, Cambridge, MA 02139), respectively). Multiple studies and continuing review of vaccine safety continue to show that these vaccines are safe. As of April 21, 2022, there have been 960 reports among people ages < 18 years under review for cases of myocarditis and pericarditis.
COMPENSATION FOR VACCINE INJURY
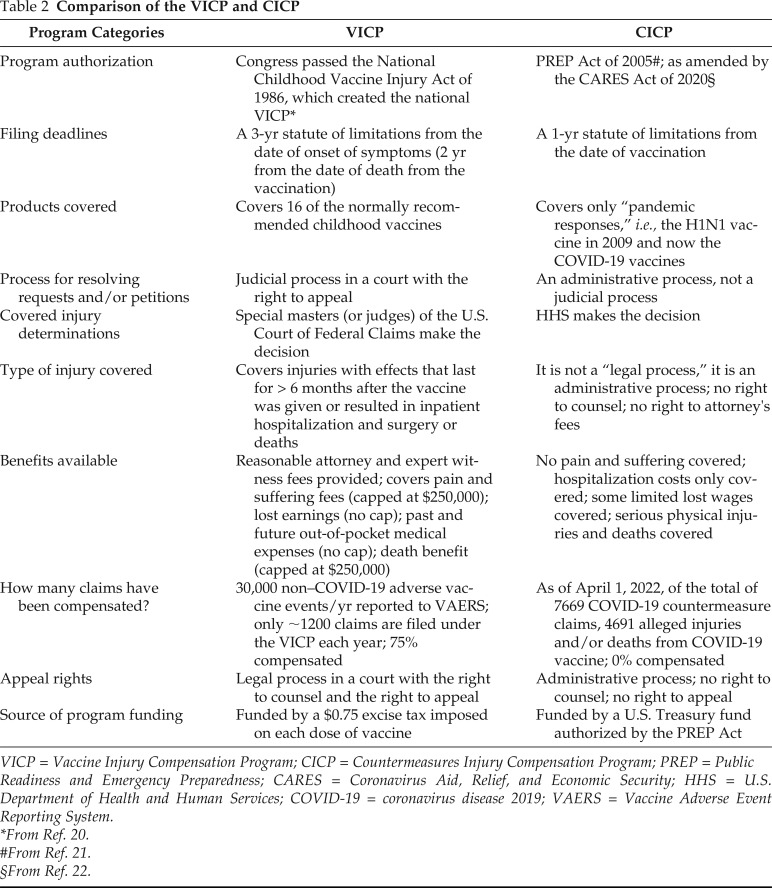
There are two federal systems that have been established for compensation of vaccine recipients who have developed serious injury as a result of receiving a vaccine.18 These include the following: (1) the Vaccine Injury Compensation Program (VICP), and (2) the Countermeasures Injury Compensation Program (CICP). The VICP, a no-fault alternative to the traditional legal system for resolving vaccine injury, was created in the 1980s, after lawsuits against vaccine companies and health-care providers threatened to cause vaccine shortages and reduce U.S. vaccination rates. The VICP covers compensation for 16 of the normally recommended childhood vaccines.
The CICP was created by Congress by the PREP (Public Readiness and Emergency Preparedness) Act of 200521 and, as amended by the CARES (Coronavirus Aid, Relief, and Economic Security) Act of 202022, covers H1/N1 and COVID-19 vaccines. Shown in Table 2 is a comparison of the VICP and CICP. It can be seen that the current CICP that covers COVID-19 vaccine injury is disproportionate to the VICP in terms of its total dimensions of the process for access to, conduct of, and potential benefits for the vaccine recipient who is injured.
Table 2.
Comparison of the VICP and CICP
VICP = Vaccine Injury Compensation Program; CICP = Countermeasures Injury Compensation Program; PREP = Public Readiness and Emergency Preparedness; CARES = Coronavirus Aid, Relief, and Economic Security; HHS = U.S. Department of Health and Human Services; COVID-19 = coronavirus disease 2019; VAERS = Vaccine Adverse Event Reporting System.
*From Ref. 20.
#From Ref. 21.
§From Ref. 22.
ROLE OF THE ALLERGIST/IMMUNOLOGIST IN MANAGEMENT OF PATIENTS WITH LONG COVID
Long COVID is a challenge for millions of patients and a growing burden on health-care providers global health-care systems. Many survivors of COVID-19 struggle with multiple symptoms, increased disability, reduced function, and poor quality of life. The allergist/immunologist can assist by providing a comprehensive and coordinated approach to the management of these patients. Because there is no known cause or identified treatment for the syndrome, the allergist/immunologist should be among the forefront of specialists advocating prevention and treatment of long COVID.
In addition to promoting comprehensive vaccination and treatment by using antiviral and anti-inflammatory biologics, allergists should establish partnerships with specialists, such as psychiatrists and psychologists, who can provide supportive emotional health care, and with other health-care professionals, such as integrative nurse practitioners, who could provide behavioral, lifestyle, and consolidative approaches (e.g., guided relaxation, breathing, and meditation techniques), which may help patients cope with their symptoms. Moreover, because of the specialized training in allergy and clinical immunology, the allergist can provide unique services that are not usually available from other types of health-care professionals such as expertise in the use of vaccines and recognition and management of adverse reactions to them.
CONCLUSION
The term “post–COVID condition,” a more recent and less anxiety-inducing moniker for the patient than long COVID, or long-haul COVID, is an umbrella term for a wide range of physical and mental health consequences experienced by some patients for ≥ 4 weeks. Although the precise mechanism for the occurrence of long COVID is unknown, there is emerging literature that describes various mechanisms to explain the pathogenesis of the condition. Of these etiologic possibilities, chronic inflammation with overproduction of inflammatory cytokines responsible for the symptoms of long COVID is most frequently cited in the literature. Compensation for patients with post–COVID conditions under the CICP is currently inadequate and in need of improvement. It is clear that some individuals, including those with mild initial symptoms of COVID-19, may suffer for many months after the initial infection from variable and debilitating symptoms similar to those seen in patients with the ME/CFS. Until a specific treatment regimen becomes available, management should focus on promoting comprehensive vaccination and rehabilitation and social services that focus on improving physical, mental, and social well-being.
Footnotes
Presented at the Eastern Allergy Conference, June 2, 2022, Palm Beach, FloridaFunding provided by the Eastern Allergy Conference
The author has no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Buonsenso D, Piazza M, Boner AL, et al. Long COVID: a proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. 2022; 43:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soriano JB, Murthy S, Marshall JC, et al. WHO Clinical Case Definition Working Group on post-COVID-19 condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022; 22:e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emergency Use ICD Codes for COVID-19 Disease Outbreak. Available online at https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak; accessed April 24, 2022.
- 4. ICD-10-CM Codes. Available online at https://www.icd10data.com/ICD10CM/Codes/U00-U85/U00-U49/U09-/U09.9; accessed April 24, 2022.
- 5. Altmann DM, Boyton RJ. Decoding the unknowns in long covid. BMJ. 2021; 372:n132. [DOI] [PubMed] [Google Scholar]
- 6. Bektas A, Schurman SH, Franceschi C, et al. A public health perspective of aging: do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short- and long-term inflammaging? Immun Ageing. 2020; 17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. 2020; 324:2251–2252. [DOI] [PubMed] [Google Scholar]
- 8. Dotan A, David P, Arnheim D, et al. The autonomic aspects of the post-COVID19 syndrome. Autoimmun Rev. 2022; 21:103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patterson BK, Francisco EB, Yogendra R, et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front Immunol. 2022; 12:746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theoharides TC. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome? Mol Neurobiol. 2022; 59:1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trougakos IP, Terpos E, Alexopoulos H, et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. 2022; 28:542-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hunt J, Blease C, Geraghty KJ. Long Covid at the crossroads: comparisons and lessons from the treatment of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Health Psychol. 2022; 13591053221084494. [DOI] [PubMed] [Google Scholar]
- 13. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)–a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021; 57:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li D, Calderone R, Nsouli TM, et al. Salivary and serum IgA and IgG responses to SARS-CoV-2-spike protein following SARS-CoV-2 infection and after immunization with COVID-19 vaccines. Allergy Asthma Proc. 2022; 43:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liston A, Humblet-Baron S, Duffy D, et al. Human immune diversity: from evolution to modernity. Nat Immunol. 2021; 22:1479–1489. [DOI] [PubMed] [Google Scholar]
- 16. Wei J, Pouwels KB, Stoesser N, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022; 28:1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steensels D, Pierlet N, Penders J, et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021; 326:1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meissner HC. A viral pandemic, vaccine safety, and compensation for adverse events. JAMA. 2021; 325:721–722. [DOI] [PubMed] [Google Scholar]
- 19. Garg M, Maralakunte M, Garg S, et al. The conundrum of 'long-COVID-19': a narrative review. Int J Gen Med. 2021; 14:2491–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The vaccine injury compensation program: addressing needs and improving practices. House Report 106–977. Available at https://www.congress.gov/106/crpt/hrpt977/CRPT-106hrpt977.pdf.
- 21. Fourth Amendment to the Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID-19 and Republication of the Declaration. 2020; 79190–79198. Available at https://www.federalregister.gov/documents/2020/12/09/2020-26977/fourth-amendment-to-the-declaration-under-the-public-readiness-and-emergency-preparedness-act-for.
- 22. Coronavirus Aid, Relief, and Economic Security (CARES) Act of 2020. Available at https://www.congress.gov/116/bills/s3548/BILLS-116s3548is.pdf. [DOI] [PubMed]
- 23. FDA News Release. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals.