Abstract
Background
The mechanism through which sodium-glucose cotransporter 2 inhibitors (SGLT2i) prevent the incidence of heart failure and/or affect cardiac structure and function remains unclear.
Methods
The EMPA-HEART trial is aimed at verifying whether empagliflozin improves myocardial contractility (left ventricle global longitudinal strain, LV-GLS) and/or cardiopulmonary fitness (peak oxygen uptake, VO2peak) in subjects with type 2 diabetes (T2D) without heart disease. Patients with T2D, normal LV systolic function (2D-Echo EF > 50%), and no heart disease were randomized to either empagliflozin 10 mg or sitagliptin 100 mg for 6 months and underwent repeated cardiopulmonary exercise tests with echocardiography and determination of plasma biomarkers.
Results
Forty-four patients completed the study, 22 per arm. Despite comparable glycaemic control, modest reductions in body weight (− 1.6; [− 2.7/− 0.5] kg, p = 0.03) and plasma uric acid (− 1.5; [− 2.3/− 0.6], p = 0.002), as well as an increase in haemoglobin (+ 0.7; [+ 0.2/+ 1.1] g/dL, p = 0.0003) were evident with empagliflozin. No difference was detectable in either LV-GLS at 1 month (empagliflozin vs sitagliptin: + 0.44; [− 0.10/+ 0.98]%, p = 0.11) and 6 months of therapy (+ 0.53; [− 0.56/+ 1.62]%), or in VO2peak (+ 0.43; [− 1.4/+ 2.3] mL/min/kg, p = 0.65). With empagliflozin, the subgroup with baseline LV-GLS below the median experienced a greater increase (time*drug p < 0.05) in LV-GLS at 1 month (+ 1.22; [+ 0.31/+ 2.13]%) and 6 months (+ 2.05; [+ 1.14/+ 2.96]%), while sitagliptin induced a modest improvement in LV-GLS only at 6 months (+ 0.92; [+ 0.21/+ 0.62]%).
Conclusions
Empagliflozin has neutral impact on both LV-GLS and exercise tolerance in subjects with T2D and normal left ventricular function. However, in patients with subclinical dysfunction (LV-GLS < 16.5%) it produces a rapid and sustained amelioration of LV contractility.
Trial registration EUDRACT Code 2016-002225-10
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01618-1.
Keywords: Empagliflozin, SGLT2, Type 2 diabetes, GLS, Speckle-tracking, Cardiovascular, Heart failure, Subclinical left ventricular dysfunction
Background
In subjects with type 2 diabetes mellitus (T2D) and at high cardiovascular risk, hospitalization for heart failure (HF) were reduced by 30–40% already after 6 months of treatment with empagliflozin independently from the presence of established HF at baseline [1]. Nonetheless, the mechanisms of the cardioprotective properties that are present irrespective of the presence of T2D, the amelioration in glycaemic control, blood pressure, and body weight, remain ill-defined [2], particularly in subjects devoid of heart and kidney disease, wherein the effect on body fluid volume regulation—considered a pillar of SGLT2i mechanism of action [3]—is unlikely to play a relevant role. Alternative pharmacological actions have been suggested, namely: improved muscle oxygen/work coupling driven by a larger availability of oxygen (through increased plasma haemoglobin), the use of more efficient metabolic substrates (ketone bodies [4]), and/or a direct effect on myocardial contractility through the inhibition of the Na/H exchanger [5]. Therefore, it is possible to hypothesize that SGLT2i might exert their positive effects on primary HF prevention particularly in those with early and mild forms of left ventricular (LV) contractility dysfunction. This condition—although clinically elusive—is extremely frequent in T2D, with a prevalence ranging from 50 to 70% when more sensitive techniques such as LV global longitudinal strain (LV-GLS) by speckle-tracking echocardiography are employed [6]. Among these nominally asymptomatic subjects, a large proportion (30–45%) shows a reduced cardiopulmonary fitness [7] with complex and uncertain pathobiology that bears an increased risk of incident symptomatic HF [8].
The difficulty in accruing clinical evidence in support for these hypotheses is possibly due to the inadequacy of the experimental design and/or of the methods employed to measure cardiopulmonary function with the necessary precision. Imaging cardiopulmonary exercise test (iCPET), being a powerful multiparametric technique capable of providing simultaneous measures of metabolic, pulmonary, cardiac, muscular, and vascular variables both at rest and during graded exercise [9], qualifies as a strategic tool. CPET is particularly useful in T2D, as patients with diabetes are at increased risk of heart failure and often show exercise intolerance early in the course of the disease, before developing clinically manifest HF [8]. Similarly, signs of left ventricular systolic and diastolic dysfunction can be detected at resting conditions early in the course of the disease. However, alterations in systo-diastolic functions during exercise can be revealed even earlier in diabetic individuals who are asymptomatic at rest, with prognostic and therapeutic meaning [7, 9] as also advocated by the most recent European guidelines for the study of cardiac dysfunctions in diabetes [10].
By using iCPET, this study aimed at verifying whether the treatment with empagliflozin is associated with an improvement in cardiac systo-diastolic functions and/or in cardiopulmonary fitness in asymptomatic T2D patients without overt heart disease and normal LV ejection fraction (LVEF > 50%). To account for the potential positive effects of improved glycemic control, we used sitagliptin as an active control, an equally effective glucose-lowering agent that has been shown to be neutral on the prevention of HF-related events [11]. As pre-specified exploratory analysis, we also verified whether the effect of empagliflozin is more evident in subjects with subtle contractility impairment (reduced LV-GLS) and whether this associates with changes in plasma biomarkers of inflammation, oxidative stress, matrix remodelling, and myocyte strain and injury.
Methods
Rationale and study design
The EMPA-HEART trial is a phase III, open label, active-controlled, parallel groups, single center, exploratory study conducted in Pisa, Italy. This is a proof-of-concept study aiming at evaluating whether the chronic treatment with the SGLT2i empagliflozin can ameliorate myocardial and cardiopulmonary functions above and beyond its effect on glycemic control, in comparison to sitagliptin, an equally effective plasma glucose lowering agent presumably neutral on cardiac function. Outpatients with T2D of either sex, age 40–80 years, on stable metformin and/or basal insulin with suboptimal glycaemic control (HbA1c 7.0–8.5%) were randomized to either Sitagliptin 100 mg or Empagliflozin 10 mg. Exclusion criteria were: (a) impaired kidney function (CK-EPI eGFR < 50 mL/min/1.76m2), (b) any heart disease defined as presence of clinically relevant cardiovascular symptom, cardiac or vascular disease or valvular defects, history of coronary artery disease or evidence of stress-induced ischemia, reduced (≤ 50%) 2D LV ejection fraction (LVEF), cardiac autonomic neuropathy, (c) any pulmonary, muscular, or orthopedic diseases potentially limiting exercise capacity. As pre-specified exploratory analysis, we evaluated whether the effect of the treatments on myocardial contractility differs in the subgroup of patients with more pronounced abnormalities at baseline (LV-GLS below the median) and whether there are treatment-related differences in the following plasma biomarkers: (a) inflammation: tumor necrosis factor-alpha (TNFα) and high-sensitive c-reactive protein (hsCRP); (b) oxidative stress: myeloperoxidase (MPO); (c) LV parietal stress: natriuretic peptides (BNP and NT-proBNP), pro-adrenomedullin (proADM); (d) cardiomyocyte damage: high-sensitive troponin T (hsTnT); and (e) extracellular matrix remodeling/fibrosis: procollagen (NT-PRO3). The rationale, study design, and the methods of the study have been previously described in detail [12].
Cardiopulmonary exercise test protocol
A symptom-limited, graded, ramp exercise test was performed in the semi-supine position using a microprocessor-controlled stress cycle ergometer (Ergoline ergoselect 2000 GmbH, Germany). A 12-lead electrocardiogram and non-invasive arterial saturation and blood pressure (BP) were monitored continuously with heart rate (HR) and brachial BP measured at rest and every minute during exercise using a validated automatic device (Omron M6 Comfort, Kyoto, Japan). The expected VO2peak, estimated on the bases of patient age, height, weight and clinical history [13], was used to adjust the ramp increments (Watt) in order to allow all the patients to reach VO2peak in 8 to 12 min. Breath-by-breath minute ventilation, carbon dioxide production (VCO2), and oxygen consumption (VO2) were measured using a dedicated cardiopulmonary test diagnostic device (Blue Cherry, Geratherm Respiratory GmbH, Germany). Patients not reaching a respiratory exchange ratio (RER) steadily > 1.0 during the exercise test were excluded from the analysis. We defined VO2peak as the highest median value of the two 30-s intervals of the last minute of exercise, as previously validated [7, 14]. The peripheral extraction, that is arterio-venous oxygen difference (Δ(a-v)O2) was estimated indirectly with a validated method [7]. Oxygen pulse was calculated as VO2peak/HR and expressed both as absolute values (mL/beat per minute) and in percentage of VO2peak. An automatic procedure was used to detect the anaerobic threshold (AT) based on the V-slope, ventilatory equivalents and end-tidal partial pressure methods; AT was verified visually and, if necessary, recalculated [13]. The chronotropic response was estimated as the change in HR from rest to peak exercise, divided by the difference between the age-predicted maximal HR and the resting HR (i.e., HR reserve). Chronotropic incompetence was defined as the failure to achieve ≥ 80% (≥ 62% if taking β-blockers or calcium-channel blockers) of the HR reserve during exercise [15].
Resting and exercise echocardiography
All patients underwent a comprehensive transthoracic echocardiography examination at rest (GE healthcare vivid e95, Milwaukee, WI, USA) according to the International Recommendations. As previously described [7], data collected at each stage (baseline, after 4 min, at the AT, and at peak effort) included: left ventricle (LV) and atrial (LA) volumes, stroke volume (SV), peak E-wave and A-wave velocities, tissue Doppler imaging (TDI)-derived S’ and e’ at the septal and lateral mitral annulus, tricuspid regurgitation velocity and systolic pulmonary artery pressure (sPAP), tricuspid annular plane systolic excursion (TAPSE); LV volumes and LVEF were calculated from the apical two- and four-chamber views using the modified Simpson’s rule. LV mass index (LVMi) was calculated according to current guidelines with 2D measures of LV indexed to body surface area. SV was calculated by multiplying the LV outflow tract area at rest by the LV outflow tract velocity–time integral measured by pulsed-wave Doppler during each activity level, as previously validated [7]. Cardiac output (CO) was calculated as the multiplication of SV and HR. Systemic vascular resistance (SVR) was calculated as the ratio of the peak mitral regurgitant velocity [m/s] to LV outflow tract time-velocity integral (TVI(LVOT)) [cm]. All measurements were reported as the average of three beats.
We measured global longitudinal strain (GLS) from the apical long-axis view and two- and four-chamber views, ensuring a frame rate > 50 Hz (GE healthcare EchoPAC BT 12). We reported the average values from the three apical views at rest and low-load effort, within the first 4 min of exercise, GLS was reported as the average of three beats and expressed in absolute values to improve readability. We excluded poorly tracked segments and patients were not analysed if more than one segment per view was deemed unacceptable.
Plasma biomarkers assays
TNFα, MPO and hsCRP were measured by ELISA kits (TNF-apha Human, High sensitivity; Myeloperoxidase Human Instant and CRP Human, produced by Invitrogen by Thermo Fisher Scientific, MA, USA). hsTnT, BNP and NT-pro BNP were assayed by ECLIA methodology using commercial kits (Elecsys Troponin T hs, Elecsys BNP, Elecsys proBNP II, respectively) from Roche Diagnostics S.p.A., Milan (Italy) on the COBAS analyser e411. Mid-regional proADM and NT-PRO3 by ELISA kits (Human MR-ProADM and Human Procollagen III N-Terminal Propeptide) produced by MYBIOSOURCE, CA (USA).
Statistical analysis
Analyses were performed using JMP Pro software version 13.2.1 (SAS Institute, Cary, NC). Values are presented as mean ± SD, or as median and interquartile range (IQR), for variables with normal and non-normal distribution, respectively. Comparisons between treatment groups were performed by the Student t-test for unpaired data for continuous variables and by the chi-square test for categorical variables. Variations from baseline to follow-up in the parameters in each of the two groups were presented as mean and [95% CI], the effect of the therapy at each follow-up assessment (1 and 6 months for LV-GLS; 6 months for the other endpoints and variables) was assessed by t-test on the differences from baseline and presented as mean [95% CI] and by ANOVA for repeated measure on the whole data set; considering the time*drug interaction effect. All tests were conducted at a two-sided (and when of borderline significance also one-sided) α level of 0.05.
Results
Baseline characteristics of the study population
According to inclusion and exclusion criteria, 106 consecutive patients were screened for the study from December 2017 to July 2020; after baseline evaluation, 37 were subsequently excluded because of definitive exclusion criteria and 13 did not participate for personal reasons. The recruitment was interrupted earlier due to lock-down imposed by COVID-19 pandemic. Fifty-six T2D subjects meeting the definitive inclusion/exclusion criteria were randomized to intervention, of which 27 were allocated to treatment with Empagliflozin 10 mg/die, and 29 to Sitagliptin 100 mg/die. During the follow-up, 3 patients abandoned the study for personal reasons and 1 patient in the Empagliflozin arm because of side effects (genital infections). At follow-up, 8 further patients were excluded because of suboptimal echocardiography images and/or incomplete or unreliable follow-up CPET data. The analysis was performed on 44 subjects, 22 in the Empagliflozin arm and 22 in the Sitagliptin arm. Patient disposition with the Consort 2010 flow diagram is shown in Additional File 1: Fig. S1.
Baseline characteristics of the study population are reported in Table 1. The population was mainly composed mainly by male adults with a relatively long duration of T2D and suboptimal glycaemic control. Lipid profile, haemoglobin, creatinine, and NT-pro-BNP showed comparable values between the groups, and no patient had peripheral artery disease (as assessed by ankle-brachial index). At baseline 2D-echoDoppler evaluation, all patients showed normal biventricular dimensions and systo-diastolic functions with no difference between the two groups (Table 1).
Table 1.
Clinical characteristics of the study population
| All patients (n = 44) | Empagliflozin (n = 22) | Sitagliptin (n = 22) | p value | |
|---|---|---|---|---|
| Clinical data | ||||
| Male (n, %) | 38 (86) | 19 (86) | 19 (86) | ns |
| Age (years) | 61.7 ± 9.7 | 61.6 ± 9.6 | 61.8 ± 10.1 | ns |
| Duration of diabetes (years) | 9.6 ± 8.0 | 7.8 ± 6.9 | 11.1 ± 8.8 | ns |
| Weight (kg) | 84.6 ± 15.3 | 83.0 ± 13.6 | 83.7 ± 12.4 | ns |
| BMI (kg/m2) | 28.7 ± 5.3 | 27.8 ± 4.7 | 29.6 ± 5.7 | ns |
| Mean BP (mmHg) | 102.6 ± 11.5 | 102.9 ± 9.9 | 102.3 ± 13.2 | ns |
| Active smokers, (n, %) | 10 (23) | 6 (27) | 4 (18) | ns |
| Hypertension (n, %) | 34 (77) | 18 (81) | 16 (72) | ns |
| Baseline therapy | ||||
| Metformin, n (%) | 40 (91) | 20 (91) | 20 (91) | ns |
| Insulin, n (%) | 11 (25) | 7 (32) | 4 (18) | ns |
| Statin, n (%) | 32 (73) | 18 (81) | 14 (63) | ns |
| ACEi/ARBs, n (%) | 27 (61) | 16 (53) | 11 (50) | ns |
| Beta-blockers, n (%) | 10 (23) | 5 (23) | 5 (23) | ns |
| CCB, n (%) | 10 (23) | 6 (27) | 4 (18) | ns |
| ASA, n (%) | 16 (36) | 4 (41) | 7 (32) | ns |
| Thiazide diuretics, n (%) | 5 (11) | 3 (14) | 2 (9) | ns |
| Furosemide, n (%) | 1 (2) | 0 (0) | 1 (5) | ns |
| Blood tests | ||||
| HbA1c (mmol/mol) | 59.2 ± 6.4 | 57.8 ± 6.5 | 60.3 ± 6.2 | ns |
| Total Cholesterol (mg/dL) | 162 ± 33 | 159 ± 29 | 165 ± 38 | ns |
| HDL-C (mg/dL) | 48 ± 12 | 49 ± 13 | 47 ± 11 | ns |
| LDL-C (mg/dL) | 97 ± 26 | 95 ± 21 | 98 ± 30 | ns |
| Triglycerides (mg/dL) | 131 ± 57 | 121 ± 59 | 142 ± 54 | ns |
| Haemoglobin (g/dL) | 14.2 ± 1.3 | 14.1 ± 1.1 | 14.3 ± 1.4 | ns |
| Creatinine (mg/dL) | 0.89 ± 0.26 | 0.86 ± 0.31 | 0.92 ± 0.19 | ns |
| eGFR (mL/min/1.73mq) | 89.6 ± 17.4 | 91.5 ± 18.5 | 87.7 ± 16.5 | ns |
| Uric acid (mg/dL) | 5.55 ± 1.45 | 6.01 ± 1.60 | 5.10 ± 1.10 | ns |
| UAlb.-UCreat.-Ratio (mg/g) | 5 (0–15) | 4 (0–7) | 8 (4–36) | ns |
| NT-proBNP (pg/mL) | 81 (27–118) | 63 (28–121) | 33 (16–76) | ns |
| Vascular and pulmonary function | ||||
| Ankle-Brachial-Index | 1.16 ± 0.10 | 1.13 ± 1.1 | 1.18 ± 1.1 | ns |
| VD/VT (%) | 16.2 ± 4.9 | 16.4 ± 3.9 | 16.1 ± 5.2 | ns |
| 2D-Echocardiography | ||||
| EDVi (mL/m2) | 51.5 ± 11.7 | 52.0 ± 12.2 | 51.0 ± 11.5 | ns |
| LVMi (g/m2) | 89.5 ± 17.3 | 89.9 ± 16.1 | 89.2 ± 18.9 | ns |
| LAVi (mL/m2) | 24.9 ± 7.5 | 24.8 ± 8.4 | 25.0 ± 6.8 | ns |
| LVEF rest (%) | 59.3 ± 4.5 | 60.5 ± 3.6 | 58.1 ± 5.1 | ns |
| E/A ratio | 0.90 ± 0.25 | 0.94 ± 0.26 | 0.86 ± 0.23 | ns |
| E/e′ (cm/sec) | 8.5 ± 2.5 | 8.3 ± 2.2 | 8.7 ± 2.7 | ns |
ACEi/ARBs, ACE inhibitors or angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; CCB, calcium channel blockers; EDVi, end diastolic volume index; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; LAVi, left atrium volume index; LVEF, left ventricular ejection fraction. LVMi, left ventricular mass index; UAlb-UCreat. Ratio, spot urine albumine-to-creatinine ratio
Changes in clinical and laboratory parameters
At 6 months follow-up, a small reduction in body weight was observed only in the empagliflozin arm, while no significant change in mean blood pressure or in resting heart rate was evident (Table 2). The two treatments produced a comparable reduction in HbA1c while an increase in plasma hemoglobin and hematocrit and a reduction in plasma uric acid were observed with empagliflozin. The remaining hematologic parameters (lipids, creatinine, ACR) did not differ from baseline to follow-up in either group (Table 2).
Table 2.
Mean changes [and 95% CI] from baseline to 6 months follow-up in clinical, biohumoral, echocardiographic, and exercise test parameters
| Empagliflozin (n = 22) | Sitagliptin (n = 22) | p value | |
|---|---|---|---|
| Clinical parameters | |||
| Weight (kg) | − 1.6 [− 2.7/− 0.5]* | 0.1 [− 1.1/1.2] | 0.0315 |
| HR at rest (beat/min) | 0.6 [− 1.6/2.8] | − 0.4 [− 4.5/3.7] | ns |
| MAP rest (mmHg) | − 5.4 [− 10.7/0.0] | − 0.22 [− 7.6/7.2] | ns |
| Biohumoral parameters | |||
| HbA1c (mmol/mol) | − 4.6 [− 7.4/− 1.8]* | − 4.9 [− 8.8/− 0.9]* | ns |
| Total Cholesterol (mg/dL) | − 8 [− 21/5] | − 15 [− 30/0] | ns |
| HDL-Cholesterol (mg/dL) | 1.3 [− 1.4/4.0] | − 1.7 [− 4.2/0.9] | ns |
| LDL-Cholesterol (mg/dL) | − 7 [− 19/6] | − 7 [− 18/3] | ns |
| Triglycerides (mg/dL) | − 2 [− 28/24] | − 14 [− 33/6] | ns |
| Haemoglobin (g/dL) | 0.7 [0.2/1.1]* | − 0.5 [− 1/− 0.1] | 0.0003 |
| Haematocrit (%) | 2.0 [0.7/3.2]* | − 1.3 [− 2.6/0.0] | 0.0006 |
| Creatinine (mg/dL) | − 0.1 [− 0.2/0.1] | − 0.0 [− 0.1/0.0] | ns |
| eGFR (mL/min/1.73mq) | 2.5 [− 3.7/8.7] | 1.4 [− 1.8/4.6] | ns |
| Uric acid (mg/dL) | − 1.5 [− 2.3/− 0.6]* | 0.2 [− 0.3/0.6] | 0.0023 |
| UAlb-UCreat-Ratio (mg/g) | 6.1 [− 1.9/14.2] | 5.0 [− 20.6/30.5] | ns |
| Echocardiography | |||
| EDVi rest (mL/m2) | 2.2 [− 0.9/5.2] | 3.6 [− 1.0/6.3] | ns |
| LVMi rest (g/m2) | 4.5 [− 1.1/10.2] | 1.1 [− 2.7/5.0] | ns |
| LAVi rest (mL/m2) | 0.5 [− 1.3/2.2] | 2.0 [− 0.4/4.3] | ns |
| CO rest, L/min | 0.0 [− 0.6/0.6] | 0.8 [− 0.3/1.4] | ns |
| CO peak, L/min | 0.7 [− 0.6/1.9] | 0.9 [− 0.3/2.1] | ns |
| LVEF rest (%) | 0.1 [− 1.3/1.6] | 2.1 [− 0.4/3.7] | ns |
| LVEF peak (%) | − 0.7 [− 2.8/1.5] | 2.0 [− 0.1/3.9] | ns |
| S’ mean rest (cm/sec) | 0.0 [− 0.8/0.9] | − 0.1 [− 1.0/0.8] | ns |
| S’ mean peak (cm/sec) | 0.4 [− 0.9/1.7] | − 0.2 [− 1.0/0.6] | ns |
| ΔS’ mean | 0.4 [− 0.8/1.5] | − 0.1 [− 1.0/0.8] | ns |
| E/e’ rest (cm/sec) | − 0.5 [− 1.3/0.4] | − 1.0 [− 2.2/0.2] | ns |
| E/e’ peak (cm/sec) | − 0.3 [− 1.5/0.9] | − 0.6 [− 1.5/0.5] | ns |
| Cardiopulmonary exercise test | |||
| Workload (W) | 5 [− 1/11] | 2 [− 5/9] | ns |
| HR at peak (beat/min) | 3.0 [− 2.1/8.0] | 1.3 [− 4.4/7.0] | ns |
| HR at peak (%max) | 1.9 [− 1.3/5.1] | 0.8 [− 2.8/4.5] | ns |
| RER peak | 0.00 [− 0.03/0.04] | 0.01 [− 0.02/0.03] | ns |
| VO2/work slope | 0.3 [− 0.5/1.1] | 0.6 [− 0.2/1.4] | ns |
| VO2 rest (mL/min/kg) | 0.5 [− 0.1/1.2] | 0.6 [− 0.1/1.4] | ns |
| VE/VCO2 slope | 0.3 [− 1.2/1.8] | 1.3 [− 0.1/2.6] | ns |
| O2 pulse peak (mL/bpm) | 0.1 [− 0.7/1.0] | 0.5 [− 0.2/1.2] | ns |
| O2 pulse peak (%VO2peak) | 2.8 [− 3.0/8.5] | 3.0 [− 1.1/7.1] | ns |
| AV O2 diff rest (mL/dL) | 0.6 [− 0.7/1.8] | 0.2 [− 1.0/1.4] | ns |
| AV O2 diff peak (mL/dL) | − 0.1 [− 0.9/0.7] | − 0.2 [− 1.3/1.0] | ns |
*Indicates a statistically significant difference within groups, p value indicates the level of statistical significance of the interaction term time*treatment at MANOVA
Resting and exercise echocardiography
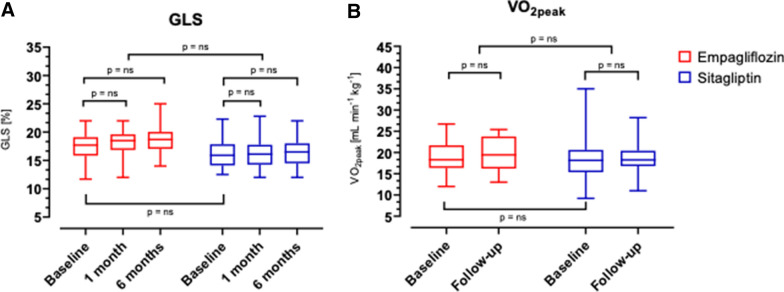
At baseline, resting and effort indices of heart function were similar in the two study groups (Additional file 2: Table S1); baseline resting LV-GLS was numerically higher in the empagliflozin group (17.3 ± 2.7 vs 15.8 ± 2.2%, p = 0.06). From baseline to 1- and 6-months follow-up, no change in resting LV-GLS was seen in any of the treatment groups (Fig. 1A); the difference between the treatments was slightly in favour of empagliflozin both at 1 month (+ 0.44 [− 0.10/+ 0.98]%) and at 6 months (+ 0.53 [− 0.56/+ 1.62]%); however, the time*drug effect at ANOVA for repeated measures was not statistically significant. The exercise-induced acute increase (from rest to 4 min of exercise) in LV-GLS was comparable in the two treatment arms both at baseline (+ 1.9 [+ 1.1/+ 2.6] vs + 1.9 [+ 1.2/+ 2.5]% for empagliflozin and sitagliptin, respectively) and at 6 months follow-up (+ 1.4 [+ 0.6/+ 2.1] vs + 2.0 [+ 1.2/+ 2.7]%). Likewise, cardiac chamber dimensions and/or geometry were not affected by either treatment, as well as Doppler and tissue-Doppler derived systo-diastolic indices (LA volume index, LVEF, LV mass index, E/A ratio, mitral anulus S’, e’, E/e’, TAPSE, sPAP) and SVR (Table 2).
Fig. 1.
Box-and-whiskers plots of a left ventricle global longitudinal strain (GLS) and b oxygen uptake at peak exercise (VO2peak) at baseline evaluation and at follow-up visits, expressed in absolute values (% and mL/min/kg, respectively)
Cardiopulmonary exercise test
At baseline, indices cardiopulmonary function were similar in the two study groups (Additional file 2: Table S1). All patients reached a maximal exercise as required by inclusion criteria, achieving a respiratory exchange ratio (RER) steadily above 1.0 (median: 1.07, IQR: [1.03–1.10]), and the duration of exercise was between 10 and 12 min as per protocol. The exercise was well tolerated without discomfort, hypertensive response, or any significant alteration in vital parameters or ECG trace. The achieved VO2peak at baseline in the whole population was 18.9 [15.8–21.3] mL/kg/min, which corresponded to 76 ± 15% of predicted maximal theoretical VO2 and was similar in the two groups (empagliflozin 18.9 ± 3.8 vs sitagliptin 18.8 ± 5.6 mL/min/kg), as was comparable the achieved peak workload (118 ± 25 vs 119 ± 22 W). From baseline to 6 months follow-up, no change in VO2peak was seen in any of the treatment groups (Fig. 1B). Also, we could not demonstrate any variation from baseline in each treatment arm or between the arms in the other main parameters derived from iCPET, namely: cardiac (cardiac output, chronotropic response, oxygen pulse), pulmonary (ventilatory efficiency, oxygen saturation, end-tidal carbon dioxide), skeletal muscle (peripheral oxygen extraction), and metabolic (RER, anaerobic threshold). The results are reported in Table 2.
Plasma biomarkers
The baseline plasma levels of the biomarkers (median [IQR]) were within the normal range and no change was observed at 6 months follow-up in either treatment arm or between the treatments. Specifically: hsCRP (mg/dL) from 0.114 [0.026–0.216] to 0.095 [0.048–0.153] with empagliflozin, from 0.177 [0.090–0.762] to 0.156 [0.081–0.405] with sitagliptin. BNP (pg/mL) from 25 [10–47] to 16 [10–46] with empagliflozin, from 12 [10–25] to 11 [10–20] with sitagliptin. TnHS (ng/mL) from 9.9 [6.7–16.4] to 10.0 [7.6–13.9] with empagliflozin, from 7.8 [5.8–27.0] to 8.2 [6.6–15.2] with sitaglitpin. ProADM (nmol/L) from 0.080 ± 0.065 to 0.150 ± 0.120 with empagliflozin, from 0.154 ± 0.198 to 0.119 ± 0.130 with sitagliptin. NT-PRO3 (ng/mL) from 5.6 [4.4–6.7] to 5.6 [4.0–7.7] with empagliflozin, from 6.7 [5.1–8.9] to 6.2 [4.7–7.6] with sitagliptin. TNFα (pg/mL) from 0.74 [0.46–0.96] to 0.79 [0.69–0.96] with empagliflozin, from 0.67 [0.59–0.88] to 0.80 [0.66–0.93] with sitagliptin. All p values > 0.05.
Subgroup analysis
As prespecified hypothesis-driven analysis, we divided each arm in two subgroups of 11 subjects according to the ranking of baseline resting LV-GLS values (median GLS empagliflozin 16.5%, median GLS sitagliptin 16.0%). The subgroups with higher LV-GLS showed no change during the study neither on empagliflozin nor sitagliptin. On the contrary, the subjects with lower baseline LV-GLS experienced an improvement in LV contractility absolute values already at 1 month after therapy with empagliflozin (+ 1.22 [+ 0.31/+ 2.13]%) followed by a further improvement at 6 months (+ 2.05 [+ 1.14/+ 2.96]%). The subjects with lower LV-GLS on sitagliptin showed no change at 1 month (+ 0.30 [− 0.13/+ 0.73]%) and a mild improvement at 6 months (+ 0.92 [+ 0.21/+ 0.62]%) (Fig. 2). The estimated differences between the changes induced by the 2 treatments by paired t-test were + 0.92 [− 0.04/+ 1.89]% (p = 0.05 for 2-side and p = 0.03 for one-side superiority of empagliflozin) at 1 month and was maintained at 6 months (+ 1.08 [+ 0.01/+ 2.17]%, p = 0.05 for 2-side and p = 0.03 for one-side superiority of empagliflozin). The ANOVA for repeated measures detected a significant effect for the interaction term time*drug (p = 0.04) as well as for the drug (p = 0.02) and for time (p < 0.0001) alone.
Fig. 2.
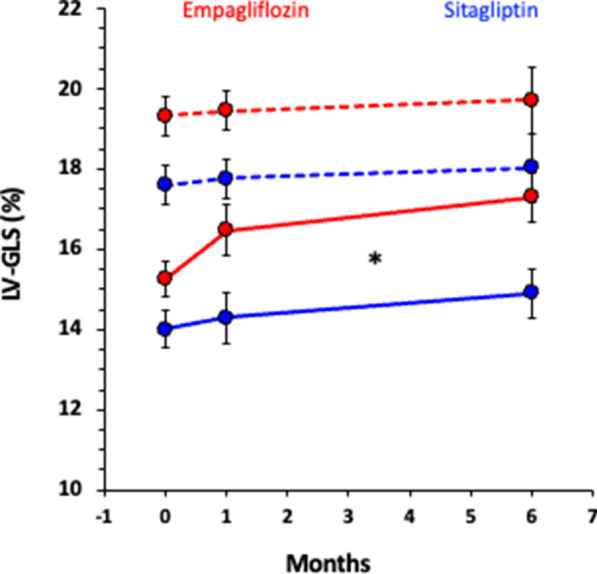
Values of left ventricle global longitudinal strain (GLS) at baseline (0), 1 month and 6 months follow-up visits during empagliflozin (red) or sitagliptin (blue) treatment. The population was divided in two subgroups depending on baseline GLS values above (continuous line) or below (dotted lines) median (median for empagliflozin group: GLS 16.4%; median for sitagliptin group: GLS 16.0%). The star indicates a statistically significant time*treatment effect at ANOVA for repeated measures
Discussion
The EMPA-HEART is a randomized trial aimed at evaluating whether the treatment with empagliflozin is associated with an amelioration of LV contractility and/or cardiopulmonary function independently of its effects on glycaemic control in T2D subjects without clinical or echocardiographic evidence of cardiac disease. In line with previous observations [16, 17], the treatment with empagliflozin was associated with a modest reduction in body weight and serum uric acid, as well as to an increase in hemoglobin and hematocrit.
No significant change in structural parameters were appreciated at resting 2D echocardiography; similarly, despite a trend towards improved values, diastolic function was also unchanged in the two intervention groups, both at rest and during exercise. SGLT2i have been inconsistently associated with an amelioration of LV structural and functional parameters in T2D subjects without overt HF and/or structural heart disease[2]. Sitagliptin has been shown to improve E/e′ by 20% in a population similar to ours, but only after 24 months [18], and empagliflozin has been reported to ameliorate diastolic function in subject with HFrEF and moderate to severe diastolic dysfunction [19]. Correspondingly, the modest reduction in LVMi reported by one uncontrolled trial [20] was not evident in our study.
With regard to systolic parameters, the crude indices provided by resting and exercise 2D LVEF did not change significantly at follow-up neither in the whole population nor in any of the treatment arms, confirming the unimportant effect of SGLT2i on this parameter in subjects without heart disease [2]. This is corroborated by the unchanged tissue Doppler S’ and speckle-tracking LV-GLS values, more sensible and less load-dependent systolic parameters than 2D LVEF (Fig. 1 and Table 2). Nevertheless, when considering subgroup analysis, while no change was observed in those with higher LV-GLS values, patients with subclinical LV contractile dysfunction (LV-GLS < 16.5%) on empagliflozin showed a significant increase in LV-GLS at follow-ups, that was evident already at 1 month and further improved at 6 months. On the contrary, in the sitagliptin arm the increase in contractility in the subgroup with lower baseline LV-GLS (< 16.0%) was evident only at 6 months and was approximately 50% smaller (Fig. 2). The similarity between the change in GLS from 1 to 6 months in both treatment groups suggests that glycaemic control per se might have had a favourable effect on myocardial contractility, as it has been recently suggested [21]. Our results imply that empagliflozin can improve LV contractility beyond its glycaemic effects in those with subclinical myocardial dysfunction. A recent publication with cardiac magnetic resonance supports this interpretation [22]. The cut-off value that we identified for a benefit during SGLT2i therapy is in accordance with a recent definition of normal LV-GLS in adults as > 18%, borderline values 16–18% and abnormal as < 16% [23].
It is known from the literature that SGLT2i are associated with a relatively heterogeneous amelioration of LV-GLS (from 2 to 11% over baseline values) despite no increase in 2D-LVEF in subjects with T2D and HF with a gradient that is proportional to the degree of baseline dysfunction [24, 25]. A 12-months long, randomized, open label clinical trial reported no effect on LV-GLS after treatment with SGLT2i (LV-GLS 17 ± 4 vs 17 ± 4%) in 40 subject with T2D, normal LVEF, and no clinical diagnosis of HF [26]; unfortunately, subgroup analysis according to baseline GLS was not performed in that study. Our results in a similar population extend the concept that empagliflozin ameliorates systolic function in T2D in proportion to baseline values [2] to include also those with early and mild subclinical contractility impairment in the absence of overt cardiac disease. Our data also indirectly confirm the high prevalence of subclinical contractility dysfunction reported in the asymptomatic T2D population (approx. 50%) [6]. Considering the prognostic value of LV-GLS [27], our finding might represent a solid rationale for verifying through a randomized double blind clinical trial whether the early use of empagliflozin can prevent or delay incident HF in this specific subgroup of patients, currently not specifically included in guidelines on the use of SGLT2i in HF prevention in T2D.
Since in T2D the condition of reduced VO2peak is associated with adverse cardiovascular outcomes [28], one may postulate that an increased cardiopulmonary function might be observed with SGLT2i therapy. In previous pilot studies lacking randomization and active control, VO2peak was increased by 24% after 6 months of therapy with empagliflozin vs “usual therapy” in T2D patients with established cardiovascular disease or at high risk [29], and by 10% in HFrEF patients with [30] and without T2D [31] after 1 month of therapy. Conversely, more rigorous studies in T2D and HFrEF failed to substantiate any improvement after SGLT2i either alone [32] or versus an active control [33]. In our study, cardiopulmonary fitness and all the major parameters influencing VO2peak—i.e., cardiac output, peripheral extraction, ventilation—were unaffected by either treatment, further sustaining the observations of a neutral effect of either drug on cardiopulmonary capacity in this population. The amelioration of glycaemic control is known to improve VO2peak in T2D with established cardiac disease [34, 35] and can justify the positive results of the non-controlled, non-randomized trials that were not confirmed when active controls were used, as it is in the present study. Interestingly, the subjects with HFrEF and concomitant therapy with loop diuretics showed a greater improvement in cardiorespiratory fitness when receiving empagliflozin [32] and this implies a synergism between the two diuretics in volume regulation as elegantly shown by Griffin et al. [36]. No patient was assuming loop diuretics in our population, and this could partly justify the negative results on VO2peak, which on the other hand confirms that volume regulation is unlikely to be the mechanism through which SGLT2i are effective in primary prevention (i.e., in patients with no congestion). We have recently shown that both effort tolerance (VO2peak) and peripheral oxygen extraction are correlated with LV contractility indices (S′ and GLS) in subjects with uncomplicated T2D [7] suggesting the presence of a subclinical myopathy involving both the heart and the skeletal muscle. Accordingly, in the present study, VO2peak values showed a trend to be lower in those with LV-GLS below the median (17.5 ± 1.0 vs 19.9 ± 1.0 mL/min/kg, p = 0.12). Nonetheless, our result of unchanged peak workload and peripheral oxygen extraction confirms the lack of clinically relevant effects of SGLT2i on skeletal muscle oxygen/work coupling in T2D subjects.
No significant change in natriuretic peptides was evident from our data, which were in the normal reference values at baseline. This confirms that volume regulation is not relevant in this study population, aligning with the available literature that failed at demonstrating a consistent reduction in natriuretic peptides with SGLT2i, with a trend towards a greater efficacy in patients with HFrEF [24] and higher baseline values of natriuretic peptides [2]. Differently from pre-clinical evidence of anti-inflammatory [37] and anti-fibrotic properties of SGLT2i [38], in this study the markers of myocardial injury, oxidative stress, matrix remodelling, and inflammation were unchanged at follow-up. Still, the neutral effect on biomarkers of matrix remodelling agrees with a recent study with cardiac magnetic resonance imaging detecting no change in myocardial fibrosis after empagliflozin therapy in T2D subjects with diabetic cardiomyopathy [39].
Conclusions
In T2D subjects free from heart disease empagliflozin has a neutral impact on aerobic fitness and LV systo-diastolic functions, both at rest and during exercise. Nevertheless, it can exert an early and sustained amelioration of myocardial contractility in those with subclinical dysfunction as defined by a mildly reduced resting LV-GLS (< 16.5%) despite normal LVEF. These data support the hypothesis that SGLT2i can directly affect myocardial contractility in selected patients with subclinical LV systolic dysfunction, possibly justifying their benefit in HF primary prevention.
Limitations
The recruitment was interrupted early due to the lock-down imposed by the COVID-19 pandemics, therefore the power of our study is lower than planned; therefore, we might have missed absolute changes in LV-GLS below 2.5% or 1.7%, which were considered relevant from a clinical and pathophysiologic point of view, respectively [12]. The data, however, are clear in showing no change in LV-GLS in each group despite a clinically relevant change (+ 2.05 [+ 1.14/+ 2.96]%) in the subjects with low baseline LV-GLS treated with empagliflozin for 6 months. The reduced sample size also forced us to restrict the secondary endpoints to only one (VO2peak) and the pre-defined exploratory analysis only to subgroup analysis according to baseline LV-GLS and to mechanism-oriented biomarkers. The a posteriori power calculation on LV-GLS measured on 44 (22 per arm) subjects, with paired comparison within each group, showed statistical power between 99 and 84% for differences ranging from 2.5 to 1.7% and the smallest difference in LV-GLS—keeping power at 95%—is 2.0%. Therefore, we acknowledge that changes smaller than 2% might be missed because of the reduced sample size. This value still is close to 1.5%, our definition of minimal clinically meaningful change. Although LV-GLS is considered an accurate method to evaluate LV contractility, there is evidence that it might be affected by the technology used, age, sex, BMI, and to some extent also by LV loading conditions [40]. In our study all these variables remained stable; therefore, while the absolute values might be difficult to interpret, the changes within subjects are robust. This was an open study, but the cardiologist performing the measurements of primary and secondary outcomes was blind to the treatment allocation.
Supplementary Information
Additional file 1: Figure S1. EMPA-HEART trial.
Additional file 2: Table S1. Cardiopulmonary exercise test, 2D Echocardiograpy, Doppler, Tissue Doppler, and Speckle tracking parameters. P-value is the result of a two-point ANOVA for repeated measures.
Acknowledgements
The Authors are grateful to all the volunteers and the personnel of the Metabolism, Nutrition, and Atherosclerosis Lab and the Cardiopulmonary Test Lab, Department of Clinical and Experimental Medicine, University of Pisa, Italy.
Abbreviations
- AT
Anaerobic threshold
- BMI
Body mass index
- BNP
Brain-derived natriuretic peptide
- BP
Blood pressure
- CO
Cardiac output
- eGFR
Estimated glomerular filtration rate
- HbA1c
Glycated hemoglobin
- HF
Heart failure
- HFpEF
Heart failure with preserved left ventricular ejection fraction
- HFrEF
Heart failure with reduced left ventricular ejection fraction
- HR
Heart rate
- hsCRP
High-sensitive C-reactive protein
- hsTnT
High-sensitivity troponin T
- iCPET
Imaging cardiopulmonary exercise test
- LA
Left atrium
- LV
Left ventricle
- LV-GLS
Left ventricle global longitudinal strain
- LVEF
Left ventricular ejection fraction
- LVMi
Left ventricular mass index
- MAP
Mean arterial pressure
- MPO
Myeloperoxidase;
- NT-PRO3
N-terminal procollagen 3
- NT-proBNP
N-terminal-proBNP
- proADM
Pro-adrenomedullin
- RER
Respiratory exchange ratio
- SGLT2i
Sodium-glucose co transporter 2 inhibitors;
- sPAP
Systolic pulmonary artery pressure
- SV
Stroke volume
- SVR
Systemic vascular resistance
- T2D
Type 2 Diabetes;
- TAPSE
Tricuspid anulus plane systolic excursion
- TNF-α
Tumor necrosis factor-alpha;
- VCO2
Carbon dioxide production
- VD/VT
Dead volume/tidal volume radio
- VE
Minute ventilation
- VE/VCO2
Ventilatory efficiency
- VO2
Oxygen uptake
- VO2peak
Oxygen uptake at peak exercise
- Δ(a-v)O2
Artero-venous oxygen difference (peripheral extraction)
Author contributions
LN: study design, screening and recruitment of patients, patient data collection, contacts with patients, performance of neurovascular tests, cardiopulmonary tests, echocardiography, randomization and prescription of drugs, database creation, literature review, draft of the manuscript, manuscript writing. NRP, IF: Echocardiography and cardiopulmonary tests, critical interpretation of data, revision of manuscript. PS: Patients management, performance of neurovascular tests, clinical measures, database creation. DT: recruitment of patients, statistics, figures, critical interpretation of data, manuscript revision. AD: recruitment of patients, critical interpretation of data, revision of manuscript. SB: laboratory tests, blood and urine samples preparation and storage. SP: performance of neurovascular tests, clinical measures, blood and urine sample collection and storage, contacts with patients, clinical data storage. IF: Echocardiography and cardiopulmonary tests, critical interpretation of data, revision of manuscript. VAB: study design, Echocardiography, and cardiopulmonary tests supervision. AN: study design, recruitment of patients, statistics, interpretation of data, revision of manuscript, tables, and figures. All authors read and approved the final manuscript.
Funding
The study is an investigator-initiated study supported at 49% by an unrestricted grant from Boehringer Ingelheim.
Availability of data and materials
The study was carried out in accordance with the most recent international GCP guidelines (CPMP/ICH/135/1995), EU Directive and guidance and the local legislation on the conduct of clinical trials. The database has been locked after the planned statistical analysis was performed. Any further modification of recorded data will be documented in a database log form. All the collected data will be stored for a maximum period of 15 years after the end of the study and then destroyed. Only the investigators of the study will have access to all the data.
Declarations
Ethics approval and consent to participate
The Local Ethic Committee approved the study protocol. All patients gave written informed consent before enrolment.
Consent for publication
All the Authors gave their consent to publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In memory of Prof. Vitantonio Di Bello who designed the study and sadly passed away on Jan 29th, 2018
Contributor Information
Lorenzo Nesti, Email: lorenzo.nesti@phd.unipi.it.
Andrea Natali, Email: andrea.natali@med.unipi.it.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Natali A, Nesti L, Trico D, Ferrannini E. Effects of GLP-1 receptor agonists and SGLT-2 inhibitors on cardiac structure and function: a narrative review of clinical evidence. Cardiovasc Diabetol. 2021;20(1):196. doi: 10.1186/s12933-021-01385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seferovic PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Cavusoglu Y, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(9):1495–1503. doi: 10.1002/ejhf.1954. [DOI] [PubMed] [Google Scholar]
- 4.Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632–644. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61(3):722–726. doi: 10.1007/s00125-017-4509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cioffi G, Giorda CB, Chinali M, Di Lenarda A, Faggiano P, Lucci D, Maggioni AP, Masson S, Mureddu GF, Tarantini L, et al. Analysis of midwall shortening reveals high prevalence of left ventricular myocardial dysfunction in patients with diabetes mellitus: the DYDA study. Eur J Prev Cardiol. 2012;19(5):935–943. doi: 10.1177/1741826711417759. [DOI] [PubMed] [Google Scholar]
- 7.Nesti L, Pugliese NR, Sciuto P, De Biase N, Mazzola M, Fabiani I, Trico D, Masi S, Natali A. Mechanisms of reduced peak oxygen consumption in subjects with uncomplicated type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):124. doi: 10.1186/s12933-021-01314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nesti L, Pugliese NR, Sciuto P, Natali A. Type 2 diabetes and reduced exercise tolerance: a review of the literature through an integrated physiology approach. Cardiovasc Diabetol. 2020;19(1):134. doi: 10.1186/s12933-020-01109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugliese NR, De Biase N, Balletti A, Filidei F, Pieroni A, D'Angelo G, Armenia S, Mazzola M, Gargani L, Del Punta L, et al. Characterisation of haemodynamic and metabolic abnormalities in the heart failure spectrum: the role of combined cardiopulmonary and exercise echocardiography stress test. Minerva Cardiol Angiol. 2021 doi: 10.23736/S2724-5683.21.05743-4. [DOI] [PubMed] [Google Scholar]
- 10.Marwick TH, Gimelli A, Plein S, Bax JJ, Charron P, Delgado V, Donal E, Lancellotti P, Levelt E, Maurovich-Horvat P, et al. Multimodality imaging approach to left ventricular dysfunction in diabetes: an expert consensus document from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23(2):e62–e84. doi: 10.1093/ehjci/jeab220. [DOI] [PubMed] [Google Scholar]
- 11.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 12.Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V. Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA-HEART trial. Cardiovasc Diabetol. 2017;16(1):130. doi: 10.1186/s12933-017-0615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Thoracic Society. American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. 2021;23(11):1858–1871. doi: 10.1002/ejhf.2337. [DOI] [PubMed] [Google Scholar]
- 15.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123(9):1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai X, Yang W, Gao X, Chen Y, Zhou L, Zhang S, Han X, Ji L. The Association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity. 2018;26(1):70–80. doi: 10.1002/oby.22066. [DOI] [PubMed] [Google Scholar]
- 17.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36(9):2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada H, Tanaka A, Kusunose K, Amano R, Matsuhisa M, Daida H, Ito M, Tsutsui H, Nanasato M, Kamiya H, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovasc Diabetol. 2017;16(1):63. doi: 10.1186/s12933-017-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Lee KH, Oh HG, Seo HJ, Jeong SJ, Kim CH. Effect of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase 4 inhibitors on cardiovascular function in patients with type 2 diabetes mellitus and coronary artery disease. J Obes Metab Syndr. 2019;28(4):254–261. doi: 10.7570/jomes.2019.28.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, Teoh H, Mazer CD, Connelly KA. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39(12):e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 21.Leung M, Wong VW, Hudson M, Leung DY. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ Cardiovasc Imaging. 2016;9(3):e003643. doi: 10.1161/CIRCIMAGING.115.003643. [DOI] [PubMed] [Google Scholar]
- 22.Thirunavukarasu S, Jex N, Chowdhary A, Hassan IU, Straw S, Craven TP, Gorecka M, Broadbent D, Swoboda P, Witte KK, et al. Empagliflozin treatment is associated with improvements in cardiac energetics and function and reductions in myocardial cellular volume in patients with type 2 diabetes. Diabetes. 2021;70(12):2810–2822. doi: 10.2337/db21-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JE, Barac A, Thavendiranathan P, Scherrer-Crosbie M. Strain imaging in cardio-oncology. JACC CardioOncol. 2020;2(5):677–689. doi: 10.1016/j.jaccao.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang IC, Cho GY, Yoon YE, Park JJ, Park JB, Lee SP, Kim HK, Kim YJ, Sohn DW. Different effects of SGLT2 inhibitors according to the presence and types of heart failure in type 2 diabetic patients. Cardiovasc Diabetol. 2020;19(1):69. doi: 10.1186/s12933-020-01042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka H, Soga F, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, et al. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol. 2020;19(1):6. doi: 10.1186/s12933-019-0985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, Kountouri A, Balampanis K, Parissis J, Andreadou I, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc. 2020;9(9):e015716. doi: 10.1161/JAHA.119.015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan H, Kunutsor S, Rauramaa R, Savonen K, Kalogeropoulos AP, Georgiopoulou VV, Butler J, Laukkanen JA. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail. 2014;16(2):180–188. doi: 10.1111/ejhf.37. [DOI] [PubMed] [Google Scholar]
- 28.Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, Fang ZY, Prins JB, Stanton T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101(13):1061–1066. doi: 10.1136/heartjnl-2014-307391. [DOI] [PubMed] [Google Scholar]
- 29.Kumar N, Garg A, Bhatt DL, Sabongui S, Gupta N, Chaudhry S, Arena R, Verma S. Empagliflozin improves cardiorespiratory fitness in type 2 diabetes: translational implications. Can J Physiol Pharmacol. 2018;96(11):1184–1187. doi: 10.1139/cjpp-2018-0359. [DOI] [PubMed] [Google Scholar]
- 30.Nunez J, Palau P, Dominguez E, Mollar A, Nunez E, Ramon JM, Minana G, Santas E, Facila L, Gorriz JL, et al. Early effects of empagliflozin on exercise tolerance in patients with heart failure: a pilot study. Clin Cardiol. 2018;41(4):476–480. doi: 10.1002/clc.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243–255. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Carbone S, Canada JM, Billingsley HE, Kadariya D, Dixon DL, Trankle CR, Buckley LF, Markley R, Vo C, de Chazal HM, et al. Effects of empagliflozin on cardiorespiratory fitness and significant interaction of loop diuretics. Diabetes Obes Metab. 2018;20(8):2014–2018. doi: 10.1111/dom.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carbone S, Billingsley HE, Canada JM, Bressi E, Rotelli B, Kadariya D, Dixon DL, Markley R, Trankle CR, Cooke R, et al. The effects of canagliflozin compared to sitagliptin on cardiorespiratory fitness in type 2 diabetes mellitus and heart failure with reduced ejection fraction: the CANA-HF study. Diabetes Metab Res Rev. 2020;36(8):e3335. doi: 10.1002/dmrr.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen R, Wiggers H, Thomsen HH, Bovin A, Refsgaard J, Abrahamsen J, Moller N, Botker HE, Norrelund H. Effect of tighter glycemic control on cardiac function, exercise capacity, and muscle strength in heart failure patients with type 2 diabetes: a randomized study. BMJ Open Diabetes Res Care. 2016;4(1):e000202. doi: 10.1136/bmjdrc-2016-000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uribe-Heredia G, Arroyo-Espliguero R, Viana-Llamas MC, Piccone-Saponara LG, Alvaro-Fernandez H, Garcia-Magallon B, Toran-Martinez C, Silva-Obregon A, Izquierdo-Alonso JL. Type 2 diabetes mellitus, glycated hemoglobin levels, and cardiopulmonary exercise capacity in patients with ischemic heart disease. J Cardiopulm Rehabil Prev. 2020;40(3):167–173. doi: 10.1097/HCR.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 36.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142(11):1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iannantuoni F. The SGLT2 Inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med. 2019 doi: 10.3390/jcm8111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang S, Verma S, Hassanabad AF, Teng G, Belke DD, Dundas JA, Guzzardi DG, Svystonyuk DA, Pattar SS, Park DSJ, et al. Direct effects of empagliflozin on extracellular matrix remodelling in human cardiac myofibroblasts: novel translational clues to explain EMPA-REG OUTCOME results. Can J Cardiol. 2020;36(4):543–553. doi: 10.1016/j.cjca.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Cohen ND, Gutman SJ, Briganti EM, Taylor AJ. Effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes: a cardiac magnetic resonance study. Intern Med J. 2019;49(8):1006–1010. doi: 10.1111/imj.14260. [DOI] [PubMed] [Google Scholar]
- 40.Abou R, van der Bijl P, Bax JJ, Delgado V. Global longitudinal strain: clinical use and prognostic implications in contemporary practice. Heart. 2020;106(18):1438–1444. doi: 10.1136/heartjnl-2019-316215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. EMPA-HEART trial.
Additional file 2: Table S1. Cardiopulmonary exercise test, 2D Echocardiograpy, Doppler, Tissue Doppler, and Speckle tracking parameters. P-value is the result of a two-point ANOVA for repeated measures.
Data Availability Statement
The study was carried out in accordance with the most recent international GCP guidelines (CPMP/ICH/135/1995), EU Directive and guidance and the local legislation on the conduct of clinical trials. The database has been locked after the planned statistical analysis was performed. Any further modification of recorded data will be documented in a database log form. All the collected data will be stored for a maximum period of 15 years after the end of the study and then destroyed. Only the investigators of the study will have access to all the data.