Abstract
Species radiations, despite immense phenotypic variation, can be difficult to resolve phylogenetically when genetic change poorly matches the rapidity of diversification. Genomic potential furnished by palaeopolyploidy, and relative roles for adaptation, random drift and hybridisation in the apportionment of genetic variation, remain poorly understood factors. Here, we study these aspects in a model radiation, Syzygium, the most species-rich tree genus worldwide. Genomes of 182 distinct species and 58 unidentified taxa are compared against a chromosome-level reference genome of the sea apple, Syzygium grande. We show that while Syzygium shares an ancient genome doubling event with other Myrtales, little evidence exists for recent polyploidy events. Phylogenomics confirms that Syzygium originated in Australia-New Guinea and diversified in multiple migrations, eastward to the Pacific and westward to India and Africa, in bursts of speciation visible as poorly resolved branches on phylogenies. Furthermore, some sublineages demonstrate genomic clines that recapitulate cladogenetic events, suggesting that stepwise geographic speciation, a neutral process, has been important in Syzygium diversification.
Subject terms: Evolutionary genetics, Phylogenomics, Polyploidy in plants, Comparative genomics
The relative importance of the mechanisms underlying species radiation remains unclear. Here, the authors combine reference genome assembly and population genetics analyses to show that neutral forces have contributed to the radiation of the most species-rich tree genus Syzygium.
Introduction
Species radiations—wherein perplexing amounts of diversity appear to have formed extremely rapidly—have featured prominently in the history of evolutionary theory1. Various underlying mechanisms for their formation have been proposed2, including adaptation2, non-adaptive processes3,4, hybridisation5,6, and polyploidy7,8, but the relative importance of these drivers remains incompletely understood. Species radiations on islands have been among the most prominently studied systems9–12. For example, the Malesian archipelago in the tropical Far East13, consisting of thousands of islands and including New Guinea and Borneo, the second and third largest islands in the world, is a biodiversity hotspot containing many radiations of plant and animal species. Among forest trees, local tree species richness across Southeast Asian forests is largely driven by a small number of highly species-rich genera14. The clove genus, Syzygium, is one of the most important of these genera, and therefore understanding diversification and its underlying drivers within Syzygium may help explain large-scale patterns of diversity in the Palaeotropics. However, Syzygium, like many other species radiations that hold immense morphological and ecological variation, has so far been difficult to resolve phylogenetically15–18, leading to the impression that evolutionary change can be a swift process that may not require substantial underlying genetic change9. Here, we employ genome-scale approaches to investigate speciation patterns and their potential drivers in the most species-rich tree genus worldwide, Syzygium19.
Syzygium, which includes 1193 species recognised worldwide20, is a genus in the myrtle family (Myrtaceae). Syzygium is restricted to tropical and subtropical regions of the Old World, where it is distributed from Africa through to India, across Southeast Asia and extending to Hawaii in the Pacific Ocean, with the centre of species diversity in Indomalesia20. The type species of Syzygium is S. caryophyllatum, a poorly known, small to medium-sized tree endemic to southern India and Sri Lanka21. The best-known species in the genus is the clove tree, Syzygium aromaticum, from which flower buds are gathered, dried, and used as a spice, a preservative and in pharmacology22. In addition, Syzygium aqueum, S. cumini, S. jambos, S. malaccense and S. samarangense are widely cultivated in the tropics for their large edible fruits23. Syzygium samarangense is cultivated commercially in Southeast Asia, where it is marketed as the wax apple, java apple, rose apple, or samarang rose apple. Apart from being used as cooking ingredients or cultivated for fruits, Syzygium species with dense and bushy crowns, such as S. antisepticum, S. australe, S. luehmannii, S. myrtifolium and S. zeylanicum, are used in the horticulture industry in Australia, Indonesia, Malaysia and Singapore for hedges, natural fences, natural sound barriers and privacy screens24.
Syzygium species are generally medium-sized to large, characteristically sub-canopy trees that are sometimes emergent, while some also form shrubs, small forest understorey treelets, swamp and mangrove forest trees, and rheophytic vegetation25. As is true of many tropical trees, Syzygium flowers are visited by a large diversity of insects and vertebrates, and their fruits are typically eaten by a variety of flying and arboreal vertebrates and even terrestrial bird, mammal and reptile browsers25. Syzygium species also occur as dominant mid-level canopy trees, affecting the ecosystems of plants, animals, and fungi in lower forest layers25. Many species co-occur; for example, there exist ca. 50 taxa on a single 52-ha. ecological plot in the Lambir Hills National Park (Sarawak, East Malaysia, Borneo26), where they display fine-scale differentiation in habitat occupancy and stature14. The genus is notorious as one of the most difficult to identify due to the paucity of clear, diagnostic morphological characters for distinguishing species;25,27,28 morphological variation in the genus can appear as continua of traits rather than collections of discrete units. Given the immense number of species assigned to Syzygium, it contributes disproportionately to the diversity of Southeast Asian tropical forests. Therefore, understanding diversification and its underlying drivers within Syzygium may help explain large-scale patterns of diversity. Thus far, however, phylogenetic studies of Syzygium have involved only a few PCR-amplified plastid and nuclear marker genes15,16. An infrageneric classification proposed in 2010 was based on three plastid loci17, and although it resolved some major clades, interrelationships within the bulk of the genus, species of Syzygium subg. Syzygium, were left largely unresolved.
Here, we sequence whole genomes to vastly increase the available data in an attempt to more fully resolve phylogenetic relationships among Syzygium species. We use Oxford Nanopore Technology (ONT) long-read sequencing29 to assemble and annotate a chromosome-scale reference genome for the sea apple, Syzygium grande23. This species was selected as a representative because it is a well-known member of the most diverse, broadly distributed group within Syzygium, and one of the most commonly cultivated shade and firebreak trees planted along streets in Singapore and Peninsular Malaysia. We examine the palaeopolyploid history of Syzygium to assess whether whole genome duplications may have played a role in speciation through sub- or neo-functionalisation events, eventually fixed by natural selection or drift processes during species transitions7. We use whole-genome sequencing of 292 Syzygium individuals and outgroups to address evolutionary relationships among the species. Both Illumina short-read assemblies, as well as mapping of the read data to the S. grande genome, are brought to bear for phylogenomic investigations of possible rapid diversification in the group.
Results and discussion
Assembly and annotation of the reference and resequenced Syzygium genomes
A chromosome-level assembly of Syzygium grande (Fig. 1a) was carried out using wtdbg230 to generate a 405,179,882 bp genome in 174 contigs (N50 of 39,560,356 bp) from more than 60 Gb of ONT long reads, and the assembly was subsequently polished with 30 Gb Illumina short reads. Finally, scaffolding into pseudo-chromosomes was carried out using Dovetail HiC technology31 to generate 11 pseudo-chromosomes (Fig. 1b).
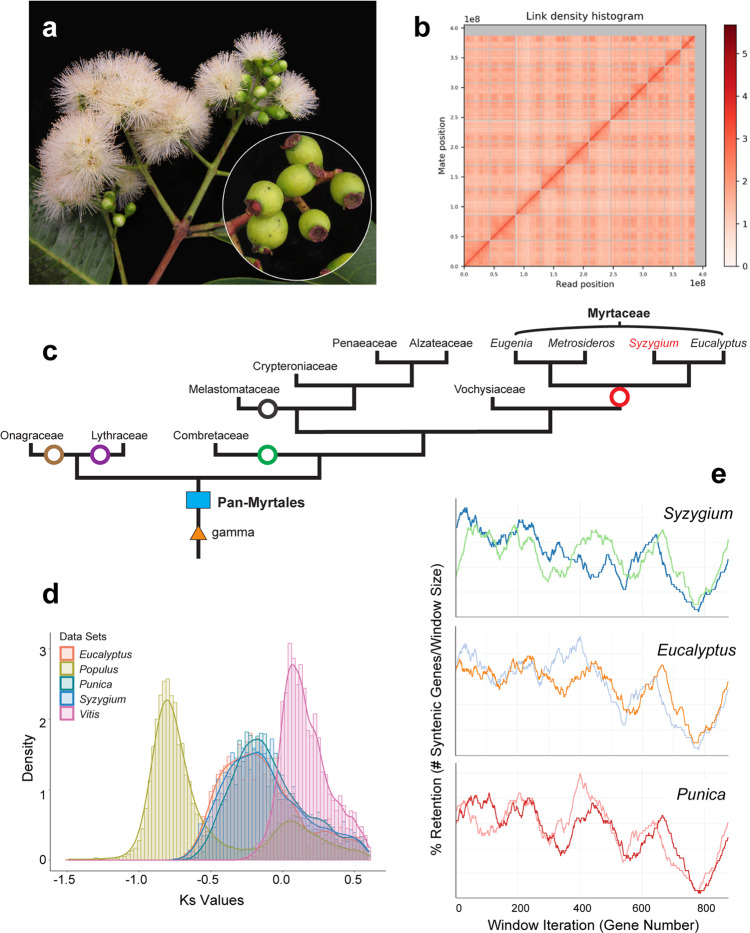
Fig. 1. Assembly and structural evolution of the Syzygium grande reference genome.
a S. grande inflorescence, flowers and fruits; the latter evoke the common name “sea apple”; b HiC contact map for the scaffolded genome, showing 11 assembled chromosomes; c Phylogeny of major lineages of Myrtales, following Maurin et al. 44. Genera of Myrtaceae used in genome structural and phylogenetic analyses are also depicted. Punica (Lythraceae) was also examined for structural evolution. Open circles represent the multiple, independent polyploidy events predicted by the 1KP study42; our results here suggest instead a single Pan-Myrtales whole genome duplication (blue rectangle) which followed the gamma hexaploidy (orange triangle) present in all core eudicots. d Synonymous substitution rate density plots for internal polyploid paralogs within Syzygium, Eucalyptus, Punica, Populus and Vitis. Modal peaks in these three Myrtales species suggest a single underlying polyploidy event. Ks asymmetries were calibrated using the gamma event present in each species. Both histograms and smoothed curves are shown. e Fractionation bias mappings of Myrtales chromosomal scaffolds, 2 each (different colours), onto Vitis vinifera chromosome 2 show similar patterns for all three Myrtales species (excluding cases of chromosomal rearrangements among the three, which are discernible as different scaffold colour switchings compared to the Vitis chromosome). X-axis shows the percent retention of fractionated gene pairs following polyploidisation; Y-axis shows the position of gene pairs along the Vitis chromosome. Photograph credit: WHL (a). Source data are provided as a Source Data file.
Following the assembly, repeat masking (Supplementary Table 1) and gene prediction were carried out using evidence from Syzygium grande RNA-seq data and protein sequences from Arabidopsis thaliana and Populus trichocarpa. Altogether 39,903 gene models were predicted with 86.6% of benchmarked universal single-copy orthologous (BUSCO 3.0.232) genes being present. In addition to the reference assembly, 30 Gb of Illumina HiSeqX sequencing data was generated for each of 289 Syzygium individuals and three outgroup taxa (two Metrosideros and one Eugenia species, both Myrtaceae; Supplementary Data 1) and assembled de novo using the MaSuRCA assembler33 (Supplementary Data 2 and Supplementary Fig. 1). The average single-copy completeness across this set of genomes was 89.23% (Supplementary Data 2), indicating that the draft assemblies were of acceptable quality.
Genome structure of Syzygium reveals that a single polyploidy event underlies all Myrtales
We used our chromosome-level assembly of Syzygium grande to re-evaluate the polyploid history of its family, Myrtaceae, and order, Myrtales. Myrtales are a diverse rosid lineage comprising approximately 13,000 species across 380 genera and 9 families34. All rosids share the gamma triplication event that occurred in the core eudicot common ancestor35–37. Sequencing of the Eucalyptus grandis (Myrtaceae) genome revealed an additional whole genome duplication (WGD) in its lineage38, and later analyses of the Punica granatum (pomegranate) genome in the related family Lythraceae suggested that this polyploidy event may have been shared39,40, occurring near the base of the order. Further work on the Psidium guajava (guava) genome came to a similar conclusion41. However, the broad, transcriptome-based 1KP project suggested that the Lythraceae and Myrtaceae WGDs might be independent events. Indeed, seven independent, lineage-specific WGDs were predicted by 1KP (their Supplementary Fig. 8) to characterise a larger lineage containing Larrea, Tribulus (both Zygophyllaceae), Combretaceae, Onagraceae, Melastomataceae, Lythraceae and Myrtaceae42.
Syntenic alignments of the Syzygium grande genome against itself revealed at least one whole genome multiplication event since the gamma palaeohexaploidy (Supplementary Fig. 2), and alignment against the Vitis vinifera genome confirmed the single lineage-specific WGD (Supplementary Fig. 3). A more detailed study against both Eucalyptus grandis and Punica granatum revealed 1:1 syntenic relationships (Supplementary Figs. 4 and 5), strongly suggesting a shared polyploid history. We investigated this further by extracting internally syntenic gene pairs in Eucalyptus grandis, Punica granatum, Vitis vinifera and Populus trichocarpa. When rate-corrected against the gamma hexaploidy event43, an ancient pan-Myrtales WGD was supported, approaching gamma in age (Fig. 1c and d). Furthermore, subgenome-wise syntenic depths and fractionation patterns were extremely similar in Syzygium grande, Eucalyptus grandis, and Punica granatum, supporting the hypothesis that a single polyploidy event underlies all Myrtales (Fig. 1e and Supplementary Fig. 6). Furthermore, alignment of Populus trichocarpa against given Syzygium grande chromosomes showed the expected 2:1 syntenic pattern indicative of an independent Salicaceae-specific WGD in the rosid order Malpighiales (Supplementary Fig. 7). Based on phylogenetic relationships recently solidified for Myrtales families44, we conclude that earlier genome-based determinations of shared polyploid status within Myrtales are correct in indicating one basal WGD, and that the transcriptome-based 1KP study erroneously inflated the number of WGDs within the clade (Fig. 1c).
Since some polyploid events such as the gamma triplication35–37 and the pan-angiosperm WGD45 co-occur with major flowering plant radiations7 (here, the core eudicots and all angiosperms, respectively), a single polyploid event shared by all Myrtales might hold implications for early diversification in the order. However, it is well-known that some large angiosperm diversifications, such as Gentianales (an even larger lineage than Myrtales at >20,000 species across 1121 genera and 5 families34), are not marked by ancestral WGDs, leaving polyploidy as a causal mechanism for diversification rather inconclusive, or at the very least an incomplete explanation.
Polyploidy within Syzygium similarly appears to play little role in its infrageneric diversification. BUSCO duplicate (D) scores suggest that the majority of species have remained at the same ploidy level following the Pan-Myrtales WGD event (Supplementary Data 2). At least one clear case of neopolyploidy is observable in S. cumini, which has the highest D score in our sample and a known haploid chromosome number of n = 2246, double the number of our S. grande pseudochromosomes.
Single nucleotide polymorphisms and single-copy nuclear genes yield well resolved major branchings within Syzygium
Species-level interrelationships within Syzygium have not yet been investigated in depth. To obtain a whole genome-level phylogeny we used the Syzygium grande genome assembly as a reference for mapping variants from three outgroup taxa and 289 independently sequenced Syzygium accessions representing at least 182 distinct species, 49 repeated species samples, and 58 additional as-yet-unidentified taxa. SNP calling yielded 1,867,173 variants across all 292 samples, from which we determined genome-wide phylogenetic relationships using RAxML47 (Fig. 2). Since the SNPs could be identified only from the relatively conserved parts of the genome, we also collected predicted universal single-copy genes from BUSCO analyses (source data are provided at Dryad, 10.5061/dryad.h18931zpw) estimate using ASTRAL48, a coalescence-based approach that incorporates individual gene trees into species tree estimation.
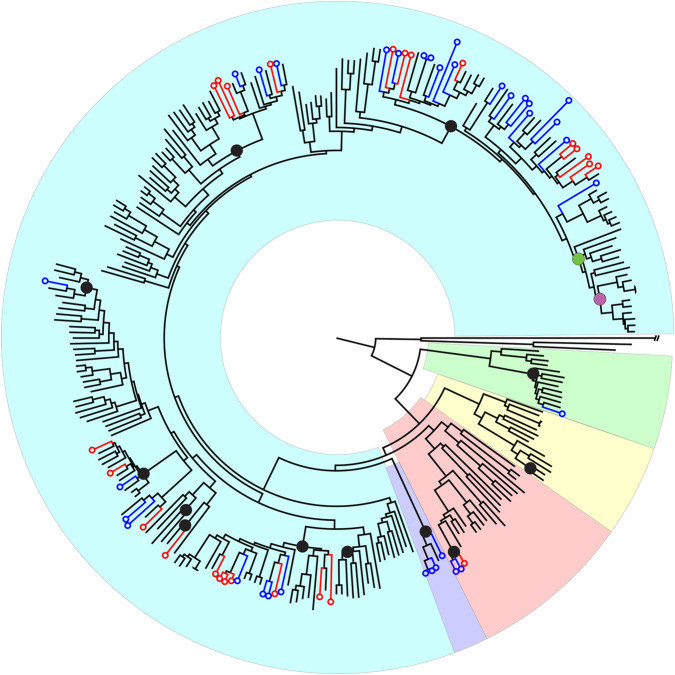
Fig. 2. Phylogenetic tree based on single nucleotide polymorphisms among all 292 resequenced Myrtaceae accessions.
Black circles represent the at-least 12 independent invasions of Sunda from Sahul. The green circle represents migration from Sunda to the Indian subcontinent, and the purple circle denotes further migration from there to Africa. Blue versus red circles at the leaves of the tree represent Syzygium accessions from Bukit Timah Nature Reserve and Danum Valley Conservation area, respectively. Background colours represent the recognised subgenera, (clockwise from the root, excluding the outgroup taxa) Syzygium subg. Sequestratum (green), S. subg. Perikion (yellow), S. subg. Acmena (red), the S. rugosum clade (purple) and S. subg. Syzygium (cyan).
Phylogenetic analysis using genome-wide SNPs (source data are provided at Dryad, 10.5061/dryad.h18931zpw) resulted in a phylogeny (Fig. 2 and Supplementary Fig. 8) that was robust and well-resolved with the outgroup-based rooting, namely Metrosideros excelsa, M. nervulosa (tribe Metrosidereae) and Eugenia reinwardtiana (tribe Myrteae). Five major clades resolved in the phylogeny were all well-supported, with most branches receiving 100% bootstrap support at the nodes, indicating strong internal consistency within the dataset. These five major clades represent the previously characterised Syzygium subg. Syzygium, S. subg. Acmena, S. subg. Perikion, S. subg. Sequestratum and a subgenus yet to be named that includes S. cf. attenuatum, S. rugosum and an unidentified species from Sulawesi labelled here as “SULAWESI2” (henceforth, we refer to this lineage as the S. rugosum clade). It is noteworthy that internal branch lengths are heterogeneous in length, indicating that the clades are differentially divergent either in time, diversification rate, population size, or all of these factors49. The largest clade in the phylogeny, having both the most recognised species and the most representative individuals in our current sample, is the Syzygium subg. Syzygium clade. Relationships within this clade are largely well resolved and supported, as are interrelationships among the five subgenera. Despite strong support, it is important to note that such an analysis generates a phylogeny that represents a genome-wide average, rather than taking into account the independent inheritance of different loci across the genome characteristic of incomplete lineage sorting or adaptive processes.
To obtain an independent view of the Syzygium species tree, we used our BUSCO single-copy gene sets (source data are provided at Dryad, 10.5061/dryad.h18931zpw) to compare the gene trees derived from independent nuclear loci in a coalescence species tree approach. We analysed two different BUSCO gene sets that differed in their completeness among accessions: the set of 229 genes containing representatives from all sequenced individuals, and a second set with 1227 genes present in ∼95% of accessions. The phylogenies obtained from both single-copy genes and genome-wide SNPs (Supplementary Fig. 8) concordantly displayed the five major, well-supported clades representing the five subgenera of Syzygium, including also their relative branching order from the outgroup root, albeit with some minor disagreement of taxon placement within clades. These corroborating results inferred from two different approaches indicate strong and consistent phylogenetic signals within our genomes. Furthermore, Syzygium interrelationships based on plastid mappings (source data are provided at Dryad, 10.5061/dryad.h18931zpw), derived by mapping the Illumina sequence reads for each accession onto the Syzygium grande plastid genome, yielded partly incongruent results that may be traceable to ancient hybridisation and plastome capture, or to incomplete lineage sorting (ILS) (Supplementary Fig. 9).
Diversification bursts characterise many terminal branchings within Syzygium phylogeny
Despite a strong overall signal supporting a bifurcating evolutionary history, the many extremely short coalescent branch lengths generated by the ASTRAL approach suggest that ILS49 may have been a confounding biological factor at various points during the Syzygium radiation. These branch lengths, which are interpretable in terms of time in generations (g) divided by effective population size (Ne)49, provide evidence that many Syzygium clades either radiated extremely rapidly, or that their ancestral population sizes were comparatively large, or both. Such g and Ne conditions are known to promote gene-tree/species-tree discordance through ILS49. We sought to investigate signatures of ILS in the data further using NeighborNet, a distance-based method based on neighbour-joining that generates phylogenetic networks50. The character incongruence that is manifested as extra edges in these networks beyond a perfectly bifurcating tree has been interpreted both in terms of interspecies admixture and/or incomplete lineage sorting phenomena51,52.
NeighborNet analysis of our genome-wide SNP data for Syzygium subg. Syzygium including a single outgroup species, S. rugosum, showed that while many of the evolutionary relationships among taxa were strongly tree-like, at least one major clade (which we informally term here the “Syzygium grande group”) likely involved a burst of lineage splits (Fig. 3a), as evidenced by the predominantly noncoding (i.e., neutrally evolving) SNPs which illustrate a highly webbed, fan-like network of splits at its base. While the stem lineage of the Syzygium grande group was strongly supported in the BUSCO and SNP trees, it is noteworthy that in the SNP analysis many parallel edges nonetheless appear along it, suggesting internal incongruence among SNPs, possibly reflecting differential inheritance with ILS (see Suh et al.52). A further, larger lineage including the Syzygium grande group and its outgroups was similarly well supported in the BUSCO and SNP trees; however, its own stem lineage contained even more parallel edges and potentially even more severe ILS (Fig. 3a).
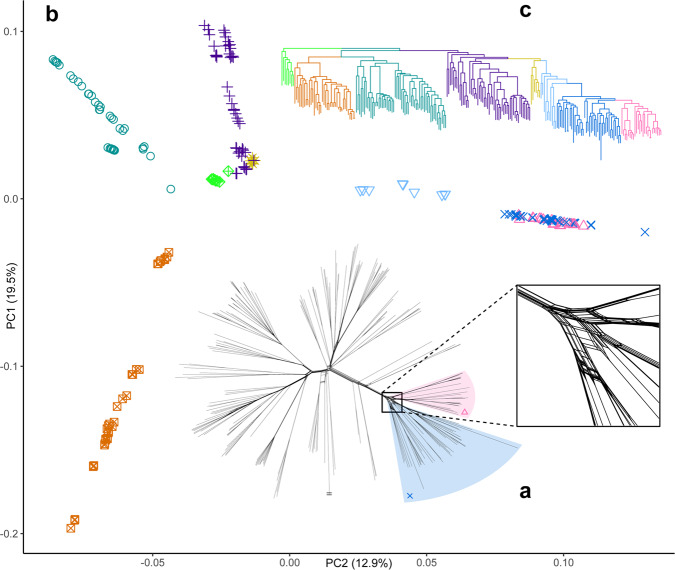
Fig. 3. Principal component analysis and phylogenetic reconstructions of single nucleotide polymorphism variation within Syzygium.
a A NeighborNet phylogenetic network shows considerable character discordance among genome-wide SNPs that may be indicative of incomplete lineage sorting. This discordance is particularly noteworthy at the highly webbed base of the Syzygium grande group (close-up view in square inset; see also the labelled network in Supplementary Fig. 89). b PCA of principal components 1 and 2 of Syzygium subg. Syzygium individuals. Clinal patterns are readily observed; the Syzygium grande group (centred around 0.10 on PC2) is comprised of a medium-blue paraphyletic grade subtending a pink terminal lineage, as shown in (c), the RAxML SNP tree, which is colour-coded following the PCA. Shading of two groups on (a) matches the colour coding on the tree as well as the colours and symbols on the PCA plot. Small matching symbols in shaded areas are shown for clarity. Edge with two horizontal lines at tip represents the outgroup taxon, Syzygium rugosum, clipped for length. Source data are provided as a Source Data file.
Incomplete lineage sorting rather than hybridisation may confound phylogenetic inferences
Next, we used the same SNP data with the ADMIXTURE software53 to search for genomic partitioning among the clades and accessions that might be attributable to admixture (introgression) or differential blockwise inheritance through extremely narrow species splits (ILS). ADMIXTURE assumes K ancestral population clusters on the data; it is not decisive regarding mechanisms underlying any K-cluster mixtures within individuals analysed. The approach was developed for population-level data wherein mixed K-clusters are most likely attributable to admixture rather than ILS through lineage splits (e.g., speciation events). However, results at the interspecific level are often interpreted uncritically as actually indicative of cross-lineage admixture54 (see ref. 55). Indeed, the K components from ADMIXTURE simply represent subsets of inherited SNP variation that could reflect any underlying mixtures, of which ILS can be one mechanistic basis (Supplementary Figs. 10 and 11). We, therefore, propose ILS to be a likely underlying causal factor for some of the K mixtures given both the short coalescence branch lengths on the ASTRAL species tree and the reticulation of the NeighborNet.
Our ADMIXTURE analysis cross-validation scores supported K = 14 as the best representation of ancestral population structure (Supplementary Fig. 12). At this K, the Syzygium grande group is almost entirely assigned to one K in the ADMIXTURE results (orange, Supplementary Fig. 11). However, at other K values (e.g., K = 10,11,12; Supplementary Figs. 10 and 11), K mixtures within this group are apparent. It is worth noting that the fold level of cross-validation affects the preferred number of components, since the optimum results in a case where for each component there is at least one representative in the test set. The outgroups to the Syzygium grande group also contain the orange-coloured cluster at K = 14, but they additionally include mixtures with other ancestral populations (Supplementary Fig. 11). These K-cluster mixtures appear to be consistent with the multiple edges underlying this larger lineage in the NeighborNet analysis. In other words, they are likely indicative of differential inheritance of genomic regions and their SNPs through ILS. To rule out admixture generating these results, we formally tested for gene flow within the Syzygium grande group using Patterson’s f3 statistic56, which tests for patterns of allele sharing (source data are provided at Dryad, 10.5061/dryad.h18931zpw). We calculated all three-way taxon comparisons of source1, source2, and target taxa to evaluate signatures of admixture. These results demonstrated no evidence for admixture, but did reveal instances where significant negative Z-scores across all possible source combinations reflected close relationships through identity by descent (described in detail by Lan et al.57) (Supplementary Figs. 13–87).
With ILS the more likely explanation for these results, we used local principal component analysis (PCA)58 to examine whether patterns of SNP-based relatedness differed instead by location along chromosomes. Clear distinctions in the sample projections on PCA components along a scaffold would indicate that different genomic blocks have different evolutionary histories, of which introgression, ILS, local selection, or even drift are suspect source mechanisms. Local PCA takes window-wise PCA projections of SNP variation and arrays differences among them on a multidimensional scaling (MDS) plot; three distinct “corners” are then selected from the MDS plot, and the corner-wise variation is pooled for final analyses58. We analysed both whole-chromosomal variations as well as repeat-masked data, the latter to ensure that distinct patterns obtained were not solely related to ambiguous mappings due to different transposable element families. The Syzygium grande group characteristically appears as a tight cluster across different corners on the 11 chromosomes (Supplementary Figs. 88–100). However, in some of these collections of windows, the group is unresolved from its closest outgroups and from the rest of Syzygium subg. Syzygium; in other corners, these outgroups are poorly distinguished from the remainder of the subgenus, while the S. grande group stays distinct. We infer that these results support the hypothesis of underlying ILS—i.e., regional block-wise genomic distinction vs. indistinction of these taxa, as reflected by the many-paralleled edges of their corresponding stem lineages in the NeighborNet result (Fig. 3a).
Principal component analysis reveals clinal patterns reflective of isolation by distance
We further studied the SNP data genome-wide using standard PCA59,60. Plots of principal components focussing on Syzygium subg. Syzygium illustrated clear clines (Fig. 3b) that mostly correspond to sublineages on the BUSCO and SNP trees (Fig. 3c and Supplementary Figs. 101–121). Several filtrations of data (Supplementary Table 2), including analyses of homozygous sites only (as well as checks for coverage that suggested no apparent biases), yielded similar results and therefore increased confidence that the clinal patterns were not artefactual (Supplementary Figs. 107–121). A simple explanation for these linear gradations is that allelic variation in Syzygium became fixed in consecutive speciation events, along an ongoing cladogenetic process. The PCA analysis highlights that different lineages within the S. grande group partly overlap (Fig. 3b and Supplementary Figs. 122–127), consistent with short internal coalescence branch lengths on the BUSCO tree. In other words, the clinal patterns may reflect a neutral process akin to isolation by distance59–62 (IBD; see ref. 63), for example, comprising serial founder events in an island-hopping model of geographic speciation3 (but see ref. 64). Similar clinal variation among Big Island (Island of Hawai’i) accessions of a closely related Myrtaceae species, Metrosideros polymorpha (see Fig. 1C of ref. 10), might also reflect simple IBD processes in its extremely young and rapidly expanding/dissecting volcanic environment.
Allopatric speciation does not necessarily require adaptive differences, only the null model of reproductive isolation and genetic drift65. The possibility of entirely neutral phenotypic clines forming in a model of progressive cladogenesis, such as we hypothesise here for diagnosable Syzygium species, may attest to IBD, and reflect environmental gradients that accompany spatial population expansion, or even involve admixture between previously isolated populations or clades63. However, many Syzygium species are sympatrically distributed, which, if the splits observed were time-coincident or nearly so, could suggest that ecological speciation66–68 (and therefore adaptive differences, such as flowering allochrony and other gene flow barriers) could also be operative. Even weak selection via such local adaptation can significantly speed up an entirely drift-based geographic speciation process65. For example, the phylogenetic results presented here show that Syzygium species sympatric in the Bukit Timah and Danum Valley forest plots are broadly distributed across the phylogenetic tree, but there are also clear clusters of species from these plots within some subclades (Fig. 2, Supplementary Note 1 and Supplementary Table 2). However, there is reason to suspect that sampling biases from within larger species ranges may influence this clustering, since Bukit Timah- or Danum-enriched clades are not entirely autochthonous to these plots, but also distributed elsewhere. For example, we sampled a S. barringtonioides specimen from Brunei that groups within an otherwise Danum cluster (both nonetheless being Bornean), and the S. chloranthum-S. cerasiforme clade that was largely sampled from Bukit Timah also contains S. ampullarium, which was collected in Borneo. As such, considerable reproductive isolation and lineage diversification likely occurred prior to many migrations into sympatric niches. The clearest inference from Fig. 2 is therefore that the Bukit Timah and Danum Syzygium floras are assemblages of phylogenetically- and time-diverse lineages.
Demographic analysis of the Syzygium grande group also implies rapid diversification
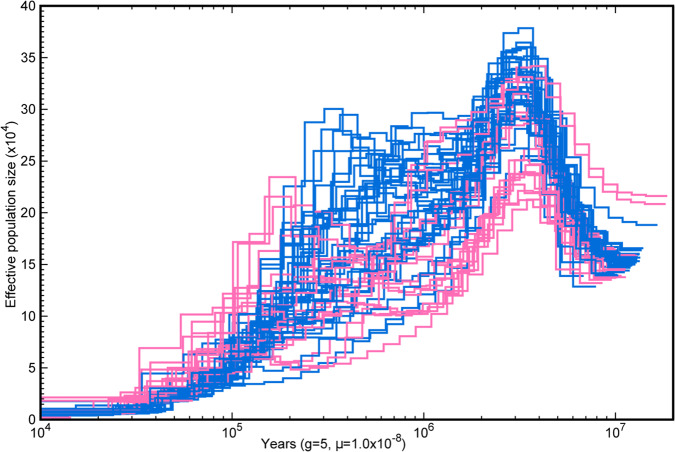
Pairwise Sequentially Markovian Coalescent (PSMC) analysis uses the two haploid genomes present in each collection of reads for a given diploid individual to estimate past effective population sizes over time. We ran PSMC demographic curves for most individuals in the closely interrelated Syzygium grande group using Illumina reads mapped against each taxon’s own MaSuRCA genome assembly. We scaled the time and Ne axes of the demographic reconstructions uniformly by employing an approximate Syzygium generation time of 5 years (roughly the median of the data in Supplementary Table 3) and a mutation rate of 1E−08, in line with previous work on woody plants69. Comparing demographic curves together, all individuals appear to follow similar trajectories wherein the genetic variation in all taxa coalesces between 9 and 20 million years ago (Mya), followed by a peak in Ne at 3–4 Mya, various Ne fluctuations/crashes in intermediate times from 1 to 0.1 Mya, followed by strong Ne collapse in recent-most time (Fig. 4). The impression that the demographies seem largely alignable in gross aspect while differing in ancient coalescence times, may reflect their joint membership in a stem lineage as well as real generation time differences among the taxa, or differences in past heterozygosity levels70. Indeed, maximum time at coalescence strongly correlates with overall heterozygosities of individuals (from SNP calls) as well as numbers of segregating sites (from reads mapping to individual genomes only; Supplementary Fig. 128).
Fig. 4. Genomic palaeodemography of Syzygium accessions from the S. grande group.
Pairwise Sequentially Markovian Coalescent analyses, coloured by groups/clades in Fig. 3. Source data are provided as a Source Data file.
Moreover, PSMC curves for many taxa in the pink terminal lineage in Fig. 3c converge together at lower Ne in ancient to intermediate times than do most individuals in the blue paraphyletic group that subtends it, which mostly follow higher Ne trajectories. These distinctions visible as early as 10 Mya suggest that the pink lineage may have begun splitting from within the blue group as early as then, while the Ne fluctuations closer to the present may represent the period of rapid cladogenesis reflected in the “fan-like” reticulate base of the S. grande group that is visible in the NeighborNet result. Phylogenetic resolution of rapid splits such as these can be particularly confounded by ILS, which by coalescent theory may itself be exacerbated by any Ne size increases. The final Ne crashes closest to the present may in turn mark the individuation of the lineages (e.g., via founder effect71) visible past the stage of the NeighborNet fan (i.e., the tips extending from its basal web).
Syzygium radiated multiple times from Sahul into Sunda and elsewhere
In a review on the origins and assembly of Malesian rainforests72, Syzygium was highlighted as a key genus for understanding the floristic evolution of the region. Formal biogeographic analyses using the BioGeoBEARS73 and RASP (Reconstruct Ancestral State in Phylogenies)74 software each demonstrate, despite limited taxon sampling of outgroups, that the genus Syzygium is of Sahul origin, i.e., centred on Australia and New Guinea (Supplementary Figs. 129 and 130; source data are provided at Dryad, 10.5061/dryad.h18931zpw). This finding is consistent with previous work on Syzygium and Myrtaceae as a whole, which similarly finds Sahul as the ancestral area75. We also generated a dated ultrametric SNP tree to provide split and crown group times for subclades and species diversifications (Supplementary Fig. 131; source data are provided at Dryad, 10.5061/dryad.h18931zpw). We used as a calibration point the minimum and maximum ages of a fossil assignable to Syzygium subg. Acmena (20.9–22.1 Mya)76. The crown group of the entire genus Syzygium is dated at 51.2 Mya, and the crown groups of subgenera Sequestratum, Perikion, Acmena, the S. rugosum clade, and Syzygium date to 34.2, 24.1, 15.8, 7.0 and 9.4 Mya, respectively (Supplementary Fig. 131). As such, Syzygium itself dates to before the Sunda-Sahul convergence which occurred ~25 Mya77, with most subgenera diversifying after the convergence.
Repeated invasions both westward and northward from Sahul that correspond with species diversifications are clearly apparent. For example, parallel migrations into Sunda occurred at least 12 times (Fig. 2), sometimes corresponding with large radiations, but only within Syzygium subg. Syzygium (Supplementary Figs. 129 and 130). The earliest migration to Sunda was by 17.1 Mya, the crown group age for the Sunda half of the first split in Syzygium subg. Sequestratum (Supplementary Fig. 131). The S. rugosum clade migrated to Sunda by 7.0 Mya, and Syzygium subg. Perikion had entered Sunda (Peninsular Malaysia) and later migrated to Sri Lanka by 3.0 Mya. Within Syzygium subg. Acmena, Sunda had been accessed by 390 Kya. Syzygium subg. Syzygium is resolved as having a Sahul origin, with a crown group age of 9.4 Mya (corresponding to the young end of PSMC curve coalescences for the S. grande group; see above and Fig. 4). Following Hall’s78 land/sea level reconstruction at 10 Mya, entry of subgenus Syzygium into Sunda, potentially via the Sula Spur, may have involved considerable island hopping from Sahul. As many as seven invasions of Sunda occurred, at least three of which (according to our sampling) resulted in hyperdiverse subclades. The earliest Sunda migrations within the type subgenus involved the hyperdiverse Syzygium pustulatum group, with a minimum crown group age of 2.8 Mya, and the large S. creaghii group, which has a similar minimum crown age of 2.5 Mya (Supplementary Fig. 131). These lineages entered Sunda following the New Guinea uplift, which began about 5 Mya79, possibly correlating with population expansions seen around this time in PSMC curves for the extremely diverse Syzygium grande group. The S. grande lineage migrated much later from Sahul into Sunda by 165 Kya, overlapping with the PSMC Ne fluctuations seen in intermediate times (Fig. 4). It subsequently radiated broadly and very recently into the North Pacific (by 14.6 Kya), the Indian subcontinent (by 21.9 Kya), and from there on to Africa (by 6.5 Kya). These recent dates correspond well with the individuation of clades within the S. grande group inferred from NeighborNet (Fig. 3a), and discussed above in reference to population crashes in PSMC analyses (Fig. 4). The Syzygium pustulatum and S. creaghii groups, which are also marked by fan-like radiations in the NeighborNet analysis (see labelled network in Supplementary Fig. 89), unlike the S. grande group, do not show considerable character incongruence suggestive of ILS at its base. The Syzygium pustulatum group and smaller and late-migrating S. jambos group (the latter having entered Sunda by 123 kya; Supplementary Fig. 131) also represent rapid diversifications into Sunda with significant tree-like structure at their stem-lineage bases in the NeighborNet analysis. The last 1 Mya in Southeast Asian biogeography was marked by cyclical sea level changes that repeatedly divided and rejoined vegetation80,81, and the minimum invasion dates for the Syzygium grande and S. jambos groups correspond with periods when sea levels were lower than today82 and therefore lowland rainforest vegetation more continuous. To summarise, parallel dispersals from Sahul into Sunda and beyond sometimes correlate with what appear to be rapid radiations that at least in one case, the Syzygium grande group, appears to have been marked by significant ILS.
Morphological transitions may accompany some Syzygium species diversifications
We thereafter sought, using Mesquite83 parsimony optimisations, to provide qualitative first approximations of morphological trait evolution and accompanying ecological variables that might correspond with these East-to-West migrations (source data are provided at Dryad, 10.5061/dryad.h18931zpw). We employed the BUSCO species tree for this exercise to minimise any topological biases that might arise from ILS. An interesting trait is the presence of a pseudocalyptrate (or “calyptrate” in Syzygium barringtonioides and S. perspicuinervium) versus free corolla (Fig. 5a–c, Supplementary Note 2 and Supplementary Figs. 132 and 133). A pseudocalyptrate corolla, which is relatively common among genera of Myrtaceae, describes a perianth that is variously fused into a cap-like structure that may protect developing stamens from predation, degradation by desiccation, or fungal rot84. As determined previously, based on PCR marker phylogenies15,16 and ontogenetic studies84, pseudocalyptrate corollas evolved convergently in several Syzygium groups. One remarkable transition from free to pseudocalyptrate corollas appears at the base of the Syzygium grande group; indeed, it was apparently fixed first in its outgroup taxa (Supplementary Fig. 133). Several evolutionary reversals thereafter to free corolla lobes occurred, including one reversal that marks a large sublineage of the Syzygium grande group including 75 species as well as S. grande itself. The Syzygium creaghii and S. jambos groups have free corollas, but the S. pustulatum group may have been primitively pseudocalyptrate. Regardless, this trait seems highly labile within Syzygium, and other than the possible exception of the S. grande group’s ancestral state, there is no clear connection with Sahul-to-Sunda diversification. Interestingly, the most-parsimonious resolution of green fruits as ancestral to this clade and some of its outgroup species accompanies diversification of the Syzygium grande group into Sunda (Fig. 5d and Supplementary Fig. 134). Later most-parsimonious state transitions from green to purplish-black fruit are also noteworthy in the group. We speculate that this combination of traits—pre-anthesis protection by pseudocalyptrae and bearing of green to purplish-black fruits that attract far-flying birds or bats85–87—may have together pre-adapted this group to broad migration.
Fig. 5. Reproductive trait diversity in the genus Syzygium, as examined to reconstruct ancestral states using Mesquite.
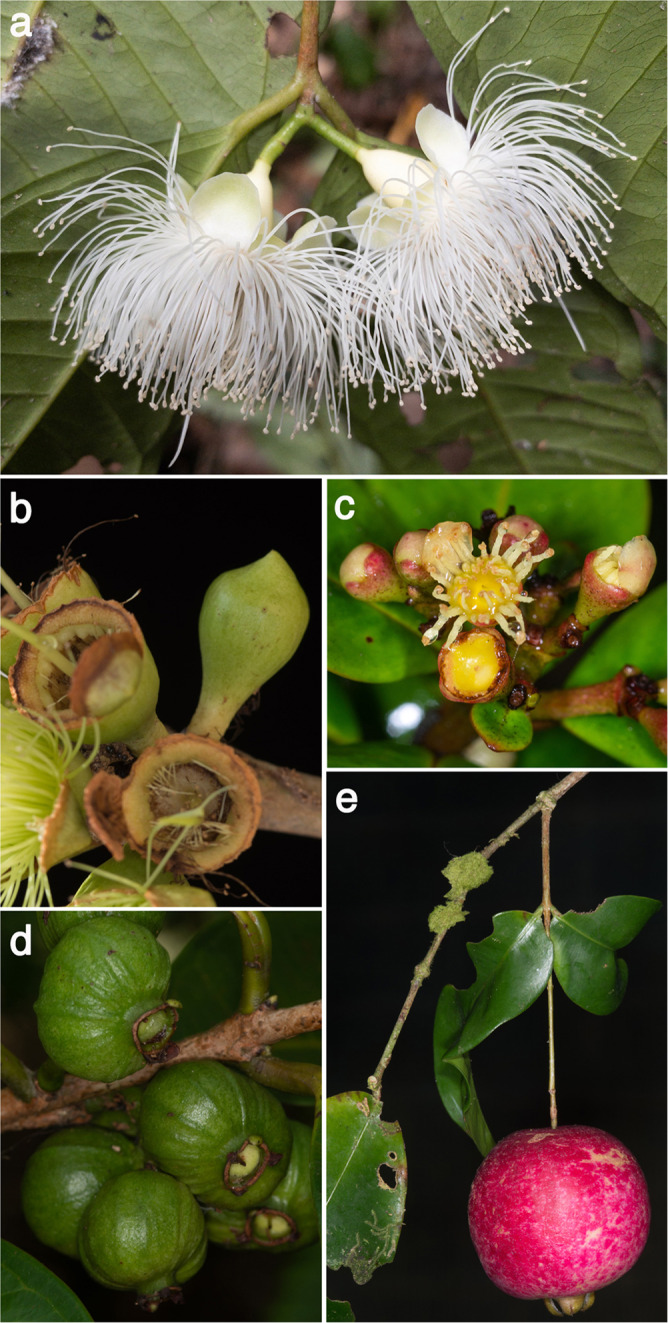
a Free petals (Syzygium pendens); b Calyptrate calyx (Syzygium paradoxum); c Pseudocalyptrate corolla (Syzygium adelphicum); d Fruits maturing green (Syzygium cf. dyerianum); e Pendulous inflorescence or infructescence (Syzygium boonjee). Photograph credits: YWL (a)–(e).
One other trait of note that marks large diversifications is the presence of pendulous inflorescences, which characterises the Syzygium creaghii group and largely marks the S. longipes group (Fig. 5e, Supplementary Note 2 and Supplementary Fig. 135). This trait is correlated with large fruits, often fleshy, which are known to reflect a specialised fruit display and dispersal strategy called flagellichory that increases fruit display for echolocating bats88, other flying/arboreal vertebrates18 or large vertebrate browsers (e.g., cassowaries89).
Implications from Syzygium for understanding species radiations
Here, we have explored species diversification patterns and their drivers in the world’s most species-rich tree genus, Syzygium. We generated a high-quality reference genome for Syzygium grande, the sea apple, and shotgun sequenced more than 15% of the species of this large genus to study their phylogenomic relationships. Through this extensive sampling of Syzygium diversity, we were able to solidify major clade relationships within the genus, currently recognised as subgenera, and, within Syzygium subg. Syzygium, provided unprecedented clarity on subclades that may become sectional units in the future. We discovered that many Syzygium species, particularly within Syzygium subg. Syzygium, likely branched from one another in rapid succession, yielding radiations of morphological and ecological diversity. One example was a group of species containing Syzygium grande itself that was marked by extremely short coalescence time intervals in our BUSCO species tree; this result was matched by highly networked edges at the base of the group in our NeighborNet analysis, which reflects underlying incongruence in the data. Since none of the f3 tests showed admixture, we interpret such webbed stem lineages in the NeighborNet network to reflect incomplete lineage sorting during rapid species radiation. PCA analysis of our samples illustrated clines of fixed allelic variation arrayed by Syzygium sublineage, possibly reflecting that a simple process of neutral geographic speciation predominated during most of the group’s cladogenesis. Plotting occurrences of species native to Singapore’s Bukit Timah Nature Reserve and East Malaysia’s Danum Valley Conservation Area illustrated that large-scale lineage diversification occurred before sympatric occupation of these habitats to generate diverse, closely associated Syzygium floras. As such, the immense radiation of the world’s largest tree genus may serve as a model for further detailed research, for example at the population level—integrating transcriptomic, proteomic, and metabolomic data—to explore actual mechanisms underlying morphological and ecological specialisation during a diversification that rivals any others under current study.
Methods
Oxford Nanopore sequencing of Syzygium grande
Young leaf tissue and twigs of Syzygium grande from a cultivated individual (Gleneagles Hospital, along Napier Road, Singapore; Low s.n. [SING]) were gathered, cleaned and flash frozen in liquid nitrogen, and then stored in −80 °C prior to extraction. About 10 g of flash frozen tissue was used for high-molecular-weight (HMW) genomic DNA isolation. The first step followed the BioNano NIBuffer nuclei isolation protocol in which frozen leaf tissue was homogenised in liquid nitrogen, followed by a nuclei lysis step using IBTB buffer with spermine and spermidine added and filtered just before use. IBTB buffer consists of Isolation Buffer (IB; 15 mM Tris, 10 mM EDTA, 130 mM KCI, 20 mM NaCl, 8%(m/V) PVP-10, pH 9.4) with 0.1% Triton X-100, and 7.5% (V/V) β-Mercaptoethanol (BME) mixed in and chilled on ice. The mixture of homogenised leaf tissue and IBTB buffer was strained to remove undissolved plant tissue. 1% Triton X-100 was added to lyse the nuclei before centrifugation at 2000×g for 10 min to pellet the nuclei. Once the nuclei pellet was obtained, we proceeded with cetyltrimethylammonium bromide (CTAB) DNA extraction with modifications for Oxford Nanopore sequencing90. The quality and concentration of HMW genomic DNA was checked using a Thermo Scientific™ NanoDrop™ Spectrophotometer, as well as on agarose gel electrophoresis following standard protocols. Genomic DNA obtained was further purified with a Qiagen® Genomic-Tip 500/G following the protocol provided by the developer.
The purified genomic DNA sample obtained was sequenced on the Oxford Nanopore Technologies (ONT) PromethION platform. We generated 60,136,770,518 bp of Nanopore reads with a read length N50 of 9382 bp and an average read quality score of 6.5. Raw ONT reads (fastq) of Syzygium grande were filtered prior to assembly using seqtk91 such that only reads 35 kb or longer were used for genome assembly, which was performed using wtdbg230 version 2.2 with flags -p19 -AS2 -e2. The genome consensus was also generated with wtdbg2. Consensus correction was performed with the input ONT reads and three rounds of racon92. The assembly generated was polished with Pilon93 using 30 Gb of 2 × 150 paired Illumina HiSeqX reads of Syzygium grande that were trimmed and filtered. The assembly of Syzygium grande comprised 1669 contigs with an N50 length of 556,915 bp. The assembly was filtered for organellar and contaminating contigs using the blobtools94 pipeline, resulting in the removal of 30 out of 1669 contigs. Next, purge haplotigs95 was used to identify 744 contigs contributing to a diploid peak, which were then removed. These contigs comprised <40 Mb of the genome assembly. This filtered primary assembly was thereafter scaffolded into chromosomes by Dovetail HiC technology31. The final scaffolded assembly size was 405,179,882 bp.
Transcriptome assembly and annotation of the Syzygium grande genome
Transcriptome assembly was carried out for 3 RNASeq libraries (S1: young leaves, S2: mature leaves, S3: twig tips; sequencing performed by NovogeneAIT) separately using an in-house custom assembly pipeline. The first step involved de novo assembly for multiple kmer values— 51, 61, 71, 81, 91, 101 using TransAbyss96 v2.0.1, and for kmer value 25 using Trinity97 v2.8.5. The second step comprised genome-guided assembly using StringTie98 v2.0. The input for this second step involved aligning the RNASeq reads against the reference genome using HISAT299 v2.1.0. The third step encompassed combining all the results from the first and the second steps using EvidentialGene100 v2018.06.18 to obtain a final high-confidence transcriptome assembly. S1 produced 57,746 transcripts (BUSCO completeness 92.9%), S2 produced 56,536 transcripts (BUSCO completeness 94.6%) and S2 produced 64,163 transcripts (BUSCO completeness 94.1%).
The genome annotation of the reference Syzygium grande genome was carried out using an in-house custom annotation pipeline. The first step involved the preparation of a de novo repeat library using RepeatModeler v1.0.11. This library was used to mask the repetitive regions in the genome assembly using RepeatMasker101 v4.0.9 resulting in 45.09% of the genome being masked. The second step was the gene prediction step, based on a modular approach using three different gene predictors gene markers, braker (using the three RNASeq libraries) and GeMoMa102 (using gene models from the model species Arabidopsis thaliana [TAIR10] and Populus trichocarpa [v3.1]). Additionally, the spliced transcript aligner PASA103 (using transcripts from the three RNASeq libraries) was used to generate evidence for gene structures. These results were then combined using the combiner tool EvidenceModeler104 to produce a single high confidence final prediction of 39,903 gene models with a BUSCO completeness score of 86.6%. A graphic workflow of these procedures is presented in Supplementary Fig. 136. Please see additional details in Supplementary Note 3.
Genome structural analyses
The chromosome-level Syzygium grande genome assembly and annotation were uploaded to the online CoGe comparative genomics platform (https://genomevolution.org/coge/GenomeInfo.pl?gid=60239)105. Syntenic dot plots and data for synonymous substitution rate (Ks) calculations were derived from CoGe SynMap105 calculations using default settings, with CodeML set to “Calculate syntenic CDS pairs and colour dots: Synonymous (Ks) substitution rates”. Ks data were collected from corresponding downloads at the “Results with synonymous/non-synonymous rate values” tabs. Each pairwise SynMap analysis (including self:self) was performed for the following species and CoGe genome IDs: Syzygium grande (id60239), Eucalyptus grandis (id28624), Punica granatum (id61248), Populus trichocarpa (id25127), Vitis vinifera (id19990). Syntenic dot plots from SynMap were further investigated for synteny relationships within and between species using the FractBias tool106. FractBias mappings for fractionation profiles between species were generated using Quota Align syntenic depth of 2:1 for Syzygium, Eucalyptus, and Punica against Vitis (analyses can be regenerated at https://genomevolution.org/r/1ig9p, https://genomevolution.org/r/1ig9r, and https://genomevolution.org/r/1ig9o, respectively), max query chromosomes = 100, max target chromosomes = 25, and “Use all genes in target genome”. For Populus against Syzygium (which can be regenerated at https://genomevolution.org/r/1ig9q), mapping of the former assembly against the latter used a Quota Align syntenic depth of 2:2 and the same options as described above for depth 2:1. Density plots (both histogram and smoothed curve) of Ks values for syntenic paralogs were generated in R107 using the tidyverse108, ggplot2109, RColorBrewer110, ggridges111, and ggpmisc112 packages. Ks peaks were calibrated by their shared gamma hexaploidy event using the method described by Wang et al. 43.
Illumina sequencing of Syzygium and outgroup individuals
A total of 289 Syzygium individuals were selected to represent the six subgenera recognised by Craven and Biffin (2010)17, across its natural distribution from Africa to the Indian subcontinent, through the Indomalaya region and into the Pacific. Three outgroup taxa in Myrtaceae, Metrosideros excelsa, M. nervulosa (tribe Metrosidereae) and Eugenia reinwardtiana (tribe Myrteae), were also sampled. Most of the 292 samples used in this study were freshly collected in the field, utilising the silica gel teabag method for preserving plant DNA113, between 2017 and 2019 either from collecting expeditions conducted in Singapore, Australia, Brunei, Indonesia (West Papua and Papua provinces) and Malaysia or from cultivated specimens in the Singapore Botanic Gardens (Singapore), Bogor Botanical Garden (Bogor, Indonesia), Cairns Botanic Gardens (Queensland, Australia) and Royal Botanic Gardens, Kew (UK).
Approximately 20 mg of silica-dried leaf tissue were sampled for genomic sequencing. Plant tissue was ground to a fine powder using Omni International Bed Rupture Homogeniser. DNA isolation was carried out at the molecular lab of the Singapore Botanic Gardens using the Qiagen DNeasy® Plant Mini Kit, following the protocol provided by the manufacturer. In rare cases, DNA yields were low when obtained from Qiagen DNeasy® Plant Mini Kit; hence for these problematic samples, the Qiagen DNeasy® Plant Maxi Kit was used instead. Quality and concentration of DNA aliquots were checked using a Thermo Scientific™ NanoDrop™ Spectrophotometer before submission to NovogeneAIT (Singapore) for QC, library construction and sequencing of 30 Gb each (150 × 150 paired ends) on an Illumina HiSeqX.
Assembly, BUSCO QC, and species tree phylogeny of the resequenced Syzygium and outgroup accessions
The 292 Illumina resequenced accessions were assembled using MaSuRCA33 v3.3.1 with library insert average length of 350 bp and a standard deviation of 100 bp. The genome completeness percentages were estimated using BUSCO v4.0.2 based on eudicots_odb10 database.
The phylogeny for the Syzygium and outgroup species was estimated using the BUSCO genes. Two species tree versions were estimated. The first tree was estimated using 229 BUSCO genes that were complete and found in all 292 species. The second tree was estimated using 1227 BUSCO genes that were present in 286 species and above.
The species tree generation was constructed using an in-house phylogeny pipeline. The first step involved extraction of BUSCO genes from all resequenced individuals, generating a multi-fasta file for each BUSCO gene containing a representation of that gene from the available species. The second step involved performing multiple sequence alignment (MSA) for each BUSCO multi-fasta file using MAFFT114 v7.407. The resulting MSA files were used to generate gene trees using RAxML47 v8.2.12. These gene trees were concatenated and sent as a single input to ASTRAL48 v5.15.1 to generate the final species tree.
Mapping the resequenced individuals to the Syzygium grande reference genome
The 30 Gb each of raw Illumina reads was trimmed to remove adapters using default settings of Trimmomatic115 version 0.38. Following trimming, the samples were mapped using bwa mem116 (version 0.7.17), and the subsequent bam files were filtered for a quality score of 20 using samtools117 view and sorted using samtools sort. Picard MarkDuplicates (version 2.7.1; https://broadinstitute.github.io/picard/) was used to remove PCR duplicates from the mapped reads. Depth and width of mapping coverage were calculated using BEDTools118 version 2.23.0.
SNP calling and statistics
SNP calling was performed using GATK version 3.8 in ERC mode for each sample. GenotypeGVCFs were used to call joint genotypes; due to RAM and time limitations, this was split into 70 intervals using the –L flag. To combine the 70 files, GatherVcfs was used to generate a VCF file. As a quality control, GATK VariantFiltration was used with the following filter expression based on GATK recommendations: ‘QD < 2.0 || FS > 60.0 || MQ < 50.0 || MQRankSum < −12.5 || ReadPosRankSum < −8.0 || SOR > 4.0’. Further filtrations were carried out in VCFtools119 (version 0.1.13) to create various datasets for downstream analyses (Supplementary Table 2). The –plink flag was applied to generate .ped and .map files, and also –recode was used to generate a filtered vcf file. SNP statistics were calculated through vcftools options –het and –singletons for dataset FRSA-1. The output was subsequently plotted using the R package ggplot (https://github.com/tidyverse/ggplot2).
RAxML SNP tree
A pseudo-alignment of SNPs was generated for phylogenetic reconstruction for dataset FRSA-1. The plink.ped file was used to convert into fasta input for RAxML. Only variable SNPs were retained for a total of 2,384,277 SNPs. A maximum likelihood tree was generated using RAxML version 8 including adjustments for ascertainment bias (—asc-corr lewis) and 500 bootstraps. Trees were viewed and edited using FigTree120.
Plastome assembly and phylogenetic tree
We filtered and removed nuclear reads from the Syzygium grande Nanopore assembly and constructed a complete chloroplast genome of 158,980 bp in length. This genome was used as a reference to examine phylogenetic relationships of Syzygium based on the plastome. Before mapping Illumina reads of 289 Syzygium individuals and three outgroup taxa (two Metrosideros and one Eugenia) to the reference, one of the Inverted Repeat regions (IRs) was removed to prevent sequence calling bias. The combined DNA alignment file of the 292 individuals was then subjected to an ML analysis using RAxML with 1000 bootstrap replicates.
NeighborNet analysis
We used the NeighborNet50,121 approach to assess incongruence within our SNP data set for Syzygium subg. Syzygium and S. rugosum as an outgroup taxon (FRSA-5). We used SplitsTree122 version 4.17.0 to calculate the network with LogDet123 distances.
ADMIXTURE analysis
We ran ADMIXTURE53 (version 1.3) for K values 5–15 and used the –cv option to find the best (lowest) K value for the number of ancestral populations (Supplementary Figs. 10 and 11). The results were then plotted using the barplot function in R.
Local PCA
Single nucleotide polymorphisms (SNPs) called against the draft assembly were transferred to the Hi-C scaffolded assembly through the use of Minimap2124, transanno (https://github.com/informationsea/transanno), and LiftoverVcf125. The BED file needed to remove SNPs from repeat regions was generated using convert2bed126. SNPs from repeat regions were removed using the VCFtools—exclude-bed option119. The VCF file was divided by the 11 pseudomolecules using HTSlib127, and converted to BCF format and indexed using BCFtools128. Local PCA was carried out using the R107 lostruct package58 and window size was chosen to include about 1000 SNPs per window as recommended by the authors.
PCA
The eigensoft129 package (version 6.1.3) was used to convert plink.map and .ped files into .ind, .geno and .snp files. Thereafter, the smartpca.perl script was used to run PCA for PC1 to PC10 under default parameters for datasets FRSA-1 (all Syzygium) and FRSA-3 (Syzygium subg. Syzygium). Taxa that were removed using the smartpca default five rounds of outlier removal shown in red (Supplementary Fig. 101). In addition, three separate PCA checks were performed to confirm that clinal results were not artefactual. PCA was run with SNPs using (i) a more stringent minimum depth of coverage of 20 (Supplementary Figs. 112–116), (ii) homozygous sites only (Supplementary Figs. 117–121), and (iii) LD correction turned on using the nsnpldregress option in the smartpca programme to control for linkage (Supplementary Figs. 107–111). In order to search for possible correlations, the PCA was coloured in numerous ways, by geography, by ecoplots, and also according to ADMIXTURE K = 14 ancestral groups (Supplementary Fig. 11), using the scatterpie package in R.
f3 statistics
Dataset FRSA-5 was used to formally test for admixture using the f3 56 statistic implemented in the qp3pop (version 650) function of the AdmixTools package (https://github.com/DReichLab/AdmixTools). A total of 7,195,530 SNPs were used to test 3,889,44 triplets, every possible combination within the Syzygium grande group. We then applied a FDR correction to the Z-scores using a custom R function developed as part of the silver birch genome project130. Next, heatmaps were plotted in R for each target.
PSMC
We used PSMC131 to infer past demographies for most members of the Syzygium grande group. To accomplish this, we mapped trimmed reads for each sample to its de novo MaSuRCA33 assembled genome, using the same mapping parameters used for S. grande reference mapping. Consensus sequences were called using samtools117 mpileup to generate diploid sequences for input to PSMC, with all parameters set to default. Demographic curves were subsequently plotted using the psmc_plot.pl script. As a first quality control, we only included samples with de novo assemblies that had N50 > 10,000 basepairs and a BUSCO completeness score >80%, which excluded additional eight samples. Following plotting, for clarity, we removed an additional six samples that deviated strongly from the general trends of the clade. The values of sum_n, the number of segregating sites, were extracted from the .psmc files for each sample. The maximum coalescence date and its corresponding Ne values were extracted from the plot files. Since strict filtering of SNPs lowers heterozygosity drastically, the dataset FRSA-GATK was used to calculate heterozygosity using the –het option in VCFtools. To plot the correlation matrix, the pairs.panels function from the R package psych was used. The parameter lm was set to true to display linear regression fit, and ci was set to true to display confidence intervals. To display R-squared values, the source code was edited. Stars were assigned to the following categories based on p-value significance (<0.001***, <0.01**, and <0.05*).
Biogeography
An ultrametric dated SNP phylogeny was generated both for phylogenetic dating and biogeographic reconstruction. The SNP tree was employed since ASTRAL species tree branch lengths are not interpretable for ultrametric conversion (see the comment from the ASTRAL developer on GitHub: https://github.com/smirarab/ASTRAL/issues/37). The function chronos() in the R package ape132 (version 3.5.2) was used to create the ultrametric tree. The model used was correlated, and the calibration applied a minimum of 20,900,000 and maximum of 22,100,000 years at the node for which Syzygium subg. Acmena and S. subg. Syzygium share a common ancestor. This calibration is based on a Syzygium fossil (S. christophelii) found in New South Wales, Australia76. The ultrametric tree was also used as input to search for and execute the best model using RASP74 and BioGeoBEARS73 (which was BAYAREALIKE + J in both cases). Each of the 292 samples was assigned to one or more of eight geographic regions (Africa, India, Mainland Asia, Sunda, Sahul, Wallacea, Zealandia, and Pacific islands) based on their distribution patterns, and RASP and BioGeoBEARS were both restricted to a maximum of six areas at each node.
Character evolution with Mesquite
States for three morphological characters—specifically (i) inflorescence habit (erect vs. pendent), (ii) shedding fused corolla present as a true calyptra, a pseudocalyptra, vs. corolla free at anthesis, and (iii) mature fruit colour (green, white or cream, black, pink, purple, red, brown, orange, yellow, blue, or grey)—were gathered from living material, herbarium specimens, published flora accounts, and species protologues. The categorical morphological characters were coded into the form of numbers 0–9 and/or letters a–z. The ASTRAL tree of BUSCO genes, and selected traits, were loaded into Mesquite83 version 3.61 and the Trace Character Evolution option with parsimony was selected to predict ancestral states.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Y.W.L. was supported by a postgraduate scholarship research grant from the Ministry of National Development, Singapore awarded through the National Parks Board, Singapore (NParks; NParks’ Garden City Fund). Principal research funding from NParks and the School of Biological Sciences (SBS), Nanyang Technological University (NTU), Singapore, is acknowledged. We thank Peter Preiser, Associate Vice President for Biomedical and Life Sciences, for facilitating NTU support, and Kenneth Er, CEO of NParks, for facilitating research funding through that organisation. V.A.A. and C.L. were funded by SBS, NTU for a one-year research leave. V.A.A. and C.L. also acknowledge support from the United States National Science Foundation (grants 2030871 and 1854550, respectively). S.R. was supported by a postdoctoral research fellowship under the NTU Strategic Plant Programme. S.R. and N.R.W.C. acknowledge funding from NTU start-up and the Academy of Finland (decisions 318288, 319947) grants to J.S. Fieldwork conducted by Y.W.L. was supported by an Indonesian Government RISTEK research permit (Application ID: 1517217008) and an Access License from the Sabah State government [JKM/MBS.1000-2/2JLD.7(84)]. T.N.C.V. is grateful to the Assemblée de la Province Nord and Assemblée de la Province Sud (New Caledonia) for facilitating relevant collection permits. A.N. was partly supported by the Research Project Promotion Grant (Strategic Research Grant No. 17SP01302) from the University of the Ryukyus, and partly by the Environment Research and Technology Development Fund (JPMEERF20204003) from the Environmental Restoration and Conservation Agency of Japan. Fieldwork in Fiji conducted by R.B. was hosted and facilitated by Elina Nabubuniyaka-Young (The Pacific Community’s Centre for Pacific Crops and Trees, Fiji). We thank the NTU-Smithsonian Partnership for tree data obtained for the Bukit Timah Nature Reserve (BTNR) long-term forest dynamics plots. Administrative support provided by Mui Hwang Khoo-Woon and Peter Ang at the molecular laboratory of the Singapore Botanic Gardens (SBG) is acknowledged. Rosie Woods and Imalka Kahandawala (DNA and Tissue Bank, Royal Botanic Gardens, Kew) facilitated additional DNA samples. Daniel Thomas (SBG) and Yan Yu (Sichuan University) commented on biogeographical analyses. NovogeneAIT in Singapore is acknowledged for personalised sequencing service.
Source data
Author contributions
Y.W.L., V.A.A., C.L., E.J.L., D.F.R.P.B., and D.J.M. conceived the study. T.P.M. generated the Oxford Nanopore Technology sequence for and assembled the reference genome. Y.W.L., S.R., N.R.W.C., S.J.F., C.M.T., C.L., and V.A.A. analysed the genomic data. Y.W.L. assembled the morphological data with contributions from HDJ on Sri Lankan taxa; Y.W.L., C.M.T., and V.A.A. performed the character evolutionary analyses. Y.W.L. and E.J.L. assembled the biogeographic data; Y.W.L., C.M.T., and V.A.A. carried out the biogeographic analyses. Y.W.L., S.R., C.M.T., C.L., and V.A.A. wrote the first draft of the paper. E.J.L., D.F.R.P.B., J.S., and D.J.M. provided comments on the first draft. Y.W.L., J.A.A., W.H.A., K.A., P.A., A.B., R.E.B., M.C., L.M.C., I.D.C., D.C., A.J.F., P.I.F., D.G., D.J.G., B.G., C.D.H., A.I., B.I., H.D.J., M.A.K., H.S.K., E.K., S.L.K., J.T.K.L., S.M.L.L., P.K.F.L., W.H.L., S.K.Y.L., R.M., W.J.F.M., F.M., W.A.M., A.N., K.M.N., M.N., S.R., R.R., H.R., V.I.S., R.S.S., S.S., L.A.T., A.T.B., T.N.C.V., J.F.W., P.W., D.S.A.W., S.W., J.W.Y., K.T.Y., G.S.W.K., D.F.R.P.B., C.L., E.J.L., and V.A.A. participated in fieldwork and/or provided plant tissue samples or other materials for this work. All authors approved the final paper version.
Peer review
Peer review information
Nature Communications thanks Brad Barbazuk, Elizabeth Stacy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The genome data generated in this study has been deposited in the NCBI database under accession code PRJNA803434 and BioSample ID SAMN29207412. The Syzygium grande genome assembly and annotation are also available on CoGe [https://genomevolution.org/coge/GenomeInfo.pl?gid=60239]. Processed data generated in this study and used for main text figures are provided in source data files. Additional processed data are available at Dryad [10.5061/dryad.h18931zpw]. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yee Wen Low, Sitaram Rajaraman, Crystal M. Tomlin.
Contributor Information
Yee Wen Low, Email: low_yee_wen@nparks.gov.sg.
Charlotte Lindqvist, Email: cl243@buffalo.edu.
Eve J. Lucas, Email: e.lucas@kew.org
Victor A. Albert, Email: vaalbert@buffalo.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-32637-x.
References
- 1.Gavrilets S, Losos JB. Adaptive radiation: contrasting theory with data. Science. 2009;323:732–737. doi: 10.1126/science.1157966. [DOI] [PubMed] [Google Scholar]
- 2.Givnish TJ. Adaptive radiation versus ‘radiation’and ‘explosive diversification’: why conceptual distinctions are fundamental to understanding evolution. N. Phytol. 2015;207:297–303. doi: 10.1111/nph.13482. [DOI] [PubMed] [Google Scholar]
- 3.Rundell RJ, Price TD. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol. Evol. 2009;24:394–399. doi: 10.1016/j.tree.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Gittenberger E. What about non-adaptive radiation? Biol. J. Linn. Soc. 1991;43:263–272. doi: 10.1111/j.1095-8312.1991.tb00598.x. [DOI] [Google Scholar]
- 5.Lamichhaney S, et al. Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature. 2015;518:371–375. doi: 10.1038/nature14181. [DOI] [PubMed] [Google Scholar]
- 6.Seehausen O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004;19:198–207. doi: 10.1016/j.tree.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Soltis DE, et al. Polyploidy and angiosperm diversification. Am. J. Bot. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- 8.Meudt HM, et al. Polyploidy on Islands: its emergence and importance for diversification. Front. Plant Sci. 2021;12:637214–637214. doi: 10.3389/fpls.2021.637214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Givnish, T. J. & Sytsma, K. J. Molecular Evolution and Adaptive Radiation (Cambridge University Press, 2000).
- 10.Choi, J. Y. et al. Ancestral polymorphisms shape the adaptive radiation of Metrosideros across the Hawaiian Islands. Proc. Natl. Acad. Sci. USA118, (2021). [DOI] [PMC free article] [PubMed]
- 11.Grant, P.R. Evolution on Islands (Oxford University Press, Oxford, 1998).
- 12.Lindqvist C, Motley TJ, Jeffrey JJ, Albert VA. Cladogenesis and reticulation in the Hawaiian endemic mints (Lamiaceae) Cladistics. 2003;19:480–495. doi: 10.1111/j.1096-0031.2003.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 13.Wallace, A. R. Alfred Russel Wallace: Letters from the Malay Archipelago (Oxford University Press, 2013).
- 14.Ashton, P. S. & Seidler, R. On the Forests of Tropical Asia: Lest the Memory Fade (Kew Publishing, 2014).
- 15.Biffin E, Craven LA, Crisp MD, Gadek PA. Molecular systematics of Syzygium and allied genera (Myrtaceae): evidence from the chloroplast genome. Taxon. 2006;55:79–94. doi: 10.2307/25065530. [DOI] [Google Scholar]
- 16.Biffin E, Harrington MG, Crisp M, Craven LA, Gadek P. Structural partitioning, paired-sites models and evolution of the ITS transcript in Syzygium and Myrtaceae. Mol. Phylogenet. Evol. 2007;43:124–139. doi: 10.1016/j.ympev.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Craven LA, Biffin E. An infrageneric classification of Syzygium (Myrtaceae) Blumea. 2010;55:94–99. doi: 10.3767/000651910X499303. [DOI] [Google Scholar]
- 18.Biffin E, et al. Evolution of exceptional species richness among lineages of fleshy-fruited Myrtaceae. Ann. Bot. 2010;106:79–93. doi: 10.1093/aob/mcq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beech E, Rivers M, Oldfield S, Smith P. GlobalTreeSearch: the first complete global database of tree species and country distributions. J. Sustain. For. 2017;36:454–489. doi: 10.1080/10549811.2017.1310049. [DOI] [Google Scholar]
- 20.Govaerts, R. et al. World Checklist of Myrtaceae (Royal Botanic Gardens, 2008).
- 21.McVaugh, R. Nomenclatural notes on Myrtaceae and related families (continuation). Taxon6, 162–167 (1956).
- 22.Nair, K. N. The genus Syzygium: Syzygium cumini and other underutilized species (CRC Press, 2017).
- 23.Kochummen, K. M. Myrtaceae. In Tree Flora of Malaya Vol. 3 (ed. Ng, F. S. P.) 119–134 (Longman Malaysia, Kuala Lumpur, 1978).
- 24.Boo, C. M., Omar-Hor, K., Ou-Yang, C. L. & Ng, C. K. 1001 Garden Plants in Singapore (National Parks, 2003).
- 25.Parnell, J., Craven, L. A. & Biffin, E. Matters of scale: dealing with one of the largest genera of angiosperms. In Reconstructing the Tree of Life: Taxonomy and Systematics of Species Rich Taxa (eds Hodkinson, T. R. & Parnell, J. A. N.) 251–273 (CRC Press LLC, 2007).
- 26.Lee, H. et al. Floristic and structural diversity of mixed dipterocarp forest in Lambir Hills National Park, Sarawak, Malaysia. J. Trop. For. Sci.14, 379–400 (2002).
- 27.Craven, L. Unravelling knots or plaiting rope: what are the major taxonomic strands in Syzygium sens. lat. (Myrtaceae) and what should be done with them? In Taxonomy: The Cornerstone of Biodiversity (eds Saw, L. G., Chua, L. S. L. & Khoo, K. C.) 75–85 (Forest Research Institute Malaysia, Kepong, 2001).
- 28.Schmid R. A resolution of the Eugenia–Syzygium controversy (Myrtaceae) Am. J. Bot. 1972;59:423–436. doi: 10.1002/j.1537-2197.1972.tb10113.x. [DOI] [Google Scholar]
- 29.Jain M, Olsen HE, Paten B, Akeson M. The Oxford Nanopore MinION: delivery of nanopore sequencing to the genomics community. Genome Biol. 2016;17:1–11. doi: 10.1186/s13059-015-0866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan J, Li H. Fast and accurate long-read assembly with wtdbg2. Nat. Methods. 2020;17:155–158. doi: 10.1038/s41592-019-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putnam NH, et al. Chromosome-scale shotgun assembly using an in vitro method for long-range linkage. Genome Res. 2016;26:342–350. doi: 10.1101/gr.193474.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 33.Zimin AV, et al. The MaSuRCA genome assembler. Bioinformatics. 2013;29:2669–2677. doi: 10.1093/bioinformatics/btt476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens, P. F. Angiosperm Phylogeny Website, Version 17http://www.mobot.org/MOBOT/research/APweb/ (2017).
- 35.Chanderbali AS, Berger BA, Howarth DG, Soltis DE, Soltis PS. Evolution of floral diversity: genomics, genes and gamma. Philos. Trans. R. Soc. B: Biol. Sci. 2017;372:20150509. doi: 10.1098/rstb.2015.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaillon O, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449:463. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- 37.Jiao Y, et al. A genome triplication associated with early diversification of the core eudicots. Genome Biol. 2012;13:1–14. doi: 10.1186/gb-2012-13-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myburg AA, et al. The genome of Eucalyptus grandis. Nature. 2014;510:356–362. doi: 10.1038/nature13308. [DOI] [PubMed] [Google Scholar]
- 39.Qin G, et al. The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. Plant J. 2017;91:1108–1128. doi: 10.1111/tpj.13625. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Z, et al. The pomegranate (Punica granatum L.) genome provides insights into fruit quality and ovule developmental biology. Plant Biotechnol. J. 2018;16:1363–1374. doi: 10.1111/pbi.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng C, et al. A chromosome‐level genome assembly provides insights into ascorbic acid accumulation and fruit softening in guava (Psidium guajava) Plant Biotechnol. J. 2021;19:717–730. doi: 10.1111/pbi.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.One Thousand Plant Transcriptomes Initiative. One thousand plant transcriptomes and the phylogenomics of green plants. Nature574, 679–685 (2019). [DOI] [PMC free article] [PubMed]
- 43.Wang X, et al. Genome alignment spanning major Poaceae lineages reveals heterogeneous evolutionary rates and alters inferred dates for key evolutionary events. Mol. Plant. 2015;8:885–898. doi: 10.1016/j.molp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Maurin, O. et al. A nuclear phylogenomic study of the angiosperm order Myrtales, exploring the potential and limitations of the universal Angiosperms353 probe set. Am. J. Bot.108, 1087–1111 (2021). [DOI] [PubMed]
- 45.Albert VA, et al. The Amborella genome and the evolution of flowering plants. Science. 2013;342:1241089. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- 46.Rice A, et al. The Chromosome Counts Database (CCDB)—a community resource of plant chromosome numbers. N. Phytol. 2015;206:19–26. doi: 10.1111/nph.13191. [DOI] [PubMed] [Google Scholar]
- 47.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirarab S, et al. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics. 2014;30:i541–i548. doi: 10.1093/bioinformatics/btu462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maddison WP. Gene trees in species trees. Syst. Biol. 1997;46:523–536. doi: 10.1093/sysbio/46.3.523. [DOI] [Google Scholar]
- 50.Bryant D, Moulton V. Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Mol. Biol. Evol. 2004;21:255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- 51.Burgon JD, et al. Phylogenomic inference of species and subspecies diversity in the Palearctic salamander genus Salamandra. Mol. Phylogenet. Evol. 2021;157:107063. doi: 10.1016/j.ympev.2020.107063. [DOI] [PubMed] [Google Scholar]
- 52.Suh A, Smeds L, Ellegren H. The dynamics of incomplete lineage sorting across the ancient adaptive radiation of neoavian birds. PLoS Biol. 2015;13:e1002224. doi: 10.1371/journal.pbio.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawson, D. J., van Dorp, L. & Falush, D. A tutorial on how not to over-interpret STRUCTURE and ADMIXTURE bar plots. Nat. Commun.9, 3258 (2018). [DOI] [PMC free article] [PubMed]
- 55.Zhang X, et al. Genomes of the banyan tree and pollinator wasp provide insights into fig-wasp coevolution. Cell. 2020;183:875–889. e17. doi: 10.1016/j.cell.2020.09.043. [DOI] [PubMed] [Google Scholar]
- 56.Patterson N, et al. Ancient admixture in human history. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lan, T. et al. Insights into bear evolution from a Pleistocene polar bear genome. Proc. Natl. Acad. Sci. USA119, e2200016119 (2022). [DOI] [PMC free article] [PubMed]
- 58.Li H, Ralph P. Local PCA shows how the effect of population structure differs along the genome. Genetics. 2019;211:289. doi: 10.1534/genetics.118.301747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reich D, Price AL, Patterson N. Principal component analysis of genetic data. Nat. Genet. 2008;40:491–492. doi: 10.1038/ng0508-491. [DOI] [PubMed] [Google Scholar]
- 60.Novembre J, Stephens M. Interpreting principal component analyses of spatial population genetic variation. Nat. Genet. 2008;40:646–649. doi: 10.1038/ng.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slatkin M. Isolation by distance in equilibrium and non‐equilibrium populations. Evolution. 1993;47:264–279. doi: 10.1111/j.1558-5646.1993.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 62.Wright S. Isolation by distance. Genetics. 1943;28:114. doi: 10.1093/genetics/28.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seeholzer GF, Brumfield RT. Isolation by distance, not incipient ecological speciation, explains genetic differentiation in an Andean songbird (Aves: Furnariidae: Cranioleuca antisiensis, Line‐cheeked Spinetail) despite near threefold body size change across an environmental gradient. Mol. Ecol. 2018;27:279–296. doi: 10.1111/mec.14429. [DOI] [PubMed] [Google Scholar]
- 64.Barton NH. Natural selection and random genetic drift as causes of evolution on islands. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 1996;351:785–795. doi: 10.1098/rstb.1996.0073. [DOI] [PubMed] [Google Scholar]
- 65.Gavrilets S. Perspective: models of speciation: what have we learned in 40 years? Evolution. 2003;57:2197–2215. doi: 10.1111/j.0014-3820.2003.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 66.Rundle HD, Nosil P. Ecological speciation. Ecol. Lett. 2005;8:336–352. doi: 10.1111/j.1461-0248.2004.00715.x. [DOI] [Google Scholar]
- 67.Schluter D. Evidence for ecological speciation and its alternative. Science. 2009;323:737–741. doi: 10.1126/science.1160006. [DOI] [PubMed] [Google Scholar]
- 68.Shafer AB, Wolf JB. Widespread evidence for incipient ecological speciation: a meta‐analysis of isolation‐by‐ecology. Ecol. Lett. 2013;16:940–950. doi: 10.1111/ele.12120. [DOI] [PubMed] [Google Scholar]
- 69.Salojärvi J, et al. Author Correction: genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2019;51:1187–1189. doi: 10.1038/s41588-019-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu G, et al. Two divergent haplotypes from a highly heterozygous lychee genome suggest independent domestication events for early and late-maturing cultivars. Nat. Genet. 2022;54:73–83. doi: 10.1038/s41588-021-00971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barton NH, Charlesworth B. Genetic revolutions, founder effects, and speciation. Annu. Rev. Ecol. Syst. 1984;15:133–164. doi: 10.1146/annurev.es.15.110184.001025. [DOI] [Google Scholar]
- 72.Kooyman, R. M. et al. Origins and assembly of Malesian rainforests. Annu. Rev. Ecol. Evol. Systemat.50, 119–143 (2019).
- 73.Matzke, N. J. BioGeoBEARS: BioGeography with Bayesian (and likelihood) evolutionary analysis in R Scripts. R package version 0.2.1https://rdrr.io/cran/BioGeoBEARS/ (2013).
- 74.Yu Y, Blair C, He X. RASP 4: ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 2020;37:604–606. doi: 10.1093/molbev/msz257. [DOI] [PubMed] [Google Scholar]
- 75.Thornhill AH, Ho SY, Kulheim C, Crisp MD. Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Mol. Phylogenet. Evol. 2015;93:29–43. doi: 10.1016/j.ympev.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Tarran M, Wilson PG, Paull R, Biffin E, Hill RS. Identifying fossil Myrtaceae leaves: the first described fossils of Syzygium from Australia. Am. J. Bot. 2018;105:1748–1759. doi: 10.1002/ajb2.1163. [DOI] [PubMed] [Google Scholar]
- 77.Hall R. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions, model and animations. J. Asian Earth Sci. 2002;20:353–431. doi: 10.1016/S1367-9120(01)00069-4. [DOI] [Google Scholar]
- 78.Hall R. Late Jurassic–Cenozoic reconstructions of the Indonesian region and the Indian Ocean. Tectonophysics. 2012;570:1–41. doi: 10.1016/j.tecto.2012.04.021. [DOI] [Google Scholar]
- 79.Toussaint EF, et al. The towering orogeny of New Guinea as a trigger for arthropod megadiversity. Nat. Commun. 2014;5:1–10. doi: 10.1038/ncomms5001. [DOI] [PubMed] [Google Scholar]
- 80.Cannon CH, Morley RJ, Bush AB. The current refugial rainforests of Sundaland are unrepresentative of their biogeographic past and highly vulnerable to disturbance. Proc. Natl Acad. Sci. USA. 2009;106:11188–11193. doi: 10.1073/pnas.0809865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stelbrink, B. A Biogeographic View on Southeast Asia's History. 271 (Humboldt-Universität zu Berlin, Lebenswissenschaftliche Fakultät, 2015).
- 82.PAGES PIWGO. Interglacials of the last 800,000 years. Rev. Geophys. 2016;54:162–219. doi: 10.1002/2015RG000482. [DOI] [Google Scholar]
- 83.Maddison, W. P. & Maddison, D. R. Mesquite: a modular system for evolutionary analysis. Version 3.61http://mesquiteproject.org (2019).
- 84.Vasconcelos TNC, Lucas EJ, Conejero M, Giaretta A, Prenner G. Convergent evolution in calyptrate flowers of Syzygieae (Myrtaceae) Bot. J. Linn. Soc. 2019;192:498–509. doi: 10.1093/botlinnean/boz105. [DOI] [Google Scholar]
- 85.Gorchov, D. L., Cornejo, F., Ascorra, C. F. & Jaramillo, M. Dietary overlap between frugivorous birds and bats in the Peruvian Amazon. Oikos74, 235–250 (1995).
- 86.Hodgkison R, Balding ST, Zubaid A, Kunz TH. Fruit Bats (Chiroptera: Pteropodidae) as seed dispersers and pollinators in a lowland Malaysian rain Forest1. Biotropica. 2003;35:491–502. doi: 10.1111/j.1744-7429.2003.tb00606.x. [DOI] [Google Scholar]
- 87.Teixeira RC, Corrêa CE, Fischer E. Frugivory by Artibeus jamaicensis (Phyllostomidae) bats in the Pantanal, Brazil. Stud. Neotrop. Fauna Environ. 2009;44:7–15. doi: 10.1080/01650520802692283. [DOI] [Google Scholar]
- 88.Kalko EK, Condon M. Echolocation, olfaction and fruit display: how bats find fruit of flagellichorous cucurbits. Funct. Ecol. 1998;12:364–372. doi: 10.1046/j.1365-2435.1998.00198.x. [DOI] [Google Scholar]
- 89.Stocker, G. & Irvine, A. Seed dispersal by cassowaries (Casuarius casuarius) in North Queensland’s rainforests. Biotropica15, 170–176 (1983).
- 90.Michael TP, et al. High contiguity Arabidopsis thaliana genome assembly with a single nanopore flow cell. Nat. Commun. 2018;9:1–8. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H. seqtk Toolkit for processing sequences in FASTA/Q formats. GitHub. 2012;767:69. [Google Scholar]
- 92.Vaser R, Sović I, Nagarajan N, Šikić M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker BJ, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laetsch DR, Blaxter ML. BlobTools: interrogation of genome assemblies. F1000Research. 2017;6:1287. doi: 10.12688/f1000research.12232.1. [DOI] [Google Scholar]
- 95.Roach MJ, Schmidt SA, Borneman AR. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinform. 2018;19:1–10. doi: 10.1186/s12859-018-2485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robertson G, et al. De novo assembly and analysis of RNA-seq data. Nat. Methods. 2010;7:909–912. doi: 10.1038/nmeth.1517. [DOI] [PubMed] [Google Scholar]
- 97.Haas BJ, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pertea M, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gilbert DG. Genes of the pig, Sus scrofa, reconstructed with EvidentialGene. PeerJ. 2019;7:e6374. doi: 10.7717/peerj.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tarailo‐Graovac, M. & Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinform.25, 4.10.1–4.10.14 (2009). [DOI] [PubMed]
- 102.Keilwagen J, Hartung F, Grau J. GeMoMa: homology-based gene prediction utilizing intron position conservation and RNA-seq data. Methods Mol. Biol. 2019;1962:161–177. doi: 10.1007/978-1-4939-9173-0_9. [DOI] [PubMed] [Google Scholar]
- 103.Haas BJ, et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic acids Res. 2003;31:5654–5666. doi: 10.1093/nar/gkg770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haas BJ, et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008;9:1–22. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lyons E, et al. Finding and comparing syntenic regions among Arabidopsis and the outgroups papaya, poplar, and grape: CoGe with rosids. Plant Physiol. 2008;148:1772–1781. doi: 10.1104/pp.108.124867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joyce BL, et al. FractBias: a graphical tool for assessing fractionation bias following polyploidy. Bioinformatics. 2017;33:552–554. doi: 10.1093/bioinformatics/btw666. [DOI] [PubMed] [Google Scholar]
- 107.Team, R. C. R: A Language and Environment For Statistical Computing (Team, R. C., 2013).
- 108.Wickham H, et al. Welcome to the Tidyverse. J. Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 109.Hadley, W. Ggplot2: Elegrant Graphics for Data Analysis (Springer, 2016).
- 110.Neuwirth, E. RColorBrewer: ColorBrewer Palettes. R package version 1.1-2https://cran.r-project.org/web/packages/RColorBrewer/index.htmlPackage (2014).
- 111.Wilke, C. O. Ridgeline plots in ‘ggplot2’[R Package Ggridges Version 0.5.3] https://cran.r-project.org/web/packages/ggridges/index.html (2021).
- 112.Aphalo, P. J. et al. ggpmisc: Miscellaneous Extensions to “ggplot2”. R package version 0.3.6https://cran.r-project.org/web/packages/ggpmisc/index.html (2020).
- 113.Wilkie P, Poulsen AD, Harris D, Forrest LL. The collection and storage of plant material for DNA extraction: the teabag method. Gardens’ Bull. Singap. 2013;65:4. [Google Scholar]
- 114.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. ArXiv preprint arXiv:1303.3997 (2013).
- 117.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rambaut, A. FigTree v1.4http://tree.bio.ed.ac.uk/software/figtree/ (2012).
- 121.Levy D, Pachter L. The neighbor-net algorithm. Adv. Appl. Math. 2011;47:240–258. doi: 10.1016/j.aam.2010.09.002. [DOI] [Google Scholar]
- 122.Huson D, Huson DH. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics (Oxford, Engl.) 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- 123.Lockhart PJ, Steel MA, Hendy MD, Penny D. Recovering evolutionary trees under a more realistic model of sequence evolution. Mol. Biol. Evol. 1994;11:605–612. doi: 10.1093/oxfordjournals.molbev.a040136. [DOI] [PubMed] [Google Scholar]
- 124.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Toolkit, P. GitHub Repositoryhttp://broadinstitute.github.io/picard (Broad Institute, 2019).
- 126.Neph S, et al. BEDOPS: high-performance genomic feature operations. Bioinformatics. 2012;28:1919–1920. doi: 10.1093/bioinformatics/bts277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonfield, J. K. et al. HTSlib: C library for reading/writing high-throughput sequencing data. Gigascience10, giab007 (2021). [DOI] [PMC free article] [PubMed]
- 128.Danecek P, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 130.Salojärvi J, et al. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2017;49:904–912. doi: 10.1038/ng.3862. [DOI] [PubMed] [Google Scholar]
- 131.Li H, Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493–496. doi: 10.1038/nature10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Popescu A-A, Huber KT, Paradis E. ape 3.0: new tools for distance-based phylogenetics and evolutionary analysis in R. Bioinformatics. 2012;28:1536–1537. doi: 10.1093/bioinformatics/bts184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The genome data generated in this study has been deposited in the NCBI database under accession code PRJNA803434 and BioSample ID SAMN29207412. The Syzygium grande genome assembly and annotation are also available on CoGe [https://genomevolution.org/coge/GenomeInfo.pl?gid=60239]. Processed data generated in this study and used for main text figures are provided in source data files. Additional processed data are available at Dryad [10.5061/dryad.h18931zpw]. Source data are provided with this paper.