Abstract
This study was conducted in the purpose of investigating the effect of Tai Chi on drug craving for women with drug disorders. One hundred and twelve women were recruited from a drug rehabilitation center in China, and 47 and 48 were finally analyzed in the control group and exercise group, respectively. The exercise group underwent a 3-month Tai Chi training, whereas the control group experienced no exercise intervention during the same time period. The drug craving was measured by the visual analog scale. In data analysis, repeated-measures were utilized to test the differences between the control and exercise group over the course of the experiment time. The mean of the craving score significantly dropped from pre-test (control: mean = 5.38, SD = 3.04; exercise: mean = 4.68, SD = 2.93) to post-test (control: mean = 4.03, SD = 2.73; exercise: mean = 1.91, SD = 1.90) in both groups (control group: t = 3.84, df = 46, p < 0.001; exercise group: t = 5.941, df = 47, p < 0.001), with more decrease witnessed in the exercise group. Repeated-measures analysis with a Huynh–Feldt correction showed the significant effect of time (F = 27.383, p < 0.001) as well as the study group by time interaction (F = 3.52, p = 0.024). Tai Chi can ameliorate the drug craving in women and it could be a supportive treatment for drug addiction.
Keywords: Addiction, Craving, Drug use, Exercise, Tai Chi
Drug addiction has become a worldwide problem. According to the World Drug Report (2018), around 275 million individuals, about 5.6% of the worldwide population, have utilized drugs at least once. Drug addiction can lead to a great variety of disorders and around 31 million people are suffering from the disorders (World Drug Report, 2018). Impaired brain function has been found to be one of the main disorders, usually accompanied by defective cognitive function, depression, and drug craving (Lundqvist, 2009; Sayette, 2016; SAMHSA 2020). Moreover, the high relapse rate, the most common trait of drug use, largely hinders the efficacy of drug addiction treatment (World Drug Report, 2018). Therefore, it is important for individuals who have drug dependence to accept effective and safe therapeutic treatment.
As the most common drug addiction treatment, medication, or behavioral techniques only show modest, even poor performance (Kampman & Jarvis 2015; Hadland et al., 2018; Acheson et al., 2022), scholars started to explore novel treatments for drug addiction, with exercise being one of them. Studies have consistently found the benefits of exercise in drug addiction treatment. Lynch et al. (2013) reviewed previous findings and concluded that exercise can not only serve to prevent drug use, but also reduce the relapse rate at the same time. Indeed, exercise is found to play an effective role in ameliorating depression or anxiety and improve cognitive ability in methamphetamine (MA)–dependent individuals (Huang et al., 2020). In fact, some studies reported that aerobic exercise even decreases drug craving in MA and cannabis (Buchowski et al., 2011; Wang et al., 2017). Taken together, previous findings have shown the advantages of exercise in drug addiction treatment.
Tai Chi (Taiji, Tai Chiquan) is a traditional Chinese mind–body health practice. By its definition, exercise benefits both physical and mental health (Hu et al., 2021; Nedeljkovic et al., 2012), with its focused and slow movement helping release muscle tension, as well as regulate and calm emotions. Some studies documented that Tai Chi might be a novel approach to treat addiction. Liu et al. (2019) reported that Tai Chi effectively reduces the level of smartphone addiction. Zhu et al. (2016) indicated that Tai Chi could improve the life quality of MA-type stimulant dependences. Moreover, both Xu et al. (2017) and Lim et al. (2019) found that one’s cognitive function is improved after certain engagement with Tai Chi training, while it is worth pointing out that cognitive function enhancing training is considered an approach for addiction treatment (Garland & Howard, 2018). However, few studies have been conducted so far to look specifically into the effect of Tai Chi on drug craving. Craving inhibition can contribute to lower relapse (Paliwal et al., 2008). Thus, this article is based on data from a randomized controlled trial assessing the effect of Tai Chi on drug craving in long-term drug-dependent women.
Methods
Study Design
This study is a randomized controlled trial, designed to test the effect of a traditional Chinese exercise, Tai Chi, on individuals with drug dependence. There were two groups, the control group and the exercise group (with the intervention of Tai Chi training), both of which accepted traditional addiction treatments, including behavioral counseling, mental health treatment, and long-term follow-up, which are included in the National Institute on Drug Abuse (2019). The specific treatments were psychological counseling, law education, and group mutual help. The exercise group was participating in Tai Chi training. All participants’ drug craving score was designed to be assessed at four time points—baseline, 1 month, 2 months, and 3 months after the baseline. All methods were performed in accordance with relevant guidelines and regulations. Approval of this study was obtained from the Local Ethic Committee of the Institute. The study was registered in the Chinese Clinical Trial Registry (ChiCTR1900026773, date of first registration: 21/10/2019) before participants’ enrollment. This study should have lasted for 6 months, from October 2019 to May 2020. However, only a 3-month-long Tai Chi training could be finished before the outbreak of COVID-19. All participants were informed of the study content and signed written informed consent prior to any assessments. If participants were under 18 years old, the consent was obtained from their parents or legal guardians and themselves.
Participants
The sample size estimation used the power calculation, conducted by G*Power (3.1.9.7). The estimated parameters were α = 0.05, power = 0.8, and size effect = 0.25. The size effect value of chronic aerobic exercise on psychological symptoms was referenced from a meta-analysis conducted by Etnier et al. (1997) and Rosenbaum et al. (2014). The estimated total sample size was 95 and considering the possible dropouts, this study recruited 112 drug-dependent women, from a local compulsory isolation rehabilitation center. The center is a government agency, with professional doctors and medical staff. The inclusion criteria were (1) women who were using illicit drugs for more than 6 months; and (2) they were in a non-acute detoxification period, more than 2 weeks since the last time using drugs, given the acute phase of MA or opioid withdrawal symptoms would last around 7–10 days (McGregor et al., 2005; NIDA 2018). The exclusion criteria were (1) having severe basic diseases; (2) cannot do Tai Chi exercise; (3) cannot finish the 6-month training; (4) less than 16 or more than 60 years old; (5) medical history against exercise training; and (6) have to use prescription medication for addiction treatment, such as methadone or naltrexone, based on the suggestion from medical staff, since managing medication would largely affect the drug craving and this could influence the effectiveness of Tai Chi. Of the 112 volunteers, 101 participants met the criteria and were randomly allocated to the control group and exercise group.
Randomization and Intervention
Participants were randomly assigned to an exercise group or a control group. The random assignments were implemented by an independent researcher who did not know the experimental procedures and the allocation outcomes were delivered in sequentially numbered, opaque, and sealed envelopes. Participants in both groups received behavioral counseling, mental health treatment, and long-term follow-up treatments, including psychological counseling, law and anti-drug education including the influence of drug use on individuals, family, and society, and mutual help in the group. The exercise group received a 3-month Tai Chi training, with two sessions per day and 5 training days per week. In the first week, a counseling class was provided to arouse participants’ interest in Tai Chi, along with a professional Tai Chi instructor coaching on-site and correcting movements during the training. The attendance of training was recorded by a researcher. In this study, Tai Chi was considered a moderate-intensity aerobic exercise, rather than a material art or kung fu. Different from normal exercises, Tai Chi requires participants to keep a peaceful mind state and stay focused during the training. Their mind needs to settle as “still water,” which is similar to a meditation process. The Tai Chi participants played in this study 24 movements and usually spent around 5 min. Participants were repeating the 24 movements for around 30 min per session. During the study, all participants were not able to access to any types of drugs, including prescription medication such as methadone.
Outcome and Measurement
The primary and only outcome of this study was the craving score, assessed by cue-induced drug craving assessment. The measurement was a visual analog scale (VAS) that is used in the psychometric field to measure subjective characteristics or attitudes that cannot be directly scaled (Crichton, 2001). The validation and reliability of VAS in addiction were verified in pain feeling and psychological measurement (Price et al., 1983; Sung & Wu, 2018) and this scale has been consistently used in cue-induced drug craving assessment (Buchowski et al., 2011; Rawson et al., 2015; Wang et al., 2017). Each individual was asked to mark their desire for drugs in a 10-cm-long line that was equally divided into 10 segments and was marked from 0 to 10 when they were shown drug-related pictures. The larger score represents a higher level of craving. For example, the left end (0) means “no craving at all” and the right end (10) means “desperate for a drug.” All participants accepted this assessment in the beginning of this study (baseline) and at the end of each month after the start of the intervention, four times in total. All four-time assessments were conducted by the same researcher, who was blinded to the groups. In addition, questionnaires collected participants’ background information and other potential risk factors including age, education, types of drugs, the age of the first time consuming drugs, the duration of using the drug (years), abstinent time (months), the frequency of using drugs (days per week), smoking, and excessive drinking (more than four times per week).
Statistical Analysis
This study used intention-to-treat analysis and utilized multiple imputations to fill the missing data, caused by dropouts (Ibrahim et al., 2012; Jakobsen et al., 2017). Participants who finished the baseline assessment were taken account into the final data analysis. An independent t-test was utilized to analyze the difference of the baselines between exercise and control groups. The difference of drug craving between groups and within the group at four time points was tested by repeated-measures analysis of variance (ANOVA). A paired t-test was utilized to confirm the difference of craving results from pre- and post-intervention. The confidence interval was 95% in this study and the differences were considered statistically significant when p value was less than 0.05. The results were presented by SPSS 25.
Results
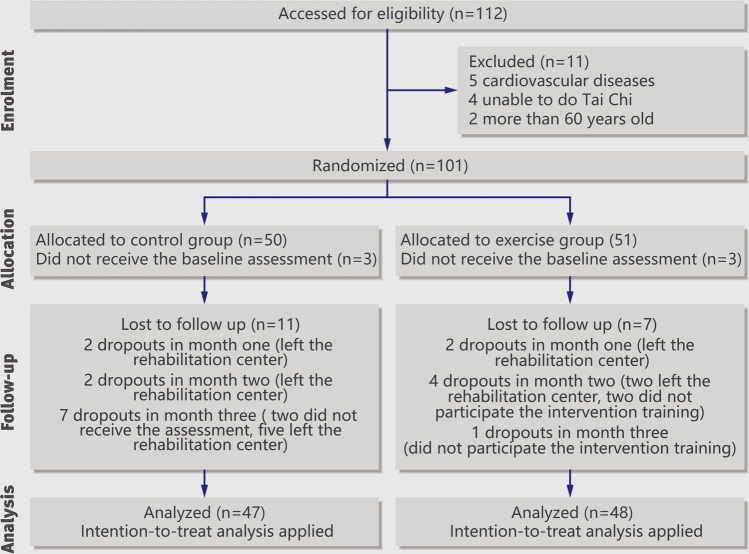
This study finished recruitment at the end of October 2019 and the intervention was from October 2019 to May 2020. There were 101 participants were randomly assigned to the exercise group and control group. Three participants in each group dropped out before receiving the baseline assessment. At the end of this study, there were 36 participants left in the control group, with two dropouts in month 1, two in month 2, and seven in month 3. In the exercise group, 41 participants left, with two dropped out in month 1, four in month 2, and one in month 3. Details of enrollment and dropouts are shown in Fig. 1. Seventy-seven participants finished the 3-month Tai Chi training, 41 in the exercise group and 36 in the control group. According to the intention-to-treat principle, data from 95 participants, aged from 17 to 55, including 18 dropouts, were analyzed, 47 from the control group and 48 from the exercise group. There was no crossover situation in this study. The baseline data and background information are shown in Table 1. Except for the duration of drug use, other indexes did not have a statistically significant difference. Of 95 participants, 69 were MA-dependent, seven being heroin-dependent, two using other illicit drugs, and the rest of them being polydrug-dependent, that is, individuals who were using more than one drug, including heroin, ecstasy, MA, or ketamine.
Fig. 1.
CONSORT flow diagram
Table 1.
Baseline demographic and clinical characteristics of participants
| Indexes | Mean (SD) | |
|---|---|---|
| Control group | Exercise group | |
| Craving scorea (baseline) | 5.38 (3.04) | 4.677 (2.93) |
| Age | 34.04 (8.73) | 32.54 (8.18) |
| Age of the first time consuming drugs (age) | 23.40 (6.72) | 23.23 (5.98) |
| The duration of using drugs (years) | 10.55 (8.42) | 8.39 (5.18)* |
| Abstinent time (months) | 12.62 (6.11) | 9.46 (6.11) |
| Frequency of using drugs (days/week) | 4.63 (3.43) | 4.20 (2.61) |
| Education (years) | 8.84 (2.90) | 9.23 (2.61) |
| Excessive drinkingb | 43% (n = 20) | 33% (n = 16) |
| Smoking | 98% (n = 46) | 90% (n = 43) |
*Statistically significant difference between control group and exercise group
aThe craving score was assessed by visual analog scale
bDrinking more than 5 times per week
SD standard deviation
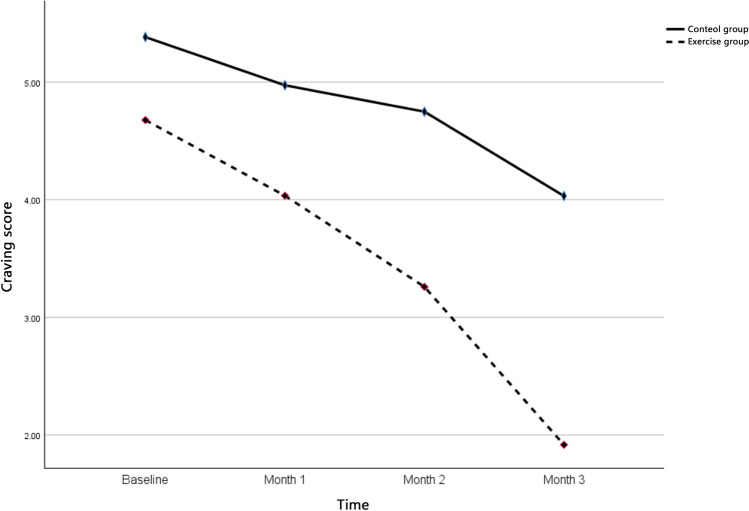
Figure 2 and Table 2 demonstrate the change of craving score in both groups. In the control group, the craving score showed a reducing trend and it decreased from baseline (mean = 5.38, SD = 3.04) to month 1 (mean = 4.97, SD = 2.88) to month 2 (mean = 4.75, SD = 2.95) to month 3 (mean = 4.03, SD = 2.73). In the exercise group, the craving score also had decreasing trend, but sharper. It dropped from baseline (mean = 4.68, SD = 2.93) to month 1 (mean = 4.03, SD = 2.98) to month 2 (mean = 3.25, SD = 2.52) to month 3 (mean = 1.92, SD = 1.91). The paired t-test examined the significant change between pre- and post-intervention in both groups (control group: t = 3.84, df = 46, p < 0.001; exercise group: t = 5.94, df = 47, p < 0.001). As shown in Table 3, the repeated-measures ANOVA with a Huynh–Feldt correction determined that the mean value of drug craving was significantly different between assessment stages (baseline, month 1, month 2, and month 3) (F = 27.383, df = 2.362, p < 0.001). The effects of time-by-group were also significant (F = 3.520, df = 2.362, p = 0.024).
Fig. 2.
The trend of mean craving score in exercise and control groups
Table 2.
Mean (SD) craving scores in exercise and control group across time
| Groups | Baseline Mean (SD) | Month 1, mean (SD) | Month 2, mean (SD) | Month 3, mean (SD) | Paired t-test* | |
|---|---|---|---|---|---|---|
| t (df) | p value | |||||
| Exercise group | 4.68 (2.93) | 4.03 (2.98) | 3.25 (2.52) | 1.92 (1.91) | 5.94 (47) | p < 0.001 |
| Control group | 5.38 (3.05) | 4.97 (2.88) | 4.75 (2.95) | 4.03 (2.73) | 3.84 (46) | p < 0.002 |
SD standard deviation
*Paired t-test compared the craving scores between pre-test (baseline) and post-test (month 3)
Table 3.
Results of repeated-measures ANOVA measuring changes in craving score across intervention study time points
| Repeated-measures ANOVA | Time | Study group × time | ||
|---|---|---|---|---|
| F (df) | p value | F (df) | p value | |
| 27.383 (2.362) | < 0.001 | 3.520 (2.362) | 0.024 | |
ANOVA analysis of variance
Discussion
Main Findings
The aim of this study is to assess the effect of Tai Chi on drug craving in long-term drug-dependent women. A comparison between the exercise group and control group was made and the results show that (1) the mean drug craving score was decreasing in both groups; and (2) repeated-measures and paired t-test showed the significant decrease of craving score across time and significant difference between groups. The results indicate that although the drug craving score in both groups was decreasing, it dropped greater and faster in the exercise group. Previous studies have not researched the effect of Tai Chi on drug craving, but research has been done on normal aerobic exercise. Wang et al. (2017) reported similar results in a study of aerobic exercise applied in MA-dependent individuals. In their 12-week study, the craving score of the exercise group dropped from around 6 to 2 and that of the control group decreased from around 6 to 5. The influence of aerobic exercise on craving was also found in cannabis-dependent adults. After a 2-week aerobic exercise training, the participants’ cannabis craving significantly decreased (Buchowski et al., 2011). Although the participants from these two studies mentioned above and the current study vary in age, sex, race, and the type of drug use, and despite the difference in exercise being involved in the study design, these studies share the common argument that aerobic exercise or Tai Chi can contribute to the reduction of drug craving.
One possible explanation of the beneficial effect of Tai Chi on drug craving would be that the impaired dopaminergic system caused by drug use made recovery during exercises. Drug use can result in the deficits of dopamine receptors and transporters (McCann et al., 2007; Volkow & Morales, 2015). The reduction of dopamine receptors can result in a state of anhedonia and this may cause more use of drugs (Heshmati & Russo, 2015; Leventhal et al., 2010). Exercises would grow the concentration of dopamine and increase the receptors of dopamine (Greenwood et al., 2011; Lynch et al., 2013). In an animal study, aerobic exercise contributed to the increase of dopamine D2 receptors in rats (Robison et al., 2018). Robertson et al. (2015) indicated that increased striatal dopamine D2/D3 receptors in MA dependences are found after structured exercise training. These findings support the idea that exercise may reduce drug craving.
Different from normal aerobic exercise, Tai Chi, the mind–body practice, requires participants to keep a peaceful and focused mind state during the whole process. Thus, Tai Chi is also called moving meditation and its mindfulness and spiritual focus are believed to be beneficial to decrease craving. The mindfulness-based approach can develop an insight that allows substance-dependent individuals to develop an aware reaction rather than an automatic response when they are confronting a desire or craving for the substance (Hjortborg & Ravn, 2020). Tang et al. (2016) reported that mindfulness meditation can improve reduced activity in the anterior cingulate cortex and adjacent prefrontal cortex to prevent and treat addiction. In clinical trials, Vidrine et al. (2016) reported that mindfulness-based addiction treatment might prevent patients from deteriorating from early lapses and subsequently progressing to a full relapse. Two previous studies demonstrated that meditation or mindfulness-based training can reduce drug craving or drug use (Lyons et al., 2019; Witkiewitz et al., 2014).
The spiritual focus, which is trained and enhanced during Tai Chi exercise (Rogers et al., 2008; Zhang et al., 2012), can also contribute to craving reduction. The role of spirituality in addiction treatment has been proved by numerous studies and acknowledgement in some recovery programs (Galanter, 2006). Spiritual power is proved to have a positive influence on alcohol addiction treatment (Tonigan et al., 2000). For example, Alcoholics Anonymous, which has helped millions of individuals, is rooted in spirituality (Warne, 2018). Attending a substance abuse help group is identified as “spiritual power” (McClure & Wilkinson, 2020). Avants et al. (2005) found that Spiritual Self-Schema therapy, a spiritual training practice, effectively decreases heroin and cocaine use. Moreover, the spiritual focus is used to relieve depression and anxiety (Borg et al., 2003; Syed Elias et al., 2019), which are one of the main disorders of drug use in the first place (Kelly & Daley, 2013).
Study Limitations
(1) This study only recruited female volunteers and the findings could not be applied to their opposite gender without an experiment. (2) The measurement VAS is a self-report assessment and it might be affected by subjective bias. Moreover, participants may deny some behaviors or experiences for their situations or some other reasons. Other scales, such as the Addiction Severity Index, Structured Clinical Interview for DSM-IV, or Semi-Structured Assessment for Drug Dependence and Alcoholism, consist of a series of questions. They may give a more comprehensive understanding of the effectiveness of treatment. VAS only has one question focusing on the drug craving and it takes much less testing time, which is more convenient for both participants and researchers. (3) In repeated-measures ANOVA, the variance of different level of drug craving score was not equal, tested by Mauchly’s Test of Sphericity, and as a result, we used Huynh–Feldt correction.
Conclusion
This study showed that Tai Chi has a positive effect on reducing drug craving in drug-dependent women. Based on the findings, Tai Chi can be used for reducing drug craving as a supportive treatment method, working as an effective approach to be combined with traditional techniques in drug addiction treatment. In the future, studies may focus on the mechanism of how exercise affects drug craving and the change of dopamine receptors.
Acknowledgements
The authors wish to thank all volunteers in this study. The authors would like to extend their sincere thanks to Teresa So for English editing service.
Author Contribution
Mu Wang was responsible for the data collection process, data analysis, and paper writing. Yanyan Chen was responsible for the project administration, writing, and reviewing the paper. Xiaoyu Zhang investigated the background information and wrote “Introduction.” Ting sun contributed to the study design and in reviewing the paper. Huazhi Li and Cunfeng Yuan were responsible for the coordination of venue and personnel, especially in cooperating data collection. Jin Lin, Zeng-Hui Ding, and Yubing Xu did the data collection and wrote the “Methods” section. Zuchang Ma and Yining Sun were responsible for the project administration and supervision, as well as writing the conclusion.
Declarations
Ethics Approval
Approval of this study was obtained from the Ethic Committee of the Institute of Intelligent Machines, Chinese Academy of Sciences, Hefei. The study was registered in the Chinese Clinical Trial Registry before participants’ enrollment and the clinical trial register number is ChiCTR1900026773 (date of first registration: 21/10/2019).
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acheson LS, Williams BH, Farrell M, McKetin R, Ezard N, Siefried KJ. Pharmacological treatment for methamphetamine withdrawal: A systematic review and meta-analysis of randomised controlled trials. Drug and Alcohol Review. 2022 doi: 10.1111/dar.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants SK, Beitel M, Margolin A. Making the shift from ‘addict self’ to ‘spiritual self’: Results from a stage I study of spiritual self-schema (3-S) therapy for the treatment of addiction and HIV risk behavior. Mental Health, Religion & Culture. 2005;8(3):167–177. doi: 10.1080/13694670500138924. [DOI] [Google Scholar]
- Borg J, Andrée B, Soderstrom H, Farde L. The serotonin system and spiritual experiences. American Journal of Psychiatry. 2003;160(11):1965–1969. doi: 10.1176/appi.ajp.160.11.1965. [DOI] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, Martin PR. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011;6(3):e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton N. Information point: Visual analogue scale. Journal of Clinical Nursing. 2001;10:697–706. doi: 10.1046/j.1365-2702.2001.00525.x. [DOI] [PubMed] [Google Scholar]
- Etnier JL, Salazar W, Landers DM, Petruzzello SJ, Han M, Nowell P. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. Journal of Sport and Exercise Psychology. 1997;19(3):249–277. doi: 10.1123/jsep.19.3.249. [DOI] [Google Scholar]
- Galanter M. Spirituality and addiction: A research and clinical perspective. American Journal on Addictions. 2006;15(4):286–292. doi: 10.1080/10550490600754325. [DOI] [PubMed] [Google Scholar]
- Garland EL, Howard MO. Mindfulness-based treatment of addiction: Current state of the field and envisioning the next wave of research. Addiction Science & Clinical Practice. 2018;13:14. doi: 10.1186/s13722-018-0115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural Brain Research. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Bagley SM, Rodean J, Silverstein M, Levy S, Larochelle MR, Samet JH, Zima BT. Receipt of timely addiction treatment and association of early medication treatment with retention in care among youths with opioid use disorder. JAMA Pediatrics. 2018;172(11):1029. doi: 10.1001/jamapediatrics.2018.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Current Behavioral Neuroscience Reports. 2015;2(3):146–153. doi: 10.1007/s40473-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortborg SK, Ravn S. Practising bodily attention, cultivating bodily awareness – A phenomenological exploration of tai chi practices. Qualitative Research in Sport, Exercise and Health. 2020;12(5):683–696. doi: 10.1080/2159676X.2019.1662475. [DOI] [Google Scholar]
- Hu Y, Kattan C, Kontos D, Zhu W, Hernandez ME. Benefits of tai ji quan practice on neuromuscular functions in older adults: A systematic review and meta-analysis. Complementary Therapies in Clinical Practice. 2021;42:101295. doi: 10.1016/j.ctcp.2020.101295. [DOI] [PubMed] [Google Scholar]
- Huang, J., Zheng, Y., Gao, D., Hu, M., & Yuan, T. (2020). Effects of exercise on depression, anxiety, cognitive control, craving, physical fitness and quality of life in methamphetamine-dependent patients. Frontiers in Psychiatry, 10. 10.3389/fpsyt.2019.00999 [DOI] [PMC free article] [PubMed]
- Ibrahim JG, Chu H, Chen MH. Missing data in clinical studies: Issues and methods. Journal of Clinical Oncology. 2012;30(26):3297–3303. doi: 10.1200/jco.2011.38.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen, J. C., Gluud, C., Wetterslev, J., & Winkel, P. (2017). When and how should multiple imputation be used for handling missing data in randomised clinical trials – A practical guide with flowcharts. BMC Medical Research Methodology, 17(1). 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed]
- Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. Journal of Addiction Medicine. 2015;9(5):358–367. doi: 10.1097/adm.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Daley DC. Integrated treatment of substance use and psychiatric disorders. Social Work in Public Health. 2013;28(3–4):388–406. doi: 10.1080/19371918.2013.774673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/s2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sun P, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Experimental and Clinical Psychopharmacology. 2010;18(6):562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K, Pysklywec A, Plante M, Demers L. The effectiveness of Tai Chi for short-term cognitive function improvement in the early stages of dementia in the elderly: A systematic literature review</p>. Clinical Interventions in Aging. 2019;14:827–839. doi: 10.2147/cia.s202055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Xiao T, Yang L, Loprinzi PD. Exercise as an alternative approach for treating smartphone addiction: A systematic review and meta-analysis of random controlled trials. International Journal of Environmental Research and Public Health. 2019;16(20):3912. doi: 10.3390/ijerph16203912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist, T. (2009). Imaging cognitive deficits in drug abuse. Behavioral Neuroscience of Drug Addiction, 247–275. 10.1007/7854_2009_26 [DOI] [PubMed]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neuroscience & Biobehavioral Reviews. 2013;37(8):1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons T, Womack VY, Cantrell WD, Kenemore T. Mindfulness-based relapse prevention in a jail drug treatment program. Substance Use & Misuse. 2019;54(1):57–64. doi: 10.1080/10826084.2018.1491054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris SA, Quintanilla D, Taetzsch A, Picard A, Letendre J, Mahler L, Lofgren I, Xu F, Delmonico MJ. The combined effects of Tai Chi, resistance training, and diet on physical function and body composition in obese older women. Journal of Aging Research. 2014;2014:1–8. doi: 10.1155/2014/657851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse (new York, n. y.) 2007;62(2):91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McClure PK, Wilkinson LR. Attending substance abuse groups and identifying as spiritual but not religious. Review of Religious Research. 2020;62(2):197–218. doi: 10.1007/s13644-020-00405-2. [DOI] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA). 2018, June. What are the long-term effects of heroin use? Retrieved from https://nida.nih.gov/publications/research-reports/heroin/what-are-long-term-effects-heroin-use on 2022, August 1.
- Nedeljkovic M, Ausfeld-Hafter B, Streitberger K, Seiler R, Wirtz PH. Taiji practice attenuates psychobiological stress reactivity – A randomized controlled trial in healthy subjects. Psychoneuroendocrinology. 2012;37(8):1171–1180. doi: 10.1016/j.psyneuen.2011.12.007. [DOI] [PubMed] [Google Scholar]
- NIDA. 2019, January 17. Treatment approaches for drug addiction drug facts. Retrieved from https://nida.nih.gov/publications/drugfacts/treatment-approaches-drug-addiction on 2022, July 27.
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: Further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug and Alcohol Dependence. 2008;93(3):252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Chudzynski J, Mooney L, Gonzales R, Ang A, Dickerson D, Cooper CB. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug and Alcohol Dependence. 2015;156:21–28. doi: 10.1016/j.drugalcdep.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA, Cooper CB, Brown AK, Mandelkern MA, London ED. Effect of exercise training on striatal dopamine D2/D3 receptors in methamphetamine users during behavioral treatment. Neuropsychopharmacology. 2015;41(6):1629–1636. doi: 10.1038/npp.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LS, Swenson S, Hamilton J, Thanos PK. Exercise reduces dopamine D1R and increases D2R in rats: Implications for addiction. Medicine & Science in Sports & Exercise. 2018;50(8):1596–1602. doi: 10.1249/mss.0000000000001627. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Larkey LK, Keller C. A review of clinical trials of Tai Chi and qigong in older adults. Western Journal of Nursing Research. 2008;31(2):245–279. doi: 10.1177/0193945908327529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness. The Journal of Clinical Psychiatry. 2014;75(9):964–974. doi: 10.4088/JCP.13r08765. [DOI] [PubMed] [Google Scholar]
- Sayette MA. The role of craving in substance use disorders: Theoretical and methodological issues. Annual Review of Clinical Psychology. 2016;12(1):407–433. doi: 10.1146/annurev-clinpsy-021815-093351. [DOI] [PubMed] [Google Scholar]
- Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): A new technique for psychological measurement. Behav Res. 2018;50:1694–1715. doi: 10.3758/s13428-018-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Retrieved from https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR1PDFW090120.pdf on 2022, August 1.
- Syed Elias SM, Petriwskyj A, Scott T, Neville C. Spiritual reminiscence therapy for older people with loneliness, anxiety and depression living in a residential aged care facility, Malaysia: A qualitative approach. Australasian Journal on Ageing. 2019;38:E25–E30. doi: 10.1111/ajag.12598. [DOI] [PubMed] [Google Scholar]
- Tang YY, Tang R, Posner MI. Mindfulness meditation improves emotion regulation and reduces drug abuse. Drug and Alcohol Dependence. 2016;163:S13–S18. doi: 10.1016/j.drugalcdep.2015.11.041. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Miller WR, Connors GJ. Project MATCH client impressions about alcoholics anonymous. Alcoholism Treatment Quarterly. 2000;18(1):25–41. doi: 10.1300/j020v18n01_02. [DOI] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, Waters AJ, Li Y, Nguyen NTT, Cao Y, Tindle HA, Fine M, Safranek LV, Wetter DW. Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2016;84(9):824–838. doi: 10.1037/ccp0000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Morales M. The brain on drugs: From reward to addiction. Cell. 2015;162(4):712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhu T, Zhou C, Chang YK. Aerobic exercise training ameliorates craving and inhibitory control in methamphetamine dependencies: A randomized controlled trial and event-related potential study. Psychology of Sport and Exercise. 2017;30:82–90. doi: 10.1016/j.psychsport.2017.02.001. [DOI] [Google Scholar]
- Warne D. Alcoholism and substance abuse. Integrative Medicine. 2018;818–828:e2. doi: 10.1016/b978-0-323-35868-2.00083-9. [DOI] [Google Scholar]
- Witkiewitz K, Warner K, Sully B, Barricks A, Stauffer C, Thompson BL, Luoma JB. Randomized trial comparing mindfulness-based relapse prevention with relapse prevention for women offenders at a residential addiction treatment center. Substance Use & Misuse. 2014;49(5):536–546. doi: 10.3109/10826084.2013.856922. [DOI] [PubMed] [Google Scholar]
- World Drug Report 2018. (2018). Executive summary conclusions and policy implications. United Nations Office on Drugs and Crime. https://www.unodc.org/wdr2018/en/topics.html (Accessed 9 April 2022)
- World Drug Report 2018. (2018). Drugs and age - Drugs and associated issues among young people and older people. United Nations Office on Drugs and Crime. https://www.unodc.org/wdr2018/en/topics.html (Accessed 9 April 2022)
- Zhang L, Layne C, Lowder T, Liu J. A review focused on the psychological effectiveness of Tai Chi on different populations. Evidence-Based Complementary and Alternative Medicine. 2012;2012:1–9. doi: 10.1155/2012/678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Xu D, Dai G, Wang F, Xu X, Zhou D. Beneficial effects of Tai Chi for amphetamine-type stimulant dependence: A pilot study. The American Journal of Drug and Alcohol Abuse. 2016;42(4):469–478. doi: 10.3109/00952990.2016.1153646. [DOI] [PubMed] [Google Scholar]