Abstract
Background
Evidence is limited on inflammation-related dietary patterns and mortality in ovarian cancer survivors.
Methods
We examined the associations between pre- and post-diagnosis dietary patterns, including change in diet from before to after diagnosis, and mortality among 1003 ovarian cancer survivors in two prospective cohort studies. Dietary pattern scores for empirical dietary inflammatory pattern (EDIP) and Alternative Healthy Eating Index (AHEI) were calculated based on food frequency questionnaires. We used Cox proportional hazard models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for ovarian cancer-specific and all-cause mortality.
Results
Pre-diagnosis EDIP score and AHEI were not associated with mortality. Among non-high grade serous cases, a higher post-diagnosis EDIP score was associated with increased risk of all-cause mortality (HR5th vs 1st quintile = 1.95, 95% CI = 1.04–3.67, p-trend = 0.06). Compared to survivors consuming a low EDIP score diet before and after diagnosis, high post-diagnosis EDIP was associated with increased risk of ovarian cancer specific mortality (pre-to-post diagnosis low/high, HR = 1.38, 95% CI = 0.99–1.92; high/high HR = 1.58, 95% CI = 1.09–2.30) and all-cause mortality (low/high HR = 1.44, 95% CI = 1.06–1.95; high/high HR = 1.55, 95% CI = 1.10–2.19).
Conclusion
Consuming a more inflammatory dietary pattern post-diagnosis was associated with increased mortality in ovarian cancer survivors, suggesting limiting the inflammatory potential of diet post-diagnosis could lead to enhanced survivorship.
Subject terms: Ovarian cancer, Epidemiology
Introduction
Ovarian cancer is the second most common cause of gynecologic malignancy death worldwide [1, 2]. While modest improvement has been observed in ovarian cancer survival in the past decades [3], the 5-year survival is still low with 49% even in high-resource countries such as the U.S. [4]. Thus, there is a pressing need to identify modifiable factors, especially those that can be implemented to improve survival following an ovarian cancer diagnosis.
Chronic inflammation plays an important role in ovarian cancer carcinogenesis and progression [5–7]. Greater systemic inflammation has been associated with worse prognosis among ovarian cancer patients [8–11]. Dietary factors can influence systemic inflammation [12]. However, evidence on whether pre- and post-diagnosis dietary patterns impact survivorship among women with ovarian cancer is limited. Four studies have examined the association between pre-diagnosis dietary patterns and survival among ovarian cancer patients [13–16] with inconsistent results, and only one study examined changes in pre- and post-diagnosis dietary patterns, reporting null associations [16]. Here, we examined the association between pre- and post-diagnosis dietary patterns, specifically those that are related to higher systemic inflammation [17, 18], and survival among women with ovarian cancer using data from two large U.S. based prospective cohorts.
Materials And Methods
Study population
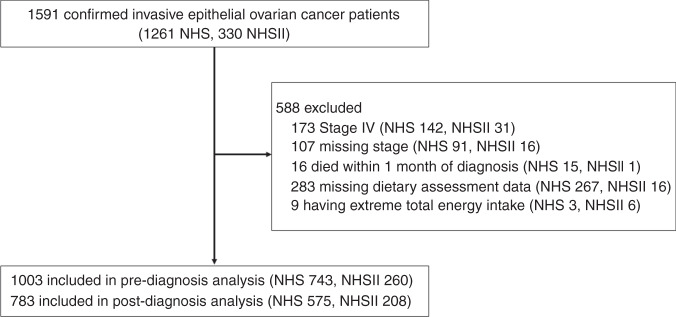
The Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII) are U.S. based, ongoing prospective cohort studies established in 1976 and 1989 respectively. NHS enrolled 121,700 female registered nurses aged 30–55 years and NHSII enrolled 116,429 female registered nurses aged 25–42 years. Details of the study designs have been previously described [19, 20]. In brief, participants completed questionnaires reporting detailed information on lifestyle, reproductive, and health-related information at enrollment and provided updated information on exposures and incident diseases every two years thereafter. The response rates have been 85–90% at each cycle. For dietary assessment, self-administered, semi-quantitative food frequency questionnaires (FFQ), which have been validated and shown to demonstrate good correlations with food records, were used [21]. In NHS, diet was assessed in 1984, 1986, and every four years thereafter. In NHSII, diet was first assessed in 1991 and every four years thereafter. We identified 1591 confirmed epithelial ovarian cancer cases (1261 from NHS 1976–2016, 330 from NHSII 1989–2017; Fig. 1). Of these cases, we excluded stage IV patients (n = 173) or those missing stage (n = 107) since this could influence post-diagnosis questionnaire return, patients who died within one month from diagnosis (n = 16), those completely missing dietary assessment data during follow-up (n = 283), and those who had implausible values for total energy intake (<500 or >3500 kcal per day; n = 9). There were 1,003 confirmed epithelial ovarian cancer cases included in the pre-diagnosis diet analysis (743 from NHS, 260 from NHSII) and783 cases included in the post-diagnosis diet analysis with dietary assessment 1–4 years after diagnosis (575 from NHS and 208 from NHSII), and 710 cases in the change in diet analysis with both pre- and post-diagnosis dietary assessments (510 from NHS and 200 from NHSII). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Completion of the questionnaire implied informed consent.
Fig. 1. Flow diagram describing the exclusions in the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII).
Extreme total energy intake is defined as those who had implausible values for total energy intake (<500 or >3500 kcal per day).
Ascertainment of ovarian cancer cases and death
New ovarian cancer cases were first self-reported by biennial questionnaires and subsequently confirmed by either medical record review by a gynecological pathologist or by linkage with the relevant cancer registry. Detailed information on disease stage, histological subtype, grade, and invasiveness were abstracted by the gynecological pathologist blinded to exposure data.
Deaths were identified by family members, the National Death Index [22], or the U.S. Postal Service and cause of death was determined by expert review of death certificates, medical records, and information from the patients’ next of kin. Ovarian cancer specific mortality (ICD version 8 codes 1830, 1831, and 1580) and all-cause mortality were examined as outcomes.
Assessment of dietary patterns and other covariates
In the current study, we examined two dietary scores: the empirical dietary inflammatory pattern (EDIP) score and the Alternate Healthy Eating Index 2010 (AHEI) as these scores have previously been related to systemic inflammation(Supplementary Table 1) [17, 18]. EDIP score is a weighted sum of 18 food groups that are predictive of circulating inflammatory biomarkers, with higher scores indicating a more pro-inflammatory diet [17, 23]. The AHEI is calculated based on 11 dietary components that are associated with reduced risk of chronic diseases [24]. A higher AHEI score indicates a dietary pattern more compliant to healthy eating, or a more anti-inflammatory diet. For pre-diagnosis diet, we calculated the cumulative average of EDIP and AHEI scores from all prior dietary assessments up to one questionnaire (i.e., 2–4 years) to ovarian cancer diagnosis, representing long-term dietary intake [25]. As sensitivity analysis, we used EDIP and AHEI scores calculated using diet assessed one cycle prior to diagnosis (recent diet), which is most comparable to diet assessed in case-control studies. For post-diagnosis diet, we calculated EDIP and AHEI scores using diet assessed one to four years after diagnosis to avoid dietary assessment during active treatment. Information on body mass index (BMI, kg/m2), smoking status, and nonsteroidal anti-inflammatory drug (NSAID) use were obtained from the biennial questionnaires. These covariates were defined from the same questionnaire relevant to the dietary pattern assessment (i.e. 2–4 years before diagnosis for pre-diagnosis, 1–4 years after diagnosis for post-diagnosis).
Statistical analysis
To examine the association between pre- and post-diagnosis dietary patterns and mortality, we used Cox proportional hazard models to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) of all-cause or ovarian cancer-specific death adjusting for age at diagnosis (continuous), calendar year at diagnosis (continuous), histology (high-grade serous/poorly differentiated or carcinosarcoma, low-grade serous, or non-serous [i.e. mucinous, endometrioid, clear cell, transitional/Brenner, or mixed subtypes], or unknown/ other histology), stage (I, II, III), smoking status (never, ever), BMI (<25, 25–29.9, ≥30 kg/m2), total energy intake (continuous), and cohort (NHS, NHSII). Post-diagnosis models were additionally adjusted for NSAID use, which included aspirin and non-aspirin NSAID use (current user, noncurrent user, and unknown) since post-diagnosis NSAID use has been associated with ovarian cancer survival [26]. As sensitivity analysis, we included those with stage 4 or unknown stage cases. The dietary pattern scores were categorized into quintiles and linear trend across quintiles was evaluated using an ordinal variable. We tested the proportional hazards assumption by calculating multiplicative interaction terms between dietary patterns and the analytic time scale and comparing models with and without the interaction terms using the likelihood ratio test. We used random-effects meta-analysis to test for heterogeneity by cohort. Since there was little evidence of significant heterogeneity by cohort, we pooled data from NHS and NHSII for all analyses (Supplementary Table 2, Supplementary Table 3). We conducted stratified analyses by histotype (i.e. high-grade serous/poorly differentiated or carcinosarcoma histology vs low-grade serous or non-serous histology). Since pre-diagnostic diet may have a different impact on survival by smoking status [14], we also conducted analyses stratified by smoking status. We tested for potential heterogeneity in associations by using the likelihood ratio test and compared models with and without interaction terms.
To examine how change in dietary pattern before and after ovarian cancer diagnosis impacted mortality, we dichotomized pre- and post-diagnosis EDIP score and AHEI at the median, with low indicating a score below the median and high indicating a score greater than or equal to the median. We created the following cross-classified change variable: Low–Low (reference), representing ovarian cancer patients who persistently consumed low EDIP score diet (less inflammatory diet) or low AHEI diet (less healthy diet) from pre-to post-diagnosis period (i.e. both scores below the median); Low-High, representing patients who consumed low EDIP score diet or low AHEI diet before diagnosis but changed towards consuming a higher EDIP score diet or higher AHEI diet after diagnosis; High–Low, representing patients who consumed high EDIP score diet or high AHEI diet before diagnosis but changed towards consuming a lower EDIP score diet or lower AHEI diet after diagnosis; High–High, representing patients who persistently consumed high EDIP score diet or high AHEI diet from the pre- to post-diagnosis period. We then used Cox proportional hazard models to examine the association between change in dietary patterns and risk of overall and ovarian cancer-specific death. All statistical analyses were conducted using SAS statistical software version 9.4 (SAS Institute Inc., Cary, North Carolina). All tests were two-sided with p-value < 0.05 considered as statistically significant.
Results
Among the 1003 ovarian cancer patients included in the pre-diagnosis diet analysis, we documented 695 total deaths (586 in NHS and 109 in NHSII) and 606 ovarian cancer-specific deaths (507 in NHS and 99 in NHSII) with median survival time of 3.7 years (IQR: 1.6–7.7) in NHS and 6.0 years (IQR: 3.1–11.5) in NHSII. Among the 783 cases included in the post-diagnosis diet analysis, we documented 496 total deaths (408 in NHS and 88 in NHSII) and 402 ovarian cancer-specific deaths (324 in NHS and 78 in NHSII) with median survival time of 5.8 years (IQR: 3.5–12.0) in NHS and 7.6 years (IQR: 5.2–13.5) in NHSII.
Baseline clinical characteristics by extreme quintiles of EDIP score and AHEI were similar at both pre- and post-diagnosis assessment (Table 1). Median age at diagnosis was 65 years in the pre-diagnosis analysis and 62 years in the post-diagnosis analysis, with most cases being stage III and high-grade serous or poorly differentiated histology. Ovarian cancer survivors consuming diet with the highest EDIP score (quintile 5), or more pro-inflammatory diet, before and after diagnosis tended to have greater BMI and were more likely to report never smoking. We observed low correlation between EDIP score and AHEI (Spearman correlation = −0.27).
Table 1.
Ovarian cancer patient characteristics by extreme quintiles of pre- and post-diagnosis empirical dietary inflammatory pattern (EDIP) score and Alternative Healthy Eating Index (AHEI), NHS and NHSII.
| EDIP score | AHEI | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-diagnosis questionnaire | Post-diagnosis questionnaire | Pre-diagnosis questionnaire | Post-diagnosis questionnaire | |||||
| Quintile 1 n = 200 | Quintile 5 n = 200 | Quintile 1 n = 156 | Quintile 5 n = 156 | Quintile 1 n = 200 | Quintile 5 n = 200 | Quintile 1 n = 139 | Quintile 5 n = 139 | |
| Diet score, median | −0.36 | 0.34 | −0.41 | 0.36 | 39.7 | 65.6 | 40.4 | 71.8 |
| Deaths from ovarian cancer, n (%) | 128 (64) | 109 (55) | 74 (47) | 82 (53) | 122 (61) | 120 (60) | 72 (52) | 64 (46) |
| Deaths from all causes, n (%) | 142 (71) | 123 (62) | 91 (58) | 101 (65) | 134 (67) | 141 (71) | 87 (63) | 78 (56) |
| Age at diagnosis, years, median (IQR) | 66 (56–73) | 61 (52–70) | 60 (52–68) | 59 (50–67) | 61 (52–68) | 66 (57–74) | 60 (51–68) | 61 (52–69) |
| Calendar year of diagnosis, years, median (IQR) | 2002 (1995–2007) | 2002 (1996–2008) | 2001 (1995–2006) | 1999 (1994–2006) | 2001 (1995–2007) | 2004 (1998–2008) | 1997 (1992–2003) | 2005 (1999–2009) |
| Overall survival time, years, median (IQR) | 3.7 (1.7–8.5) | 5.6 (2.1–11.7) | 6.8 (3.7–14.9) | 7.0 (3.9–14.3) | 4.5 (1.8−8.2) | 3.7 (1.7–9.0) | 7.3 (4.2–16.3) | 6.2 (4.3–11.9) |
| Stage, n (%) | ||||||||
| I | 41 (21) | 53 (27) | 43 (28) | 45 (29) | 46 (23) | 48 (24) | 38 (27) | 41 (30) |
| II | 15 (8) | 21 (11) | 12 (8) | 18 (12) | 26 (13) | 15 (8) | 17 (12) | 12 (9) |
| III | 144 (72) | 126 (63) | 101 (65) | 93 (60) | 128 (64) | 137 (69) | 84 (60) | 86 (62) |
| Histotype, n (%) | ||||||||
| High-grade serous or poorly differentiated | 140 (70) | 108 (54) | 101 (65) | 88 (56) | 115 (58) | 129 (65) | 91 (65) | 91 (65) |
| Mucinous | 6 (3) | 6 (3) | 3 (2) | 5 (3) | 11 (6) | 4 (2) | 4 (3) | 2 (1) |
| Endometrioid | 21 (11) | 40 (20) | 23 (15) | 35 (22) | 38 (19) | 25 (13) | 29 (21) | 18 (13) |
| Clear cell | 14 (7) | 13 (7) | 13 (8) | 9 (6) | 12 (6) | 16 (8) | 7 (5) | 14 (10) |
| Other or unknown | 19 (10) | 33 (17) | 16 (10) | 19 (12) | 24 (12) | 26 (13) | 8 (6) | 14 (10) |
| Body mass index, kg/m2, median (IQR) | 24.0 (22.3–26.9) | 28.3 (23.6–33.1) | 24.0 (21.8–27.1) | 27.4 (23.4–31.9) | 27.0 (22.9–33.0) | 24.2 (22.2–28.2) | 25.8 (22.3–30.7) | 24.4 (21.5–28.2) |
| Ever smoker, n (%) | 123 (62) | 81 (41) | 89 (57) | 79 (51) | 89 (45) | 108 (54) | 73 (53) | 71 (51) |
| Current NSAIDs use, n (%) | 117 (59) | 111 (56) | 81 (52) | 79 (51) | 117 (59) | 116 (58) | 72 (52) | 80 (58) |
IQR interquartile range, NHS Nurses’ Health Study; NHSII, Nurses’ Health Study II, NSAID non-steroidal anti-inflammatory drug.
Neither pre-diagnosis EDIP score nor AHEI were significantly associated with ovarian cancer-specific mortality or all-cause mortality among women with ovarian cancer when all cases were combined (Table 2). We observed similar results when examining pre-diagnosis dietary patterns assessed one cycle prior to diagnosis (Supplementary Table 4) or included ovarian cancer cases with stage 4 or unknown stage (Supplementary Table 5). Similarly, post-diagnosis AHEI were not significantly associated with all-cause or ovarian cancer-specific mortality among women with ovarian cancer in primary or sensitivity analyses. However, higher post-diagnosis EDIP score was suggestively associated with increased risk of all-cause (HR5th vs 1st quintile = 1.21, 95% CI = 0.91–1.62, p-trend = 0.12) and ovarian cancer specific mortality (HR5th vs 1st quintile = 1.24, 95% CI = 0.90–1.71, p-trend = 0.11). To assess a potential threshold effect, we collapsed quintiles 3 to 5 and compared to quintile 1 and observed a suggestive but not statistically significant positive associations (all-cause mortality: HR = 1.22, 95% CI = 0.96–1.56; ovarian cancer specific mortality: HR = 1.23, 95% CI = 0.94–1.60).
Table 2.
Association between pre-diagnosis and post-diagnosis dietary scores and mortality among women with ovarian cancer, NHS and NHSII.
| Ovarian cancer-specific mortality | All-cause mortality | ||||
|---|---|---|---|---|---|
| Total cases, n | Deaths, n | HR (95% CI) | Deaths, n (%) | HR (95% CI) | |
| Pre-diagnosis EDIP score | |||||
| Quintile 1 | 200 | 128 | ref | 142 | ref |
| Quintile 2 | 201 | 125 | 0.93 (0.72–1.19) | 145 | 0.97 (0.77–1.23) |
| Quintile 3 | 201 | 123 | 0.91 (0.71–1.17) | 141 | 0.95 (0.75–1.21) |
| Quintile 4 | 201 | 121 | 0.95 (0.74–1.23) | 144 | 1.04 (0.82–1.33) |
| Quintile 5 | 200 | 109 | 0.85 (0.65–1.11) | 123 | 0.84 (0.65–1.09) |
| p-trend | – | – | 0.32 | – | 0.38 |
| Post-diagnosis EDIP score | |||||
| Quintile 1 | 156 | 74 | ref | 91 | ref |
| Quintile 2 | 157 | 80 | 1.05 (0.76–1.45) | 100 | 1.09 (0.81–1.45) |
| Quintile 3 | 157 | 85 | 1.30 (0.95–1.79) | 100 | 1.26 (0.94–1.69) |
| Quintile 4 | 157 | 81 | 1.22 (0.88–1.69) | 104 | 1.25 (0.93–1.67) |
| Quintile 5 | 156 | 82 | 1.24 (0.90–1.71) | 101 | 1.21 (0.91–1.62) |
| p-trend | – | – | 0.11 | – | 0.12 |
| Pre-diagnosis AHEI | |||||
| Quintile 1 | 200 | 122 | ref | 134 | ref |
| Quintile 2 | 201 | 125 | 0.79 (0.61–1.02) | 143 | 0.81 (0.63–1.03) |
| Quintile 3 | 201 | 123 | 0.87 (0.67–1.12) | 140 | 0.89 (0.70–1.14) |
| Quintile 4 | 201 | 116 | 0.73 (0.56–0.94) | 137 | 0.77 (0.61–0.99) |
| Quintile 5 | 200 | 120 | 0.95 (0.73–1.22) | 141 | 0.99 (0.77–1.26) |
| p-trend | – | – | 0.52 | – | 0.86 |
| Post-diagnosis AHEI | |||||
| Quintile 1 | 139 | 72 | ref | 87 | ref |
| Quintile 2 | 136 | 66 | 1.02 (0.73–1.43) | 86 | 1.14 (0.84–1.55) |
| Quintile 3 | 139 | 74 | 1.20 (0.86–1.66) | 89 | 1.30 (0.96–1.76) |
| Quintile 4 | 139 | 70 | 1.05 (0.75–1.48) | 88 | 1.16 (0.86–1.58) |
| Quintile 5 | 139 | 64 | 1.12 (0.79–1.60) | 78 | 1.26 (0.92–1.74) |
| p-trend | – | – | 0.50 | – | 0.17 |
Models adjusted for age at diagnosis, calendar year at diagnosis, histology, stage, smoking status, body mass index (<25, 25–29, 30+), and total energy intake. Post-diagnosis model additionally adjusted for nonsteroidal anti-inflammatory drug (NSAID) use, which included aspirin and non-aspirin NSAID use.
Pre-diagnosis diet is the cumulative average of the dietary scores up to 1 cycle prior to diagnosis. Post-diagnosis diet was assessed 1 to 4 years after diagnosis.
Post-diagnosis AHEI analyses included 692 ovarian cancer cases.
AHEI alternative healthy eating index, CI confidence interval, EDIP empirical dietary inflammatory pattern score, HR hazard ratio, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II.
When we stratified by histotype, among the non-high grade serous cases, a higher post-diagnosis EDIP score (indicating a more pro-inflammatory diet) was associated with increased risk of all-cause mortality (HR5th vs 1st quintile = 1.95, 95% CI = 1.04–3.67, p-trend = 0.06, p-het vs. high grade serous/poorly differentiated histology = 0.33) but was not statistically significant for ovarian cancer-specific mortality (HR5th vs 1st quintile = 1.77, 95% CI = 0.79–3.96, p-trend = 0.24, p-het = 0.97; Table 3) or for the pre-diagnosis EDIP score. Pre- and post-diagnosis AHEI were not associated with mortality when stratified by histotype (Supplementary Table 6). When we included ovarian cancer cases with stage 4 or unknown stage we observed similar results among the non-high grade serous cases with a higher post-diagnosis EDIP score being associated with increased risk of all-cause mortality (HR5th vs 1st quintile = 2.04, 95% CI = 1.10–3.79, p-trend = 0.03) but no significant association for ovarian cancer specific mortality (HR5th vs 1st quintile = 1.80, 95% CI = 0.83–3.89, p-trend = 0.19; Supplementary Table 7). When we stratified by smoking status, we did not observe significant differences in HRs for mortality between never and ever smokers for pre-diagnosis EDIP score or AHEI (data not shown). For post-diagnosis diet, we observed significant differences in all-cause mortality by smoking status (p-het = 0.03) with positive associations between post-diagnosis EDIP score and all-cause mortality risk observed among ever smokers (Supplementary Table 8).
Table 3.
Association between pre-diagnosis and post-diagnosis EDIP score and mortality among women with ovarian cancer by histotype, NHS and NHSII.
| Ovarian cancer-specific mortality | All-cause mortality | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-grade serous or poorly differentiated histology | Non-serous or low-grade serous histology | High-grade serous or poorly differentiated histology | Non-serous or low-grade serous histology | |||||||||||
| Total cases, n | Deaths, n | HR (95% CI) | Total cases, n | Deaths, n | HR (95% CI) | p-het | Total cases, n | Deaths, n | HR (95% CI) | Total cases, n | Deaths, n | HR (95% CI) | p-het | |
| Pre-diagnosis EDIP score | ||||||||||||||
| Quintile 1 | 140 | 102 | ref | 57 | 23 | ref | 0.40 | 140 | 110 | ref | 57 | 29 | ref | 0.14 |
| Quintile 2 | 145 | 102 | 0.83 (0.63–1.10) | 53 | 21 | 1.20 (0.64–2.24) | 145 | 114 | 0.84 (0.64–1.09) | 53 | 28 | 1.45 (0.84–2.51) | ||
| Quintile 3 | 134 | 97 | 0.88 (0.66–1.16) | 61 | 21 | 0.87 (0.47–1.59) | 134 | 107 | 0.89 (0.68–1.16) | 61 | 29 | 1.03 (0.61–1.75) | ||
| Quintile 4 | 137 | 93 | 0.91 (0.68–1.21) | 54 | 20 | 1.25 (0.67–2.34) | 137 | 104 | 0.93 (0.70–1.23) | 54 | 32 | 1.53 (0.90–2.60) | ||
| Quintile 5 | 108 | 70 | 0.78 (0.57–1.07) | 85 | 34 | 1.17 (0.65–2.11) | 108 | 77 | 0.77 (0.57–1.04) | 85 | 41 | 1.11 (0.65–1.90) | ||
| p-trend | – | – | 0.26 | – | – | 0.64 | – | – | – | 0.24 | – | – | 0.67 | – |
| Post-diagnosis EDIP score | ||||||||||||||
| Quintile 1 | 101 | 59 | ref | 53 | 13 | ref | 0.97 | 101 | 70 | ref | 53 | 19 | ref | 0.33 |
| Quintile 2 | 105 | 67 | 1.03 (0.71–1.47) | 47 | 10 | 1.42 (0.59–3.40) | 105 | 77 | 0.95 (0.68–1.33) | 47 | 19 | 2.20 (1.13–4.31) | ||
| Quintile 3 | 110 | 67 | 1.29 (0.90–1.85) | 42 | 15 | 1.59 (0.74–3.44) | 110 | 74 | 1.17 (0.83–1.63) | 42 | 23 | 1.91(1.02–3.55) | ||
| Quintile 4 | 103 | 69 | 1.22 (0.85–1.75) | 52 | 11 | 1.25 (0.53–2.97) | 103 | 78 | 1.12 (0.80–1.57) | 52 | 25 | 2.05 (1.07–3.90) | ||
| Quintile 5 | 88 | 61 | 1.23 (0.85–1.76) | 65 | 19 | 1.77 (0.79–3.96) | 88 | 68 | 1.15 (0.82–1.61) | 65 | 31 | 1.95 (1.04–3.67) | ||
| p-trend | – | – | 0.15 | – | – | 0.24 | – | – | – | 0.24 | – | – | 0.06 | – |
Non-serous histology subtype refers to mucinous, endometrioid, clear cell, transitional/Brenner, or mixed subtypes of ovarian cancer.
Pre-diagnosis diet is the cumulative average of the dietary scores up to 1 cycle prior to diagnosis. Post-diagnosis diet was assessed 1–4 years after diagnosis.
Models adjusted for age at diagnosis, calendar year at diagnosis, histology, stage, smoking status, body mass index (<25, 25–29, 30 + ), total energy intake. Post-diagnosis model additionally adjusted for nonsteroidal anti-inflammatory drug (NSAID) use, which include aspirin and non-aspirin NSAID use.
AHEI alternative healthy eating index, CI confidence interval, EDIP empirical dietary inflammatory pattern score, HR hazard ratio, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, p-het p-heterogeneity.
Next, we examined the change in dietary patterns before and after diagnosis and mortality among ovarian cancer patients (Table 4). Compared to those who had a persistently low score on the EDIP (consistently consumed a low inflammatory diet), those who persistently scored high on the EDIP (consistently consumed a high inflammatory diet) had increased risk of ovarian cancer specific (HRhigh/high vs low/low = 1.58, 95% CI = 1.09–2.30) and all-cause mortality (HRhigh/high vs low/low = 1.55, 95% CI = 1.10–2.19). Ovarian cancer survivors who changed from a low EDIP score pre-diagnosis to high post-diagnosis had a suggestive increased risk of ovarian cancer specific (HRlow/high vs low/low = 1.38, 95% CI = 0.99–1.92) and increased risk of all-cause death (HRlow/high vs low/low = 1.44, 95% CI = 1.06–1.95) compared to those who consistently consumed an anti-inflammatory diet; decreasing EDIP score from pre- to post-diagnosis was not associated with mortality. We did not observe associations between change in AHEI from before to after diagnosis and mortality among ovarian cancer survivors.
Table 4.
Change in dietary patterns from pre-diagnosis to post-diagnosis and mortality among women with ovarian cancer, NHS and NHSII.
| Ovarian cancer specific mortality | All-cause mortality | ||||
|---|---|---|---|---|---|
| Total cases, n | Deaths, n | HR (95% CI) | Deaths, n | HR (95% CI) | |
| EDIP score | |||||
| Low–Low | 257 | 132 | ref | 157 | ref |
| Low–High | 98 | 54 | 1.38 (0.99–1.92) | 66 | 1.44 (1.06–1.95) |
| High–Low | 98 | 45 | 1.07 (0.71–1.61) | 57 | 1.16 (0.79–1.68) |
| High–High | 257 | 138 | 1.58 (1.09–2.30) | 166 | 1.55 (1.10–2.19) |
| AHEI | |||||
| Low–Low | 229 | 117 | ref | 142 | ref |
| Low–High | 81 | 37 | 1.04 (0.71–1.53) | 41 | 0.97 (0.68–1.39) |
| High–Low | 81 | 43 | 0.92 (0.60–1.43) | 52 | 0.89 (0.60–1.32) |
| High–High | 230 | 116 | 1.10 (0.71–1.70) | 143 | 1.08 (0.73–1.60) |
EDIP score and AHEI were dichotomized at the median. Low–Low, the reference category, represents participants who persistently consumed low EDIP score diet or AHEI (below the median) from pre- to post-diagnosis period.
Models were adjusted for age at diagnosis, calendar year at diagnosis, histology, stage, smoking status, body mass index (<25, 25–29, 30+), total energy intake, nonsteroidal anti-inflammatory drug (NSAID) use, pre-diagnosis cumulative average EDIP score or AHEI.
AHEI alternative healthy eating index, CI confidence interval, EDIP empirical dietary inflammatory pattern score, HR hazard ratio, NHS Nurses’ Health Study, NHSII Nurses’ Health Study II, p-het p-heterogeneity.
Discussion
In our prospective cohort with more than 1000 women diagnosed with ovarian cancer, pre-diagnosis inflammatory dietary patterns were not associated with mortality after diagnosis. However, we observed that consuming a more pro-inflammatory diet post-diagnosis was associated with a suggestive increase in mortality among ovarian cancer survivors overall and among those with non-serous tumors. Furthermore, when examining change in dietary patterns before and after ovarian cancer diagnosis, consuming a more pro-inflammatory diet post-diagnosis was associated with increased risk of both ovarian cancer-specific and all-cause death compared to those who persistently consumed a less inflammatory diet before and after diagnosis.
Inflammation-related dietary patterns have been reported to be associated with an increased risk of all-cause mortality and worse prognosis in multiple cancer types [27–32]. However, in ovarian cancer, only two case-control studies have examined the association between pre-diagnosis pro-inflammatory dietary patterns (assessed using the dietary inflammatory index) and mortality and both reported null associations overall [14, 15], which is in line with our findings. One prospective cohort study and one case-control study examined the association between pre-diagnosis high-quality diet (assessed using HEI or AHEI) and mortality among ovarian cancer survivors with conflicting results; the case-control study reported a null association (HR3rd vs 1st tertile = 1.12, 95% CI = 0.84–1.51) [16] and the prospective cohort study reported an inverse association with all-cause mortality (HR3rd vs 1st tertile = 0.73, 95% CI = 0.55–0.97) [13]. Three other studies examining individual dietary components reported higher intake of vegetables pre-diagnosis was associated with lower risk of all-cause death [33–35]. All studies except one were case-control studies in which recall bias may have impacted their pre-diagnosis dietary exposure assessment. Our study differed from prior studies as we had repeated measurement of diet assessed prospectively both before and after diagnosis and used cumulative average of pre-diagnosis diet as the primary exposure which is more likely to reflect the long-term average diet and less prone to measurement error. We also used an empirical diet score that directly predicts systemic inflammation in healthy individuals [17].
To our knowledge, this is the first study to report that consuming a more pro-inflammatory diet post-diagnosis was associated with increased risk of all-cause mortality among ovarian cancer survivors with non-high grade serous histology. However, we did not observe statistically significant heterogeneity in the associations by histotype, possibly due to limited statistical power. Since some of the non high-grade serous histology (e.g. endometrioid, low grade serous) are more likely to be diagnosed at an earlier stage compared to high-grade serous ovarian cancer cases and therefore have better survival outcomes in general [36], exposure to pro-inflammatory diet after diagnosis may be more relevant to these ovarian cancer survivors and could increase their risk of death from other chronic diseases [27, 28]. It is also possible that factors that impact ovarian cancer progression may differ by histotype given the difference in etiology [37], although evidence is still limited on post-diagnosis factors associated with ovarian cancer survival. Only one study, the African-American Cancer Epidemiology Study (AACES) reported that pre-diagnosis exposure to greater pro-inflammatory diet being associated with increased risk of all-cause mortality among women with high-grade serous cancer [14]. Additionally in AACES, pre-diagnosis exposure to greater pro-inflammatory diet was positively associated with mortality among women who had ever reported cigarette smoking, but not among never smokers. In our data, we did not observe meaningful differences by cigarette smoking status for pre-diagnosis diet. However, we observed that post-diagnosis exposure to greater pro-inflammatory diet was positively associated with all-cause mortality among ever smokers but not for never smokers. We previously reported post-diagnosis smoking being associated with increased mortality compared to never smoking [38]. Smoking enhances progression and metastasis of ovarian cancer cells [39] and could also alter drug metabolism [40], leading to increased mortality among ovarian cancer survivors undergoing treatment. Thus, additional exposure to greater systemic pro-inflammation through post-diagnosis diet could accelerate tumor progression and thereby leading to worse prognosis. Furthermore, smoking and greater consumption of pro-inflammatory diet is associated with increased all-cause mortality [29, 41] and therefore ovarian cancer survivors exposed to both could be at greater risk of all-cause mortality compared to those who are exposed to neither.
Interestingly, when we examined change between pre- and post-diagnosis diet in relation to survival, ovarian cancer survivors who consistently consumed a higher pro-inflammatory dietary pattern had a greater risk of both all-cause and ovarian cancer-specific death versus those who consistently consumed an anti-inflammatory diet. One prior study examined change in dietary patterns, including AHEI, before and after ovarian cancer diagnosis and reported null associations [16]. However, this study did not examine pro-inflammatory dietary patterns and had participants recall pre-diagnosis diet. Our results on change in diet before and after diagnosis somewhat conflicts with our results on post-diagnosis pro-inflammatory diet and mortality, which could be due to a threshold effect. Another possible reason for this observation is that those survivors categorized as consuming lower (or higher) EDIP score diet at both pre- and post-diagnosis may have truly consumed a lower (or higher) pro-inflammatory diet at post-diagnosis since dietary patterns are generally consistent over time [42–44], and therefore the analysis on change in diet may have had less misclassification in the reference group. While replication is needed on larger, independent datasets, our results suggest limiting increase in the inflammatory potential of diet post-diagnosis may be beneficial for ovarian cancer survivors.
We previously reported that pro-inflammatory dietary pattern was not associated with ovarian cancer risk [45], however, in the current study we observed that post-diagnosis pro-inflammatory dietary patterns may be associated with mortality. Interestingly, studies have reported differences in factors associated with ovarian cancer risk and survival, which suggests that factors that impact carcinogenesis may be different from factors that stimulate cancer progression and possibly treatment resistance. Inflammation can stimulate ovarian cancer progression and treatment resistance. Pro-inflammatory cytokines such as TNF-α enhances ovarian cancer metastasis [46, 47] and IL-6 promotes chemoresistance in ovarian cancer [48]. Thus, it is plausible that post-diagnosis exposures to a more pro-inflammatory diet may lead to worse prognosis in women with ovarian cancer.
The reasons why we only observed significant observation for EDIP score and not for AHEI is not entirely clear. However, since the EDIP score was derived based on food groups most predictive of systemic inflammatory biomarkers, it is possible that EDIP score is capturing more precisely the dietary exposure that modulate systemic inflammation, which may be more relevant to progression and survival outcomes for ovarian cancer.
Major strengths of the present study include the prospective design, access to detailed, repeated information on diet and other covariates assessed prior to and after ovarian cancer diagnosis (minimizing the potential impact of change in diet due to preclinical symptoms and recall bias), long follow-up period, and availability of cause of death. We used cumulative average of dietary patterns as the pre-diagnosis exposure, using the repeated diet assessments rather than a single diet assessment, as this has been shown to reduce measurement error and reflect long-term diet [25]. Importantly, our study also had post-diagnosis diet information as well as detailed covariate information allowing us to examine the change in dietary patterns before and after ovarian cancer diagnosis in relation to mortality. However, due to the limited sample size in our study, we could not conduct a more detailed stratified analysis by individual histologic subtypes. It is likely that our analysis was limited with power to detect statistically significant associations especially in the stratified analyses. Our findings may have limited generalizability since all ovarian cancer survivors were healthcare professionals residing in the U.S. with the majority being white. While these results suggest adhering a less pro-inflammatory diet post-diagnosis may improve survival outcomes among ovarian cancer patients, validation of our results in larger, more diverse, independent studies is necessary.
In conclusion, our findings from two large prospective cohort studies provide evidence that pre-diagnosis inflammation-associated dietary patterns are not associated with mortality among ovarian cancer survivors. While the magnitude of associations observed are not large, which suggests that other factors, including clinical characteristics such as tumor histology and patient characteristics such as age, may have larger impact on survival outcomes compared to pre- or post-diagnosis diet, our results support that reducing pro-inflammatory diet among ovarian cancer survivors could have a small but positive impact on survival. If confirmed, these results suggest advising ovarian cancer survivors to adhere to an anti-inflammatory diet could lead to enhanced survivorship.
Supplementary information
Supplementary Table1, Supplementary Table2, Supplementary Table3, Supplementary Table4, Supplementary Table5, Supplementary Table6, Supplementary Table7, Supplementary Table8
Acknowledgements
We would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Author contributions
NS, KLT, SST, HRH contributed to the study concept and design. NS conducted the statistical analysis and drafted the initial manuscript. All authors contributed to the data interpretation and provided critical revisions of the final manuscript. All authors approved the final manuscript.
Funding
This work was supported in part by the National Institutes of Health (UM1 CA186107, P01 CA87969, U01 CA176726). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. NS was supported by the Rivkin Scientific Scholars Award and Department of Defense W81XWH2110320.
Data availability
The data generated in this study are not publicly available but are available upon request. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu).
Competing interests
SST received a grant from BMS that is unrelated to this work. The authors declare no competing interest.
Ethics approval and consent to participate
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Completion of the questionnaire implied informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01901-8.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA: A Cancer J Clinicians. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 3.Yi M, Li T, Niu M, Luo S, Chu Q, Wu K. Epidemiological trends of women’s cancers from 1990 to 2019 at the global, regional, and national levels: a population-based study. Biomark Res. 2021;9:55–55. doi: 10.1186/s40364-021-00310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2021. Atlanta: American Cancer Society; 2021.
- 5.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. JNCI. 1999;91:1459–67. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 6.Poole EM, Lee IM, Ridker PM, Buring JE, Hankinson SE, Tworoger SS. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor α receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256–64. doi: 10.1093/aje/kwt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peres LC, Mallen AR, Townsend MK, Poole EM, Trabert B, Allen NE, et al. High levels of C-reactive protein are associated with an increased risk of ovarian cancer: results from the ovarian cancer cohort consortium. Cancer Res. 2019;79:5442–51. doi: 10.1158/0008-5472.CAN-19-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savant, SS, Sriramkumar, S, O’Hagan, HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel). 2018;10. [DOI] [PMC free article] [PubMed]
- 9.Hefler LA, Concin N, Hofstetter G, Marth C, Mustea A, Sehouli J, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14:710–4. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Gu JH, Guo CS, Li XH, Yang WC. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival of epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Oncotarget. 2017;8:46414–24. doi: 10.18632/oncotarget.16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao XP, Liu YH, Liu ZY, Wang LJ, Jing CX, Zhu S, et al. Pretreatment lymphocyte-to-monocyte ratio as a predictor of survival among patients with ovarian cancer: a meta-analysis. Cancer Manag Res. 2019;11:1907–20. doi: 10.2147/CMAR.S184970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galland L. Diet and inflammation. Nutr Clin Pr. 2010;25:634–40. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 13.Thomson CA, Crane TE, Wertheim BC, Neuhouser ML, Li W, Snetselaar LG, et al. Diet quality and survival after ovarian cancer: results from the Women’s Health Initiative. J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed]
- 14.Peres LC, Hebert JR, Qin B, Guertin KA, Bandera EV, Shivappa N, et al. Prediagnostic proinflammatory dietary potential is associated with all-cause mortality among African-American women with high-grade serous ovarian carcinoma. J Nutr. 2019;149:1606–16. doi: 10.1093/jn/nxz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagle CM, Ibiebele T, Shivappa N, Hébert JR, DeFazio A, Webb PM. The association between the inflammatory potential of diet and risk of developing, and survival following, a diagnosis of ovarian cancer. Eur J Nutr. 2019;58:1747–56. doi: 10.1007/s00394-018-1779-x. [DOI] [PubMed] [Google Scholar]
- 16.Al Ramadhani RM, Nagle CM, Ibiebele TI, Grant P, Friedlander M, DeFazio A, et al. Pre- and post-diagnosis diet quality and ovarian cancer survival. Cancer Epidemiol Biomark Prev. 2021;30:229–32. doi: 10.1158/1055-9965.EPI-20-1036. [DOI] [PubMed] [Google Scholar]
- 17.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146:1560–70. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SX, Hodge AM, MacInnis RJ, Bassett JK, Ueland PM, Midttun Ø, et al. Inflammation-related marker profiling of dietary patterns and all-cause mortality in the Melbourne Collaborative Cohort Study. J Nutr. 2021;151:2908–16. doi: 10.1093/jn/nxab231. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 20.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Spiegelman D, et al. Physical activity and breast cancer risk in a cohort of young women. J Natl Cancer Inst. 1998;90:1155–60. doi: 10.1093/jnci/90.15.1155. [DOI] [PubMed] [Google Scholar]
- 21.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185:570–84. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, et al. Test of the national death index. Am J Epidemiol. 1984;119:837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 23.Tabung FK, Smith-Warner SA, Chavarro JE, Fung TT, Hu FB, Willett WC, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. 2017;147:1567–77. doi: 10.3945/jn.117.248377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee JJ, Mattei J, Hughes MD, Hu FB, Willett WC. Dietary diabetes risk reduction score, race and ethnicity, and risk of type 2 diabetes in women. Diabetes Care. 2015;38:596–603. doi: 10.2337/dc14-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merritt MA, Rice MS, Barnard ME, Hankinson SE, Matulonis UA, Poole EM, et al. Pre-diagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): a cohort study. Lancet Oncol. 2018;19:1107–16. doi: 10.1016/S1470-2045(18)30373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong X, Guo L, Zhang L, Li Y, He R, Cheng G. Inflammatory potential of diet and risk of cardiovascular disease or mortality: a meta-analysis. Sci Rep. 2017;7:6367. doi: 10.1038/s41598-017-06455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng FE, Shivappa N, Tang Y, Mann JR, Hebert JR. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: findings from NHANES III. Eur J Nutr. 2017;56:1085–93. doi: 10.1007/s00394-016-1158-4. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Arellano A, Martínez-González MA, Ramallal R, Salas-Salvadó J, Hébert JR, Corella D, et al. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin Nutr. 2019;38:1221–31. doi: 10.1016/j.clnu.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, et al. Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women’s Health Initiative. Br J Cancer. 2016;114:1277–85. doi: 10.1038/bjc.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zucchetto A, Gini A, Shivappa N, Hébert JR, Stocco C, Dal Maso L, et al. Dietary inflammatory index and prostate cancer survival. Int J Cancer. 2016;139:2398–404. doi: 10.1002/ijc.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan C, Morales-Oyarvide V, Khalaf N, Perez K, Tabung FK, Ho GYF, et al. Prediagnostic inflammation and pancreatic cancer survival. J Natl Cancer Inst. 2021;113:1186–93. doi: 10.1093/jnci/djab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Playdon MC, Nagle CM, Ibiebele TI, Ferrucci LM, Protani MM, Carter J, et al. Pre-diagnosis diet and survival after a diagnosis of ovarian cancer. Br J Cancer. 2017;116:1627–37. doi: 10.1038/bjc.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagle CM, Purdie DM, Webb PM, Green A, Harvey PW, Bain CJ. Dietary influences on survival after ovarian cancer. Int J Cancer. 2003;106:264–9. doi: 10.1002/ijc.11204. [DOI] [PubMed] [Google Scholar]
- 35.Dolecek TA, McCarthy BJ, Joslin CE, Peterson CE, Kim S, Freels SA, et al. Prediagnosis food patterns are associated with length of survival from epithelial ovarian cancer. J Am Diet Assoc. 2010;110:369–82. doi: 10.1016/j.jada.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Peres LC, Cushing-Haugen KL, Köbel M, Harris HR, Berchuck A, Rossing MA, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst. 2019;111:60–8. doi: 10.1093/jnci/djy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurman RJ, Shih Ie,M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer-shifting the paradigm. Hum Pathol. 2011;42:918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Townsend MK, Simmons V, Terry KL, Matulonis UA, Tworoger SS. Prediagnosis and postdiagnosis smoking and survival following diagnosis with ovarian cancer. Int J Cancer. 2020;147:736–46. doi: 10.1002/ijc.32773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon SY, Go RE, Heo JR, Kim CW, Hwang KA, Choi KC. Effects of cigarette smoke extracts on the progression and metastasis of human ovarian cancer cells via regulating epithelial-mesenchymal transition. Reprod Toxicol. 2016;65:1–10. doi: 10.1016/j.reprotox.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Kelemen LE, Warren GW, Koziak JM, Köbel M, Steed H. Smoking may modify the association between neoadjuvant chemotherapy and survival from ovarian cancer. Gynecol Oncol. 2016;140:124–30. doi: 10.1016/j.ygyno.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Müezzinler A, Mons U, Gellert C, Schöttker B, Jansen E, Kee F, et al. Smoking and all-cause mortality in older adults: results from the CHANCES Consortium. Am J Prev Med. 2015;49:e53–63. doi: 10.1016/j.amepre.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Maunsell E, Drolet M, Brisson J, Robert J, Deschênes L. Dietary change after breast cancer: extent, predictors, and relation with psychological distress. J Clin Oncol. 2002;20:1017–25. doi: 10.1200/JCO.2002.20.4.1017. [DOI] [PubMed] [Google Scholar]
- 43.Salminen EK, Lagström HK, Heikkilä S, Salminen S. Does breast cancer change patients’ dietary habits? Eur J Clin Nutr. 2000;54:844–8. doi: 10.1038/sj.ejcn.1601103. [DOI] [PubMed] [Google Scholar]
- 44.Harris HR, Bergkvist L, Wolk A. Folate intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Res Treat. 2012;132:243–50. doi: 10.1007/s10549-011-1838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabung FK, Huang T, Giovannucci EL, Smith-Warner SA, Tworoger SS, Poole EM. The inflammatory potential of diet and ovarian cancer risk: results from two prospective cohort studies. Br J Cancer. 2017;117:907–11. doi: 10.1038/bjc.2017.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau TS, Chan LK, Wong EC, Hui CW, Sneddon K, Cheung TH, et al. A loop of cancer-stroma-cancer interaction promotes peritoneal metastasis of ovarian cancer via TNFα-TGFα-EGFR. Oncogene. 2017;36:3576–87. doi: 10.1038/onc.2016.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355–62. doi: 10.1158/0008-5472.CAN-05-0957. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, et al. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295:110–23. doi: 10.1016/j.canlet.2010.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table1, Supplementary Table2, Supplementary Table3, Supplementary Table4, Supplementary Table5, Supplementary Table6, Supplementary Table7, Supplementary Table8
Data Availability Statement
The data generated in this study are not publicly available but are available upon request. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu).